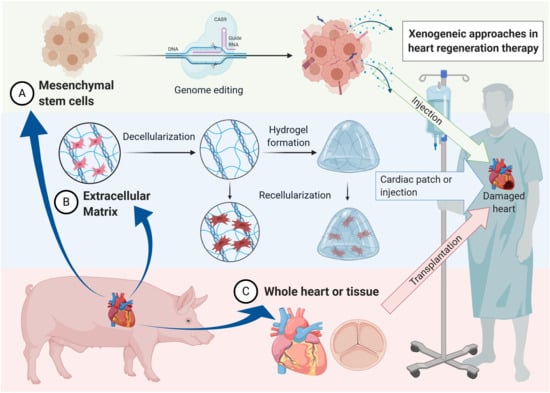
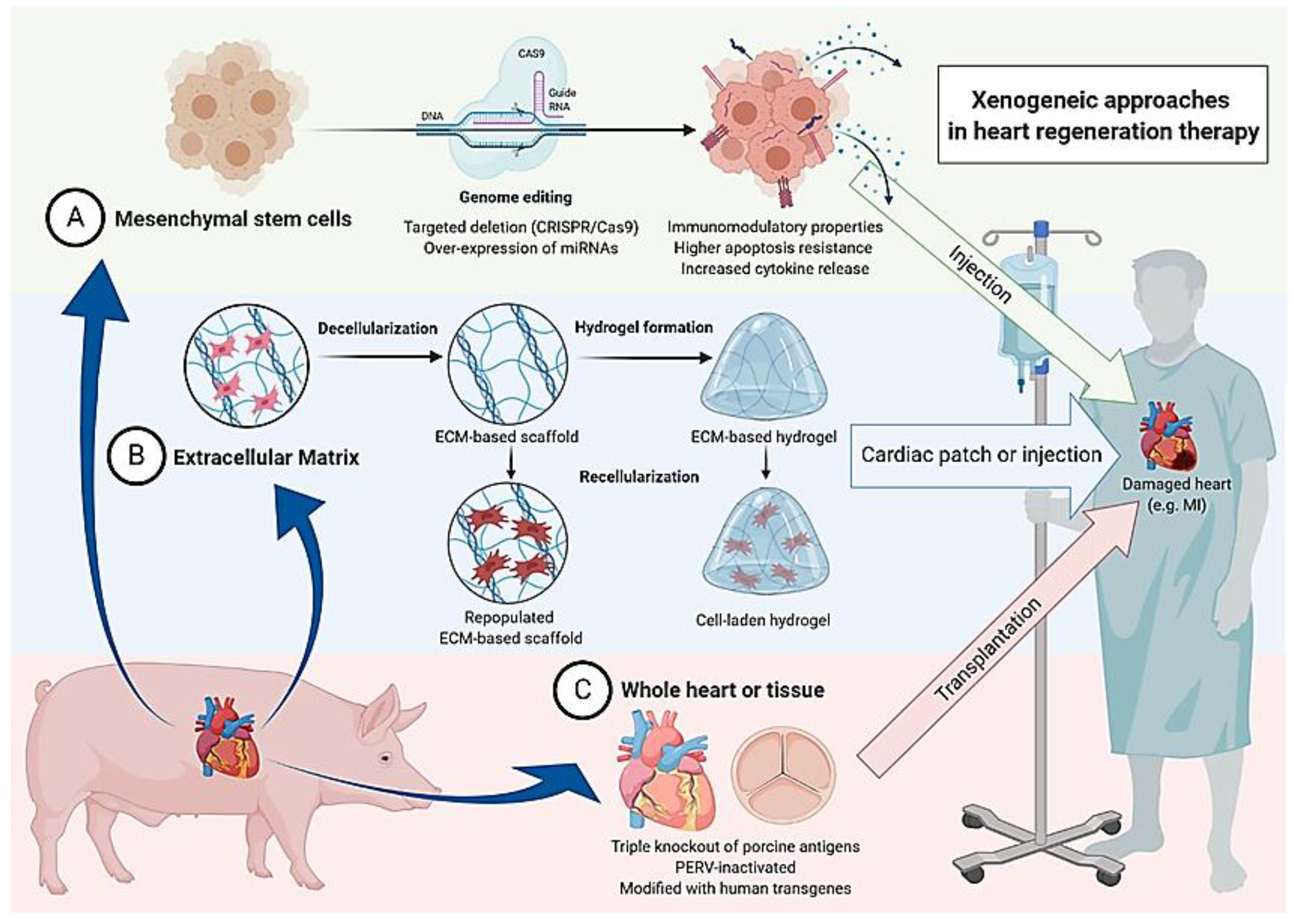
Xenogeneic and Stem Cell-Based Therapy for Cardiovascular Diseases: Genetic Engineering of Porcine Cells and Their Applications in Heart Regeneration
Abstract
1. Cardiac Wound Healing and the Road to Xenogeneic Cell Therapy
2. Overcoming the Immunological Barrier and Graft Rejection
2.1. Multilayered Immunological Challenges
2.2. Genetic Engineering to Overcome the Barriers
3. Infection Risks in Xenogeneic Cell Therapy
4. Stem Cells and Their Applications in Cardiovascular Regeneration
4.1. Porcine Mesenchymal Stem Cells as a Promising Candidate in Cardiac Regeneration
4.2. Delivery and Final Fate
4.3. Genetic Engineering to Improve Efficiency of Mesenchymal Stem Cells
5. Future Prospects for Xenogeneic Cell Therapy
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ANG-1 | angiopoietin 1 |
| ATMSC | adipose tissue-derived mesenchymal stem cell |
| ATSC | adipose tissue-derived stem cell |
| CDC | cardiosphere-derived cells |
| CRISPR | clustered regularly interspaced short palindromic repeat |
| ECM | extracellular matrix |
| ERK1/2 | extracellular signal-regulated kinase 1/2 |
| ESC | embryonal stem cell |
| FGFβ | fibroblast growth factor β |
| G-CSF | granulocyte colony-stimulating factor |
| GTKO | galactosyltransferase knockout |
| HEV | hepatitis E virus |
| HGF | hepatocyte growth factor |
| HLA | human leukocyte antigen |
| IGF-1 | Insulin-like growth factor 1 |
| IL-8 | interleukin-8 |
| iPSCs | induced pluripotent stem cells |
| MHC | major histocompatibility complex |
| MI | myocardial infarction |
| MSC | mesenchymal stromal cells |
| PCMV | porcine cytomegalovirus |
| PCV | porcine circovirus |
| PDGF | platelet-derived growth factor |
| PD-L1 | programmed cell death ligand 1 |
| PERV | porcine endogenous retroviruses |
| PI3K | phosphoinositide 3-kinase |
| ROS | reactive oxygen species |
| SDF-1 | stromal cell-derived factor 1 |
| SLA | swine leukocyte antigen |
| TALEN | transcription activator-like effector nucleases |
| TGFβ | transforming growth factor β |
| TKO | triple knockout |
| TLR4 | toll-like receptor 4 |
| VEGF | vascular endothelial growth factor |
References
- Czubryt, M.P. Common threads in cardiac fibrosis, infarct scar formation, and wound healing. Fibrogenesis Tissue Repair 2012, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Schächinger, V.; Erbs, S.; Elsässer, A.; Haberbosch, W.; Hambrecht, R.; Hölschermann, H.; Yu, J.; Corti, R.; Mathey, D.G.; Hamm, C.W.; et al. Intracoronary bone marrow–derived progenitor cells in acute myocardial infarction. N. Engl. J. Med. 2006, 355, 1210–1221. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Fang, W.; Ye, F.; Liu, Y.-H.; Qian, J.; Shan, S.; Zhang, J.; Chunhua, R.Z.; Liao, L.; Lin, S.; et al. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am. J. Cardiol. 2004, 94, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Makkar, R.R.; Smith, R.R.; Cheng, K.; Malliaras, K.; Thomson, L.E.J.; Berman, D.; Czer, L.S.C.; Marbán, L.; Mendizabal, A.; Johnston, P.V.; et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): A prospective, randomised phase 1 trial. Lancet 2012, 379, 895–904. [Google Scholar] [CrossRef]
- Chugh, A.R.; Beache, G.; Loughran, J.H.; Mewton, N.; Elmore, J.B.; Kajstura, J.; Pappas, P.; Tatooles, A.; Stoddard, M.F.; Lima, J.A.C.; et al. Administration of cardiac stem cells in patients with ischemic cardiomyopathy (the SCIPIO Trial): Surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation 2012, 126, S54–S64. [Google Scholar] [CrossRef]
- Malik, N. Allogeneic Versus Autologous Stem-Cell Therapy; BioPharm International: San Mateo, CA, USA, 2012; Volume 25. [Google Scholar]
- Lelovas, P.P.; Kostomitsopoulos, N.G.; Xanthos, T.T. A comparative anatomic and physiologic overview of the porcine heart. J. Am. Assoc. Lab. Anim. Sci. 2014, 53, 7. [Google Scholar]
- Liu, X.; Federlin, K.F.; Bretzel, R.G.; Hering, B.J.; Brendel, M.D. Persistent reversal of diabetes by transplantation of fetal pig proislets into nude mice. Diabetes 1991, 40, 858–866. [Google Scholar] [CrossRef]
- Tze, W.J.; Tai, J.; Cheung, S.S.; Bissada, N.; Tsang, A. A diabetic rabbit model for pig islet xenotransplantation. Transplantation 1993, 56, 1348–1352. [Google Scholar] [CrossRef]
- Groth, C.G.; Korsgren, O.; Tibell, A.; Tollemar, J.; Möller, E.; Bolinder, J.; Ostman, J.; Reinholt, F.P.; Hellerström, C.; Andersson, A. Transplantation of porcine fetal pancreas to diabetic patients. Lancet Lond. Engl. 1994, 344, 1402–1404. [Google Scholar] [CrossRef]
- Kordower, J.H.; Freeman, T.B.; Snow, B.J.; Vingerhoets, F.J.; Mufson, E.J.; Sanberg, P.R.; Hauser, R.A.; Smith, D.A.; Nauert, G.M.; Perl, D.P. Neuropathological evidence of graft survival and striatal reinnervation after the transplantation of fetal mesencephalic tissue in a patient with Parkinson’s disease. N. Engl. J. Med. 1995, 332, 1118–1124. [Google Scholar] [CrossRef]
- Isacson, O.; Deacon, T.W.; Pakzaban, P.; Galpern, W.R.; Dinsmore, J.; Burns, L.H. Transplanted xenogeneic neural cells in neurodegenerative disease models exhibit remarkable axonal target specificity and distinct growth patterns of glial and axonal fibres. Nat. Med. 1995, 1, 1189–1194. [Google Scholar] [CrossRef]
- Valdés-González, R.A.; Dorantes, L.M.; Garibay, G.N.; Bracho-Blanchet, E.; Mendez, A.J.; Dávila-Pérez, R.; Elliott, R.B.; Terán, L.; White, D.J.G. Xenotransplantation of porcine neonatal islets of Langerhans and Sertoli cells: A 4-year study. Eur. J. Endocrinol. 2005, 153, 419–427. [Google Scholar] [CrossRef]
- Wang, W.; Mo, Z.; Ye, B.; Hu, P.; Liu, S.; Yi, S. A clinical trial of xenotransplantation of neonatal pig islets for diabetic patients. J. Cent. South Univ. Med Ed. 2011, 36, 1134–1140. [Google Scholar] [CrossRef]
- Valdes-Gonzalez, R.; Rodriguez-Ventura, A.L.; White, D.J.G.; Bracho-Blanchet, E.; Castillo, A.; Ramírez-González, B.; López-Santos, M.G.; León-Mancilla, B.H.; Dorantes, L.M. Long-term follow-up of patients with type 1 diabetes transplanted with neonatal pig islets. Clin. Exp. Immunol. 2010, 162, 537–542. [Google Scholar] [CrossRef]
- Zhu, H.; Lu, L.; Liu, X.; Yu, L.; Lyu, Y.; Wang, B. Treatment of diabetes with encapsulated pig islets: An update on current developments. J. Zhejiang Univ. Sci. B 2015, 16, 329–343. [Google Scholar] [CrossRef]
- Galili, U. Evolution of α1,3Galactosyltransferase and of the α-Gal Epitope. In α-Gal and Anti-Gal: α1,3-Galactosyltransferase, α-Gal Epitopes, and the Natural Anti-Gal Antibody Subcellular Biochemistry; Subcellular Biochemistry; Galili, U., Avila, J.L., Eds.; Springer: Boston, MA, USA, 1999; pp. 1–23. ISBN 978-1-4615-4771-6. [Google Scholar]
- Galili, U. Interaction of the natural anti-Gal antibody with α-galactosyl epitopes: A major obstacle for xenotransplantation in humans. Immunol. Today 1993, 14, 480–482. [Google Scholar] [CrossRef]
- Lexer, G.; Cooper, D.; Rose, A.G.; Wicomb, W.N.; Rees, J.; Keraan, M.; Du Toit, E. Hyperacute rejection in a discordant (pig to baboon) cardiac xenograft model. J. Heart Transpl. 1986, 5, 411–418. [Google Scholar]
- Kobayashi, T.; Cooper, D.K. Anti-Gal, alpha-Gal epitopes, and xenotransplantation. Subcell. Biochem. 1999, 32, 229–257. [Google Scholar] [CrossRef]
- Bannett, A.D.; McAlack, R.F.; Raja, R.; Baquero, A.; Morris, M. Experiences with known ABO-mismatched renal transplants. Transpl. Proc. 1987, 19, 4543–4546. [Google Scholar]
- Taniguchi, S.; Neethling, F.A.; Korchagina, E.Y.; Bovin, N.; Ye, Y.; Kobayashi, T.; Niekrasz, M.; Li, S.; Koren, E.; Oriol, R.; et al. In vivo immunoadsorption of antipig antibodies in baboons using a specific Gal(alpha)1-3Gal column. Transplantation 1996, 62, 1379–1384. [Google Scholar] [CrossRef]
- Houser, S.L.; Kuwaki, K.; Knosalla, C.; Dor, F.J.M.F.; Gollackner, B.; Cheng, J.; Shimizu, A.; Schuurman, H.-J.; Cooper, D.K.C. Thrombotic microangiopathy and graft arteriopathy in pig hearts following transplantation into baboons. Xenotransplantation 2004, 11, 416–425. [Google Scholar] [CrossRef]
- Bühler, L.; Basker, M.; Alwayn, I.P.; Goepfert, C.; Kitamura, H.; Kawai, T.; Gojo, S.; Kozlowski, T.; Ierino, F.L.; Awwad, M.; et al. Coagulation and thrombotic disorders associated with pig organ and hematopoietic cell transplantation in nonhuman primates. Transplantation 2000, 70, 1323–1331. [Google Scholar] [CrossRef]
- Yamada, K.; Sachs, D.H.; DerSimonian, H. Human anti-porcine xenogeneic T cell response. Evidence for allelic specificity of mixed leukocyte reaction and for both direct and indirect pathways of recognition. J. Immunol. 1995, 155, 5249–5256. [Google Scholar]
- Murray, A.G.; Khodedoust, M.M.; Pober, J.S.; Bothwell, A.L.M. Porcine aortic endothelial cells activate human T cells: Direct presentation of MHC antigens and costimulation by ligands for human CD2 and CD28. Immunity 1994, 1, 57–63. [Google Scholar] [CrossRef]
- Dorling, A.; Lombardi, G.; Binns, R.; Lechler, R.I. Detection of primary direct and indirect human anti-porcine T cell responses using a porcine dendritic cell population. Eur. J. Immunol. 1996, 26, 1378–1387. [Google Scholar] [CrossRef]
- Kirk, A.D.; Harlan, D.M.; Armstrong, N.N.; Davis, T.A.; Dong, Y.; Gray, G.S.; Hong, X.; Thomas, D.; Fechner, J.H.; Knechtle, S.J. CTLA4-Ig and anti-CD40 ligand prevent renal allograft rejection in primates. Proc. Natl. Acad. Sci. USA 1997, 94, 8789–8794. [Google Scholar] [CrossRef]
- Iwase, H.; Ekser, B.; Satyananda, V.; Bhama, J.; Hara, H.; Ezzelarab, M.; Klein, E.; Wagner, R.; Long, C.; Thacker, J.; et al. Pig-to-baboon heterotopic heart transplantation--exploratory preliminary experience with pigs transgenic for human thrombomodulin and comparison of three costimulation blockade-based regimens. Xenotransplantation 2015, 22, 211–220. [Google Scholar] [CrossRef]
- Lin, H.; Bolling, S.F.; Linsley, P.S.; Wei, R.Q.; Gordon, D.; Thompson, C.B.; Turka, L.A. Long-term acceptance of major histocompatibility complex mismatched cardiac allografts induced by CTLA4Ig plus donor-specific transfusion. J. Exp. Med. 1993, 178, 1801–1806. [Google Scholar] [CrossRef]
- Knosalla, C.; Ryan, D.J.J.; Moran, K.; Gollackner, B.; Schuler, W.; Sachs, D.H.; Awwad, M.; Schuurman, H.-J.; Cooper, D.K.C. Initial experience with the human anti-human CD154 monoclonal antibody, ABI793, in pig-to-baboon xenotransplantation. Xenotransplantation 2004, 11, 353–360. [Google Scholar] [CrossRef]
- Elwood, E.T.; Larsen, C.P.; Cho, H.R.; Corbascio, M.; Ritchie, S.C.; Alexander, D.Z.; Tucker-Burden, C.; Linsley, P.S.; Aruffo, A.; Hollenbaugh, D.; et al. Prolonged acceptance of concordant and discordant xenografts with combined CD40 and C28 pathway blockade. Transplantation 1998, 65, 1422–1428. [Google Scholar] [CrossRef]
- Lehnert, A.M.; Mottram, P.L.; Han, W.; Walters, S.N.; Patel, A.T.; Hawthorne, W.J.; Cowan, P.J.; d’Apice, A.J.F.; O’Connell, P.J. Blockade of the CD28 and CD40 pathways result in the acceptance of pig and rat islet xenografts but not rat cardiac grafts in mice. Transpl. Immunol. 2001, 9, 51–56. [Google Scholar] [CrossRef]
- Wu, G.; Pfeiffer, S.; Schröder, C.; Zhang, T.; Nguyen, B.N.; Lea, W.; Kelishadi, S.; Atkinson, J.B.; Schuurman, H.-J.; White, D.J.G.; et al. Co-stimulation blockade targeting CD154 and CD28/B7 modulates the induced antibody response after a pig-to-baboon cardiac xenograft. Xenotransplantation 2005, 12, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Dalmasso, A.P.; Vercellotti, G.M.; Platt, J.L.; Bach, F.H. Inhibition of complement-mediated endothelial cell cytotoxicity by decay-accelerating factor. Potential for prevention of xenograft hyperacute rejection. Transplantation 1991, 52, 530–533. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.H.; Kadner, A.; Chen, R.H.; Farivar, R.S. Human membrane cofactor protein (MCP, CD 46) protects transgenic pig hearts from hyperacute rejection in primates. Xenotransplantation 2001, 8, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Phelps, C.J.; Koike, C.; Vaught, T.D.; Boone, J.; Wells, K.D.; Chen, S.-H.; Ball, S.; Specht, S.M.; Polejaeva, I.A.; Monahan, J.A.; et al. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science 2003, 299, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Kolber-Simonds, D.; Lai, L.; Watt, S.R.; Denaro, M.; Arn, S.; Augenstein, M.L.; Betthauser, J.; Carter, D.B.; Greenstein, J.L.; Hao, Y.; et al. Production of -1,3-galactosyltransferase null pigs by means of nuclear transfer with fibroblasts bearing loss of heterozygosity mutations. Proc. Natl. Acad. Sci. USA 2004, 101, 7335–7340. [Google Scholar] [CrossRef] [PubMed]
- Kuwaki, K.; Tseng, Y.-L.; Dor, F.J.M.F.; Shimizu, A.; Houser, S.L.; Sanderson, T.M.; Lancos, C.J.; Prabharasuth, D.D.; Cheng, J.; Moran, K.; et al. Heart transplantation in baboons using α1,3-galactosyltransferase gene-knockout pigs as donors: Initial experience. Nat. Med. 2005, 11, 29–31. [Google Scholar] [CrossRef]
- Ezzelarab, M.; Garcia, B.; Azimzadeh, A.; Sun, H.; Lin, C.C.; Hara, H.; Kelishadi, S.; Zhang, T.; Lin, Y.J.; Tai, H.-C.; et al. The innate immune response and activation of coagulation in α1,3-galactosyltransferase gene-knockout xenograft recipients. Transplantation 2009, 87, 805–812. [Google Scholar] [CrossRef]
- Hara, H.; Long, C.; Lin, Y.J.; Tai, H.-C.; Ezzelarab, M.; Ayares, D.; Cooper, D.K.C. In vitro investigation of pig cells for resistance to human antibody-mediated rejection. Transpl. Int. Off. J. Eur. Soc. Organ Transpl. 2008, 21, 1163–1174. [Google Scholar] [CrossRef]
- McGregor, C.G.A.; Ricci, D.; Miyagi, N.; Stalboerger, P.G.; Du, Z.; Oehler, E.A.; Tazelaar, H.D.; Byrne, G.W. Human CD55 expression blocks hyperacute rejection and restricts complement activation in gal knockout cardiac xenografts. Transplantation 2012, 93, 686–692. [Google Scholar] [CrossRef]
- Padler-Karavani, V.; Varki, A. Potential impact of the non-human sialic acid n-glycolylneuraminic acid on transplant rejection risk. Xenotransplantation 2011, 18, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Lutz, A.J.; Li, P.; Estrada, J.L.; Sidner, R.A.; Chihara, R.K.; Downey, S.M.; Burlak, C.; Wang, Z.-Y.; Reyes, L.M.; Ivary, B.; et al. Double knockout pigs deficient in N-glycolylneuraminic acid and galactose α-1,3-galactose reduce the humoral barrier to xenotransplantation. Xenotransplantation 2013, 20, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Estrada, J.; Martens, G.; Li, P.; Adams, A.; Newell, K.; Ford, M.; Butler, J.; Sidner, R.; Tector, M.; Tector, A. Evaluation of human and nonhuman primate antibody binding to pig cells lacking GGTA1/CMAH/β4GalNT2 genes. Xenotransplantation 2015, 22, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Phelps, C.J.; Ball, S.F.; Vaught, T.D.; Vance, A.M.; Mendicino, M.; Monahan, J.A.; Walters, A.H.; Wells, K.D.; Dandro, A.S.; Ramsoondar, J.J.; et al. Production and characterization of transgenic pigs expressing porcine CTLA4-Ig. Xenotransplantation 2009. [Google Scholar] [CrossRef]
- Hara, H.; Witt, W.; Crossley, T.; Long, C.; Isse, K.; Fan, L.; Phelps, C.J.; Ayares, D.; Cooper, D.K.C.; Dai, Y.; et al. Human dominant-negative class II transactivator transgenic pigs – effect on the human anti-pig T-cell immune response and immune status. Immunology 2013, 140, 39–46. [Google Scholar] [CrossRef]
- Plege, A.; Borns, K.; Baars, W.; Schwinzer, R. Suppression of human T-cell activation and expansion of regulatory T cells by pig cells overexpressing PD-ligands. Transplantation 2009, 87, 975–982. [Google Scholar] [CrossRef]
- Ding, Q.; Lu, L.; Zhou, X.; Zhou, Y.; Chou, K.-Y. Human PD-L1-overexpressing porcine vascular endothelial cells induce functionally suppressive human CD4+CD25hiFoxp3+ Treg cells. J. Leukoc. Biol. 2011, 90, 77–86. [Google Scholar] [CrossRef]
- Lin, Y.J.; Hara, H.; Tai, H.-C.; Long, C.; Tokita, D.; Yeh, P.; Ayares, D.; Morelli, A.E.; Cooper, D.K.C. Suppresive efficacy and proliferative capacity of human regulatory t cells in allogeneic and xenogeneic responses. Transplantation 2008, 86, 1452–1462. [Google Scholar] [CrossRef]
- Li, J.; Ezzelarab, M.B.; Ayares, D.; Cooper, D.K.C. The potential role of genetically-modified pig mesenchymal stromal cells in xenotransplantation. Stem Cell Rev. 2014, 10, 79–85. [Google Scholar] [CrossRef]
- Mohiuddin, M.M.; Singh, A.K.; Corcoran, P.C.; Hoyt, R.F.; Thomas, M.L.; Ayares, D.; Horvath, K.A. Genetically engineered pigs and target-specific immunomodulation provide significant graft survival and hope for clinical cardiac xenotransplantation. J. Thorac. Cardiovasc. Surg. 2014, 148, 1106–1113. [Google Scholar] [CrossRef]
- Lin, C.; Ezzelarab, M.; Hara, H.; Long, C.; Lin, C.; Dorling, A.; Cooper, D. Atorvastatin or transgenic expression of tfpi inhibits coagulation initiated by anti-nongal igg binding to porcine aortic endothelial cells. J. Thromb. Haemost. JTH 2010, 8, 2001–2010. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.K.C.; Ezzelarab, M.; Iwase, H.; Hara, H. Perspectives on the optimal genetically-engineered pig in 2018 for initial clinical trials of kidney or heart xenotransplantation. Transplantation 2018, 102, 1974–1982. [Google Scholar] [CrossRef]
- Längin, M.; Mayr, T.; Reichart, B.; Michel, S.; Buchholz, S.; Guethoff, S.; Dashkevich, A.; Baehr, A.; Egerer, S.; Bauer, A.; et al. Consistent success in life-supporting porcine cardiac xenotransplantation. Nature 2018, 564, 430–433. [Google Scholar] [CrossRef] [PubMed]
- Reichart, B.; Längin, M.; Radan, J.; Mokelke, M.; Buttgereit, I.; Ying, J.; Fresch, A.K.; Mayr, T.; Issl, L.; Buchholz, S.; et al. Pig-to-non-human primate heart transplantation: The final step toward clinical xenotransplantation? J. Heart Lung Transpl. 2020, 39, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Bach, F.H.; Fishman, J.A.; Daniels, N.; Proimos, J.; Anderson, B.; Carpenter, C.B.; Forrow, L.; Robson, S.C.; Fineberg, H.V. Uncertainty in xenotransplantation: Individual benefit versus collective risk. Nat. Med. 1998, 4, 141–144. [Google Scholar] [CrossRef]
- Weiss, R.A. Transgenic pigs and virus adaptation. Nature 1998, 391, 327–328. [Google Scholar] [CrossRef]
- Fishman, J.A. Prevention of infection in xenotransplantation: Designated pathogen-free swine in the safety equation. Xenotransplantation 2020, 27, e12595. [Google Scholar] [CrossRef]
- Denner, J. Sensitive detection systems for infectious agents in xenotransplantation. Xenotransplantation 2020, e12594. [Google Scholar] [CrossRef]
- Lu, L.; Li, C.; Hagedorn, C.H. Phylogenetic analysis of global hepatitis E virus sequences: Genetic diversity, subtypes and zoonosis. Rev. Med. Virol. 2006, 16, 5–36. [Google Scholar] [CrossRef]
- Denner, J. Xenotransplantation and hepatitis E virus. Xenotransplantation 2015, 22, 167–173. [Google Scholar] [CrossRef]
- Ramanan, P.; Razonable, R.R. Cytomegalovirus infections in solid organ transplantation: A review. Infect. Chemother. 2013, 45, 260–271. [Google Scholar] [CrossRef]
- Denner, J.; Längin, M.; Reichart, B.; Krüger, L.; Fiebig, U.; Mokelke, M.; Radan, J.; Mayr, T.; Milusev, A.; Luther, F.; et al. Impact of porcine cytomegalovirus on long-term orthotopic cardiac xenotransplant survival. bioRxiv 2020. [Google Scholar] [CrossRef]
- Krüger, L.; Längin, M.; Reichart, B.; Fiebig, U.; Kristiansen, Y.; Prinz, C.; Kessler, B.; Egerer, S.; Wolf, E.; Abicht, J.-M.; et al. Transmission of porcine circovirus 3 (PCV3) by xenotransplantation of pig hearts into baboons. Viruses 2019, 11, 650. [Google Scholar] [CrossRef]
- Denner, J. How active are porcine endogenous retroviruses (PERVs)? Viruses 2016, 8, 215. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Patience, C.; Magre, S.; Weiss, R.A.; Banerjee, P.T.; Le Tissier, P.; Stoye, J.P. Host range and interference studies of three classes of pig endogenous retrovirus. J. Virol. 1998, 72, 9986–9991. [Google Scholar] [CrossRef]
- Le Tissier, P.; Stoye, J.P.; Takeuchi, Y.; Patience, C.; Weiss, R.A. Two sets of human-tropic pig retrovirus. Nature 1997, 389, 681–682. [Google Scholar] [CrossRef]
- Martin, U.; Winkler, M.E.; Id, M.; Radeke, H.; Arseniev, L.; Takeuchi, Y.; Simon, A.R.; Patience, C.; Haverich, A.; Steinhoff, G. Productive infection of primary human endothelial cells by pig endogenous retrovirus (PERV). Xenotransplantation 2000, 7, 138–142. [Google Scholar] [CrossRef]
- Yang, L.; Güell, M.; Niu, D.; George, H.; Lesha, E.; Grishin, D.; Aach, J.; Shrock, E.; Xu, W.; Poci, J.; et al. Genome-wide inactivation of porcine endogenous retroviruses (PERVs). Science 2015, 350, 1101–1104. [Google Scholar] [CrossRef]
- Niu, D.; Wei, H.-J.; Lin, L.; George, H.; Wang, T.; Lee, I.-H.; Zhao, H.-Y.; Wang, Y.; Kan, Y.; Shrock, E.; et al. Inactivation of porcine endogenous retrovirus in pigs using CRISPR-Cas9. Science 2017, 357, 1303–1307. [Google Scholar] [CrossRef]
- Blum, B.; Benvenisty, N. The tumorigenicity of human embryonic stem cells. Adv. Cancer Res. 2008, 100, 133–158. [Google Scholar] [CrossRef]
- Beltrami, A.P.; Barlucchi, L.; Torella, D.; Baker, M.; Limana, F.; Chimenti, S.; Kasahara, H.; Rota, M.; Musso, E.; Urbanek, K.; et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 2003, 114, 763–776. [Google Scholar] [CrossRef]
- Marcello, R.; Padin-Iruegas, M.E.; Yu, M.; Antonella, D.A.; Silvia, M.; João, F.; Emanuela, F.; Raffaella, R.; Arcarese Michael, L.; Mitchell Thomas, S.; et al. Local activation or implantation of cardiac progenitor cells rescues scarred infarcted myocardium improving cardiac function. Circ. Res. 2008, 103, 107–116. [Google Scholar] [CrossRef]
- Orlic, D.; Kajstura, J.; Chimenti, S.; Jakoniuk, I.; Anderson, S.M.; Li, B.; Pickel, J.; McKay, R.; Nadal-Ginard, B.; Bodine, D.M.; et al. Bone marrow cells regenerate infarcted myocardium. Nature 2001, 410, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Rota, M.; Kajstura, J.; Hosoda, T.; Bearzi, C.; Vitale, S.; Esposito, G.; Iaffaldano, G.; Padin-Iruegas, M.E.; Gonzalez, A.; Rizzi, R.; et al. Bone marrow cells adopt the cardiomyogenic fate in vivo. Proc. Natl. Acad. Sci. USA 2007, 104, 17783–17788. [Google Scholar] [CrossRef]
- Jianhua, Z.; Wilson, G.F.; Soerens, A.G.; Koonce, C.H.; Junying, Y.; Palecek, S.P.; Thomson, J.A.; Kamp, T.J. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ. Res. 2009, 104, e30–e41. [Google Scholar] [CrossRef]
- Zhu, W.; Zhao, M.; Mattapally, S.; Chen, S.; Zhang, J. CCND2 overexpression enhances the regenerative potency of human induced pluripotent stem cell–derived cardiomyocytes: remuscularization of injured ventricle. Circ. Res. 2018, 122, 88–96. [Google Scholar] [CrossRef]
- Hoashi, T.; Matsumiya, G.; Miyagawa, S.; Ichikawa, H.; Ueno, T.; Ono, M.; Saito, A.; Shimizu, T.; Okano, T.; Kawaguchi, N.; et al. Skeletal myoblast sheet transplantation improves the diastolic function of a pressure-overloaded right heart. J. Thorac. Cardiovasc. Surg. 2009, 138, 460–467. [Google Scholar] [CrossRef][Green Version]
- Haider, H.K.; Tan, A.C.K.; Aziz, S.; Chachques, J.C.; Sim, E.K.W. Myoblast transplantation for cardiac repair: A clinical perspective. Mol. J. Am. Soc. Gene 2004, 9, 14–23. [Google Scholar] [CrossRef]
- Wollert, K.C.; Helmut, D. Clinical applications of stem cells for the heart. Circ. Res. 2005, 96, 151–163. [Google Scholar] [CrossRef]
- Duelen, R.; Sampaolesi, M. Stem cell technology in cardiac regeneration: A pluripotent stem cell promise. EBioMedicine 2017, 16, 30–40. [Google Scholar] [CrossRef]
- Rikhtegar, R.; Pezeshkian, M.; Dolati, S.; Safaie, N.; Afrasiabi Rad, A.; Mahdipour, M.; Nouri, M.; Jodati, A.R.; Yousefi, M. Stem cells as therapy for heart disease: IPSCs, ESCs, CSCs, and skeletal myoblasts. Biomed. Pharm. 2019, 109, 304–313. [Google Scholar] [CrossRef]
- Piero, A.; Jan, K.; Annarosa, L.; Roberto, B. Life and death of cardiac stem cells. Circulation 2006, 113, 1451–1463. [Google Scholar] [CrossRef]
- Kucia, M.; Dawn, B.; Hunt, G.; Guo, Y.; Wysoczynski, M.; Majka, M.; Ratajczak, J.; Rezzoug, F.; Ildstad, S.T.; Bolli, R.; et al. Cells expressing early cardiac markers reside in the bone marrow and are mobilized into the peripheral blood after myocardial infarction. Circ. Res. 2004, 95, 1191–1199. [Google Scholar] [CrossRef]
- Murry, C.E.; Soonpaa, M.H.; Reinecke, H.; Nakajima, H.; Nakajima, H.O.; Rubart, M.; Pasumarthi, K.B.S.; Ismail Virag, J.; Bartelmez, S.H.; Poppa, V.; et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature 2004, 428, 664–668. [Google Scholar] [CrossRef]
- Van Berlo, J.H.; Kanisicak, O.; Maillet, M.; Vagnozzi, R.J.; Karch, J.; Lin, S.-C.J.; Middleton, R.C.; Marbán, E.; Molkentin, J.D. C-kit+ cells minimally contribute cardiomyocytes to the heart. Nature 2014, 509, 337–341. [Google Scholar] [CrossRef]
- Nguyen, P.K.; Rhee, J.-W.; Wu, J.C. Adult stem cell therapy and heart failure, 2000 to 2016: A systematic review. JAMA Cardiol. 2016, 1, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Mirotsou, M.; Jayawardena, T.M.; Schmeckpeper, J.; Gnecchi, M.; Dzau, V.J. Paracrine mechanisms of stem cell reparative and regenerative actions in the heart. J. Mol. Cell. Cardiol. 2011, 50, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Li, T.-S.; Cheng, K.; Malliaras, K.; Smith, R.R.; Zhang, Y.; Sun, B.; Matsushita, N.; Blusztajn, A.; Terrovitis, J.; Kusuoka, H.; et al. Direct comparison of different stem cell types and subpopulations reveals superior paracrine potency and myocardial repair efficacy with cardiosphere-derived cells. J. Am. Coll. Cardiol. 2012, 59, 942–953. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Hass, R.; Kasper, C.; Böhm, S.; Jacobs, R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun. Signal. 2011, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Monsanto, M.M.; White, K.S.; Kim, T.; Wang, B.J.; Fisher, K.; Ilves, K.; Khalafalla, F.G.; Casillas, A.; Broughton, K.; Mohsin, S.; et al. Concurrent isolation of 3 distinct cardiac stem cell populations from a single human heart biopsy. Circ. Res. 2017, 121, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Hare, J.M.; Fishman, J.E.; Gerstenblith, G.; DiFede Velazquez, D.L.; Zambrano, J.P.; Suncion, V.Y.; Tracy, M.; Ghersin, E.; Johnston, P.V.; Brinker, J.A.; et al. Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy. JAMA 2012, 308, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ezzelarab, M.B.; Cooper, D.K.C. Do mesenchymal stem cells function across species barriers? Relevance for xenotransplantation. Xenotransplantation 2012, 19, 273–285. [Google Scholar] [CrossRef]
- Medicetty, S.; Bledsoe, A.R.; Fahrenholtz, C.B.; Troyer, D.; Weiss, M.L. Transplantation of pig stem cells into rat brain: Proliferation during the first 8 weeks. Exp. Neurol. 2004, 190, 32–41. [Google Scholar] [CrossRef]
- Eguchi, H.; Kuroiwa, Y.; Matsui, A.; Sada, M.; Nagaya, N.; Kawano, S. Intra-bone marrow cotransplantation of donor mesenchymal stem cells in pig-to-NOD/SCID mouse bone marrow transplantation facilitates short-term xenogeneic hematopoietic engraftment. Transpl. Proc. 2008, 40, 574–577. [Google Scholar] [CrossRef]
- Nakamura, Y.; Wang, X.; Xu, C.; Asakura, A.; Yoshiyama, M.; From, A.H.L.; Zhang, J. Xenotransplantation of long-term-cultured swine bone marrow-derived mesenchymal stem cells. Stem Cells Dayt. Ohio 2007, 25, 612–620. [Google Scholar] [CrossRef]
- Bharti, D.; Shivakumar, S.B.; Subbarao, R.B.; Rho, G.-J. Research advancements in porcine derived mesenchymal stem cells. Curr. Stem Cell Res. Ther. 2016, 11, 78–93. [Google Scholar] [CrossRef]
- Karpov, A.A.; Udalova, D.V.; Pliss, M.G.; Galagudza, M.M. Can the outcomes of mesenchymal stem cell-based therapy for myocardial infarction be improved? Providing weapons and armour to cells. Cell Prolif. 2016, 50. [Google Scholar] [CrossRef]
- Bagno, L.; Hatzistergos, K.E.; Balkan, W.; Hare, J.M. Mesenchymal stem cell-based therapy for cardiovascular disease: Progress and challenges. Mol. Ther. 2018, 26, 1610–1623. [Google Scholar] [CrossRef]
- Moscoso, I.; Centeno, A.; López, E.; Rodriguez-Barbosa, J.I.; Santamarina, I.; Filgueira, P.; Sánchez, M.J.; Domínguez-Perles, R.; Peñuelas-Rivas, G.; Domenech, N. Differentiation “in vitro” of primary and immortalized porcine mesenchymal stem cells into cardiomyocytes for cell transplantation. Transpl. Proc. 2005, 37, 481–482. [Google Scholar] [CrossRef]
- Quevedo, H.C.; Hatzistergos, K.E.; Oskouei, B.N.; Feigenbaum, G.S.; Rodriguez, J.E.; Valdes, D.; Pattany, P.M.; Zambrano, J.P.; Hu, Q.; McNiece, I.; et al. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc. Natl. Acad. Sci. USA 2009, 106, 14022–14027. [Google Scholar] [CrossRef] [PubMed]
- Gallina, C.; Turinetto, V.; Giachino, C. A new paradigm in cardiac regeneration: The mesenchymal stem cell secretome. Stem Cells Int. 2015, 2015, 765846. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Shen, R.; Song, L.; Lu, M.; Wang, J.; Zhao, S.; Tang, Y.; Meng, X.; Li, Z.; He, Z.-X. Bone marrow mesenchymal stem cells (BM-MSCs) improve heart function in swine myocardial infarction model through paracrine effects. Sci. Rep. 2016, 6, 28250. [Google Scholar] [CrossRef]
- Natsumeda, M.; Florea, V.; Rieger, A.C.; Tompkins, B.A.; Banerjee, M.N.; Golpanian, S.; Fritsch, J.; Landin, A.M.; Kashikar, N.D.; Karantalis, V.; et al. A combination of allogeneic stem cells promotes cardiac regeneration. J. Am. Coll. Cardiol. 2017, 70, 2504–2515. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.R.; Hatzistergos, K.E.; Addicott, B.; McCall, F.; Carvalho, D.; Suncion, V.; Morales, A.R.; Silva, J.D.; Sussman, M.A.; Heldman, A.W.; et al. Enhanced effect of human cardiac stem cells and bone marrow mesenchymal stem cells to reduce infarct size and restore cardiac function after myocardial infarction. Circulation 2013, 127, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Karantalis, V.; Suncion-Loescher, V.Y.; Bagno, L.; Golpanian, S.; Wolf, A.; Sanina, C.; Premer, C.; Kanelidis, A.J.; McCall, F.; Wang, B.; et al. Synergistic effects of combined cell therapy for chronic ischemic cardiomyopathy. J. Am. Coll. Cardiol. 2015, 66, 1990–1999. [Google Scholar] [CrossRef] [PubMed]
- Tompkins, B.A.; Wayne, B.; Johannes, W.; Mariann, G.; Georg, G.; Francisco, F.; Hare, J.M. Preclinical studies of stem cell therapy for heart disease. Circ. Res. 2018, 122, 1006–1020. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.J.H.; Yang, X.; Don, C.W.; Minami, E.; Liu, Y.-W.; Weyers, J.J.; Mahoney, W.M.; Van Biber, B.; Cook, S.M.; Palpant, N.J.; et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 2014, 510, 273–277. [Google Scholar] [CrossRef]
- Keyang, Z.; Qiang, W.; Cheng, N.; Peng, Z.; Zhiwei, Z.; Yan, W.; Yingchao, W.; Yinchuan, X.; Minjian, K.; Haifeng, C.; et al. Lack of remuscularization following transplantation of human embryonic stem cell-derived cardiovascular progenitor cells in infarcted nonhuman primates. Circ. Res. 2018, 122, 958–969. [Google Scholar] [CrossRef]
- Shiba, Y.; Gomibuchi, T.; Seto, T.; Wada, Y.; Ichimura, H.; Tanaka, Y.; Ogasawara, T.; Okada, K.; Shiba, N.; Sakamoto, K.; et al. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature 2016, 538, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Yue-Jin, Y.; Hai-Yan, Q.; Ji, H.; Jian-Jun, L.; Run-Lin, G.; Ke-Fei, D.; Guo-Sheng, Y.; Willerson, J.T.; Yong-Jian, G. Combined therapy with simvastatin and bone marrow–derived mesenchymal stem cells increases benefits in infarcted swine hearts. Arter. Thromb. Vasc. Biol. 2009, 29, 2076–2082. [Google Scholar] [CrossRef][Green Version]
- Bobi, J.; Solanes, N.; Fernández-Jiménez, R.; Galán-Arriola, C.; Dantas, A.P.; Fernández-Friera, L.; Gálvez-Montón, C.; Rigol-Monzó, E.; Agüero, J.; Ramírez, J.; et al. Intracoronary administration of allogeneic adipose tissue—Derived mesenchymal stem cells improves myocardial perfusion but not left ventricle function, in a translational model of acute myocardial infarction. J. Am. Heart Assoc. Cardiovasc. Cereb. Dis. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Mauricio, G.; Moscoso, I.; Martín-Cancho, M.-F.; Crisóstomo, V.; Prat-Vidal, C.; Báez-Díaz, C.; Sánchez-Margallo, F.M.; Bernad, A. Combined administration of mesenchymal stem cells overexpressing IGF-1 and HGF enhances neovascularization but moderately improves cardiac regeneration in a porcine model. Stem Cell Res. Ther. 2016, 7, 94. [Google Scholar] [CrossRef] [PubMed]
- Perea-Gil, I.; Prat-Vidal, C.; Gálvez-Montón, C.; Roura, S.; Llucià-Valldeperas, A.; Soler-Botija, C.; Iborra-Egea, O.; Díaz-Güemes, I.; Crisóstomo, V.; Sánchez-Margallo, F.M.; et al. A cell-enriched engineered myocardial graft limits infarct size and improves cardiac function: Pre-clinical study in the porcine myocardial infarction model. JACC Basic Transl. Sci. 2016, 1, 360–372. [Google Scholar] [CrossRef] [PubMed]
- Amado, L.C.; Saliaris, A.P.; Schuleri, K.H.; John, S.M.; Xie, J.-S.; Cattaneo, S.; Durand, D.J.; Fitton, T.; Kuang, J.Q.; Stewart, G.; et al. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc. Natl. Acad. Sci. USA 2005, 102, 11474–11479. [Google Scholar] [CrossRef]
- Ward, M.R.; Connelly, K.A.; Vijayaraghavan, R.; Vaags, A.K.; Graham, J.J.; Foltz, W.; Hough, M.R.; Stewart, D.J.; Dick, A. eNOS overexpressing bone marrow cells are safe and effective in a porcine model of myocardial regeneration following acute myocardial infarction. Cardiovasc. Ther. 2013, 31, e72–e78. [Google Scholar] [CrossRef]
- Kim, M.C.; Kim, Y.S.; Kang, W.S.; Lee, K.H.; Cho, M.; Hong, M.H.; Lim, K.S.; Jeong, M.H.; Ahn, Y. Intramyocardial injection of stem cells in pig myocardial infarction model: The first trial in korea. J. Korean Med. Sci. 2017, 32, 1708–1712. [Google Scholar] [CrossRef]
- Shake, J.G.; Gruber, P.J.; Baumgartner, W.A.; Senechal, G.; Meyers, J.; Redmond, J.M.; Pittenger, M.F.; Martin, B.J. Mesenchymal stem cell implantation in a swine myocardial infarct model: Engraftment and functional effects. Ann. Thorac. Surg. 2002, 73, 1919–1926. [Google Scholar] [CrossRef]
- Yang, Z.; Ma, D.; Wang, W.; Xu, S.; Zhang, Y.; Chen, B.; Zhou, F.; Zhu, T.; Wang, L.; Jia, E.; et al. Neovascularization and cardiomyocytes regeneration in acute myocardial infarction after bone marrow stromal cell transplantation: Comparison of infarct-relative and noninfarct-relative arterial approaches in swine. Clin. Chim. Acta 2007, 381, 114–118. [Google Scholar] [CrossRef]
- Pak, H.-N.; Qayyum, M.; Kim, D.T.; Hamabe, A.; Miyauchi, Y.; Lill, M.C.; Frantzen, M.; Takizawa, K.; Chen, L.S.; Fishbein, M.C.; et al. Mesenchymal stem cell injection induces cardiac nerve sprouting and increased tenascin expression in a swine model of myocardial infarction. J. Cardiovasc. Electrophysiol. 2003, 14, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Weil, B.R.; Suzuki, G.; Leiker, M.M.; Fallavollita, J.A.; Canty, J.M. Comparative efficacy of intracoronary allogeneic mesenchymal stem cells and cardiosphere-derived cells in swine with hibernating myocardium. Circ. Res. 2015, 117, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Golpanian, S.; Schulman, I.H.; Ebert, R.F.; Heldman, A.W.; DiFede, D.L.; Yang, P.C.; Wu, J.C.; Bolli, R.; Perin, E.C.; Moyé, L.; et al. Concise review: Review and perspective of cell dosage and routes of administration from preclinical and clinical studies of stem cell therapy for heart disease. Stem Cells Transl. Med. 2016, 5, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Sheng, C.C.; Zhou, L.; Hao, J. Current stem cell delivery methods for myocardial repair. Biomed. Res. Int. 2013. [Google Scholar] [CrossRef]
- Hou, D.; Youssef, E.A.-S.; Brinton, T.J.; Zhang, P.; Rogers, P.; Price, E.T.; Yeung, A.C.; Johnstone, B.H.; Yock, P.G.; March, K.L. Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: Implications for current clinical trials. Circulation 2005, 112, I150–I156. [Google Scholar] [CrossRef]
- Roura, S.; Gálvez-Montón, C.; Mirabel, C.; Vives, J.; Bayes-Genis, A. Mesenchymal stem cells for cardiac repair: Are the actors ready for the clinical scenario? Stem Cell Res. Ther. 2017, 8, 238. [Google Scholar] [CrossRef]
- Perea-Gil, I.; Prat-Vidal, C.; Bayes-Genis, A. In vivo experience with natural scaffolds for myocardial infarction: The times they are a-changin’. Stem Cell Res. Ther. 2015, 6. [Google Scholar] [CrossRef]
- Nguyen, A.H.; Marsh, P.; Schmiess-Heine, L.; Burke, P.J.; Lee, A.; Lee, J.; Cao, H. Cardiac tissue engineering: State-of-the-art methods and outlook. J. Biol. Eng. 2019, 13, 57. [Google Scholar] [CrossRef]
- Gálvez-Montón, C.; Bragós, R.; Soler-Botija, C.; Díaz-Güemes, I.; Prat-Vidal, C.; Crisóstomo, V.; Sánchez-Margallo, F.M.; Llucià-Valldeperas, A.; Bogónez-Franco, P.; Perea-Gil, I.; et al. Noninvasive assessment of an engineered bioactive graft in myocardial infarction: Impact on cardiac function and scar healing. Stem Cells Transl. Med. 2017, 6, 647–655. [Google Scholar] [CrossRef]
- Shah, M.; Pawan, K.C.; Copeland, K.M.; Liao, J.; Zhang, G. A thin layer of decellularized porcine myocardium for cell delivery. Sci. Rep. 2018, 8, 16206. [Google Scholar] [CrossRef]
- Wainwright, J.M.; Hashizume, R.; Fujimoto, K.L.; Remlinger, N.T.; Pesyna, C.; Wagner, W.R.; Tobita, K.; Gilbert, T.W.; Badylak, S.F. Right ventricular outflow tract repair with a cardiac biologic scaffold. Cells Tissues Organs 2012, 195, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Gálvez-Montón, C.; Fernandez-Figueras, M.T.; Martí, M.; Soler-Botija, C.; Roura, S.; Perea-Gil, I.; Prat-Vidal, C.; Llucià-Valldeperas, A.; Raya, Á.; Bayes-Genis, A. Neoinnervation and neovascularization of acellular pericardial-derived scaffolds in myocardial infarcts. Stem Cell Res. Ther. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Mewhort, H.E.M.; Turnbull, J.D.; Satriano, A.; Chow, K.; Flewitt, J.A.; Andrei, A.-C.; Guzzardi, D.G.; Svystonyuk, D.A.; White, J.A.; Fedak, P.W.M. Epicardial infarct repair with bioinductive extracellular matrix promotes vasculogenesis and myocardial recovery. J. Heart Lung Transpl. 2016, 35, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Spinali, K.L.; Schmuck, E.G. Natural sources of extracellular matrix for cardiac repair. In Cardiac Extracellular Matrix: Fundamental Science to Clinical Applications; Advances in Experimental Medicine and Biology; Schmuck, E.G., Hematti, P., Raval, A.N., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 115–130. ISBN 978-3-319-97421-7. [Google Scholar]
- Brewster, B.D.; Rouch, J.D.; Wang, M.; Meldrum, D.R. Toll-like receptor 4 ablation improves stem cell survival after hypoxic injury. J. Surg. Res. 2012, 177, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Abarbanell, A.M.; Herrmann, J.L.; Weil, B.R.; Manukyan, M.C.; Poynter, J.A.; Meldrum, D.R. TLR4 inhibits mesenchymal stem cell (MSC) STAT3 activation and thereby exerts deleterious effects on MSC–mediated cardioprotection. PLoS ONE 2010, 5, e14206. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.; Kim, Y.S.; Ahn, Y.; Jeong, M.H.; Hong, M.H.; Joo, S.Y.; Nam, K.I.; Cho, J.G.; Kang, P.M.; Park, J.C. The effects of mesenchymal stem cells transduced with Akt in a porcine myocardial infarction model. Cardiovasc. Res. 2006, 70, 530–542. [Google Scholar] [CrossRef]
- Mao, Q.; Lin, C.; Gao, J.; Liang, X.; Gao, W.; Shen, L.; Kang, L.; Xu, B. Mesenchymal stem cells overexpressing integrin-linked kinase attenuate left ventricular remodeling and improve cardiac function after myocardial infarction. Mol. Cell. Biochem. 2014, 397, 203–214. [Google Scholar] [CrossRef]
- Hsiao, S.T.-F.; Asgari, A.; Lokmic, Z.; Sinclair, R.; Dusting, G.J.; Lim, S.Y.; Dilley, R.J. Comparative analysis of paracrine factor expression in human adult mesenchymal stem cells derived from bone marrow, adipose, and dermal tissue. Stem Cells Dev. 2012, 21, 2189–2203. [Google Scholar] [CrossRef]
- Hnatiuk, A.P.; Ong, S.; Olea, F.D.; Locatelli, P.; Riegler, J.; Lee, W.H.; Jen, C.H.; De Lorenzi, A.; Giménez, C.S.; Laguens, R.; et al. Allogeneic mesenchymal stromal cells overexpressing mutant human hypoxia-inducible factor 1-α (HIF1-α) in an ovine model of acute myocardial infarction. J. Am. Heart Assoc. Cardiovasc. Cereb. Dis. 2016, 5. [Google Scholar] [CrossRef]
- Kim, S.H.; Moon, H.-H.; Kim, H.A.; Hwang, K.-C.; Lee, M.; Choi, D. Hypoxia-inducible vascular endothelial growth factor-engineered mesenchymal stem cells prevent myocardial ischemic injury. Mol. Ther. J. Am. Soc. Gene 2011, 19, 741–750. [Google Scholar] [CrossRef]
- Cornu, T.I.; Mussolino, C.; Cathomen, T. Refining strategies to translate genome editing to the clinic. Nat. Med. 2017, 23, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Dakhlallah, D.; Zhang, J.; Yu, L.; Marsh, C.B.; Angelos, M.G.; Khan, M. MicroRNA-133a engineered mesenchymal stem cells augment cardiac function and cell survival in the infarct heart. J. Cardiovasc. Pharm. 2015, 65, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Shin, S.; Lee, J.; Seo, H.-H.; Lim, K.H.; Kim, H.; Choi, J.-W.; Kim, S.W.; Lee, S.; Lim, S.; et al. MicroRNA-mediated down-regulation of apoptosis signal-regulating kinase 1 (ASK1) attenuates the apoptosis of human mesenchymal stem cells (MSCs) transplanted into infarcted heart. Int. J. Mol. Sci. 2016, 17, 1752. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Huang, M.; Nguyen, P.K.; Gong, Y.; Li, Z.; Jia, F.; Lan, F.; Liu, J.; Nag, D.; Robbins, R.C.; et al. Novel microRNA pro-survival cocktail for improving engraftment and function of cardiac progenitor cell transplantation. Circulation 2011, 124, S27–S34. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Zhu, X.; Hu, X.-Q.; Fang, Z.-F.; Tang, L.; Lu, X.-L.; Zhou, S.-H. Mesenchymal stem cells modified with miR-126 release angiogenic factors and activate Notch ligand Delta-like-4, enhancing ischemic angiogenesis and cell survival. Int. J. Mol. Med. 2013, 31, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.-H.; Lee, S.-Y.; Lee, C.Y.; Kim, R.; Kim, P.; Oh, S.; Lee, H.; Lee, M.Y.; Kim, J.; Kim, L.K.; et al. Exogenous miRNA-146a enhances the therapeutic efficacy of human mesenchymal stem cells by increasing vascular endothelial growth factor secretion in the ischemia/reperfusion-injured heart. J. Vasc. Res. 2017, 54, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy, K.T.; Jones, D.C.; Sperber, H.; Madan, A.; Fischer, K.A.; Rodriguez, M.L.; Pabon, L.; Zhu, W.-Z.; Tulloch, N.L.; Yang, X.; et al. Let-7 family of microRNA is required for maturation and adult-like metabolism in stem cell-derived cardiomyocytes. Proc. Natl. Acad. Sci. USA 2015, 112, E2785–E2794. [Google Scholar] [CrossRef]
- Shen, X.; Pan, B.; Zhou, H.; Liu, L.; Lv, T.; Zhu, J.; Huang, X.; Tian, J. Differentiation of mesenchymal stem cells into cardiomyocytes is regulated by miRNA-1-2 via WNT signaling pathway. J. Biomed. Sci. 2017, 24, 29. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Liu, F.; Liu, W.; Wang, Y.; Zhu, B.; Yu, B. MiR-499 induces cardiac differentiation of rat mesenchymal stem cells through wnt/β-catenin signaling pathway. Biochem. Biophys. Res. Commun. 2012, 420, 875–881. [Google Scholar] [CrossRef]
- Crisostomo, V.; Baez, C.; Abad, J.L.; Sanchez, B.; Alvarez, V.; Rosado, R.; Gómez-Mauricio, G.; Gheysens, O.; Blanco-Blazquez, V.; Blazquez, R.; et al. Dose-dependent improvement of cardiac function in a swine model of acute myocardial infarction after intracoronary administration of allogeneic heart-derived cells. Stem Cell Res. Ther. 2019, 10, 152. [Google Scholar] [CrossRef]
- Fernández-Avilés, F.; Sanz-Ruiz, R.; Bogaert, J.; Casado Plasencia, A.; Gilaberte, I.; Belmans, A.; Fernández-Santos, M.E.; Charron, D.; Mulet, M.; Yotti, R.; et al. Safety and efficacy of intracoronary infusion of allogeneic human cardiac stem cells in patients with st-segment elevation myocardial infarction and left ventricular dysfunction. Circ. Res. 2018, 123, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Lemcke, H.; Voronina, N.; Steinhoff, G.; David, R. Recent progress in stem cell modification for cardiac regeneration. Stem Cells Int. 2018, 2018, 1909346. [Google Scholar] [CrossRef] [PubMed]
- Bahsoun, S.; Coopman, K.; Akam, E.C. The impact of cryopreservation on bone marrow-derived mesenchymal stem cells: A systematic review. J. Transl. Med. 2019, 17, 397. [Google Scholar] [CrossRef] [PubMed]
- Stevens, S. Genome engineering for xenotransplantation. Genet. Eng. Glimpse Tech. Appl. 2019. [Google Scholar] [CrossRef]
- Wilmut, I.; Beaujean, N.; de Sousa, P.A.; Dinnyes, A.; King, T.J.; Paterson, L.A.; Wells, D.N.; Young, L.E. Somatic cell nuclear transfer. Nature 2002, 419, 583–586. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, P.E.; Meyer, E.H.; Kooreman, N.G.; Diecke, S.; Dey, D.; Sanchez-Freire, V.; Hu, S.; Ebert, A.; Odegaard, J.; Mordwinkin, N.; et al. Transplanted terminally differentiated induced pluripotent stem cells are accepted by immune mechanisms similar to self-tolerance. Nat. Commun. 2014, 5, 3903. [Google Scholar] [CrossRef]
- Bravery, C.A. Do human leukocyte antigen-typed cellular therapeutics based on induced pluripotent stem cells make commercial sense? Stem Cells Dev. 2014, 24, 1–10. [Google Scholar] [CrossRef]
- Nakatsuji, N.; Nakajima, F.; Tokunaga, K. HLA-haplotype banking and iPS cells. Nat. Biotechnol. 2008, 26, 739–740. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Liu, C.-L.; Ting, C.-Y.; Chiu, Y.-T.; Cheng, Y.-C.; Nicholson, M.W.; Hsieh, P.C.H. Human iPSC banking: Barriers and opportunities. J. Biomed. Sci. 2019, 26, 87. [Google Scholar] [CrossRef]
- Yoshihara, M.; Oguchi, A.; Murakawa, Y. Genomic instability of iPSCs and challenges in their clinical applications. In Stem Cells: Therapeutic Applications; Advances in Experimental Medicine and Biology; Ratajczak, M.Z., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 23–47. ISBN 978-3-030-31206-0. [Google Scholar]
- Wu, J.; Platero-Luengo, A.; Sakurai, M.; Sugawara, A.; Gil, M.A.; Yamauchi, T.; Suzuki, K.; Bogliotti, Y.S.; Cuello, C.; Valencia, M.M.; et al. Interspecies chimerism with mammalian pluripotent stem cells. Cell 2017, 168, 473–486.e15. [Google Scholar] [CrossRef]
- Koplin, J.; Wilkinson, D. Moral uncertainty and the farming of human-pig chimeras. J. Med. Ethics 2019, 45, 440–446. [Google Scholar] [CrossRef]
- Pawan, K.C.; Hong, Y.; Zhang, G. Cardiac tissue-derived extracellular matrix scaffolds for myocardial repair: Advantages and challenges. Regen. Biomater. 2019, 6, 185–199. [Google Scholar] [CrossRef]
- Huang, K.; Ozpinar, E.W.; Su, T.; Tang, J.; Shen, D.; Qiao, L.; Hu, S.; Li, Z.; Liang, H.; Mathews, K.; et al. An off-the-shelf artificial cardiac patch improves cardiac repair after myocardial infarction in rats and pigs. Sci. Transl. Med. 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.D.; Dequach, J.A.; Gaetani, R.; Ungerleider, J.; Elhag, D.; Nigam, V.; Behfar, A.; Christman, K.L. Human versus porcine tissue sourcing for an injectable myocardial matrix hydrogel. Biomater. Sci. 2014, 2014, 60283D. [Google Scholar] [CrossRef] [PubMed]
- Traverse, J.H.; Henry, T.D.; Nabil, D.; Patel, A.N.; Carl, P.; Schaer, G.L.; DeQuach, J.A.; Kinsey, A.M.; Paul, C.; Christman, K.L. First-in-man study of a cardiac extracellular matrix hydrogel in early and late myocardial infarction patients. JACC Basic Transl. Sci. 2019, 4, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Schuurman, H.-J. Regulatory aspects of clinical xenotransplantation. Int. J. Surg. 2015, 23, 312–321. [Google Scholar] [CrossRef] [PubMed]
| Cell Type and Cell Number | Delivery Method | Clinical Condition | Main Outcome | Study |
|---|---|---|---|---|
| Autologous MSC 3 × 107 cells | Direct left ventricular injection | Acute MI | Improved cardiac function Reduced oxidative stress and inflammatory response | [114] |
| Allogeneic ATMSCs 1 × 107 cells | Intracoronary infusion | Acute MI | Unaltered cardiac function Unaltered scar size Promoted revascularization | [115] |
| Allogeneic ATMSCs 5 × 107 cells Overexpressing IGF-1 or HGF | Intramyocardial injection | Acute MI | Unaltered cardiac function Promoted revascularization Reduced inflammation Enhanced fibrosis | [116] |
| Allogeneic ATDPCs 1.75 × 106 cells | Recellularized myocardial scaffold | Acute MI | Improved cardiac function Reduced infarct size Promoted revascularization Attenuated fibrosis | [117] |
| Xenogeneic MSCs 1 × 106 cells | Intramyocardial injection | Acute MI | Improved cardiac function Unaltered infarct size Improved preservation of capillarity | [99] |
| Allogeneic MSCs 1.4 × 107 cells | Intramyocardial injection | Subacute MI | Unaltered cardiac function Reduced scar size | [118] |
| Autologous BMCs eNOS-transfected 1 × 107 cells | Intramyocardial injection | Subacute MI | Improved cardiac function Unaltered scar size Unaltered fibrosis | [119] |
| ATSCs 1 × 107 cells | Intramyocardial injection | Subacute MI | Improved cardiac function Reduced infarct size | [120] |
| Autologous MSC 6 × 107 cells | Intramyocardial injection | Chronic MI | Unaltered cardiac function Unaltered scar size Attenuation of LV wall thinning | [121] |
| Autologous BMCs 5 × 106 | Left or right coronary artery infusion | Chronic MI | Improved cardiac function Promoted revascularization | [122] |
| Allogeneic MSC 2 × 108 cells | Intramyocardial injection | Chronic MI | Improved cardiac function Reduced scar size | [104] |
| Allogeneic MSC 1.5 × 107 cells | Recellularized collagen scaffold | Chronic MI | Improved cardiac function Reduced scar size Promoted revascularization | [123] |
| Allogeneic MSCs and CDCs 3.5 × 107 cells | Intracoronary infusion | Chronic ischemic cardiomyopathy (coronary stenosis) | Improved cardiac function Unaltered perfusion Improved remote wall thickening | [124] |
| Allogeneic MSCs and CSCs 2 × 108 cells | Transendocardial injection | Chronic ischemic cardiomyopathy | Improved cardiac function Reduced scar size Low-grade inflammatory infiltrates | [107] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galow, A.-M.; Goldammer, T.; Hoeflich, A. Xenogeneic and Stem Cell-Based Therapy for Cardiovascular Diseases: Genetic Engineering of Porcine Cells and Their Applications in Heart Regeneration. Int. J. Mol. Sci. 2020, 21, 9686. https://doi.org/10.3390/ijms21249686
Galow A-M, Goldammer T, Hoeflich A. Xenogeneic and Stem Cell-Based Therapy for Cardiovascular Diseases: Genetic Engineering of Porcine Cells and Their Applications in Heart Regeneration. International Journal of Molecular Sciences. 2020; 21(24):9686. https://doi.org/10.3390/ijms21249686
Chicago/Turabian StyleGalow, Anne-Marie, Tom Goldammer, and Andreas Hoeflich. 2020. "Xenogeneic and Stem Cell-Based Therapy for Cardiovascular Diseases: Genetic Engineering of Porcine Cells and Their Applications in Heart Regeneration" International Journal of Molecular Sciences 21, no. 24: 9686. https://doi.org/10.3390/ijms21249686
APA StyleGalow, A.-M., Goldammer, T., & Hoeflich, A. (2020). Xenogeneic and Stem Cell-Based Therapy for Cardiovascular Diseases: Genetic Engineering of Porcine Cells and Their Applications in Heart Regeneration. International Journal of Molecular Sciences, 21(24), 9686. https://doi.org/10.3390/ijms21249686