Adiponectin Exerts Peripheral Inhibitory Effects on the Mouse Gastric Smooth Muscle through the AMPK Pathway
Abstract
1. Introduction
2. Results
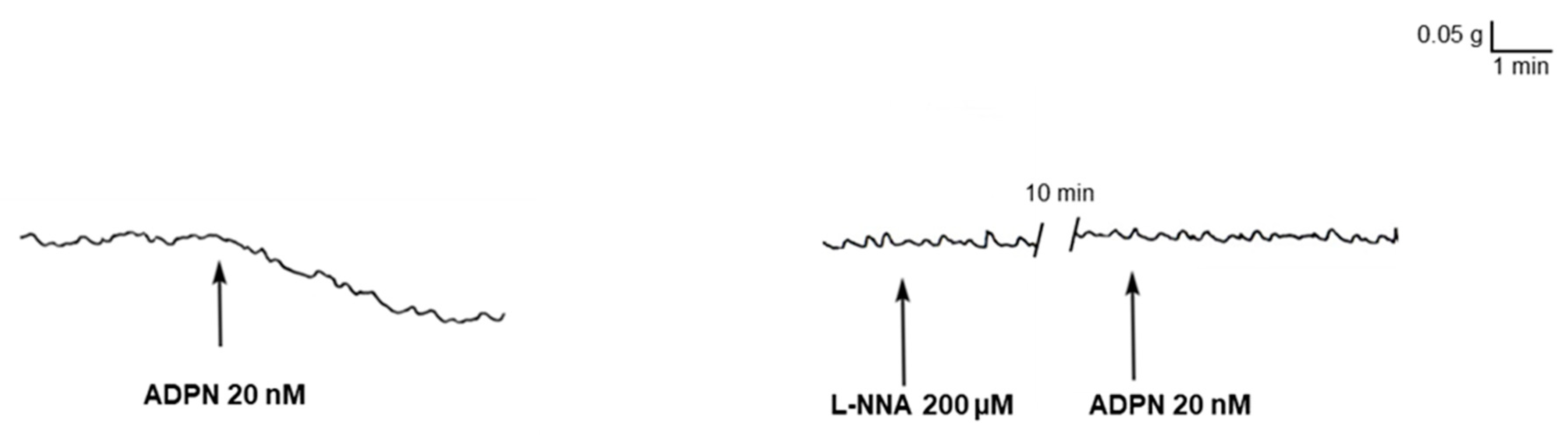
2.1. L-NNA Prevents the Mechanical Inhibitory Effect of ADPN on the Gastric Fundus
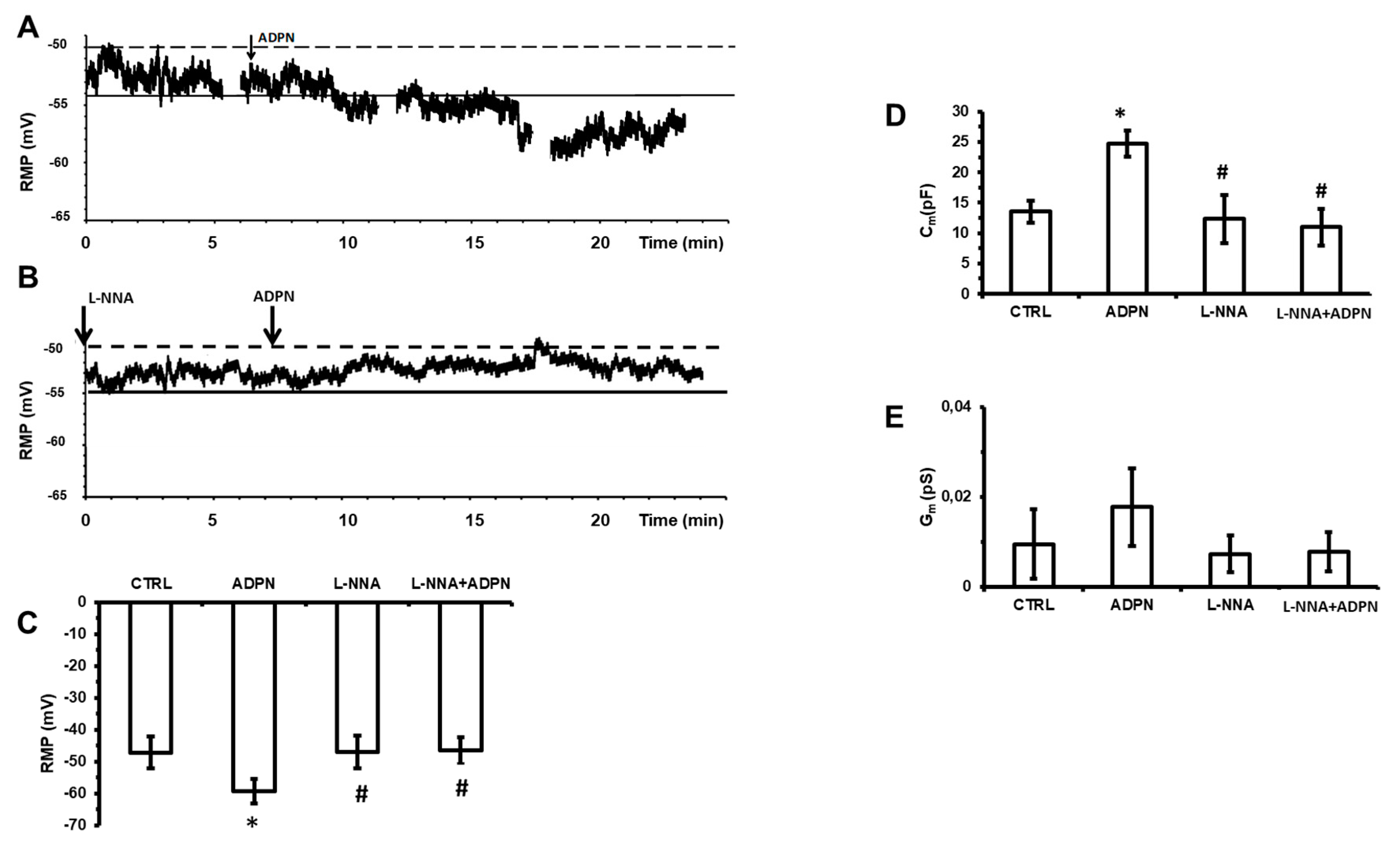
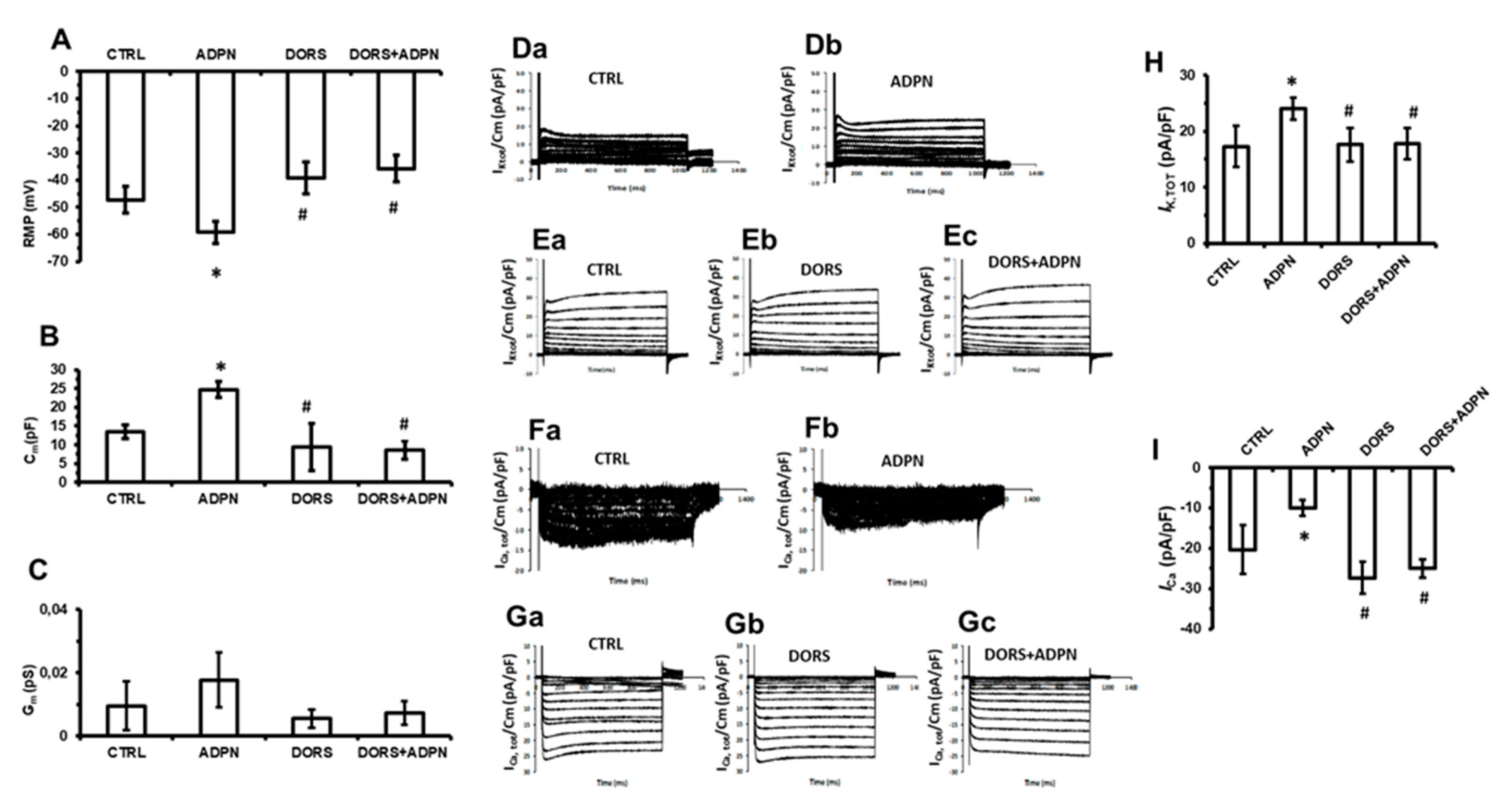
2.2. L-NNA Hampers the Effects of ADPN on the Resting Membrane Potential and on the Membrane Passive Properties of SMCs
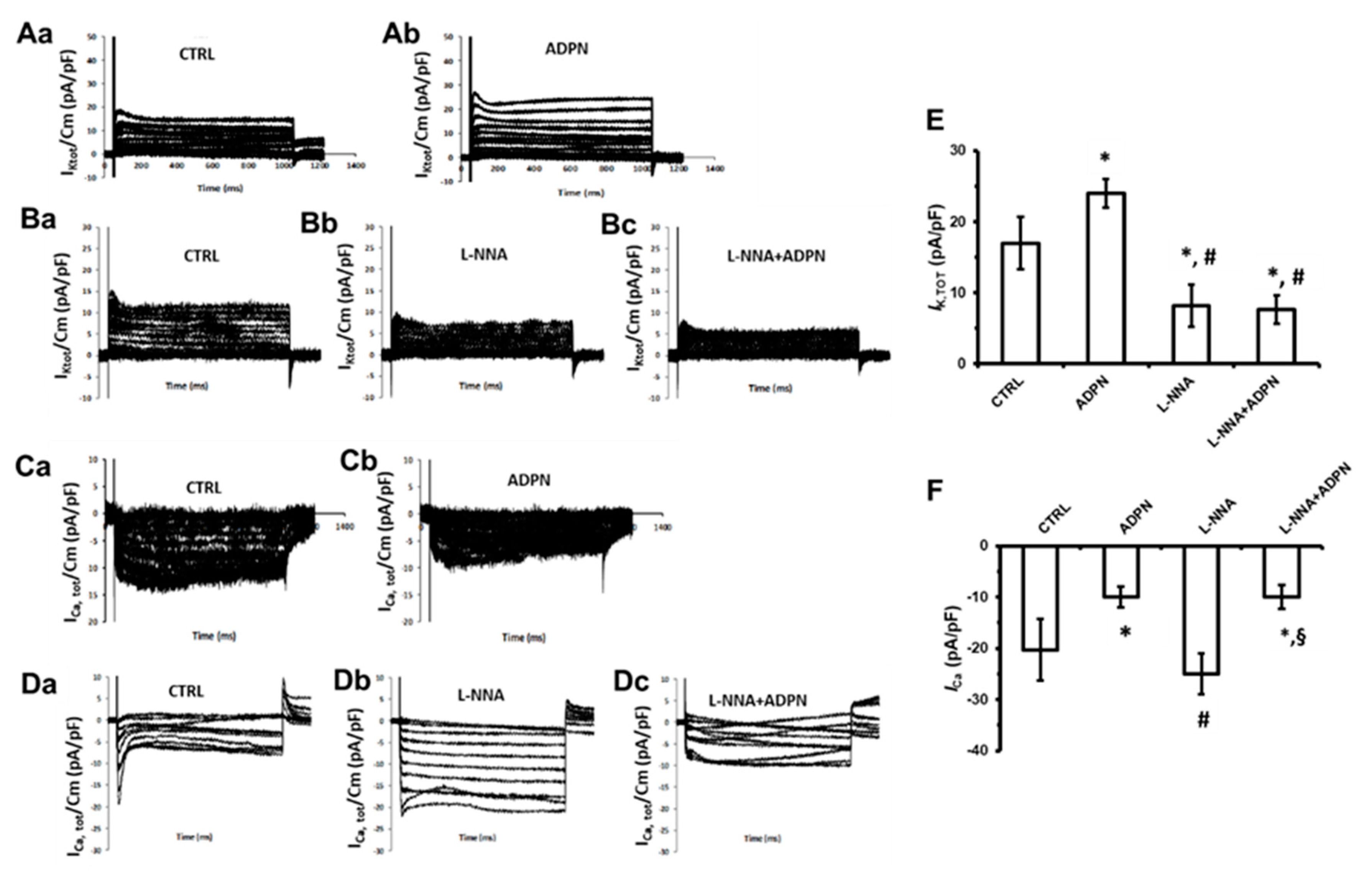
2.3. Effects of ADPN on the Ion Currents in the Presence of L-NNA
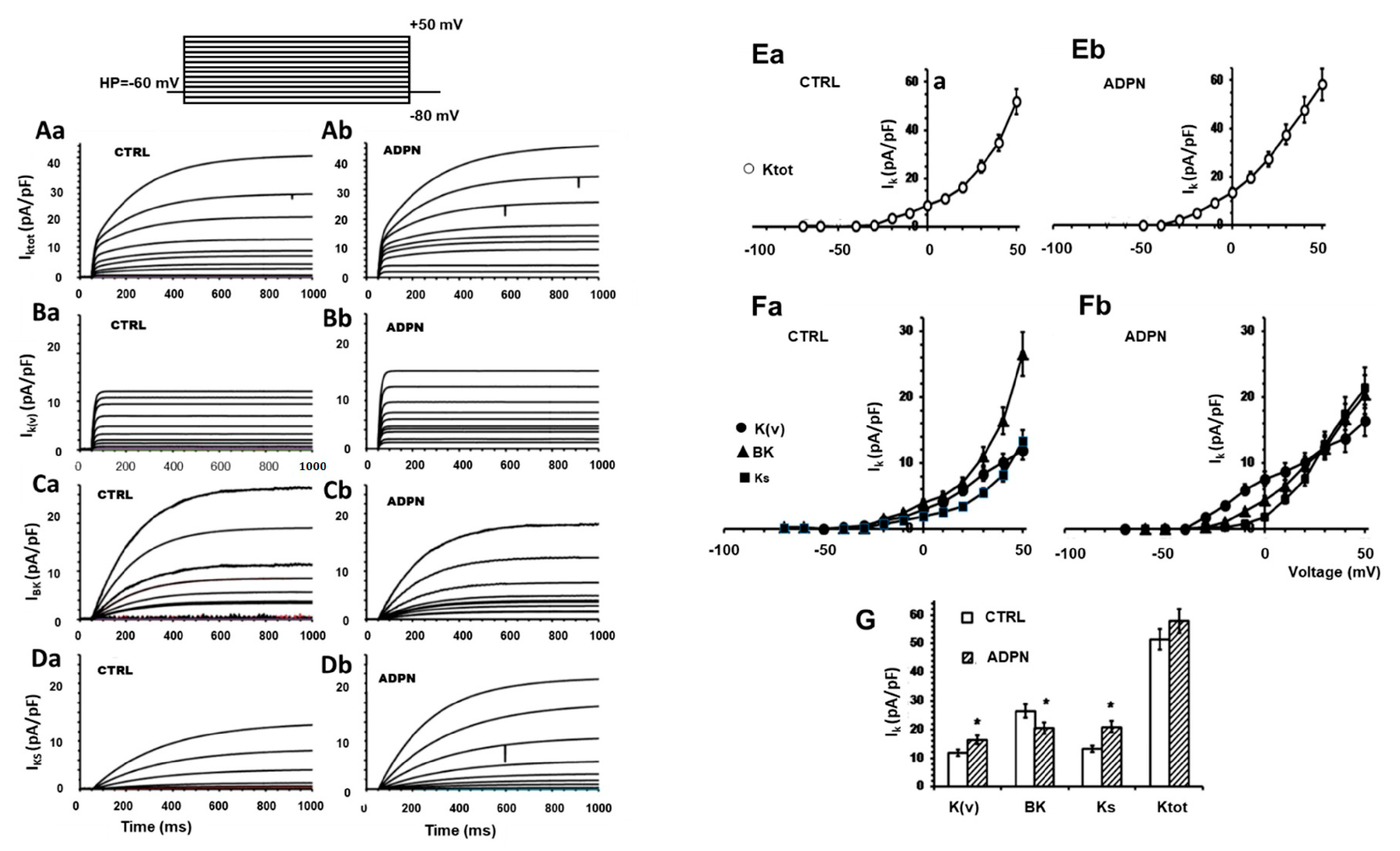
2.3.1. Effects of ADPN on the Different Types of Voltage-Dependent Outward K+ Currents
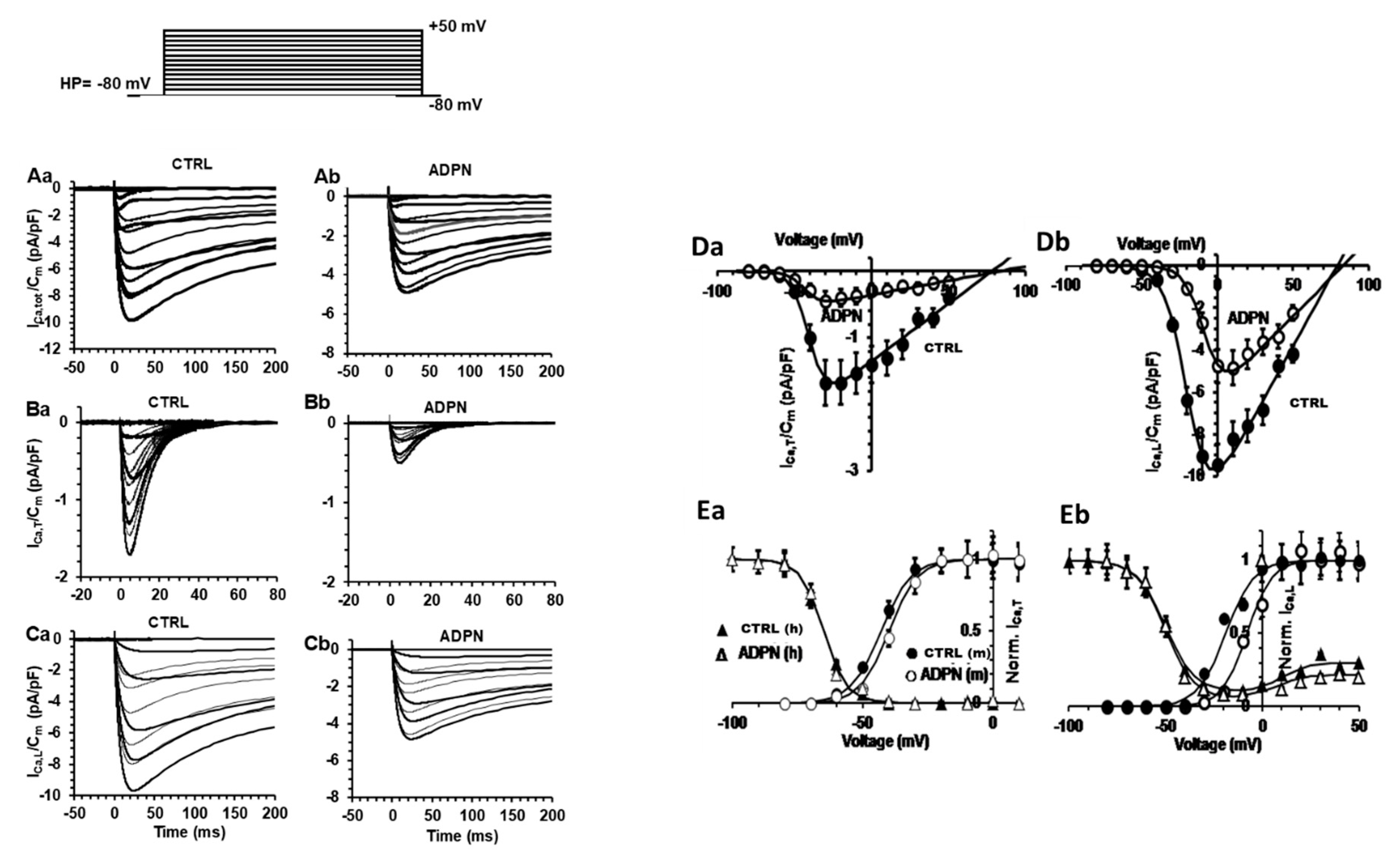
2.3.2. Effects of ADPN on the Different Types of Voltage-Dependent Inward Ca2+ Current
2.3.3. L-NNA Prevents the Effect of ADPN on K+ Channels but Not on ca2+ Channels
2.4. Dorsomorphin Hampers the Effects of ADPN on the Electrophysiological Properties and Ion Currents of SMCs
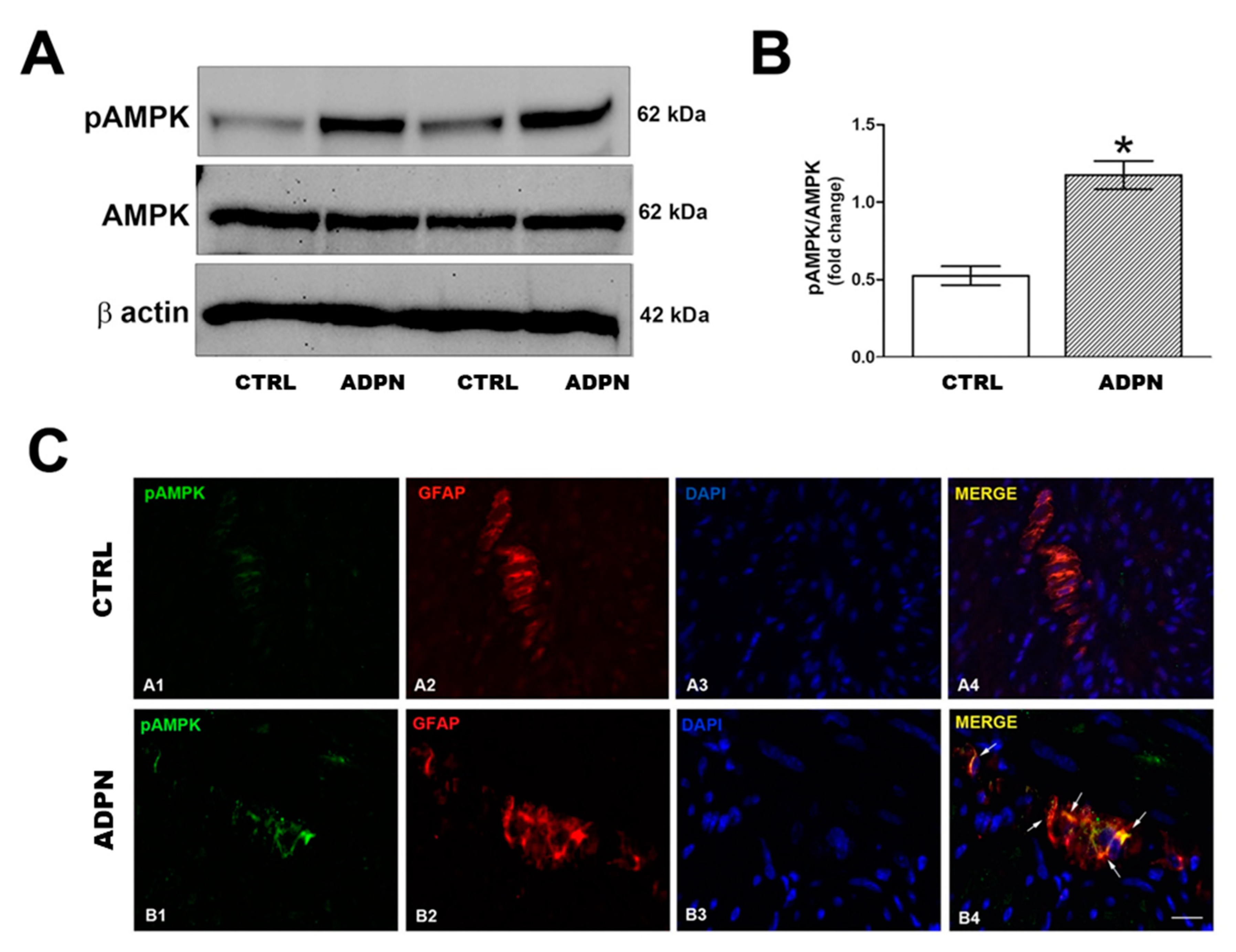
2.5. ADPN Increases AMPK Signaling
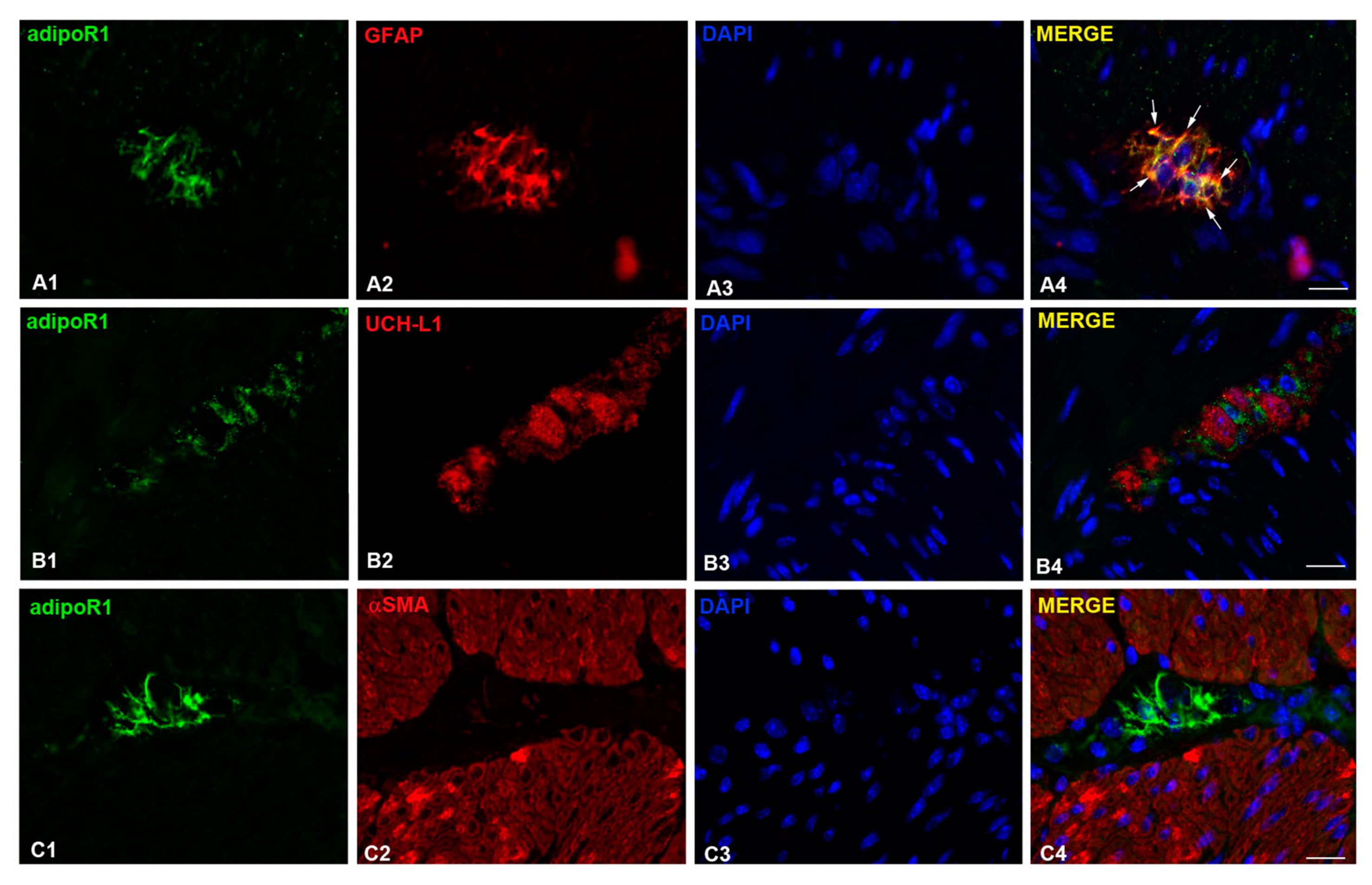
2.6. AdipoR1 Detection
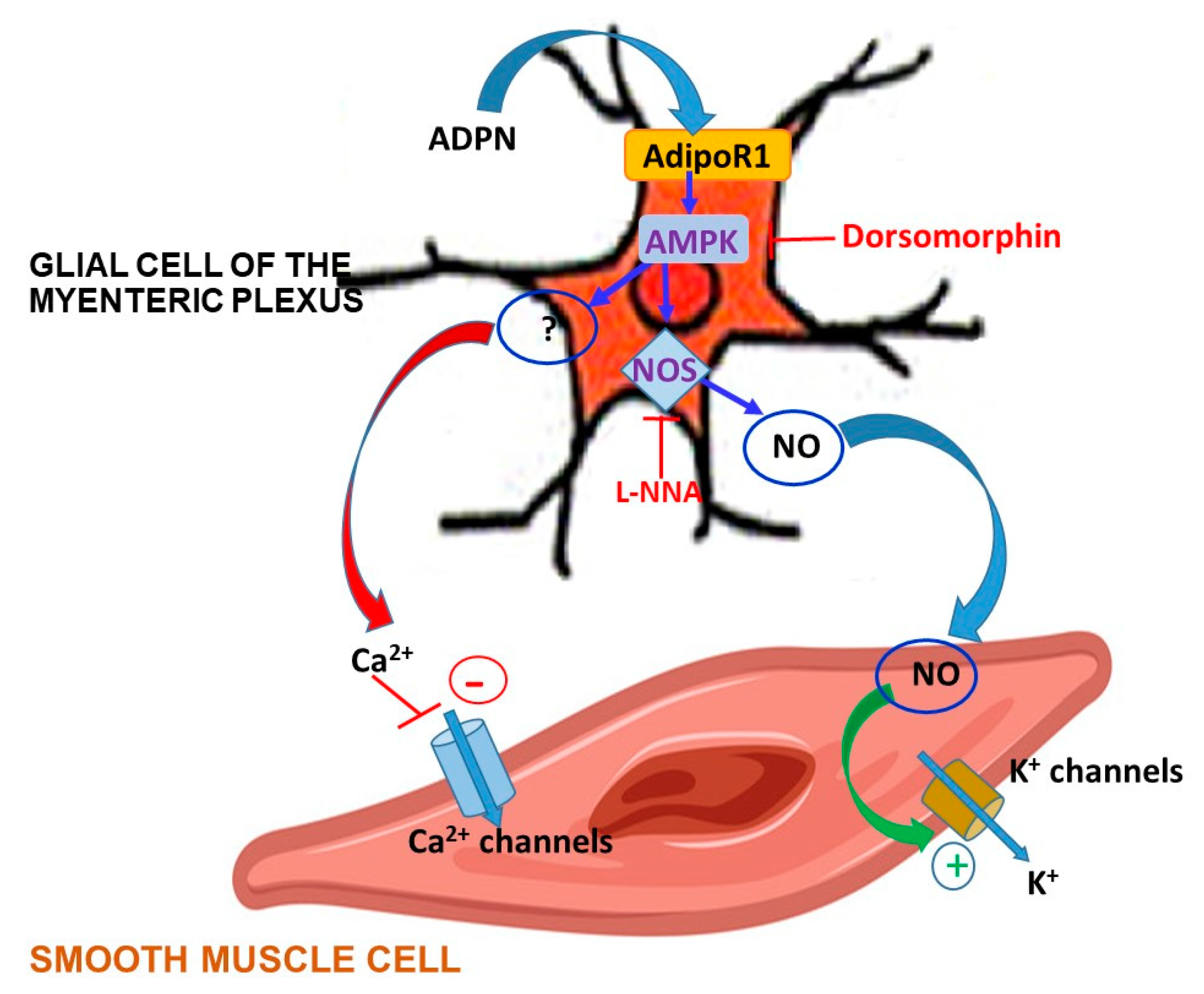
3. Discussion
4. Materials and Methods
4.1. Ethical Approval
4.2. Animals
4.3. Mechanical Experiments
4.4. Electrophysiological Experiments
Pulse Protocols of Stimulation
4.5. Western Blotting
4.6. Immunofluorescence Analysis
4.7. Data Analysis and Statistical Tests
4.8. Drugs
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADPN | Adiponectin |
| Cm | Cell linear capacitance |
| CTRL | Control |
| Gm | Membrane conductance |
| Gm/Cm | Specific membrane conductance |
| HP | Holding potential |
| ka | Steepness factor of activation |
| kh | Steepness factor of inactivation |
| Ia | Current activation |
| ICa | Ca2+ current |
| IK | Voltage-dependent delayed rectifier K+ currents |
| RMP | Resting membrane potential |
| SMC | Smooth muscle cell |
| Va | Half-maximal activation voltage |
| Vh | Half-maximal inactivation voltage |
| Vrev | Apparent reversal potential |
References
- Fasshauer, M.; Blüher, M. Adipokines in health and disease. Trends Pharmacol. Sci. 2015, 36, 461–470. [Google Scholar] [CrossRef]
- Choi, H.M.; Doss, H.M.; Kim, K.S. Multifaceted Physiological Roles of Adiponectin in Inflammation and Diseases. Int. J. Mol. Sci. 2020, 21, 1219. [Google Scholar] [CrossRef]
- Idrizaj, E.; Garella, R.; Squecco, R.; Baccari, M.C. Adipocytes-released Peptides Involved in the Control of Gastrointestinal Motility. Curr. Protein Pept. Sci. 2019, 20, 614–629. [Google Scholar] [CrossRef]
- Camilleri, M. Peripheral mechanisms in appetite regulation. Gastroenterology 2015, 148, 1219–1233. [Google Scholar] [CrossRef]
- Idrizaj, E.; Garella, R.; Castellini, G.; Mohr, H.; Pellegata, N.S.; Francini, F.; Ricca, V.; Squecco, R.; Baccari, M.C. Adiponectin affects the mechanical responses in strips from the mouse gastric fundus. World J. Gastroenterol. 2018, 24, 4028–4035. [Google Scholar] [CrossRef]
- Idrizaj, E.; Garella, R.; Castellini, G.; Francini, F.; Ricca, V.; Baccari, M.C.; Squecco, R. Adiponectin Decreases Gastric Smooth Muscle Cell Excitability in Mice. Front. Physiol. 2019, 10, 1000. [Google Scholar] [CrossRef]
- Bova, S.; Cavalli, M.; Cima, L.; Luciani, S.; Saponara, S.; Sgaragli, G.; Cargnelli, G.; Fusi, F. Relaxant and Ca2+ channel blocking properties of norbormide on rat non-vascular smooth muscles. Eur. J. Pharmacol. 2003, 470, 185–191. [Google Scholar] [CrossRef]
- Si, X.; Huang, L.; Luo, H.; Shi, R. Inhibitory effects of somatostatin on cholecystokinin octapeptide induced bile regurgitation under stress: Ionic and molecular mechanisms. Regul. Pept. 2009, 156, 34–41. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Cai, Z.X.; Li, P.; Cai, C.Y.; Qu, C.L.; Guo, H.S. Effect of dendroaspis natriuretic peptide (DNP) on L-type calcium channel current and its pathway. Regul. Pept. 2010, 164, 120–125. [Google Scholar] [CrossRef]
- Rich, A.; Kenyon, J.L.; Hume, J.R.; Overtuf, K.; Horowitz, B.; Sanders, K.M. Dihydropyridine-sensitive calcium channels expressed in canine colonic smooth muscle cells. Am. J. Physiol. 1993, C745–C754. [Google Scholar] [CrossRef]
- Kovac, J.R.; Preiksaitis, H.G.; Sims, S.M. Functional and molecular analysis of L-type calcium channels in human esophagus and lower esophageal sphincter smooth muscle. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 289, G998–G1006. [Google Scholar] [CrossRef]
- Xiong, Z.; Sperelakis, N.; Noffsinger, A.; Fenoglio-Preiser, C. Ca2+ currents in human colonic smooth muscle cells. Am. J. Physiol. 1995, 269, G378–G385. [Google Scholar] [CrossRef]
- Hart, P.J.; Overturf, K.E.; Russell, S.N.; Carl, A.; Hume, J.R.; Sanders, K.M.; Horowitz, B. Cloning and expression of a Kv1.2 class delayed rectifier K+ channel from canine colonic smooth muscle. Proc. Natl. Acad. Sci. USA 1993, 90, 9659–9663. [Google Scholar] [CrossRef]
- Overturf, K.E.; Russel, S.N.; Carl, A.; Vogalis, F.; Hart, P.J.; Hume, J.R.; Sanders, K.M.; Horowitz, B. Cloning and characterization of a Kv1.5 delayed rectifier K+ channel from vascular and visceral smooth muscles. Am. J. Physiol. 1994, C1231–C1238. [Google Scholar] [CrossRef]
- Schmalz, F.; Kinsella, J.; Koh, S.D.; Vogalis, F.; Schneider, A.; Flynn, E.R.; Kenyon, J.L.; Horowitz, B. Molecular identification of a component of delayed rectifier current in gastrointestinal smooth muscle. Am. J. Physiol. 1998, 274, G901–G911. [Google Scholar] [CrossRef]
- Koh, S.D.; Ward, S.M.; Dick, G.M.; Epperson, A.; Bonner, H.P.; Sanders, K.M.; Horowitz, B.; Kenyon, J.L. Contribution of delayed rectifier potassium currents to the electrical activity of murine colonic smooth muscle. J. Physiol. 1999, 515, 475–487. [Google Scholar] [CrossRef]
- Benham, C.D.; Bolton, T.D.; Lang, R.J.; Takewaki, T. The mechanism of action of Ba2+ and TEA on single Ca2+-activated K+-channels in arterial and intestinal smooth muscle cell membranes. Pflügers Arch. 1985, 403, 120–127. [Google Scholar] [CrossRef]
- Carl, A.; Sanders, K.M. Ca2+-activated K channels of canine colonic myocytes. Am. J. Physiol. 1989, 257, C470–C480. [Google Scholar] [CrossRef]
- Vogalis, F.; Goyal, R.K. Activation of small conductance Ca2+-dependent K+ channels by purinergic agonists in smooth muscle cells of the mouse ileum. J. Physiol. 1997, 502, 497–508. [Google Scholar] [CrossRef]
- Koh, S.D.; Dick, G.M.; Sanders, K.M. Small-conductance Ca2+ dependent K+ channels activated by ATP in murine colonic smooth muscle. Am. J. Physiol. 1997, 273, C2010–C2021. [Google Scholar] [CrossRef]
- Kadowaki, T.; Yamauchi, T.; Kubota, N. The physiological and pathophysiological role of adiponectin and adiponectin receptors in the peripheral tissues and CNS. FEBS Lett. 2008, 582, 74–80. [Google Scholar] [CrossRef] [PubMed]
- González, C.R.; Caminos, J.E.; Gallego, R.; Tovar, S.; Vázquez, M.J.; Garcés, M.F.; Lopez, M.; García-Caballero, T.; Tena-Sempere, M.; Nogueiras, R.; et al. Adiponectin receptor 2 is regulated by nutritional status, leptin and pregnancy in a tissue-specific manner. Physiol. Behav. 2010, 12, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Daisuke, N.; Yasunobu, H. The role of AMP-activated protein kinase in the cardiovascular system. Hypertens. Res. 2010, 33, 22–28. [Google Scholar] [CrossRef]
- Musi, N.; Goodyear, L.J. Insulin resistance and improvements in signal transduction. Endocrine 2006, 29, 73–80. [Google Scholar] [CrossRef]
- de Morentin, P.B.M.; Urisarri, A.; Couce, M.L.; López, M. Molecular mechanisms of appetite and obesity: A role for brain AMPK. Clin. Sci. 2016, 130, 1697–1709. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Cheng, K.K. Hypothalamic AMPK as a Mediator of Hormonal Regulation of Energy Balance. Int. J. Mol. Sci. 2018, 19, 3552. [Google Scholar] [CrossRef] [PubMed]
- Coope, A.; Milanski, M.; Araújo, E.P.; Tambascia, M.; Saad, M.J.; Geloneze, B.; Velloso, L.A. AdipoR1mediates the anorexigenic and insulin/leptin-like actions of adiponectin in the hypothalamus. FEBS Lett. 2008, 582, 1471–1476. [Google Scholar] [CrossRef]
- Squecco, R.; Garella, R.; Luciani, G.; Francini, F.; Baccari, M.C. Muscular effects of orexin A on the mouse duodenum: Mechanical and electrophysiological studies. J. Physiol. 2011, 589, 5231–5246. [Google Scholar] [CrossRef]
- Lynch, F.M.; Withers, S.B.; Yao, Z.; Werner, M.E.; Edwards, G.; Weston, A.H.; Heagerty, A.M. Perivascular adipose tissue-derived adiponectin activates BK(Ca) channels to induce anticontractile responses. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H786–H795. [Google Scholar] [CrossRef]
- Park, S.Y.; Choi, J.H.; Ryu, H.S.; Pak, Y.K.; Park, K.S.; Lee, H.K.; Lee, W. C1q tumor necrosis factor alpha-related protein isoform 5 is increased in mitochondrial DNA-depleted myocytes and activates AMP-activated protein kinase. J. Biol. Chem. 2009, 284, 27780–27789. [Google Scholar] [CrossRef]
- He, Y.; Liu, B.; Yao, P.; Shao, Y.; Cheng, Y.; Zhao, J.; Wu, J.; Zhao, Z.W.; Huang, W.; Christopher, T.A.; et al. Adiponectin inhibits cardiac arrest/cardiopulmonary resuscitation-induced apoptosis in brain by increasing autophagy involved in AdipoR1-AMPK signaling. Mol. Med. Rep. 2020, 22, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Niemann, B.; Li, L.; Siegler, D.; Siegler, B.H.; Knapp, F.; Hanna, J.; Aslam, M.; Kracht, M.; Schulz, R.; Rohrbach, S. CTRP9 Mediates Protective Effects in Cardiomyocytes via AMPK- and Adiponectin Receptor-Mediated Induction of Anti-Oxidant Response. Cells 2020, 9, 1229. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Porwal, K.; Rajak, S.; Sinha, R.A.; Chattopadhyay, N. Selective dietary polyphenols induce differentiation of human osteoblasts by adiponectin receptor 1-mediated reprogramming of mitochondrial energy metabolism. Biomed. Pharmacother. 2020, 127, 110207. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Montagani, M.; Funahashi, T.; Shimimura, L.; Quon, M.J. Adiponectin stimulates production of nitric oxid in vascular endothelial cells. J. Biol. Chem. 2003, 278, 45021–45026. [Google Scholar] [CrossRef]
- Han, G.; Ma, H.; Chintala, R.; Miyake, K.; Fulton, D.J.; Barman, S.A.; White, R.E. Nongenomic, endothelium-independent effects of estrogen on human coronary smooth muscle are mediated by type I (neuronal) NOS and PI3-kinase-Akt signaling. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H314–H321. [Google Scholar] [CrossRef]
- Shin, M.J.; Lee, Y.P.; Kim, D.W.; An, J.J.; Jang, S.H.; Cho, S.M.; Sheen, S.H.; Lee, H.R.; Kweon, H.Y.; Kang, S.W.; et al. Transduced PEP-1-AMPK inhibits the LPS-induced expression of COX-2 and iNOS in Raw264.7 cells. BMB Rep. 2010, 43, 40–45. [Google Scholar] [CrossRef]
- Squecco, R.; Garella, R.; Idrizaj, E.; Nistri, S.; Francini, F.; Baccari, M.C. Relaxin Affects smooth muscle biophysical properties and mechanical activity of the female mouse colon. Endocrinology 2015, 156, 4398–4410. [Google Scholar] [CrossRef]
- Garella, R.; Idrizaj, E.; Traini, C.; Squecco, R.; Vannucchi, M.G.; Baccari, M.C. Glucagon-like peptide-2 modulates the nitrergic neurotransmission in strips from the mouse gastric fundus. World J. Gastroenterol. 2017, 23, 7211–7220. [Google Scholar] [CrossRef]
- Nour-Eldine, W.; Ghantous, C.M.; Zibara, K.; Dib, L.; Issaa, H.; Itani, H.A.; El-Zein, N.; Zeidan, A. Adiponectin attuates angiotensin II- induced vascular smooth, muscle cell remodeling through nitric oxide and the Rhoa/ROCK pathway. Front. Pharmacol. 2016, 7, 86. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, Y.; Zhang, S.; Zhao, Y.; Yin, X.; Wang, W.; Ma, X.; Liu, H. The role of NO-cGMP pathway inhibition in vascular endothelial-dependent smooth muscle relaxation disorder of AT1-AA positive rats: Protective effects of adiponectin. Nitric Oxide 2019, 87, 10–22. [Google Scholar] [CrossRef]
- Cersosimo, E.; Xu, X.; Terasawa, T.; Dong, L.Q. Anti-inflammatory and anti-proliferative action of adiponectin mediated by insulin signaling cascade in human vascular smooth muscle cells. Mol. Biol. Rep. 2020, 47, 6561–6572. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, T.; Yamauchi, T. Adiponectin and Adiponectin Receptors. Endocr. Rev. 2005, 26, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Ahima, R.S. Adipose Tissue as an Endocrine Organ. Obesity 2006, 5, 242S–249S. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.M. Regulation and function of AMPK in physiology and diseases. Exp. Mol. Med. 2016, 48, e245. [Google Scholar] [CrossRef] [PubMed]
- Ruan, H.; Dong, L.Q. Adiponectin signaling and function in insulin target tissues. J. Mol. Cell Biol. 2016, 8, 101–109. [Google Scholar] [CrossRef]
- Schneider, S.; Wright, C.M.; Heuckeroth, R.O. Unexpected Roles for the Second Brain: Enteric Nervous System as Master Regulator of Bowel Function. Annu. Rev. Physiol. 2019, 81, 235–259. [Google Scholar] [CrossRef]
- Idrizaj, E.; Garella, R.; Squecco, R.; Baccari, M.C. Can adiponectin have an additional effect on the regulation of food intake by inducing gastric motor changes? World J. Gastroenterol. 2020, 26, 2472–2478. [Google Scholar] [CrossRef]
- Duca, F.A.; Covasa, M. Current and emerging concepts on the role on peripheral signals in the control of food intake and development of obesity. Br. J. Nutr. 2012, 108, 778–793. [Google Scholar] [CrossRef]
- Idrizaj, E.; Garella, R.; Francini, F.; Squecco, R.; Baccari, M.C. Relaxin influences ileal muscular activity through a dual signaling pathway in mice. World J. Gastroenterol. 2018, 24, 882–893. [Google Scholar] [CrossRef]
- Baglioni, S.; Cantini, G.; Poli, G.; Francalanci, M.; Squecco, R.; Di Franco, A.; Borgogni, E.; Frontera, S.; Nesi, G.; Liotta, F.; et al. Functional differences in visceral and subcutaneous fat pads originate from differences in the adipose stem cell. PLoS ONE 2012, 7, e36569. [Google Scholar] [CrossRef]
- Squecco, R.; Sassoli, C.; Nuti, F.; Martinesi, M.; Chellini, F.; Nosi, D.; Zecchi-Orlandini, S.; Francini, F.; Formigli, L.; Meacci, E. Sphingosine 1-phosphate induces myoblast differentiation through Cx43 protein expression: A role for a gap junction-dependent and -independent function. Mol. Biol. Cell 2006, 17, 4896–4910. [Google Scholar] [CrossRef]
- Formigli, L.; Sassoli, C.; Squecco, R.; Bini, F.; Martinesi, M.; Chellini, F.; Luciani, G.; Sbrana, F.; Zecchi-Orlandini, S.; Francini, F.; et al. Regulation of transient receptor potential canonical channel 1 (TRPC1) by sphingosine 1-phosphate in C2C12 myoblasts and its relevance for a role of mechanotransduction in skeletal muscle differentiation. J. Cell Sci. 2009, 122, 1322–1333. [Google Scholar] [CrossRef]
| Cm (pF) | Gm (pS) | RMP (mV) | IK (pA/pF) | ICa (pA/pF) | |
|---|---|---|---|---|---|
| CTRL | 13.5 ± 1.8 | 0.009 ± 0.002 | −47.2 ± 5.0 | 17.1 ± 3.7 | −20.3 ± 6.0 |
| (n = 52) | (n = 52) | (n = 50) | (n = 30) | (n = 20) | |
| ADPN | 24.7 ± 2.2 * | 0.0175 ± 0.002 | −59.4 ± 3.9 * | 23.9 ± 2.1 * | −10.1 ± 2 * |
| (n = 17) | (n = 16) | (n = 16) | (n = 9) | (n = 7) | |
| L-NNA | 12.3 ± 3.9 # | 0.0072 ± 0.001 | −47.0 ± 5.1 # | 8.3 ± 2.9 *,# | −24.9 ± 4.1 # |
| (n = 18) | (n = 18) | (n = 17) | (n = 10) | (n = 7) | |
| L-NNA + ADPN | 10.9 ± 3.1 # | 0.0078 ± 0.001 | −46.6 ± 4.3 # | 7.8 ± 1.8 *,# | −10.0 ± 2.3 *,§ |
| (n = 18) | (n = 18) | (n = 17) | (n = 10) | (n = 7) | |
| DORS | 9.5 ± 6.7 # | 0.005 ± 0.001 | −39.2 ± 5.9 # | 17.6 ± 3.0 # | −27.3 ± 4.1 # |
| (n = 16) | (n = 13) | (n = 14) | (n = 7) | (n = 6) | |
| DORS + ADPN | 8.5 ± 2.4 # | 0.007 ± 0.002 | −35.7 ± 4.9 # | 17.8 ± 2.8 # | −25 ± 2.3 # |
| (n = 16) | (n = 13) | (n = 14) | (n = 7) | (n = 6) |
| ICa,T | ICa,L | |||
|---|---|---|---|---|
| Parameters | CTRL | ADPN | CTRL | ADPN |
| ICa,p/Cm (pA/pF) | 1.7 ± 0.2 | 0.5 ± 0.1 *** | 9.42 ± 0.6 | 5.2 ± 0.4 ** |
| Gm/Cm (pS/pF) | 18 ± 5 | 5.5 ± 9 *** | 46 ± 5.1 | 32 ± 3.8 ** |
| Vthr (mV) | −54.8 ± 2 | −53.0 ± 2 | −50.2 ± 3 | −48.7 ± 3 |
| Vp (mV) | −25.1 ± 2 | −30.2 ± 1.6 ** | 0.5 ± 0.07 | 10.2 ± 1 *** |
| Va (mV) | −42.1 ± 3 | −40.0 ± 2 | −18.1 ± 2 | −10.2 ± 2 *** |
| ka (mV) | 7.2 ± 0.4 | 7.1 ± 0.5 | 7.6 ± 0.3 | 8.1 ± 0.4 |
| Vrev (mV) | 76.5 ± 6 | 80.1 ± 7 | 79.4 ± 6 | 81.7 ± 7 |
| Vh (mV) | −64.7 ± 6 | −64.8 ± 6 | −51 ± 4 | −53 ± 5 |
| kh (mV) | 4.5 ± 0.5 | 4.3 ± 0.4 | 7.5 ± 0.5 | 7.4 ± 0.5 |
| tp (ms) | 5.5 ± 0.5 | 5.8 ± 0.5 | 22.7 ± 2 | 21.8 ± 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Idrizaj, E.; Garella, R.; Nistri, S.; Dell’Accio, A.; Cassioli, E.; Rossi, E.; Castellini, G.; Ricca, V.; Squecco, R.; Baccari, M.C. Adiponectin Exerts Peripheral Inhibitory Effects on the Mouse Gastric Smooth Muscle through the AMPK Pathway. Int. J. Mol. Sci. 2020, 21, 9617. https://doi.org/10.3390/ijms21249617
Idrizaj E, Garella R, Nistri S, Dell’Accio A, Cassioli E, Rossi E, Castellini G, Ricca V, Squecco R, Baccari MC. Adiponectin Exerts Peripheral Inhibitory Effects on the Mouse Gastric Smooth Muscle through the AMPK Pathway. International Journal of Molecular Sciences. 2020; 21(24):9617. https://doi.org/10.3390/ijms21249617
Chicago/Turabian StyleIdrizaj, Eglantina, Rachele Garella, Silvia Nistri, Alfonso Dell’Accio, Emanuele Cassioli, Eleonora Rossi, Giovanni Castellini, Valdo Ricca, Roberta Squecco, and Maria Caterina Baccari. 2020. "Adiponectin Exerts Peripheral Inhibitory Effects on the Mouse Gastric Smooth Muscle through the AMPK Pathway" International Journal of Molecular Sciences 21, no. 24: 9617. https://doi.org/10.3390/ijms21249617
APA StyleIdrizaj, E., Garella, R., Nistri, S., Dell’Accio, A., Cassioli, E., Rossi, E., Castellini, G., Ricca, V., Squecco, R., & Baccari, M. C. (2020). Adiponectin Exerts Peripheral Inhibitory Effects on the Mouse Gastric Smooth Muscle through the AMPK Pathway. International Journal of Molecular Sciences, 21(24), 9617. https://doi.org/10.3390/ijms21249617








