Abstract
Aquaporins (AQPs) are universal membrane integrated water channel proteins that selectively and reversibly facilitate the movement of water, gases, metalloids, and other small neutral solutes across cellular membranes in living organisms. Compared with other organisms, plants have the largest number of AQP members with diverse characteristics, subcellular localizations and substrate permeabilities. AQPs play important roles in plant water relations, cell turgor pressure maintenance, the hydraulic regulation of roots and leaves, and in leaf transpiration, root water uptake, and plant responses to multiple biotic and abiotic stresses. They are also required for plant growth and development. In this review, we comprehensively summarize the expression and roles of diverse AQPs in the growth and development of various vegetative and reproductive organs in plants. The functions of AQPs in the intracellular translocation of hydrogen peroxide are also discussed.
1. Introduction
Aquaporins (AQPs) are ubiquitous membrane channel proteins that selectively and reversibly facilitate water movement across plasmalemma and organelle membranes in plants and other organisms [1,2]. In growing plants, water is constantly absorbed by roots, flows axially through xylem vessels and moves radially through apoplastic, symplastic and transcellular pathways to leaves, and evaporates through stomata. In the transcellular pathway, water traverses cellular membranes either by simple diffusion or more frequently by AQP-formed pores [1,2]. The rapid movement of water through the biomembrane is essential for the maintenance of cellular water homeostasis as well as for the accomplishment of various metabolic activities under many circumstances, for example during cell elongation, root water absorption, leaf movement, stomatal opening and closure, flowering and fertilization. Therefore, AQPs are greatly important for plant growth, development and survival [1,2,3,4].
AQPs transport not only water and H2O2, but also other neutral molecules with important physiological significance such as carbon dioxide (CO2), glycerol, ammonium, urea, and metalloids like boric acid, silicic acid and arsenic acid [2]. Some AQPs may also have the ability to conduct monovalent cations [5,6]. AQPs have been found to fulfill functions in responses to a variety of stresses like drought, osmotic stress, high salinity, cold, anoxia, nutrient unavailability and pathogen attack; in growth and development including seed germination, root growth, stem (shoot) elongation, leaf expansion, and reproductive organ development; and in the signal transduction of multiple hormones such as auxin, gibberellins (GAs), ethylene and abscisic acid (ABA) in plants [1,2,3,7].
In recent years, many review papers covering the structure, classification, localization, substrate specificity, trafficking, functions and regulation of AQPs have been published [2,3,7,8,9,10]. However, the roles of AQPs in the growth and development of diverse plant organs have not been comprehensively reviewed. Here, we mainly discuss the expression and functions of various AQPs during plant growth and development.
2. Classification, Characteristics and Regulation of Plant AQPs
AQPs belong to the membrane integrated major intrinsic superfamily proteins (MIPs) with small molecule weights (about 26 to 34 kDa), and are present in nearly all living organisms. Besides AQPs, MIPs contain two subfamily proteins: glycerol facilitators (GLPs/GlpFs) and aquaglyceroporins (GLAs). GLPs transfer glycerol and neutral molecules, and GLAs transport water, glycerol and other small solutes. All MIPs are comprised of six membrane-spanning alpha helices, five inter-helical loops, an Ala-Glu-Phe (AEF or AEFXXT) motif, two highly conserved Asp-Pro-Ala (NPA) motifs and an aromatic/arginine (ar/R) region. Both the NPA motifs and ar/R selectivity filter are important for determining the substrate selectivity of AQPs [8,11].
In cellular membranes, AQPs form homo- or hetero-tetramer pores, and each of the four subunits can generate a water channel pore for permeability of substrate molecules [2,3,8]. Based on their intracellular locations and sequence similarities, AQPs are categorized into five major subfamilies in higher plants: the plasma membrane intrinsic proteins (PIPs), tonoplast intrinsic proteins (TIPs), nodulin 26-like intrinsic proteins (NIPs), small basic intrinsic proteins (SIPs) and uncategorized intrinsic proteins (XIPs) [12]. Among these, PIPs and TIPs are predominant, and mainly mediate water flow across cells and subcellular compartments in plants. PIPs can be further divided into PIP1 and PIP2 subgroups. Each subgroup contains different isoforms named PIP1;1, and PIP1;2, etc. Similar to PIPs, TIPs are further classified into TIP1 (formerly called γ-TIP, similarly hereinafter), TIP2 (δ-TIP), TIP3 (α-TIP or β-TIP), TIP4 (ε-TIP) and TIP5 (ξ-TIP) subtypes, and the isoforms within these subtypes are designated as TIP1;1, TIP1;2, and so on. NIPs are also divided into NIPIs, NIPIIs and NIPIIIs based on their channel pore structures. NIPIs transport water, glycerol and lactic acid, while both NIPIIs and NIPIIIs are mainly permeable to metalloids such as silicic acid, boric acid, arsenic acid, selenite and germanic acid [13].
At present, AQP family members have been identified or investigated in more than 100 plant species [14,15]. Compared with animals and other organisms, plants contain the largest number of AQP homologs. Moreover, AQPs in plants are highly diverse in species, subcellular localizations, spatiotemporal expression patterns, solute permeability and functions [1,2,3,7]. The functional efficiency of an AQP is determined by its amounts and activity (opened or closed states, or gating) in the membranes, as well as its specific permeability for substrates. The abundance of an AQP is regulated by transcriptional or post-transcriptional processes. The activity of an AQP is controlled by posttranslational modifications (for example, phosphorylation and methylation), pH, Ca2+, interactions between one AQP with other AQPs or proteins, while the substrate specificity of an AQP is governed by its architecture [2,3,16,17,18,19]. The localizations, substrate permeability, properties and regulations of AQPs in plants have been widely reviewed in recent years [2,20,21].
3. Roles of AQPs in Vegetative Growth
3.1. Seed Germination
Seed germination is fundamental for seedling establishment. It commences with imbibition, followed by rehydration of the endosperm and embryo, cell vacuolation (large vacuole biosynthesis), metabolic activation, reserve mobilization, the elongation of the embryonic axis and radicle emergence. Most of these processes are inseparable from AQP-facilitated water uptake and transport in seeds [22,23,24,25].
Expression analysis revealed that many TIP1, TIP2 and PIP genes are strongly expressed or noticeably upregulated in mRNA and/or protein levels, whereas TIP3s are significantly downregulated during seed germination in many plants, highlighting their possible roles in germination [23]. The upregulated genes mainly include AtTIP1;1, AtTIP1;2, AtTIP2;1, AtTIP2;2, AtPIP1;1, AtPIP1;2, AtPIP1;3, AtPIP1;4, AtPIP2;1, AtPIP2;2 and AtPIP2;7 in Arabidopsis [25,26,27,28]; OsTIP1;1, OsTIP1;2, OsPIP1;1, OsPIP1;2, OsPIP1;3, OsPIP2;1, OsPIP2;4, OsPIP2;5, OsPIP2;7 and OsPIP2;8 in rice (Oryza sativa) [29,30]; PsTIP1;1 and PsPIP1;1 in pea (Pisum sativum) [31]; Bnγ-TIP2, BnPIP1 and BnPIP1;4 in oilseed rape (Brassica napus) [32,33]; and VfTIP1;1, VfTIP2;1, VfTIP2;2 and VfPIP2;1 in broad bean (Vicia faba var. minor) [34]. In contrast, transcripts and/or proteins of AtTIP3;1 and AtTIP3;2 of Arabidopsis [26,28], OsTIP3;1 and OsTIP3;2 of rice [30], and VfTIP3;1 and VfTIP3;2 of broad bean [34] dramatically decrease or vanish during seed germination (Figure S1).
Genetic evidence further reveals that AQPs are required for seed germination. For instance, in rice, knock-down of OsPIP1;3 leads to a clear reduction in seed germination rate under normal conditions [29]. Moderate or low overexpression of OsPIP1;1 also significantly enhances the seed germination rate and the activity of α-amylase [35] (Figure 1) (Table 1).
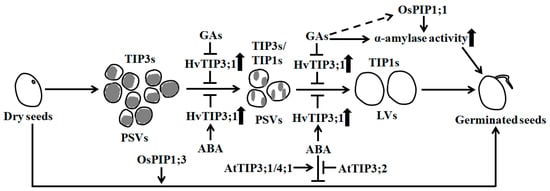
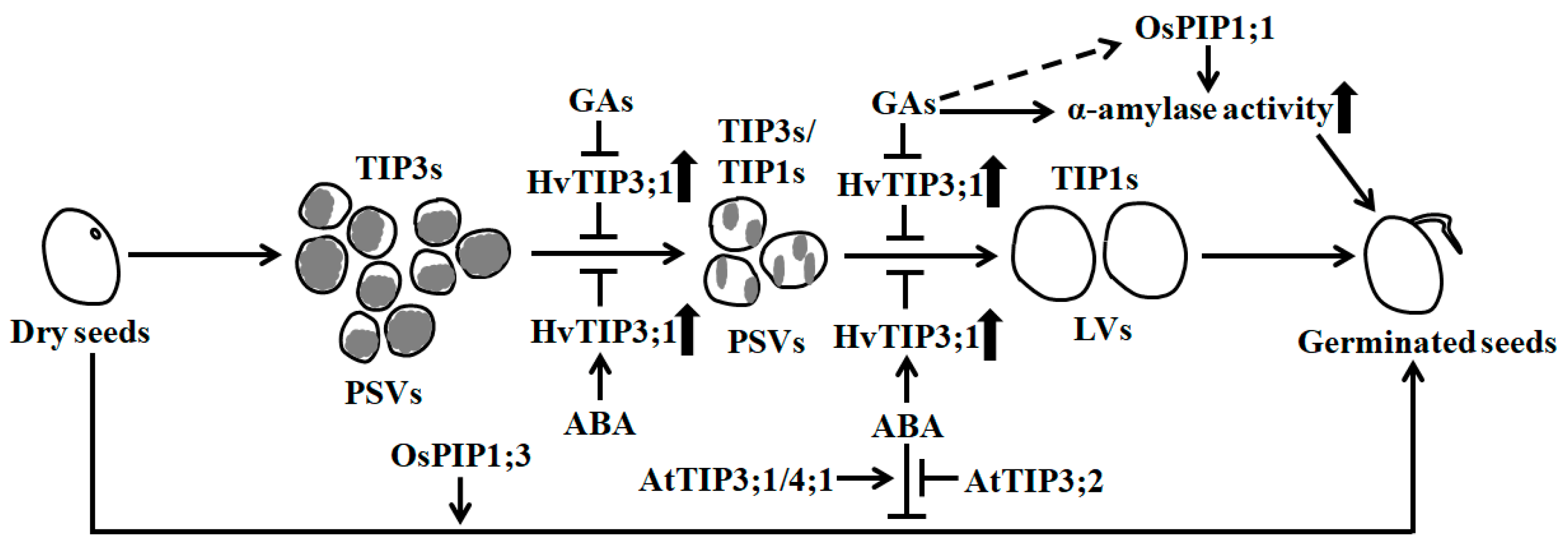
Figure 1.
Aquaporins are required for the germination of orthodox seeds through affecting vacuolation and α-amylase activity. During seed germination, vacuolation occurs. TIP3s are enriched in small protein storage vacuoles (PSVs), whereas TIP1s accumulate in large vacuoles (LV). TIP3s negatively affect the formation of LVs. Gibberellins (GAs) activate α-amylase activity and promote the conversion of PSVs to LVs by inhibiting HvTIP3;1 expression, but abscisic acid (ABA) has the opposite effect during barley seed germination [38]. AtTIP3;1 and AtTIP4;1 have positive effects, whereas AtTIP3;2 has negative effects on ABA-inhibited seed germination [39]. Rice OsPIP1;1 has a beneficial role in seed germination by inducing α-amylase activity, and OsPIP1;3 plays a positive role during seed germination [35]. Long solid arrows show the transition of seed germination, and short and thick up arrows indicate increases in the abundance of HvTIP3;1 and the activities of α-amylase. Short and thin solid arrows reveal positive regulation, and bars represent negative regulation. The dotted arrow represents unidentified regulation and the gray in PSVs shows the storage nutrients.

Table 1.
The roles of AQPs in the growth of vegetative organs.
In germinating seeds, some TIPs like TIP1s have been shown to function in vacuolar biosynthesis and in facilitating water flow into vacuoles, causing the mobilization of reserve substances, the establishment and sustainment of cell turgor pressure and the promotion of embryo cell elongation. Many PIPs such as PIP1s and PIP2s are found to act in water exchange between extracellular and cytoplasmic compartments, and are a requisite for water balance maintenance in the cytoplasm [22,23,36]. In dry orthodox seeds of higher plants, large amounts of nutrients like storage proteins have accumulated in many small protein storage vacuoles (PSVs) of cells. During germination, accompanied by the rapid flow of water into seeds through AQPs, the PSVs are fused and converted into large central lytic vacuoles (LVs), leading to the activation of multiple hydrolytic enzymes and a significant increase in cellular turgor pressure [23,37] (Figure 1). Thus, cell elongation is favored. TIP3s, the seed-specific expressed proteins, have been proven to be abundant in PSVs, while TIP1s are highly accumulated in LVs. During the formation of LVs in seed germination, the contents of TIP1s pronouncedly increase, while those of TIP3s drastically decrease or disappear [26,30,34]. The abundance of TIP1s are positively correlated to the formation of LVs, but those of TIP3s are negatively correlated [23,34] (Figure 1). Nevertheless, TIP3s are also found to favor optimal water uptake during the early stage of seed germination in Vicia faba [37].
In barley (Hordeum vulgare), the transformation of small PSVs into LVs in the aleurone cells of seed endosperm is modulated by gibberellins (GAs) and abscisic acid (ABA), two controllers of seed germination [60]. GAs promote the conversion of PSVs to LVs during seed germination, but ABA prevents it. GAs also specifically inhibit the expression of HvTIP3;1 and HvTIP1;2, while ABA prominently enhances the transcription of HvTIP3;1. The increase and deletion of HvTIP3;1 transcripts individually result in the delay of GA-induced vacuolation (coalescence of PSVs) and the stimulation of vacuolation, thereby inhibiting and promoting seed germination, respectively [38] (Figure 1). These results demonstrate that the transcriptional changes in HvTIP3;1, which are tightly controlled by GAs and ABA, are important for vacuolation and seed germination. However, the mechanism for HvTIP3;1 functioning in vacuolation is currently unknown. In Arabidopsis, genetic evidence shows that AtTIP3;1 and AtTIP4;1 are positive regulators of the ABA response, whereas AtTIP3;2 is a negative regulator of the ABA response during seed germination [39] (Figure 1). Thus, AtTIP3;1/4;1 and AtTIP3;2 antagonistically affect ABA-inhibited seed germination.
AQPs may also serve roles in the germination of recalcitrant seeds. In horse chestnut, AhTIP2, AhTIP3;1, AhPIP1 and AhPIP2 are enriched during seed germination. Active vacuoles that are maintained in the embryonic axis cells of hypocotyls and radicles after seed shedding initially enlarge, followed by vacuolation during seed germination. Moreover, cell vacuolation is accompanied by the activation of vacuolar acid invertase (breaking down the storage compound sucrose). After growth initiation, vacuole enlargement is facilitated by an AQP-mediated increase in water inflow [61].
3.2. Root Growth
Primary roots come from radicles in seeds. After seed germination, the growth of radicles is accompanied by the generation of LVs through the fusion of provacuoles in orthodox seed plants, or through the enlargement of preserved vacuoles in recalcitrant seed plants [23,61]. The formation of LVs is a prerequisite for cell elongation in that LVs act as osmotic compartments and produce turgor pressure to stimulate cell expansion. Evidence reveals that TIPs and PIPs are important for the syntheses of provacuoles and LVs through transferring water into vacuoles and cytoplasms during post-germinative root growth [22,23]. Consistently, VfTIP2;1 and VfTIP2;2 are highly expressed during post-germinative root growth in broad bean. Furthermore, the expression patterns of the two AQPs are in parallel with the formation of vacuoles from provacuoles in the meristems [34].
Apart from being expressed in radicles, PIPs and TIPs are enriched in the growing regions of roots or the root tip, where more growing cells and tissues exist [62,63,64]. In maize (Zea mays), ZmPIP1;2, ZmPIP2;4 and ZmTIP1;1 are highly expressed in the root growing zone [65]. Transcripts of ZmPIP1;1, ZmPIP1;5, ZmPIP2;1, ZmPIP2;5 and ZmPIP2;6 are abundant in the root tip, and the expression of most of the genes is developmentally regulated [66]. In barley, HvPIP1;2, HvPIP2;2, HvPIP2;5, HvTIP1;1 and HvTIP2;3 are strongly expressed in the growing tissue of roots [62]. VvPIP1;1, VvPIP1;2/1;4, VvPIP1;3/1;5, VvPIP2;1, VvPIP2;2, VvPIP2;3 and VvPIP2;4 are also dominantly expressed in the root tip of grapevine (Vitis vinifera), and the average transcript abundances of VvPIP1;1, VvPIP1;2/1;4 and VvPIP1;3/1;5 in the meristematic zone are 4–100 fold higher than in older root zones [63,67,68]. Moreover, the expression patterns of these VvPIPs are very similar to their homologs in Arabidopsis, including AtPIP1;1; AtPIP1;2, AtPIP1;3, AtPIP2;1; AtPIP2;3 and AtPIP2;6 [63,69] (Figure S1). The presence of high amounts of PIPs and TIPs in growing tissues might imply that these AQPs play important roles during root cell growth.
The attributes of PIPs and TIPs affecting root growth were further confirmed by transgenic experiments. In Arabidopsis, antisense plants with decreased expression of AtPIP1a and AtPIP1b have remarkably abundant roots compared to the control plants under normal condition [40], indicating that the two PIP1s have negative effects on root development, or the increased roots compensate for reduced cellular water permeability. Conversely, the overexpression of Vicia faba VfPIP1 in Arabidopsis significantly promotes the growth of primary and lateral roots (LRs) [41]. Transgenic Arabidopsis plants overexpressing Jojoba (Simmondsia chinensis) ScPIP1 also exhibit longer roots and better growth status than the wild type (WT) plants under normal growth condition [42]. The reason for this may be that both VfPIP1 and ScPIP1 are homologous proteins in the plasma membrane, likely having similar structures and functions in root growth. Besides PIPs, transgenic durum wheat (Triticum turgidum subsp. durum) cv. Maali expressing wheat TdPIP2;1, and transgenic Arabidopsis plants overexpressing of Panax ginseng PgTIP1, display increased root growth [43,44] (Figure 2a).
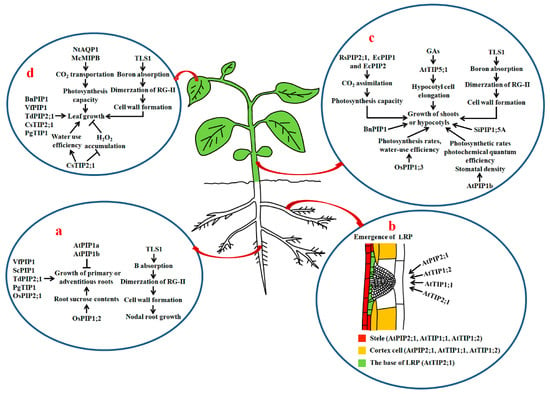
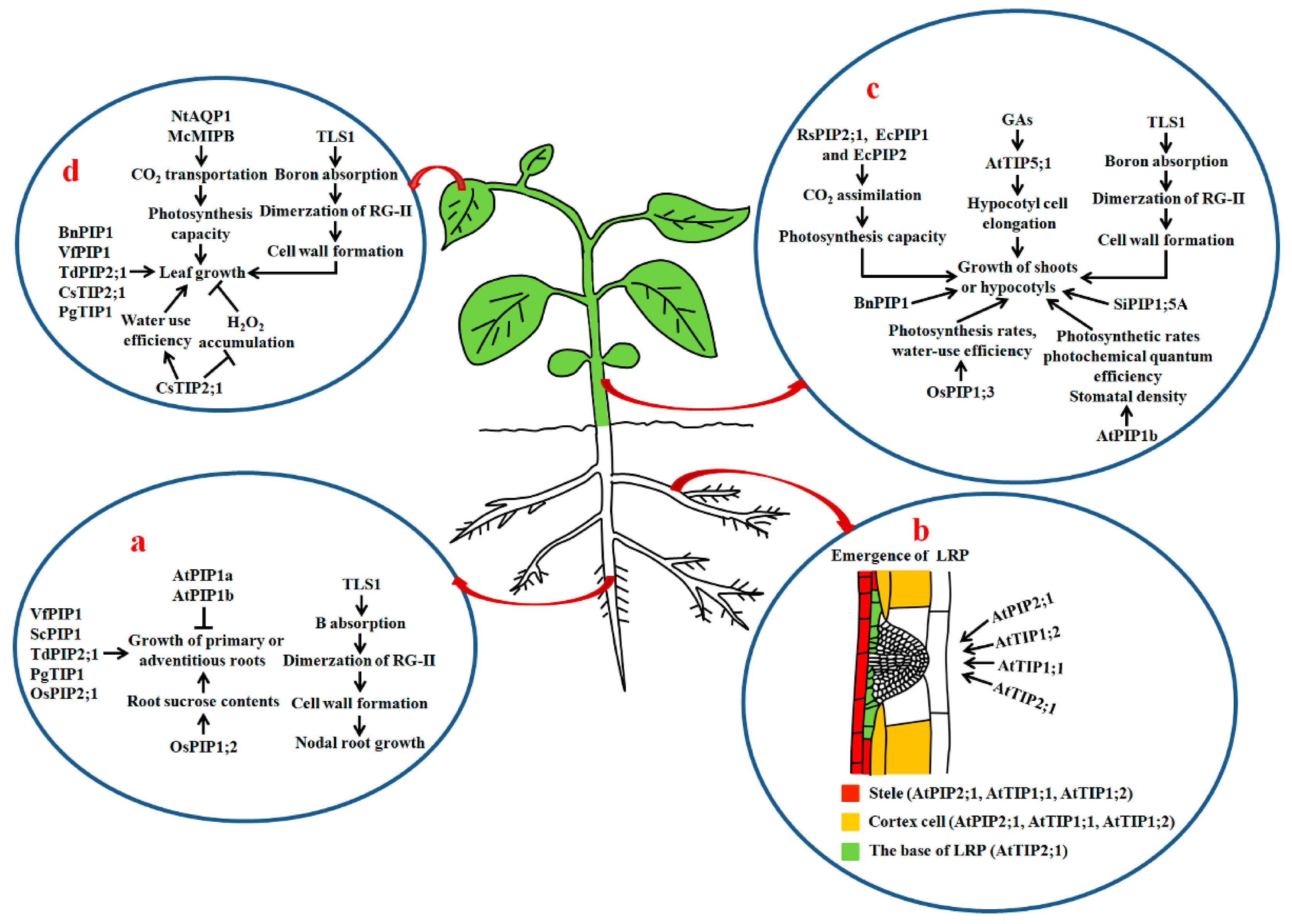
Figure 2.
The roles of AQPs in the growth of roots, shoots, hypocotyls and leaves in plants. (a) Roles of AQPs in growth of primary roots, adventitious roots and nodal roots; (b) effects of PIPs and TIPs on lateral root formation. The expression or localization of AtPIP2;1, AtTIP1;1, AtTIP1;2 and AtTIP2;1 is shown in color; (c) roles of AQPs in shoot growth and hypocotyl elongation; (d) actions of AQPs in leaf growth. Thick up arrows represent the increase in Lpr, the thin arrows show positive regulation, and bars show negative regulation. GAs: gibberellins; Lpr: root hydraulic conductivity; LRP: lateral root primordium; RG-II: rhamnogalacturonan II; TLS1: maize tassel-less1.
Péret et al. demonstrated that auxin stimulates LR development through regulating the spatial and temporal distribution of AQP-dependent water transport in root tissues of Arabidopsis. Auxin decreases AtPIP2;1 expression in cortical cells, but increases AtPIP2;8 expression at the base of the lateral root primordium (LRP) and underlying stele when LRPs emerge. Both overexpression and disruption of AtPIP2;1 causes defective LRs to emerge, suggesting that tight and spatiotemporal control of the AQP-mediated water flow in root tissues by auxin is necessitated for LR branching [45]. Recently, Reinhardt et al. reported that loss-of-function mutations of AtTIP1;1, AtTIP1;2 and AtTIP2;1 markedly inhibit the emergence of LRPs and decrease the number of LRs in Arabidopsis. They also found that the distributions and contents of the AtTIPs in the tonoplast of LRP cells modulate LR development. During LR formation, AtTIP2;1 is initially expressed in some defined cells at the base, then actively expressed at the flanks in LRPs. Moreover, disruptions of AtTIP1;1 and AtTIP1;2 in LRPs clearly delay the emergence of LRs [46] (Figure 2b) (Table 1). Accordingly, both the PIPs and TIPs act in the establishment of LRP patterning and the formation of LRs via finely controlling water flow at the sites of LR emergence in plants.
In rice, the overexpression of OsPIP1;2 leads to substantial enhancements of leaf CO2 conductance, photosynthetic performance and sucrose transport activity in the shoot phloem, and of root sucrose contents and root length [47]. Noteworthily, phloem sucrose transport from shoot to root exerts essential effects on root growth [70]. Moreover, sucrose influences root development as a signaling molecule and as an energy source [71]. Therefore, AQPs can play a role in root growth through facilitating leaf CO2 diffusion and phloem sucrose transport in plants (Figure 2a) (Table 1).
Root growth is mostly determined by cell division, elongation and differentiation in the root tip of plants. These cellular behaviors, especially cell elongation, are largely dependent upon the proper hydraulic conductivity of roots (Lpr) and root cells (Lprc), which are regulated by the functions of AQPs [2,3,72]. Pharmacological data reveal that AQPs contribute to the Lpr up to 64% in Arabidopsis [73], 70–80% in wheat (Triticum aestivum) [74], 60–70% in maize [65,75], 57% in tomato (Solanum lycopersicum) [76], and >90% in barley [62]. The mutation of AtPIP2;2 in Arabidopsis has been shown to decrease Lprc by about 25–30% [77]. Null mutant atpip1;2 displays a 20–30% reduction in Lpr [78]. Likewise, the inhibition of pea PsPIP2;1 expression by the VIGS method causes a significant blockage of water transport, and about a 29% and 20% reduction of Lpr and Lprc, respectively [79].
In maize, the Lprc values in two transgenic lines overexpressing ZmPIP2;5 increase by 67% and 69%, whereas Lprc values in a Zmpip2;5 knockout line decrease by 63% compared with the control [80]. The knock-down of OsPIP2;1 by RNAi in rice also leads to a decline in Lpr by approximately four fold. Compared to WT, the RNAi plants have markedly lowered root total length, surface area, root volume and fewer root tips [48] (Figure 2a) (Table 1).
Consistent with the roles of AQPs in the adjustment of Lpr and Lprc, and of root growth, Lpr in the meristematic and elongation zones is about 10 times greater than that in the secondary growth zone in grapevine fine roots. The inhibitory effects of the AQP blocker H2O2 on Lpr in the meristematic and elongation zones are far greater than those in the secondary growth zone [63]. Similarly, the Lprc in the growth zone is about 100 times higher than in the mature root region during gravitropic bending in pea roots [81].
Additionally, AQPs can exert effects on root growth by impacting the effective absorption of water and some nutrients from soil, and the subsequent distributions of these substances in plants [1,2,3,77,82]. It is known that the driving forces for water uptake and transport come from the potential differences of water along not only xylem vessels (the apoplastic and symplastic paths), but also transcellular routes due to the hydraulic barriers in the apoplasts of roots. AQPs play important roles in influencing the rapid flow of water via transcellular pathways and the maintenance of proper Lpr, thereby facilitating root water uptake and transportation [2,3,83,84,85]. AQPs are also helpful for root absorption and the transport of nutrients since some AQPs themselves have the capacity to transfer multiple nutrients like ammonium, urea and boric acid [1,2,86].
Besides PIPs and TIPs, NIPs play a role in root growth. In maize, a mutant tassel-less1 (tls1) has been characterized. The nodal roots of the mutant are clearly shorter than those of normal siblings. In addition, disruption of TLS1 results in thinning cell walls in the xylem, a reduced Casparian strip and less uniform cell shape (Figure 2a). Further studies revealed that TLS1 is an AtNIP5;1 homolog, and capable of transporting water and boric acid. Loss-of-function mutation of TLS1 causes boron deprivation, reduced dimerization of the pectic polysaccharide rhamnogalacturonan II (RG-II) of cell walls and disorder in meristem function [49] (Table 1).
3.3. Hypocotyls and Stems
The growth of hypocotyls and stems is dominantly attributed to cell elongation, which is regulated by AQP-dependent changes in hydraulic conductivity and cell turgor pressure. In Arabidopsis, AtTIP1;1 has been found to be highly expressed in hypocotyls, and its expression pattern is correlated with cell expansion [87]. Similarly, strong expression of TIP1;1 genes has been found in the growing tissues of shoots in multiple plants like maize, tulip (Tulipa gesneriana), cauliflower (Brassica oleracea) and oilseed rape [64]. PsPIP1;1, PsPIP2;1 and PsTIP1;1 are also preferentially expressed in shoots (stems) of pea seedlings [31]. Additionally, in castor bean (Ricinus communis), the transcripts and proteins of RcPIP1;1, RcPIP2;1 and RcTIP1;1 are very abundant in hypocotyl tissues. The abundance of RcPIP2;1 mRNA is positively correlated with the elongation activity of hypocotyls [88]. Besides, Muto et al. provided evidence that the transcripts of OsPIP1;1, OsPIP1;2, OsPIP2;1, OsPIP2;6, OsTIP1;1, OsTIP2;2 and OsSIP1;1 are enriched in the growing internodes in rice [89]. Both OsTIP1;1 and OsTIP2;2 have water channel activity when expressed in yeast cells. The transcription levels of SvPIP2;1 are also very high in the elongating stem in Setaria viridis [90] (Figure S1). Based on these observations, it is plausible that these PIPs, SIPs and especially TIPs as water transporters function in hypocotyl and stem growth.
Genetic experiments further demonstrated that AQPs are required for the elongation of hypocotyls and stems in plants. It has been reported that overexpression of AtPIP1b in tobacco observably increases the height and number of shoot internodes and stem diameter of transgenic plants under favorable growth conditions, but not under drought or salt stresses. Moreover, transpiration rates, photosynthesis rates, photochemical quantum efficiency and stomatal density of the transgenic lines are prominently higher than those of the control plants [50]. Transgenic tobacco plants expressing antisense BnPIP1 show thicker and shorter stems under normal conditions [51]. Also, the ectopic expression of radish (Raphanus sativus) RasPIP2;1 in Eucalyptus trees evidently stimulates the increase in assimilation of CO2 and shoot growth, whereas downregulation of Eucalyptus PIP1 and PIP2 by introducing RsPIP1;1 in Eucalyptus causes a clear inhibition of CO2 assimilation and shoot growth [52]. Similarly, the ectopic expression of rice OsPIP1;3 in tobacco (Nicotiana benthamiana) significantly promotes shoot growth. The transgenic plants show higher photosynthesis rates, Lpr, water use efficiency and biomass compared with the controls [53] (Figure 2c). These PIPs are specifically expressed in the plasma membrane, and might facilitate rapid water movement into or out of the cytoplasm to affect shoot growth through the alterations of leaf photosynthesis capacity, Lpr or stomatal development.
Pang et al. investigated the role of AtTIP5;1 in Arabidopsis, and found that the overexpression of AtTIP5;1 markedly increases hypocotyl cell elongation under normal or excess boron conditions [54]. Furthermore, AtTIP5;1 acts as an downstream target of GA signaling, positively influencing hypocotyl cell elongation in Arabidopsis [55]. In maize, the mutation of TLS1 (the homolog of AtNIP5;1) causes a clear reduction of seedling height, suggesting that TLS1-mediated boron transport and cell wall synthesis are important for shoot elongation [49]. These findings reveal that PIPs, TIPs and NIPs exert favorable effects in hypocotyl or shoot growth. However, transgenic tomato plants overexpressing Saussurea involucrata SiPIP1;5A exhibit dwarf phenotypes [56] (Figure 2c) (Table 1), hinting that different AQP isoforms may have distinct roles in affecting shoot elongation in plants.
AQPs also act in the modulation of the relative growth of shoots and roots. For example, Kaldenhoff et al. found that downregulation of PIP1a and PIP1b in Arabidopsis leads to a five-fold decrease in root abundance and a remarkable reduction of shoot/root ratio. Consistently, Aharon et al. demonstrated that the overexpression of Arabidopsis PIP1b in tobacco clearly enhances the shoot/root mass ratio due to a nearly 50% increase in shoot weight [50]. Transgenic rice plants overexpressing barley HvPIP2;1 also show about a 150% increase of shoot/root ratio compared with the control [91]. The reason for this may be that relatively small number of roots in plants with high activities of AQPs is sufficient to supply water for shoot growth. However, overexpressing OsPIP2;4 in two rice cultivars (Giza178 and IR64) does not cause marked changes in the shoot/root ratio [92], suggesting that the effects of AQPs on shoot/root ratio may depend upon AQP species and plant genotypes.
3.4. Leaf Growth
The leaf is the key plant organ where photosynthesis, respiration and transpiration occur. Leaf growth and development greatly depend upon the successful accomplishment of these metabolisms, which involve continuous transport of much water and CO2 in leaf cells and are heavily impacted by the expression and activities of AQPs [93,94]. Transcription studies indicate that changes in the expression of AtTIP1;1 are correlated with cell expansion in Arabidopsis leaves [87]. In maize, ZmPIP1;1, ZmPIP1;2, ZmPIP1;3, ZmPIP2;1 and ZmPIP2;2 are actively expressed in the elongation zones of leaves. Moreover, the expression of six ZmPIP1s (ZmPIP1;1, ZmPIP1;2, ZmPIP1;3, ZmPIP1;4, ZmPIP1;5, ZmPIP1;6) and six ZmPIP2s (ZmPIP2;1, ZmPIP2;2, ZmPIP2;3, ZmPIP2;4; ZmPIP2;5, ZmPIP2;6) is largely dependent on leaf developmental stages. The expression patterns of these genes are correlated with cell water permeability [95]. Besse et al. investigated the relationship between the expression of 23 AQP genes with leaf development in barley, and found that seven genes (HvPIP1;1, HvPIP1;5, HvPIP2;2, HvPIP2;5, HvTIP1;1, HvTIP2;3, and HvNIP1;1) are strongly expressed in the elongation zone (Figure S1). These data provide important clues about the involvement of AQPs in leaf growth [96].
Transgenic experiments give further evidence that PIPs and TIPs are necessary for leaf growth. For instance, transgenic tobacco plants expressing antisense BnPIP1 display deformed leaves. Furthermore, the leaf veins of antisense plants seem to extrude above the leaf surface compared with those of WT plants [51]. Overexpression of Vicia faba VfPIP1 in Arabidopsis causes a clear promotion of leaf growth [41]. Likewise, the overexpression of wheat TdPIP2;1 in durum wheat cv. Maali enhances leaf growth [43]. Overexpression of Panax ginseng PgTIP1 in Arabidopsis markedly increases the size of leaf mesophyll cells, most likely due to the roles of PgTIP1 in the promotion of water permeability of tonoplasts and cell enlargement [44]. The ectopic expression of citrus CsTIP2;1, a highly expressed gene in leaves, in tobacco also pronouncedly promotes leaf growth under normal growth conditions. Additionally, the CsTIP2;1 transgenic lines have improved water condition and water use efficiency, enhanced mesophyll cell expansion, midrib aquiferous parenchyma abundance, and decreased H2O2 accumulation in leaves [57] (Figure 2d) (Table 1).
AQPs contribute to leaf growth likely through enlarging the hydraulic conductivity of leaves (Lpl) in growing tissues [3,94]. It has been documented that AQPs account for about 25–50% of leaf Lpl in sunflower (Helianthus annuus), grapevine, diverse deciduous trees and Arabidopsis using AQP inhibitors [78,97,98,99]. Transgenic experiments disturbing PIP genes in Arabidopsis also revealed that approximately 35% of whole rosette Lpl and approximately 50% of Lpl are attributed to AQPs [100]. Similarly, silencing PsPIP2;1 by VIGS leads to a 29% reduction of Lpl and the hydraulic conductivity of leaf cells (Lplc) in pea plants [79]. High Lpl is commonly associated with greater leaf elongation rates [3,94,101]. Instantaneous leaf growth patterns are also considered to be largely driven by hydraulic relations in mangrove Avicennia marina [102].
In addition, AQPs play positive roles in leaf growth by affecting CO2 transportation, as numerous AQPs have the capacity to transport CO2 and are of importance for photosynthesis and leaf growth [9,103]. For instance, NtAQP1 is a CO2 permeable PIP in tobacco [104]. Overexpression of NtAQP1 in tobacco leads to marked increases in membrane permeability for CO2 and water, leaf mesophyll CO2 conductance, photosynthesis capacity and the promotion of leaf growth of the transgenic lines, whereas the suppression of NtAQP1 expression causes decreases in the photosynthesis rate [58,105]. Moreover, NtAQP1 can restore the Arabidopsis hexokinase 1-mediated growth inhibition of leaves and plants when expressed in tomato via positively influencing the conductance of CO2 [106]. In Arabidopsis, AtPIP1;2 controls the permeability to CO2. Overexpressing AtPIP1;2 in tobacco noticeably enhances transpiration rates and the photosynthesis efficiency of transgenic plants, whilst mutations in AtPIP1;2 decrease photosynthesis rates [107,108,109]. Similarly, overexpression of barley HvPIP2;1 in rice results in enhanced CO2 conductance and CO2 assimilation in leaves [110], and expressing Mesembryanthemum crystallinum AQP McMIPB in tobacco causes enhanced photosynthesis rates, mesophyll conductance to CO2 and leaf growth [59]. Transgenic rice lines overexpressing OsPIP1;2 also display notable increased biomass, yield, net CO2 assimilation, photosynthetic capacity and phloem sucrose transport compared with controls [47] (Table 1). Other AQPs that serve as CO2 transporters are HvPIP2;1, HvPIP2;2, HvPIP2;3 and HvPIP2;5 in barley [110,111,112], OsPIP1;1 in rice [113], ZmPIP1;5 and ZmPIP1;6 in maize [114], TcAQP1 in Terfezia claveryi [115], and PIP1;1 and PIP1;3 in Populus tremula × alba [116]. It has been addressed that isoleucine (I-254) at the C-terminal end of the E-loop of HvPIP2;3 is an essential amino acid residue for CO2 permeability in barley [111].
AQPs also play roles in leaf growth by impacting Lpr. It was reported that acid load, treatment with H2O2 or anoxia results in clear decreases of Lpr, which cause a significant decline in cell turgor in the elongating zone of leaves and xylem water potentials, and the resultant reduction of leaf elongation rates [75]. Similarly, fine-root Lpr is positively correlated with leaf area and transpiration in vines, and changes in AQP activities affected by drought and ABA impact Lpr variation and leaf growth [68,117]. Caldeira et al. also demonstrated that rhythmic leaf growth of maize under continuous light is tightly linked to the fluctuations of hydraulic conductance and of the expression of many PIPs (ZmPIP1;1, ZmPIP1;2, ZmPIP1;3, ZmPIP1;5, ZmPIP1;6, ZmPIP2;1, ZmPIP2;2, ZmPIP2;3, ZmPIP2;4, ZmPIP2;5 and ZmPIP2;6) in roots [118]. The underlying mechanism may be that changes in Lpr affected by PIPs or other AQPs cause alterations in the hydraulic conductivity of leaves and whole plants, thus stimulating leaf growth.
Besides, AQPs function in leaf growth via facilitating the transportation of boric acid. TLS1, a NIP in maize, has been shown to transport boric acid. Compared with normal siblings, mutant tls1 displays shorter and narrower leaves at the middle and lower parts of the plants. Moreover, leaf development is significantly defective in the mutant at the floral transition time [49] (Figure 2d) (Table 1).
4. Roles of AQPs in Reproductive Development, Seed Development and Dormancy
Reproduction and seed development are crucial for the survival and population multiplication of angiosperms. They involve flowering (generation of flower buds, formation of various floral organs including sepals, petals, stamens and carpels, flower opening), pollination, pollen germination and pollen tube growth, fertilization, formation of the zygote, and growth and development of embryos, seeds and fruits. During these processes, AQPs are important players in facilitating the transport of water and nutrients across cellular membranes.
4.1. Flower Bud Development, Petal Expansion and Flower Opening
Flower buds from fruit trees in temperate and boreal regions typically undergo endodormancy in winter, during which many complex activities involving water consumption are taken place, and AQPs may be required. Transcriptional studies revealed that the expression of the PpδTIP1 and PpPIP2 genes is pronouncedly enhanced in peach (Prunus persica) flower buds from November to January or February, the stage of bud endodormancy [119]. Yue et al. identified 20 AQP genes in tea (Camellia sinensis) plants and found that the expression levels of CsPIP2;4, CsPIP2;5, CsTIP1;1, CsTIP1;4, CsSIP2;1 and CsXIP are lower in dormant buds but higher in active buds. These findings signify that the AQP-mediated movement of water and some nutrients likely play roles in bud development.
In maize, the tls1 mutant of ZmNIP, a gene encoding the boric acid transporter, shows abnormal phenotypes during inflorescence development. Compared with normal siblings, the mutant plants have no tassel or defective tassels, and produce no ear or disabled ears. Further studies revealed that the disruption of TLS1 results in early defects in apical and axillary meristems in the inflorescence [49].
In rose, RhPIP2;1 is predominantly expressed in petal epidermal cells, and its expression is strongly linked to petal expansion. Silencing of RhPIP2;1 by RNAi leads to significant suppression of petal expansion [120]. Further investigations showed that ethylene regulates petal growth by impacting the expression of RhPIP2;1. Ethylene is generally regarded as a negative regulator of organ expansion [121]. Treatment with ethylene causes marked inhibition of petal cell expansion and a reduction of water content in rose petals. Moreover, ethylene downregulates the expression of RhPIP2;1, and ethylene-treated flowers are similar to those of RhPIP2;1-silenced plants in terms of the anatomical characteristics of the petals. Hence, ethylene regulates petal expansion and may rely on the roles of RhPIP2;1 [120]. In addition, RhPIP1;1 can interact with RhPIP2;1 to evidently increase the activity of RhPIP2;1, although RhPIP1;1 alone is incapable of transporting water. Furthermore, suppression of RhPIP1;1 markedly inhibits petal growth [122] (Figure 3a) (Table 2). RhTIP1;1 has also been shown to be preferentially expressed in rose petals. Its expression is highly correlated with the flowering process, and negatively regulated by ethylene [123].
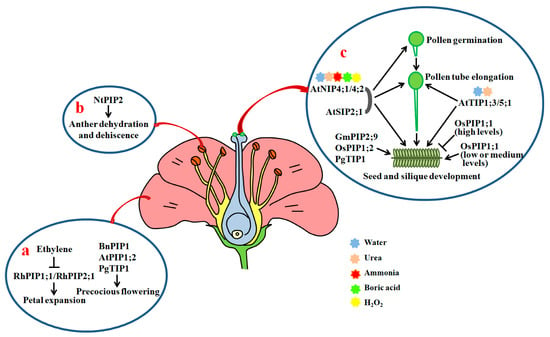
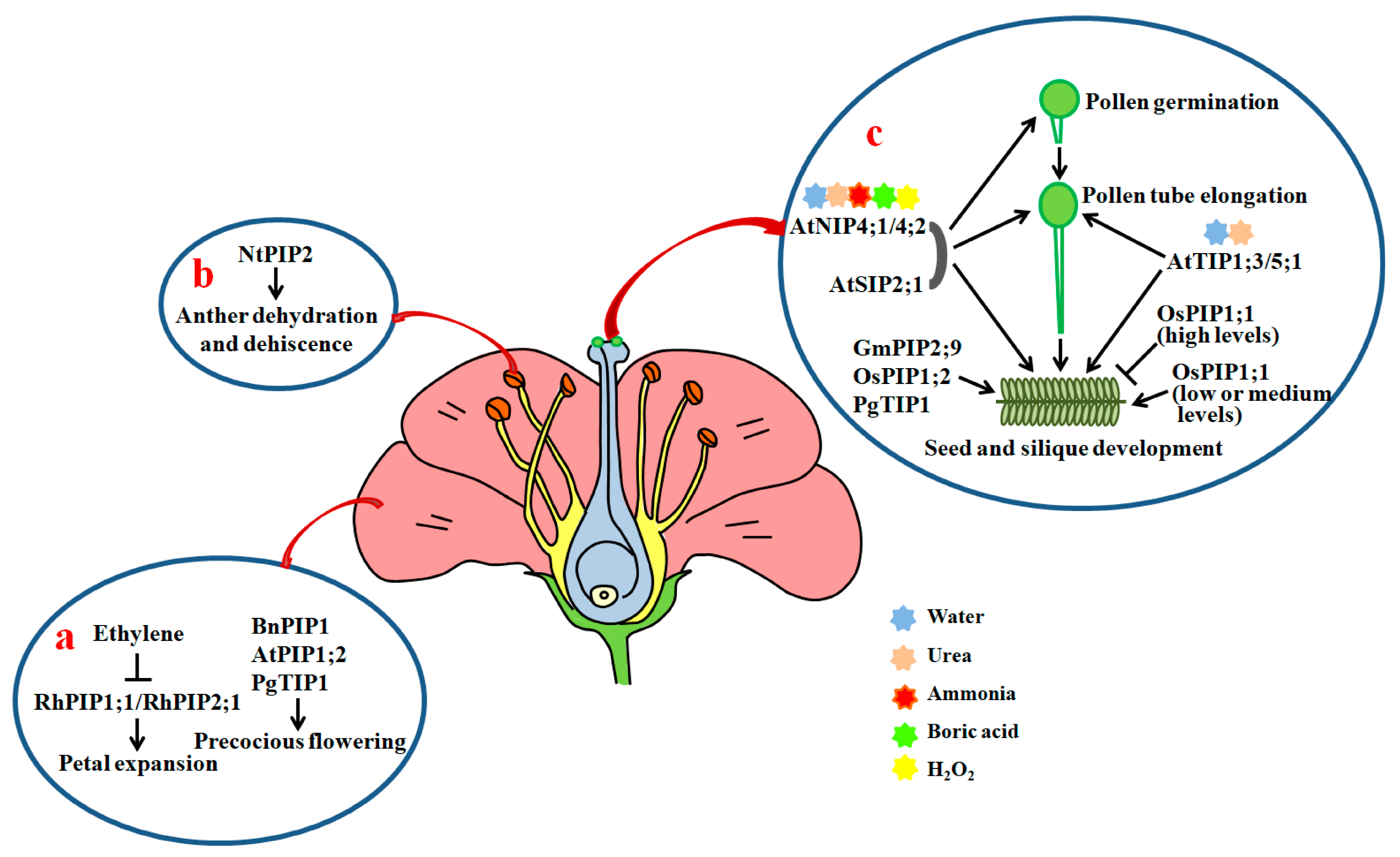
Figure 3.
Roles of AQPs in petal expansion, anther dehydration, pollen development, hydration, germination, pollen tube elongation and seed development. (a) Roles of AQPs in petal cell expansion and flowering; (b) effects of NtPIP2 on anther dehydration and dehiscence; (c) AtTIP1;3, AtTIP5;1, AtNIP4;1, AtNIP4;2 and AtSIP2;1 play positive roles in the promotion of pollen hydration, pollen development, pollen tube elongation, and seed and silique development through facilitating the diffusion of water, urea and other small solutes. Arrows show positive regulation and bars show negative regulation.

Table 2.
The roles of AQPs during reproductive organ development.
AQPs may also exert effects on flower opening. In tulip, TgPIP2;1 and TgPIP2;2 are abundantly expressed in petals. Petal opening and closing are accompanied by water transport, and are modulated by the reversible phosphorylation of a PIP, most likely TgPIP2;2 [132,133,134]. In tea plants, the transcription of many CsAQPs increases at the beginning of flower expansion, and remains at high levels during flower opening [135]. Likewise, AQP genes DcPIP1;1, DcPIP1;3, DcPIP2;1, DcPIP2;2, DcPIP2;5, DcTIP1;4, DcTIP2;2, DcTIP4;1, DcNIP6;1 and DcSIP1;1 are strongly expressed during all flower opening stages in carnation (Dianthus caryophyllus), and high expression levels of DcPIP1;1 and DcPIP2;1 are maintained throughout the flower opening process [136,137] (Figure S1). It has been found that expressing antisense BnPIP1 in tobacco causes a clear delay in blossom time [51]. Similarly, the Arabidopsis mutant atpip1;2 shows delayed flowering time in comparison with the WT [109]. Transgenic Arabidopsis plants overexpressing ginseng PgTIP1 also exhibit the phenotype of precocious flowering as compared with WT plants [44], indicating that PIPs and TIPs have favorable roles in flowering (Figure 3a) (Table 2).
4.2. Anther Dehydration, Pollen Development, Hydration and Germination, and Pollen Tube Elongation
In tobacco, NtPIP1 and NtPIP2 have shown to be actively expressed in the anther. NtPIP2 protein levels are regulated during anther development. Moreover, downregulation of NtPIP2 by RNAi results in a clear delay in anther dehydration and dehiscence in comparison with control plants [124,138] (Figure 3b). In rice, OsPIP1;1 and OsPIP4;1 are strongly expressed in the anther [35,139] (Figure S1). PIP1s are also expressed in the anther in Brassica sp. [140]. These findings indicate that PIPs are necessary for the dehydration and dehiscence of anthers.
Recently, Sato and Maeshima found that AtSIP2;1 is localized in the endoplasmic reticulum (ER). A mutation in AtSIP2;1 results in a significant reduction in the pollen germination rate in comparison with WT. Moreover, the pollen tube lengths of atsip2;1 are remarkably shorter than those of the WT, and a majority of pollen tubes from atsip2;1 stop elongating in the mid-region of pistils. Seeds in the bottom region of atsip2;1 siliques are also scarce, and siliques from atsip2;1 are evidently shorter than those from WT (Figure 3c). Further, the transcriptional levels of a key ER stress (misfolded protein accumulation in the ER caused by the disorder between the protein folding capacity of the ER and the client protein load) induced gene Binding protein 3 in pollen in atsip2;1 are markedly higher than those in WT [125]. This means that AtSIP2;1 exerts positive effects on pollen germination and pollen tube elongation, probably via the alleviation of ER stress in Arabidopsis.
In Arabidopsis, AtTIP1;3 and AtTIP5;1 have been found to be specifically expressed in pollen. The transcripts of AtTIP1;3 are primarily enriched in vegetative cells, while those of AtTIP5;1 are abundant in sperm cells or vegetative cells of pollen [126,141]. The elongation of pollen tubes from single mutant attip1;3 and attip5;1, and double mutant tip1;3/tip5;1 is inhibited compared with the control under nitrogen (N) deprivation conditions. Furthermore, loss-of-function mutations of AtTIP1;3 and AtTIP5;1 lead to clear increases in the abnormal rate of barren siliques (Figure 3c). Therefore, both AtTIP1;3 and AtTIP5;1 are required for pollen development and pollen tube growth [141]. Since AtTIP1;3 and AtTIP5;1 act as water and urea channels to remobilize N in mature pollen, the two AQPs may function via the transportation of N in Arabidopsis [126,142].
AtNIP4;1 and AtNIP4;2 are also pollen-specific water channel proteins, having permeability to ammonia, urea, boric acid, and H2O2 in addition to water. AtNIP4;1 is active in mature pollen and pollen tubes with low transcription activity. AtNIP4;2 is exclusively expressed in pollen tubes, and its expression levels significantly increase during pollen tube growth. Reductions in the expression of AtNIP4;1 and AtNIP4;2 by RNAi markedly inhibit pollen germination and pollen tube elongation, and decrease seed fertility. Further studies showed that the water permeable activities of AtNIP4;1 and AtNIP4;2 are regulated by phosphorylation at Ser-267 of the C termini by a pollen-specific calcium-dependent protein kinase 34 [127,143] (Figure 3c). Thus, the kinase-modulated AtNIP4;1 and AtNIP4;2 are important for pollen germination and pollen tube elongation.
In rice, overexpression of OsPIP1;1 at very high levels markedly decreases fertility, whereas expression of the gene at low or medium levels raises seed yield but does not affect single grain weight [35], suggesting that OsPIP1;1 acts in a seed setting probably via affecting pollen germination in the stigma or pollen tube growth (Table 2). The underlying mechanism remains to be determined.
4.3. Fruit Development and Ripening
Fruits are rich in water and various compounds, which are stored within the vacuoles. Fruit growth and development result from cell division, especially cell expansion. Cell expansion largely depends upon AQP-mediated water uptake into the vacuole, which is driven by the high osmotic pressure generated by the compounds. Therefore, AQPs may play important roles in fruit development [144,145]. In pear, TIPs are the most abundant proteins in fruits, and TIP proteins and γ-TIP transcripts are enriched in young fruits. Similarly, peach γ-TIP is highly expressed in the early stage and at the end of fruit growth, and changes in the expression of γ-TIP appears to be associated with the fruit growth rate [144]. In watermelon (Citrullus lanatus), the mRNA of 12 ClAQP genes is identified in at least one developmental stage of fruits. Among these, ClTIP1;2 and ClTIP1;3 are strongly expressed at four key stages of fruit development [146]. These data provide clues that TIPs are likely more important for fruit growth than other types of AQPs in pear, peach and watermelon.
In tomato, LePIP1;1, LePIP1;4 and LePIP1;5 are predominantly expressed during fruit development [147]. Both FaPIP1;1 and FaPIP2;1 are strongly expressed during fruit ripening in strawberry (Fragaria × ananassa) “Camarosa” cultivar. The expression of FaPIP1;1 is fruit-specific and correlated with fruit ripening [148]. In apple, two genes (MdPIP1a and MdPIP1b) are highly expressed at the fruit expanding stage [149]. The expression levels of MdPIP1;3 increase with two peaks, in accordance with the two cell expansion periods during fruit development. Additionally, overexpression of MdPIP1;3 in tomatoes markedly promotes fruit growth during the expansion stage. Transgenic tomatoes are also significantly bigger and heavier than WT tomatoes [128] (Table 2). These results indicate that PIPs may be the main participators of fruit development in the three plants.
Intriguingly, in tomato cultivar “Micro-Tom”, the transcripts of SlTIP3;1, SlTIP3;2, SlPIP1;1, SlPIP1;2, SlPIP1;7, SlNIP2;1, SlNIP4;1, SlNIP5;1, SlNIP6;1, SlSIP2;1 and SlXIP1;1 are rich in fruits, and most of the genes are expressed in a fruit development-specific manner [150]. In grape plants, the transcription of several AQPs like VvTIP1;2, VvTIP2;1, VvPIP1;2, VvPIP1;3, VvPIP2;1, VvPIP2;3 relies on berry developmental stage. The expression of most of the genes is strong in young berries, and downregulated during berry ripening, while that of VvTIP1;2, VvTIP1;3, VvPIP2;3 and VvPIP2;5 is upregulated at the onset of ripening and later during maturation of the berry. Moreover, the highly expressed AQP genes in young berries are frequently seen in dividing and elongating cells, and in the cells participating in water and solute transport [151,152]. Hu et al. identified 47 AQP genes in banana plants and analyzed the expression patterns of 40 genes at different stages of fruit development and ripening. Twenty-five and thirty genes were found to be expressed in the “FJ” and “BX” varieties, respectively, during all stages of fruit development. MaPIP2;10, MaPIP1;6, MaPIP2;7, and MaSIP1;1 in “FJ”, and MaTIP4;1 and MaPIP2;5 in “BX” show high expression at all tested stages [153] (Supplementary Figure S1). Likewise, 12 CsPIP genes revealed to be differentially expressed during fruit development in cucumber plants, and CsTIP1;1, CsTIP2;1 and CsPIP1;3 are expressed in dynamic and fruit-specific patterns [154,155]. It appears that TIPs and PIPs, as well as NIPs, SIPs and XIPs, are modulators of fruit development in these plants.
4.4. Seed Development and Dormancy
Generally, seed development can be divided into three stages: the early cell division and embryogenesis after the double fertilization, seed maturation (including cell expansion, differentiation and the accumulation of storage compounds in the embryo), and late maturation of seeds including the acquisition of desiccation tolerance [156,157]. During the first two stages, substantial changes in the water and solute contents occur in seeds, and high amounts of water and nutrients are required for cell division, elongation and differentiation. As essential facilitators of water and solutes, AQPs may be involved in the transportation of these substances required for seed development. At the late stages of maturation, especially for orthodox seeds, AQPs serve roles in rapid water efflux, leading to seed dehydration and the accumulation of large amounts of dry mass [25,39].
Transcription analyses revealed that many AQP genes are actively expressed in diverse seed tissues and at different stages of seed development in plants. In Arabidopsis, transcripts of AtPIP1;2, AtPIP1;3, AtPIP1;4, AtPIP1;5, AtPIP2;1, AtPIP2;2/2;3, AtPIP2;5, AtPIP2;7/AtPIP2;8, AtTIP1;1, AtTIP2;2, AtTIP3;1, AtSIP1;1, AtSIP1;2 and AtSIP2;1 are abundant in one or more tissues, and at certain stages of seed development [25]. Also, a number of genes are strongly expressed in the early periods of seed development, like ScPIP2s in the ovules of Solanum chacoense; PtNIP1;1 in the suspensors of Pinus taeda; PsPIP1;1, PsPIP2;1, PsTIP1;1 and PsNIP1;1 in the expanding cotyledons and seed coats of pea [31]; LePIP1;1, LePIP1;2, LePIP1;4, LePIP1;5 and LePIP2;1 in tomato [147]; PvPIP1;1, PvPIP2;2 and PvPIP2;3 in seed coats of French bean [22,158]; and OsPIP1;1, OsPIP1;2, OsPIP2;1, OsPIP2;2, OsPIP2;6, OsTIP2;2, OsTIP4;2, and OsNIP1;1 in rice [159], pointing to the potential roles of these AQPs in seed development (Figure S1).
In soybean (Glycine max), GmPIP2;9 is highly expressed in developing pods and the seed hilum, where assimilation and water transport occur. GmPIP2;9 has high water channel activity. Moreover, overexpression of GmPIP2;9 causes pronounced increases in the pod number, seed number, and seed weight per plant, indicating that GmPIP2;9 may facilitate water flow in pod walls from the seed coat, and play a positive role in developing seeds, seed setting and filling [129]. In rice, overexpression of OsPIP1;2 markedly enhances the number of spikelets per panicle and yield, reflecting the roles of the AQP in seed development. The major mechanism for this may be that OsPIP1;2 favors mesophyll CO2 conductance, further causing the enhancements of net CO2 assimilation rate, photosynthetic capacity and phloem sucrose transport, but not due to OsPIP1;2 facilitation of water transport in leaves [47]. Besides, overexpression of Panax ginseng PgTIP1 in Arabidopsis promotes the development of seeds [44] (Table 2).
AQPs are also of importance for seed maturation. During the maturation of orthodox seeds, up to 90% of water is lost. Concomitantly, LVs convert into numerous PSVs, and large quantities of protein reserves are accumulated in the PSVs. Accompanied by the formation of PSVs, compositions of TIP3s significantly increase, while those of TIP1s noticeably decrease in seeds. Moreover, the expression of TIP3s and TIP1s genes is remarkably altered [28,160]. In Arabidopsis, the transcripts and/or proteins of AtTIP3;1 and AtTIP3;2 are found to be abundant during seed maturation [26,28]. Moreover, disruptions of both AtTIP3;1 and AtTIP3;2 genes clearly reduce seed longevity and increase the accumulation of H2O2 in seeds. The promoters of AtTIP3;1 and AtTIP3;2 can be bound and activated by the transcription factor ABA insensitive 3, a master regulator of seed maturation, in the presence of ABA [130]. Thus, AtTIP3;1 and AtTIP3;2 act as positive modulators of seed maturation and seed longevity by transporting water and H2O2 under the control of ABA.
In rice, the transcripts and protein contents of OsPIP2;1, OsTIP2;2 and OsTIP3;1 are very abundant in the mid-grain filling stage of seeds. OsPIP2;1 mainly accumulates in the starchy endosperm, nucellar projection, nucellar epidermis, and dorsal vascular bundles, while OsTIP3;1 is enriched in the aleurone layer and starchy endosperm during rice grain filling [159]. Besides, barley HvTIP3;1 has been shown to be specifically expressed after the middle ripening stage of seed development, with its expression reaching a peak during seed desiccation. HvTIP3;1 proteins accumulate in aleurone cells and in the outer layers of developing seeds, and have water permeability when co-expressed with HvTIP1;2 in Xenopus oocytes [161]. AhTIP3;1 is also implicated in the formation of small vacuoles in the maturing seeds of horse chestnut [61]. Therefore, PIP2s, TIP2s and TIP3s may function in seed maturation by transporting water and solutes.
AQPs also play roles in seed dormancy. Footitt et al. provide genetic evidence that AtTIP3;1, AtTIP3;2 and AtTIP4;1 are involved in the modulation of seed dormancy. All three TIPs have inhibitory roles in primary dormancy induction. AtTIP3;1 and AtTIP3;2 act antagonistically in the induction of secondary dormancy. AtTIP3;2 and AtTIP4;1 exert repressed effects, but AtTIP3;1 has promoted effects on the secondary dormancy induction during dormancy cycling in Arabidopsis. Gene and protein expression of AtTIP3;1 and AtTIP3;2 is also changed during seasonal dormancy cycling. The expression levels increase when seed dormancy levels enhance, and vice versa [39] (Table 2).
4.5. Fiber Elongation
Cotton fibers are unicellular trichomes that originate from the ovule epidermis. Their formation is due to the rapid growth of fiber cells. AQPs function in fiber elongation. It is reported that the genes GhγTIP1, GhPIP1;2, GhPIP2;3, GhPIP2;4, GhPIP2;5 and GhPIP2;6 are preferentially expressed in fibers in Gossypium hirsutum, and the transcripts of the latter four GhPIP2s reach peak values in the stage of fiber cell rapid elongation. Moreover, the overexpression of GhPIP2;3, GhPIP2;4 or GhPIP2;6 in yeast markedly promotes longitudinal growth of the host cells. Knockdown of GhPIP2s in cotton by RNAi clearly inhibits fiber elongation. In addition, GhPIP2;3, GhPIP2;4, GhPIP2;5 and GhPIP2;6 are able to form heterotetramers, and their water channel activities increase accordingly [162]. Transcriptome analyses also revealed that the expression of seven PIPs, four TIPs and two NIPs in cotton short fiber mutants Li1 and Li2 remarkably decreases in developing fibers as compared with WT plants. Additionally, the osmotic pressure of fiber cells in the mutants is markedly reduced during the rapid cell elongation of fibers [163] (Table 2). These findings highlight the possible roles of the PIPs, TIPs and NIPs in the osmoregulation of fiber growth in cotton.
The roles of AQPs in fiber cell elongation are also confirmed in Milkweed (Calotropis procera), a kind of plant generating long seed trichomes. In the plant, the CpPIP2 gene is strongly expressed in trichomes, and peaks in its expression when trichome cells elongate at the highest rate. Furthermore, overexpression of CpPIP2 in tobacco prominently increases the number of trichomes on leaves and stems [131] (Table 2).
5. AQPs Serve Roles in Plant Growth and Development through Transferring ROS across Membranes
Reactive oxygen species (ROS) such as the superoxide radical and H2O2 are toxic byproducts of cellular metabolism in plants. They also act as signal molecules to regulate stomatal movement, programmed cell death, hormone signal transduction, plant responses to diverse environmental stimuli, as well as growth and development, including seed development and germination, primary root elongation, lateral root branching, root hair formation, shoot growth and flower development [164,165,166,167,168,169,170]. Several lines of evidence suggest that plant AQPs can conduct H2O2 across the biological membrane. For instance, Arabidopsis AtTIP1;1, AtTIP1;2, AtTIP2;3, AtPIP2;1, AtPIP2;2, AtPIP2;4, AtPIP2;5, AtPIP2;7 and AtNIP1;2 are permeable to H2O2 in yeast cells [171,172,173,174]. It has also been found that rice OsNIP3;2, OsNIP3;3 and OsTIP2;2, barley HvPIP2;5, HvTIP2;2, HvTIP2;3 and HvTIP5;1, maize ZmPIP2;5, tulip TgTIP1;1 and TgTIP1;2, tobacco NtXIP1;1, tomato SlXIP1;1 and potato StXIP1;1 transport H2O2 in yeast [175,176,177,178,179]. Moreover, AtPIP2;1, AtPIP1;4 and other AtPIP2s facilitate H2O2 transportation in Arabidopsis [180,181,182].
AQPs may play essential roles in the detoxification and signaling of ROS through facilitating intracellular translocation of H2O2 during growth and development. In plants, H2O2 is mainly generated in the apoplast, plasma membrane, chloroplast, mitochondria, peroxisome and ER [183]. AtPIP1;4, has been shown to transfer apoplast H2O2 induced by bacterial pathogen and pathogen-associated molecular patterns (PAMPs) to the cytoplasm to activate systemic acquired resistance and immune responses in Arabidopsis [181]. Likewise, AtPIP2;1 mediates the transportation of H2O2 from the apoplast to the cytoplasm of guard cells during ABA- and PAMP flg22-triggered stomatal closure [182]. In addition, AtPIP2;1 favors the movement of both H2O2 and water during stomatal closure [182,184]. AtPIP2;4 and spinach SoPIP2;1 also transport both H2O and H2O2 [185], implying that many water permeable AQPs are H2O2 transporters in plants. Indeed, five out of 13 Arabidopsis AtPIPs can conduct H2O2 in yeast [173]. It seems that some AQPs are localized in the chloroplast envelope and aid the transport of H2O2 from the inside to the outside of the chloroplast, since the AQP inhibitor acetazolamide significantly suppresses H2O2 release from isolated chloroplasts under high light [186]. H2O2 derived from the chloroplast has been found to affect the level of nuclear H2O2 and gene expression [187]. Accordingly, changes in the activities of chloroplast envelope AQPs likely impact light signaling and plant growth. Some AQPs like AtTIP5;1 have been documented or predicted to be localized to the mitochondria in plants [126,188], yet it is unclear whether these AQPs transfer H2O2 across the mitochondrial membrane. To date, no AQP has been detected in plant peroxisomes. However, evidence reveals that porin-like channels or proteins exist in the peroxisomal membrane in castor bean, sunflower and cotton plants. These porins transfer small metabolites like succinate, malate and aspartate through the membrane [189,190]. They probably facilitate H2O2 transport across peroxisomal membrane under specific conditions. Several AQPs like Arabidopsis SIP1;1, SIP1;2 and SIP2;1 are localized to the ER, and the first two AQPs, but not the third, show permeability to water. SIP2;1 acts in pollen germination and pollen tube growth possibly through the alleviation of ER stress, and is an ortholog of mammalian AQP11 [125]. Recently, AQP11 has been demonstrated to transport H2O2 out of the ER [191]. It is possible that SIP2;1 is a H2O2 transporter during pollen development. Whether SIP2;1 is permeable to H2O2 deserves further investigation.
In the presence of ABA, the H2O2 transport activity of AtPIP2;1 is activated by the brassinosteroid insensitive 1-associated receptor kinase 1 and/or open stomata 1/Snf1-related protein kinase 2.6-mediated phosphorylation of Ser121 [182,184]. The activities and expression of AQPs are also modulated by H2O2 levels. H2O2 treatment clearly inhibits the water transport capacity of AQPs in maize [192]. H2O2 application also changes AQP expression in Arabidopsis [173], indicating that a feedback regulation of AQP-facilitated H2O2 diffusion may exist. Besides, alterations in the H2O2 level can influence the subcellular localization of an AQP. H2O2 treatment results in the redistribution of AtPIP2;1 from the plasma membrane to the endomembane [193]. Therefore, the distribution and permeability of AQPs and H2O2 levels are dynamic and mutually affected, and AQPs may play roles in growth and development as ROS signaling transducers and modulators of ROS homoeostasis in plants.
6. Concluding Remarks and Perspectives
AQPs are widely distributed in the plasma membrane and other organellar membranes, and play versatile roles in nearly every aspect of plant growth and development by affecting the water homeostasis of cells and whole plants, leaf photosynthesis capacity and cell wall formation, among others. Accumulating evidence suggests that TIPs are essential for cell expansion since they predominantly contribute to the formation of large central vacuoles and the buildup of cell turgor pressure, the driving force for cell extension. PIPs seem to function in cell division in plants [2,36,63]. By contrast, NIPs are required for cell division and cell elongation primarily via the transportation of nutrients like boric acid. Boron participates in cell wall expansion and is crucial for cell formation and cellular functions. Yet, information on the involvement of SIPs and XIPs in growth and development is relatively scarce.
In the past few decades, much progress has been achieved in understanding the functions and regulatory mechanisms of various AQPs. Enormous transcriptional evidence and a great number of forward and reverse genetic data concerning the roles of AQPs in plant growth and development have been accumulated. However, it is still very hard to define the precise contributions of individual AQPs to growth and development in plants because water is ubiquitous and transported along not only the AQP-involved transcellular pathway, but also xylem vessels, and the apoplastic and symplastic routes. Moreover, a plant commonly has a number of AQP members which operate redundantly and mutually affect each other [2,161]. AQPs can also interplay with other proteins or exogenous AQPs (ectopic expression of AQPs), and the expression and activities of numerous AQPs are regulated by many mechanisms [3,194]. Consequently, the effects of one AQP on growth and development almost integrate with those of other AQP homologs or proteins. Therefore, researchers need to develop new technologies and methods to unveil the specific roles and underlying mechanisms of individual AQPs in plant growth and development.
It is well known that plant growth and development are ultimately controlled by various phytohormones. AQPs commonly act downstream of hormones signaling and are downstream targets of some hormones such as auxin, GA, ABA and ethylene in root formation, seed germination or petal expansion [38,45,195]. However, the detailed mechanisms for most of the AQPs are still not clear. Moreover, it remains to explore whether and how other hormones like brassinostroid, cytokinin and salicylic acid modulate plant growth and development through affecting the expression and activities of AQPs.
As described above, AQPs play important roles in plant growth and development under diverse environmental stimuli. The effects of RhPIP2;1 in the balance between growth and survival is emerging [196]. Yet, our knowledge about the role of AQPs in the coordination of different growth and stress signals through the exchange of water and solutes across biological membranes, and the underlying mechanisms, is largely lacking.
At present, AQPs have been considered to be important candidate genes for engineering to improve crop stress tolerance and yields [42,197]. Therefore, much effort should be made to better understand the complex regulatory mechanisms of AQPs to optimize the efficient utilization of water and nutrients for healthy plant development, especially under stress conditions. We believe that the specific functional mechanisms of AQPs will be elucidated with the development of various omics technologies and other new technologies like CRISPR–Cas9 in the future.
Supplementary Materials
Supplementary materials can be found at https://www.mdpi.com/1422-0067/21/24/9485/s1.
Author Contributions
F.H., Y.W. and L.S. wrote the manuscript. Z.Z. and F.L. contributed to the correction of the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (31870248 and 31801274).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Abbreviations
| ABA | Abscisic acid |
| AEF or AEFXXT | Ala-Glu-Phe |
| AQPs | Aquaporins |
| ar/R | Aromatic/arginine |
| CO2 | Carbon dioxide |
| ER | Endoplasmic reticulum |
| GAs | Gibberellins |
| GLAs | Aquaglyceroporins |
| GLPs/GlpFs | Glycerol-facilitators |
| gs | Stomatal conductance |
| H2O2 | Hydrogen peroxide |
| Lpl | Hydraulic conductivity of leaves |
| Lpr | Hydraulic conductivity of roots |
| Lprc | Hydraulic conductivity of root cells |
| LR | Lateral root |
| LRPs | Lateral root primordia |
| LVs | Lytic vacuoles |
| MIPs | Intrinsic superfamily proteins |
| N | Nitrogen |
| NIPs | Nodulin 26-like intrinsic proteins |
| NPA | Asp-Pro-Ala |
| PAMP | Pathogen-associated molecular pattern |
| PEG | Polyethylene glycol |
| PIPs | Plasma membrane intrinsic proteins |
| ROS | Reactive oxygen species |
| Si | Silicon |
| SIPs | Small basic intrinsic proteins |
| TIPs | Tonoplast intrinsic proteins |
| WT | Wild type |
| XIPs | Uncategorized intrinsic proteins |
References
- Maurel, C.; Verdoucq, L.; Luu, D.-T.; Santoni, V. Plant aquaporins: Membrane channels with multiple integrated functions. Annu. Rev. Plant Biol. 2008, 59, 595–624. [Google Scholar] [CrossRef] [PubMed]
- Maurel, C.; Boursiac, Y.; Luu, D.T.; Santoni, V.R.; Shahzad, Z.; Verdoucq, L. Aquaporins in plants. Physiol. Rev. 2015, 95, 1321–1358. [Google Scholar] [CrossRef] [PubMed]
- Chaumont, F.; Tyerman, S.D. Aquaporins: Highly regulated channels controlling plant water relations. Plant Physiol. 2014, 164, 1600–1618. [Google Scholar] [CrossRef] [PubMed]
- Kaldenhoff, R.; Ribas-Carbo, M.; Sans, J.F.; Lovisolo, C.; Heckwolf, M.; Uehlein, N. Aquaporins and plant water balance. Plant Cell Environ. 2008, 31, 658–666. [Google Scholar] [CrossRef]
- Byrt, C.S.; Zhao, M.; Kourghi, M.; Bose, J.; Henderson, S.W.; Qiu, J.; Gilliham, M.; Schultz, C.; Schwarz, M.; Ramesh, S.A.; et al. Non-selective cation channel activity of aquaporin AtPIP2;1 regulated by Ca2+ and pH. Plant Cell Environ. 2017, 40, 802–815. [Google Scholar] [CrossRef] [PubMed]
- Kourghi, M.; Nourmohammadi, S.; Pei, J.V.; Qiu, J.; McGaughey, S.; Tyerman, S.D.; Byrt, C.S.; Yool, A.J. Divalent cations regulate the ion conductance properties of diverse classes of aquaporins. Int. J. Mol. Sci. 2017, 18, 2323. [Google Scholar] [CrossRef]
- Afzal, Z.; Howton, T.C.; Sun, Y.; Mukhtar, M.S. The roles of aquaporins in plant stress responses. J. Dev. Biol. 2016, 4, 9. [Google Scholar] [CrossRef]
- Deshmukh, R.K.; Nguyen, H.T.; Belanger, R.R. Editorial: Aquaporins: Dynamic role and regulation. Front. Plant Sci. 2017, 8, 1420. [Google Scholar] [CrossRef]
- Pawlowicz, I.; Masajada, K. Aquaporins as a link between water relations and photosynthetic pathway in abiotic stress tolerance in plants. Gene 2019, 687, 166–172. [Google Scholar] [CrossRef]
- Singh, R.K.; Deshmukh, R.; Muthamilarasan, M.; Rani, R.; Prasad, M. Versatile roles of aquaporin in physiological processes and stress tolerance in plants. Plant Physiol. Biochem. 2020, 149, 178–189. [Google Scholar] [CrossRef]
- Mitani-Ueno, N.; Yamaji, N.; Zhao, F.J.; Ma, J.F. The aromatic/arginine selectivity filter of NIP aquaporins plays a critical role in substrate selectivity for silicon, boron, and arsenic. J. Exp. Bot. 2011, 62, 4391–4398. [Google Scholar] [CrossRef] [PubMed]
- Bienert, M.D.; Bienert, G.P. Plant Aquaporins and Metalloids; Springer: Cham, Switzerland, 2017; pp. 297–332. [Google Scholar]
- Deshmukh, R.; Bélanger, R.R.; Hartley, S. Molecular evolution of aquaporins and silicon influx in plants. Funct. Ecol. 2015, 30, 1277–1285. [Google Scholar] [CrossRef]
- Bezerra-Neto, J.P.; de Araújo, F.C.; Ferreira-Neto, J.R.; da Silva, M.D.; Pandolfi, V.; Aburjaile, F.F.; Sakamoto, T.; de Oliveira Silva, R.L.; Kido, E.A.; Barbosa Amorim, L.L.; et al. Plant aquaporins: Diversity, evolution and biotechnological applications. Curr. Protein Pept. Sci. 2019, 20, 368–395. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, R.; Sonah, H.; Belanger, R. New evidence defining the evolutionary path of aquaporins regulating silicon uptake in land plants. J. Exp. Bot. 2020, 71, 6775–6788. [Google Scholar] [CrossRef]
- Gerbeau, P.; Amodeo, G.; Henzler, T.; Santoni, V.; Ripoche, P.; Maurel, C. The water permeability of Arabidopsis plasma membrane is regulated by divalent cations and pH. Plant J. 2002, 30, 71–81. [Google Scholar] [CrossRef]
- Alleva, K.; Niemietz, C.M.; Sutka, M.; Maurel, C.; Parisi, M.; Tyerman, S.D.; Amodeo, G. Plasma membrane of Beta vulgaris storage root shows high water channel activity regulated by cytoplasmic pH and a dual range of calcium concentrations. J. Exp. Bot. 2006, 57, 609–621. [Google Scholar] [CrossRef]
- Kim, D.Y.; Scalf, M.; Smith, L.M.; Vierstra, R.D. Advanced proteomic analyses yield a deep catalog of ubiquitylation targets in Arabidopsis. Plant Cell 2013, 25, 1523–1540. [Google Scholar] [CrossRef]
- Luang, S.; Hrmova, M. Structural basis of the permeation function of plant aquaporins. In Plant Aquaporins. Signaling and Communication in Plants; Chaumont, F., Tyerman, S., Eds.; Springer: Cham, Switzerland, 2017; pp. 1–28. [Google Scholar]
- Fox, A.R.; Maistriaux, L.C.; Chaumont, F. Toward understanding of the high number of plant aquaporin isoforms and multiple regulation mechanisms. Plant Sci. 2017, 264, 179–187. [Google Scholar] [CrossRef]
- Laloux, T.; Junqueira, B.; Maistriaux, L.C.; Ahmed, J.; Jurkiewicz, A.; Chaumont, F. Plant and mammal aquaporins: Same but dfferent. Int. J. Mol. Sci. 2018, 19, 521. [Google Scholar] [CrossRef]
- Obroucheva, N.V. Aquaporins in seeds. Seed Sci. Res. 2013, 23, 213–216. [Google Scholar] [CrossRef]
- Obroucheva, N.V.; Sinkevich, I.A.; Lityagina, S.V.; Novikova, G.V. Water relations in germinating seeds. Russ. J. Plant Physiol. 2017, 64, 625–633. [Google Scholar] [CrossRef]
- Penfield, S. Seed dormancy and germination. Curr. Biol. 2017, 27, R874–R878. [Google Scholar] [CrossRef] [PubMed]
- Hoai, P.T.T.; Tyerman, S.D.; Schnell, N.; Tucker, M.; McGaughey, S.A.; Qiu, J.; Groszmann, M.; Byrt, C.S. Deciphering aquaporin regulation and roles in seed biology. J. Exp. Bot. 2020, 71, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Willigen, C.V.; Postaire, O.; Tournaire-Roux, C.; Boursiac, Y.; Maurel, C. Expression and inhibition of aquaporins in germinating Arabidopsis seeds. Plant Cell Physiol. 2006, 47, 1241–1250. [Google Scholar] [CrossRef]
- Hunter, P.R.; Craddock, C.P.; Benedetto, S.D.; Roberts, L.M.; Frigerio, L. Fluorescent reporter proteins for the tonoplast and the vacuolar lumen identify a single vacuolar compartment in Arabidopsis cells. Plant Physiol. 2007, 145, 1371–1382. [Google Scholar] [CrossRef]
- Gattolin, S.; Sorieul, M.; Frigerio, L. Mapping of tonoplast intrinsic proteins in maturing and germinating Arabidopsis seeds reveals dual localization of embryonic TIPs to the tonoplast and plasma membrane. Mol. Plant 2011, 4, 180–189. [Google Scholar] [CrossRef]
- Liu, H.Y.; Yu, X.; Cui, D.Y.; Sun, M.H.; Sun, W.N.; Tang, Z.C.; Kwak, S.S.; Su, W.A. The role of water channel proteins and nitric oxide signaling in rice seed germination. Cell Res. 2007, 17, 638–649. [Google Scholar] [CrossRef]
- Li, G.W.; Peng, Y.H.; Yu, X.; Zhang, M.H.; Cai, W.M.; Sun, W.N.; Su, W.A. Transport functions and expression analysis of vacuolar membrane aquaporins in response to various stresses in rice. J. Plant Physiol. 2008, 165, 1879–1888. [Google Scholar] [CrossRef]
- Schuurmans, J.A.; Dongen, J.T.V.; Rutjens, B.P.; Boonman, A.; Pieterse, C.M.; Borstlap, A.C. Members of the aquaporin family in the developing pea seed coat include representatives of the PIP, TIP, and NIP subfamilies. Plant Mol. Biol. 2003, 53, 655–667. [Google Scholar] [CrossRef]
- Gao, Y.P.; Young, L.; Bonham-Smith, P.; Gusta, L.V. Characterization and expression of plasma and tonoplast membrane aquaporins in primed seeds of Brassica napus during germination under stress conditions. Plant Mol. Biol. 1999, 40, 635–644. [Google Scholar] [CrossRef]
- Ge, F.W.; Tao, P.; Zhang, Y.; Wang, J.B. Characterization of AQP gene expressions in Brassica napus during seed germination and in response to abiotic stresses. Biol. Plant 2014, 58, 274–282. [Google Scholar] [CrossRef]
- Novikova, G.V.; Tournaire-Roux, C.; Sinkevich, I.A.; Lityagina, S.V.; Maurel, C.; Obroucheva, N. Vacuolar biogenesis and aquaporin expression at early germination of broad bean seeds. Plant Physiol. Biochem. 2014, 82, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Fukumoto, T.; Matsumoto, T.; Gena, P.; Frascaria, D.; Kaneko, T.; Katsuhara, M.; Zhong, S.; Sun, X.; Zhu, Y.; et al. Aquaporin OsPIP1;1 promotes rice salt resistance and seed germination. Plant Physiol. Biochem. 2013, 63, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Obroucheva, N.V.; Sin’kevich, I.A. Aquaporins and cell growth. Russ. J. Plant Physiol. 2010, 57, 153–165. [Google Scholar] [CrossRef]
- Béré, E.; Lahbib, K.; Merceron, B.; Fleurat-Lessard, P.; Boughanmi, N.G. α-TIP aquaporin distribution and size tonoplast variation in storage cells of Vicia faba cotyledons at seed maturation and germination stages. J. Plant Physiol. 2017, 216, 145–151. [Google Scholar] [CrossRef]
- Lee, S.E.; Yim, H.K.; Lim, M.N.; Yoon, I.; Kim, J.; Hwang, Y.S. Abscisic acid prevents the coalescence of protein storage vacuoles by upregulating expression of a tonoplast intrinsic protein gene in barley aleurone. J. Exp. Bot. 2015, 66, 1191–1203. [Google Scholar] [CrossRef][Green Version]
- Footitt, S.; Clewes, R.; Feeney, M.; Finch-Savage, W.E.; Frigerio, L. Aquaporins influence seed dormancy and germination in response to stress. Plant Cell Environ. 2019, 42, 2325–2339. [Google Scholar] [CrossRef]
- Kaldenhoff, R.; Grote, K.; Zhu, J.J.; Zimmermann, U. Significance of plasmalemma aquaporins for water transport in Arabidopsis thaliana. Plant J. 1998, 14, 121–128. [Google Scholar] [CrossRef]
- Cui, X.H.; Hao, F.S.; Chen, H.; Chen, J.; Wang, X.C. Expression of the Vicia faba VfPIP1 gene in Arabidopsis thaliana plants improves their drought resistance. J. Plant Res. 2008, 121, 207–214. [Google Scholar] [CrossRef]
- Wang, X.; Gao, F.; Bing, J.; Sun, W.; Feng, X.; Ma, X.; Zhou, Y.; Zhang, G. Overexpression of the Jojoba aquaporin gene, ScPIP1, enhances drought and salt tolerance in transgenic Arabidopsis. Int. J. Mol. Sci. 2019, 20, 153. [Google Scholar] [CrossRef]
- Ayadi, M.; Brini, F.; Masmoudi, K. Overexpression of a wheat aquaporin gene, TdPIP2;1, enhances salt and drought tolerance in transgenic durum wheat cv. Maali. Int. J. Mol. Sci. 2019, 20, 2389. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Peng, Y.; Li, G.; Arora, R.; Tang, Z.; Su, W.; Cai, W. Isolation and functional characterization of PgTIP1, a hormone-autotrophic cells-specific tonoplast aquaporin in ginseng. J. Exp. Bot. 2007, 58, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Péret, B.; Li, G.; Zhao, J.; Band, L.R.; Voss, U.; Postaire, O.; Luu, D.T.; Ines, O.D.; Casimiro, I.; Lucas, M.; et al. Auxin regulates aquaporin function to facilitate lateral root emergence. Nat. Cell Biol. 2012, 14, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, H.; Hachez, C.; Bienert, M.D.; Beebo, A.; Swarup, K.; Voss, U.; Bouhidel, K.; Frigerio, L.; Schjoerring, J.K.; Bennett, M.J.; et al. Tonoplast aquaporins fcilitate lateral root emergence. Plant Physiol. 2016, 170, 1640–1654. [Google Scholar] [CrossRef]
- Xu, F.; Wang, K.; Yuan, W.; Xu, W.; Shuang, L.; Kronzucker, H.J.; Chen, G.; Miao, R.; Zhang, M.; Ding, M.; et al. Overexpression of rice aquaporin OsPIP1;2 improves yield by enhancing mesophyll CO2 conductance and phloem sucrose transport. J. Exp. Bot. 2019, 70, 671–681. [Google Scholar] [CrossRef]
- Ding, L.; Uehlein, N.; Kaldenhoff, R.; Guo, S.; Zhu, Y.; Kai, L. Aquaporin PIP2;1 affects water transport and root growth in rice (Oryza sativa L.). Plant Physiol. Biochem. 2019, 139, 152–160. [Google Scholar] [CrossRef]
- Durbak, A.R.; Phillips, K.A.; Pike, S.; O’Neill, M.A.; Mares, J.; Gallavotti, A.; Malcomber, S.T.; Gassmann, W.; McSteen, P. Transport of boron by the tassel-less1 aquaporin is critical for vegetative and reproductive development in maize. Plant Cell 2014, 26, 2978–2995. [Google Scholar] [CrossRef]
- Aharon, R.; Shahak, Y.; Wininger, S.; Bendov, R.; Kapulnik, Y.; Galili, G. Overexpression of a plasma membrane aquaporin in transgenic tobacco improves plant vigor under favorable growth conditions but not under drought or salt stress. Plant Cell 2003, 15, 439–447. [Google Scholar] [CrossRef]
- Yu, Q.; Hu, Y.; Li, J.; Wu, Q.; Lin, Z. Sense and antisense expression of plasma membrane aquaporin BnPIP1 from Brassica napus in tobacco and its effects on plant drought resistance. Plant Sci. 2005, 169, 647–656. [Google Scholar] [CrossRef]
- Tsuchihira, A.; Hanba, Y.T.; Kato, N.; Doi, T.; Kawazu, T.; Maeshima, M. Effect of overexpression of radish plasma membrane aquaporins on water-use efficiency, photosynthesis and growth of Eucalyptus trees. Tree Physiol. 2010, 30, 417–430. [Google Scholar] [CrossRef]
- Liu, S.; Fukumoto, T.; Gena, P.; Feng, P.; Sun, Q.; Li, Q.; Matsumoto, T.; Kaneko, T.; Zhang, H.; Zhang, Y.; et al. Ectopic expression of a rice plasma membrane intrinsic protein (OsPIP1;3) promotes plant growth and water uptake. Plant J. 2020, 102, 779–796. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.Q.; Li, L.J.; Ren, F.; Lu, P.L.; Wei, P.C.; Cai, J.H.; Xin, L.G.; Zhang, J.; Chen, J.; Wang, X.C. Overexpression of the tonoplast aquaporin AtTIP5;1 conferred tolerance to boron toxicity in Arabidopsis. J. Genet. Genom. 2010, 37, 389–397. [Google Scholar] [CrossRef]
- Pang, Y.Q.; Li, J.T.; Qi, B.S.; Tian, M.; Sun, L.R.; Wang, X.C.; Hao, F.S. Aquaporin AtTIP5;1 as an essential target of gibberellins promotes hypocotyl cell elongation in Arabidopsis thaliana under excess boron stress. Funct. Plant Biol. 2018, 45, 305. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xia, W.; Zang, H.; Dai, B.; Zhang, Y.; Feng, Y.; Wang, A.; Lin, Z.; Liu, H.; Zhu, J. Expression analysis of aquaporin genes in Saussurea involucrata rosette leaves and functional analysis of upregulated SiPIP1;5A under low-temperature stress. Environ. Exp. Bot. 2020, 171, 103958. [Google Scholar] [CrossRef]
- Martins, C.P.; Neves, D.M.; Cidade, L.C.; Mendes, A.F.; Silva, D.C.; Almeida, A.F.; Coelho-Filho, M.A.; Gesteira, A.S.; Soares-Filho, W.S.; Costa, M.G. Expression of the citrus CsTIP2;1 gene improves tobacco plant growth, antioxidant capacity and physiological adaptation under stress conditions. Planta 2017, 245, 951–963. [Google Scholar] [CrossRef]
- Sade, N.; Gebretsadik, M.; Seligmann, R.; Schwartz, A.; Wallach, R.; Moshelion, M. The role of tobacco Aquaporin1 in improving water use efficiency, hydraulic conductivity, and yield production under salt stress. Plant Physiol. 2010, 152, 245–254. [Google Scholar] [CrossRef]
- Kawase, M.; Hanba, Y.T.; Katsuhara, M. The photosynthetic response of tobacco plants overexpressing ice plant aquaporin McMIPB to a soil water deficit and high vapor pressure deficit. J. Plant Res. 2013, 126, 517–527. [Google Scholar] [CrossRef]
- Shu, K.; Liu, X.D.; Xie, Q.; He, Z.H. Two faces of one seed: Hormonal regulation of dormancy and germination. Mol. Plant 2016, 9, 34–45. [Google Scholar] [CrossRef]
- Obroucheva, N.V.; Lityagina, S.V.; Novikova, G.V.; Sin’kevich, I.A. Vacuolar status and water relations in embryonic axes of recalcitrant Aesculus hippocastanum seeds during stratification and early germination. AoB Plants 2012, 2012, pls008. [Google Scholar] [CrossRef]
- Knipfer, T.; Besse, M.; Verdeil, J.L.; Fricke, W. Aquaporin-facilitated water uptake in barley (Hordeum vulgare L.) roots. J. Exp. Bot. 2011, 62, 4115–4126. [Google Scholar] [CrossRef]
- Gambetta, G.A.; Fei, J.; Rost, T.L.; Knipfer, T.; Matthews, M.A.; Shackel, K.A.; Walker, M.A.; McElrone, A.J. Water uptake along the length of grapevine fine roots: Developmental anatomy, tissue-specific aquaporin expression, and pathways of water transport. Plant Physiol. 2013, 163, 1254–1265. [Google Scholar] [CrossRef]
- Fricke, W.; Knipfer, T. Plant aquaporins and cell elongation. In Plant Aquaporins. Signaling and Communication in Plants; Chaumont, F., Tyerman, S., Eds.; Springer: Cham, Switzerland, 2017; pp. 107–131. [Google Scholar]
- Hukin, D.; Doering-Saad, C.; Thomas, C.R.; Pritchard, J. Sensitivity of cell hydraulic conductivity to mercury is coincident with symplasmic isolation and expression of plasmalemma aquaporin genes in growing maize roots. Planta 2002, 215, 1047–1056. [Google Scholar]
- Hachez, C.; Moshelion, M.; Zelazny, E.; Cavez, D.; Chaumont, F. Localization and quantification of plasma membrane aquaporin expression in maize primary root: A clue to understanding their role as cellular plumbers. Plant Mol. Biol. 2006, 62, 305–323. [Google Scholar] [CrossRef] [PubMed]
- Vandeleur, R.K.; Mayo, G.; Shelden, M.C.; Gilliham, M.; Kaiser, B.N.; Tyerman, S.D. The role of plasma membrane intrinsic protein aquaporins in water transport through roots: Diurnal and drought stress responses reveal different strategies between isohydric and anisohydric cultivars of grapevine. Plant Physiol. 2009, 149, 445–460. [Google Scholar] [CrossRef] [PubMed]
- Gambetta, G.A.; Manuck, C.M.; Drucker, S.T.; Shaghasi, T.; Fort, K.; Matthews, M.A.; McElrone, A.J. The relationship between root hydraulics and scion vigour across Vitis rootstocks: What role do root aquaporins play? J. Exp. Bot. 2012, 63, 6445–6455. [Google Scholar] [CrossRef] [PubMed]
- Brady, S.M.; Orlando, D.A.; Lee, J.Y.; Wang, J.Y.; Koch, J.; Dinneny, J.R.; Mace, D.; Ohler, U.; Benfey, P.N. A high-resolution root spatiotemporal map reveals dominant expression patterns. Science 2007, 318, 801–806. [Google Scholar] [CrossRef]
- Salerno, G.L.; Curatti, L. Origin of sucrose metabolism in higher plants: When, how and why? Trends Plant Sci. 2003, 8, 63–69. [Google Scholar] [CrossRef]
- Chiou, T.J.; Bush, D.R. Sucrose is a signal molecule in assimilate partitioning. Proc. Natl. Acad. Sci. USA 1998, 95, 4784–4788. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J.S.; Silk, W.K. Hydraulics of plant growth. Func. Plant Biol. 2004, 31, 761–773. [Google Scholar] [CrossRef]
- Sutka, M.; Li, G.; Boudet, J.; Boursiac, Y.; Doumas, P.; Maurel, C. Natural variation of root hydraulics in Arabidopsis grown in normal and salt-stressed conditions. Plant Physiol. 2011, 155, 1264–1276. [Google Scholar] [CrossRef]
- Bramley, H.; Turner, N.C.; Turner, D.W.; Tyerman, S.D. Roles of morphology, anatomy, and aquaporins in determining contrasting hydraulic behavior of roots. Plant Physiol. 2009, 150, 348–364. [Google Scholar] [CrossRef] [PubMed]
- Ehlert, C.; Maurel, C.; Tardieu, F.; Simonneau, T. Aquaporin-mediated reduction in maize root hydraulic conductivity impacts cell turgor and leaf elongation even without changing transpiration. Plant Physiol. 2009, 150, 1093–1104. [Google Scholar] [CrossRef] [PubMed]
- Maggio, A.; Joly, R.J. Effects of mercuric chloride on the hydraulic conductivity of tomato root systems. Plant Physiol. 1995, 109, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Javot, H.; Lauvergeat, V.; Santoni, V.; Martin-Laurent, F.; Guclu, J.; Vinh, J.; Heyes, J.; Franck, K.I.; Schaffner, A.R.; Bouchez, D.; et al. Role of a single aquaporin isoform in root water uptake. Plant Cell 2003, 15, 509–522. [Google Scholar] [CrossRef] [PubMed]
- Postaire, O.; Tournaire-Roux, C.; Grondin, A.; Boursiac, Y.; Morillon, R.; Schäffner, A.R.; Maurel, C. A PIP1 aquaporin contributes to hydrostatic pressure-induced water transport in both the root and rosette of Arabidopsis. Plant Physiol. 2010, 152, 1418–1430. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Ye, G.; Qian, Z.; Ye, Q. Virus-induced plasma membrane aquaporin Pspip2;1 silencing inhibits plant water transport of Pisum sativum. Bot. Stud. 2016, 57, 15. [Google Scholar] [CrossRef]
- Ding, L.; Milhiet, T.; Couvreur, V.; Nelissen, H.; Meziane, A.; Parent, B.; Aesaert, S.; Van Lijsebettens, M.; Inze, D.; Tardieu, F.; et al. Modification of the expression of the aquaporin ZmPIP2;5 affects water relations and plant growth. Plant Physiol. 2020, 182, 2154–2165. [Google Scholar] [CrossRef]
- Miyamoto, N.; Ookawa, T.; Takahashi, H.; Hirasawa, T. Water uptake and hydraulic properties of elongation cells in hydrotropically bending roots of Pisum sativum L. Plant Cell Physiol. 2002, 43, 393–401. [Google Scholar] [CrossRef]
- Scharwies, J.D.; Dinneny, J.R. Water transport, perception, and response in plants. J. Plant Res. 2019, 132, 311–324. [Google Scholar] [CrossRef]
- Knipfer, T.; Fricke, W. Root pressure and a solute reflection coefficient close to unity exclude a purely apoplastic pathway of radial water transport in barley (Hordeum vulgare). New Phytol. 2010, 187, 159–170. [Google Scholar] [CrossRef]
- Knipfer, T.; Fricke, W. Water uptake by seminal and adventitious roots in relation to whole-plant water flow in barley (Hordeum vulgare L.). J. Exp. Bot. 2011, 62, 717–733. [Google Scholar] [CrossRef] [PubMed]
- Gambetta, G.A.; Knipfer, T.; Fricke, W.; McElrone, A.J. Aquaporins and root water uptake. In Plant Aquaporins. Signaling and Communication in Plants; Chaumont, F., Tyerman, S., Eds.; Springer: Cham, Switzerland, 2017; pp. 133–153. [Google Scholar]
- Wang, M.; Ding, L.; Gao, L.; Li, Y.; Shen, Q.; Guo, S. The interactions of aquaporins and mineral nutrients in higher plants. Int. J. Mol. Sci. 2016, 17, 1229. [Google Scholar] [CrossRef] [PubMed]
- Ludevid, D.; Höfte, H.; Himelblau, E.; Chrispeels, M.J. The expression pattern of the tonoplast intrinsic protein γ-TIP in Arabidopsis thaliana is correlated with cell enlargement. Plant Physiol. 1992, 100, 1633–1639. [Google Scholar] [CrossRef] [PubMed]
- Eisenbarth, D.A.; Weig, A.R. Dynamics of aquaporins and water relations during hypocotyl elongation in Ricinus communis L. seedlings. J. Exp. Bot. 2005, 56, 1831–1842. [Google Scholar] [CrossRef] [PubMed]
- Muto, Y.; Segami, S.; Hayashi, H.; Sakurai, J.; Murai-Hatano, M.; Hattori, Y.; Ashikari, M.; Maeshima, M. Vacuolar proton pumps and aquaporins involved in rapid internode elongation of deepwater rice. Biosci. Biotechnol. Biochem. 2011, 75, 114–122. [Google Scholar] [CrossRef] [PubMed]
- McGaughey, S.A.; Osborn, H.L.; Chen, L.; Pegler, J.L.; Tyerman, S.D.; Furbank, R.T.; Byrt, C.S.; Grof, C.P. Roles of aquaporins in Setaria viridis stem development and sugar storage. Front. Plant Sci. 2016, 7, 1815. [Google Scholar] [CrossRef]
- Katsuhara, M.; Koshio, K.; Shibasaka, M.; Hayashi, Y.; Hayakawa, T.; Kasamo, K. Over-expression of, a.barley aquaporin increased the shoot/root ratio and raised salt sensitivity in transgenic rice plants. Plant Cell Physiol. 2003, 44, 1378–1383. [Google Scholar] [CrossRef]
- Nada, R.M.; Abogadallah, G.M. Contrasting root traits and native regulation of aquaporin differentially determine the outcome of overexpressing a single aquaporin (OsPIP2;4) in two rice cultivars. Protoplasma 2020, 257, 583–595. [Google Scholar] [CrossRef]
- Moshelion, M.; Halperin, O.; Wallach, R.; Oren, R.; Way, D.A. Role of aquaporins in determining transpiration and photosynthesis in water-stressed plants: Crop water-use efficiency, growth and yield. Plant Cell Environ. 2014, 38, 1785–1793. [Google Scholar] [CrossRef]
- Heinen, R.B.; Ye, Q.; Chaumont, F. Role of aquaporins in leaf physiology. J. Exp. Bot. 2009, 60, 2971–2985. [Google Scholar] [CrossRef]
- Hachez, C.; Heinen, R.B.; Draye, X.; Chaumont, F. The expression pattern of plasma membrane aquaporins in maize leaf highlights their role in hydraulic regulation. Plant Mol. Biol. 2008, 68, 337–353. [Google Scholar] [CrossRef] [PubMed]
- Besse, M.; Knipfer, T.; Miller, A.J.; Verdeil, J.L.; Jahn, T.P.; Fricke, W. Developmental pattern of aquaporin expression in barley (Hordeum vulgare L.) leaves. J. Exp. Bot. 2011, 62, 4127–4142. [Google Scholar] [CrossRef] [PubMed]
- Aasamaa, K.; Sober, A. Seasonal courses of maximum hydraulic conductance in shoots of six temperate deciduous tree species. Funct. Plant Biol. 2005, 32, 1077–1087. [Google Scholar] [CrossRef] [PubMed]
- Nardini, A.; Salleo, S. Water stress-induced modifications of leaf hydraulic architecture in sunflower: Co-ordination with gas exchange. J. Exp. Bot. 2005, 56, 3093–3101. [Google Scholar] [CrossRef] [PubMed]
- Pou, A.; Medrano, H.; Flexas, J.; Tyerman, S.D. A putative role for TIP and PIP aquaporins in dynamics of leaf hydraulic and stomatal conductances in grapevine under water stress and re-watering. Plant Cell Environ. 2013, 36, 828–843. [Google Scholar] [CrossRef]
- Sade, N.; Shatil-Cohen, A.; Attia, Z.; Maurel, C.; Boursiac, Y.; Kelly, G.; Granot, D.; Yaaran, A.; Lerner, S.; Moshelion, M. The role of plasma membrane aquaporins in regulating the bundle sheath-mesophyll continuum and leaf hydraulics. Plant Physiol. 2014, 166, 1609–1620. [Google Scholar] [CrossRef]
- Volkov, V.; Hachez, C.; Moshelion, M.; Draye, X.; Chaumont, F.; Fricke, W. Water permeability differs between growing and non-growing barley leaf tissues. J. Exp. Bot. 2007, 58, 377–390. [Google Scholar] [CrossRef][Green Version]
- Hilty, J.; Pook, C.; Leuzinger, S. Water relations determine short time leaf growth patterns in the mangrove Avicennia marina (Forssk.) Vierh. Plant Cell Environ. 2019, 42, 527–535. [Google Scholar] [CrossRef]
- Groszmann, M.; Osborn, H.L.; Evans, J.R. Carbon dioxide and water transport through plant aquaporins. Plant Cell Environ. 2017, 40, 938–961. [Google Scholar] [CrossRef]
- Uehlein, N.; Lovisolo, C.; Siefritz, F.; Kaldenhoff, R. The tobacco aquaporin NtAQP1 is a membrane CO2 pore with physiological functions. Nature 2003, 425, 734–737. [Google Scholar] [CrossRef]
- Flexas, J.; Ribas-Carbo, M.; Hanson, D.T.; Bota, J.; Otto, B.; Cifre, J.; McDowell, N.; Medrano, H.; Kaldenhoff, R. Tobacco aquaporin NtAQP1 is involved in mesophyll conductance to CO2 in vivo. Plant J. 2006, 48, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Kelly, G.; Sade, N.; Attia, Z.; Secchi, F.; Zwieniecki, M.; Holbrook, N.M.; Levi, A.; Alchanatis, V.; Moshelion, M.; Granot, D. Relationship between hexokinase and the aquaporin PIP1 in the regulation of photosynthesis and plant growth. PLoS ONE 2014, 9, e87888. [Google Scholar] [CrossRef] [PubMed]
- Heckwolf, M.; Pater, D.; Hanson, D.T.; Kaldenhoff, R. The Arabidopsis thaliana aquaporin AtPIP1;2 is a physiologically relevant CO2 transport facilitator. Plant J. 2011, 67, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Uehlein, N.; Sperling, H.; Heckwolf, M.; Kaldenhoff, R. The Arabidopsis aquaporin PIP1;2 rules cellular CO2 uptake. Plant Cell Environ. 2012, 35, 1077–1083. [Google Scholar] [CrossRef]
- Boudichevskaia, A.; Heckwolf, M.; Kaldenhoff, R. T-DNA insertion in aquaporin gene AtPIP1;2 generates transcription profiles reminiscent of a low CO2 response. Plant Cell Environ. 2015, 38, 2286–2298. [Google Scholar] [CrossRef]
- Hanba, Y.T.; Shibasaka, M.; Hayashi, Y.; Hayakawa, T.; Kasamo, K.; Terashima, I.; Katsuhara, M. Overexpression of the barley aquaporin HvPIP2;1 increases internal CO2 conductance and CO2 assimillation in the leaves of transgenic rice plants. Plant Cell Physiol. 2004, 45, 521–529. [Google Scholar] [CrossRef]
- Mori, I.C.; Rhee, J.; Shibasaka, M.; Sasano, S.; Kaneko, T.; Horie, T.; Katsuhara, M. CO2 transport by PIP2 aquaporins of barley. Plant Cell Physiol. 2014, 55, 251–257. [Google Scholar] [CrossRef]
- Katsuhara, M.; Hanba, Y.T. Barley plasma membrane intrinsic proteins (PIP Aquaporins) as water and CO2 transporters. Pflugers Archiv. 2008, 456, 687–691. [Google Scholar] [CrossRef]
- Ding, L.; Gao, L.; Liu, W.; Wang, M.; Gu, M.; Ren, B.; Xu, G.; Shen, Q.; Guo, S. Aquaporin plays an important role in mediating chloroplastic CO2 concentration under high-N supply in rice (Oryza sativa) plants. Physiol. Plant 2016, 156, 215–226. [Google Scholar] [CrossRef]
- Heinen, R.B.; Bienert, G.P.; Cohen, D.; Chevalier, A.S.; Uehlein, N.; Hachez, C.; Kaldenhoff, R.; Thiec, D.L.; Chaumont, F. Expression and characterization of plasma membrane aquaporins in stomatal complexes of Zea mays. Plant Mol. Biol. 2014, 86, 335–350. [Google Scholar] [CrossRef]
- Navarro-Ródenas, A.; Ruíz-Lozano, J.M.; Kaldenhoff, R.; Morte, A. The aquaporin TcAQP1 of the desert Truffle terfezia claveryi is a membrane pore for water and CO2 transport. Mol. Plant Microbe Interact. 2012, 25, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Secchi, F.; Zwieniecki, M.A. The physiological response of Populus tremula x alba leaves to the down-regulation of PIP1 aquaporin gene expression under no water stress. Front. Plant Sci. 2013, 4, 507. [Google Scholar] [CrossRef] [PubMed]
- Parent, B.; Hachez, C.; Redondo, E.; Simonneau, T.; Chaumont, F.; Tardieu, F. Drought and abscisic acid effects on aquaporin content translate into changes in hydraulic conductivity and leaf growth rate: A trans-scale approach. Plant Physiol. 2009, 149, 2000–2012. [Google Scholar] [CrossRef] [PubMed]
- Caldeira, C.F.; Jeanguenin, L.; Chaumont, F.; Tardieu, F. Circadian rhythms of hydraulic conductance and growth are enhanced by drought and improve plant performance. Nat. Commun. 2014, 5, 5365. [Google Scholar] [CrossRef]
- Yooyongwech, S.; Horigane, A.K.; Yoshida, M.; Yamaguchi, M.; Sekozawa, Y.; Sugaya, S.; Gemma, H. Changes in aquaporin gene expression and magnetic resonance imaging of water status in peach tree flower buds during dormancy. Physiol. Plant 2008, 134, 522–533. [Google Scholar] [CrossRef]
- Ma, N.; Xue, J.; Li, Y.; Liu, X.; Dai, F.; Jia, W.; Luo, Y.; Gao, J. Rh-PIP2;1, a rose aquaporin gene, is involved in ethylene-regulated petal expansion. Plant Physiol. 2008, 148, 894–907. [Google Scholar] [CrossRef]
- Dubois, M.; Broeck, L.V.D.; Inze, D. The pivotal role of ethylene in plant growth. Trends Plant Sci. 2018, 23, 311–323. [Google Scholar] [CrossRef]
- Chen, W.; Yin, X.; Wang, L.; Tian, J.; Yang, R.; Liu, D.; Yu, Z.; Ma, N.; Gao, J. Involvement of rose aquaporin RhPIP1;1 in ethylene-regulated petal expansion through interaction with RhPIP2;1. Plant Mol. Biol. 2013, 83, 219–233. [Google Scholar] [CrossRef]
- Xue, J.; Yang, F.; Gao, J. Isolation of Rh-TIP1;1, an aquaporin gene and its expression in rose flowers in response to ethylene and water deficit. Postharvest Biol. Technol. 2009, 51, 407–413. [Google Scholar] [CrossRef]
- Bots, M.; Vergeldt, F.; Wolters-Arts, M.; Weterings, K.; van As, H.; Mariani, C. Aquaporins of the PIP2 class are required for efficient anther dehiscence in tobacco. Plant Physiol. 2005, 137, 1049–1056. [Google Scholar] [CrossRef]
- Sato, R.; Maeshima, M. The ER-localized aquaporin SIP2;1 is involved in pollen germination and pollen tube elongation in Arabidopsis thaliana. Plant Mol. Biol. 2019, 100, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Soto, G.; Fox, R.; Ayub, N.; Alleva, K.; Guaimas, F.; Erijman, E.J.; Mazzella, A.; Amodeo, G.; Muschietti, J. TIP5;1 is an aquaporin specifically targeted to pollen mitochondria and is probably involved in nitrogen remobilization in Arabidopsis thaliana. Plant J. 2010, 64, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Giorgio, J.A.D.; Bienert, G.P.; Ayub, N.D.; Yaneff, A.; Barberini, M.L.; Mecchia, M.A.; Amodeo, G.; Soto, G.C.; Muschietti, J.P. Pollen-specific aquaporins NIP4;1 and NIP4;2 are required for pollen development and pollination in Arabidopsis thaliana. Plant Cell 2016, 28, 1053–1077. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Q.T.; Lei, Q.; Feng, C.; Zheng, X.; Zhou, F.; Li, L.; Liu, X.; Wang, Z.; Kong, J. Ectopically expressing MdPIP1;3, an aquaporin gene, increased fruit size and enhanced drought tolerance of transgenic tomatoes. BMC Plant Biol. 2017, 17, 246. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Dong, C.; Liu, R.; Zhou, B.; Wang, C.; Shou, H. Roles of soybean plasma membrane intrinsic protein GmPIP2;9 in drought tolerance and seed development. Front. Plant Sci. 2018, 9, 530. [Google Scholar] [CrossRef]
- Mao, Z.; Sun, W. Arabidopsis seed-specific vacuolar aquaporins are involved in maintaining seed longevity under the control of Abscisic Acid Insensitive 3. J. Exp. Bot. 2015, 66, 4781–4794. [Google Scholar] [CrossRef]
- Aslam, U.; Khatoon, A.; Cheema, H.M.; Bashir, A. Identification and characterization of plasma membrane aquaporins isolated from fiber cells of Calotropis procera. J. Zhejiang Univ. Sci. B 2013, 14, 586–595. [Google Scholar] [CrossRef][Green Version]
- Azad, A.K.; Sawa, Y.; Ishikawa, T.; Shibata, H. Phosphorylation of plasma membrane aquaporin regulates temperature-dependent opening of tulip petals. Plant Cell Physiol. 2004, 45, 608–617. [Google Scholar] [CrossRef]
- Azad, A.K.; Sawa, Y.; Ishikawa, T.; Shibata, H. Characterization of protein phosphatase 2A acting on phosphorylated plasma membrane aquaporin of tulip petals. Biosci. Biotechnol. Biochem. 2014, 68, 1170–1174. [Google Scholar] [CrossRef]
- Azad, A.K.; Katsuhara, M.; Sawa, Y.; Ishikawa, T.; Shibata, H. Characterization of four plasma membrane aquaporins in tulip petals: A putative homolog is regulated by phosphorylation. Plant Cell Physiol. 2008, 49, 1196–1208. [Google Scholar] [CrossRef][Green Version]
- Yue, C.; Cao, H.; Wang, L.; Zhou, Y.; Hao, X.; Zeng, J.; Wang, X.; Yang, Y. Molecular cloning and expression analysis of tea plant aquaporin (AQP) gene family. Plant Physiol. Biochem. 2014, 83, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Bendahmane, M.; Fu, X. Genome-wide identification and characterization of aquaporins and their role in the flower opening processes in Carnation (Dianthus caryophyllus). Molecules 2018, 23, 1895. [Google Scholar] [CrossRef] [PubMed]
- Morita, S.; Sugiyama, S.; Tateishi, A.; Satoh, S. Identification and characterization of plasma membrane intrinsic protein (PIP) aquaporin genes in petals of opening carnation flowers. Horticult. J. 2017, 86, 78–86. [Google Scholar] [CrossRef]
- Bots, M.; Feron, R.; Uehlein, N.; Weterings, K.; Kaldenhoff, R.; Mariani, T. PIP1 and PIP2 aquaporins are differentially expressed during tobacco anther and stigma development. J. Exp. Bot. 2005, 56, 113–121. [Google Scholar] [CrossRef]
- Sakurai, J.; Ishikawa, F.; Yamaguchi, T.; Uemura, M.; Maeshima, M. Identification of 33 rice aquaporin genes and analysis of their expression and function. Plant Cell Physiol. 2005, 46, 1568–1577. [Google Scholar] [CrossRef]
- Dixit, R.; Rizzo, C.; Nasrallah, M.; Nasrallah, J. The Brassica MIP-MOD gene encodes a functional water channel that is expressed in the stigma epidermis. Plant Mol. Biol. 2001, 45, 51–62. [Google Scholar] [CrossRef]
- Wudick, M.M.; Luu, D.T.; Tournaire-Roux, C.; Sakamoto, W.; Maurel, C. Vegetative and sperm cell-specific aquaporins of Arabidopsis highlight the vacuolar equipment of pollen and contribute to plant reproduction. Plant Physiol. 2014, 164, 1697–1706. [Google Scholar] [CrossRef]
- Soto, G.; Alleva, K.; Mazzella, M.A.; Amodeo, G.; Muschietti, J.P. AtTIP1;3 and AtTIP5;1, the only highly expressed Arabidopsis pollen-specific aquaporins, transport water and urea. FEBS Lett. 2008, 582, 4077–4082. [Google Scholar] [CrossRef]
- Giorgio, J.A.P.D.; Barberini, M.L.; Amodeo, G.; Muschietti, J.P. Pollen aquaporins: What are they there for? Plant Signal. Behav. 2016, 11, e1217375. [Google Scholar] [CrossRef]
- Shiratake, K.; Martinoia, E. Transporters in fruit vacuole. Plant Biotechnol. 2007, 24, 127–133. [Google Scholar] [CrossRef]
- Katsuhara, M.; Hanba, Y.T.; Shiratake, K.; Maeshima, M. Expanding roles of plant aquaporins in plasma membranes and cell organelles. Funct. Plant Biol. 2008, 35, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tao, J.; Ahammed, G.J.; Li, J.; Yang, Y. Genome-wide identification and expression analysis of aquaporin gene family related to abiotic stress in watermelon. Genome 2019, 62, 643–656. [Google Scholar] [CrossRef] [PubMed]
- Shiota, H.; Sudoh, T.; Tanaka, I. Expression analysis of genes encoding plasma membrane aquaporins during seed and fruit development in tomato. Plant Sci. 2006, 171, 277–285. [Google Scholar] [CrossRef]
- Alleva, K.; Marquez, M.; Villarreal, N.; Mut, P.; Bustamante, C.; Bellati, J.; Martinez, G.; Civello, M.; Amodeo, G. Cloning, functional characterization, and co-expression studies of a novel aquaporin (FaPIP2;1) of strawberry fruit. J. Exp. Bot. 2010, 61, 3935–3945. [Google Scholar] [CrossRef]
- Hu, C.G.; Hao, H.J.; Honda, C.; Kita, M.; Moriguchi, T. Putative PIP1 genes isolated from apple: Expression analyses during fruit development and under osmotic stress. J. Exp. Bot. 2003, 54, 2193–2194. [Google Scholar] [CrossRef]
- Reuscher, S.; Akiyama, M.; Mori, C.; Aoki, K.; Shibata, D.; Shiratake, K. Genome-wide identification and expression analysis of aquaporins in tomato. PLoS ONE 2013, 8, e79052. [Google Scholar] [CrossRef]
- Fouquet, R.; Leon, C.; Ollat, N.; Barrieu, F. Identification of grapevine aquaporins and expression analysis in developing berries. Plant Cell Rep. 2008, 27, 1541–1550. [Google Scholar] [CrossRef]
- Wong, D.C.J.; Zhang, L.; Merlin, I.; Castellarin, S.D.; Gambetta, G.A. Structure and transcriptional regulation of the major intrinsic protein gene family in grapevine. BMC Genom. 2018, 19, 248. [Google Scholar] [CrossRef]
- Hu, W.; Hou, X.; Huang, C.; Yan, Y.; Tie, W.; Ding, Z.; Wei, Y.; Liu, J.; Miao, H.; Lu, Z.; et al. Genome-wide identification and expression analyses of aquaporin gene family during development and abiotic stress in banana. Int. J. Mol. Sci. 2015, 16, 19728–19751. [Google Scholar] [CrossRef]
- Shi, J.; Wang, J.; Li, R.; Li, D.; Xu, F.; Sun, Q.; Zhao, B.; Mao, A.J.; Guo, Y.D. Expression patterns of genes encoding plasma membrane aquaporins during fruit development in cucumber (Cucumis sativus L.). Plant Physiol. Biochem. 2015, 96, 329–336. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Yang, L.; Liu, N.; Yang, J.; Zhou, X.K.; Xia, Y.C.; He, Y.; He, Y.Q.; Gong, H.J.; Ma, D.F.; et al. Genome-wide identification, structure characterization, and expression pattern profiling of aquaporin gene family in cucumber. BMC Plant Biol. 2019, 19, 345. [Google Scholar] [CrossRef] [PubMed]
- Baud, S.; Boutin, J.P.; Miquel, M.; Lepiniec, L.; Rochat, C. An integrated overview of seed development in Arabidopsis thaliana ecotype WS. Plant Physiol. Biochem. 2002, 40, 51–160. [Google Scholar] [CrossRef]
- Weijers, D.; Jurgens, G. Auxin and embryo axis formation: The ends in sight? Curr. Opin. Plant Biol. 2005, 8, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Setz, N.; Niemietz, C.; Qu, H.; Offler, C.E.; Tyerman, S.D.; Patrick, J.W. Aquaporins and unloading of phloem-imported water in coats of developing bean seeds. Plant Cell Environ. 2007, 30, 1566–1577. [Google Scholar] [CrossRef]
- Hayashi, H.; Ishikawa-Sakurai, J.; Murai-Hatano, M.; Ahamed, A.; Uemura, M. Aquaporins in developing rice grains. Biosci. Biotechnol. Biochem. 2015, 79, 1422–1429. [Google Scholar] [CrossRef]
- Feeney, M.; Kittelmann, M.; Menassa, R.; Hawes, C.; Frigerio, L. Protein storage vacuoles originate from remodeled preexisting vacuoles in Arabidopsis thaliana. Plant Physiol. 2018, 177, 241–254. [Google Scholar] [CrossRef]
- Utsugi, S.; Shibasaka, M.; Maekawa, M.; Katsuhara, M. Control of the water transport activity of barley HvTIP3;1 specifically expressed in seeds. Plant Cell Physiol. 2015, 56, 1831–1840. [Google Scholar] [CrossRef][Green Version]
- Li, D.D.; Ruan, X.M.; Zhang, J.; Wu, Y.J.; Wang, X.L.; Li, X.B. Cotton plasma membrane intrinsic protein 2s (PIP2s) selectively interact to regulate their water channel activities and are required for fibre development. New Phytol. 2013, 199, 695–707. [Google Scholar] [CrossRef]
- Naoumkina, M.; Thyssen, G.N.; Fang, D.D. RNA-seq analysis of short fiber mutants Ligon-lintless-1 (Li1) and-2 (Li2) revealed important role of aquaporins in cotton (Gossypium hirsutum L.) fiber elongation. BMC Plant Biol. 2015, 15, 65. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, H.; Sun, L.; Jiao, Y.; Zhang, G.; Miao, C.; Hao, F. NADPH oxidase AtrbohD and AtrbohF function in ROS-dependent regulation of Na+/K+ homeostasis in Arabidopsis under salt stress. J. Exp. Bot. 2012, 63, 305–317. [Google Scholar] [CrossRef]
- Jiao, Y.; Sun, L.; Song, Y.; Wang, L.; Liu, L.; Zhang, L.; Liu, B.; Li, N.; Miao, C.; Hao, F. AtrbohD and AtrbohF positively regulate abscisic acid-inhibited primary root growth by affecting Ca2+ signalling and auxin response of roots in Arabidopsis. J. Exp. Bot. 2013, 64, 4183–4192. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Miao, Y.; Song, C.P. Behind the scenes: The roles of reactive oxygen species in guard cells. New Phytol. 2014, 201, 1121–1140. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Sun, L.; Zhang, L.; Song, Y.; Hu, P.; Li, C.; Hao, F.S. AtrbohD and AtrbohF negatively regulate lateral root development by changing the localized accumulation of superoxide in primary roots of Arabidopsis. Planta 2015, 241, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.J.; Zhou, Y.H.; Shi, K.; Zhou, J.; Foyer, C.H.; Yu, J.Q. Interplay between reactive oxygen species and hormones in the control of plant development and stress tolerance. J. Exp. Bot. 2015, 66, 2839–2856. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Sun, L.; Ma, L.; Hao, F.S. Both AtrbohD and AtrbohF are essential for mediating responses to oxygen deficiency in Arabidopsis. Plant Cell Rep. 2017, 36, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Mhamdi, A.; Breusegem, F.V. Reactive oxygen species in plant development. Development 2018, 145, dev164376. [Google Scholar] [CrossRef]
- Bienert, G.P.; Moller, A.L.; Kristiansen, K.A.; Schulz, A.; Moller, I.M.; Schjoerring, J.K.; Jahn, T.P. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J. Biol. Chem. 2007, 282, 1183–1192. [Google Scholar] [CrossRef]
- Dynowski, M.; Schaaf, G.; Loque, D.; Moran, O.; Ludewig, U. Plant plasma membrane water channels conduct the signalling molecule H2O2. Biochem. J. 2008, 414, 53–61. [Google Scholar] [CrossRef]
- Hooijmaijers, C.; Rhee, J.Y.; Kwak, K.J.; Chung, G.C.; Horie, T.; Katsuhara, M.; Kang, H. Hydrogen peroxide permeability of plasma membrane aquaporins of Arabidopsis thaliana. J. Plant Res. 2012, 125, 147–153. [Google Scholar] [CrossRef]
- Bienert, G.P.; Chaumont, F. Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim. Biophys. Acta 2014, 1840, 1596–1604. [Google Scholar] [CrossRef]
- Katsuhara, M.; Sasano, S.; Horie, T.; Matsumoto, T.; Rhee, J.; Shibasaka, M. Functional and molecular characteristics of rice and barley NIP aquaporins transporting water, hydrogen peroxide and arsenite. Plant Biotechnol. 2014, 31, 213–219. [Google Scholar] [CrossRef]
- Rhee, J.; Horie, T.; Sasano, S.; Nakahara, Y.; Katsuhara, M. Identification of an H2O2 permeable PIP aquaporin in barley and a serine residue promoting H2O2 transport. Physiol. Plant 2017, 159, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Bienert, G.P.; Heinen, R.B.; Berny, M.C.; Chaumont, F. Maize plasma membrane aquaporin ZmPIP2;5, but not ZmPIP1;2, facilitates transmembrane diffusion of hydrogen peroxide. Biochim. Biophys. Acta Biomembr. 2014, 1838, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Azad, A.K.; Yoshikawa, N.; Ishikawa, T.; Sawa, Y.; Shibata, H. Substitution of a single amino acid residue in the aromatic/arginine selectivity filter alters the transport profiles of tonoplast aquaporin homologs. Biochim. Biophys. Acta 2012, 1818, 1–11. [Google Scholar] [CrossRef]
- Bienert, G.P.; Bienert, M.D.; Jahn, T.P.; Boutry, M.; Chaumont, F. Solanaceae XIPs are plasma membrane aquaporins that facilitate the transport of many uncharged substrates. Plant J. 2011, 66, 306–317. [Google Scholar] [CrossRef]
- Jang, J.Y.; Rhee, J.Y.; Chung, G.C.; Kang, H. Aquaporin as a membrane transporter of hydrogen peroxide in plant response to stresses. Plant Signal. Behav. 2012, 7, 1180–1181. [Google Scholar] [CrossRef]
- Tian, S.; Wang, X.; Li, P.; Wang, H.; Ji, H.; Xie, J.; Qiu, Q.; Shen, D.; Dong, H. Plant aquaporin AtPIP1;4 links apoplastic H2O2 induction to disease immunity pathways. Plant Physiol. 2016, 171, 1635–1650. [Google Scholar] [CrossRef]
- Rodrigues, O.; Reshetnyak, G.; Grondin, A.; Saijo, Y.; Leonhardt, N.; Maurel, C.; Verdoucq, L. Aquaporins facilitate hydrogen peroxide entry into guard cells to mediate ABA- and pathogen-triggered stomatal closure. Proc. Natl. Acad. Sci. USA 2017, 114, 9200–9205. [Google Scholar] [CrossRef]
- Smirnoff, N.; Arnaud, D. Hydrogen peroxide metabolism and functions in plants. New Phytol. 2019, 221, 1197–1214. [Google Scholar] [CrossRef]
- Grondin, A.; Rodrigues, O.; Verdoucq, L.; Merlot, S.; Leonhardt, N.; Maurel, C. Aquaporins contribute to ABA-triggered stomatal closure through OST1-mediated phosphorylation. Plant Cell 2015, 27, 1945–1954. [Google Scholar] [CrossRef]
- Wang, H.; Schoebel, S.; Schmitz, F.; Dong, H.; Hedfalk, K. Characterization of aquaporin-driven hydrogen peroxide transport. Biochim. Biophys. Acta 2020, 1862, 183065. [Google Scholar] [CrossRef] [PubMed]
- Mubarakshina Borisova, M.M.; Kozuleva, M.A.; Rudenko, N.N.; Naydov, I.A.; Klenina, I.B.; Ivanov, B.N. Photosynthetic electron flow to oxygen and diffusion of hydrogen peroxide through the chloroplast envelope via aquaporins. Biochim. Biophys. Acta 2012, 1817, 1314–1321. [Google Scholar] [CrossRef] [PubMed]
- Exposito-Rodriguez, M.; Laissue, P.P.; Yvon-Durocher, G.; Smirnoff, N.; Mullineaux, P.M. Photosynthesis-dependent H2O2 transfer from chloroplasts to nuclei provides a high-light signalling mechanism. Nat. Commun. 2017, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Møller, I.M.; Rao, R.S.P.; Jiang, Y.; Thelen, J.J.; Xu, D. Proteomic and bioinformatic profiling of transporters in higher plant mitochondria. Biomolecules 2020, 10, 1190. [Google Scholar] [CrossRef] [PubMed]
- Reumann, S.; Bettermann, M.; Benz, R.; Heldt, H.W. Evidence for the presence of a porin in the membrane of glyoxysomes of castor bean. Plant Physiol. 1997, 115, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Corpas, F.J.; Sandalio, L.M.; Brown, M.J.; Río, L.A.D.; Trelease, R.N. Identification of porin-like polypeptide(s) in the boundary membrane of oilseed glyoxysomes. Plant Cell Physiol. 2000, 41, 1218–1228. [Google Scholar] [CrossRef]
- Bestetti, S.; Galli, M.; Sorrentino, I.; Pinton, P.; Rimessi, A.; Sitia, R.; Medraño-Fernandeza, I. Human aquaporin-11 guarantees efficient transport of H2O2 across the endoplasmic reticulum membrane. Redox Biol. 2019, 28, 101326. [Google Scholar] [CrossRef]
- Kim, Y.X.; Steudle, E. Gating of aquaporins by light and reactive oxygen species in leaf parenchyma cells of the midrib of Zea mays. J. Exp. Bot. 2009, 60, 547–556. [Google Scholar] [CrossRef]
- Wudick, M.M.; Li, X.; Valentini, V.; Geldner, N.; Chory, J.; Lin, J.; Maurel, C.; Luu, D.T. Subcellular redistribution of root aquaporins induced by hydrogen peroxide. Mol. Plant 2015, 8, 1103–1114. [Google Scholar] [CrossRef]
- Li, G.; Santoni, V.; Maurel, C. Plant aquaporins: Roles in plant physiology. Biochim. Biophys. Acta 2014, 1840, 1574–1582. [Google Scholar] [CrossRef]
- Pei, H.; Ma, N.; Tian, J.; Luo, J.; Chen, J.; Li, J.; Zheng, Y.; Chen, X.; Fei, Z.; Gao, J. An NAC transcription factor controls ethylene-regulated cell expansion in flower petals. Plant Physiol. 2013, 163, 775–791. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Feng, M.; Chen, W.; Zhou, X.; Lu, J.; Wang, Y.; Li, Y.; Jiang, C.Z.; Gan, S.S.; Ma, N.; et al. In rose, transcription factor PTM balances growth and drought survival via PIP2;1 aquaporin. Nat. Plants 2019, 5, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Shekoofa, A.; Sinclair, T.R. Aquaporin activity to improve crop drought tolerance. Cells 2018, 7, 123. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).