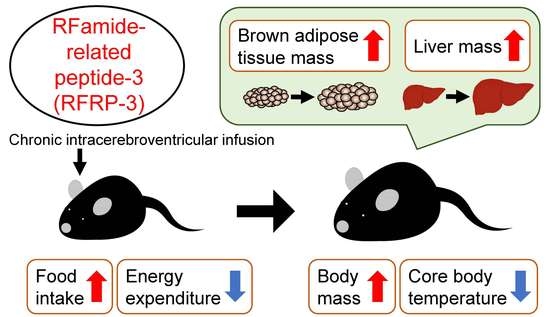
Effects of Chronic Intracerebroventricular Infusion of RFamide-Related Peptide-3 on Energy Metabolism in Male Mice
Abstract
1. Introduction
2. Results
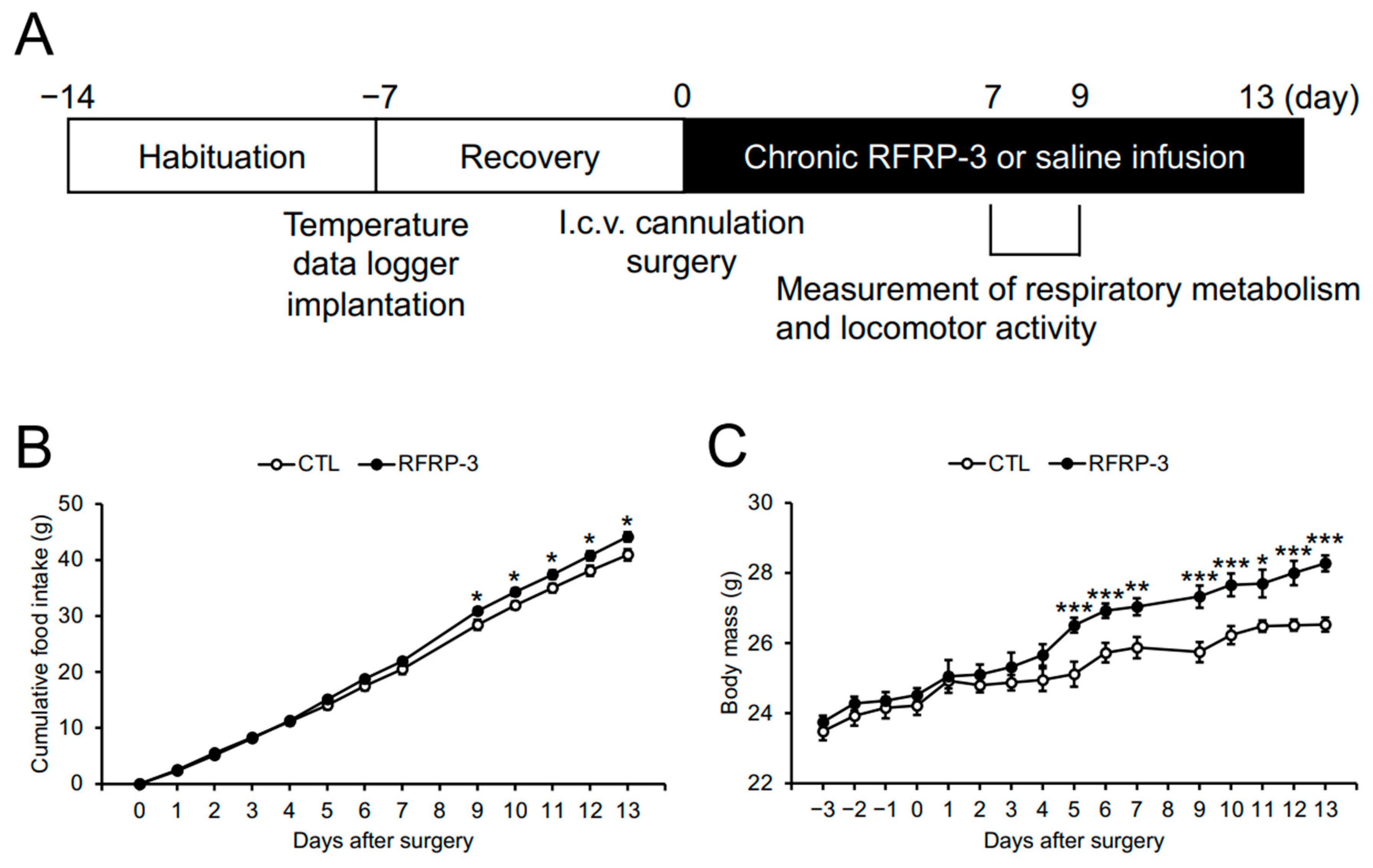
2.1. Effects of Chronic I.C.V. Infusion of RFRP-3 on Food Intake and Body Mass
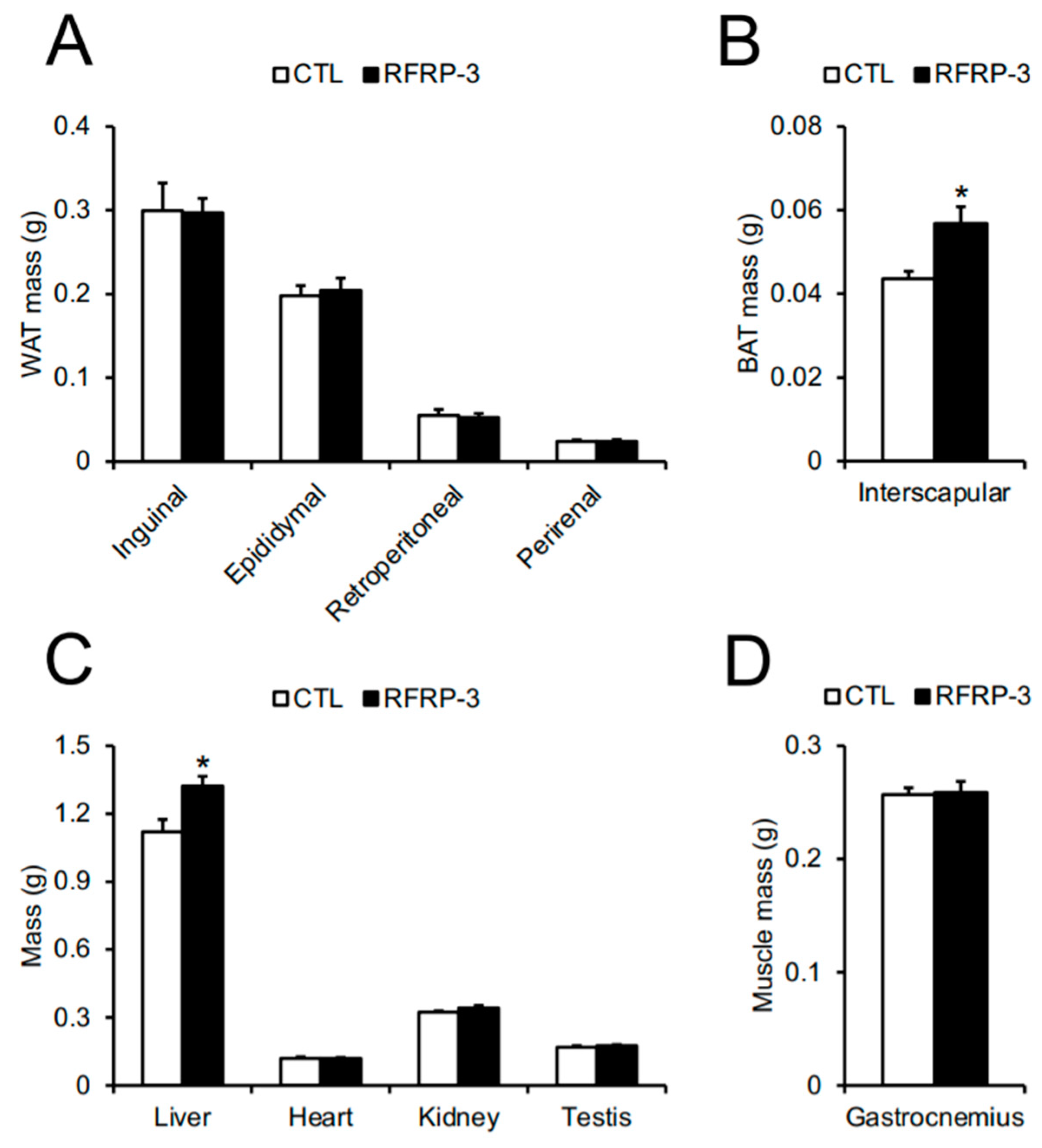
2.2. Effects of Chronic I.C.V. Infusion of RFRP-3 on Body Composition
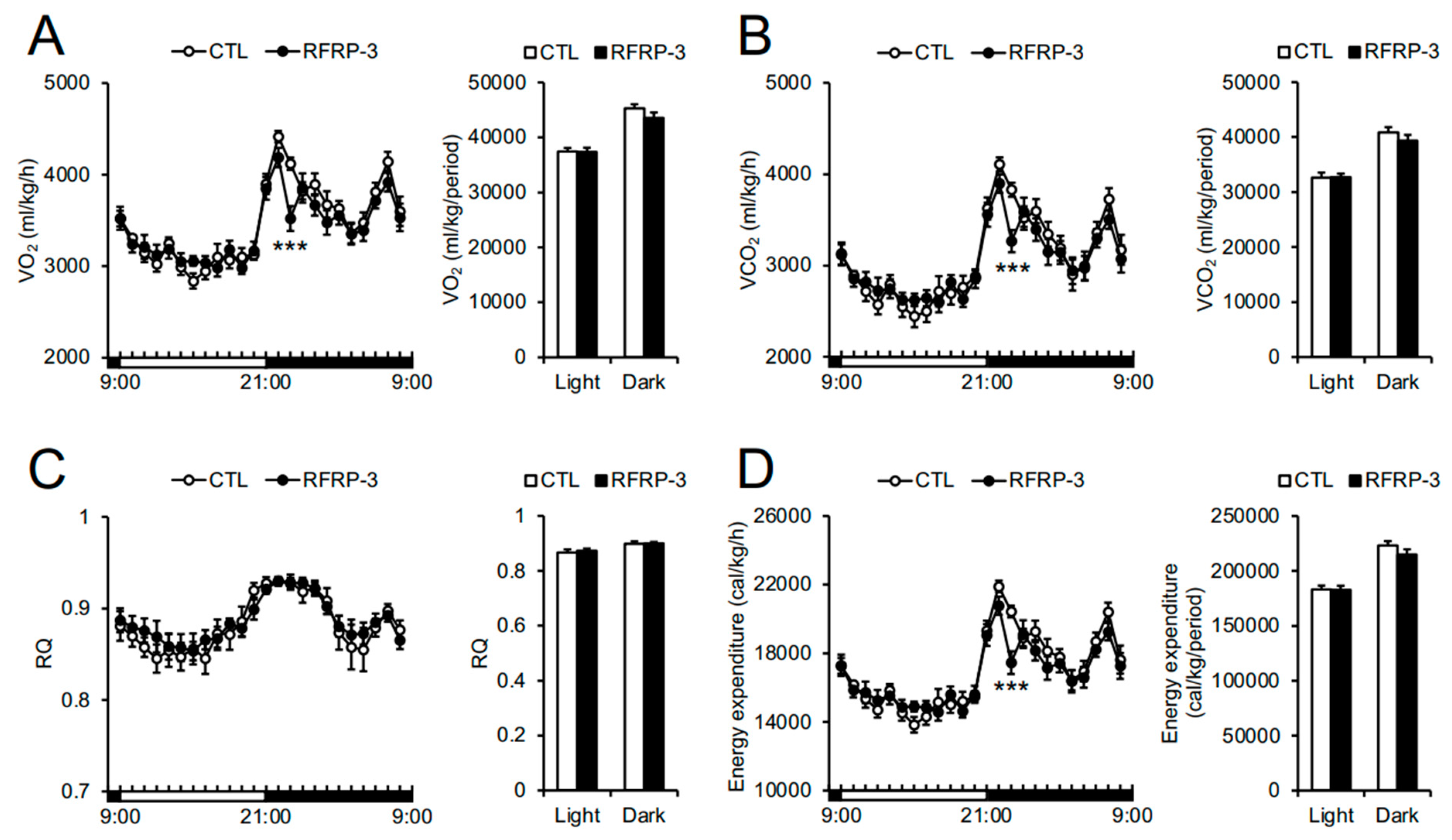
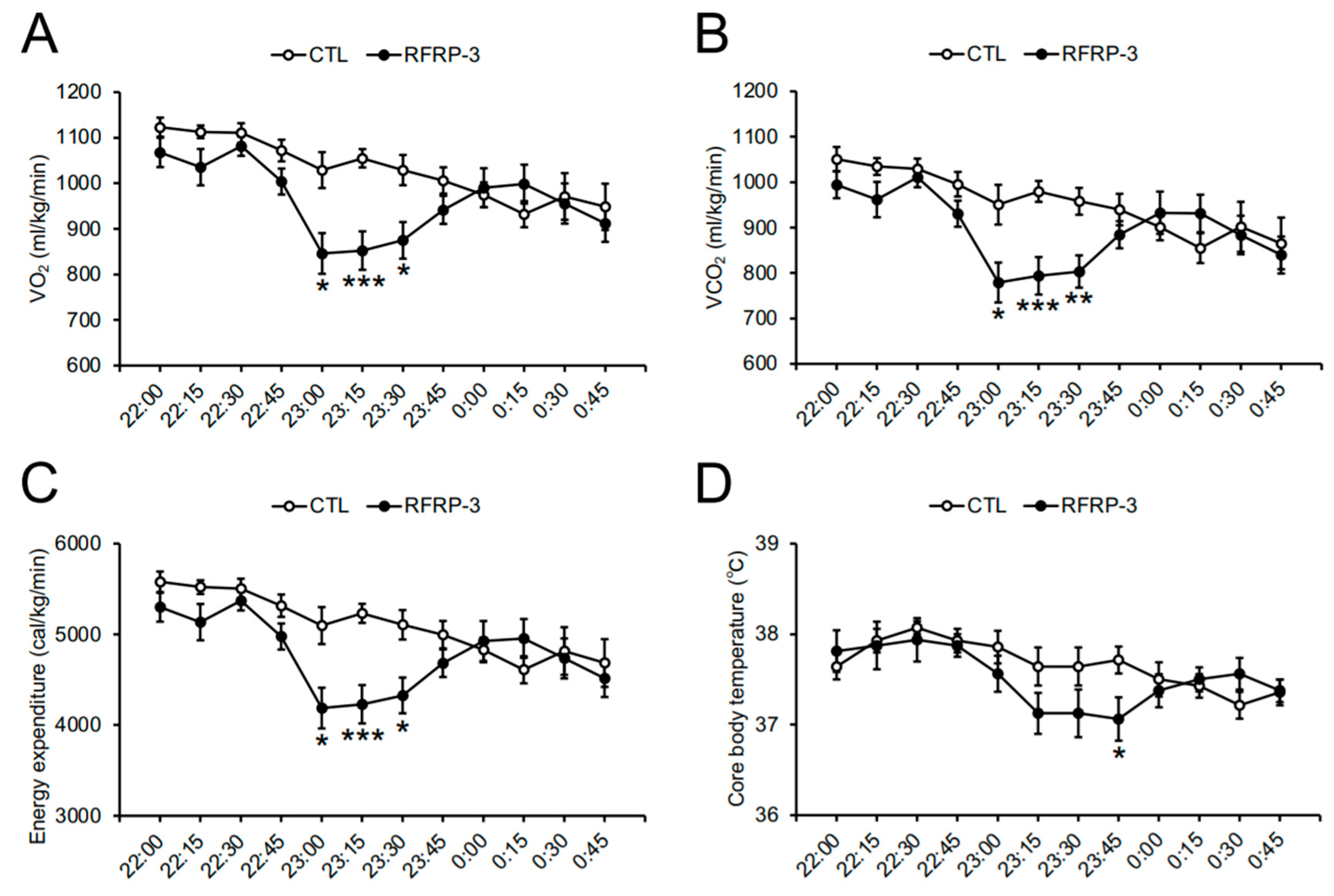
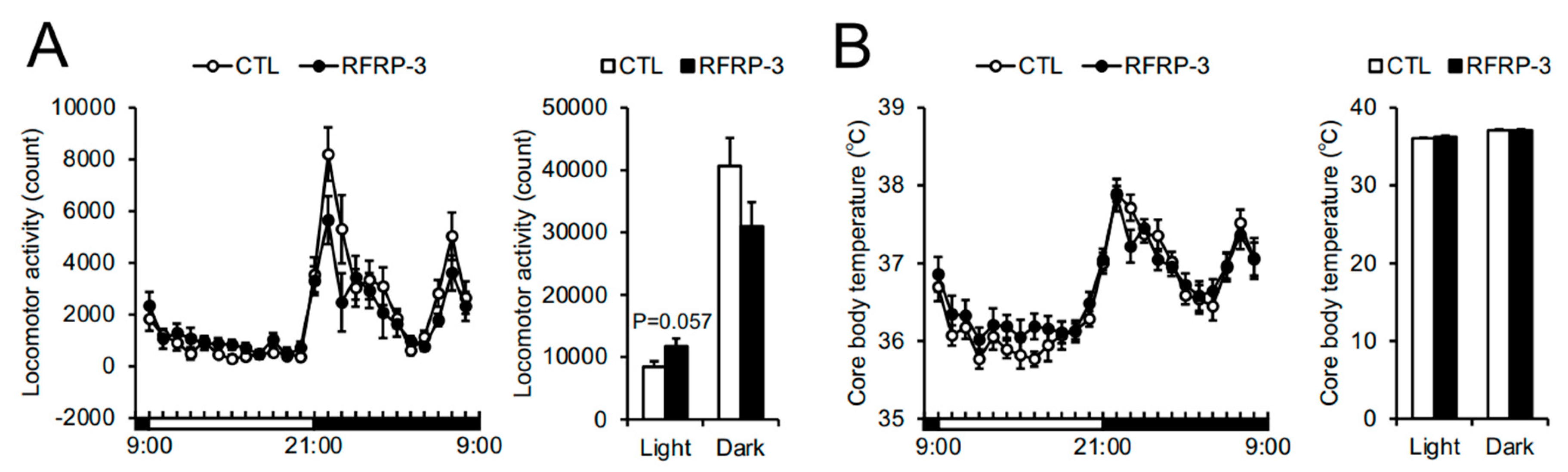
2.3. Effects of Chronic I.C.V. Infusion of RFRP-3 on Respiratory Metabolism, Locomotor Activity, and Core Body Temperature
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Production of RFRP-3
4.3. Measurement of Core Body Temperature
4.4. Chronic I.C.V. Infusion
4.5. Indirect Calorimetry and Locomotor Activity
4.6. qRT-PCR
4.7. Blood Chemistry
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACC | Acetyl-CoA carboxylase |
| ACTB | β-actin |
| AgRP | Agouti-related peptide |
| Arc | Arcuate nucleus |
| ATGL | Adipose triglyceride lipase |
| BAT | Brown adipose tissue |
| CD36 | Cluster of differentiation 36 |
| ChREBPα, β | Carbohydrate-responsive element-binding protein α, β |
| CIDEA | Cell death-inducing DNA fragmentation factor-like effector A |
| COX4 | Cytochrome c oxidase subunit 4 |
| CPT1a | Carnitine palmitoyltransferase 1a |
| DIO2 | Type II iodothyronine deiodinase |
| DMH | Dorsomedial hypothalamus |
| FAS | Fatty acid synthase |
| FSH | Follicle-stimulating hormone |
| G6Pase | Glucose-6-phosphatase |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| GH | Growth hormone |
| GnIH | Gonadotropin-inhibitory hormone |
| GnRH | Gonadotropin-releasing hormone |
| GPAT1 | Glycerol-3-phosphate acyltransferase 1 |
| GPR147 | G protein-coupled receptor 147 |
| HPG | Hypothalamic–pituitary–gonadal |
| HSL | Hormone-sensitive lipase |
| i.c.v. | Intracerebroventricular |
| iWAT | Inguinal white adipose tissue |
| LH | Luteinizing hormone |
| NPY | Neuropeptide Y |
| PEPCK | Phosphoenolpyruvate carboxykinase |
| PGC1α | Peroxisome proliferator-activated receptor γ coactivator 1α |
| POMC | Proopiomelanocortin |
| PPARα, γ | Peroxisome proliferator-activated receptor α, γ |
| PRL | Prolactin |
| PVN | Paraventricular nucleus |
| RFRP | RFamide-related peptide |
| RPS18 | Ribosomal protein S18 |
| RQ | Respiratory quotient |
| SCD1 | Stearoyl-CoA desaturase 1 |
| SLC2A | Solute carrier family 2 member |
| T4 | Thyroxine |
| TNFα | Tumor necrosis factor α |
| TRH | Thyrotropin-releasing hormone |
| TSH | Thyroid-stimulating hormone |
| UCP1 | Uncoupling protein 1 |
| VCO2 | CO2 production |
| VO2 | O2 consumption |
| WAT | White adipose tissue |
References
- Boland, M.P.; Lonergan, P.; O’Callaghan, D. Effect of nutrition on endocrine parameters, ovarian physiology, and oocyte and embryo development. Theriogenology 2001, 55, 1323–1340. [Google Scholar] [CrossRef]
- Huss-ashmore, R. Fat and fertility: Demographic implications of differential fat storage. Yearb. Phys. Anthropol. 1980, 23, 65–92. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.O.; Wyatt, H.R.; Peters, J.C. Energy balance and obesity. Circulation 2012, 126, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Barsh, G.S.; Schwartz, M.W. Genetic approaches to studying energy balance: Perception and integration. Nat. Rev. Genet. 2002, 3, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Halaas, J.L.; Boozer, C.; Blair-West, J.; Fidahusein, N.; Denton, D.A.; Friedman, J.M. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc. Natl. Acad. Sci. USA. 1997, 94, 8878–8883. [Google Scholar] [CrossRef]
- Nakazato, M.; Murakami, N.; Date, Y.; Kojima, M.; Matsuo, H.; Kangawa, K.; Matsukura, S. A role for ghrelin in the central regulation of feeding. Nature 2001, 409, 194–198. [Google Scholar] [CrossRef]
- Chehab, F.F.; Lim, M.E.; Lu, R. Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat. Genet. 1996, 12, 318–320. [Google Scholar] [CrossRef]
- Schally, A.V.; Arimura, A.; Kastin, A.J.; Matsuo, H.; Baba, Y.; Redding, T.W.; Nair, R.M.; Debeljuk, L.; White, W.F. Gonadotropin-releasing hormone: One polypeptide regulates secretion of luteinizing and follicle-stimulating hormones. Science 1971, 173, 1036–1038. [Google Scholar] [CrossRef]
- Amoss, M.; Burgus, R.; Blackwell, R.; Vale, W.; Fellows, R.; Guillemin, R. Purification, amino acid composition and N-terminus of the hypothalamic luteinizing hormone releasing factor (LRF) of ovine origin. Biochem. Biophys. Res. Commun. 1971, 44, 205–210. [Google Scholar] [CrossRef]
- Tena-Sempere, M. GPR54 and kisspeptin in reproduction. Hum. Reprod. Update. 2006, 12, 631–639. [Google Scholar] [CrossRef]
- Harter, C.J.L.; Kavanagh, G.S.; Smith, J.T. The role of kisspeptin neurons in reproduction and metabolism. J. Endocrinol. 2018, 238, R173–R183. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, K.; Saigoh, E.; Ukena, K.; Teranishi, H.; Fujisawa, Y.; Kikuchi, M.; Ishii, S.; Sharp, P.J. A novel avian hypothalamic peptide inhibiting gonadotropin release. Biochem. Biophys. Res. Commun. 2000, 275, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Clarke, I.J.; Qi, Y.; Puspita Sari, I.; Smith, J.T. Evidence that RF-amide related peptides are inhibitors of reproduction in mammals. Front. Neuroendocrinol. 2009, 30, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Kriegsfeld, L.J.; Mei, D.F.; Bentley, G.E.; Ubuka, T.; Mason, A.O.; Inoue, K.; Ukena, K.; Tsutsui, K.; Silver, R. Identification and characterization of a gonadotropin-inhibitory system in the brains of mammals. Proc. Natl. Acad. Sci. USA 2006, 103, 2410–2415. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, K. Phylogenetic aspects of gonadotropin-inhibitory hormone and its homologs in vertebrates. Ann. N. Y. Acad. Sci. 2010, 1200, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Ukena, K.; Iwakoshi, E.; Minakata, H.; Tsutsui, K. A novel rat hypothalamic RFamide-related peptide identified by immunoaffinity chromatography and mass spectrometry. FEBS Lett. 2002, 512, 255–258. [Google Scholar] [CrossRef]
- Tsutsui, K.; Ukena, K. Hypothalamic LPXRF-amide peptides in vertebrates: Identification, localization and hypophysiotropic activity. Peptides 2006, 27, 1121–1129. [Google Scholar] [CrossRef]
- Ukena, K.; Tsutsui, K. A new member of the hypothalamic RF-amide peptide family, LPXRF-amide peptides: Structure, localization, and function. Mass. Spectrom. Rev. 2005, 24, 469–486. [Google Scholar] [CrossRef]
- Clarke, I.J.; Sari, I.P.; Qi, Y.; Smith, J.T.; Parkington, H.C.; Ubuka, T.; Iqbal, J.; Li, Q.; Tilbrook, A.; Morgan, K.; et al. Potent action of RFamide-related peptide-3 on pituitary gonadotropes indicative of a hypophysiotropic role in the negative regulation of gonadotropin secretion. Endocrinology 2008, 149, 5811–5821. [Google Scholar] [CrossRef]
- Hinuma, S.; Shintani, Y.; Fukusumi, S.; Iijima, N.; Matsumoto, Y.; Hosoya, M.; Fujii, R.; Watanabe, T.; Kikuchi, K.; Terao, T.; et al. New neuropeptides containing carboxy-terminal RFamide and their receptor in mammals. Nat. Cell. Biol. 2000, 2, 703–708. [Google Scholar] [CrossRef]
- Yoshida, H.; Habata, Y.; Hosoya, M.; Kawamata, Y.; Kitada, C.; Hinuma, S. Molecular properties of endogenous RFamide-related peptide-3 and its interaction with receptors. Biochim. Biophys. Acta 2003, 1593, 151–157. [Google Scholar] [CrossRef]
- Fukusumi, S.; Habata, Y.; Yoshida, H.; Iijima, N.; Kawamata, Y.; Hosoya, M.; Fujii, R.; Hinuma, S.; Kitada, C.; Shintani, Y.; et al. Characteristics and distribution of endogenous RFamide-related peptide-1. Biochim. Biophys. Acta 2001, 1540, 221–232. [Google Scholar] [CrossRef]
- Ducret, E.; Anderson, G.M.; Herbison, A.E. RFamide-related peptide-3, a mammalian gonadotropin-inhibitory hormone ortholog, regulates gonadotropin-releasing hormone neuron firing in the mouse. Endocrinology 2009, 150, 2799–2804. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Dumalska, I.; Morozova, E.; van den Pol, A.N.; Alreja, M. Gonadotropin inhibitory hormone inhibits basal forebrain vGluT2-gonadotropin-releasing hormone neurons via a direct postsynaptic mechanism. J. Physiol. 2009, 587, 1401–1411. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.A.; Tsutsui, K.; Fraley, G.S. Rat RFamide-related peptide-3 stimulates GH secretion, inhibits LH secretion, and has variable effects on sex behavior in the adult male rat. Horm. Behav. 2007, 51, 171–180. [Google Scholar] [CrossRef]
- Johnson, M.A.; Fraley, G.S. Rat RFRP-3 alters hypothalamic GHRH expression and growth hormone secretion but does not affect KiSS-1 gene expression or the onset of puberty in male rats. Neuroendocrinology 2008, 88, 305–315. [Google Scholar] [CrossRef]
- Anderson, G.M.; Relf, H.L.; Rizwan, M.Z.; Evans, J.J. Central and peripheral effects of RFamide-related peptide-3 on luteinizing hormone and prolactin secretion in rats. Endocrinology 2009, 150, 1834–1840. [Google Scholar] [CrossRef]
- Murakami, M.; Matsuzaki, T.; Iwasa, T.; Yasui, T.; Irahara, M.; Osugi, T.; Tsutsui, K. Hypophysiotropic role of RFamide-related peptide-3 in the inhibition of LH secretion in female rats. J. Endocrinol. 2008, 199, 105–112. [Google Scholar] [CrossRef]
- Kadokawa, H.; Shibata, M.; Tanaka, Y.; Kojima, T.; Matsumoto, K.; Oshima, K.; Yamamoto, N. Bovine C-terminal octapeptide of RFamide-related peptide-3 suppresses luteinizing hormone (LH) secretion from the pituitary as well as pulsatile LH secretion in bovines. Domest. Anim. Endocrinol. 2009, 36, 219–224. [Google Scholar] [CrossRef]
- Sari, I.P.; Rao, A.; Smith, J.T.; Tilbrook, A.J.; Clarke, I.J. Effect of RF-amide-related peptide-3 on luteinizing hormone and follicle-stimulating hormone synthesis and secretion in ovine pituitary gonadotropes. Endocrinology 2009, 150, 5549–5556. [Google Scholar] [CrossRef]
- Pineda, R.; Garcia-Galiano, D.; Sanchez-Garrido, M.A.; Romero, M.; Ruiz-Pino, F.; Aguilar, E.; Dijcks, F.A.; Blomenröhr, M.; Pinilla, L.; van Noort, P.I.; et al. Characterization of the inhibitory roles of RFRP3, the mammalian ortholog of GnIH, in the control of gonadotropin secretion in the rat: In vivo and in vitro studies. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E39–E46. [Google Scholar] [CrossRef] [PubMed]
- Bonini, J.A.; Jones, K.A.; Adham, N.; Forray, C.; Artymyshyn, R.; Durkin, M.M.; Smith, K.E.; Tamm, J.A.; Boteju, L.W.; Lakhlani, P.P.; et al. Identification and characterization of two G protein-coupled receptors for neuropeptide FF. J. Biol. Chem. 2000, 275, 39324–39331. [Google Scholar] [CrossRef] [PubMed]
- León, S.; García-Galiano, D.; Ruiz-Pino, F.; Barroso, A.; Manfredi-Lozano, M.; Romero-Ruiz, A.; Roa, J.; Vázquez, M.J.; Gaytan, F.; Blomenrohr, M.; et al. Physiological roles of gonadotropin-inhibitory hormone signaling in the control of mammalian reproductive axis: Studies in the NPFF1 receptor null mouse. Endocrinology 2014, 155, 2953–2965. [Google Scholar] [CrossRef] [PubMed]
- McConn, B.; Wang, G.; Yi, J.; Gilbert, E.R.; Osugi, T.; Ubuka, T.; Tsutsui, K.; Chowdhury, V.S.; Furuse, M.; Cline, M.A. Gonadotropin-inhibitory hormone-stimulation of food intake is mediated by hypothalamic effects in chicks. Neuropeptides 2014, 48, 327–334. [Google Scholar] [CrossRef]
- Tachibana, T.; Sato, M.; Takahashi, H.; Ukena, K.; Tsutsui, K.; Furuse, M. Gonadotropin-inhibiting hormone stimulates feeding behavior in chicks. Brain Res. 2005, 1050, 94–100. [Google Scholar] [CrossRef]
- Huo, K.; Li, X.; Hu, W.; Song, X.; Zhang, D.; Zhang, X.; Chen, X.; Yuan, J.; Zuo, J.; Wang, X. RFRP-3, the mammalian ortholog of GnIH, is a novel modulator involved in food intake and glucose homeostasis. Front. Endocrinol. 2020, 11, 194. [Google Scholar] [CrossRef]
- Gospodarska, E.; Kozak, L.P.; Jaroslawska, J. Isolation and identification of endogenous RFamide-related peptides 1 and 3 in the mouse hypothalamus. J. Neuroendocrinol. 2019, 31, e12668. [Google Scholar] [CrossRef]
- Ukena, K.; Tsutsui, K. Distribution of novel RFamide-related peptide-like immunoreactivity in the mouse central nervous system. Neurosci. Lett. 2001, 300, 153–156. [Google Scholar] [CrossRef]
- Sohn, J.W.; Elmquist, J.K.; Williams, K.W. Neuronal circuits that regulate feeding behavior and metabolism. Trends Neurosci. 2013, 36, 504–512. [Google Scholar] [CrossRef]
- Clarke, I.J.; Smith, J.T.; Henry, B.A.; Oldfield, B.J.; Stefanidis, A.; Millar, R.P.; Sari, I.P.; Chng, K.; Fabre-Nys, C.; Caraty, A.; et al. Gonadotropin-inhibitory hormone is a hypothalamic peptide that provides a molecular switch between reproduction and feeding. Neuroendocrinology 2012, 95, 305–316. [Google Scholar] [CrossRef]
- Green, C.J.; Hodson, L. The influence of dietary fat on liver fat accumulation. Nutrients 2014, 6, 5018–5033. [Google Scholar] [CrossRef] [PubMed]
- Chao, P.T.; Yang, L.; Aja, S.; Moran, T.H.; Bi, S. Knockdown of NPY expression in the dorsomedial hypothalamus promotes development of brown adipocytes and prevents diet-induced obesity. Cell. Metab. 2011, 13, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Bi, S. Hypothalamic regulation of brown adipose tissue thermogenesis and energy homeostasis. Front. Endocrinol. 2015, 6, 136. [Google Scholar] [CrossRef] [PubMed]
- Chechi, K.; Carpentier, A.C.; Richard, D. Understanding the brown adipocyte as a contributor to energy homeostasis. Trends Endocrinol. Metab. 2013, 24, 408–420. [Google Scholar] [CrossRef]
- Shimizu, I.; Aprahamian, T.; Kikuchi, R.; Shimizu, A.; Papanicolaou, K.N.; MacLauchlan, S.; Maruyama, S.; Walsh, K. Vascular rarefaction mediates whitening of brown fat in obesity. J. Clin. Investig. 2014, 124, 2099–2112. [Google Scholar] [CrossRef] [PubMed]
- Ubuka, T.; Bentley, G.E.; Ukena, K.; Wingfield, J.C.; Tsutsui, K. Melatonin induces the expression of gonadotropin-inhibitory hormone in the avian brain. Proc. Natl. Acad. Sci. USA 2005, 102, 3052–3057. [Google Scholar] [CrossRef] [PubMed]
- Revel, F.G.; Saboureau, M.; Pévet, P.; Simonneaux, V.; Mikkelsen, J.D. RFamide-related peptide gene is a melatonin-driven photoperiodic gene. Endocrinology 2008, 149, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Ubuka, T.; Inoue, K.; Fukuda, Y.; Mizuno, T.; Ukena, K.; Kriegsfeld, L.J.; Tsutsui, K. Identification, expression, and physiological functions of Siberian hamster gonadotropin-inhibitory hormone. Endocrinology 2012, 153, 373–385. [Google Scholar] [CrossRef]
- Poling, M.C.; Luo, E.Y.; Kauffman, A.S. Sex differences in steroid receptor coexpression and circadian-timed activation of kisspeptin and RFRP-3 neurons may contribute to the sexually dimorphic basis of the LH surge. Endocrinology 2017, 158, 3565–3578. [Google Scholar] [CrossRef]
- Ebihara, S.; Marks, T.; Hudson, D.J.; Menaker, M. Genetic control of melatonin synthesis in the pineal gland of the mouse. Science 1986, 231, 491–493. [Google Scholar] [CrossRef]
- Fu, L.Y.; van den Pol, A.N. Kisspeptin directly excites anorexigenic proopiomelanocortin neurons but inhibits orexigenic neuropeptide Y cells by an indirect synaptic mechanism. J. Neurosci. 2010, 30, 10205–10219. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, J.S.; Coleman, H.A.; Enriori, P.J.; Parkington, H.C.; Li, Q.; Pereira, A.; Cowley, M.A.; Clarke, I.J. Paradoxical effect of gonadotrophin-inhibiting hormone to negatively regulate neuropeptide Y neurones in mouse arcuate nucleus. J. Neuroendocrinol. 2013, 25, 1308–1317. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Morrison, S.F. Central efferent pathways mediating skin cooling-evoked sympathetic thermogenesis in brown adipose tissue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R127–R136. [Google Scholar] [CrossRef] [PubMed]
- Ubuka, T.; Ukena, K.; Sharp, P.J.; Bentley, G.E.; Tsutsui, K. Gonadotropin-inhibitory hormone inhibits gonadal development and maintenance by decreasing gonadotropin synthesis and release in male quail. Endocrinology 2006, 147, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Ancel, C.; Bentsen, A.H.; Sébert, M.E.; Tena-Sempere, M.; Mikkelsen, J.D.; Simonneaux, V. Stimulatory effect of RFRP-3 on the gonadotrophic axis in the male Syrian hamster: The exception proves the rule. Endocrinology 2012, 153, 1352–1363. [Google Scholar] [CrossRef]
- Ancel, C.; Inglis, M.A.; Anderson, G.M. Central RFRP-3 stimulates LH secretion in male mice and has cycle stage-dependent inhibitory effects in females. Endocrinology 2017, 158, 2873–2883. [Google Scholar] [CrossRef]
- Masuda, K.; Ooyama, H.; Shikano, K.; Kondo, K.; Furumitsu, M.; Iwakoshi-Ukena, E.; Ukena, K. Microwave-assisted solid-phase peptide synthesis of neurosecretory protein GL composed of 80 amino acid residues. J. Pept. Sci. 2015, 21, 454–460. [Google Scholar] [CrossRef]
- Bartolomucci, A.; Bresciani, E.; Bulgarelli, I.; Rigamonti, A.E.; Pascucci, T.; Levi, A.; Possenti, R.; Torsello, A.; Locatelli, V.; Muller, E.E.; et al. Chronic intracerebroventricular injection of TLQP-21 prevents high fat diet induced weight gain in fast weight-gaining mice. Genes Nutr. 2009, 4, 49–57. [Google Scholar] [CrossRef]
- Lusk, G. The Elements of the Science of Nutrition, 4th ed.; W.B. Saunders Company: Philadelphia, PA, USA, 1928. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆Ct Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
| Serum Components | CTL | RFRP-3 |
|---|---|---|
| Glucose (mg/dL) | 126 ± 7.0 | 146 ± 6.3* |
| Free Fatty Acids (mEq/L) | 2.25 ± 0.19 | 1.82 ± 0.26 |
| Triglyceride (mg/dL) | 113 ± 10.4 | 123 ± 13.8 |
| Cholesterol (mg/mL) | 99 ± 5.0 | 104 ± 4.4 |
| Thyroxine (T4) (µg/dL) | 0.43 ± 0.18 | 2.03 ± 0.84 |
| Insulin (ng/mL) | 0.77 ± 0.19 | 0.79 ± 0.10 |
| Gene | Sense Primer (5′ to 3′) | Antisense Primer (5′ to 3′) |
|---|---|---|
| Npy | TATCTCTGCTCGTGTGTTTG | GATTGATGTAGTGTCGCAGA |
| Agrp | TGTTCCCAGAGTTCCCAGGTC | GCATTGAAGAAGCGGCAGTAGCAC |
| Pomc | AGCTGCCTTTCCGCGACA | ATCTATGGAGGTCTGAAGCA |
| Trh | TCGTGCTAACTGGTATCCCC | CCCAAATCTCCCCTCTCTTC |
| Gnrh | AGCACTGGTCCTATGGGTTG | CCTGGCTTCCTCTTCAATCA |
| Rfrp | TGGAAGGACCATAGATGAGAAA | GCTGTTGTTCTCCCAAACCT |
| Gpr147 | CTTTCCGTGAGAAGCTGACC | GAGCATCCAGCATGAAGTGA |
| Prl | GGCTACACCTGAAGACAAGGAACAA | TGTTCCTCAATCTCTTTGGCTCTTG |
| Gh | GGAGGCTAGTGCTTTTCCCG | AGGCACGCTCGAACTCTTTG |
| Lhβ | TGGCCGCAGAGAATGAGTTC | ACTCGGACCATGCTAGGACA |
| Fshβ | GGAGAGCAATCTGCTGCCAT | GCCGAGCTGGGTCCTTATAC |
| Tshβ | CACCATCTGTGCTGGGTATTG | CATCCTGGTATTTCCACCGTTC |
| Acc | TCCGCACTGACTGTAACCACAT | TGCTCCGCACAGATTCTTCA |
| Fas | AGGGGTCGACCTGGTCCTCA | GCCATGCCCAGAGGGTGGTT |
| Scd1 | CTGTACGGGATCATACTGGTTC | GCCGTGCCTTGTAAGTTCTG |
| Atgl | AACACCAGCATCCAGTTCAA | GGTTCAGTAGGCCATTCCTC |
| Hsl | GCTGGGCTGTCAAGCACTGT | GTAACTGGGTAGGCTGCCAT |
| Pparγ | GCCCTTTGGTGACTTTATGGA | GCAGCAGGTTGTCTTGGATG |
| Pgc1α | GCAACATGCTCAAGCCAAAC | TGCAGTTCCAGAGAGTTCCA |
| Ucp1 | CAAAAACAGAAGGATTGCCGAAA | TCTTGGACTGAGTCGTAGAGG |
| Dio2 | CCACCTTCTTGACTTTGCCA | GGTGAGCCTCATCAATGTATAC |
| Cidea | CTTATCAGCAAGACTCTGGATG | GAAGGTGACTCTGGCTATTC |
| Cox4 | TGAGCCTGATTGGCAAGAGA | CGAAGCTCTCGTTAAACTGG |
| Gpat1 | TCATCCAGTATGGCATTCTCACA | GCAAGGCCAGGACTGACATC |
| Chrebpα | CGACACTCACCCACCTCTTC | TTGTTCAGCCGGATCTTGTC |
| Chrebpβ | TCTGCAGATCGCGTGGAG | CTTGTCCCGGCATAGCAAC |
| Cpt1a | CCTGGGCATGATTGCAAAG | GGACGCCACTCACGATGTT |
| Gapdh | AAGGTCATCCCAGAGCTGAA | CTGCTTCACCACCTTCTTGA |
| Cd36 | TCCTCTGACATTTGCAGGTCTATC | AAAGGCATTGGCTGGAAGAA |
| Pepck | GTGCTGGAGTGGATGTTCGG | CTGGCTGATTCTCTGTTTCAGG |
| G6pase | ACTGTGGGCATCAATCTCCTC | CGGGACAGACAGACGTTCAGC |
| Slc2a2 | GGCTAATTTCAGGACTGGTT | TTTCTTTGCCCTGACTTCCT |
| Pparα | TCGAATATGTGGGGACAAGG | GACAGGCACTTGTGAAAACG |
| Tnfα | GCCTCTTCTCATTCCTGCTTG | CTGATGAGAGGGAGGCCATT |
| Slc2a4 | GTAACTTCATTGTCGGCATGG | AGCTGAGATCTGGTCAAACG |
| Actb | GGCACCACACCTTCTACAAT | AGGTCTCAAACATGATCTGG |
| Rps18 | CCTGAGAAGTTCCAGCACAT | TTCTCCAGCCCTCTTGGTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moriwaki, S.; Narimatsu, Y.; Fukumura, K.; Iwakoshi-Ukena, E.; Furumitsu, M.; Ukena, K. Effects of Chronic Intracerebroventricular Infusion of RFamide-Related Peptide-3 on Energy Metabolism in Male Mice. Int. J. Mol. Sci. 2020, 21, 8606. https://doi.org/10.3390/ijms21228606
Moriwaki S, Narimatsu Y, Fukumura K, Iwakoshi-Ukena E, Furumitsu M, Ukena K. Effects of Chronic Intracerebroventricular Infusion of RFamide-Related Peptide-3 on Energy Metabolism in Male Mice. International Journal of Molecular Sciences. 2020; 21(22):8606. https://doi.org/10.3390/ijms21228606
Chicago/Turabian StyleMoriwaki, Shogo, Yuki Narimatsu, Keisuke Fukumura, Eiko Iwakoshi-Ukena, Megumi Furumitsu, and Kazuyoshi Ukena. 2020. "Effects of Chronic Intracerebroventricular Infusion of RFamide-Related Peptide-3 on Energy Metabolism in Male Mice" International Journal of Molecular Sciences 21, no. 22: 8606. https://doi.org/10.3390/ijms21228606
APA StyleMoriwaki, S., Narimatsu, Y., Fukumura, K., Iwakoshi-Ukena, E., Furumitsu, M., & Ukena, K. (2020). Effects of Chronic Intracerebroventricular Infusion of RFamide-Related Peptide-3 on Energy Metabolism in Male Mice. International Journal of Molecular Sciences, 21(22), 8606. https://doi.org/10.3390/ijms21228606