MicroRNA-23a-3p Down-Regulation in Active Pulmonary Tuberculosis Patients with High Bacterial Burden Inhibits Mononuclear Cell Function and Phagocytosis through TLR4/TNF-α/TGF-β1/IL-10 Signaling via Targeting IRF1/SP1
Abstract
1. Introduction
2. Results
2.1. Demographics of the Participants
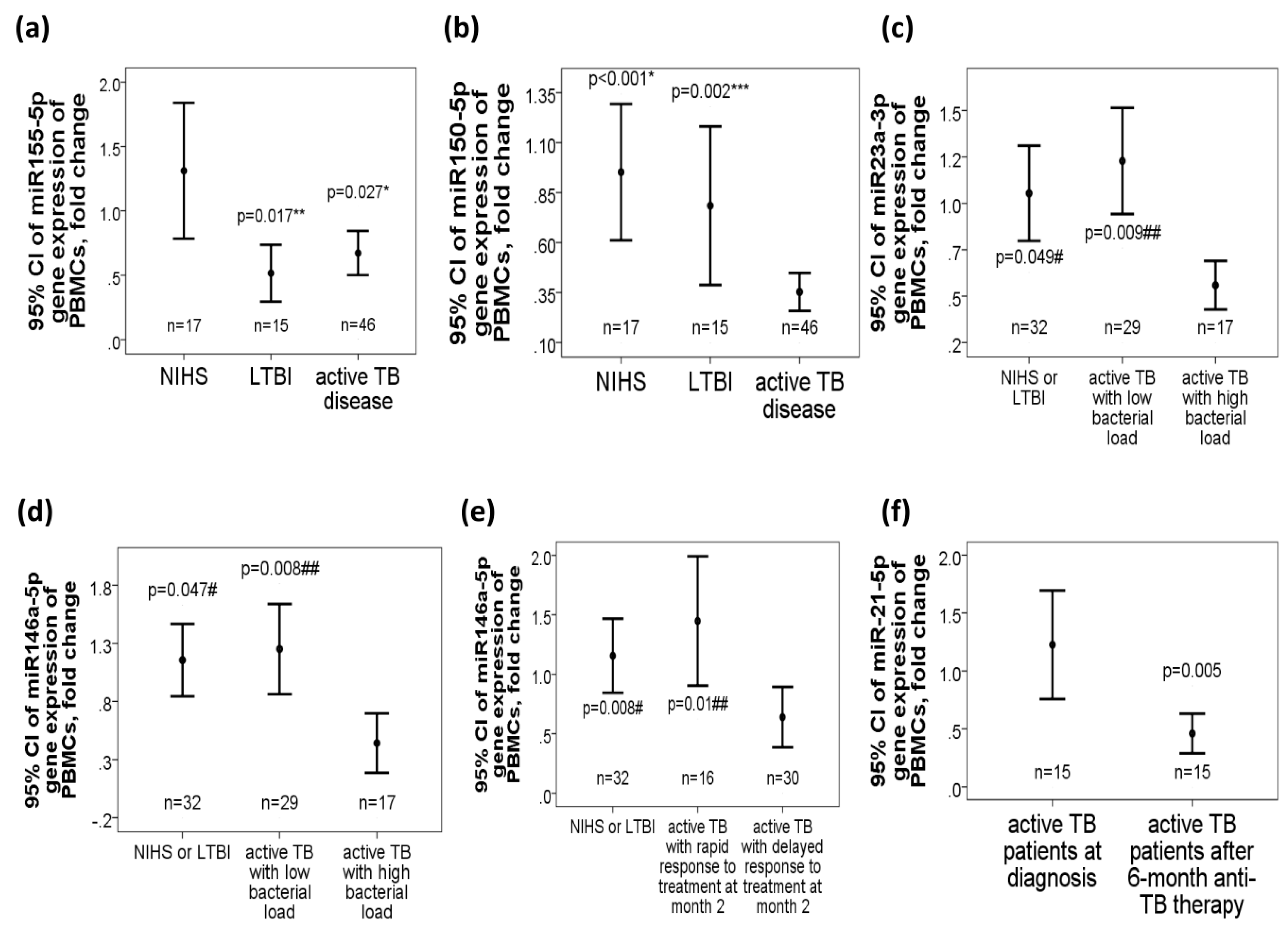
2.2. Decreased miR-155-5p, miR-150-5p, miR-23a-3p, and miR-146a-5p Gene Expressions in Active TB Patients, Especially in Those with High Bacterial Burden
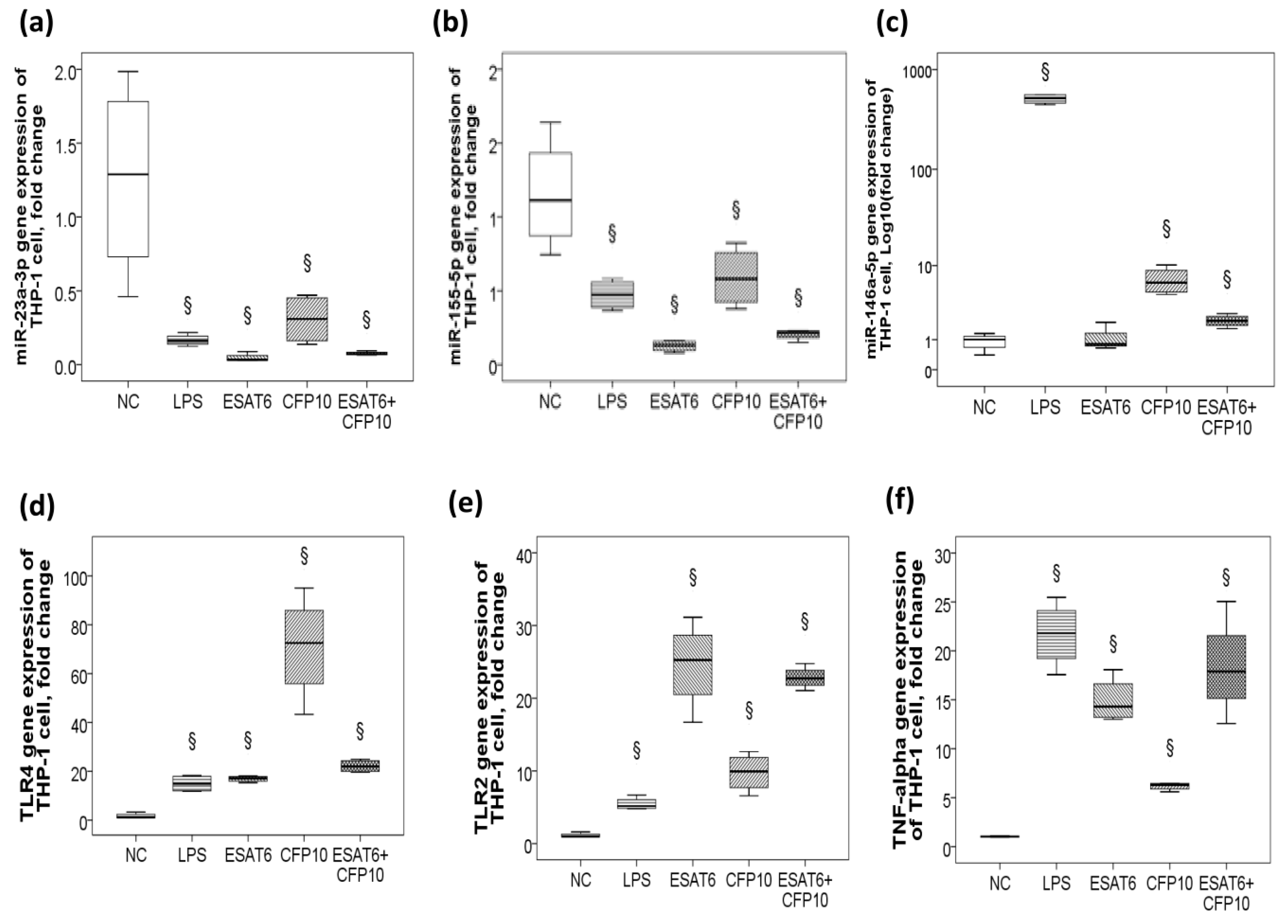
2.3. Decreased miR-23a-3p/miR-155-5p Gene Expression and Increased MiR-146a-5p Gene Expression in Response to ESAT-6 and CFP-10 Stimuli In Vitro
2.4. Predicted Target Genes of miR-23a-3p
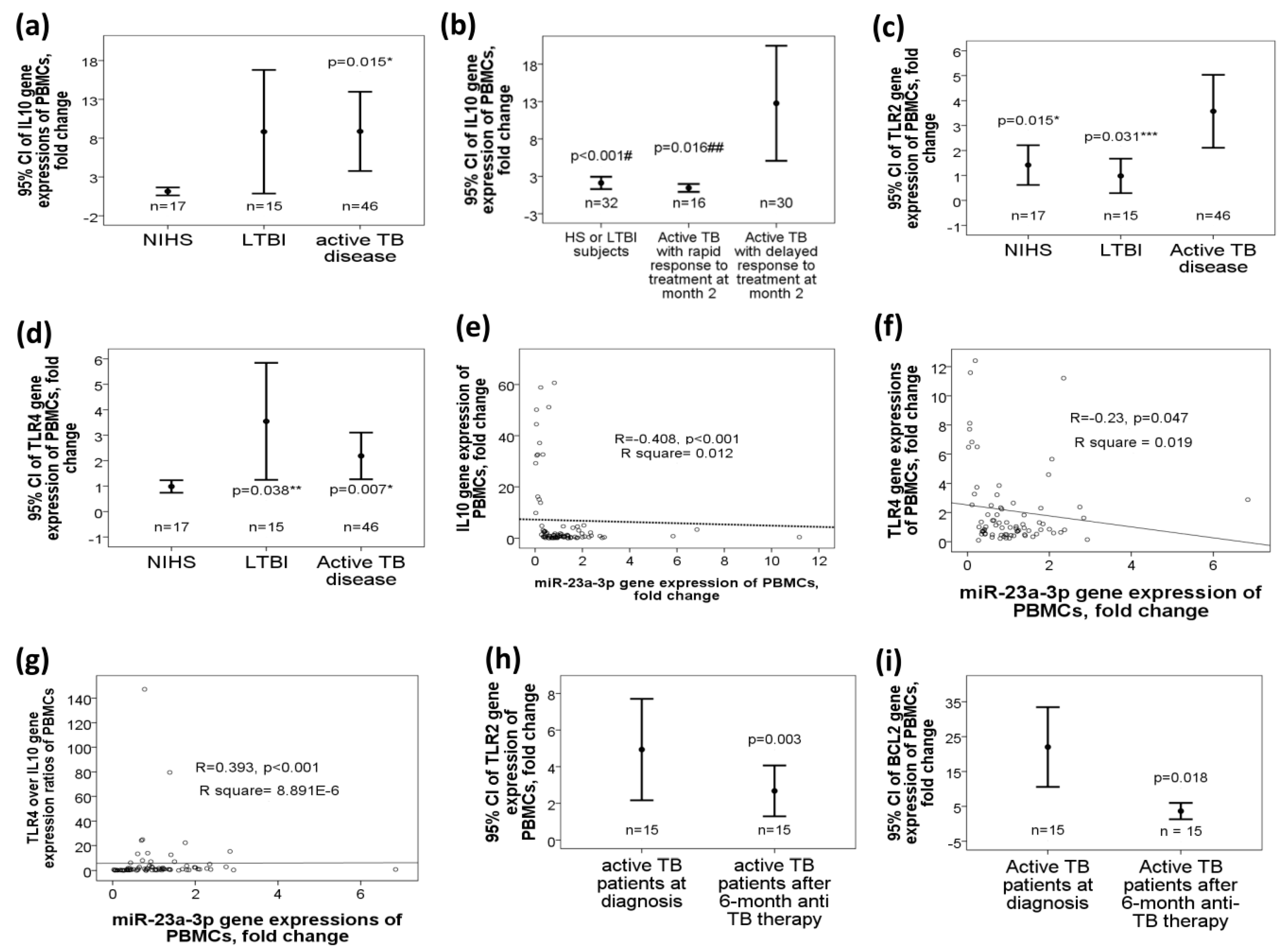
2.5. Increased Target Gene Expressions of miR-23a-3p, Including IL10, TLR4, and TLR2 Genes, in Active TB Patients
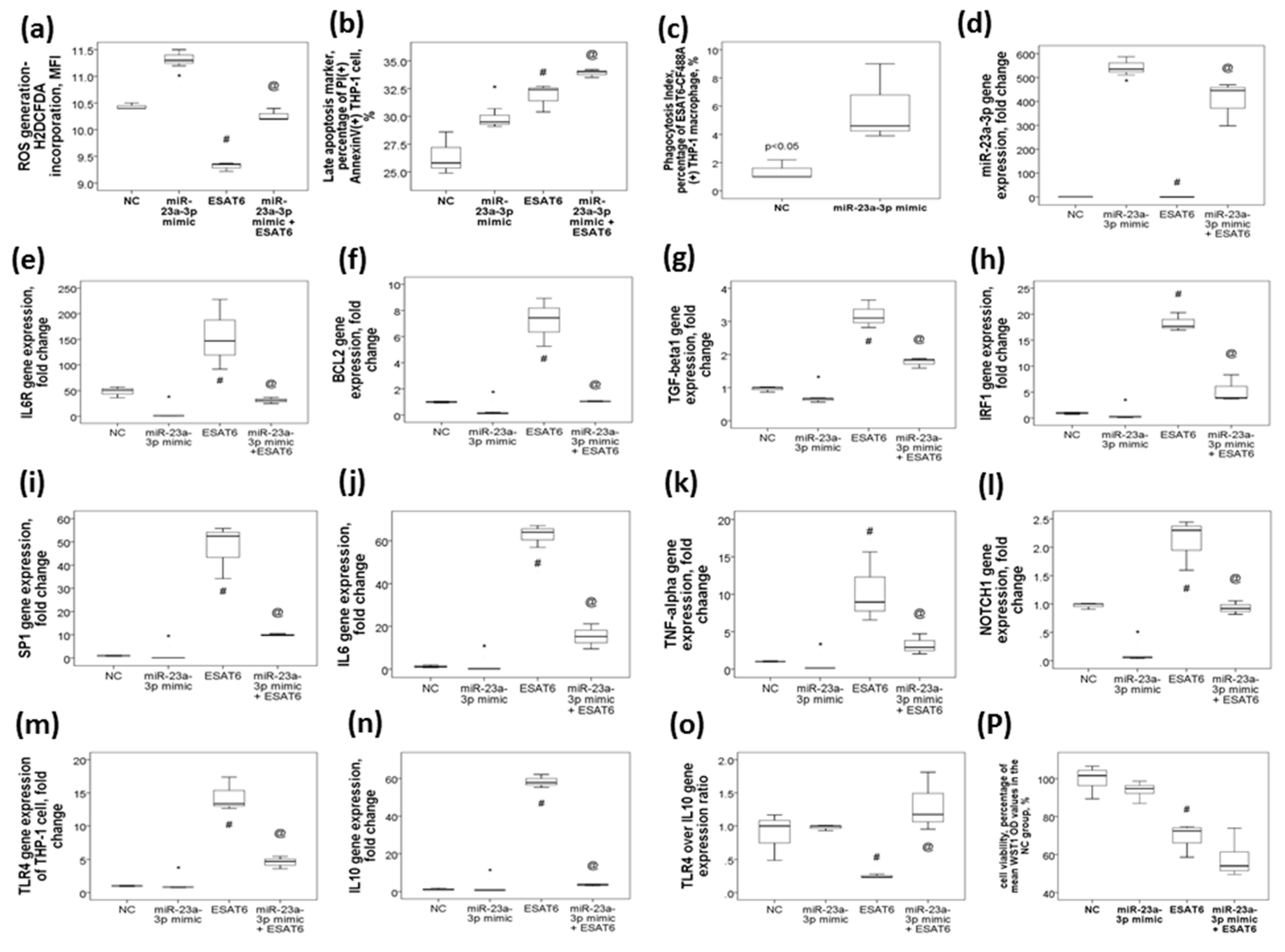
2.6. MiR-23a-3p Transfection in THP-1 Cells Resulted in Increased ROS Production, Late Apoptosis, and Phagocytosis in Association with the Reversal of ESAT6-Induced Up-Regulations of The IL6R, BCL2, TGF-β1, IRF1, SP1, IL6, TNF-α, NOTCH1, TLR4, and IL10 Genes
3. Discussion
4. Materials and Methods
4.1. Study Subjects
4.2. Isolation of Leukocyte RNA and Protein from Blood Leukocyte Samples
4.3. Analysis of miR Gene Expressions
4.4. Analysis of Target Gene mRNA Expressions of Isolated PBMCs Using Quantitative Reverse-Transcriptase Polymerase Chain Reaction (RT-PCR)
4.5. In Vitro Cell Culture Model under M.tb-Specific Antigen Stimuli
4.6. Transfection of miRNA-23a-3p Mimic in THP-1 cells
4.7. Measurement of THP-1 Intracellular Reactive Oxygen Species (ROS)
4.8. Measurement of Cell Apoptosis by Flow Cytometry Analysis
4.9. Phagocytosis Assay
4.10. Measurement of Cell Viability (Mitochondrial Activity) by WST-1
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Mtb | Mycobacterium tuberculosis |
| LTBI | Latent TB infection |
| miR | microRNA |
| TLR | Toll-like receptor |
| PBMC | Peripheral blood mononuclear cell |
| NIHS | Non-infected healthy subject |
| AFB | Acid fast bacilli |
| CXR | Chest radiography |
| RT-PCR | Reverse transcriptase-polymerase chain reaction |
| ESAT6 | 6 kDa early secretory antigenic target |
| CFP10 | 10 kDa culture filtrate antigen |
| NC | Nonsense sequence normal control |
| ROS | Reactive oxygen species |
References
- Marimani, M.; Ahmad, A.; Duse, A. The role of epigenetics, bacterial and host factors in progression of Mycobacterium tuberculosis infection. Tuberculosis 2018, 113, 200–214. [Google Scholar] [CrossRef]
- Lin, P.L.; Flynn, J.L. The End of the Binary Era: Revisiting the Spectrum of Tuberculosis. J. Immunol. 2018, 201, 2541–2548. [Google Scholar] [CrossRef]
- Yang, T.; Ge, B. miRNAs in immune responses to Mycobacterium tuberculosis infection. Cancer Lett. 2018, 431, 22–30. [Google Scholar] [CrossRef]
- Sabir, N.; Hussain, T.; Shah, S.Z.A.; Peramo, A.; Zhao, D.; Zhou, X. miRNAs in Tuberculosis: New Avenues for Diagnosis and Host-Directed Therapy. Front. Microbiol. 2018, 9, 602. [Google Scholar] [CrossRef] [PubMed]
- Sepehri, Z.; Kiani, Z.; Kohan, F.; Ghavami, S. Toll-Like Receptor 4 as an Immune Receptor against Mycobacterium tuberculosis: A Systematic Review. Lab. Med. 2019, 50, 117–129. [Google Scholar] [CrossRef]
- Gopalakrishnan, A.; Salgame, P. Toll-like receptor 2 in host defense against Mycobacterium tuberculosis: To be or not to be-that is the question. Curr. Opin. Immunol. 2016, 42, 76–82. [Google Scholar] [CrossRef]
- Zhi, H.; Yuan, N.; Wu, J.P.; Lu, L.M.; Chen, X.Y.; Wu, S.K.; Mai, B.X. MicroRNA-21 attenuates BDE-209-induced lipid accumulation in THP-1 macrophages by downregulating Toll-like receptor 4 expression. Food Chem. Toxicol. 2019, 125, 71–77. [Google Scholar] [CrossRef]
- Tan, Y.; Yu, L.; Zhang, C.; Chen, K.; Lu, J.; Tan, L. miRNA-146a attenuates inflammation in an in vitro spinal cord injury model via inhibition of TLR4 signaling. Exp. Ther. Med. 2018, 16, 3703–3709. [Google Scholar] [CrossRef]
- Gu, X.; Gao, Y.; Mu, D.G.; Fu, E.Q. MiR-23a-5p modulates mycobacterial survival and autophagy during mycobacterium tuberculosis infection through TLR2/MyD88/NF-kappaB pathway by targeting TLR2. Exp. Cell Res. 2017, 354, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Bala, S.; Csak, T.; Kodys, K.; Catalano, D.; Ambade, A.; Furi, I.; Lowe, P.; Cho, Y.; Iracheta-Vellve, A.; Szabo, G. Alcohol-induced miR-155 and HDAC11 inhibit negative regulators of the TLR4 pathway and lead to increased LPS responsiveness of Kupffer cells in alcoholic liver disease. J. Leukoc. Biol. 2017, 102, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Ye, E.A.; Steinle, J.J. miR-146a Attenuates Inflammatory Pathways Mediated by TLR4/NF-kappaB and TNFalpha to Protect Primary Human Retinal Microvascular Endothelial Cells Grown in High Glucose. Mediat. Inflamm. 2016, 2016, 3958453. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.M.O.; Fernandes, M.; Araujo, R.; Sousa, H.; Ribeiro, J.; Bastos, M.; Oliveira, P.A.; Carmo, D.; Casaca, F.; Silva, S.; et al. Dysregulated expression of microRNA-150 in human papillomavirus-induced lesions of K14-HPV16 transgenic mice. Life Sci. 2017, 175, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Ghorpade, D.S.; Leyland, R.; Kurowska-Stolarska, M.; Patil, S.A.; Balaji, K.N. MicroRNA-155 is required for Mycobacterium bovis BCG-mediated apoptosis of macrophages. Mol. Cell. Biol. 2012, 32, 2239–2253. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, K.; Zhou, L.; Wu, Y.; Zhu, M.; Lai, X.; Chen, T.; Feng, L.; Li, M.; Huang, C.; et al. MicroRNA-155 promotes autophagy to eliminate intracellular mycobacteria by targeting Rheb. PLoS Pathog. 2013, 9, e1003697. [Google Scholar] [CrossRef]
- Wang, J.; Wu, M.; Wen, J.; Yang, K.; Li, M.; Zhan, X.; Feng, L.; Li, M.; Huang, X. MicroRNA-155 induction by Mycobacterium bovis BCG enhances ROS production through targeting SHIP1. Mol. Immunol. 2014, 62, 29–36. [Google Scholar] [CrossRef]
- Iwai, H.; Funatogawa, K.; Matsumura, K.; Kato-Miyazawa, M.; Kirikae, F.; Kiga, K.; Sasakawa, C.; Miyoshi-Akiyama, T.; Kirikae, T. MicroRNA-155 knockout mice are susceptible to Mycobacterium tuberculosis infection. Tuberculosis (Edinb) 2015, 95, 246–250. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, Q.; Zhou, Y.; Duan, Y.; Gao, Q. Inhibition of IFN-gamma-Induced Nitric Oxide Dependent Antimycobacterial Activity by miR-155 and C/EBPbeta. Int. J. Mol. Sci. 2016, 17, 535. [Google Scholar] [CrossRef]
- Wagh, V.; Urhekar, A.; Modi, D. Levels of microRNA miR-16 and miR-155 are altered in serum of patients with tuberculosis and associate with responses to therapy. Tuberculosis (Edinb) 2017, 102, 24–30. [Google Scholar] [CrossRef]
- da Silva, M.V.; Tiburcio, M.G.; Machado, J.R.; Silva, D.A.; Rodrigues, D.B.; Rodrigues, V.; Oliveira, C.J. Complexity and Controversies over the Cytokine Profiles of T Helper Cell Subpopulations in Tuberculosis. J. Immunol. Res. 2015, 2015, 639107. [Google Scholar] [CrossRef]
- Kim, T.W.; Lee, S.J.; Oh, B.M.; Lee, H.; Uhm, T.G.; Min, J.K.; Park, Y.J.; Yoon, S.R.; Kim, B.Y.; Kim, J.W.; et al. Epigenetic modification of TLR4 promotes activation of NF-kappaB by regulating methyl-CpG-binding domain protein 2 and Sp1 in gastric cancer. Oncotarget 2016, 7, 4195–4209. [Google Scholar] [CrossRef]
- Cooper, Z.A.; Singh, I.S.; Hasday, J.D. Febrile range temperature represses TNF-alpha gene expression in LPS-stimulated macrophages by selectively blocking recruitment of Sp1 to the TNF-alpha promoter. Cell Stress Chaperones 2010, 15, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Presser, L.D.; McRae, S.; Waris, G. Activation of TGF-beta1 promoter by hepatitis C virus-induced AP-1 and Sp1: Role of TGF-beta1 in hepatic stellate cell activation and invasion. PLoS ONE 2013, 8, e56367. [Google Scholar] [CrossRef] [PubMed]
- Burocchi, A.; Pittoni, P.; Gorzanelli, A.; Colombo, M.P.; Piconese, S. Intratumor OX40 stimulation inhibits IRF1 expression and IL-10 production by Treg cells while enhancing CD40L expression by effector memory T cells. Eur. J. Immunol. 2011, 41, 3615–3626. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.A.; Huang, L.; Zheng, L.J.; Fu, K.; Wang, J.; Hu, F.D.; Liao, R.Y. Ginsenoside (Rg-1) promoted the wound closure of diabetic foot ulcer through iNOS elevation via miR-23a/IRF-1 axis. Life Sci. 2019, 233, 116525. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Liang, Z.; Du, Q.; Yang, M.; Geller, D.A. MicroRNA-23a downregulates the expression of interferon regulatory factor-1 in hepatocellular carcinoma cells. Oncol. Rep. 2016, 36, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, B.; Jiang, F.; Wang, D.; Liu, H.; Yan, Y.; Dong, H.; Wang, F.; Gong, B.; Zhu, Y.; et al. A feedback loop consisting of microRNA 23a/27a and the beta-like globin suppressors KLF3 and SP1 regulates globin gene expression. Mol. Cell. Biol. 2013, 33, 3994–4007. [Google Scholar] [CrossRef]
- Cui, M.; Yao, X.; Lin, Y.; Zhang, D.; Cui, R.; Zhang, X. Interactive functions of microRNAs in the miR-23a-27a-24-2 cluster and the potential for targeted therapy in cancer. J. Cell. Physiol. 2020, 235, 6–16. [Google Scholar] [CrossRef]
- Roufayel, R.; Kadry, S. Expression of miR-23a by apoptotic regulators in human cancer: A review. Cancer Biol. Ther. 2017, 18, 269–276. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, S.Q.; Li, C.; Lykken, E.; Jiang, S.; Wong, E.; Gong, Z.; Tao, Z.; Zhu, B.; Wan, Y.; et al. MicroRNA-23a Curbs Necrosis during Early T Cell Activation by Enforcing Intracellular Reactive Oxygen Species Equilibrium. Immunity 2016, 44, 568–581. [Google Scholar] [CrossRef]
- Peng, P.; Li, Z.; Liu, X. Reduced Expression of miR-23a Suppresses A20 in TLR-stimulated Macrophages. Inflammation 2015, 38, 1787–1793. [Google Scholar] [CrossRef]
- Teng, Y.; Miao, J.; Shen, X.; Yang, X.; Wang, X.; Ren, L.; Wang, X.; Chen, J.; Li, J.; Chen, S.; et al. The modulation of MiR-155 and MiR-23a manipulates Klebsiella pneumoniae Adhesion on Human pulmonary Epithelial cells via Integrin alpha5beta1 Signaling. Sci. Rep. 2016, 6, 31918. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Xu, X.Y.; Shen, Y.; Li, J. miR-23a-3p and miR-23a-5p target CiGadd45ab to modulate inflammatory response and apoptosis in grass carp. Fish Shellfish. Immunol. 2020, 98, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Choo, K.B.; Soon, Y.L.; Nguyen, P.N.; Hiew, M.S.; Huang, C.J. MicroRNA-5p and -3p co-expression and cross-targeting in colon cancer cells. J. Biomed. Sci. 2014, 21, 95. [Google Scholar] [CrossRef]
- Yi, Z.; Fu, Y.; Ji, R.; Li, R.; Guan, Z. Altered microRNA signatures in sputum of patients with active pulmonary tuberculosis. PLoS ONE 2012, 7, e43184. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Tan, H.Y.; Feng, Y.G.; Zhang, C.; Chen, F.; Feng, Y. microRNA-23a in Human Cancer: Its Roles, Mechanisms and Therapeutic Relevance. Cancers (Basel) 2018, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Stelekati, E.; Kurachi, M.; Yu, S.; Cai, Z.; Manne, S.; Khan, O.; Yang, X.; Wherry, E.J. miR-150 Regulates Memory CD8 T Cell Differentiation via c-Myb. Cell Rep. 2017, 20, 2584–2597. [Google Scholar] [CrossRef]
- Ban, Y.H.; Oh, S.C.; Seo, S.H.; Kim, S.M.; Choi, I.P.; Greenberg, P.D.; Chang, J.; Kim, T.D.; Ha, S.J. miR-150-Mediated Foxo1 Regulation Programs CD8(+) T Cell Differentiation. Cell Rep. 2017, 20, 2598–2611. [Google Scholar] [CrossRef]
- Moran, J.; Ramirez-Martinez, G.; Jimenez-Alvarez, L.; Cruz, A.; Perez-Patrigeon, S.; Hidalgo, A.; Orozco, L.; Martinez, A.; Padilla-Noriega, L.; Avila-Moreno, F.; et al. Circulating levels of miR-150 are associated with poorer outcomes of A/H1N1 infection. Exp. Mol. Pathol. 2015, 99, 253–261. [Google Scholar] [CrossRef]
- Chen, R.F.; Yang, K.D.; Lee, I.K.; Liu, J.W.; Huang, C.H.; Lin, C.Y.; Chen, Y.H.; Chen, C.L.; Wang, L. Augmented miR-150 expression associated with depressed SOCS1 expression involved in dengue haemorrhagic fever. J. Infect. 2014, 69, 366–374. [Google Scholar] [CrossRef]
- Ghorpade, D.S.; Holla, S.; Kaveri, S.V.; Bayry, J.; Patil, S.A.; Balaji, K.N. Sonic hedgehog-dependent induction of microRNA 31 and microRNA 150 regulates Mycobacterium bovis BCG-driven toll-like receptor 2 signaling. Mol. Cell. Biol. 2013, 33, 543–556. [Google Scholar] [CrossRef]
- Li, M.; Wang, J.; Fang, Y.; Gong, S.; Li, M.; Wu, M.; Lai, X.; Zeng, G.; Wang, Y.; Yang, K.; et al. microRNA-146a promotes mycobacterial survival in macrophages through suppressing nitric oxide production. Sci. Rep. 2016, 6, 23351. [Google Scholar] [CrossRef]
- Li, S.; Yue, Y.; Xu, W.; Xiong, S. MicroRNA-146a represses mycobacteria-induced inflammatory response and facilitates bacterial replication via targeting IRAK-1 and TRAF-6. PLoS ONE 2013, 8, e81438. [Google Scholar] [CrossRef] [PubMed]
- Kathirvel, M.; Saranya, S.; Mahadevan, S. Expression levels of candidate circulating microRNAs in pediatric tuberculosis. Pathog. Glob. Health 2020, 114, 262–270. [Google Scholar]
- Chakrabarty, S.; Kumar, A.; Raviprasad, K.; Mallya, S.; Satyamoorthy, K.; Chawla, K. Host and MTB genome encoded miRNA markers for diagnosis of tuberculosis. Tuberculosis (Edinb) 2019, 116, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, G.; Deng, X.; Yu, Q.; Hu, Y.; Sun, H.; Wang, Z.; Chen, H.; Jia, C.; Wang, D. Analysis of miRNA expression profiling in human macrophages responding to Mycobacterium infection: Induction of the immune regulator miR-146a. J. Infect. 2014, 68, 553–561. [Google Scholar] [CrossRef]
- Ravimohan, S.; Kornfeld, H.; Weissman, D.; Bisson, G.P. Tuberculosis and lung damage: From epidemiology to pathophysiology. Eur. Respir. Rev. 2018, 27, 147. [Google Scholar] [CrossRef] [PubMed]
- Nie, M.; Yu, S.; Peng, S.; Fang, Y.; Wang, H.; Yang, X. miR-23a and miR-27a promote human granulosa cell apoptosis by targeting SMAD5. Biol. Reprod. 2015, 93, 98. [Google Scholar] [CrossRef]
- Rothchild, A.C.; Sissons, J.R.; Shafiani, S.; Plaisier, C.; Min, D.; Mai, D.; Gilchrist, M.; Peschon, J.; Larson, R.P.; Bergthaler, A.; et al. MiR-155-regulated molecular network orchestrates cell fate in the innate and adaptive immune response to Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 2016, 113, E6172–E6181. [Google Scholar] [CrossRef]
| Non-Infected Healthy Subjects N = 17 | Subjects with Latent TB Infection N = 15 | Patients with Active Pulmonary TB Disease N = 46 | p Value | |
|---|---|---|---|---|
| Age, years | 53.86 ± 17.73 | 59 ± 10.55 | 59.7 ± 14.77 | 0.428 |
| Male sex, n (%) | 5 (35.7) | 7 (46.7) | 32 (69.6) | 0.012 |
| Co-morbidity, n (%) | ||||
| Hypertension | 5 (29.4) | 4 (26.7) | 9 (19.6) | 0.666 |
| Diabetes mellitus | 2 (11.8) | 2 (13.3) | 16 (34.8) | 0.085 |
| COPD/Asthma | 0 (0) | 1 (6.7) | 6 (13) | 0.258 |
| Chronic hepatitis | 2 (11.8) | 1 (6.7) | 8 (17.4) | 0.557 |
| Chronic kidney disease | 2 (11.8) | 1 (6.7) | 2 (4.3) | 0.565 |
| Heart failure | 2 (11.8) | 1 (6.7) | 2 (4.3) | 0.565 |
| Alcoholism, n (%) | 0 (0) | 0 (0) | 5 (10.9) | 0.196 |
| Current Smoker, n (%) | 3 (17.6) | 2 (13.3) | 14 (30.4) | 0.312 |
| Sputum smear at diagnosis, n (%) | ||||
| Acid fast bacilli 0 | 20 (43.5) | |||
| Acid fast bacilli 1+ | 9 (19.6) | |||
| Acid fast bacilli 2+ | 4 (8.7) | |||
| Acid fast bacilli 3+ | 5 (10.9) | |||
| Acid fast bacilli 4+ | 8 (17.4) | |||
| Drug-resistant TB, n (%) | 4 (8.6) | |||
| CXR at diagnosis, n (%) | ||||
| Far advanced lesions | 20 (43.5) | |||
| Minimal to moderate | 26 (56.5) | |||
| Pleural effusion | 9 (19.6) | |||
| Delayed resolution after 2-month anti-TB therapy | 25 (54.3) | |||
| Systemic symptoms, n (%) | 14 (30.4) | |||
| Fever | 10 (21.7) | |||
| Body weight loss | 7 (15.2) |
| Gene Name | Primer | Sequence |
|---|---|---|
| TLR2 | forward | 5′- CGTTCTCTCAGGTGACTGCTC -3′ |
| reverse | 5′- CCTTTGGATCCTGCTTGC-3 | |
| TLR4 | forward | 5′-TGGAAGTTGAACGAATGGAATGTG-3′ |
| reverse | 5′-ACCAGAACTGCTACAACAGATACT-3′ | |
| TNF-α | forward | 5′- CCCCAGGGACCTCTCTCTAA-3′ |
| reverse | 5′CTCAGCTTGAGGGTTTGCTAC-3′ | |
| IL6R | forward | 5′- AGCCTCCCAGTGCAAGATTC-3′ |
| reverse | 5′- GGTATTGTCAGACCCCAGGC-3′ | |
| IRF1 | forward | 5′- CATCCCGCCTGAACTTG -3′ |
| reverse | 5′- AGGCTGGTCTCGAACTC -3′ | |
| SP1 | forward | 5′- CGACCTTCTGGTATCTTGT-3′ |
| reverse | 5′- CTTGCTTTCTATCAGATCAGGG-3′ | |
| IL10 | forward | 5′-GCTGGAGGACTTTAAGGGTTACCT-3′ |
| reverse | 5′-CTTGATGTCTGGGTCTTGGTTCT-3′ | |
| TGF-β1 | forward | 5′-CAAGGGCTACCATGCCAACT-3′ |
| reverse | 5′-AGGGCCAGGACCTTGCTG-3′ | |
| NOTCH1 | forward | 5′-GAGGCGTGGCAGACTATGC-3′ |
| reverse | 5′-CTTGTACTCCGTCAGCGTGA-3′ | |
| IL6 | forward | 5′-ACTCACCTCTTCAGAACGAATTG-3′ |
| reverse | 5′-CCATCTTTGGAAGGTTCAGGTTG-3′ | |
| BCL2 | forward | 5′-TTGTGGCCTTCTTTGAGTTCGGTG-3′ |
| reverse | 5′-GGTGCCGGTTCAGGTACTCAGTCA-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-C.; Lee, C.P.; Hsiao, C.-C.; Hsu, P.-Y.; Wang, T.-Y.; Wu, C.-C.; Chao, T.-Y.; Leung, S.-Y.; Chang, Y.-P.; Lin, M.-C. MicroRNA-23a-3p Down-Regulation in Active Pulmonary Tuberculosis Patients with High Bacterial Burden Inhibits Mononuclear Cell Function and Phagocytosis through TLR4/TNF-α/TGF-β1/IL-10 Signaling via Targeting IRF1/SP1. Int. J. Mol. Sci. 2020, 21, 8587. https://doi.org/10.3390/ijms21228587
Chen Y-C, Lee CP, Hsiao C-C, Hsu P-Y, Wang T-Y, Wu C-C, Chao T-Y, Leung S-Y, Chang Y-P, Lin M-C. MicroRNA-23a-3p Down-Regulation in Active Pulmonary Tuberculosis Patients with High Bacterial Burden Inhibits Mononuclear Cell Function and Phagocytosis through TLR4/TNF-α/TGF-β1/IL-10 Signaling via Targeting IRF1/SP1. International Journal of Molecular Sciences. 2020; 21(22):8587. https://doi.org/10.3390/ijms21228587
Chicago/Turabian StyleChen, Yung-Che, Chiu Ping Lee, Chang-Chun Hsiao, Po-Yuan Hsu, Ting-Ya Wang, Chao-Chien Wu, Tung-Ying Chao, Sum-Yee Leung, Yu-Ping Chang, and Meng-Chih Lin. 2020. "MicroRNA-23a-3p Down-Regulation in Active Pulmonary Tuberculosis Patients with High Bacterial Burden Inhibits Mononuclear Cell Function and Phagocytosis through TLR4/TNF-α/TGF-β1/IL-10 Signaling via Targeting IRF1/SP1" International Journal of Molecular Sciences 21, no. 22: 8587. https://doi.org/10.3390/ijms21228587
APA StyleChen, Y.-C., Lee, C. P., Hsiao, C.-C., Hsu, P.-Y., Wang, T.-Y., Wu, C.-C., Chao, T.-Y., Leung, S.-Y., Chang, Y.-P., & Lin, M.-C. (2020). MicroRNA-23a-3p Down-Regulation in Active Pulmonary Tuberculosis Patients with High Bacterial Burden Inhibits Mononuclear Cell Function and Phagocytosis through TLR4/TNF-α/TGF-β1/IL-10 Signaling via Targeting IRF1/SP1. International Journal of Molecular Sciences, 21(22), 8587. https://doi.org/10.3390/ijms21228587






