Nox2 Upregulation and p38α MAPK Activation in Right Ventricular Hypertrophy of Rats Exposed to Long-Term Chronic Intermittent Hypobaric Hypoxia
Abstract
1. Introduction
2. Results
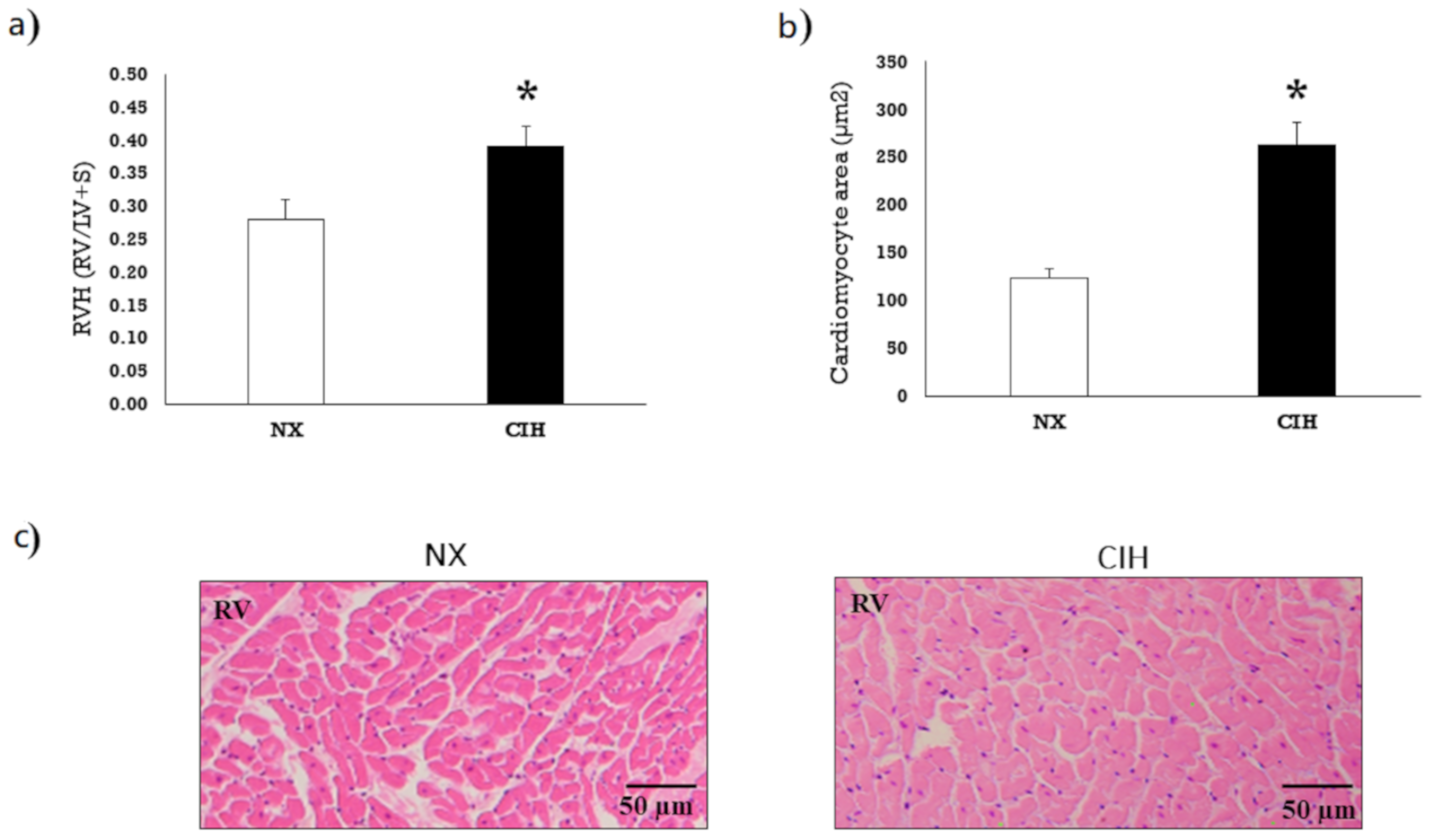
2.1. Hematocrit and Ventricular Hypertrophy
2.2. LOX-1, Nox2, Nox4 and p22phox Expression
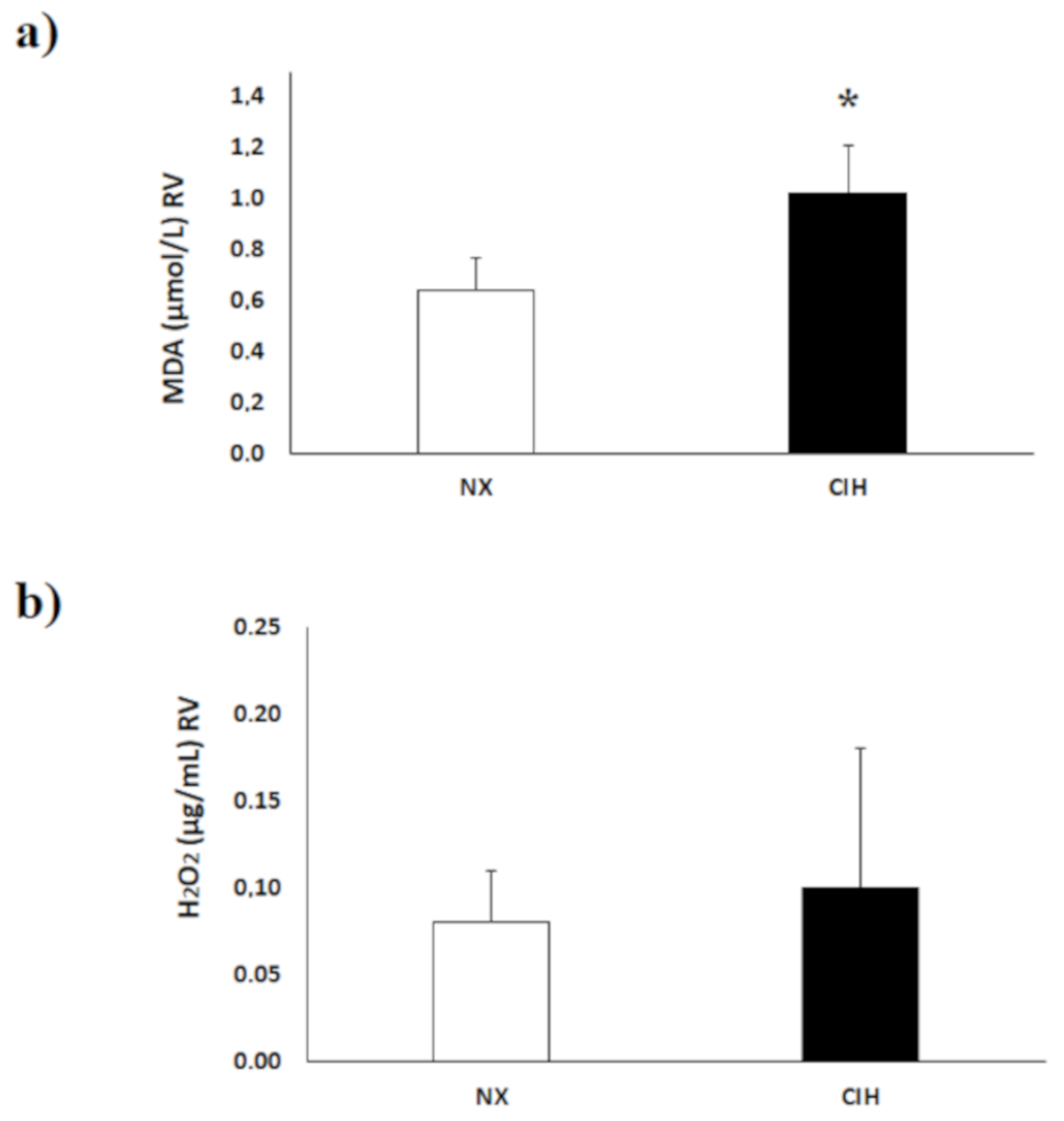
2.3. Lipid Peroxidation (MDA) Level and H2O2 Concentration
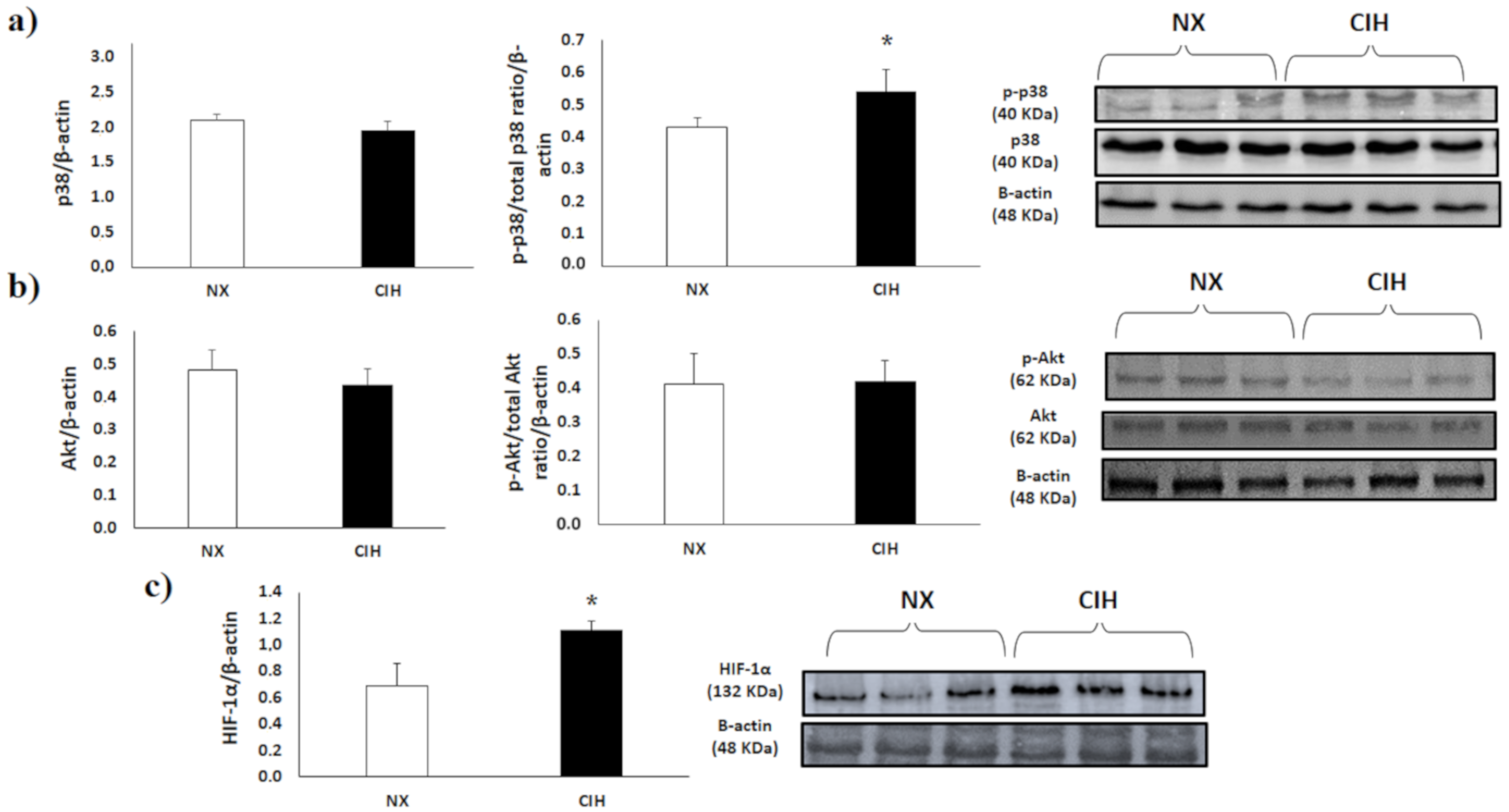
2.4. p38α MAPK, Akt and HIF-1α
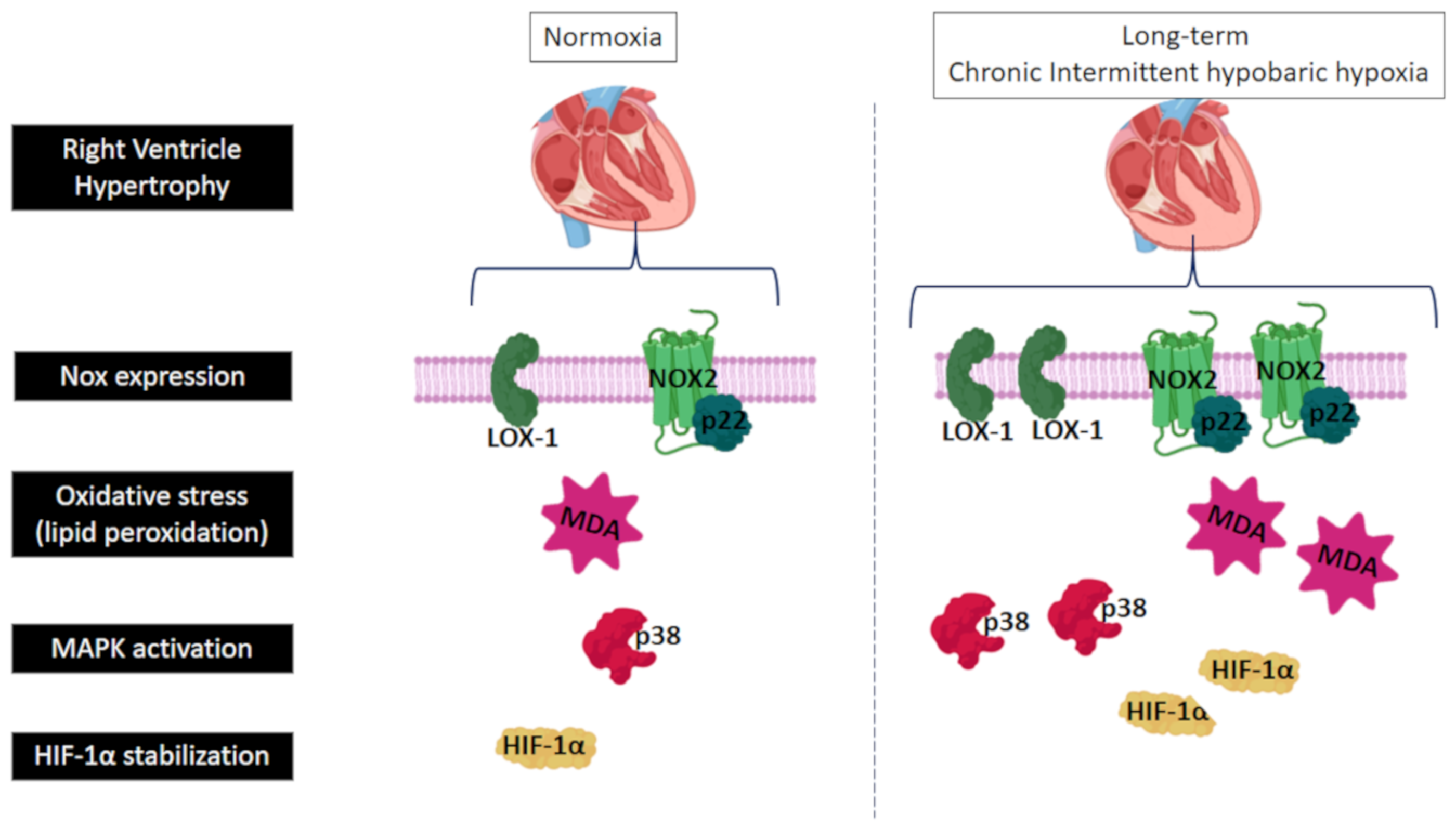
3. Discussion
4. Materials and Methods
4.1. Study Groups
4.2. Animal Model
4.3. Biomedical Variables
4.4. Ventricular Hypertrophy
4.5. Lipid Peroxidation
4.6. Hydrogen Peroxide (H2O2) Determination
4.7. Western Blot Analysis
4.8. Data Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| RV | Right ventricle |
| CIH | Long-term chronic intermittent hypobaric hypoxia |
| RVH | Right ventricle hypertrophy |
| CIH | Long-term chronic intermittent hypobaric hypoxia |
| TAC | Transverse aortic constriction |
| NADPH oxidase | Nicotinamide adenine dinucleotide phosphate oxidase |
| LOX-1 | Lectin-like oxidized low-density lipoprotein receptor-1 |
| PKC | Protein kinase C |
| MEKK-2 | MAP kinase kinase kinase-2 |
| HIF-1α | Hypoxia-inducible factor-1α |
| H2O2 | Hydrogen peroxide |
| MAPKs | Mitogen activated protein kinases |
| ROS | Reactive oxygen species |
| O2.− | Superoxide |
| OH | Hydroxyl radical |
| NX | Normobaric normoxia |
| BW | Body weight |
| Hct | Hematocrit |
| RV | Right ventricle |
| MDA | Malondialdehyde |
| TCA | Trichloroacetic acid |
| TBA | Thiobarbituric acid |
| BSA | Bovine serum albumin |
References
- Bärtsch, P.; Gibbs, J.S. Effect of altitude on the heart and the lungs. Circulation 2007, 116, 2191–2202. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.G. Human genetic adaptation to high altitude. High Alt. Med. Biol. 2001, 2, 257–279. [Google Scholar] [CrossRef] [PubMed]
- Richalet, J.P.; Donoso, M.V.; Jiménez, D.; Antezana, A.M.; Hudson, C.; Cortès, G.; Osorio, J.; Leòn, A. Chilean miners commuting from sea level to 4500 m: A prospective study. High Alt. Med. Biol. 2002, 3, 159–166. [Google Scholar] [CrossRef] [PubMed]
- West, J.B. Commuting to high altitude: Value of oxygen enrichment of room air. High Alt. Med. Biol. 2002, 3, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Q.; Jing, Z.C. High-altitude pulmonary hypertension. Eur. Respir. Rev. 2009, 18, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Dunham-Snary, K.J.; Wu, D.; Sykes, E.A.; Thakrar, A.; Parlow, L.R.G.; Mewburn, J.D.; Parlow, J.L.; Archer, S.L. Hypoxic pulmonary vasoconstriction: From molecular mechanisms to medicine. Chest 2017, 151, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Chen, J.; Luo, Y.; Liu, F.; Xu, G.; Gao, Y. Silencing of STIM1 attenuates hypoxia-induced PASMCs proliferation via inhibition of the SOC/Ca2+/NFAT pathway. Respir. Res. 2013, 14, 2. [Google Scholar] [CrossRef] [PubMed]
- Von Euler, U.S.; Liljestrand, G. Observations on the pulmonary arterial blood pressure in the cat. Acta Physiol. Scand. 1946, 12, 301–320. [Google Scholar] [CrossRef]
- Moudgil, R.; Michelakis, E.D.; Archer, S.L. Hypoxic pulmonary vasoconstriction. J. Appl. Physiol. 2005, 98, 390–403. [Google Scholar] [CrossRef] [PubMed]
- Peñaloza, D.; Sime, F. Chronic cor pulmonale due to loss of altitude acclimatization (chronic mountain sickness). Am. J. Med. 1971, 50, 728–743. [Google Scholar] [CrossRef]
- Penaloza, D.; Arias-Stella, J. The heart and pulmonary circulation at high altitudes. Circulation 2007, 115, 1132–1146. [Google Scholar] [CrossRef]
- León-Velarde, F.; Villafuerte, F.C.; Richalet, J.P. Chronic mountain sickness and the heart. Prog. Cardiovasc. Dis. 2010, 52, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Sarybaev, A.S.; Palasiewicz, G.; Usupbaeva, D.A.; Plywaczewski, R.; Maripov, A.M.; Sydykov, A.S.; Mirrakhimov, M.M.; Le Roux, H.; Kadyrov, T.; Zielinski, J. Effects of intermittent exposure to high altitude on pulmonary hemodynamics: A prospective study. High Alt. Med. Biol. 2003, 4, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Brito, J.; Siques, P.; Arribas, S.M.; López de Pablo, A.L.; González, M.C.; Naveas, N.; Arriaza, K.; Flores, K.; León-Velarde, F.; Pulido, R.; et al. Adventitial alterations are the main features in pulmonary artery remodeling due to long-term chronic intermittent hypobaric hypoxia in rats. BioMed Res. Int. 2015, 2015, 169841. [Google Scholar] [CrossRef]
- Brito, J.; Siques, P.; López, R.; Romero, R.; León-Velarde, F.; Flores, K.; Lüneburg, N.; Hannemann, J.; Böger, R.H. Long-term intermittent work at high altitude: Right heart functional and morphological status and associated cardiometabolic factors. Front. Physiol. 2018, 9, 248. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K.; Tajima, F.; Nakamura, A.; Yagura, S.; Ookawara, T.; Yamashita, H.; Suzuki, K.; Taniguchi, N.; Ohno, H. Effects of hypobaric hypoxia on antioxidant enzymes in rats. J. Physiol. 1995, 489, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, J.A.; Simoni, J.; Escudero, E.; Hurtado, M.E.; Swenson, E.R.; Wesson, D.E.; Schreiner, G.F.; Schoene, R.B.; Johnson, R.J.; Hurtado, A. Increased oxidative stress following acute and chronic high altitude exposure. High Alt. Med. Biol. 2004, 5, 61–69. [Google Scholar] [CrossRef]
- Li, J.M.; Gall, N.P.; Grieve, D.J.; Chen, M.; Shah, A.M. Activation of NADPH oxidase during progression of cardiac hypertrophy to failure. Hypertension 2002, 40, 477–484. [Google Scholar] [CrossRef]
- Lu, Z.; Xu, X.; Hu, X.; Zhu, G.; Zhang, P.; Van Deel, E.D.; French, J.P.; Fassett, J.T.; Oury, T.D.; Bache, R.J.; et al. Extracellular superoxide dismutase deficiency exacerbates pressure overload-induced left ventricular hypertrophy and dysfunction. Hypertension 2008, 51, 19–25. [Google Scholar] [CrossRef]
- Zhu, T.T.; Zhang, W.F.; Luo, P.; Qian, Z.X.; Li, F.; Zhang, Z.; Hu, C.P. LOX-1 promotes right ventricular hypertrophy in hypoxia-exposed rats. Life Sci. 2017, 174, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Guzy, R.D.; Hoyos, B.; Robin, E.; Chen, H.; Liu, L.; Mansfield, K.D.; Simon, M.C.; Hammerling, U.; Schumacker, P.T. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 2005, 1, 401–408. [Google Scholar] [CrossRef]
- Li, K.; He, C. Gastric mucosal lesions in Tibetans with high-altitude polycythemia show increased HIF-1A expression and ROS production. BioMed Res. Int. 2019, 2019, 6317015. [Google Scholar] [CrossRef] [PubMed]
- Fujio, Y.; Nguyen, T.; Wencker, D.; Kitsis, R.N.; Walsh, K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation 2000, 101, 660–667. [Google Scholar] [CrossRef]
- Emerling, B.M.; Platanias, L.C.; Black, E.; Nebreda, A.R.; Davis, R.J.; Chandel, N.S. Mitochondrial reactive oxygen species activation of p38 mitogen-activated protein kinase is required for hypoxia signaling. Mol. Cell. Biol. 2005, 25, 4853–4862. [Google Scholar] [CrossRef]
- Son, Y.; Kim, S.; Chung, H.T.; Pae, H.O. Reactive oxygen species in the activation of MAP kinases. Methods Enzymol. 2013, 528, 27–48. [Google Scholar] [CrossRef]
- Sag, C.M.; Santos, C.X.; Shah, A.M. Redox regulation of cardiac hypertrophy. J. Mol. Cell. Cardiol. 2014, 73, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Octavia, Y.; Brunner-La Rocca, H.P.; Moens, A.L. NADPH oxidase-dependent oxidative stress in the failing heart: From pathogenic roles to therapeutic approach. Free Radic. Biol. Med. 2012, 52, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Lasseègue, B.; Sorescu, D.; Szoöcs, K.; Yin, Q.; Akers, M.; Zhang, Y.; Grant, S.L.; Lambeth, J.D.; Griendling, K.K. Novel gp91 phox homologues in vascular smooth muscle cells. Circ. Res. 2001, 88, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Chen, X. The human Nox4: Gene, structure, physiological function and pathological significance. J. Drug Target 2015, 23, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Hingtgen, S.D.; Tian, X.; Yang, J.; Dunlay, S.M.; Peek, A.S.; Wu, Y.; Sharma, R.V.; Engelhardt, J.F.; Davisson, R.L. Nox2-containing NADPH oxidase and Akt activation play a key role in angiotensin II-induced cardiomyocyte hypertrophy. Physiol. Genom. 2006, 26, 180–191. [Google Scholar] [CrossRef]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.Y. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxid. Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef]
- Rodiño-Janeiro, B.K.; Paradela-Dobarro, B.; Castiñeiras-Landeira, M.I.; Raposeiras-Roubín, S.; González-Juanatey, J.R.; Alvarez, E. Current status of NADPH oxidase research in cardiovascular pharmacology. Vasc. Health Risk Manag. 2013, 9, 401–428. [Google Scholar] [CrossRef]
- Forteza, R.; Salathe, M.; Miot, F.; Forteza, R.; Conner, G.E. Regulated hydrogen peroxide production by Duox in human airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 2005, 32, 462–469. [Google Scholar] [CrossRef]
- Ogura, S.; Shimosawa, T.; Mu, S.; Sonobe, T.; Kawakami-Mori, F.; Wang, H.; Uetake, Y.; Yoshida, K.; Yatomi, Y.; Shirai, M.; et al. Oxidative stress augments pulmonary hypertension in chronically hypoxic mice overexpressing the oxidized LDL receptor. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H155–H162. [Google Scholar] [CrossRef]
- Taye, A.; El-Sheikh, A.A. Lectin-like oxidized low-density lipoprotein receptor 1 pathways. Eur. J. Clin. Investig. 2013, 43, 740–745. [Google Scholar] [CrossRef]
- Monge, C.; León-Velarde, F. El Reto Fisiológico de Vivir en los Andes; IFEA, UPCH: Lima, Peru, 2003. [Google Scholar]
- Reeves, J.T.; Leon-Velarde, F. Chronic mountain sickness: Recent studies of the relationship between hemoglobin concentration and oxygen transport. High Alt. Med. Biol. 2004, 5, 147–155. [Google Scholar] [CrossRef]
- Villafuerte, F.C.; Cárdenas, R.; Monge, C.-C. Optimal hemoglobin concentration and high altitude: A theoretical approach for Andean men at rest. J. Appl. Physiol. 2004, 96, 1581–1588. [Google Scholar] [CrossRef]
- León-Velarde, F.; Maggiorini, M.; Reeves, J.T.; Aldashev, A.; Asmus, I.; Bernardi, L.; Ge, R.L.; Hackett, P.; Kobayashi, T.; Moore, L.G.; et al. Consensus statement on chronic and subacute high altitude diseases. High Alt. Med. Biol. 2005, 6, 147–157. [Google Scholar] [CrossRef]
- Siqués, P.; Brito, J.; León-Velarde, F.; Barrios, L.; Cruz, J.J.D.L.; López, V.; Herruzo, R. Time course of cardiovascular and hematological responses in rats exposed to chronic intermittent hypobaric hypoxia (4600 m). High Alt. Med. Biol. 2006, 7, 72–80. [Google Scholar] [CrossRef]
- Groeneveldt, J.A.; de Man, F.S.; Westerhof, B.E. The right treatment for the right ventricle. Curr. Opin. Pulm. Med. 2019, 25, 410–417. [Google Scholar] [CrossRef]
- Brito, J.; Siqués, P.; León-Velarde, F.; De La Cruz, J.J.; López, V.; Herruzo, R. Chronic intermittent hypoxia at high altitude exposure for over 12 years: Assessment of hematological, cardiovascular, and renal effects. High Alt. Med. Biol. 2007, 8, 236–244. [Google Scholar] [CrossRef]
- Lüneburg, N.; Siques, P.; Brito, J.; Arriaza, K.; Pena, E.; Klose, H.; Leon-Velarde, F.; Böger, R.H. Long-term chronic intermittent hypobaric hypoxia in rats causes an imbalance in the asymmetric dimethylarginine/nitric oxide pathway and ROS activity: A possible synergistic mechanism for altitude pulmonary hypertension? Pulm. Med. 2016, 2016, 65785789. [Google Scholar] [CrossRef]
- Bailey, D.; Davies, B.; Young, I.S.; Hullin, D.A.; Seddon, P.S. A potential role of free radical-mediated skeletal muscle soreness in pathophysiology of acute mountain sickness. Aviat. Space Environ. Med. 2001, 72, 513–521. [Google Scholar] [CrossRef]
- Joanny, P.; Steinberg, J.; Robach, P.; Richalet, J.P.; Gortan, C.; Gardette, B.; Jammes, Y. Operation everest III (Comex’97): The effect of simulated severe hypobaric hypoxia on lipid peroxidation and antioxidant defence systems in human blood at rest and after maximal exercise. Resuscitation 2001, 49, 307–314. [Google Scholar] [CrossRef]
- Moller, P.; Loft, S.; Lundby, C.; Olsen, N.V. Acute hypoxia and hypoxic exercise induce DNA strand breaks and oxidative DNA damage in humans. FASEB J. 2001, 15, 1181–1186. [Google Scholar] [CrossRef]
- Gutteridge, J.M.C.; West, M.; Eneff, K.; Floyd, R.A. Bleomycin-iron damage to DNA with formation of 8-hydroxydeoxyguanosine and base propenals. Indications that xanthine oxidase generates superoxide from DNA degradation products. Free Radic. Res. Commun. 1990, 10, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Takac, I.; Schröder, K.; Zhang, L.; Lardy, B.; Anilkumar, N.; Lambeth, J.D.; Shah, A.M.; Morel, F.; Brandes, R.P. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J. Biol. Chem. 2011, 286, 13304–13313. [Google Scholar] [CrossRef]
- Sciarretta, S.; Zhai, P.; Shao, D.; Zablocki, D.; Nagarajan, N.; Terada, L.S.; Volpe, M.; Sadoshima, J. Activation of NADPH oxidase 4 in the endoplasmic reticulum promotes cardiomyocyte autophagy and survival during energy stress through the protein kinase RNA-activated-like endoplasmic reticulum kinase/eukaryotic initiation factor 2α/activating transcription factor 4 pathway. Circ. Res. 2013, 113, 1253–1264. [Google Scholar] [CrossRef]
- Bengtsson, S.H.; Gulluyan, L.M.; Dusting, G.J.; Drummond, G.R. Novel isoforms of NADPH oxidase in vascular physiology and pathophysiology. Clin. Exp. Pharmacol. Physiol. 2003, 30, 849–854. [Google Scholar] [CrossRef]
- Barman, S.A.; Chen, F.; Su, Y.; Dimitropoulou, C.; Wang, Y.; Catravas, J.D.; Han, W.; Orfi, L.; Szantai-Kis, C.; Keri, G.; et al. NADPH oxidase 4 is expressed in pulmonary artery adventitia and contributes to hypertensive vascular remodeling. Arterioscler. Thromb Vasc. Biol. 2014, 34, 1704–1715. [Google Scholar] [CrossRef]
- Jarman, E.R.; Khambata, V.S.; Cope, C.; Jones, P.; Roger, J.; Ye, L.Y.; Duggan, N.; Head, D.; Pearce, A.; Press, N.J.; et al. An inhibitor of NADPH oxidase-4 attenuates established pulmonary fibrosis in a rodent disease model. Am. J. Respir. Cell Mol. Biol. 2014, 50, 158–169. [Google Scholar] [CrossRef]
- Haber, F.; Weiss, J. Über die Katalyse des Hydroperoxydes. Naturwissenschaften 1932, 20, 948–950. [Google Scholar] [CrossRef]
- Kehrer, J.P. The Haber-Weiss reaction and mechanisms of toxicity. Toxicology 2000, 149, 43–50. [Google Scholar] [CrossRef]
- Gutteridge, J.M. Lipid peroxidation initiated by superoxide-dependent hydroxyl radicals using complexed iron and hydrogen peroxide. FEBS Lett. 1984, 172, 245–249. [Google Scholar] [CrossRef]
- Yu, B.; Meng, F.; Yang, Y.; Liu, D.; Shi, K. NOX2 antisense attenuates hypoxia-induced oxidative stress and apoptosis in cardiomyocyte. Int. J. Med. Sci. 2016, 13, 646–652. [Google Scholar] [CrossRef][Green Version]
- Lü, J.; Mehta, J.L. LOX-1: A critical player in the genesis and progression of myocardial ischemia. Cardiovasc. Drugs Ther. 2011, 25, 431–440. [Google Scholar] [CrossRef]
- Takano, H.; Zou, Y.; Hasegawa, H.; Akazawa, H.; Nagai, T.; Komuro, I. Oxidative stress-induced signal transduction pathways in cardiac myocytes: Involvement of ROS in heart diseases. Antioxid. Redox Signal. 2004, 5, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Takimoto, E.; Kass, D.A. Role of oxidative stress in cardiac hypertrophy and remodeling. Hypertension 2007, 49, 241–248. [Google Scholar] [CrossRef]
- Oka, T.; Akazawa, H.; Naito, A.T.; Komuro, I. Angiogenesis and cardiac hypertrophy. Circ. Res. 2014, 114, 565–571. [Google Scholar] [CrossRef]
- Folden, D.V.; Gupta, A.; Sharma, A.C.; Li, S.Y.; Saari, J.T.; Ren, J. Malondialdehyde inhibits cardiac contractile function in ventricular myocytesviaa p38 mitogen-activated protein kinase-dependent mechanism. Br. J. Pharmacol. 2003, 139, 1310–1316. [Google Scholar] [CrossRef]
- Yu, M.; Zheng, Y.; Sun, H.X.; Yu, D.J. Inhibitory effects of enalaprilat on rat cardiac fibroblast proliferation via ROS/P38MAPK/TGF-β1 signaling pathway. Molecules 2012, 17, 2738–2751. [Google Scholar] [CrossRef]
- Kumar, S.; Blake, S.M. Pharmacological potential of p38 MAPK inhibitors. In Inhibitors of Protein Kinases and Protein Phosphates. Handbook of Experimental Pharmacology; Pinna, L.A., Cohen, P.T., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 65–83. [Google Scholar]
- Kojonazarov, B.; Novoyatleva, T.; Boehm, M.; Happe, C.; Sibinska, Z.; Tian, X.; Sajjad, A.; Luitel, H.; Kriechling, P.; Posern, G.; et al. p38 MAPK inhibition improves heart function in pressure-loaded right ventricular hypertrophy. Am. J. Respir. Cell Mol. Biol. 2017, 57, 603–614. [Google Scholar] [CrossRef]
- Gao, N.; Jiang, B.H.; Leonard, S.S.; Corum, L.; Zhang, Z.; Roberts, J.R.; Antonini, J.; Zheng, J.Z.; Flynn, D.C.; Castranova, V.; et al. p38 signaling-mediated hypoxia-inducible factor 1α and vascular endothelial growth factor induction by Cr(VI) in DU145 human prostate carcinoma cells. J. Biol. Chem. 2002, 277, 45041–45048. [Google Scholar] [CrossRef]
- Koodie, L.; Ramakrishnan, S.; Roy, S. Morphine suppresses tumor angiogenesis through a HIF-1α/p38MAPK pathway. Am. J. Pathol. 2010, 177, 984–997. [Google Scholar] [CrossRef]
- McCarthy, J.; Lochner, A.; Opie, L.H.; Sack, M.N.; Essop, M.F. PKCε promotes cardiac mitochondrial and metabolic adaptation to chronic hypobaric hypoxia by GSK3β inhibition. J. Cell. Physiol. 2011, 226, 2457–2468. [Google Scholar] [CrossRef]
- Uenoyama, M.; Ogata, S.; Nakanishi, K.; Kanazawa, F.; Hiroi, S.; Tominaga, S.; Seo, A.; Matsui, T.; Kawai, T.; Suzuki, S. Protein kinase C mRNA and protein expressions in hypobaric hypoxia-induced cardiac hypertrophy in rats. Acta Physiol. 2010, 198, 431–440. [Google Scholar] [CrossRef]
- Song, X.; Qian, X.; Shen, M.; Jiang, R.; Wagner, M.B.; Ding, G.; Chen, G.; Shen, B. Protein kinase C promotes cardiac fibrosis and heart failure by modulating galectin-3 expression. Biochim. Biophys. Acta 2015, 1853, 513–521. [Google Scholar] [CrossRef]
- Brown, R.D.; Ambler, S.K.; Li, M.; Sullivan, T.M.; Henry, L.N.; Crossno, J.T., Jr.; Long, C.S.; Garrington, T.P.; Stenmark, K.R. MAP kinase kinase kinase-2 (MEKK2) regulates hypertrophic remodeling of the right ventricle in hypoxia-induced pulmonary hypertension. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H269–H281. [Google Scholar] [CrossRef]
- Gorr, M.W.; Sriram, K.; Chinn, A.M.; Muthusamy, A.; Insel, P.A. Transcriptomic profiles reveal differences between the right and left ventricle in normoxia and hypoxia. Physiol. Rep. 2020, 8, e14344. [Google Scholar] [CrossRef]
- Muthuramu, I.; Singh, N.; Amin, R.; Nefyodova, E.; Debasse, M.; Van Horenbeeck, I.; Jacobs, F.; De Geest, B. Selective homocysteine-lowering gene transfer attenuates pressure overload-induced cardiomyopathy via reduced oxidative stress. J. Mol. Med. 2015, 93, 609–618. [Google Scholar] [CrossRef]
- Muthuramu, I.; Amin, R.; Postnov, A.; Mishra, M.; Aboumsallem, J.P.; Dresselaers, T.; Himmelreich, U.; Van Veldhoven, P.P.; Gheysens, O.; Jacobs, F.; et al. Cholesterol-lowering gene therapy counteracts the development of non-ischemic cardiomyopathy in mice. Mol. Ther. 2017, 25, 2513–2525. [Google Scholar] [CrossRef]
- Brito, J.; Siqués, P.; León-Velarde, F.; Cruz, J.J.D.L.; Barlaro, T.; López, V.; Herruzo, R. Varying exposure regimes to long term chronic intermittent hypoxia exert different outcomes and morphological effects on Wistar rats at 4600 m. Toxicol. Environ. Chem. 2008, 90, 169–179. [Google Scholar] [CrossRef]
- Kay, J.M. Effect of intermittent normoxia on chronic hypoxic pulmonary hypertension, right ventricular hypertrophy, and polycythemia in rats. Am. Rev. Respir. Dis. 1980, 121, 993–1001. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pena, E.; Siques, P.; Brito, J.; Arribas, S.M.; Böger, R.; Hannemann, J.; León-Velarde, F.; González, M.C.; López, M.R.; López de Pablo, Á.L. Nox2 Upregulation and p38α MAPK Activation in Right Ventricular Hypertrophy of Rats Exposed to Long-Term Chronic Intermittent Hypobaric Hypoxia. Int. J. Mol. Sci. 2020, 21, 8576. https://doi.org/10.3390/ijms21228576
Pena E, Siques P, Brito J, Arribas SM, Böger R, Hannemann J, León-Velarde F, González MC, López MR, López de Pablo ÁL. Nox2 Upregulation and p38α MAPK Activation in Right Ventricular Hypertrophy of Rats Exposed to Long-Term Chronic Intermittent Hypobaric Hypoxia. International Journal of Molecular Sciences. 2020; 21(22):8576. https://doi.org/10.3390/ijms21228576
Chicago/Turabian StylePena, Eduardo, Patricia Siques, Julio Brito, Silvia M. Arribas, Rainer Böger, Juliane Hannemann, Fabiola León-Velarde, M. Carmen González, M. Rosario López, and Ángel Luis López de Pablo. 2020. "Nox2 Upregulation and p38α MAPK Activation in Right Ventricular Hypertrophy of Rats Exposed to Long-Term Chronic Intermittent Hypobaric Hypoxia" International Journal of Molecular Sciences 21, no. 22: 8576. https://doi.org/10.3390/ijms21228576
APA StylePena, E., Siques, P., Brito, J., Arribas, S. M., Böger, R., Hannemann, J., León-Velarde, F., González, M. C., López, M. R., & López de Pablo, Á. L. (2020). Nox2 Upregulation and p38α MAPK Activation in Right Ventricular Hypertrophy of Rats Exposed to Long-Term Chronic Intermittent Hypobaric Hypoxia. International Journal of Molecular Sciences, 21(22), 8576. https://doi.org/10.3390/ijms21228576










