Current Status and Application of Metformin for Prostate Cancer: A Comprehensive Review
Abstract
1. Introduction
2. Association between Prostate Cancer and Metabolic Syndrome
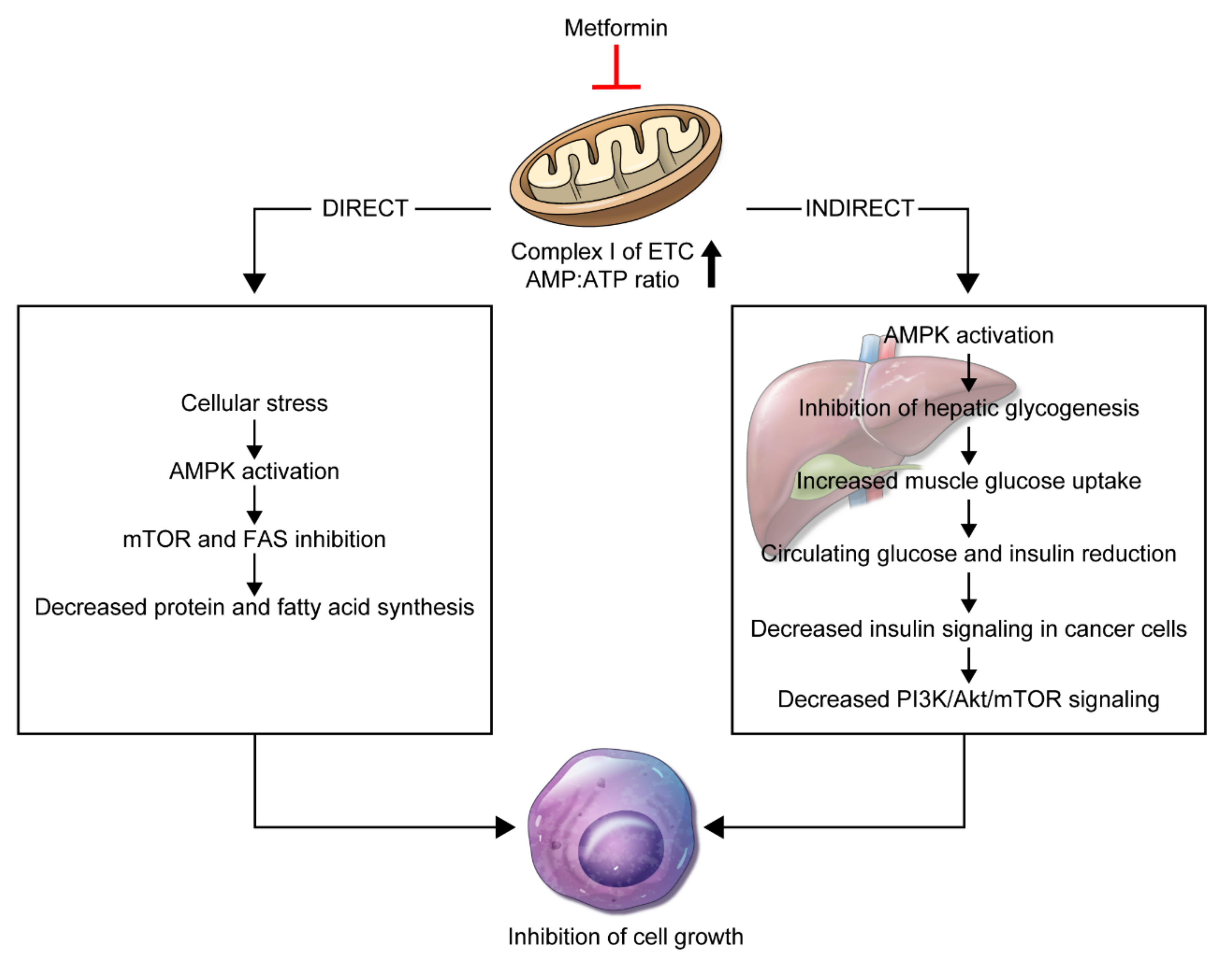
3. Mechanism of Action of Metformin on Anti-Cancer Effects
3.1. Direct Effect
3.2. Indirect Effect
3.3. Biologic Effects
4. Effect of Metformin on Prostate Cancer Incidence
5. Effect of Metformin on Recurrence-Free Survival
6. Effect of Metformin on Overall Survival
7. Effect of Metformin on Cancer-Specific Survival
8. Clinical Trials
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Franciosi, M.; Lucisano, G.; Lapice, E.; Strippoli, G.F.; Pellegrini, F.; Nicolucci, A. Metformin therapy and risk of cancer in patients with type 2 diabetes: Systematic review. PLoS ONE 2013, 8, e71583. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Economou, F.; Palimeri, S.; Christakou, C. Metformin in polycystic ovary syndrome. Ann. N. Y. Acad. Sci. USA 2010, 1205, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Zhou, J.; Gorak, E.J.; Quddus, F. Metformin is associated with survival benefit in cancer patients with concurrent type 2 diabetes: A systematic review and meta-analysis. Oncologist 2013, 18, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, S.; Diamond, E.J.; Stagger, S.; Stone, N.N.; Stock, R.G. Serum insulin level, disease stage, prostate specific antigen (psa) and gleason score in prostate cancer. Br. J. Cancer 2002, 87, 726–728. [Google Scholar] [CrossRef]
- Ma, J.; Li, H.; Giovannucci, E.; Mucci, L.; Qiu, W.; Nguyen, P.L.; Gaziano, J.M.; Pollak, M.; Stampfer, M.J. Prediagnostic body-mass index, plasma c-peptide concentration, and prostate cancer-specific mortality in men with prostate cancer: A long-term survival analysis. Lancet Oncol. 2008, 9, 1039–1047. [Google Scholar] [CrossRef]
- He, K.; Hu, H.; Ye, S.; Wang, H.; Cui, R.; Yi, L. The effect of metformin therapy on incidence and prognosis in prostate cancer: A systematic review and meta-analysis. Sci. Rep. 2019, 9, 2218. [Google Scholar] [CrossRef]
- Zhang, P.; Li, H.; Tan, X.; Chen, L.; Wang, S. Association of metformin use with cancer incidence and mortality: A meta-analysis. Cancer Epidemiol. 2013, 37, 207–218. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Li, S. The prognostic value of metformin for cancer patients with concurrent diabetes: A systematic review and meta-analysis. Diabetes Obes. Metab. 2014, 16, 707–710. [Google Scholar] [CrossRef]
- Hirata, Y.; Shiota, M.; Kobayashi, T.; Kashiwagi, E.; Takeuchi, A.; Inokuchi, J.; Tatsugami, K.; Eto, M. Prognostic significance of diabetes mellitus and dyslipidemia in men receiving androgen-deprivation therapy for metastatic prostate cancer. Prostate Int. 2019, 7, 166–170. [Google Scholar] [CrossRef]
- Kim, W.J.; Kim, J. Looking to the metabolic landscapes for prostate health monitoring. Prostate Int. 2017, 5, 85–88. [Google Scholar] [CrossRef]
- De Nunzio, C.; Aronson, W.; Freedland, S.J.; Giovannucci, E.; Parsons, J.K. The correlation between metabolic syndrome and prostatic diseases. Eur. Urol. 2012, 61, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Laukkanen, J.A.; Laaksonen, D.E.; Niskanen, L.; Pukkala, E.; Hakkarainen, A.; Salonen, J.T. Metabolic syndrome and the risk of prostate cancer in finnish men: A population-based study. Cancer Epidemiol. Biomarkers Prev. 2004, 13, 1646–1650. [Google Scholar] [PubMed]
- Lund Haheim, L.; Wisloff, T.F.; Holme, I.; Nafstad, P. Metabolic syndrome predicts prostate cancer in a cohort of middle-aged norwegian men followed for 27 years. Am. J. Epidemiol. 2006, 164, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Beebe-Dimmer, J.L.; Nock, N.L.; Neslund-Dudas, C.; Rundle, A.; Bock, C.H.; Tang, D.; Jankowski, M.; Rybicki, B.A. Racial differences in risk of prostate cancer associated with metabolic syndrome. Urology 2009, 74, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Collaborative Normal-Tension Glaucoma Study Group. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am. J. Ophthalmol. 1998, 126, 487–497. [Google Scholar]
- Haggstrom, C.; Stocks, T.; Ulmert, D.; Bjorge, T.; Ulmer, H.; Hallmans, G.; Manjer, J.; Engeland, A.; Nagel, G.; Almqvist, M.; et al. Prospective study on metabolic factors and risk of prostate cancer. Cancer 2012, 118, 6199–6206. [Google Scholar] [CrossRef]
- Hammarsten, J.; Damber, J.E.; Haghsheno, M.A.; Mellstrom, D.; Peeker, R. A stage-dependent link between metabolic syndrome components and incident prostate cancer. Nat. Rev. Urol. 2018, 15, 321–333. [Google Scholar] [CrossRef]
- Tande, A.J.; Platz, E.A.; Folsom, A.R. The metabolic syndrome is associated with reduced risk of prostate cancer. Am. J. Epidemiol. 2006, 164, 1094–1102. [Google Scholar] [CrossRef]
- Stocks, T.; Hergens, M.P.; Englund, A.; Ye, W.; Stattin, P. Blood pressure, body size and prostate cancer risk in the swedish construction workers cohort. Int. J. Cancer 2010, 127, 1660–1668. [Google Scholar] [CrossRef]
- Kintzel, P.E.; Chase, S.L.; Schultz, L.M.; O’Rourke, T.J. Increased risk of metabolic syndrome, diabetes mellitus, and cardiovascular disease in men receiving androgen deprivation therapy for prostate cancer. Pharmacotherapy 2008, 28, 1511–1522. [Google Scholar] [CrossRef]
- Nguyen, P.L.; Alibhai, S.M.; Basaria, S.; D’Amico, A.V.; Kantoff, P.W.; Keating, N.L.; Penson, D.F.; Rosario, D.J.; Tombal, B.; Smith, M.R. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur. Urol. 2015, 67, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Lubik, A.A.; Gunter, J.H.; Hendy, S.C.; Locke, J.A.; Adomat, H.H.; Thompson, V.; Herington, A.; Gleave, M.E.; Pollak, M.; Nelson, C.C. Insulin increases de novo steroidogenesis in prostate cancer cells. Cancer Res. 2011, 71, 5754–5764. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, J.; Gray, P.K.; Hahn, N.; Hayes, J.; Myers, L.J.; Carney-Doebbeling, C.; Sweeney, C.J. Presence of the metabolic syndrome is associated with shorter time to castration-resistant prostate cancer. Ann. Oncol. 2011, 22, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Dowling, R.J.; Zakikhani, M.; Fantus, I.G.; Pollak, M.; Sonenberg, N. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res. 2007, 67, 10804–10812. [Google Scholar] [CrossRef] [PubMed]
- Kahn, B.B.; Alquier, T.; Carling, D.; Hardie, D.G. Amp-activated protein kinase: Ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005, 1, 15–25. [Google Scholar] [CrossRef]
- Renehan, A.G.; Zwahlen, M.; Egger, M. Adiposity and cancer risk: New mechanistic insights from epidemiology. Nat. Rev. Cancer 2015, 15, 484–498. [Google Scholar] [CrossRef]
- Brunmair, B.; Staniek, K.; Gras, F.; Scharf, N.; Althaym, A.; Clara, R.; Roden, M.; Gnaiger, E.; Nohl, H.; Waldhausl, W.; et al. Thiazolidinediones, like metformin, inhibit respiratory complex i: A common mechanism contributing to their antidiabetic actions? Diabetes 2004, 53, 1052–1059. [Google Scholar] [CrossRef]
- Zakikhani, M.; Dowling, R.; Fantus, I.G.; Sonenberg, N.; Pollak, M. Metformin is an amp kinase-dependent growth inhibitor for breast cancer cells. Cancer Res. 2006, 66, 10269–10273. [Google Scholar] [CrossRef]
- Inoki, K.; Zhu, T.; Guan, K.L. Tsc2 mediates cellular energy response to control cell growth and survival. Cell 2003, 115, 577–590. [Google Scholar] [CrossRef]
- Mahalingam, D.; Sankhala, K.; Mita, A.; Giles, F.J.; Mita, M.M. Targeting the mtor pathway using deforolimus in cancer therapy. Future Oncol. 2009, 5, 291–303. [Google Scholar] [CrossRef]
- Kalender, A.; Selvaraj, A.; Kim, S.Y.; Gulati, P.; Brule, S.; Viollet, B.; Kemp, B.E.; Bardeesy, N.; Dennis, P.; Schlager, J.J.; et al. Metformin, independent of ampk, inhibits mtorc1 in a rag gtpase-dependent manner. Cell Metab. 2010, 11, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. Ampk: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Shaw, R.J.; Lamia, K.A.; Vasquez, D.; Koo, S.H.; Bardeesy, N.; Depinho, R.A.; Montminy, M.; Cantley, L.C. The kinase lkb1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 2005, 310, 1642–1646. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Zheng, Y.; Xiao, Y.; Zhou, P.; Tan, H. Meta-analysis of studies using metformin as a reducer for liver cancer risk in diabetic patients. Medicine (Baltimore) 2017, 96, e6888. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, P.J.; Ennis, M.; Pritchard, K.I.; Trudeau, M.E.; Koo, J.; Taylor, S.K.; Hood, N. Insulin- and obesity-related variables in early-stage breast cancer: Correlations and time course of prognostic associations. J. Clin. Oncol. 2012, 30, 164–171. [Google Scholar] [CrossRef]
- Wolpin, B.M.; Meyerhardt, J.A.; Chan, A.T.; Ng, K.; Chan, J.A.; Wu, K.; Pollak, M.N.; Giovannucci, E.L.; Fuchs, C.S. Insulin, the insulin-like growth factor axis, and mortality in patients with nonmetastatic colorectal cancer. J. Clin. Oncol. 2009, 27, 176–185. [Google Scholar] [CrossRef]
- De Meyts, P. Insulin and its receptor: Structure, function and evolution. Bioessays 2004, 26, 1351–1362. [Google Scholar] [CrossRef]
- Cui, Y.; Andersen, D.K. Diabetes and pancreatic cancer. Endocr. Relat. Cancer 2012, 19, F9–F26. [Google Scholar] [CrossRef]
- Salani, B.; Maffioli, S.; Hamoudane, M.; Parodi, A.; Ravera, S.; Passalacqua, M.; Alama, A.; Nhiri, M.; Cordera, R.; Maggi, D. Caveolin-1 is essential for metformin inhibitory effect on igf1 action in non-small-cell lung cancer cells. FASEB J. 2012, 26, 788–798. [Google Scholar] [CrossRef]
- Hamoudane, M.; Maffioli, S.; Cordera, R.; Maggi, D.; Salani, B. Caveolin-1 and polymerase i and transcript release factor: New players in insulin-like growth factor-i receptor signaling. J. Endocrinol. Invest. 2013, 36, 204–208. [Google Scholar]
- Salani, B.; Del Rio, A.; Marini, C.; Sambuceti, G.; Cordera, R.; Maggi, D. Metformin, cancer and glucose metabolism. Endocr. Relat. Cancer 2014, 21, R461–R471. [Google Scholar] [CrossRef] [PubMed]
- Koppenol, W.H.; Bounds, P.L.; Dang, C.V. Otto warburg’s contributions to current concepts of cancer metabolism. Nat. Rev. Cancer 2011, 11, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Salani, B.; Marini, C.; Rio, A.D.; Ravera, S.; Massollo, M.; Orengo, A.M.; Amaro, A.; Passalacqua, M.; Maffioli, S.; Pfeffer, U.; et al. Metformin impairs glucose consumption and survival in calu-1 cells by direct inhibition of hexokinase-ii. Sci. Rep. 2013, 3, 2070. [Google Scholar] [CrossRef] [PubMed]
- Whitburn, J.; Edwards, C.M.; Sooriakumaran, P. Metformin and prostate cancer: A new role for an old drug. Curr. Urol. Rep. 2017, 18, 46. [Google Scholar] [CrossRef] [PubMed]
- Culig, Z.; Santer, F.R. Androgen receptor signaling in prostate cancer. Cancer Metastasis Rev. 2014, 33, 413–427. [Google Scholar] [CrossRef]
- Demir, U.; Koehler, A.; Schneider, R.; Schweiger, S.; Klocker, H. Metformin anti-tumor effect via disruption of the mid1 translational regulator complex and ar downregulation in prostate cancer cells. BMC Cancer 2014, 14, 52. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, G.; Tong, D.; Parmar, H.; Hasenmayer, D.; Yuan, W.; Zhang, D.; Jiang, J. Metformin represses androgen-dependent and androgen-independent prostate cancers by targeting androgen receptor. Prostate 2015, 75, 1187–1196. [Google Scholar] [CrossRef]
- Preston, M.A.; Riis, A.H.; Ehrenstein, V.; Breau, R.H.; Batista, J.L.; Olumi, A.F.; Mucci, L.A.; Adami, H.O.; Sorensen, H.T. Metformin use and prostate cancer risk. Eur. Urol. 2014, 66, 1012–1020. [Google Scholar] [CrossRef]
- Haring, A.; Murtola, T.J.; Talala, K.; Taari, K.; Tammela, T.L.; Auvinen, A. Antidiabetic drug use and prostate cancer risk in the finnish randomized study of screening for prostate cancer. Scand. J. Urol. 2017, 51, 5–12. [Google Scholar] [CrossRef]
- Ruiter, R.; Visser, L.E.; van Herk-Sukel, M.P.; Coebergh, J.W.; Haak, H.R.; Geelhoed-Duijvestijn, P.H.; Straus, S.M.; Herings, R.M.; Stricker, B.H. Lower risk of cancer in patients on metformin in comparison with those on sulfonylurea derivatives: Results from a large population-based follow-up study. Diabetes Care 2012, 35, 119–124. [Google Scholar] [CrossRef]
- Feng, T.; Sun, X.; Howard, L.E.; Vidal, A.C.; Gaines, A.R.; Moreira, D.M.; Castro-Santamaria, R.; Andriole, G.L.; Freedland, S.J. Metformin use and risk of prostate cancer: Results from the reduce study. Cancer Prev. Res. (Phila) 2015, 8, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.B.; Eurich, D.T.; Majumdar, S.R.; Johnson, J.A. Metformin and the risk of prostate cancer across racial/ethnic groups: A population-based cohort study. Prostate Cancer Prostatic Dis. 2017, 20, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.P.; Lehman, D.M.; Lam, Y.F.; Kuhn, J.G.; Mahalingam, D.; Weitman, S.; Lorenzo, C.; Downs, J.R.; Stuart, E.A.; Hernandez, J.; et al. Metformin for reducing racial/ethnic difference in prostate cancer incidence for men with type ii diabetes. Cancer Prev. Res. (Phila) 2016, 9, 779–787. [Google Scholar] [CrossRef]
- Raval, A.D.; Mattes, M.D.; Madhavan, S.; Pan, X.; Wei, W.; Sambamoorthi, U. Association between metformin use and cancer stage at diagnosis among elderly medicare beneficiaries with preexisting type 2 diabetes mellitus and incident prostate cancer. J. Diabetes Res. 2016, 2016, 2656814. [Google Scholar] [CrossRef] [PubMed]
- Nordstrom, T.; Clements, M.; Karlsson, R.; Adolfsson, J.; Gronberg, H. The risk of prostate cancer for men on aspirin, statin or antidiabetic medications. Eur. J. Cancer 2015, 51, 725–733. [Google Scholar] [CrossRef]
- Onitilo, A.A.; Stankowski, R.V.; Berg, R.L.; Engel, J.M.; Glurich, I.; Williams, G.M.; Doi, S.A. Type 2 diabetes mellitus, glycemic control, and cancer risk. Eur. J. Cancer Prev. 2014, 23, 134–140. [Google Scholar] [CrossRef]
- Tseng, C.H. Metformin significantly reduces incident prostate cancer risk in taiwanese men with type 2 diabetes mellitus. Eur. J. Cancer 2014, 50, 2831–2837. [Google Scholar] [CrossRef]
- Chen, C.B.; Eskin, M.; Eurich, D.T.; Majumdar, S.R.; Johnson, J.A. Metformin, asian ethnicity and risk of prostate cancer in type 2 diabetes: A systematic review and meta-analysis. BMC Cancer 2018, 18, 65. [Google Scholar] [CrossRef]
- Kasuga, M.; Ueki, K.; Tajima, N.; Noda, M.; Ohashi, K.; Noto, H.; Goto, A.; Ogawa, W.; Sakai, R.; Tsugane, S.; et al. Report of the japan diabetes society/japanese cancer association joint committee on diabetes and cancer. Cancer Sci. 2013, 104, 965–976. [Google Scholar] [CrossRef]
- Nomiyama, T.; Kawanami, T.; Irie, S.; Hamaguchi, Y.; Terawaki, Y.; Murase, K.; Tsutsumi, Y.; Nagaishi, R.; Tanabe, M.; Morinaga, H.; et al. Exendin-4, a glp-1 receptor agonist, attenuates prostate cancer growth. Diabetes 2014, 63, 3891–3905. [Google Scholar] [CrossRef]
- Lin, C.; Cai, X.; Yang, W.; Lv, F.; Nie, L.; Ji, L. Glycemic control and the incidence of neoplasm in patients with type 2 diabetes: A meta-analysis of randomized controlled trials. Endocrine 2020. [Google Scholar] [CrossRef] [PubMed]
- Danzig, M.R.; Kotamarti, S.; Ghandour, R.A.; Rothberg, M.B.; Dubow, B.P.; Benson, M.C.; Badani, K.K.; McKiernan, J.M. Synergism between metformin and statins in modifying the risk of biochemical recurrence following radical prostatectomy in men with diabetes. Prostate Cancer Prostatic Dis. 2015, 18, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Taussky, D.; Preisser, F.; Karakiewicz, P.I.; Tilki, D.; Lambert, C.; Bahary, J.P.; Delouya, G.; Wistaff, R.; Laskine, M.; Nguyen, P.V.; et al. Impact of diabetes and metformin use on prostate cancer outcome of patients treated with radiation therapy: Results from a large institutional database. Can. J. Urol. 2018, 25, 9509–9515. [Google Scholar] [PubMed]
- Ranasinghe, W.K.B.; Williams, S.; Ischia, J.; Wetherell, D.; Baldwin, G.; Shulkes, A.; Sengupta, S.; Bolton, D.; Patel, O. Metformin may offer no protective effect in men undergoing external beam radiation therapy for prostate cancer. BJU Int. 2019, 123 (Suppl 5), 36–42. [Google Scholar] [CrossRef]
- Stopsack, K.H.; Ziehr, D.R.; Rider, J.R.; Giovannucci, E.L. Metformin and prostate cancer mortality: A meta-analysis. Cancer Causes Control 2016, 27, 105–113. [Google Scholar] [CrossRef]
- Xiao, Y.; Zheng, L.; Mei, Z.; Xu, C.; Liu, C.; Chu, X.; Hao, B. The impact of metformin use on survival in prostate cancer: A systematic review and meta-analysis. Oncotarget 2017, 8, 100449–100458. [Google Scholar] [CrossRef]
- Hwang, I.C.; Park, S.M.; Shin, D.; Ahn, H.Y.; Rieken, M.; Shariat, S.F. Metformin association with lower prostate cancer recurrence in type 2 diabetes: A systematic review and meta-analysis. Asian Pac. J. Cancer Prev. 2015, 16, 595–600. [Google Scholar] [CrossRef]
- Xu, H.; Aldrich, M.C.; Chen, Q.; Liu, H.; Peterson, N.B.; Dai, Q.; Levy, M.; Shah, A.; Han, X.; Ruan, X.; et al. Validating drug repurposing signals using electronic health records: A case study of metformin associated with reduced cancer mortality. J. Am. Med. Inform Assoc. 2015, 22, 179–191. [Google Scholar] [CrossRef]
- Randazzo, M.; Beatrice, J.; Huber, A.; Grobholz, R.; Manka, L.; Wyler, S.F.; Chun, F.F.; Recker, F.; Kwiatkowski, M. Influence of metformin use on psa values, free-to-total psa, prostate cancer incidence and grade and overall survival in a prospective screening trial (erspc aarau). World J. Urol. 2015, 33, 1189–1196. [Google Scholar] [CrossRef]
- Spratt, D.E.; Zhang, C.; Zumsteg, Z.S.; Pei, X.; Zhang, Z.; Zelefsky, M.J. Metformin and prostate cancer: Reduced development of castration-resistant disease and prostate cancer mortality. Eur. Urol. 2013, 63, 709–716. [Google Scholar] [CrossRef]
- Bensimon, L.; Yin, H.; Suissa, S.; Pollak, M.N.; Azoulay, L. The use of metformin in patients with prostate cancer and the risk of death. Cancer Epidemiol. Biomarkers Prev. 2014, 23, 2111–2118. [Google Scholar] [CrossRef] [PubMed]
- Zaorsky, N.G.; Shaikh, T.; Ruth, K.; Sharda, P.; Hayes, S.B.; Sobczak, M.L.; Hallman, M.A.; Smaldone, M.C.; Chen, D.Y.; Horwitz, E.M. Prostate cancer patients with unmanaged diabetes or receiving insulin experience inferior outcomes and toxicities after treatment with radiation therapy. Clin. Genitourin. Cancer 2017, 15, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.J.; Klotz, L.H.; Venkateswaran, V. The effect of metformin use during docetaxel chemotherapy on prostate cancer specific and overall survival of diabetic patients with castration resistant prostate cancer. J. Urol. 2017, 197, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Joentausta, R.M.; Kujala, P.M.; Visakorpi, T.; Tammela, T.L.; Murtola, T.J. Tumor features and survival after radical prostatectomy among antidiabetic drug users. Prostate Cancer Prostatic Dis. 2016, 19, 367–373. [Google Scholar] [CrossRef]
- Taira, A.V.; Merrick, G.S.; Galbreath, R.W.; Morris, M.; Butler, W.M.; Adamovich, E. Metformin is not associated with improved biochemical free survival or cause-specific survival in men with prostate cancer treated with permanent interstitial brachytherapy. J. Contemp. Brachytherapy 2014, 6, 254–261. [Google Scholar] [CrossRef]
- Biernacka, K.M.; Persad, R.A.; Bahl, A.; Gillatt, D.; Holly, J.M.; Perks, C.M. Hyperglycaemia-induced resistance to docetaxel is negated by metformin: A role for igfbp-2. Endocr. Relat. Cancer 2017, 24, 17–30. [Google Scholar] [CrossRef]
- Chevalier, B.; Pasquier, D.; Lartigau, E.F.; Chargari, C.; Schernberg, A.; Jannin, A.; Mirabel, X.; Vantyghem, M.C.; Escande, A. Metformin: (future) best friend of the radiation oncologist? Radiother. Oncol. 2020, 151, 95–105. [Google Scholar] [CrossRef]
- Margel, D.; Urbach, D.R.; Lipscombe, L.L.; Bell, C.M.; Kulkarni, G.; Austin, P.C.; Fleshner, N. Metformin use and all-cause and prostate cancer-specific mortality among men with diabetes. J. Clin. Oncol. 2013, 31, 3069–3075. [Google Scholar] [CrossRef]
- Rothermundt, C.; Hayoz, S.; Templeton, A.J.; Winterhalder, R.; Strebel, R.T.; Bartschi, D.; Pollak, M.; Lui, L.; Endt, K.; Schiess, R.; et al. Metformin in chemotherapy-naive castration-resistant prostate cancer: A multicenter phase 2 trial (sakk 08/09). Eur. Urol. 2014, 66, 468–474. [Google Scholar] [CrossRef]
- Devalingam, M.; Hanni, S.; Christos, F.; Joel, M.; John, S.; Paromita, D.; Ofelia, R.; Sureshkumar, M.; John, G.K.; Michael, N.P.; et al. Metformin to treat prostate cancer (PCa) and prevent metabolic syndrome associated with androgen deprivation therapy (ADT): Results of a randomized double-blind placebo-controlled study of metformin in non-diabetic men initiating ADT for advanced PCa. J. Clin. Oncol. 2017, 35 (Suppl. 15). [Google Scholar] [CrossRef]
- US National Library of Medicine. Metformin in Castration-Resistant Prostate Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT01215032 (accessed on 16 November 2019).
- Mark, M.; Klingbiel, D.; Mey, U.; Winterhalder, R.; Rothermundt, C.; Gillessen, S.; von Moos, R.; Pollak, M.; Manetsch, G.; Strebel, R.; et al. Impact of addition of metformin to abiraterone in metastatic castration-resistant prostate cancer patients with disease progressing while receiving abiraterone treatment (metab-pro): Phase 2 pilot study. Clin. Genitourin. Cancer 2019, 17, e323–e328. [Google Scholar] [CrossRef] [PubMed]
- US National Library of Medicine. Metformin Prostate Cancer Adjuvant Trial. Available online: https://clinicaltrials.gov/ct2/show/NCT02176161 (accessed on 16 November 2019).
- US National Library of Medicine. Enzalutamide and Metformin Hydrochloride in Treating Patients With Hormone-Resistant Prostate Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT0233916875 (accessed on 16 November 2019).
- US National Library of Medicine. SAKK 08/15—PROMET—Salvage Radiotherapy +/- Metformin for Patients With Prostate Cancer After Prostatectomy. Available online: https://clinicaltrials.gov/ct2/show/NCT02945813 (accessed on 16 November 2019).
| Author (Country) | Period | No. Cases/ Controls | Risk Estimates (95% CI) | Adjusted Variables |
|---|---|---|---|---|
| Preston (Denmark) [48] | 1989–2011 | 12,226/122,260 | Adjusted OR: 0.84 (0.74–0.96) | CCI, diabetic complications, marital status, use of PPI, statin, 5αRI |
| Haring (Finland) [49] | 1996–1999 | 7681/1080 | HR: 0.80 (0.67–0.96) | age, use of antihypertensives, cholesterol-lowering drugs, 5αRI, α-blockers, NSAIDs, aspirin |
| Ruiter (Netherlands) [50] | 1998–2008 | 52,698/32,591 | HR: 0.90 (0.88–0.91) | Age, sex, number of unique other drugs, number of hospitalizations, calendar time |
| Feng (REDUCE Study Group) [51] | 2003–2009 | 194/205 | Overall OR: 1.19 (0.72–1.99) Low-grade PCa OR: 1.01 (0.57–1.81)High-grade PCa OR: 1.83 (0.75–4.46) | Age, race, geographic region, PSA, prostate volume, digital rectal examination, BMI, family history of PCa, coronary artery disease, smoking, aspirin, NSAIDs, statin |
| Chen (Canada) [52] | 1994–2012 | 35,829/44,172 | Adjusted HR in non-Chinese men aged 50–59: 0.86 (0.74–1.00) aged 60–69: 1.00 (0.90–1.12) aged >70: 1.13 (0.99–1.29) | Socioeconomic class, use of other diabetes medications, dipeptidyl peptidase IV inhibitors, glucagon-like peptide 1 receptor agonists, thiazolidinediones, insulin |
| Wang (US) [53] | 2003–2012 | 23,130/36,776 | Non-Hispanic white HR: 0.91 (0.82–1.01) African American HR: 1.10 (0.94–1.27) Hispanics HR: 0.63 (0.49–0.80) | Age, race, CCI, BMI, LDL, HbA1c, PSA |
| Raval (US) [54] | 2008–2009 | 948/1704 | Unadjusted OR: 0.69 (0.49–0.95) Adjusted OR: 0.68 (0.48–0.97) | Age, race, marital status. |
| Nordstrom (Sweden) [55] | 2007–2012 | 7678/177,989 | Any PCa OR: 1.013 (0.816–1.257) High-grade PCaOR: 1.207 (0.936–1.557) | Age, log-transformed PSA level, PSA quotient, comorbidity, educational level, medication use |
| Onitilo (Australia) [56] | 1995–2009 | NA | HR: 0.86 (0.72−1.03) | HbA1c, glucose-lowering medication use (insulin, metformin, sulfonylurea), age, BMI, insurance status, comorbidities, smoking history, location of residence |
| Tseng (Taiwan) [57] | 1998–2002 | 186,212/209,269 | HR: 0.362 (0.345–0.380) | Age, PSA, comorbidities, obesity, other cancers |
| Author (Country) | Population | Period | No. Cases/Controls | Risk Estimates (95% CI) | Adjusted Variables |
|---|---|---|---|---|---|
| Mayer (Canada) [73] | Patients receiving docetaxel chemotherapy | 2005–2012 | 359/2473 | CSM HR: 0.96, 95% CI: 0.79–1.16 OM HR: 0.94, 95% CI: 0.82–1.08 | Age, use of statins and COX-2i, socio-economic status, urban/rural designation |
| Zaorsky (US) [72] | Patients receiving radiation therapy | 1998–2013 | 251/2352 | CSM sub-HR: 2.13 (0.90–5.08) OM sub-HR: 0.99 (0.65–1.52) | Age, comorbidities, PSA (log-transformed), Gleason score, T stage, ADT |
| Joentausta (Finland) [74] | Patients receiving radical prostatectomy | 1995–2009 | 28/136 | OM age-adjusted HR: 1.98 (0.86–4.53) multivariate-adjusted HR: 1.53 (0.57–4.08) | Age, use of 5αRI, preoperative PSA |
| Xu (US) [77] | Patients diagnosed with PCa, mixed therapy | 1995–2010 | 3029/5910 | OM (adjusted HR) Vanderbilt group: 1.04 (0.66–1.67) Mayo group: 0.69 (0.52–0.93) | Age, sex, race, BMI, tobacco use, insulin, cancer type, CCI |
| Randazzo (Switzerland) [69] | PSA-screened cohort, mixed therapy | 1998–2003 | 150/4164 | CSM OR: 27.94 (1.73–448.8) OM HR: 2.14 (1.19–3.87) | Age, PSA, family history, IPSS |
| Bensimon (UK) [71] | Patients newly diagnosed with non-metastatic PCa, mixed therapy | 1998–2009 | 78/194 | CSM overall RR: 1.09 (0.51–2.33) cumulative duration >938 days RR: 3.20 (1.00–10.24) OM post-diagnostic use of metformin RR: 0.79 (0.50–1.23) | Age, BMI, CCI, smoking, PSA, Gleason score, HbA1c excessive alcohol use, use of anti-diabetic agents (metformin, sulfonylureas, thiazolidinediones, insulins) |
| Spratt (Canada) [70] | Patients receiving radiation therapy | 1992–2008 | 157/162 | CSM (adjusted HR) non-diabetic group: 2.68 (0.85–8.44) * diabetic non-metformin group: 5.15 (1.53–17.35) * OS Non-diabetic group: 1.38 (0.90–2.11) * diabetic non-metformin group: 2.25 (1.38–3.66) * | Age, risk group, T stage, Gleason score, PSA, neoadjuvant ADT, BMI |
| Taira (US) [75] | Patients receiving interstitial brachytherapy | 1995–2010 | 126/2172 | OM HR: NA (p = 0.873) | Age, %positive biopsy, T stage, Gleason score, PSA, ADT duration, BMI, comorbidities |
| Margel (US) [78] | Patients newly diagnosed with PCa, mixed therapy | 1997–2008 | 1251/2586 | CSM HR: 0.76 (0.64–0.89) | Age, Johns Hopkins ACG Case-Mix System weighted sum of adjusted diagnostic groups, year of cohort entry, socioeconomic status, COX-2i, statins |
| Combination Agents | Clinical Phase | Identifier | Indication | Study End-Points | Status |
|---|---|---|---|---|---|
| Metformin monotherapy | II | NCT01243385 [79] | CRPC | PFS, OS, and safety | Completed |
| Metformin monotherapy | II | NCT01620593 [80] | Metastatic PCa | PFS, OS, PSA response, and safety | Completed |
| Metformin monotherapy | II | NCT01215032 [81] | CRPC | PSA response | Completed |
| Metformin + abiraterone | II | NCT01677897 [82] | Pre-chemotherapy CRPC progressing on abiraterone | PFS, OS | Completed |
| Metformin monotherapy | II | NCT02176161 [83] | Radical prostatectomy patients with high-risk pathology, prior RT with or without increasing PSA | PSADT | Ongoing |
| Metformin + enzalutamide | I | NCT02339168 [84] | CRPC | PSA response | Ongoing |
| Metformin + salvage RT | II | NCT02945813 [85] | Biochemical failure following radical prostatectomy | Time to progression, PFS, OS | Ongoing |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, H.K.; Lee, Y.H.; Koo, K.C. Current Status and Application of Metformin for Prostate Cancer: A Comprehensive Review. Int. J. Mol. Sci. 2020, 21, 8540. https://doi.org/10.3390/ijms21228540
Ahn HK, Lee YH, Koo KC. Current Status and Application of Metformin for Prostate Cancer: A Comprehensive Review. International Journal of Molecular Sciences. 2020; 21(22):8540. https://doi.org/10.3390/ijms21228540
Chicago/Turabian StyleAhn, Hyun Kyu, Young Hwa Lee, and Kyo Chul Koo. 2020. "Current Status and Application of Metformin for Prostate Cancer: A Comprehensive Review" International Journal of Molecular Sciences 21, no. 22: 8540. https://doi.org/10.3390/ijms21228540
APA StyleAhn, H. K., Lee, Y. H., & Koo, K. C. (2020). Current Status and Application of Metformin for Prostate Cancer: A Comprehensive Review. International Journal of Molecular Sciences, 21(22), 8540. https://doi.org/10.3390/ijms21228540





