CSF Biomarkers Reflecting Protein Pathology and Axonal Degeneration Are Associated with Memory, Attentional, and Executive Functioning in Early-Stage Parkinson′s Disease
Abstract
1. Introduction
2. Results
2.1. Cognitive Functioning in Early-Stage PD
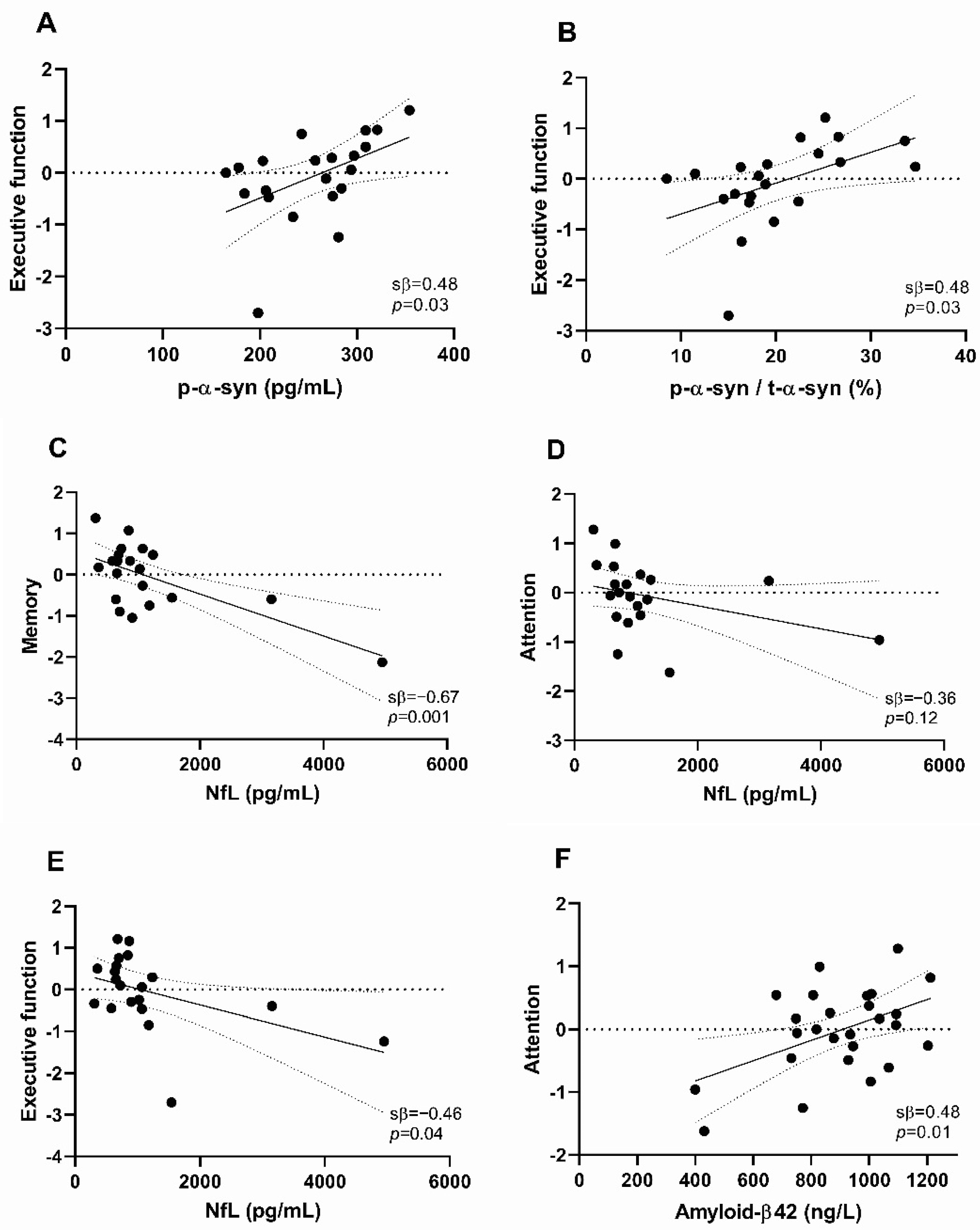
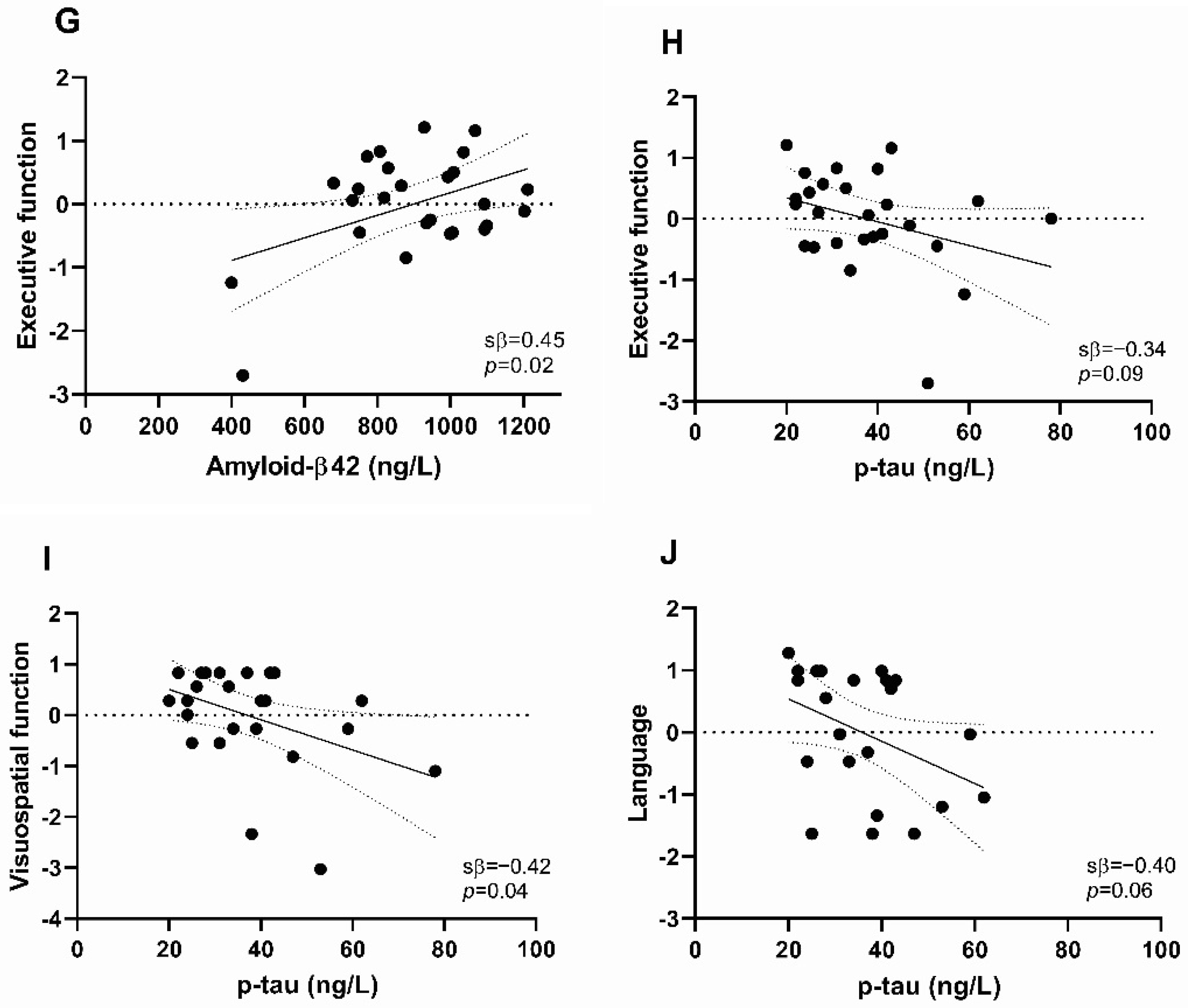
2.2. Associations of CSF Biomarker Levels with Cognitive Performance
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Neuropsychological Assessment
4.3. CSF Biomarkers
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Aβ42 | amyloid-β42 |
| AD | Alzheimer′s disease |
| CSF | cerebrospinal fluid |
| ELISA | enzyme-linked immunosorbent assays |
| HY | modified Hoehn & Yahr classification |
| NfL | neurofilament light chain |
| MDS | Movement Disorder Society |
| MMSE | Mini-Mental State Examination |
| p-α-syn | α-synuclein phosphorylated at residue Ser 129 |
| PD | Parkinson′s disease |
| PD-MCI | Parkinson′s disease mild cognitive impairment |
| p-tau | tau phosphorylated at threonine 181 |
| RAVLT | Rey Auditory Verbal Learning Test |
| t-α-syn | total α-synuclein |
| t-tau | total tau |
| TMT | Trail Making Test |
| UPDRS III | Unified Parkinson′s Disease Rating Scale Part III |
| VAT | Visual Association Test |
References
- Lawson, R.A.; Yarnall, A.J.; Duncan, G.W.; Khoo, T.K.; Breen, D.P.; Barker, R.A.; Collerton, D.; Taylor, J.-P.; Burn, D.J. Severity of mild cognitive impairment in early Parkinson’s disease contributes to poorer quality of life. Park. Relat. Disord. 2014, 20, 1071–1075. [Google Scholar] [CrossRef] [PubMed]
- Litvan, I.; Goldman, J.G.; Tröster, A.I.; Schmand, B.A.; Weintraub, D.; Petersen, R.C.; Mollenhauer, B.; Adler, C.H.; Marder, K.; Williams-Gray, C.H.; et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Mov. Disord. 2012, 27, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.F.; Larsen, J.P.; Tysnes, O.-B.; Alves, G. Natural course of mild cognitive impairment in Parkinson disease. Neurology 2017, 88, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Kehagia, A.A.; Barker, R.A.; Robbins, T.W. Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson’s disease. Lancet Neurol. 2010, 9, 1200–1213. [Google Scholar] [CrossRef]
- Muslimović, D.; Post, B.; Speelman, J.D.; Schmand, B. Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology 2005, 65, 1239–1245. [Google Scholar] [CrossRef]
- Elgh, E.; Domellöf, M.; Linder, J.; Edström, M.; Stenlund, H.; Forsgren, L. Cognitive function in early Parkinson’s disease: A population-based study. Eur. J. Neurol. 2009, 16, 1278–1284. [Google Scholar] [CrossRef]
- Yarnall, A.J.; Breen, D.P.; Duncan, G.W.; Khoo, T.K.; Coleman, S.Y.; Firbank, M.J.; Nombela, C.; Winder-Rhodes, S.; Evans, J.R.; Rowe, J.B.; et al. Characterizing mild cognitive impairment in incident Parkinson disease: The ICICLE-PD Study. Neurology 2014, 82, 308–316. [Google Scholar] [CrossRef]
- Weintraub, D.; Simuni, T.; Caspell-Garcia, C.; Coffey, C.; Lasch, S.; Siderowf, A.; Aarsland, D.; Barone, P.; Burn, D.; Chahine, L.M.; et al. Cognitive performance and neuropsychiatric symptoms in early, untreated Parkinson’s disease. Mov. Disord. 2015, 30, 919–927. [Google Scholar] [CrossRef]
- Pfeiffer, H.C.V.; Lokkegaard, A.; Zoetmulder, M.; Friberg, L.; Werdelin, L. Cognitive impairment in early-stage non-demented Parkinson’s disease patients. Acta Neurol. Scand. 2013, 129, 307–318. [Google Scholar] [CrossRef]
- Aarsland, D.; Creese, B.; Politis, M.; Chaudhuri, K.R.; Ffytche, D.H.; Weintraub, D.; Ballard, C. Cognitive decline in Parkinson disease. Nat. Rev. Neurol. 2017, 13, 217–231. [Google Scholar] [CrossRef]
- Stav, A.L.; Aarsland, D.; Kunszt Johansen, K.; Hessen, E.; Auning, E.; Fladby, T. Amyloid-beta and alpha-synuclein cerebrospinal fluid biomarkers and cognition in early Parkinson′s disease. Parkinsonism Relat. Disord. 2015, 21, 758–764. [Google Scholar] [CrossRef]
- Alves, G.; Bronnick, K.; Aarsland, D.; Blennow, K.; Zetterberg, H.; Ballard, C.; Wilhelm Kurz, M.; Andreasson, U.; Tysnes, O.-B.; Larsen, J.P.; et al. CSF amyloid-beta and tau proteins, and cognitive performance, in early and untreated Parkinson′s disease: The Norwegian ParkWest study. J. Neurol. Neurosurg. Psychiatry 2010, 81, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Skogseth, R.E.; Bronnick, K.; Pereira, J.B.; Mollenhauer, B.; Weintraub, D.; Fladby, T.; Aarsland, D. Associations between Cerebrospinal Fluid Biomarkers and Cognition in Early Untreated Parkinson’s Disease. J. Park. Dis. 2015, 5, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Majbour, N.K.; Vaikath, N.N.; Van Dijk, K.D.; Ardah, M.T.; Varghese, S.; Vesterager, L.B.; Montezinho, L.P.; Poole, S.; Safieh-Garabedian, B.; Tokuda, T.; et al. Oligomeric and phosphorylated alpha-synuclein as potential CSF biomarkers for Parkinson’s disease. Mol. Neurodegener. 2016, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Olsson, B.; Portelius, E.; Cullen, N.C.; Sandelius, Å.; Zetterberg, H.; Andreasson, U.; Höglund, K.; Irwin, D.J.; Grossman, M.; Weintraub, D.; et al. Association of Cerebrospinal Fluid Neurofilament Light Protein Levels With Cognition in Patients With Dementia, Motor Neuron Disease, and Movement Disorders. JAMA Neurol. 2019, 76, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Bäckström, D.; Domellöf, M.E.; Linder, J.; Olsson, B.; Öhrfelt, A.; Trupp, M.; Zetterberg, H.; Blennow, K.; Forsgren, L. Cerebrospinal Fluid Patterns and the Risk of Future Dementia in Early, Incident Parkinson Disease. JAMA Neurol. 2015, 72, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Morfini, G.A.; Langhamer, L.B.; He, Y.; Brady, S.T.; Kordower, J.H. Alterations in axonal transport motor proteins in sporadic and experimental Parkinson’s disease. Brain 2012, 135, 2058–2073. [Google Scholar] [CrossRef]
- Aarsland, D.; Bronnick, K.; Williams-Gray, C.; Weintraub, D.; Marder, K.; Kulisevsky, J.; Burn, D.; Barone, P.; Pagonabarraga, J.; Allcock, L.; et al. Mild cognitive impairment in Parkinson disease: A multicenter pooled analysis. Neurology 2010, 75, 1062–1069. [Google Scholar] [CrossRef]
- Gaetani, L.; Salvadori, N.; Lisetti, V.; Eusebi, P.; Mancini, A.; Gentili, L.; Borrelli, A.; Portaccio, E.; Sarchielli, P.; Blennow, K.; et al. Cerebrospinal fluid neurofilament light chain tracks cognitive impairment in multiple sclerosis. J. Neurol. 2019, 266, 2157–2163. [Google Scholar] [CrossRef]
- Rolstad, S.; Berg, A.I.; Eckerström, C.; Johansson, B.; Wallin, A. Differential Impact of Neurofilament Light Subunit on Cognition and Functional Outcome in Memory Clinic Patients with and without Vascular Burden. J. Alzheimer’s Dis. 2015, 45, 873–881. [Google Scholar] [CrossRef]
- Leverenz, J.B.; Watson, G.S.; Shofer, J.; Zabetian, C.P.; Zhang, J.; Montine, T.J. Cerebrospinal fluid biomarkers and cognitive performance in non-demented patients with Parkinson’s disease. Park. Relat. Disord. 2011, 17, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Compta, Y.; Martí, M.J.; Ibarretxe-Bilbao, N.; Junque, C.; Valldeoriola, F.; Muñoz, E.; Ezquerra, M.; Ríos, J.; Tolosa, E. Cerebrospinal tau, phospho-tau, and beta-amyloid and neuropsychological functions in Parkinson’s disease. Mov. Disord. 2009, 24, 2203–2210. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, K.D.; Jongbloed, W.; Heijst, J.A.; Teunissen, C.E.; Groenewegen, H.J.; Berendse, H.W.; Van De Berg, W.D.; Veerhuis, R. Cerebrospinal fluid and plasma clusterin levels in Parkinson’s disease. Park. Relat. Disord. 2013, 19, 1079–1083. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.J.; Daniel, S.E.; Kilford, L.; Lees, A.J. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry 1992, 55, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Verhage, F. Intelligence and Age: Study with Dutch People Aged 12–77; Van Gorcum: Assen, The Netherlands, 1964. [Google Scholar]
- Lindeboom, J.; Schmand, B.; Tulner, L.; Walstra, G.; Jonker, C. Visual association test to detect early dementia of the Alzheimer type. J. Neurol. Neurosurg. Psychiatry 2002, 73, 126–133. [Google Scholar] [CrossRef]
- Shin, M.-S.; Park, S.-Y.; Park, S.-R.; Seol, S.-H.; Kwon, J.S. Clinical and empirical applications of the Rey–Osterrieth Complex Figure Test. Nat. Protoc. 2006, 1, 892–899. [Google Scholar] [CrossRef]
- Dalrymple-Alford, J.C.; Ba, L.L.; Macaskill, M.; Ma, C.G.; Melzer, T.R.; Porter, R.J.; Watts, R.; Anderson, T.J. Characterizing mild cognitive impairment in Parkinson’s disease. Mov. Disord. 2011, 26, 629–636. [Google Scholar] [CrossRef]
- Teunissen, C.E.; Petzold, A.; Bennett, J.L.; Berven, F.S.; Brundin, L.; Comabella, M.; Franciotta, D.; Frederiksen, J.L.; Fleming, J.O.; Furlan, R.; et al. A consensus protocol for the standardization of cerebrospinal fluid collection and biobanking. Neurology 2009, 73, 1914–1922. [Google Scholar] [CrossRef]
- Oosterveld, L.P.; Verberk, I.M.; Majbour, N.K.; El-Agnaf, O.M.; Weinstein, H.C.; Berendse, H.W.; Teunissen, C.E.; Van De Berg, W.D. CSF or Serum Neurofilament Light Added to α-Synuclein Panel Discriminates Parkinson’s From Controls. Mov. Disord. 2019, 35, 288–295. [Google Scholar] [CrossRef]
- Mollenhauer, B.; Parnetti, L.; Rektorova, I.; Kramberger, M.G.; Pikkarainen, M.; Schulz-Schaeffer, W.J.; Aarsland, D.; Svenningsson, P.; Farotti, L.; Verbeek, M.M.; et al. Biological confounders for the values of cerebrospinal fluid proteins in Parkinson’s disease and related disorders. J. Neurochem. 2016, 139, 290–317. [Google Scholar] [CrossRef]

| Demographics | n | |
|---|---|---|
| Female/male (n) | 26 | 14/12 |
| Age (years) | 26 | 63 ± 13 (41–84) |
| Education (number per Verhage level 1/2/3/4/5/6/7) | 26 | 0/1/1/3/8/5/8 |
| Disease duration (years) | 26 | 2 (1–5) |
| Hoehn and Yahr stage (nr. per stage 1/1.5/2/2.5/3/4/5) | 26 | 2/3/13/8/0/0/0 |
| UPDRS III score | 26 | 22 (6–38) |
| MMSE score | 26 | 29 (23–30) |
| BDI score | 22 | 8 (0–29) |
| BAI score | 22 | 10 (1–21) |
| CSF Biomarker Levels | ||
| t-α-syn (pg/mL) | 21 | 1271 (723–1950) |
| p-α-syn (pg/mL) | 21 | 268 (165–354) |
| p-α-syn/t-α-syn (%) | 21 | 18.9 (8.5–34.7) |
| NfL (pg/mL) | 20 | 858 (307–4948) |
| Amyloid-β42 (ng/L) | 26 | 931 (400−1211) |
| t-tau (ng/L) | 26 | 186.5 (95.0–369.0) |
| p-tau (ng/L) | 26 | 35.5 (20.0–78.0) |
| Cognitive Domain | Test | n | Test Score | t-score a |
|---|---|---|---|---|
| Memory | RAVLT delayed recall (nr. of words correct) | 26 | 7.8 ± 3.4 (2–15) | 43.3 ± 11.6 (24–66) |
| VAT A trial 1 (nr. of objects correct) | 26 | 5.6 ± 0.6 (4–6) | 43.7 ± 2.3 (37–45) | |
| Domain z-score b | 26 | 0.00 ± 0.80 (−2.13–1.37) | NA | |
| Attention | TMT A (sec) | 26 | 45 ± 20 (19–116) | 43.6 ± 8.9 (26–58) |
| Digit Span forward (nr. of series correct) | 25 | 13 ± 3 (6–19) | 49.8 ± 17.1 (16–75) | |
| Domain z-score b | 26 | −0.02 ± 0.72 (−2.32–1.08) | NA | |
| Executive functions | Category fluency (nr. of words correct) | 26 | 24 ± 5 (14–32) | 50.7 ± 8.8 (35–69) |
| Stroop Color Word (sec part III / sec part II) | 24 | 1.8 ± 0.4 (1.2–3.2) | 50.0 ± 8.2 (32–67) | |
| Domain z-score b | 26 | 0.00 ± 0.81 (−2.70–1.21) | NA | |
| Visuospatial function | Rey copy (elements correct) | 25 | 33 ± 3.6 (22–36) | 38.6 ± 3.4 (28–40) |
| Domain z-score c | 25 | 0.00 ± 1.00 (−3.03–0.83) | NA | |
| Language | Boston Naming Test (nr. of objects correct) | 23 | 78 ± 7 (67–87) | 47.5 ± 7.8 (36–65) |
| Domain z-score c | 23 | 0.00 ±1.00 (−1.63–1.28) | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oosterveld, L.P.; Kuiper, T.I.; Majbour, N.K.; Verberk, I.M.W.; van Dijk, K.D.; Twisk, J.W.R.; El-Agnaf, O.M.; Teunissen, C.E.; Weinstein, H.C.; Klein, M.; et al. CSF Biomarkers Reflecting Protein Pathology and Axonal Degeneration Are Associated with Memory, Attentional, and Executive Functioning in Early-Stage Parkinson′s Disease. Int. J. Mol. Sci. 2020, 21, 8519. https://doi.org/10.3390/ijms21228519
Oosterveld LP, Kuiper TI, Majbour NK, Verberk IMW, van Dijk KD, Twisk JWR, El-Agnaf OM, Teunissen CE, Weinstein HC, Klein M, et al. CSF Biomarkers Reflecting Protein Pathology and Axonal Degeneration Are Associated with Memory, Attentional, and Executive Functioning in Early-Stage Parkinson′s Disease. International Journal of Molecular Sciences. 2020; 21(22):8519. https://doi.org/10.3390/ijms21228519
Chicago/Turabian StyleOosterveld, Linda P., Tessa I. Kuiper, Nour K. Majbour, Inge M. W. Verberk, Karin D. van Dijk, Jos W. R. Twisk, Omar M. El-Agnaf, Charlotte E. Teunissen, Henry C. Weinstein, Martin Klein, and et al. 2020. "CSF Biomarkers Reflecting Protein Pathology and Axonal Degeneration Are Associated with Memory, Attentional, and Executive Functioning in Early-Stage Parkinson′s Disease" International Journal of Molecular Sciences 21, no. 22: 8519. https://doi.org/10.3390/ijms21228519
APA StyleOosterveld, L. P., Kuiper, T. I., Majbour, N. K., Verberk, I. M. W., van Dijk, K. D., Twisk, J. W. R., El-Agnaf, O. M., Teunissen, C. E., Weinstein, H. C., Klein, M., Berendse, H. W., & van de Berg, W. D. J. (2020). CSF Biomarkers Reflecting Protein Pathology and Axonal Degeneration Are Associated with Memory, Attentional, and Executive Functioning in Early-Stage Parkinson′s Disease. International Journal of Molecular Sciences, 21(22), 8519. https://doi.org/10.3390/ijms21228519





