Triplex Real-Time PCR Approach for the Detection of Crucial Fungal Berry Pathogens—Botrytis spp., Colletotrichum spp. and Verticillium spp.
Abstract
1. Introduction
2. Results
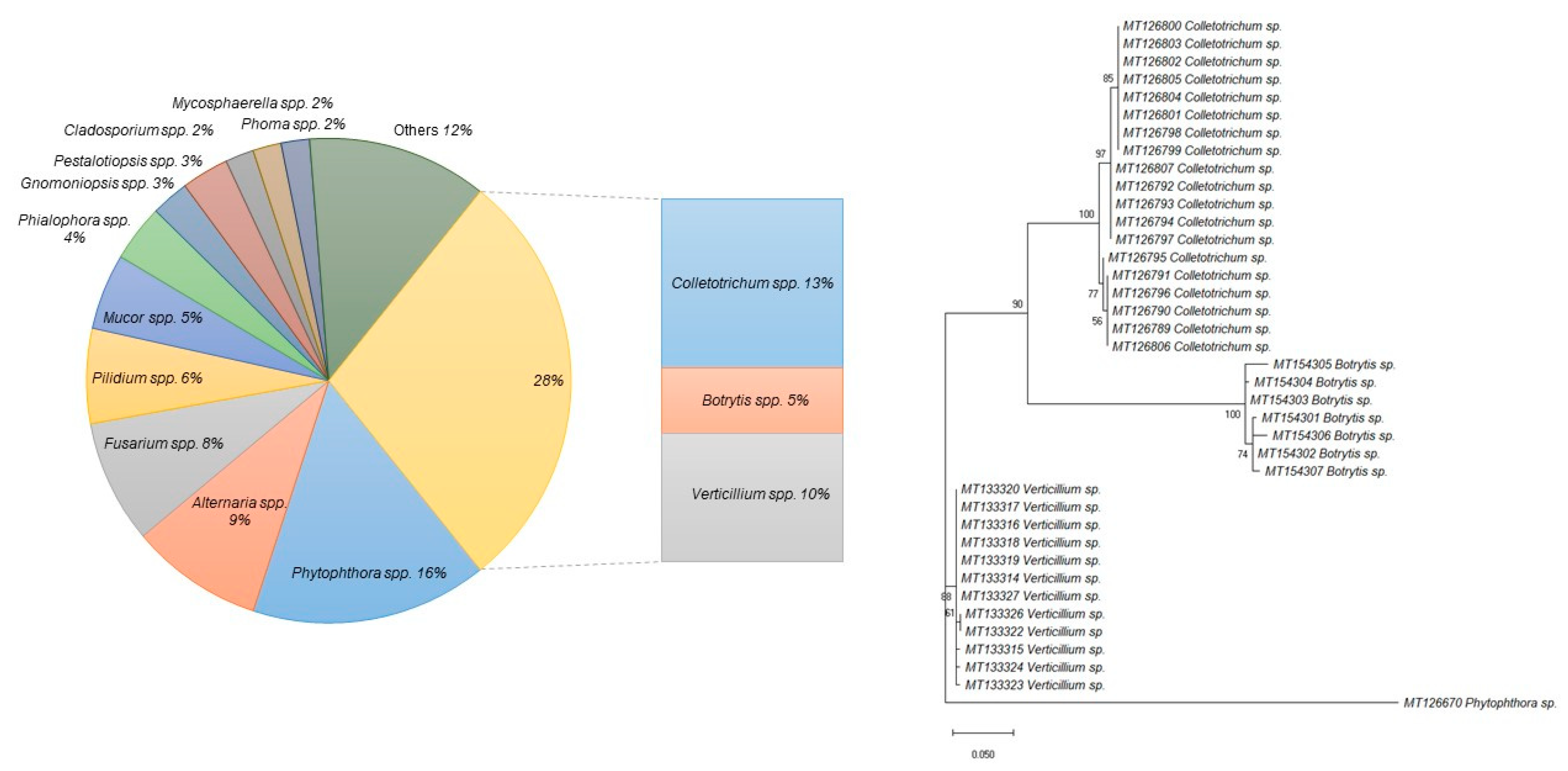
2.1. Phytopathogenic Microorganisms
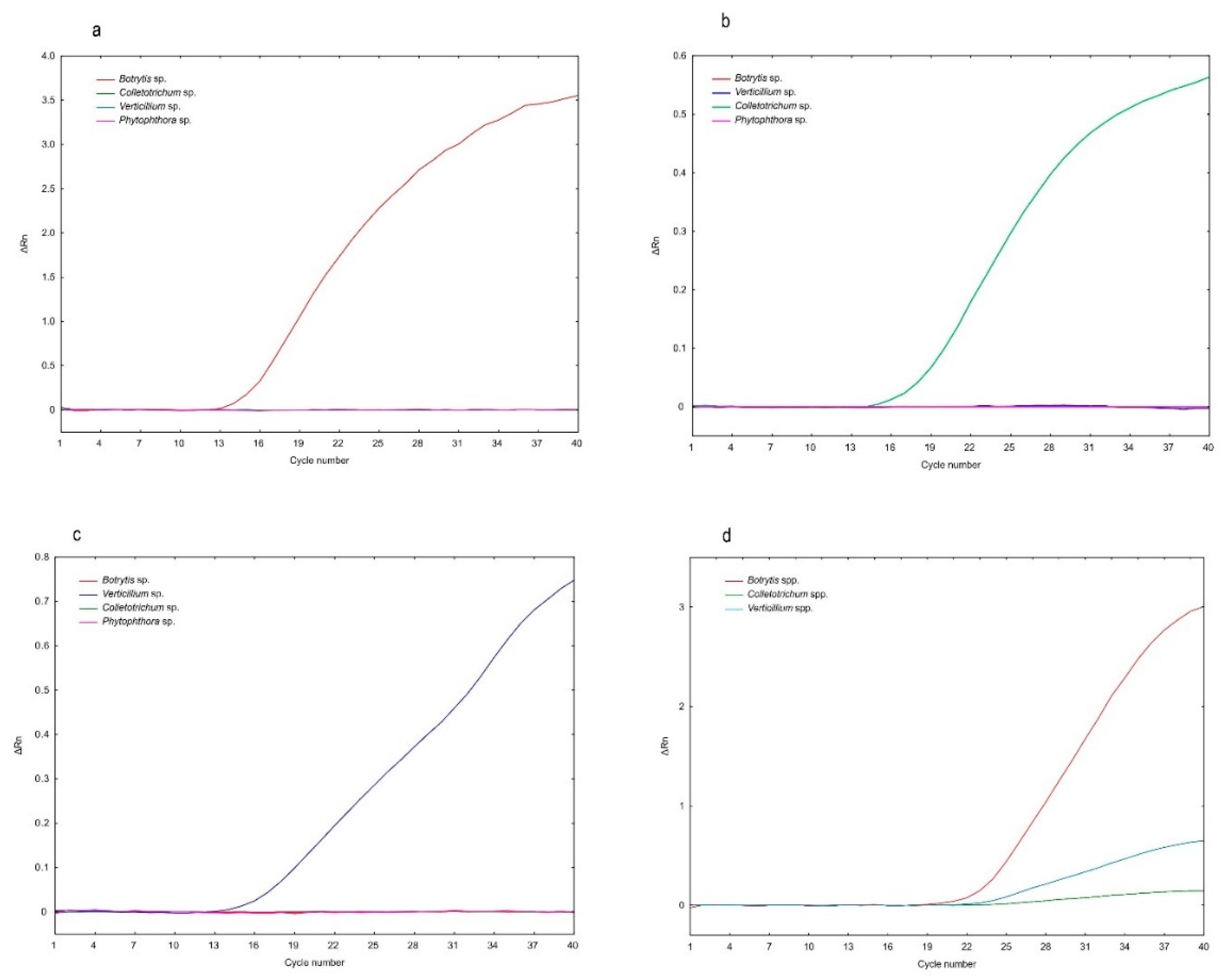
2.2. Specificity of the Fluorogenic Probes
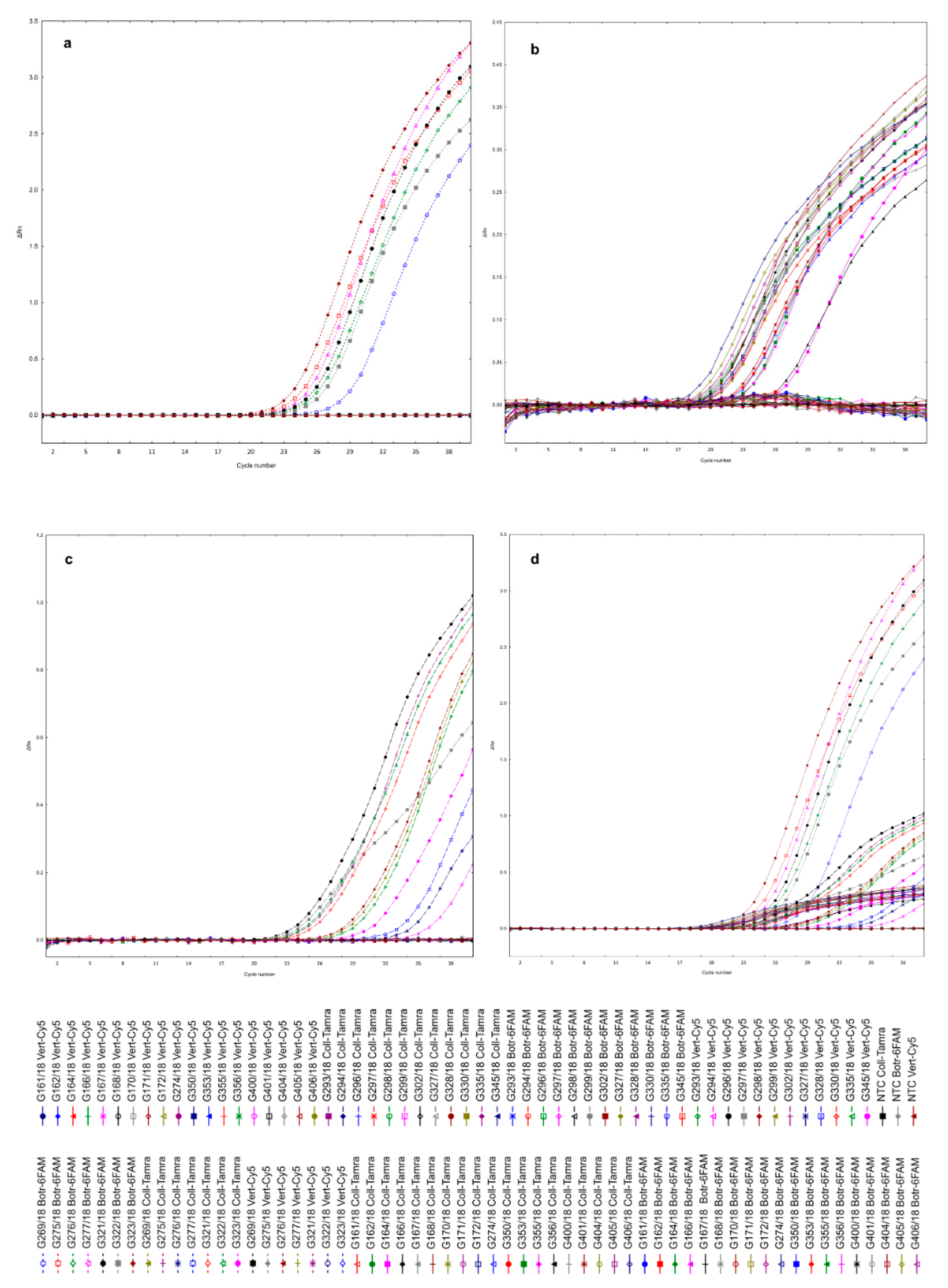
2.3. Optimization of Multiplex Real-Time PCR
2.4. Detection Limit of Multiplex Reaction
2.5. Detection of Fungal Pathogens in Artificially Infested Environmental Samples
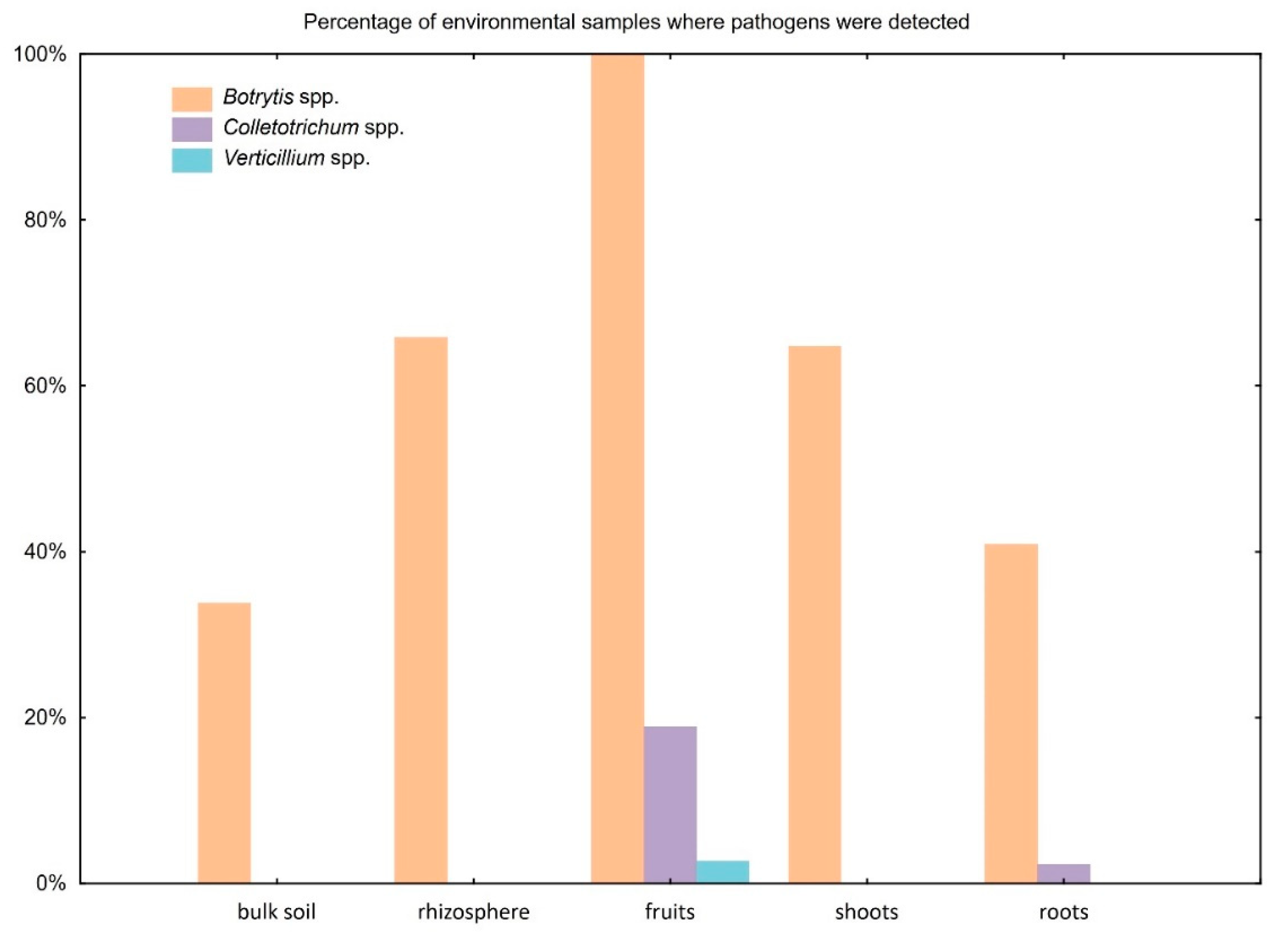
2.6. Validation Assay in Environmental Samples
3. Discussion
4. Materials and Methods
4.1. Phytopathogenic Organisms
4.2. Isolation of DNA
4.2.1. DNA Isolation from Pure Culture of Fungi
4.2.2. DNA Isolation from Environmental Samples
4.3. Oligonucleotide Primers and Probes Design
4.4. Development of the Multiplex Real-Time PCR Assay
4.4.1. Specificity of the Fluorogenic Probes
4.4.2. Optimization of the Multiplex Real-Time PCR
4.5. Detection Limit of Multiplex Reactions
4.6. Detection of Fungal Pathogens in Artificially Infested Environmental Samples
4.7. Validation Assay in Environmental Samples
4.8. Software, Data Collection and Analysis
4.9. Data Availability
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Osbourn, A.E. Verticillium wilt of strawberry. Proc. Natl. Acad. Sci. USA 2001, 98, 14187–14188. [Google Scholar] [CrossRef] [PubMed]
- Blanco, C.; De Los Santos, B.; Romero, F. Relationship between concentrations of Botrytis cinerea conidia in air, environmental conditions, and the incidence of grey mould in strawberry flowers and fruits. Eur. J. Plant Pathol. 2006, 114, 415–425. [Google Scholar] [CrossRef]
- Droby, S.; Lichter, A. Post-harvest Botrytis infection: Etiology, development and managment. In Botrytis: Biology, Pathology and Control; Elad, Y., Williamson, B., Tudzynski, P., Delen, N., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 349–368. [Google Scholar]
- Kozhar, O.; Peever, T.L. How does Botrytis cinerea infect red raspberry? Phytopathology 2018, 108, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef]
- Prusky, D. Pathogen quiescence in postharvest diseases. Annu. Rev. Phytopathol 1996, 34, 413–434. [Google Scholar] [CrossRef]
- Polashock, J.J.; Caruso, F.L.; Oudemans, P.V.; McManus, P.S.; Crouch, J.A. The North American cranberry fruit rot fungal community: A systematic overview using morphological and phylogenetic affinities. Plant Pathol. 2009, 58, 1116–1127. [Google Scholar] [CrossRef]
- Arbefeville, S.; Harris, A.; Ferrieri, P. Comparison of sequencing the D2 region of the large subunit ribosomal RNA gene (MicroSEQ®) versus the internal transcribed spacer (ITS) regions using two public databases for identification of common and uncommon clinically relevant fungal species. J. Microbiol. Methods 2017, 140, 40–46. [Google Scholar] [CrossRef]
- Saiki, R.K.; Stoffel, S.; Scharf, S.J.; Higuchi, R.; Horn, G.T.; Mullis, K.B.; Erlich, H.A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science 1988, 239, 487–491. [Google Scholar] [CrossRef]
- Higuchi, R.; Fockler, C.; Dollinger, G.; Watson, R. Kinectic PCR analysis: Real-time monitoring of DNA amplification reactions. Nat. Biotechnol. 1993, 11, 1026–1030. [Google Scholar] [CrossRef]
- Higuchi, R.; Dollinger, G.; Walsh, S.P.; Griffith, R. Simultaneous amplification and detection of specific DNA sequences. Nat. Biotechnol. 1992, 10, 413–417. [Google Scholar] [CrossRef]
- Livak, K.J.; Flood, S.J.A.; Marmaro, J.; Giusti, W.; Deetz, K. Oligonucleotides with Fluorescent Dyes at System Useful for Detecting PCR Product and Nucleic Acid Hybridization. PCR Methods Appl. 1995, 4, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Reich, J.D.; Alexander, T.W.; Chatterton, S. A multiplex PCR assay for the detection and quantification of Sclerotinia sclerotiorum and Botrytis cinerea. Lett. Appl. Microbiol. 2016, 62, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, e63. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Dong, D.; Yang, Z.; Zou, D.; Chen, Z.; Yuan, J.; Huang, L. Polymerase Spiral Reaction (PSR): A novel isothermal nucleic acid amplification method. Sci. Rep. 2015, 5, 12723. [Google Scholar] [CrossRef]
- Banér, J.; Nilsson, M.; Mendel-Hartvig, M.; Landegren, U. Signal amplification of padlock probes by rolling circle replication. Nucleic Acids Res. 1998, 26, 5073–5078. [Google Scholar] [CrossRef]
- Fang, R.; Li, X.; Hu, L.; You, Q.; Li, J.; Wu, J.; Xu, P.; Zhong, H.; Luo, Y.; Mei, J.; et al. Cross-priming amplification for rapid detection of Mycobacterium tuberculosis in sputum specimens. J. Clin. Microbiol. 2009, 47, 845–847. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.D.; Lee, S.B.; Taylor, J.W.; Innis, M.A.; Gelfand, D.H.; Sninsky, J. PCR Protoc. A Guid. to Methods and Applications. In Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics; Academic Press Inc.: London, UK, 1990; pp. 315–322. [Google Scholar]
- Yu, J.M.; Cafarov, I.H.; Babadoost, M. Morphology, molecular identity, and pathogenicity of Verticillium dahliae and V. longisporum asssociated with internally discolored horseradish roots. Plant Dis. 2016, 100, 749–757. [Google Scholar] [CrossRef]
- Özer, G.; Bayraktar, H. First report of Verticillium dahliae causing verticillium wilt on Goji berry in Turkey. J. Plant Pathol. 2016, 98, 682. [Google Scholar]
- Wurzbacher, C.; Larsson, E.; Bengtsson-Palme, J.; Van den Wyngaert, S.; Svantesson, S.; Kristiansson, E.; Kagami, M.; Nilsson, R.H. Introducing ribosomal tandem repeat barcoding for fungi. Mol. Ecol. Resour. 2019, 19, 118–127. [Google Scholar] [CrossRef]
- Lievens, B.; Brouwer, M.; Vanachter, A.C.R.C.; Lévesque, C.A.; Cammue, B.P.A.; Thomma, B.P.H.J. Design and development of a DNA array for rapid detection and identification of multiple tomato vascular wilt pathogens. FEMS Microbiol. Lett. 2003, 223, 113–122. [Google Scholar] [CrossRef]
- Knüfer, J.; Lopisso, D.T.; Koopmann, B.; Karlovsky, P.; von Tiedemann, A. Assessment of latent infection with Verticillium longisporum in field-grown oilseed rape by qPCR. Eur. J. Plant Pathol. 2017, 147, 819–831. [Google Scholar] [CrossRef]
- Bilodeau, G.J.; Koike, S.T.; Uribe, P.; Martin, F.N. Development of an assay for rapid detection and quantification of Verticillium dahliae in soil. Phytopathology 2012, 102, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Suarez, M.B.; Walsh, K.; Boonham, N.; O’Neill, T.M.; Pearson, S.; Barker, I. Development of real-time PCR (TaqMan®) assays for the detection and quantification of Botrytis cinerea in planta. Plant Physiol. Biochem. 2005, 43, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Debode, J.; van Hemelrijck, W.; Baeyen, S.; Creemers, P.; Heungens, K.; Maes, M. Quantitative detection and monitoring of Colletotrichum acutatum in strawberry leaves using real-time PCR. Plant Pathol. 2009, 58, 504–514. [Google Scholar] [CrossRef]
- Pertile, G.; Panek, J.; Oszust, K.; Siczek, A.; Frąc, M. Intraspecific functional and genetic diversity of Petriella setifera. PeerJ 2018, 6, e4420. [Google Scholar] [CrossRef]
- Issakainen, J.; Jalava, J.; Hyvönen, J.; Sahlberg, N.; Pirness, T.; Campbell, C.K. Relationships of Scopulariopsis based on LSU rDNA sequences. Med. Mycol. 2003, 41, 31–42. [Google Scholar] [CrossRef]
- Brown, S.P.; Rigdon-Huss, A.R.; Jumpponen, A. Analyses of ITS and LSU gene regions provide congruent results on fungal community responses. Fungal Ecol. 2014, 9, 65–68. [Google Scholar] [CrossRef]
- Rozynek, P.; Gilges, S.; Brüning, T.; Wilhelm, M. Quality test of the microseq D2 LSU fungal sequencing kit for the identification of fungi. Int. J. Hyg. Environ. Health 2004, 207, 297–299. [Google Scholar] [CrossRef]
- Tamura, K.; Nei, M. Estimation of the Number of Nucleotide Substitutions in the Control Region of Mitochondrial DNA in Humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Malarczyk, D.; Panek, J.; Frąc, M. Alternative molecular-based diagnostic methods of plant pathogenic fungi affecting berry crops—A review. Molecules 2019, 24, 1200. [Google Scholar] [CrossRef] [PubMed]
- Frąc, M.; Jezierska-Tys, S.; Yaguchi, T. Occurrence, detection, and molecular and metabolic characterization of heat-resistant fungi in soils and plants and their risk to human health. Adv. Agron. 2015, 132, 161–204. [Google Scholar]
- Feng, C.; Mansouri, S.; Bluhm, B.H.; du Toit, L.J.; Correll, J.C. Multiplex real-time PCR assays for detection of four seedborne spinach pathogens. J. Appl. Microbiol. 2014, 117, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Maurer, K.A.; Radišek, S.; Berg, G.; Seefelder, S. Real-time PCR assay to detect Verticillium albo-atrum and V. dahliae in hops: Development and comparison with a standard PCR method. J. Plant Dis. Prot. 2013, 120, 105–114. [Google Scholar] [CrossRef]
- Lievens, B.; Brouwer, M.; Vanachter, A.C.R.C.; Cammue, B.P.A.; Thomma, B.P.H.J. Real-time PCR for detection and quantification of fungal and oomycete tomato pathogens in plant and soil samples. Plant Sci. 2006, 171, 155–165. [Google Scholar] [CrossRef]
- Ceccherini, M.T.; Luchi, N.; Pantani, O.L.; Ascher, J.; Capretti, P.; Pietramellara, G. Upward movement of Verticillium dahliae from soil to olive plants detected by qPCR. World J. Microbiol. Biotechnol. 2013, 29, 1961–1967. [Google Scholar] [CrossRef]
- Ozyilmaz, U.; Benlioglu, K.; Yildiz, A.; Benlioglu, H.S. Effects of soil amendments combined with solarization on the soil microbial community in strawberry cultivation using quantitative real-time PCR. Phytoparasitica 2016, 44, 661–680. [Google Scholar] [CrossRef]
- Tzelepis, G.; Bejai, S.; Sattar, M.N.; Schwelm, A.; Ilbäck, J.; Fogelqvist, J.; Dixelius, C. Detection of Verticillium species in Swedish soils using real-time PCR. Arch. Microbiol. 2017, 199, 1383–1389. [Google Scholar] [CrossRef]
- Pasche, J.S.; Mallik, I.; Anderson, N.R.; Gudmestad, N.C. Development and validation of a real-time PCR assay for the quantification of Verticillium dahliae in potato. Plant Dis. 2013, 97, 608–618. [Google Scholar] [CrossRef]
- Atallah, Z.K.; Bae, J.; Jansky, S.H.; Rouse, D.I.; Stevenson, W.R. Multiplex Real-Time Quantitative PCR to Detect and Quantify Verticillium dahliae Colonization in Potato Lines that Differ in Response to Verticillium Wilt. Phytopathology 2007, 97, 865–872. [Google Scholar] [CrossRef]
- Garrido, C.; Carbú, M.; Fernández-Acero, F.J.; Boonham, N.; Colyer, A.; Cantoral, J.M.; Budge, G. Development of protocols for detection of Colletotrichum acutatum and monitoring of strawberry anthracnose using real-time PCR. Plant Pathol. 2009, 58, 43–51. [Google Scholar] [CrossRef]
- Schena, L.; Abdelfattah, A.; Mosca, S.; Li Destri Nicosia, M.G.; Agosteo, G.E.; Cacciola, S.O. Quantitative detection of Colletotrichum godetiae and C. acutatum sensu stricto in the phyllosphere and carposphere of olive during four phenological phases. Eur. J. Plant Pathol. 2017, 149, 337–347. [Google Scholar] [CrossRef]
- Yang, H.-C.; Haudenshield, J.S.; Hartman, G.L. Multiplex Real-time PCR Detection and Differentiation of Colletotrichum Species Infecting Soybean. Plant Dis. 2015, 99, 1559–1568. [Google Scholar] [CrossRef] [PubMed]
- Yaguchi, T.; Imanishi, Y.; Matzuzawa, T.; Hosoya, K.; Hitomi, J.; Nakayama, M. Method for Identifying Heat-Resistant Fungi of the Genus Neosartorya. J. Food Prot. 2012, 75, 1806–1813. [Google Scholar] [CrossRef] [PubMed]
- Pertile, G.; Frąc, M.; Fornal, E.; Oszust, K.; Gryta, A.; Yaguchi, T. Molecular and metabolic strategies for postharvest detection of heat-resistant fungus Neosartorya fisheri and its discrimination from Aspergillus fumigatus. Postharvest Biol. Technol. 2020, 161, 111082. [Google Scholar] [CrossRef]
- Frac, M.; Oszust, K.; Lipiec, J.; Jezierska-Tys, S.; Nwaichi, E.O. Soil microbial functional and fungal diversity as influenced by municipal sewage sludge accumulation. Int. J. Environ. Res. Public. Health 2014, 11, 8891–8908. [Google Scholar] [CrossRef]
- Altshul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: A multiple sequence alignment method with reduced time and space complexity. BMC Bioinform. 2004, 5, 113. [Google Scholar] [CrossRef]
- Zhang, Z.; Schwartz, S.; Wagner, L.; Miller, W. A greedy algorithm for aligning DNA sequences. J. Comput. Biol. 2000, 7, 203–214. [Google Scholar] [CrossRef]
- Benson, D.A.; Cavanaugh, M.; Clark, K.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Sayers, E.W. GenBank. Nucleic Acids Res. 2013, 41, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3-new capabilities and interfaces. Nucleic Acids Res. 2012, 40, 1–12. [Google Scholar] [CrossRef] [PubMed]

| Primer or Probe Name | Sequence | Product Size (bp) | Excitation/Fluorescence Reading (nm) |
|---|---|---|---|
| D2LSU_F | 5′-AGACCGATAGCGmACAAG-3′ | N/A | N/A |
| D2LSU_R | 5′-CTTGGTCCGTGTTTCAAG-3′ | N/A | N/A |
| Botr-6FAM | 5′-6FAM-TCAGGGTCTCGTACCCTGTGTACTT-BHQ1-3′ | 322 | 495/520 |
| Coll-Tamra | 5′-Tamra(R)-TGTGACCAGACTTGCGTCCGGTGAA-BHQ2-3′ | 321 | 544/576 |
| Vert-Cy5 | 5′-Cy5-TGGTTCAACCAGGTCCATGACCT-BHQ2-3′ | 320 | 643/667 |
| Conc. of Fluorogenic Probes | Botrytis spp. (G277/18) | Colletotrichum spp. (G171/18) | Verticillium spp. (G296/18) | |||
| Cqmean | SD | Cqmean | SD | Cqmean | SD | |
| 0.1 µM | 14.343 | 0.268 | 18.925 | 0.482 | 17.749 | 0.512 |
| 0.15 µM | 14.084 | 0.377 | 19.286 | 0.152 | 18.235 | 0.417 |
| 0.2 µM | 12.792 | 0.895 | 18.576 | 0.329 | 16.942 | 0.440 |
| 0.25 µM | 13.930 | 0.615 | 18.921 | 0.733 | 16.838 | 0.590 |
| 0.3 µM | 14.093 | 0.533 | 18.814 | 0.185 | 20.576 | 5.697 |
| 0.35 µM | 13.569 | 0.455 | 18.832 | 0.431 | 14.898 | – |
| Conc. of Primers | Cqmean | SD | Cqmean | SD | Cqmean | SD |
| 0.1 µM | 21.421 | 4.103 | – | – | – | – |
| 0.2 µM | 15.451 | 0.095 | 18.915 | 0.467 | 17.459 | 1.398 |
| 0.3 µM | 16.478 | 0.172 | 18.777 | 0.142 | 16.565 | 0.146 |
| 0.4 µM | 15.178 | 0.090 | 19.067 | 0.104 | 17.654 | 0.487 |
| 0.5 µM | 15.315 | 0.321 | 18.740 | 0.164 | 16.872 | 0.846 |
| 0.6 µM | 15.389 | 0.215 | 18.633 | 0.040 | 15.994 | 0.909 |
| 0.7 µM | 15.795 | 0.205 | 18.821 | 0.264 | 19.326 | – |
| 0.8 µM | 14.963 | 0.364 | 18.718 | 0.233 | 21.984 | 10.268 |
| DNA Conc. | Botrytis spp. (G277/18) | Colletotrichum spp. (G171/18) | Verticillium spp. (G296/18) | |||
|---|---|---|---|---|---|---|
| Cqmean | SD | Cqmean | SD | Cqmean | SD | |
| 5 pg/µL | 28.222 | 1.659 | 32.144 | 1.106 | 30.659 | 0.902 |
| 2.5 pg/µL | 28.076 | 0.543 | 31.955 | 0.155 | 30.888 | 0.196 |
| 1.25 pg/µL | 29.278 | 0.691 | 33.200 | 0.859 | 31.969 | 0.817 |
| 625 fg/µL | 30.668 | 0.852 | 34.470 | 1.659 | 32.628 | 0.056 |
| 312 fg/µL | 31.600 | 0.950 | 35.028 | 1.072 | 34.037 | 0.938 |
| 156 fg/µL | 32.841 | – | 36.699 | 0.731 | 36.391 | 0.223 |
| 78 fg/µL | 33.750 | 2.542 | 34.012 | – | 35.219 | 1.524 |
| 39 fg/µL | 33.276 | 0.286 | – | – | 36.267 | 0.610 |
| Time of Incubation | Spores per 1 g of Soil | Botrytis spp. (G277/18) | Colletotrichum spp. (G171/18) | Verticillium spp. (G296/18) |
| 24 h | 500 | 25.513 | – | 27.732 |
| 1000 | 24.980 | – | 26.673 | |
| 5000 | 24.278 | 24.936 | 24.306 | |
| 10,000 | 28.166 | – | 22.491 | |
| 48 h | 500 | 24.731 | – | 27.004 |
| 1000 | 24.642 | – | 26.356 | |
| 5000 | 24.790 | – | 24.479 | |
| 10,000 | 25.684 | 24.320 | 23.261 | |
| Time of Incubation | Spores per 1 g of Strawberry | Botrytis spp. (G277/18) | ||
| 0 h | 100 | 21.672 | ||
| 1000 | 19.289 | |||
| 10,000 | 20.106 | |||
| 100,000 | 19.242 | |||
| 72 h | 100 | 15.980 | ||
| 1000 | 19.810 | |||
| 10,000 | 21.032 | |||
| 100,000 | 19.597 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malarczyk, D.G.; Panek, J.; Frąc, M. Triplex Real-Time PCR Approach for the Detection of Crucial Fungal Berry Pathogens—Botrytis spp., Colletotrichum spp. and Verticillium spp. Int. J. Mol. Sci. 2020, 21, 8469. https://doi.org/10.3390/ijms21228469
Malarczyk DG, Panek J, Frąc M. Triplex Real-Time PCR Approach for the Detection of Crucial Fungal Berry Pathogens—Botrytis spp., Colletotrichum spp. and Verticillium spp. International Journal of Molecular Sciences. 2020; 21(22):8469. https://doi.org/10.3390/ijms21228469
Chicago/Turabian StyleMalarczyk, Dominika G., Jacek Panek, and Magdalena Frąc. 2020. "Triplex Real-Time PCR Approach for the Detection of Crucial Fungal Berry Pathogens—Botrytis spp., Colletotrichum spp. and Verticillium spp." International Journal of Molecular Sciences 21, no. 22: 8469. https://doi.org/10.3390/ijms21228469
APA StyleMalarczyk, D. G., Panek, J., & Frąc, M. (2020). Triplex Real-Time PCR Approach for the Detection of Crucial Fungal Berry Pathogens—Botrytis spp., Colletotrichum spp. and Verticillium spp. International Journal of Molecular Sciences, 21(22), 8469. https://doi.org/10.3390/ijms21228469






