Seed Priming: A Feasible Strategy to Enhance Drought Tolerance in Crop Plants
Abstract
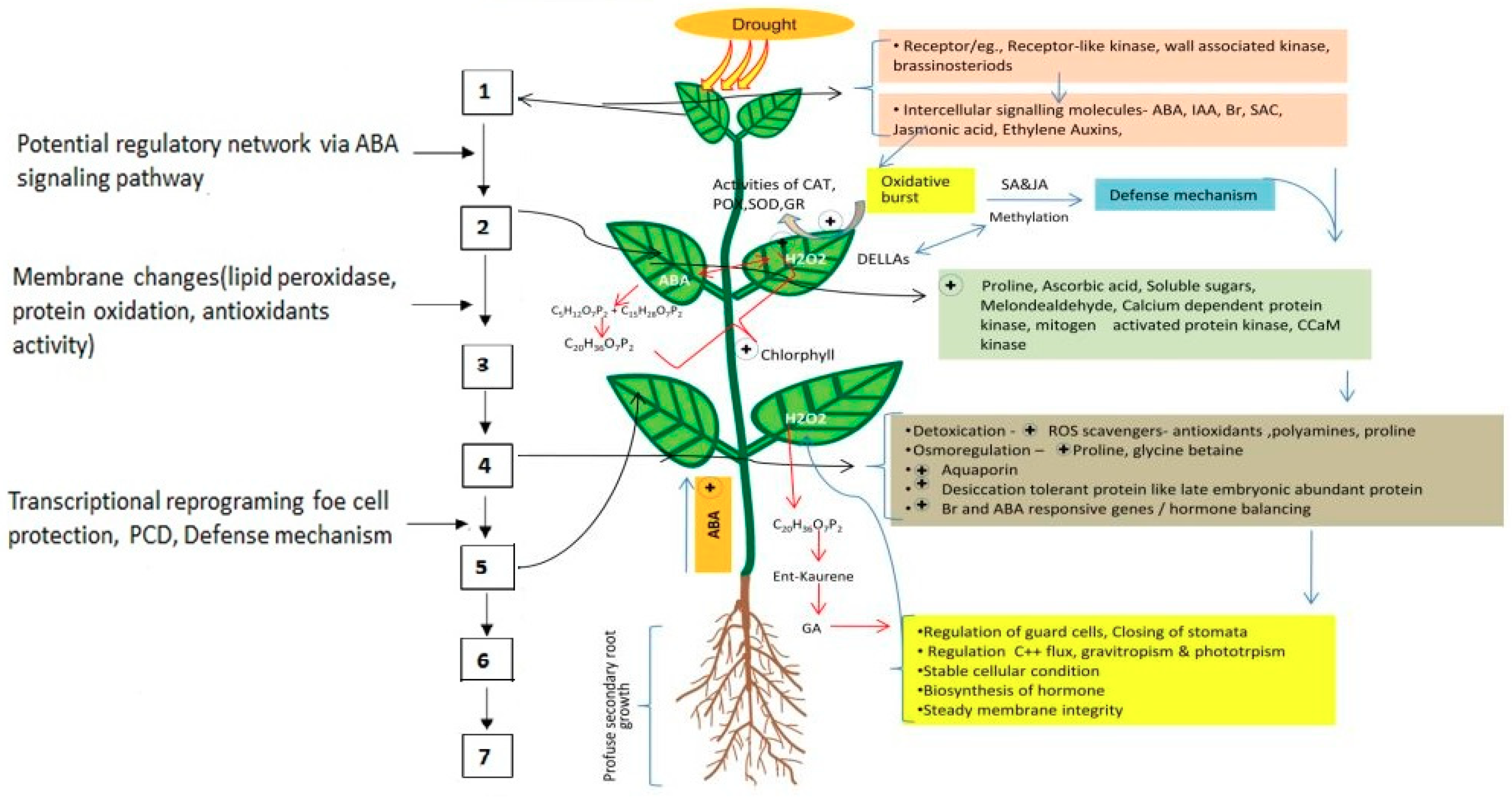
:1. Introduction
2. Plant Responses to Drought Stress
2.1. Morphological Response
2.2. Physiological Response
2.3. Biochemical Changes
2.4. Molecular Changes
2.5. Metabolomic Changes
3. Seed Priming
3.1. Hydropriming
3.2. Osmopriming
3.3. Chemical Priming
3.4. Biological Priming
3.5. Hormonal Priming
3.6. Solid Matrix Priming
3.7. Nutripriming
4. Mechanisms of Seed-Priming-Induced Drought Tolerance in Crop Plants
5. Research Gap and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lobell, D.B.; Schlenker, W.; Costa-Roberts, J. Climate trends and global crop production since 1980. J. Sci. 2011, 333, 616–620. [Google Scholar] [CrossRef] [Green Version]
- Trenberth, K.; Dai, A.; van der Schrier, G. Global warming and changes in drought. Nat. Clim. Chang. 2014, 4, 17–22. [Google Scholar] [CrossRef]
- FAO. 2018. Available online: http://www.faonews/story/en/item/1106977/icode/ (accessed on 20 May 2020).
- Kasim, W.A.; Osman, M.E.; Omar, M.N.; El-Daim, I.A.A.; Bejai, S.; Meijer, J. Control of drought stress in wheat using plant growth promoting bacteria. J. Plant Growth Regul. 2013, 32, 122–130. [Google Scholar] [CrossRef]
- Kaya, M.D.; Okcub, G.; Ataka, M.; Cikilic, Y.; Kolsaricia, O. Seed treatments to overcome salt and drought stress during germination in sunflower (Helianthus annuus L.). Eur. J. Agron. 2006, 24, 291–295. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop Production under Drought and Heat Stress: Plant Responses and Management Options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bareke, T. Biology of seed development and germination physiology. Adv. Plants Agric. Res. 2018, 8, 336–346. [Google Scholar] [CrossRef]
- Okcu, G.; Kaya, M.D.; Atak, M. Effects of salt and drought stresses on germination and seedling growth of pea (Pisum sativum L.). Turk. J. Agric. For. 2005, 29, 237–242. [Google Scholar]
- Wu, L.M.; Fang, Y.; Yang, H.N.; Bai, L.Y. Effects of drought-stress on seed germination and growth physiology of quinclorac-resistant Echinochloa crusgalli. PLoS ONE 2019, 14, e0214480. [Google Scholar] [CrossRef]
- Islam, M.M.E.; Kayesh, E.; Zaman, T.A.; Urmi, M.M. Haque Evaluation of Rice (Oryza sativa L.) Genotypes for Drought Tolerance at Germination and Early Seedling Stage. Agriculturists 2018, 16, 44–54. [Google Scholar] [CrossRef] [Green Version]
- Humera, R.M.; Hammad, N.D.; Hafeez, A.S.; Bushra, S. Screening of sunflower (Helianthus annus L.) accessions under drought stress conditions, an experimental assay. J. Soil Sci. Plant Nutr. 2017, 17, 662–671. [Google Scholar] [CrossRef]
- Barnabas, B.; Jager, K.; Feher, A. The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ. 2008, 31, 11–38. [Google Scholar] [CrossRef] [PubMed]
- Abdelraheem, A.; Esmaeili, N.; Connell, M.O.; Zhang, J. Progress and perspective on drought and salt stress tolerance in cotton. Ind. Crop. Prod. 2019, 130, 118–129. [Google Scholar] [CrossRef]
- Hussain, M.; Malik, M.A.; Farooq, M.; Ashraf, M.Y.; Cheema, M.A. Improving Drought tolerance by exogenous application of glycine betaine and salicylic acid in sunflower. J. Agron. Crop Sci. 2008, 194, 193–199. [Google Scholar] [CrossRef]
- Nair, A.S.; Abraham, T.K.; Jaya, D.S. Studies on the changes in lipid peroxidation and antioxidants in drought stress induced Cowpea (Vigna unguiculata L.) varieties. J. Environ. Biol. 2008, 29, 689–691. [Google Scholar]
- Pallavi, S.; Ambuj, B.J.; Rama, S.D.; Mohammad, P. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Basu, S.; Ramegowda, V.; Kumar, A.; Pereira, A. Plant adaptation to drought stress. Res. Rev. 2016, 5, 1554. [Google Scholar] [CrossRef]
- Muhammad, A.; Shafaqat, A.; Lei, K.Q.; Rizwan, Z.; Zhongwei, T.; Dong, J.; Jhon, L.S.; Tingbo, D. Physiological and biochemical changes during drought and recovery periods at tillering and jointing stages in wheat (Triticum aestivum L.). Sci. Rep. 2018, 8, 4615. [Google Scholar] [CrossRef]
- Tani, E.; Chronopoulou, E.G.; Labrou, N.E.; Sarri, E.; Goufa, Μ.; Vaharidi, X.; Tornesaki, A.; Psychogiou, M.; Bebeli, P.J.; Abraham, E.M. Growth, Physiological, Biochemical, and Transcriptional Responses to Drought Stress in Seedlings of Medicago sativa L., Medicago arborea L. and Their Hybrid (Alborea). Agronomy 2019, 9, 38. [Google Scholar] [CrossRef] [Green Version]
- Demirevska, K.; Zasheva, D.; Dimitrov, R.; Simova-Stoilova, L.; Stamenova, M.; Feller, U. Drought stress effects on Rubisco in wheat: Changes in the Rubisco large subunit. Acta Physiol. Plant. 2009, 31, 1129–1138. [Google Scholar] [CrossRef]
- Venkateswarlu, B.; Shanker, A. Climate change and agriculture: Adaptation and mitigation stategies. Indian J. Agron. 2009, 54, 226–230. [Google Scholar]
- Vurukonda, S.S.K.P.; Vardharajula, S.; Shrivastava, M.; Ali, S.Z. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res. 2016, 184, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Anwar, S.; Yu, S.; Sun, M.; Yang, Z.; Gao, Z.Q. Development of Drought-Tolerant Transgenic Wheat: Achievements and Limitations. Int. J. Mol. Sci. 2019, 20, 3350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chanjuan, L. Genetically Modified Crops with Drought Tolerance: Achievements, Challenges, and Perspectives; Springer: Geneva, Switzerland, 2016; pp. 531–547. [Google Scholar] [CrossRef]
- Ferrante, A.; Mariani, L. Agronomic Management for Enhancing Plant Tolerance to Abiotic Stresses: High and Low Values of Temperature, Light Intensity, and Relative Humidity. Horticulturae 2018, 4, 21. [Google Scholar] [CrossRef] [Green Version]
- Hussain, M.M.; Farooq, D.J. Evaluating the role of seed priming in improving drought tolerance of pigmented and non-pigmented rice. J. Agron. Lee Crop Sci. 2017, 203, 269–276. [Google Scholar] [CrossRef]
- Brocklehurst, P.A.; Dearman, J. Interaction between seed priming treatments and nine seed lots of carrot, celery and onion II. Seedling emergence and plant growth. Ann. Appl. Biol. 2008, 102, 583–593. [Google Scholar] [CrossRef]
- Hussain, S.; Khan, F.; Hussain, H.A.; Nie, L. Physiological and biochemical mechanisms of seed priming-Induced chilling tolerance in rice cultivars. Front. Plant Sci. 2016, 7, 116. [Google Scholar] [CrossRef] [Green Version]
- Farooq, M.A.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef] [Green Version]
- Jisha, K.C.; Puthur, J.T. Seed priming with BABA (β-amino butyric acid) a cost-effective method of abiotic stress tolerance in Vigna radiata (L.) Wilczek. Protoplasma 2016, 253, 277. [Google Scholar] [CrossRef] [PubMed]
- Samota, M.K.; Sasi, M.; Awana, M.; Yadav, O.P.; Amitha, S.V.; Tyagi, A.; Kumar, S.; Singh, A. Elicitor-Induced Biochemical and Molecular Manifestations to Improve Drought Tolerance in Rice (Oryzasativa, L.) through Seed-Priming. Front. Plant Sci. 2017, 8, 934. [Google Scholar] [CrossRef] [Green Version]
- Kavitha Mary, J.P.; Marimuthu, K.; Sivakumar, U. Seed priming effect of arbuscular mycorrhizal fungi against induced drought in rice. J. Pharmacogn. Phytochem. 2018, 7, 1742–1746. Available online: https://www.phytojournal.com/archives/2018/vol7issue2/PartY/7-2-113-703.pdf (accessed on 27 June 2020).
- Tabassum, T.; Farooq, M.; Ahmad, R.; Zohaib, A.; Wahid, A.; Shahid, M. Terminal drought and seed priming improves drought tolerance in wheat. Physiol. Mol. Biol. Plants 2018, 24, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, N.; Alizadeh, O.; Sharaf Zadeh, S.; Aref, F.; Ordookhani, K. Evaluation of Auxin priming and plant growth promoting Rhizobacteria on yield and yield components of wheat under drought stress. Eurasia J. Biosci. 2019, 13, 711–716. [Google Scholar]
- Ali, M.O.; Sarkar, A.; Rahman, M.M.; Gahoonia, T.S. Improvement of lentil yield through seed priming in Bangladesh. J. Lentil Res. 2005, 2, 54–59. [Google Scholar]
- Sajjan, A.; Dhanelappagol, M.S.; Jolli, R.B. Seed quality enhancement through seed priming in pigeonpea [Cajanus cajan (L.) Millsp.]. Leg Res. 2017, 40, 173–177. [Google Scholar]
- Bhowmick, M.; Duary, B.; Biswas, P.K.; Rakshit, A.; Adhikari, B. Seed Priming, Row Spacing and Foliar Nutrition in Relation to Growth and Yield of Chickpea under Rainfed Condition Introduction. SATSA Mukhapatra Annu. Tech. Issue 2013, 17, 114–119. [Google Scholar]
- Shariatmadari, M.H.; Parsa, M.; Nezami, A.; Kafi, M. The effects of hormonal priming on emergence, growth and yield of chickpea under drought stress in glasshouse and field. Biosci. Res. 2017, 14, 34–41. Available online: https://www.isisnBR-14-2017/34-41-14(1)2017BR-1404.pdf (accessed on 15 January 2020).
- Kumeera, B.; Swapnil, M.; Chaurasia, A.K.; Ramteke, P.W. Effect of seed priming with inorganics on growth, yield and physiological parameters of chickpea (Cicer arietinum L.) under drought. Pharma Innov. J. 2018, 7, 411–414. [Google Scholar]
- Farooq, M.; Ullah, A.; Lee, D.J.; Alghamdi, S.S. Terminal drought-priming improves the drought tolerance in desi and kabulichickpea. Int. J. Agric. Biol. 2018, 30, 1129–1136. [Google Scholar]
- Shankrayya, R.G.; Teggelli, M.P. Studies on Climate Smart Intervention on Induction of Drought Tolerance by Seed Priming with CaCl2 in Chickpea Growth, Yield and Quality Parameters. Int. J. Curr. Microbiol. App. Sci. 2018, 7, 3510–3514. [Google Scholar] [CrossRef]
- Puthiyottil, P. Priming of Abelmoschus esculentus (L.) Moench (okra) seeds with liquid phosphobacterium: An approach to mitigate drought stress. Trop. Plant Res. 2015, 2, 276–281. [Google Scholar]
- Yan, M. Seed priming stimulate germination and early seedling growth of Chinese cabbage under drought stress. S. Afr. J. Bot. 2015, 99, 88–92. [Google Scholar] [CrossRef]
- Moghanibashi, M.; Karimmojeni, H.; Nikneshan, P. Seed treatment to overcome drought and salt stress during germination of sunflower (Helianthus annuus L.). J. Agrobiol. 2013, 30, 89–96. [Google Scholar]
- Castanares, L.; Bouzo, C. Effect of different priming treatments and priming durations on melon germination behaviour under suboptimal conditions. Open Agric. 2018, 3, 386–392. [Google Scholar] [CrossRef]
- Iseri, O.D.; Sahin, F.; Hberal, M. Sodium chloride priming improves salinity responses of tomato at seedling stage. J. Plant Nutr. 2014, 37, 374–392. [Google Scholar] [CrossRef]
- Padgham, J. Agricultural Development under a Changing Climate: Opportunities and Challenges for Adaptation; Agriculture and Rural Development & Environmental Departments, The World Bank: Washington, DC, USA, 2009. [Google Scholar]
- Huber, A.E.; Bauerle, T.L. Long-distance plant signaling pathways in response multiple stressors: The gap in knowledge. J. Exp. Bot. 2016, 67, 2063–2079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, Y.; Du, Y.; Wang, J.; Wu, A.; Qiao, S.; Xu, B.; Zhang, S.; Siddique, K.H.M.; Chen, Y. Moderate drought stress affected root growth and grain yield in old, modern and newly released cultivars of winter wheat. Front. Plant Sci. 2017, 8, 672. [Google Scholar] [CrossRef] [Green Version]
- Kooyers, N.J. The evolution of drought escape and avoidance in natural herbaceous populations. Plant Sci. 2015, 234, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Manavalan, L.P.; Guttikonda, S.K.; Tran, L.S.P.; Nguyen, H.T. Physiological and molecular approaches to improve drought resistance in soybean. Plant Cell Physiol. 2009, 50, 1260–1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Li, P.; Xu, G.C.; Xiao, L.; Ren, Z.P.; Li, Z.B. Growth, Morphological, and Physiological Responses to Drought Stress in Bothriochloa ischaemum. Front. Plant Sci. 2017, 8, 230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.B.; Suh, M.C. Recent advances in cuticular wax biosynthesis and its regulation in Arabidopsis. Mol. Plant 2013, 6, 246–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.S.; Kanwal, B.; Nazir, S. Metabolic engineering of the chloroplast genome reveals that the yeast ArDH gene confers enhanced tolerance to salinity and drought in plants. Front. Plant Sci. 2015, 6, 725. [Google Scholar] [CrossRef] [Green Version]
- Blum, A. Osmotic adjustment is a prime drought stress adaptive engine in support of plant production: Osmotic adjustment and plant production. Plant Cell Environ. 2017, 40, 4–10. [Google Scholar] [CrossRef]
- Liu, H.Y.; Yu, X.; Cui, D.Y.; Sun, M.H.; Sun, W.N.; Tang, Z.C. The role of water channel proteins and nitric oxide signaling in rice seed germination. Cell Res. 2007, 17, 638–649. [Google Scholar] [CrossRef]
- Anket, S.; Babar, S.; Vinod, K.; Sukhmeen, K.K.; Gagan, P.S.S.; Aditi, S.B.; Neha, H.; Dhriti, K.; Renu, B.; Bingsong, Z. Phytohormones Regulate Accumulation of Osmolytes under Abiotic Stress. Biomolecules 2019, 9, 285. [Google Scholar] [CrossRef] [Green Version]
- Anjum, A.; Xiao-yu, X.; Long, C.W.; Muhammad, F.S.; Chen, M.; Wang, L. Morphological, physiological and biochemical responses of plants to drought stress Shakeel. Afr. J. Agric. Res. 2011, 6, 2026–2032. [Google Scholar] [CrossRef]
- Kudoyarova, G.R.; Dodd, I.C.; Veselov, D.S.; Rothwell, S.A.; Veselov, S.Y. Common and specific responses to availability of mineral nutrients and water. J. Exp. Bot. 2015, 66–68, 2133–2144. [Google Scholar] [CrossRef] [Green Version]
- Sims, L.; Pastor, J.; Lee, T.; Dewey, B. Nitrogen phosphorus and light effects on growth and allocation of biomass and nutrients in wild rice. Oecologia 2012, 170, 65–67. [Google Scholar] [CrossRef]
- Kwon, M.Y.; Woo, S.Y. Plants responses to drought and shade environments. Afr. J. Biotechnol. 2016, 15, 29–31. [Google Scholar] [CrossRef] [Green Version]
- Gargallo, G.A.; Sardans, J.; Perez, T. Opposite metabolic responses of shoots and roots to drought. Sci. Rep. 2015, 4, 6829. [Google Scholar] [CrossRef] [Green Version]
- Mangena, P. Effect of Hormonal Seed Priming on Germination, Growth, Yield and Biomass Allocation in Soybean Grown under Induced Drought Stress. Indian J. Agric. Sci. 2019, A-441. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Manivannan, P.; Wahid, A.; Farooq, M.; Al-Juburi, H.J.; Somasundaram, R.; Panneerselvam, R. Drought stress in plants: A review on morphological characteristics and pigments composition. Int. J. Agric. Biol. 2009, 11, 100–105. [Google Scholar]
- Mabulwana, P.T. Determination of Drought Stress Tolerance among Soybean Varieties Using Morphological and Physiological Markers. Master’s Thesis, University of Limpopo, Sovenga, South Africa, 2013. [Google Scholar]
- Baret, F.; Madec, S.; Irfan, K.; Lopez, J.; Comar, A.; Hemmerlé, M.; Dutartre, D.; Praud, S.; Tixier, M.H. Leaf-rolling in maize crops: From leaf scoring to canopy-level measurements for phenotyping. J. Exp. Bot. 2018, 69, 2705–2716. [Google Scholar] [CrossRef]
- Jenks, M.A.; Andersen, L.; Teusink, R.S.; Williams, M.H. Leaf cuticular waxes of potted rose cultivars as affected by plant development, drought and paclobutrazol treatments. Physiol. Plant. 2001, 112, 62–70. [Google Scholar] [CrossRef]
- Omprakash, R.; Gobu, P.B.; Murlimanohar, B.; Kumar, N.C. Resistance/Tolerance Mechanism under Water Deficit (Drought) Condition in Plants. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 66–78. [Google Scholar] [CrossRef]
- Khan, A.; Pan, X.; Najeeb, U.; Tan, D.; Fahad, S.; Zahoor, R.; Luo, H. Coping with drought: Stress and adaptive mechanisms, and management through cultural and molecular alternatives in cotton as vital constituents for plant stress resilience and fitness. Biol. Res. 2018, 51, 47. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Gopi, R.; Sankar, B.; Gomathinayagam, M.; Panneerselvam, R. Differential responses in water use efficiency in two varieties of Catharanthus roseus under drought stress. C. R. Biol. 2008, 1, 42–47. [Google Scholar] [CrossRef]
- Sun, X.K.; Fan, Z.P.; Wang, H.; Bai, J.; Zhang, Y.; Deng, D.Z. Photosynthetic characteristics and water use efficiency of three broad-leaved tree species in the horqin sandland. J. Arid Land Res. Environ. 2008, 10, 188–194. [Google Scholar]
- Blum, A.; Sullivan, C.Y. The Effect of Plant Size on Wheat Response to Agents of Drought Stress. I. Root Drying. Aust. J. Plant Physiol. 1997, 24, 35–41. [Google Scholar] [CrossRef]
- Xu, W.Z.; Xu, B.C.; Duan, D.P.; Niu, F.R. Study on the photosynthetic characteristics of Bothriochloa ischaemum under different water and nutrient conditions 1. Diurnal variation of photosynthesis. Acta Agrestia Sin. 2010, 5, 629–635. [Google Scholar]
- Ttrai, Z.A.; Sanoubar, R.; Pluhar, Z.; Mancarella, S.; Orsini, F.; Gianquinto, G. Morphological and Physiological Plant Responses to Drought Stress in Thymus citriodorus. Int. J. Agron. 2016, 2016, 4165750. [Google Scholar] [CrossRef] [Green Version]
- Rollins, J.A.; Habte, E.; Templer, S.E.; Colby, T.; Schmidt, J.; Von, K.M. Leaf proteome alterations in the context of physiological and morphological responses to drought and heat stress in barley (Hordeum vulgare L.). J. Exp. Bot. 2013, 64, 3201–3212. [Google Scholar] [CrossRef] [Green Version]
- Khosroshahi, M.Z.; Mahmoud, E.A.; Ahmad, E.; Ali, I. Morphological Changes in Response to Drought Stress in Cultivated and Wild Almond Species. Int. J. Hortic. Sci. Tech. 2014, 1, 79–92. [Google Scholar] [CrossRef]
- Sun, J.; Gu, J.; Zeng, J.; Han, S.; Song, A.; Chen, F.; Fang, W.; Jiang, J.; Chen, S. Changes in leaf morphology, antioxidant activity and photosynthesis capacity in two different drought-tolerant cultivars of chrysanthemum during and after water stress. Sci. Hortic. 2013, 161, 249–258. [Google Scholar] [CrossRef]
- Ying, Y.; Yue, Y.; Huang, X.; Wang, H.; Mei, L.; Yu, W.; Zheng, B.; Wu, J. Salicylic acid induces physiological and biochemical changes in three Red bayberry (Myric rubra) genotypes under water stress. Plant Growth Regul. 2013, 71, 181–189. [Google Scholar] [CrossRef]
- Rivas, R.; Falcao, H.M.; Ribeiro, R.V.; Machado, E.C.; Pimentel, C.; Santos, M.G. Drought tolerance in cowpea species is driven by less sensitivity of leaf gas exchange to water deficit and rapid recovery of photosynthesis after rehydration. S. Afr. J. Bot. 2016, 103, 101–107. [Google Scholar] [CrossRef]
- Chen, Y.E.; Zhang, C.M.; Su, Y.Q.; Ma, J.; Zhang, Z.W.; Yuan, M.; Zhang, H.Y.; Yuan, S. Responses of photosystem II and antioxidative systems to high light and high temperature co-stress in wheat. Environ. Exp. Bot. 2017, 135, 45–55. [Google Scholar] [CrossRef]
- Albert, K.; Mikkelsen, T.N.; Michelsen, A.; Ro-Poulsen, H.; Van Der Linden, L. Interactive effects of drought, elevated CO2 and warming on photosynthetic capacity and photosystem performance in temperate heath plants. J. Plant Physiol. 2011, 168, 1550–1561. [Google Scholar] [CrossRef]
- Tattini, M.; Velikova, V.; Vickers, C.; Brunetti, C.; Di Ferdinando, M.; Trivellini, A.; Fineschi, S.; Agati, G.; Ferrini, F.; Loreto, F. Isoprene production in transgenic tobacco alters isoprenoid, non-structural carbohydrate and phenylpropanoid metabolism, and protects photosynthesis from drought stress. Plant Cell Environ. 2014, 37, 1950–1964. [Google Scholar] [CrossRef] [Green Version]
- Rana, R.M.; Rehman, S.; Ahmed, J.; Bilal, M. A comprehensive overview of recent advances in drought stress tolerance research in wheat (Triticum aestivum L.). Asian J. Agric. Biol. 2013, 1, 29–37. [Google Scholar]
- Zhang, Q. Strategies for developing Green Super Rice. Proc. Natl. Acad. Sci. USA 2004, 104, 16402–16409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, A.; Kumar, V.; Shahzad, B.; Ramakrishnan, M.; Sidhu, G.P.S.; Bali, A.S.; Handa, N.; Kapoor, D.; Yadav, P.; Khanna, K.; et al. Photosynthetic Response of Plants under Different Abiotic Stresses: A Review. J. Plant Growth Regul. 2020, 39, 509–531. [Google Scholar] [CrossRef]
- Kim, Y.; Chung, Y.S.; Lee, E.; Tripathi, P.; Heo, S.; Kim, K.-H. Root response to drought stress in rice (Oryza Sativa L). Int. J. Mol. Sci. 2020, 21, 1513. [Google Scholar] [CrossRef] [Green Version]
- Roblero, M.D.J.M.; Joel, P.P.; Ma, T.C.L.; Jaime, S.C. Oxygen in the root zone and its effects on plants. Rev. Mex. Agric. 2020, 11, 4. [Google Scholar]
- Batra, N.G.; Sharma, V.; Kumari, N. Drought-induced changes in chlorophyll florescence, photosynthetic pigments and thylakoid membrane proteins of vigna radiata. J. Plant Interact. 2014, 9, 712–721. [Google Scholar] [CrossRef] [Green Version]
- Brestic, M.; Zivcak, M. PSII Fluorescence Techniques for Measurement of Drought and High Temperature Stress Signal in Crop Plants: Protocols and Applications. In Molecular Stress Physiology of Plants; Rout, G., Das, A., Eds.; Springer: New Delhi, India, 2013. [Google Scholar] [CrossRef]
- Rowe, J.H.; Topping, J.F.; Liu, J.; Lindsey, K. Abscisic acid regulates root growth under osmotic stress conditions via an interacting hormonal network with cytokinin, ethylene and auxin. New Phytol. 2016, 211, 225–239. [Google Scholar] [CrossRef] [Green Version]
- Ullah, A.; Manghwar, H.; Shaban, M.; Khan, A.H.; Akbar, A.; Ali, U.; Ali, E.; Fahad, S. Phytohormones enhanced drought tolerance in plants: A coping strategy. Environ. Sci. Pollut. Res. 2018, 25, 33103–33118. [Google Scholar] [CrossRef]
- Rivero, R.M.; Kojima, M.; Gepstein, A.; Sakakibara, H.; Mittler, R.; Gepstein, S.; Blumwald, E. Delayed leaf senescence induces extreme drought tolerance in a flowering plant. Proc. Natl. Acad. Sci. USA 2007, 104, 19631–19636. [Google Scholar] [CrossRef] [Green Version]
- Tarchoune, C.; Sgherri, R.; Izzo, M.; Lachaal, Z.; Ouerghi, F.; Navari, I. Antioxidative responses of Ocimum basilicum to sodium chloride or sodium sulphate salinization. Plant Physiol. Biochem. 2010, 48, 772–777. [Google Scholar] [CrossRef]
- Chen, J.; Yang, L.; Yan, X.; Liu, Y.; Wang, R.; Fan, T.; Ren, Y.; Tang, X.; Xiao, F.; Liu, Y.; et al. Zinc-Finger Transcription Factor ZAT6 Positively Regulates Cadmium Tolerance through the Glutathione-Dependent Pathway in Arabidopsis. Plant Physiol. 2016, 171, 707–719. [Google Scholar] [CrossRef]
- Daszkowska-Golec, A.; Szarejko, I. Open or close the gate—Stomata action under the control of phytohormones in drought stress conditions. Front. Plant Sci. 2013, 4, 138. [Google Scholar] [CrossRef] [Green Version]
- Brodribb, T.J.; McAdam, S.A. Evolution of the stomatal regulation of plant water content. Plant Physiol. 2017, 174, 639–649. [Google Scholar] [CrossRef] [Green Version]
- De, O.C.; Arbona, V.; Gomez, C. A Jasmonoyl isoleucine accumulation is needed for abscisic acid build-up in roots of Arabidopsis under water stress conditions. Plant Cell Environ. 2015, 38, 2157–2170. [Google Scholar] [CrossRef] [Green Version]
- Szabados, L.; Savoure, A. Proline: A Multifunctional Amino Acid. Trends Plant Sci. 2009, 15, 89–97. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Wang, B.; Hu, T.; Chen, H.; Li, H.; Zhang, W.; Zhong, Y.; Hu, H. Combined action of an antioxidant defence system and osmolytes on drought tolerance and post-drought recovery of Phoebe zhennan S. Lee saplings. Acta Physiol. Plant. 2015, 37, 84. [Google Scholar] [CrossRef]
- Kaur, G.; Asthir, B. Proline: A key player in plant abiotic stress tolerance. Biol. Plant. 2017, 59, 609–619. [Google Scholar] [CrossRef]
- Giri, J. Glycinebetaine and abiotic stress tolerance in plants. Plant Signal. Behav. 2011, 6, 1746–1751. [Google Scholar] [CrossRef]
- Nakashima, K.; Yamaguchi-Shinozaki, K.; Shinozaki, K. The Molecular responses to drought stress transcriptional regulatory network in the drought response and its crosstalk in abiotic stress responses including drought, cold, and heat. Front. Plant Sci. 2014, 5, 170. [Google Scholar] [CrossRef] [Green Version]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.S. Response of plants to water stress. Front. Plant Sci. 2014, 5, 86. [Google Scholar] [CrossRef] [Green Version]
- Nishiyama, R.; Watanabe, Y.; Leyva-Gonzalez, M.A.; Van Ha, C.; Fujita, Y.; Tanaka, M.; Seki, M.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Herrera-Estrella, L.; et al. Arabidopsis AHP2, AHP3, and AHP5 histidine phosphotransfer proteins function as redundant negative regulators of drought stress response. Proc. Natl. Acad. Sci. USA 2013, 110, 4840–4845. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Lee, K.; Hwang, H.; Hwang, H.; Bhatnagar, N.; Kim, d.; Yoon, I.; Byun, M.; Kim, S.; Jung, K.; et al. Over expression of PYL5 in rice enhances drought tolerance, inhibits growth, and modulates gene expression. J. Exp. Bot. 2014, 65, 453–464. [Google Scholar] [CrossRef] [Green Version]
- Maqbool, M.A.; Aslam, M.; Ali, H. Breeding for improved drought tolerance in Chickpea (Cicer arietinum L.). Plant Breed. 2017, 136, 300–318. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi-Shinozaki, K.; Shinozaki, K. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu. Rev. Plant Biol. 2006, 57, 781–803. [Google Scholar] [CrossRef] [Green Version]
- Luchi, M.; Kobayashi, T.; Taji, M.; Naramoto, M.; Seki, T.; Kato, S.; Tabata, Y.; Kakubari, K.; Yamaguchi-Shinozaki, K. Shinozaki. Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. Plant J. 2001, 27, 325–333. [Google Scholar]
- Todaka, D.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Recent advances in the dissection of drought-stress regulatory networks and strategies for development of drought-tolerant transgenic rice plants. Front. Plant Sci. 2015, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.Q.; Meng, X.P.; Zhang, Y.; Xia, M.; Wang, X.P. Over-expression of OsDREB genes lead to enhanced drought tolerance in rice. Biotechnol. Lett. 2008, 30, 2191–2198. [Google Scholar] [CrossRef]
- Cui, M.; Zhang, W.; Zhang, Q.; Xu, Z.; Zhu, Z.; Duan, F.; Wu, R. Induced over-expression of the transcription factor OsDREB2A improves drought tolerance in rice. Plant Physiol. Biochem. 2011, 49, 1384–1391. [Google Scholar] [CrossRef] [PubMed]
- Lenka, S.K.; Katiyar, A.; Chinnusamy, V.; Bansal, K.C. Comparative analysis of drought-responsive transcriptome in Indica rice genotypes with contrasting drought tolerance. Plant Biotechnol. J. 2011, 9, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Grelet, J.; Benamar, A.; Teyssier, E.; Avelange Macherel, M.H.; Grunwald, D.; Macherel, D. Identification in pea seed mitochondria of a late-embryogenesis abundant protein able to protect enzymes from drying. Plant Physiol. 2005, 137, 157–167. [Google Scholar] [CrossRef] [Green Version]
- Hanin, M.; Brini, F.; Ebel, C.; Toda, Y.; Takeda, S.; Masmoudi, K. Plant dehydrins and stress tolerance: Versatile proteins for complex mechanisms. Plant Signal. Behav. 2011, 6, 1503–1509. [Google Scholar] [CrossRef]
- Xu, D.; Duan, X.; Wang, B.; Hong, B.; Ho, T.; Wu, R. Expression of a late embryogenesis abundant protein gene, hva1, from barley confers tolerance to water deficit and salt stress in transgenic rice. Plant Physiol. 1996, 110, 249–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, B.; Huang, Y.; Tang, N.; Xiong, L. Overexpression of a LEA gene in rice improves drought resistance under the field conditions. Appl. Genet. 2007, 115, 35–46. [Google Scholar] [CrossRef]
- Duan, J.; Cai, W. OsLEA3-2, an Abiotic Stress Induced Gene of Rice Plays a Key Role in Salt and Drought Tolerance. PLoS ONE 2012, 7, e45117. [Google Scholar] [CrossRef] [Green Version]
- Shriram, V.; Kumar, V.; Devarumath, R.M.; Khare, T.S.; Wani, S.H. MicroRNAs As Potential Targets for Abiotic Stress Tolerance in Plants. Front. Plant Sci. 2016, 7, 817. [Google Scholar] [CrossRef]
- Ferdous, J.; Hussain, S.S.; Shi, B. Role of microRNAs in plant drought tolerance. Plant Biotechnol. J. 2016, 13, 293–305. [Google Scholar] [CrossRef]
- Hackenberg, M.; Gustafson, P.; Langridge, P.; Shi, B. Differential expression of microRNAs and other small RNAs in barley between water and drought conditions. Plant Biotechnol. J. 2015, 13, 2–13. [Google Scholar] [CrossRef] [Green Version]
- Joshi, R.; Wani, S.H.; Singh, B.; Bohra, A.; Dar, Z.A.; Lone, A.A.; Pareek, A.; Singla-Pareek, S.L. Transcription Factors and Plants Response to Drought Stress: Current Understanding and Future Directions. Front. Plant Sci. 2016, 7, 1029. [Google Scholar] [CrossRef] [Green Version]
- Gahlaut, V.; Jaiswal, V.; Kumar, A.; Gupta, P.K. Transcription factors involved in drought tolerance and their possible role in developing drought tolerant cultivars with emphasis on wheat (Triticumaestivum, L.). Theor. Appl. Genet. 2016, 129, 2019–2042. [Google Scholar] [CrossRef]
- Zeng, J.; Zhu, X.; Haider, M.S.; Wang, X.; Zhang, C.; Wang, C. Genome wide identification and analysis of the type-B authentic response regulator gene family in peach (Prunus persica). Cytogenet. Genome Res. 2017, 151, 41–49. [Google Scholar] [CrossRef]
- Li, J.; Besseau, S.; Toronen, P.; Sipari, N.; Kollist, H.; Holm, L.; Palva, E.T. Defense-related transcription factors WRKY70 and WRKY54 modulate osmotic stress tolerance by regulating stomatal aperture in Arabidopsis. New Phytol. 2013, 200, 457–472. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Hu, W.; Zhou, R.; Wang, L.; Wang, X.; Wang, Q.; Feng, Z.; Li, Y.; Qiu, D.; He, G.; et al. The Brachypodium distachyon BdWRKY36 gene confers tolerance to drought stress in transgenic tobacco plants. Plant Cell Rep. 2015, 34, 23–35. [Google Scholar] [CrossRef]
- Liu, S.; Wang, X.; Wang, H.; Xin, H.; Yang, X.; Yan, J.; Li, J. Genome-wide analysis of ZmDREB genes and their association with natural variation in drought tolerance at seedling stage of Zea mays L. PLoS Genet. 2013, 9, e1003790. [Google Scholar] [CrossRef] [Green Version]
- Mao, H.; Wang, H.; Liu, S.; Li, Z.; Yang, X.; Yan, J.; Li, J.; Tran, L.S.P.; Qin, F. A transposable element in a NAC gene is associated with drought tolerance in maize seedlings. Nat. Commun. 2015, 6, 8326. [Google Scholar] [CrossRef] [Green Version]
- Xiaoyang, G.; Zeyu, X.; Tiegang, Y.; Xingali, M.; Yang, Z.; Zhiqiang, W.; Yongzhe, R.; Tongbao, L. Metabolomics response for drought stress tolerance in which Chinese wheat genotypes (Triticum aestivum.). Plants 2020, 9, 250. [Google Scholar] [CrossRef] [Green Version]
- Jacobo-Velázquez, D.A.; González-Agüero, M.; Cisneros-Zevallos, L. Cross-talk between signaling pathways: The link between plant secondary metabolite production and wounding stress response. Sci. Rep. 2015, 5, 8608. [Google Scholar] [CrossRef] [Green Version]
- Isah, T. Stress and defense response in plant secondary metabolites production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef] [Green Version]
- Suseela, V.; Tharayil, N.; Xing, B.; Dukes, J.S. Warming and drought differentially influence the production and resorption of elemental and metabolic nitrogen pools in Quercusrubra. Glob. Chang. Biol. 2015, 21, 4177–4195. [Google Scholar] [CrossRef]
- Peterbauer, T.; Richter, A. Biochemistry and physiology of raffinose family oligosaccharides and galactosylcyclitols in seeds. Seed Sci. Res. 2001, 11, 185–197. [Google Scholar] [CrossRef]
- Farrant, J.M.; Cooper, K.; Nell, H. Desiccation Tolerance in Plant Stress Physiology; Shabala, S., Ed.; CAB International: Wallingford, UK, 2012; pp. 238–265. [Google Scholar]
- Suguiyama, V.F.; Silva, E.A.; Meirelles, S.T.; Centeno, D.C.; Braga, M.R. Leaf metabolite profile of the Brazilian resurrection plant Barbaceniapurpurea Hook. (Velloziaceae) shows two time-dependent responses during desiccation and recovering. Front. Plant Sci. 2014, 5, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muhammad, Z.I.; Samina, J.N.A.; Zahid, H.S.; Hafiz, M.R.; Zubair, Z.; Ishita, A.; Atle, M.B.; Jam, N.A. Gene mining for proline based signaing proteins in cell wall of Arabidopsis thaliana. Front. Plant Sci. 2017, 8, 23. [Google Scholar] [CrossRef] [Green Version]
- Kalamaki, M.S.; Merkouropoulos, G.; Kanellis, A.K. Can ornithine accumulation modulate abiotic stress tolerance in Arabidopsis? Plant Signal. Behav. 2009, 4, 1099–1101. [Google Scholar] [CrossRef] [Green Version]
- Lea, P.J.; Sodek, L.; Parry, M.A.; Shewry, P.R.; Halford, N.G. Asparagine in plants. Ann. Appl. Biol. 2007, 150, 1–26. [Google Scholar] [CrossRef]
- Andrea, R.M.; Andreo, C.S.; Lara, M.V. Deciphering the mechanisms involved in Portulaca oleracea C4 response to drought: Metabolic changes including crassulacean acid-like metabolism induction and reversal upon re-watering. Physiol. Plant. 2014, 152, 414–430. [Google Scholar] [CrossRef]
- Farooq, M.; Basra, S.M.A.; Wahid, A. Priming of field-sown rice seed enhances germination, seedling establishment, allometry and yield. Plant Growth Regul. 2006, 49, 285–294. [Google Scholar] [CrossRef]
- Rouhi, H.R.; Aboutalebian, M.A.; Sharif-Zadeh, F. Effects of hydro and osmopriming on drought stress tolerance during germination in four grass species. Int. J. Agrisience 2011, 1, 107–114. [Google Scholar]
- Paparella, S.; Araujo, S.S.; Rossi, G.; Wijaya, S.M.; Carbonera, D.; Balestrazzi, A. Seed priming: State of the art and new perspectives. Plant Cell Rep. 2015, 34, 1281–1293. [Google Scholar] [CrossRef]
- Afzal, I.S.; Rauf, S.M.A.; Murtaza, G. Halopriming improves vigor, metabolism of reserves and ionic contents in wheat seedlings under salt stress. Plant Soil Environ. 2008, 54, 382–388. [Google Scholar] [CrossRef] [Green Version]
- Jafar, M.Z.; Farooq, M.; Cheema, M.A.; Afzal, I.; Basra, S.M.A.; Wahid, M.A.; Aziz, T.; Shahid, M. Improving the performance of wheat by seed priming under saline conditions. J Agron. Crop Sci. 2012, 198, 38–45. [Google Scholar] [CrossRef]
- Delavari, P.M.; Baghizadeh, A.; Enteshari, S.H.; Kalantari, K.M.; Yazdanpanah, A.; Mousavi, E.A. The Effects of salicylic acid on some of biochemical and morphological characteristic of Ocimum basilicum under salinity stress. Aust. J. Basic Appl. Sci. 2010, 4, 4832–4845. [Google Scholar]
- Singh, H.; Jassal, R.K.; Kang, J.S.; Sandhu, S.S.; Kang, H.; Grewal, K. Seed priming techniques in field crops—A review. Agric. Rev. 2015, 36, 251–264. [Google Scholar] [CrossRef] [Green Version]
- Zulueta-Rodríguez, R.; Hernández-Montiel, L.G.; Murillo-Amador, B.; Rueda-Puente, E.O.; Capistrán, L.L.; Diéguez, E.T.; Cordoba, M. Effect of Hydropriming and Biopriming on Seed Germination and Growth of Two Mexican Fir Tree Species in Danger of Extinction. Forests 2015, 6, 3109–3122. [Google Scholar] [CrossRef] [Green Version]
- Kaur, S.; Gupta, A.K.; Kaur, N. Effect of osmo-and hydropriming of chickpea seeds on seedling growth and carbohydrate metabolism under water deficit stress. Plant Growth Regul. 2002, 37, 17–22. [Google Scholar] [CrossRef]
- Damalas, C.A.; Koutroubas, S.D.; Fotiadis, S.; Damalas, A.; Koutroubas, D. Hydro-Priming Effects on Seed Germination and Field Performance of Faba Bean in Spring Sowing. Agriculture 2019, 9, 201. [Google Scholar] [CrossRef] [Green Version]
- Nahid, K.; Rosimah, N.; Parisa, A.; Rambod, A.; Narges, A. Hydro Priming Stimulates Seedling Growth and Establishment of Malaysian Indica rice (MR219) under Drought Stress. Acta Sci. Agric. 2018, 2, 9–16. [Google Scholar]
- Adinde, J.; Omeje, T.E.; Uche, O.J.; Agu, C.J. Impact of hydropriming duration on seed germination and emergence indices of sweet basil. J. Agric. Sci. Prac. 2020, 5, 1–7. [Google Scholar] [CrossRef]
- Rambod, A.; Noor, S.S.; Mahmood, M.; Zetty, N.B.U.; Narges, A.; Mahbod, S.; Parisa, A. Quantitative assessment of indica rice germination to hydropriming, hormonal priming and polyethylene glycol priming. Chil. J. Agric. Res. 2016, 76, 392–400. [Google Scholar]
- Lutts, S.; Paolo, B.; Lukasz, W.S.K.S.; Robert, P. Seed priming: New comprehensive approaches for an old empirical technique. In New Challenges in Seed Biology-Basic and Translational Research Driving Seed Technology; Intech Open: Rijeka, Croatia, 2016; pp. 1–46. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Dahiru, R.; Musa, M.; Haliru, B.S. Effects of osmo-priming duration on germination, emergence and early growth of cowpea (Vigna unguiculata (L.) Walp.) in the Sudan savanna Nigeria. Int. J. Agron. 2014, 4, 841238. [Google Scholar]
- Katarzyna, K.; Beata, P.M.; Ewelina, R. Reactive oxygen species as potential drivers of the seed aging process. Plants 2019, 8, 174. [Google Scholar] [CrossRef] [Green Version]
- Moradi, A.; Younesi, O. Effects of osmo-and hydro-priming on seed parameters of grain sorghum (Sorghum bicolor L.). Aust. J. Basic Appl. Sci. 2009, 3, 1696–1700. [Google Scholar]
- Mirmazloum, I.; Kiss, A.; Erdelyi, E.; Marta, L.; Nemeth, E.Z.; Radacsi, P. The Effect of Osmopriming on Seed Germination and Early Seedling Characteristics of Carum carvi L. Agriculture 2020, 10, 94. [Google Scholar] [CrossRef] [Green Version]
- Jisha, K.C.; Vijayakumari, K.; Puthur, J.T. Seed priming for abiotic stress tolerance: An overview. Acta Physiol. Plant 2013, 35, 1381–1396. [Google Scholar] [CrossRef]
- Demir, I.; Ozuaydın, I.; Yasar, F.; Van, S.J. Effect of smoke-derived butenolide priming treatment on pepper and salvia seeds in relation to transplant quality and catalase activity. S. Afr. J. Bot. 2012, 78, 83–87. [Google Scholar] [CrossRef] [Green Version]
- Hameed, A.; Iqbal, N. Chemo-priming with Mannose, Mannitol and H2O2 Mitigate Drought Stress in Wheat, Cereal Research Communications. Cereal Res. Commun. 2014, 42, 450–462. [Google Scholar] [CrossRef] [Green Version]
- Dutta, P. Seed Priming: New Vistas and Contemporary Perspectives; Springer: Singapore, 2018; pp. 3–22. [Google Scholar] [CrossRef]
- Upadhyaya, H.; Begum, L.; Dey, B.; Nath, P.K.; Panda, S.K. Impact of calcium phosphate nanopar-ticles on rice plant. J. Plant Sci. Phytopathol. 2017, 1, 1–10. [Google Scholar]
- Mahmood, A.; Turgay, O.C.; Farooq, M.; Hayat, R. Seed biopriming with plant growth promoting rhizobacteria: A review. FEMS Microbiol. Ecol. 2016, 92, fiw112. [Google Scholar] [CrossRef]
- Timmusk, S.; Abd, E.I.A.; Copolovici, L.; Tanilas, T.; Kannaste, A.; Behers, L.; Nevo, E.; Seisenbaeva, G.; Stenstrom, E.; Niinemets, U. Drought-tolerance of wheat improved by rhizosphere bacteria from harsh environments: Enhanced biomass production and reduced emissions of stress volatiles. PLoS ONE 2014, 9, e96086. [Google Scholar] [CrossRef] [Green Version]
- Rakshit, A.; Sunita, K.; Pal, S.; Singh, A.; Singh, H.B. Bio-priming mediated nutrient use efficiency of crop species. In Nutrient Use Efficiency: From Basics to Advances; Springer: New Delhi, India, 2015; pp. 181–191. [Google Scholar]
- Reddy, P.P. Bio-priming of seeds. In Recent Advances in Crop Protection; Springer: New Delhi, India, 2012; pp. 83–90. [Google Scholar]
- Harman, G.E. Overview of mechanism and uses of Trichoderma spp. Phytopathology 2006, 96, 190–194. [Google Scholar] [CrossRef] [Green Version]
- Shukla, N.; Awasthi, R.P.; Rawat, L.; Kumar, J. Seed biopriming with drought tolerant isolates of Trichoderma harzianum promote growth and drought tolerance in Triticum aestivum. Ann. Appl. Biol. 2014, 166, 171–182. [Google Scholar] [CrossRef]
- Bakhtavar, M.A.; Afzal, I.; Basra, S.M.A.; Ahmad, A.-U.-H.; Noor, M.A. Physiological Strategies to Improve the Performance of Spring Maize (Zea mays L.) Planted under Early and Optimum Sowing Conditions. PLoS ONE 2015, 10, e0124441. [Google Scholar] [CrossRef]
- Wei, L.X.; Lv, B.S.; Li, X.W.; Wang, M.M.; Ma, H.Y.; Yang, H.Y.; Yang, R.F.; Piao, Z.Z.; Wang, Z.H.; Lou, J.H. Priming of rice (Oryza sativa L.) seedlings with abscisic acid enhances seedling survival, plant growth, and grain yield in saline-alkaline paddy fields. Field Crop. Res. 2017, 203, 86–93. [Google Scholar] [CrossRef]
- Zheng, M.; Tao, Y.; Hussain, S.; Jiang, Q.; Peng, S.; Huang, J.; Cui, K.; Nie, L. Seed priming in dry direct-seeded rice: Consequences for emergence, seedling growth and associated metabolic events under drought stress. Plant Growth Regul. 2016, 78, 167–178. [Google Scholar] [CrossRef]
- Umezawa, T.; Nakashima, K.; Miyakawa, T.; Kuromori, T.; Tanokura, M.; Shinozaki, K.; Yamaguchi, K. Molecular basis of the core regulatory network in ABA responses: Sensing, signaling and transport. Plant Cell Physiol. 2010, 51, 1821–1839. [Google Scholar] [CrossRef] [PubMed]
- Ansari, O.; Azadi, M.S.; Sharif, Z.F.; Younesi, E. Effect of hormone priming on germination characteristics and enzyme activity of mountain rye (Secale montanum) seeds under drought stress conditions. J. Stress Physiol. Biochem. 2013, 9, 61–71. [Google Scholar]
- Grzesik, M.; Nowak, J. Effects of matriconditioning and hydropriming on (Helichrysum bracteatum L.) seed germination, seedling emergence and stress tolerance. Seed Sci. Technol. 1998, 26, 363–376. [Google Scholar]
- Guan, Y.; Hu, J.; Wang, X.; Shao, C. Seed priming with chitosan improves maize germination and seedling growth in relation to physiological changes under low temperature stress. J. Zhejiang Univ. Sci. B 2009, 10, 427–433. [Google Scholar] [CrossRef] [Green Version]
- Kubik, K.K.; Eastin, J.A.; Eskridge, K.M. Solid matrix priming of tomato and pepper. In Proceedings of the International Conference on Stand Establishment for Horticultural Crops, Lancaster, PA, USA, 1988; pp. 86–96. [Google Scholar]
- Iqbal, S.; Farooq, M.; Alam Cheema, S.; Afzal, I. Boron Seed Priming Improves the Seedling Emergence, Growth, Grain Yield and Grain Biofortification of Bread Wheat. Int. J. Agric. Biol. 2016, 19, 177–182. [Google Scholar] [CrossRef]
- Rehman, A.; Farooq, M.; Naveed, M.; Nawaz, A.; Shahzad, B. Seed priming of Zn with endophytic bacteria improves the productivity and grain biofortification of bread wheat. Eur. J. Agron. 2018, 94, 98–107. [Google Scholar] [CrossRef]
- Farooq, M.; Ullah, A.; Rehman, A.; Nawaz, A.; Nadeem, A.; Wakeel, A.; Nadeem, F.; Siddique, K.H.M. Application of zinc improves the productivity and biofortification of fine grain aromatic rice grown in dry seeded and puddled transplanted production systems. Field Crop. Res. 2018, 216, 53–62. [Google Scholar] [CrossRef]
- Ullah, A.; Farooq, M.; Hussain, M.; Wakeel, A. Zinc seed priming improves stand establishment, tissue zinc concentration and early seedling growth of chickpea. J. Anim. Plant Sci. 2019, 29, 1049–1053. [Google Scholar]
- Rehman, A.; Farooq, M.; Cheema, Z.A.; Wahid, A. Role of boron in leaf elongation and tillering dynamics in fine grain aromatic rice. J. Plant Nutr. 2013, 36, 42–54. [Google Scholar] [CrossRef]
- Cakmak, I. The role of potassium in alleviating detrimental effects of abiotic stresses in plants. J. Plant Nutr. Soil Sci. 2005, 168, 521–530. [Google Scholar] [CrossRef]
- Shivay, Y.S.; Prasad, R.; Pal, M. Genetic variability for zinc use efficiency in chickpea as influenced by zinc fertilization. Int. J. Bio-Resour. Stress Manag. 2014, 5, 31–36. [Google Scholar] [CrossRef]
- Farooq, M.A.; Gogoi, N.; Barthakur, S.; Baroowa, B.; Bharadwaj, N.; Alghamdi, S.S.; Siddique, K.H.M. Drought stress in grain legumes during reproduction and grain filling. J. Agron. Crop. Sci. 2017, 203, 81–102. [Google Scholar] [CrossRef]
- Waqas, M.; Korres, N.E.; Khan, M.D.; Nizami, A.-S.; Deeba, F.; Ali, I.; Hussain, H. Advances in the Concept and Methods of Seed Priming; Springer: Singapore, 2019; pp. 11–41. [Google Scholar] [CrossRef]
- Kranner, I.; Minibayeva, F.V.; Beckett, R.P.; Seal, C.E. What is stress? Concepts, definitions and applications in seed science. New Phytol. 2010, 188, 655–673. [Google Scholar] [CrossRef]
- Koller, D.; Hadass, A. Water Relations in the Germination of Seeds; Springer: Berlin, Germany, 1982; Volume 12, pp. 401–431. [Google Scholar] [CrossRef]
- Stanley, L.; Paolo, B.; Wojtyla, L.; Kubala, S.; Pace, R. Seed priming: New comprehensive approaches for an old empirical technique. In New Challenges in Seed Biology—Basic and Translational Research Driving Seed Technology; Araujo, S., Balestrazzi, A., Eds.; InTechOpen: Winchester, UK, 2016; pp. 1–49. Available online: http://hdl.handle.net/2078.1/177458 (accessed on 11 January 2020). [CrossRef] [Green Version]
- Mustafa, H.S.; Mahmood, T.; Ullah, A.; Sharif, A.; Bhatti, A.N.; Nadeem, M.; Ali, R. Role of seed priming to enhance growth and development of crop plants against biotic and abiotic stress. Bull. Biol. All. Sci. 2017, 2, 1–11. [Google Scholar]
- Ventura, L.; Dona, M.; Macovei, A.; Carbonera, D.; Buttafava, A.; Mondoni, A.; Rossi, G.; Balestrazzi, A. Understanding the molecular pathways associated with seed vigor. Plant Physiol. Biochem. 2012, 60, 196–206. [Google Scholar] [CrossRef]
- Hameed, A.; Sheikh, M.A.; Farooq, T.; Basra, S.; Jamil, A. Chitosan priming enhances the seed germination, antioxidants, hydrolytic enzymes, soluble proteins and sugars in wheat seeds. Agrochimica 2013, 57, 97–110. [Google Scholar]
- Gallardo, K.; Job, C.; Groot, S.P.C.; Puype, M.; Demol, H.; Vandekerckhove, J.; Job, D. Proteomics of Arabidopsis seed germination and priming. In The Biology of Seeds: Recent Research Advances, Proceedings of the Seventh International Workshop on Seeds, Salamanca, Spain, 16 May 2002; CABI: Wallingford, UK, 2003. [Google Scholar] [CrossRef]
- Meena, R.P.; Sendhil, R.; Tripathi, S.; Chander, S.; Chhokar, R.; Sharma, R. Hydro-priming of seed improves the water use efficiency, grain yield and net economic return of wheat under different moisture regimes. SAARC J. Agric. 2013, 11, 149–159. [Google Scholar] [CrossRef] [Green Version]
- Raj, A.; Raj, S. Seed priming: An approach towards agricultural sustainability. J. Appl. Nat. Sci. 2019, 11, 227–234. [Google Scholar] [CrossRef]
- Yan, H.; Jia, S.; Mao, P. Melatonin Priming Alleviates Aging-Induced Germination Inhibition by Regulating β-oxidation, Protein Translation, and Antioxidant Metabolism in Oat (Avena sativa L.) Seeds. Int. J. Mol. Sci. 2020, 21, 1898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De, A.E.J.; De, G.R.; Van, M.M.; Engler, G. In situ hybridization to mRNA of Arabidopsis tissue sections. Methods 2001, 23, 325–334. [Google Scholar]
- Borges, A.A.; Jimenez, A.D.; Exposito, R.M.; Sandalio, L.M.; Perez, J.A. Priming crops against biotic and abiotic stresses: MSB as a tool for studying mechanisms. Front. Plant Sci. 2014, 5, 642. [Google Scholar] [CrossRef] [Green Version]
- Gomes, D.; Agasse, A.; Thiebaud, P.; Delrot, S.; Geros, H.; Chaumont, F. Aquaporins are multifunctional water and solute transporters highly divergent in living organisms. Biochim. Biophys. Acta 2009, 1788, 1213–1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alleva, K.; Chara, O.; Amodeo, G. Aquaporins: Another piece in the osmotic puzzle. FEBS Lett. 2010, 586, 2991–2999. [Google Scholar] [CrossRef] [Green Version]
- Vander, W.; Postaire, C.; Tournaire-Roux, O.C.; Boursiac, Y.; Maurel, C. Expression and inhibition of aquaporins in germinating Arabidopsis seeds. Plant Cell Physiol. 2006, 47, 1241–1250. [Google Scholar] [CrossRef] [Green Version]
- Ge, F.W.; Tao, P.; Zhang, Y.; Wang, J.B. Characterization of AQP gene expressions in Brassica napus during seed germination and in response to abiotic stresses. Biol. Plant. 2014, 58, 274–282. [Google Scholar] [CrossRef]
- Kubala, S.; Garnczarska, M.; Wojtyla, Ł.; Clippe, A.; Kosmala, A.; Zmienko, A.; Lutts, S.; Quinet, M. Deciphering priming-induced improvement of rapeseed (Brassica napus L.) germination through an integrated transcriptomic and proteomic approach. Plant Sci. 2015, 231, 94–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mawlong, I.; Ali, K.; Srinivasan, R.; Rai, R.D.; Tyagi, A. Functional validation of a drought-responsive AP2/ERF family transcription factor-encoding gene from rice in Arabidopsis. Mol. Breed. 2015, 35, 163. [Google Scholar] [CrossRef]
- Gholami, H.; Farhadi, R.; Rahimi, M.; Zeinalikharaji, A.; Askari, A. Effect of growth hormones on physiology characteristics and essential oil of basil under drought stress condition. J. Am. Sci. 2013, 9, 61–63. [Google Scholar]
- Pastor, V.; Luna, E.; Mauch, M.B.; Ton, J.; Flors, V. Primed plants do not forget. Environ. Exp. Bot. 2013, 94, 46–56. [Google Scholar] [CrossRef]
- Xiao, Y.M.; Luo, R.P.; Hayes, J.; Kim, S.N.; Ding, M.; Liao, A.K. Structure basis for directional R-loop formation and substrate handover mechanisms in type I CRISPR-Cas system cryo-EM structures of type I CRISPR-Cas system resolve the mechanisms governing the PAM-dependent R-loop formation, Cas3 recruitment, and subst. Cell 2017, 170, 48–60.e11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conrath, U. Molecular aspects of defence priming. Trends Plant Sci. 2011, 16, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Fromm, M.; Avramova, Z. Multiple exposures to drought ‘train’ transcriptional responses in Arabidopsis. Nat. Commun. 2012, 3, 740. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Topbjerg, H.B.; Jiang, D.; Liu, F. Drought priming at vegetative stage improves the antioxidant capacity and photosynthesis performance of wheat exposed to a short-term low temperature stress at jointing stage. Plant Soil. 2015, 393, 307–318. [Google Scholar] [CrossRef]
- Wang, X.; Vignjevic, M.; Liu, F.; Jacobsen, S.; Jiang, D.; Wollenweber, B. Drought priming at vegetative growth stages improves tolerance to drought and heat stresses occurring during grain filling in spring wheat. Plant Growth Regul. 2015, 75, 677–687. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, J.; Huang, X.; Guo, D.; Lou, H.; Hou, Z.; Su, M.; Liang, R.; Xie, C.; You, M.; et al. Differential effects of a post-anthesis heat stress on wheat (Triticum Aestivum, L.) grain proteome determine by iTRAQ. Sci. Rep. 2017, 7, 3468. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhang, X.; Chen, J.; Wang, X.; Cai, J.; Zhou, Q.; Dai, T.; Cao, W.; Jiang, D. Parental drought-priming enhances tolerance to post-anthesis drought offspring of wheat. Front. Plant Sci. 2018, 9, 261. [Google Scholar] [CrossRef]
- Lata, C.; Prasad, M. Role of DREB in regulation of abiotic stress response in plants. J. Exp. Bot. 2011, 62, 4731–4748. [Google Scholar] [CrossRef] [Green Version]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef] [Green Version]
- Hirayama, T.; Shinozaki, K. Research on plant abiotic stress responses in the post-genome era: Past, present and future. Plant J. 2010, 61, 1041–1052. [Google Scholar] [CrossRef]
- Jakab, G.; Ton, J.; Flors, V.; Zimmerli, L.; Metraux, J.P.; Mauch, M.B. Enhancing Arabidopsis salt and drought stress tolerance by chemical priming for its abscisic acid responses. Plant Physiol. 2005, 139, 267–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nosalewicz, A.; Siecinska, J.; Smiech, M.; Nosalewicz, M.; Wiącek, D.; Pecio, A. Transgenerational effects of temporal drought stress on spring barley morphology and functioning. Environ. Exp. Bot. 2016, 131, 120–127. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marthandan, V.; Geetha, R.; Kumutha, K.; Renganathan, V.G.; Karthikeyan, A.; Ramalingam, J. Seed Priming: A Feasible Strategy to Enhance Drought Tolerance in Crop Plants. Int. J. Mol. Sci. 2020, 21, 8258. https://doi.org/10.3390/ijms21218258
Marthandan V, Geetha R, Kumutha K, Renganathan VG, Karthikeyan A, Ramalingam J. Seed Priming: A Feasible Strategy to Enhance Drought Tolerance in Crop Plants. International Journal of Molecular Sciences. 2020; 21(21):8258. https://doi.org/10.3390/ijms21218258
Chicago/Turabian StyleMarthandan, Vishvanathan, Rathnavel Geetha, Karunanandham Kumutha, Vellaichamy Gandhimeyyan Renganathan, Adhimoolam Karthikeyan, and Jegadeesan Ramalingam. 2020. "Seed Priming: A Feasible Strategy to Enhance Drought Tolerance in Crop Plants" International Journal of Molecular Sciences 21, no. 21: 8258. https://doi.org/10.3390/ijms21218258
APA StyleMarthandan, V., Geetha, R., Kumutha, K., Renganathan, V. G., Karthikeyan, A., & Ramalingam, J. (2020). Seed Priming: A Feasible Strategy to Enhance Drought Tolerance in Crop Plants. International Journal of Molecular Sciences, 21(21), 8258. https://doi.org/10.3390/ijms21218258




