Inhibition of Src Family Kinases Ameliorates LPS-Induced Acute Kidney Injury and Mitochondrial Dysfunction in Mice
Abstract
:1. Introduction
2. Results
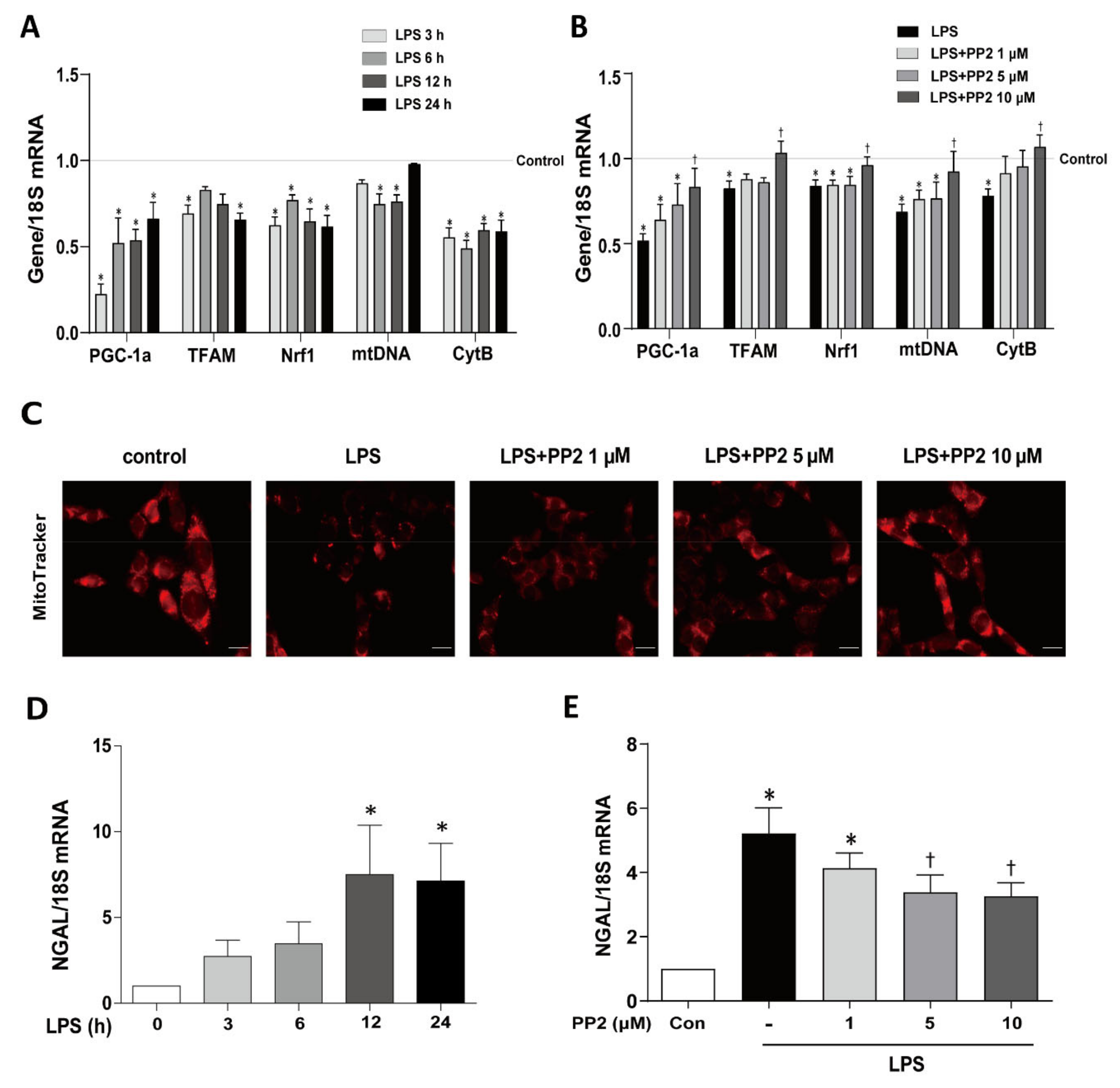
2.1. PP2 Ameliorates LPS-Induced Mitochondrial Dysfunction and Tubular Injury in mProx Cells
2.2. PP2 Improves Kidney Function and Attenuates Kidney Tubular Injury in LPS-Induced AKI
2.3. PP2 Ameliorates Inflammation in LPS-Induced AKI
2.4. PP2 Attenuates Oxidative Stress in LPS-Induced AKI
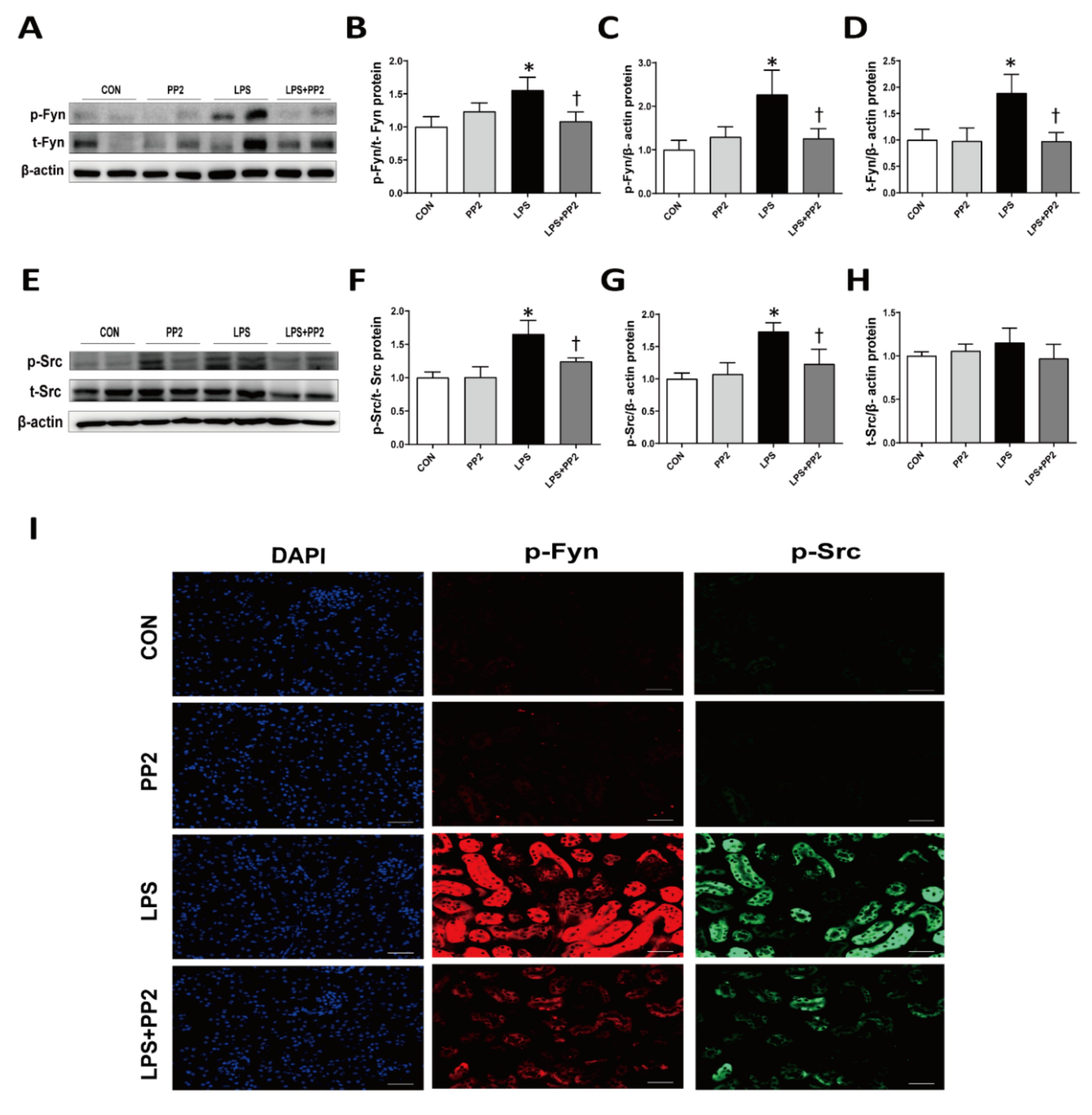
2.5. SFK, Mainly Fyn and Src, Are Increased in LPS-Induced AKI
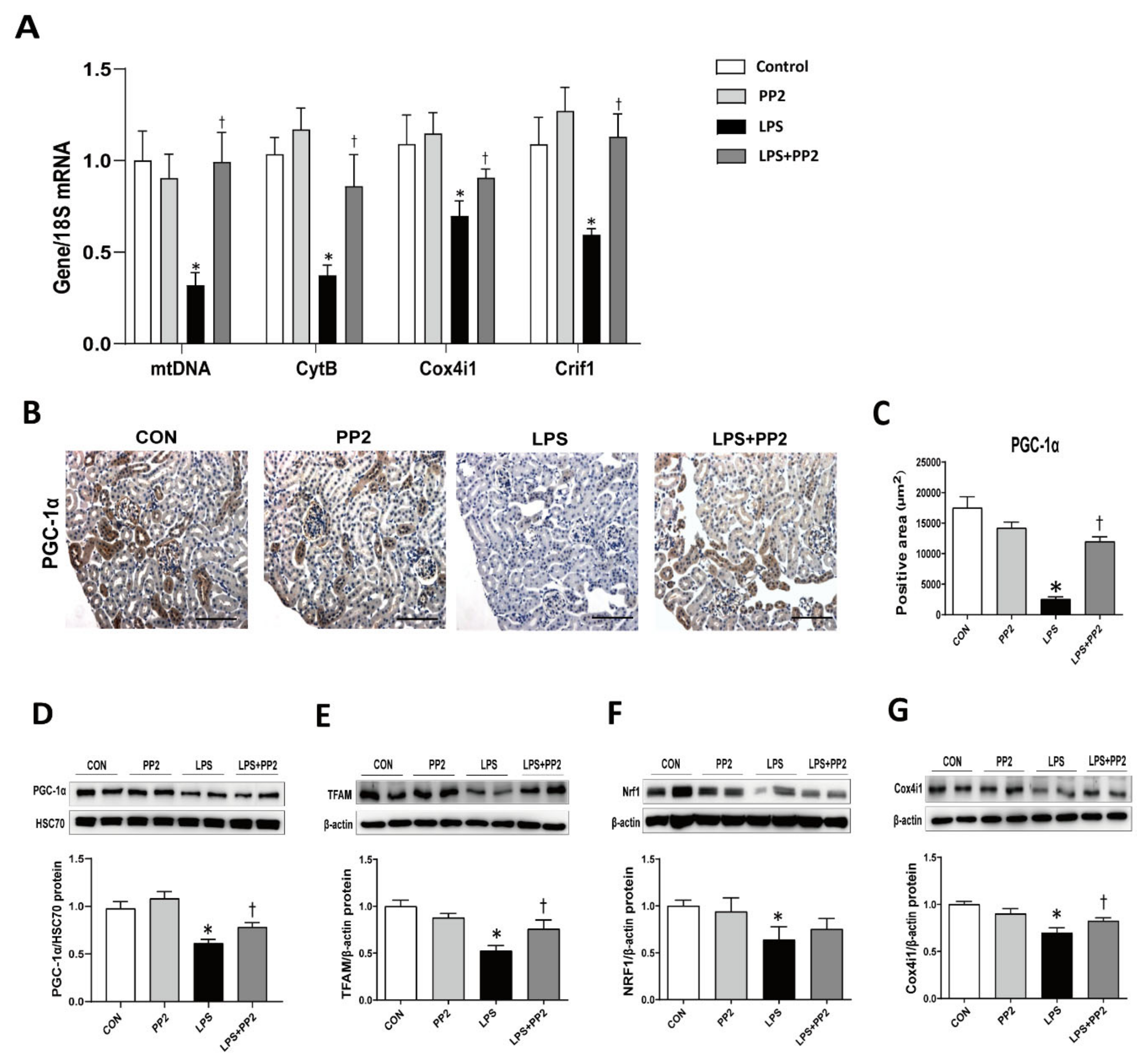
2.6. PP2 Attenuates Mitochondrial Dysfunction in LPS-Induced AKI
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture
4.3. Animals
4.4. Measurements of Blood Parameters
4.5. Histology and Immunohistochemistry
4.6. Immunofluorescence Staining
4.7. Quantitative Real-Time Reverse Transcriptase Polymerase Chain Reaction (RT-PCR)
4.8. Western Blot Analysis
4.9. Measurement of Cellular ROS
4.10. Terminal Transferase-dUTP-Nick-End Labeling (TUNEL) Assay
4.11. Measurement of Mitochondria
4.12. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- International Society of Nephrology. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. 2012, 2, 122–123. [Google Scholar] [CrossRef] [Green Version]
- Lewington, A.J.; Cerda, J.; Mehta, R.L. Raising awareness of acute kidney injury: A global perspective of a silent killer. Kidney Int. 2013, 84, 457–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seller-Perez, G.; Mas-Font, S.; Perez-Calvo, C.; Villa-Diaz, P.; Celaya-Lopez, M.; Herrera-Gutierrez, M.E. Acute kidney injury: Renal disease in the ICU. Med. Intensiva 2016, 40, 374–382. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Wei, Q.; Liu, J.; Yi, M.; Liu, Y.; Liu, H.; Sun, L.; Peng, Y.; Liu, F.; Venkatachalam, M.A.; et al. AKI on CKD: Heightened injury, suppressed repair, and the underlying mechanisms. Kidney Int. 2017, 92, 1071–1083. [Google Scholar] [CrossRef]
- Togel, F.; Westenfelder, C. Recent advances in the understanding of acute kidney injury. F1000Prime Rep. 2014, 6, 83–88. [Google Scholar] [CrossRef]
- Stasi, A.; Intini, A.; Divella, C.; Franzin, R.; Montemurno, E.; Grandaliano, G.; Ronco, C.; Fiaccadori, E.; Pertosa, G.B.; Gesualdo, L.; et al. Emerging role of Lipopolysaccharide binding protein in sepsis-induced acute kidney injury. Nephrol. Dial. Transplant. 2017, 32, 24–31. [Google Scholar] [CrossRef] [Green Version]
- Peerapornratana, S.; Manrique-Caballero, C.L.; Gómez, H.; Kellum, J.A. Acute kidney injury from sepsis: Current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. 2019, 96, 1083–1099. [Google Scholar] [CrossRef]
- Weisbord, S.D.; Palevsky, P.M. Design of clinical trials in acute kidney injury: Lessons from the past and future directions. Semin. Nephrol. 2016, 36, 42–52. [Google Scholar] [CrossRef]
- Conger, J.D. Interventions in clinical acute renal failure: What are the data? Am. J. Kidney Dis. 1995, 26, 565–576. [Google Scholar] [CrossRef]
- Thomas, S.M.; Brugge, J.S. Cellular functions regulated by Src family kinases. Annu. Rev. Cell Dev. Biol. 1997, 13, 513–609. [Google Scholar] [CrossRef] [Green Version]
- Verma, R.; Wharram, B.; Kovari, I.; Kunkel, R.; Nihalani, D.; Wary, K.K.; Wiggins, R.C.; Killen, P.; Holzman, L.B. Fyn binds to and phosphorylates the kidney slit diaphragm component Nephrin. J. Biol. Chem. 2003, 278, 20716–20723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sen, B.; Johnson, F.M. Regulation of SRC family kinases in human cancers. J. Signal Transduct. 2011, 2011, 865819–865833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.; Shi, Y.; Deng, X.; Su, Y.; Du, C.; Wei, J.; Ren, Y.; Wu, M.; Hou, Y.; Duan, H. Inhibition of c-Src/p38 MAPK pathway ameliorates renal tubular epithelial cells apoptosis in db/db mice. Mol. Cell. Endocrinol. 2015, 417, 27–35. [Google Scholar] [CrossRef]
- Taniguchi, K.; Xia, L.; Goldberg, H.J.; Lee, K.W.; Shah, A.; Stavar, L.; Masson, E.A.; Momen, A.; Shikatani, E.A.; John, R.; et al. Inhibition of Src kinase blocks high glucose-induced EGFR transactivation and collagen synthesis in mesangial cells and prevents diabetic nephropathy in mice. Diabetes 2013, 62, 3874–3886. [Google Scholar] [CrossRef] [Green Version]
- Seo, H.Y.; Jeon, J.H.; Jung, Y.A.; Jung, G.S.; Lee, E.J.; Choi, Y.K.; Park, K.G.; Choe, M.S.; Jang, B.K.; Kim, M.K.; et al. Fyn deficiency attenuates renal fibrosis by inhibition of phospho-STAT3. Kidney Int. 2016, 90, 1285–1297. [Google Scholar] [CrossRef] [PubMed]
- Dorotea, D.; Lee, S.; Lee, S.J.; Lee, G.; Son, J.B.; Choi, H.G.; Ahn, S.M.; Ha, H. KF-1607, A Novel pan src kinase inhibitor, attenuates obstruction-induced tubulointerstitial fibrosis in mice. Biomol. Ther. 2020. [Google Scholar] [CrossRef]
- Uddin, M.J.; Dorotea, D.; Pak, E.S.; Ha, H. Fyn kinase: A potential therapeutic target in acute kidney injury. Biomol. Ther. 2020, 28, 213–221. [Google Scholar] [CrossRef]
- Xiong, C.; Zang, X.; Zhou, X.; Liu, L.; Masucci, M.V.; Tang, J.; Li, X.; Liu, N.; Bayliss, G.; Zhao, T.C.; et al. Pharmacological inhibition of Src kinase protects against acute kidney injury in a murine model of renal ischemia/reperfusion. Oncotarget 2017, 8, 31238–31253. [Google Scholar] [CrossRef]
- McBride, H.M.; Neuspiel, M.; Wasiak, S. Mitochondria: More than just a powerhouse. Curr. Biol. 2006, 16, R551–R560. [Google Scholar] [CrossRef] [Green Version]
- Osellame, L.D.; Blacker, T.S.; Duchen, M.R. Cellular and molecular mechanisms of mitochondrial function. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 711–723. [Google Scholar] [CrossRef] [Green Version]
- Sanchis-Gomar, F.; Garcia-Gimenez, J.L.; Gomez-Cabrera, M.C.; Pallardo, F.V. Mitochondrial biogenesis in health and disease. Molecular and therapeutic approaches. Curr. Pharm. Des. 2014, 20, 5619–5633. [Google Scholar] [CrossRef]
- Bhargava, P.; Schnellmann, R.G. Mitochondrial energetics in the kidney. Nat. Rev. Nephrol. 2017, 13, 629–646. [Google Scholar] [CrossRef]
- Tran, M.; Parikh, S.M. Mitochondrial biogenesis in the acutely injured kidney. Nephron Clin. Pract. 2014, 127, 42–45. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, J.; Tian, J.; Virzi, G.M.; Digvijay, K.; Cueto, L.; Yin, Y.; Rosner, M.H.; Ronco, C. Mitochondria in Sepsis-Induced AKI. J. Am. Soc. Nephrol. 2019, 30, 1151–1161. [Google Scholar] [CrossRef]
- Ventura-Clapier, R.; Garnier, A.; Veksler, V. Transcriptional control of mitochondrial biogenesis: The central role of PGC-1alpha. Cardiovasc. Res. 2008, 79, 208–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, M.; Tam, D.; Bardia, A.; Bhasin, M.; Rowe, G.C.; Kher, A.; Zsengeller, Z.K.; Akhavan-Sharif, M.R.; Khankin, E.V.; Saintgeniez, M.; et al. PGC-1alpha promotes recovery after acute kidney injury during systemic inflammation in mice. J. Clin. Investig. 2011, 121, 4003–4014. [Google Scholar] [CrossRef] [Green Version]
- Tibaldi, E.; Brunati, A.M.; Massimino, M.L.; Stringaro, A.; Colone, M.; Agostinelli, E.; Arancia, G.; Toninello, A. Src-Tyrosine kinases are major agents in mitochondrial tyrosine phosphorylation. J. Cell. Biochem. 2008, 104, 840–849. [Google Scholar] [CrossRef]
- Salvi, M.; Brunati, A.M.; Bordin, L.; La Rocca, N.; Clari, G.; Toninello, A. Characterization and location of Src-dependent tyrosine phosphorylation in rat brain mitochondria. Biochim. Biophys. Acta 2002, 1589, 181–195. [Google Scholar] [CrossRef]
- Augereau, O.; Claverol, S.; Boudes, N.; Basurko, M.J.; Bonneu, M.; Rossignol, R.; Mazat, J.P.; Letellier, T.; Dachary-Prigent, J. Identification of tyrosine-phosphorylated proteins of the mitochondrial oxidative phosphorylation machinery. Cell. Mol. Life Sci. 2005, 62, 1478–1488. [Google Scholar] [CrossRef]
- Hunter, C.A.; Koc, H.; Koc, E.C. c-Src kinase impairs the expression of mitochondrial OXPHOS complexes in liver cancer. Cell. Signal. 2020, 72, 109651–109662. [Google Scholar] [CrossRef]
- Okutani, D.; Lodyga, M.; Han, B.; Liu, M. Src protein tyrosine kinase family and acute inflammatory responses. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 291, L129–L141. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, S. Src family kinases in chronic kidney disease. Am. J. Physiol. Renal. Physiol. 2017, 313, F721–F728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plotnikov, E.Y.; Pevzner, I.B.; Zorova, L.D.; Chernikov, V.P.; Prusov, A.N.; Kireev, I.I.; Silachev, D.N.; Skulachev, V.P.; Zorov, D.B. Mitochondrial damage and mitochondria-targeted antioxidant protection in LPS-induced acute kidney injury. Antioxidants 2019, 8, 176. [Google Scholar] [CrossRef] [Green Version]
- Arany, I.; Megyesi, J.K.; Kaneto, H.; Price, P.M.; Safirstein, R.L. Cisplatin-induced cell death is EGFR/src/ERK signaling dependent in mouse proximal tubule cells. Am. J. Physiol. Renal. Physiol. 2004, 287, F543–F549. [Google Scholar] [CrossRef] [Green Version]
- Cardone, L.; Carlucci, A.; Affaitati, A.; Livigni, A.; DeCristofaro, T.; Garbi, C.; Varrone, S.; Ullrich, A.; Gottesman, M.E.; Avvedimento, E.V.; et al. Mitochondrial AKAP121 binds and targets protein tyrosine phosphatase D1, a novel positive regulator of src signaling. Mol. Cell. Biol. 2004, 24, 4613–4626. [Google Scholar] [CrossRef] [Green Version]
- Itoh, S.; Lemay, S.; Osawa, M.; Che, W.; Duan, Y.; Tompkins, A.; Brookes, P.S.; Sheu, S.S.; Abe, J. Mitochondrial Dok-4 recruits Src kinase and regulates NF-kappaB activation in endothelial cells. J. Biol. Chem. 2005, 280, 26383–26396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basile, D.P.; Anderson, M.D.; Sutton, T.A. Pathophysiology of acute kidney injury. Compr. Physiol. 2012, 2, 1303–1353. [Google Scholar] [CrossRef] [Green Version]
- Kinsey, G.R.; Li, L.; Okusa, M.D. Inflammation in acute kidney injury. Nephron Exp. Nephrol. 2008, 109, e102–e107. [Google Scholar] [CrossRef]
- Lennmyr, F.; Ericsson, A.; Gerwins, P.; Akterin, S.; Ahlström, H.; Terént, A. Src family kinase-inhibitor PP2 reduces focal ischemic brain injury. Acta Neurol. Scand. 2004, 110, 175–179. [Google Scholar] [CrossRef]
- Severgnini, M.; Takahashi, S.; Tu, P.; Perides, G.; Homer, R.J.; Jhung, J.W.; Bhavsar, D.; Cochran, B.H.; Simon, A.R. Inhibition of the Src and Jak kinases protects against lipopolysaccharide-induced acute lung injury. Am. J. Respir. Crit. Care Med. 2005, 171, 858–867. [Google Scholar] [CrossRef] [PubMed]
- Giannoni, E.; Buricchi, F.; Raugei, G.; Ramponi, G.; Chiarugi, P. Intracellular reactive oxygen species activate Src tyrosine kinase during cell adhesion and anchorage-dependent cell growth. Mol. Cell. Biol. 2005, 25, 6391–6403. [Google Scholar] [CrossRef] [Green Version]
- Giannoni, E.; Chiarugi, P. Redox circuitries driving Src regulation. Antioxid. Redox Signal. 2014, 20, 2011–2025. [Google Scholar] [CrossRef]
- Chowdhury, A.K.; Watkins, T.; Parinandi, N.L.; Saatian, B.; Kleinberg, M.E.; Usatyuk, P.V.; Natarajan, V. Src-mediated tyrosine phosphorylation of p47phox in hyperoxia-induced activation of NADPH oxidase and generation of reactive oxygen species in lung endothelial cells. J. Biol. Chem. 2005, 280, 20700–20711. [Google Scholar] [CrossRef] [Green Version]
- Takikita-Suzuki, M.; Haneda, M.; Sasahara, M.; Owada, M.K.; Nakagawa, T.; Isono, M.; Takikita, S.; Koya, D.; Ogasawara, K.; Kikkawa, R. Activation of Src kinase in platelet-derived growth factor-B-dependent tubular regeneration after acute ischemic renal injury. Am. J. Pathol. 2003, 163, 277–286. [Google Scholar] [CrossRef] [Green Version]
- Płóciennikowska, A.; Hromada-Judycka, A.; Borzęcka, K.; Kwiatkowska, K. Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2015, 72, 557–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, P.; Angelini, D.J.; Yang, S.; Xia, G.; Cross, A.S.; Mann, D.; Bannerman, D.D.; Vogel, S.N.; Goldblum, S.E. TLR4 signaling is coupled to SRC family kinase activation, tyrosine phosphorylation of zonula adherens proteins, and opening of the paracellular pathway in human lung microvascular endothelia. J. Biol. Chem. 2008, 283, 13437–13449. [Google Scholar] [CrossRef] [Green Version]
- Sohn, M.; Kim, K.; Uddin, M.J.; Lee, G.; Hwang, I.; Kang, H.; Kim, H.; Lee, J.H.; Ha, H. Delayed treatment with fenofibrate protects against high-fat diet-induced kidney injury in mice: The possible role of AMPK autophagy. Am. J. Physiol. Renal. Physiol. 2017, 312, F323–F334. [Google Scholar] [CrossRef] [Green Version]
- Ha, H.; Yu, M.R.; Kim, K.H. Melatonin and taurine reduce early glomerulopathy in diabetic rats. Free Radic. Biol. Med. 1999, 26, 944–950. [Google Scholar] [CrossRef]
- Kwon, G.; Uddin, M.J.; Lee, G.; Jiang, S.; Cho, A.; Lee, J.H.; Lee, S.R.; Bae, Y.S.; Moon, S.H.; Lee, S.J.; et al. A novel pan-Nox inhibitor, APX-115, protects kidney injury in streptozotocin-induced diabetic mice: Possible role of peroxisomal and mitochondrial biogenesis. Oncotarget 2017, 8, 74217–74232. [Google Scholar] [CrossRef] [Green Version]
- Uddin, M.J.; Pak, E.S.; Ha, H. Carbon monoxide releasing molecule-2 protects mice against acute kidney injury through inhibition of ER stress. Korean J. Physiol. Pharmacol. 2018, 22, 567–575. [Google Scholar] [CrossRef] [Green Version]





| Gene | Forward (5′→3′) | Reverse (5′→3′) |
|---|---|---|
| 18S | CGAAAGCATTTGCCAAGAAT | AGTCGGCATCGTTTATGGTC |
| Cox4i1 | TCGATCGTGACTGGGTGGCCA | GCCGAGGGAGTGAGGGAGGC |
| Crif1 | AGCTAACGCCCCGCTATGTG | ATGGTCGCTAAGCTCGGGTA |
| CytB | AAGAGCACCTGGGTGATCCTGCA | CGTGCATCCGTAGAGTGCCCG |
| Fyn | CTTTGGGGGTGTGAACTCCT | TTCTGCCTGGATGGAGTCAA |
| Hck | AGGGGTTAGGACTGGGAACA | CCCCAGAGATTTTGGACCCC |
| ICAM-1 | CTTCCAGCTACCATGCCAAA | CTTCAGAGGCAGGAAACAGG |
| iNOS | GGCAGCCTGTGAGACCTTTG | CATTGGAAGTGAAGCGTTTCG |
| Lck | ACGATCTCGGGGATCATGG | GAGATCTTGCTGTCCAGTGGG |
| Lyn | AGCTCCAGAGGCCATCAACT | CACATCTGCGTTGGTTCTCC |
| mtDNA | CCACTTCATCTTACCATTTA | ATCTGCATCTGAGTTTAATC |
| NGAL | GGCCAGTTCACTCTGGGAAA | TGGCGAACTGGTTGTAGTCC3 |
| NRF-1 | CAACAGGGAAGAAACGGAAA | GCACCACATTCTCCAAAGGT |
| PGC-1α | TCGATGTGTCGCCTTCTTG | ACGAGAGCGCATCCTTTGG |
| Src | TCCACACCTCTCCGAAGCAA | CATGCTGATGGCCTGTGTCA |
| TFAM | ATTCCGAAGTGTTTTTCCAGCA | TCTGAAAGTTTTGCATCTGGGT |
| TNF-α | CGTCAGCCGATTTGCTATCT | CGGACTCCGCAAAGTCTAAG |
| Yes | TGGGAATCAGCGAGGTATTT | ACATTGTCACCCCTCACCTC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pak, E.S.; Uddin, M.J.; Ha, H. Inhibition of Src Family Kinases Ameliorates LPS-Induced Acute Kidney Injury and Mitochondrial Dysfunction in Mice. Int. J. Mol. Sci. 2020, 21, 8246. https://doi.org/10.3390/ijms21218246
Pak ES, Uddin MJ, Ha H. Inhibition of Src Family Kinases Ameliorates LPS-Induced Acute Kidney Injury and Mitochondrial Dysfunction in Mice. International Journal of Molecular Sciences. 2020; 21(21):8246. https://doi.org/10.3390/ijms21218246
Chicago/Turabian StylePak, Eun Seon, Md Jamal Uddin, and Hunjoo Ha. 2020. "Inhibition of Src Family Kinases Ameliorates LPS-Induced Acute Kidney Injury and Mitochondrial Dysfunction in Mice" International Journal of Molecular Sciences 21, no. 21: 8246. https://doi.org/10.3390/ijms21218246
APA StylePak, E. S., Uddin, M. J., & Ha, H. (2020). Inhibition of Src Family Kinases Ameliorates LPS-Induced Acute Kidney Injury and Mitochondrial Dysfunction in Mice. International Journal of Molecular Sciences, 21(21), 8246. https://doi.org/10.3390/ijms21218246







