Intussusceptive Angiogenesis and Peg–Socket Junctions between Endothelial Cells and Smooth Muscle Cells in Early Arterial Intimal Thickening
Abstract
:1. Introduction
2. Results
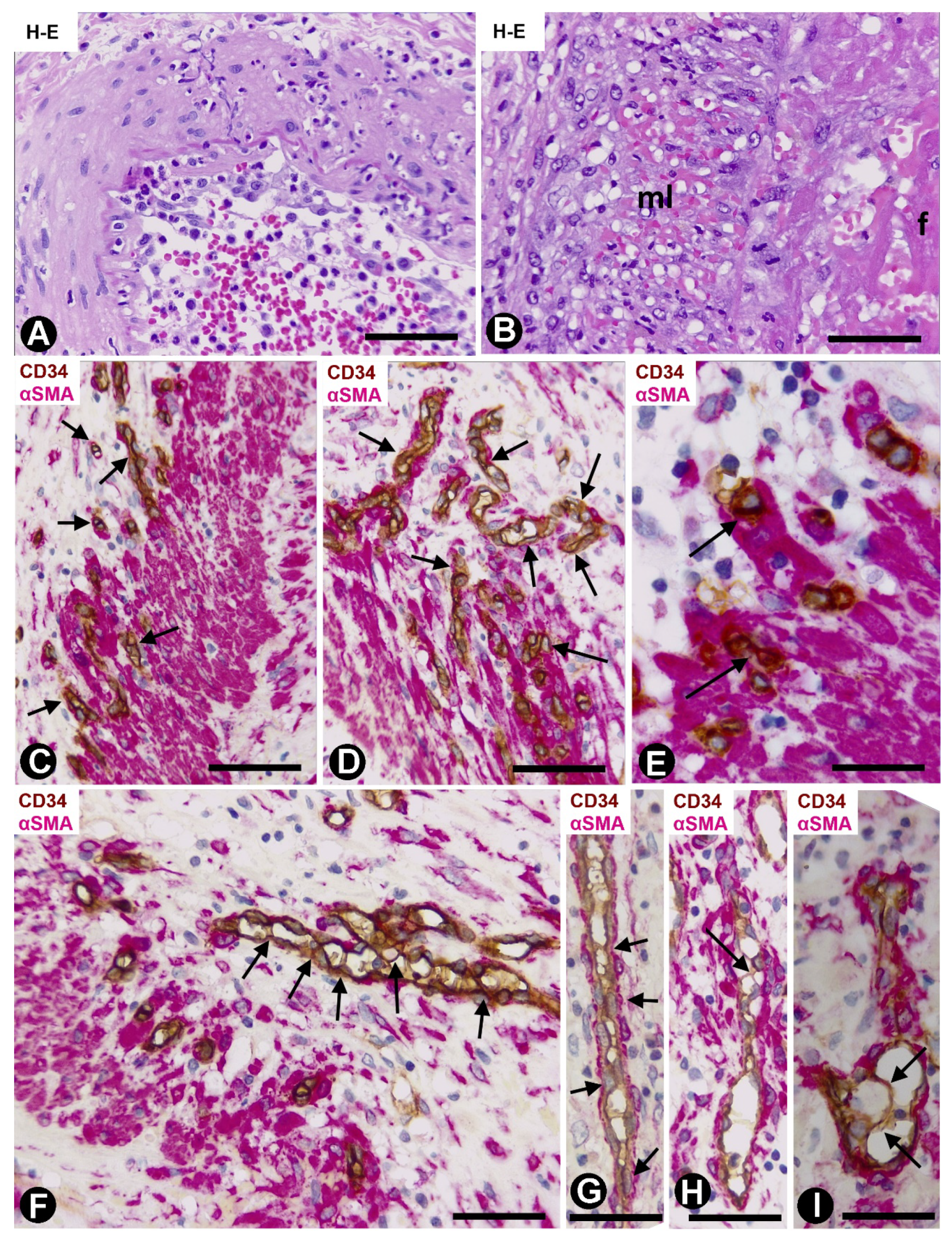
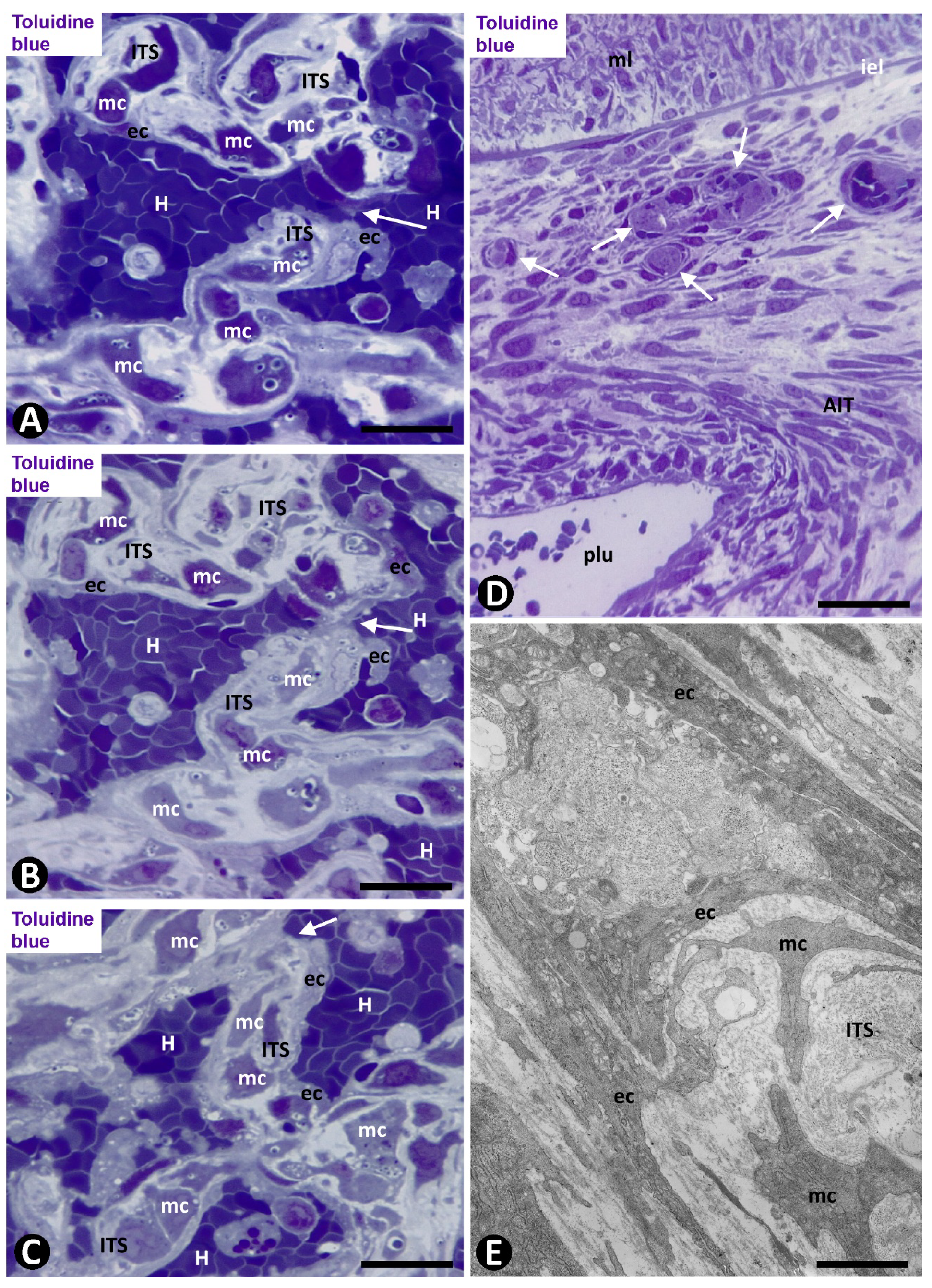
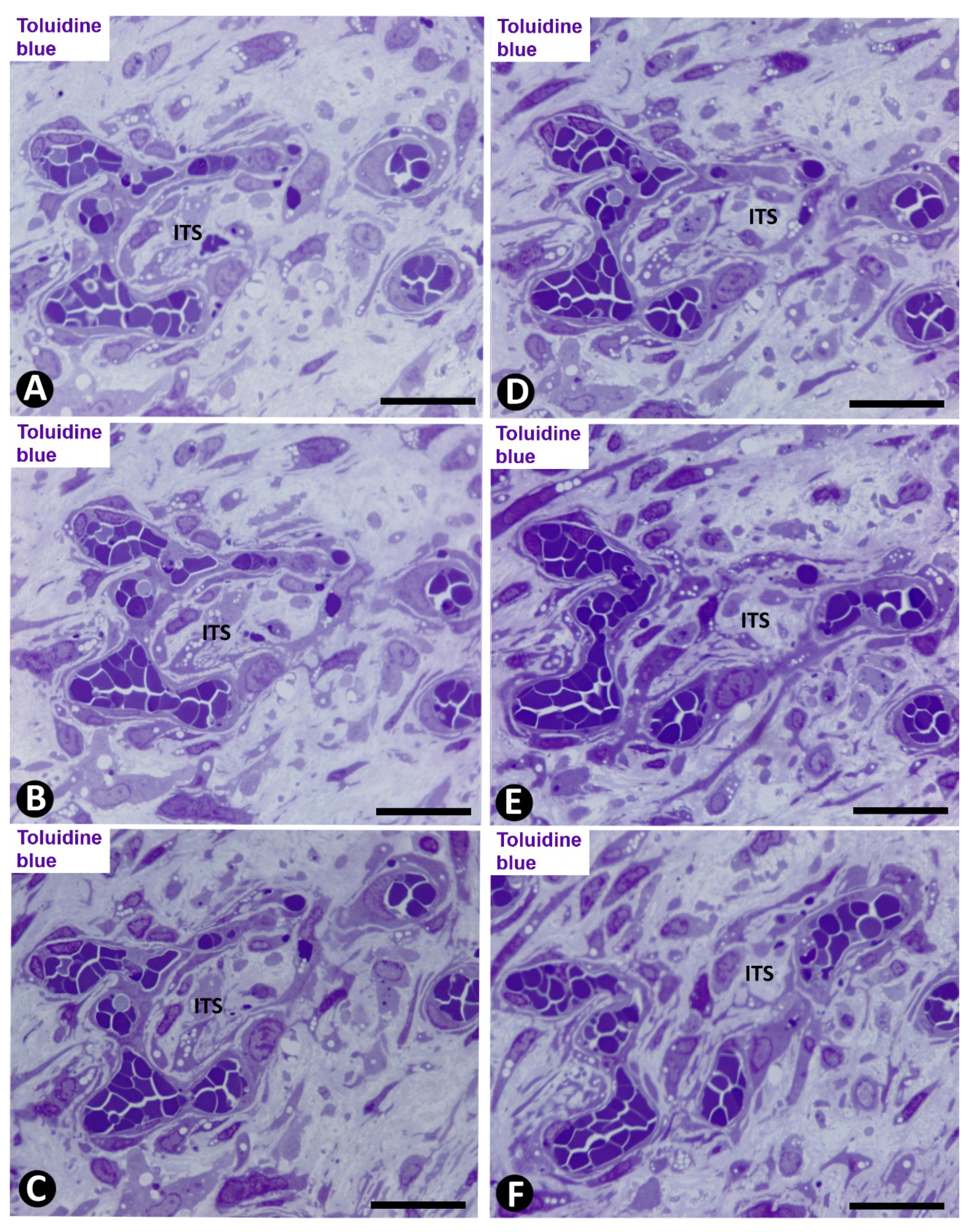
2.1. Angiogenesis in Arteries of the Human Gallbladder with Vasculitis and AIT Formation in Acute Cholecystitis
2.1.1. Arteries in Gallbladders with Acute Cholecystitis and Urgent Cholecystectomy
2.1.2. Arteries in Acute Cholecystitis with Delayed Cholecystectomy
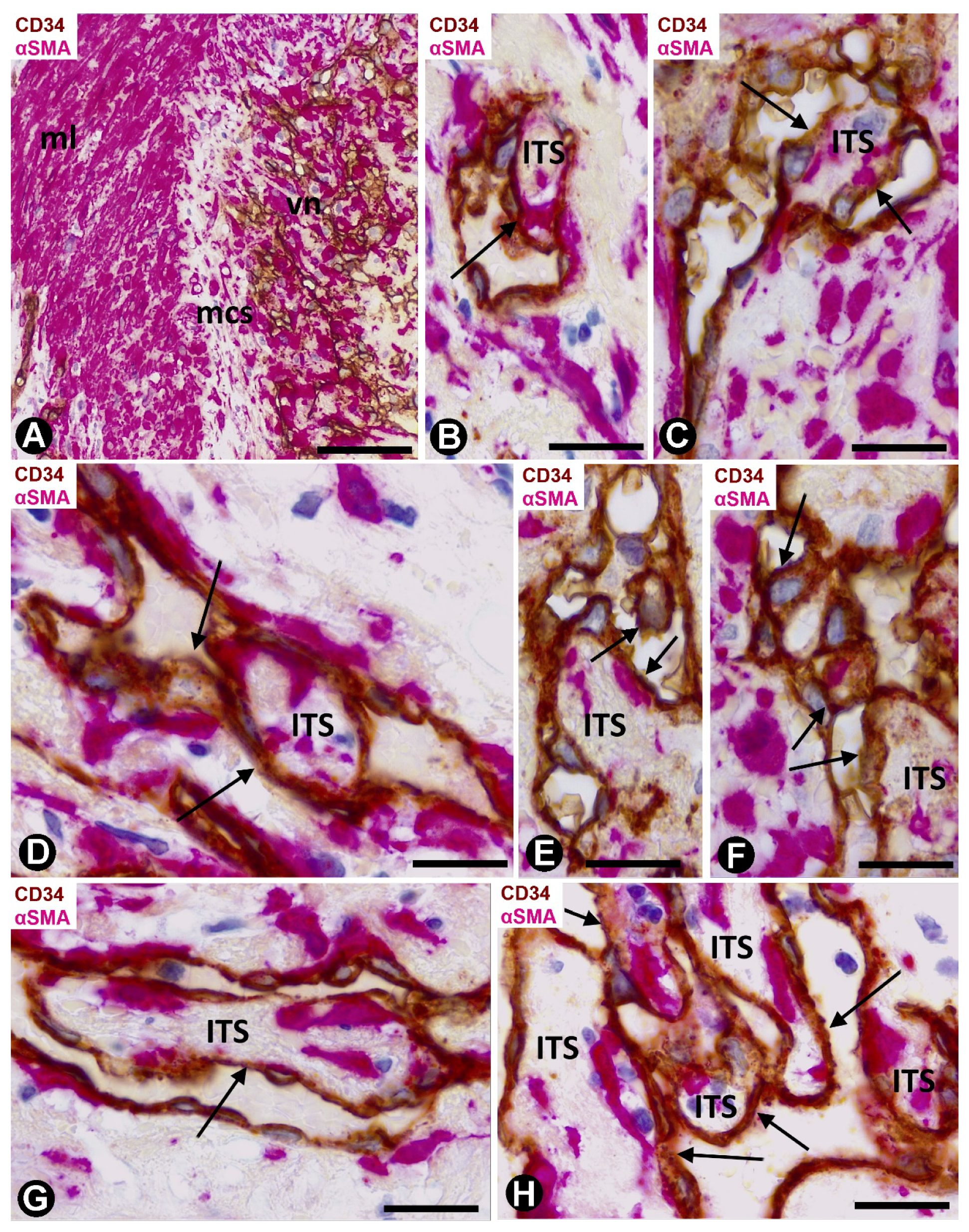
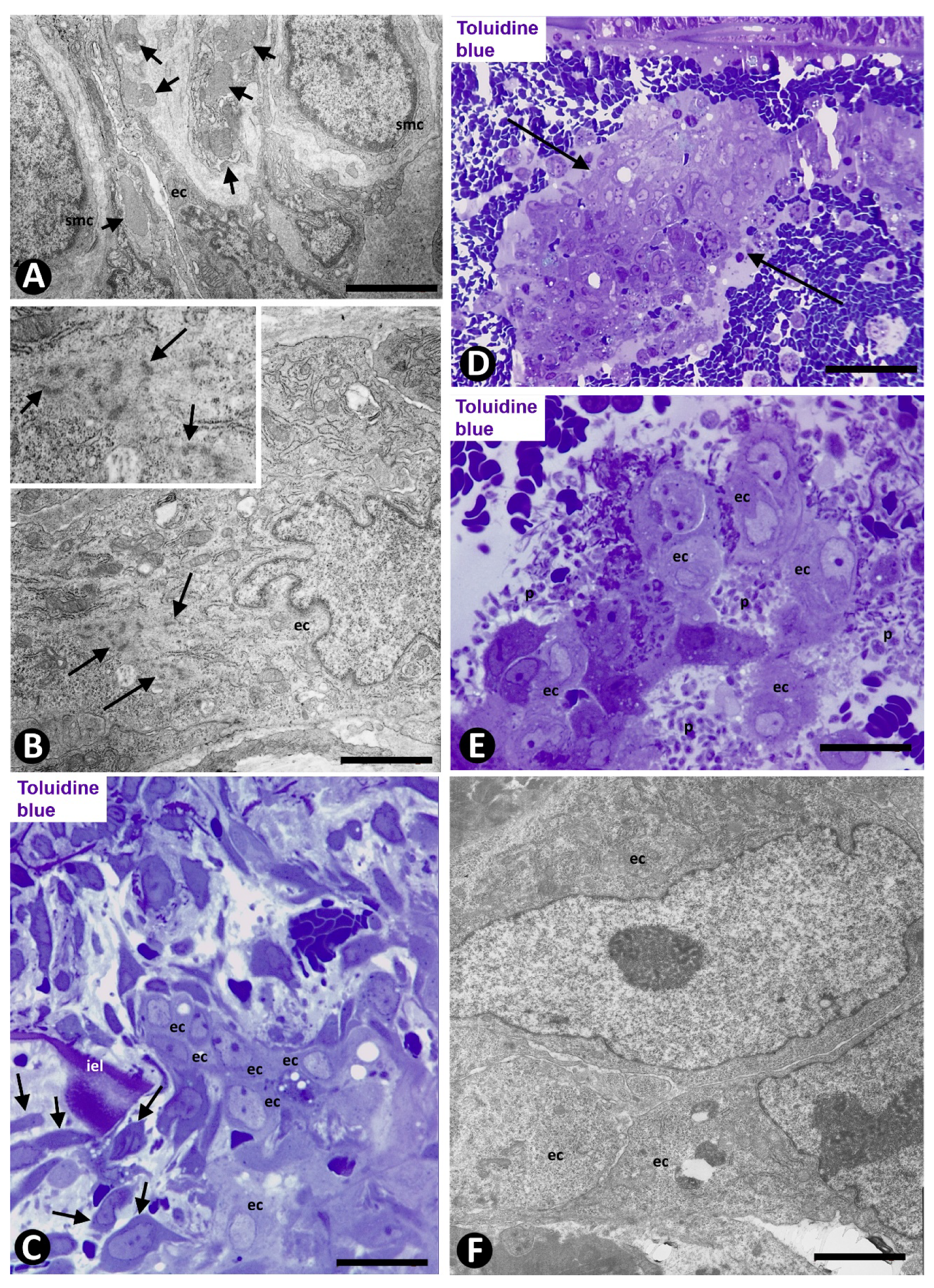
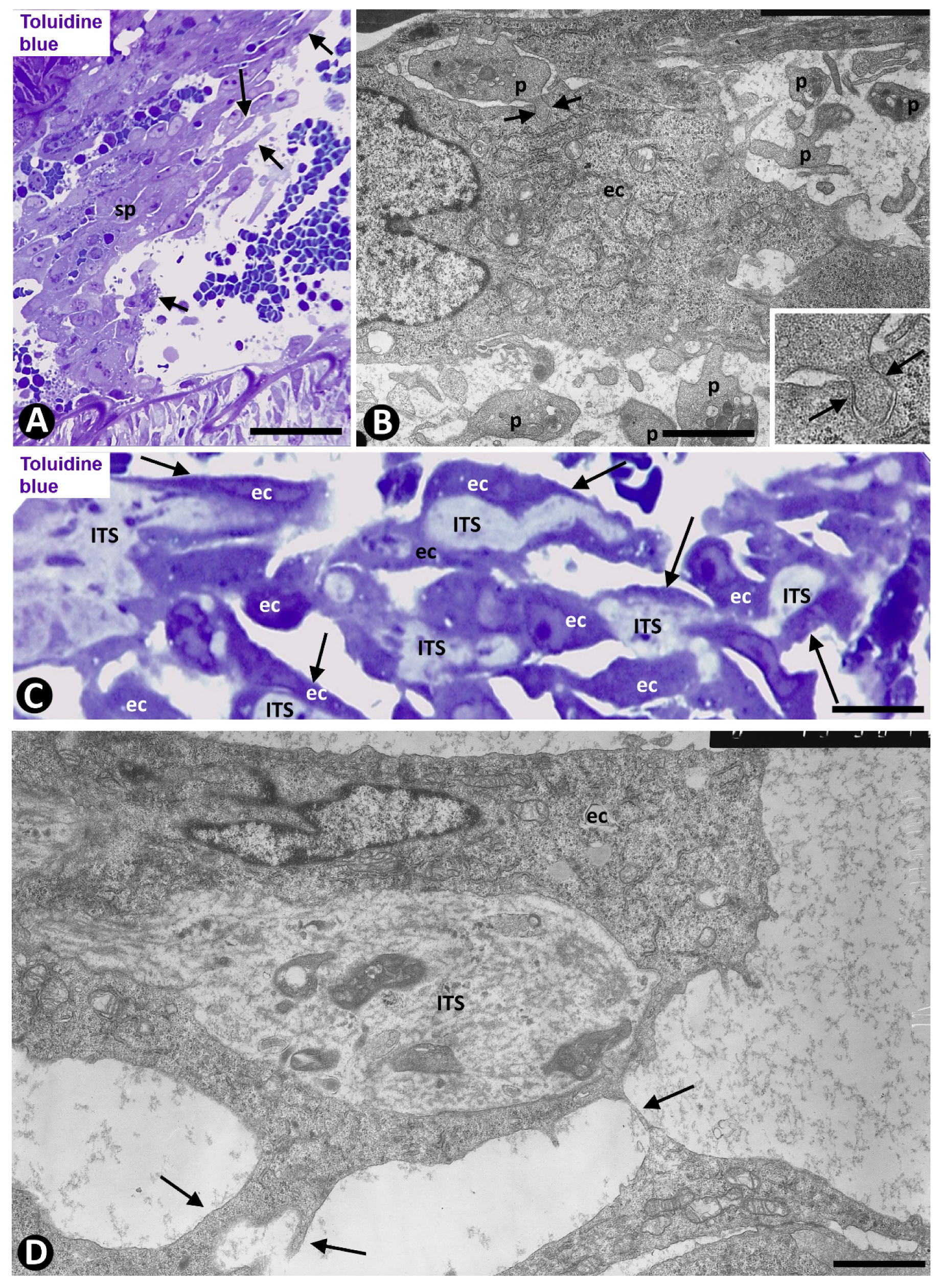
2.2. Angiogenesis in Experimental Occluded Arterial Segments with Intimal Thickening Formation
3. Discussion
4. Materials and Methods
4.1. Human Samples
4.2. Experimental Procedures
4.3. Techniques in Conventional Light Microscopy, Immunohistochemistry, and Electron Microscopy
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tibaut, M.; Caprnda, M.; Kubatka, P.; Sinkovič, A.; Valentova, V.; Filipova, S.; Gazdikova, K.; Gaspar, L.; Mozos, I.; Egom, E.E.; et al. Markers of Atherosclerosis: Part 1—Serological Markers. Heart Lung Circ. 2019, 28, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Tibaut, M.; Caprnda, M.; Kubatka, P.; Sinkovič, A.; Valentova, V.; Filipova, S.; Gazdikova, K.; Gaspar, L.; Mozos, I.; Egom, E.E.; et al. Markers of Atherosclerosis: Part 2—Genetic and Imaging Markers. Heart Lung Circ. 2019, 28, 678–689. [Google Scholar] [CrossRef] [PubMed]
- Brezinski, M.; Willard, F.; Rupnick, M. Inadequate intimal angiogenesis as a source of coronary plaque instability: Implications for healing. Circulation 2019, 140, 1857–1859. [Google Scholar] [CrossRef] [PubMed]
- Fong, G.H. Potential contributions of intimal and plaque hypoxia to atherosclerosis. Cur. Atheros. Rep. 2015, 17, 510. [Google Scholar] [CrossRef] [PubMed]
- Moreno, P.R.; Purushothaman, K.R.; Zias, E.; Sanz, J.; Fuster, V. Neovascularization in human atherosclerosis. Cur. Mol. Med. 2006, 6, 457–477. [Google Scholar] [CrossRef] [Green Version]
- Moulton, K.S.; Vakili, K.; Zurakowski, D.; Soliman, M.; Butterfield, C.; Sylvin, E.; Lo, K.M.; Gillies, S.; Javaherian, K.; Folkman, J. Inhibition of plaque neovascularization reduces macrophage accumulation and progression of advanced atherosclerosis. Proc. Natl. Acad. Sci. USA 2003, 100, 4736–4741. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Lu, X.; Shi, G.P. Vasa vasorum in atherosclerosis and clinical significance. Int. J. Mol. Sci. 2015, 16, 11574–11608. [Google Scholar] [CrossRef] [Green Version]
- Ravindran, D.; Cartland, S.P.; Bursill, C.A.; Kavurma, M.M. Broad-spectrum chemokine inhibition blocks inflammation-induced angiogenesis, but preserves ischemia-driven angiogenesis. FASEB 2019, 33, 13423–13434. [Google Scholar] [CrossRef] [Green Version]
- Diaz-Flores, L.; Dominguez, C. Relation between arterial intimal thickening and the vasa-vasorum. Virchows Arch. A 1985, 406, 165–177. [Google Scholar] [CrossRef]
- Díaz-Flores, L.; Gutiérrez, R.; García-Suárez, M.P.; Sáez, F.J.; Gutiérrez, E.; Valladares, F.; Carrasco, J.L.; Díaz-Flores, L., Jr.; Madrid, J.F. Morphofunctional basis of the different types of angiogenesis and formation of postnatal angiogenesis-related secondary structures. Histol. Histopathol. 2017, 32, 1239–1279. [Google Scholar] [CrossRef]
- Díaz-Flores, L.; Gutiérrez, R.; González-Gómez, M.; García, M.P.; Carrasco, J.L.; Díaz-Flores, L., Jr.; Madrid, J.F.; Álvarez-Argüelles, H. Participation of intussusceptive angiogenesis in the morphogenesis of lobular capillary hemangioma. Sci. Rep. 2020, 10, 4987. [Google Scholar] [CrossRef] [Green Version]
- Djonov, V.G.; Kurz, H.; Burri, P.H. Optimality in the developing vascular system: Branching remodeling by means of intussusception as an efficient adaptation mechanism. Dev. Dyn. 2002, 224, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Hlushchuk, R.; Makanya, A.N.; Djonov, V. Escape mechanisms after antiangiogenic treatment, or why are the tumors growing again? Int. J. Dev. Biol. 2011, 55, 563–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karthik, S.; Djukic, T.; Kim, J.D.; Zuber, B.; Makanya, A.; Odriozola, A.; Hlushchuk, R.; Filipovic, N.; Jin, S.W.; Djonov, V. Synergistic interaction of sprouting and intussusceptive angiogenesis during zebrafish caudal vein plexus development. Sci. Rep. 2018, 8, 9840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konerding, M.A.; Gibney, B.C.; Houdek, J.P.; Chamoto, K.; Ackermann, M.; Lee, G.S.; Lin, M.; Tsuda, A.; Mentzer, S.J. Spatial dependence of alveolar angiogenesis in postpneumonectomy lung growth. Angiogenesis 2012, 15, 23–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peebo, B.B.; Fagerholm, P.; Traneus-Röckert, C.; Lagali, N. Cellular level characterization of capillary regression in inflammatory angiogenesis using an in vivo corneal model. Angiogenesis 2011, 14, 393–405. [Google Scholar] [CrossRef] [Green Version]
- Burri, P.H. Intussusceptive microvascular growth, a new mechanism of capillary network formation. EXS 1992, 61, 32–39. [Google Scholar] [CrossRef]
- Burri, P.H.; Tarek, M.R. A novel mechanism of capillary growth in the rat pulmonary microcirculation. Anat. Rec. 1990, 228, 35–45. [Google Scholar] [CrossRef]
- Burri, P.H.; Hlushchuk, R.; Djonov, V. Intussusceptive angiogenesis: Its emergence, its characteristics, and its significance. Dev. Dyn. 2004, 231, 474–488. [Google Scholar] [CrossRef]
- Díaz-Flores, L.; Gutiérrez, R.; García, M.P.; González-Gómez, M.; Sáez, F.J.; Díaz-Flores, L., Jr.; Carrasco, J.L.; Madrid, J.F. Sinusoidal hemangioma and intravascular papillary endothelial hyperplasia: Interrelated processes that share a histogenetic piecemeal angiogenic mechanism. Acta Histochem. 2018, 120, 255–262. [Google Scholar] [CrossRef]
- Díaz-Flores, L.; Gutiérrez, R.; González-Gómez, M.; García, P.; Sáez, F.J.; Díaz-Flores, L., Jr.; Carrasco, J.L.; Madrid, J.F. Segmentation of dilated hemorrhoidal veins in hemorrhoidal disease. Cells Tissues Organs 2018, 205, 120–128. [Google Scholar] [CrossRef]
- Díaz-Flores, L.; Gutiérrez, R.; Gayoso, S.; García, M.P.; González-Gómez, M.; Díaz-Flores, L., Jr.; Sánchez, R.; Carrasco, J.L.; Madrid, J.F. Intussusceptive angiogenesis and its counterpart intussusceptive lymphangiogenesis. Histol. Histopathol. 2020, 18222. [Google Scholar] [CrossRef]
- Djonov, V.; Schmid, M.; Tschanz, S.A.; Burri, P.H. Intussusceptive angiogenesis: Its role in embryonic vascular network formation. Circ. Res. 2000, 86, 286–292. [Google Scholar] [CrossRef]
- Djonov, V.G.; Galli, A.B.; Burri, P.H. Intussusceptive arborization contributes to vascular tree formation in the chick chorio-allantoic membrane. Anat. Embryol. 2000, 202, 347–357. [Google Scholar] [CrossRef]
- Djonov, V.; Baum, O.; Burri, P.H. Vascular remodeling by intussusceptive angiogenesis. Cell Tissue Res. 2003, 314, 107–117. [Google Scholar] [CrossRef]
- Makanya, A.N.; Hlushchuk, R.; Djonov, V.G. Intussusceptive angiogenesis and its role in vascular morphogenesis, patterning, and remodeling. Angiogenesis 2009, 12, 113–123. [Google Scholar] [CrossRef] [Green Version]
- Paku, S.; Dezso, K.; Bugyik, E.; Tóvári, J.; Tímár, J.; Nagy, P.; Laszlo, V.; Klepetko, W.; Döme, B. A new mechanism for pillar formation during tumor-induced intussusceptive angiogenesis: Inverse sprouting. Am. J. Pathol. 2011, 179, 1573–1585. [Google Scholar] [CrossRef]
- Patan, S.; Munn, L.L.; Tanda, S.; Roberge, S.; Jain, R.K.; Jones, R.C. Vascular morphogenesis and remodeling in a model of tissue repair: Blood vessel formation and growth in the ovarian pedicle after ovariectomy. Circ. Res. 2001, 89, 723–731. [Google Scholar] [CrossRef]
- Patan, S.; Tanda, S.; Roberge, S.; Jones, R.C.; Jain, R.K.; Munn, L.L. Vascular morphogenesis and remodeling in a human tumor xenograft: Blood vessel formation and growth after ovariectomy and tumor implantation. Circ. Res. 2001, 89, 732–739. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.T. Gallbladder vasculitis. J. Clin. Gastroenterol. 1989, 11, 537–540. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, J.; Tan, C.D.; Rodríguez, E.R.; Hoffman, G.S. Single-organ gallbladder vasculitis: Characterization and distinction from systemic vasculitis involving the gallbladder. An analysis of 61 patients. Medicine 2014, 93, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Burri, P.H.; Djonov, V. Intussusceptive angiogenesis-the alternative to capillary sprouting. Mol. Asp. Med. 2002, 23, S1–27. [Google Scholar] [CrossRef]
- De Spiegelaere, W.; Casteleyn, C.; Van den Broeck, W.; Plendl, J.; Bahramsoltani, M.; Simoens, P.; Djonov, V.; Cornillie, P. Intussusceptive angiogenesis: A biologically relevant form of angiogenesis. J. Vasc. Res. 2012, 49, 390–404. [Google Scholar] [CrossRef]
- Díaz-Flores, L.; Gutiérrez, R.; García, M.P.; González-Gómez, M.; Díaz-Flores, L., Jr.; Carrasco, J.L. Intussusceptive lymphangiogenesis in the sinuses of developing human foetal lymph nodes. Ann. Anat. 2019, 226, 73–83. [Google Scholar] [CrossRef]
- Díaz-Flores, L.; Gutiérrez, R.; García, M.P.; González-Gómez, M.; Díaz-Flores, L., Jr.; Carrasco, J.L.; Álvarez-Argüelles, H. Intussusceptive lymphangiogenesis in vascular transformation of lymph node sinuses. Act. Histochem. 2019, 121, 392–399. [Google Scholar] [CrossRef]
- Djonov, V.; Andres, A.C.; Ziemiecki, A. Vascular remodelling during the normal and malignant life cycle of the mammary gland. Microsc. Res. Tech. 2001, 52, 182–189. [Google Scholar] [CrossRef]
- Grubić Rotkvić, P.; Cigrovski Berković, M.; Rotkvić, L.; Bulj, N. Prevention of cardiac allograft vasculopathy—A new possible indication for SGLT-2 inhibitors? Med. Hypoth. 2020, 137, 109594. [Google Scholar] [CrossRef]
- Narula, N.; Dannenberg, A.J.; Olin, J.W.; Bhatt, D.L.; Johnson, K.W.; Nadkarni, G.; Min, J.; Torii, S.; Poojary, P.; Anand, S.S.; et al. Pathology of peripheral artery disease in patients with critical limb ischemia. J. Am. Coll. Cardiol. 2018, 72, 2152–2163. [Google Scholar] [CrossRef]
- Umezu, R.; Koga, J.I.; Matoba, T.; Katsuki, S.; Wang, L.; Hasuzawa, N.; Nomura, M.; Tsutsui, H.; Egashira, K. Macrophage (Drp1) dynamin-related protein 1 accelerates intimal thickening after vascular injury. Arterios. Thromb. Vasc Biol. 2020, 40, e214–e226. [Google Scholar] [CrossRef]
- Bhardwaj, S.; Roy, H.; Heikura, T.; Ylä-Herttuala, S. VEGF-A, VEGF-D and VEGF-D (DeltaNDeltaC) induced intimal hyperplasia in carotid arteries. Eur. J. Clin. Investig. 2005, 35, 669–676. [Google Scholar] [CrossRef]
- Allsopp, G.; Gamble, H.J. An electron microscopic study of the pericytes of the developing capillaries in human fetal brain and muscle. J. Anat. 1979, 128, 155–168. [Google Scholar] [PubMed]
- Bigler, M.; Koutsantonis, D.; Odriozola, A.; Halm, S.; Tschanz, S.A.; Zakrzewicz, A.; Weichert, A.; Baum, O. Morphometry of skeletal muscle capillaries: The relationship between capillary ultrastructure and ageing in humans. Acta Physiol. 2016, 218, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Flores, L.; Gutiérrez, R.; Varela, H.; Rancel, N.; Valladares, F. Microvascular pericytes: A review of their morphological and functional characteristics. Histol. Histopathol. 1991, 6, 269–286. [Google Scholar]
- Díaz-Flores, L.; Gutiérrez, R.; Madrid, J.F.; Varela, H.; Valladares, F.; Acosta, E.; Martín-Vasallo, P.; Díaz-Flores, L., Jr. Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol. Histopathol. 2009, 24, 909–969. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Flores, L.; Gutiérrez, R.; García, M.P.; Díaz-Flores, L., Jr.; Valladares, F.; Madrid, J.F. Ultrastructure of myopericytoma: A continuum of transitional phenotypes of myopericytes. Ultrastruct. Pathol. 2012, 36, 189–194. [Google Scholar] [CrossRef]
- Díaz-Flores, L., Jr.; Gutiérrez, R.; Madrid, J.F.; Sáez, F.J.; Valladares, F.; Villar, J.; Díaz-Flores, L. Peg-and-socket junctions between smooth muscle cells and endothelial cells in femoral veins are stimulated to angiogenesis by prostaglandin E₂ and glycerols. Histol. Histopathol. 2011, 26, 623–630. [Google Scholar] [CrossRef]
- De Moor, L.; Merovci, I.; Baetens, S.; Verstraeten, J.; Kowalska, P.; Krysko, D.V.; De Vos, W.H.; Declercq, H. High-throughput fabrication of vascularized spheroids for bioprinting. Biofabrication 2018, 10, 035009. [Google Scholar] [CrossRef]
- Heiss, M.; Hellström, M.; Kalén, M.; May, T.; Weber, H.; Hecker, M.; Augustin, H.G.; Korff, T. Endothelial cell spheroids as a versatile tool to study angiogenesis in vitro. FASEB 2015, 29, 3076–3084. [Google Scholar] [CrossRef] [PubMed]
- Laschke, M.W.; Menger, M.D. Spheroids as vascularization units: From angiogenesis research to tissue engineering applications. Biotechnol. Adv. 2017, 35, 782–791. [Google Scholar] [CrossRef]
- van Engeland, N.; Pollet, A.; den Toonder, J.; Bouten, C.; Stassen, O.; Sahlgren, C.M. A biomimetic microfluidic model to study signalling between endothelial and vascular smooth muscle cells under hemodynamic conditions. Lab Chip 2018, 18, 1607–1620. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Flores, L.; Gutiérrez, R.; García, M.D.P.; Sáez, F.J.; Díaz-Flores, L., Jr.; Madrid, J.F. Piecemeal mechanism combining sprouting and intussusceptive angiogenesis in intravenous papillary formation induced by PGE2 and glycerol. Anat. Rec. 2017, 300, 1781–1792. [Google Scholar] [CrossRef] [Green Version]
- Patan, S.; Álvarez, M.J.; Schittny, J.C.; Burri, P.H. Intussusceptive microvascular growth: A common alternative to capillary sprouting. Arch. Histol. Cytol. 1992, 55, 65–75. [Google Scholar] [CrossRef] [Green Version]
- Patan, S.; Haenni, B.; Burri, P.H. Evidence for intussusceptive capillary growth in the chicken chorio-allantoic membrane (CAM). Anat. Embryol. 1993, 187, 121–130. [Google Scholar] [CrossRef]
- Patan, S.; Munn, L.L.; Jain, R.K. Intussusceptive microvascular growth in a human colon adenocarcinoma xenograft: A novel mechanism of tumor angiogenesis. Microvasc. Res. 1996, 51, 260–272. [Google Scholar] [CrossRef]
- Patan, S.; Haenni, B.; Burri, P.H. Implementation of intussusceptive microvascular growth in the chicken chorioallantoic membrane (CAM). Microvasc. Res. 1997, 53, 33–52. [Google Scholar] [CrossRef]
- Patan, S. Lycat and cloche at the switch between blood vessel growth and differentiation? Circ. Res. 2008, 102, 1005–1007. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Flores, L.; Gutiérrez, R.; García, M.; Carrasco, J.L.; Sáez, F.J.; Díaz-Flores, L., Jr.; González-Gómez, M.; Madrid, J.F. Intussusceptive lymphangiogenesis in lymphatic malformations/lymphangiomas. Anat. Rec. 2019, 302, 2003–2013. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, M.K.; Finsterwalder, R.; Leb, H.; Resch, U.; Neumüller, K.; de Martin, R.; Petzelbauer, P. Three-dimensional coculture model to analyze the cross talk between endothelial and smooth muscle cells. Tissue Eng. Part C Methods 2017, 23, 38–49. [Google Scholar] [CrossRef] [Green Version]
- Hamaoka-Okamoto, A.; Suzuki, C.; Yahata, T.; Ikeda, K.; Nagi-Miura, N.; Ohno, N.; Arai, Y.; Tanaka, H.; Takamatsu, T.; Hamaoka, K. The involvement of the vasa vasorum in the development of vasculitis in animal models of Kawasaki disease. Pediatric Rheumatol. 2014, 12, 12. [Google Scholar] [CrossRef] [Green Version]
- Sakurai, Y. Autoimmune Aspects of Kawasaki Disease. J. Investig. Alergol. Clin. Immunol. 2019, 29, 251–261. [Google Scholar] [CrossRef] [Green Version]
- Ackermann, M.; Tsuda, A.; Secomb, T.W.; Mentzer, S.J.; Konerding, M.A. Intussusceptive remodeling of vascular branch angles in chemically-induced murine colitis. Microvasc. Res. 2013, 87, 75–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurz, H.; Burri, P.H.; Djonov, V.G. Angiogenesis and vascular remodeling by intussusception: From form to function. News Physiol. Sci. 2003, 18, 65–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, G.S.; Filipovic, N.; Lin, M.; Gibney, B.C.; Simpson, D.C.; Konerding, M.A.; Tsuda, A.; Mentzer, S.J. Intravascular pillars and pruning in the extraembryonic vessels of chick embryos. Dev. Dyn. 2011, 240, 1335–1343. [Google Scholar] [CrossRef] [Green Version]
- Tsuda, A.; Turhan, A.; Konerding, M.; Ravnic, D.; Hanidziar, D.; Lin, M.; Mentzer, S.J. Bimodal oscillation frequencies of blood flow in the inflammatory colon microcirculation. Anat. Rec. 2009, 292, 65–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turhan, A.; Konerding, M.A.; Tsuda, A.; Ravnic, D.J.; Hanidziar, D.; Lin, M.; Mentzer, S.J. Bridging mucosal vessels associated with rhythmically oscillating blood flow in murine colitis. Anat. Rec. 2008, 291, 74–82. [Google Scholar] [CrossRef]
- Alamri, A.; Burzangi, A.S.; Coats, P.; Watson, D.G. Untargeted metabolic profiling cell-based approach of pulmonary artery smooth muscle cells in response to high glucose and the effect of the antioxidant vitamins D and E. Metabolites 2018, 8, 87. [Google Scholar] [CrossRef] [Green Version]
- Lehners, M.; Dobrowinski, H.; Feil, S.; Feil, R. cGMP signaling and vascular smooth muscle cell plasticity. J. Cardiovasc. Dev. Dis. 2018, 5, 20. [Google Scholar] [CrossRef] [Green Version]
- Lin, P.Y.; Sung, P.H.; Chung, S.Y.; Hsu, S.L.; Chung, W.J.; Sheu, J.J.; Hsueh, S.K.; Chen, K.H.; Wu, R.W.; Yip, H.K. Hyperbaric oxygen therapy enhanced circulating levels of endothelial progenitor cells and angiogenesis biomarkers, blood flow, in ischemic areas in patients with peripheral arterial occlusive disease. J. Clin. Med. 2018, 7, 548. [Google Scholar] [CrossRef] [Green Version]
- Sato, K.; Nitta, M.; Ogawa, A. A microfluidic cell stretch device to investigate the effects of stretching stress on artery smooth muscle cell proliferation in pulmonary arterial hypertension. Inventions 2019, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Sato, K.; Yamashita, T.; Shirai, R.; Shibata, K.; Okano, T.; Yamaguchi, M.; Mori, Y.; Hirano, T.; Watanabe, T. Adropin contributes to anti-atherosclerosis by suppressing monocyte-endothelial cell adhesion and smooth muscle cell proliferation. Int. J. Mol. Sci. 2018, 19, 1293. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Flores, L.; Gutiérrez, R.; García, M.P.; González-Gómez, M.; Díaz-Flores, L., Jr.; Álvarez-Argüelles, H.; Carrasco, J.L. Presence/absence and specific location of resident cd34+ stromal cells/telocytes condition stromal cell development in repair and tumors. Front. Cell Dev. Biol. 2020, 8, 544845. [Google Scholar] [CrossRef] [PubMed]
- Madrid, J.F.; Díaz-Flores, L.; Gutiérrez, R.; Varela, H.; Valladares, F.; Rancel, N.; Rodríguez, F. Participation of angiogenesis from rat femoral veins in the neovascularization of adjacent occluded arteries. Histol. Histopathol. 1998, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Flores, L.; Gutiérrez, R.; García, M.P.; Gayoso, S.; Carrasco, J.L.; Díaz-Flores, L., Jr.; González-Gómez, M.; Madrid, J.F. Intussusceptive Angiogenesis and Peg–Socket Junctions between Endothelial Cells and Smooth Muscle Cells in Early Arterial Intimal Thickening. Int. J. Mol. Sci. 2020, 21, 8049. https://doi.org/10.3390/ijms21218049
Díaz-Flores L, Gutiérrez R, García MP, Gayoso S, Carrasco JL, Díaz-Flores L Jr., González-Gómez M, Madrid JF. Intussusceptive Angiogenesis and Peg–Socket Junctions between Endothelial Cells and Smooth Muscle Cells in Early Arterial Intimal Thickening. International Journal of Molecular Sciences. 2020; 21(21):8049. https://doi.org/10.3390/ijms21218049
Chicago/Turabian StyleDíaz-Flores, Lucio, Ricardo Gutiérrez, Mª Pino García, Sara Gayoso, José Luís Carrasco, Lucio. Díaz-Flores, Jr., Miriam González-Gómez, and Juan Francisco Madrid. 2020. "Intussusceptive Angiogenesis and Peg–Socket Junctions between Endothelial Cells and Smooth Muscle Cells in Early Arterial Intimal Thickening" International Journal of Molecular Sciences 21, no. 21: 8049. https://doi.org/10.3390/ijms21218049
APA StyleDíaz-Flores, L., Gutiérrez, R., García, M. P., Gayoso, S., Carrasco, J. L., Díaz-Flores, L., Jr., González-Gómez, M., & Madrid, J. F. (2020). Intussusceptive Angiogenesis and Peg–Socket Junctions between Endothelial Cells and Smooth Muscle Cells in Early Arterial Intimal Thickening. International Journal of Molecular Sciences, 21(21), 8049. https://doi.org/10.3390/ijms21218049




