Circulatory Rejuvenated EPCs Derived from PAOD Patients Treated by CD34+ Cells and Hyperbaric Oxygen Therapy Salvaged the Nude Mouse Limb against Critical Ischemia
Abstract
:1. Introduction
2. Results
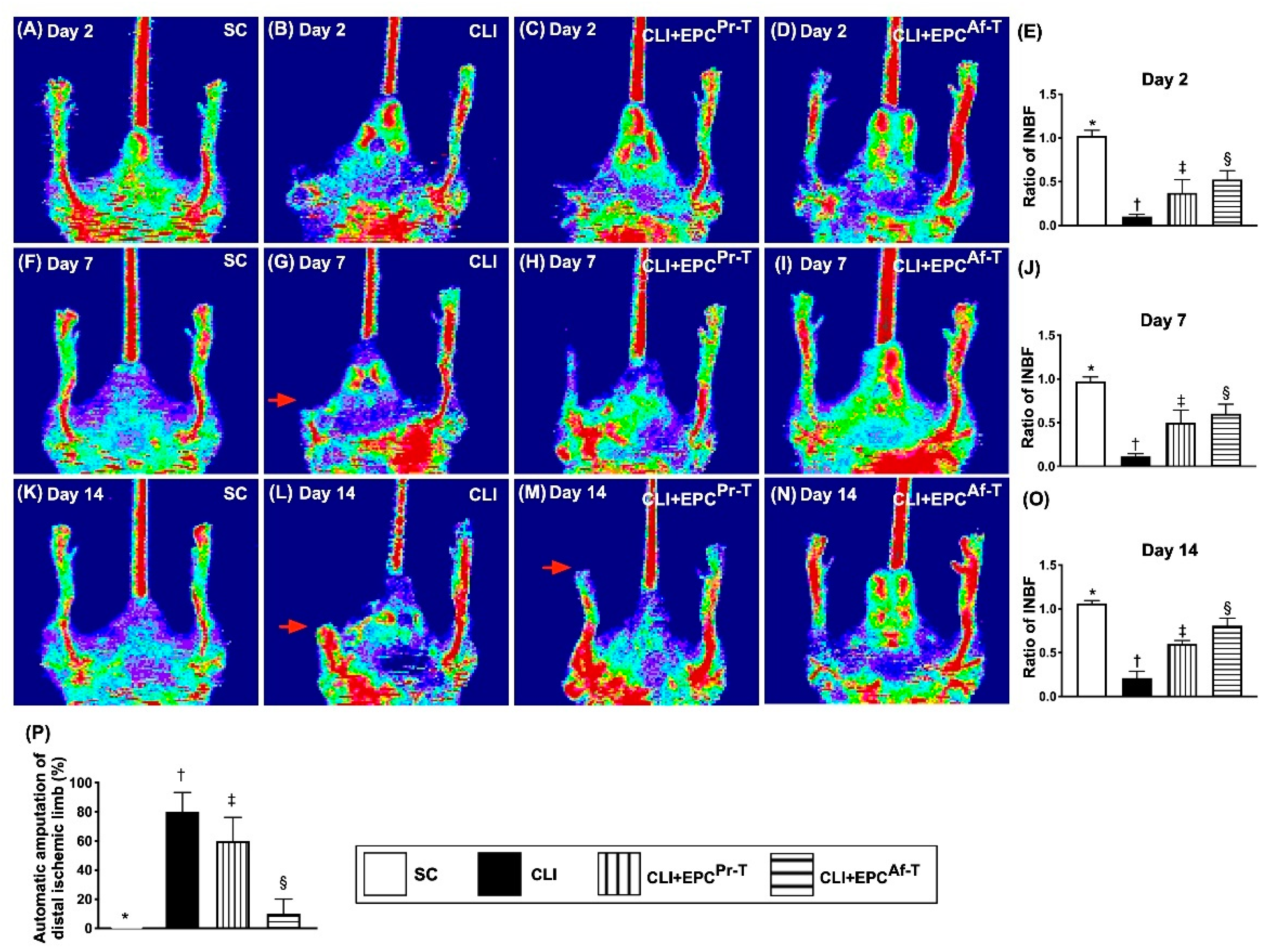
2.1. Ischemic/Normal Blood Flow (INBF) Ratio Measured by Laser Doppler Scan at Days 2, 7 and 14 after Left Femoral Artery Ligated and Totally Removed and Automatic Amputation of Distal Critical Ischemic Limb
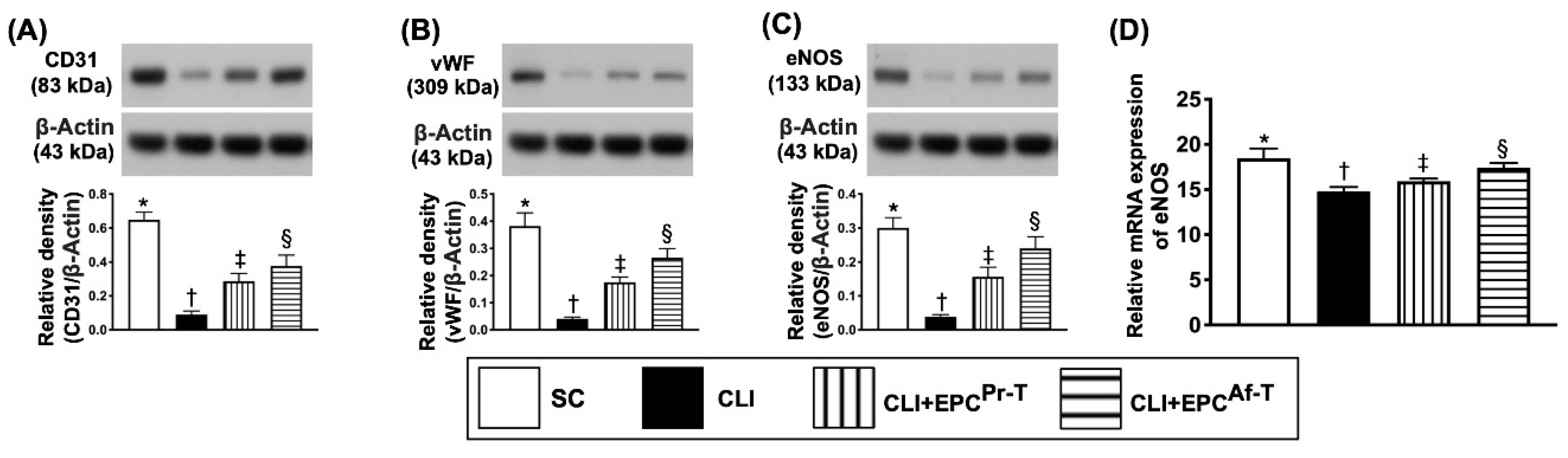
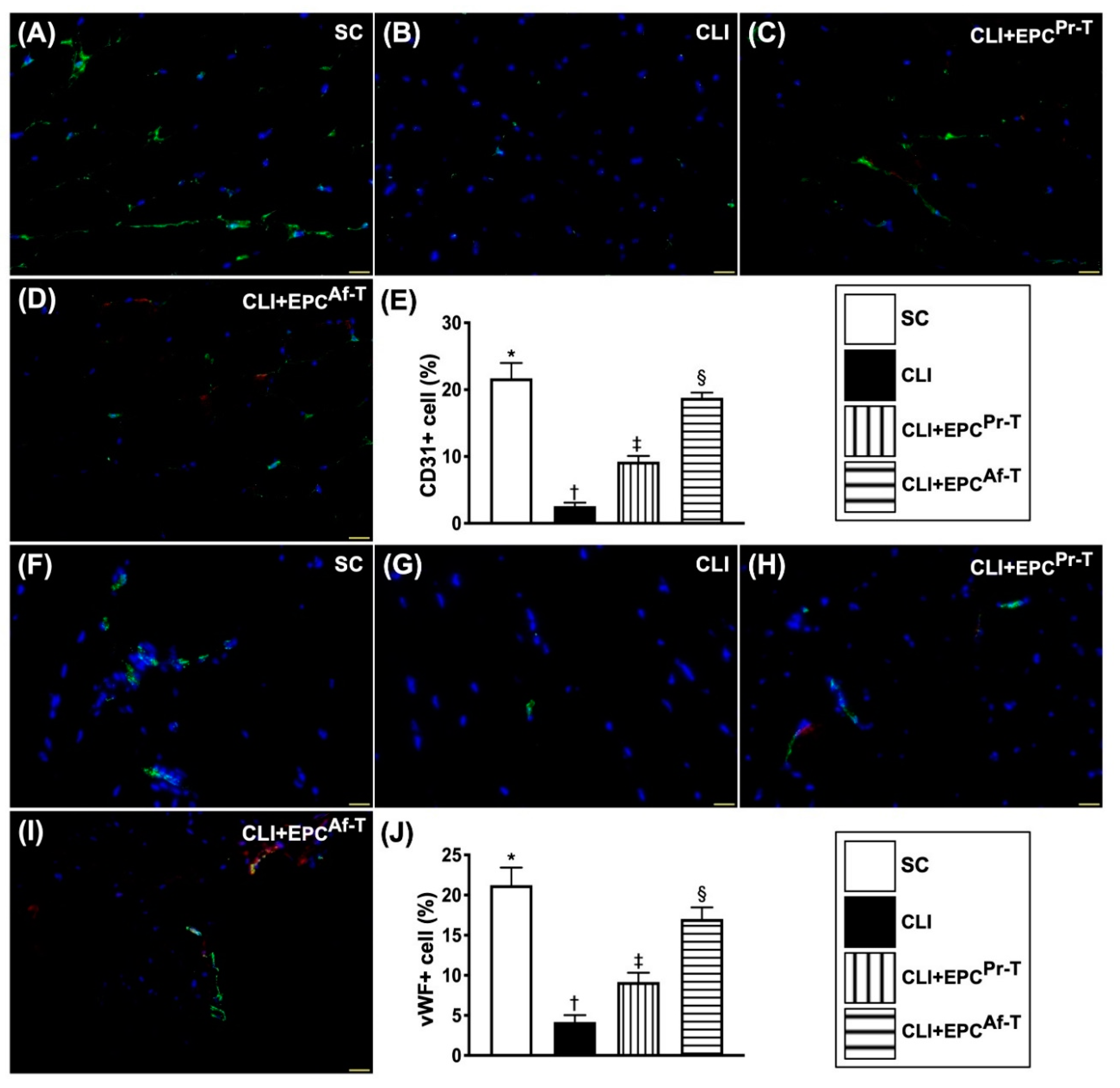
2.2. The Protein Expressions of Endothelial Cell Functional Integrity in CLI Zone by Day 28 after CLI Procedure
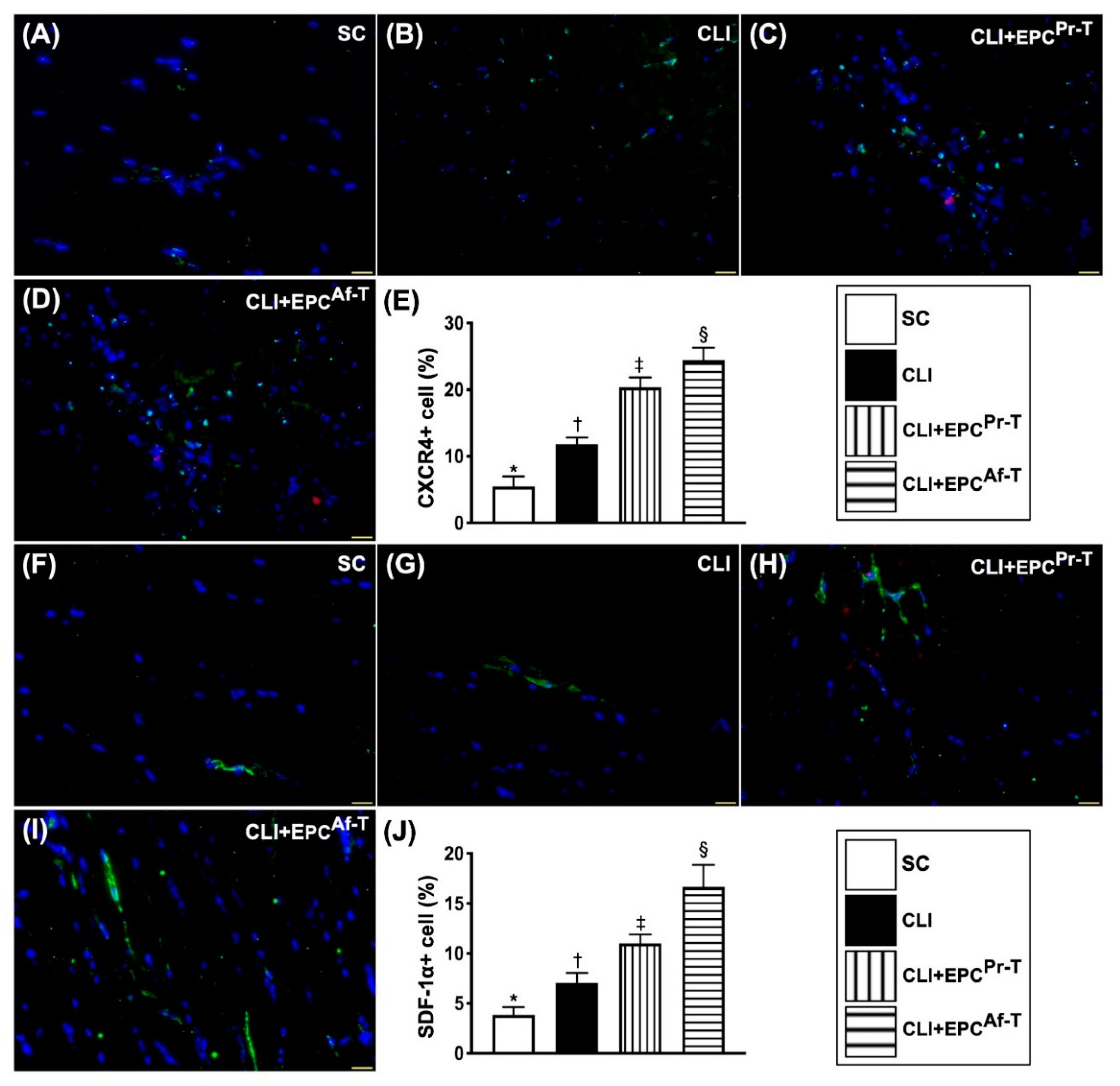
2.3. Protein Expressions of Angiogenesis in CLI Zone by Day 28 after CLI Procedure
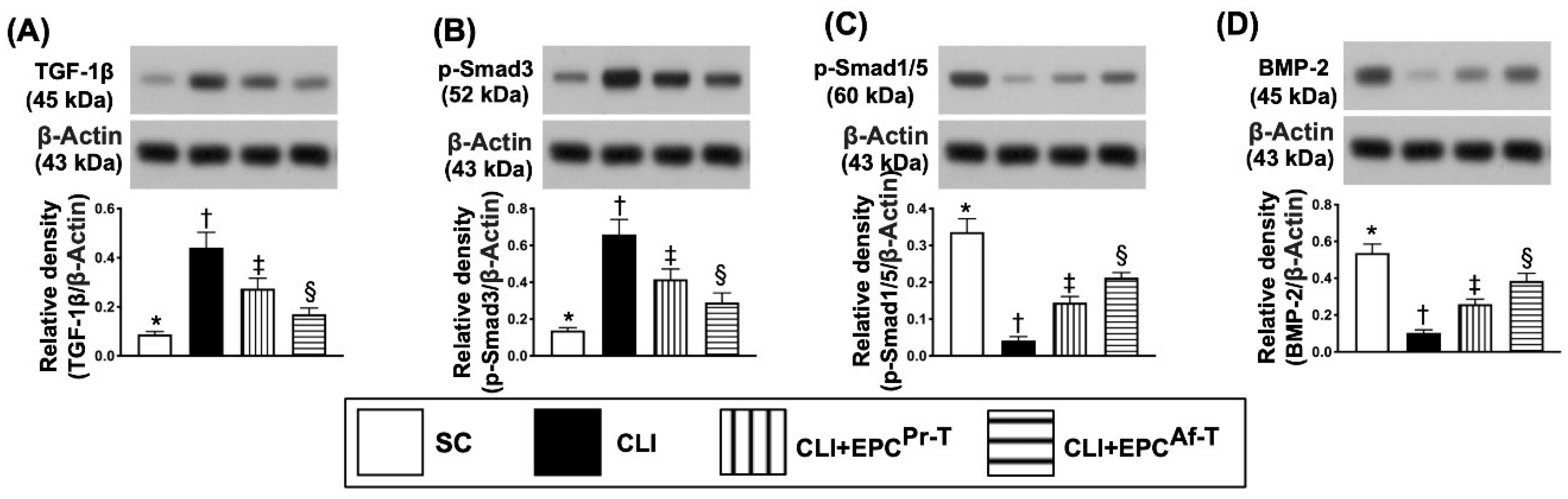
2.4. Protein Expressions of Fibrotic and Antifibrotic Biomarkers in CLI Zone by Day 28 after CLI Procedure
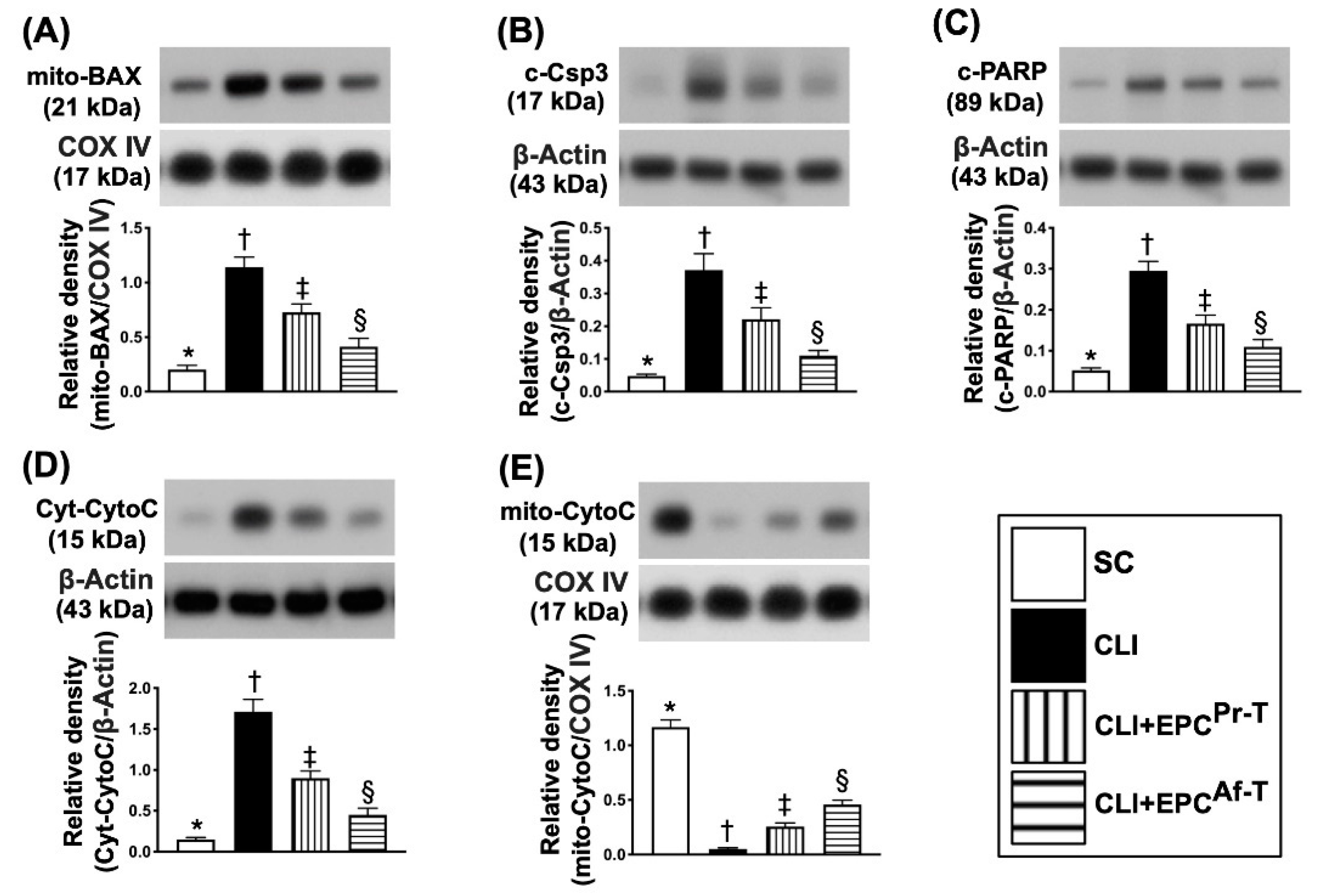
2.5. Protein Expressions of Apoptotic, Mitochondrial-Damaged and Mitochondrial-Integrity Biomarkers in CLI Zone by Day 28 after CLI Procedure
2.6. Cellular Expressions of Endothelial Cell and EPC Surface Markers in CLI Zone by Day 28 after CLI Procedure
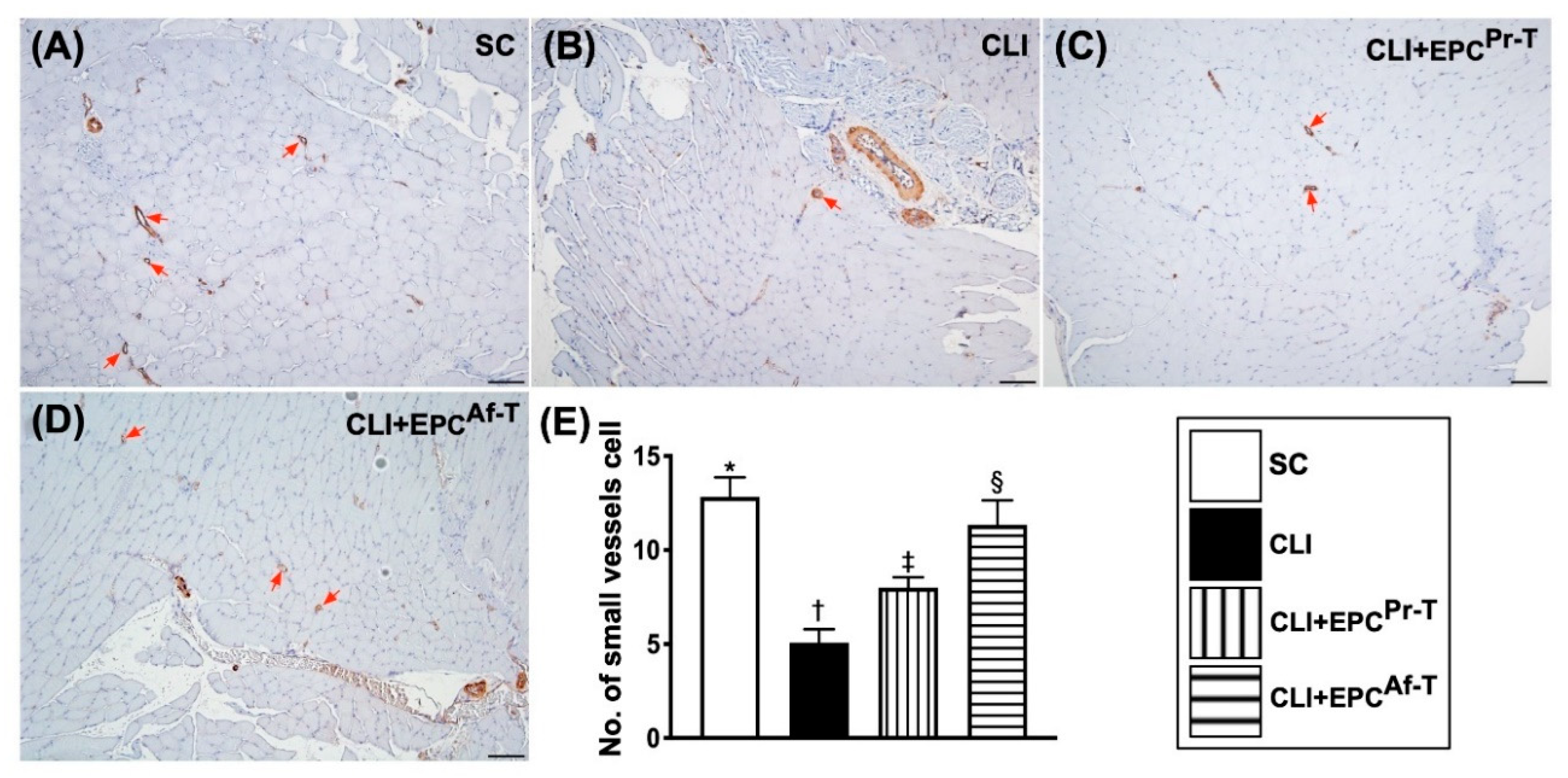
2.7. Small Vessel Density in CLI Zone by Day 28 after CLI Procedure
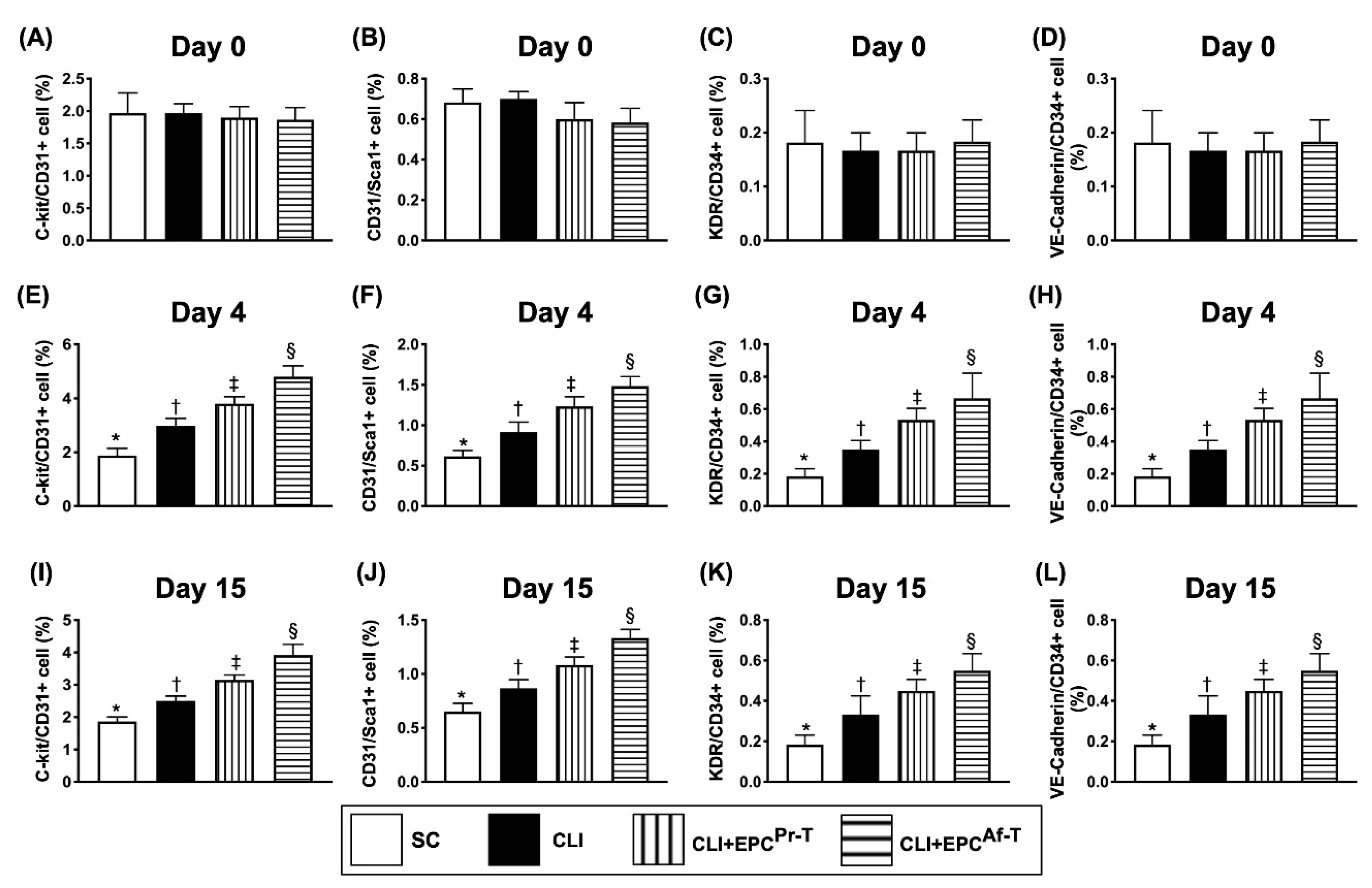
2.8. Time Courses of Circulatory Endothelial Progenitor Cells among the Groups
3. Discussion
Study Limitation
4. Materials and Methods
4.1. Ethics and Study Design
4.2. Procedure and Protocol for Isolation of Autologous CD34+ Cells and Intra-Superficial Femoral Artery Infusion
4.3. Procedure and Protocol of Hyperbaric Oxygen Therapy
4.4. Ethics of Animal Model Study
4.5. Animal Model of CLI, Animal Grouping and Strategic Treatments
4.6. Peripheral Blood Collected and Cultured for Endothelial Progenitor Cells
4.7. Measurement of Blood Flow with Laser Doppler
4.8. Vessel Density in CLI Area
4.9. Western Blot Analysis
4.10. Immunohistochemical (IHC) and Immunofluorescent (IF) Staining
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hiatt, W.R. Medical Treatment of Peripheral Arterial Disease and Claudication. N. Engl. J. Med. 2001, 344, 1608–1621. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, A.T.; Criqui, M.H.; Treat-Jacobson, D.; Regensteiner, J.G.; Creager, M.A.; Olin, J.W.; Krook, S.H.; Hunninghake, D.B.; Comerota, A.J.; Walsh, M.E.; et al. Peripheral Arterial Disease Detection, Awareness, and Treatment in Primary Care. JAMA 2001, 286, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Ouriel, K. Peripheral arterial disease. Lancet 2001, 358, 1257–1264. [Google Scholar] [CrossRef]
- Dormandy, J.; Heeck, L.; Vig, S. The natural history of claudication: Risk to life and limb. Semin. Vasc. Surg. 1999, 12, 123–137. [Google Scholar] [PubMed]
- Muluk, S.C.; Muluk, V.S.; Kelley, M.E.; Whittle, J.C.; Tierney, J.A.; Webster, M.W.; Makaroun, M.S. Outcome events in patients with claudication: A 15-year study in 2777 patients. J. Vasc. Surg. 2001, 33, 251–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dormandy, J.A.; Charbonnel, B.; A Eckland, D.J.; Erdmann, E.; Massi-Benedetti, M.; Moules, I.K.; Skene, A.M.; Tan, M.H.; Lefèbvre, P.J.; Murray, G.D.; et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): A randomised controlled trial. Lancet 2005, 366, 1279–1289. [Google Scholar] [CrossRef]
- Rena, O.; Garavoglia, M.; Francini, M.; Bellora, P.; Oliaro, A.; Casadio, C. Solitary pericardial hydatid cyst. J. Cardiovasc. Surg. 2004, 45, 77–80. [Google Scholar]
- Sheu, J.-J.; Lin, P.-Y.; Sung, P.-H.; Chen, Y.-C.; Leu, S.; Chen, Y.-L.; Tsai, T.-H.; Chai, H.-T.; Chua, S.; Chang, H.-W.; et al. Levels and values of lipoprotein-associated phospholipase A2, galectin-3, RhoA/ROCK, and endothelial progenitor cells in critical limb ischemia: Pharmaco-therapeutic role of cilostazol and clopidogrel combination therapy. J. Transl. Med. 2014, 12, 101. [Google Scholar] [CrossRef] [Green Version]
- Diehm, C.; Allenberg, J.R.; Pittrow, D.; Mahn, M.; Tepohl, G.; Haberl, R.L.; Darius, H.; Burghaus, I.; Trampisch, H.J.; German Epidemiological Trial on Ankle Brachial Index Study Group. Mortality and Vascular Morbidity in Older Adults with Asymptomatic Versus Symptomatic Peripheral Artery Disease. Circulation 2009, 120, 2053–2061. [Google Scholar] [CrossRef] [Green Version]
- Dormandy, J.; Heeck, L.; Vig, S. The fate of patients with critical leg ischemia. Semin. Vasc. Surg. 1999, 12, 142–147. [Google Scholar]
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G.; Group, T.I.W. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J. Vasc. Surg. 2007, 45, S5–S67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradbury, A.W.; Adam, D.J.; Bell, J.; Forbes, J.F.; Fowkes, F.G.R.; Gillespie, I.; Raab, G.; Ruckley, C.V. Multicentre randomised controlled trial of the clinical and cost-effectiveness of a bypass-surgery-first versus a balloon-angioplasty-first revascularisation strategy for severe limb ischaemia due to infrainguinal disease. The Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial. Health Technol. Assess. 2010, 14, 1–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Sayed, H.F. Bypass surgery for lower extremity limb salvage: Vein bypass. Methodist DeBakey Cardiovasc. J. 2013, 8, 37–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vartanian, S.M.; Conte, M.S. Surgical Intervention for Peripheral Arterial Disease. Circ. Res. 2015, 116, 1614–1628. [Google Scholar] [CrossRef]
- Thukkani, A.K.; Kinlay, S. Endovascular Intervention for Peripheral Artery Disease. Circ. Res. 2015, 116, 1599–1613. [Google Scholar] [CrossRef] [Green Version]
- Leu, S.; Sun, C.-K.; Sheu, J.-J.; Chang, L.-T.; Yuen, C.-M.; Yen, C.-H.; Chiang, C.-H.; Ko, S.-F.; Pei, S.-N.; Chua, S.; et al. Autologous bone marrow cell implantation attenuates left ventricular remodeling and improves heart function in porcine myocardial infarction: An echocardiographic, six-month angiographic, and molecular—Cellular study. Int. J. Cardiol. 2011, 150, 156–168. [Google Scholar] [CrossRef]
- Losordo, D.W.; Henry, T.D.; Davidson, C.; Lee, J.S.; Costa, M.A.; Bass, T.; Mendelsohn, F.; Fortuin, F.D.; Pepine, C.J.; Traverse, J.H.; et al. Intramyocardial, Autologous CD34+ Cell Therapy for Refractory Angina. Circ. Res. 2011, 109, 428–436. [Google Scholar] [CrossRef] [Green Version]
- Lee, F.-Y.; Chen, Y.-L.; Sung, P.-H.; Ma, M.-C.; Pei, S.-N.; Wu, C.-J.; Yang, C.-H.; Fu, M.; Ko, S.-F.; Leu, S.; et al. Intracoronary Transfusion of Circulation-Derived CD34+ Cells Improves Left Ventricular Function in Patients With End-Stage Diffuse Coronary Artery Disease Unsuitable for Coronary Intervention. Crit. Care Med. 2015, 43, 2117–2132. [Google Scholar] [CrossRef]
- Sung, P.-H.; Lee, F.-Y.; Tong, M.-S.; Chiang, J.Y.; Pei, S.-N.; Ma, M.-C.; Li, Y.-C.; Chen, Y.-L.; Wu, C.-J.; Sheu, J.-J.; et al. The Five-Year Clinical and Angiographic Follow-Up Outcomes of Intracoronary Transfusion of Circulation-Derived CD34+ Cells for Patients with End-Stage Diffuse Coronary Artery Disease Unsuitable for Coronary Intervention—Phase I Clinical Trial. Crit. Care Med. 2018, 46, e411–e418. [Google Scholar] [CrossRef]
- Quyyumi, A.A.; Vasquez, A.; Kereiakes, D.J.; Klapholz, M.; Schaer, G.L.; Abdel-Latif, A.; Frohwein, S.; Henry, T.D.; Schatz, R.A.; Dib, N.; et al. PreSERVE-AMI: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial of Intracoronary Administration of Autologous CD34+ Cells in Patients With Left Ventricular Dysfunction Post STEMI. Circ. Res. 2017, 120, 324–331. [Google Scholar] [CrossRef] [Green Version]
- Peeters Weem, S.M.; Teraa, M.; de Borst, G.J.; Verhaar, M.C.; Moll, F.L. Bone Marrow derived Cell Therapy in Critical Limb Ischemia: A Meta-analysis of Randomized Placebo Controlled Trials. Eur. J. Vasc. Endovasc. Surg. 2015, 50, 775–783. [Google Scholar] [CrossRef]
- Rigato, M.; Monami, M.; Fadini, G.P. Autologous Cell Therapy for Peripheral Arterial Disease: Systematic Review and Meta-Analysis of Randomized, Nonrandomized, and Noncontrolled Studies. Circ. Res. 2017, 120, 1326–1340. [Google Scholar] [CrossRef] [PubMed]
- Slovut, D.P.; Sullivan, T.M. Critical limb ischemia: Medical and surgical management. Vasc. Med. 2008, 13, 281–291. [Google Scholar] [CrossRef]
- Thom, S.R. Hyperbaric Oxygen: Its Mechanisms and Efficacy. Plast. Reconstr. Surg. 2011, 127 (Suppl. 1), 131S–141S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, P.Y.; Sung, P.H.; Chung, S.Y.; Hsu, S.L.; Chung, W.J.; Sheu, J.J.; Hsueh, S.K.; Chen, K.H.; Wu, R.W.; Yip, H.K. Hyperbaric Oxygen Therapy Enhanced Circulating Levels of Endothelial Progenitor Cells and Angiogenesis Biomarkers, Blood Flow, in Ischemic Areas in Patients with Peripheral Arterial Occlusive Disease. J. Clin. Med. 2018, 7, 548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorman, J.F.; Stansell, G.B.; Douglass, F.M. Limitations of Hyperbaric Oxygenation in Occlusive Arterial Disease. Circulation 1965, 32, 936–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manax, W.G.; Bloch, J.H.; Longerbeam, J.K.; Lillehei, R.C. Successful 24 Hour In Vitro Preservation of Canine Kidneys by the Combined Use of Hyperbaric Oxygenation and Hypothermia. Surgery 1964, 56, 275–282. [Google Scholar] [PubMed]
- Schreml, S.; Szeimies, R.; Prantl, L.; Karrer, S.; Landthaler, M.; Babilas, P. Oxygen in acute and chronic wound healing. Br. J. Dermatol. 2010, 163, 257–268. [Google Scholar] [CrossRef]
- Yeh, K.-H.; Sheu, J.-J.; Lin, Y.-C.; Sun, C.-K.; Chang, L.-T.; Kao, Y.-H.; Yen, C.-H.; Shao, P.-L.; Tsai, T.-H.; Chen, Y.-L.; et al. Benefit of combined extracorporeal shock wave and bone marrow-derived endothelial progenitor cells in protection against critical limb ischemia in rats*. Crit. Care Med. 2012, 40, 169–177. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-L.; Tsai, T.-H.; Wallace, C.G.; Chen, Y.-L.; Huang, T.-H.; Sung, P.-H.; Yuen, C.-M.; Sun, C.-K.; Lin, K.-C.; Chai, H.-T.; et al. Intra-carotid arterial administration of autologous peripheral blood-derived endothelial progenitor cells improves acute ischemic stroke neurological outcomes in rats. Int. J. Cardiol. 2015, 201, 668–683. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-C.; Sheu, J.-J.; Chiang, J.Y.; Shao, P.-L.; Wu, S.-C.; Sung, P.-H.; Li, Y.-C.; Chen, Y.-L.; Huang, T.-H.; Chen, K.-H.; et al. Circulatory Rejuvenated EPCs Derived from PAOD Patients Treated by CD34+ Cells and Hyperbaric Oxygen Therapy Salvaged the Nude Mouse Limb against Critical Ischemia. Int. J. Mol. Sci. 2020, 21, 7887. https://doi.org/10.3390/ijms21217887
Chen Y-C, Sheu J-J, Chiang JY, Shao P-L, Wu S-C, Sung P-H, Li Y-C, Chen Y-L, Huang T-H, Chen K-H, et al. Circulatory Rejuvenated EPCs Derived from PAOD Patients Treated by CD34+ Cells and Hyperbaric Oxygen Therapy Salvaged the Nude Mouse Limb against Critical Ischemia. International Journal of Molecular Sciences. 2020; 21(21):7887. https://doi.org/10.3390/ijms21217887
Chicago/Turabian StyleChen, Yin-Chia, Jiunn-Jye Sheu, John Y. Chiang, Pei-Lin Shao, Shun-Cheng Wu, Pei-Hsun Sung, Yi-Chen Li, Yi-Ling Chen, Tien-Hung Huang, Kuan-Hung Chen, and et al. 2020. "Circulatory Rejuvenated EPCs Derived from PAOD Patients Treated by CD34+ Cells and Hyperbaric Oxygen Therapy Salvaged the Nude Mouse Limb against Critical Ischemia" International Journal of Molecular Sciences 21, no. 21: 7887. https://doi.org/10.3390/ijms21217887
APA StyleChen, Y.-C., Sheu, J.-J., Chiang, J. Y., Shao, P.-L., Wu, S.-C., Sung, P.-H., Li, Y.-C., Chen, Y.-L., Huang, T.-H., Chen, K.-H., & Yip, H.-K. (2020). Circulatory Rejuvenated EPCs Derived from PAOD Patients Treated by CD34+ Cells and Hyperbaric Oxygen Therapy Salvaged the Nude Mouse Limb against Critical Ischemia. International Journal of Molecular Sciences, 21(21), 7887. https://doi.org/10.3390/ijms21217887




