Heterogeneous Presynaptic Distribution of Munc13 Isoforms at Retinal Synapses and Identification of an Unconventional Bipolar Cell Type with Dual Expression of Munc13 Isoforms: A Study Using Munc13-EXFP Knock-in Mice
Abstract
:1. Introduction
2. Results
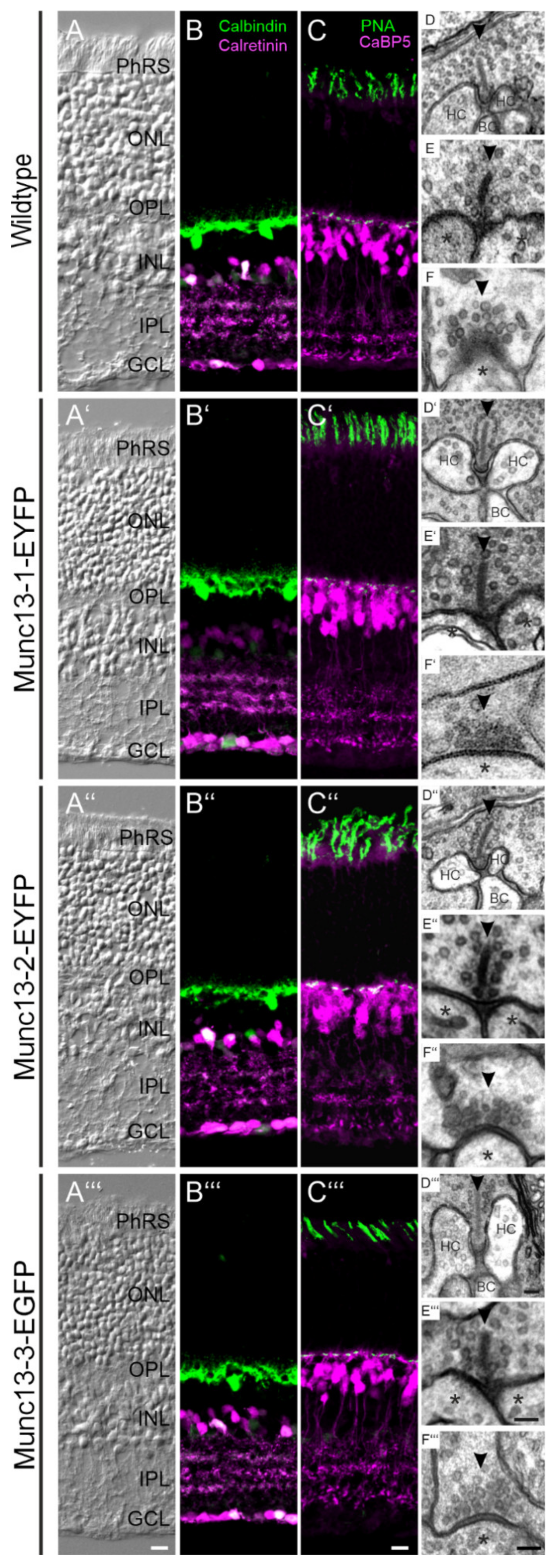
2.1. Retinal Anatomy, Neuronal Morphology, and Synaptic Ultrastructure Are Normal in Munc13-EXFP KI Mice
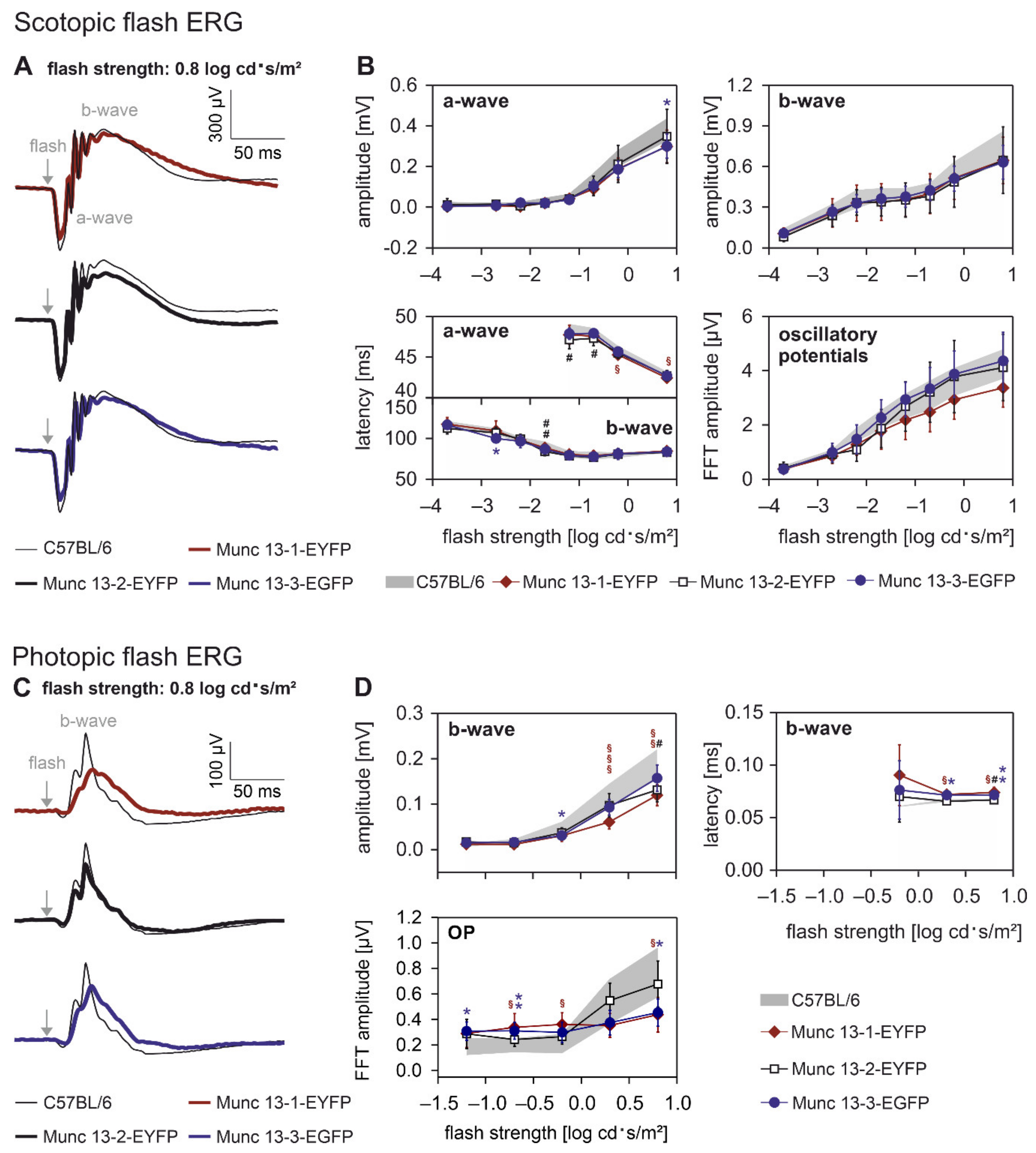
2.2. Retinal Function Is slightly Altered in Munc13-EXFP KI Mice
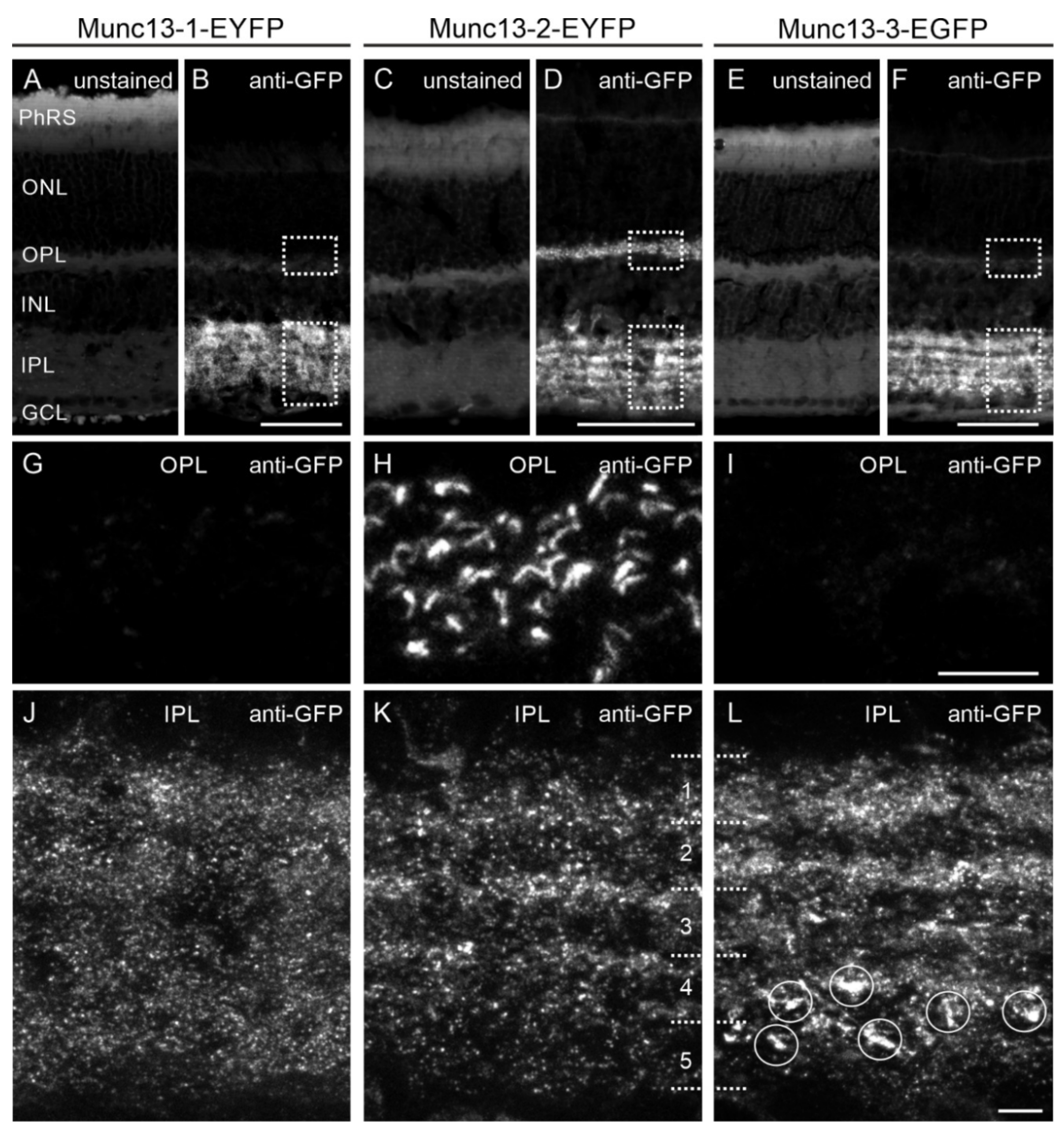
2.3. Analysis of Munc13 Fusion Protein Expression in Munc13-EXFP KI Retinae
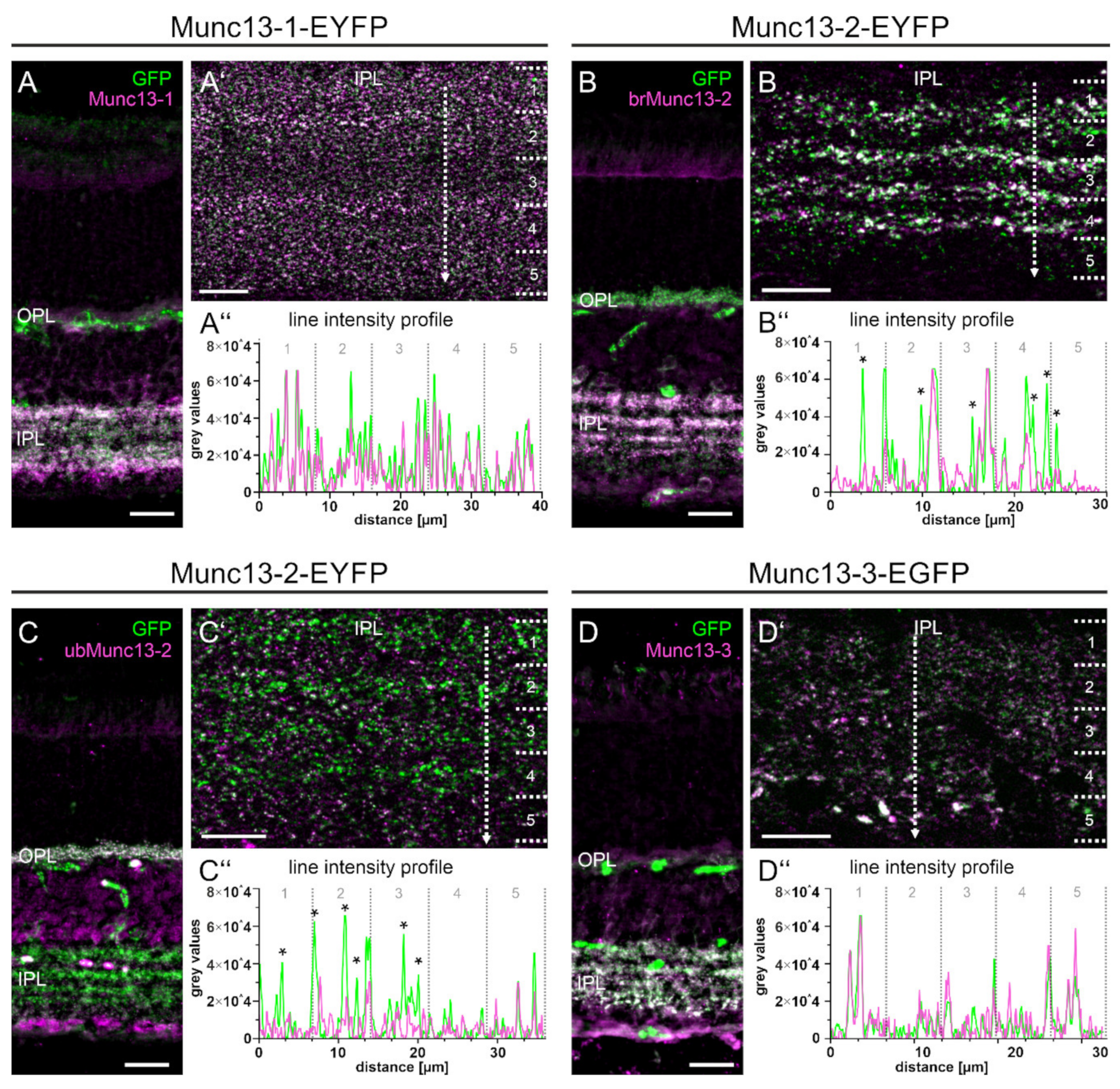
2.4. The Munc13-EXFP Fusion Protein Signals Match the Munc13 Isoform-Specific Antibody Signals
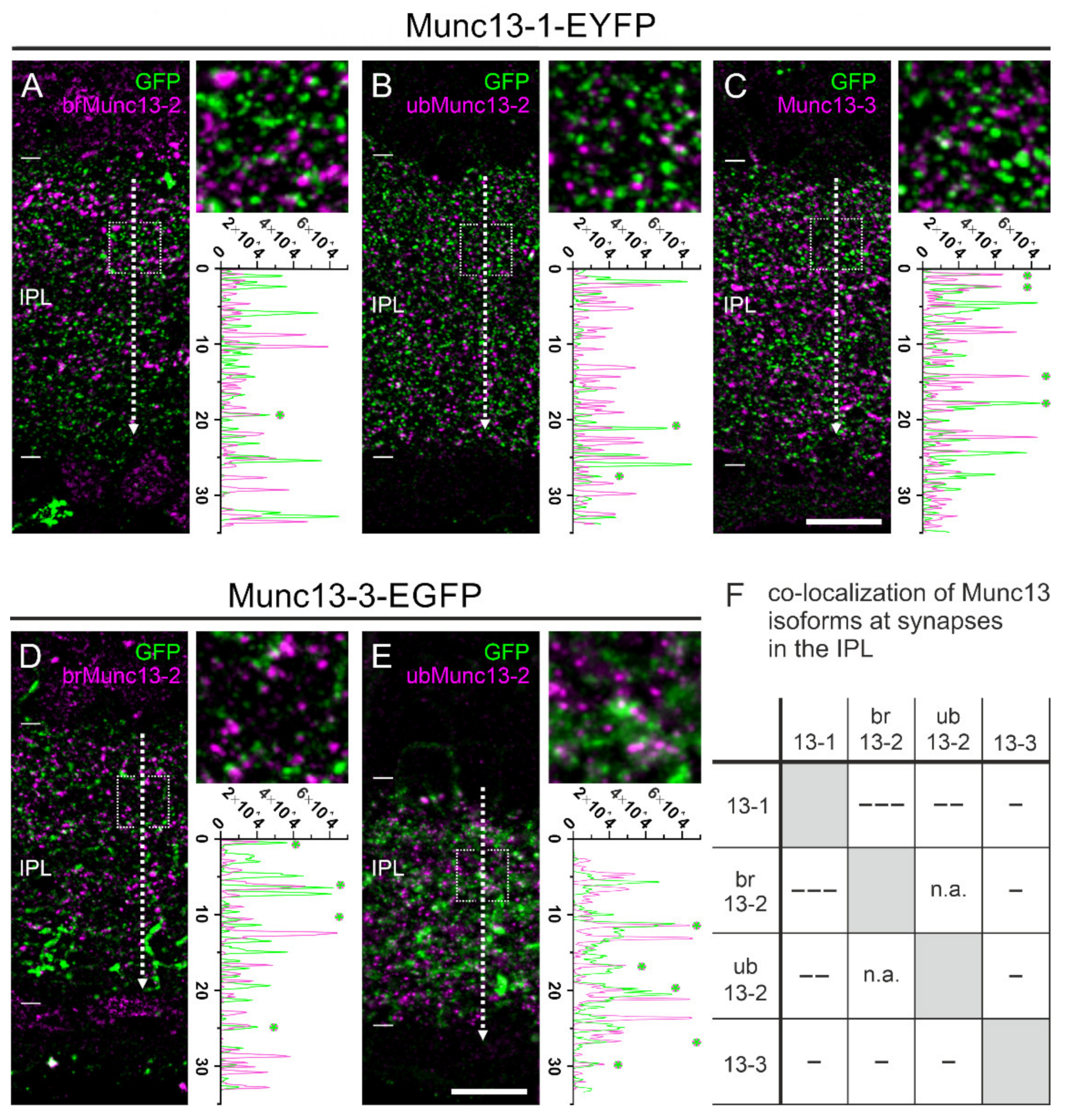
2.5. The Majority of Retinal Synapses Contains Only One Munc13 Isoform
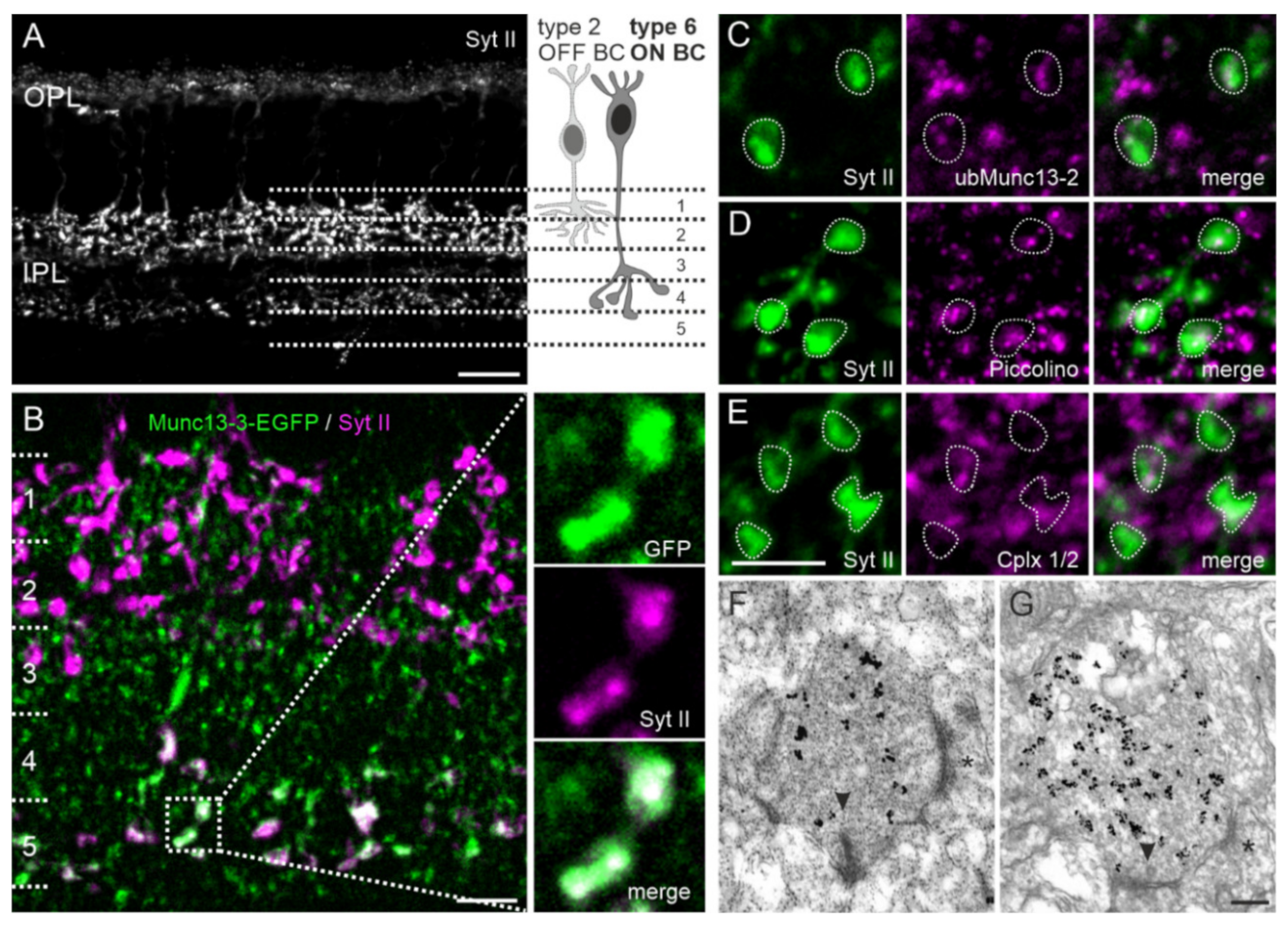
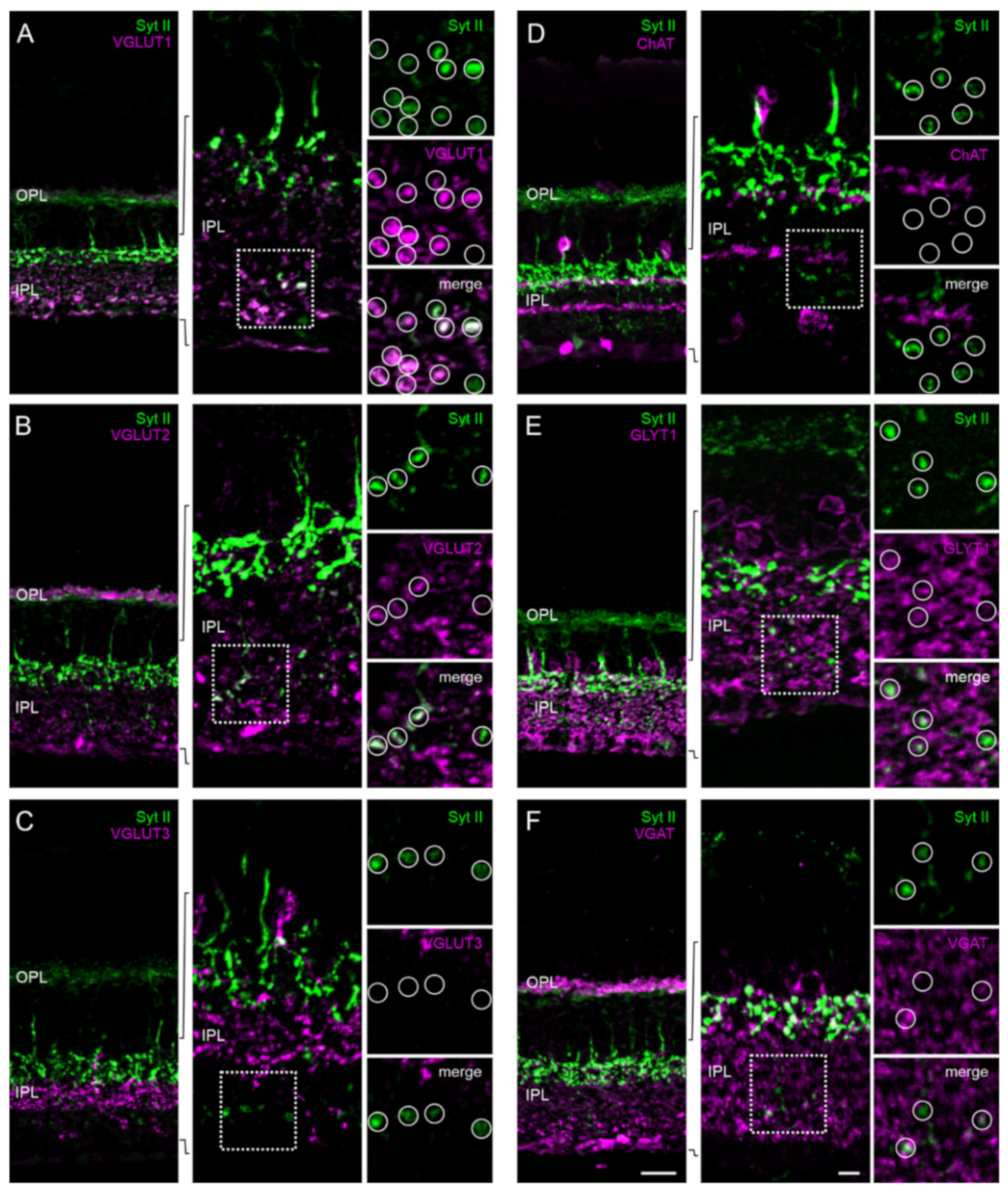
2.6. Type 6 ON Bipolar Cells Contain Munc13-3 in Addition to ubMunc13-2
3. Discussion
3.1. Munc13-EXFP KI Mouse Lines, a Tool for In Vivo and In Vitro Retinal Studies?
3.2. Mutually Exclusive Presence of Munc13 Isoforms at Retinal Synapses
3.3. Dual Expression of Munc13 Isoforms in Synaptic Terminals of Type 6 ON Bipolar Cells
4. Materials and Methods
4.1. Animals
4.2. Antibodies
4.3. Immunofluorescence Staining and Light Microscopy
4.4. Best Fix Electron Microscopy
4.5. Preembedding Immunoelectron Microscopy
4.6. Electroretinographic (ERG) Recordings
4.7. Fluorescence Intensity Profile Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | amacrine cell |
| BC | bipolar cell |
| CaBP5 | calcium binding protein 5 |
| EXFP | EGFP and its derivative EYFP |
| GCL | ganglion cell layer |
| HC | horizontal cell |
| INL | inner nuclear layer |
| IPL | inner plexiform layer |
| KI | knock-in |
| ONL | outer nuclear layer |
| OP | oscillatory potentials |
| OPL | outer plexiform layer |
| PhRS | photoreceptor segments |
| PNA | peanut agglutinin |
References
- Day, R.N.; Davidson, M.W. The fluorescent protein palette: Tools for cellular imaging. Chem. Soc. Rev. 2009, 38, 2887–2921. [Google Scholar] [CrossRef] [Green Version]
- Kalla, S.; Stern, M.; Basu, J.; Varoqueaux, F.; Reim, K.; Rosenmund, C.; Ziv, N.E.; Brose, N. Molecular dynamics of a presynaptic active zone protein studied in Munc13-1-enhanced yellow fluorescent protein knock-in mutant mice. J. Neurosci. 2006, 26, 13054–13066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brose, N.; Hofmann, K.; Hata, Y.; Sudhof, T.C. Mammalian homologues of Caenorhabditis elegans unc-13 gene define novel family of C2-domain proteins. J. Biol. Chem. 1995, 270, 25273–25280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koch, H.; Hofmann, K.; Brose, N. Definition of Munc13-homology-domains and characterization of a novel ubiquitously expressed Munc13 isoform. Biochem. J. 2000, 349, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Sudhof, T.C. The presynaptic active zone. Neuron 2012, 75, 11–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Y.; Ailenberg, M.; Silverman, M. Cloning of a novel gene in the human kidney homologous to rat munc13s: Its potential role in diabetic nephropathy. Kidney Int. 1998, 53, 1689–1695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betz, A.; Thakur, P.; Junge, H.J.; Ashery, U.; Rhee, J.S.; Scheuss, V.; Rosenmund, C.; Rettig, J.; Brose, N. Functional interaction of the active zone proteins Munc13-1 and RIM1 in synaptic vesicle priming. Neuron 2001, 30, 183–196. [Google Scholar] [CrossRef] [Green Version]
- Augustin, I.; Betz, A.; Herrmann, C.; Jo, T.; Brose, N. Differential expression of two novel Munc13 proteins in rat brain. Biochem. J. 1999, 337 Pt 3, 363–371. [Google Scholar] [CrossRef]
- Augustin, I.; Rosenmund, C.; Sudhof, T.C.; Brose, N. Munc13-1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature 1999, 400, 457–461. [Google Scholar] [CrossRef]
- Varoqueaux, F.; Sigler, A.; Rhee, J.S.; Brose, N.; Enk, C.; Reim, K.; Rosenmund, C. Total arrest of spontaneous and evoked synaptic transmission but normal synaptogenesis in the absence of Munc13-mediated vesicle priming. Proc. Natl. Acad. Sci. USA 2002, 99, 9037–9042. [Google Scholar] [CrossRef] [Green Version]
- Rosenmund, C.; Sigler, A.; Augustin, I.; Reim, K.; Brose, N.; Rhee, J.S. Differential control of vesicle priming and short-term plasticity by Munc13 isoforms. Neuron 2002, 33, 411–424. [Google Scholar] [CrossRef] [Green Version]
- Breustedt, J.; Gundlfinger, A.; Varoqueaux, F.; Reim, K.; Brose, N.; Schmitz, D. Munc13-2 differentially affects hippocampal synaptic transmission and plasticity. Cereb. Cortex 2010, 20, 1109–1120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Cooper, B.; Kalla, S.; Varoqueaux, F.; Young, S.M., Jr. The Munc13 proteins differentially regulate readily releasable pool dynamics and calcium-dependent recovery at a central synapse. J. Neurosci. 2013, 33, 8336–8351. [Google Scholar] [CrossRef] [PubMed]
- Cooper, B.; Hemmerlein, M.; Ammermuller, J.; Imig, C.; Reim, K.; Lipstein, N.; Kalla, S.; Kawabe, H.; Brose, N.; Brandstatter, J.H.; et al. Munc13-independent vesicle priming at mouse photoreceptor ribbon synapses. J. Neurosci. 2012, 32, 8040–8052. [Google Scholar] [CrossRef] [Green Version]
- Wassle, H. Parallel processing in the mammalian retina. Nat. Rev. Neurosci. 2004, 5, 747–757. [Google Scholar] [CrossRef]
- Brandstatter, J.H.; Koulen, P.; Wassle, H. Diversity of glutamate receptors in the mammalian retina. Vision Res. 1998, 38, 1385–1397. [Google Scholar] [CrossRef] [Green Version]
- Wassle, H.; Koulen, P.; Brandstatter, J.H.; Fletcher, E.L.; Becker, C.M. Glycine and GABA receptors in the mammalian retina. Vision Res. 1998, 38, 1411–1430. [Google Scholar] [CrossRef] [Green Version]
- Kalloniatis, M.; Tomisich, G. Amino acid neurochemistry of the vertebrate retina. Prog. Retin. Eye Res. 1999, 18, 811–866. [Google Scholar] [CrossRef]
- Thoreson, W.B.; Witkovsky, P. Glutamate receptors and circuits in the vertebrate retina. Prog. Retin. Eye Res. 1999, 18, 765–810. [Google Scholar] [CrossRef]
- Regus-Leidig, H.; Brandstatter, J.H. Structure and function of a complex sensory synapse. Acta Physiol. 2012, 204, 479–486. [Google Scholar] [CrossRef]
- Schmitz, F.; Augustin, I.; Brose, N. The synaptic vesicle priming protein Munc13-1 is absent from tonically active ribbon synapses of the rat retina. Brain Res. 2001, 895, 258–263. [Google Scholar] [CrossRef]
- Haverkamp, S.; Wassle, H. Immunocytochemical analysis of the mouse retina. J. Comp. Neurol. 2000, 424, 1–23. [Google Scholar] [CrossRef]
- Rogers, J.H. Calretinin: A gene for a novel calcium-binding protein expressed principally in neurons. J. Cell Biol. 1987, 105, 1343–1353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanks, J.C.; Johnson, L.V. Selective lectin binding of the developing mouse retina. J. Comp. Neurol. 1983, 221, 31–41. [Google Scholar] [CrossRef]
- Haeseleer, F.; Sokal, I.; Verlinde, C.L.; Erdjument-Bromage, H.; Tempst, P.; Pronin, A.N.; Benovic, J.L.; Fariss, R.N.; Palczewski, K. Five members of a novel Ca(2+)-binding protein (CABP) subfamily with similarity to calmodulin. J. Biol. Chem. 2000, 275, 1247–1260. [Google Scholar] [CrossRef] [Green Version]
- Nusinowitz, S.; Heckenlively, J. Evaluating retinal function in the mouse retina with the electroretinogram. Princ. Pract. Clin. Electrophysiol. Vis. 2006, 899–901. [Google Scholar]
- Wachtmeister, L. Oscillatory potentials in the retina: What do they reveal. Prog. Retin. Eye Res. 1998, 17, 485–521. [Google Scholar] [CrossRef]
- Ramon, Y.; Cajal, S. La retine des vertebres. La Cell 1893, 9, 119–259. [Google Scholar]
- Varoqueaux, F.; Sons, M.S.; Plomp, J.J.; Brose, N. Aberrant morphology and residual transmitter release at the Munc13-deficient mouse neuromuscular synapse. Mol. Cell Biol. 2005, 25, 5973–5984. [Google Scholar] [CrossRef] [Green Version]
- Stevens, C.F.; Sullivan, J.M. The synaptotagmin C2A domain is part of the calcium sensor controlling fast synaptic transmission. Neuron 2003, 39, 299–308. [Google Scholar] [CrossRef] [Green Version]
- Fox, M.A.; Sanes, J.R. Synaptotagmin I and II are present in distinct subsets of central synapses. J. Comp. Neurol. 2007, 503, 280–296. [Google Scholar] [CrossRef] [PubMed]
- Wassle, H.; Puller, C.; Muller, F.; Haverkamp, S. Cone contacts, mosaics, and territories of bipolar cells in the mouse retina. J. Neurosci. 2009, 29, 106–117. [Google Scholar] [CrossRef]
- Regus-Leidig, H.; Ott, C.; Lohner, M.; Atorf, J.; Fuchs, M.; Sedmak, T.; Kremers, J.; Fejtova, A.; Gundelfinger, E.D.; Brandstatter, J.H. Identification and immunocytochemical characterization of Piccolino, a novel Piccolo splice variant selectively expressed at sensory ribbon synapses of the eye and ear. PLoS ONE 2013, 8, e70373. [Google Scholar] [CrossRef] [PubMed]
- Muller, T.M.; Gierke, K.; Joachimsthaler, A.; Sticht, H.; Izsvak, Z.; Hamra, F.K.; Fejtova, A.; Ackermann, F.; Garner, C.C.; Kremers, J.; et al. A Multiple Piccolino-RIBEYE Interaction Supports Plate-Shaped Synaptic Ribbons in Retinal Neurons. J. Neurosci. 2019, 39, 2606–2619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reim, K.; Wegmeyer, H.; Brandstatter, J.H.; Xue, M.; Rosenmund, C.; Dresbach, T.; Hofmann, K.; Brose, N. Structurally and functionally unique complexins at retinal ribbon synapses. J. Cell Biol. 2005, 169, 669–680. [Google Scholar] [CrossRef] [Green Version]
- Tsukamoto, Y.; Omi, N. Classification of Mouse Retinal Bipolar Cells: Type-Specific Connectivity with Special Reference to Rod-Driven AII Amacrine Pathways. Front. Neuroanat. 2017, 11, 92. [Google Scholar] [CrossRef]
- Okawa, H.; Yu, W.Q.; Matti, U.; Schwarz, K.; Odermatt, B.; Zhong, H.; Tsukamoto, Y.; Lagnado, L.; Rieke, F.; Schmitz, F.; et al. Dynamic assembly of ribbon synapses and circuit maintenance in a vertebrate sensory system. Nat. Commun. 2019, 10, 2167. [Google Scholar] [CrossRef]
- Haverkamp, S.; Wassle, H. Characterization of an amacrine cell type of the mammalian retina immunoreactive for vesicular glutamate transporter 3. J. Comp. Neurol. 2004, 468, 251–263. [Google Scholar] [CrossRef]
- Wassle, H.; Regus-Leidig, H.; Haverkamp, S. Expression of the vesicular glutamate transporter vGluT2 in a subset of cones of the mouse retina. J. Comp. Neurol. 2006, 496, 544–555. [Google Scholar] [CrossRef]
- Johnson, J.; Tian, N.; Caywood, M.S.; Reimer, R.J.; Edwards, R.H.; Copenhagen, D.R. Vesicular neurotransmitter transporter expression in developing postnatal rodent retina: GABA and glycine precede glutamate. J. Neurosci. 2003, 23, 518–529. [Google Scholar] [CrossRef]
- Hutchins, J.B. Acetylcholine as a neurotransmitter in the vertebrate retina. Exp. Eye Res. 1987, 45, 1–38. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, W.Q.; Hoshino, A.; Huang, J.; Rieke, F.; Reh, T.A.; Wong, R.O.L. Development of ON and OFF cholinergic amacrine cells in the human fetal retina. J. Comp. Neurol. 2019, 527, 174–186. [Google Scholar] [CrossRef] [PubMed]
- McIntire, S.L.; Reimer, R.J.; Schuske, K.; Edwards, R.H.; Jorgensen, E.M. Identification and characterization of the vesicular GABA transporter. Nature 1997, 389, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Cueva, J.G.; Haverkamp, S.; Reimer, R.J.; Edwards, R.; Wassle, H.; Brecha, N.C. Vesicular gamma-aminobutyric acid transporter expression in amacrine and horizontal cells. J. Comp. Neurol. 2002, 445, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Vaney, D.I.; Nelson, J.C.; Pow, D.V. Neurotransmitter coupling through gap junctions in the retina. J. Neurosci. 1998, 18, 10594–10602. [Google Scholar] [CrossRef] [Green Version]
- Eulenburg, V.; Knop, G.; Sedmak, T.; Schuster, S.; Hauf, K.; Schneider, J.; Feigenspan, A.; Joachimsthaler, A.; Brandstatter, J.H. GlyT1 determines the glycinergic phenotype of amacrine cells in the mouse retina. Brain Struct. Funct. 2018, 223, 3251–3266. [Google Scholar] [CrossRef]
- Geppert, M.; Sudhof, T.C. RAB3 and synaptotagmin: The yin and yang of synaptic membrane fusion. Annu. Rev. Neurosci. 1998, 21, 75–95. [Google Scholar] [CrossRef]
- Sudhof, T.C. The synaptic vesicle cycle. Annu. Rev. Neurosci. 2004, 27, 509–547. [Google Scholar] [CrossRef] [Green Version]
- Schoch, S.; Gundelfinger, E.D. Molecular organization of the presynaptic active zone. Cell Tissue Res. 2006, 326, 379–391. [Google Scholar] [CrossRef]
- Betz, A.; Ashery, U.; Rickmann, M.; Augustin, I.; Neher, E.; Sudhof, T.C.; Rettig, J.; Brose, N. Munc13-1 is a presynaptic phorbol ester receptor that enhances neurotransmitter release. Neuron 1998, 21, 123–136. [Google Scholar] [CrossRef] [Green Version]
- Betz, A.; Okamoto, M.; Benseler, F.; Brose, N. Direct interaction of the rat unc-13 homologue Munc13-1 with the N terminus of syntaxin. J. Biol. Chem. 1997, 272, 2520–2526. [Google Scholar] [CrossRef] [Green Version]
- Andrews-Zwilling, Y.S.; Kawabe, H.; Reim, K.; Varoqueaux, F.; Brose, N. Binding to Rab3A-interacting molecule RIM regulates the presynaptic recruitment of Munc13-1 and ubMunc13-2. J. Biol. Chem. 2006, 281, 19720–19731. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Hu, B.; Zieba, A.; Neumann, N.G.; Kasper-Sonnenberg, M.; Honsbein, A.; Hultqvist, G.; Conze, T.; Witt, W.; Limbach, C.; et al. A protein interaction node at the neurotransmitter release site: Domains of Aczonin/Piccolo, Bassoon, CAST, and rim converge on the N-terminal domain of Munc13-1. J. Neurosci. 2009, 29, 12584–12596. [Google Scholar] [CrossRef] [Green Version]
- Vogl, C.; Cooper, B.H.; Neef, J.; Wojcik, S.M.; Reim, K.; Reisinger, E.; Brose, N.; Rhee, J.S.; Moser, T.; Wichmann, C. Unconventional molecular regulation of synaptic vesicle replenishment in cochlear inner hair cells. J. Cell Sci. 2015, 128, 638–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schnell, U.; Dijk, F.; Sjollema, K.A.; Giepmans, B.N. Immunolabeling artifacts and the need for live-cell imaging. Nat. Methods 2012, 9, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Augustin, I.; Korte, S.; Rickmann, M.; Kretzschmar, H.A.; Sudhof, T.C.; Herms, J.W.; Brose, N. The cerebellum-specific Munc13 isoform Munc13-3 regulates cerebellar synaptic transmission and motor learning in mice. J. Neurosci. 2001, 21, 10–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, W.; Laboulaye, M.A.; Tran, N.M.; Whitney, I.E.; Benhar, I.; Sanes, J.R. Mouse Retinal Cell Atlas: Molecular Identification of over Sixty Amacrine Cell Types. J. Neurosci. 2020, 40, 5177–5195. [Google Scholar] [CrossRef]
- MacNeil, M.A.; Masland, R.H. Extreme diversity among amacrine cells: Implications for function. Neuron 1998, 20, 971–982. [Google Scholar] [CrossRef] [Green Version]
- Diamond, J.S. Inhibitory Interneurons in the Retina: Types, Circuitry, and Function. Annu. Rev. Vis. Sci. 2017, 3, 1–24. [Google Scholar] [CrossRef]
- Ishiyama, S.; Schmidt, H.; Cooper, B.H.; Brose, N.; Eilers, J. Munc13-3 superprimes synaptic vesicles at granule cell-to-basket cell synapses in the mouse cerebellum. J. Neurosci. 2014, 34, 14687–14696. [Google Scholar] [CrossRef] [Green Version]
- Johnson, J.; Sherry, D.M.; Liu, X.; Fremeau, R.T., Jr.; Seal, R.P.; Edwards, R.H.; Copenhagen, D.R. Vesicular glutamate transporter 3 expression identifies glutamatergic amacrine cells in the rodent retina. J. Comp. Neurol. 2004, 477, 386–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Chen, L.; Chen, M.; Ye, M.; Seal, R.P.; Zhou, Z.J. An unconventional glutamatergic circuit in the retina formed by vGluT3 amacrine cells. Neuron 2014, 84, 708–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Zhang, Y.; Chen, M.; Zhou, Z.J. Segregated Glycine-Glutamate Co-transmission from vGluT3 Amacrine Cells to Contrast-Suppressed and Contrast-Enhanced Retinal Circuits. Neuron 2016, 90, 27–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tien, N.W.; Kim, T.; Kerschensteiner, D. Target-Specific Glycinergic Transmission from VGluT3-Expressing Amacrine Cells Shapes Suppressive Contrast Responses in the Retina. Cell Rep. 2016, 15, 1369–1375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dick, O.; tom Dieck, S.; Altrock, W.D.; Ammermuller, J.; Weiler, R.; Garner, C.C.; Gundelfinger, E.D.; Brandstatter, J.H. The presynaptic active zone protein bassoon is essential for photoreceptor ribbon synapse formation in the retina. Neuron 2003, 37, 775–786. [Google Scholar] [CrossRef] [Green Version]
- Lohner, M.; Babai, N.; Muller, T.; Gierke, K.; Atorf, J.; Joachimsthaler, A.; Peukert, A.; Martens, H.; Feigenspan, A.; Kremers, J.; et al. Analysis of RIM Expression and Function at Mouse Photoreceptor Ribbon Synapses. J. Neurosci. 2017, 37, 7848–7863. [Google Scholar] [CrossRef]
- Eldred, W.D.; Zucker, C.; Karten, H.J.; Yazulla, S. Comparison of fixation and penetration enhancement techniques for use in ultrastructural immunocytochemistry. J. Histochem. Cytochem. 1983, 31, 285–292. [Google Scholar] [CrossRef] [Green Version]
- Harazny, J.; Scholz, M.; Buder, T.; Lausen, B.; Kremers, J. Electrophysiological deficits in the retina of the DBA/2J mouse. Doc. Ophthalmol. 2009, 119, 181–197. [Google Scholar] [CrossRef]
- Joachimsthaler, A.; Tsai, T.I.; Kremers, J. Electrophysiological Studies on The Dynamics of Luminance Adaptation in the Mouse Retina. Vision 2017, 1, 23. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, T.; Hiner, M.; Rueden, C.; Miura, K.; Eglinger, J.; Chef, B. Tferr/Scripts: BAR 1.5.1; CERN European Organization for Nuclear Research: Geneva, Switzerland, 2017. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gierke, K.; von Wittgenstein, J.; Hemmerlein, M.; Atorf, J.; Joachimsthaler, A.; Kremers, J.; Cooper, B.H.; Varoqueaux, F.; Regus-Leidig, H.; Brandstätter, J.H. Heterogeneous Presynaptic Distribution of Munc13 Isoforms at Retinal Synapses and Identification of an Unconventional Bipolar Cell Type with Dual Expression of Munc13 Isoforms: A Study Using Munc13-EXFP Knock-in Mice. Int. J. Mol. Sci. 2020, 21, 7848. https://doi.org/10.3390/ijms21217848
Gierke K, von Wittgenstein J, Hemmerlein M, Atorf J, Joachimsthaler A, Kremers J, Cooper BH, Varoqueaux F, Regus-Leidig H, Brandstätter JH. Heterogeneous Presynaptic Distribution of Munc13 Isoforms at Retinal Synapses and Identification of an Unconventional Bipolar Cell Type with Dual Expression of Munc13 Isoforms: A Study Using Munc13-EXFP Knock-in Mice. International Journal of Molecular Sciences. 2020; 21(21):7848. https://doi.org/10.3390/ijms21217848
Chicago/Turabian StyleGierke, Kaspar, Julia von Wittgenstein, Maike Hemmerlein, Jenny Atorf, Anneka Joachimsthaler, Jan Kremers, Benjamin H. Cooper, Frederique Varoqueaux, Hanna Regus-Leidig, and Johann Helmut Brandstätter. 2020. "Heterogeneous Presynaptic Distribution of Munc13 Isoforms at Retinal Synapses and Identification of an Unconventional Bipolar Cell Type with Dual Expression of Munc13 Isoforms: A Study Using Munc13-EXFP Knock-in Mice" International Journal of Molecular Sciences 21, no. 21: 7848. https://doi.org/10.3390/ijms21217848




