Abstract
Health-care systems that develop rapidly and efficiently may increase the lifespan of humans. Nevertheless, the older population is more fragile, and is at an increased risk of disease development. A concurrently growing number of surgeries and transplantations have caused antibiotics to be used much more frequently, and for much longer periods of time, which in turn increases microbial resistance. In 1945, Fleming warned against the abuse of antibiotics in his Nobel lecture: “The time may come when penicillin can be bought by anyone in the shops. Then there is the danger that the ignorant man may easily underdose himself and by exposing his microbes to non-lethal quantities of the drug make them resistant”. After 70 years, we are witnessing the fulfilment of Fleming’s prophecy, as more than 700,000 people die each year due to drug-resistant diseases. Naturally occurring antimicrobial peptides protect all living matter against bacteria, and now different peptidomimetic strategies to engineer innovative antibiotics are being developed to defend humans against bacterial infections.
1. Introduction
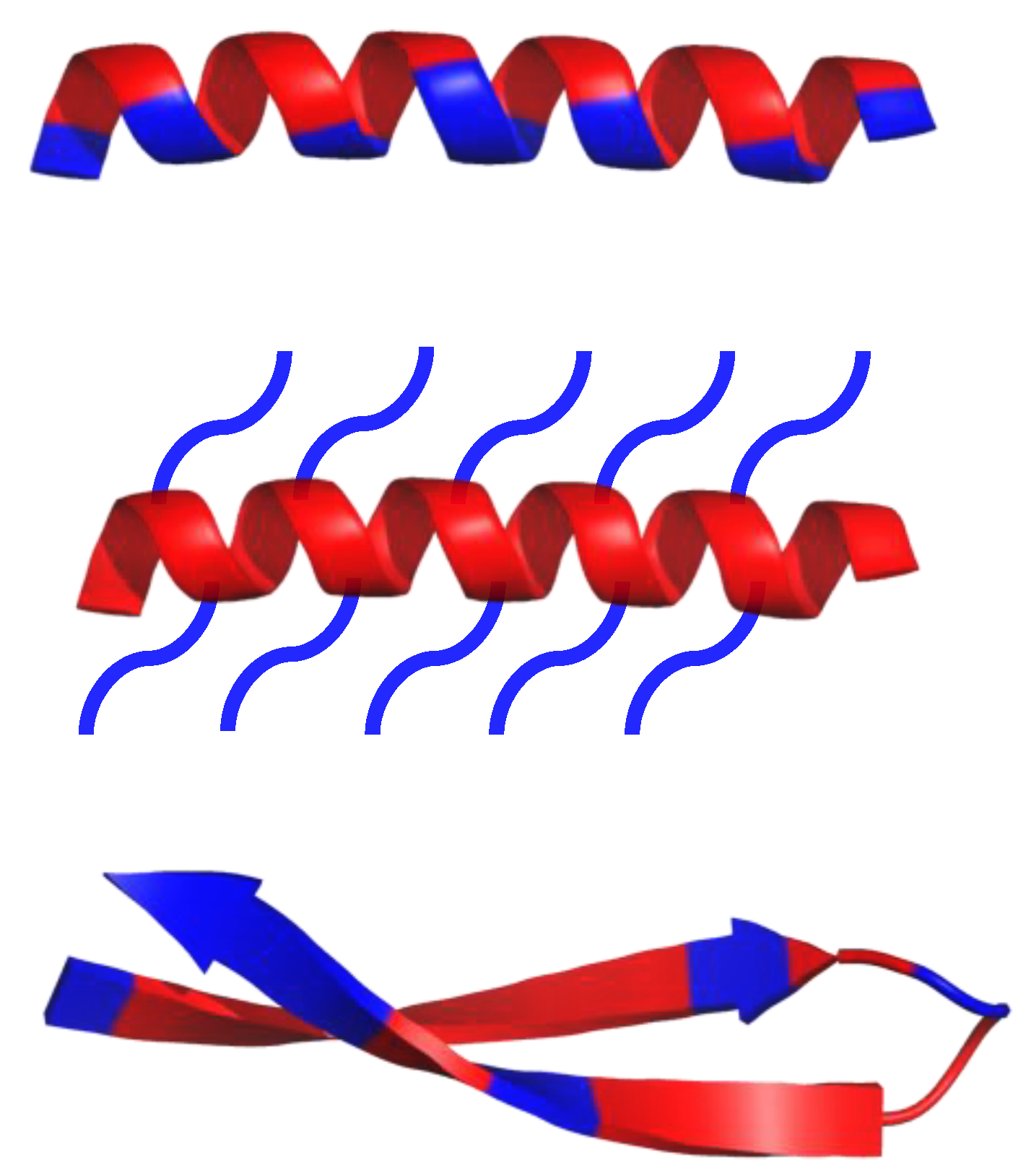
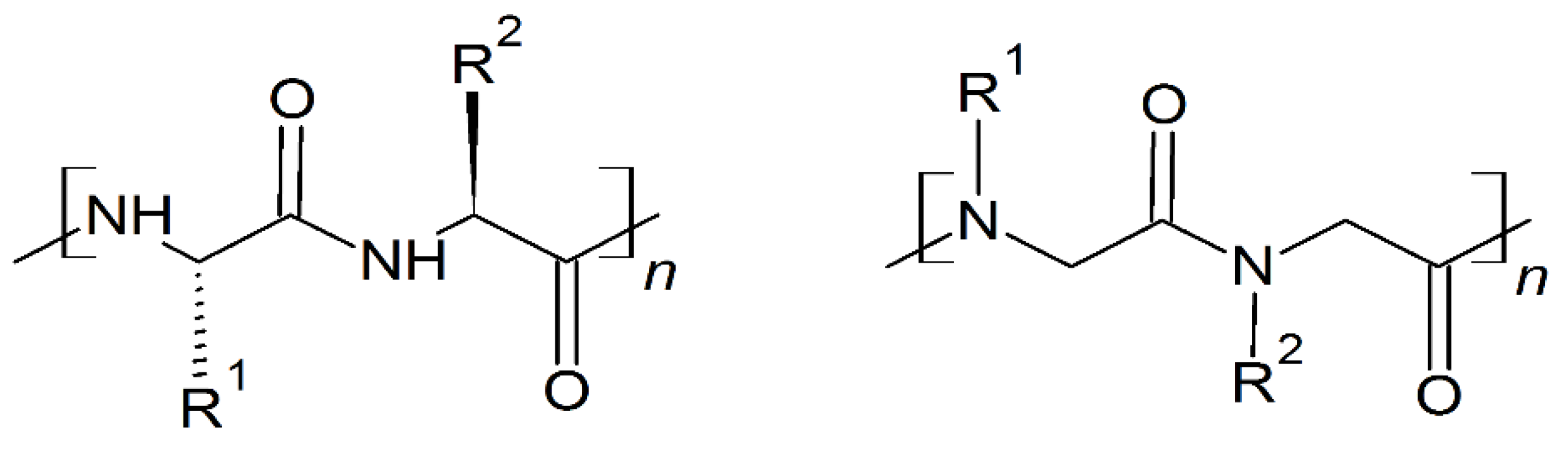
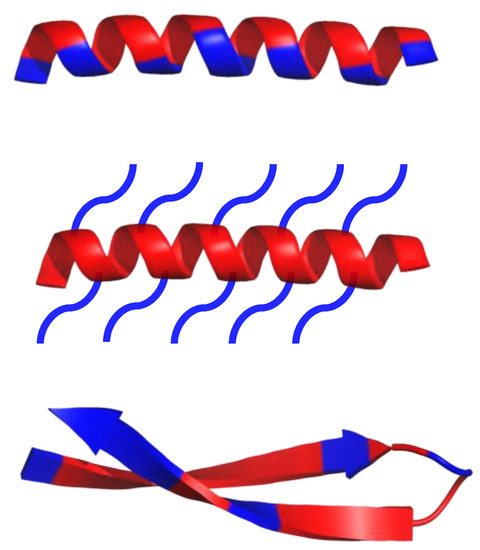
Antimicrobial peptides (AMPs), also known as host defense peptides (HDPs), are produced by all living matter as a critical part of the innate immune system [1,2,3]. Their existence was discovered in 1939, the year gramicidin was isolated from the bacteria, Bacillus brevis; some resources, however, claim that the discovery of lysozyme in the 1920s should be treated as the first AMP instance, due to lysozyme’s non enzymatic, bactericidal second mode of action [2]. As of 2019, over 3000 AMPs have been isolated across all kingdoms [4]. Most of them have variable sequence length—from 10 up to 60 amino acid residues, mainly in L-configuration [4]. AMPs are expressed in many cell types in response to the activation of the Toll-like receptor (TLR) signaling pathway [1]. They are predominantly comprised of two types of amino acids residues—cationic residues such as Arg, Lys, and His, and hydrophobic residues (mainly aliphatic and aromatic) [1]. Both cationic and hydrophobic residues engage in the antimicrobial mechanism of action. Furthermore, AMPs can be classified into three groups, based on their structures (Scheme 1): α-helical, β-sheets, and extended peptides. α-helical peptides are the largest group of AMPs with characteristic qualities such as amphipathic properties, and the ability to possess a tertiary structure with a hinge in the middle of a chain [1,4]. β-sheets peptides feature one to five disulfide bridges that help to stabilize their bioactive conformation. Peptides rich in amino acids such as proline, arginine, tryptophan or glycine usually have a linear structure [1,4].
Scheme 1.
Schematic representation of α-helical and β-sheets, common secondary structure of amphipathic peptides. Hydrophobic amino acid residues are colored red, while hydrophilic residues are colored blue.
HDPs have a wide range of antibacterial activities, and low susceptibility to antimicrobial resistance [5,6,7,8,9,10,11,12]. Nevertheless, the use of HDPs is limited due to their low resistance to proteases, and their increased cost of preparation [8,13,14]. Different peptides [15,16,17,18], peptoids [19,20,21,22,23], polymethacrylate derivatives [24], polynorbornene derivatives [25], polycarbonate derivatives [26], peptide polymers [27,28,29,30,31,32], and polymers made by controlled living radical polymerization (CLRP) [33] were developed to mimic the antibacterial properties of HDPs, and to ameliorate their shortcomings [34].
The widely developed peptide-drug research has led to the introduction of around 60 FDA approved peptide therapeutics in the market [35]. Over 140 are in clinical trials, and over 500 are in the preclinical stages. Although numerous biomimetic and de novo designed AMPs are at different stages of clinical trials [36,37], only eight AMPs received FDA approval, i.e., daptomycin (approved in 2003) and oritavancin (approved in 2014) [37].
In this review paper, we describe innovative peptidomimetics strategies and we present successful examples of new antimicrobial peptide-mimicking antibiotics. The aim of this review is to gather the latest advances in the research of new antibiotics for those who are engineering future antibacterial strategies.
2. Samsons Hair of AMPs
There are three main factors—charge, hydrophobicity and amphipathicity, which determine the activity of AMPs, and they are all related to their intrinsic properties.
The positive charge of AMPs (between 2+ and 9+) is one of the most essential factors responsible for their antibacterial activity [38]. Positively charged AMPs outcompete the binding of native Mg(II) and Ca(II) ions to lipopolysaccharides (LPS), and destabilize the outer membrane of Gram-negative bacteria [39]. Consequently, the destabilized regions enable the peptide to pass into the cell. Once through, AMPs bind to the cytoplasmic membrane and cause depolarization and pore formation, resulting in cell death [40]. In the case of Gram-positive bacteria, AMPs must overcome the outer wall with two main obstacles, peptidoglycan and teichoic acids, before an AMP interacts with the cytoplasmic membrane. AMPs overcome peptidoglycan because it is able to penetrate through the relatively porous peptidoglycan. The porous nature of peptidoglycan makes it easy for small molecules (< 50 kDa) to penetrate through it, and furthermore, it does not possess a negative charge, which could prevent the penetration of positively charged AMPs through it [40,41,42]. To overcome the anionic teichoic acids, they can either act as a ladder that will help positively charged AMPs to travel to the cytoplasmic membrane, or the can act as “cages” that entrap AMPs inside the bacteria, thus reducing their local concentration on the membrane The behaviors of teichoic acids vary based on the type of AMP and the type of bacteria [42]. After crossing the outer wall, AMPs can disrupt the integrity of the cytoplasmic membrane, leading to membrane disruption and dislocation of peripheral membrane proteins [42].
The hydrophobicity of AMPs is another important factor. It is assumed that hydrophobic residues help to insert peptides into the bacterial membrane and further impair the membrane [4,42]. It was also revealed that hydrophobic residues account for 40–60% of all amino acids in AMPs [38]. Moreover, the hydrophobicity is strongly correlated with antimicrobial activity, where increasing the hydrophobicity to a certain level improves antimicrobial activity [1,38]. The relationship between hydrophobicity and antimicrobial activity is also proven to be the case for peptidomimetics such as peptoids, where amino acid side chains are linked to the peptide backbone through the amide nitrogen rather than to the α-carbons [43]. However, a higher hydrophobicity also increases hemolytic activities, resulting in unwanted toxicity to eukaryotic cells [1,38]. Studies with model peptides have shown that a continuous region of 4-6 hydrophobic residues is sufficient to sustain the antimicrobial activity of peptides, while simultaneously reducing the hemolytic activity [44].
The amphipathicity of AMPs, created by the segregation of hydrophobic and polar residues on the opposite sites of the backbone, is another factor related to their activity [1]. Amphipathicity is considered the strongest indicator of AMP activity, and is described by the hydrophobic moment, defined as the vector sum of the hydrophobicity of each amino acid located in the helix [1,45]. The mechanism by, which amphipathicity works is the result of previous factors. The positively charged regions of AMPs enable binding to the anionic phospholipid head groups of the bacterial membrane. Meanwhile, the hydrophobic regions of peptides invade the lipid bilayer and interact with the hydrophobic acyl chains of phospholipids, which results in membrane penetration [38]. Several studies have shown the importance of amphipathicity in the process of AMPs binding to membranes, and the negative impact on activity against Gram-positive bacteria and fungi, when amphipathic character of the peptide was eliminated [13,46,47,48].
3. AMPs: Achilles’ Heel
Despite the recent advancements in AMP research, there are still major challenges to overcome. One of the main obstacles in AMP applications is the proteolytic instability of peptide drugs [49,50]. Peptides are targeted by numerous proteolytic enzymes located in bodily fluids and tissues of the host. Moreover, AMPs can be recognized as an antigen and targeted by host immune system [51,52]. One of the countermeasures for their potential immunogenicity could be glycosylating AMPs with a glycosyl profile similar to that of the host. For instance, covering AMPs with polysialic acid (derived from Neisseria meningitidis or E. coli that shares structural mimicry with host cell lectins), or its analogues, can lessen the immunological response [52]. Nevertheless, some bacteria have already developed resistance to AMPs. For example, LL-37, a human antimicrobial peptide, can be digested by proteases synthesized by P. aeruginosa, Enterococcus faecalis, Proteus mirabilis, S. aureus, and S. Pyogenes [50,53]. Synthesizing proteases to target specific antimicrobial peptides is one of the many ways bacteria participate in this evolutionary “arms race”. Other methods used by bacteria to cope with AMPs, including the use of efflux pumps, lowering the binding affinity, or reducing the anionic charge of lipopolysaccharides on their surface, are exhaustively discussed in [50,54].
The high toxicity of AMPs in eukaryotic cells is another one of their shortcomings. This high toxicity can lead to hemolysis, nephrotoxicity, and neurotoxicity [1,55,56,57]. More studies need to focus on the pharmacokinetics and pharmacodynamics of AMPs in order to determine a proper drug dosage that will maintain a balance between positive and negative outcomes [2].
The bioavailability of AMPs is rather low. Peptides are hardly absorbed by the intestinal mucosa, and their pharmacological distribution requires additional financial input. In recent years, much progress has been made in this field—e.g., nano-carriers not only increase the bioavailability, but also lower cytotoxicity and reduce degradation of a compound, leading to increased efficiency [37]. Despite different efforts, the entire process of AMPs design and discovery (costs of synthesis and screening) is relatively high and does not guarantee the expected outcome [58]. The high production cost of AMPs (as for 2006, producing 1 g of peptide using solid-phase peptide synthesis (SPPS) costs USD 100–600) limits the development and testing of new AMPs, even if recent improvements in SPPS lowered the costs of synthesis [8].
AMPs, as other peptide drugs, can induce immune responses and cause allergies [59]. Immunogenicity depends on the host immune system, but is also correlated with the peptide’s dose, duration and frequency of treatment, route of administration, and the patient’s pathologies [60,61,62,63]. Immunogenic response can affect the peptide-drug efficiency and even lead to allergy and/or hypersensitivity [63]. Bering that in mind, it is important to study the immunogenic and allergic activity of AMPs. In vitro tests together with mathematical calculations of allergens similarities, with the use of specific databases of allergenic compounds [64], i.e., http://www.allergome.org, can give nowadays reliable results [65].
4. Bacterial Membranes vs. Human Cell Membranes
AMPs’ selectivity depends on the intrinsic differences in the cell wall of prokaryotes and eukaryotes [66]. Since most bacterial plasma membranes are rich in anionic phospholipids (such as phosphatidyl-glycerol (PG), cardiolipin (CL), and phosphatidylserine (PS)) and since the outer monolayers of eukaryotic membranes are composed of zwitterionic (overall neutral) lipids, the prokaryotic membranes are more electronegative than eukaryotic membranes [66]. AMPs’ selectivity towards either zwitterionic or negatively charged lipids is crucial in determining their bioactivity, toxicity, and potential use as drugs [67]. Furthermore, in some cases, the high electronegative nature of a membrane helps AMPs to adopt active conformations and interact with the membrane.
The cell-walls of Gram-positive and Gram-negative bacteria vary significantly. In Gram-positive bacteria, the cell wall is comprised of multiple layers of peptidoglycan composed of a poly-[N-acetylglucosamine-N-acetylmuramic acid] backbone, with a thickness of 30–100 nm [68]. The peptidoglycan wall determines the shape of bacteria, prevents cell lysis (due to high internal osmotic pressure), and defends the cell from environmental hazards such as antibiotics. The cell wall is interspersed by two types of anionic polymers—teichoic acids, which are linked to peptidoglycan, and lipoteichoic acids that are anchored to the cell membrane. Anionic polymers play an important role in ion homeostasis, regulate cell morphology, division and autolytic activity, and protect bacteria against the host’s defense mechanisms and antibiotics [69]. When compared to Gram-negative bacteria, Gram-positive bacteria have a much higher content of negatively charged lipids, predominantly phosphatidylglycerol (PG) and cardiolipin (CL) [66].
The envelope of Gram-negative bacteria can be divided into three layers: the outer membrane (OM), the peptidoglycan cell-wall, and the cytoplasmic or inner membrane (IM). The outer membrane is composed mainly of glycolipids, and principally, of lipopolysaccharides (LPS). LPS are composed of three domains: lipid A, the core oligosaccharide, and the O-antigen [70]. LPS create a permeability barrier and protect the bacterial cell against various environmental factors such as antibiotics and bile salts. However, the LPS are primary bacterial components encountered in the host immune system and play an important role in bacterial pathogenicity (the host organism can treat them as a pathogen-associated molecular pattern; PAMP). The inhibition of LPS biosynthesis could also trigger stress response pathways and affect the assembly and function of membrane proteins [71]. In addition to LPS, the outer membrane contains lipoproteins and β-barrel proteins that can act as gated channels for vitamin transport. In Gram-negative bacteria, the peptidoglycan cell-wall plays the same role as in Gram-positive bacteria, but it is thinner—ranging from 2.5 to 7 nm (1–3 layers of peptidoglycan) [72]. The inner membrane of bacteria serves as a platform for all membrane-associated functions of eukaryotic organelles, which include energy production, lipid biosynthesis, protein secretion, and the transport of molecules [73].
Despite all the differences in the cell wall structure, there are three models, which describe how AMPs destabilize the bacterial membrane (Table 1). In the barrel-stave model, a variable number of peptides are inserted perpendicularly into the bilayer, forming a barrel-like ring with a central lumen acting as a pore [74]. In the carpet model, peptides are positioned parallel to the bilayer, and form carpet-like structure on the membrane surface. After reaching a concentration threshold of adsorbed AMP, accumulated AMPs change membrane fluidity and reduce the barrier properties of the membrane, producing a “detergent-like” effect (formation of micelles), and resulting in membrane disruption [74]. In the last model, called toroidal-pore model, the peptides are inserted perpendicularly into the bilayer and induce a bend in the membrane. This results in a formation of a pore that constitutes partially peptides and partially phospholipid head groups. The difference between toroidal pores and barrel-stave pores is that peptides are always intercalated with the lipids head groups when forming a pore.

Table 1.
Summary of the three most widely accepted mechanisms of antimicrobial peptides (AMPs).
Although, AMPs’ main antimicrobial activity is membrane disruption, several studies have shown that antimicrobial peptides exhibit other mechanisms of action. AMPs can inhibit the synthesis of cell-walls, nucleic acids and proteins, and they can inhibit cell division. Moreover, AMPs are able to target organelles of eukaryotic pathogens [81]. Many of these aspects are broadly described in the literature [82]. For instance, recent results showed that IARR-Anal10, the analog of mBjAMP1, exhibits no effect on the permeability or integrity of the bacterial outer membrane, and acts intracellularly rather than at the cell membrane. Moreover, IARR-Anal10 can bind bacterial DNA [82].
5. Antibacterial Peptides in the Management of Oral Infections with Biofilm Production
The oral cavity is unique in the level of microbial diversity it holds, and it harbors about 1000 microbial species. Most of them have physiological functions in oral tissue, and the majority of oral microbes are considered commensals [83]. Others are associated with oral diseases and can be divided into two, ideal groups: “always” pathogenic and “potentially” pathogenic.
A pathogen is a microorganism capable of causing disease in healthy individuals. It can enter the host organism, establish a niche within which to replicate, and disseminate to reach a new host. It is now possible to identify different genes associated with pathogenicity factors inside the pathogen genome. Compounds that harm the host are naturally expressed, and they are not associated with stress conditions in the oral cavity.
An opportunistic pathogen does not cause disease in healthy individuals, but it can cause pathological states in “at-risk” patients, for instance, during a dysbiosis status in the oral tissues. In this context, an opportunistic bacterium could be useful or even essential during human life, but it can also become a pathogen under different chemical and biological stimuli released by the host’s tissues [84,85,86].
Periodontitis and periimplantitis are two of the most pervasive pathologies in dental medicine. According to recent publications and WHO reports, millions of peoples in the industrialized countries are toothless, and over hundred-and-twenty million are missing at least one tooth. Moreover, from 70 to 90% of people over 60 years old have dentures. Periodontitis is an infectious disease caused by microbes, the majority of which is represented by Gram-negative anaerobic rods [8], capable of harming periodontal tissues by direct products (e.g., proteases), and mark an unusual inflammatory response, which leads to the destruction of the underlying tissue and tooth loss in adults [9].
In the same way, peri-implantitis (PI) is a specific infective-inflammatory site-specific disease that occurs in dental osseointegrated implants. The onset of this infection is represented by the adherence and proliferation of the periodontal anaerobic bacteria on implant surfaces and it is strongly linked to an inflammatory process that occurs and may be limited to the soft-tissues, or is associated with the progressive loss of osseointegration, mucositis, and peri-implantitis, respectively. Peri-implantitis shows a remarkable impact on health status and related costs in industrialized countries. Recent reports indicate that the percentages of clinically evident PI range from 30 to 60%, and different efforts are undertaken to treat this infectious disease [10].
Nowadays, periodontal therapy follows two main procedures—the with or without surgery approach. The first one is used to improve access to the root surface; nevertheless, is not effective if bacteria invade periodontal tissues [11,12]. In other cases, adjunctive systemic antimicrobial therapy remains the treatment of choice [13]. However, factors such as the mode of antimicrobial action, the susceptibility of periodontal pathogens, the dosage, correct management and use in the treatment of periodontal disease, as well as the mechanism of bacterial resistance to each antimicrobial, are still discussed among dentists and microbiologists [14].
Antimicrobial resistance is a natural phenomenon of bacterial survival, but at the same time, it represents a worldwide public health risk, particularly in patients who have chronic periodontitis or periimplantitis, and who are frequently infected with multidrug-resistant strains. For instance, Prevotella intermedia isolated from oral cavity has shown resistance to clindamycin, amoxicillin, doxycycline, or metronidazole [15]. In a clinical investigation performed by Ram et al., about 70% of peri-implantitis patients exhibited strains that are drug-resistant in vitro to one or more of the tested antibiotics [16]. In this context, new approaches are required to combat the spread of drug-resistant bacteria in the oral cavity, especially given that oral microbes are also associated to degenerative systemic diseases in other tissues [17].
Oral cavity microorganisms are present either as planktonic cells in saliva, or as sessile and incorporated into a complex biological matrix called a biofilm. In the mouth, the biofilms represent the conditions of severe oral pathologies such as dental caries, periodontal diseases, and periimplantitis.
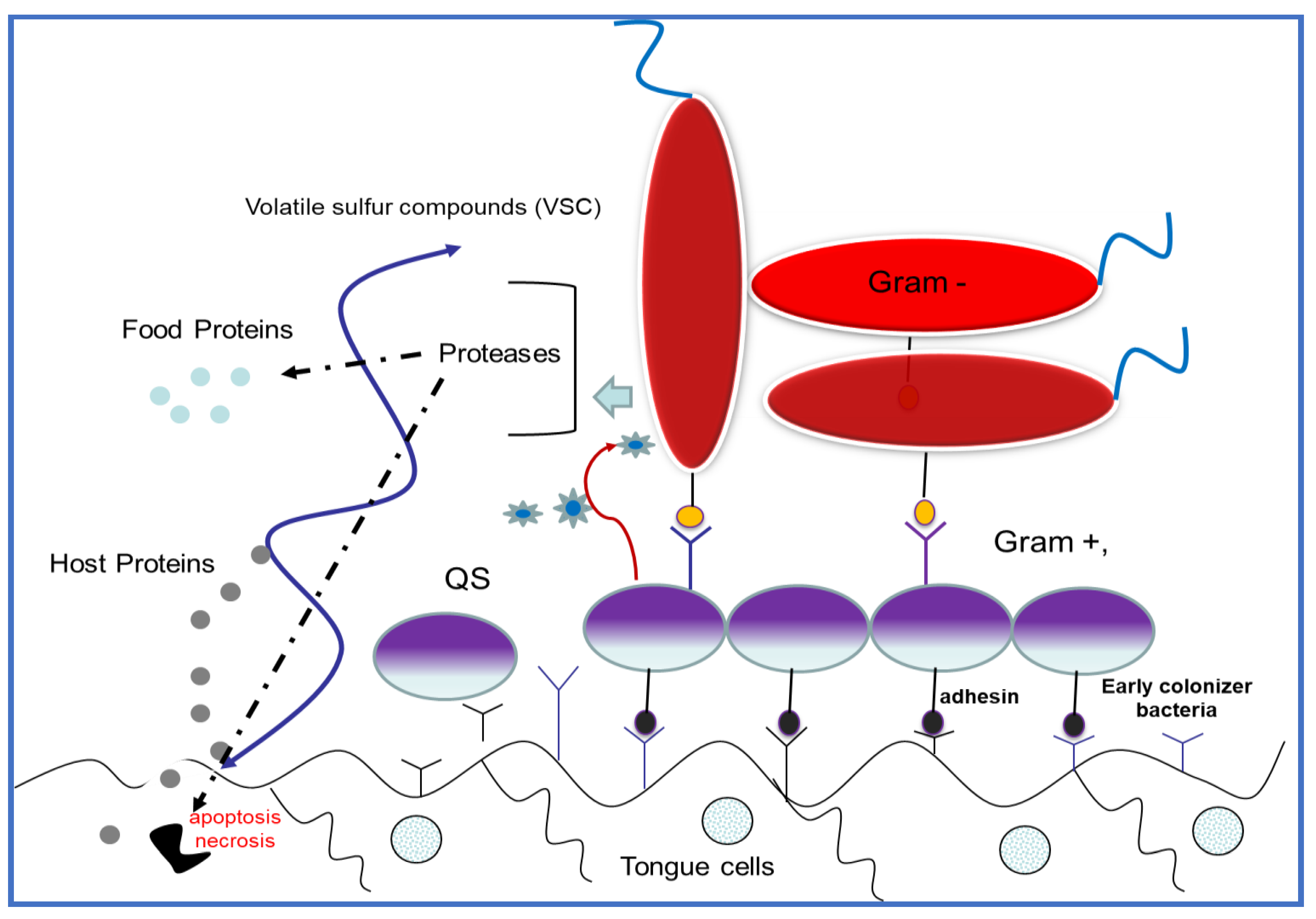
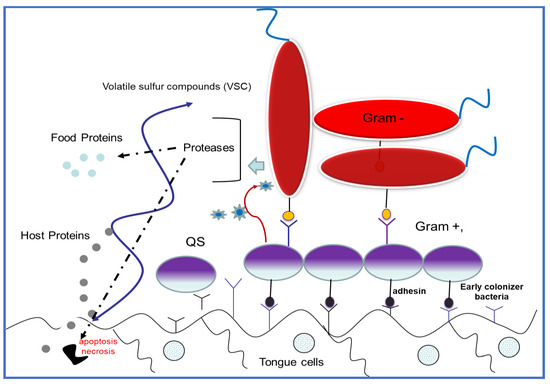
The resident bacteria cells in the biofilm are encased in an exopolymeric complex matrix with amyloid-like properties. Biofilm is comprised of exopolysaccharides, lipids, proteins, and cDNA. This extracellular matrix provides mechanical rigidity and protects bacteria from the external environment, often acting as waterproof barrier (Scheme 2). The resident bacteria strictly regulate the biofilm functions via a coordinated gene expression network, including growth direction, metabolic pathways, and pathogenic compounds for the host. In this way, biofilm represent an exceptional survival strategy for pathogens, and together with corresponding changes in gene expression, can protect the bacteria from disinfectant agents or antibiotics, and from the immunity system. On the other hand, biofilm sessile conditions allow bacteria to adhere to the oral tissues e.g., teeth surface, in opposing salivary flux.
Scheme 2.
Schematic structure of an oral biofilm complex. Early colonizer bacteria attach to the oral tissues covered with host cell external proteins. Early colonizer bacteria “communicate” with late colonizer microorganisms (i.e., periodontal pathogens) by using different acceptor–receptor interactions among the quorum sensing (QS) pathway.
In the oral cavity, we can observe diverse biofilms located in different tissues/organs: the tongue, a dental apparatus, or in prostheses i.e., implants and orthodontic appliances. These bacterial complexes possess great phenotypic adaptability, genetic resistance, and as already discussed, are resistant to antimicrobial treatment. It is often the extracellular biofilm matrix that physically restricts the diffusion of antimicrobial agents, even if does not seem to be a predominant mechanism of antimicrobial resistance.
The oral infective diseases are host–pathogen interactions with complicated biochemical networks, and in the last decade they have been uncovered with metagenomic and proteomic analyses. Surprisingly, the resident oral bacteria can assist the pathogens, or they interact by turning opportunistic bacterium into the pathogen phenotype by a wide variety of mechanisms, such as evasion of the immune response, cell–cell signaling, metabolic interactions, etc. [87].
Inside biofilms, bacterial cells use a communication pathway called quorum sensing (QS) to coordinate the population density and growth direction. In oral bacteria, QS also modulates virulence and pathogenic functions [88], and studies on QS signaling molecules to produce a “quorum-quenching” effect that could lead to bacterial communication interference [89], and new modes of antibacterial action.
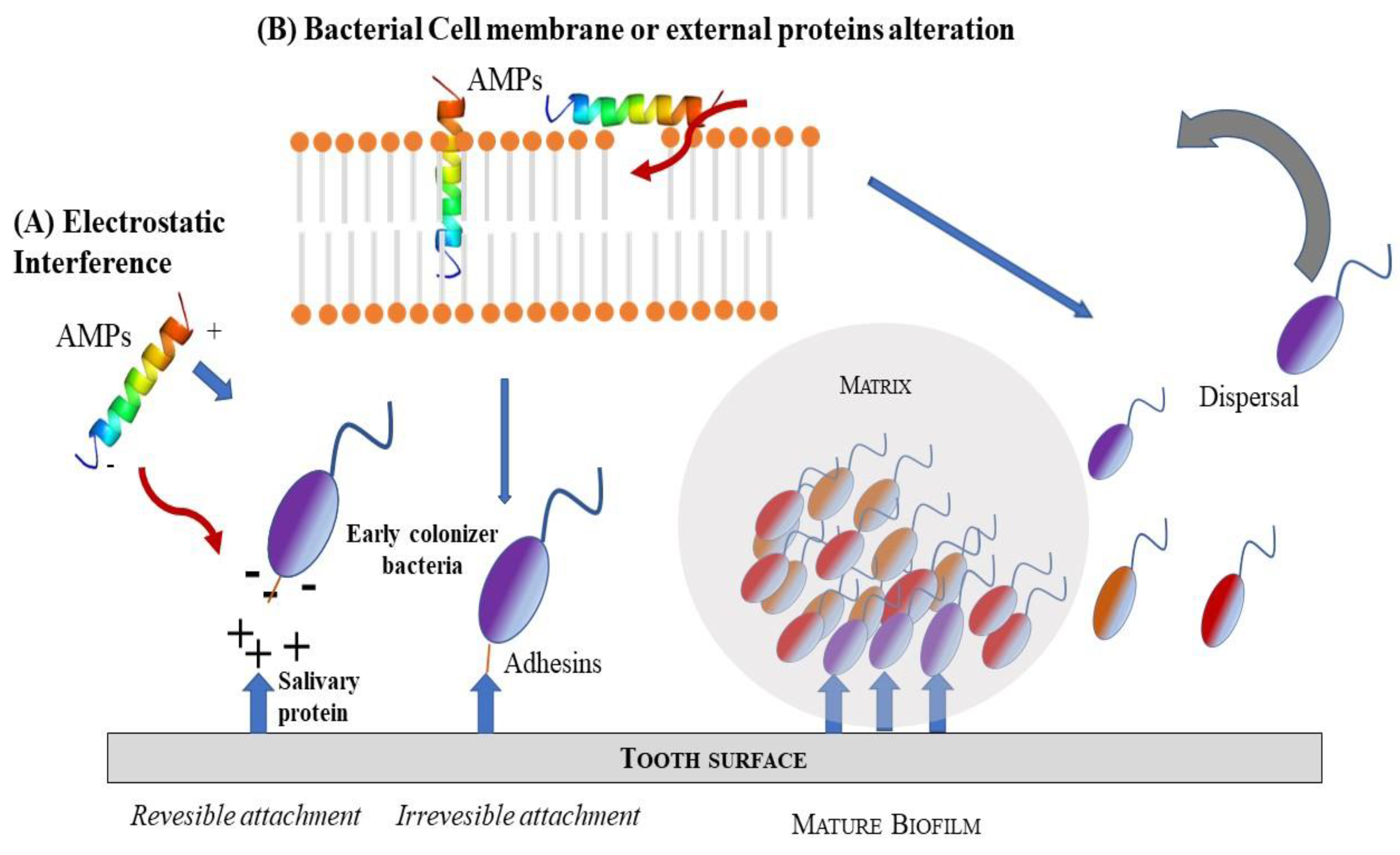
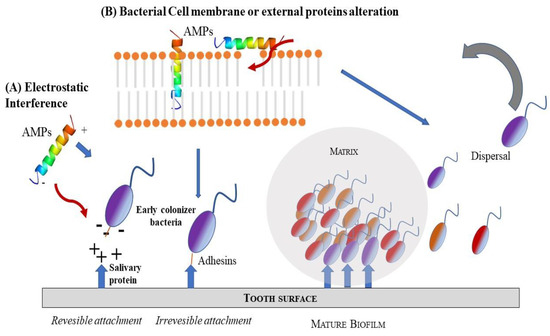
Recent studies have better defined the functions of antibiofilm peptides [26,27] (Scheme 3). Clinical studies examined the expression of AMPs in oral tissues and in crevicular fluid or saliva and correlated them with the clinical index of periodontal diseases and related microbiological analysis pathogens. Among different AMPs, Cathelicidin, α- and β-defensins 1–3 were strongly linked to periodontal status [90,91].
Scheme 3.
Schematic representation of two possible mode of AMPs action as antimicrobial agents: (A) electrostatic interference; (B) bacteria cell membrane or external protein alteration.
In the oral cavity, human AMPs are small cationic peptides, synthesized in the oral epithelium and salivary glands, and serve as defensive tools. After a look through the antimicrobial peptides databases (APD) [92], over 40 different salivary AMPs were found. These peptides are, on average, 43 amino acids long, with a medium net charge of 4.83. Oral AMPs include peptides of different biological roles: Defensins [93], Histatins, Cathelicidins, Adrenomedullin, Statherin, C-C Chemokine, Azurocidin, and Neuropeptides [94,95].
Oral peptides have evolved together with the oral microbiota and have acquired particular properties. Some AMPs families can both kill periodontal bacteria and neutralize the lipopolysaccharide (LPS) of Gram-negative bacteria. Cationic AMPs, as adrenomedullin, α-defensins (HNP), β-defensins, cathelicidin, ll-37, histatins 1 and 3, statherin, C–C motif chemokine 28 (CCL28), Azurocidin, are protected against bacterial proteases (i.e., P. gingivalis gingipains) by salivary proteins [79]. Other AMPs are involved in the bacterial agglutination, small salivary mucin-7 (MUC7) or can inhibit bacterial growth by acting as divalent cation scavengers (ion chelating agents) [96]. In the periodontal aetiologic process, the AMPs mechanism of inhibition of bacterial proteases, MPs hBD-2 and CCL20, or the peroxidase activity is particularly interesting. In this process, different active compounds against A. actinomycetemcomitans, P. gingivalis and oral streptococci [97,98] are produced.
Dysregulation of AMPs in the oral tissues is related to the pathogenesis of periodontal diseases. This aspect is observed in the patients with systemic diseases (rheumatoid arthritis, diabetes mellitus), obesity, and tobacco smokers, all of which are at high risk of periodontal pathologies. It is suggested that AMPs may act as a putative factor in these mentioned conditions and periodontal diseases.
Even if numerous in vitro experiments have shown AMPs activities versus oral pathogens bacteria, it is not clear if the AMPs exert direct antibacterial activity The concentration of AMPs in the crevicular fluid is lower than the MIC value, and some authors suggest that the primary role of oral AMPs is not linked to antibacterial activity, but inversely may serve to maintain oral microbiota homeostasis. Future solutions could be antimicrobial peptides cocktails that combat a subset of oral pathogens by modulating commensals/pathogens and by avoiding oral dysbiosis [99,100,101,102,103,104,105,106].
6. Mimicking Amino Acid Sequence
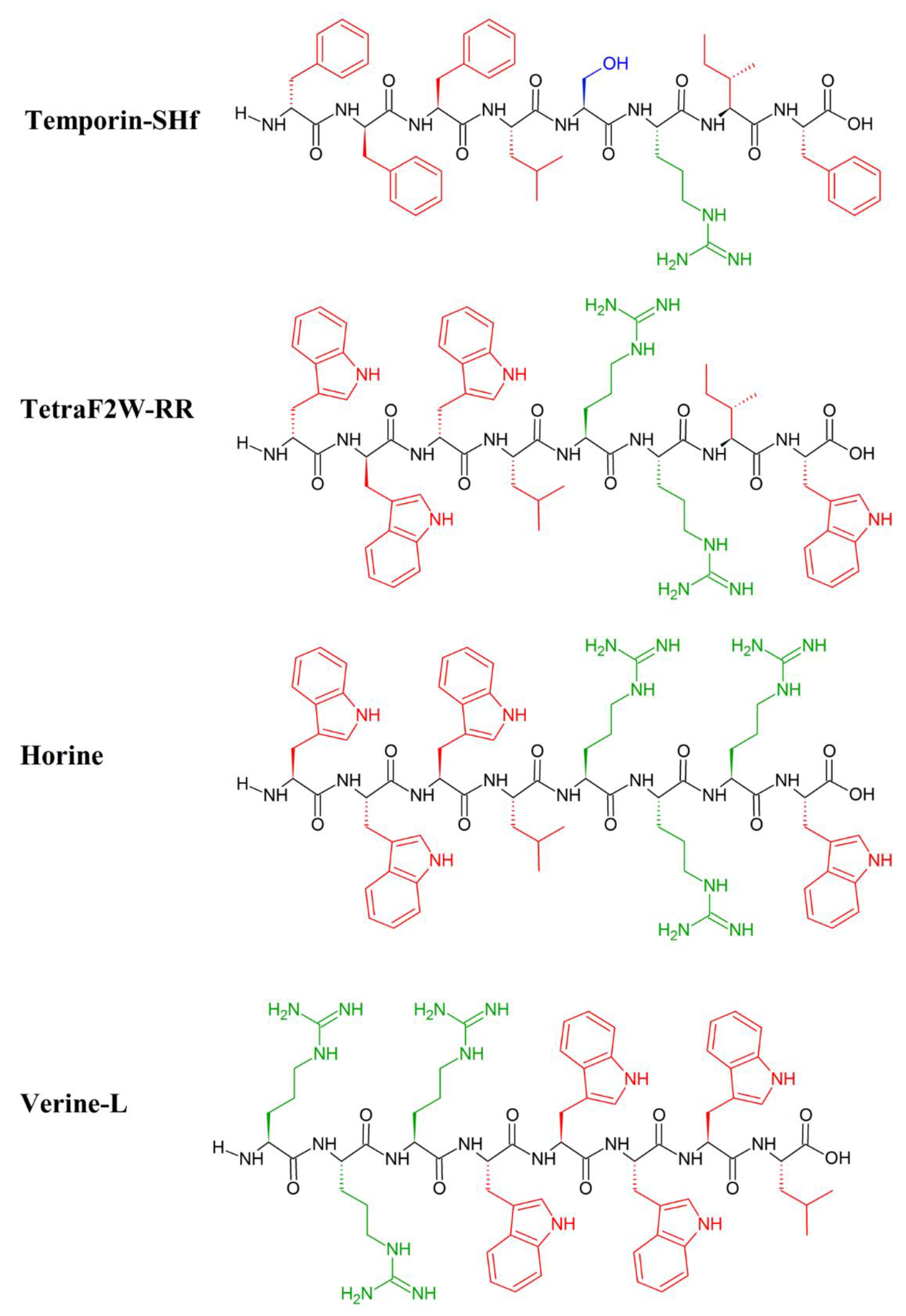
The inspiration for the AMP-mimicking peptides comes mostly from nature. Temporin-SHf (FFFLSRIF; Figure 1) is a short (8 amino acids long) unstructured [107] and highly hydrophobic peptide found in the frog’s skin, with moderate antimicrobial activity (MICE.coli(μM) = 25–30; MICS.aureus(μM) = 12.5) [107]. The tuning of its antimicrobial properties has led to the identification of an analogue peptide, TetraF2W-RR (WWWLRRIW; Figure 1), with enhanced antimicrobial (MICE.coli(μM) = 25; MICS.aureus(μM) = 1.6–3.1) and antibiofilm activity against methicillin-resistant Staphylococcus aureus (MRSA) in free and immobilized forms. Substituting phenylalanine residues with tryptophan, enhanced the antimicrobial activity, particularly against MRSA (MIC(μM) = 1.6–3.1) [108]. Horine (WWWLRRRW) and Verine-L (RRRWWWWL) are the ultimate uprade of Temporine (Figure 1), and have also enhanced the antimicrobial activity against S. aureus (MICHorine = 4 μM, MICVerine-L = 4 μM) and E. coli (MICHorine = 32 μM, MICVerine-L = 4 μM) [109].
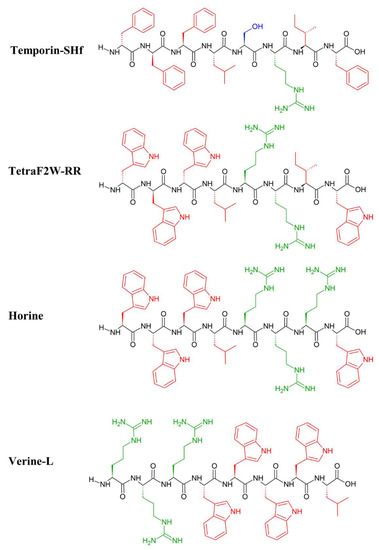
Figure 1.
Primary structure of Temporin_SHf and its artificial analogues: TetraF2W-RR, Horine and Verine-L. Amino acid side chains are colored in red (hydrophobic), blue (hydrophilic) and green (positively charged).
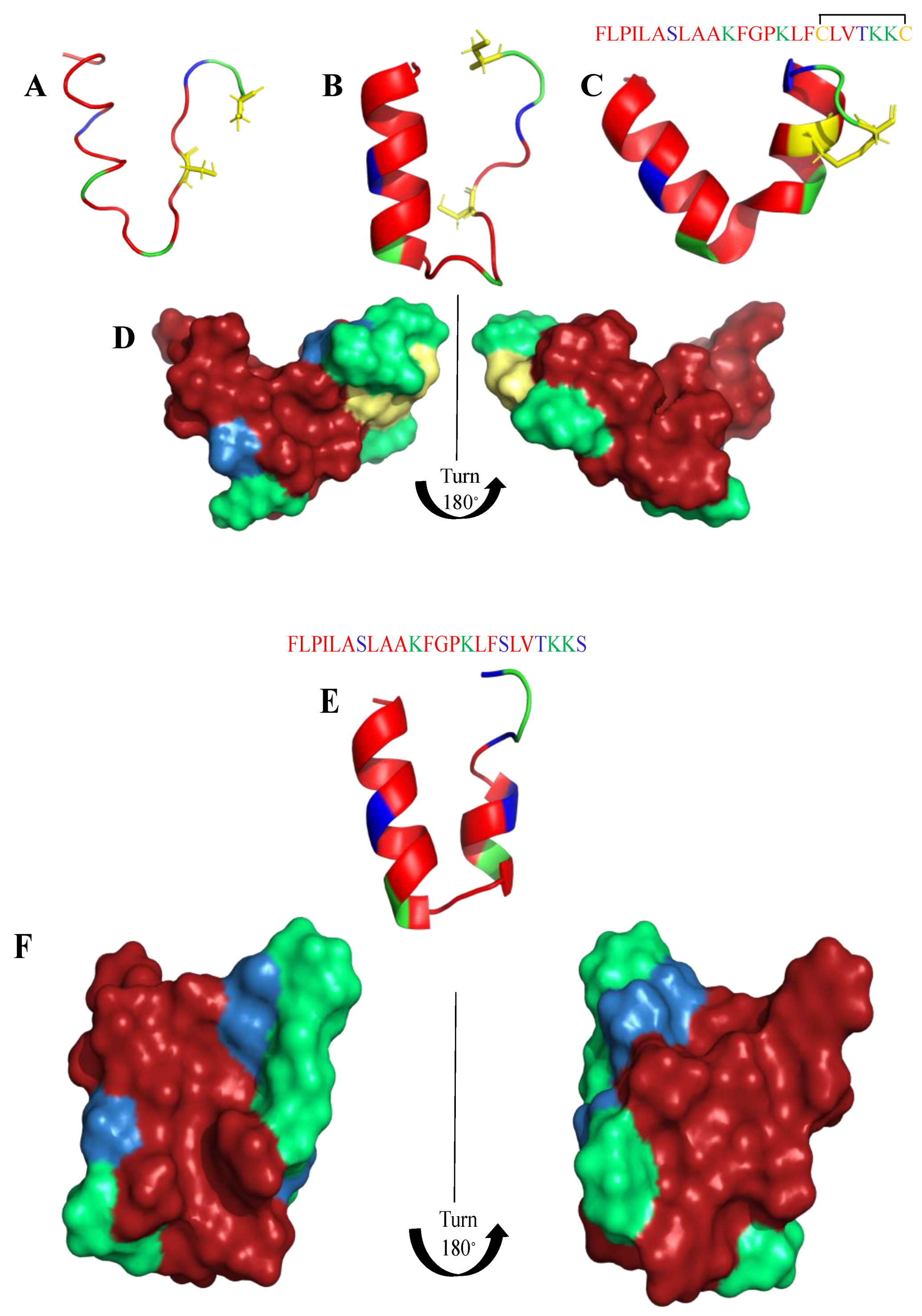
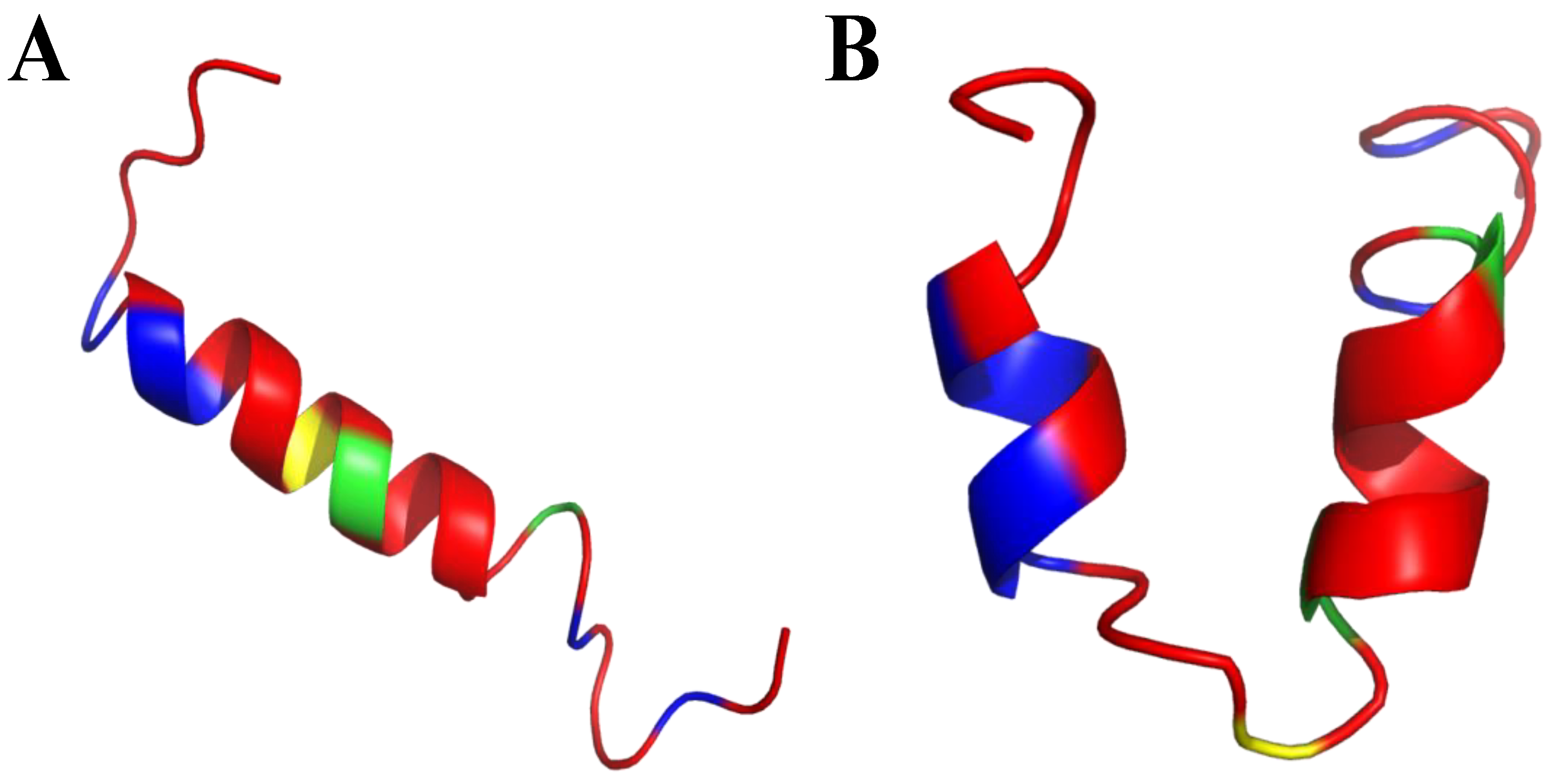
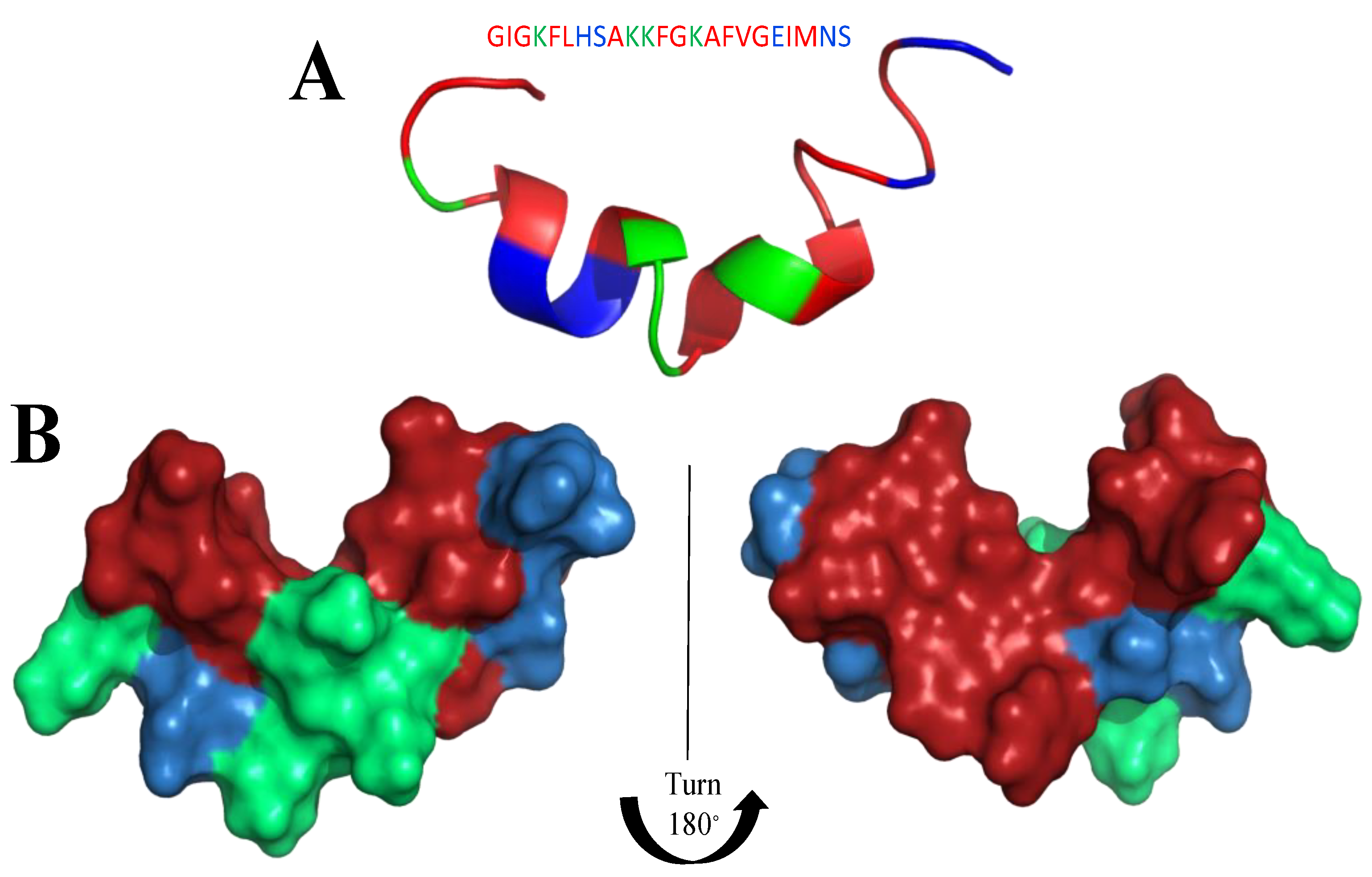
Frogs, such as Rana temporaria and Litoria aurea, secrete numerous closely related antimicrobial peptides as an effective chemical dermal defense [110]. Short peptides, isolated from frogs of the Rana genus (Anura), have intriguing antimicrobial activity against pathogenic microorganisms, and even drug-resistant strains. Each peptide has a heptapeptide loop, and reduction of the C-terminal disulfide bridge maintains the loop-like conformation and has no significant effect on hemolytic and antimicrobial activity [111]. In contrast, the substitution of the C-terminal cysteines and disulfide bridge by metathesized vinylic allylglycine residues resulted in increased antimicrobial potency [112]. Brevinin-1BYa (FLPILASLAAKFGPKLFCLVTKKC) was first isolated from skin secretions of the foothill yellow-legged frog Rana boylii, and showed broad-spectrum activity, and was particularly effective against opportunistic yeast pathogens [113]. Brevinin-1BYa has no secondary structure in water, while assuming a flexible helix–hinge–helix motif with C-terminal intramolecular disulfide bridge in membrane-mimicking micelles (Figure 2A–D) [113]. Indeed, in the I-Tasser calculations [114,115] (Figure 2A,B) the unfolded and partially folded structure can be obtained. Notably, residues 1, 4, 5, 7, 8, 10, 11 are potential chlorophylla binding sides, while residues 2, 3, 6, and 7 are nucleic acids binding sides. Nevertheless, the therapeutic potential of brevinin-1BYa is limited by its high hemolytic activity against human erythrocytes (LD50 = 10 μM) [116]. [C18S,C24S]brevinin-1BYa (Figure 2E,F) involves substitution of the conserved cysteine residues by serine residues, which results in an acyclic analogue eightfold reduction in hemolytic activity with retained high potency against Gram-positive bacteria, including strains of S. aureus MRSA (MIC = 5 μM). However, activities against Gram-negative bacteria and yeast species were reduced [117]. The non-linear relationship between the hydrophobicity of α-helical AMPs and their hemolytic activity is well documented [118]. It is possible that the [C18S,C24S] analogue fails to maintain a non-bonded loop structure akin to that of the native peptide with reduced cysteines as the Ser24 residue is not sufficiently hydrophobic to associate with the largely hydrophobic region of the helix [116]. I-Tasser calculation show that [C18S,C24S] analogue forms a helical structure; nevertheless, the C-end remains unfolded (Figure 2E,F), and the overall 3D structure of the peptide differs from that of brevinin-1BYa.
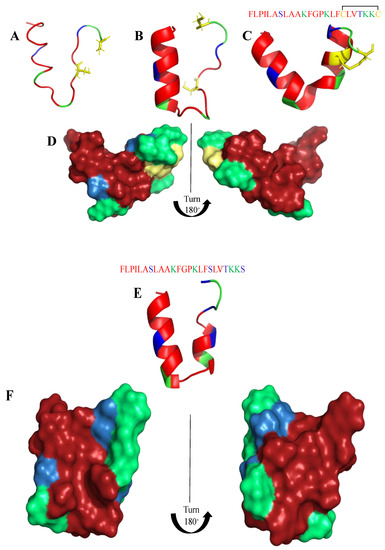
Figure 2.
(A,B) I-Tasser simulation of brevinin-1BYa ternary structure. (C,D) PDB (PDB: 6G4U) ternary structure of brevinin-1BYa. (E,F) I-Tasser simulation of [C18S,C24S]brevinin-1BYa ternary structure. Amino acids are colored in red (hydrophobic), blue (hydrophilic), green (positively charged) and yellow (cysteine).
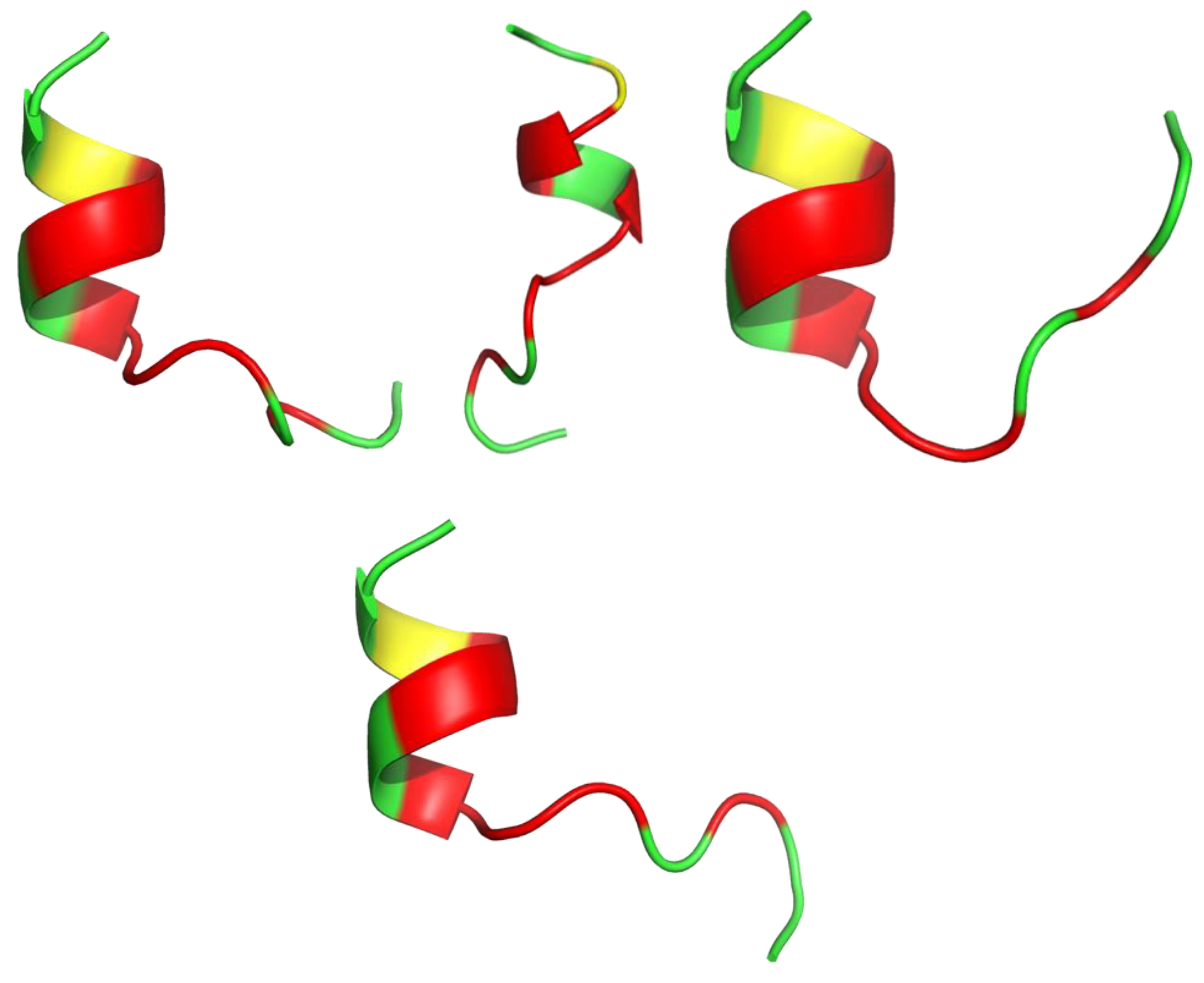
Snake venom toxins have valuable potential in the design and synthesis of novel antimicrobials. Lys49 phospholipase A2s (PLA2s) are multifunctional snake toxins able to induce a huge variety of therapeutic effects and consequently serve as templates for new drug leads. Almeida et al. synthesized five oligopeptides mimicking regions of the antibacterial Lys49 PLA2 toxin (CoaTx-II isolated from Crotalus oreganus abyssus snake venom). The 13 amino acid peptide pC-CoaTxII, corresponding to residues 115-129 of CoaTx-II (KKYRIYPKFLCKK), was able to reproduce the promising bactericidal effect of the toxin against multi-resistant clinical isolates of Gram-negative bacteria (MICP. Aeruginosa(μM) = 5.95). Even though, the molecular dynamics (MD) simulations showed that pC-CoaTxII is unstructured [119], the I-Tasser calculation (Figure 3) showed the peptide’s susceptibility to form a helical shape. A possible antimicrobial mechanism of pC-CoaTxII is through strong interaction with anionic lipid membranes such as those in bacteria. In silico studies suggested formation of a water channel across the membrane upon peptide insertion, eventually leading to bacterial cell disruption and death [119].
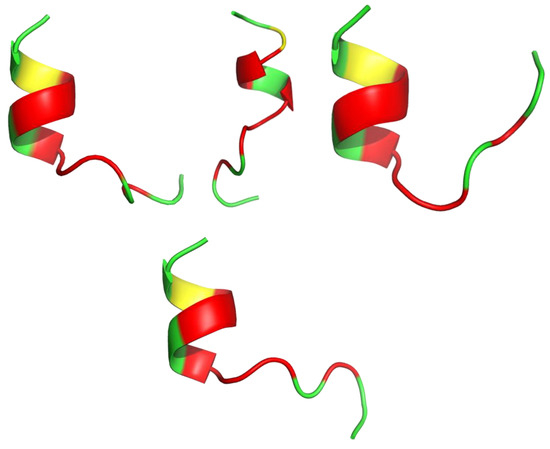
Figure 3.
Four models of the antibacterial synthetic peptide pC-CoaTxII structure calculated with I-Tasser. Amino acids are colored in red (hydrophobic), green (positively charged) and yellow (cysteine).
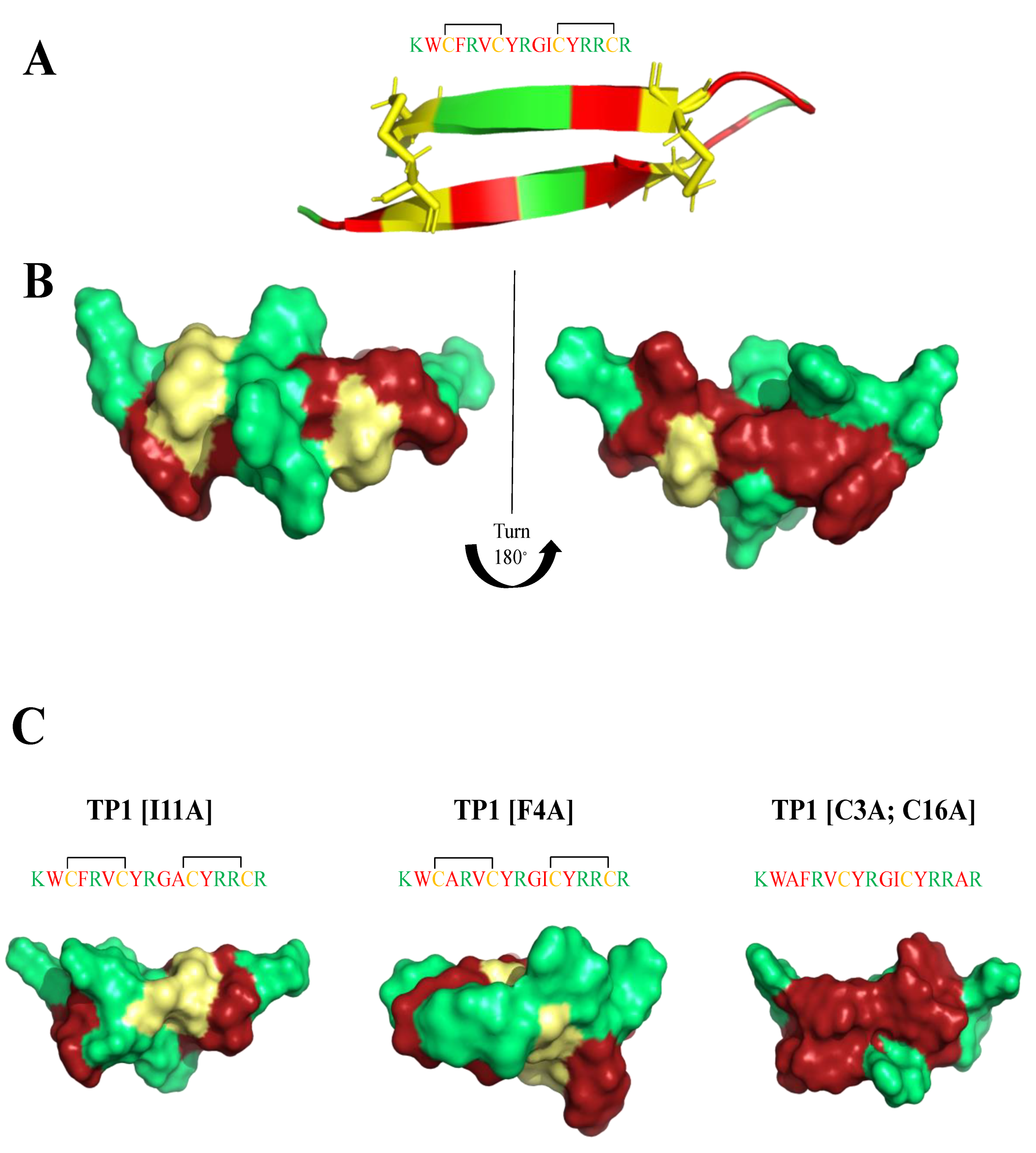
Tachyplesin-1 (TP1) is a 17 amino acid AMP (KWCFRVCYRGICYRRCR) extracted from the hemocytes of the horseshoe crab Tachypleus tridentatus [120]. It forms a β-hairpin structure (Figure 4), both in aqueous solution and in lipid-mimicking environments [121,122], and has two disulfide bridges (Cys3-Cys16, Cys7-Cys12) that play an important role in broad spectrum antimicrobial activity [123]. The reduction in antimicrobial activity, when Cys residues are removed from the peptide’s sequence, is presumably linked to the subsequent loss of β-sheet stacking [124,125,126]. TP1 exhibits potent activity against Gram-positive (MICB. pseudomallei(μM) = 62) and Gram-negative (MICE.coli(μM) = 22) bacteria, as well as fungi. It did not induce resistance in short-term studies [127], but caused decreased susceptibility under long-term continuous selection conditions [124]. However, TP1 is highly toxic toward mammalian cells (IC50HEPG2(μM) = 110), making it unsuitable for therapeutic development. Edwards et al. [7] prepared a systematic study of a clear structure−function relationship for each amino acid in the sequence, while modulating charge and hydrophobicity by residue modification and truncation of the peptide. They were able to assess the effects of amino acid replacements on antimicrobial activity, cytotoxicity, and hemolytic activity. Moreover, they evaluated the membrane binding affinity and stability of the most interesting peptides. Three modified peptides: (TP1[F4A]), (TP1[I11A]), and (TP1[C3A,C16A]) maintained the β-hairpin secondary structure motif (Figure 4), and possessed substantially improved therapeutic indexes (26-to 64-fold) over the progenitor peptide, exhibiting MICE.coli(μg/mL) = 0.125–4; MICK.pneumoniae(μg/mL) = 0.25–16; MICA.baumanii(μg/mL) = 0.25–0.5; MICP.aeruginosa(μg/mL) = 0.25–2; MICB.subtilis(μg/mL) = 0.125–0.25; MICS.aureus(μg/mL) = 2–8; MICC.albicans(μg/mL) = 2–8; MICC.neofomans(μg/mL) = 0.25–4 values and were considerably less hemolyticly toxic. (TP1[F4A]) and (TP1[I11A]), not only conserve the β-hairpin secondary structure motif, but also are highly stable in mouse and human plasma. Of noteworthy mention, I-Tasser calculation showed the presence of potential magnesium and zinc binding sides in TP1 analogues.
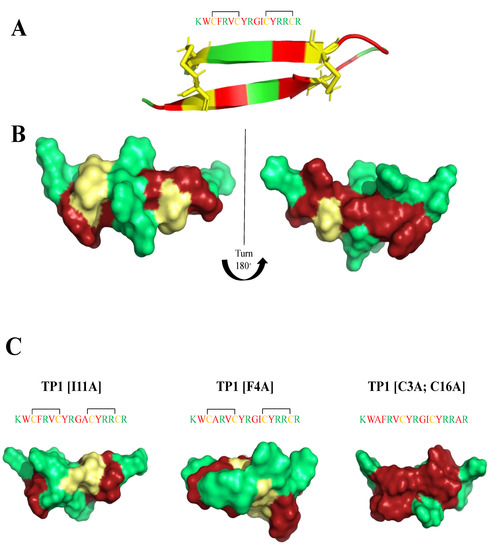
Figure 4.
(A,B) Tachyplesin-1 (TP1) structure (PDB:1WO0). (C) I-Tasser calculations of TP1 analogues structure. Amino acids are colored in red (hydrophobic), green (positively charged) and yellow (cysteine).
The design of a library of peptide antibiotics is usually done using a parent peptide (naturally occurring or designed in the laboratory), followed by structure−activity relationship (SAR) studies and fine-tuning the activity of the peptide [11]. A synthetic antimicrobial peptide library based on the human autophagy 16 polypeptide has been developed by Varnava et al. [128]. The fatty acids conjugation (inspired by lipopeptide antibiotics e.g., polymyxin [129]) and N-Acetylation [130,131,132,133] strategies were used to enhance the activity and serum stability of AMPs. The fine-tuning of structure and length of the fatty acid component of the antimicrobial lipopeptide battacin [134] resulted in peptides library with enhanced potency with respect to the parent Atg1, a human autophagy polypeptide. A 21-residue fragment of Atg16 conjugated to 4-methylhexanoic acid (K30; Figure 5) emerged as the most potent antibacterial agent (MICC.albicans(μM) = 30–60; MICP.aeruginosa(μM) = 60–120; MICE.coli(μM) = 6.4–12.8; MICS.aureus(μM) = 0.9–1.8). Moreover, the relationship between the K30 structure (Figure 5) and function in the bacterial membrane was determined. The negatively charged bacterial membrane anchor the K30 peptide, and subsequent interactions with the hydrophobic residues of the peptide, causing the K30 peptide to adopt a helix−loop−helix structure. The folded structure is able to penetrate the nonpolar acyl chain of the lipid molecules, where it subsequently causes membrane disintegration leading to cell lysis [128].
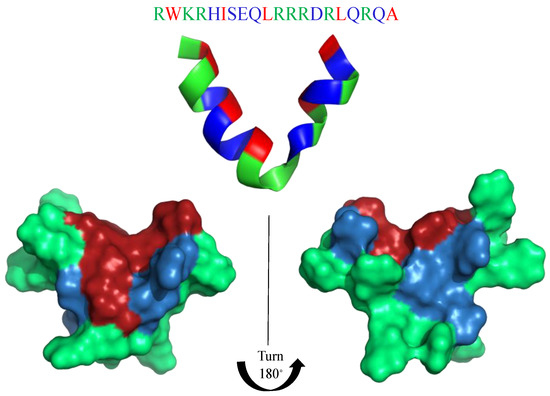
Figure 5.
K30 structure calculated with I-Tasser. Amino acids are colored in red (hydrophobic), blue (hydrophilic), and green (positively charged).
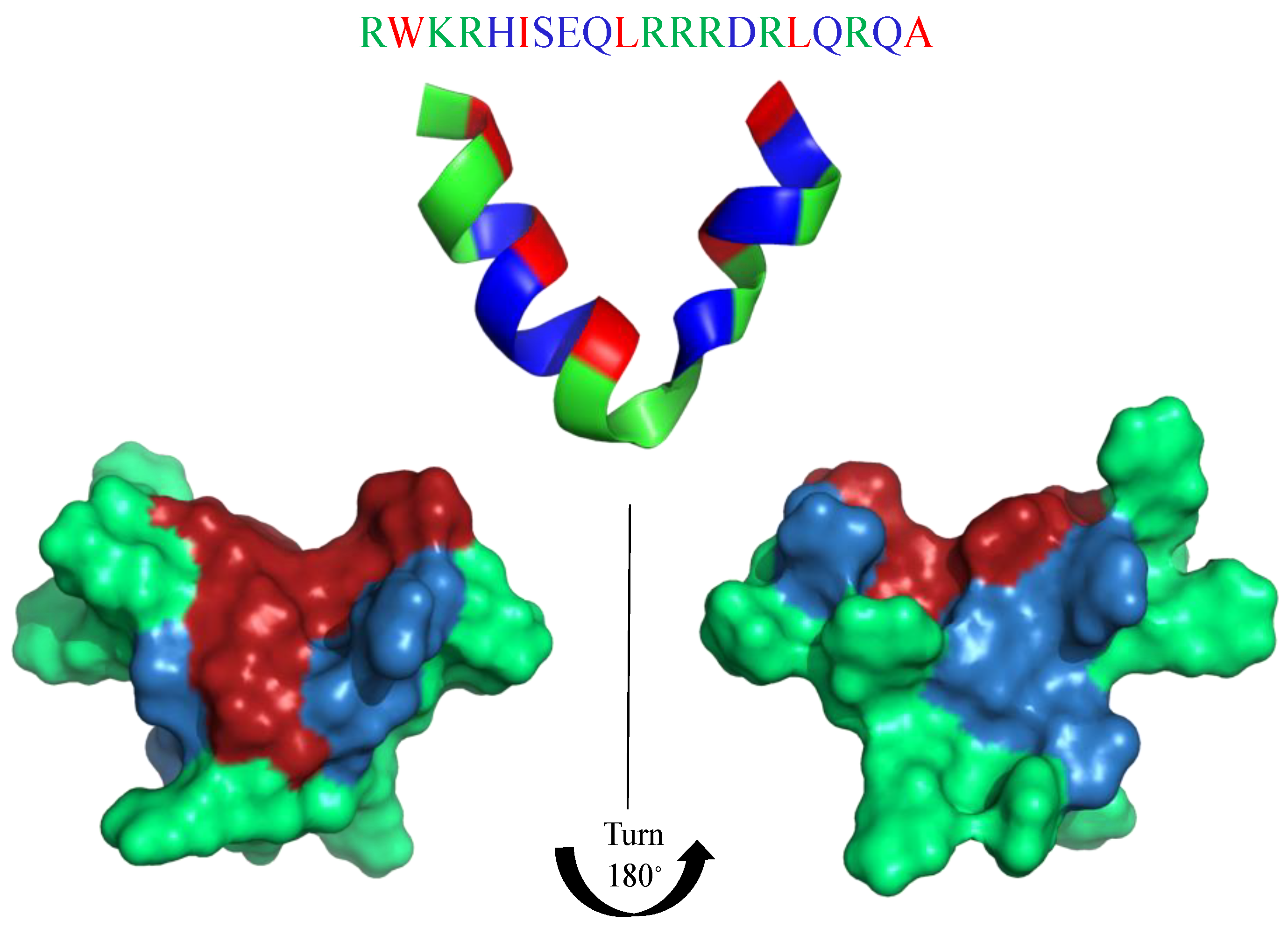
Interference with bacterial virulence is a promising alternative approach or a complementary adjunct to traditional antimicrobial therapy [135]. Antivirulence strategies focus on pathogenicity factors and bypass the pressure on the bacterium to develop resistance. The MgtC membrane protein has been proposed as an attractive antimicrobial target, while it is involved in the ability of several major bacterial pathogens (e.g., Pseudomonas aeruginosa) to survive inside macrophages. Moussouni et al. [135] developed an antivirulence strategy targeting MgtC, by taking advantage of a natural antagonistic peptide, MgtR (ILFVADSLQMIPLCLRIWVALKINILFSVL). Heterologous expression of MgtR in P. aeruginosa PAO1 was shown to reduce its ability to survive within macrophages, while exogenously added synthetic MgtR peptide lowered the intramacrophage survival of wild-type P. aeruginosa PAO1, thus mimicking the phenotype of an MgtC mutant as well as the effect of endogenously produced MgtR peptide [135]. Similar to AMP-mimicking peptides, MgtR has a high susceptibility to form a helical structure. The crystal structure not known at the present time, nevertheless, the I-Tasser structure prediction shows the possibility to form a central helix or a helix–strand–helix structure (Figure 6).
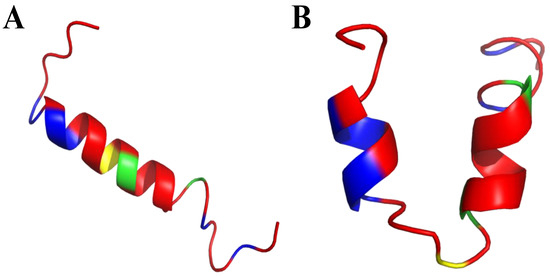
Figure 6.
I-Tasser prediction of MgtR secondary structure. (A) one alpha-helix motif in the middle of MgtR secondary structure. (B) two short alpha-helix motifs in the middle of MgtR secondary structure. Amino acids are colored in red (hydrophobic), blue (hydrophilic), green (positively charged) and yellow (cysteine).
7. Amino Acid Conjugated Polymers
Synthetic homo- and co-polymers have low toxicity to host cells and at the same time simulate the functions of AMPs [25,136]. In contrast to AMPs, synthetic antibacterial polymers have a versatile chemical structure, scalability, and lower synthesis costs [137,138]. The polymers are bilateral—on one side cationic residues act with the negatively charged bacterial cell-wall, on the other side hydrophobic domains interact with the lipophilic layers of the bacterial cell-wall leading to the disruption of the cell membrane [28,139,140,141]. Many peptide-mimetic antibacterial polymers are already reported in the literature in recent decades, and are mainly derivatives of polymethacrylates [142,143], polyacrylates [144,145], polynorbornenes [146,147], poly(β-lactam)s [28,139], polymaleimides [148,149], and polycarbonates [150,151]. Extensive studies were dedicated to probing the correlation between antimicrobial activity and polymer amphiphilicity [143,152], structure (random or block) [136,153], type of cationic charge [154], molecular weight [145], and spaced arm (distance from polymer backbone to pendant cationic center) [1].
Systematic optimization of the (co)polymer composition, chain length, hydrophobicity, and cationic charge has generated selected examples that are also highly biocompatible (non-hemolytic and non-cytotoxic in vitro). The extensive review on biomimetic antimicrobial polymers together with polymer chemistry was published by Ergene et al. in 2018 [138], and here, we discuss some recent advances in effective antimicrobial polymer discovery.
Two key parameters are known to affect the efficacy of AMP mimics: molecular weight and amphipathic balance. Polymers of lower molecular weight have greater antimicrobial activity [155], while correct amphipathic balance is necessary to impart both antibacterial activity and selectivity versus bacterial membranes [25]. If the hydrophobicity is too high, selectivity is reduced, and eukaryotic cell death occurs. Researchers increased hydrophobicity through copolymerization of cationic monomers with hydrophobic monomers with alkyl tails of different lengths, which generally resulted in increased bacterial cell death at the expense of selectivity [156]. Multiple polymer backbone structures, including methacrylates [157], β-lactams [158], norbornenes [159], and methacrylamides [160], with varying solubility and inherent polarity have been evaluated as AMP mimics.
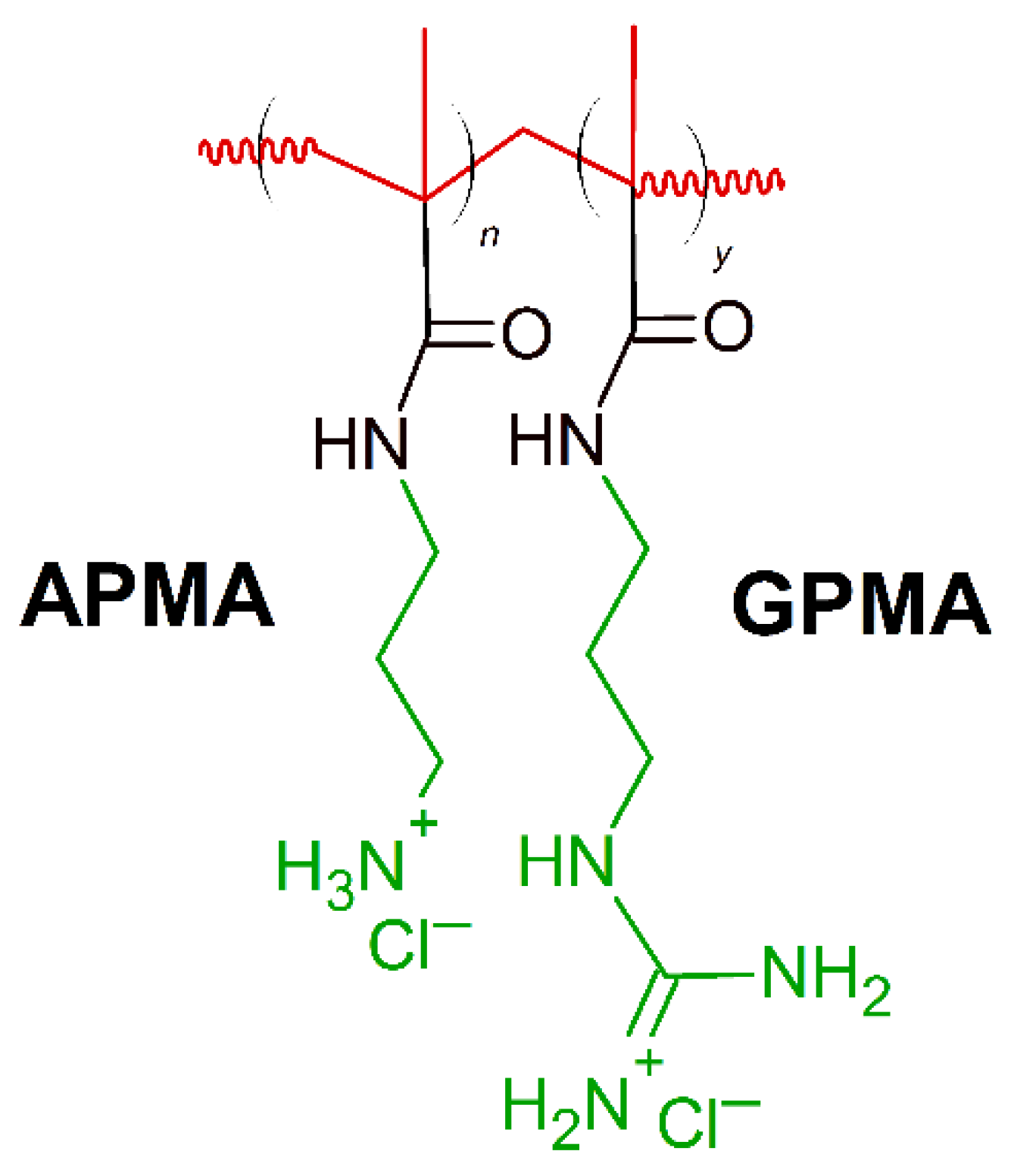
Positively charged peptides- lysine and arginine are present in the primary sequence of different AMPs constituting 6–8% incidence of each amino acid [138]. Synthetic polymers containing moieties mimicking lysine and arginine components found in AMPs have been reported to show effectiveness against specific bacteria. Exley at al. [161] created a series of copolymers containing lysine-mimicking aminopropyl methacrylamide (APMA) and arginine-mimicking guanadinopropyl methacrylamide (GPMA) (Figure 7). Copolymers were prepared with varying ratios of the co-monomers (APMA and GPMA), with a degree of polymerization of 35−40 and narrow molecular weight distribution to simulate naturally occurring AMPs. The APMA homopolymer demonstrated the greatest antimicrobial activity against E. coli, S. aureus and P. aeruginosa (MIC(μg/mL) = 500) and the lowest toxicity to mammalian cells. At the same time, the antimicrobial activity of the APMA homopolymer was least affected by changes in salt concentration of both type of the polymers tested [161]. On the contrary, addition of GPMA units in the polymer, lower the antimicrobial activity. Considering MIC ∼100 μg/mL as high antimicrobial activity, and MIC ranging from 500 to 1000 μg/mL as moderate activity, the APMAhomo polymer showed promising results, but needs some future improvements.
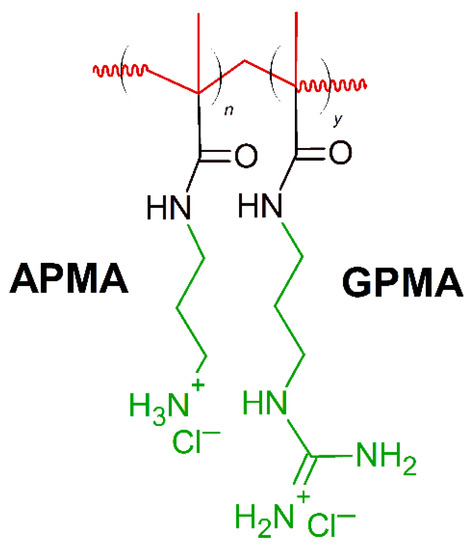
Figure 7.
Schematic representation of aminopropyl methacrylamide (APMA)-stat-guanadinopropyl methacrylamide (GPMA) polymer. Red: hydrophobic chain, green: positively charged residues.
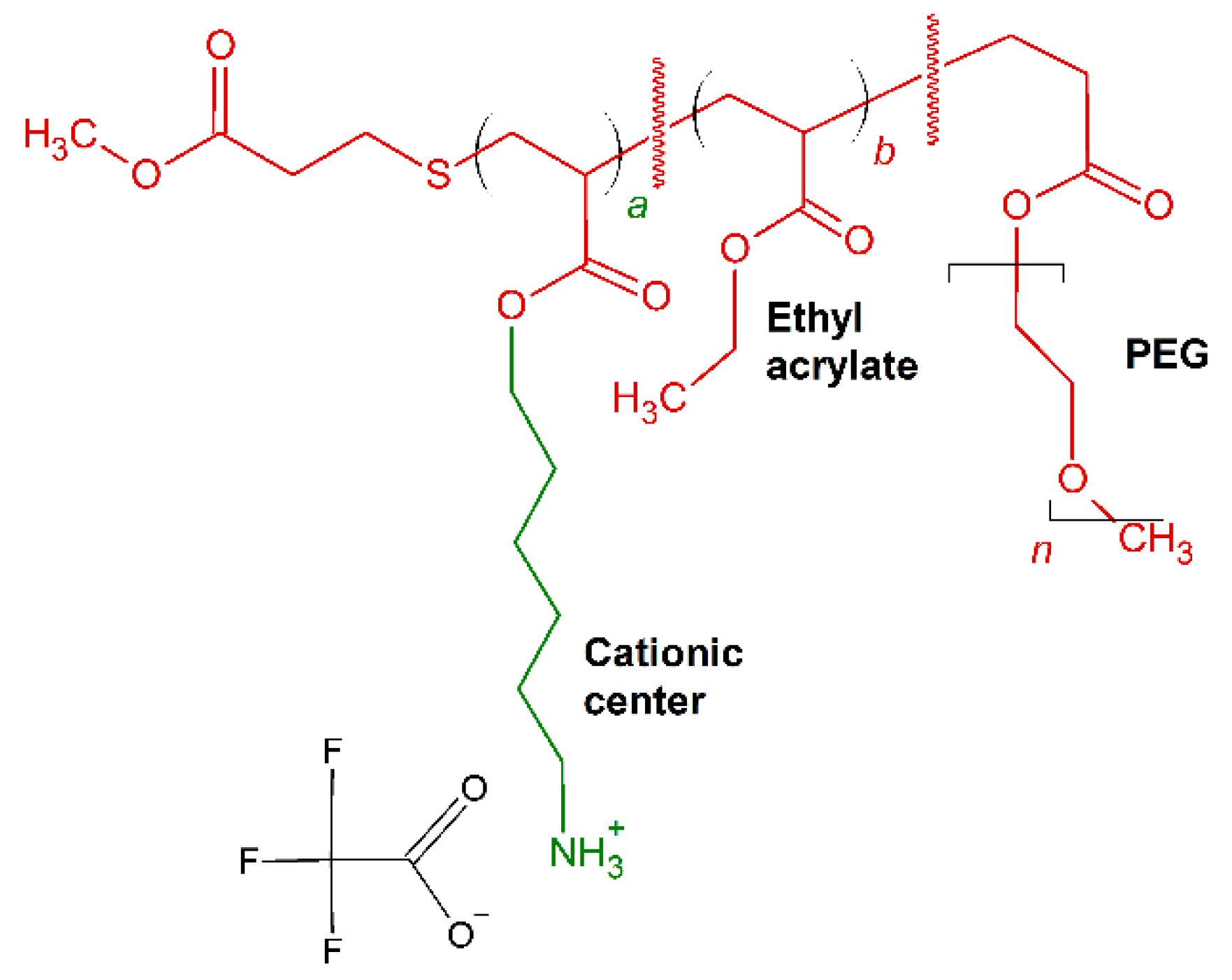
Brittin et al. [162] optimized the cationic charged placement, amphiphilic balance and PEGylation content in synthesis of acrylate-based random ternary copolymers comprised of same center cationic, ethyl and poly(oligoethyleneglycol) side chains [162]. The resultant molecules (Figure 8) showed effective antimicrobial activity with low MIC (μg/mL) against E. coli and B. subtilis, ranging between 22 and 44 (μg/mL) and between 2.5 and 10 (μg/mL), respectively.
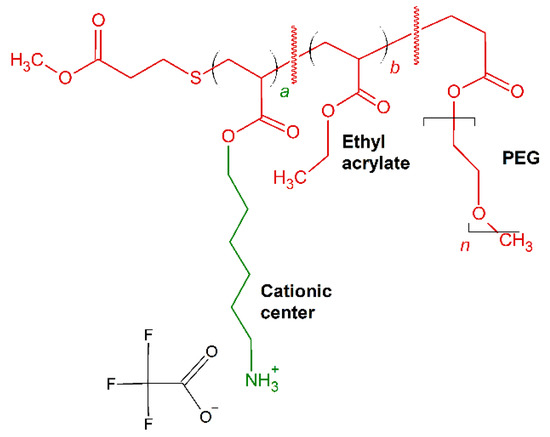
Figure 8.
Schematic representation of P1–P4 polymers. In red: hydrophobic residues, in green: positively charged chains.
Antimicrobial polycarbonates containing primary amino groups can effectively kill bacteria as shown by Nimmagadda et al. [150]. Their single, di-block and random copolymers containing hydrophobic (0–10) and hydrophilic (10–20) units (Figure 9) were revealed to be more effective against different multidrug resistant Gram-positive bacteria strains (MICS.aureus(μM) = 5–25; MICS. epidermidis(μM) = 5–25; MICE. faecalis(μM) = 5–25) respect Maganin II (MICS.aureus(μM) = 16; MICS. epidermidis(μM) > 50; MICE. faecalis(μM) > 50). The amphiphilic units are essential for bacterial killing by bacterial membrane disruption, and random block polymers are more potent than di-block polymers, probably due to stable nanostructures of di-block polymers, which prevent them from interacting with bacterial membranes more effectively.
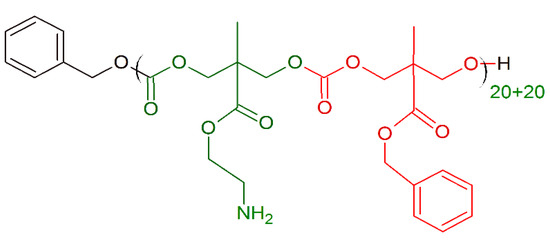
Figure 9.
Schematic representation of the polycarbonate polymer with primary amino groups. Green: positively charged monomer; red: hydrophobic monomer.
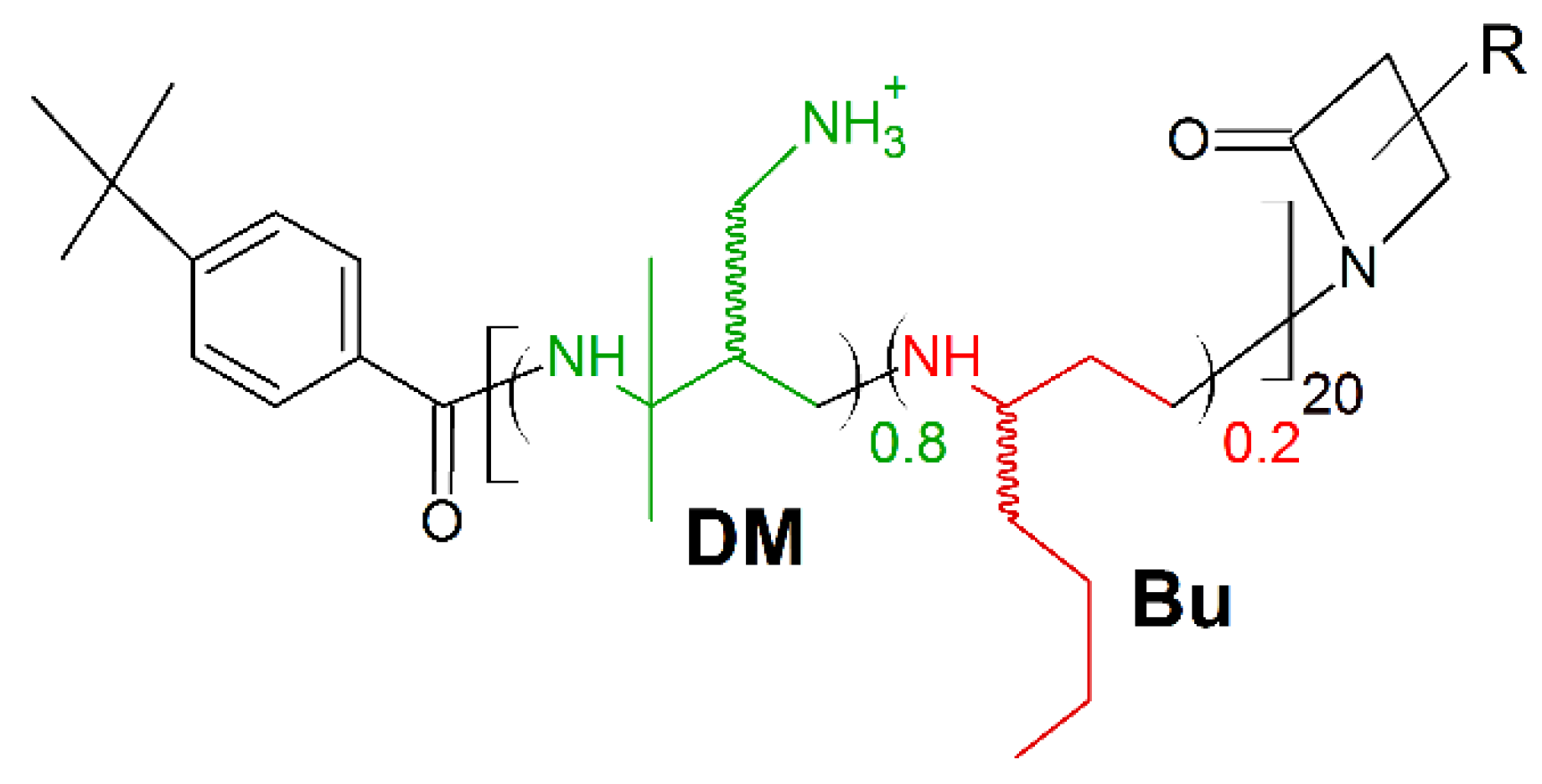
A new frontier in host defense peptides mimicking is β-peptide polymers. Their potent antimicrobial activity and excellent biocompatibility, together with high stability upon protease associated biodegradation are well ascertained [163,164]. Recently, Chen et al. presented the new potent antimicrobial peptide polymer named 80:20 DM:Bu (Figure 10). It is composed of two subunits, one hydrophilic/cationic subunit (named DM) and one hydrophobic subunit (named Bu), to have both cationic charges and amphiphilicity as synthetic mimics of HDPs [27,28,32]. The polymer displayed fast, potent, and broad-spectrum activities upon multiple multi-drug resistant (MDR) strains of bacteria (MICS. aureus (μg/mL) = 12.5; MICs.haemolyticus (μg/mL) = 3.13; MIC P.aeruginosa (μg/mL) = 12.5; MICE.coli (μg/mL) = 50), resistant to ampicillin (MICS. aureus (μg/mL) > 200; MICs.haemolyticus (μg/mL) > 200; MIC P.aeruginosa (μg/mL) > 200; MICE.coli (μg/mL) > 200) and streptomycin (MICS. aureus (μg/mL) = 25; MICs.haemolyticus (μg/mL) > 200; MIC P.aeruginosa (μg/mL) > 200; MICE.coli (μg/mL) = 100). Moreover, peptide polymer 80:20 DM:Bu exerted satisfactory activity against K. pneumoniae (MIC (μg/mL) = 50–100) and A. baumannii (MIC (μg/mL) = 12.5–50).
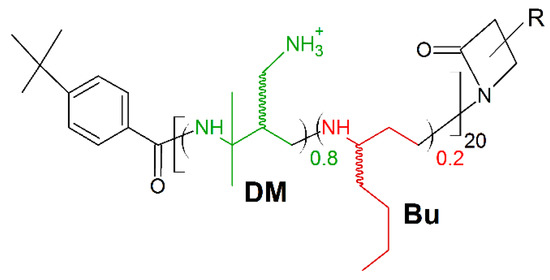
Figure 10.
Schematic representation of 80:20 DM:Bu polymer. Green: positively charged monomer; red: hydrophobic monomer.
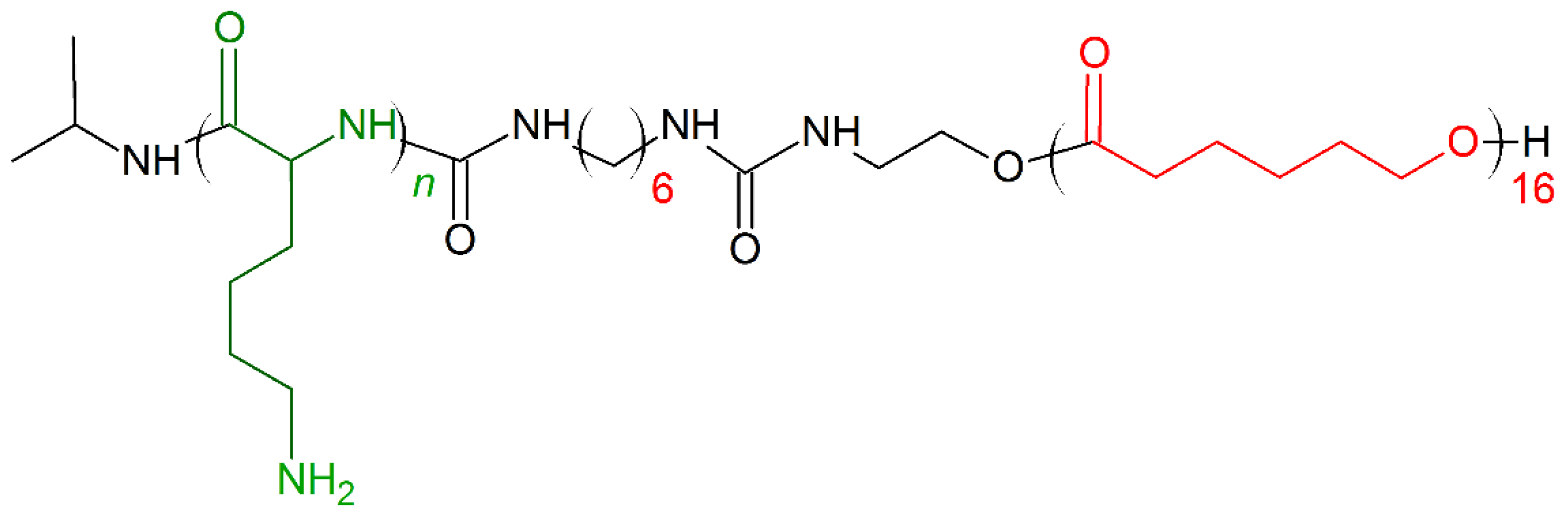
In 2019, Zhou et al. [165] presented a new concept of designing novel biocompatible antibacterial copolymers and expanded the categories of next-generation antibacterial agents without inducing drug resistance. The series of di-block copolymers were inspired by Poly(ε-caprolactone) (PCL) natural AMPs. Poly(ε-caprolactone) (PCL) is a biodegradable copolymer with controlled degradability, biocompatibility, and miscibility with other polymers, properties [166,167,168] excellent for biomedicine applications [166]. PCL (hydrophobic component) was coupled with polylysine (Kn) to form diblock copolymers named PCL16-b-Kn (Figure 11). Three polymers PCL16-b-K11, PCL16-b-K20, PCL16-b-K27 showed excellent antimicrobial activity against E. coli (MIC (μg/mL) = 8–32) and S. aureus (MIC (μg/mL) = 8–16). The membrane disruption mechanism and cytoplasm leakage were observed for both E. coli and S. aureus treated with PCL16-b-K20 copolymer. The cytotoxicity tests conducted on human dermal fibroblasts for 72 h showed that even at 500 μg/mL PCL16-b-K20 concentration (fifty times higher than MIC activity), more than 70% of the normal human cells were still alive [165]. It was also shown that PCL16-b-Kn copolymer vesicles exhibit the value of a potential application as multifunctional drug-carrier systems with antibacterial capability in cancer therapy [169].
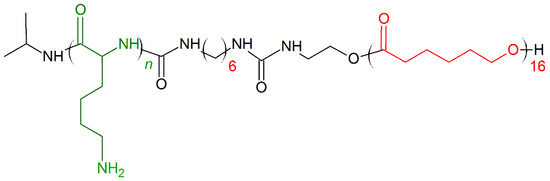
Figure 11.
Schematic representation of Poly(ε-caprolactone) (PCL)16-b-Kn polymer. Green: positively charged monomer; red: hydrophobic monomers.
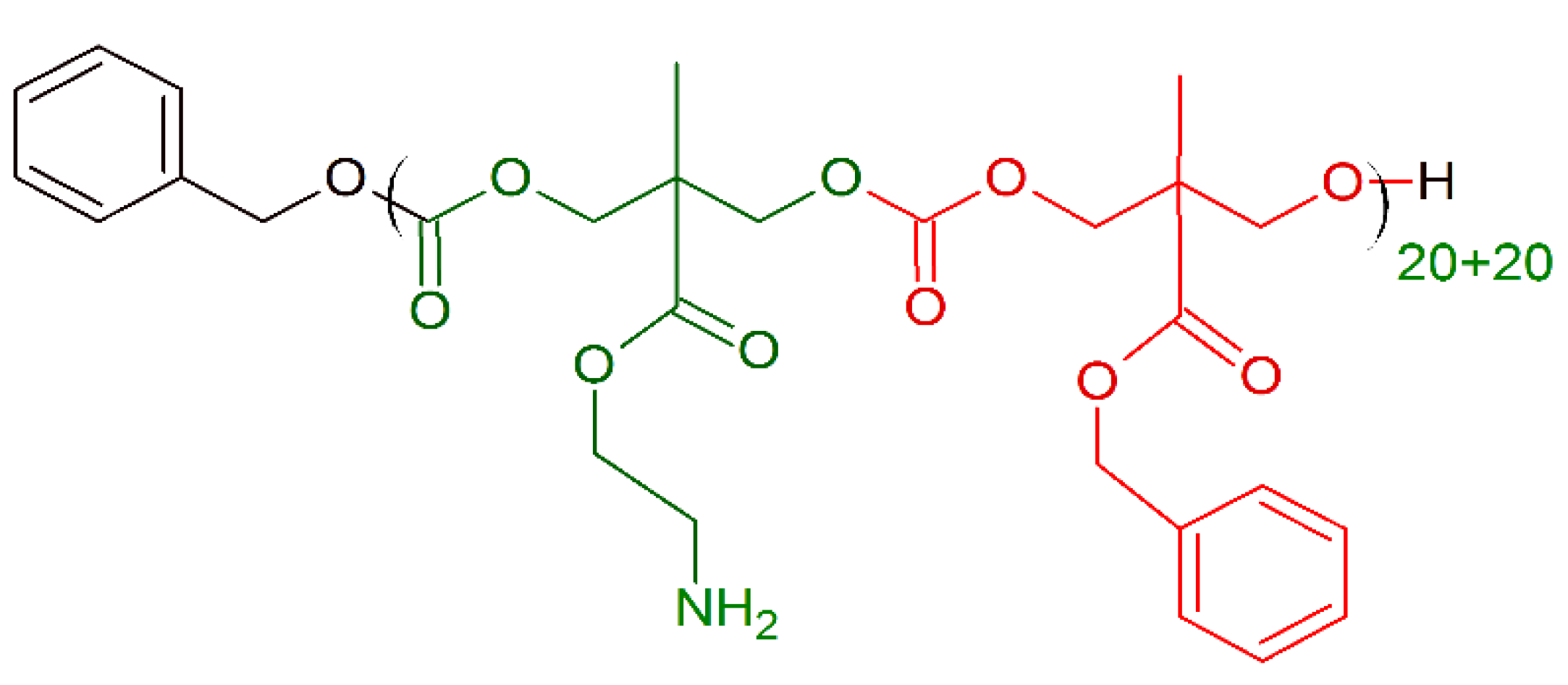
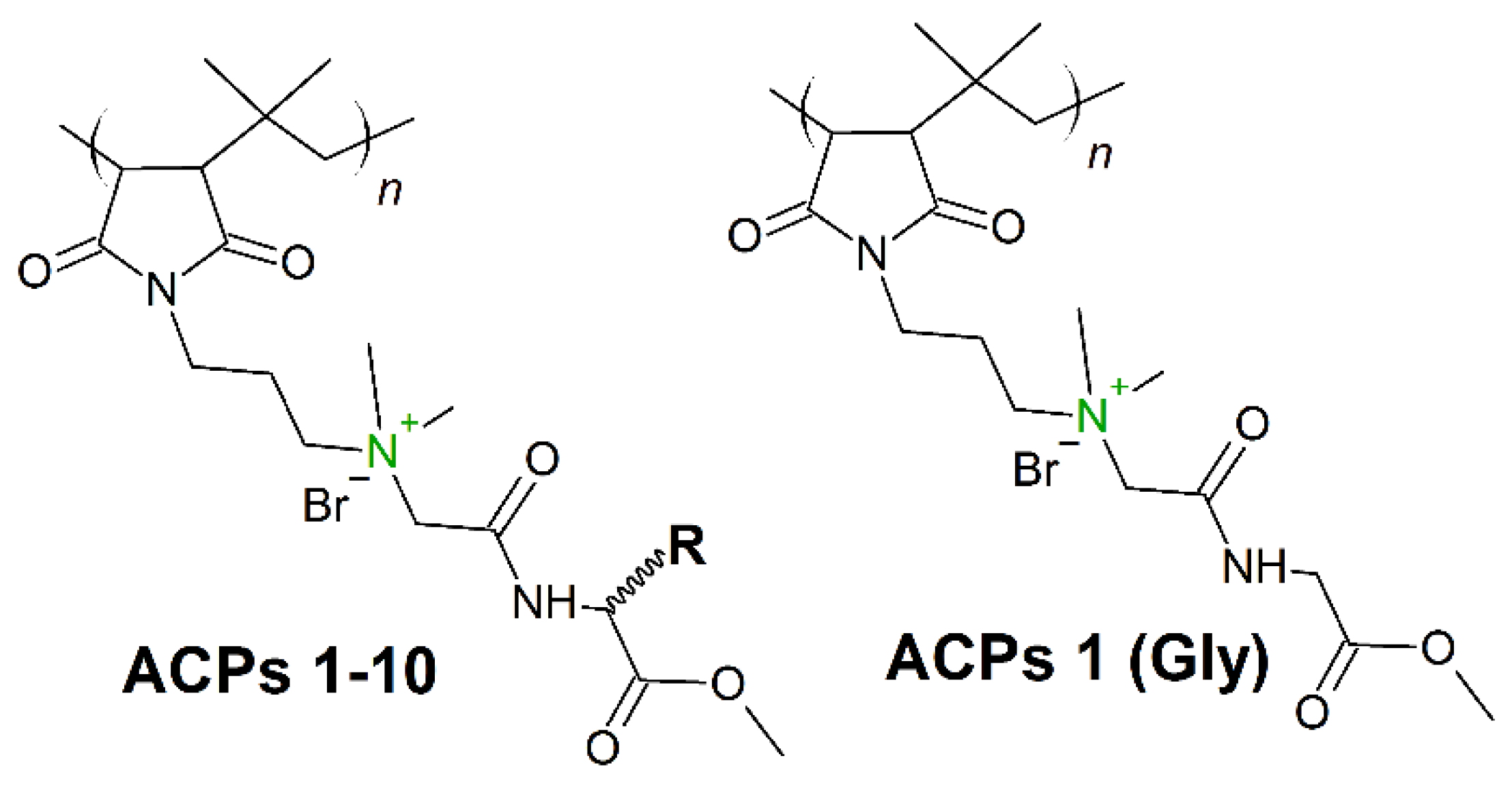
Hydrophobicity modulation through incorporation of amino acids in cationic polymers can provide a significant opportunity to design new amino acid conjugated polymers (ACPs) with potent antibacterial activity and minimum toxicity toward mammalian cells. In 2019, Barman et al. [170] presented a class of ACPs with tunable antibacterial activity through a simple post-polymer-functionalization strategy. Permanent cationic charge was present in every repeating unit (Figure 12), whereby the hydrophobicity of the entire molecule was tuned through incorporation of different amino acids. The amino acid alteration had a strong influence on antibacterial efficacy, and as the amino acid side-chain hydrophobicity was increased, both the antibacterial activity (against broad spectrum of bacteria) and toxicity increased. ACP including a glycine residue (ACP-1 (Gly), Figure 12) showed very good activity (MICA.baumannii (μg/mL) = 8−16) against both drug-sensitive and drug-resistant strains, including clinical isolates. Moreover, after 14 continuous passages there was no propensity for bacterial resistance development against this polymer.
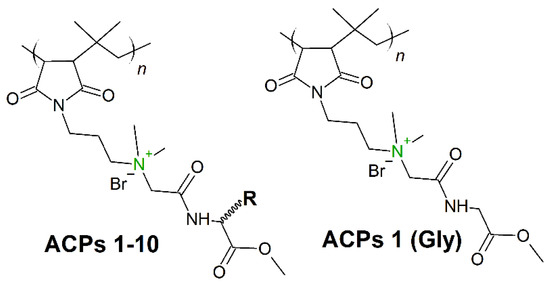
Figure 12.
Schematic representation of 1-10 amino acid conjugated polymers (ACPs). In green: permanent cationic charge.
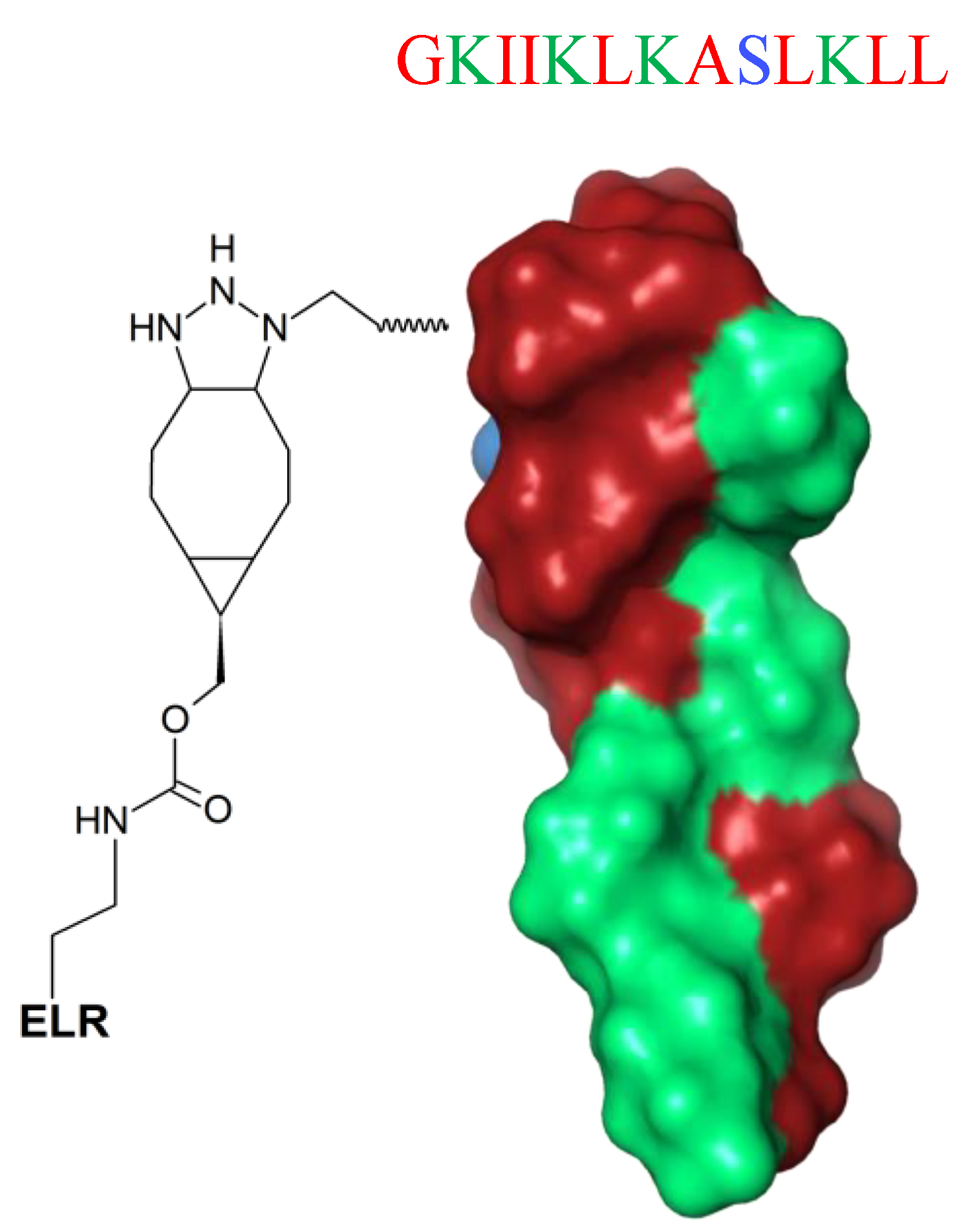
Fixing AMPs on surfaces is a new strategy to enhance their stability and increase local concentration and biological availability. Nevertheless, immobilized AMPs have lower ability to interact with multiple targets [171]. A possible solution is attachment of the peptides to the substrate via a spacer, which increases the flexibility and enhances the functional conformation of AMPs, thereby ameliorating the overall antimicrobial effect [172]. Such a strategy is a new horizon in the fabrication of biocoatings. Recently, Acosta et al. [172] proposed an effective antibiofilm coatings based on protein-engineered polymers and antimicrobial peptides for preventing implant-associated infections [172]. The unstructured GL13K (GKIIKLKASLKLL-NH2) peptide (Figure 13) was hybridized with elastin-like recombinamers (ELRs) and subsequently tethered to commercially pure titanium discs, and their biological response was successfully tested against the biofilm activity of oral microorganisms (S. gordonii and P. gingivalis). This innovative protein-engineered molecular platform could be used for immobilization of different AMPs on an ECM-mimicking polymer for the development of antibiofilm and cytocompatible coatings on titanium.
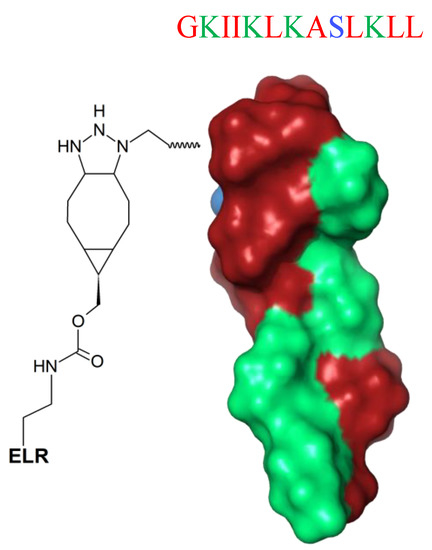
Figure 13.
Schematic representation of the GL13K peptide hybridized with cyclooctyne-modified elastin-like recombinamer (ELR) (spacer). GL13K secondary structure was calculated with I-Tasser. In blue: hydrophilic amino acids; in red: hydrophobic amino acids; in green: positively charged amino acids.
8. Mimicking the Structure of AMPs
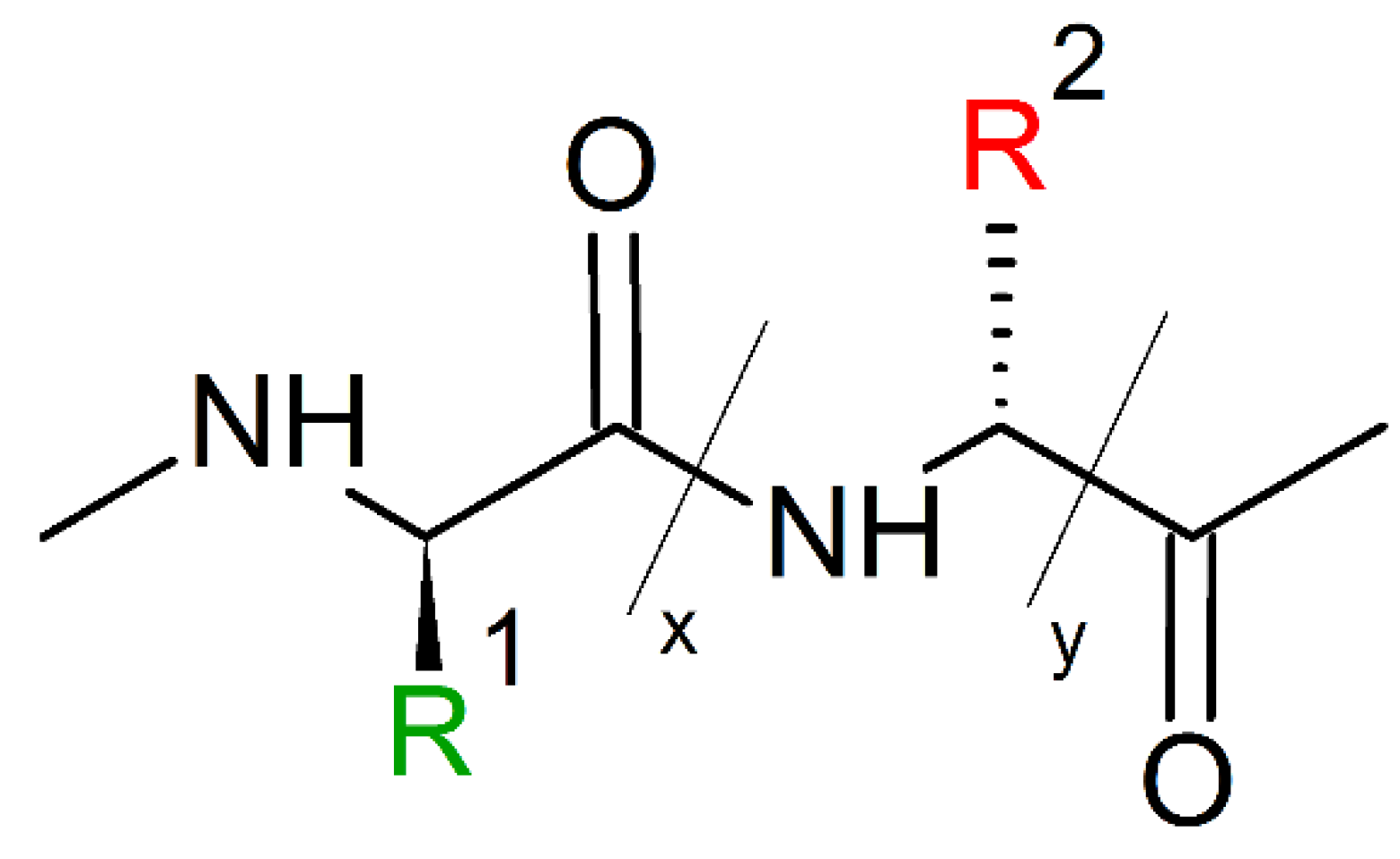
Peptoids are non-natural, sequence-specific peptidomimetic oligomers based on a protein-like backbone, but with a side chain appendage at the amide nitrogen (Scheme 4) [23].
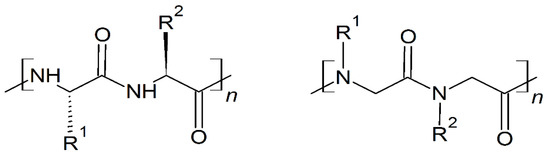
Scheme 4.
Chemical structures of peptides (left) and peptoids (right).
Synthetic polymers mimicking antimicrobial peptides have huge therapeutics potential; nevertheless, designing an ideal peptoid drug is complicated because there are many variables that influence the activity and function of the oligomer. The most important factors to consider are the cationic charge, conformational constraint via macrocyclization, and hydrophobicity, which are directly correlated with membrane interactions and biological activities in vitro. Peptoid optimization strategies in use are in vitro trial-and-error approaches [173], high-throughput ligand screening on large peptoid libraries [174], and computational approaches with prediction of peptoid efficacy in vitro [175,176,177]. The synchrotron liquid surface X-ray scattering studies of molecule structure–activity relationships have a new impact on studies of the mechanism of action by peptoid antimicrobials, and suggest optimization strategies for future therapeutics based on peptoids [178]. For instance, substitution of lysine to guanidine groups, increases the cationic charge and guides peptide–peptoid chimeras toward phosphatidylglycerol in bacterial membranes [179]. Macrocyclic peptoids reveal superior in vitro efficacy over linear peptoids, with identical monomer composition, against Gram-positive and Gram-negative bacteria [180]. The optimal hydrophobic parameters, balancing membrane specificity and at the same time low cytotoxicity, have been proposed to form helical peptoids [20].
Of noteworthy mention, peptoids adopt a helical structure [181], and are resistant to proteolytic degradation [182]. Helical peptidomimetic oligomers (foldamers) with a structure similar to that of linear, cationic, facially amphipathic helical antibacterial peptides such as magainins (class of AMPs found in the African clawed frog Xenopus laevis [183]) have garnered particular interest [181]. For example, certain amphipathic β-peptide helices are comparable to magainins (Figure 14) in antibacterial activity and selectivity [163]. Parameters that may have an influence on activities (oligomer length, charge, hydrophobicity, chirality, secondary structure and side chain nature), the mode of action of these peptide-mimetics and their selectivity for bacteria versus mammalian cells have been scrutinized over the last ten years [20,173,184,185,186].
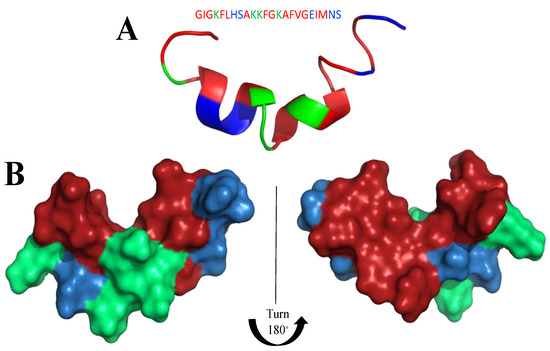
Figure 14.
Structure of Magainin-2 (PDB: 2LSA) as a representative of the magainin protein family. (A) Ribbon model (B) Surface model: left and right side. In blue: hydrophilic amino acids, in green: positively charged amino acids; in red: hydrophobic amino acids.
The structure–activity relationship among 22 cationic amphipathic peptoids was studied by Mojsoska et al. [187]. All studied peptoids had an overall net charge of +4 and were 8 to 9 residues long; however, the hydrophobicity and charge distribution along the abiotic backbone varied. Changes, such as replacing Ntrp with monomers with specific aromatic side chains, rearranging the charge distribution along the peptoid backbone, and shortening the length, significantly impacted the overall hydrophobicity profiles of the peptoids. Peptoids with high hydrophobicity do not always appear as the most potent against E. coli and P. aeruginosa, while against S. aureus, there is a linear relationship between their hydrophobicity and potency. In addition, increased hydrophobicity caused by introducing highly aromatic residues, such as the N-(2,2-diphenylethyl)glycine (Ndpe) monomer, is strongly correlated with a loss of antibacterial specificity, resulting in high toxicity in mammalian cells.
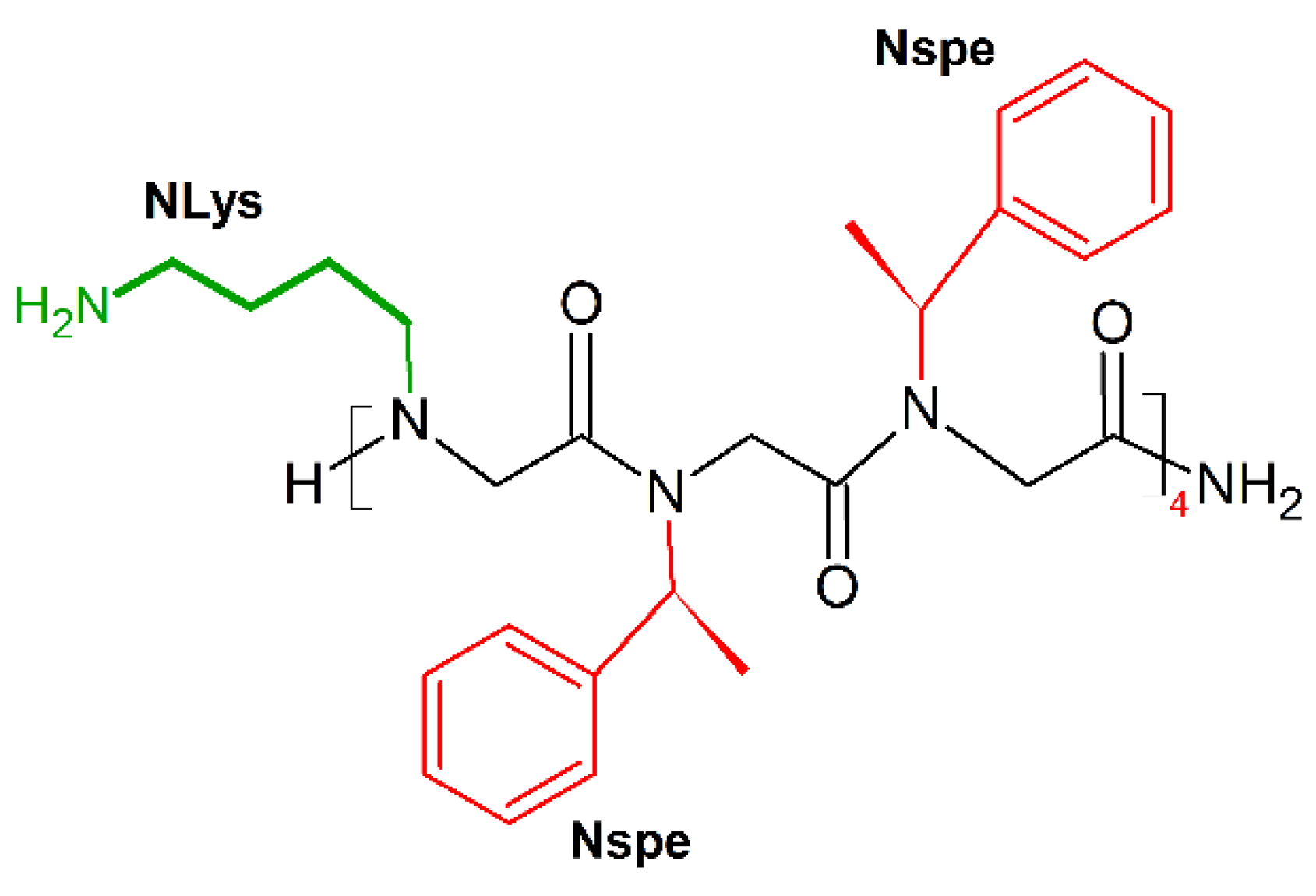
Barron and colleagues showed that the threefold periodicity of the poly-proline type I-like (PPI-like) helix of α-peptoids [188] enabled mimicry of the magainin helical structure [23]. The new amphipathic helix was built by periodic incorporation of a cationic side chain (NLys) every three residues, the remaining positions being occupied by the aromatic a-chiral (S)-phenylethyl side chain (Nspe), which helps to format the helix structure and provides hydrophobic helical faces. The dodecamer (NLys-Nspe-Nspe)4 (Figure 15) showed excellent broad-spectrum antibacterial activities (MICE.coli (μM) = 3.5–14), but also displayed potent cytotoxicity toward carcinoma cells (LC50(breast cancer)(μM) = 5; LC50(prostate cancer)(μM) = 5; LC50(ovarian cancer)(μM) = 6; LC50(fetal lung fibroblast)(μM) = 8; LC50(primary dermal fibroblast)(μM) = 8) [189].
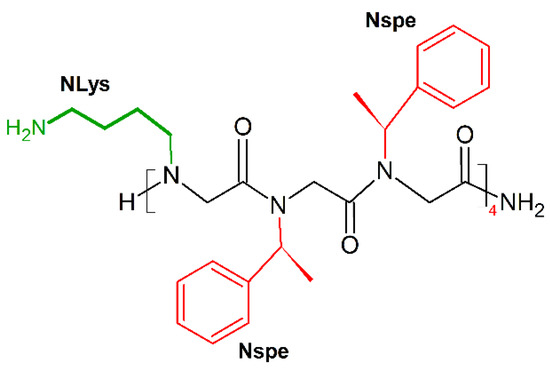
Figure 15.
Chemical structure of (NLys-Nspe-Nspe)4 peptoid. In green: positively charged side chain; in red: hydrophobic side chain.
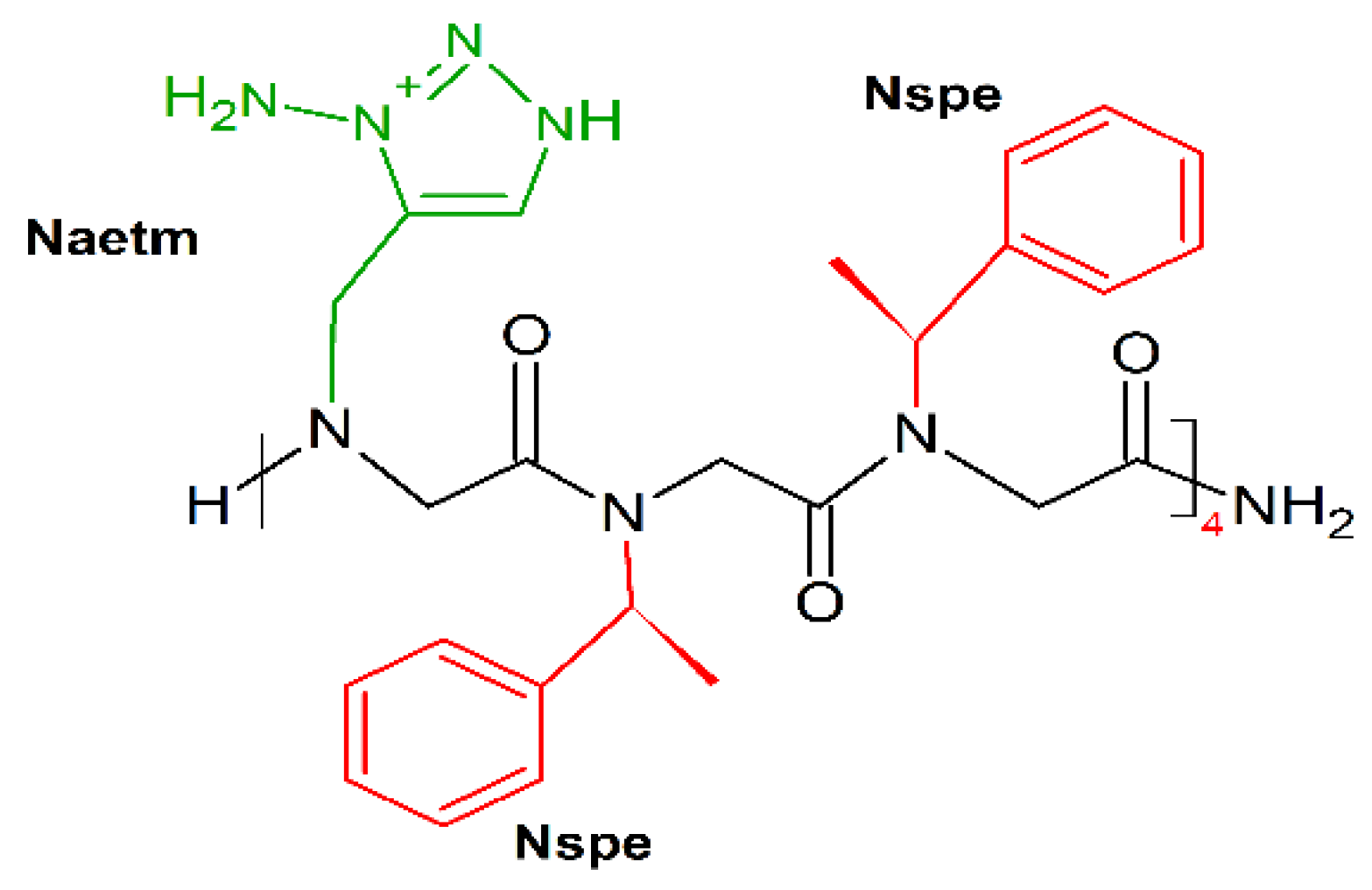
Following this mimicking trend, Shyam et al. prepared a series of 1,2,3-triazolium-based cationic amphipathic peptoid oligomers that mimic antimicrobial helical peptides [190]. They explored the potential of the triazolium group as a cationic moiety and helix inducer to develop potent antimicrobial helical peptoids. Several triazolium-based oligomers, even of short length, selectively killed bacteria over mammalian cells. Among tested oligomers H-(Naetm-Nspe-Nspe)4-NH2 (Figure 16) was the most effective (MICE.coli (μM) = 6.3–50; MICE.fecalis (μM) = 11; MICS.aureus (μM) = 10).
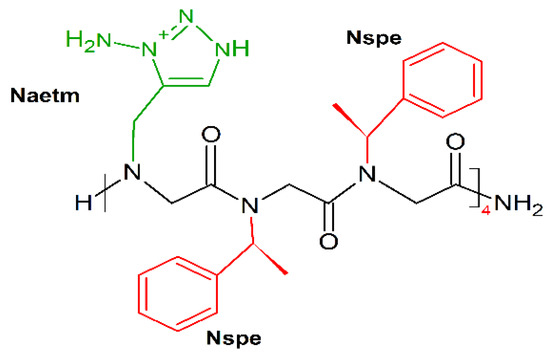
Figure 16.
Chemical structure of H-(Naetm-Nspe-Nspe)4-NH2 peptoid. In green: positively charged side chain; in red: hydrophobic side chain.
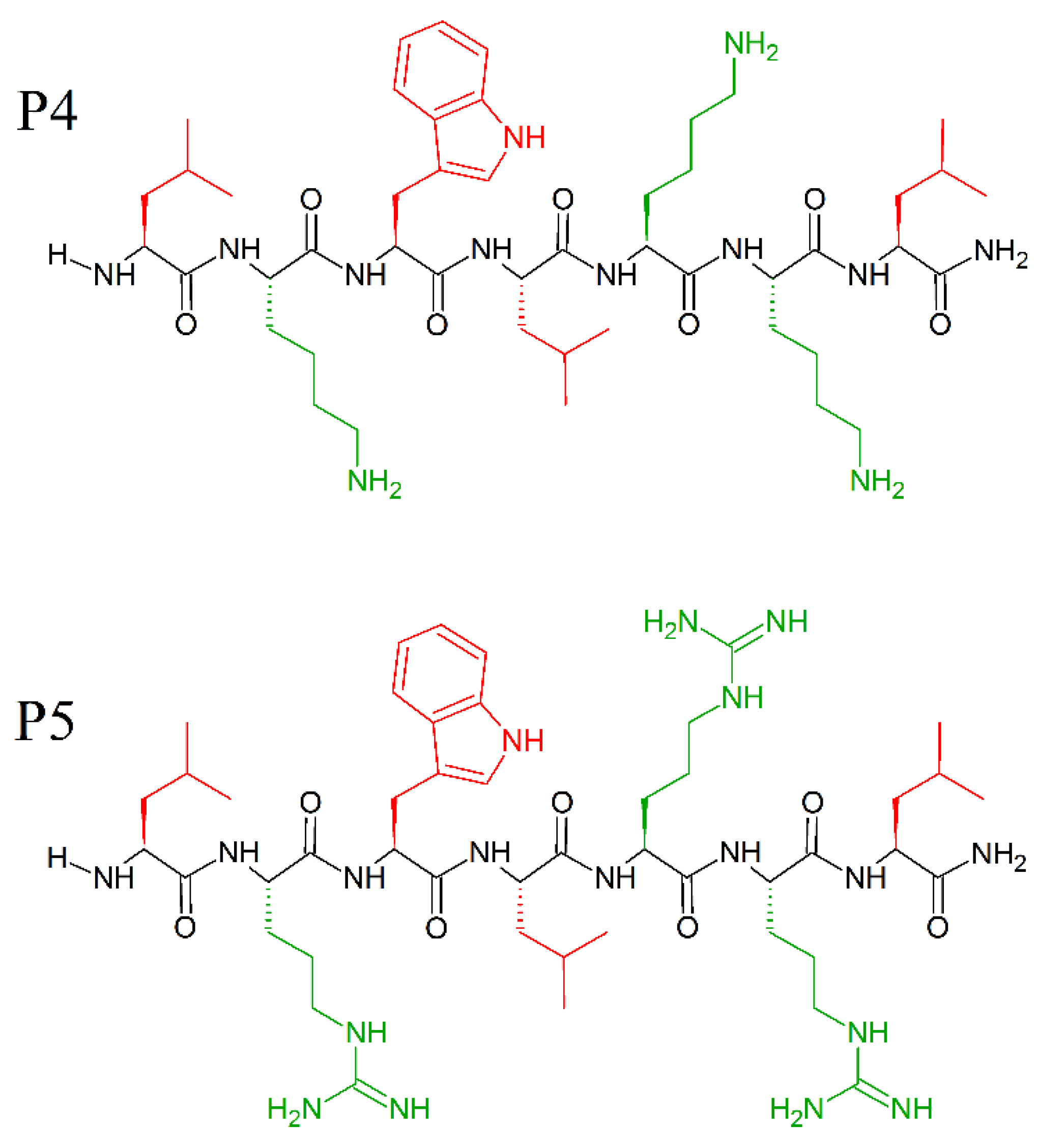
The amino acids—leucine and lysine—have strong helix-promoting abilities [191] and have been used for the construction of prototype α-helical antimicrobial peptides, usually referred to as “LK peptides”. Among them, 14- and 15-mer peptides with amphipathic helix, were found to be the most effective against bacteria [44,192], while shorter peptides were inactive. Attaining a secondary structure and amphipathicity are two determining factors for the antimicrobial activity of the LK peptides. Indeed, Monroc et. al. demonstrated that 4−10-residue-long LK peptides have antimicrobial activity only when cyclized [193], due to structural rigidity, and increased binding to the bacteria membranes. Additionally, the presence of numerous Trp residues stabilize the helical structure [194] and increase the affinity of the naturally occurring AMPs peptide for the membrane [195]. Even single tryptophan substitution at certain positions of inactive fragments of the amphipathic helical AMPs can significantly enhance their antimicrobial activity [195]. Following these instructions, and keeping in mind the role of C-terminal amidation on the activity of AMPs and the prevalence of tryptophan residues in several natural and synthetic AMPs, Pandit et al. prepared short peptides, cheaper in synthesis, and just as effective as long AMPs [196], with P5 peptide (MICE.coli (μM) = 10; MICP.aeruginosa (μM) = 10; MICK. pneumonie(μM) = 10; MICS.typhi(μM) = 7.5; MICS.aureus(μM) = 50; MICC. albicans(μM) = 50; MICC.grubii(μM) = 15) slightly more effective than the P4 peptide (MICE.coli (μM) = 50; MICP.aeruginosa (μM) = 50; MICK. pneumonie(μM) = 50; MICS.typhi(μM) = 15; MICS.aureus(μM) = 50; MICC. albicans(μM) = 50; MICC.grubii(μM) = 25). Moreover, the peptides P4 (LKWLKKL-NH2) and P5 (LRWLRRL-NH2) (Figure 17) were found to be non-hemolytic and non-cytotoxic to human cell lines. Surprisingly, these small peptides did not adopt any specific secondary structure in the free or sodium dodecyl sulphate (SDS) micellar bound state, and do not need secondary structures to exhibit antimicrobial activity. Electrostatic interaction is enough for P4 and P5 to attach to the membrane, and its successive deformation and lysis [196].
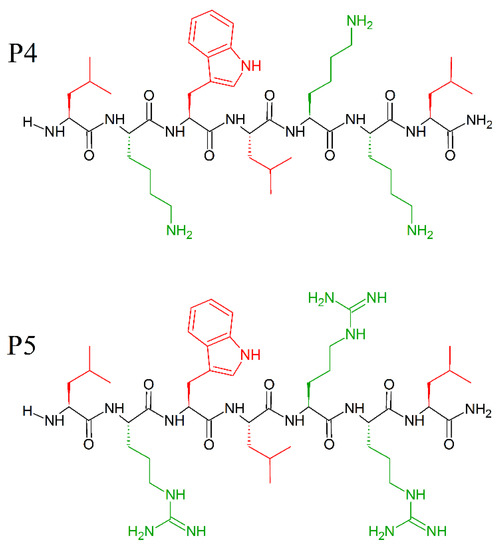
Figure 17.
Chemical structures of P4 (LKWLKKL-NH2) and P5 (LRWLRL-NH2) peptides. In red: hydrophobic side chains; in green: positively charged side chains.
Cationic antimicrobial poly(α-amino acid)s (APAAs) mimic structures and antimicrobial properties of the AMPs were extensively described in a recent review of Shen et al. [197]. APAAs are easy to synthesize, and have prolonged antimicrobial activity, low cytotoxicity, and enhanced stability to protease degradation. A series of random co-polypeptides with various chain lengths (5 to 200 residues) and hydrophobic contents (1–50 mol%) synthesized by Wyrsta et al. [198] mimic the cationic and amphiphilic nature (Figure 18) of many natural antimicrobial peptides. The most reactive samples have high hydrophobic contents and intermediate chain lengths, and in particular, peptides consisting of Lys/Leu with α-helix conformation exhibit good selectivity and activity to the microbial-mimicked membrane.
Figure 18.
Schematic representation of cationic antimicrobial poly(α-amino acid)s (APAAs) mimic structures. R1(green) = -(CH2)4NH3+Br−; R2(red) = -CH3; -CH(CH3)2; -CH(CH3)CH2CH3; -CH2CH(CH3)2; -CH2C6H5.
Moreover, the surface-initiated N-Carboxyanhydride (NCA) polymerization strategies used for APAA synthesis have also been proposed to generate dense polypeptides brushes on surfaces of a wide range of organic and inorganic substrates, including gold, carbon nanotubes, macroporous polymeric templates, magnetite nanoparticles, and silica nanoparticles [199]. Thus, surface-grafting technologies could be used to fabricate APAAs coatings on biomedical devices, and solve the critical problem of device-related infections [200].
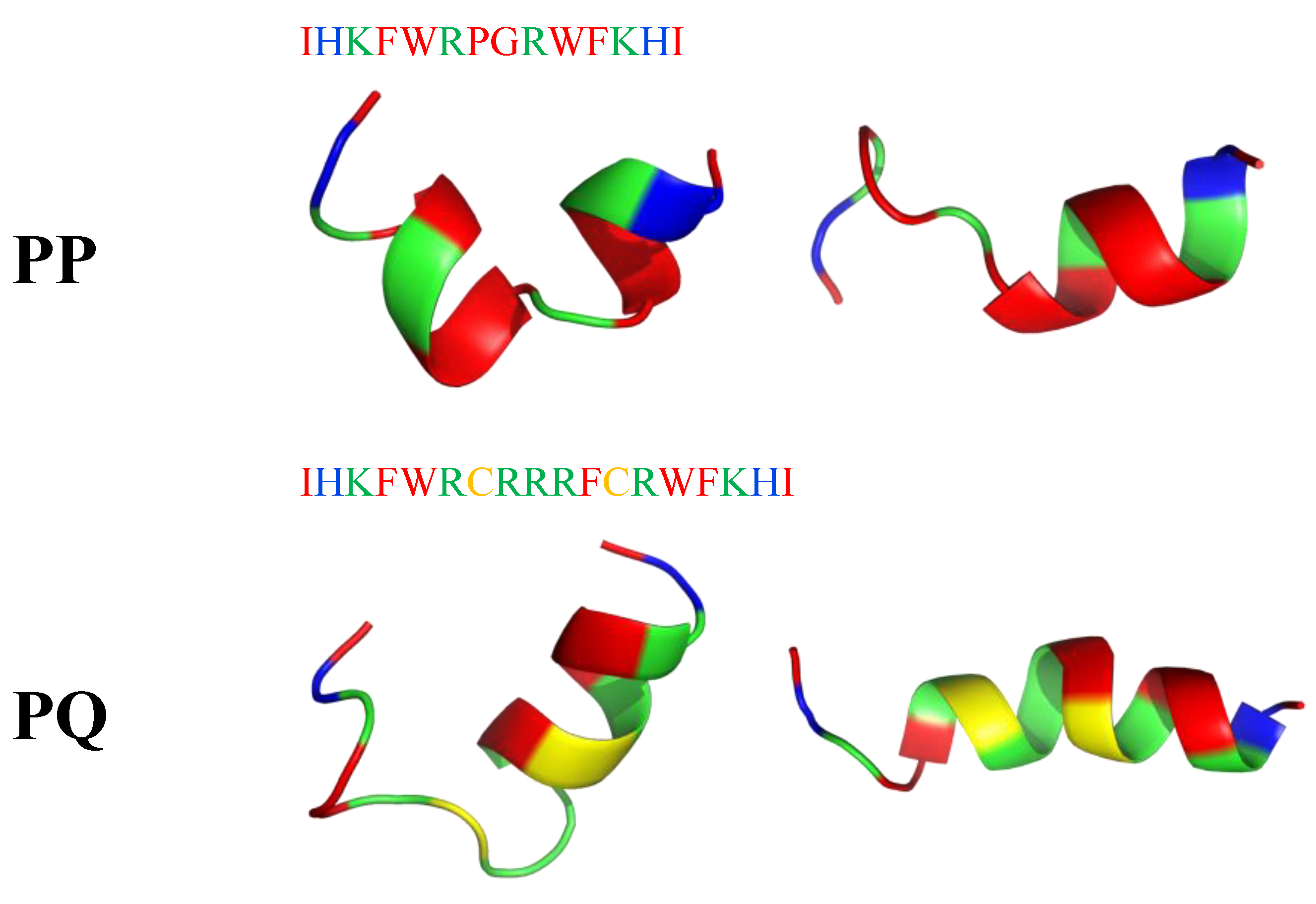
Recently, short peptides combining α-helix and β-turn sequence-motif in a symmetric-end template showed enhanced cell selectivity, antibacterial activity (MICE.coli(μM) = 2–32; MICB.pyocyaneum(μM) = 2–16; MICS.pullorum(μM) = 2–32; MICS.aureus(μM) = 8–32; MICS.faecalis(μM) = 8–32) and salt-resistance. Two peptides PQ (IHKFWRCRRRFCRWFKHI-NH2) and PP (IHKFWRPGRWFKHI-NH2) tended to form an α-helical structure upon interacting with a membrane-mimicking environment (Figure 19). According to I-Tasser calculation, PP has a manganese binding site (K3, R6, P7 residues), while PQ has a magnesium binding site (R10, C12 and R13 residues). Moreover, they showed significant cell selectivity toward bacterial cells over eukaryotic cells. Their activities were mostly maintained in the presence of different conditions (salts, serum, heat, and pH), which indicated their stability in vivo [201].
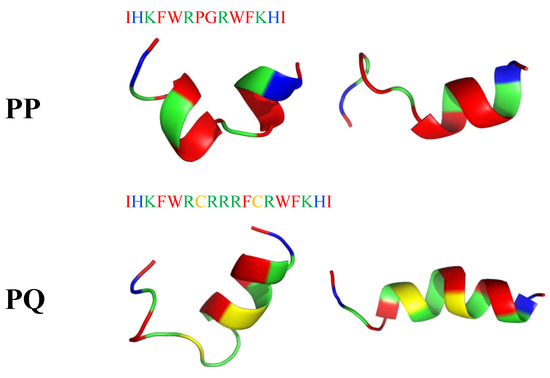
Figure 19.
Two possible secondary structures of PP and PQ peptides calculated with I-Tasser. In blue: hydrophilic amino acids; in green: positively charged amino acids; in red: hydrophobic amino acids.
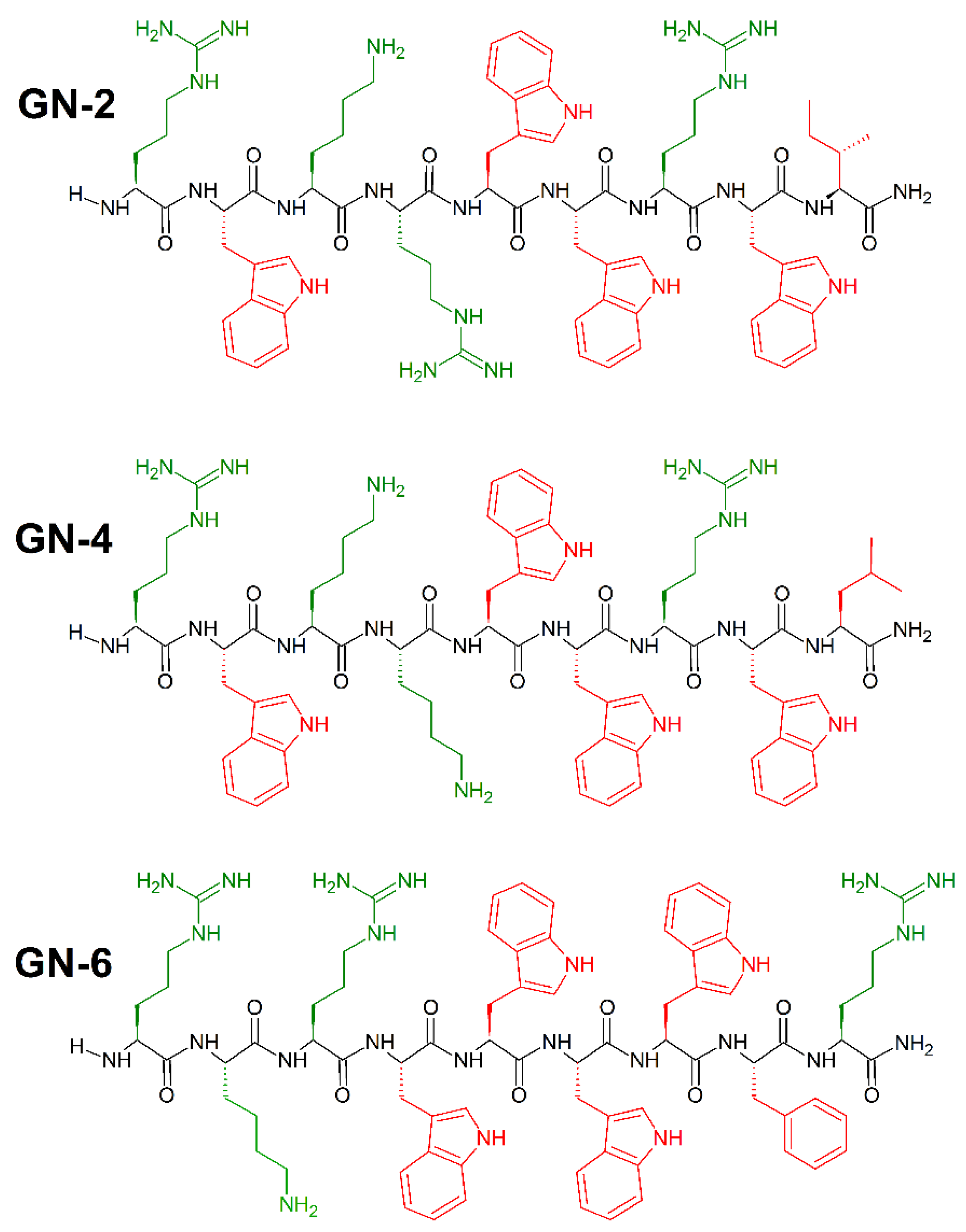
Synthetic peptides with high antibacterial activity and low toxicity can be identified with high accuracy using cheminformatics and machine learning, and without the use of an original template sequence [202]. To achieve that, Fjell et al. [203] used a quantitative structure–activity relationship (QSAR) approach utilizing artificial neural networks (ANN), and built computational models of peptide activity based on data from over 1400 random sequences, biased to contain amino acids believed from substitution analyses to be important for antibacterial activity. In addition, they posed an innovative method of generating candidate peptide sequences using the heuristic evolutionary programming method of genetic algorithms (GA), which provided a large (19-fold) improvement in identification of novel antibacterial peptides. To demonstrate the effectiveness of these techniques in identifying drug candidates, an in silico screening of 100,000 peptides was performed. The 10 most active peptides from this optimization were selected for in vivo study, all having a positive charge of 14, a uniform chain length of 9 amino acids and at least three tryptophan residues [204]. Potent broad spectrum activity was observed for GN-2 (RWKRWWRWI-CONH2), GN-4 (RWKKWWRWL-CONH2), and GN-6 RKRWWWWFR-CONH2 peptides (Figure 20) against E. coli (MICGN−2(μL/mL) = 6.2; MICGN−4(μL/mL) = 6.2; MICGN−6(μL/mL) = 12.5), P. aeruginosa (MICGN−2(μL/mL) = 3.1; MICGN−4(μL/mL) = 3.1; MICGN−6(μL/mL) = 6.2), and S. aureus (MICGN−2(μL/mL) = 3.1; MICGN−4(μL/mL) = 3.1; MICGN−6(μL/mL) = 3.1).
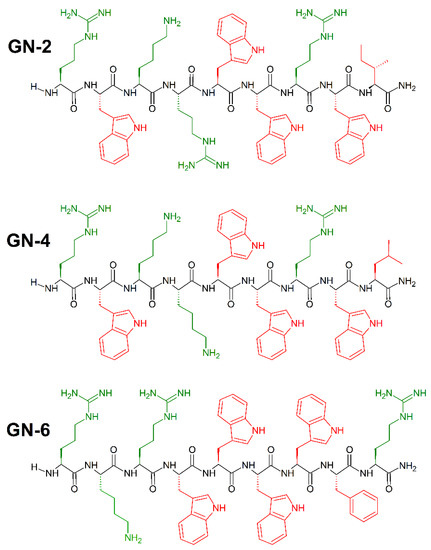
Figure 20.
Chemical structures of GN-2, GN-4 and GN-6 peptides. In green: positively charged amino acids; in red: hydrophobic amino acids.
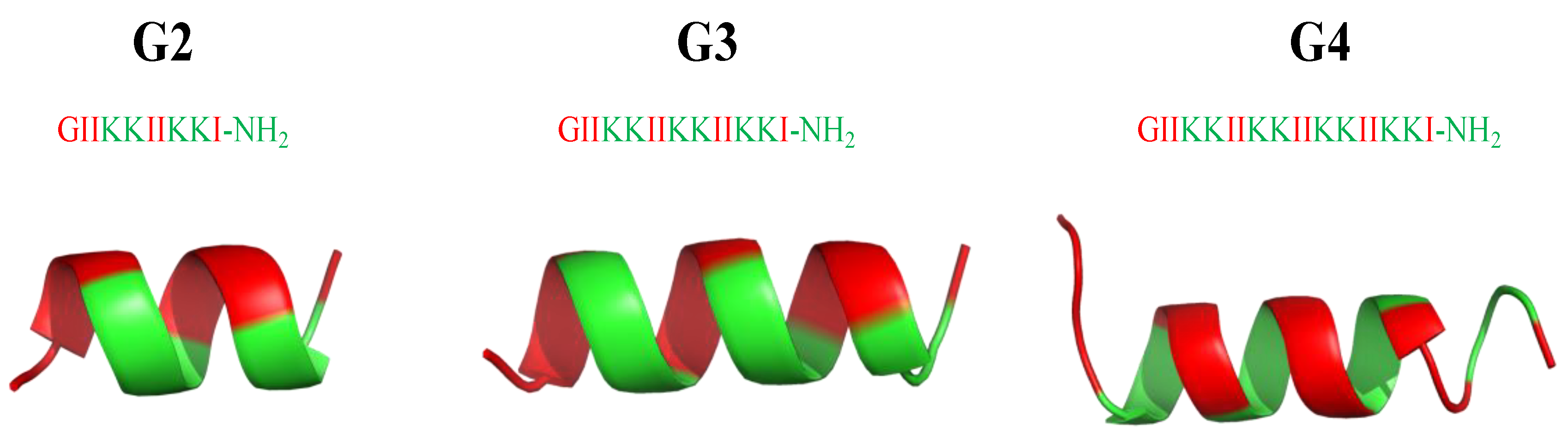
From an AMP database [205] with a total of 2619 AMP sequences, AMPs have an average of +3.2 net positive charges and 32.7 amino acids, with glycine (G), lysine (K), and leucine (L) being the most abundant amino acids, and α-helices and β-sheets being the most common secondary structures. For the development of potent and biocompatible AMPs, α-helical cationic AMPs (αCAMPs) have been the most popular species for investigation [36]. Extensive studies have focused on developing new AMPs by either modifying natural ones or following the strategy of artificial design to produce efficient and cost-effective versions. Among other approaches, one key factor lies in altering amino acid sequences to produce AMPs with different amphiphilicities [206,207,208]. Following this trend, a series of surfactant-like αCAMPs based on the general formula of G(IIKK)nI-NH2 (n = 2−4, and the AMPs are denoted as G2, G3, and G4, respectively) [209] were synthesized. Among them (Figure 21), G3 is optimal with potent bioactivity (MICE.coli(μM) = 8; MICB. subtilis(μM) = 2) and low cytotoxicity (IC50 (μM) = 15 (HeLa cells); = 25 HL60 cells) to the host mammalian cells. Hydrophobic modifications of G3 have been created by replacing the amino acid residues at the peptide N-terminals or C-terminals or the side chains [210,211]. The high selectivity and associated features are attributed to two design tactics: the use of Ile residues rather than Leu and the perturbation of the hydrophobic face of the helical structure with the insertion of a positively charged Lys residue. Moreover, studies for anticancer applications have demonstrated that the stronger the hydrophobicity—the higher the bioactivity, but also the higher the toxicity [209].
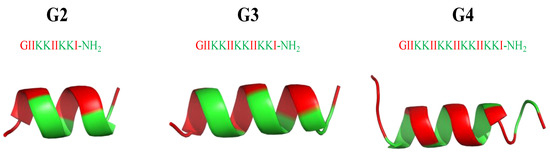
Figure 21.
Secondary structure of G2, G3 and G4 peptides calculated with I-Tasser. In green: positively charged amino acids; in red: hydrophobic amino acids.
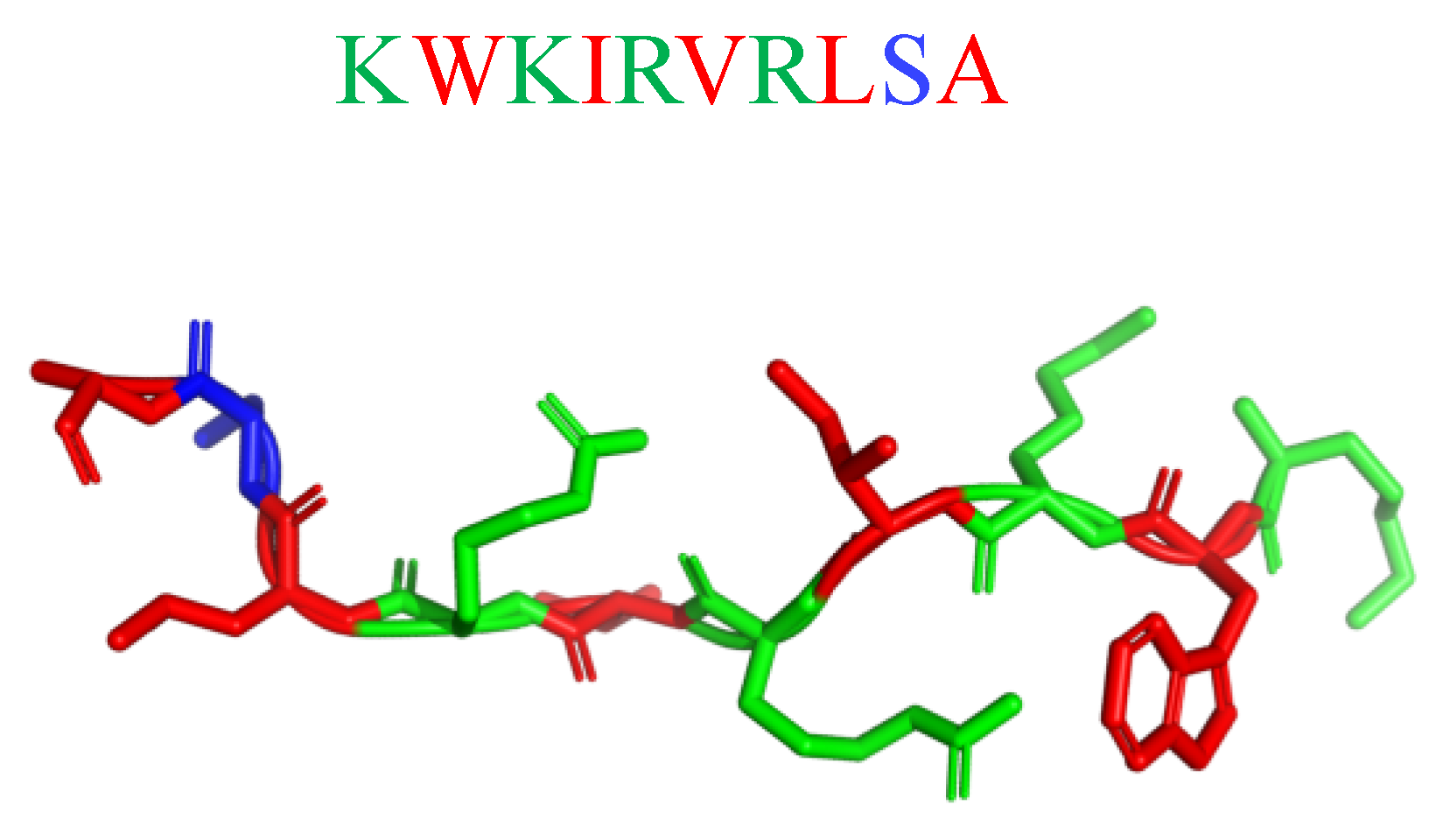
Potent in vitro activity of AMPs often does not translate into in vivo effectiveness due to the interference of the host microenvironment with peptide stability/availability. Hence, mimicking the complex environment found in biofilm-associated infections is essential to predicting the clinical potential of novel AMP-based antimicrobials [212]. The antibiofilm activity of the semisynthetic peptide lin-SB056-1 (KWKIRVRLSA-NH2; Figure 22) and its dendrimeric derivative (lin-SB056-1)2-K ([KWKIRVRLSA]2-K) against Pseudomonas aeruginosa in an in vivo-like three-dimensional lung epithelial cell model, and an in vitro wound model (consisting of an artificial dermis and blood components at physiological levels) gave rewarding results. [212]. When alone, Lin-SB056-1 was moderately effective (MICP.aeruginosa(μM) = 38.5) in reducing P. aeruginosa biofilm formation in 3D lung epithelial cells, but its antibiofilm activity was significantly increased in association with the chelating agent EDTA. The combination of lin-SB056-1 at 38.5 mM with EDTA (0.3 to 1.25 mM), know metal chelating agent, resulted in the reduction of the initial bacterial inoculum to the limit of detection (10 CFU/mL). The dimeric derivative (lin-SB056-1)2-K demonstrated an enhanced biofilm-inhibitory activity (MICP.aeruginosa(μM) = 19.25) as compared to both lin-SB056-1 and the lin-SB056-1/EDTA combination, reducing the number of biofilm-associated bacteria up to 3-Log units at concentrations causing less than 20% cell death [212].
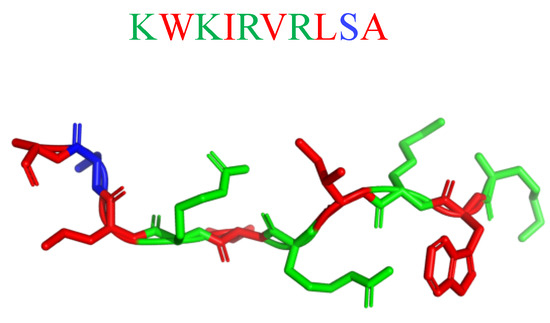
Figure 22.
Secondary structure of lin-SB056-1 calculated with I-Tasser. In blue: hydrophilic amino acids; in green: positively charged amino acids; in red: hydrophobic amino acids.
9. Unnatural Amino Acids
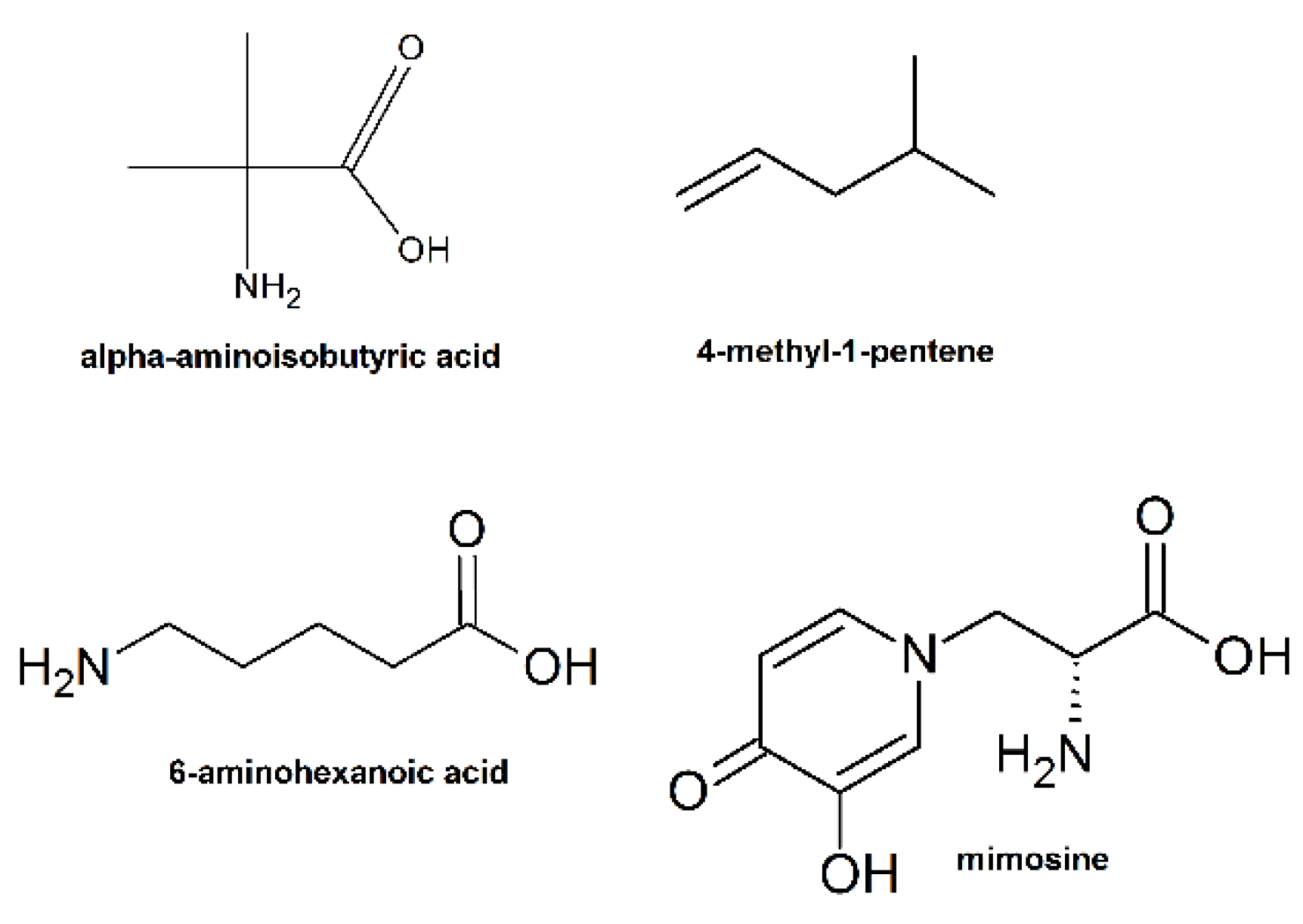
Introduction of fluorinated amino acids or other non-natural amino acids such as α-aminoisobutyric acid (Figure 23) in AMPs were proved to improve their resistance to protease [213,214]. A possible mechanism is that the abnormal structure of AMPs can impede the access of protease to the amide backbone. In addition, many peptidomimetics were introduced in order to avoid the undesired degradation of the α-polypeptides in vivo. For example, Gellman and co-workers designed a series of cationic and amphiphilic β-amino acid oligomers and polyamides to mimic the natural AMPs, which exhibited low hemolysis, high antibacterial activity, and extreme stability [215].
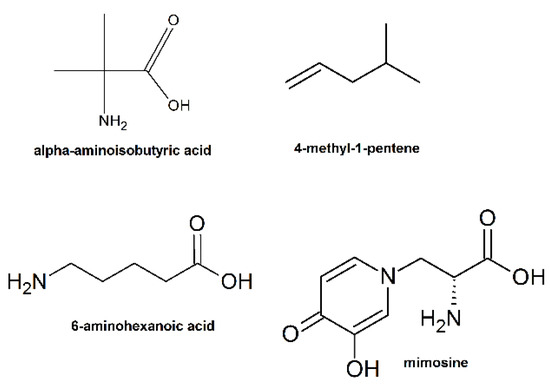
Figure 23.
Examples of unnatural amino acids used for the synthesis of AMPs.
Alkene and maleic anhydride copolymer mimic amphiphilic structure and antimicrobial properties of natural antimicrobial peptides. Szkudlarek et al. used 4-methyl-1-pentene (Figure 23) as a hydrophobic co-monomer (structure similarity with leucine) and maleic anhydride (leaves ample space for further design of the hydrophilic part by means of chemical modification) at constant 1:1 ratio to ensure the hydrophobic and cationic part similar to those in Leu:Lys 1:1 LK-peptides [216]. The C3 copolymer showed rewarding antimicrobial activity against Gram-negative (MICE.coli(μg/mL) = 20) and Gram-positive bacteria (MICS.epidermidisi(μg/mL) = 20).
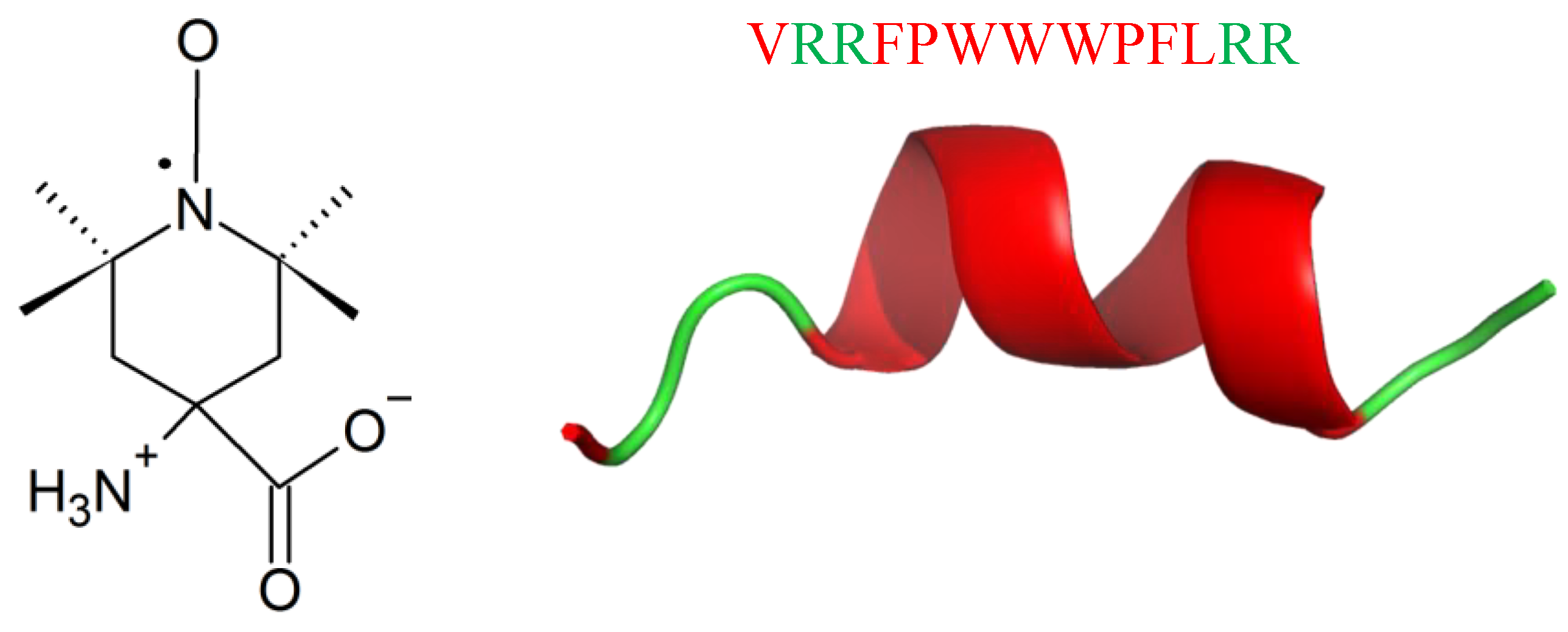
Labelling with the paramagnetic amino acid TOAC (Figure 24) is a new strategy in the studies of conformation, dynamics, orientation, and physicochemical properties of AMPs [217]. Unexpectedly, labelling with TOAC increases activity against Gram-positive bacteria, as it was shown for Tritrpticin (TRP3; Figure 24), an AMP against bacteria and fungi [218,219,220,221,222,223]. TRP3 is 13-residues long (VRRFPWWWPFLRR) with a net positive charge of (+4) at physiological pH. It exhibits ion channel-like activity in planar membranes, and permeabilizes bacterial cytoplasmic membranes [218], leading to leakage of the cell contents [222,224]. Recombinational studies showed that replacing both P or residues by A, or removal of P5 led to peptide conformational changes, higher membrane permeabilization, and ultimately, increased antimicrobial activity [222,223,225]. Labelling TRP3 with TOAC (TOAC-VRRFPWWWPFLRR (T1) and VRRF-TOAC-WWWPFLRR (T2)) led to the increase in antibacterial activity against M. luteus (MICT1(μM) < 0.38; MICT2(μM) < 0.39) and E.coli (MICT1(μM) = 4; MICT2(μM) = 1.3), respect to TRP3 (MICM.luteus(μM) = 1.1; MICE.coli(μM) = 1.9) TOAC presented a greater freedom of motion at the N-terminus rather than at the internal position. Analogously to TRP3, both TOAC-labelled peptides, showed prominent cation selectivity [217].
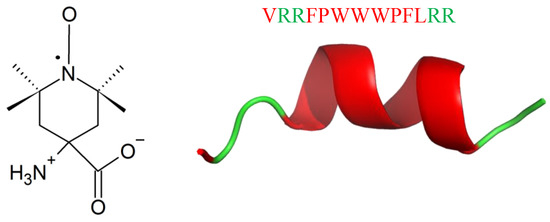
Figure 24.
Chemical structure of TOAC (left) and secondary structure of TRP3 calculated with I-Tasser. In green: positively charged amino acids; in red: hydrophobic amino acids.
Most AMPs targeting membrane bilayers are cationic antimicrobial peptides (CAMPs) [226] with numerous arginine or lysine residues (generally from 2 to 9), which account for the positive net charge. CAMPs have an amphiphilic structure, in which cationic and hydrophobic residues are clustered in different spatial regions. Nevertheless, CAMPs are highly sensitive to proteases. In order to overcome their short half-life, different CAMP-mimicking peptides were protected on N- and C-end by 6-aminohexanoic acid residues (Figure 22). All peptides display high activity toward a broad spectrum of pathogens (MICE.coli (μM) = 10, MICP.aeruginosa (μM) = 1.25–20; MICB.spizizenii (μM) = 10–40; MICS.aureus (μM) = 5–40) and have high stability [227].
A clever solution for protease resistance is the replacement of L-amino acids with their D counterparts. This strategy was successfully used by Qui et al. [228] who replaced the amino acid residues or the cationic lysine residue with the corresponding D-amino acids in protonectin (Prt; Figure 25). Protonectin (ILGTILGLLKGL–NH2) was originally isolated from the venom of the neotropical social wasp Agelaia pallipes [229], and has potent antifungal and antibacterial activity with membrane active action mode [230,231]. Qui et al. showed that both the D-enantiomer of protonectin (D-prt) and D-Lys-protonectin (D-Lys-prt) have strong antimicrobial activity against bacteria (MICS.aureus(μM) = 4–128; MICE.coli(μM) = 2–8; MICB.subtilis(μM) = 8–128; MICS.epidermis(μM) = 8–16; MICK.penumoniae(μM) = 8–258) and fungi (MICSakazaii(μM) = 8–64). Moreover, D-prt showed strong stability against trypsin, chymotrypsin and the human serum, while D-Lys-prt only showed strong stability against trypsin. D-Lys-prt still kept typical α-helical structure in the membrane mimicking environment, while D-prt showed left hand α-helical structure. In addition, all D-amino acid substituted analogues or partially D-amino acid substituted analogues could act on bacteria with mechanism of protonectin, and disrupt the integrity of membrane while leading to the cell death [228].
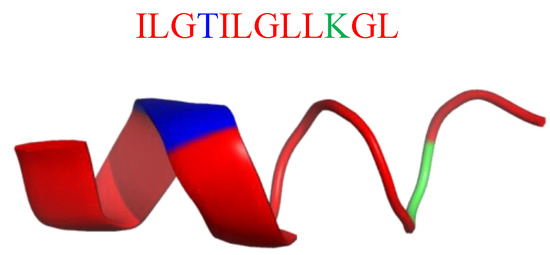
Figure 25.
Secondary structure of protonectin (PDB: 6N68). In blue: hydrophilic amino acids; in green: positively charged amino acids; in red: hydrophobic amino acids.
10. Mimicking Peptide Bonds
Peptidomimetics can mimic primary, secondary, and even tertiary structures of peptides and proteins, and therefore they have been developed for biomolecular recognition and modulation of protein interactions. In addition, peptidomimetics display advantages over conventional peptides, including resistance to enzymatic hydrolysis, improved bioavailability, and enhanced chemo-diversity [232]. The past decade showed the fast progress in developing biomimetic oligomers, including β-peptides [233] peptoids [234], α-aminoxy-peptides [235], α/β-peptides [236], azapeptides [237], oligoureas [238], aromatic oligoamides [239]. Nonetheless, the development and application of peptidomimetics is still limited due to the availability of backbones and molecular frameworks.
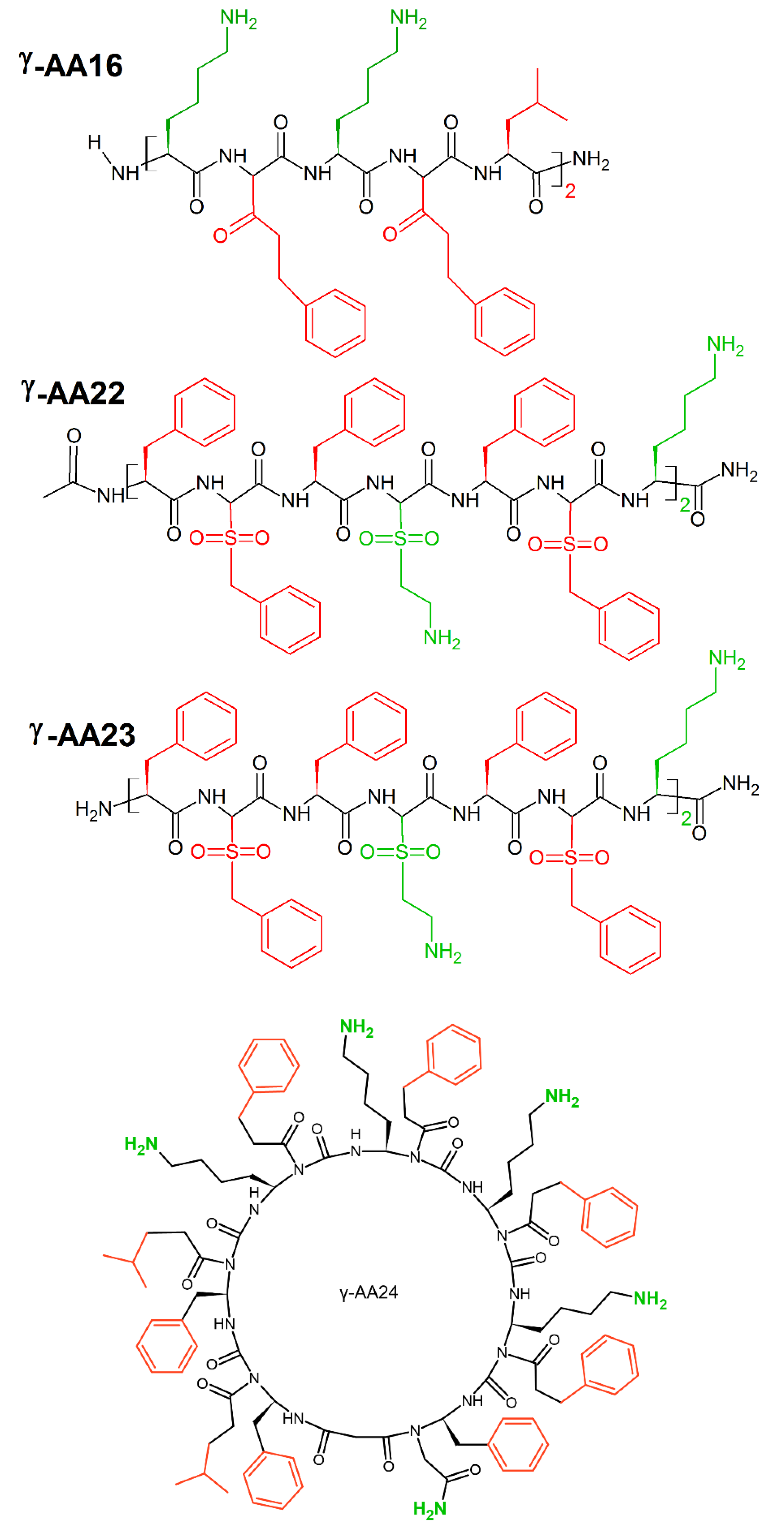
A new class of peptidomimetics, “γ-AApeptides”, based on the chiral γ-PNA backbone was developed by Shi et al. [17]. These γ-AApeptides are oligomers of γ-substituted-N-acylated-N-aminoethyl amino acids resistant to proteolytic degradation and possess the potential to enhance chemo-diversity γ-AApeptides could mimic the primary structure of peptides, as they project the same number of side chains as peptides of the same lengths. Moreover, γ-AApeptides can fold into discrete secondary structures, such as helical and β-turn-like structures, and they can mimic host-defense peptides and display potent and broad-spectrum activity toward a panel of drug resistant bacterial pathogens. Linear γ-AApeptides link amphiphilic building blocks (containing one hydrophobic and one cationic group in each building block) together, and the sequence may adjust the conformation to adopt a globally amphipathic structure on the surface of bacterial membranes. Indeed, γ-AA16 (Figure 26) kills bacteria (MICE.coli, E.faecalis(μg/mL) = 5) through the disruption of bacterial membranes. Sulfono-γ-AApeptides form helical structures [240], that mimic cationic host-defense peptides e.g., magainin 2. Among them, γ-AA22 (MICE.coli, E.faecalis(μg/mL) = 2) and γ-AA23 (MICE.coli(μg/mL) = 4; (MICE.faecalis(μg/mL) = 2) (Figure 26) possess excellent antimicrobial activity [241]. Cyclic γ-AApeptides successfully mimic cyclic peptide antibiotics. This is particularly seen for γ-AA24 (MICE.faecalis(μg/mL) = 5) (Figure 26), which displays broad-spectrum antimicrobial activity [18].
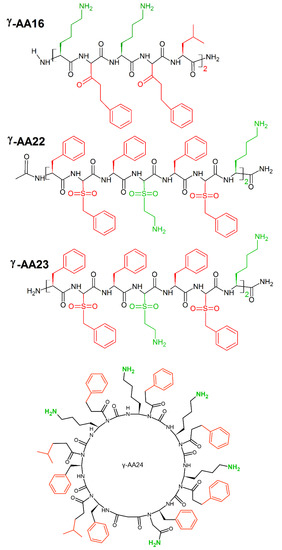
Figure 26.
Chemical structures of linear γ-AA16, γ-AA22, γ-AA23 and cyclin γ-AA16 peptides. In green: positively charged amino acids; in red: hydrophobic amino acids.
11. Enhancing AMPs Activity with Metal Ions
Enhancement of antibiotic activity through complexation with metal ions is well ascertained. Copper (II), zinc (II) and silver (I) metal adducts with different antibiotics were extensively studied in recent years [242,243,244,245]. Cu(II) and Ag(I) themselves have antimicrobial activity [246], but together with antibiotics e.g., ampicillin, penicillin G and Cefuroxime, they present synergistic effects against Gram-positive (MRSA) bacteria (S. aureus). Zn(II) is an essential cofactor for metallo-β-lacamases (enzyme resistant to β-lactan antibiotics), and together with ampicillin and penicillin G shows a high synergistic effect [245].
Not surprisingly, AMPs also bind metal ions (30% of proteins in the living cell coordinate at least one metal ion [247]) and their activity against bacteria can be enhanced upon metal binding. AMPs with a metal chelating ability can simply compete with bacteria for their essential metal ions and lead to the bacteria’s death under starvation. Psoriasin enters bacterial cells and sequesters Zn(II) ions [248], while Microplusin binds copper(II) ions, making them unavailable for the pathogen [249]. Metal binding can also have toxic effects. For instance, copper(II)/Colistin adducts cleave RNA molecules and have dangerous side effects [250].
AMPs change the structure and charge upon metal binding, leading to acquisition and/or enhancement of antibacterial activity [248]. Binding Zn(II) to Calcitermin increases the positive charge and facilitate the interactions with negatively charged bacterial membranes [251]. A similar effect can be observed in zinc(II)/Histidine rich glycoprotein (HRG) abundant in human plasma [252]. Histatins compose a wide group of short, cationic salivary peptides secreted by the parotid and sub-mandibular salivary gland. The C-terminal 16 amino acids fragment is rich in histidine residues, giving an opportunity for complex formation with copper(II) and zinc ions. Indeed, histatin 1 and 3 complexes with metal ions were already established [253], even if the exact functioning of metal adducts is still under study.
Mimicking a metal binding motif is a new strategy in enhancing AMPs activity. One of the most studied strategies is the use of Amino Terminal Copper and Nickel (ATCUN) binding motif. ATCUN is a three-amino acid sequence, finishing with histidine residues (XXH), with high affinity to divalent transition metal ions. The ATCUN motif is present in human albumin, the most abundant metal and drug carrier in plasma [254], but is also found in other metal-binding proteins (e.g., histatins) [255]. Recently, Agbale et al. [256] engineered ATCUN motifs into the native sequence of two AMPs: CM15 and citropin1.1. The incorporation of metal binding motifs changed the antimicrobial activity of the peptides against a panel of carbapenem-resistant enterococci (CRE) bacteria, including carbapenem-resistant Klebsiella pneumoniae (KpC+) and Escherichia coli (KpC+). The antimicrobial activity was modulated according to the type of ATCUN variant utilized. For instance, CM15 modulated by incorporation of the GGH and VIH ATCUN motifs had 4-fold and 8-fold increased potency against carbapenem-resistant K. pneumoniae (KpC+ 1825971), respectively, with respect to the potency of the original peptide. It is noteworthy that there is no need to incorporate Cu(II) metals within the ATCUN-AMP complex drugs since the motif is able to scavenge metal ions in the plasma or target organism. This should avoid many regulatory bottlenecks associated with the use of metals in drugs [257].
Incorporation of mimosine (MIM) residues in the peptide backbone is another rewarding strategy in enhancing antimicrobial activity upon metal binding. Mimosine is a non-protein amino acid with various properties, such as antibacterial, anti-inflammatory, anti-cancer, and anti-virus. Due to its structural similarity with deferiprone (DFP), mimosine is an excellent chelator of Cu(II), Cd(II), Al(III), Fe(III), Ga(III), Gd(III), and In(III) metal ions, as well as actinides and lanthanides. Recent studies showed that in silico design of mimosine-containing peptides is an effective tool in prediction of metal/complexes [258], which increases the antimicrobial activity of free mimosine peptides. For instance, iron(III) complex with 6-amino acid peptide with three MIM residues (H-Mim-Gly-ProGly-Mim-Gly-Gly-Mim-OH) showed 15 times higher activity against S. aureus and B. cereus strains relative to the free peptide [96].
12. Bacteriocins
Bacteriocins comprise a large and functionally diverse family of AMPs produced by a variety of bacteria and play a critical role in mediating microbial interactions and in maintaining microbial diversity [259]. Most Gram-positive bacteria produce bacteriocins, which inhibit the growth of alike or closely related bacterial strains with a narrow spectrum of activity. Some bacteriocins have activity against pathogenic and opportunistic bacteria (including multidrug-resistant species), not discriminating between antibiotic resistant and sensitive strains [260]. Moreover, bacteriocins shown antiviral, antiprotozoal and anticancer activity [261]. Therefore, bacteriocins are considered as alternatives to conventional antibiotics, and represent promising candidates as antibiotic synergists, or alternatives to enhancing the therapeutic effects of current infection treatments and decrease the prevalence of resistant strains.
The term bacteriocins is referred to as a ribosomally-produced peptides composed of 20-60 amino acids, mostly cationic and hydrophobic [262,263]. Initially, bacteriocins were divided into three classes differing in function, molecular weight, amino acid sequence and physicochemical properties. Class I bacteriocins consist of small (<10 kDa) and heat-stable peptides that are post-translationally modified, resulting in the non-standard amino acids, such as lanthionine and β-methyllanthionine [264]. Class II bacteriocins are small (<10 kDa), temperature- and pH-resistant peptides. Subsequently, class II bacteriocins are divided into subclasses based on structure and modifications: subclass IIa bacteriocins (known as pediocin-like bacteriocins, consist of the anti-listerial one-peptide with one or two disulfide bonds, and a conservative N-terminal motif). Subclass IIb bacteriocins are two-peptide bacteriocins, subclass IIc bacteriocins are cyclic bacteriocins. Class III bacteriocins (>30 kDa) are heat-sensitive, protein-like bacteriocins produced by both Gram-positive and Gram-negative bacteria [263,265]. A fourth class of bacteriocins, initially described by Klaenhammer 1993 [266], has been aborted and renamed as bacteriolysins, which comprise large complexes with carbohydrate and lipid moieties such as leuconocin S and lactococcin 27 [267]. Given the complexity and diversity of bacteriocin, as well as the identification of novel ones, the classification scheme is constantly evolving.
Bacteriocins act in different modes, but most of them interact initially with a specific receptor on the target cell and form pores in bacterial cell-membrane, resulting in a dissipation of proton-motive force that leads to cell death [264]. Class I bacteriocins kill target microorganisms by pore formation in the cell membrane and by inhibition of cell-wall peptidoglycan biosynthesis. Class II bacteriocins, such as pediocin and lactococcin, bind to a membrane protein component of the mannose phosphotransferase system to interact with the membranes. The membrane permeabilization allows bacteriocins to enter the cytoplasm, where they destabilize DNA/RNA integrity, protein and cell wall synthesis and enzyme activity.
In some cases, Gram-negative bacteria resist bacteriocins due to the outer membrane, which acts as an effective barrier. In such cases, some metal chelating agents and environmental conditions (acidic pH, high salt concentration, temperature variations) may destabilize the outer membrane and enables bacteriocins to act towards Gram-negative bacteria [268]. The use of bacteriocins in association with chemical compounds or physical treatments extends their activity spectrum on Gram-negative bacteria and counteract the emergence of resistant bacterial strains [269].
Bacteriocins are already used in our daily life. Lactic acid bacteria (LAB) produce bacteriocins applied in food preservation alone, or in combination with other preservation methods (hurdle technology), to increase the shelf-life and the safety of the foods. Nisin, produced by Lactococcus lactis, is authorized as a food additive in the EU (E234) under Annex II of Regulation (EC) 1333/2008 for use in several food categories (clotted cream, mascarpone, ripened and processed cheese and cheese products, pasteurized liquid eggs and semolina and tapioca puddings and similar products). Nisin inhibits different Gram-positive pathogens alone, among them are the particularly dangerous Clostridium botulinum, Listeria monocytogenes, Staphylococcus aureus, Bacillus and Enterococcus. When used in combination with other antimicrobials, Nisin is also active towards Gram-negative bacteria [270].
Bacteriocins are promising defenses against pathogenic bacteria, but before market introduction, there is still much to know about their biosynthesis and mode of action. Genomics, peptidomics and proteomics are used to understand the molecular mechanistic of their production, regulation, immunity, and mode of action, and help to decrease cytotoxic effects and increase the target selectivity.
13. Conclusions
In front of Fleming’s fearsome prophecies about resistant bacteria, we can find consolation in Pasteur’s optimism “If it is a terrifying thought that life is at the mercy of the multiplication of these minute bodies it is a consoling hope that Science will not always remain powerless before such enemies.” The antimicrobial peptides provide new and promising therapeutic approaches, especially in chronic diseases, like oral cavity pathologies, where they can effectively interfere in the early steps of biofilm formation, and at the same time block the inflammatory effects of bacterial toxins. Human antimicrobial peptides are often unable to exhibit toxicity at physiological concentrations, while other organisms’ AMPs express toxicity in the human body. Clever AMPs-mimicking strategies lead to the development of innovative antimicrobial peptides that express antibacterial activity without harmful side effects. In this review, we gathered the most successful AMPs-mimicking strategies of the last decade to be used as a manual for the future synthesis of new peptides.
Author Contributions
J.I.L. coordinated project and described sections dedicated to different peptidomimicking strategies, K.S. described sections dedicated to known AMPs, A.S., C.C., S.F. and G.O. presented growing potential of AMPs in oral infections and revised entire manuscript, B.P. presented bacteriocins as a new class of AMPs, M.P. described sections dedicated to the selectivity of AMPs, M.J. coordinated project and revised entire manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
K.S. and M.J. would like to thank King Abdullah University of Science and Technology (KAUST) for financial support.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Wang, J.; Dou, X.; Song, J.; Lyu, Y.; Zhu, X.; Xu, L.; Li, W.; Shan, A. Antimicrobial peptides: Promising alternatives in the post feeding antibiotic era. Med. Res. Rev. 2019, 39, 831–859. [Google Scholar] [CrossRef] [PubMed]
- Lewies, A.; Du Plessis, L.H.; Wentzel, J.F. Antimicrobial peptides: The Achilles’ heel of antibiotic resistance? Probiotics Antimicrob. Proteins 2019, 11, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Maróti, G.; Kereszt, A.; Kondorosi, E.; Mergaert, P. Natural roles of antimicrobial peptides in microbes, plants and animals. Res. Microbiol. 2011, 162, 363–374. [Google Scholar] [CrossRef]
- Kuppusamy, R.; Willcox, M.; Black, D.S.; Kumar, N. Short cationic peptidomimetic antimicrobials. Antibiotics 2019, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Boman, H.G. Antibacterial peptides: Key components needed in immunity. Cell 1991, 65, 205–207. [Google Scholar] [CrossRef]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef]
- Edwards, I.A.; Elliott, A.G.; Kavanagh, A.M.; Blaskovich, M.A.; Cooper, M.A. Structure–Activity and Toxicity Relationships of the Antimicrobial Peptide Tachyplesin-1. ACS Infect. Dis. 2017, 3, 917–926. [Google Scholar] [CrossRef]
- Hancock, R.E.; Sahl, H.-G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef]
- Lee, E.Y.; Zhang, C.; Di Domizio, J.; Jin, F.; Connell, W.; Hung, M.; Malkoff, N.; Veksler, V.; Gilliet, M.; Ren, P. Helical antimicrobial peptides assemble into protofibril scaffolds that present ordered dsDNA to TLR9. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Ma, Z.; Han, J.; Chang, B.; Gao, L.; Lu, Z.; Lu, F.; Zhao, H.; Zhang, C.; Bie, X. Membrane-active amphipathic peptide WRL3 with in vitro antibiofilm capability and in vivo efficacy in treating methicillin-resistant Staphylococcus aureus burn wound infections. ACS Infect. Dis. 2017, 3, 820–832. [Google Scholar] [CrossRef]
- Mishra, B.; Reiling, S.; Zarena, D.; Wang, G. Host defense antimicrobial peptides as antibiotics: Design and application strategies. Curr. Opin. Chem. Biol. 2017, 38, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Bray, B.L. Large-scale manufacture of peptide therapeutics by chemical synthesis. Nat. Rev. Drug Discov. 2003, 2, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Sieprawska-Lupa, M.; Mydel, P.; Krawczyk, K.; Wójcik, K.; Puklo, M.; Lupa, B.; Suder, P.; Silberring, J.; Reed, M.; Pohl, J. Degradation of human antimicrobial peptide LL-37 by Staphylococcus aureus-derived proteinases. Antimicrob. Agents Chemother. 2004, 48, 4673–4679. [Google Scholar] [CrossRef]
- Choi, S.; Isaacs, A.; Clements, D.; Liu, D.; Kim, H.; Scott, R.W.; Winkler, J.D.; DeGrado, W.F. De novo design and in vivo activity of conformationally restrained antimicrobial arylamide foldamers. Proc. Natl. Acad. Sci. USA 2009, 106, 6968–6973. [Google Scholar] [CrossRef]
- Porter, E.A.; Wang, X.; Lee, H.-S.; Weisblum, B.; Gellman, S.H. Non-haemolytic β-amino-acid oligomers. Nature 2000, 404, 565. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Teng, P.; Sang, P.; She, F.; Wei, L.; Cai, J. γ-AApeptides: Design, structure, and applications. Acc. Chem. Res. 2016, 49, 428–441. [Google Scholar] [CrossRef]
- Wu, H.; Niu, Y.; Padhee, S.; Wang, R.E.; Li, Y.; Qiao, Q.; Bai, G.; Cao, C.; Cai, J. Design and synthesis of unprecedented cyclic γ-AApeptides for antimicrobial development. Chem. Sci. 2012, 3, 2570–2575. [Google Scholar] [CrossRef]
- Bremner, J.B.; Keller, P.A.; Pyne, S.G.; Boyle, T.P.; Brkic, Z.; David, D.M.; Garas, A.; Morgan, J.; Robertson, M.; Somphol, K. Binaphthyl-Based Dicationic Peptoids with Therapeutic Potential. Angew. Chem. Int. Ed. 2010, 49, 537–540. [Google Scholar] [CrossRef]
- Chongsiriwatana, N.P.; Patch, J.A.; Czyzewski, A.M.; Dohm, M.T.; Ivankin, A.; Gidalevitz, D.; Zuckermann, R.N.; Barron, A.E. Peptoids that mimic the structure, function, and mechanism of helical antimicrobial peptides. Proc. Natl. Acad. Sci. USA 2008, 105, 2794–2799. [Google Scholar] [CrossRef]
- Ghosh, C.; Manjunath, G.B.; Akkapeddi, P.; Yarlagadda, V.; Hoque, J.; Uppu, D.S.; Konai, M.M.; Haldar, J. Small molecular antibacterial peptoid mimics: The simpler the better! J. Med. Chem. 2014, 57, 1428–1436. [Google Scholar] [CrossRef] [PubMed]
- Olsen, C.A.; Ziegler, H.L.; Nielsen, H.M.; Frimodt-Møller, N.; Jaroszewski, J.W.; Franzyk, H. Antimicrobial, Hemolytic, and Cytotoxic Activities of β-Peptoid–Peptide Hybrid Oligomers: Improved Properties Compared to Natural AMPs. ChemBioChem 2010, 11, 1356–1360. [Google Scholar] [CrossRef] [PubMed]
- Patch, J.A.; Barron, A.E. Helical peptoid mimics of magainin-2 amide. J. Am. Chem. Soc. 2003, 125, 12092–12093. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Poon, Y.F.; Li, W.; Zhu, H.-Y.; Yeap, S.H.; Cao, Y.; Qi, X.; Zhou, C.; Lamrani, M.; Beuerman, R.W. A polycationic antimicrobial and biocompatible hydrogel with microbe membrane suctioning ability. Nat. Mater. 2011, 10, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Ilker, M.F.; Nüsslein, K.; Tew, G.N.; Coughlin, E.B. Tuning the hemolytic and antibacterial activities of amphiphilic polynorbornene derivatives. J. Am. Chem. Soc. 2004, 126, 15870–15875. [Google Scholar] [CrossRef]
- Chin, W.; Zhong, G.; Pu, Q.; Yang, C.; Lou, W.; De Sessions, P.F.; Periaswamy, B.; Lee, A.; Liang, Z.C.; Ding, X. A macromolecular approach to eradicate multidrug resistant bacterial infections while mitigating drug resistance onset. Nat. Commun. 2018, 9, 1–14. [Google Scholar] [CrossRef]
- Chakraborty, S.; Liu, R.; Hayouka, Z.; Chen, X.; Ehrhardt, J.; Lu, Q.; Burke, E.; Yang, Y.; Weisblum, B.; Wong, G.C. Ternary nylon-3 copolymers as host-defense peptide mimics: Beyond hydrophobic and cationic subunits. J. Am. Chem. Soc. 2014, 136, 14530–14535. [Google Scholar] [CrossRef]
- Mowery, B.P.; Lindner, A.H.; Weisblum, B.; Stahl, S.S.; Gellman, S.H. Structure—activity relationships among random nylon-3 copolymers that mimic antibacterial host-defense peptides. J. Am. Chem. Soc. 2009, 131, 9735–9745. [Google Scholar] [CrossRef]
- Qian, Y.-X.; Zhang, D.-F.; Wu, Y.-M.; Chen, Q.; Liu, R.-H. The design, synthesis and biological activity study of nylon-3 polymers as mimics of host defense peptides. ACTA Polym. Sin. 2016, 10, 1300–1311. [Google Scholar]
- Xiong, M.; Lee, M.W.; Mansbach, R.A.; Song, Z.; Bao, Y.; Peek, R.M.; Yao, C.; Chen, L.-F.; Ferguson, A.L.; Wong, G.C. Helical antimicrobial polypeptides with radial amphiphilicity. Proc. Natl. Acad. Sci. USA 2015, 112, 13155–13160. [Google Scholar] [CrossRef]
- Xiong, M.; Han, Z.; Song, Z.; Yu, J.; Ying, H.; Yin, L.; Cheng, J. Bacteria-assisted activation of antimicrobial polypeptides by a random-coil to helix transition. Angew. Chem. Int. Ed. 2017, 56, 10826–10829. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ma, P.; Xie, J.; Zhang, S.; Xiao, X.; Qiao, Z.; Shao, N.; Zhou, M.; Zhang, W.; Dai, C. Host defense peptide mimicking poly-β-peptides with fast, potent and broad spectrum antibacterial activities. Biomater. Sci. 2019, 7, 2144–2151. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.-K.; Lam, S.J.; Ho, K.K.; Kumar, N.; Qiao, G.G.; Egan, S.; Boyer, C.; Wong, E.H. Rational design of single-chain polymeric nanoparticles that kill planktonic and biofilm bacteria. ACS Infect. Dis. 2017, 3, 237–248. [Google Scholar] [CrossRef]
- Hartlieb, M.; Williams, E.G.; Kuroki, A.; Perrier, S.; Locock, K.E. Antimicrobial Polymers: Mimicking Amino Acid Functionali ty, Sequence Control and Three-dimensional Structure of Host-defen se Peptides. Curr. Med. Chem. 2017, 24, 2115–2140. [Google Scholar] [CrossRef]
- Fosgerau, K.; Hoffmann, T. Peptide therapeutics: Current status and future directions. Drug Discov. Today 2015, 20, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Zhang, J.; Hu, X.; Li, Z.; Fa, K.; Liu, H.; Waigh, T.A.; McBain, A.; Lu, J.R. Hydrophobic Control of the Bioactivity and Cytotoxicity of de Novo-Designed Antimicrobial Peptides. ACS Appl. Mater. Interfaces 2019, 11, 34609–34620. [Google Scholar] [CrossRef]
- Carratalá, J.V.; Serna, N.; Villaverde, A.; Vázquez, E.; Ferrer-Miralles, N. Nanostructured antimicrobial peptides: The last push towards clinics. Biotechnol. Adv. 2020, 44, 107603. [Google Scholar] [CrossRef]
- Takahashi, D.; Shukla, S.K.; Prakash, O.; Zhang, G. Structural determinants of host defense peptides for antimicrobial activity and target cell selectivity. Biochimie 2010, 92, 1236–1241. [Google Scholar] [CrossRef]
- Clifton, L.A.; Skoda, M.W.; Le Brun, A.P.; Ciesielski, F.; Kuzmenko, I.; Holt, S.A.; Lakey, J.H. Effect of divalent cation removal on the structure of gram-negative bacterial outer membrane models. Langmuir 2015, 31, 404–412. [Google Scholar] [CrossRef]
- Shai, Y. Mode of action of membrane active antimicrobial peptides. Biopolym. Pept. Sci. Sect. 2002, 66, 236–248. [Google Scholar] [CrossRef]
- Malanovic, N.; Lohner, K. Gram-positive bacterial cell envelopes: The impact on the activity of antimicrobial peptides. Biochim. Biophys. Acta Biomembr. 2016, 1858, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Malanovic, N.; Lohner, K. Antimicrobial peptides targeting gram-positive bacteria. Pharmaceuticals 2016, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Andreev, K.; Martynowycz, M.W.; Huang, M.L.; Kuzmenko, I.; Bu, W.; Kirshenbaum, K.; Gidalevitz, D. Hydrophobic interactions modulate antimicrobial peptoid selectivity towards anionic lipid membranes. Biochim. Biophys. Acta BBA Biomembr. 2018, 1860, 1414–1423. [Google Scholar] [CrossRef] [PubMed]
- Blondelle, S.E.; Houghten, R.A. Design of model amphipathic peptides having potent antimicrobial activities. Biochemistry 1992, 31, 12688–12694. [Google Scholar] [CrossRef]
- Eisenberg, D.; Weiss, R.M.; Terwilliger, T.C. The hydrophobic moment detects periodicity in protein hydrophobicity. Proc. Natl. Acad. Sci. USA 1984, 81, 140–144. [Google Scholar] [CrossRef]
- Giangaspero, A.; Sandri, L.; Tossi, A. Amphipathic α helical antimicrobial peptides. A systematic study of the effects of structural and physical properties on biological activity. Eur. J. Biochem. 2001, 268, 5589–5600. [Google Scholar] [CrossRef]
- Iavicoli, P.; Rossi, F.; Lamarre, B.; Bella, A.; Ryadnov, M.G.; Calzolai, L. Modulating charge-dependent and folding-mediated antimicrobial interactions at peptide—lipid interfaces. Eur. Biophys. J. 2017, 46, 375–382. [Google Scholar] [CrossRef]
- Findlay, B.; Zhanel, G.G.; Schweizer, F. Cationic amphiphiles, a new generation of antimicrobials inspired by the natural antimicrobial peptide scaffold. Antimicrob. Agents Chemother. 2010, 54, 4049–4058. [Google Scholar] [CrossRef]
- Otvos, L., Jr.; Wade, J.D. Current challenges in peptide-based drug discovery. Front. Chem. 2014, 2, 62. [Google Scholar] [CrossRef]
- Guilhelmelli, F.; Vilela, N.; Albuquerque, P.; Derengowski, L.; Silva-Pereira, I.; Kyaw, C. Antibiotic development challenges: The various mechanisms of action of antimicrobial peptides and of bacterial resistance. Front. Microbiol. 2013, 4, 353. [Google Scholar] [CrossRef]
- Eckert, R. Road to clinical efficacy: Challenges and novel strategies for antimicrobial peptide development. Future Microbiol. 2011, 6, 635–651. [Google Scholar] [CrossRef] [PubMed]
- Bednarska, N.G.; Wren, B.W.; Willcocks, S.J. The importance of the glycosylation of antimicrobial peptides: Natural and synthetic approaches. Drug Discov. Today 2017, 22, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Schmidtchen, A.; Frick, I.M.; Andersson, E.; Tapper, H.; Björck, L. Proteinases of common pathogenic bacteria degrade and inactivate the antibacterial peptide LL-37. Mol. Microbiol. 2002, 46, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Andersson, D.I.; Hughes, D.; Kubicek-Sutherland, J.Z. Mechanisms and consequences of bacterial resistance to antimicrobial peptides. Drug Resist. Updates 2016, 26, 43–57. [Google Scholar] [CrossRef]
- Gai, Z.; Samodelov, S.L.; Kullak-Ublick, G.A.; Visentin, M. Molecular mechanisms of colistin-induced nephrotoxicity. Molecules 2019, 24, 653. [Google Scholar] [CrossRef]
- Radermacher, S.; Schoop, V.; Schluesener, H. Bactenecin, a leukocytic antimicrobial peptide, is cytotoxic to neuronal and glial cells. J. Neurosci. Res. 1993, 36, 657–662. [Google Scholar] [CrossRef]
- Gordon, Y.J.; Romanowski, E.G.; McDermott, A.M. A review of antimicrobial peptides and their therapeutic potential as anti-infective drugs. Curr. Eye Res. 2005, 30, 505–515. [Google Scholar] [CrossRef]
- Craik, D.J.; Fairlie, D.P.; Liras, S.; Price, D. The future of peptide-based drugs. Chem. Biol. Drug Des. 2013, 81, 136–147. [Google Scholar] [CrossRef]
- Deptuła, M.; Wardowska, A.; Dzierżyńska, M.; Rodziewicz-Motowidło, S.; Pikuła, M. Antibacterial peptides in dermatology–strategies for evaluation of allergic potential. Molecules 2018, 23, 414. [Google Scholar] [CrossRef]
- Corominas, M.; Gastaminza, G.; Lobera, T. Hypersensitivity reactions to biological drugs. J. Investig. Allergol. Clin. Immunol. 2014, 24, 212–225. [Google Scholar]
- Moussa, E.M.; Panchal, J.P.; Moorthy, B.S.; Blum, J.S.; Joubert, M.K.; Narhi, L.O.; Topp, E.M. Immunogenicity of therapeutic protein aggregates. J. Pharm. Sci. 2016, 105, 417–430. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, M.; Bird, C.; Dilger, P.; Gaines-Das, R.; Thorpe, R. Strategies for detection, measurement and characterization of unwanted antibodies induced by therapeutic biologicals. J. Immunol. Methods 2003, 278, 1–17. [Google Scholar] [CrossRef]
- Fathallah, A.M.; Bankert, R.B.; Balu-Iyer, S.V. Immunogenicity of subcutaneously administered therapeutic proteins—a mechanistic perspective. AAPS J. 2013, 15, 897–900. [Google Scholar] [CrossRef] [PubMed]
- Mari, A.; Rasi, C.; Palazzo, P.; Scala, E. Allergen databases: Current status and perspectives. Curr. Allergy Asthma Rep. 2009, 9, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Pikuła, M.; Zieliński, M.; Specjalski, K.; Barańska-Rybak, W.; Dawgul, M.; Langa, P.; Jassem, E.; Kamysz, W.; Trzonkowski, P. In vitro evaluation of the allergic potential of antibacterial peptides: Camel and citropin. Chem. Biol. Drug Des. 2016, 87, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Bobone, S.; Stella, L. Selectivity of Antimicrobial Peptides: A Complex Interplay of Multiple Equilibria. In Antimicrobial Peptides; Springer: Berlin/Heidelberg, Germany, 2019; pp. 175–214. [Google Scholar]
- Maturana, P.; Martinez, M.; Noguera, M.E.; Santos, N.; Disalvo, E.A.; Semorile, L.; Maffia, P.C.; Hollmann, A. Lipid selectivity in novel antimicrobial peptides: Implication on antimicrobial and hemolytic activity. Colloids Surf. B Biointerfaces 2017, 153, 152–159. [Google Scholar] [CrossRef]
- Dörr, T.; Moynihan, P.J.; Mayer, C. Bacterial cell wall structure and dynamics. Front. Microbiol. 2019, 10, 2051. [Google Scholar] [CrossRef]
- Tomasz, A.; McDonnell, M.; Westphal, M.; Zanati, E. Coordinated incorporation of nascent peptidoglycan and teichoic acid into pneumococcal cell walls and conservation of peptidoglycan during growth. J. Biol. Chem. 1975, 250, 337–341. [Google Scholar]
- Bertani, B.; Ruiz, N. Function and Biogenesis of Lipopolysaccharides. EcoSal Plus 2018. [Google Scholar] [CrossRef]
- Zhang, G.; Meredith, T.C.; Kahne, D. On the essentiality of lipopolysaccharide to Gram-negative bacteria. Curr. Opin. Microbiol. 2013, 16, 779–785. [Google Scholar] [CrossRef]
- Gan, L.; Chen, S.; Jensen, G.J. Molecular organization of Gram-negative peptidoglycan. Proc. Natl. Acad. Sci. USA 2008, 105, 18953–18957. [Google Scholar] [CrossRef] [PubMed]
- Gerondakis, S.; Siebenlist, U. Roles of the NF-κB pathway in lymphocyte development and function. Cold Spring Harb. Perspect. Biol. 2010, 2, a000182. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, V.; Feio, M.J.; Bastos, M. Role of lipids in the interaction of antimicrobial peptides with membranes. Prog. Lipid Res. 2012, 51, 149–177. [Google Scholar] [CrossRef] [PubMed]
- Bocchinfuso, G.; Palleschi, A.; Orioni, B.; Grande, G.; Formaggio, F.; Toniolo, C.; Park, Y.; Hahm, K.S.; Stella, L. Different mechanisms of action of antimicrobial peptides: Insights from fluorescence spectroscopy experiments and molecular dynamics simulations. J. Pept. Sci. Off. Publ. Eur. Pept. Soc. 2009, 15, 550–558. [Google Scholar] [CrossRef]
- Leontiadou, H.; Mark, A.E.; Marrink, S.J. Antimicrobial peptides in action. J. Am. Chem. Soc. 2006, 128, 12156–12161. [Google Scholar] [CrossRef]
- Bogdanova, L.R.; Valiullina, Y.A.; Faizullin, D.A.; Kurbanov, R.K.; Ermakova, E.A. Spectroscopic, zeta potential and molecular dynamics studies of the interaction of antimicrobial peptides with model bacterial membrane. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 242, 118785. [Google Scholar] [CrossRef]
- Lohner, K. Membrane-active Antimicrobial Peptides as Template Structures for Novel Antibiotic Agents. Curr. Top. Med. Chem. 2017, 17, 508–519. [Google Scholar] [CrossRef]
- Aisenbrey, C.; Marquette, A.; Bechinger, B. The Mechanisms of Action of Cationic Antimicrobial Peptides Refined by Novel Concepts from Biophysical Investigations. Adv. Exp. Med. Biol. 2019, 1117, 33–64. [Google Scholar]
- Zeth, K.; Sancho-Vaello, E. The Human Antimicrobial Peptides Dermcidin and LL-37 Show Novel Distinct Pathways in Membrane Interactions. Front. Chem. 2017, 5, 86. [Google Scholar] [CrossRef]
- Yount, N.Y.; Bayer, A.S.; Xiong, Y.Q.; Yeaman, M.R. Advances in antimicrobial peptide immunobiology. Pept. Sci. Orig. Res. Biomol. 2006, 84, 435–458. [Google Scholar] [CrossRef]
- Le, C.-F.; Fang, C.-M.; Sekaran, S.D. Intracellular targeting mechanisms by antimicrobial peptides. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Yin, J.; Zhao, J.; Ma, S.R.; Wang, H.R.; Wang, M.; Chen, W.; Wei, W.Q. Microbial Similarity and Preference for Specific Sites in Healthy Oral Cavity and Esophagus. Front. Microbiol. 2018, 9, 1603. [Google Scholar] [CrossRef] [PubMed]
- Kitada, K.; de Toledo, A.; Oho, T. Increase in detectable opportunistic bacteria in the oral cavity of orthodontic patients. Int. J. Dent. Hyg. 2009, 7, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Tets, G.V.; Vikina, D.S.; Vecherkovskaia, M.F.; Domorad, A.A.; Kharlamova, V.V.; Tets, V.V. New approaches to oral cavity opportunistic microbiota study. Stomatologiia 2013, 92, 14–16. [Google Scholar]
- Feazel, L.M.; Baumgartner, L.K.; Peterson, K.L.; Frank, D.N.; Harris, J.K.; Pace, N.R. Opportunistic pathogens enriched in showerhead biofilms. Proc. Natl. Acad. Sci. USA 2009, 106, 16393–16399. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Pabst, B.; Klapper, I.; Stewart, P.S. General theory for integrated analysis of growth, gene, and protein expression in biofilms. PLoS ONE 2013, 8, e83626. [Google Scholar] [CrossRef]
- Muras, A.; Mayer, C.; Otero-Casal, P.; Exterkate, R.A.M.; Brandt, B.W.; Crielaard, W.; Otero, A.; Krom, B.P. Short-Chain N-Acylhomoserine Lactone Quorum-Sensing Molecules Promote Periodontal Pathogens in In Vitro Oral Biofilms. Appl. Environ. Microbiol. 2020, 86. [Google Scholar] [CrossRef]
- Cho, Y.J.; Song, H.Y.; Ben Amara, H.; Choi, B.K.; Eunju, R.; Cho, Y.A.; Seol, Y.; Lee, Y.; Ku, Y.; Rhyu, I.C.; et al. In Vivo Inhibition of Porphyromonas gingivalis Growth and Prevention of Periodontitis With Quorum-Sensing Inhibitors. J. Periodontol. 2016, 87, 1075–1082. [Google Scholar] [CrossRef]
- Ertugrul, A.S.; Sahin, H.; Dikilitas, A.; Alpaslan, N.Z.; Bozoglan, A.; Tekin, Y. Gingival crevicular fluid levels of human beta-defensin-2 and cathelicidin in smoker and non-smoker patients: A cross-sectional study. J. Periodontal Res. 2014, 49, 282–289. [Google Scholar] [CrossRef]
- Turkoglu, O.; Emingil, G.; Kutukculer, N.; Atilla, G. Gingival crevicular fluid levels of cathelicidin LL-37 and interleukin-18 in patients with chronic periodontitis. J. Periodontol. 2009, 80, 969–976. [Google Scholar] [CrossRef]
- Available online: http://aps.unmc.edu/AP/database/mysql.php (accessed on 27 September 2020).
- Greer, A.; Zenobia, C.; Darveau, R.P. Defensins and LL-37: A review of function in the gingival epithelium. Periodontology 2000 2013, 63, 67–79. [Google Scholar] [CrossRef] [PubMed]
- de Sousa-Pereira, P.; Amado, F.; Abrantes, J.; Ferreira, R.; Esteves, P.J.; Vitorino, R. An evolutionary perspective of mammal salivary peptide families: Cystatins, histatins, statherin and PRPs. Arch. Oral Biol. 2013, 58, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, Z.; Naseem, M.; Sheikh, Z.; Najeeb, S.; Shahab, S.; Zafar, M.S. Oral antimicrobial peptides: Types and role in the oral cavity. Saudi Pharm. J. SPJ Off. Publ. Saudi Pharm. Soc. 2016, 24, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Lachowicz, J.I.; Dalla Torre, G.; Cappai, R.; Randaccio, E.; Nurchi, V.M.; Bachor, R.; Szewczuk, Z.; Jaremko, L.; Jaremko, M.; Pisano, M.B.; et al. Metal self-assembly mimosine peptides with enhanced antimicrobial activity: Towards a new generation of multitasking chelating agents. Dalton Trans. 2020, 49, 2862–2879. [Google Scholar] [CrossRef]
- Puklo, M.; Guentsch, A.; Hiemstra, P.S.; Eick, S.; Potempa, J. Analysis of neutrophil-derived antimicrobial peptides in gingival crevicular fluid suggests importance of cathelicidin LL-37 in the innate immune response against periodontogenic bacteria. Oral Microbiol. Immunol. 2008, 23, 328–335. [Google Scholar] [CrossRef]
- Orru, G.; Marini, M.F.; Ciusa, M.L.; Isola, D.; Cotti, M.; Baldoni, M.; Piras, V.; Pisano, E.; Montaldo, C. Usefulness of real time PCR for the differentiation and quantification of 652 and JP2 Actinobacillus actinomycetemcomitans genotypes in dental plaque and saliva. BMC Infect. Dis. 2006, 6, 98. [Google Scholar] [CrossRef]
- Abriani, A.; Hamad, C. Activated antimicrobial peptides due to periodontal bacteria in synovial fluid - The link between psoriatic arthritis and periodontitis? Med. Hypotheses 2020, 144, 109967. [Google Scholar] [CrossRef]
- Jourdain, M.L.; Velard, F.; Pierrard, L.; Sergheraert, J.; Gangloff, S.C.; Braux, J. Cationic antimicrobial peptides and periodontal physiopathology: A systematic review. J. Periodontal Res. 2019, 54, 589–600. [Google Scholar] [CrossRef]
- Li, S.; Schmalz, G.; Schmidt, J.; Krause, F.; Haak, R.; Ziebolz, D. Antimicrobial peptides as a possible interlink between periodontal diseases and its risk factors: A systematic review. J. Periodontal Res. 2018, 53, 145–155. [Google Scholar] [CrossRef]
- Turkoglu, O.; Gurkan, A.; Emingil, G.; Afacan, B.; Toz, H.; Kutukculer, N.; Atilla, G. Are antimicrobial peptides related to cyclosporine A-induced gingival overgrowth? Arch. Oral Biol. 2015, 60, 508–515. [Google Scholar] [CrossRef]
- Hussain, M.; Stover, C.M.; Dupont, A. P. gingivalis in Periodontal Disease and Atherosclerosis—Scenes of Action for Antimicrobial Peptides and Complement. Front. Immunol. 2015, 6, 45. [Google Scholar] [CrossRef] [PubMed]
- Guncu, G.N.; Yilmaz, D.; Kononen, E.; Gursoy, U.K. Salivary Antimicrobial Peptides in Early Detection of Periodontitis. Front. Cell. Infect. Microbiol. 2015, 5, 99. [Google Scholar] [CrossRef] [PubMed]
- Dommisch, H.; Jepsen, S. Diverse functions of defensins and other antimicrobial peptides in periodontal tissues. Periodontology 2000 2015, 69, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Bedran, T.B.; Mayer, M.P.; Spolidorio, D.P.; Grenier, D. Synergistic anti-inflammatory activity of the antimicrobial peptides human beta-defensin-3 (hBD-3) and cathelicidin (LL-37) in a three-dimensional co-culture model of gingival epithelial cells and fibroblasts. PLoS ONE 2014, 9, e106766. [Google Scholar] [CrossRef]
- Abbassi, F.; Lequin, O.; Piesse, C.; Goasdoué, N.; Foulon, T.; Nicolas, P.; Ladram, A. Temporin-SHf, a new type of phe-rich and hydrophobic ultrashort antimicrobial peptide. J. Biol. Chem. 2010, 285, 16880–16892. [Google Scholar] [CrossRef]
- Mishra, B.; Lushnikova, T.; Golla, R.M.; Wang, X.; Wang, G. Design and surface immobilization of short anti-biofilm peptides. Acta Biomater. 2017, 49, 316–328. [Google Scholar] [CrossRef]
- Bozelli, J.C.; Yune, J.; Dang, X.; Narayana, J.L.; Wang, G.; Epand, R.M. Membrane activity of two short Trp-rich amphipathic peptides. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183280. [Google Scholar] [CrossRef]
- Manzo, G.; Ferguson, P.M.; Hind, C.K.; Clifford, M.; Gustilo, V.B.; Ali, H.; Bansal, S.S.; Bui, T.T.; Drake, A.F.; Atkinson, R.A.; et al. Temporin L and aurein 2.5 have identical conformations but subtly distinct membrane and antibacterial activities. Sci. Rep. 2019, 9, 10934. [Google Scholar] [CrossRef]
- Suh, J.-Y.; Lee, K.-H.; Chi, S.-W.; Hong, S.-Y.; Choi, B.-W.; Moon, H.-M.; Choi, B.-S. Unusually stable helical kink in the antimicrobial peptide—A derivative of gaegurin. FEBS Lett. 1996, 392, 309–312. [Google Scholar] [CrossRef]
- Hossain, M.A.; Guilhaudis, L.; Sonnevend, A.; Attoub, S.; Van Lierop, B.J.; Robinson, A.J.; Wade, J.D.; Conlon, J.M. Synthesis, conformational analysis and biological properties of a dicarba derivative of the antimicrobial peptide, brevinin-1BYa. Eur. Biophys. J. 2011, 40, 555–564. [Google Scholar] [CrossRef]
- Timmons, P.B.; O’Flynn, D.; Conlon, J.M.; Hewage, C.M. Structural and positional studies of the antimicrobial peptide brevinin-1BYa in membrane-mimetic environments. J. Pept. Sci. 2019, 25. [Google Scholar] [CrossRef]
- Zhang, Y. I-TASSER: Fully automated protein structure prediction in CASP8. Proteins Struct. Funct. Bioinform. 2009, 77, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Yang, J.; Zhang, Y. COFACTOR: An accurate comparative algorithm for structure-based protein function annotation. Nucleic Acids Res. 2012, 40, W471–W477. [Google Scholar] [CrossRef] [PubMed]
- Timmons, P.B.; O’Flynn, D.; Conlon, J.M.; Hewage, C.M. Insights into conformation and membrane interactions of the acyclic and dicarba-bridged brevinin-1BYa antimicrobial peptides. Eur. Biophys. J. 2019, 48, 701–710. [Google Scholar] [CrossRef]
- Pál, T.; Abraham, B.; Sonnevend, Á.; Jumaa, P.; Conlon, J.M. Brevinin-1BYa: A naturally occurring peptide from frog skin with broad-spectrum antibacterial and antifungal properties. Int. J. Antimicrob. Agents 2006, 27, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.-Y.; Hong, S.-Y.; Lee, K.-H. Structure-activity analysis of brevinin 1E amide, an antimicrobial peptide from Rana esculenta. Biochim. Biophys. Acta BBA Protein Struct. Mol. Enzymol. 1998, 1387, 239–248. [Google Scholar] [CrossRef]
- Almeida, J.R.; Mendes, B.; Lancellotti, M.; Marangoni, S.; Vale, N.; Passos, Ó.; Ramos, M.J.; Fernandes, P.A.; Gomes, P.; Da Silva, S.L. A novel synthetic peptide inspired on Lys49 phospholipase A2 from Crotalus oreganus abyssus snake venom active against multidrug-resistant clinical isolates. Eur. J. Med. Chem. 2018, 149, 248–256. [Google Scholar] [CrossRef]
- Nakamura, T.; Furunaka, H.; Miyata, T.; Tokunaga, F.; Muta, T.; Iwanaga, S.; Niwa, M.; Takao, T.; Shimonishi, Y. Tachyplesin, a class of antimicrobial peptide from the hemocytes of the horseshoe crab (Tachypleus tridentatus). Isolation and chemical structure. J. Biol. Chem. 1988, 263, 16709–16713. [Google Scholar]
- Kawano, K.; Yoneya, T.; Miyata, T.; Yoshikawa, K.; Tokunaga, F.; Terada, Y.; Iwanaga, S. Antimicrobial peptide, tachyplesin I, isolated from hemocytes of the horseshoe crab (Tachypleus tridentatus). NMR determination of the beta-sheet structure. J. Biol. Chem. 1990, 265, 15365–15367. [Google Scholar]
- Kushibiki, T.; Kamiya, M.; Aizawa, T.; Kumaki, Y.; Kikukawa, T.; Mizuguchi, M.; Demura, M.; Kawabata, S.-I.; Kawano, K. Interaction between tachyplesin I, an antimicrobial peptide derived from horseshoe crab, and lipopolysaccharide. Biochim. Biophys. Acta BBA Proteins Proteom. 2014, 1844, 527–534. [Google Scholar] [CrossRef]
- Tamamura, H.; Ikoma, R.; Niwa, M.; Funakoshi, S.; Murakami, T.; Fujii, N. Antimicrobial activity and conformation of tachyplesin I and its analogs. Chem. Pharm. Bull. 1993, 41, 978–980. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Hu, J.; Ke, F. Experimental induction of bacterial resistance to the antimicrobial peptide tachyplesin I and investigation of the resistance mechanisms. Antimicrob. Agents Chemother. 2016, 60, 6067–6075. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Guan, W.; Jin, G.; Zhao, H.; Jiang, X.; Dai, J. Mechanism of tachyplesin I injury to bacterial membranes and intracellular enzymes, determined by laser confocal scanning microscopy and flow cytometry. Microbiol. Res. 2015, 170, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Brogden, N.K.; Brogden, K.A. Will new generations of modified antimicrobial peptides improve their potential as pharmaceuticals? Int. J. Antimicrob. Agents 2011, 38, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Dai, J.; Guan, W.; Jin, G.; Huang, Z.L.; Zhang, L.; Dang, J.Z.; Zhang, Y. Tachyplesin I induce drug resistance in bacteria in vitro. J. Anim. Vet. Adv. 2012, 11, 939–945. [Google Scholar]
- Varnava, K.G.; Mohid, S.A.; Calligari, P.; Stella, L.; Reynison, J.; Bhunia, A.; Sarojini, V. Design, synthesis, antibacterial potential, and structural characterization of N-acylated derivatives of the human autophagy 16 polypeptide. Bioconjugate Chem. 2019, 30, 1998–2010. [Google Scholar] [CrossRef]
- Tsubery, H.; Ofek, I.; Cohen, S.; Fridkin, M. N-terminal modifications of polymyxin B nonapeptide and their effect on antibacterial activity. Peptides 2001, 22, 1675–1681. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Matsumoto, H.; Hashimoto, K.; Teraguchi, S.; Takase, M.; Hayasawa, H. N-Acylated and D enantiomer derivatives of a nonamer core peptide of lactoferricin B showing improved antimicrobial activity. Antimicrob. Agents Chemother. 1999, 43, 1267–1269. [Google Scholar] [CrossRef] [PubMed]
- Majerle, A.; Kidrič, J.; Jerala, R. Enhancement of antibacterial and lipopolysaccharide binding activities of a human lactoferrin peptide fragment by the addition of acyl chain. J. Antimicrob. Chemother. 2003, 51, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Chu-Kung, A.F.; Nguyen, R.; Bozzelli, K.N.; Tirrell, M. Chain length dependence of antimicrobial peptide–fatty acid conjugate activity. J. Colloid Interface Sci. 2010, 345, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Chu-Kung, A.F.; Bozzelli, K.N.; Lockwood, N.A.; Haseman, J.R.; Mayo, K.H.; Tirrell, M.V. Promotion of peptide antimicrobial activity by fatty acid conjugation. Bioconjugate Chem. 2004, 15, 530–535. [Google Scholar] [CrossRef] [PubMed]
- De Zoysa, G.H.; Cameron, A.J.; Hegde, V.V.; Raghothama, S.; Sarojini, V. Antimicrobial peptides with potential for biofilm eradication: Synthesis and structure activity relationship studies of battacin peptides. J. Med. Chem. 2015, 58, 625–639. [Google Scholar] [CrossRef] [PubMed]
- Moussouni, M.; Nogaret, P.; Garai, P.; Ize, B.; Vivès, E.; Blanc-Potard, A.B. Activity of a synthetic peptide targeting MgtC on Pseudomonas aeruginosa intramacrophage survival and biofilm formation. Front. Cell. Infect. Microbiol. 2019, 9, 84. [Google Scholar] [CrossRef] [PubMed]
- Oda, Y.; Kanaoka, S.; Sato, T.; Aoshima, S.; Kuroda, K. Block versus random amphiphilic copolymers as antibacterial agents. Biomacromolecules 2011, 12, 3581–3591. [Google Scholar] [CrossRef]
- Engler, A.C.; Wiradharma, N.; Ong, Z.Y.; Coady, D.J.; Hedrick, J.L.; Yang, Y.-Y. Emerging trends in macromolecular antimicrobials to fight multi-drug-resistant infections. Nano Today 2012, 7, 201–222. [Google Scholar] [CrossRef]
- Ergene, C.; Yasuhara, K.; Palermo, E.F. Biomimetic antimicrobial polymers: Recent advances in molecular design. Polym. Chem. 2018, 9, 2407–2427. [Google Scholar] [CrossRef]
- Mowery, B.P.; Lee, S.E.; Kissounko, D.A.; Epand, R.F.; Epand, R.M.; Weisblum, B.; Stahl, S.S.; Gellman, S.H. Mimicry of antimicrobial host-defense peptides by random copolymers. J. Am. Chem. Soc. 2007, 129, 15474–15476. [Google Scholar] [CrossRef]
- Palermo, E.F.; Lee, D.-K.; Ramamoorthy, A.; Kuroda, K. Role of cationic group structure in membrane binding and disruption by amphiphilic copolymers. J. Phys. Chem. B 2011, 115, 366–375. [Google Scholar] [CrossRef]
- Avery, C.W.; Palermo, E.F.; McLaughlin, A.; Kuroda, K.; Chen, Z. Investigations of the interactions between synthetic antimicrobial polymers and substrate-supported lipid bilayers using sum frequency generation vibrational spectroscopy. Anal. Chem. 2011, 83, 1342–1349. [Google Scholar] [CrossRef]
- Kuroda, K.; DeGrado, W.F. Amphiphilic polymethacrylate derivatives as antimicrobial agents. J. Am. Chem. Soc. 2005, 127, 4128–4129. [Google Scholar] [CrossRef]
- Kuroda, K.; Caputo, G.A.; DeGrado, W.F. The role of hydrophobicity in the antimicrobial and hemolytic activities of polymethacrylate derivatives. Chem. A Eur. J. 2009, 15, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Punia, A.; He, E.; Lee, K.; Banerjee, P.; Yang, N.-L. Cationic amphiphilic non-hemolytic polyacrylates with superior antibacterial activity. Chem. Commun. 2014, 50, 7071–7074. [Google Scholar] [CrossRef] [PubMed]
- Punia, A.; Debata, P.R.; Banerjee, P.; Yang, N.-L. Structure–property relationships of antibacterial amphiphilic polymers derived from 2-aminoethyl acrylate. RSC Adv. 2015, 5, 95300–95306. [Google Scholar] [CrossRef]
- Sgolastra, F.; Deronde, B.M.; Sarapas, J.M.; Som, A.; Tew, G.N. Designing mimics of membrane active proteins. Acc. Chem. Res. 2013, 46, 2977–2987. [Google Scholar] [CrossRef]
- Al-Badri, Z.M.; Som, A.; Lyon, S.; Nelson, C.F.; Nüsslein, K.; Tew, G.N. Investigating the effect of increasing charge density on the hemolytic activity of synthetic antimicrobial polymers. Biomacromolecules 2008, 9, 2805–2810. [Google Scholar] [CrossRef]
- Uppu, D.; Konai, M.; Baul, U.; Singh, P.; Siersma, T.; Samaddar, S.; Vemparala, S.; Hamoen, L.; Narayana, C.; Haldar, J. Isosteric substitution in cationic-amphiphilic polymers reveals an important role for hydrogen bonding in bacterial membrane interactions. Chem. Sci. 2016, 7, 4613–4623. [Google Scholar] [CrossRef]
- Uppu, D.S.; Samaddar, S.; Hoque, J.; Konai, M.M.; Krishnamoorthy, P.; Shome, B.R.; Haldar, J. Side chain degradable cationic–amphiphilic polymers with tunable hydrophobicity show in vivo activity. Biomacromolecules 2016, 17, 3094–3102. [Google Scholar] [CrossRef]
- Nimmagadda, A.; Liu, X.; Teng, P.; Su, M.; Li, Y.; Qiao, Q.; Khadka, N.K.; Sun, X.; Pan, J.; Xu, H. Polycarbonates with potent and selective antimicrobial activity toward gram-positive bacteria. Biomacromolecules 2017, 18, 87–95. [Google Scholar] [CrossRef]
- Yang, C.; Lou, W.; Zhong, G.; Lee, A.; Leong, J.; Chin, W.; Ding, B.; Bao, C.; Tan, J.P.; Pu, Q. Degradable antimicrobial polycarbonates with unexpected activity and selectivity for treating multidrug-resistant Klebsiella pneumoniae lung infection in mice. Acta Biomater. 2019, 94, 268–280. [Google Scholar] [CrossRef]
- Kenawy, E.-R.; Worley, S.; Broughton, R. The chemistry and applications of antimicrobial polymers: A state-of-the-art review. Biomacromolecules 2007, 8, 1359–1384. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, J.; Zhang, Y.; Yan, H.; Liu, K. Antimicrobial and hemolytic activities of copolymers with cationic and hydrophobic groups: A comparison of block and random copolymers. Macromol. Biosci. 2011, 11, 1499–1504. [Google Scholar] [CrossRef] [PubMed]
- Gelman, M.A.; Weisblum, B.; Lynn, D.M.; Gellman, S.H. Biocidal activity of polystyrenes that are cationic by virtue of protonation. Org. Lett. 2004, 6, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Lienkamp, K.; Madkour, A.E.; Musante, A.; Nelson, C.F.; Nusslein, K.; Tew, G.N. Antimicrobial polymers prepared by ROMP with unprecedented selectivity: A molecular construction kit approach. J. Am. Chem. Soc. 2008, 130, 9836–9843. [Google Scholar] [CrossRef]
- Ikeda, T.; Yamaguchi, H.; Tazuke, S. New polymeric biocides: Synthesis and antibacterial activities of polycations with pendant biguanide groups. Antimicrob. Agents Chemother. 1984, 26, 139–144. [Google Scholar] [CrossRef]
- Palermo, E.F.; Kuroda, K. Chemical structure of cationic groups in amphiphilic polymethacrylates modulates the antimicrobial and hemolytic activities. Biomacromolecules 2009, 10, 1416–1428. [Google Scholar] [CrossRef]
- Epand, R.F.; Mowery, B.P.; Lee, S.E.; Stahl, S.S.; Lehrer, R.I.; Gellman, S.H.; Epand, R.M. Dual mechanism of bacterial lethality for a cationic sequence-random copolymer that mimics host-defense antimicrobial peptides. J. Mol. Biol. 2008, 379, 38–50. [Google Scholar] [CrossRef]
- Gabriel, G.J.; Madkour, A.E.; Dabkowski, J.M.; Nelson, C.F.; Nüsslein, K.; Tew, G.N. Synthetic mimic of antimicrobial peptide with nonmembrane-disrupting antibacterial properties. Biomacromolecules 2008, 9, 2980–2983. [Google Scholar] [CrossRef] [PubMed]
- Palermo, E.F.; Sovadinova, I.; Kuroda, K. Structural determinants of antimicrobial activity and biocompatibility in membrane-disrupting methacrylamide random copolymers. Biomacromolecules 2009, 10, 3098–3107. [Google Scholar] [CrossRef]
- Exley, S.E.; Paslay, L.C.; Sahukhal, G.S.; Abel, B.A.; Brown, T.D.; McCormick, C.L.; Heinhorst, S.; Koul, V.; Choudhary, V.; Elasri, M.O.; et al. Antimicrobial Peptide Mimicking Primary Amine and Guanidine Containing Methacrylamide Copolymers Prepared by Raft Polymerization. Biomacromolecules 2015, 16, 3845–3852. [Google Scholar] [CrossRef]
- Brittin, J.; Fry, M.R.; Punia, A.; Johnson, K.A.; Sengupta, A. Antibacterial and hemolytic properties of acrylate-based random ternary copolymers comprised of same center cationic, ethyl and poly(oligoethylene glycol) side chains. Eur. Polym. J. 2020, 132. [Google Scholar] [CrossRef]
- Porter, E.A.; Weisblum, B.; Gellman, S.H. Mimicry of host-defense peptides by unnatural oligomers: Antimicrobial β-peptides. J. Am. Chem. Soc. 2002, 124, 7324–7330. [Google Scholar] [CrossRef] [PubMed]
- Frackenpohl, J.; Arvidsson, P.I.; Schreiber, J.V.; Seebach, D. The outstanding biological stability of β-and γ-peptides toward proteolytic enzymes: An in vitro investigation with fifteen peptidases. ChemBioChem 2001, 2, 445–455. [Google Scholar] [CrossRef]
- Zhou, X.; He, J.; Zhou, C. Strategies from nature: Polycaprolactone-based mimetic antimicrobial peptide block copolymers with low cytotoxicity and excellent antibacterial efficiency. Polym. Chem. 2019, 10, 945–953. [Google Scholar] [CrossRef]
- Sinha, V.; Bansal, K.; Kaushik, R.; Kumria, R.; Trehan, A. Poly-ϵ-caprolactone microspheres and nanospheres: An overview. Int. J. Pharm. 2004, 278, 1–23. [Google Scholar] [CrossRef]
- Wei, X.; Gong, C.; Gou, M.; Fu, S.; Guo, Q.; Shi, S.; Luo, F.; Guo, G.; Qiu, L.; Qian, Z. Biodegradable poly (ɛ-caprolactone)—poly (ethylene glycol) copolymers as drug delivery system. Int. J. Pharm. 2009, 381, 1–18. [Google Scholar] [CrossRef]
- Zhou, S.; Deng, X.; Yang, H. Biodegradable poly (ε-caprolactone)-poly (ethylene glycol) block copolymers: Characterization and their use as drug carriers for a controlled delivery system. Biomaterials 2003, 24, 3563–3570. [Google Scholar] [CrossRef]
- Qian, Y.; Zhou, X.; He, J.; Zhou, C. Polycaprolactone-based mimetic antimicrobial peptide copolymers vesicles as an effective drug-carrier for cancer therapy. Polymers 2019, 11, 1783. [Google Scholar] [CrossRef]
- Barman, S.; Konai, M.M.; Samaddar, S.; Haldar, J. Amino Acid Conjugated Polymers: Antibacterial Agents Effective against Drug-Resistant Acinetobacter baumannii with No Detectable Resistance. ACS Appl. Mater. Interfaces 2019, 11, 33559–33572. [Google Scholar] [CrossRef]
- Costa, F.; Gomes, P.; Martins, M.C.L. Peptides and Proteins as Biomaterials for Tissue Regeneration and Repair; Woodhead Publishing: Cambridge, UK, 2018; pp. 329–345. [Google Scholar]
- Acosta, S.; Ibañez-Fonseca, A.; Aparicio, C.; Rodríguez-Cabello, J.C. Antibiofilm coatings based on protein-engineered polymers and antimicrobial peptides for preventing implant-associated infections. Biomater. Sci. 2020, 8, 2866–2877. [Google Scholar] [CrossRef]
- Mojsoska, B.; Zuckermann, R.N.; Jenssen, H. Structure-activity relationship study of novel peptoids that mimic the structure of antimicrobial peptides. Antimicrob. Agents Chemother. 2015, 59, 4112–4120. [Google Scholar] [CrossRef]
- Thakkar, A.; Cohen, A.S.; Connolly, M.D.; Zuckermann, R.N.; Pei, D. High-throughput sequencing of peptoids and peptide—peptoid hybrids by partial Edman degradation and mass spectrometry. J. Comb. Chem. 2009, 11, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Szleifer, I. Structural and dynamical characteristics of peptoid oligomers with achiral aliphatic side chains studied by molecular dynamics simulation. J. Phys. Chem. B 2011, 115, 10967–10975. [Google Scholar] [CrossRef] [PubMed]
- Mirijanian, D.T.; Mannige, R.V.; Zuckermann, R.N.; Whitelam, S. Development and use of an atomistic CHARMM-based forcefield for peptoid simulation. J. Comput. Chem. 2014, 35, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Prakash, A.; Baer, M.D.; Mundy, C.J.; Pfaendtner, J. Peptoid backbone flexibilility dictates its interaction with water and surfaces: A molecular dynamics investigation. Biomacromolecules 2018, 19, 1006–1015. [Google Scholar] [CrossRef]
- Andreev, K.; Martynowycz, M.W.; Gidalevitz, D. Peptoid drug discovery and optimization via surface X-ray scattering. Biopolymers 2019, 110. [Google Scholar] [CrossRef]
- Andreev, K.; Bianchi, C.; Laursen, J.S.; Citterio, L.; Hein-Kristensen, L.; Gram, L.; Kuzmenko, I.; Olsen, C.A.; Gidalevitz, D. Guanidino groups greatly enhance the action of antimicrobial peptidomimetics against bacterial cytoplasmic membranes. Biochim. Biophys. Acta BBA Biomembr. 2014, 1838, 2492–2502. [Google Scholar] [CrossRef]
- Huang, M.L.; Shin, S.B.Y.; Benson, M.A.; Torres, V.J.; Kirshenbaum, K. A comparison of linear and cyclic peptoid oligomers as potent antimicrobial agents. ChemMedChem 2012, 7, 114–122. [Google Scholar] [CrossRef]
- Gellman, S.H. Foldamers: A manifesto. Acc. Chem. Res. 1998, 31, 173–180. [Google Scholar] [CrossRef]
- Miller, S.M.; Simon, R.J.; Ng, S.; Zuckermann, R.N.; Kerr, J.M.; Moos, W.H. Comparison of the proteolytic susceptibilities of homologous L-amino acid, D-amino acid, and N-substituted glycine peptide and peptoid oligomers. Drug Dev. Res. 1995, 35, 20–32. [Google Scholar] [CrossRef]
- Ibrahim, O.O. Classification of Antimicrobial Peptides Bacteriocins, and the Nature of Some Bacteriocins with Potential Applications in Food Safety and Bio-Pharmaceuticals. EC Microbiol. 2019, 15, 591–608. [Google Scholar]
- Olsen, C.A.; Bonke, G.; Vedel, L.; Adsersen, A.; Witt, M.; Franzyk, H.; Jaroszewski, J.W. α-peptide/β-peptoid chimeras. Org. Lett. 2007, 9, 1549–1552. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Ren, G.; Liu, H.; Miao, Z.; Park, M.; Wang, Y.; Miller, T.M.; Barron, A.E.; Cheng, Z. In vivo biodistribution and small animal PET of 64Cu-Labeled antimicrobial peptoids. Bioconjugate Chem. 2012, 23, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Fisher, K.J.; Turkett, J.A.; Corson, A.E.; Bicker, K.L. Peptoid Library Agar Diffusion (PLAD) assay for the high-throughput identification of antimicrobial peptoids. ACS Comb. Sci. 2016, 18, 287–291. [Google Scholar] [CrossRef]
- Mojsoska, B.; Carretero, G.; Larsen, S.; Mateiu, R.V.; Jenssen, H. Peptoids successfully inhibit the growth of gram negative E. coli causing substantial membrane damage. Sci. Rep. 2017, 7, 42332. [Google Scholar] [CrossRef] [PubMed]
- Kirshenbaum, K.; Barron, A.E.; Goldsmith, R.A.; Armand, P.; Bradley, E.K.; Truong, K.T.; Dill, K.A.; Cohen, F.E.; Zuckermann, R.N. Sequence-specific polypeptoids: A diverse family of heteropolymers with stable secondary structure. Proc. Natl. Acad. Sci. USA 1998, 95, 4303–4308. [Google Scholar] [CrossRef]
- Huang, W.; Seo, J.; Willingham, S.B.; Czyzewski, A.M.; Gonzalgo, M.L.; Weissman, I.L.; Barron, A.E. Learning from host-defense peptides: Cationic, amphipathic peptoids with potent anticancer activity. PLoS ONE 2014, 9, e90397. [Google Scholar] [CrossRef]
- Shyam, R.; Charbonnel, N.; Job, A.; Blavignac, C.; Forestier, C.; Taillefumier, C.; Faure, S. 1,2,3-Triazolium-Based Cationic Amphipathic Peptoid Oligomers Mimicking Antimicrobial Helical Peptides. ChemMedChem 2018, 13, 1513–1516. [Google Scholar] [CrossRef]
- Chou, P.Y.; Fasman, G.D. Conformational parameters for amino acids in helical, β-sheet, and random coil regions calculated from proteins. Biochemistry 1974, 13, 211–222. [Google Scholar] [CrossRef]
- Béven, L.; Castano, S.; Dufourcq, J.; Wieslander, Å.; Wróblewski, H. The antibiotic activity of cationic linear amphipathic peptides: Lessons from the action of leucine/lysine copolymers on bacteria of the class Mollicutes. Eur. J. Biochem. 2003, 270, 2207–2217. [Google Scholar] [CrossRef]
- Monroc, S.; Badosa, E.; Feliu, L.; Planas, M.; Montesinos, E.; Bardají, E. De novo designed cyclic cationic peptides as inhibitors of plant pathogenic bacteria. Peptides 2006, 27, 2567–2574. [Google Scholar] [CrossRef]
- Epand, R.M.; Vogel, H.J. Diversity of antimicrobial peptides and their mechanisms of action. Biochim. Biophys. Acta Bba Biomembr. 1999, 1462, 11–28. [Google Scholar] [CrossRef]
- Won, H.S.; Park, S.H.; Kim, H.E.; Hyun, B.; Kim, M.; Lee, B.J.; Lee, B.J. Effects of a tryptophanyl substitution on the structure and antimicrobial activity of C-terminally truncated gaegurin 4. Eur. J. Biochem. 2002, 269, 4367–4374. [Google Scholar] [CrossRef] [PubMed]
- Pandit, G.; Ilyas, H.; Ghosh, S.; Bidkar, A.P.; Mohid, S.A.; Bhunia, A.; Satpati, P.; Chatterjee, S. Insights into the Mechanism of Antimicrobial Activity of Seven-Residue Peptides. J. Med. Chem. 2018, 61, 7614–7629. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; He, P.; Xiao, C.; Chen, X. From Antimicrobial Peptides to Antimicrobial Poly(α-amino acid)s. Adv. Healthc. Mater. 2018, 7, e1800354. [Google Scholar] [CrossRef] [PubMed]
- Wyrsta, M.D.; Cogen, A.L.; Deming, T.J. A parallel synthetic approach for the analysis of membrane interactive copolypeptides. J. Am. Chem. Soc. 2001, 123, 12919–12920. [Google Scholar] [CrossRef]
- Borase, T.; Heise, A. Hybrid Nanomaterials by Surface Grafting of Synthetic Polypeptides Using N-Carboxyanhydride (NCA) Polymerization. Adv. Mater. 2016, 28, 5725–5731. [Google Scholar] [CrossRef]
- Cloutier, M.; Mantovani, D.; Rosei, F. Antibacterial coatings: Challenges, perspectives, and opportunities. Trends Biotechnol. 2015, 33, 637–652. [Google Scholar] [CrossRef]
- Dong, N.; Chou, S.; Li, J.; Xue, C.; Li, X.; Cheng, B.; Shan, A.; Xu, L. Short symmetric-end antimicrobial peptides centered on β-turn amino acids unit improve selectivity and stability. Front. Microbiol. 2018, 9, 2832. [Google Scholar] [CrossRef]
- Fjell, C.D.; Jenssen, H.; Hilpert, K.; Cheung, W.A.; Pante, N.; Hancock, R.E.; Cherkasov, A. Identification of novel antibacterial peptides by chemoinformatics and machine learning. J. Med. Chem. 2009, 52, 2006–2015. [Google Scholar] [CrossRef]
- Fjell, C.D.; Jenssen, H.; Cheung, W.A.; Hancock, R.E.; Cherkasov, A. Optimization of antibacterial peptides by genetic algorithms and cheminformatics. Chem. Biol. Drug Des. 2011, 77, 48–56. [Google Scholar] [CrossRef]
- Godballe, T.; Mojsoska, B.; Nielsen, H.M.; Jenssen, H. Antimicrobial activity of GN peptides and their mode of action. Biopolymers 2016, 106, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, X.; Wang, Z. APD3: The antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016, 44, D1087–D1093. [Google Scholar] [CrossRef] [PubMed]
- Stark, M.; Liu, L.-P.; Deber, C.M. Cationic hydrophobic peptides with antimicrobial activity. Antimicrob. Agents Chemother. 2002, 46, 3585–3590. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Guarnieri, M.T.; Vasil, A.I.; Vasil, M.L.; Mant, C.T.; Hodges, R.S. Role of peptide hydrophobicity in the mechanism of action of α-helical antimicrobial peptides. Antimicrob. Agents Chemother. 2007, 51, 1398–1406. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.M.; Edwards, M.A.; Li, J.; Yip, C.M.; Deber, C.M. Roles of hydrophobicity and charge distribution of cationic antimicrobial peptides in peptide-membrane interactions. J. Biol. Chem. 2012, 287, 7738–7745. [Google Scholar] [CrossRef]
- Hu, J.; Chen, C.; Zhang, S.; Zhao, X.; Xu, H.; Zhao, X.; Lu, J.R. Designed antimicrobial and antitumor peptides with high selectivity. Biomacromolecules 2011, 12, 3839–3843. [Google Scholar] [CrossRef]
- Chen, C.; Hu, J.; Yang, C.; Zhang, Y.; Wang, F.; Mu, Q.; Pan, F.; Xu, H.; Lu, J.R. Amino acid side chains affect the bioactivity of designed short peptide amphiphiles. J. Mater. Chem. B 2016, 4, 2359–2368. [Google Scholar] [CrossRef]
- Chen, C.; Yang, C.; Chen, Y.; Wang, F.; Mu, Q.; Zhang, J.; Li, Z.; Pan, F.; Xu, H.; Lu, J.R. Surface physical activity and hydrophobicity of designed helical peptide amphiphiles control their bioactivity and cell selectivity. ACS Appl. Mater. Interfaces 2016, 8, 26501–26510. [Google Scholar] [CrossRef]
- Grassi, L.; Batoni, G.; Ostyn, L.; Rigole, P.; Van Den Bossche, S.; Rinaldi, A.C.; Maisetta, G.; Esin, S.; Coenye, T.; Crabbé, A. The antimicrobial peptide lin-SB056-1 and its dendrimeric derivative prevent pseudomonas aeruginosabiofilm formation in physiologically relevant models of chronic infections. Front. Microbiol. 2019, 10, 198. [Google Scholar] [CrossRef]
- Meng, H.; Kumar, K. Antimicrobial activity and protease stability of peptides containing fluorinated amino acids. J. Am. Chem. Soc. 2007, 129, 15615–15622. [Google Scholar] [CrossRef]
- Zikou, S.; Koukkou, A.I.; Mastora, P.; Sakarellos-Daitsiotis, M.; Sakarellos, C.; Drainas, C.; Panou-Pomonis, E. Design and synthesis of cationic Aib-containing antimicrobial peptides: Conformational and biological studies. J. Pept. Sci. Off. Publ. Eur. Pept. Soc. 2007, 13, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Chen, X.; Falk, S.P.; Masters, K.S.; Weisblum, B.; Gellman, S.H. Nylon-3 polymers active against drug-resistant Candida albicans biofilms. J. Am. Chem. Soc. 2015, 137, 2183–2186. [Google Scholar] [CrossRef] [PubMed]
- Szkudlarek, M.; Heine, E.; Keul, H.; Beginn, U.; Möller, M. Synthesis, characterization, and antimicrobial properties of peptides mimicking copolymers of maleic anhydride and 4-methyl-1-pentene. Int. J. Mol. Sci. 2018, 19, 2617. [Google Scholar] [CrossRef] [PubMed]
- Bozelli, J.C.; Salay, L.C.; Arcisio-Miranda, M.; Procopio, J.; Riciluca, K.C.T.; Silva Junior, P.I.; Nakaie, C.R.; Schreier, S. A comparison of activity, toxicity, and conformation of tritrpticin and two TOAC-labeled analogues. Effects on the mechanism of action. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183110. [Google Scholar] [CrossRef]
- Arias, M.; Jensen, K.V.; Nguyen, L.T.; Storey, D.G.; Vogel, H.J. Hydroxy-tryptophan containing derivatives of tritrpticin: Modification of antimicrobial activity and membrane interactions. Biochim. Biophys. Acta Bba Biomembr. 2015, 1848, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Cirioni, O.; Giacometti, A.; Silvestri, C.; Della Vittoria, A.; Licci, A.; Riva, A.; Scalise, G. In vitro activities of tritrpticin alone and in combination with other antimicrobial agents against Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2006, 50, 3923–3925. [Google Scholar] [CrossRef]
- Lawyer, C.; Pai, S.; Watabe, M.; Borgia, P.; Mashimo, T.; Eagleton, L.; Watabe, K. Antimicrobial activity of a 13 amino acid tryptophan-rich peptide derived from a putative porcine precursor protein of a novel family of antibacterial peptides. FEBS Lett. 1996, 390, 95–98. [Google Scholar] [CrossRef]
- Nguyen, L.T.; de Boer, L.; Zaat, S.A.; Vogel, H.J. Investigating the cationic side chains of the antimicrobial peptide tritrpticin: Hydrogen bonding properties govern its membrane-disruptive activities. Biochim. Biophys. Acta BBA Biomembr. 2011, 1808, 2297–2303. [Google Scholar] [CrossRef]
- Schibli, D.J.; Nguyen, L.T.; Kernaghan, S.D.; Rekdal, Ø.; Vogel, H.J. Structure-function analysis of tritrpticin analogs: Potential relationships between antimicrobial activities, model membrane interactions, and their micelle-bound NMR structures. Biophys. J. 2006, 91, 4413–4426. [Google Scholar] [CrossRef]
- Yang, S.-T.; Shin, S.Y.; Kim, Y.-C.; Kim, Y.; Hahm, K.-S.; Kim, J.I. Conformation-dependent antibiotic activity of tritrpticin, a cathelicidin-derived antimicrobial peptide. Biochem. Biophys. Res. Commun. 2002, 296, 1044–1050. [Google Scholar] [CrossRef]
- Schibli, D.J.; Epand, R.F.; Vogel, H.J.; Epand, R.M. Tryptophan-rich antimicrobial peptides: Comparative properties and membrane interactions. Biochem. Cell Biol. 2002, 80, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Salay, L.C.; Procopio, J.; Oliveira, E.; Nakaie, C.R.; Schreier, S. Ion channel-like activity of the antimicrobial peptide tritrpticin in planar lipid bilayers. FEBS Lett. 2004, 565, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Ageitos, J.; Sánchez-Pérez, A.; Calo-Mata, P.; Villa, T. Antimicrobial peptides (AMPs): Ancient compounds that represent novel weapons in the fight against bacteria. Biochem. Pharmacol. 2017, 133, 117–138. [Google Scholar] [CrossRef] [PubMed]
- Oliva, R.; Chino, M.; Pane, K.; Pistorio, V.; De Santis, A.; Pizzo, E.; D’Errico, G.; Pavone, V.; Lombardi, A.; Del Vecchio, P.; et al. Exploring the role of unnatural amino acids in antimicrobial peptides. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Zhu, R.; Zhao, Y.; An, X.; Jia, F.; Peng, J.; Ma, Z.; Zhu, Y.; Wang, J.; Su, J.; et al. Antimicrobial activity and stability of protonectin with D-amino acid substitutions. J. Pept. Sci. 2017, 23, 392–402. [Google Scholar] [CrossRef]
- Mendes, M.A.; de Souza, B.M.; Marques, M.R.; Palma, M.S. Structural and biological characterization of two novel peptides from the venom of the neotropical social wasp Agelaia pallipes pallipes. Toxicon 2004, 44, 67–74. [Google Scholar] [CrossRef]
- Wang, K.; Dang, W.; Yan, J.; Chen, R.; Liu, X.; Yan, W.; Zhang, B.; Xie, J.; Zhang, J.; Wang, R. Membrane perturbation action mode and structure-activity relationships of Protonectin, a novel antimicrobial peptide from the venom of the neotropical social wasp Agelaia pallipes pallipes. Antimicrob. Agents Chemother. 2013, 57, 4632–4639. [Google Scholar] [CrossRef]
- Wang, K.; Dang, W.; Xie, J.; Zhu, R.; Sun, M.; Jia, F.; Zhao, Y.; An, X.; Qiu, S.; Li, X. Antimicrobial peptide protonectin disturbs the membrane integrity and induces ROS production in yeast cells. Biochim. Biophys. Acta Bba Biomembr. 2015, 1848, 2365–2373. [Google Scholar] [CrossRef]
- Patch, J.A.; Barron, A.E. Mimicry of bioactive peptides via non-natural, sequence-specific peptidomimetic oligomers. Curr. Opin. Chem. Biol. 2002, 6, 872–877. [Google Scholar] [CrossRef]
- Cheng, R.P.; Gellman, S.H.; DeGrado, W.F. β-Peptides: From structure to function. Chem. Rev. 2001, 101, 3219–3232. [Google Scholar] [CrossRef]
- Laursen, J.S.; Engel-Andreasen, J.; Olsen, C.A. β-Peptoid Foldamers at Last. Acc. Chem. Res. 2015, 48, 2696–2704. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, Y.-D.; Yang, D. α-Aminoxy acids: New possibilities from foldamers to anion receptors and channels. Acc. Chem. Res. 2008, 41, 1428–1438. [Google Scholar] [CrossRef] [PubMed]
- Horne, W.S.; Johnson, L.M.; Ketas, T.J.; Klasse, P.J.; Lu, M.; Moore, J.P.; Gellman, S.H. Structural and biological mimicry of protein surface recognition by α/β-peptide foldamers. Proc. Natl. Acad. Sci. USA 2009, 106, 14751–14756. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Song, J.-W.; Choi, Y.-S.; Park, H.-M.; Lee, K.-B. A theoretical study of conformational properties of N-methyl azapeptide derivatives. J. Am. Chem. Soc. 2002, 124, 11881–11893. [Google Scholar] [CrossRef]
- Claudon, P.; Violette, A.; Lamour, K.; Decossas, M.; Fournel, S.; Heurtault, B.; Godet, J.; Mély, Y.; Jamart-Grégoire, B.; Averlant-Petit, M.C. Consequences of isostructural main-chain modifications for the design of antimicrobial foldamers: Helical mimics of host-defense peptides based on a heterogeneous amide/urea backbone. Angew. Chem. Int. Ed. 2010, 49, 333–336. [Google Scholar] [CrossRef]
- Chandramouli, N.; Ferrand, Y.; Lautrette, G.; Kauffmann, B.; Mackereth, C.D.; Laguerre, M.; Dubreuil, D.; Huc, I. Iterative design of a helically folded aromatic oligoamide sequence for the selective encapsulation of fructose. Nat. Chem. 2015, 7, 334–341. [Google Scholar] [CrossRef]
- Wu, H.; Qiao, Q.; Hu, Y.; Teng, P.; Gao, W.; Zuo, X.; Wojtas, L.; Larsen, R.W.; Ma, S.; Cai, J. Sulfono-γ-AApeptides as a New Class of Nonnatural Helical Foldamer. Chem. A Eur. J. 2015, 21, 2501–2507. [Google Scholar] [CrossRef]
- Li, Y.; Wu, H.; Teng, P.; Bai, G.; Lin, X.; Zuo, X.; Cao, C.; Cai, J. Helical antimicrobial sulfono-γ-AApeptides. J. Med. Chem. 2015, 58, 4802–4811. [Google Scholar] [CrossRef]
- Anacona, J.R.; Rodriguez, A. Synthesis and antibacterial activity of ceftriaxone metal complexes. Transit. Met. Chem. 2005, 30, 897–901. [Google Scholar] [CrossRef]
- Chohan, Z.H.; Supuran, C.T.; Scozzafava, A. Metalloantibiotics: Synthesis and antibacterial activity of cobalt (II), copper (II), nickel (II) and zinc (II) complexes of kefzol. J. Enzym. Inhib. Med. Chem. 2004, 19, 79–84. [Google Scholar] [CrossRef]
- Anacona, J.R.; Osorio, I. Synthesis and antibacterial activity of copper (II) complexes with sulphathiazole and cephalosporin ligands. Transit. Met. Chem. 2008, 33, 517–521. [Google Scholar] [CrossRef]
- Möhler, J.S.; Kolmar, T.; Synnatschke, K.; Hergert, M.; Wilson, L.A.; Ramu, S.; Elliott, A.G.; Blaskovich, M.A.; Sidjabat, H.E.; Paterson, D.L. Enhancement of antibiotic-activity through complexation with metal ions-Combined ITC, NMR, enzymatic and biological studies. J. Inorg. Biochem. 2017, 167, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Medici, S.; Peana, M.; Crisponi, G.; Nurchi, V.M.; Lachowicz, J.I.; Remelli, M.; Zoroddu, M.A. Silver coordination compounds: A new horizon in medicine. Coord. Chem. Rev. 2016, 327, 349–359. [Google Scholar] [CrossRef]
- Waldron, K.J.; Robinson, N.J. How do bacterial cells ensure that metalloproteins get the correct metal? Nat. Rev. Microbiol. 2009, 7, 25–35. [Google Scholar] [CrossRef]
- Łoboda, D.; Kozłowski, H.; Rowińska-Żyrek, M. Antimicrobial peptide–metal ion interactions–a potential way of activity enhancement. New J. Chem. 2018, 42, 7560–7568. [Google Scholar] [CrossRef]
- Silva, F.D.; Rezende, C.A.; Rossi, D.C.; Esteves, E.; Dyszy, F.H.; Schreier, S.; Gueiros-Filho, F.; Campos, C.B.; Pires, J.R.; Daffre, S. Structure and mode of action of microplusin, a copper II-chelating antimicrobial peptide from the cattle tick Rhipicephalus (Boophilus) microplus. J. Biol. Chem. 2009, 284, 34735–34746. [Google Scholar] [CrossRef]
- Jeong, S.-H.; Song, Y.-K.; Cho, J.-H. Risk assessment of ciprofloxacin, flavomycin, olaquindox and colistin sulfate based on microbiological impact on human gut biota. Regul. Toxicol. Pharmacol. 2009, 53, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Cole, A.M.; Kim, Y.-H.; Tahk, S.; Hong, T.; Weis, P.; Waring, A.J.; Ganz, T. Calcitermin, a novel antimicrobial peptide isolated from human airway secretions. FEBS Lett. 2001, 504, 5–10. [Google Scholar] [CrossRef]
- Wrzesinski, J.; Błaszczyk, L.; Wrońska, M.; Kasprowicz, A.; Stokowa-Sołtys, K.; Nagaj, J.; Szafraniec, M.; Kulinski, T.; Jeżowska-Bojczuk, M.; Ciesiołka, J. Mapping the interactions of selected antibiotics and their C u2+ complexes with the antigenomic delta ribozyme. FEBS J. 2013, 280, 2652–2664. [Google Scholar] [CrossRef]
- Oppenheim, F.; Xu, T.; McMillian, F.; Levitz, S.; Diamond, R.; Offner, G.; Troxler, R. Histatins, a novel family of histidine-rich proteins in human parotid secretion. Isolation, characterization, primary structure, and fungistatic effects on Candida albicans. J. Biol. Chem. 1988, 263, 7472–7477. [Google Scholar]
- Al-Harthi, S.; Lachowicz, J.I.; Nowakowski, M.E.; Jaremko, M.; Jaremko, Ł. Towards the functional high-resolution coordination chemistry of blood plasma human serum albumin. J. Inorg. Biochem. 2019, 198, 110716. [Google Scholar] [CrossRef] [PubMed]
- Jeżowska-Bojczuk, M.; Stokowa-Sołtys, K. Peptides having antimicrobial activity and their complexes with transition metal ions. Eur. J. Med. Chem. 2018, 143, 997–1009. [Google Scholar] [CrossRef] [PubMed]
- Agbale, C.M.; Sarfo, J.K.; Galyuon, I.K.; Juliano, S.A.; Silva, G.G.; Buccini, D.F.; Cardoso, M.H.; Torres, M.D.; Angeles-Boza, A.M.; de la Fuente-Nunez, C. Antimicrobial and Antibiofilm Activities of Helical Antimicrobial Peptide Sequences Incorporating Metal-Binding Motifs. Biochemistry 2019, 58, 3802–3812. [Google Scholar] [CrossRef] [PubMed]
- Harford, C.; Sarkar, B. Amino terminal Cu (II)-and Ni (II)-binding (ATCUN) motif of proteins and peptides: Metal binding, DNA cleavage, and other properties. Acc. Chem. Res. 1997, 30, 123–130. [Google Scholar] [CrossRef]
- Mujika, J.; Dalla Torre, G.; Lachowicz, J.; Lopez, X. In silico design of mimosine containing peptides as new efficient chelators of aluminum. RSC Adv. 2019, 9, 7688–7697. [Google Scholar] [CrossRef]
- Perez, R.H.; Zendo, T.; Sonomoto, K. Circular and leaderless bacteriocins: Biosynthesis, mode of action, applications, and prospects. Front. Microbiol. 2018, 9, 2085. [Google Scholar] [CrossRef]
- Cotter, P.D.; Ross, R.P.; Hill, C. Bacteriocins—A viable alternative to antibiotics? Nat. Rev. Microbiol. 2013, 11, 95–105. [Google Scholar] [CrossRef]
- Drider, D.; Bendali, F.; Naghmouchi, K.; Chikindas, M.L. Bacteriocins: Not only antibacterial agents. Probiotics Antimicrob. Proteins 2016, 8, 177–182. [Google Scholar] [CrossRef]
- Sahl, H.-G.; Bierbaum, G. Lantibiotics: Biosynthesis and biological activities of uniquely modified peptides from gram-positive bacteria. Annu. Rev. Microbiol. 1998, 52, 41–79. [Google Scholar] [CrossRef]
- Cotter, P.D.; Hill, C.; Ross, R.P. Bacteriocins: Developing innate immunity for food. Nat. Rev. Microbiol. 2005, 3, 777–788. [Google Scholar] [CrossRef]
- Cleveland, J.; Montville, T.J.; Nes, I.F.; Chikindas, M.L. Bacteriocins: Safe, natural antimicrobials for food preservation. Int. J. Food Microbiol. 2001, 71, 1–20. [Google Scholar] [CrossRef]
- Héchard, Y.; Sahl, H.-G. Mode of action of modified and unmodified bacteriocins from Gram-positive bacteria. Biochimie 2002, 84, 545–557. [Google Scholar] [CrossRef]
- Klaenhammer, T.R. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol. Rev. 1993, 12, 39–85. [Google Scholar] [CrossRef]
- Carolissen-Mackay, V.; Arendse, G.; Hastings, J.W. Purification of bacteriocins of lactic acid bacteria: Problems and pointers. Int. J. Food Microbiol. 1997, 34, 1–16. [Google Scholar] [CrossRef]
- Galvez, A.; Burgos, M.; Lopez, R.; Pulido, R. Natural antimicrobials for food biopreservation. In Food Biopreservation; Springer: New York, NY, USA; Heidelberg, Germany; Dordrecht, The Netherland; London, UK, 2014; pp. 3–11. [Google Scholar]
- Prudêncio, C.V.; Dos Santos, M.T.; Vanetti, M.C.D. Strategies for the use of bacteriocins in Gram-negative bacteria: Relevance in food microbiology. J. Food Sci. Technol. 2015, 52, 5408–5417. [Google Scholar] [CrossRef]
- Pisano, M.B.; Fadda, M.E.; Melis, R.; Ciusa, M.L.; Viale, S.; Deplano, M.; Cosentino, S. Molecular identification of bacteriocins produced by Lactococcus lactis dairy strains and their technological and genotypic characterization. Food Control 2015, 51, 1–8. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).