RAGE Mediates Cholesterol Efflux Impairment in Macrophages Caused by Human Advanced Glycated Albumin
Abstract
1. Introduction
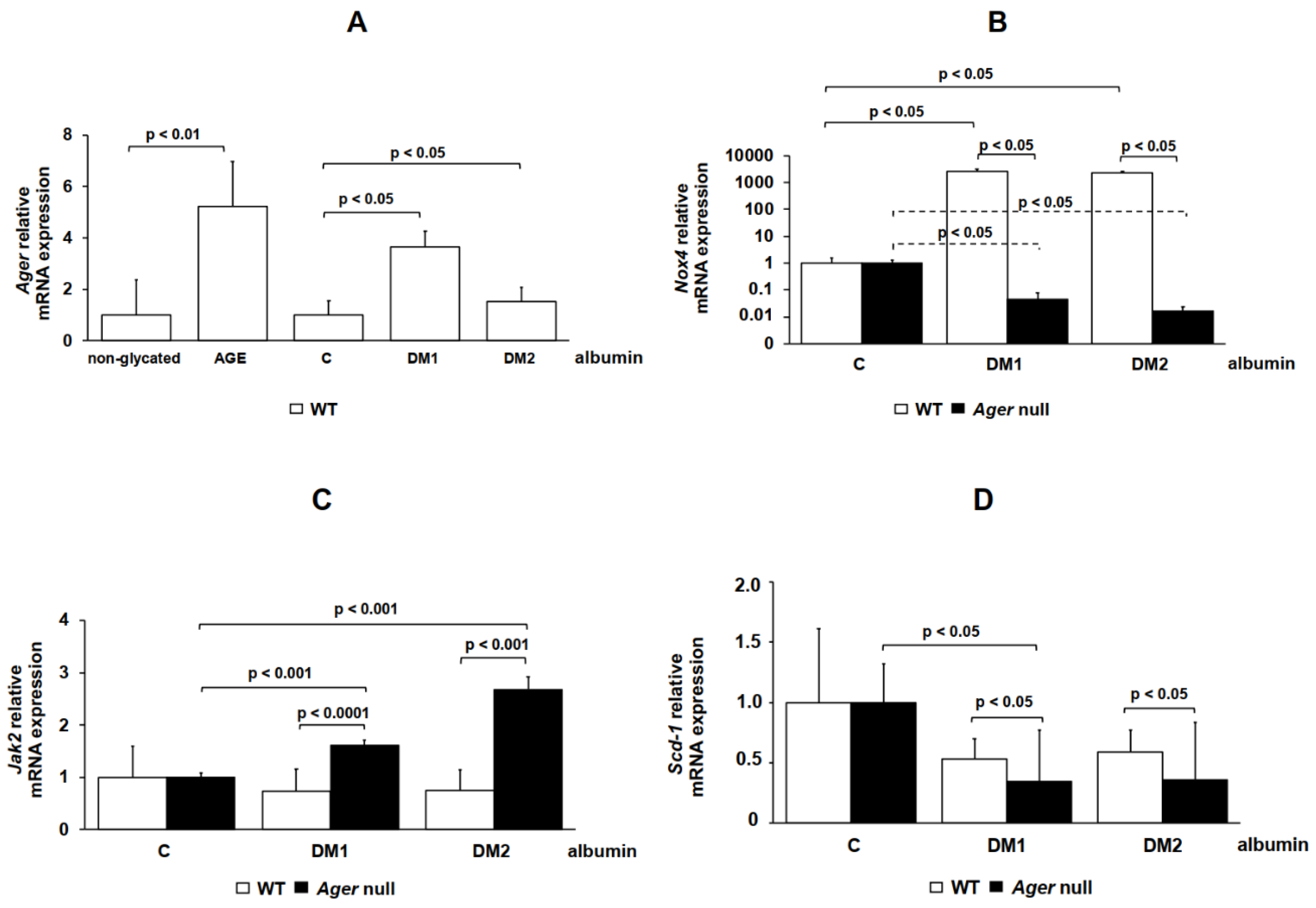
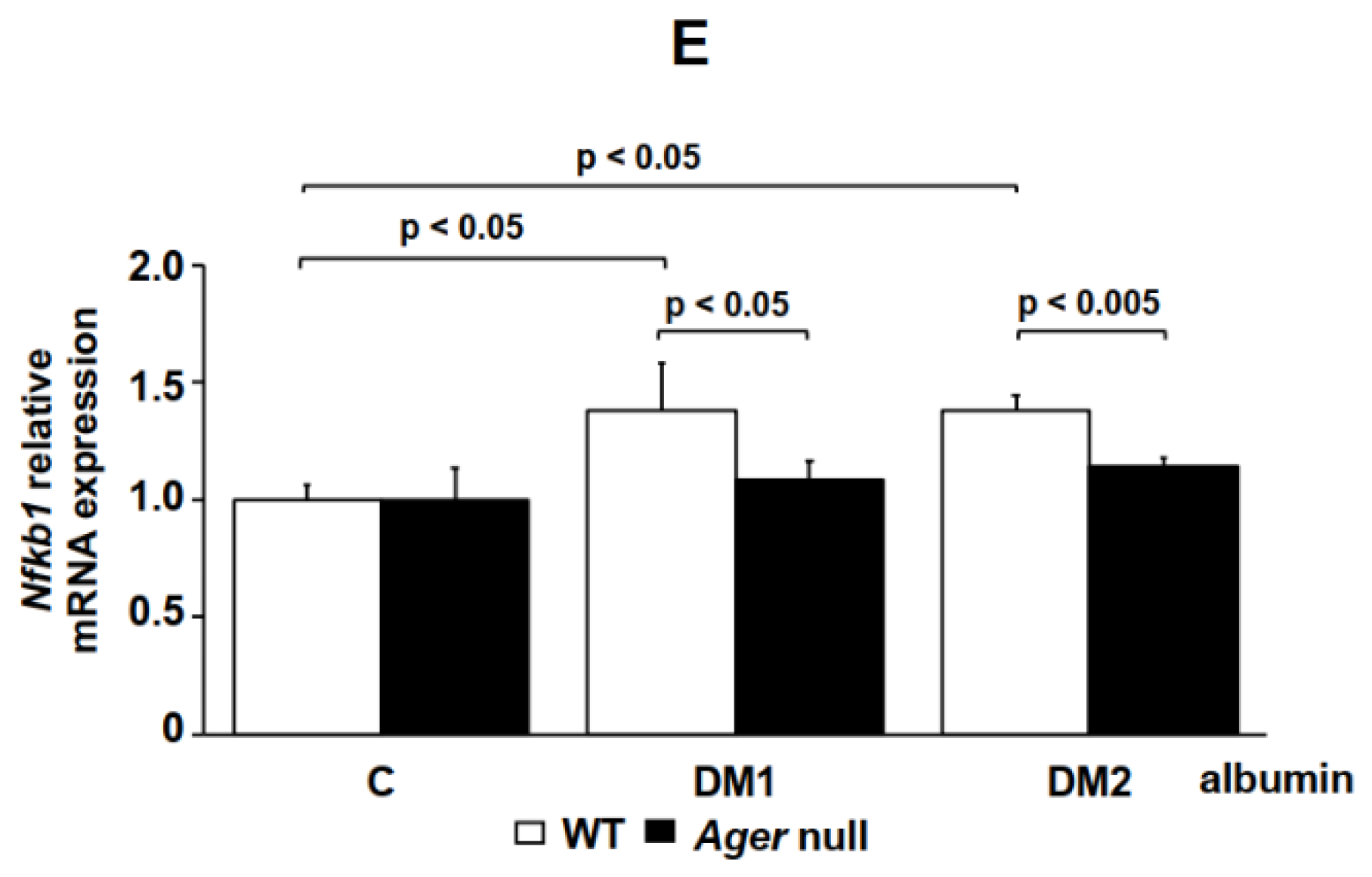
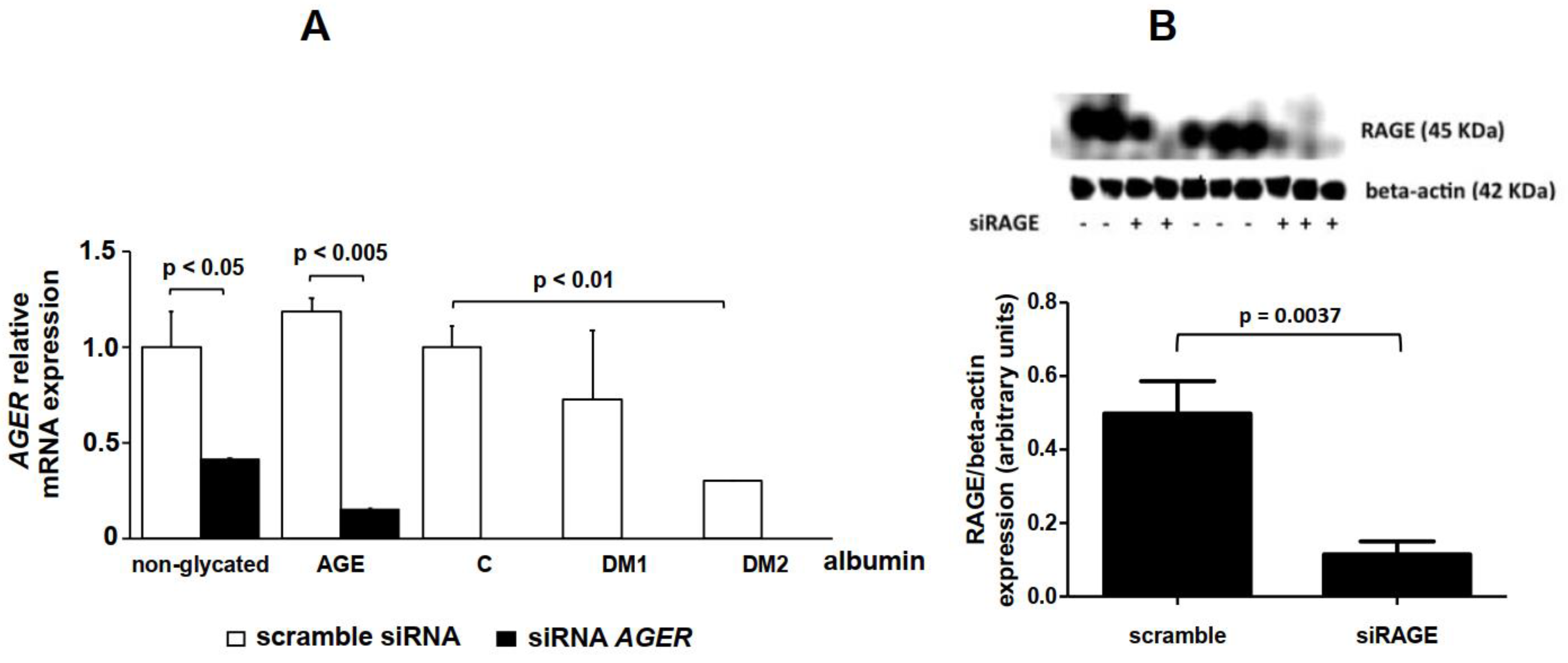
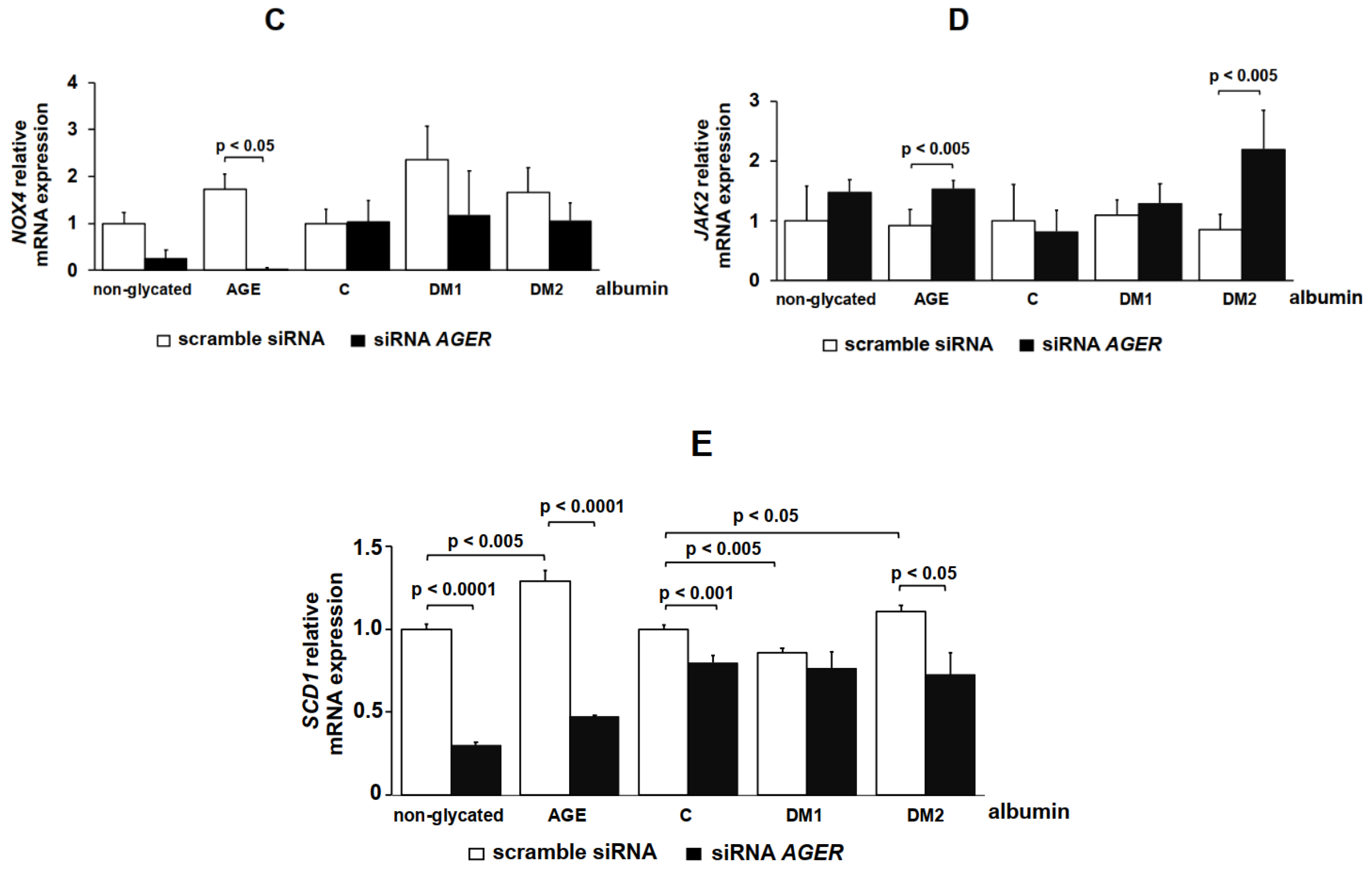
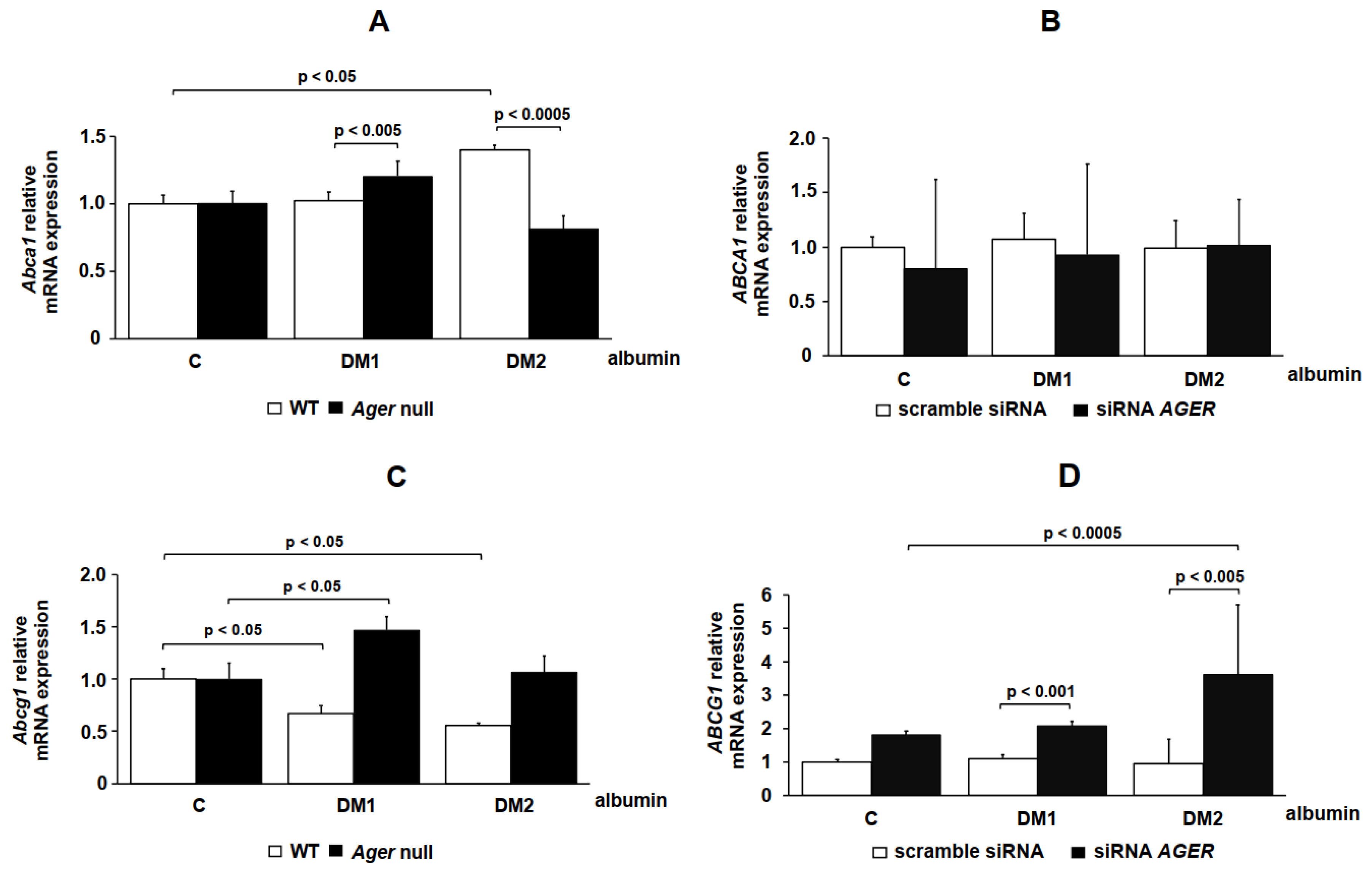
2. Results
3. Discussion
4. Materials and Methods
4.1. Isolation and Purification of Serum Albumin
4.2. In Vitro Advanced Glycation of Human Albumin
4.3. Total AGE Measurement
4.4. Isolation of Plasma Lipoprotein
4.5. L929 Cell Culture
4.6. Isolation of Mouse Bone Marrow Cells
4.7. Cholesterol Efflux Assay
4.8. Intracellular Lipid Staining
4.9. AGER siRNA
4.10. Real-Time Quantitative PCR
4.11. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABCA1 | ATP-binding cassette transporter A1 |
| ABCG1 | ATP-binding cassette transporter G1 |
| AGEs | Advanced glycation end products |
| AGE albumin | glycated albumin |
| BMDM | bone marrow-derived macrophages |
| CSF-1 | colony-stimulating factor-1 |
| C | non-DM subjects |
| DM | diabetes mellitus |
| DM1 | type 1 diabetic subjects |
| DM2 | type 2 diabetic subjects |
| FAFA | fatty acid free albumin |
| HMBG1 | high-mobility group protein 1 |
| IPO8 | importin 8 |
| Jak2 | janus kinase 2 |
| NFKB1 | NF-κB |
| Nox4 | NADPH oxidase 4 |
| RAGE, AGER gene | receptor for AGE |
| RCT | reverse cholesterol transport |
| Scd1 | stearoyl-Coenzyme A desaturase 1 |
| WT | wild-type |
References
- Khera, A.V.; Cuchel, M.; De La Llera-Moya, M.; Rodrigues, A.; Burke, M.F.; Jafri, K.; French, B.C.; Phillips, J.A.; Mucksavage, M.L.; Wilensky, R.L.; et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N. Engl. J. Med. 2011, 364, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Rosenson, R.S.; Brewer, H.B., Jr.; Davidson, W.S.; Fayad, Z.A.; Fuster, V.; Goldstein, J.; Hellerstein, M.; Jiang, X.C.; Phillips, M.C.; Rader, D.J.; et al. Cholesterol efflux and atheroprotection: Advancing the concept of reverse cholesterol transport. Circulation 2012, 125, 1905–1919. [Google Scholar] [CrossRef] [PubMed]
- Vlassara, H.; Uribarri, J. Advanced glycation end products (AGE) and diabetes: Cause, effect, or both? Curr. Diabetes Rep. 2014, 14, 453. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.P.; Pinto, R.S.; Moyses, Z.P.; Nakandakare, E.R.; Quintao, E.C.; PASSARELLI, M. Aminoguanidine and metformin prevent the reduced rate of HDL-mediated cell cholesterol efflux induced by formation of advanced glycation end products. Int. J. Biochem. Cell Biol. 2006, 38, 392–403. [Google Scholar] [CrossRef]
- Daffu, G.; Shen, X.; Senatus, L.; Thiagarajan, D.; Abedini, A.; Hurtado Del Pozo, C.; Rosario, R.; Song, F.; Friedman, R.A.; Ramasamy, R.; et al. RAGE Suppresses ABCG1-Mediated Macrophage Cholesterol Efflux in Diabetes. Diabetes 2015, 64, 4046–4060. [Google Scholar] [CrossRef] [PubMed]
- Okuda, L.S.; Castilho, G.; Rocco, D.D.; Nakandakare, E.R.; Catanozi, S.; Passarelli, M. Advanced glycated albumin impairs HDL anti-inflammatory activity and primes macrophages for inflammatory response that reduces reverse cholesterol transport. Biochim. Biophys. Acta 2012, 1821, 1485–1492. [Google Scholar] [CrossRef]
- Castilho, G.; Sartori, C.H.; Machado-Lima, A.; Nakandakare, E.R.; Correa-Giannella, M.L.; Roverso, M.; Porcu, S.; Lapolla, A.; Traldi, P.; Passarelli, M. Glycated human serum albumin isolated from poorly controlled diabetic patients impairs cholesterol efflux from macrophages: An investigation by mass spectrometry. Eur. J. Mass Spectrom. 2015, 21, 233–244. [Google Scholar] [CrossRef]
- Isoda, K.; Folco, E.J.; Shimizu, K.; Libby, P. AGE-BSA decreases ABCG1 expression and reduces macrophage cholesterol efflux to HDL. Atherosclerosis 2007, 192, 298–304. [Google Scholar] [CrossRef]
- Dattilo, B.M.; Fritz, G.; Leclerc, E.; Kooi, C.W.; Heizmann, C.W.; Chazin, W.J. The extracellular region of the receptor for advanced glycation end products is composed of two independent structural units. Biochemistry 2007, 46, 6957–6970. [Google Scholar] [CrossRef]
- Schmidt, A.M.; Yan, S.D.; Wautier, J.L.; Stern, D. Activation of receptor for advanced glycation end products: A mechanism for chronic vascular dysfunction in diabetic vasculopathy and atherosclerosis. Circ. Res. 1999, 84, 489–497. [Google Scholar] [CrossRef]
- Kawai, T.; Kamide, K.; Ito, N.; Onishi, M.; Oguro, R.; Takeya, Y.; Tatara, Y.; Maekawa, Y.; Katsuya, T.; Ohishi, M.; et al. 374 T/A polymorphism in RAGE gene is associated with onset of diabetes mellitus, atherosclerosis, and renal dysfunction in patients with hypertension. Clin. Exp. Hypertens. 2013, 35, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Park, L.; Raman, K.G.; Lee, K.J.; Lu, Y.; Ferran, L.J., Jr.; Chow, W.S.; Stern, D.; Schmidt, A.M. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat. Med. 1998, 4, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Li, S.H.; Liu, S.M.; Szmitko, P.E.; He, X.Q.; Fedak, P.W.; Verma, S. C-Reactive protein upregulates receptor for advanced glycation end products expression in human endothelial cells. Hypertension 2006, 48, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Grimm, S.; Ott, C.; Horlacher, M.; Weber, D.; Hohn, A.; Grune, T. Advanced-glycation-end-product-induced formation of immunoproteasomes: Involvement of RAGE and Jak2/STAT1. Biochem. J. 2012, 448, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Machado-Lima, A.; Iborra, R.T.; Pinto, R.S.; Sartori, C.H.; Oliveira, E.R.; Nakandakare, E.R.; Stefano, J.T.; Giannella-Neto, D.; Correa-Giannella, M.L.; Passarelli, M. Advanced glycated albumin isolated from poorly controlled type 1 diabetes mellitus patients alters macrophage gene expression impairing ABCA-1-mediated reverse cholesterol transport. Diabetes Metab. Res. Rev. 2013, 29, 66–76. [Google Scholar] [CrossRef]
- Machado-Lima, A.; Iborra, R.T.; Pinto, R.S.; Castilho, G.; Sartori, C.H.; Oliveira, E.R.; Okuda, L.S.; Nakandakare, E.R.; Giannella-Neto, D.; Machado, U.F.; et al. In type 2 diabetes mellitus glycated albumin alters macrophage gene expression impairing ABCA1-mediated cholesterol efflux. J. Cell. Physiol. 2015, 230, 1250–1257. [Google Scholar] [CrossRef]
- Selvin, E.; Rawlings, A.M.; Lutsey, P.L.; Maruthur, N.; Pankow, J.S.; Steffes, M.; Coresh, J. Fructosamine and Glycated Albumin and the Risk of Cardiovascular Outcomes and Death. Circulation 2015, 132, 269–277. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, H.; Schmidt, A.M.; Zhang, C. AGE/RAGE produces endothelial dysfunction in coronary arterioles in type 2 diabetic mice. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H491–H498. [Google Scholar] [CrossRef]
- Ramasamy, R.; Shekhtman, A.; Schmidt, A.M. The multiple faces of RAGE—Opportunities for therapeutic intervention in aging and chronic disease. Expert Opin. Ther. Targets 2016, 20, 431–446. [Google Scholar] [CrossRef]
- Myoishi, M.; Hao, H.; Minamino, T.; Watanabe, K.; Nishihira, K.; Hatakeyama, K.; Asada, Y.; Okada, K.; Ishibashi-Ueda, H.; Gabbiani, G.; et al. Increased endoplasmic reticulum stress in atherosclerotic plaques associated with acute coronary syndrome. Circulation 2007, 116, 1226–1233. [Google Scholar] [CrossRef]
- Iborra, R.T.; Machado-Lima, A.; Castilho, G.; Nunes, V.S.; Abdalla, D.S.; Nakandakare, E.R.; Passarelli, M. Advanced glycation in macrophages induces intracellular accumulation of 7-ketocholesterol and total sterols by decreasing the expression of ABCA-1 and ABCG-1. Lipids Health Dis. 2011, 10, 172. [Google Scholar] [CrossRef]
- Castilho, G.; Okuda, L.S.; Pinto, R.S.; Iborra, R.T.; Nakandakare, E.R.; Santos, C.X.; Laurindo, F.R.; Passarelli, M. ER stress is associated with reduced ABCA-1 protein levels in macrophages treated with advanced glycated albumin-reversal by a chemical chaperone. Int. J. Biochem. Cell Biol. 2012, 44, 1078–1086. [Google Scholar] [CrossRef]
- Iborra, R.T.; Machado-Lima, A.; Okuda, L.S.; Pinto, P.R.; Nakandakare, E.R.; Machado, U.F.; Correa-Giannella, M.L.; Pickford, R.; Woods, T.; Brimble, M.A.; et al. AGE-albumin enhances ABCA1 degradation by ubiquitin-proteasome and lysosomal pathways in macrophages. J. Diabetes Complicat. 2018, 32, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.K.; Shen, Y.; Shen, W.F.; Pu, L.J.; Meng, H.; Zhang, R.Y.; Zhang, Q.; Chen, Q.J.; De Caterina, R.; Lu, L. Elevated glycated albumin and reduced endogenous secretory receptor for advanced glycation endproducts levels in serum predict major adverse cardio-cerebral events in patients with type 2 diabetes and stable coronary artery disease. Int. J. Cardiol. 2015, 197, 241–247. [Google Scholar] [CrossRef]
- Di Pino, A.; Urbano, F.; Zagami, R.M.; Filippello, A.; Di Mauro, S.; Piro, S.; Purrello, F.; Rabuazzo, A.M. Low Endogenous Secretory Receptor for Advanced Glycation End-Products Levels Are Associated With Inflammation and Carotid Atherosclerosis in Prediabetes. J. Clin. Endocrinol. Metab. 2016, 101, 1701–1709. [Google Scholar] [CrossRef] [PubMed]
- Heier, M.; Margeirsdottir, H.D.; Gaarder, M.; Stensaeth, K.H.; Brunborg, C.; Torjesen, P.A.; Seljeflot, I.; Hanssen, K.F.; Dahl-Jorgensen, K. Soluble RAGE and atherosclerosis in youth with type 1 diabetes: A 5-year follow-up study. Cardiovasc. Diabetol. 2015, 14, 126. [Google Scholar] [CrossRef]
- Tang, C.; Vaughan, A.M.; Oram, J.F. Janus kinase 2 modulates the apolipoprotein interactions with ABCA1 required for removing cellular cholesterol. J. Biol. Chem. 2004, 279, 7622–7628. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.S.; Guh, J.Y.; Chen, H.C.; Hung, W.C.; Lai, Y.H.; Chuang, L.Y. Role of receptor for advanced glycation end-product (RAGE) and the JAK/STAT-signaling pathway in AGE-induced collagen production in NRK-49F cells. J. Cell. Biochem. 2001, 81, 102–113. [Google Scholar] [CrossRef]
- Shaw, S.S.; Schmidt, A.M.; Banes, A.K.; Wang, X.; Stern, D.M.; Marrero, M.B. S100B-RAGE-mediated augmentation of angiotensin II-induced activation of JAK2 in vascular smooth muscle cells is dependent on PLD2. Diabetes 2003, 52, 2381–2388. [Google Scholar] [CrossRef][Green Version]
- Wautier, M.P.; Chappey, O.; Corda, S.; Stern, D.M.; Schmidt, A.M.; Wautier, J.L. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E685–E694. [Google Scholar] [CrossRef]
- Yeh, C.H.; Sturgis, L.; Haidacher, J.; Zhang, X.N.; Sherwood, S.J.; Bjercke, R.J.; Juhasz, O.; Crow, M.T.; Tilton, R.G.; Denner, L. Requirement for p38 and p44/p42 mitogen-activated protein kinases in RAGE-mediated nuclear factor-kappaB transcriptional activation and cytokine secretion. Diabetes 2001, 50, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Hao, M.; Luo, Y.; Liang, C.P.; Silver, D.L.; Cheng, C.; Maxfield, F.R.; Tall, A.R. Stearoyl-CoA desaturase inhibits ATP-binding cassette transporter A1-mediated cholesterol efflux and modulates membrane domain structure. J. Biol. Chem. 2003, 278, 5813–5820. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kurdi-Haidar, B.; Oram, J.F. LXR-mediated activation of macrophage stearoyl-CoA desaturase generates unsaturated fatty acids that destabilize ABCA1. J. Lipid Res. 2004, 45, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, R.; Yokoyama, S. Helical apolipoproteins stabilize ATP-binding cassette transporter A1 by protecting it from thiol protease-mediated degradation. J. Biol. Chem. 2002, 277, 22426–22429. [Google Scholar] [CrossRef]
- Yokoyama, S.; Arakawa, R.; Wu, C.A.; Iwamoto, N.; Lu, R.; Tsujita, M.; Abe-Dohmae, S. Calpain-mediated ABCA1 degradation: Post-translational regulation of ABCA1 for HDL biogenesis. Biochim. Biophys. Acta 2012, 1821, 547–551. [Google Scholar] [CrossRef]
- Machado, J.T.; Iborra, R.T.; Fusco, F.B.; Castilho, G.; Pinto, R.S.; Machado-Lima, A.; Nakandakare, E.R.; Seguro, A.C.; Shimizu, M.H.; Catanozi, S.; et al. N-acetylcysteine prevents endoplasmic reticulum stress elicited in macrophages by serum albumin drawn from chronic kidney disease rats and selectively affects lipid transporters, ABCA-1 and ABCG-1. Atherosclerosis 2014, 237, 343–352. [Google Scholar] [CrossRef]
- Belmokhtar, K.; Robert, T.; Ortillon, J.; Braconnier, A.; Vuiblet, V.; Boulagnon-Rombi, C.; Diebold, M.D.; Pietrement, C.; Schmidt, A.M.; Rieu, P.; et al. Signaling of Serum Amyloid A Through Receptor for Advanced Glycation End Products as a Possible Mechanism for Uremia-Related Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 800–809. [Google Scholar] [CrossRef]
- Basu, S.K.; Goldstein, J.L.; Anderson, G.W.; Brown, M.S. Degradation of cationized low density lipoprotein and regulation of cholesterol metabolism in homozygous familial hypercholesterolemia fibroblasts. Proc. Natl. Acad. Sci. USA 1976, 73, 3178–3182. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 1193, 265–275. [Google Scholar]
- Englen, M.D.; Valdez, Y.E.; Lehnert, N.M.; Lehnert, B.E. Granulocyte/macrophage colony-stimulating factor is expressed and secreted in cultures of murine L929 cells. J. Immunol. Methods 1995, 184, 281–283. [Google Scholar] [CrossRef]
- Bu, D.X.; Rai, V.; Shen, X.; Rosario, R.; Lu, Y.; D’agati, V.; Yan, S.F.; Friedman, R.A.; Nuglozeh, E.; Schmidt, A.M. Activation of the ROCK1 branch of the transforming growth factor-beta pathway contributes to RAGE-dependent acceleration of atherosclerosis in diabetic ApoE-null mice. Circ. Res. 2010, 106, 1040–1051. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Mueller, R. Real-time reverse transcription PCR (qRT-PCR) and its potential use in clinical diagnosis. Clin. Sci. 2005, 109, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Kubista, M.; Andrade, J.M.; Bengtsson, M.; Forootan, A.; Jonak, J.; Lind, K.; Sindelka, R.; Sjoback, R.; Sjogreen, B.; Strombom, L.; et al. The real-time polymerase chain reaction. Mol. Aspects Med. 2006, 27, 95–125. [Google Scholar] [CrossRef] [PubMed]


| Control (n = 7) | DM 1 (n = 7) | DM 2 (n = 9) | |
|---|---|---|---|
| Female/Male | 5/2 | 5/2 | 6/3 |
| Age (years) | 28 ± 2 | 26 ± 3 | 63 ± 3 ** |
| Weight (kg) | 74.9 ± 8.5 | 64.3 ± 4 | 73.9 ± 4.2 |
| BMI (kg/m2) | 25.5 ± 1.8 | 23 ± 1.2 | 30.1 ± 1.7 |
| Duration of DM (years) | - | 14 ± 2 | 14 ± 2 |
| Total cholesterol (mg/dL) | 170 ± 8 | 156 ± 7 | 187 ± 8 |
| HDL-c (mg/dL) | 63 ± 7 | 55 ± 4 | 55 ± 10 |
| LDL-c (mg/dL) | 87 ± 7 | 85 ± 8 | 98 ± 5 |
| Triglycerides (mg/dL) | 103 ± 16 | 80 ± 14 | 197 ± 37 * |
| Urinary albumin (mg/dL) | 5.6 ± 1.2 | 13.5 ± 3.5 | 10.9 ± 2.3 |
| Glucose (mg/dL) | 80 ± 2 | 170 ± 39 * | 192 ± 25 * |
| HbA1c (%) | 5.3 ± 0.1 | 9.6 ± 0.4 ** | 10.2 ± 0.4 ** |
| Fructosamine (μmol/L) | 245 ± 11.7 | 433 ± 34 ** | 351 ± 10.7 ** |
| Total AGE (mU AGE/mg of albumin) | 12.7 ± 1.5 | 38.5 ± 1.4 ** | 35.6 ± 0.6 ** |
| GENE | Human ID | Mouse ID |
|---|---|---|
| AGER (RAGE) | Hs00153957_m1 (FAM 75 μL 20×) | Mm01134790_g1 (FAM 75 μL 20×) |
| NOX4 (NADPH oxidase 4) | Hs00418356_m1 (FAM 75 μL 20×) | Mm00479246_m1 (FAM 75 μL 20×) |
| JAK2 (Janus kinase 2) | Hs00234567_m1 (FAM 75 μL 20×) | Mm01208489_m1 (FAM 75 μL 20×) |
| SCD1 (Stearoyl-CoA desaturase-1) | Hs01682761_m1 (FAM 75 μL 20×) | Mm00772290_m1 (FAM 75 μL 20×) |
| NFKB1 (NF-kappaB) | Hs00765730_m1 (FAM 75 μL 20×) | Mm00476361_m1 (FAM 75 μL 20×) |
| ABCA1 (ATP binding cassette subfamily A member 1) | Hs01059118_m1 (FAM 75 μL 20×) | Mm00442646_m1 (FAM 75 μL 20×) |
| ABCG1 (ATP binding cassette subfamily G member 1) | Hs00245154_m1 (FAM 75 μL 20×) | Mm00437390_m1 (FAM 75 μL 20×) |
| IPO8 (Importin 8) | Hs00183533_m1 (VIC 75 μL 20×) | Mm01255158_m1 (VIC 75 μL 20×) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado-Lima, A.; López-Díez, R.; Iborra, R.T.; Pinto, R.d.S.; Daffu, G.; Shen, X.; Nakandakare, E.R.; Machado, U.F.; Corrêa-Giannella, M.L.C.; Schmidt, A.M.; et al. RAGE Mediates Cholesterol Efflux Impairment in Macrophages Caused by Human Advanced Glycated Albumin. Int. J. Mol. Sci. 2020, 21, 7265. https://doi.org/10.3390/ijms21197265
Machado-Lima A, López-Díez R, Iborra RT, Pinto RdS, Daffu G, Shen X, Nakandakare ER, Machado UF, Corrêa-Giannella MLC, Schmidt AM, et al. RAGE Mediates Cholesterol Efflux Impairment in Macrophages Caused by Human Advanced Glycated Albumin. International Journal of Molecular Sciences. 2020; 21(19):7265. https://doi.org/10.3390/ijms21197265
Chicago/Turabian StyleMachado-Lima, Adriana, Raquel López-Díez, Rodrigo Tallada Iborra, Raphael de Souza Pinto, Gurdip Daffu, Xiaoping Shen, Edna Regina Nakandakare, Ubiratan Fabres Machado, Maria Lucia Cardillo Corrêa-Giannella, Ann Marie Schmidt, and et al. 2020. "RAGE Mediates Cholesterol Efflux Impairment in Macrophages Caused by Human Advanced Glycated Albumin" International Journal of Molecular Sciences 21, no. 19: 7265. https://doi.org/10.3390/ijms21197265
APA StyleMachado-Lima, A., López-Díez, R., Iborra, R. T., Pinto, R. d. S., Daffu, G., Shen, X., Nakandakare, E. R., Machado, U. F., Corrêa-Giannella, M. L. C., Schmidt, A. M., & Passarelli, M. (2020). RAGE Mediates Cholesterol Efflux Impairment in Macrophages Caused by Human Advanced Glycated Albumin. International Journal of Molecular Sciences, 21(19), 7265. https://doi.org/10.3390/ijms21197265








