Transient Changes of Metabolism at the Pronuclear Stage in Mice Influences Skeletal Muscle Phenotype in Adulthood
Abstract
1. Introduction
2. Results
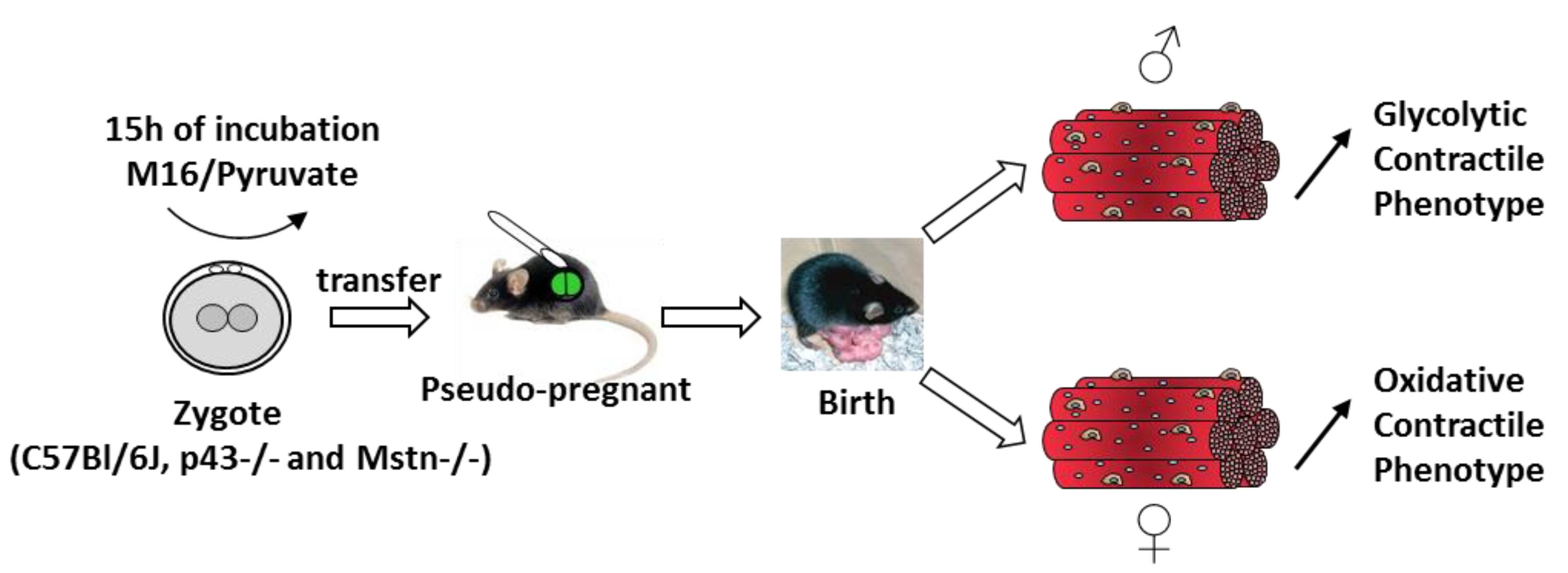
2.1. Experimental Design
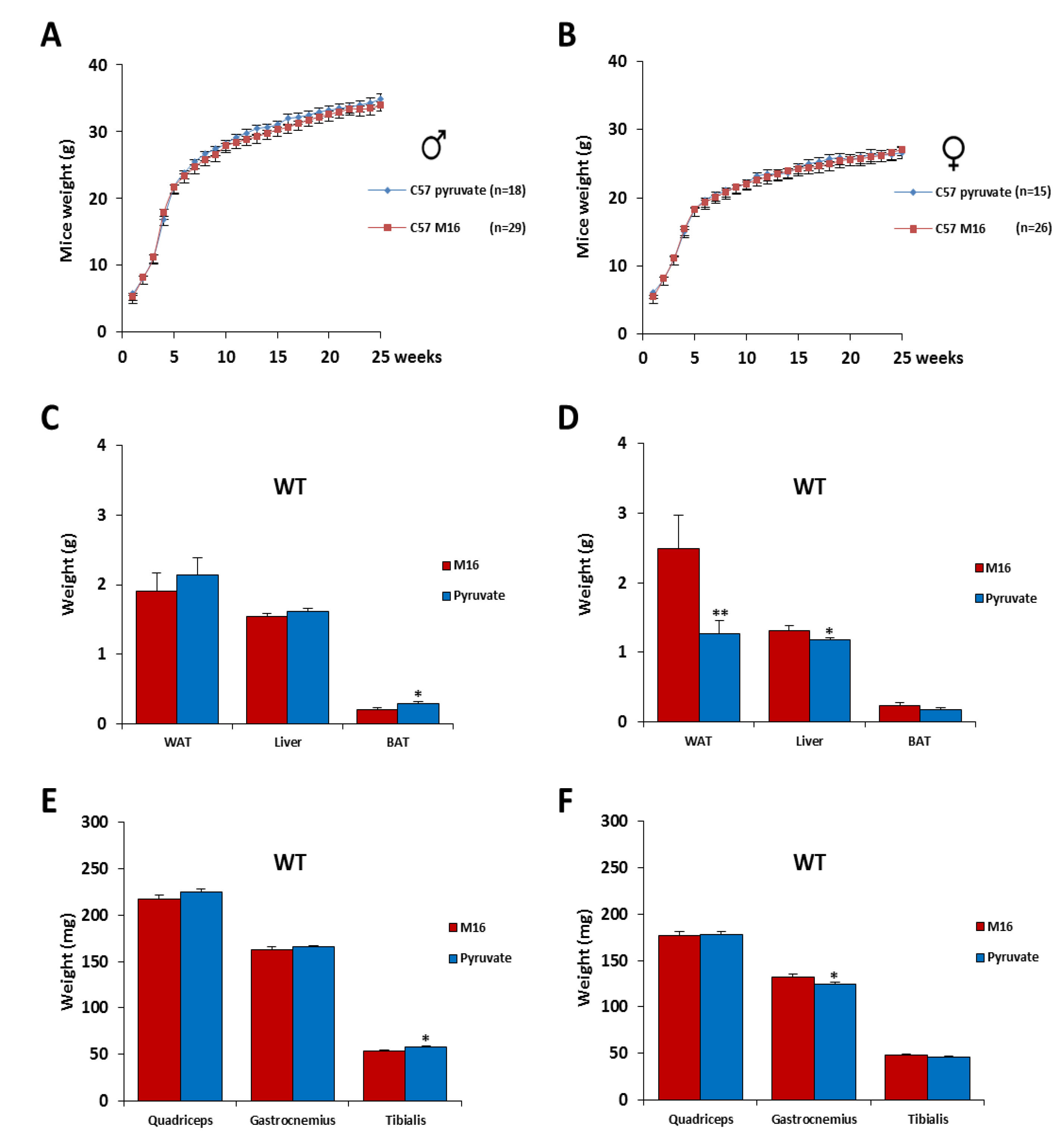
2.2. Post-Natal Growth of C57Bl6 Mice Was Not Affected by a Short Exposure in M16/Pyruvate Medium at 1 Cell Embryo Stage. Wild Type (WT)
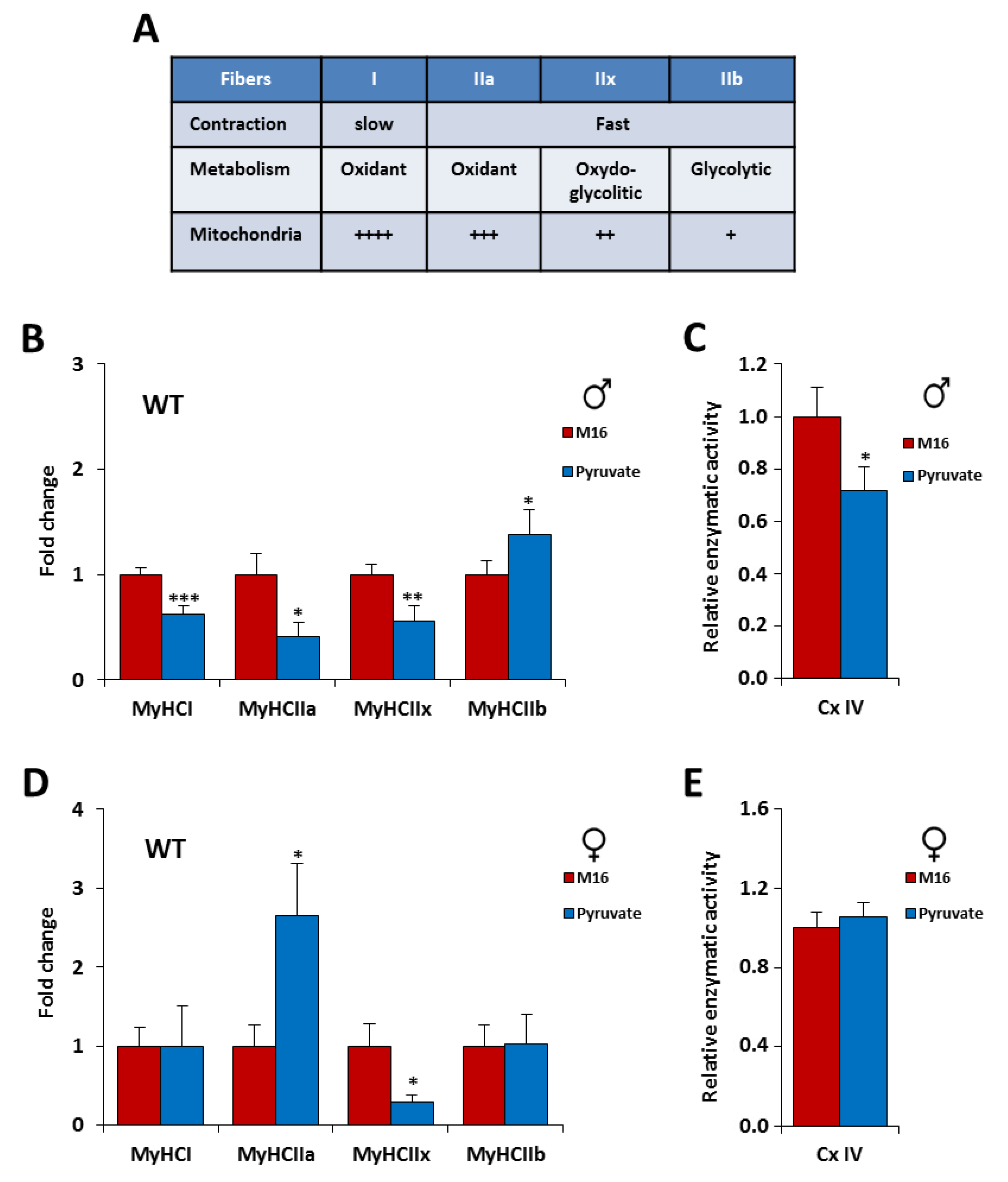
2.3. The Contractile Phenotype of the Skeletal Muscle is Highly Sensitive to a Short Exposure in M16/Pyruvate Medium at 1-Cell Embryo Stage
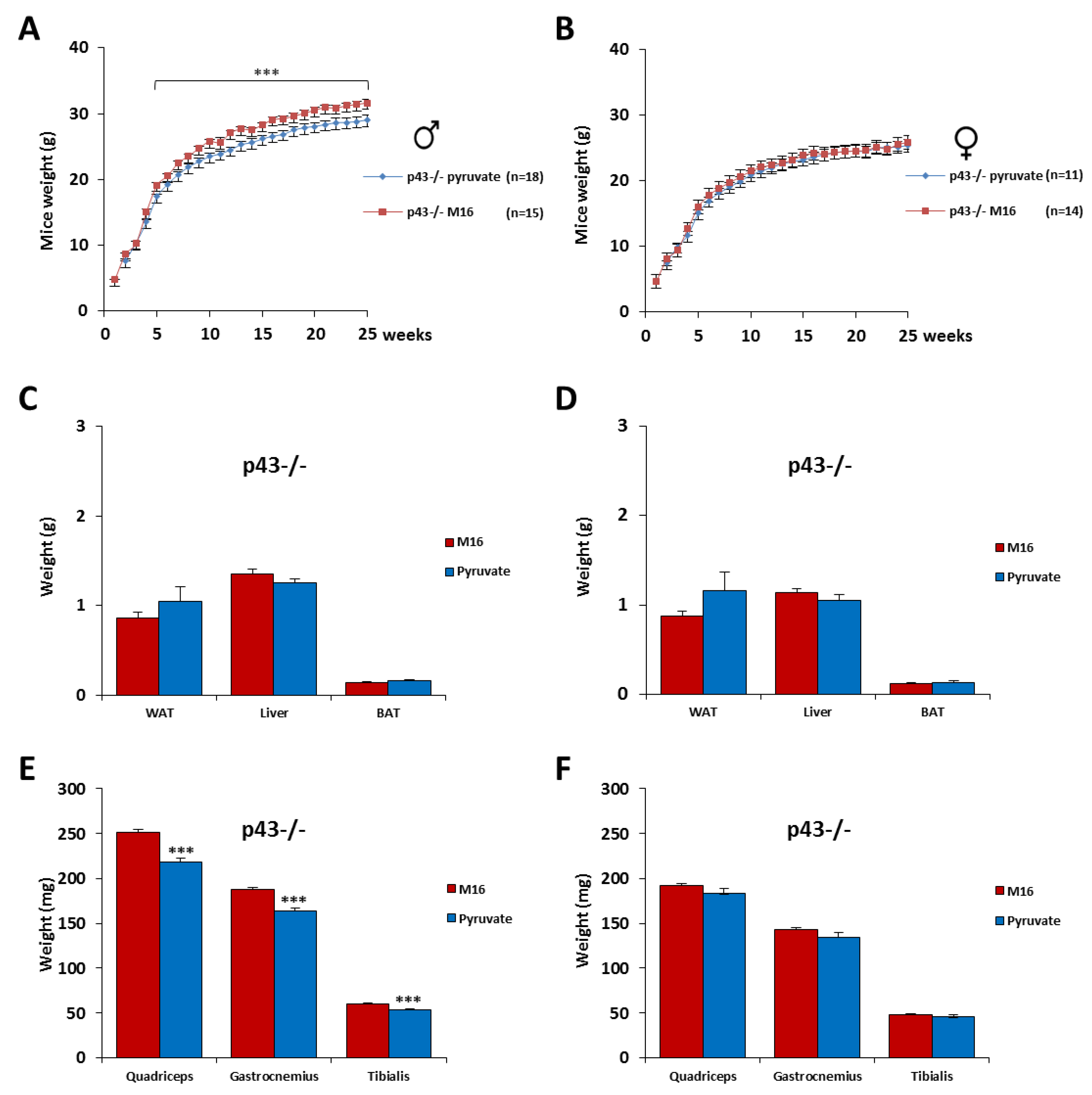
2.4. Skeletal Muscle Hypertrophy Was Fully Abrogated for p43−/− Male Mice by a Short Exposure in M16/Pyruvate Medium at 1 Cell Embryo Stage
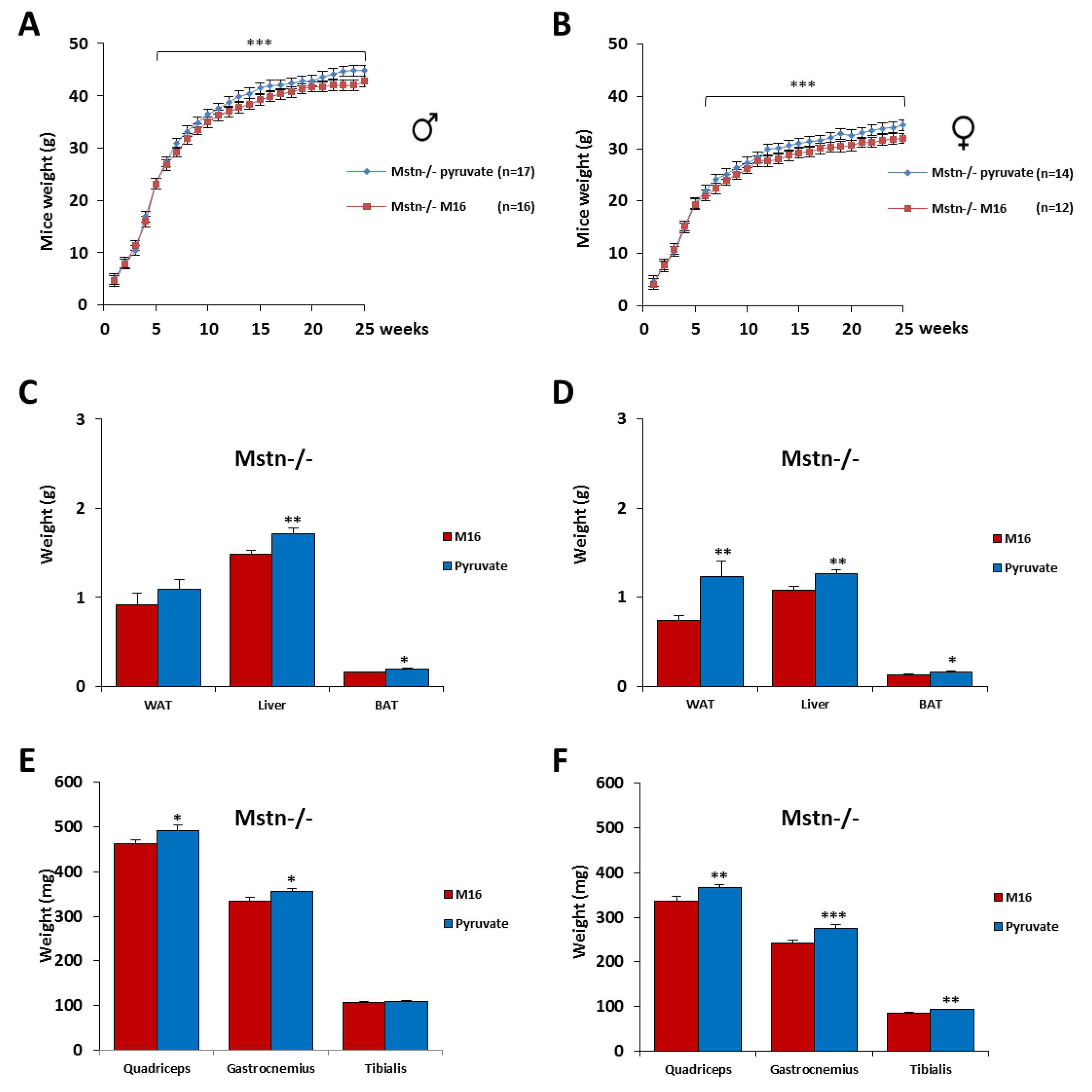
2.5. Skeletal Muscle Hypertrophy Was Increased in Mstn−/− Mice of Both Genders by a Short Exposure in M16/Pyruvate Medium at 1 Cell Embryo Stage
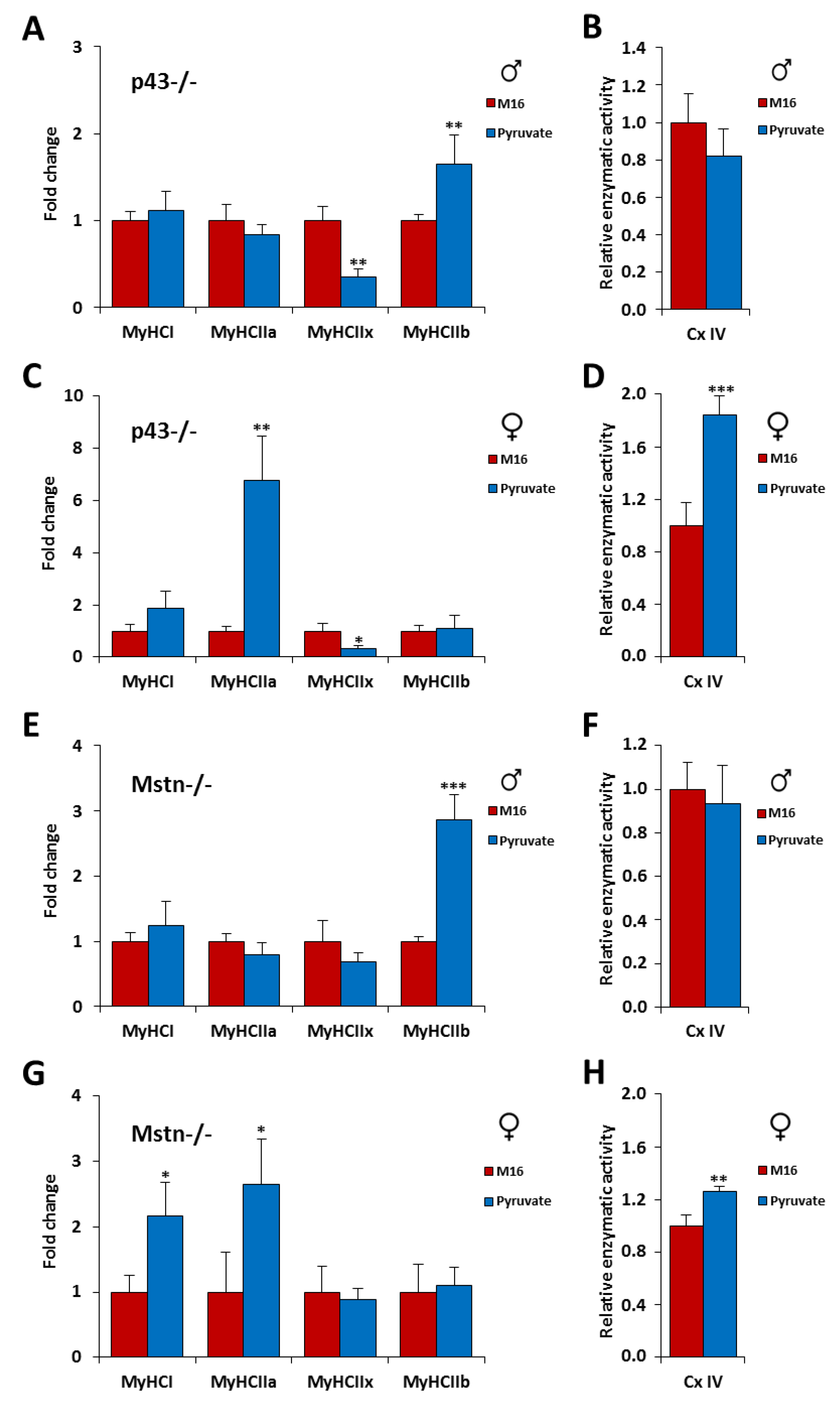
2.6. Short Exposure in M16/Pyruvate Medium at 1 Cell Embryo Stage Also Alters Contractile Features of Skeletal Muscle in p43−/− and Mstn−/− Mice
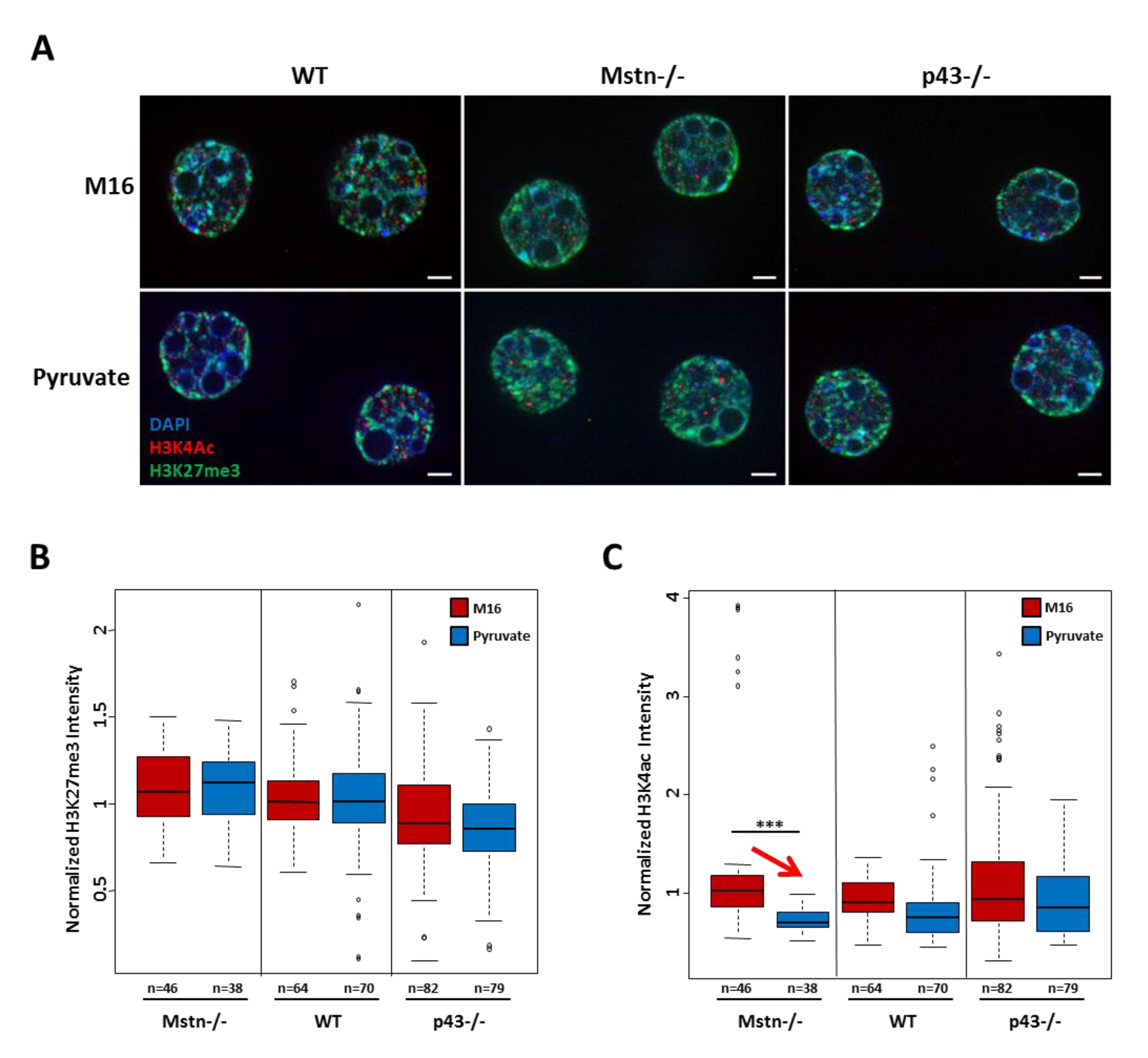
2.7. Influence of Culture Medium at 1 Cell Embryo Stage on H3K27me and H3K4ac Marks
3. Discussion
4. Materials and Methods
4.1. Animals and Ethics Statement
4.2. Embryo Production, Collection, and Treatment
4.3. Embryo Transfer and Post-Natal Growth
4.4. Gene Expression Studies
4.5. Mitochondrial Complex IV Activity
4.6. Immunostaining of Post-Translational Modifications of Histones and Fluorescence Intensity Measurements
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McPherron, A.C.; Lawler, A.M.; Lee, S.-J. Regulation of skeletal muscle mass in mice by a new TGF-p superfamily member. Nature 1997, 387, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Wrutniak, C.; Cassar-Malek, I.; Marchal, S.; Rascle, A.; Heusser, S.; Keller, J.-M.; Fléchon, J.; Dauça, M.; Samarut, J.; Ghysdael, J.; et al. A 43-kDa Protein Related to c-Erb A α1 Is Located in the Mitochondrial Matrix of Rat Liver. J. Boil. Chem. 1995, 270, 16347–16354. [Google Scholar] [CrossRef] [PubMed]
- Casas, F.; Daury, L.; Grandemange, S.; Busson, M.; Seyer, P.; Hatier, R.; Carazo, Á.; Cabello, G.; Wrutniak-Cabello, C. Endocrine regulation of mitochondrial activity: Involvement of truncated RXRα and c-Erb Aαl proteins. FASEB J. 2003, 17, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Casas, F.; Domenjoud, L.; Rochard, P.; Hatier, R.; Rodier, A.; Daury, L.; Bianchi, A.; Krémarik-Bouillaud, P.; Becuwe, P.; Keller, J.; et al. A 45 kDa protein related to PPARgamma2, induced by peroxisome proliferators, is located in the mitochondrial matrix. FEBS Lett. 2000, 478, 4–8. [Google Scholar] [CrossRef]
- Casas, F.; Rochard, P.; Rodier, A.; Cassar-Malek, I.; Marchal-Victorion, S.; Wiesner, R.J.; Gerard, C.; Wrutniak, C. A Variant Form of the Nuclear Triiodothyronine Receptor c-ErbAα1 Plays a Direct Role in Regulation of Mitochondrial RNA Synthesis. Mol. Cell. Boil. 1999, 19, 7913–7924. [Google Scholar] [CrossRef] [PubMed]
- Casas, F.; Pessemesse, L.; Grandemange, S.; Seyer, P.; Gueguen, N.; Baris, O.; Lepourry, L.; Cabello, G.; Wrutniak-Cabello, C. Overexpression of the Mitochondrial T3 Receptor p43 Induces a Shift in Skeletal Muscle Fiber Types. PLoS ONE 2008, 3, e2501. [Google Scholar] [CrossRef]
- Pessemesse, L.; Schlernitzauer, A.; Sar, C.; Levin, J.; Grandemange, S.; Seyer, P.; Favier, F.B.; Kaminski, S.; Cabello, G.; Wrutniak-Cabello, C.; et al. Depletion of the p43 mitochondrial T3 receptor in mice affects skeletal muscle development and activity. FASEB J. 2011, 26, 748–756. [Google Scholar] [CrossRef]
- Casas, F.; Pessemesse, L.; Grandemange, S.; Seyer, P.; Baris, O.; Gueguen, N.; Ramonatxo, C.; Perrin, F.; Fouret, G.; Lepourry, L.; et al. Overexpression of the Mitochondrial T3 Receptor Induces Skeletal Muscle Atrophy during Aging. PLoS ONE 2009, 4, e5631. [Google Scholar] [CrossRef]
- Pessemesse, L.; Tintignac, L.; Blanchet, E.; Cortade, F.; Jublanc, E.; Demangel, R.; Py, G.; Sar, C.; Cabello, G.; Wrutniak-Cabello, C.; et al. Regulation of mitochondrial activity controls the duration of skeletal muscle regeneration in response to injury. Sci. Rep. 2019, 9, 12249. [Google Scholar] [CrossRef]
- Chason, R.J.; Csokmay, J.; Segars, J.H.; DeCherney, A.H.; Armant, D.R. Environmental and epigenetic effects upon preimplantation embryo metabolism and development. Trends Endocrinol. Metab. 2011, 22, 412–420. [Google Scholar] [CrossRef]
- Barker, D.J.P. The origins of the developmental origins theory. J. Intern. Med. 2007, 261, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Banrezes, B.; Sainte-Beuve, T.; Canon, E.; Schultz, R.M.; Cancela, J.; Ozil, J.-P. Adult Body Weight Is Programmed by a Redox-Regulated and Energy-Dependent Process during the Pronuclear Stage in Mouse. PLoS ONE 2011, 6, e29388. [Google Scholar] [CrossRef] [PubMed]
- Ecker, D.J.; Stein, P.; Xu, Z.; Williams, C.J.; Kopf, G.S.; Bilker, W.B.; Abel, T.; Schultz, R.M. Long-term effects of culture of preimplantation mouse embryos on behavior. Proc. Natl. Acad. Sci. USA 2004, 101, 1595–1600. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Gonzalez, R.; Moreira, P.; Bilbao, A.; Jiménez, A.; Pérez-Crespo, M.; Ramírez, M.A.; De Fonseca, F.R.; Pintado, B.; Gutierrez-Adan, A. Long-term effect of in vitro culture of mouse embryos with serum on mRNA expression of imprinting genes, development, and behavior. Proc. Natl. Acad. Sci. USA 2004, 101, 5880–5885. [Google Scholar] [CrossRef]
- Fernández-Gonzalez, R.; Moreira, P.N.; Pérez-Crespo, M.; Sánchez-Martín, M.; Ramirez, M.A.; Pericuesta, E.; Bilbao, A.; Bermejo-Álvarez, P.; Hourcade, J.D.D.; De Fonseca, F.R.; et al. Long-Term Effects of Mouse Intracytoplasmic Sperm Injection with DNA-Fragmented Sperm on Health and Behavior of Adult Offspring1. Boil. Reprod. 2008, 78, 761–772. [Google Scholar] [CrossRef]
- Kwong, W.Y.; Osmond, C.; Fleming, T.P. Support for Barker hypothesis upheld in rat model of maternal undernutrition during the preimplantation period: Application of integrated ’random effects’ statistical model. Reprod. Biomed. Online 2004, 8, 574–576. [Google Scholar] [CrossRef]
- Young, L.E.; Sinclair, K.D.; Wilmut, I. Large offspring syndrome in cattle and sheep. Rev. Reprod. 1998, 3, 155–163. [Google Scholar] [CrossRef]
- Donjacour, A.; Liu, X.; Lin, W.; Simbulan, R.; Rinaudo, P. In vitro fertilization affects growth and glucose metabolism in a sex-specific manner in an outbred mouse model. Boil. Reprod. 2014, 90, 80. [Google Scholar] [CrossRef]
- Dumollard, R.; Carroll, J.; Duchen, M.R.; Campbell, K.; Swann, K. Mitochondrial function and redox state in mammalian embryos. Semin. Cell Dev. Boil. 2009, 20, 346–353. [Google Scholar] [CrossRef]
- Feil, D.; Lane, M.; Roberts, C.T.; Kelley, R.L.; Edwards, L.J.; Thompson, J.G.; Kind, K.L. Effect of culturing mouse embryos under different oxygen concentrations on subsequent fetal and placental development. J. Physiol. 2006, 572, 87–96. [Google Scholar] [CrossRef]
- Lane, M.; Gardner, D. Amino acids and vitamins prevent culture-induced metabolic perturbations and associated loss of viability of mouse blastocysts. Hum. Reprod. 1998, 13, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Leese, H.J. Quiet please, do not disturb: A hypothesis of embryo metabolism and viability. BioEssays 2002, 24, 845–849. [Google Scholar] [CrossRef] [PubMed]
- Rexhaj, E.; Paoloni-Giacobino, A.; Rimoldi, S.F.; Fuster, D.G.; Anderegg, M.; Somm, E.; Bouillet, E.; Allemann, Y.; Sartori, C.; Scherrer, U. Mice generated by in vitro fertilization exhibit vascular dysfunction and shortened life span. J. Clin. Investig. 2013, 123, 5052–5060. [Google Scholar] [CrossRef] [PubMed]
- Feuer, S.K.; Donjacour, A.; Simbulan, R.K.; Lin, W.; Liu, X.; Maltepe, E.; Rinaudo, P. Sexually dimorphic effect of in vitro fertilization (IVF) on adult mouse fat and liver metabolomes. Endocrinology 2014, 155, 4554–4567. [Google Scholar] [CrossRef]
- Feuer, S.; Liu, X.; Donjacour, A.; Simbulan, R.; Maltepe, E.; Rinaudo, P. Common and specific transcriptional signatures in mouse embryos and adult tissues induced by in vitro procedures. Reproduction 2016. [Google Scholar] [CrossRef]
- Feuer, S.K.; Rinaudo, P.F. Physiological, metabolic and transcriptional postnatal phenotypes of in vitro fertilization (IVF) in the mouse. J. Dev. Orig. Health Dis. 2017, 8, 403–410. [Google Scholar] [CrossRef]
- Cantone, I.; Fisher, A.G. Epigenetic programming and reprogramming during development. Nat. Struct. Mol. Boil. 2013, 20, 282–289. [Google Scholar] [CrossRef]
- Bannister, A.J.; Kouzarides, T. Regulation of chromatin by histone modifications. Cell Res. 2011, 21, 381–395. [Google Scholar] [CrossRef]
- Kouzarides, T. Chromatin Modifications and Their Function. Cell 2007, 128, 693–705. [Google Scholar] [CrossRef]
- Peterson, C.L.; Laniel, M.-A. Histones and histone modifications. Curr. Boil. 2004, 14, R546–R551. [Google Scholar] [CrossRef]
- Dumollard, R.; Ward, Z.; Carroll, J.; Duchen, M.R. Regulation of redox metabolism in the mouse oocyte and embryo. Development 2006, 134, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Booth, F.W.; Thomason, D.B. Molecular and cellular adaptation of muscle in response to exercise: Perspectives of various models. Physiol. Rev. 1991, 71, 541–585. [Google Scholar] [CrossRef] [PubMed]
- Olson, E.N.; Williams, R. Calcineurin signaling and muscle remodeling. Cell 2000, 101, 689–692. [Google Scholar] [CrossRef]
- Bloemberg, D.; Quadrilatero, J. Rapid Determination of Myosin Heavy Chain Expression in Rat, Mouse, and Human Skeletal Muscle Using Multicolor Immunofluorescence Analysis. PLoS ONE 2012, 7, e35273. [Google Scholar] [CrossRef] [PubMed]
- Nagaraj, R.; Sharpley, M.S.; Chi, F.; Braas, D.; Zhou, Y.; Kim, R.; Clark, A.T.; Banerjee, U. Nuclear Localization of Mitochondrial TCA Cycle Enzymes as a Critical Step in Mammalian Zygotic Genome Activation. Cell 2017, 168, 210–223.e11. [Google Scholar] [CrossRef]
- Gardner, D.; Lane, M. Culture and selection of viable blastocysts: A feasible proposition for human IVF? Hum. Reprod. Updat. 1997, 3, 367–382. [Google Scholar] [CrossRef]
- Leese, H.J. Metabolism of the preimplantation mammalian embryo. Oxf. Rev. Reprod. Boil. 1991, 13, 35–72. [Google Scholar]
- Gardner, D.K.; Leese, H.J. The role of glucose and pyruvate transport in regulating nutrient utilization by preimplantation mouse embryos. Development 1988, 104, 423–429. [Google Scholar]
- Blanchet, E.; Bertrand, C.; Annicotte, J.-S.; Schlernitzauer, A.; Pessemesse, L.; Levin, J.; Fouret, G.; Feillet-Coudray, C.; Bonafos, B.; Fajas, L.; et al. Mitochondrial T3 receptor p43 regulates insulin secretion and glucose homeostasis. FASEB J. 2011, 26, 40–50. [Google Scholar] [CrossRef]
- Watkins, A.J.; Wilkins, A.; Cunningham, C.; Perry, V.H.; Seet, M.J.; Osmond, C.; Eckert, J.J.; Torrens, C.; Cagampang, F.R.; Cleal, J.K.; et al. Low protein diet fed exclusively during mouse oocyte maturation leads to behavioural and cardiovascular abnormalities in offspring. J. Physiol. 2008, 586, 2231–2244. [Google Scholar] [CrossRef]
- Gardner, D.; Larman, M.G.; Thouas, G.A. Sex-related physiology of the preimplantation embryo. Mol. Hum. Reprod. 2010, 16, 539–547. [Google Scholar] [CrossRef]
- Feuer, S.K.; Liu, X.; Donjacour, A.; Lin, W.; Simbulan, R.K.; Giritharan, G.; Piane, L.D.; Kolahi, K.; Ameri, K.; Maltepe, E.; et al. Use of a mouse in vitro fertilization model to understand the developmental origins of health and disease hypothesis. Endocrinology 2014, 155, 1956–1969. [Google Scholar] [CrossRef] [PubMed]
- Giritharan, G.; Li, M.; De Sebastiano, F.; Esteban, F.J.; Horcajadas, J.; Lloyd, K.; Donjacour, A.; Maltepe, E.; Rinaudo, P.; Di Sebastiano, F. Effect of ICSI on gene expression and development of mouse preimplantation embryos. Hum. Reprod. 2010, 25, 3012–3024. [Google Scholar] [CrossRef] [PubMed]
- Giritharan, G.; Talbi, S.; Donjacour, A.; Di Sebastiano, F.; Dobson, A.T.; Rinaudo, P. Effect of in vitro fertilization on gene expression and development of mouse preimplantation embryos. Reproduction 2007, 134, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, P.; Giritharan, G.; Talbi, S.; Dobson, A.T.; Schultz, R.M. Effects of oxygen tension on gene expression in preimplantation mouse embryos. Fertil. Steril. 2006, 86, 1265.e1–1265.e36. [Google Scholar] [CrossRef] [PubMed]
- Beaujean, N. Histone post-translational modifications in preimplantation mouse embryos and their role in nuclear architecture. Mol. Reprod. Dev. 2013, 81, 100–112. [Google Scholar] [CrossRef]
- Harvey, A.J. Mitochondria in early development: Linking the microenvironment, metabolism and the epigenome. Reproduction 2019, 157, R159–R179. [Google Scholar] [CrossRef]
- Wiese, M.; Bannister, A.J. Two genomes, one cell: Mitochondrial-nuclear coordination via epigenetic pathways. Mol. Metab. 2020, 38, 100942. [Google Scholar] [CrossRef]
- Ryall, J.G.; Cliff, T.; Dalton, S.; Sartorelli, V. Metabolic Reprogramming of Stem Cell Epigenetics. Cell Stem Cell 2015, 17, 651–662. [Google Scholar] [CrossRef]
- Houtkooper, R.H.; Pirinen, E.; Auwerx, J. Sirtuins as regulators of metabolism and healthspan. Nat. Rev. Mol. Cell Boil. 2012, 13, 225–238. [Google Scholar] [CrossRef]
- Brown, J.J.; Whittingham, D.G. The roles of pyruvate, lactate and glucose during preimplantation development of embryos from F1 hybrid mice in vitro. Development 1991, 112, 99–105. [Google Scholar] [PubMed]
- Leese, H.J. Metabolism of the preimplantation embryo: 40 years on. Reproduction 2012, 143, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Whittingham, D.G. Culture of mouse ova. J. Reprod. Fertil. Suppl. 1971, 14, 7–21. [Google Scholar] [PubMed]
- Wharton, D.C.; Tzagoloff, A. Cytochrome oxidase from beef heart mitochondria. Methods Enzymol. 1967, 10, 245–250. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertrand-Gaday, C.; Letheule, M.; Blanchet, E.; Vernus, B.; Pessemesse, L.; Bonnet-Garnier, A.; Bonnieu, A.; Casas, F. Transient Changes of Metabolism at the Pronuclear Stage in Mice Influences Skeletal Muscle Phenotype in Adulthood. Int. J. Mol. Sci. 2020, 21, 7203. https://doi.org/10.3390/ijms21197203
Bertrand-Gaday C, Letheule M, Blanchet E, Vernus B, Pessemesse L, Bonnet-Garnier A, Bonnieu A, Casas F. Transient Changes of Metabolism at the Pronuclear Stage in Mice Influences Skeletal Muscle Phenotype in Adulthood. International Journal of Molecular Sciences. 2020; 21(19):7203. https://doi.org/10.3390/ijms21197203
Chicago/Turabian StyleBertrand-Gaday, Christelle, Martine Letheule, Emilie Blanchet, Barbara Vernus, Laurence Pessemesse, Amélie Bonnet-Garnier, Anne Bonnieu, and François Casas. 2020. "Transient Changes of Metabolism at the Pronuclear Stage in Mice Influences Skeletal Muscle Phenotype in Adulthood" International Journal of Molecular Sciences 21, no. 19: 7203. https://doi.org/10.3390/ijms21197203
APA StyleBertrand-Gaday, C., Letheule, M., Blanchet, E., Vernus, B., Pessemesse, L., Bonnet-Garnier, A., Bonnieu, A., & Casas, F. (2020). Transient Changes of Metabolism at the Pronuclear Stage in Mice Influences Skeletal Muscle Phenotype in Adulthood. International Journal of Molecular Sciences, 21(19), 7203. https://doi.org/10.3390/ijms21197203




