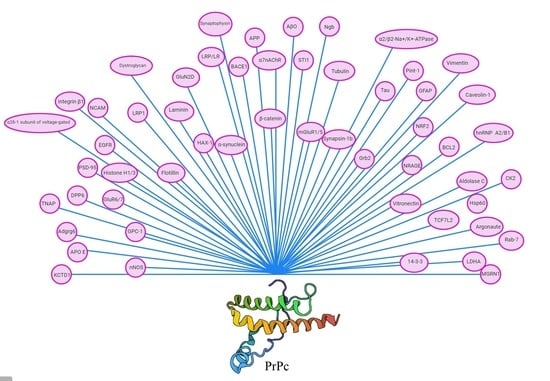
Cellular Prion Protein (PrPc): Putative Interacting Partners and Consequences of the Interaction
Abstract
1. Introduction
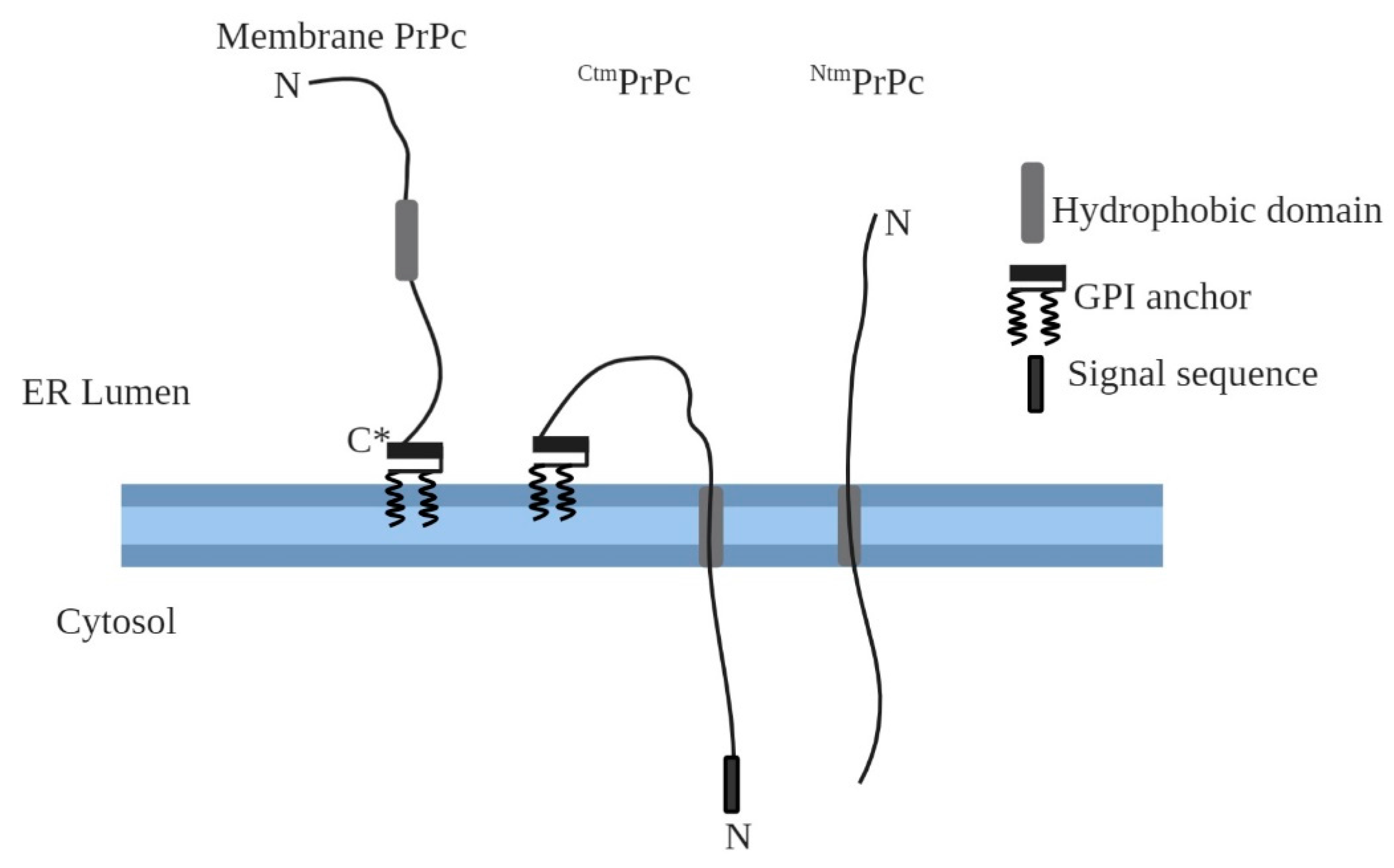
2. Transmembrane Forms of Cellular Prion Protein (PrPc)
3. Itracellular Partners of PrPc
3.1. Neuroglobin
3.2. Tubulin
3.3. Tau
3.4. Synapsin-1b
3.5. Growth Factor Receptor-Bound Protein 2
3.6. Prion Interactor 1
3.7. Glial Fibrillary Acidic Protein
3.8. Heterogeneous Nuclear Ribonucleoproteins and Aldolase C
3.9. Nuclear Factor Erythroid 2-Related Factor 2
3.10. B Cell Lymphoma 2
3.11. 14-3-3 Proteins
3.12. Neurotrophin Receptor-Interacting MAGE Homolog
3.13. Casein Kinase II
3.14. Mahogunin Ring Finger 1
3.15. Chaperones
3.16. Members of the Rab Family of Small GTPases
3.17. Argonaute
3.18. Lactate Dehydrogenase A
3.19. Vimentin
3.20. Wnt Signaling Pathway Proteins
4. The Interaction Partners of PrPc in the Plasma Membrane
4.1. Stress-Inducible Phosphoprotein 1
4.2. Nicotinic Acetylcholine Receptors
4.3. Alzheimer’s Disease-Related Proteins
4.4. Laminin
4.5. GluN2D Subunit of NMDAR
4.6. Low-Density Lipoprotein Receptor-Related Protein 1
4.7. Neural Cell Adhesion Molecule
4.8. Vitronectin
4.9. Caveolin-1
4.10. Integrin β1
4.11. Flotillin
4.12. Epidermal Growth Factor Receptor
4.13. Kainate Receptor GluR6/7 and Postsynaptic Density Protein 95
4.14. Dipeptidyl Peptidase-Like Protein 6
4.15. α2δ-1 Subunit of Voltage-Gated Calcium Channels
4.16. Tissue Nonspecific Alkaline Phosphatase
4.17. Na+/K+-ATPase
4.18. Glypican-1
4.19. Gpr126 (Adgrg6)
4.20. Neuronal Nitric Oxide Synthase (nNOS) and Dystroglycan
4.21. Potassium Channel Tetramerization Domain Containing 1
5. PrPc in the Immune System
6. Polymorphisms and Mutations on the PRNP Gene
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Acevedo-Morantes, C.Y.; Wille, H. The Structure of Human Prions: From Biology to Structural Models—Considerations and Pitfalls. Viruses 2014, 6, 3875–3892. [Google Scholar] [CrossRef] [PubMed]
- Orlean, P.; Menon, A.K. Thematic review series: Lipid Posttranslational Modifications.GPI anchoring of protein in yeast and mammalian cells, or: How we learned to stop worrying and love glycophospholipids. J. Lipid Res. 2007, 48, 993–1011. [Google Scholar] [CrossRef]
- Béland, M.; Roucou, X. The prion protein unstructured N-terminal region is a broad spectrum molecular sensor with diverse and contrasting potential functions. J. Neurochem. 2011, 120. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, T.; Fisher, S.; Olofsson, S.; Endo, T.; Groth, D.; Tarentino, A.; Borchelt, D.R.; Teplow, D.; Hood, L.; Burlingame, A.; et al. Asparagine-linked glycosylation of the scrapie and cellular prion proteins. Arch. Biochem. Biophys. 1989, 274, 1–13. [Google Scholar] [CrossRef]
- Wulf, M.-A.; Senatore, A.; Aguzzi, A. The biological function of the cellular prion protein: An update. BMC Boil. 2017, 15, 34. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, C.J.; Zhang, K.; Munn, A.L.; Wiegmans, A.; Wei, M.Q. Prion protein scrapie and the normal cellular prion protein. Prion 2015, 10, 63–82. [Google Scholar] [CrossRef]
- Prusiner, S. Novel proteinaceous infectious particles cause scrapie. Science 1982, 216, 136–144. [Google Scholar] [CrossRef]
- McKinley, M.P.; Bolton, D.C.; Prusiner, S.B. A protease-resistant protein is a structural component of the Scrapie prion. Cell 1983, 35, 57–62. [Google Scholar] [CrossRef]
- Prusiner, S.B.; Groth, D.F.; Bolton, D.C.; Kent, S.B.; Hood, L.E. Purification and structural studies of a major scrapie prion protein. Cell 1984, 38, 127–134. [Google Scholar] [CrossRef]
- Kanaani, J.; Prusiner, S.B.; Diacovo, J.; Baekkeskov, S.; Legname, G. Recombinant prion protein induces rapid polarization and development of synapses in embryonic rat hippocampal neurons in vitro. J. Neurochem. 2005, 95, 1373–1386. [Google Scholar] [CrossRef]
- Linden, R.; Martins, V.R.; Prado, M.A.M.; Cammarota, M.; Izquierdo, I.; Brentani, R.R. Physiology of the Prion Protein. Physiol. Rev. 2008, 88, 673–728. [Google Scholar] [CrossRef] [PubMed]
- Bounhar, Y.; Zhang, Y.; Goodyer, C.G.; Leblanc, A.C. Prion Protein Protects Human Neurons against Bax-mediated Apoptosis. J. Boil. Chem. 2001, 276, 39145–39149. [Google Scholar] [CrossRef]
- Roucou, X.; Giannopoulos, P.N.; Zhang, Y.; Jodoin, J.; Goodyer, C.G.; Leblanc, A.C. Cellular prion protein inhibits proapoptotic Bax conformational change in human neurons and in breast carcinoma MCF-7 cells. Cell Death Differ. 2005, 12, 783–795. [Google Scholar] [CrossRef] [PubMed]
- Vassallo, N.; Herms, J. Cellular prion protein function in copper homeostasis and redox signalling at the synapse. J. Neurochem. 2003, 86, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.R.; Qin, K.; Herms, J.; Madlung, A.; Manson, J.C.; Strome, R.; Fraser, P.E.; Kruck, T.; Von Bohlen, A.; Schulz-Schaeffer, W.; et al. The cellular prion protein binds copper in vivo. Nature 1997, 390, 684–687. [Google Scholar] [CrossRef]
- Laurén, J.; Gimbel, D.A.; Nygaard, H.B.; Gilbert, J.W.; Strittmatter, S.M. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-β oligomers. Nature 2009, 457, 1128–1132. [Google Scholar] [CrossRef]
- Leighton, P.L.A.; Nadolski, N.J.; Morrill, A.; Hamilton, S.; Allison, W.T. An ancient conserved role for prion protein in learning and memory. Boil. Open 2018, 7, bio025734. [Google Scholar] [CrossRef]
- Roguski, A.; Gill, A.C. The Role of the Mammalian Prion Protein in the Control of Sleep. Pathogens 2017, 6, 58. [Google Scholar] [CrossRef]
- Stahl, N.; Baldwin, M.; Hecker, R.; Pan, K.-M.; Burlingame, A.; Prusiner, S. Glycosylinositol Phospholipid Anchors of the Scrapie and Cellular Prion Proteins Contain Sialic Acid. Biochemistry 1992, 31, 5043–5053. [Google Scholar] [CrossRef]
- Vana, K.; Zuber, C.; Nikles, D.; Weiss, S. Novel Aspects of Prions, Their Receptor Molecules, and Innovative Approaches for TSE Therapy. Cell. Mol. Neurobiol. 2006, 27, 107–128. [Google Scholar] [CrossRef]
- Hajj, G.N.M.; Lopes, M.H.; Mercadante, A.F.; Veiga, S.S.; Da Silveira, R.B.; Dos Santos, T.G.; Ribeiro, K.C.B.; Juliano, M.A.; Jacchieri, S.G.; Zanata, S.M.; et al. Cellular prion protein interaction with vitronectin supports axonal growth and is compensated by integrins. J. Cell Sci. 2007, 120, 1915–1926. [Google Scholar] [CrossRef] [PubMed]
- Nandi, P.K. Interaction of prion peptide HuPrP106–126 with nucleic acid. Arch. Virol. 1997, 142, 2537–2545. [Google Scholar] [CrossRef] [PubMed]
- Budnik, V.; Ruiz-Canada, C.; Wendler, F. Extracellular vesicles round off communication in the nervous system. Nat. Rev. Neurosci. 2016, 17, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Heisler, F.F.; Pechmann, Y.; Wieser, I.; Altmeppen, H.C.; Veenendaal, L.; Muhia, M.; Schiweizer, M.; Glatzel, M.; Krasemann, S.; Kneussel, M. Muskelin Coordinates PrPC Lysosome versus Exosome Targeting and Impacts Prion Disease Progression. Neuron 2018, 99, 1155–1169.e9. [Google Scholar] [CrossRef]
- Godsave, S.F.; Wille, H.; Kujala, P.; Latawiec, D.; DeArmond, S.J.; Serban, A.; Prusiner, S.B.; Peters, P.J. Cryo-immunogold electron microscopy for prions: Toward identification of a conversion site. J. Neurosci. 2008, 28, 12489–12499. [Google Scholar] [CrossRef]
- Yost, C.S.; Lopez, C.D.; Prusiner, S.B.; Myers, R.M.; Lingappa, V.R. Non-hydrophobic extracytoplasmic determinant of stop transfer in the prion protein. Nature 1990, 343, 669–672. [Google Scholar] [CrossRef]
- Ivanova, L.; Barmada, S.; Kummer, T.; Harris, D.A. Mutant Prion Proteins Are Partially Retained in the Endoplasmic Reticulum. J. Boil. Chem. 2001, 276, 42409–42421. [Google Scholar] [CrossRef]
- Siskova, Z.; Mahad, D.J.; Pudney, C.; Campbell, G.R.; Cadogan, M.; O’Connor, V.; Asuni, A.; Perry, V.H. Morphological and Functional Abnormalities in Mitochondria Associated with Synaptic Degeneration in Prion Disease. Am. J. Pathol. 2010, 177, 1411–1421. [Google Scholar] [CrossRef]
- Faris, R.; Moore, R.A.; Ward, A.; Race, B.; Dorward, D.W.; Hollister, J.R.; Fischer, E.R.; Priola, S.A. Cellular prion protein is present in mitochondria of healthy mice. Sci. Rep. 2017, 7, 41556. [Google Scholar] [CrossRef]
- Sorice, M.; Mattei, V.; Tasciotti, V.; Manganelli, V.; Garofalo, T.; Misasi, R. Trafficking of PrPc to mitochondrial raft-like microdomains during cell apoptosis. Prion 2012, 6, 354–358. [Google Scholar] [CrossRef]
- Mattei, V.; Matarrese, P.; Garofalo, T.; Tinari, A.; Gambardella, L.; Ciarlo, L.; Manganelli, V.; Tasciotti, V.; Misasi, R.; Malorni, W.; et al. Recruitment of cellular prion protein to mitochondrial raft-like microdomains contributes to apoptosis execution. Mol. Boil. Cell 2011, 22, 4842–4853. [Google Scholar] [CrossRef] [PubMed]
- Lechauve, C.; Rezaei, H.; Célier, C.; Kiger, L.; Corral-Debrinski, M.; Noinville, S.; Chauvierre, C.; Hamdane, D.; Pato, C.; Marden, M.C. Neuroglobin and Prion Cellular Localization: Investigation of a Potential Interaction. J. Mol. Boil. 2009, 388, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Palladino, P.; Scaglione, G.L.; Arcovito, A.; Vitale, R.M.; Amodeo, P.; Vallone, B.; Brunori, M.; Benedetti, E.; Rossi, F. Neuroglobin-prion protein interaction: What’s the function? J. Pept. Sci. 2011, 17, 387–391. [Google Scholar] [CrossRef]
- Dong, C.-F.; Shi, S.; Wang, X.-F.; An, R.; Li, P.; Chen, J.-M.; Wang, X.; Wang, G.-R.; Shan, B.; Zhang, B.-Y.; et al. The N-terminus of PrP is responsible for interacting with tubulin and fCJD related PrP mutants possess stronger inhibitive effect on microtubule assembly in vitro. Arch. Biochem. Biophys. 2008, 470, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Osiecka, K.M.; Nieznanska, H.; Skowronek, K.; Karolczak, J.; Schneider, G.; Nieznański, K. Prion protein region 23-32 interacts with tubulin and inhibits microtubule assembly. Proteins: Struct. Funct. Bioinform. 2009, 77, 279–296. [Google Scholar] [CrossRef] [PubMed]
- Hachiya, N.S.; Watanabe, K.; Yamada, M.; Sakasegawa, Y.; Kaneko, K. Anterograde and retrograde intracellular trafficking of fluorescent cellular prion protein. Biochem. Biophys. Res. Commun. 2004, 315, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhang, J.; Yao, H.; Wang, X.; Li, F.; Chen, L.; Gao, C.; Gao, J.; Nie, K.; Zhou, W.; et al. Study on interaction between microtubule associated protein tau and prion protein. Sci. China Ser. C Life Sci. 2006, 49, 473–479. [Google Scholar] [CrossRef]
- Wang, X.-F.; Dong, C.-F.; Zhang, J.; Wan, Y.-Z.; Li, F.; Huang, Y.-X.; Han, L.; Shan, B.; Gao, C.; Han, J.; et al. Human tau protein forms complex with PrP and some GSS- and fCJD-related PrP mutants possess stronger binding activities with tau in vitro. Mol. Cell. Biochem. 2007, 310, 49–55. [Google Scholar] [CrossRef]
- Osiecka, K.M.; Nieznanska, H.; Skowronek, K.; Jozwiak, J.; Nieznański, K. Tau inhibits tubulin oligomerization induced by prion protein. Biochim. Biophys. Acta Bioenerg. 2011, 1813, 1845–1853. [Google Scholar] [CrossRef]
- Ávila, J.; Lucas, J.J.; Pérez, M.; Hernandez, F. Role of Tau Protein in Both Physiological and Pathological Conditions. Physiol. Rev. 2004, 84, 361–384. [Google Scholar] [CrossRef]
- Spielhaupter, C.; Schatzl, H.M. PrPC directly interacts with proteins involved in signaling pathways. J. Biol. Chem. 2001, 276, 44604–44612. [Google Scholar] [CrossRef] [PubMed]
- Sisó, S.; Puig, B.; Varea, R.; Vidal, E.; Acín, C.; Prinz, M.; Montrasio, F.; Badiola, J.; Aguzzi, A.; Pumarola, M.; et al. Abnormal synaptic protein expression and cell death in murine scrapie. Acta Neuropathol. 2002, 103, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Clinton, J.; Forsyth, C.; Royston, M.C.; Roberts, G.W. Synaptic degeneration is the primary neuropathological feature in prion disease. NeuroReport 1993, 4, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Lysek, D.A.; Wüthrich, K. Prion Protein Interaction with the C-Terminal SH3 Domain of Grb2 Studied Using NMR and Optical Spectroscopy. Biochemistry 2004, 43, 10393–10399. [Google Scholar] [CrossRef] [PubMed]
- Oesch, B.; Teplow, D.B.; Stahl, N.; Serban, D.; Hood, L.E.; Prusiner, S.B. Identification of cellular proteins binding to the scrapie prion protein. Biochemistry 1990, 29, 5848–5855. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Scallet, A.C.; Kascsak, R.J.I.; Carp, R. Astrocytosis and amyloid deposition in scrapie-infected hamsters. Brain Res. 1998, 809, 277–287. [Google Scholar] [CrossRef]
- Dong, C.-F.; Wang, X.-F.; Wang, X.; Shi, S.; Wang, G.-R.; Shan, B.; An, R.; Li, X.-L.; Zhang, B.-Y.; Han, J.; et al. Molecular interaction between prion protein and GFAP both in native and recombinant forms in vitro. Med. Microbiol. Immunol. 2007, 197, 361–368. [Google Scholar] [CrossRef]
- Strom, A.; Diecke, S.; Hunsmann, G.; Stuke, A.W. Identification of prion protein binding proteins by combined use of far-Western immunoblotting, two dimensional gel electrophoresis and mass spectrometry. Proteomics 2006, 6, 26–34. [Google Scholar] [CrossRef]
- Yehiely, F.; Perry, B.J.; Carlson, G.A.; Bamborough, P.; Da Costa, M.; Thinakaran, G.; Cohen, F.E.; Prusiner, S.B. Identification of Candidate Proteins Binding to Prion Protein. Neurobiol. Dis. 1997, 3, 339–355. [Google Scholar] [CrossRef]
- Kurschner, C.; Morgan, J.I. The cellular prion protein (PrP) selectively binds to Bcl-2 in the yeast two-hybrid system. Mol. Brain Res. 1995, 30, 165–168. [Google Scholar] [CrossRef]
- Kurschner, C.; Morgan, J.I. Analysis of interaction sites in homo- and heteromeric complexes containing Bcl-2 family members and the cellular prion protein. Mol. Brain Res. 1996, 37, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Rambold, A.S.; Miesbauer, M.; Rapaport, D.; Bartke, T.; Baier, M.; Winklhofer, K.F.; Tatzelt, J. Association of Bcl-2 with Misfolded Prion Protein Is Linked to the Toxic Potential of Cytosolic PrP. Mol. Boil. Cell 2006, 17, 3356–3368. [Google Scholar] [CrossRef]
- Richard, M.; Biacabe, A.-G.; Streichenberger, N.; Ironside, J.W.; Mohr, M.; Kopp, N.; Perret-Liaudet, A. Immunohistochemical localization of 14.3.3 ζ protein in amyloid plaques in human spongiform encephalopathies. Acta Neuropathol. 2003, 105, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Satoh, J.-I.; Onoue, H.; Arima, K.; Yamamura, T. The 14-3-3 Protein Forms a Molecular Complex with Heat Shock Protein Hsp60 and Cellular Prion Protein. J. Neuropathol. Exp. Neurol. 2005, 64, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Fede, D.G.; Giaccone, G.; Limido, L.; Mangieri, M.; Suardi, S.; Puoti, G.; Morbin, M.; Mazzoleni, G.; Ghetti, B.; Tagliavini, F. The epsilon isoform of 14-3-3 protein is a component of the prion protein amyloid deposits of Gerstmann-Straussler-Scheinker disease. J. Neuropathol. Exp. Neurol. 2007, 66, 124–130. [Google Scholar]
- Mei, G.-Y.; Li, Y.; Wang, G.-R.; Zhang, B.; Tian, C.; Chen, C.; Zhou, R.-M.; Wang, X.; Li, X.-L.; Wang, K.-X.; et al. Molecular interaction between PrP protein and the signal protein 14-3-3 beta. BDXB Chin. J. Virol. 2009, 25, 208–212. [Google Scholar]
- Bragason, B.T.; Palsdottir, A. Interaction of PrP with NRAGE, a protein involved in neuronal apoptosis. Mol. Cell. Neurosci. 2005, 29, 232–244. [Google Scholar] [CrossRef]
- Meggio, F.; Negro, A.; Sarno, S.; Ruzzene, M.; Bertoli, A.; Sorgato, M.C.; Pinna, L.A. Bovine prion protein as a modulator of protein kinase CK2. Biochem. J. 2000, 352, 191–196. [Google Scholar] [CrossRef]
- Negro, A.; Meggio, F.; Bertoli, A.; Battistutta, R.; Sorgato, M.; Pinna, L.A. Susceptibility of the Prion Protein to Enzymic Phosphorylation. Biochem. Biophys. Res. Commun. 2000, 271, 337–341. [Google Scholar] [CrossRef]
- Zamponi, E.; Buratti, F.; Cataldi, G.; Caicedo, H.H.; Song, Y.; Jungbauer, L.M.; Ladu, M.J.; Bisbal, M.; Lorenzo, A.; Ma, J.; et al. Prion protein inhibits fast axonal transport through a mechanism involving casein kinase 2. PLoS ONE 2017, 12, e0188340. [Google Scholar] [CrossRef]
- Silvius, D.; Pitstick, R.; Ahn, M.; Meishery, D.; Oehler, A.; Barsh, G.S.; DeArmond, S.J.; Carlson, G.A.; Gunn, T.M. Levels of the Mahogunin Ring Finger 1 E3 Ubiquitin Ligase Do Not Influence Prion Disease. PLoS ONE 2013, 8, e55575. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, A.; Amanullah, A.; Chhangani, D.; Mishra, R.; Prasad, A.; Mishra, A. Mahogunin Ring Finger-1 (MGRN1), a Multifaceted Ubiquitin Ligase: Recent Unraveling of Neurobiological Mechanisms. Mol. Neurobiol. 2015, 53, 4484–4496. [Google Scholar] [CrossRef] [PubMed]
- Edenhofer, F.; Rieger, R.; Famulok, M.; Wendler, W.; Weiss, S.; Winnacker, E.L. Prion protein PrPC interacts with molecular chaperones of the Hsp60 family. J. Virol. 1996, 70, 4724–4728. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Leal, J.B.; Seabra, M.C. Evolution of the rab family of small GTP-binding proteins. J. Mol. Boil. 2001, 313, 889–901. [Google Scholar] [CrossRef]
- Zerial, M.; McBride, H.M. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Boil. 2001, 2, 107–117. [Google Scholar] [CrossRef]
- Gibbings, D.; Leblanc, P.; Jay, F.; Pontier, D.; Michel, F.; Schwab, Y.; Alais, S.; Lagrange, T.; Voinnet, O. Human prion protein binds Argonaute and promotes accumulation of microRNA effector complexes. Nat. Struct. Mol. Boil. 2012, 19, 517–524. [Google Scholar] [CrossRef]
- Watts, J.C.; Hairu, H.; Yu, B.; Sepehr, E.; Amy, H.W.; Tujin, S.; Nathalie, D.; Agnes, L.; Rebecca, Y.; Lei, X.; et al. Interactome analyses identify ties of PrPC and its mammalian paralogs to oligomannosidic N-glycans and endoplasmic reticulum-derived chaperones. PLoS Pathog. 2009, 5, e1000608. [Google Scholar] [CrossRef]
- Ramljak, S.; Schmitz, M.; Zafar, S.; Wrede, A.; Schenkel, S.; Asif, A.R.; Carimalo, J.; Doeppner, T.R.; Schulz-Schaeffer, W.J.; Weise, J.; et al. Cellular prion protein directly interacts with and enhances lactate dehydrogenase expression under hypoxic conditions. Exp. Neurol. 2015, 271, 155–167. [Google Scholar] [CrossRef]
- Zafar, S.; Von Ahsen, N.; Oellerich, M.; Zerr, I.; Schulz-Schaeffer, W.J.; Armstrong, V.W.; Asif, A.R. Proteomics Approach to Identify the Interacting Partners of Cellular Prion Protein and Characterization of Rab7a Interaction in Neuronal Cells. J. Proteome Res. 2011, 10, 3123–3135. [Google Scholar] [CrossRef]
- Gimenez, A.P.L.; Richter, L.M.L.; Atherino, M.C.; Beirão, B.C.B.; Fávaro, C.; Costa, M.D.M.; Zanata, S.M.; Malnic, B.; Mercadante, A.F. Identification of novel putative-binding proteins for cellular prion protein and a specific interaction with the STIP1 homology and U-Box-containing protein 1. Prion 2015, 9, 355–366. [Google Scholar] [CrossRef]
- Besnier, L.S.; Cardot, P.; Da Rocha, B.; Simon, A.; Loew, D.; Klein, C.; Riveau, B.; Lacasa, M.; Clair, C.; Rousset, M.; et al. The cellular prion protein PrPC is a partner of the Wnt pathway in intestinal epithelial cells. Mol. Boil. Cell 2015, 26, 3313–3328. [Google Scholar] [CrossRef]
- Strom, A.; Wang, G.-S.; Picketts, D.J.; Reimer, R.; Stuke, A.W.; Scott, F.W. Cellular prion protein localizes to the nucleus of endocrine and neuronal cells and interacts with structural chromatin components. Eur. J. Cell Boil. 2011, 90, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.-Y.; Li, X.-L.; Shi, Q.; Wang, Z.-Y.; Guo, Y.; Pan, M.-M.; Tian, C.; Zhu, S.-Y.; Chen, C.; Gong, H.-S.; et al. A Novel PrP Partner HS-1 Associated Protein X-1 (HAX-1) Protected the Cultured Cells Against the Challenge of H2O2. J. Mol. Neurosci. 2011, 45, 216–228. [Google Scholar] [CrossRef]
- Burmester, T.; Reinhardt, S.; Weich, B.; Hankeln, T. A vertebrate globin expressed in the brain. Nature 2000, 407, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.-Y.; Zhou, S.; Lou, Z.-Y.; Zhu, C.; Zheng, X.-P.; Hu, X. Translocation and neuroprotective properties of transactivator-of-transcription protein-transduction domain–neuroglobin fusion protein in primary cultured cortical neurons. Biotechnol. Appl. Biochem. 2008, 49, 25. [Google Scholar] [CrossRef] [PubMed]
- Amos, L.A.; Schlieper, D. Microtubules and maps. Adv. Protein. Chem. 2005, 71, 257–298. [Google Scholar]
- Nieznański, K.; Nieznanska, H.; Skowronek, K.; Osiecka, K.M.; Stępkowski, D. Direct interaction between prion protein and tubulin. Biochem. Biophys. Res. Commun. 2005, 334, 403–411. [Google Scholar] [CrossRef]
- Hachiya, N.S.; Watanabe, K.; Sakasegawa, Y.; Kaneko, K. Microtubules-associated intracellular localization of the NH2-terminal cellular prion protein fragment. Biochem. Biophys. Res. Commun. 2004, 313, 818–823. [Google Scholar] [CrossRef]
- Muroyama, A.; Lechler, T. Microtubule organization, dynamics and functions in differentiated cells. Development 2017, 144, 3012–3021. [Google Scholar] [CrossRef]
- Weingarten, M.D.; Lockwood, A.H.; Hwo, S.Y.; Kirschner, M.W. A protein factor essential for microtubule assembly. Proc. Natl. Acad. Sci. USA 1975, 72, 1858–1862. [Google Scholar] [CrossRef]
- Alonso, A.D.; Cohen, L.S.; Corbo, C.; Morozova, V.; Elidrissi, A.; Phillips, G.; Kleiman, F.E. Hyperphosphorylation of Tau Associates With Changes in Its Function Beyond Microtubule Stability. Front. Cell. Neurosci. 2018, 12. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Xiaopeng, D. Dysfunction of microtubule-associated proteins of MAP2/tau family in Prion disease. Prion 2012, 6, 334–338. [Google Scholar]
- Navone, F.; Greengard, P.; De Camilli, P. Synapsin I in nerve terminals: Selective association with small synaptic vesicles. Science 1984, 226, 1209–1211. [Google Scholar] [CrossRef] [PubMed]
- Takenawa, T.; Miki, H.; Matuoka, K. Signaling through Grb2/Ash-control of the Ras pathway and cytoskeleton. Curr. Top. Microbiol. Immunol. 1998, 228, 325–342. [Google Scholar] [PubMed]
- Soriano, J.V.; Liu, N.; Gao, Y.; Yao, Z.-J.; Ishibashi, T.; Underhill, C.; Burke, T.R.; Bottaro, D.P. Inhibition of angiogenesis by growth factor receptor bound protein 2-Src homology 2 domain bound antagonists. Mol. Cancer Ther. 2004, 3, 1289–1299. [Google Scholar]
- Eddleston, M.; Mucke, L. Molecular profile of reactive astrocytes—Implications for their role in neurologic disease. Neuroscience 1993, 54, 15–36. [Google Scholar] [CrossRef]
- Gomi, H.; Yokoyama, T.; Fujimoto, K.; Ikeda, T.; Katoh, A.; Itoh, T.; Itohara, S. Mice devoid of the glial fibrillary acidic protein develop normally and are susceptible to scrapie prions. Neuron 1995, 14, 29–41. [Google Scholar] [CrossRef]
- Dormont, D.; Delpech, B.; Delpech, A.; Courcel, M.N.; Viret, J.; Markovits, P.; Court, L. Hyperproduction of glial fibrillary acidic protein (GFA) during development of experimental scrapie in mice. C. R. Acad. Sci. Ser. III 1981, 293, 53–56. [Google Scholar]
- MacKenzie, A. Immunohistochemical demonstration of glial fibrillary acidic protein in scrapie. J. Comp. Pathol. 1983, 93, 251–259. [Google Scholar] [CrossRef]
- Gomi, H.; Yokoyama, T.; Itohara, S. Role of GFAP in morphological retention and distribution of reactive astrocytes induced by scrapie encephalopathy in mice. Brain Res. 2010, 1312, 156–167. [Google Scholar] [CrossRef]
- Kozu, T.; Henrich, B.; Schäfer, K.P. Structure and expression of the gene (HNRPA2B1) encoding the human hnRNP protein A2/B1. Genomics 1995, 25, 365–371. [Google Scholar] [CrossRef]
- Gao, Y.; Tatavarty, V.; Korza, G.; Levin, M.K.; Carson, J.H. Multiplexed Dendritic Targeting of α Calcium Calmodulin-dependent Protein Kinase II, Neurogranin, and Activity-regulated Cytoskeleton-associated Protein RNAs by the A2 Pathway. Mol. Boil. Cell 2008, 19, 2311–2327. [Google Scholar] [CrossRef]
- Thompson, R.; Kynoch, P.A.; Willson, V.J. Cellular localization of aldolase C subunits in human brain. Brain Res. 1982, 232, 489–493. [Google Scholar] [CrossRef]
- Cañete-Soler, R.; Reddy, K.S.; Tolan, D.R.; Zhai, J. Aldolases A and C Are Ribonucleolytic Components of a Neuronal Complex That Regulates the Stability of the Light-Neurofilament mRNA. J. Neurosci. 2005, 25, 4353–4364. [Google Scholar] [CrossRef] [PubMed]
- Dodson, M.; Redmann, M.; Rajasekaran, N.S.; Darley-Usmar, V.; Zhang, J. KEAP1-NRF2 signalling and autophagy in protection against oxidative and reductive proteotoxicity. Biochem. J. 2015, 469, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Cichon, A.-C.; Brown, D.R. Nrf-2 regulation of prion protein expression is independent of oxidative stress. Mol. Cell. Neurosci. 2014, 63, 31–37. [Google Scholar] [CrossRef]
- Wong, W.W.-L.; Puthalakath, H. Bcl-2 family proteins: The sentinels of the mitochondrial apoptosis pathway. IUBMB Life 2008, 60, 390–397. [Google Scholar] [CrossRef]
- Hardwick, J.M.; Soane, L. Multiple Functions of BCL-2 Family Proteins. Cold Spring Harb. Perspect. Boil. 2013, 5, a008722. [Google Scholar] [CrossRef]
- Fu, H.; Subramanian, R.R.; Masters, S.C. 14-3-3 proteins: Structure, function, and regulation. Annu. Rev. Pharmacol. Toxicol. 2000, 40, 617–647. [Google Scholar] [CrossRef]
- Berg, D.; Holzmann, C.; Riess, O. 14-3-3 proteins in the nervous system. Nat. Rev. Neurosci. 2003, 4, 752–762. [Google Scholar] [CrossRef]
- Muayqil, T.; Gronseth, G.; Camicioli, R. Evidence-based guideline: Diagnostic accuracy of CSF 14-3-3 protein in sporadic Creutzfeldt-Jakob disease: Report of the guideline development subcommittee of the American Academy of Neurology. Neurology 2012, 79, 1499–1506. [Google Scholar] [CrossRef] [PubMed]
- Harris, V.K.; Sadiq, S.A. Disease Biomarkers in Multiple Sclerosis. Mol. Diagn. Ther. 2009, 13, 225–244. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Song, Q.-Q.; Sun, P.; Zhang, J.; Wang, X.; Song, J.; Li, G.-Q.; Liu, Y.-H.; Mei, G.-Y.; Shi, Q.; et al. Interaction between 14-3-3β and PrPC influences the dimerization of 14-3-3 and fibrillization of PrPC106–126. Int. J. Biochem. Cell Boil. 2014, 47, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Salehi, A.H.; Xanthoudakis, S.; Barker, P.A. NRAGE, a p75 Neurotrophin Receptor-interacting Protein, Induces Caspase Activation and Cell Death through a JNK-dependent Mitochondrial Pathway. J. Boil. Chem. 2002, 277, 48043–48050. [Google Scholar] [CrossRef]
- Götz, C.; Montenarh, M. Protein kinase CK2 in development and differentiation. Biomed. Rep. 2016, 6, 127–133. [Google Scholar] [CrossRef]
- Litchfield, D.W. Protein kinase CK2: Structure, regulation and role in cellular decisions of life and death. Biochem. J. 2003, 369, 1–15. [Google Scholar] [CrossRef]
- Chen, J.; Gao, C.; Shi, Q.; Wang, G.; Lei, Y.; Shan, B.; Zhang, B.; Dong, C.; Shi, S.; Wang, X.; et al. Casein kinase II interacts with prion protein in vitro and forms complex with native prion protein in vivo. Acta Biochim. Biophys. Sin. 2008, 40, 1039–1047. [Google Scholar] [CrossRef][Green Version]
- Chen, J.-M.; Gao, C.; Shi, Q.; Shan, B.; Lei, Y.-J.; Dong, C.-F.; An, R.; Wang, G.-R.; Zhang, B.-Y.; Han, J.; et al. Different expression patterns of CK2 subunits in the brains of experimental animals and patients with transmissible spongiform encephalopathies. Arch. Virol. 2008, 153, 1013–1020. [Google Scholar] [CrossRef]
- Canton, D.A.; Litchfield, D.W. The shape of things to come: An emerging role for protein kinase CK2 in the regulation of cell morphology and the cytoskeleton. Cell. Signal. 2006, 18, 267–275. [Google Scholar] [CrossRef]
- Kim, B.Y.; Olzmann, J.A.; Barsh, G.S.; Chin, L.-S.; Li, L. Spongiform Neurodegeneration-associated E3 Ligase Mahogunin Ubiquitylates TSG101 and Regulates Endosomal Trafficking. Mol. Boil. Cell 2007, 18, 1129–1142. [Google Scholar] [CrossRef]
- Amit, I.; Yakir, L.; Katz, M.; Zwang, Y.; Marmor, M.D.; Citri, A.; Shtiegman, K.; Alroy, I.; Tuvia, S.; Reiss, Y.; et al. Tal, a Tsg101-specific E3 ubiquitin ligase, regulates receptor endocytosis and retrovirus budding. Genes Dev. 2004, 18, 1737–1752. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liao, J.; Ruland, J.; Mak, T.W.; Cohen, S.N. A TSG101/MDM2 regulatory loop modulates MDM2 degradation and MDM2/p53 feedback control. Proc. Natl. Acad. Sci. USA 2001, 98, 1619–1624. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.H.; Cohen, S.N. Human MDM2 isoforms translated differentially on constitutive versus p53-regulated transcripts have distinct functions in the p53/MDM2 and TSG101/MDM2 feedback control loops. Mol. Cell Biol. 2007, 27, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J. Proteins as molecular chaperones. Nature 1987, 328, 378–379. [Google Scholar] [CrossRef]
- Martins, V.R.; Graner, E.; Garcia-Abreu, J.; De Souza, S.J.; Mercadante, A.F.; Veiga, S.S.; Zanata, S.M.; Moura-Neto, V.; Brentani, R.R. Complementary hydropathy identifies a cellular prion protein receptor. Nat. Med. 1997, 3, 1376–1382. [Google Scholar] [CrossRef]
- Sun, G.; Guo, M.; Shen, A.; Mei, F.; Peng, X.; Gong, R.; Guo, D.-Y.; Wu, J.; Tien, P.; Xiao, G. Bovine PrPC directly interacts with αB-crystalline. FEBS Lett. 2005, 579, 5419–5424. [Google Scholar] [CrossRef][Green Version]
- Dulle, J.E.; Fort, P.E. Crystallins and neuroinflammation: The glial side of the story. Biochim. Biophys. Acta Bioenerg. 2015, 1860, 278–286. [Google Scholar] [CrossRef]
- Leach, M.R.; Williams, D.B. Calnexin and Calreticulin, Molecular Chaperones of the Endoplasmic Reticulum. In Wilms Tumor: Clinical and Molecular Characterization; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2003; pp. 49–62. [Google Scholar]
- Gething, M.-J. Role and regulation of the ER chaperone BiP. Semin. Cell Dev. Boil. 1999, 10, 465–472. [Google Scholar] [CrossRef]
- Capellari, S.; Zaidi, S.I.A.; Urig, C.B.; Perry, G.; Smith, M.A.; Petersen, R.B. Prion Protein Glycosylation Is Sensitive to Redox Change. J. Boil. Chem. 1999, 274, 34846–34850. [Google Scholar] [CrossRef]
- Marzec, M.; Eletto, D.; Argon, Y. GRP94: An HSP90-like protein specialized for protein folding and quality control in the endoplasmic reticulum. Biochim. Biophys. Acta Bioenerg. 2012, 1823, 774–787. [Google Scholar] [CrossRef]
- Ohtsuka, K.; Hata, M.; Ohtsuka, M.H.K. Molecular chaperone function of mammalian Hsp70 and Hsp40—A review. Int. J. Hyperth. 2000, 16, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Saibil, H.R. Chaperone machines for protein folding, unfolding and disaggregation. Nat. Rev. Mol. Cell Boil. 2013, 14, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.J.; Fort, P.E. Heat Shock Proteins Regulatory Role in Neurodevelopment. Front. Mol. Neurosci. 2018, 12. [Google Scholar] [CrossRef] [PubMed]
- Stenmark, H. Rab GTPases as coordinators of vesicle traffic. Nat. Rev. Mol. Cell Boil. 2009, 10, 513–525. [Google Scholar] [CrossRef]
- Feng, Y.; Press, B.; Chen, W.; Zimmerman, J.; Wandinger-Ness, A. Expression and properties of Rab7 in endosome function. Enzym. Eng. Evol. Gen. Methods 2001, 329, 175–187. [Google Scholar] [CrossRef]
- Feng, Y.; Press, B.; Wandinger-Ness, A. Rab 7: An important regulator of late endocytic membrane traffic. J. Cell Boil. 1995, 131, 1435–1452. [Google Scholar] [CrossRef]
- Edinger, A.L.; Cinalli, R.M.; Thompson, C.B. Rab7 Prevents Growth Factor-Independent Survival by Inhibiting Cell-Autonomous Nutrient Transporter Expression. Dev. Cell 2003, 5, 571–582. [Google Scholar] [CrossRef]
- Rosales, K.R.; Peralta, E.R.; Guenther, G.G.; Wong, S.Y.; Edinger, A.L. Rab7 Activation by Growth Factor Withdrawal Contributes to the Induction of Apoptosis. Mol. Boil. Cell 2009, 20, 2831–2840. [Google Scholar] [CrossRef]
- Harrison, R.E.; Bucci, C.; Vieira, O.V.; Schroer, T.A.; Grinstein, S. Phagosomes Fuse with Late Endosomes and/or Lysosomes by Extension of Membrane Protrusions along Microtubules: Role of Rab7 and RILP. Mol. Cell. Boil. 2003, 23, 6494–6506. [Google Scholar] [CrossRef]
- Shim, S.Y.; Karri, S.; Law, S.; Schatzl, H.M.; Gilch, S. Prion infection impairs lysosomal degradation capacity by interfering with rab7 membrane attachment in neuronal cells. Sci. Rep. 2016, 6, 21658. [Google Scholar] [CrossRef]
- Meister, G. Argonaute proteins: Functional insights and emerging roles. Nat. Rev. Genet. 2013, 14, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Peters, L.; Meister, G. Argonaute Proteins: Mediators of RNA Silencing. Mol. Cell 2007, 26, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Hutvagner, G.; Simard, M.J. Argonaute proteins: Key players in RNA silencing. Nat. Rev. Mol. Cell Boil. 2008, 9, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Macrae, I.J.; Ma, E.; Zhou, M.; Robinson, C.V.; Doudna, J.A. In vitro reconstitution of the human RISC-loading complex. Proc. Natl. Acad. Sci. USA 2008, 105, 512–517. [Google Scholar] [CrossRef]
- El-Shami, M.; Pontier, D.; Lahmy, S.; Braun, L.; Picart, C.; Vega, D.; Hakimi, M.-A.; Jacobsen, S.E.; Cooke, R.; Lagrange, T. Reiterated WG/GW motifs form functionally and evolutionarily conserved ARGONAUTE-binding platforms in RNAi-related components. Genes Dev. 2007, 21, 2539–2544. [Google Scholar] [CrossRef]
- Gould, G.W.; Lippincott-Schwartz, J. New roles for endosomes: From vesicular carriers to multi-purpose platforms. Nat. Rev. Mol. Cell Boil. 2009, 10, 287–292. [Google Scholar] [CrossRef]
- Sivaramakrishnan, M.; Sivapathasundharam, B.; Jananni, M. Evaluation of lactate dehydrogenase enzyme activity in saliva and serum of oral submucous fibrosis patients. J. Oral Pathol. Med. 2014, 44, 449–452. [Google Scholar] [CrossRef]
- Schurr, A.; Dong, W.-Q.; Reid, K.H.; West, C.A.; Rigor, B.M. Lactic acidosis and recovery of neuronal function following cerebral hypoxia in vitro. Brain Res. 1988, 438, 311–314. [Google Scholar] [CrossRef]
- Schurr, A.; Payne, R.S.; Miller, J.J.; Rigor, B.M. Brain lactate, not glucose, fuels the recovery of synaptic function from hypoxia upon reoxygenation: An in vitro study. Brain Res. 1997, 744, 105–111. [Google Scholar] [CrossRef]
- Schurr, A.; Payne, R.S.; Miller, J.J.; Tseng, M.T.; Rigor, B.M. Blockade of lactate transport exacerbates delayed neuronal damage in a rat model of cerebral ischemia. Brain Res. 2001, 895, 268–272. [Google Scholar] [CrossRef]
- Valvona, C.; Fillmore, H.L.; Nunn, P.B.; Pilkington, G.J. The Regulation and Function of Lactate Dehydrogenase A: Therapeutic Potential in Brain Tumor. Brain Pathol. 2015, 26, 3–17. [Google Scholar] [CrossRef]
- Berthet, C.; Lei, H.; Thevenet, J.; Gruetter, R.; Magistretti, P.J.; Hirt, L. Neuroprotective Role of Lactate after Cerebral Ischemia. Br. J. Pharmacol. 2009, 29, 1780–1789. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Puschmann, T.B.; Marasek, P.; Inagaki, M.; Pekna, M.; Wilhelmsson, U.; Pekny, M. Increased Neuronal Differentiation of Neural Progenitor Cells Derived from Phosphovimentin-Deficient Mice. Mol. Neurobiol. 2017, 55, 5478–5489. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, H.; Chen, L.; Wang, J.; Lv, Y.; Yang, X.; Zhang, B.Y.; Tian, C.; Shi, Q.; Dong, X.P.; et al. Remarkable impairment of Wnt/beta-catenin signaling in the brains of the mice infected with scrapie agents. J. Neurochem. 2016, 136, 731–740. [Google Scholar] [CrossRef]
- Khosravani, H.; Zhang, Y.; Tsutsui, S.; Hameed, S.; Altier, C.; Hamid, J.; Lina, C.; Michelle, V.; Zenobia, A.; Frank, R.; et al. Prion protein attenuates excitotoxicity by inhibiting NMDA receptors. J. Cell Biol. 2008, 181, 551–565. [Google Scholar] [CrossRef] [PubMed]
- Haitao, Y.; Shigeki, T.; Shahid, H.; Thomas, J.K.; Lina, C.; Peng, X.; Jordan, D.T.; Stuart, A.L.; Peter, K.S.; Gerald, W.Z. Abeta neurotoxicity depends on interactions between copper ions, prion protein, and N-methyl-D-aspartate receptors. Proc. Natl. Acad. Sci. USA 2012, 109, 1737–1742. [Google Scholar]
- Chiarini, L.B.; Freitas, A.R.O.; Zanata, S.M.; Brentani, R.R.; Martins, V.R.; Linden, R. Cellular prion protein transduces neuroprotective signals. EMBO J. 2002, 21, 3317–3326. [Google Scholar] [CrossRef] [PubMed]
- Zanata, S.M.; Lopes, M.H.; Mercadante, A.F.; Hajj, G.N.M.; Chiarini, L.B.; Nomizo, R.; Freitas, A.R.O.; Cabral, A.L.B.; Lee, K.S.; Juliano, M.A.; et al. Stress-inducible protein 1 is a cell surface ligand for cellular prion that triggers neuroprotection. EMBO J. 2002, 21, 3307–3316. [Google Scholar] [CrossRef]
- Lopes, M.H.; Hajj, G.N.M.; Muras, A.G.; Mancini, G.L.; Castro, R.M.P.S.; Ribeiro, K.C.B.; Brentani, R.R.; Linden, R.; Martins, V.R. Interaction of Cellular Prion and Stress-Inducible Protein 1 Promotes Neuritogenesis and Neuroprotection by Distinct Signaling Pathways. J. Neurosci. 2005, 25, 11330–11339. [Google Scholar] [CrossRef]
- Fabiana, A.C.; Marilene, H.L.; Glaucia, N.M.H.; Cleiton, F.M.; Camila, P.A.; Ana, C.M.; Mônica, P.B.V.; Tatiana, A.A.; Andre, R.M.; Suzette, A.P.; et al. Endocytosis of prion protein is required for ERK1/2 signaling induced by stress-inducible protein 1. J. Neurosci. 2008, 28, 6691–6702. [Google Scholar]
- Beraldo, F.H.; Arantes, C.P.; Dos Santos, T.G.; Queiroz, N.G.T.; Young, K.; Rylett, R.; Markus, R.; Prado, M.A.M.; Martins, V.R. Role of α7 Nicotinic Acetylcholine Receptor in Calcium Signaling Induced by Prion Protein Interaction with Stress-inducible Protein 1. J. Boil. Chem. 2010, 285, 36542–36550. [Google Scholar] [CrossRef]
- Jeong, J.-K.; Park, S.-Y. Neuroprotective effect of cellular prion protein (PrPC) is related with activation of alpha7 nicotinic acetylcholine receptor (α7nAchR)-mediated autophagy flux. Oncotarget 2015, 6, 24660–24674. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, D.M.; Acharya, M.; Leighton, P.L.A.; Wang, H.; Daude, N.; Wohlgemuth, S.; Shi, B.; Allison, W.T. Amyloid Beta Precursor Protein and Prion Protein Have a Conserved Interaction Affecting Cell Adhesion and CNS Development. PLoS ONE 2012, 7, e51305. [Google Scholar] [CrossRef] [PubMed]
- Edward, T.P.; Nicole, T.W.; Ishrut, H.; Elizabeth, A.E.; Christopher, B.E.; Jean, C.M.; Herbert, N.B.; Anthony, J.T.; Nigel, M.H. Cellular prion protein regulates beta-secretase cleavage of the Alzheimer’s amyloid precursor protein. Proc. Natl. Acad. Sci. USA 2007, 104, 11062–11067. [Google Scholar]
- Chen, S.; Yadav, S.P.; Surewicz, W.K. Interaction between human prion protein and amyloid-beta (Abeta) oligomers: Role OF N-terminal residues. J. Biol. Chem. 2010, 285, 26377–26383. [Google Scholar] [CrossRef]
- Gimbel, D.A.; Nygaard, H.B.; Coffey, E.E.; Gunther, E.C.; Laurén, J.; Gimbel, Z.A.; Strittmatter, S.M. Memory impairment in transgenic Alzheimer mice requires cellular prion protein. J. Neurosci. 2010, 30, 6367–6374. [Google Scholar] [CrossRef]
- Um, J.W.; Nygaard, H.B.; Heiss, J.K.; Kostylev, M.A.; Stagi, M.; Vortmeyer, A.; Wisniewski, T.; Gunther, E.C.; Strittmatter, S.M. Alzheimer amyloid-β oligomer bound to postsynaptic prion protein activates Fyn to impair neurons. Nat. Neurosci. 2012, 15, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Dohler, F.; Sepulveda-Falla, D.; Krasemann, S.; Altmeppen, H.; Schlüter, H.; Hildebrand, D.; Zerr, I.; Matschke, J.; Glatzel, M. High molecular mass assemblies of amyloid-β oligomers bind prion protein in patients with Alzheimer’s disease. Brain 2014, 137, 873–886. [Google Scholar] [CrossRef] [PubMed]
- Flavio, H.B.; Camila, P.A.; Tiago, G.S.; Cleiton, F.M.; Martin, R.; Gláucia, N.H.; Kil, S.L.; Ana, C.M.; Fabiana, A.C.; Gabriel, L.M.; et al. Metabotropic glutamate receptors transduce signals for neurite outgrowth after binding of the prion protein to laminin gamma1 chain. FASEB J. 2011, 25, 265–279. [Google Scholar]
- Kunishima, N.; Shimada, Y.; Tsuji, Y.; Sato, T.; Yamamoto, M.; Kumasaka, T.; Nakanishi, S.; Jingami, H.; Morikawa, K. Structural basis of glutamate recognition by a dimeric metabotropic glutamate receptor. Nature 2000, 407, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, T.; Satoh, K.; Homma, T.; Nakagaki, T.; Yamaguchi, N.; Atarashi, R.; Sudo, Y.; Uezono, Y.; Ishibashi, D.; Nishida, N. Prion protein interacts with the metabotropic glutamate receptor 1 and regulates the organization of Ca2+ signaling. Biochem. Biophys. Res. Commun. 2020, 525, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Neng-Wei, H.; Andrew, J.N.; Dainan, Z.; Alexandra, J.M.; Tiernan, O.M.; Silvia, A.P.; Cassandra, T.; John, C.; Dominic, M.W.; Michael, J.R.; et al. mGlu5 receptors and cellular prion protein mediate amyloid-beta-facilitated synaptic long-term depression in vivo. Nat. Commun. 2014, 5, 3374. [Google Scholar]
- Ji, W.U.; Adam, C.K.; Mikhail, K.; Jacqueline, K.H.; Massimiliano, S.; Hideyuki, T.; Meghan, E.K.; Alexander, V.; Thomas, W.; Anthony, J.K.; et al. Metabotropic glutamate receptor 5 is a coreceptor for Alzheimer aβ oligomer bound to cellular prion protein. Neuron 2013, 79, 887–902. [Google Scholar]
- Rieger, R.; Edenhofer, F.; Lasmézas, C.I.; Weiss, S.; Lasm, C.I. The human 37-kDa laminin receptor precursor interacts with the prion protein in eukaryotic cells. Nat. Med. 1997, 3, 1383–1388. [Google Scholar] [CrossRef] [PubMed]
- Gauczynski, S.; Peyrin, J.-M.; Haik, S.; Leucht, C.; Hundt, C.; Rieger, R.; Krasemann, S.; Deslys, J.; Dormont, M.; Lasmézas, C.I.; et al. The 37-kDa/67-kDa laminin receptor acts as the cell-surface receptor for the cellular prion protein. EMBO J. 2001, 20, 5863–5875. [Google Scholar] [CrossRef]
- Hundt, C.; Peyrin, J.-M.; Haik, S.; Gauczynski, S.; Leucht, C.; Rieger, R.; Riley, M.L.; Deslys, J.; Dormont, M.; Lasmézas, C.I.; et al. Identification of interaction domains of the prion protein with its 37-kDa/67-kDa laminin receptor. EMBO J. 2001, 20, 5876–5886. [Google Scholar] [CrossRef]
- Urrea, L.; Ferrer, I.; Gavin, R.; Del-Rio, J.A. The cellular prion protein (PrPC) as neuronal receptor for alpha-synuclein. Prion 2017, 11, 226–233. [Google Scholar] [CrossRef][Green Version]
- Graner, E.; Mercadante, A.F.; Zanata, S.M.; Forlenza, O.V.; Cabral, A.L.; Veiga, S.S.; Juliano, M.A.; Roesler, R.; Walz, R.; Minetti, A.; et al. Cellular prion protein binds laminin and mediates neuritogenesis. Mol. Brain Res. 2000, 76, 85–92. [Google Scholar] [CrossRef]
- Graner, E.; Mercadante, A.F.; Zanata, S.M.; Martins, V.R.; Jay, D.G.; Brentani, R.R. Laminin-induced PC-12 cell differentiation is inhibited following laser inactivation of cellular prion protein. FEBS Lett. 2000, 482, 257–260. [Google Scholar] [CrossRef]
- Tiago, G.S.; Flavio, H.B.; Glaucia, N.M.H.; Marilene, H.L.; Martin, R.; Fernanda, C.S.L.; Valeriy, G.O.; Vania, F.P.; Marco, A.M.P.; Vilma, R.M. Laminin-gamma1 chain and stress inducible protein 1 synergistically mediate PrPC-dependent axonal growth via Ca2+ mobilization in dorsal root ganglia neurons. J. Neurochem. 2013, 124, 210–223. [Google Scholar]
- Taylor, D.R.; Hooper, N.M. The low-density lipoprotein receptor-related protein 1 (LRP1) mediates the endocytosis of the cellular prion protein. Biochem. J. 2007, 402, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Parkyn, C.J.; Vermeulen, E.G.M.; Mootoosamy, R.C.; Sunyach, C.; Jacobsen, C.; Oxvig, C.; Moestrup, S.K.; Liu, Q.; Bu, G.; Jen, A.; et al. LRP1 controls biosynthetic and endocytic trafficking of neuronal prion protein. J. Cell Sci. 2008, 121, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Schmitt-Ulms, G.; Legname, G.; Baldwin, M.A.; Ball, H.L.; Bradon, N.; Bosque, P.J.; Crossin, K.L.; Edelman, G.M.; DeArmond, S.J.; Cohen, F.E.; et al. Binding of neural cell adhesion molecules (N-CAMs) to the cellular prion protein. J. Mol. Boil. 2001, 314, 1209–1225. [Google Scholar] [CrossRef] [PubMed]
- Santuccione, A.; Sytnyk, V.; Leshchyns’Ka, I.; Schachner, M. Prion protein recruits its neuronal receptor NCAM to lipid rafts to activate p59fyn and to enhance neurite outgrowth. J. Cell Boil. 2005, 169, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Harmey, J.; Doyle, D.; Brown, V.; Rogers, M. The Cellular Isoform of the Prion Protein, PrPC, Is Associated with Caveolae in Mouse Neuroblastoma (N2a) Cells. Biochem. Biophys. Res. Commun. 1995, 210, 753–759. [Google Scholar] [CrossRef]
- Vey, M.; Pilkuhn, S.; Wille, H.; Nixon, R.; DeArmond, S.J.; Smart, E.J.; Anderson, R.G.W.; Taraboulos, A.; Prusiner, S.B. Subcellular colocalization of the cellular and scrapie prion proteins in caveolae-like membranous domains. Proc. Natl. Acad. Sci. USA 1996, 93, 14945–14949. [Google Scholar] [CrossRef]
- Mattia, T.; Enzo, S.; Cristiana, G.; Spartaco, S.; Massimo, R.; Patrizia, L.; Vittorio, T. Cellular prion protein and caveolin-1 interaction in a neuronal cell line precedes Fyn/Erk 1/2 signal transduction. J. Biomed. Biotechnol. 2006, 5, 69469. [Google Scholar]
- Pantera, B.; Bini, C.; Cirri, P.; Paoli, P.; Camici, G.; Manao, G.; Caselli, A. PrPcactivation induces neurite outgrowth and differentiation in PC12 cells: Role for caveolin-1 in the signal transduction pathway. J. Neurochem. 2009, 110, 194–207. [Google Scholar] [CrossRef]
- Bodrikov, V.; Solis, G.P.; Stuermer, C.A. Prion Protein Promotes Growth Cone Development through Reggie/Flotillin-Dependent N-Cadherin Trafficking. J. Neurosci. 2011, 31, 18013–18025. [Google Scholar] [CrossRef]
- Solis, G.P.; Schrock, Y.; Hülsbusch, N.; Wiechers, M.; Plattner, H.; Stuermer, C.A. Reggies/flotillins regulate E-cadherin–mediated cell contact formation by affecting EGFR trafficking. Mol. Boil. Cell 2012, 23, 1812–1825. [Google Scholar] [CrossRef]
- Llorens, F.; Carulla, P.; Villa, A.; Torres, J.M.; Fortes, P.; Ferrer, I.; Del Río, J.A. PrPC regulates epidermal growth factor receptor function and cell shape dynamics in Neuro2a cells. J. Neurochem. 2013, 127. [Google Scholar] [CrossRef]
- Martellucci, S.; Manganelli, V.; Santacroce, C.; Santilli, F.; Piccoli, L.; Sorice, M.; Mattei, V. Role of Prion protein-EGFR multimolecular complex during neuronal differentiation of human dental pulp-derived stem cells. Prion 2018, 12, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Rangel, A.; Burgaya, F.; Gavín, R.; Soriano, E.; Aguzzi, A.; Del Río, J.A. Enhanced susceptibility ofPrnp-deficient mice to kainate-induced seizures, neuronal apoptosis, and death: Role of AMPA/kainate receptors. J. Neurosci. Res. 2007, 85, 2741–2755. [Google Scholar] [CrossRef] [PubMed]
- Patricia, C.; Ana, B.; Alejandra, R.; Rosalina, G.; Isidro, F.; Carme, C.; José, A.R.; Franc, L. Neuroprotective role of PrPC against kainate-induced epileptic seizures and cell death depends on the modulation of JNK3 activation by GluR6/7-PSD-95 binding. Mol. Biol. Cell 2011, 22, 3041–3054. [Google Scholar]
- Choi, H.J.; Kang, K.S.; Fukui, M.; Zhu, B.T. Critical role of the JNK-p53-GADD45α apoptotic cascade in mediating oxidative cytotoxicity in hippocampal neurons. Br. J. Pharmacol. 2010, 162, 175–192. [Google Scholar] [CrossRef]
- Mercer, R.C.C.; Ma, L.; Watts, J.; Strome, R.; Wohlgemuth, S.; Yang, J.; Cashman, N.R.; Coulthart, M.B.; Schmitt-Ulms, G.; Jhamandas, J.; et al. The Prion Protein Modulates A-type K+ Currents Mediated by Kv4.2 Complexes through Dipeptidyl Aminopeptidase-like Protein 6. J. Boil. Chem. 2013, 288, 37241–37255. [Google Scholar] [CrossRef]
- Assunta, S.; Simona, C.; Claudia, V.; Elena, R.; Raffaella, M.; Steven, B.C.; Ilaria, B.; Susanna, M.; Mara, C.; Edoardo, M. Mutant PrPC suppresses glutamatergic neurotransmission in cerebellar granule neurons by impairing membrane delivery of VGCC alpha(2)delta-1 Subunit. Neuron 2012, 74, 300–313. [Google Scholar]
- Bouybayoune, I.; Mantovani, S.; Del Gallo, F.; Bertani, I.; Restelli, E.; Comerio, L.; Tapella, L.; Baracchi, F.; Fernández-Borges, N.; Mangieri, M.; et al. Transgenic Fatal Familial Insomnia Mice Indicate Prion Infectivity-Independent Mechanisms of Pathogenesis and Phenotypic Expression of Disease. PLoS Pathog. 2015, 11, e1004796. [Google Scholar] [CrossRef]
- Ermonval, M.; Baudry, A.; Baychelier, F.; Pradines, E.; Pietri, M.; Oda, K.; Schneider, B.; Mouillet-Richard, S.; Launay, J.-M.; Kellermann, O. The Cellular Prion Protein Interacts with the Tissue Non-Specific Alkaline Phosphatase in Membrane Microdomains of Bioaminergic Neuronal Cells. PLoS ONE 2009, 4, e6497. [Google Scholar] [CrossRef]
- Kleene, R.; Loers, G.; Langer, J.; Frobert, Y.; Buck, F.; Schachner, M. Prion Protein Regulates Glutamate-Dependent Lactate Transport of Astrocytes. J. Neurosci. 2007, 27, 12331–12340. [Google Scholar] [CrossRef]
- Taylor, D.R.; Whitehouse, I.J.; Hooper, N.M. Glypican-1 Mediates Both Prion Protein Lipid Raft Association and Disease Isoform Formation. PLoS Pathog. 2009, 5, e1000666. [Google Scholar] [CrossRef]
- Radovanovic, I.; Braun, N.; Giger, O.T.; Mertz, K.; Miele, G.; Prinz, M.; Navarro, B.; Aguzzi, A. Truncated Prion Protein and Doppel Are Myelinotoxic in the Absence of Oligodendrocytic PrPC. J. Neurosci. 2005, 25, 4879–4888. [Google Scholar] [CrossRef] [PubMed]
- Küffer, A.; Lakkaraju, A.K.K.; Mogha, A.; Petersen, S.; Airich, K.; Doucerain, C.; Marpakwar, R.; Bakirci, P.; Senatore, A.; Monnard, A.; et al. The prion protein is an agonistic ligand of the G protein-coupled receptor Adgrg6. Nature 2016, 536, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Keshet, G.; Bar-Peled, O.; Yaffe, D.; Nudel, U.; Gabizon, R. The Cellular Prion Protein Colocalizes with the Dystroglycan Complex in the Brain. J. Neurochem. 2002, 75, 1889–1897. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Lei, Y.-J.; Han, J.; Shi, Q.; Chen, L.; Guo, Y.; Gao, Y.-J.; Chen, J.-M.; Jiang, H.-Y.; Zhou, W.; et al. Recombinant Neural Protein PrP Can Bind with Both Recombinant and Native Apolipoprotein E In Vitro. Acta Biochim. Biophys. Sin. 2006, 38, 593–601. [Google Scholar] [CrossRef]
- Huang, T.; Xu, J.; Xiang, J.; Lu, Y.; Chen, R.; Huang, L.; Xiao, G.; Sun, G. PrPC interacts with potassium channel tetramerization domain containing 1 (KCTD1) protein through the PrP51-136 region containing octapeptide repeats. Biochem. Biophys. Res. Commun. 2012, 417, 182–186. [Google Scholar] [CrossRef]
- Picard, D. Heat-shock protein 90, a chaperone for folding and regulation. Cell. Mol. Life Sci. 2002, 59, 1640–1648. [Google Scholar] [CrossRef]
- Chen, S. Hop as an Adaptor in the Heat Shock Protein 70 (Hsp70) and Hsp90 Chaperone Machinery. J. Boil. Chem. 1998, 273, 35194–35200. [Google Scholar] [CrossRef]
- Roffé, M.; Beraldo, F.H.; Bester, R.; Nunziante, M.; Bach, C.; Mancini, G.; Gilch, S.; Vorberg, I.; Castilho, B.; Martins, V.R.; et al. Prion protein interaction with stress-inducible protein 1 enhances neuronal protein synthesis via mTOR. Proc. Natl. Acad. Sci. USA 2010, 107, 13147–13152. [Google Scholar] [CrossRef]
- Sakudo, A.; Lee, D.-C.; Li, S.; Nakamura, T.; Matsumoto, Y.; Saeki, K.; Itohara, S.; Ikuta, K.; Onodera, T. PrP cooperates with STI1 to regulate SOD activity in PrP-deficient neuronal cell line. Biochem. Biophys. Res. Commun. 2005, 328, 14–19. [Google Scholar] [CrossRef]
- Sargent, P.B. The Diversity of Neuronal Nicotinic Acetylcholine Receptors. Annu. Rev. Neurosci. 1993, 16, 403–443. [Google Scholar] [CrossRef]
- Zdanowski, R.; Krzyzowska, M.; Ujazdowska, D.; Lewicka, A.; Lewicki, S. Role of α7 nicotinic receptor in the immune system and intracellular signaling pathways. Central Eur. J. Immunol. 2015, 40, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Broide, R.S.; Leslie, F.M. The α7 nicotinic acetylcholine receptor in neuronal plasticity. Mol. Neurobiol. 1999, 20, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Santos, R.; Hung, S.-Y.; Huang, W.-P.; Liou, H.-C.; Fu, W.-M. Faculty Opinions recommendation of Autophagy protects neuron from Abeta-induced cytotoxicity. Fac. Opin. Post-Publ. Peer Rev. Biomed. Lit. 2010, 5. [Google Scholar] [CrossRef]
- Thomsen, M.; Hansen, H.; Timmerman, M.; Mikkelsen, J. Cognitive Improvement by Activation of α7 Nicotinic Acetylcholine Receptors: From Animal Models to Human Pathophysiology. Curr. Pharm. Des. 2010, 16, 323–343. [Google Scholar] [CrossRef] [PubMed]
- Srivareerat, M.; Tran, T.T.; Salim, S.; Aleisa, A.M.; Alkadhi, K.A. Chronic nicotine restores normal Aβ levels and prevents short-term memory and E-LTP impairment in Aβ rat model of Alzheimer’s disease. Neurobiol. Aging 2011, 32, 834–844. [Google Scholar] [CrossRef]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef]
- Zheng, H.; Koo, E.H. Biology and pathophysiology of the amyloid precursor protein. Mol. Neurodegener. 2011, 6, 27. [Google Scholar] [CrossRef]
- Haas, L.T.; Kostylev, M.A.; Strittmatter, S.M. Therapeutic Molecules and Endogenous Ligands Regulate the Interaction between Brain Cellular Prion Protein (PrPC) and Metabotropic Glutamate Receptor 5 (mGluR5). J. Boil. Chem. 2014, 289, 28460–28477. [Google Scholar] [CrossRef]
- Ferraguti, F.; Crepaldi, L.; Nicoletti, F. Metabotropic Glutamate 1 Receptor: Current Concepts and Perspectives. Pharmacol. Rev. 2008, 60, 536–581. [Google Scholar] [CrossRef]
- Larson, M.; Sherman, M.A.; Amar, F.; Nuvolone, M.; Schneider, J.A.; Bennett, D.A.; Aguzzi, A.; Lesne, S. The complex PrPC-Fyn couples human oligomeric Aβ with pathological tau changes in Alzheimer’s disease. J. Neurosci. 2012, 32, 16857–16871. [Google Scholar] [CrossRef] [PubMed]
- Rushworth, J.V.; Jarosz-Griffiths, H.H.; Watt, N.T.; Hooper, N.M. Prion Protein-mediated Toxicity of Amyloid-β Oligomers Requires Lipid Rafts and the Transmembrane LRP1. J. Boil. Chem. 2013, 288, 8935–8951. [Google Scholar] [CrossRef] [PubMed]
- Ostapchenko, V.G.; Beraldo, F.H.; Mohammad, A.H.; Xie, Y.F.; Hirata, P.H.; Magalhaes, A.C.; Guillaume, L.; Hongbin, L.; Andrzej, M.; Jillian, C.B.; et al. The prion protein ligand, stress-inducible phosphoprotein 1, regulates amyloid-beta oligomer toxicity. J. Neurosci. 2013, 33, 16552–16564. [Google Scholar] [CrossRef]
- Goniotaki, D.; Lakkaraju, A.K.K.; Shrivastava, A.N.; Bakirci, P.; Sorce, S.; Senatore, A.; Marpakwar, R.; Hornemann, S.; Gasparini, F.; Triller, A.; et al. Inhibition of group-I metabotropic glutamate receptors protects against prion toxicity. PLoS Pathog. 2017, 13, e1006733. [Google Scholar] [CrossRef] [PubMed]
- Leucht, C.; Simoneau, S.; Rey, C.; Vana, K.; Rieger, R.; Lasmézas, C.I.; Weiss, S. The 37 kDa/67 kDa laminin receptor is required for PrP Sc propagation in scrapie-infected neuronal cells. EMBO Rep. 2003, 4, 290–295. [Google Scholar] [CrossRef]
- Damien, L.; Caroline, D.; Mathéa, P.; Elodie, P.; Sophie, B.; Jacques, C.; Hector, A.-O.; Sophie, M.-R.; Jean-Marie, L.; Odile, K.L.D.; et al. Neuritogenesis: The prion protein controls beta1 integrin signaling activity. FASEB J. 2012, 26, 678–690. [Google Scholar]
- Meade, R.M.; Fairlie, D.P.; Mason, J.M. Alpha-synuclein structure and Parkinson’s disease—lessons and emerging principles. Mol. Neurodegener. 2019, 14. [Google Scholar] [CrossRef]
- Ferreira, D.G.; Temido-Ferreira, M.; Miranda, H.V.; Batalha, V.L.; Coelho, J.E.; Szegö, É.M.; Marques-Morgado, I.; Vaz, S.H.; Rhee, J.S.; Schmitz, M.; et al. α-synuclein interacts with PrPC to induce cognitive impairment through mGluR5 and NMDAR2B. Nat. Neurosci. 2017, 20, 1569–1579. [Google Scholar] [CrossRef]
- Burgeson, R.E.; Chiquet, M.; Deutzmann, R.; Ekblom, P.; Engel, J.; Kleinman, H.; Martin, G.R.; Meneguzzi, G.; Paulsson, M.; Sanes, J.; et al. A new nomenclature for the laminins. Matrix Boil. 1994, 14, 209–211. [Google Scholar] [CrossRef]
- Grimpe, B.; Dong, S.; Doller, C.; Temple, K.; Malouf, A.T.; Silver, J. The critical role of basement membrane-independent laminin γ 1 chain during axon regeneration in the CNS. J. Neurosci. 2002, 22, 3144–3160. [Google Scholar] [CrossRef]
- Newcomer, J.W.; Farber, N.B.; Olney, J.W. NMDA receptor function, memory, and brain aging. Dialog- Clin. Neurosci. 2000, 2, 219–232. [Google Scholar]
- Jahr, C.E.; Stevens, C.F. Calcium permeability of the N-methyl-D-aspartate receptor channel in hippocampal neurons in culture. Proc. Natl. Acad. Sci. USA 1993, 90, 11573–11577. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, P.; Bellone, C.; Zhou, Q. NMDA receptor subunit diversity: Impact on receptor properties, synaptic plasticity and disease. Nat. Rev. Neurosci. 2013, 14, 383–400. [Google Scholar] [CrossRef] [PubMed]
- Hunt, D.L.; Castillo, P.E. Synaptic plasticity of NMDA receptors: Mechanisms and functional implications. Curr. Opin. Neurobiol. 2012, 22, 496–508. [Google Scholar] [CrossRef]
- Morris, R.G.M.; Moser, E.I.; Riedel, G.; Martin, S.; Sandin, J.; Day, M.; O’Carroll, C. Elements of a neurobiological theory of the hippocampus: The role of activity-dependent synaptic plasticity in memory. Philos. Trans. R. Soc. B Boil. Sci. 2003, 358, 773–786. [Google Scholar] [CrossRef]
- Li, C.-T.; Yang, K.-C.; Lin, W.-C. Glutamatergic Dysfunction and Glutamatergic Compounds for Major Psychiatric Disorders: Evidence from Clinical Neuroimaging Studies. Front. Psychiatry 2019, 9. [Google Scholar] [CrossRef]
- Gielen, M.; Retchless, B.S.; Mony, L.; Johnson, J.W.; Paoletti, P. Mechanism of differential control of NMDA receptor activity by NR2 subunits. Nature 2009, 459, 703–707. [Google Scholar] [CrossRef]
- Gasperini, L.; Meneghetti, E.; Pastore, B.; Benetti, F.; Legname, G. Prion protein and copper cooperatively protect neurons by modulating NMDA receptor through S-nitrosylation. Antioxid. Redox Signal. 2015, 22, 772–784. [Google Scholar] [CrossRef]
- Choi, Y.-B.; Tenneti, L.; Le, D.A.; Ortiz, J.; Bai, G.; Chen, H.-S.V.; Lipton, S.A. Molecular basis of NMDA receptor-coupled ion channel modulation by S-nitrosylation. Nat. Neurosci. 2000, 3, 15–21. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W. Nitric oxide synthases: Regulation and function. Eur. Hear. J. 2011, 33, 829–837. [Google Scholar] [CrossRef]
- Stys, P.K.; You, H.; Zamponi, G.W. Copper-dependent regulation of NMDA receptors by cellular prion protein: Implications for neurodegenerative disorders. J. Physiol. 2012, 590, 1357–1368. [Google Scholar] [CrossRef] [PubMed]
- Biasini, E.; Unterberger, U.; Solomon, I.H.; Massignan, T.; Senatore, A.; Bian, H.; Voigtlaender, T.; Bowman, F.P.; Bonetto, V.; Chiesa, R.; et al. A mutant prion protein sensitizes neurons to glutamate-induced excitotoxicity. J. Neurosci. 2013, 33, 2408–2418. [Google Scholar] [CrossRef] [PubMed]
- Thellung, S.; Gatta, E.; Pellistri, F.; Corsaro, A.; Villa, V.; Vassalli, M.; Robello, M.; Florio, T. Excitotoxicity Through NMDA Receptors Mediates Cerebellar Granule Neuron Apoptosis Induced by Prion Protein 90-231 Fragment. Neurotox. Res. 2012, 23, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Nykjaer, A.; Willnow, T.E. The low-density lipoprotein receptor gene family: A cellular Swiss army knife? Trends Cell Boil. 2002, 12, 273–280. [Google Scholar] [CrossRef]
- Strickland, D.K.; Gonias, S.L.; Argraves, W.S. Diverse roles for the LDL receptor family. Trends Endocrinol. Metab. 2002, 13, 66–74. [Google Scholar] [CrossRef]
- Gonias, S.L.; Campana, W.M. LDL Receptor–Related Protein-1. Am. J. Pathol. 2014, 184, 18–27. [Google Scholar] [CrossRef]
- Julia, H.-R.; Séverine, M.-L.; Théo, Z.H.; Elodie, P.; Aurélie, A.-B.; Benoît, S.; Anne, B.; Jean-Marie, L.; Sophie, M.-R. A PrPC-caveolin-Lyn complex negatively controls neuronal GSK3beta and serotonin 1B receptor. Sci. Rep. 2014, 4, 4881. [Google Scholar]
- Jarosz-Griffiths, H.H.; Whitehouse, I.J.; Baybutt, H.; Brown, D.; Kellett, K.A.B.; Jackson, C.D.; Turner, A.J.; Piccardo, P.; Manson, J.C.; Hooper, N.M. Prion Protein Interacts with BACE1 Protein and Differentially Regulates Its Activity toward Wild Type and Swedish Mutant Amyloid Precursor Protein. J. Boil. Chem. 2011, 286, 33489–33500. [Google Scholar] [CrossRef]
- Griffiths, H.H.; Whitehouse, I.J.; Hooper, N.M. Regulation of amyloid-beta production by the prion protein. Prion 2012, 6, 217–222. [Google Scholar] [CrossRef]
- Kleene, R.; Schachner, M. Glycans and neural cell interactions. Nat. Rev. Neurosci. 2004, 5, 195–208. [Google Scholar] [CrossRef]
- Walsh, F.S.; Doherty, P. Neural Cell Adhesion Molecules of the Immunoglobulin Superfamily: Role in Axon Growth and Guidance. Annu. Rev. Cell Dev. Boil. 1997, 13, 425–456. [Google Scholar] [CrossRef] [PubMed]
- Weledji, E.P.; Assob, J.C.N. The ubiquitous neural cell adhesion molecule (N-CAM). Ann. Med. Surg. 2014, 3, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Barbas, J.A.; Chaix, J.C.; Steinmetz, M.; Goridis, C. Differential splicing and alternative polyadenylation generates distinct NCAM transcripts and proteins in the mouse. EMBO J. 1988, 7, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Schvartz, I.; Seger, D.; Shaltiel, S. Vitronectin. Int. J. Biochem. Cell Boil. 1999, 31, 539–544. [Google Scholar] [CrossRef]
- Marti´nez-Morales, J.-R.; Martí, E.; Frade, J.M.; Rodri´guez-Te´bar, A. Developmentally regulated vitronectin influences cell differentiation, neuron survival and process outgrowth in the developing chicken retina. Neuroscience 1995, 68, 245–253. [Google Scholar] [CrossRef]
- Chidlow, J.H.; Sessa, W. Caveolae, caveolins, and cavins: Complex control of cellular signalling and inflammation. Cardiovasc. Res. 2010, 86, 219–225. [Google Scholar] [CrossRef]
- Fridolfsson, H.N.; Roth, D.M.; Insel, P.A.; Patel, H.H. Regulation of intracellular signaling and function by caveolin. FASEB J. 2014, 28, 3823–3831. [Google Scholar] [CrossRef]
- Fu, G.; Wang, W.; Luo, B.-H. Overview: Structural Biology of Integrins. Breast Cancer 2011, 757, 81–99. [Google Scholar] [CrossRef]
- Guan, J.-L. Integrin signaling through FAK in the regulation of mammary stem cells and breast cancer. IUBMB Life 2010, 62, 268–276. [Google Scholar] [CrossRef]
- Stuermer, C.A. The reggie/flotillin connection to growth. Trends Cell Boil. 2010, 20, 6–13. [Google Scholar] [CrossRef]
- Munderloh, C.; Solis, G.P.; Bodrikov, V.; Jaeger, F.A.; Wiechers, M.; Málaga-Trillo, E.; Stuermer, C.A. Reggies/Flotillins Regulate Retinal Axon Regeneration in the Zebrafish Optic Nerve and Differentiation of Hippocampal and N2a Neurons. J. Neurosci. 2009, 29, 6607–6615. [Google Scholar] [CrossRef] [PubMed]
- Meister, M.; Tikkanen, R. Endocytic Trafficking of Membrane-Bound Cargo: A Flotillin Point of View. Membranes 2014, 4, 356–371. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Z.; Peng, H.; Zeng, K. Recent advances on the roles of epidermal growth factor receptor in psoriasis. Am. J. Transl. Res. 2019, 11, 520–528. [Google Scholar] [PubMed]
- Zhang, M.; Mu, X.-Y.; Jiang, S.; Liu, Q.-L.; Li, D.-W. Relationship between epidermal growth factor receptor gene expression and radiosensitivity of non-small-cell lung cancer cells. Zhonghua zhong liu za zhi Chin. J. Oncol. 2013, 35, 94–97. [Google Scholar]
- Pinheiro, P.S.; Mulle, C. Kainate receptors. Cell Tissue Res. 2006, 326, 457–482. [Google Scholar] [CrossRef] [PubMed]
- Frerking, M.; Nicoll, R.A. Synaptic kainate receptors. Curr. Opin. Neurobiol. 2000, 10, 342–351. [Google Scholar] [CrossRef]
- Niethammer, M.; Kim, E.; Sheng, M. Interaction between the C terminus of NMDA receptor subunits and multiple members of the PSD-95 family of membrane-associated guanylate kinases. J. Neurosci. 1996, 16, 2157–2163. [Google Scholar] [CrossRef]
- Kim, E.; Cho, K.-O.; Rothschild, A.; Sheng, M. Heteromultimerization and NMDA Receptor-Clustering Activity of Chapsyn-110, a Member of the PSD-95 Family of Proteins. Neuron 1996, 17, 103–113. [Google Scholar] [CrossRef]
- Chen, X.; Nelson, C.D.; Li, X.; Winters, C.A.; Azzam, R.; Sousa, A.A.; Leapman, R.D.; Gainer, H.; Sheng, M.; Reese, T.S. PSD-95 is required to sustain the molecular organization of the postsynaptic density. J. Neurosci. 2011, 31, 6329–6338. [Google Scholar] [CrossRef]
- Tezuka, T.; Umemori, H.; Akiyama, T.; Nakanishi, S.; Yamamoto, T. PSD-95 promotes Fyn-mediated tyrosine phosphorylation of the N-methyl-D-aspartate receptor subunit NR2A. Proc. Natl. Acad. Sci. USA 1999, 96, 435–440. [Google Scholar] [CrossRef]
- Jerng, H.H.; Pfaffinger, P.J. Modulatory mechanisms and multiple functions of somatodendritic A-type K+ channel auxiliary subunits. Front. Cell. Neurosci. 2014, 8. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A. Voltage-gated calcium channels. Cold Spring Harb Perspect Biol. 2011, 3, a003947. [Google Scholar] [CrossRef]
- Bauer, C.S.; Van Minh, A.T.; Kadurin, I.; Dolphin, A.C. A new look at calcium channel α2δ subunits. Curr. Opin. Neurobiol. 2010, 20, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, J.S.; Ferron, L.; Kadurin, I.; Pratt, W.S.; Dolphin, A.C. Functional exofacially tagged N-type calcium channels elucidate the interaction with auxiliary alpha2delta-1 subunits. Proc. Natl. Acad. Sci. USA 2014, 111, 8979–8984. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, M.; Bittner, T.; Mitteregger, G.; Haider, N.; Moosmang, S.; Kretzschmar, H.; Herms, J. Loss of the cellular prion protein affects the Ca2+ homeostasis in hippocampal CA1 neurons. J. Neurochem. 2006, 98, 1876–1885. [Google Scholar] [CrossRef] [PubMed]
- Dossena, S.; Imeri, L.; Mangieri, M.; Garofoli, A.; Ferrari, L.; Senatore, A.; Restelli, E.; Balducci, C.; Fiordaliso, F.; Salio, M.; et al. Mutant Prion Protein Expression Causes Motor and Memory Deficits and Abnormal Sleep Patterns in a Transgenic Mouse Model. Neuron 2008, 60, 598–609. [Google Scholar] [CrossRef]
- Chiesa, R.; Piccardo, P.; Ghetti, B.; Harris, D.A. Neurological Illness in Transgenic Mice Expressing a Prion Protein with an Insertional Mutation. Neuron 1998, 21, 1339–1351. [Google Scholar] [CrossRef]
- Moss, D. Alkaline Phosphatase Isoenzymes. Enzyme 1975, 20, 20–34. [Google Scholar] [CrossRef]
- Low, M.G.; Zilversmit, D.B. Role of phosphatidylinositol in attachment of alkaline phosphatase to membranes. Biochemistry 1980, 19, 3913–3918. [Google Scholar] [CrossRef]
- Narisawa, S.; Yadav, M.C.; Millán, J.L. In Vivo Overexpression of Tissue-Nonspecific Alkaline Phosphatase Increases Skeletal Mineralization and Affects the Phosphorylation Status of Osteopontin. J. Bone Miner. Res. 2013, 28, 1587–1598. [Google Scholar] [CrossRef]
- Kaplan, J.H. Biochemistry of Na,K-ATPase. Annu. Rev. Biochem. 2002, 71, 511–535. [Google Scholar] [CrossRef]
- David, G. Integral membrane heparan sulfate proteoglycans. FASEB J. 1993, 7, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, L.; Maurel, P.; Rauch, U.; Margolis, R.; Margolis, R. Cloning of a major heparan sulfate proteoglycan from brain and identification as the rat form of glypican. Biochem. Biophys. Res. Commun. 1992, 188, 395–401. [Google Scholar] [CrossRef]
- Hijazi, N.; Kariv-Inbal, Z.; Gasset, M.; Gabizon, R. PrPScIncorporation to Cells Requires Endogenous Glycosaminoglycan Expression. J. Boil. Chem. 2005, 280, 17057–17061. [Google Scholar] [CrossRef] [PubMed]
- Horonchik, L.; Tzaban, S.; Ben-Zaken, O.; Yedidia, Y.; Rouvinski, A.; Papy-Garcia, D.; Barritault, D.; Vlodavsky, I.; Taraboulos, A. Heparan Sulfate Is a Cellular Receptor for Purified Infectious Prions. J. Boil. Chem. 2005, 280, 17062–17067. [Google Scholar] [CrossRef] [PubMed]
- Amit, M.; Breanne, L.H.; Dan, C.; Jessica, J.; Nicholas, E.S.; Ueli, S.; Xianhua, P.; Valeria, C.; Kelly, R.M. Gpr126/Adgrg6 Has Schwann Cell Autonomous and Nonautonomous Functions in Peripheral Nerve Injury and Repair. J. Neurosci. 2016, 36, 12351–12367. [Google Scholar]
- Ravenscroft, G.; Nolent, F.; Rajagopalan, S.; Meireles, A.M.; Paavola, K.J.; Gaillard, D.; Alanio, E.; Buckland, M.; Arbuckle, S.; Krivanek, M.; et al. Mutations of GPR126 Are Responsible for Severe Arthrogryposis Multiplex Congenita. Expand. Spectr. BAF-Relat. Disord. Novo Var. SMARCC2 Cause Syndr. Intellect. Disabil. Dev. Delay 2015, 96, 955–961. [Google Scholar] [CrossRef]
- Hashida-Okumura, A.; Okumura, N.; Iwamatsu, A.; Buijs, R.M.; Romijn, H.J.; Nagai, K. Interaction of Neuronal Nitric-oxide Synthase with α1-Syntrophin in Rat Brain. J. Boil. Chem. 1999, 274, 11736–11741. [Google Scholar] [CrossRef][Green Version]
- Isaacs, J.D.; Jackson, G.S.; Altmann, D.M. The role of the cellular prion protein in the immune system. Clin. Exp. Immunol. 2006, 146, 1–8. [Google Scholar] [CrossRef]
- Termini, C.M.; Gillette, J.M. Tetraspanins Function as Regulators of Cellular Signaling. Front. Cell Dev. Boil. 2017, 5. [Google Scholar] [CrossRef]
- Guo, M.; Huang, T.; Cui, Y.; Pan, B.; Shen, A.; Sun, Y.; Yi, Y.; Wang, Y.; Xiao, G.; Sun, G. PrPC interacts with tetraspanin-7 through bovine PrP154–182 containing alpha-helix 1. Biochem. Biophys. Res. Commun. 2008, 365, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Perot, B.P.; Ménager, M.M. Tetraspanin 7 and its closest paralog tetraspanin 6: Membrane organizers with key functions in brain development, viral infection, innate immunity, diabetes and cancer. Med Microbiol. Immunol. 2020, 209, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, L.; Bruni, A.C. Mutations in Prion Protein Gene: Pathogenic Mechanisms in C-Terminal vs. N-Terminal Domain, a Review. Int. J. Mol. Sci. 2019, 20, 3606. [Google Scholar] [CrossRef] [PubMed]
- Minikel, E.; Vallabh, S.M.; Lek, M.; Estrada, K.; Samocha, K.E.; Sathirapongsasuti, J.F.; McLean, C.Y.; Tung, J.Y.; Yu, L.P.C.; Gambetti, P.; et al. Quantifying prion disease penetrance using large population control cohorts. Sci. Transl. Med. 2016, 8, 322ra9. [Google Scholar] [CrossRef]
- Scialo, C.; De Cecco, E.; Manganotti, P.; Legname, G. Prion and Prion-Like Protein Strains: Deciphering the Molecular Basis of Heterogeneity in Neurodegeneration. Viruses 2019, 11, 261. [Google Scholar] [CrossRef]
- Benetti, F.; Legname, G. New insights into structural determinants of prion protein folding and stability. Prion 2015, 9, 119–124. [Google Scholar] [CrossRef]
- Walmsley, A.R.; Zeng, F.; Hooper, N.M. The N-terminal Region of the Prion Protein Ectodomain Contains a Lipid Raft Targeting Determinant. J. Boil. Chem. 2003, 278, 37241–37248. [Google Scholar] [CrossRef]
- Turnbaugh, J.A.; Westergard, L.; Unterberger, U.; Biasini, E.; Harris, D. The N-Terminal, Polybasic Region Is Critical for Prion Protein Neuroprotective Activity. PLoS ONE 2011, 6, e25675. [Google Scholar] [CrossRef]
- Ghetti, B.; Piccardo, P.; Zanusso, G. Dominantly inherited prion protein cerebral amyloidosis—A modern view of Gerstmann–Sträussler–Scheinker. Handb. Clin. Neurol. 2018, 153, 243–269. [Google Scholar] [CrossRef]
- Nitrini, R.; Da Silva, L.S.T.; Rosemberg, S.; Caramelli, P.; Carrilho, P.E.M.; Iughetti, P.; Passos-Bueno, M.R.; Zatz, M.; Albrecht, S.; Leblanc, A. Prion disease resembling frontotemporal dementia and parkinsonism linked to chromosome 17. Arq. Neuro-Psiquiatr. 2001, 59, 161–164. [Google Scholar] [CrossRef]
- Hall, D.A.; Leehey, M.A.; Filley, C.M.; Steinbart, E.; Montine, T.; Schellenberg, G.D.; Bosque, P.; Nixon, R.; Bird, T. PRNP H187R mutation associated with neuropsychiatric disorders in childhood and dementia. Neurol 2005, 64, 1304–1306. [Google Scholar] [CrossRef] [PubMed]
- Woulfe, J.; Kertesz, A.; Frohn, I.; Bauer, S.; George-Hyslop, P.S.; Bergeron, C. Gerstmann-Sträussler-Scheinker disease with the Q217R mutation mimicking frontotemporal dementia. Acta Neuropathol. 2005, 110, 317–319. [Google Scholar] [CrossRef] [PubMed]
- Clerici, F.; Elia, A.; Girotti, F.; Contri, P.; Mariani, C.; Tagliavini, F.; Di Fede, G. Atypical presentation of Creutzfeldt–Jakob disease: The first Italian case associated with E196K mutation in the PRNP gene. J. Neurol. Sci. 2008, 275, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Giovagnoli, A.R.; Di Fede, G.; Aresi, A.; Reati, F.; Rossi, G.; Tagliavini, F. Atypical frontotemporal dementia as a new clinical phenotype of Gerstmann-Straussler-Scheinker disease with the PrP-P102L mutation. Description of a previously unreported Italian family. Neurol. Sci. 2008, 29, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Samaia, H.B.; Mari, J.D.J.; Vallada, H.; Moura, R.P.; Simpson, A.J.G.; Brentania, R.R.; Brentani, H. A prion-linked psychiatric disorder. Nature 1997, 390, 241. [Google Scholar] [CrossRef]
- Collinge, J.; Brown, J.; Hardy, J.; Mullan, M.; Rossor, M.; Baker, H.; Crow, T.J.; Lofthouse, R.; Poulter, M.; Ridley, R.; et al. Inherited prion disease with 144 base pair gene insertion: 2. Clinical and pathological features. Brain 1992, 115, 687–710. [Google Scholar] [CrossRef]
- Finckh, U.; Müller-Thomsen, T.; Mann, U.; Eggers, C.; Marksteiner, J.; Meins, W.; Binetti, G.; Alberici, A.; Hock, C.; Nitsch, R.M.; et al. High Prevalence of Pathogenic Mutations in Patients with Early-Onset Dementia Detected by Sequence Analyses of Four Different Genes. Am. J. Hum. Genet. 1999, 66, 110–117. [Google Scholar] [CrossRef]
- Hayes, S.; Malacrida, B.; Kiely, M.; Kiely, P.A. Studying protein–protein interactions: Progress, pitfalls and solutions. Biochem. Soc. Trans. 2016, 44, 994–1004. [Google Scholar] [CrossRef]
- Reidenbach, A.G.; Minikel, E.; Zhao, H.; Guzman, S.G.; Leed, A.J.; Mesleh, M.F.; Kordasiewicz, H.; Schreiber, S.L.; Vallabh, S.M. Characterization of the Prion Protein Binding Properties of Antisense Oligonucleotides. Biomolecules 2019, 10, 1. [Google Scholar] [CrossRef]
- Schätzl, H.M.; Da Costa, M.; Taylor, L.; Cohen, F.E.; Prusiner, S.B. Prion protein gene variation among primates. J. Mol. Boil. 1997, 265, 257. [Google Scholar] [CrossRef]
- Wopfner, F.; Weidenhöfer, G.; Schneider, R.; Von Brunn, A.; Gilch, S.; Schwarz, T.F.; Werner, T.; Schätzl, H.M. Analysis of 27 mammalian and 9 avian PrPs reveals high conservation of flexible regions of the prion protein 1 1Edited by A. R. Fersht. J. Mol. Boil. 1999, 289, 1163–1178. [Google Scholar] [CrossRef] [PubMed]
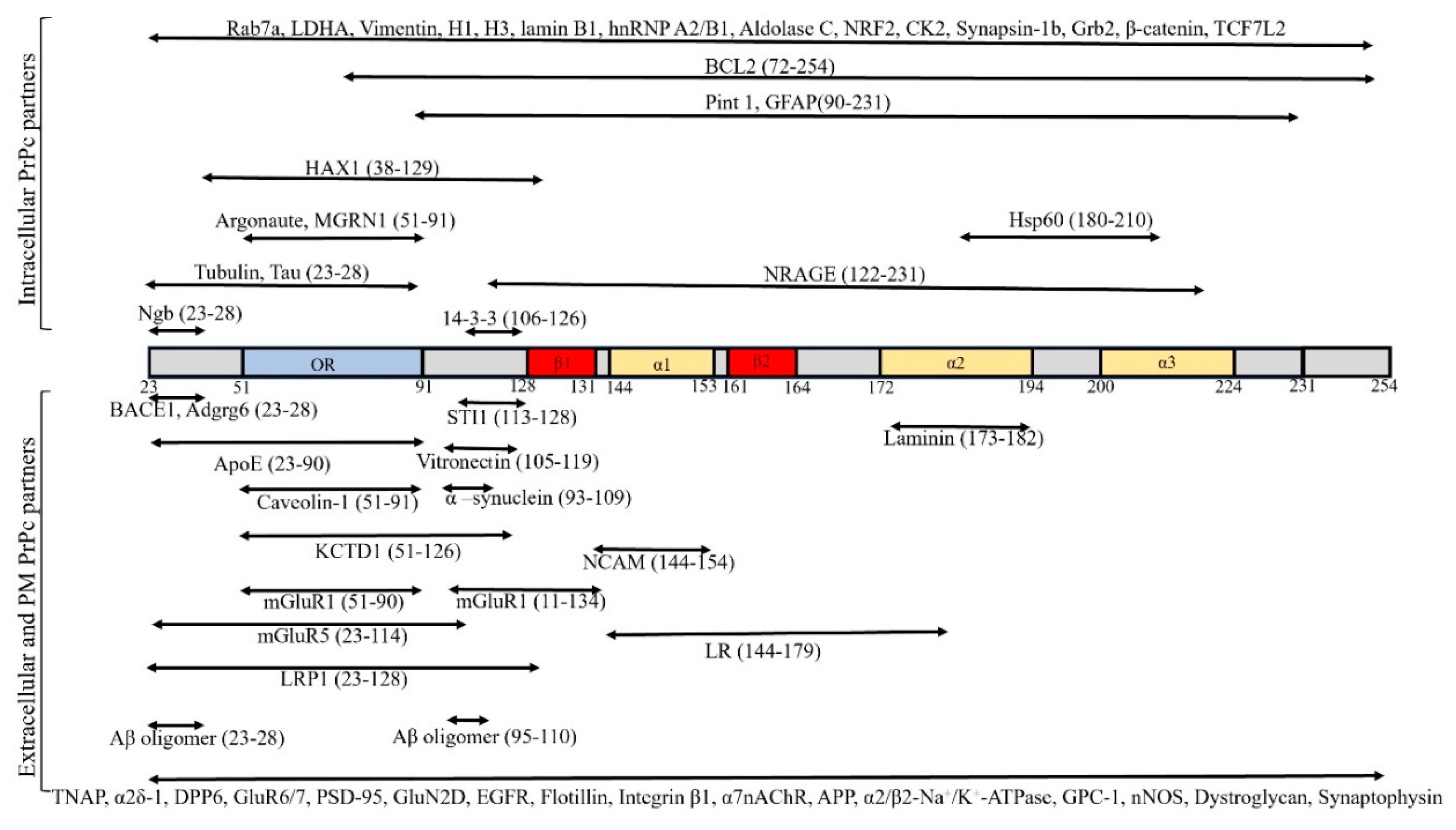


| PrPc Interacting Protein/Function | Cellular Localization | Effect of Interaction | Technique | References |
|---|---|---|---|---|
| Neuroglobin (Ngb)/hemoglobin | Cytoplasm | PrPc aggregation | Immunostaining, docking | [32,33] |
| Tubulin/cytoskeletal protein | Cytoplasm | Inhibit microtubule polymerization, Tubulin oligomerization, Retrograde and anterograde transport of PrPc | Co-IP, pull-down, co-fractionation, crosslinking | [34,35,36] |
| Tau/microtubule-associated protein | Cytoplasm | Reduction of PrPc induced oligomerization of tubulin | Co-IP, pull-down | [37,38,39,40] |
| Synapsin-1b/neuron-specific phosphoprotein | Cytoplasm | Unknown | Cofractionation, Y2H, Co-IP | [41,42,43] |
| Growth factor receptor-bound protein 2 (Grb2)/growth factor receptor | Cytoplasm, Nucleus | Unknown | Cofractionation, Y2H, Co-IP | [41,44] |
| Pint-1/unknown | Cytoplasm | Unknown | Y2H, Co-IP | [41] |
| Glial fibrillary acidic protein (GFAP)/intermediate filament | Cytoplasm | Unknown | Co-IP, pull-down, overlay | [45,46,47] |
| Heterogeneous nuclear ribonucleoproteins (hnRNP) A2/B1/RNA-binding protein | Cytoplasm, Nucleus | Unknown | Co-IP, overlay | [48] |
| Aldolase C/metabolic enzyme | Cytoplasm | Unknown | Co-IP, overlay | [48] |
| Nuclear factor erythroid 2-related factor 2 (NRF2)/transcription factor | Cytoplasm, nucleus | Unknown | Screen of bacteriophage expression library of brain cDNA | [49] |
| B cell lymphoma 2 (BCL2)/apoptosis regulator | Cytoplasm | PrPc aggregation, Inhibits BCL2 anti-apoptotic function | Co-IP, Y2H, affinity | [50,51,52] |
| 14-3-3 protein/phosphorylation-dependent scaffold protein | Cytoplasm | Unknown | Co-IP, pull-down, overlay | [53,54,55,56] |
| Neurotrophin receptor-interacting MAGE homolog (NRAGE)/cell-death inducer | Cytoplasm | Aggregation, changing mitochondrial membrane potential | Co-IP, pull-down, Y2H | [57] |
| Casein kinase II (CK2)/serine-threonine kinase | Cytoplasm, nucleus, extracellular matrix | PrPc phosphorylation, regulates CK2 enzymatic activity | Co-IP, pull-down, overlay, surface plasmon resonance | [58,59,60] |
| Mahogunin ring finger 1 (MGRN1)/ubiquitin ligase | Cytoplasm | Aggregation, disruption of mahogunin function, neurodegeneration | Pull-down, Co-IP, Y2H | [61,62] |
| Heat shock protein 60 (Hsp60)/chaperon | Mitochondria | Unknown | Y2H | [63] |
| Members of the Rab family of small GTPases/guanosine triphosphate proteins | Cytoplasm | Intracellular PrPc trafficking | Co-IP | [64,65] |
| Argonaute/small RNA binding protein | Cytoplasm | Posttranscription cytoplasmic gene-silencing mechanism | Pull-down, electron microscopy | [66] |
| Lactate dehydrogenase A (LDHA)/oxidoreductases enzyme | Cytoplasm | Activation of LDHA, neuroprotection | Co-IP | [67,68] |
| Vimentin/cytoskeletal protein | Cytoplasm | Regulate intracellular transportation | Co-IP | [69,70] |
| β-catenin/transcriptional coactivator | Cytoplasm, nucleus | Transcriptional regulation | Co-IP | [71] |
| Transcription factor 7-like 2 (TCF7L2)/transcription factor | Cytoplasm, nucleus | Transcriptional regulation | Co-IP | [71] |
| Histone H1/3, lamin B1/structural chromatin components | Nucleus | Transcriptional regulation | Far-Western blot | [72] |
| HS-1-associated protein X1 (HAX-1)/apoptosis regulator | Cytoplasm | Oxidative stress, antiapoptosis | Co-IP, microarray | [73] |
| PrPc Interacting Protein/Function | Cellular Localization | Effect of Interaction | Technique | References |
|---|---|---|---|---|
| Stress-inducible phosphoprotein 1 (STI1)/extracellular ligands | PM | Ca2+ influx induction, neuritogenesis, neuroprotection | Co-IP, pull-down, complementary hydropathy | [148,149,150,151] |
| Nicotinic acetylcholine receptor (α7nAChR)/neurotransmitter receptors | PM | Mediate autophagic flux, Ca2+ signaling | Co-IP | [152,153] |
| Amyloid beta precursor protein (APP)/cell adhesion receptors | PM | Cell adhesion, CNS development | Co-IP | [154] |
| β-site amyloid precursor protein cleaving enzyme 1 (BACE1)/membrane-bound aspartic protease | PM | APP cleavage | Co-IP | [155] |
| Amyloid beta oligomer (AβO)/ extracellular ligand | PM | Neurotoxicity, LTP suppression, NMDAR phosphorylation, Tau phosphorylation | Co-IP, immunostaining | [16,156,157,158,159] |
| Metabotropic glutamate receptor 1 (mGluR1)/neurotransmitter receptors | PM | Neurite outgrowth | Co-IP, immunostaining | [160,161,162] |
| Metabotropic glutamate receptor 5 (mGluR5)/neurotransmitter receptors | PM | AβO -mediate neurotoxicity, promoting long term depression and inhibiting long-term potentiation, neurite outgrowth | Co-IP, immunostaining | [160,163,164] |
| Laminin receptor (LRP/LR)/ cell adhesion receptors | PM | PrPc internalization, Cell survival | Co-IP, Y2H | [165,166,167] |
| α-synuclein/neurotransmitter release regulator | PM | Ca2+ influx | Co-IP | [168] |
| Laminin/Extracellular matrix protein | Extracellular matrix | Modulate neuronal plasticity and memory | Pull-down | [169,170,171] |
| N-methyl-D-aspartate (NMDA) (GluN2D)/neurotransmitter receptors | PM | Neuroprotection | Co-IP | [146,147] |
| Low-density lipoprotein receptor-related protein 1 (LRP1)/endocytic receptor | PM, Intracellular compartments | Endocytosis and trafficking of PrPc, stimulation of neurotransmitter release | Co-IP | [172,173] |
| Neural cell adhesion molecule (NCAM)/ cell adhesion receptors | PM | Neuritogenesis, neurons development | Co-IP, immunostaining, pull-down | [174,175] |
| Vitronectin/extracellular matrix protein | Extracellular matrix | Axonal growth | Immunostaining, pull-down | [21] |
| Caveolin-1/scaffold proteins | PM | Regulate exosome secretion, regulate Src kinase Fyn activation | Co-IP, immunostaining, pull-down | [176,177,178,179] |
| Integrin β1/cell adhesion receptors | PM | Neuritogenesis | Co-IP, immunostaining, pull-down, overlay | [21,179] |
| Flotillin/scaffold proteins | PM | Neurons differentiation by trafficking of N-cadherin, trafficking of EGFR | Co-IP, immunostaining, electron microscopy | [180,181] |
| Epidermal growth factor receptor (EGFR)/growth factor receptors | PM | Neuritogenesis | Co-IP | [182,183] |
| Postsynaptic density protein 95 (PSD-95)/scaffolding protein | PM | Protection against excitotoxicity | Co-IP, cDNA library | [146,182,183,184,185,186] |
| Kainate receptor GluR6/7/ ionotropic glutamate receptors | PM | Protection against excitotoxicity | Co-IP | [146,182,183,184,185,186] |
| Dipeptidyl peptidase-like protein 6 (DPP6)/potassium channel | PM | Downregulation of neuronal membrane excitability | Co_IP | [187] |
| α2δ-1 subunit of voltage-gated/calcium channel | PM | Ca2+ influx | Co-IP, immunostaining | [188,189] |
| Tissue nonspecific alkaline phosphatase (TNAP)/ectoenzyme | PM | Laminin dephosphorylation | Co-IP, immunostaining, mass spectrometry | [190] |
| α2/β2-Na+/K+-ATPase/neurotransmitter receptors | PM | Lactate transportation in astrocytic | Co-IP, immunoaffinity chromatography, pull-down | [191] |
| Glypican-1 (GPC-1)/growth factor regulator | PM | Lipid raft localization of PrPc | Co-IP, immunostaining | [192] |
| G protein-coupled receptor (Adgrg6)/adhesion G protein-coupled receptor | PM | Myelin maintenance in the peripheral nerves | Co-IP, immunostaining, electron microscopy | [193,194] |
| Neuronal nitric oxide synthase (nNOS)/flavo-hemoprotein | PM | Modulate nNOS activity | Co-IP, immunostaining | [195] |
| Dystroglycan/integral membrane glycoprotein | PM | Unknown | Co-IP, immunostaining | [195] |
| Synaptophysin/Integral membrane glycoprotein | Synaptic vesicles | Unknown | Co-IP, immunostaining | [195] |
| ApoE/cholesterol carrier | Extracellular matrix | Unknown | Co-IP, pull-down | [196] |
| Potassium channel tetramerization domain containing 1 (KCTD1)/ion channel | PM | Unknown | Co-IP, Y2H | [197] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miranzadeh Mahabadi, H.; Taghibiglou, C. Cellular Prion Protein (PrPc): Putative Interacting Partners and Consequences of the Interaction. Int. J. Mol. Sci. 2020, 21, 7058. https://doi.org/10.3390/ijms21197058
Miranzadeh Mahabadi H, Taghibiglou C. Cellular Prion Protein (PrPc): Putative Interacting Partners and Consequences of the Interaction. International Journal of Molecular Sciences. 2020; 21(19):7058. https://doi.org/10.3390/ijms21197058
Chicago/Turabian StyleMiranzadeh Mahabadi, Hajar, and Changiz Taghibiglou. 2020. "Cellular Prion Protein (PrPc): Putative Interacting Partners and Consequences of the Interaction" International Journal of Molecular Sciences 21, no. 19: 7058. https://doi.org/10.3390/ijms21197058
APA StyleMiranzadeh Mahabadi, H., & Taghibiglou, C. (2020). Cellular Prion Protein (PrPc): Putative Interacting Partners and Consequences of the Interaction. International Journal of Molecular Sciences, 21(19), 7058. https://doi.org/10.3390/ijms21197058