Abstract
The bone microenvironment is an ideal fertile soil for both primary and secondary tumors to seed. The occurrence and development of osteosarcoma, as a primary bone tumor, is closely related to the bone microenvironment. Especially, the metastasis of osteosarcoma is the remaining challenge of therapy and poor prognosis. Increasing evidence focuses on the relationship between the bone microenvironment and osteosarcoma metastasis. Many elements exist in the bone microenvironment, such as acids, hypoxia, and chemokines, which have been verified to affect the progression and malignance of osteosarcoma through various signaling pathways. We thoroughly summarized all these regulators in the bone microenvironment and the transmission cascades, accordingly, attempting to furnish hints for inhibiting osteosarcoma metastasis via the amelioration of the bone microenvironment. In addition, analysis of the cross-talk between the bone microenvironment and osteosarcoma will help us to deeply understand the development of osteosarcoma. The cellular and molecular protagonists presented in the bone microenvironment promoting osteosarcoma metastasis will accelerate the exploration of novel therapeutic strategies towards osteosarcoma.
1. Introduction
The bone microenvironment is composed of bone marrow and a mineralized extracellular matrix. Bone marrow contains two different cell types: hematopoietic stem cells with hematopoietic function and bone marrow mesenchymal stem cells, which are responsible for differentiating into non-blood cells components in bone including mesenchymal stem cells (MSCs), osteoblasts, osteoclasts, osteocytes, fibroblasts, fat cells, etc. [1,2,3]. The bone microenvironment provides an ideal site for many distinct cancers to thrive. For example, some secondary tumors which originate in other sites tend to diffuse into the bone including breast cancer, prostate cancer, etc. [4,5,6]. The concept of the “vicious cycle” has been present since the 1990’s to describe this close connection between metastatic tumor cells and bone cells [7]. Tumor cells invade bone, resulting in the loss of balance between bone formation and bone resorption [4,5].
The bone microenvironment is also a fertile soil and a complex biological system that facilitates the metastasis of many cancers including osteosarcoma (OS) [8,9]. In recent years, to understand the progress of OS, a number of studies have focused on the relationship between OS and the bone microenvironment, for example, MSCs, which are one of the most important members in the bone microenvironment [10]. Here, we summarized the research approaches toward the bone microenvironment and OS metastasis. We reviewed the promoting effect of the main components in the bone microenvironment on OS metastasis such as mesenchymal stem cells, hypoxia, acidosis, and chemokine. Meanwhile, we were particularly concerned with the signaling pathways that were activated by these factors in the bone microenvironment and the consequences for the progress of OS metastasis.
OS is the main malignant primary bone tumor in young adults and children. The metaphysis regions of long bones, the most active sites in bone, are major sites of OS growth [11]. The large tumor heterogeneity in OS creates some difficult issues for tumor therapy such as identification of reliable biomarkers, recognizing the mechanism of recurrence, and identifying which cell type causes OS [12].The conventional form of OS can be broken down to distinctive subtypes based on histological analysis: osteoblastic, fibroblastic, chondroblastic, epithelioid, giant-cell rich, small-cell and telangiectatic types [13]. Although the morphological characteristics are different, the mainstream research believes that OS emerges in osteoids generated from mesenchymal stem cells as well as pre-osteoblasts or osteoblast precursors [14]. For example, some papers reported that the deletion of TP53 and Rb can cause OS transformation of osteoblasts [15,16,17]. The loss of Rb can trigger the transformation of MSCs into OS, and the overexpression of C-myc also has similar consequences for MSCs [18]. The molecular pathogenesis of OS is complicated, but despite this, there are some key genes that have been studied and can give us a little inspiration. The TP53 and Rb are the genes with the highest frequencies of absence and mutations in human OS and transgenic mouse models. Both TP53 and Rb are the tumor suppressor genes and involved in cell cycle regulation, while TP53 is involved in cell apoptosis. There are other genes with aberrant expression in OS, including c-myc, AP-1, c-fos, TWIST, MMP, IGF-1, etc. [19,20,21,22].
Tumor metastasis is the primary problem for tumor therapy [23]. Most OS infiltrate the surrounding tissue, and even metastasize to the lung when they are found. Lung metastasis is the main challenge for OS therapy; however, due to the introduction of chemotherapy, the five-year survival rate of OS has increased to about 70% since the 1970s, but the five-year survival rate still remains as low as 20–30% after lung metastasis [24]. The study of how the spread and metastasis of OS is affected by the tumor microenvironment is not yet in-depth. Malignant OS cells form a complex mixture with other normal cells (MSCs, fibroblasts, osteoblasts, and myeloid immune cells) and some chemical factors (hypoxia, acidosis). This special tumor microenvironment of the complex mixture is a perfect place for OS to develop and metastasize. Understanding this special bone microenvironment, the mechanism of OS metastasis can be better understood, and it is possible to find a therapeutic target for the treatment of OS metastasis.
2. Bone Microenvironment and OS Metastasis
2.1. Mesenchymal Stem Cells and OS Metastasis
The most important factors in the bone microenvironment, which is considered to promote OS metastasis, are MSCs (Figure 1). MSCs are pluripotent stem cells and highly associated with tumor development, metastasis, and drug resistance, and they are a source of OS [25]. For example, the deficiency of TP53 and Rb gene, the aneuploidization and genomic loss of P16/Cdkn2a are common causes of the transition of MSCs to OS cells [18,26]. However, OS-derived MSCs have no characteristics of tumors but are highly similar to normal MSCs [10]. Despite all this, a growing number of researchers suggest that MSCs existing in the bone microenvironment can promote the invasion and metastasis of OS. In rat OS model, after 3 weeks and 5 weeks subcutaneous injection of rat OS COS1NR cells, the intravenous injection of MSCs had no effect on tumor growth, whereas, it promoted pulmonary metastasis significantly [27]. Meanwhile, gene expression analysis showed that the focal adhesion, cytokine–cytokine receptor and extracellular matrix–receptor pathway significantly changed in MSCs compared to COS1NR cells [27]. The pathway molecules that dramatically altered were highly related to the tumor metastasis and angiogenesis such as the CXCL12/CXCR4 axis, MMP-2, and MMP-9 [27,28,29] (Table 1). Some reports find that the interaction between MSCs and OS tumor cells is bidirectional (Figure 1). For example, tumor cells can modulate their microenvironment which, in turn, becomes more beneficial to tumor growth through metabolic reprogramming [30]. In this situation, MSCs are the modulators of OS metabolism. In addition, MSCs can secrete more lactic acid by upregulated lactate monocarboxylate transporters [30]. This process is driven by the oxidative stress induced by OS to improve the mitochondrial activity of OS and then lead to the OS metastasis [30].
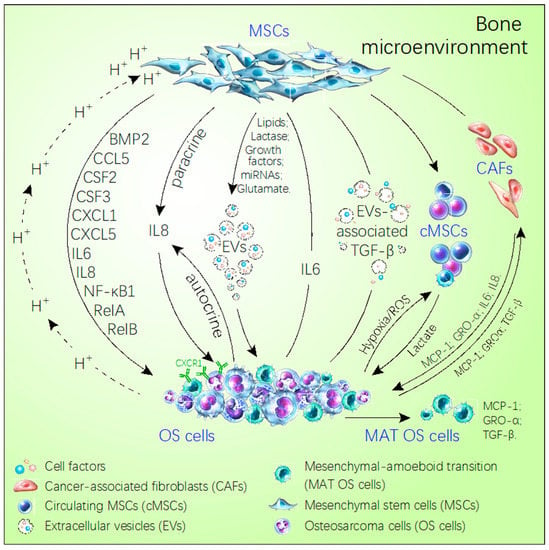
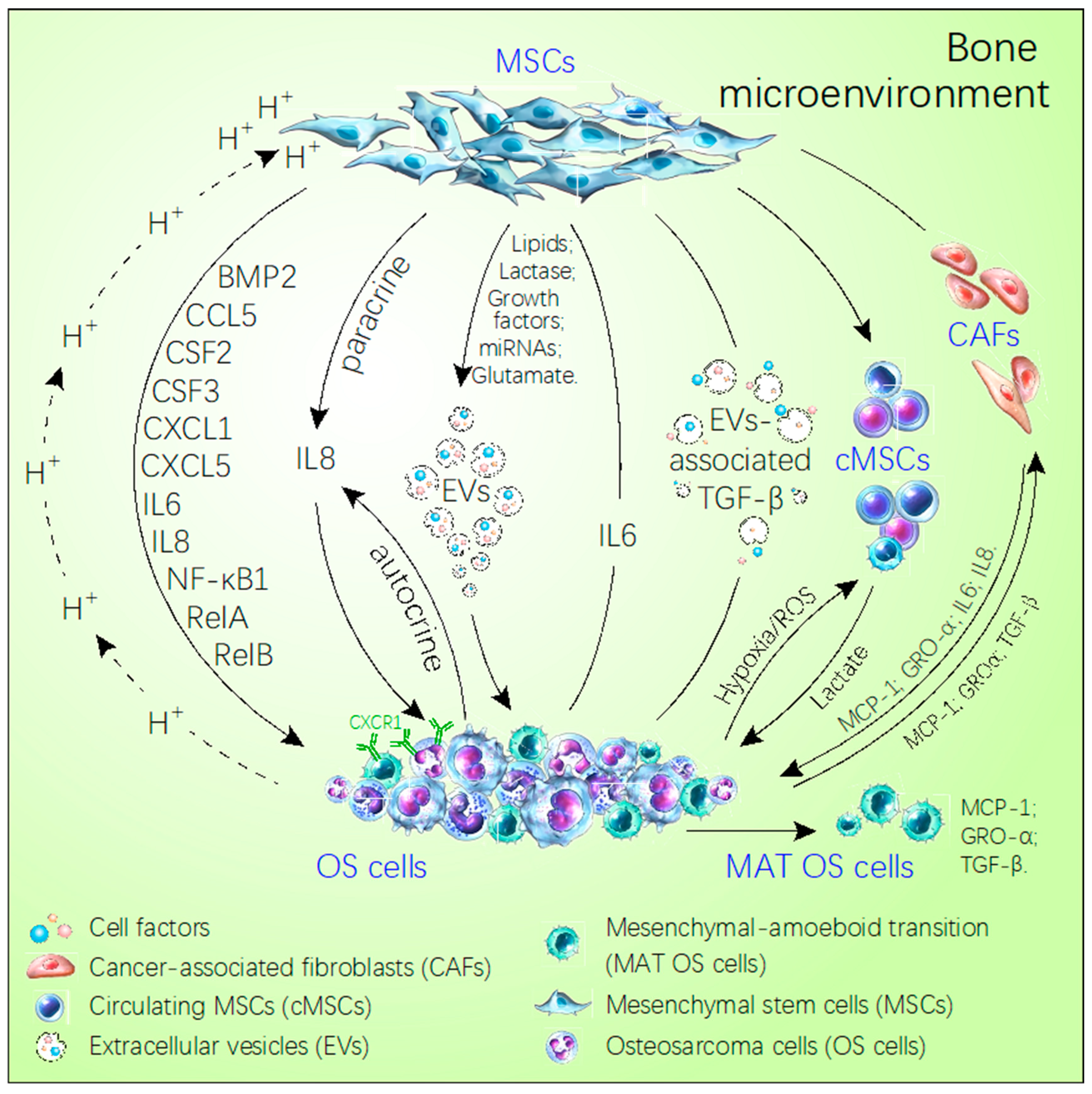
Figure 1.
Schematic diagram of the cross-talk between MSCs and OS cells in the bone microenvironment. MSCs directly secrete some factors or choose the extracellular vesicles as the carrier to transport miRNAs, growth factor, lipids, glutamate, lactase, which promote OS metastasis. Meanwhile, OS utilizes the acidic environment, hypoxia and extracellular vesicles inducing the MSCs secreting cell factors to facilitate their own growth and metastasis, like IL-8, IL-6, RelA, RelB, NF-κB1, CSF2/GM-CSF, CSF3/G-CSF, BMP2, CCL5, CXCL5, CXCL1. In addition, OS via secreted MCP-1, GRO-α, and TGF-β induces MSCs trans-differentiating the cancer-associated stem cells to express more MCP-1. GRO-α, IL-6, and IL-8 promote the MAT of OS. The solid arrows refer to the direction of the cell or factor from the MSCs. The dotted arrows refer to the direction of cells and factors from OS.

Table 1.
Chemokines and other factors that affect OS metastasis in the bone microenvironment.
Reports about tumor-derived extracellular vesicles (EVs) reveal the direct interaction of MSCs and OS cells [33,36] (Table 1). The preclinical mouse model also indicates the Es-mediated cross-talk between OS cells and MSCs [38]. The EVs carrying a membrane-associated form of TGF-β stimulate the IL-6 expression in MSCs, which is called tumor extracellular vesicle-educated mesenchymal stem cells (TEMSCs) [33]. Compared to the normal MSCs, TEMSCs can activate STAT3 expression in OS and facilitate the pulmonary metastasis [33]. Meanwhile, the activation of STAT3 also increases the drug resistance of OS [33]. MicroRNA derived from EVs, such as hsa-miR-195 and has-miR-148a, can also increases the aggressiveness and metastasis of OS through targeting MMP1 and PTK2 [36,37] (Table 1).
MSCs are driven by oxidative stress induced by OS and undergo metabolism reprogramming. Lactate production is increased which promotes the migration ability of OS cells [30]. OS cells can acidify the microenvironment where they are embedded. This highly acidic environment triggers MSCs to secrete many different types of factors, including mitogenic, clonogenic, chemotactic, and pro-migratory factors via activating the NF-κB pathway (Table 2) [31]. These factors, in turn, promote invasion and metastasis of OS. For example, IL-8 secreted by MSCs can activate the CXCR1, a member of the chemokine receptor family, and further improves p-Akt expression leading to anoikis resistance of OS cell and the advancement of pulmonary metastasis [39]. OS cells also can activate the CXCR1 through autocrine of IL-8 and then promote their own pulmonary metastasis through the Akt pathway [39]. On the other hand, the contact of MSCs and OS cells can promote the tumor invasion and migration through inducing the MAT in OS cells (Figure 1) [32]. Firstly, OS cells secrete chemokines, including MCP-1 (CCL2), TGF-β, and GRO-α (CXCL1), in the microenvironment [32]. Then MSCs are recruited into contact with OS cells, trans-differentiate into cancer-fibroblasts, and secret more MCP-1(CCL2), GRO-α (CXCL1), TGF-β, IL-6, and IL-8 in the microenvironment [32]. These cytokines active the small GTPase RhoA to induce the MAT in the OS cells finally [32] (Table 1).

Table 2.
The hypoxia and acidosis in the bone microenvironment affect OS metastasis (↑: Up-regulation).
2.2. Effect of Hypoxia and Acidosis Environment on OS Metastasis
Hypoxia and acidic condition are common features of the bone microenvironment. Aberrant expressions of a number of genes induced by hypoxia and acidic conditions promote the metastasis of OS (Table 2). Hypoxia triggers the expression of Hypoxia-inducible factor (HIF) which is the main focus of research in OS metastasis [35,41,43,44,45,46,47]. Recently, miR-20b and miR-33b were reported to directly target HIF1α [35,41]. By decreasing the expression of HIF1α, they could inhibit the invasion and proliferation of MG63 and U2OS cells [35]. HIF2α and HIF2PUT are upregulated in clinical OS samples [45]. Moreover, upregulation of HIF2α and HIF2PUT have significantly correlated with OS at the clinical stage, distant metastasis, and poor survival rate [45]. Angiogenesis is necessary in the metastasis progress. LncRNA MALAT1 is found to induce the pro-angiogenic function and is highly associated to the hypoxia responses and OS development [43]. Meanwhile MALAT1 participates in the mTOR/HIF1α loop [43]. In hypoxia microenvironment of OS, ANGPTL2 is induced in patient samples, and its expression was controlled by HIF1α [46]. Moreover, overexpression of ANGPTL4 facilitates the migration and proliferation and improves the osteoclastogenesis and bone resorption [44]. Additionally, SENP1 is upregulated and positively accommodates the HIF expression to promote the invasion and EMT process in OS cells [47].
The balance of pH in bone microenvironment is important to keep normal bone formation and bone resorption. The acidosis microenvironment promotes OS progress by activation of mesenchymal stem cells [31]. Moreover, after short time stimulation of acid, sequencing data show that the expression of stress factors, growth factors, and immunoregulatory molecules are upregulated including NFκB1, RelB, RelA, CSF3, IL-1A, IL-23A, IL-1RN, IL-6, IL-8, CXCL1,CXCL2, CXCR4, CXCL5, CCL5, CCR7 CSF2/GM-CSF, CSF3/G-CSF, BMP-2, and MMP-2. The levels of IL-6 and IL-8 are the highest in all the factors (Table 2) [31]. GM-CSF and G-CSF promote the colony formation and are related to the immunoreaction [31]. In addition, CXCL1, CXCL5, and CCL5 affect OS growth and metastasis [31].
2.3. Chemokines and OS Metastasis
Chemokines are composed of four subfamilies including CCL, CXCL, CX3C, and XCL [48]. The functions of chemokines are closely related to immune cells which can guide their migration [49]. Recently, increasing research show that chemokines can communicate with OS in the bone microenvironment [50,51]. Clinicopathological analysis shows that CXCR4, receptor of CXCL12, and MMP-9 are overexpressed in OS with lung metastasis patients compared to the patients without metastasis [52]. CXCL12/CXCR4/CXCR7 was activated when a co-culture of bone marrow mesenchymal stem cells and OS cells (Table 1) [53]. At the same time, the activation of CXCL12/CXCR4/CXCR7 in the co-culture model boosts the OS invasion [53]. Importantly, the CCL5/CCR5 axis not only enhances the migration of OS via αvβ3 but also facilitates tumor angiogenesis via PKC delta/c-Scr/HIF-1α pathway [54] (Table 1). The levels of IL-8 in the serum of OS patients are higher than normal patients [55]. IL-8 from OS cells autocrine or from MSCs paracrine promote OS cells invasion and pulmonary metastasis through the IL-8/CXCR1/Akt signaling pathway [34,39] (Table 1). As previously described, the acidic conditions induce various chemokines’ expression in MSCs to promote progression of OS.
2.4. Functions of Extracellular Vesicles in the Tumor Microenvironment
EVs in the tumor microenvironment, as a medium of communication among cells, play an important role in tumor development and metastasis. EVs can carry miRNA, cytokines, and small molecule proteins, such as TGFβ and MMP [56,57]. MSCs-derived EVs promote OS growth by activation of the Hedgehog and PI3K/AKT signal pathways [58,59]. Meanwhile, OS-derived EVs modulate the transformation of MSCs by regulating the hypomethylation of LINE-1 in MSCs [60]. Moreover, OS-derived EVs change the bone microenvironment remodeling through influenced genes’ expression [60,61]. EVs derived from highly metastatic OS clonal variants induce metastasis of poorly metastatic clones in mouse model [62]. More descriptions of EVs has been comprehensively summarized by Perut [63].
3. Signal Pathways in OS Metastasis
3.1. PI3K/Akt Signaling Pathway
PI3K/Akt is one of the most important intracellular signal transduction pathways that regulates cell motility, growth, proliferation, adhesion, and cell survival [64]. An increasing number of studies show that PI3K/Akt signaling pathway components are abnormally expressed in human cancer including OS [65]. Immunostaining analysis in primary OS cases show that PI3K/Akt signaling is highly and significantly related with poor prognosis [66]. Moreover, activation of Akt is highly implicated in lung metastasis. There are many reports showing that aberrant expression of proteins can active the PI3K/Akt signaling pathway facilitating the progression of OS [67,68,69,70,71,72,73,74,75] (Figure 2). Intracellular adhesion molecule-1 (ICAM-1), a surface glycoprotein, takes part in cell–ECM adhesion and promotes metastasis in cancers [67]. The Fractalkine/CX3CR1 axis can induce ICAM-1 expression to promote cell migration of OS [67]. The process is mediated by the PI3K/Akt/NF-κB signaling pathway. In the PI3K/Akt/NF-κB cascade, Fractalkine/CX3CR1 axis can phosphorylate Akt through PI3K, and the phosphorylation of Akt can active the NF-κB further; finally, the NF-κB as the transcription factor, facilitates the expression of ICAM-1 [67]. The IL-8/CXCR1 axis can directly activate the Akt signaling to enhance the resistance of OS to anoikis [39]. The apoptosis-related imprinted gene, tumor-suppressing STF cDNA 3 (TSSC3) and a prognostic marker for OS patients, can induce the autophagy in OS and inhibit the cell migration and invasion in vitro and in vivo via depressing the src-dependent PI3K/Akt/mTOR signaling pathway [68]. Overexpression of the Fibulin-4, an extracellular matrix protein playing a key role in stabilizing the matrix structure, can also active the PI3K/Akt/mTOR signaling pathway to promote OS cell invasion and metastasis [69]. Meanwhile, EMT, an important cellular process in cancer cell metastasis, is accelerated with the upregulation of Fibulin-4 [69]. The PI3K/Akt/mTOR signaling pathway is also active via the high expression of GPNMB and promotes OS cell metastasis and proliferation [70]. Transcription factors also take effect on the activation of PI3K/Akt such as overexpression of zinc finger transcription factor ZIC2, which can activate PI3K/Akt and promote the viability, migration, and invasion of OS cells [71].
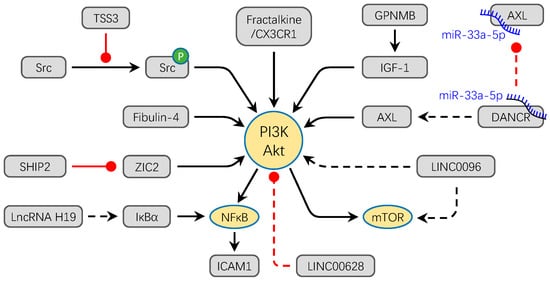
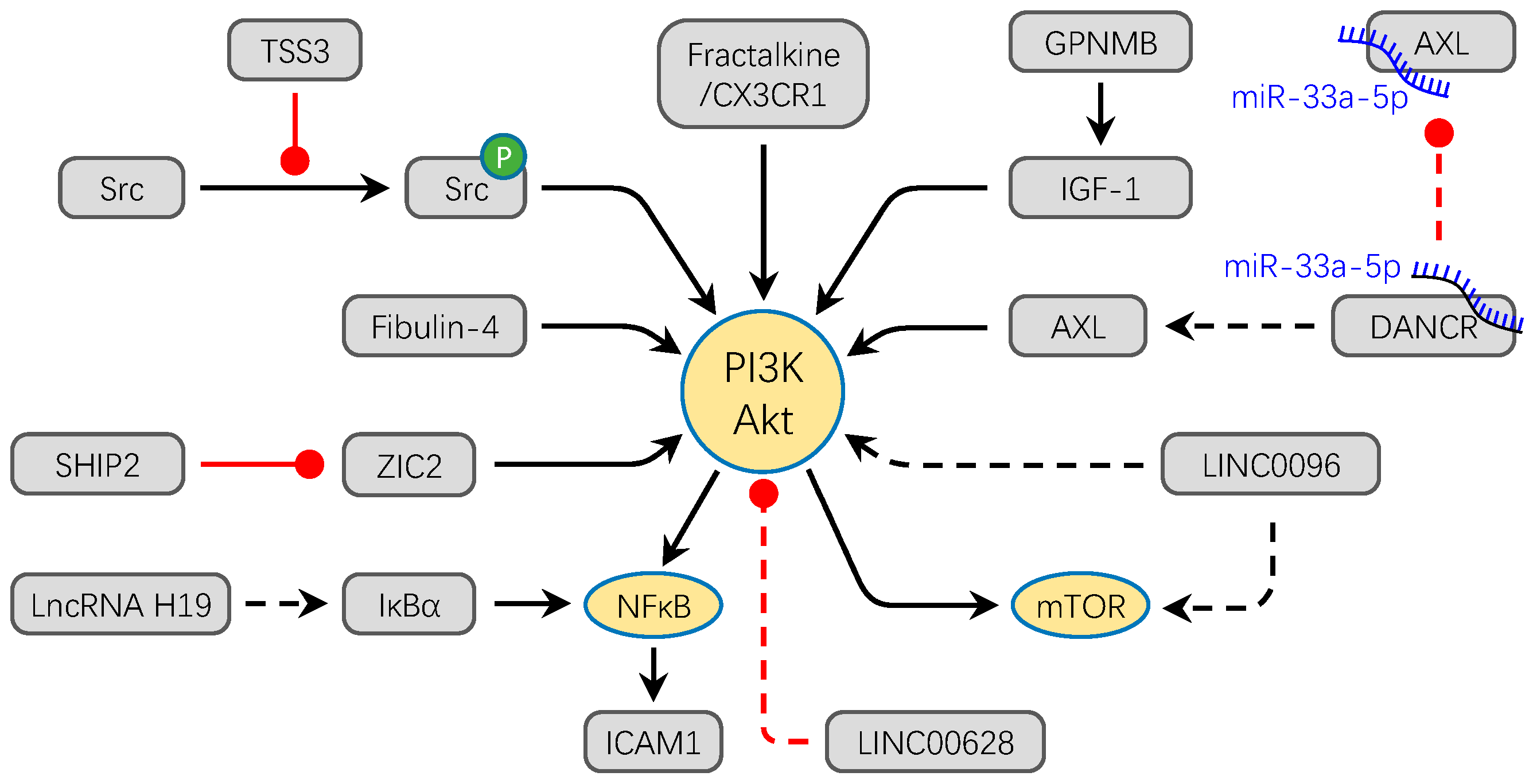
Figure 2.
The PI3K/Akt signaling pathway converges to facilitate the progression OS. Most factors directly activate PI3K/Akt signaling pathway to promote the migration and invasion of OS. For example, overexpression of Fibulin-4, Fractalkine/CX3CR1, ZIC2, LINC0096, and lncRNA H19 promote the osteosarcoma metastasis through the activation of PI3K/Akt signal pathway. Meanwhile, GPNMB and DANCR/miR-33a-5p activate the PI3K/Akt pathway by promoting the expression of IGF-1 and AX, respectively. TSSC3 inhibits the phosphorylation of Src to activate the PI3K/Akt signaling pathway. Conversely, overexpression of LINC00628 inhibits the phosphorylation of PI3K and Akt. The solid lines refer to protein molecules and the dotted lines refer to non-coding RNAs in this figure. Black arrows show the promotion of molecules, while red dots show the inhibition of molecules.
Non-coding RNA is also an important regulator in activation of the PI3K/Akt pathway. Silence of LncRNA H19 and LINC00968 can decrease the activation of the PI3K/Akt/mTOR signaling pathway in vitro [72]. Overexpression of LINC00628 obviously suppresses the related protein expression level of the PI3K/Akt signaling pathway [73]. The LncDANCR via competitive combination of miR-33a-5p promotes the expression of AXL and then facilitates the invasion and metastasis of OS in vitro and in vivo through the PI3K/Akt signaling pathway [74]. Moreover, the PI3K/Akt signaling pathway has the potential as a therapy target. For example, Celastrol can inhibit OS cell line metastasis via inactivation of the PI3K/Akt/NF-κB signaling pathway in vitro [75].
3.2. Wnt/β-Catenin Signaling Pathway
There are three different Wnt signaling pathways: the canonical Wnt pathway, the noncanonical planar cell polarity pathway, and the non-canonical Wnt/calcium pathway [76]. The canonical Wnt signaling pathway is the Wnt/β-catenin pathway including many molecules such as TCF/LEF, APC, Axin, GSK-3β, CK1α, and LPR5/6. The activation of the Wnt signaling pathway is frequently detected in most cancers like OS [77]. Many different molecules can enhance OS’s malignancy through activating the Wnt/β-catenin signaling pathway.
Non-coding RNAs have been star molecules in recent reports. Overexpression of miR-135b, an oncogenic miR in OS, can promote OS invasion and metastasis in vitro and in vivo through activating the Wnt/β-catenin signaling pathway via directly targeting GSK-3β, APC, β-TrCP, and CK1α [78]. The Notch signaling pathway also can be activated by Wnt/β-catenin-dependent and -independent mechanisms in OS cells simultaneously [78]. Meanwhile inhibition of miR-135b not only decreases the activation of the Wnt/β-catenin and Notch signaling pathway but also depresses OS metastasis and reduces recurrence in OS xenografts models [78]. Similarly, the Wnt/β-catenin signaling pathway is activated directly or indirectly by miR-183, miR-184, miR-146b-5p, etc., (Table 3) to promote OS metastasis and invasion [79,80,81,82,83,84,85,86]. Meanwhile, the activation of Wnt/β-catenin can increase the expression of Runx2 to facilitate the metastasic-related genes expression in OS [87].

Table 3.
Non-coding RNAs related with Wnt/β-catenin pathway promote the OS metastasis and invasion (↑: Up-regulation).
Wnt/β-catenin is related to EMT. The signaling cascade of Wnt/β-catenin induced by tumor-suppressing STF cDNA3 downsregulation results in the accumulation of the β-catenin and snail, finally enhancing the upregulation of Wnt target genes and mesenchymal genes to promote EMT [88]. The Wnt antagonist, APCDDI, is downregulated by methylation in the promoter to activate the Wnt/β-catenin signaling pathway elevating the ability of invasion and migration of OS cells in vitro and in vivo [89].
In summary, the Wnt/β-catenin signaling pathway is widely activated in OS and highly related to the invasion and metastasis of OS. Therefore, an increasing number of studies focus on the Wnt/β-catenin signaling pathway, and it would be a suitable therapy target in the future treatment of OS.
3.3. MAPK/ERK Signaling Pathway
MAPK/ERK is a complex cascade of signaling pathway that transfers signals from a receptor on a cell’s surface to the DNA in a cell’s nucleus, involving various cellular processes such as cell motility, survival, apoptosis, differentiation, and proliferation [89]. The MAPK/ERK signaling pathway includes many components such as ERK1/2/3/4/5, JNK1/2/3, SAPK, and P38 [89]. Any mutation in the components of this pathway can result in the stop or start of the signaling and along with tumorigenesis [90]. Paris saponin VII (PS VII), Delphinidin can effectively inhibit the activation of the MAPK/ERK signaling pathway and further suppress the OS invasion, migration and promote apoptosis, which indicates that MAPK/ERK signaling pathway is activated in OS [91,92]. Over expressions of MAP2K6, MAP4K3, and DUSP1 are correlated with poor clinical prognoses [93]. Inversely, patients with decreasing expression of MAP4K3 can achieve a better treatment effect [93]. Moreover, the expression of DUSP1, one of the protein phosphatases which inhibits MAPKs through dephosphorylation, is significantly increased in metastasis patients and post-chemotherapy patients, suggesting that DUSP1 gene is related to OS metastasis and drug resistance [93]. Continuously, there are some other cellular molecules, such as ONZIN, macrophage migration inhibitory factor (MIF) activates the MAPK/ERK signaling pathway via directly or indirectly phosphorylating ERK1/2 to promoting OS invasion and metastasis [94,95]. For example, MIF expression is increased both in serum and OS tissues of patients along with poor survival rate and highly lung metastasis rate [95]. MIF promotes metastasis and proliferation of OS cells by activating the RAS/MAPK signaling pathway via enhancing p-ERK1/2, p-SRC, p-MEK1/2, and upregulation of RAS-GTP [95]. Moreover, silence of MIF decreases the p-ERK level and inhibits the ability of metastasis and proliferation in vitro and suppresses OS lung metastasis in vivo and raises the sensitivity of OS cells to chemotherapy drugs [95]. Higher levels of ERK1/2 and STAT3 has been confirmed to be associated with poor prognosis of OS [96]. STATs, as members of the JAK–STAT signaling pathway, are considered as oncogenes in OS [97]. The downregulation of the JAK–STAT signaling pathway by miR-125b and miR-126 suppresses migration, invasion, and proliferation in OS cell lines [98,99].
3.4. Hedgehog Signaling Pathway
The Hedgehog (Hh) signaling pathway, composed of twelve-transmembrane Patched protein (PTc), seven-transmembrane protein smoothened (SMO), and glioma-associated oncogene homologs (GLI), is a conserved signaling pathway participating in cell migration, differentiation, growth, and polarity [100]. The function of Hh signaling in OS metastasis has been reviewed explicitly elsewhere [101]. Briefly, the Hh pathway is activated broadly in many different human cancers including OS [101,102]. The activation of the Hh pathway mediated by various agents facilitates OS invasion and metastasis [101]. Ribosomal protein S3 (RPS3), Smad, Yes-associated protein 1, and LncRNA H19 modulates the Hh/Gli pathway to improve the OS invasion and metastasis [103,104,105]. Some data show that the Smo antagonist and Degalactotigonin suppresses OS progression by inactivation of the Hh/Gli pathway [106,107]. Moreover, the Hh/Gli pathway can promote OS metastasis by interacting with other signaling pathways such as the PI3K/AKT pathway and the Wnt pathway [106,107]. Additionally, details of the treatment strategies targeting the Hh signaling pathway have been review by Yaoet et al. [101].
3.5. Notch Signaling Pathway
The Notch signaling pathway is a conserved pathway modulated cell–cell communication, which is important for regulating cell processes [108]. The Notch proteins are transferred on the cell surface to modulate cellular signal transmission. Many data show that the Notch pathway is positive in various cancers including OS [109]. The Notch pathway is activated with the decrease in DTX1 [110]. At the same time, the depletion of DTX1 results in the improvement of Notch1 in OS cells [110]. Moreover, the activation and recycling rate of Notch is suppressed by the activation of PI5P4Kγ [110]. Some other reports show that LncRNAs and microRNAs play important roles in activating the Notch pathway. The Notch pathway is activated through the upregulation of Notch2 which modulates LncRNA SNHG12 sponging miR-195-5p in OS [111]. Moreover, SNHG12 is highly expressed in OS tissues and cell lines and is significantly related with the poor prognosis in OS patients [111]. The motilities of OS cells are suppressed by the silence of SNHG12 [111]. Lnc CRNDE is significantly highly expressed in OS samples from patients, particularly in metastasis patients [112]. Overexpression of CRNDE promotes the upregulation of Notch1 activating the Notch pathway and induces the EMT progress in OS cells [112]. In other words, the Notch pathway may have a close relationship with EMT in OS metastasis. Clinical data and animal model suggest that miR-135b, similar to oncogenic genes, improves pulmonary metastasis, cancer cell stemness, and tumor recurrence in OS [78]. Meanwhile, miR-135b activates the Notch pathway and Wnt/β-catenin pathway through inhibiting the negative modulator of these pathway in cancer stem cells of OS [78]. For example, miR-135b targets GSK-3β, APC, β-TrCP, and CK1α in Wnt/β-catenin pathway and inhibits the TET3 in Notch pathway [78]. The combined treatment of chemotherapy and natural compounds (traditional Chinese medicine) reveals that the Notch pathway is a therapeutic target in OS metastasis and development [113].
4. The Bone Microenvironment as a Promising Treatment
The treatment of OS, usually conducted with the combination of chemotherapy and surgery, has not been significantly improved in recent decades. The development of OS is closely related to the bone microenvironment. There may be a new therapeutic strategy that uses the bone microenvironment as target. Although targeting a certain type of cell is impractical due to the heterogeneity of OS, improving the physical conditions of the bone microenvironment may achieve a certain therapeutic effect. Tumor hypoxia is one of the main reasons for treatment failure. TH-302, as a developed hypoxia-activated DNA cross-linking pro-drug, shows potent hypoxia-dependent cytotoxicity. TH-302, combined with doxorubicin, reduced lung metastases of OS [114]. Acidic conditions in the tumor microenvironment increased tumor resistance. Doxorubicin cytotoxicity, compared with the standard pH7.4, was reduced in pH6.5 condition. Combining omeprazole, a proton pump inhibitor targeting lysosomal acidity, with doxorubicin significantly reduce tumor volume and body weight loss by improving the doxorubicin cytotoxicity [115]. As described above, EVs, as a messenger of communication among cells in the microenvironment, promotes the development and metastasis of OS. To prevent the production of EVs or directly target the EVs in tumor microenvironment may be also an effective strategy to treat OS. However, current research on EVs is mainly focused on using it as a drug or delivery system [116].
5. Conclusions and Perspectives
Previous studies showed that the bone microenvironment promotes the OS metastasis or malignancy. In this review, we briefly reviewed the OS and bone microenvironment; mainly discussed the function of mesenchymal stem cells, acidosis, hypoxia, and chemokines in the microenvironment to promote the OS migration, invasion, and lung metastasis; and extensively summarized the signaling pathways activated in the OS that facilitate the metastasis of OS including the PI3K/Akt, Wnt/β-Catenin, MAPK/ERK, Hedgehog, and Notch signaling pathways. We found that the function of mesenchymal stem cells and chemokines are hot spots in the research of OS and also are the most important factors in the bone microenvironment to promote the progress of OS. There are always some cross-talks between different pathways in tumor occurrence and development, which provides a comprehensive understanding of tumor occurrence and development. Furthermore, the IL-8/CXCR1 axis and CXCL12/CXCR4/CXCR7 axis are commonly activated in the bone microenvironment when OS metastasis occur [29,31,33,39,53]. IL-8 and CXCL12, as the secreted molecules, are detected easily through peripheral blood. IL-8 and CXCL12 may be biomarkers of OS metastasis and IL-8/CXCR1 axis and CXCL12/CXCR4/CXCR7 axis may be as the potential therapeutic targets for OS metastasis in the future.
Author Contributions
C.Y. and Y.T. prepared the original draft, which was then equally edited by Z.C., F.Z., and P.S. and was finally supervised by Y.L. and A.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China(81801871), the Shaanxi Provincial Key R&D Program (2018KWZ-10), Special Fund for Technological Innovation of Shaanxi Province (2019QYPY-207), and the Fundamental Research Funds for the Central Universities (3102018zy053).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ANGPTL4 | Angiopoietin-like 4 |
| AXL | Tyrosine-protein kinase receptor UFO |
| CK1α | Casein kinase I α |
| CRNDE | Colorectal Neoplasia Differentially Expressed |
| DANCR | Differentiation Antagonizing Non-Protein Coding RNA |
| DTX1 | Protein deltex-1 |
| DUSP1 | Dual specificity protein phosphatase 1 |
| ECM | Extracellular matrices |
| GPNMB | Glycoprotein non-metastatic melanoma protein B |
| HIF2PUT | Hypoxia-inducible factor-2α promoter upstream transcript |
| MALAT1 | Metastasis associated lung adenocarcinoma transcript 1 |
| TWIST | twist family bHLH transcription factor 1 |
| MCP-1 | Monocyte chemoattractant protein 1 |
| ONZIN | placenta specific 8 |
| PTK2 | Protein tyrosine kinase 2 |
| SENP1 | Sentrin-specific protease 1 |
| SNHG12 | small nucleolar RNA host gene 12 |
| TET3 | Tet Methylcytosine Dioxygenase 3 |
| TSSC3 | tumor- suppressing STF cDNA 3 |
| ZIC2 | Zinc finger protein 2 |
| β-TrcP | β-transducin repeats-containing proteins |
References
- Zheng, Y.; Zhou, H.; Dunstan, C.R.; Sutherland, R.L.; Seibel, M.J. The role of the bone microenvironment in skeletal metastasis. J. Bone Oncol. 2013, 2, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Junttila, M.R.; de Sauvage, F.J. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature 2013, 501, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Belli, C.; Trapani, D.; Viale, G.; D’Amico, P.; Duso, B.A.; Della Vigna, P.; Orsi, F.; Curigliano, G. Targeting the microenvironment in solid tumors. Cancer Treat. Rev. 2018, 65, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Buenrostro, D.; Mulcrone, P.L.; Owens, P.; Sterling, J.A. The Bone Microenvironment: A Fertile Soil for Tumor Growth. Curr. Osteoporos. Rep. 2016, 14, 151–158. [Google Scholar] [CrossRef]
- Hui, L.; Chen, Y. Tumor microenvironment: Sanctuary of the devil. Cancer Lett. 2015, 368, 7–13. [Google Scholar] [CrossRef]
- Seton-Rogers, S. Mapping dysfunction of the bone marrow stroma in leukaemia. Nat. Rev. Cancer 2019, 19, 368. [Google Scholar] [CrossRef]
- Yoneda, T.; Sasaki, A.; Mundy, G.R. Osteolytic bone metastasis in breast cancer. Breast Cancer Res. Treat. 1994, 32, 73–84. [Google Scholar] [CrossRef]
- Alfranca, A.; Martinez-Cruzado, L.; Tornin, J.; Abarrategi, A.; Amaral, T.; de Alava, E.; Menendez, P.; Garcia-Castro, J.; Rodriguez, R. Bone microenvironment signals in osteosarcoma development. Cell. Mol. Life Sci. 2015, 72, 3097–3113. [Google Scholar] [CrossRef]
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun. Signal. 2020, 18, 59. [Google Scholar] [CrossRef]
- Le Nail, L.R.; Brennan, M.; Rosset, P.; Deschaseaux, F.; Piloquet, P.; Pichon, O.; Le Caignec, C.; Crenn, V.; Layrolle, P.; Herault, O.; et al. Comparison of Tumor- and Bone Marrow-Derived Mesenchymal Stromal/Stem Cells from Patients with High-Grade Osteosarcoma. Int. J. Mol. Sci. 2018, 19, 707. [Google Scholar] [CrossRef]
- Cortini, M.; Avnet, S.; Baldini, N. Mesenchymal stroma: Role in osteosarcoma progression. Cancer Lett. 2017, 405, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Botter, S.M.; Neri, D.; Fuchs, B. Recent advances in osteosarcoma. Curr. Opin. Pharmacol. 2014, 16, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.; Khoury, J.D.; Hoffer, F.A.; Wu, J.; Billups, C.A.; Heck, R.K.; Quintana, J.; Poe, D.; Rao, B.N.; Daw, N.C. Telangiectatic osteosarcoma: The St. Jude Children’s Research Hospital’s experience. Cancer 2007, 109, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- Ragland, B.D.; Bell, W.C.; Lopez, R.R.; Siegal, G.P. Cytogenetics and molecular biology of osteosarcoma. Lab. Invest. 2002, 82, 365–373. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Walkley, C.R.; Qudsi, R.; Sankaran, V.G.; Perry, J.A.; Gostissa, M.; Roth, S.I.; Rodda, S.J.; Snay, E.; Dunning, P.; Fahey, F.H.; et al. Conditional mouse osteosarcoma, dependent on p53 loss and potentiated by loss of Rb, mimics the human disease. Genes Dev. 2008, 22, 1662–1676. [Google Scholar] [CrossRef]
- Mutsaers, A.J.; Ng, A.J.; Baker, E.K.; Russell, M.R.; Chalk, A.M.; Wall, M.; Liddicoat, B.J.; Ho, P.W.; Slavin, J.L.; Goradia, A.; et al. Modeling distinct osteosarcoma subtypes in vivo using Cre:lox and lineage-restricted transgenic shRNA. Bone 2013, 55, 166–178. [Google Scholar] [CrossRef]
- Berman, S.D.; Calo, E.; Landman, A.S.; Danielian, P.S.; Miller, E.S.; West, J.C.; Fonhoue, B.D.; Caron, A.; Bronson, R.; Bouxsein, M.L.; et al. Metastatic osteosarcoma induced by inactivation of Rb and p53 in the osteoblast lineage. Proc. Natl. Acad. Sci. USA 2008, 105, 11851–11856. [Google Scholar] [CrossRef]
- Rubio, R.; Abarrategi, A.; Garcia-Castro, J.; Martinez-Cruzado, L.; Suarez, C.; Tornin, J.; Santos, L.; Astudillo, A.; Colmenero, I.; Mulero, F.; et al. Bone environment is essential for osteosarcoma development from transformed mesenchymal stem cells. Stem Cells 2014, 32, 1136–1148. [Google Scholar] [CrossRef]
- He, J.P.; Hao, Y.; Wang, X.L.; Yang, X.J.; Shao, J.F.; Guo, F.J.; Feng, J.X. Review of the molecular pathogenesis of osteosarcoma. Asian Pac. J. Cancer Prev. 2014, 15, 5967–5976. [Google Scholar] [CrossRef]
- Broadhead, M.L.; Clark, J.C.; Myers, D.E.; Dass, C.R.; Choong, P.F. The molecular pathogenesis of osteosarcoma: A review. Sarcoma 2011, 2011, 959248. [Google Scholar] [CrossRef]
- Denduluri, S.K.; Wang, Z.; Yan, Z.; Wang, J.; Wei, Q.; Mohammed, M.K.; Haydon, R.C.; Luu, H.H.; He, T.C. Molecular pathogenesis and therapeutic strategies of human osteosarcoma. J. Biomed. Res. 2015, 30. [Google Scholar] [CrossRef]
- Kansara, M.; Thomas, D.M. Molecular pathogenesis of osteosarcoma. DNA Cell Biol. 2007, 26, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Bielack, S.S.; Kempf-Bielack, B.; Delling, G.; Exner, G.U.; Flege, S.; Helmke, K.; Kotz, R.; Salzer-Kuntschik, M.; Werner, M.; Winkelmann, W.; et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: An analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J. Clin. Oncol. 2002, 20, 776–790. [Google Scholar] [CrossRef] [PubMed]
- Mirabello, L.; Troisi, R.J.; Savage, S.A. Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the Surveillance, Epidemiology, and End Results Program. Cancer 2009, 115, 1531–1543. [Google Scholar] [CrossRef] [PubMed]
- Ehnman, M.; Chaabane, W.; Haglund, F.; Tsagkozis, P. The Tumor Microenvironment of Pediatric Sarcoma: Mesenchymal Mechanisms Regulating Cell Migration and Metastasis. Curr. Oncol. Rep. 2019, 21, 90. [Google Scholar] [CrossRef]
- Mohseny, A.B.; Szuhai, K.; Romeo, S.; Buddingh, E.P.; Briaire-de Bruijn, I.; de Jong, D.; van Pel, M.; Cleton-Jansen, A.M.; Hogendoorn, P.C. Osteosarcoma originates from mesenchymal stem cells in consequence of aneuploidization and genomic loss of Cdkn2. J. Pathol. 2009, 219, 294–305. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Honoki, K.; Fujii, H.; Tohma, Y.; Kido, A.; Mori, T.; Tsujiuchi, T.; Tanaka, Y. Mesenchymal stem cells promote tumor engraftment and metastatic colonization in rat osteosarcoma model. Int. J. Oncol. 2012, 40, 163–169. [Google Scholar]
- Zhang, M.; Zhang, X. Association of MMP-2 expression and prognosis in osteosarcoma patients. Int. J. Clin. Exp. Pathol. 2015, 8, 14965–14970. [Google Scholar]
- Neklyudova, O.; Arlt, M.J.; Brennecke, P.; Thelen, M.; Gvozdenovic, A.; Kuzmanov, A.; Robl, B.; Botter, S.M.; Born, W.; Fuchs, B. Altered CXCL12 expression reveals a dual role of CXCR4 in osteosarcoma primary tumor growth and metastasis. J. Cancer Res. Clin. Oncol. 2016, 142, 1739–1750. [Google Scholar] [CrossRef]
- Bonuccelli, G.; Avnet, S.; Grisendi, G.; Salerno, M.; Granchi, D.; Dominici, M.; Kusuzaki, K.; Baldini, N. Role of mesenchymal stem cells in osteosarcoma and metabolic reprogramming of tumor cells. Oncotarget 2014, 5, 7575–7588. [Google Scholar] [CrossRef]
- Avnet, S.; Di Pompo, G.; Chano, T.; Errani, C.; Ibrahim-Hashim, A.; Gillies, R.J.; Donati, D.M.; Baldini, N. Cancer-associated mesenchymal stroma fosters the stemness of osteosarcoma cells in response to intratumoral acidosis via NF-κB activation. Int. J. Cancer 2017, 140, 1331–1345. [Google Scholar] [CrossRef] [PubMed]
- Pietrovito, L.; Leo, A.; Gori, V.; Lulli, M.; Parri, M.; Becherucci, V.; Piccini, L.; Bambi, F.; Taddei, M.L.; Chiarugi, P. Bone marrow-derived mesenchymal stem cells promote invasiveness and transendothelial migration of osteosarcoma cells via a mesenchymal to amoeboid transition. Mol. Oncol. 2018, 12, 659–676. [Google Scholar] [CrossRef] [PubMed]
- Baglio, S.R.; Lagerweij, T.; Perez-Lanzon, M.; Ho, X.D.; Leveille, N.; Melo, S.A.; Cleton-Jansen, A.M.; Jordanova, E.S.; Roncuzzi, L.; Greco, M.; et al. Blocking Tumor-Educated MSC Paracrine Activity Halts Osteosarcoma Progression. Clin. Cancer Res. 2017, 23, 3721–3733. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, X.; Miao, W.; Wang, B.; Qiu, Y. CXCL8 promotes the invasion of human osteosarcoma cells by regulation of PI3K/Akt signaling pathway. APMIS 2017, 125, 773–780. [Google Scholar] [CrossRef]
- Liu, M.; Wang, D.; Li, N. MicroRNA-20b Downregulates HIF-1α and Inhibits the Proliferation and Invasion of Osteosarcoma Cells. Oncol. Res. 2016, 23, 257–266. [Google Scholar] [CrossRef]
- Vallabhaneni, K.C.; Hassler, M.Y.; Abraham, A.; Whitt, J.; Mo, Y.Y.; Atfi, A.; Pochampally, R. Mesenchymal Stem/Stromal Cells under Stress Increase Osteosarcoma Migration and Apoptosis Resistance via Extracellular Vesicle Mediated Communication. PLoS ONE 2016, 11, e0166027. [Google Scholar] [CrossRef]
- Geng, S.; Zhang, X.; Chen, J.; Liu, X.; Zhang, H.; Xu, X.; Ma, Y.; Li, B.; Zhang, Y.; Bi, Z.; et al. The tumor suppressor role of miR-124 in osteosarcoma. PLoS ONE 2014, 9, e91566. [Google Scholar] [CrossRef]
- Lagerweij, T.; Perez-Lanzon, M.; Baglio, S.R. A Preclinical Mouse Model of Osteosarcoma to Define the Extracellular Vesicle-mediated Communication Between Tumor and Mesenchymal Stem Cells. J. Vis. Exp. 2018, e56932. [Google Scholar] [CrossRef]
- Du, L.; Han, X.G.; Tu, B.; Wang, M.Q.; Qiao, H.; Zhang, S.H.; Fan, Q.M.; Tang, T.T. CXCR1/Akt signaling activation induced by mesenchymal stem cell-derived IL-8 promotes osteosarcoma cell anoikis resistance and pulmonary metastasis. Cell Death Dis. 2018, 9, 714. [Google Scholar] [CrossRef]
- McMahon, S.; Charbonneau, M.; Grandmont, S.; Richard, D.E.; Dubois, C.M. Transforming growth factor beta1 induces hypoxia-inducible factor-1 stabilization through selective inhibition of PHD2 expression. J. Biol. Chem. 2006, 281, 24171–24181. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, C.; Wang, K.; Liu, X.; Liu, Q. MicroRNA-33b Inhibits the Proliferation and Migration of Osteosarcoma Cells via Targeting Hypoxia-Inducible Factor-1α. Oncol. Res. 2017, 25, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ren, T.; Huang, Y.; Bao, X.; Sun, K.; Shen, D.; Guo, W. BMPR2 and HIF1-α overexpression in resected osteosarcoma correlates with distant metastasis and patient survival. Chin. J. Cancer Res. 2017, 29, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.C.; Tang, C.; Dong, Y.; Zhang, J.; Yuan, T.; Tao, S.C.; Li, X.L. Targeting the long noncoding RNA MALAT1 blocks the pro-angiogenic effects of osteosarcoma and suppresses tumour growth. Int. J. Biol. Sci. 2017, 13, 1398–1408. [Google Scholar] [CrossRef]
- Zhang, T.; Kastrenopoulou, A.; Larrouture, Q.; Athanasou, N.A.; Knowles, H.J. Angiopoietin-like 4 promotes osteosarcoma cell proliferation and migration and stimulates osteoclastogenesis. BMC Cancer 2018, 18, 536. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; He, X.; Xue, R.; Zhang, Y.; Zhang, X.; Lu, J.; Zhang, Z.; Xue, L. Combined over-expression of the hypoxia-inducible factor 2α gene and its long non-coding RNA predicts unfavorable prognosis of patients with osteosarcoma. Pathol. Res. Pract. 2016, 212, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Odagiri, H.; Kadomatsu, T.; Endo, M.; Masuda, T.; Morioka, M.S.; Fukuhara, S.; Miyamoto, T.; Kobayashi, E.; Miyata, K.; Aoi, J.; et al. The secreted protein ANGPTL2 promotes metastasis of osteosarcoma cells through integrin α5β1, p38 MAPK, and matrix metalloproteinases. Sci. Signal. 2014, 7, ra7. [Google Scholar] [CrossRef]
- Wang, X.; Liang, X.; Liang, H.; Wang, B. SENP1/HIF-1α feedback loop modulates hypoxia-induced cell proliferation, invasion, and EMT in human osteosarcoma cells. J. Cell. Biochem. 2018, 119, 1819–1826. [Google Scholar] [CrossRef]
- Charo, I.F.; Ransohoff, R.M. The many roles of chemokines and chemokine receptors in inflammation. N. Engl. J. Med. 2006, 354, 610–621. [Google Scholar] [CrossRef]
- Zlotnik, A.; Burkhardt, A.M.; Homey, B. Homeostatic chemokine receptors and organ-specific metastasis. Nat. Rev. Immunol. 2011, 11, 597–606. [Google Scholar] [CrossRef]
- Li, Y.; Flores, R.; Yu, A.; Okcu, M.F.; Murray, J.; Chintagumpala, M.; Hicks, J.; Lau, C.C.; Man, T.K. Elevated expression of CXC chemokines in pediatric osteosarcoma patients. Cancer 2011, 117, 207–217. [Google Scholar] [CrossRef]
- Pradelli, E.; Karimdjee-Soilihi, B.; Michiels, J.F.; Ricci, J.E.; Millet, M.A.; Vandenbos, F.; Sullivan, T.J.; Collins, T.L.; Johnson, M.G.; Medina, J.C.; et al. Antagonism of chemokine receptor CXCR3 inhibits osteosarcoma metastasis to lungs. Int. J. Cancer 2009, 125, 2586–2594. [Google Scholar] [CrossRef]
- Ren, Z.; Liang, S.; Yang, J.; Han, X.; Shan, L.; Wang, B.; Mu, T.; Zhang, Y.; Yang, X.; Xiong, S.; et al. Coexpression of CXCR4 and MMP9 predicts lung metastasis and poor prognosis in resected osteosarcoma. Tumour Biol. 2016, 37, 5089–5096. [Google Scholar] [CrossRef]
- Han, Y.; Wu, C.; Wang, J.; Liu, N. CXCR7 maintains osteosarcoma invasion after CXCR4 suppression in bone marrow microenvironment. Tumour Biol. 2017, 39, 1010428317701631. [Google Scholar] [CrossRef]
- Wang, S.W.; Liu, S.C.; Sun, H.L.; Huang, T.Y.; Chan, C.H.; Yang, C.Y.; Yeh, H.I.; Huang, Y.L.; Chou, W.Y.; Lin, Y.M.; et al. CCL5/CCR5 axis induces vascular endothelial growth factor-mediated tumor angiogenesis in human osteosarcoma microenvironment. Carcinogenesis 2015, 36, 104–114. [Google Scholar] [CrossRef]
- Kushlinskii, N.E.; Timofeev, Y.S.; Solov’ev, Y.N.; Gerstein, E.S.; Lyubimova, N.V.; Bulycheva, I.V. Components of the RANK/RANKL/OPG system, IL-6, IL-8, IL-16, MMP-2, and calcitonin in the sera of patients with bone tumors. Bull. Exp. Biol. Med. 2014, 157, 520–523. [Google Scholar] [CrossRef]
- Jerez, S.; Araya, H.; Hevia, D.; Irarrazaval, C.E.; Thaler, R.; van Wijnen, A.J.; Galindo, M. Extracellular vesicles from osteosarcoma cell lines contain miRNAs associated with cell adhesion and apoptosis. Gene 2019, 710, 246–257. [Google Scholar] [CrossRef]
- Lan, M.; Zhu, X.P.; Cao, Z.Y.; Liu, J.M.; Lin, Q.; Liu, Z.L. Extracellular vesicles-mediated signaling in the osteosarcoma microenvironment: Roles and potential therapeutic targets. J. Bone Oncol. 2018, 12, 101–104. [Google Scholar] [CrossRef]
- Qi, J.; Zhou, Y.; Jiao, Z.; Wang, X.; Zhao, Y.; Li, Y.; Chen, H.; Yang, L.; Zhu, H.; Li, Y. Exosomes Derived from Human Bone Marrow Mesenchymal Stem Cells Promote Tumor Growth Through Hedgehog Signaling Pathway. Cell Physiol. Biochem. 2017, 42, 2242–2254. [Google Scholar] [CrossRef]
- Lin, S.; Zhu, B.; Huang, G.; Zeng, Q.; Wang, C. Microvesicles derived from human bone marrow mesenchymal stem cells promote U2OS cell growth under hypoxia: The role of PI3K/AKT and HIF-1α. Hum. Cell 2019, 32, 64–74. [Google Scholar] [CrossRef]
- Mannerstrom, B.; Kornilov, R.; Abu-Shahba, A.G.; Chowdhury, I.M.; Sinha, S.; Seppanen-Kaijansinkko, R.; Kaur, S. Epigenetic alterations in mesenchymal stem cells by osteosarcoma-derived extracellular vesicles. Epigenetics 2019, 14, 352–364. [Google Scholar] [CrossRef]
- Garimella, R.; Washington, L.; Isaacson, J.; Vallejo, J.; Spence, M.; Tawfik, O.; Rowe, P.; Brotto, M.; Perez, R. Extracellular Membrane Vesicles Derived from 143B Osteosarcoma Cells Contain Pro-Osteoclastogenic Cargo: A Novel Communication Mechanism in Osteosarcoma Bone Microenvironment. Transl. Oncol. 2014, 7, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Macklin, R.; Wang, H.; Loo, D.; Martin, S.; Cumming, A.; Cai, N.; Lane, R.; Ponce, N.S.; Topkas, E.; Inder, K.; et al. Extracellular vesicles secreted by highly metastatic clonal variants of osteosarcoma preferentially localize to the lungs and induce metastatic behaviour in poorly metastatic clones. Oncotarget 2016, 7, 43570–43587. [Google Scholar] [CrossRef] [PubMed]
- Perut, F.; Roncuzzi, L.; Baldini, N. The Emerging Roles of Extracellular Vesicles in Osteosarcoma. Front. Oncol. 2019, 9, 1342. [Google Scholar] [CrossRef] [PubMed]
- Engelman, J.A. Targeting PI3K signalling in cancer: Opportunities, challenges and limitations. Nat. Rev. Cancer 2009, 9, 550–562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yu, X.H.; Yan, Y.G.; Wang, C.; Wang, W.J. PI3K/Akt signaling in osteosarcoma. Clin. Chim. Acta 2015, 444, 182–192. [Google Scholar] [CrossRef]
- Zhao, G.; Cai, C.; Yang, T.; Qiu, X.; Liao, B.; Li, W.; Ji, Z.; Zhao, J.; Zhao, H.; Guo, M.; et al. MicroRNA-221 induces cell survival and cisplatin resistance through PI3K/Akt pathway in human osteosarcoma. PLoS ONE 2013, 8, e53906. [Google Scholar] [CrossRef]
- Liu, J.F.; Tsao, Y.T.; Hou, C.H. Fractalkine/CX3CL1 induced intercellular adhesion molecule-1-dependent tumor metastasis through the CX3CR1/PI3K/Akt/NF-κB pathway in human osteosarcoma. Oncotarget 2017, 8, 54136–54148. [Google Scholar] [CrossRef]
- Zhao, G.S.; Gao, Z.R.; Zhang, Q.; Tang, X.F.; Lv, Y.F.; Zhang, Z.S.; Zhang, Y.; Tan, Q.L.; Peng, D.B.; Jiang, D.M.; et al. TSSC3 promotes autophagy via inactivating the Src-mediated PI3K/Akt/mTOR pathway to suppress tumorigenesis and metastasis in osteosarcoma, and predicts a favorable prognosis. J. Exp. Clin. Cancer Res. 2018, 37, 188. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, S.; Chen, J.; Liu, H.; Lu, J.; Jiang, H.; Huang, A.; Chen, Y. Fibulin-4 promotes osteosarcoma invasion and metastasis by inducing epithelial to mesenchymal transition via the PI3K/Akt/mTOR pathway. Int. J. Oncol. 2017, 50, 1513–1530. [Google Scholar] [CrossRef]
- Jin, R.; Jin, Y.Y.; Tang, Y.L.; Yang, H.J.; Zhou, X.Q.; Lei, Z. GPNMB silencing suppresses the proliferation and metastasis of osteosarcoma cells by blocking the PI3K/Akt/mTOR signaling pathway. Oncol. Rep. 2018, 39, 3034–3040. [Google Scholar] [CrossRef]
- Huang, S.; Jin, A. ZIC2 promotes viability and invasion of human osteosarcoma cells by suppressing SHIP2 expression and activating PI3K/AKT pathways. J. Cell. Biochem. 2018, 119, 2248–2257. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Yuan, D.; Sun, P.; Liu, W.; Wu, P.F.; Liu, H.; Yu, G.Y. LINC00968 functions as an oncogene in osteosarcoma by activating the PI3K/AKT/mTOR signaling. J. Cell. Physiol. 2018, 233, 8639–8647. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Wu, J.X.; Zhang, Y.; Che, H.; Yang, L. LncRNA LINC00628 overexpression inhibits the growth and invasion through regulating PI3K/Akt signaling pathway in osteosarcoma. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 5857–5866. [Google Scholar] [PubMed]
- Jiang, N.; Wang, X.; Xie, X.; Liao, Y.; Liu, N.; Liu, J.; Miao, N.; Shen, J.; Peng, T. lncRNA DANCR promotes tumor progression and cancer stemness features in osteosarcoma by upregulating AXL via miR-33a-5p inhibition. Cancer Lett. 2017, 405, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wang, Q.; Zhou, X.; Fu, C.; Cheng, M.; Guo, R.; Liu, H.; Zhang, B.; Dai, M. Celastrol negatively regulates cell invasion and migration ability of human osteosarcoma via downregulation of the PI3K/Akt/NF-κB signaling pathway in vitro. Oncol. Lett. 2016, 12, 3423–3428. [Google Scholar] [CrossRef]
- Clevers, H. Wnt/beta-catenin signaling in development and disease. Cell 2006, 127, 469–480. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Liu, G.; Rus, I.A.; Yao, S.; Chen, Y.; Akiri, G.; Grumolato, L.; Aaronson, S.A. High-frequency canonical Wnt activation in multiple sarcoma subtypes drives proliferation through a TCF/β-catenin target gene, CDC25A. Cancer Cell 2011, 19, 601–612. [Google Scholar] [CrossRef]
- Jin, H.; Luo, S.; Wang, Y.; Liu, C.; Piao, Z.; Xu, M.; Guan, W.; Li, Q.; Zou, H.; Tan, Q.Y.; et al. miR-135b Stimulates Osteosarcoma Recurrence and Lung Metastasis via Notch and Wnt/beta-Catenin Signaling. Mol. Ther. Nucleic Acids 2017, 8, 111–122. [Google Scholar] [CrossRef]
- Yang, X.; Wang, L.; Wang, Q.; Li, L.; Fu, Y.; Sun, J. MiR-183 inhibits osteosarcoma cell growth and invasion by regulating LRP6-Wnt/beta-catenin signaling pathway. Biochem. Biophys. Res. Commun. 2018, 496, 1197–1203. [Google Scholar] [CrossRef]
- Xu, E.; Zhao, J.; Ma, J.; Wang, C.; Zhang, C.; Jiang, H.; Cheng, J.; Gao, R.; Zhou, X. miR-146b-5p promotes invasion and metastasis contributing to chemoresistance in osteosarcoma by targeting zinc and ring finger 3. Oncol. Rep. 2016, 35, 275–283. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, L.; Lv, Z.; Li, Q.; Gong, W.; Wu, H. MicroRNA-342-3p Inhibits the Proliferation, Migration, and Invasion of Osteosarcoma Cells by Targeting Astrocyte-Elevated Gene-1 (AEG-1). Oncol. Res. 2017, 25, 1505–1515. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Li, C.B.; Yuan, B.T.; Qi, W.; Li, H.L.; Shen, X.Z.; Zhao, G.; Wang, J.T.; Liu, Y.J. MicroRNA-26a induces osteosarcoma cell growth and metastasis via the Wnt/beta-catenin pathway. Oncol. Lett. 2016, 11, 1592–1596. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.B.; Zhang, Z.C.; Han, G.S.; Han, J.Z.; Qiu, D.P. Overexpression of miR-214 promotes the progression of human osteosarcoma by regulating the Wnt/β-catenin signaling pathway. Mol. Med. Rep. 2017, 15, 1884–1892. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Yang, G.; Jiang, P.; Zhang, L.; Wang, J.; Sun, S. Long non-coding RNA Sox4 promotes proliferation and migration by activating Wnt/β-catenin signaling pathway in osteosarcoma. Pharmazie 2017, 72, 537–542. [Google Scholar]
- Jiang, Z.; Jiang, C.; Fang, J. Upregulated lnc-SNHG1 contributes to osteosarcoma progression through sequestration of miR-577 and activation of WNT2B/Wnt/β-catenin pathway. Biochem. Biophys. Res. Commun. 2018, 495, 238–245. [Google Scholar] [CrossRef]
- Zhao, H.; Hou, W.; Tao, J.; Zhao, Y.; Wan, G.; Ma, C.; Xu, H. Upregulation of lncRNA HNF1A-AS1 promotes cell proliferation and metastasis in osteosarcoma through activation of the Wnt/β-catenin signaling pathway. Am. J. Transl. Res. 2016, 8, 3503–3512. [Google Scholar]
- Vega, O.A.; Lucero, C.M.J.; Araya, H.F.; Jerez, S.; Tapia, J.C.; Antonelli, M.; Salazar-Onfray, F.; Las Heras, F.; Thaler, R.; Riester, S.M.; et al. Wnt/beta-Catenin Signaling Activates Expression of the Bone-Related Transcription Factor RUNX2 in Select Human Osteosarcoma Cell Types. J. Cell. Biochem. 2017, 118, 3662–3674. [Google Scholar] [CrossRef]
- Lv, Y.F.; Dai, H.; Yan, G.N.; Meng, G.; Zhang, X.; Guo, Q.N. Downregulation of tumor suppressing STF cDNA 3 promotes epithelial-mesenchymal transition and tumor metastasis of osteosarcoma by the Wnt/GSK-3β/β-catenin/Snail signaling pathway. Cancer Lett. 2016, 373, 164–173. [Google Scholar] [CrossRef]
- Han, W.; Liu, J. Epigenetic silencing of the Wnt antagonist APCDD1 by promoter DNA hyper-methylation contributes to osteosarcoma cell invasion and metastasis. Biochem. Biophys. Res. Commun. 2017, 491, 91–97. [Google Scholar] [CrossRef]
- McCain, J. The MAPK (ERK) Pathway: Investigational Combinations for the Treatment of BRAF-Mutated Metastatic Melanoma. P&T A Peer-Rev. J. Formul. Manag. 2013, 38, 96–108. [Google Scholar]
- Cheng, G.; Gao, F.; Sun, X.; Bi, H.; Zhu, Y. Paris saponin VII suppresses osteosarcoma cell migration and invasion by inhibiting MMP-2/9 production via the p38 MAPK signaling pathway. Mol. Med. Rep. 2016, 14, 3199–3205. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.M.; Park, B.S.; Kang, H.K.; Park, H.R.; Yu, S.B.; Kim, I.R. Delphinidin induces apoptosis and inhibits epithelial-to-mesenchymal transition via the ERK/p38 MAPK-signaling pathway in human osteosarcoma cell lines. Environ. Toxicol. 2018, 33, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Lopes, L.J.S.; Tesser-Gamba, F.; Petrilli, A.S.; de Seixas Alves, M.T.; Garcia-Filho, R.J.; Toledo, S.R.C. MAPK pathways regulation by DUSP1 in the development of osteosarcoma: Potential markers and therapeutic targets. Mol. Carcinog. 2017, 56, 1630–1641. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, Q.; Li, G.; Li, L.; Liang, S.; Zhang, Y.; Liu, J.; Fan, Z.; Li, L.; Zhou, B.; et al. ONZIN Upregulation by Mutant p53 Contributes to Osteosarcoma Metastasis Through the CXCL5-MAPK Signaling Pathway. Cell. Physiol. Biochem. 2018, 48, 1099–1111. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, X.; Li, W.; Li, M.; Tu, T.; Ba, X.; Wu, Y.; Huang, Z.; Fan, G.; Zhou, G.; et al. Macrophage migration inhibitory factor promotes osteosarcoma growth and lung metastasis through activating the RAS/MAPK pathway. Cancer Lett. 2017, 403, 271–279. [Google Scholar] [CrossRef]
- Salas, S.; Jiguet-Jiglaire, C.; Campion, L.; Bartoli, C.; Frassineti, F.; Deville, J.L.; Maues De Paula, A.; Forest, F.; Jézéquel, P.; Gentet, J.C.; et al. Correlation between ERK1 and STAT3 expression and chemoresistance in patients with conventional osteosarcoma. BMC Cancer 2014, 14, 606. [Google Scholar] [CrossRef]
- Pencik, J.; Pham, H.T.; Schmoellerl, J.; Javaheri, T.; Schlederer, M.; Culig, Z.; Merkel, O.; Moriggl, R.; Grebien, F.; Kenner, L. JAK-STAT signaling in cancer: From cytokines to non-coding genome. Cytokine 2016, 87, 26–36. [Google Scholar] [CrossRef]
- Jiang, R.; Zhang, C.; Liu, G.; Gu, R.; Wu, H. MicroRNA-126 Inhibits Proliferation, Migration, Invasion, and EMT in Osteosarcoma by Targeting ZEB1. J. Cell. Biochem. 2017, 118, 3765–3774. [Google Scholar] [CrossRef]
- Liu, L.H.; Li, H.; Li, J.P.; Zhong, H.; Zhang, H.C.; Chen, J.; Xiao, T. miR-125b suppresses the proliferation and migration of osteosarcoma cells through down-regulation of STAT3. Biochem. Biophys. Res. Commun. 2011, 416, 31–38. [Google Scholar] [CrossRef]
- Lum, L.; Beachy, P.A. The Hedgehog response network: Sensors, switches, and routers. Science 2004, 304, 1755–1759. [Google Scholar] [CrossRef]
- Yao, Z.; Han, L.; Chen, Y.; He, F.; Sun, B.; Kamar, S.; Zhang, Y.; Yang, Y.; Wang, C.; Yang, Z. Hedgehog signalling in the tumourigenesis and metastasis of osteosarcoma, and its potential value in the clinical therapy of osteosarcoma. Cell Death Dis. 2018, 9, 701. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Takebe, N.; Lorusso, P. Targeting the Hedgehog pathway in cancer. Ther. Adv. Med. Oncol. 2010, 2, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.H.; Wang, W.; Yeung, W.; Deng, Y.; Yuan, P.; Mak, K.K. Hedgehog signaling induces osteosarcoma development through Yap1 and H19 overexpression. Oncogene 2014, 33, 4857–4866. [Google Scholar] [CrossRef]
- Mohseny, A.B.; Cai, Y.; Kuijjer, M.; Xiao, W.; van den Akker, B.; de Andrea, C.E.; Jacobs, R.; ten Dijke, P.; Hogendoorn, P.C.; Cleton-Jansen, A.M. The activities of Smad and Gli mediated signalling pathways in high-grade conventional osteosarcoma. Eur. J. Cancer 2012, 48, 3429–3438. [Google Scholar] [CrossRef] [PubMed]
- Nagao-Kitamoto, H.; Setoguchi, T.; Kitamoto, S.; Nakamura, S.; Tsuru, A.; Nagata, M.; Nagano, S.; Ishidou, Y.; Yokouchi, M.; Kitajima, S.; et al. Ribosomal protein S3 regulates GLI2-mediated osteosarcoma invasion. Cancer Lett. 2015, 356 Pt B, 855–861. [Google Scholar] [CrossRef]
- Lo, W.W.; Wunder, J.S.; Dickson, B.C.; Campbell, V.; McGovern, K.; Alman, B.A.; Andrulis, I.L. Involvement and targeted intervention of dysregulated Hedgehog signaling in osteosarcoma. Cancer 2014, 120, 537–547. [Google Scholar] [CrossRef]
- Zhao, Z.; Jia, Q.; Wu, M.S.; Xie, X.; Wang, Y.; Song, G.; Zou, C.Y.; Tang, Q.; Lu, J.; Huang, G.; et al. Degalactotigonin, a Natural Compound from Solanum nigrum L., Inhibits Growth and Metastasis of Osteosarcoma through GSK3β Inactivation-Mediated Repression of the Hedgehog/Gli1 Pathway. Clin. Cancer Res. 2018, 24, 130–144. [Google Scholar] [CrossRef]
- Artavanis-Tsakonas, S.; Rand, M.D.; Lake, R.J. Notch signaling: Cell fate control and signal integration in development. Science 1999, 284, 770–776. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, Y.; Nolo, R.; Zweidler-McKay, P.A.; Hughes, D.P. Regulation of NOTCH signaling by reciprocal inhibition of HES1 and Deltex 1 and its role in osteosarcoma invasiveness. Oncogene 2010, 29, 2916–2926. [Google Scholar] [CrossRef]
- Zheng, L.; Conner, S.D. PI5P4Kγ functions in DTX1-mediated Notch signaling. Proc. Natl. Acad. Sci. USA 2018, 115, E1983–E1990. [Google Scholar] [CrossRef]
- Zhou, S.; Yu, L.; Xiong, M.; Dai, G. LncRNA SNHG12 promotes tumorigenesis and metastasis in osteosarcoma by upregulating Notch2 by sponging miR-195-5p. Biochem. Biophys. Res. Commun. 2018, 495, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Tang, Y.; Xing, W.; Dong, W.; Wang, Z. LncRNA, CRNDE promotes osteosarcoma cell proliferation, invasion and migration by regulating Notch1 signaling and epithelial-mesenchymal transition. Exp. Mol. Pathol. 2018, 104, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P.; Kaushik, G.; Subramaniam, D.; Dandawate, P.; Neville, K.; Chastain, K.; Anant, S. Natural compounds targeting major cell signaling pathways: A novel paradigm for osteosarcoma therapy. J. Hematol. Oncol. 2017, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Liapis, V.; Labrinidis, A.; Zinonos, I.; Hay, S.; Ponomarev, V.; Panagopoulos, V.; DeNichilo, M.; Ingman, W.; Atkins, G.J.; Findlay, D.M.; et al. Hypoxia-activated pro-drug TH-302 exhibits potent tumor suppressive activity and cooperates with chemotherapy against osteosarcoma. Cancer Lett. 2015, 357, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Avnet, S.; Lemma, S.; Cortini, M.; Pellegrini, P.; Perut, F.; Zini, N.; Kusuzaki, K.; Chano, T.; Grisendi, G.; Dominici, M.; et al. Altered pH gradient at the plasma membrane of osteosarcoma cells is a key mechanism of drug resistance. Oncotarget 2016, 7, 63408–63423. [Google Scholar] [CrossRef]
- Wiklander, O.P.B.; Brennan, M.; Lötvall, J.; Breakefield, X.O.; El Andaloussi, S. Advances in therapeutic applications of extracellular vesicles. Sci. Transl. Med. 2019, 11, eaav8521. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).