How Kinesin-1 Utilize the Energy of Nucleotide: The Conformational Changes and Mechanochemical Coupling in the Unidirectional Motion of Kinesin-1
Abstract
1. Introduction
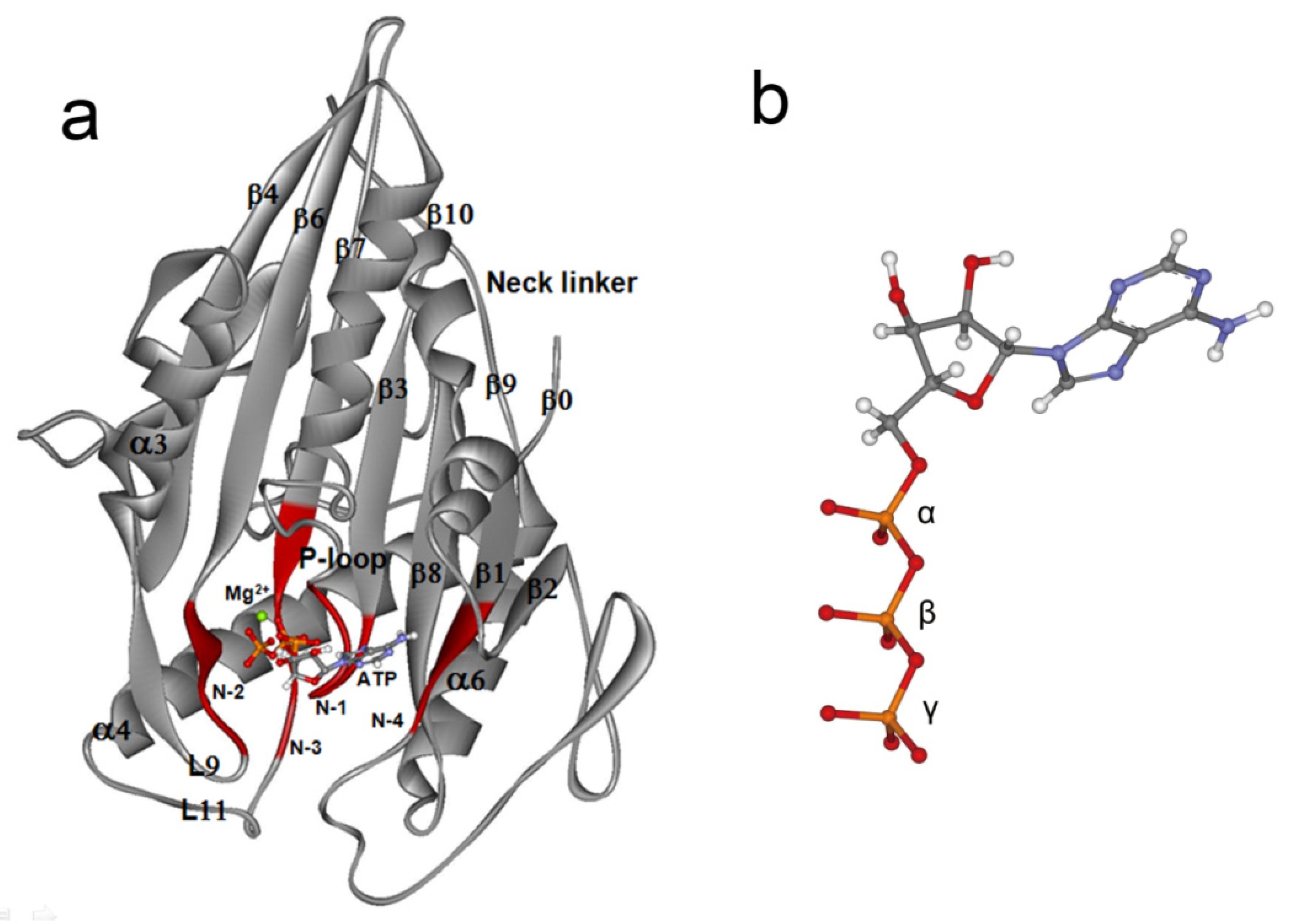
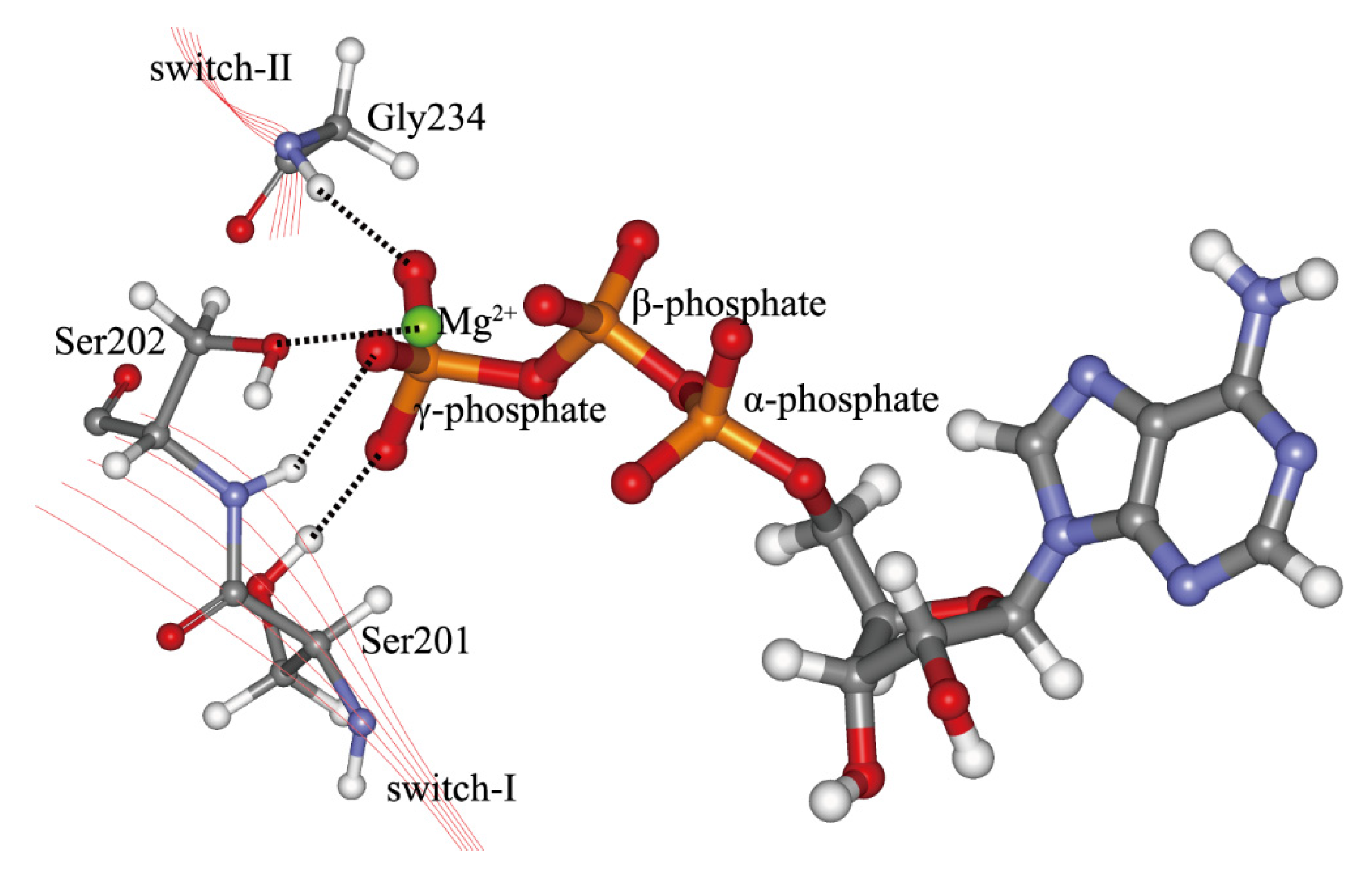
2. Interactions Between ATP Molecule and Motor Domain of Kinesin-1
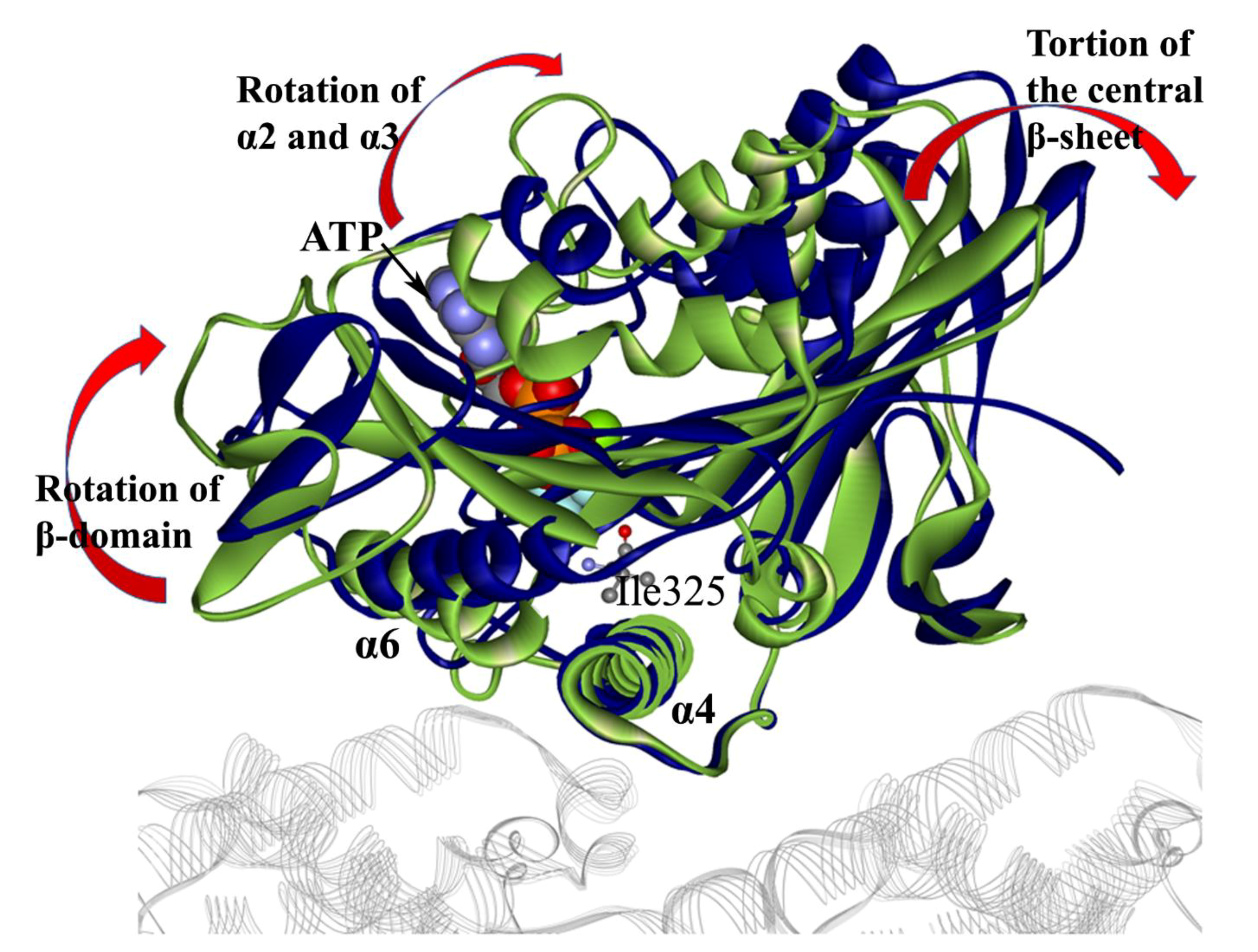
3. Motor-Domain Rotation Induced by ATP Binding Initiates the Force-Generation Process of Kinesin-1
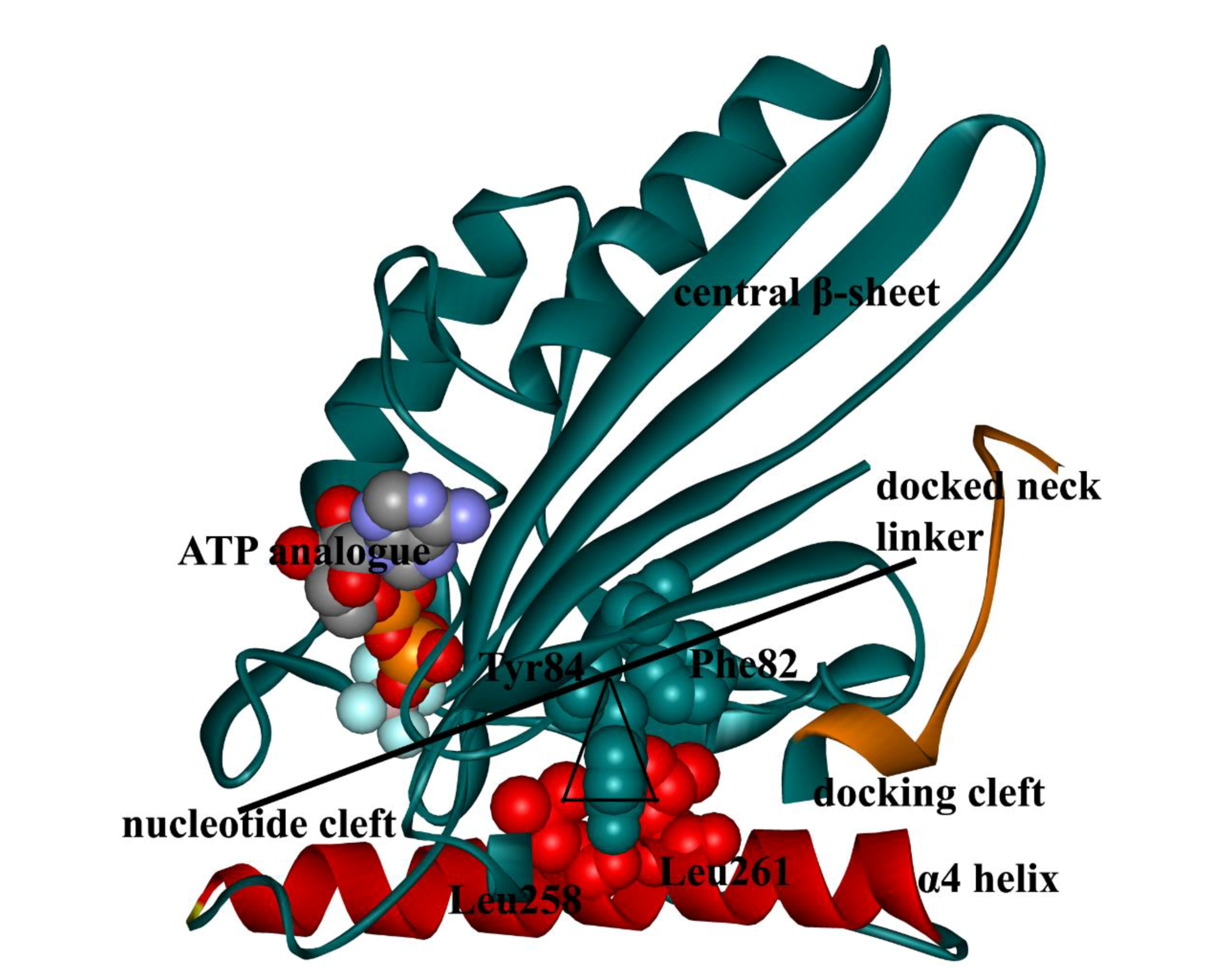
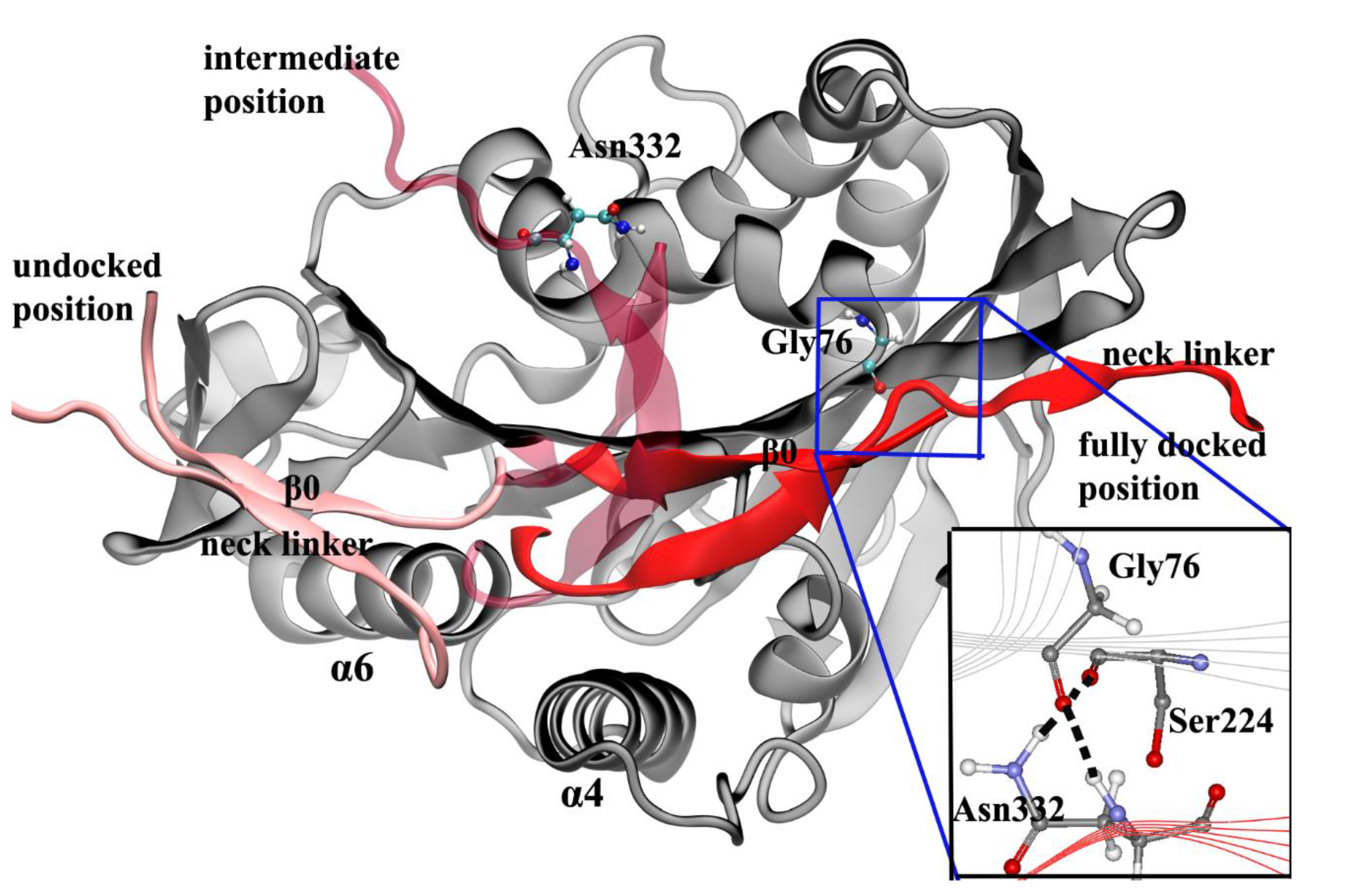
4. Docking Movement of Kinesin-1 Neck Linker to Motor Domain
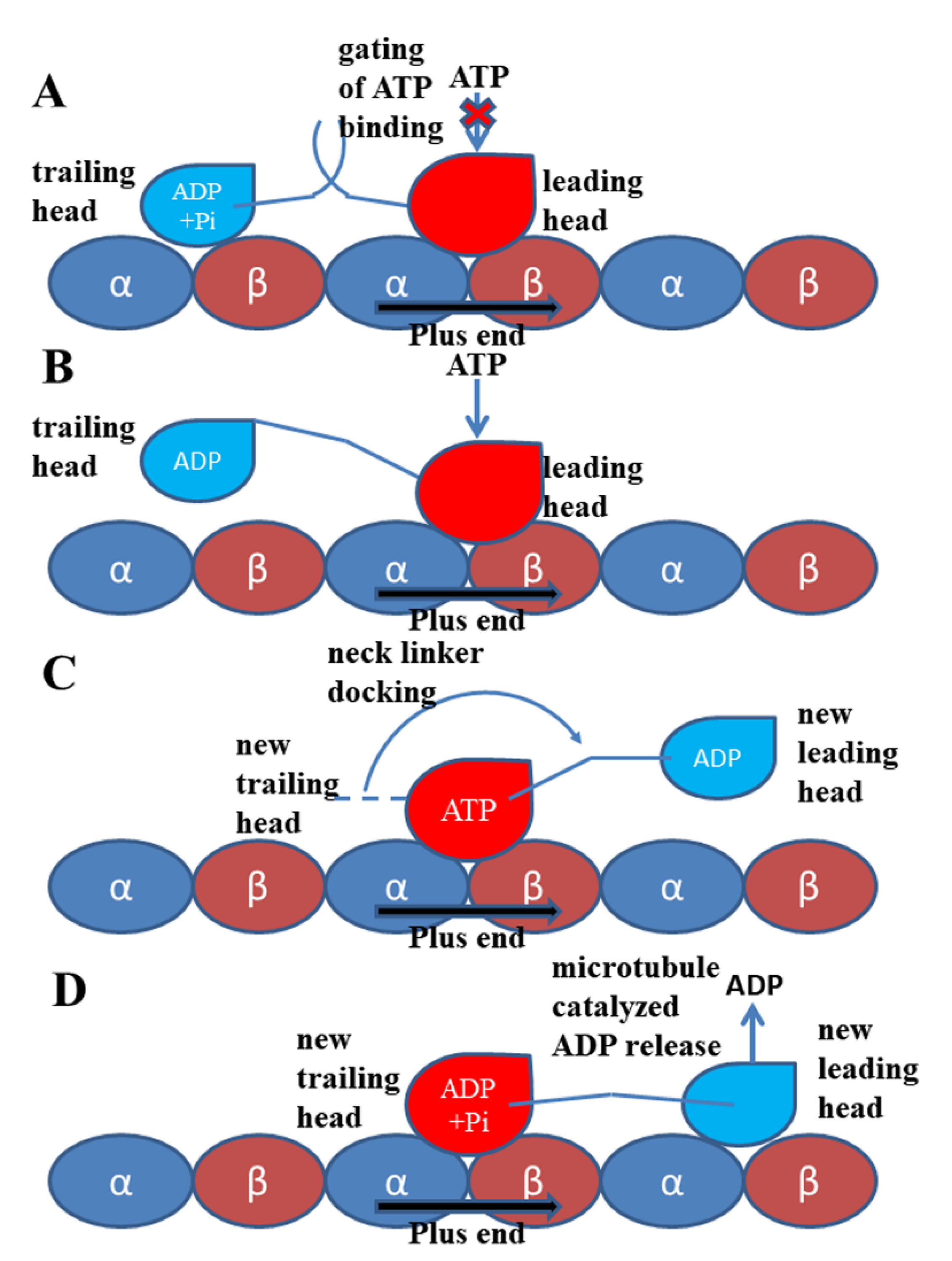
5. Coupling Regulation of Kinesin-1 Mechanochemical Cycle
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ATP | adenosine triphosphate |
| ADP | adenosine diphosphate |
| MD | molecular dynamics |
| SMD | steered molecular dynamics |
| QM/MM | quantum-mechanical/molecular-mechanical |
| PDB | protein data bank |
| EM | electron microscope |
| CNB | cover-neck bundle |
| MCAK | mitotic centromere-associated kinesin |
References
- Lawrence, C.J.; Kelly Dawe, R.; Christie, K.R.; Cleveland, D.W.; Dawson, S.C.; Endow, S.A.; Goldstein, L.S.B.; Goodson, H.V.; Hirokawa, N.; Howard, J.; et al. A standardized kinesin nomenclature. J. Cell Biol. 2004, 167, 19–22. [Google Scholar] [CrossRef]
- Vale, R.D. The molecular motor toolbox for intracellular transport. Cell 2003, 112, 467–480. [Google Scholar] [CrossRef]
- Hirokawa, N.; Noda, Y.; Tanaka, Y.; Niwa, S. Kinesin superfamily motor proteins and intracellular transport. Nat. Rev. Mol. Cell Biol. 2009, 10, 682–696. [Google Scholar] [CrossRef] [PubMed]
- Hirokawa, N.; Tanaka, Y. Kinesin superfamily proteins (KIFs): Various functions and their relevance for important phenomena in life and diseases. Exp. Cell Res. 2015, 334, 16–25. [Google Scholar] [CrossRef]
- Brady, S.T. A novel brain ATPase with properties expected for the fast axonal transport motor. Nature 1985, 317, 73–75. [Google Scholar] [CrossRef] [PubMed]
- Vale, R.D.; Reese, T.S.; Sheetz, M.P. Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell 1985, 42, 39–50. [Google Scholar] [CrossRef]
- Guo, S.K.; Shi, X.X.; Wang, P.Y.; Xie, P. Run length distribution of dimerized kinesin-3 molecular motors: Comparison with dimeric kinesin-1. Sci. Rep. UK 2019, 9, 16973. [Google Scholar] [CrossRef]
- Cockburn, J.J.B.; Hesketh, S.J.; Mulhair, P.; Thomsen, M.; O’Connell, M.J.; Way, M. Insights into kinesin-1 activation from the crystal structure of KLC2 bound to JIP3. Structure 2018, 26, 1486–1498. [Google Scholar] [CrossRef]
- Rice, S.; Lin, A.W.; Safer, D.; Hart, C.L.; Naber, N.; Carragher, B.O.; Cain, S.M.; Pechatnikova, E.; Wilson-Kubalek, E.M.; Whittaker, M.; et al. A structural change in the kinesin motor protein that drives motility. Nature 1999, 402, 778–784. [Google Scholar] [CrossRef]
- Vale, R.D.; Milligan, R.A. The way things move: Looking under the hood of molecular motor proteins. Science 2000, 288, 88–95. [Google Scholar] [CrossRef]
- Case, R.B.; Rice, S.; Hart, C.L.; Ly, B.; Vale, R.D. Role of the kinesin neck linker and catalytic core in microtubule-based motility. Curr. Biol. 2000, 10, 157–160. [Google Scholar] [CrossRef]
- Sindelar, C.V.; Budny, M.J.; Rice, S.; Naber, N.; Fletterick, R.; Cooke, R. Two conformations in the human kinesin power stroke defined by X-ray crystallography and EPR spectroscopy. Nat. Struct. Biol. 2002, 9, 844–848. [Google Scholar] [CrossRef] [PubMed]
- Tomishige, M.; Stuurman, N.; Vale, R.D. Single-molecule observations of neck linker conformational changes in the kinesin motor protein. Nat. Struct. Mol. Biol. 2006, 13, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Pandey, H.; Al-Bassam, J.; Gheber, L. Bidirectional motility of kinesin-5 motor proteins: Structural determinants, cumulative functions and physiological roles. Cell. Mol. Life Sci. 2018, 75, 1757–1771. [Google Scholar] [CrossRef]
- Yamagishi, M.; Shigematsu, H.; Yokoyama, T.; Kikkawa, M.; Sugawa, M.; Aoki, M.; Shirouzu, M.; Yajima, J.; Nitta, R. Structural basis of backwards motion in kinesin-1-kinesin-14 chimera: Implication for kinesin-14 motility. Structure 2016, 24, 1–13. [Google Scholar] [CrossRef]
- Desai, A.; Verma, S.; Mitchison, T.J.; Walczak, C.E. Kin I kinesins are microtubule-destabilizing enzymes. Cell 1999, 96, 69–78. [Google Scholar] [CrossRef]
- Hunter, A.W.; Caplow, M.; Coy, D.L.; Hancock, W.O.; Diez, S.; Wordeman, L.; Howard, J. The kinesin-related protein MCAK is a microtubule depolymerase that forms an ATP-hydrolyzing complex at microtubule ends. Mol. Cell 2003, 11, 445–457. [Google Scholar] [CrossRef]
- Helenius, J.; Brouhard, G.; Kalaidzidis, Y.; Diez, S.; Howard, J. The depolymerizing kinesin MCAK uses lattice diffusion to rapidly target microtubule ends. Nature 2006, 441, 115–119. [Google Scholar] [CrossRef]
- Walczak, C.E.; Gayek, S.; Ohi, R. Microtubule-depolymerizing kinesins. Annu. Rev. Cell Dev. Biol. 2013, 29, 417–441. [Google Scholar] [CrossRef]
- Schnitzer, M.J.; Block, S.M. Kinesin hydrolyses one ATP per 8-nm step. Nature 1997, 388, 386–390. [Google Scholar] [CrossRef]
- Coy, D.L.; Wagenbach, M.; Howard, J. Kinesin takes one 8-nm step for each ATP that it hydrolyzes. J. Biol. Chem. 1999, 274, 3667–3671. [Google Scholar] [CrossRef]
- Cochran, J.C. Kinesin motor enzymology: Chemistry, structure, and physics of nanoscale molecular machines. Biophys. Rev. 2015, 7, 269–299. [Google Scholar] [CrossRef] [PubMed]
- Sack, S.; Müller, J.; Marx, A.; Thormählen, M.; Mandelkow, E.-M.; Brady, S.T.; Mandelkow, E. X-ray structure of motor and neck domains from rat brain kinesin. Biochemistry 1997, 36, 16155–16165. [Google Scholar] [CrossRef] [PubMed]
- Kozielski, F.; Sack, S.; Marx, A.; Thormählen, M.; Schönbrunn, E.; Biou, V.; Thompson, A.; Mandelkow, E.-M.; Mandelkow, E. The crystal structure of dimeric kinesin and implications for microtubule-dependent motility. Cell 1997, 91, 985–994. [Google Scholar] [CrossRef]
- Kull, F.J.; Sablin, E.P.; Lau, R.; Fletterick, R.J.; Vale, R.D. Crystal structure of the kinesin motor domain reveals a structural similarity to myosin. Nature 1996, 380, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Sablin, E.P.; Kull, F.J.; Cooke, R.; Vale, R.D.; Fletterick, R.J. Crystal structure of the motor domain of the kinesin-related motor ncd. Nature 1996, 380, 555–559. [Google Scholar] [CrossRef]
- Smith, C.A.; Rayment, I. Active site comparisons highlight structural similarities between myosin and other P-loop proteins. Biophys. J. 1996, 70, 1590–1602. [Google Scholar] [CrossRef]
- Walker, J.E.; Saraste, M.; Runswick, M.J.; Gay, N.J. Distantly related sequences in the α- and β-subunits of ATP synthase, myosin, kinases, and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982, 1, 945–951. [Google Scholar] [CrossRef]
- Saraste, M.; Sibbald, P.R.; Wittinghofer, A. The P-loop-a common motif in ATP- and GTP-binding proteins. Trends Biol. Sci. 1990, 15, 430–434. [Google Scholar] [CrossRef]
- Sack, S.; Kull, F.J.; Mandelkow, E. Motor proteins of the kinesin family-Structures, variations, and nucleotide binding sites. Eur. J. Biochem. 1999, 262, 1–11. [Google Scholar] [CrossRef]
- Kull, F.J.; Endow, S.A. Kinesin: Switch I & II and the motor mechanism. J. Cell Sci. 2002, 115, 15–23. [Google Scholar] [PubMed]
- Parke, C.L.; Wojcik, E.J.; Kim, S.Y.; Worthylake, D.K. ATP hydrolysis in Eg5 kinesin involves a catalytic two-water mechanism. J. Biol. Chem. 2010, 285, 5859–5867. [Google Scholar] [CrossRef] [PubMed]
- Hwang, W.; Lang, M.J.; Karplus, M. Kinesin motility is driven by subdomain dynamics. eLife 2017, 6, e28948. [Google Scholar] [CrossRef] [PubMed]
- Vale, R.D. Switches, latches, and amplifiers: Common themes of G proteins and molecular motors. J. Cell Biol. 1996, 135, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Skiniotis, G.; Surrey, T.; Altmann, S.; Gross, H.; Song, Y.H.; Mandelkow, E.; Hoenger, A. Nucleotide-induced conformations in the neck region of dimeric kinesin. EMBO J. 2003, 22, 1518–1528. [Google Scholar] [CrossRef]
- Skiniotis, G.; Cochran, J.C.; Müller, J.; Mandelkow, E.; Gilbert, S.P.; Hoenger, A. Modulation of kinesin binding by the C-termini of tubulin. EMBO J. 2004, 23, 989–999. [Google Scholar] [CrossRef]
- Sindelar, C.V.; Downing, K.H. The beginning of kinesin’s force-generating cycle visualized at 9-Å resolution. J. Cell Biol. 2007, 177, 377–385. [Google Scholar] [CrossRef]
- Sindelar, C.V.; Downing, K.H. An atomic-level mechanism for activation of the kinesin molecular motors. Proc. Natl. Acad. Sci. USA 2010, 107, 4111–4116. [Google Scholar] [CrossRef]
- Sindelar, C.V. A seesaw model for intermolecular gating in the kinesin motor protein. Biophys. Rev. 2011, 3, 85–100. [Google Scholar] [CrossRef]
- Kikkawa, M.; Hirokawa, N. High-resolution cryo-EM maps show the nucleotide binding pocket of KIF1A in open and closed conformations. EMBO J. 2006, 25, 4187–4194. [Google Scholar] [CrossRef]
- Goulet, A.; Behnke-Parks, W.M.; Sindelar, C.V.; Major, J.; Rosenfeld, S.S.; Moores, C.A. The structural basis of force generation by the mitotic motor Kinesin-5. J. Biol. Chem. 2012, 287, 44654–44666. [Google Scholar] [CrossRef]
- Geng, Y.; Liu, S.; Ji, Q.; Yan, S. Mechanical amplification mechanism of kinesin’s beta-domain. Arch. Biochem. Biophys. 2014, 543, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Kikkawa, M.; Sablin, E.P.; Okada, Y.; Yajima, H.; Fletterick, R.J.; Hirokawa, N. Switch-based mechanism of kinesin motors. Nature 2001, 411, 439–445. [Google Scholar] [CrossRef]
- Sablin, E.P.; Fletterick, R.J. Nucleotide switches in molecular motors: Structural analysis of kinesins and myosins. Curr. Opin. Struct. Biol. 2001, 11, 716–724. [Google Scholar] [CrossRef]
- Fisher, A.J.; Smith, C.A.; Thoden, J.B.; Smith, R.; Sutoh, K.; Holden, H.M.; Rayment, I. X-ray structures of the myosin motor domain of dictyostelium discoideum complexed with MgADP·BeFx and MgADP·AlF4−. Biochemistry 1995, 34, 8960–8972. [Google Scholar] [CrossRef] [PubMed]
- Minehardt, T.J.; Cooke, R.; Pate, E.; Kollman, P.A. Molecular dynamics study of the energetic, mechanistic, and structural implications of a closed phosphate tube in ncd. Biophys. J. 2001, 80, 1151–1168. [Google Scholar] [CrossRef][Green Version]
- McGrath, M.J.; Kuo, I.F.; Hayashi, S.; Takada, S. Adenosine triphosphate hydrolysis mechanism in kinesin studied by combined quantum-mechanical/molecular-mechanical metadynamics simulations. J. Am. Chem. Soc. 2013, 135, 8908–8919. [Google Scholar] [CrossRef] [PubMed]
- Hoeng, J.C.; Dawson, S.C.; House, S.A.; Sagolla, M.S.; Pham, J.K.; Mancuso, J.J.; Löwe, J.; Cande, W.Z. High-resolution crystal structure and in vivo function of a kinesin-2 homologue in Giardia intestinalis. Mol. Biol. Cell 2008, 19, 3124–3137. [Google Scholar] [CrossRef] [PubMed]
- Nitta, R.; Okada, Y.; Hirokawa, N. Structural model for strain-dependent microtubule activation of Mg-ADP release from kinesin. Nat. Struct. Mol. Biol. 2008, 15, 1067–1075. [Google Scholar] [CrossRef]
- Chang, Q.; Nitta, R.; Inoue, S.; Hirokawa, N. Structural basis for the ATP-induced isomerization of kinesin. J. Mol. Biol. 2013, 425, 1869–1880. [Google Scholar] [CrossRef]
- Turner, J.; Anderson, R.; Guo, J.; Beraud, C.; Fletterick, R.; Sakowicz, R. Crystal structure of the mitotic spindle kinesin Eg5 reveals a novel conformation of the neck-linker. J. Biol. Chem. 2001, 276, 25496–25502. [Google Scholar] [CrossRef] [PubMed]
- Atherton, J.; Yu, I.M.; Cook, A.; Muretta, J.M.; Joseph, A.; Major, J.; Sourigues, Y.; Clause, J.; Topf, M.; Rosenfeld, S.S.; et al. The divergent mitotic kinesin MKLP2 exhibits atypical structure and mechanochemistry. eLife 2017, 6, e27793. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Saez, I.; Yen, T.; Wade, R.H.; Kozielski, F. Crystal structure of the motor domain of the human kinetochore protein CENP-E. J. Mol. Biol. 2004, 340, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Nitta, R.; Morikawa, M.; Yajima, H.; Inoue, S.; Shigematsu, H.; Kikkawa, M.; Hirokawa, N. Motility and microtubule depolymerization mechanisms of the Kinesin-8 motor, KIF19A. eLife 2016, 5, e18101. [Google Scholar] [CrossRef]
- Zhu, H.; Tempel, W.; He, H.; Shen, Y.; Wang, J.; Brothers, G.; Landry, R.; Arrowsmith, C.H.; Edwards, A.M.; Sundstrom, M.; et al. Crystal structure of the human KIF9 motor domain in complex with ADP. PDB 2010, unpublished. [Google Scholar] [CrossRef]
- Cochran, J.C.; Sindelar, C.V.; Mulko, N.K.; Collins, K.A.; Kong, S.E.; Hawley, R.S.; Kull, F.J. ATPase cycle of the nonmotile kinesin NOD allows microtubule end tracking and drives chromosome movement. Cell 2009, 136, 110–122. [Google Scholar] [CrossRef]
- Klejnot, M.; Falnikar, A.; Ulaganathan, V.; Cross, R.A.; Baas, P.W.; Kozielski, F. The crystal structure and biochemical characterization of Kif15: A bifunctional molecular motor involved in bipolar spindle formation and neuronal development. Acta Crystallogr. D. Biol. Crystallogr. 2014, 70, 123–133. [Google Scholar] [CrossRef]
- Wang, W.; Cantos-Fernandes, S.; Lv, Y.; Kuerban, H.; Ahmad, S.; Wang, C.; Gigant, B. Insight into microtubule disassembly by kinesin-13s from the structure of Kif2C bound to tubulin. Nat. Commun. 2017, 8, 70. [Google Scholar] [CrossRef]
- Sablin, E.P.; Case, R.B.; Dai, S.C.; Hart, C.L.; Ruby, A.; Vale, R.D.; Fletterick, R.J. Direction determination in the minus-end-directed kinesin motor ncd. Nature 1998, 395, 813–816. [Google Scholar] [CrossRef]
- Block, S.M. Kinesin motor mechanics: Binding, stepping, tracking, gating, and limping. Biophys. J. 2007, 92, 2986–2995. [Google Scholar] [CrossRef]
- Gigant, B.; Wang, W.; Dreier, B.; Jiang, Q.; Pecqueur, L.; Plückthun, A.; Wang, C.; Knossow, M. Structure of a kinesin-tubulin complex and implications for kinesin motility. Nat. Struct. Mol. Biol. 2013, 20, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Wang, W.; Jiang, Q.; Wang, C.; Knossow, M.; Gigant, B. The structure of apo-kinesin bound to tubulin links the nucleotide cycle to movement. Nat. Comm. 2014, 5, 5364. [Google Scholar] [CrossRef] [PubMed]
- Asenjo, A.B.; Krohn, N.; Sosa, H. Configuration of the two kinesin motor domains during ATP hydrolysis. Nat. Struct. Biol. 2003, 10, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Asenjo, A.B.; Sosa, H. A mobile kinesin-head intermediate during the ATP-waiting state. Proc. Natl. Acad. Sci. USA 2009, 106, 5657–5662. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Geng, Y.; Lü, L.; Ma, Y.; Lü, G.; Zhang, H.; Ji, Q. Anchor effect of interactions between kinesin’s nucleotide-binding pocket and microtubule. Cel. Mol. Bioeng. 2017, 10, 162–173. [Google Scholar] [CrossRef]
- Atherton, J.; Farabella, I.; Yu, I.; Rosenfeld, S.S.; Houdusse, A.; Topf, M.; Moores, C.A. Conserved mechanisms of microtubule-stimulated ADP release, ATP binding, and force generation in transport kinesins. eLife 2014, 3, e03680. [Google Scholar] [CrossRef]
- Shang, Z.; Zhou, K.; Xu, C.; Csencsits, R.; Cochran, J.C.; Sindelar, C.V. High-resolution structures of kinesin on microtubules provide a basis for nucleotide-gated force-generation. eLife 2014, 3, e04686. [Google Scholar] [CrossRef]
- Wang, W.; Cao, L.; Wang, C.; Gigant, B.; Knossow, M. Kinesin, 30 years later: Recent insights from structural studies. Protein Sci. 2015, 24, 1047–1056. [Google Scholar] [CrossRef]
- Morikawa, M.; Yajima, H.; Nitta, R.; Inoue, S.; Ogura, T.; Sato, C.; Hirokawa, N. X-ray and cryo-EM structures reveal mutual conformational changes of Kinesin and GTP-state microtubules upon binding. EMBO J. 2015, 34, 1270–1286. [Google Scholar] [CrossRef]
- Muretta, J.M.; Jun, Y.; Gross, S.P.; Major, J.; Thomas, D.D.; Rosenfeld, S.S. The structural kinetics of switch-1 and the neck linker explain the functions of kinesin-1 and Eg5. Proc. Natl. Acad. Sci. USA 2015, 112, E6606–E6613. [Google Scholar] [CrossRef]
- Cao, L.; Cantos-Fernandes, S.; Gigant, B. The structural switch of nucleotide-free kinesin. Sci. Rep. UK 2017, 7, 42558. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Liu, X.; Shang, Z.; Sindelar, C.V. Structural basis of cooperativity in kinesin revealed by 3D reconstruction of a two-head-bound state on microtubules. eLife 2017, 6, e24490. [Google Scholar] [CrossRef] [PubMed]
- Scarabelli, G.; Grant, B.J. Mapping the structural and dynamical features of kinesin motor domains. PLoS Comput. Biol. 2013, 9, e1003329. [Google Scholar] [CrossRef]
- Krukau, A.; Knecht, V.; Lipowsky, R. Allosteric control of kinesin’s motor domain by tubulin: A molecular dynamics study. Phys. Chem. Chem. Phys. 2014, 16, 6189–6198. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ma, Y.; Li, T.; Jin, Y.; Geng, Y.; Ji, Q. Shaft function of kinesin-1’s α4 helix in the processive movement. Cell. Mol. Bioeng. 2019, 12, 345–354. [Google Scholar] [CrossRef]
- Liu, F.; Ji, Q.; Wang, H.; Wang, J. Mechanochemical model of the power stroke of the single-headed motor protein KIF1A. J. Phys. Chem. B 2018, 122, 11002–11013. [Google Scholar] [CrossRef]
- Rice, S.; Cui, Y.; Sindelar, C.; Naber, N.; Matuska, M.; Vale, R.; Cooke, R. Thermodynamic properties of the kinesin neck-region docking to the catalytic core. Biophys. J. 2003, 84, 1844–1854. [Google Scholar] [CrossRef]
- Asenjo, A.B.; Weinberg, Y.; Sasa, H. Nucleotide binding and hydrolysis induces a disorder-order transition in the kinesin neck-linker region. Nat. Struct. Mol. Biol. 2006, 13, 648–654. [Google Scholar] [CrossRef]
- Budaitis, B.G.; Jariwala, S.; Reinemann, D.N.; Schimeret, K.I.; Scarabelli, G.; Grant, B.J.; Sept, D.; Lang, M.J.; Verhey, K.J. Neck linker docking is critical for Kinesin-1 force generation in cells but at a cost to motor speed and processivity. eLife 2019, 8, e44146. [Google Scholar] [CrossRef]
- Hwang, W.; Karplus, M. Structural basis for power stroke vs. Brownian ratchet mechanisms of motor proteins. Proc. Natl. Acad. Sci. USA 2019, 116, 19777–19785. [Google Scholar] [CrossRef]
- Asbury, C.L.; Fehr, A.N.; Block, S.M. Kinesin moves by an asymmetric hand-over-hand mechanism. Science 2003, 302, 2130–2134. [Google Scholar] [CrossRef] [PubMed]
- Kaseda, K.; Higuchi, H.; Hirose, K. Alternate fast and slow stepping of a heterodimeric kinesin molecule. Nat. Cell Biol. 2003, 5, 1079–1082. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, A.; Tomishige, M.; Vale, R.D.; Selvin, P.R. Kinesin walks hand-over-hand. Science 2004, 303, 676–678. [Google Scholar] [CrossRef]
- Xie, P. Mechanism of processive movement of monomeric and dimeric kinesin molecules. Int. J. Biol. Sci. 2010, 6, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Hyeon, C.; Onuchic, J.N. Mechanical control of the directional stepping dynamics of the kinesin motor. Proc. Natl. Acad. Sci. USA 2007, 104, 17382–17387. [Google Scholar] [CrossRef]
- Zhang, Z.; Thirumalai, D. Dissecting the kinematics of the kinesin step. Structure 2012, 20, 628–640. [Google Scholar] [CrossRef]
- Geng, Y.; Li, T.; Ji, Q.; Yan, S. Simulation study of interactions between kinesin’s neck linker and motor domain. Cel. Mol. Bioeng. 2014, 7, 99–105. [Google Scholar] [CrossRef]
- Geng, Y.; Ji, Q.; Liu, S.; Yan, S. Initial conformation of kinesin’s neck linker. Chin. Phys. B 2014, 23, 108701. [Google Scholar] [CrossRef]
- Geng, Y.; Zhang, H.; Lyu, G.; Ji, Q. Initiation mechanism of kinesin’s neck linker docking process. Chin. Phys. Lett. 2017, 34, 028701. [Google Scholar] [CrossRef]
- Hwang, W.; Lang, M.J.; Karplus, M. Force generation in kinesin hinges on cover-neck bundle formation. Structure 2008, 16, 62–71. [Google Scholar] [CrossRef]
- Khalil, A.S.; Appleyard, D.C.; Labno, A.K.; Georges, A.; Karplus, M.; Belcher, A.; Hwang, W.; Lang, M.J. Kinesin’s cover-neck bundle folds forward to generate force. Proc. Natl. Acad. Sci. USA 2008, 105, 19247–19252. [Google Scholar] [CrossRef] [PubMed]
- Hesse, W.R.; Steiner, M.; Wohlever, M.L.; Kamm, R.D.; Hwang, W.; Lang, M.J. Modular aspects of kinesin force generation machinery. Biophys. J. 2013, 104, 1969–1978. [Google Scholar] [CrossRef] [PubMed]
- Rice, S.E. Kinesin-5 seems to step to its own unique tune, but really it’s a cover. Biophys. J. 2013, 104, 1846–1848. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Qin, J.; Geng, Y.; Lü, G.; Ji, Q.; Fang, H. Protection-against-water-attack determined difference between strengths of backbone hydrogen bonds in kinesin’s neck zipper region. Chin. Phys. B 2018, 27, 028704. [Google Scholar] [CrossRef]
- Shi, X.; Guo, S.; Wang, P.; Chen, H.; Xie, P. All-atom molecular dynamics simulations reveal how kinesin transits from one-head-bound to two-heads-bound state. Proteins 2019, 88, 545–557. [Google Scholar] [CrossRef]
- Milic, B.; Andreasson, J.O.L.; Hancock, W.O.; Block, M. Kinesin processivity is gated by phosphate release. Proc. Natl. Acad. Sci. USA 2014, 111, 14136–14140. [Google Scholar] [CrossRef]
- Andreasson, J.O.L.; Milic, B.; Chen, G.Y.; Guydosh, N.R.; Hancock, W.O.; Block, S.M. Examining kinesin processivity within a general gating framework. eLife 2015, 4, e07403. [Google Scholar] [CrossRef]
- Mickolajczyk, K.J.; Deffenbaugh, N.C.; Arroyo, J.O.; Andrecka, J.; Kukura, P.; Hancock, W.O. Kinetics of nucleotide-dependent structural transitions in the kinesin-1 hydrolysis cycle. Proc. Natl. Acad. Sci. USA 2015, 112, E7186–E7193. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Hancock, W.O.; Howard, J. Kinesin’s processivity results from mechanical and chemical coordination between the ATP hydrolysis cycles of the two motor domains. Proc. Natl. Acad. Sci. USA 1999, 96, 13147–13152. [Google Scholar] [CrossRef]
- Rosenfeld, S.S.; Xing, J.; Jefferson, G.M.; Cheung, H.C.; King, P.H. Measuring kinesin’s first step. J. Biol. Chem. 2002, 277, 36731–36739. [Google Scholar] [CrossRef]
- Schief, W.R.; Clark, R.H.; Crevenna, A.H.; Howard, J. Inhibition of kinesin motility by ADP and phosphate supports a hand-over-hand mechanism. Proc. Natl. Acad. Sci. USA 2004, 101, 1183–1188. [Google Scholar] [CrossRef]
- Crevel, I.M.; Nyitrai, M.; Alonso, M.C.; Weiss, S.; Geeves, M.A.; Cross, R.A. What kinesin does at roadblocks: The coordination mechanism for molecular walking. EMBO J. 2004, 23, 23–32. [Google Scholar] [CrossRef]
- Sablin, E.P.; Fletterick, R.J. Coordination between motor domains in processive kinesins. J. Biol. Chem. 2004, 279, 15707–15710. [Google Scholar] [CrossRef]
- Hancock, W.O. The kinesin-1 chemomechanical cycle: Stepping toward a consensus. Biophys. J. 2016, 110, 1216–1225. [Google Scholar] [CrossRef]
- Coy, D.L.; Hancock, W.O.; Wagenbach, M.; Howard, J. Kinesin’s tail domain is an inhibitory regulator of the motor domain. Nat. Cell Biol. 1999, 1, 288–292. [Google Scholar] [CrossRef]
- Hackney, D.D.; Stock, M.F. Kinesin’s IAK tail domain inhibits initial microtubule-stimulated ADP release. Nat. Cell Biol. 2000, 2, 257–260. [Google Scholar] [CrossRef]
- Hackney, D.D.; Stock, M.F. Kinesin tail domains and Mg2+ directly inhibit release of ADP from head domains in the absence of microtubules. Biochemistry 2008, 47, 7770–7778. [Google Scholar] [CrossRef]
- Hackney, D.D.; Baek, N.; Snyder, A.C. Half-site inhibition of dimeric kinesin head domains by monomeric tail domains. Biochemistry 2009, 48, 3448–3456. [Google Scholar] [CrossRef]
- Kaan, H.Y.; Hackney, D.D.; Kozielski, F. The structure of the kinesin-1 motor-tail complex reveals the mechanism of autoinhibition. Science 2011, 333, 883–885. [Google Scholar] [CrossRef]
- Kuznetsov, S.A.; Gelfand, V.I. Bovine brain kinesin is a microtubule-activated ATPase. Proc. Natl. Acad. Sci. USA 1986, 83, 8530–8534. [Google Scholar] [CrossRef] [PubMed]
- Hackney, D.D. Kinesin ATPase: Rate-limiting ADP release. Proc. Natl. Acad. Sci. USA 1988, 85, 6314–6318. [Google Scholar] [CrossRef] [PubMed]
- Kikkawa, M. The role of microtubules in processive kinesin movement. Trends Cell Biol. 2008, 18, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Vale, R.D.; Tomishige, M. How kinesin waits between steps. Nature 2007, 450, 750–754. [Google Scholar] [CrossRef]
- Tomishige, M.; Vale, R.D. Controlling kinesin by reversible disulfide cross-linking: Identifying the motility-producing conformational change. J. Cell Biol. 2000, 151, 1081–1092. [Google Scholar] [CrossRef]
- Hackney, D.D.; Stock, M.F.; Moore, J.; Patterson, R.A. Modulation of kinesin half-site ADP release and kinetic processivity by a spacer between the head groups. Biochemistry 2003, 42, 12011–12018. [Google Scholar] [CrossRef]
- Rosenfeld, S.S.; Fordyce, P.M.; Jefferson, G.M.; King, P.H.; Block, S.M. Stepping and stretching. How kinesin uses internal strain to walk processively. J. Biol. Chem. 2003, 278, 18550–18556. [Google Scholar] [CrossRef]
- Uemura, S.; Ishiwata, S. Loading direction regulates the affinity of ADP for kinesin. Nat. Struct. Biol. 2003, 10, 308–311. [Google Scholar] [CrossRef]
- Hyeon, C.; Onuchic, J.N. Internal strain regulates the nucleotide binding site of the kinesin leading head. Proc. Natl. Acad. Sci. USA 2007, 104, 2175–2180. [Google Scholar] [CrossRef]
- Yildiz, A.; Tonishige, M.; Gennerich, A.; Vale, R.D. Intramolecular strain coordinates kinesin stepping behavior along microtubules. Cell 2008, 134, 1030–1041. [Google Scholar] [CrossRef]
- Clancy, B.E.; Behnke-Parks, W.M.; Andreasson, J.O.; Rosenfeld, S.S.; Block, S.M. A universal pathway for kinesin stepping. Nat. Struct. Mol. Biol. 2011, 18, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, V.; Hancock, W.O. Insights into the mechanical properties of the kinesin neck linker domain from sequence analysis and molecular dynamics simulations. Cell. Mol. Bioeng. 2009, 2, 177–189. [Google Scholar] [CrossRef]
- Shastry, S.; Hancock, W.O. Interhead tension determines processivity across diverse N-terminal kinesins. Proc. Natl. Acad. Sci. USA 2011, 108, 16253–16258. [Google Scholar] [CrossRef] [PubMed]
- Guydosh, N.R.; Block, S.M. Backsteps induced by nucleotide analogs suggest the front head of kinesin is gated by strain. Proc. Natl. Acad. Sci. USA 2006, 103, 8054–8059. [Google Scholar] [CrossRef]
- Dogan, M.Y.; Can, S.; Cleary, D.B.; Purde, V.; Yildiz, A. Kinesin’s front head is gated by the backward orientation of its neck linker. Cell Rep. 2015, 10, 1967–1973. [Google Scholar] [CrossRef]
- Sosa, H.; Peterman, E.J.G.; Moerner, W.E.; Goldstein, L.S.B. ADP-induced rocking of the kinesin motor domain revealed by single-molecule fluorescence polarization microscopy. Nat. Struct. Biol. 2001, 8, 540–544. [Google Scholar] [CrossRef]
- Toprak, E.; Yildiz, A.; Hoffman, M.T.; Rosenfeld, S.S.; Selvin, P.R. Why kinesin is so processive. Proc. Natl. Acad. Sci. USA 2009, 106, 12717–12722. [Google Scholar] [CrossRef]
- Cross, R.A.; McAinsh, A. Prime movers: The mechanochemistry of mitotic kinesins. Nat. Rev. Mol. Cell Biol. 2014, 15, 257–271. [Google Scholar] [CrossRef]
- Friel, C.T.; Howard, J. The kinesin-13 MCAK has an unconventional ATPase cycle adapted for microtubule depolymerization. EMBO J. 2011, 30, 3928–3939. [Google Scholar] [CrossRef]
- Henrichs, V.; Grycoval, L.; Barinka, C.; Nahacka, Z.; Neuzil, J.; Diez, S.; Rohlena, J.; Braun, M.; Lansky, Z. Mitochondria-adaptor TRAK1 promotes kinesin-1 driven transport in crowded environments. Nat. Commun. 2020, 11, 3123. [Google Scholar] [CrossRef]
- Konishi, Y.; Setou, M. Tubulin tyrosination navigates the kinesin-1 motor domain to axons. Nat. Neurosci. 2009, 12, 559–567. [Google Scholar] [CrossRef]
- Dunn, S.; Morrison, E.E.; Liverpool, T.B.; Molina-Paris, C.; Cross, R.A.; Alonso, M.C.; Peckham, M. Differential trafficking of Kif5c on tyrosinated and detyrosinated microtubules in live cells. J. Cell Sci. 2008, 121, 1085–1095. [Google Scholar] [CrossRef]
- Sirajuddin, M.; Rice, L.M.; Vale, R.D. Regulation of microtubule motors by tubulin isotypes and post-translational modifications. Nat. Cell Biol. 2014, 16, 335–344. [Google Scholar] [CrossRef]

| Kinesin Family 1 | N-1 | N-2 | N-3 | N-4 |
|---|---|---|---|---|
| Kinesin-1 2 | G Q T S S G K T H | N E H S S R | D L A G S E | R F R P |
| Kinesin-2 3 | G Q T G A G K T Y | N D T S S R | D L A G S E | R C R P |
| Kinesin-3 4 | G Q T G S G K S Y | N D T S S R | D L A G S E | R V R A |
| Kinesin-4 5 | G Q T G S G K T Y | N S Q S S R | D L A G S E | R C R P |
| Kinesin-5 6 | G Q T G T G K T F | N A Y S S R | D L A G S E | R C R P |
| Kinesin-6 7 | G V T N S G K T Y | N Q Q S S R | D L A G S E | R I R P |
| Kinesin-7 8 | G Q T A S G K T Y | N Q R S S R | D L A G S E | R V R P |
| Kinesin-8 9 | G P T G C G K T Y | N Q T S S R | D L A G S E | R V R P |
| Kinesin-9 10 | G Q T G A G K T Y | N K N S S R | D L A G S E | R V K P |
| Kinesin-10 11 | G Q T G T G K S Y | N S N S S R | D L A G S E | R E A P |
| Kinesin-12 12 | G Q T G S G K T F | N R E S S R | D L A G S E | R I R P |
| Kinesin-13 13 | G Q T G S G K T H | N S N S S R | D L A G S E | R K R P |
| Kinesin-14 14 | G Q T G S G K T Y | N E R S S R | D L A G S E | R I R P |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, J.; Zhang, H.; Geng, Y.; Ji, Q. How Kinesin-1 Utilize the Energy of Nucleotide: The Conformational Changes and Mechanochemical Coupling in the Unidirectional Motion of Kinesin-1. Int. J. Mol. Sci. 2020, 21, 6977. https://doi.org/10.3390/ijms21186977
Qin J, Zhang H, Geng Y, Ji Q. How Kinesin-1 Utilize the Energy of Nucleotide: The Conformational Changes and Mechanochemical Coupling in the Unidirectional Motion of Kinesin-1. International Journal of Molecular Sciences. 2020; 21(18):6977. https://doi.org/10.3390/ijms21186977
Chicago/Turabian StyleQin, Jingyu, Hui Zhang, Yizhao Geng, and Qing Ji. 2020. "How Kinesin-1 Utilize the Energy of Nucleotide: The Conformational Changes and Mechanochemical Coupling in the Unidirectional Motion of Kinesin-1" International Journal of Molecular Sciences 21, no. 18: 6977. https://doi.org/10.3390/ijms21186977
APA StyleQin, J., Zhang, H., Geng, Y., & Ji, Q. (2020). How Kinesin-1 Utilize the Energy of Nucleotide: The Conformational Changes and Mechanochemical Coupling in the Unidirectional Motion of Kinesin-1. International Journal of Molecular Sciences, 21(18), 6977. https://doi.org/10.3390/ijms21186977




