Hippocampal Impairment Triggered by Long-Term Lead Exposure from Adolescence to Adulthood in Rats: Insights from Molecular to Functional Levels
Abstract
1. Introduction
2. Results
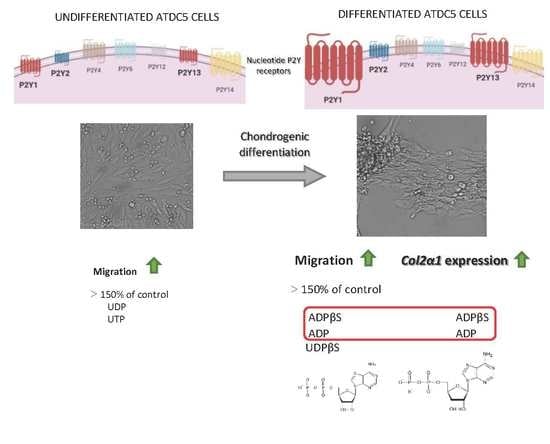
2.1. Long-Term Exposure to Pb Promotes Short-Term Memory Impairment in the Object Recognition Task
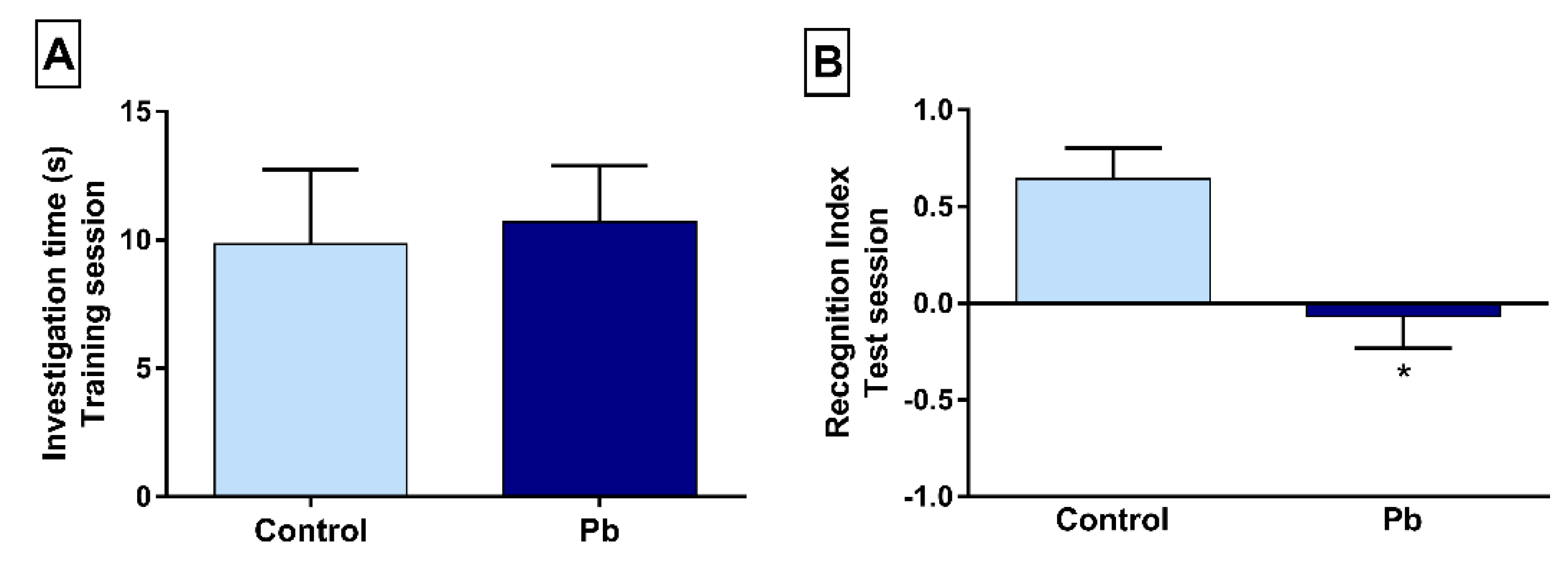
2.2. Long-Term Exposure to Pb Causes Long-Term Memory Impairment
2.3. Exposure to Pb Increases Metal Concentration in the Hippocampus
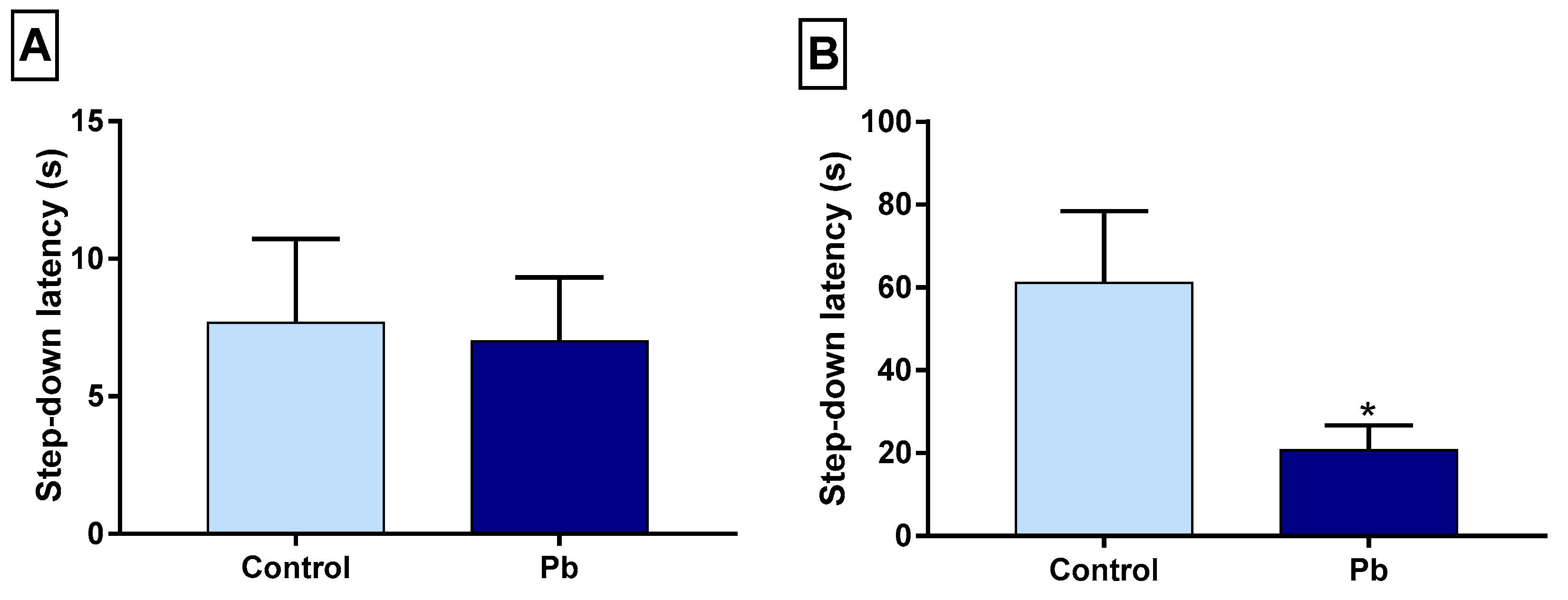
2.4. Effects of Long-Term Exposure to Pb on Oxidative Balance in the Hippocampus
2.5. The Hippocampal Proteome was Significantly Changed after Pb Exposure
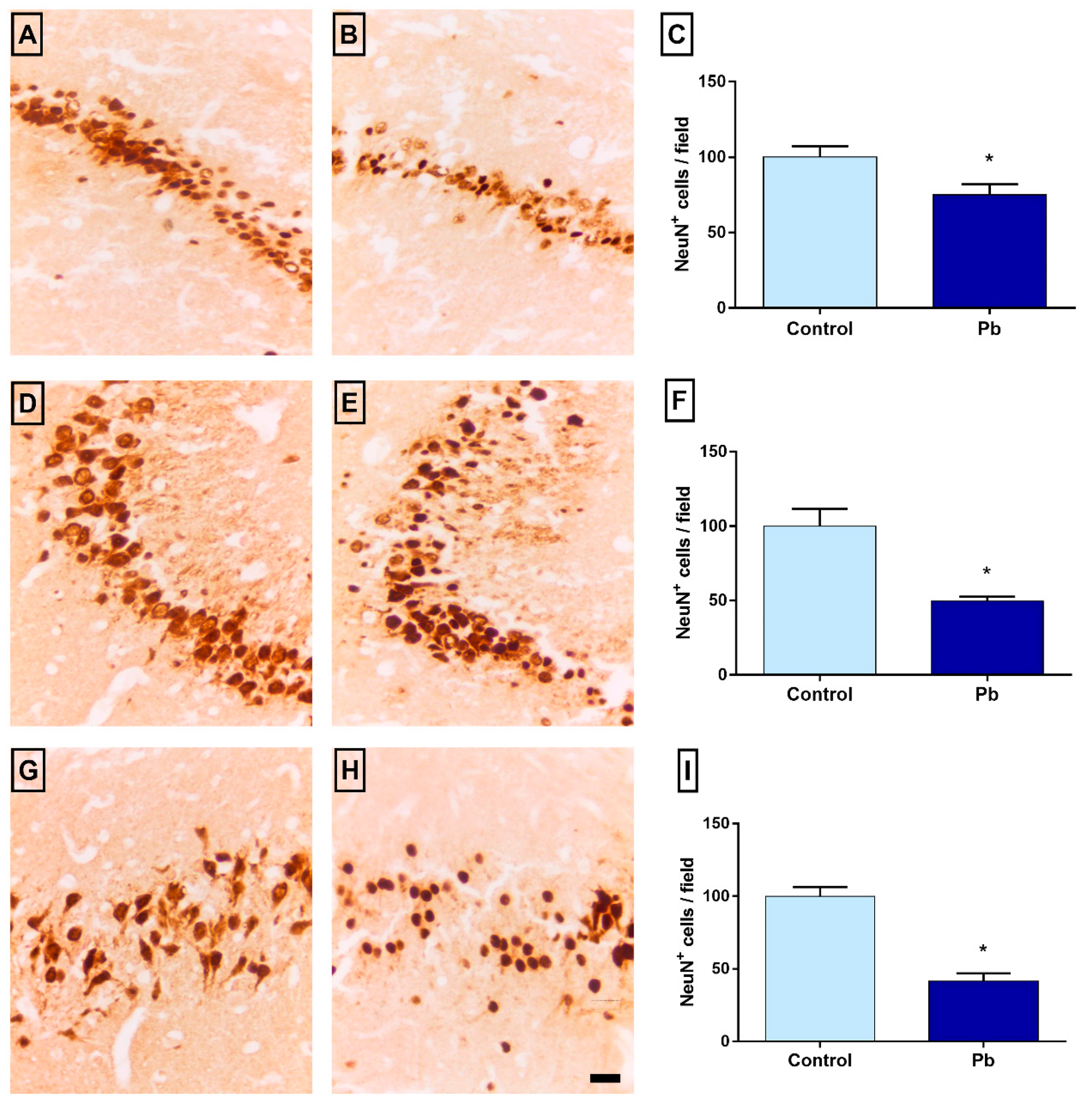
2.6. Long-Term Exposure to Pb Promotes Neuronal Loss
3. Discussion
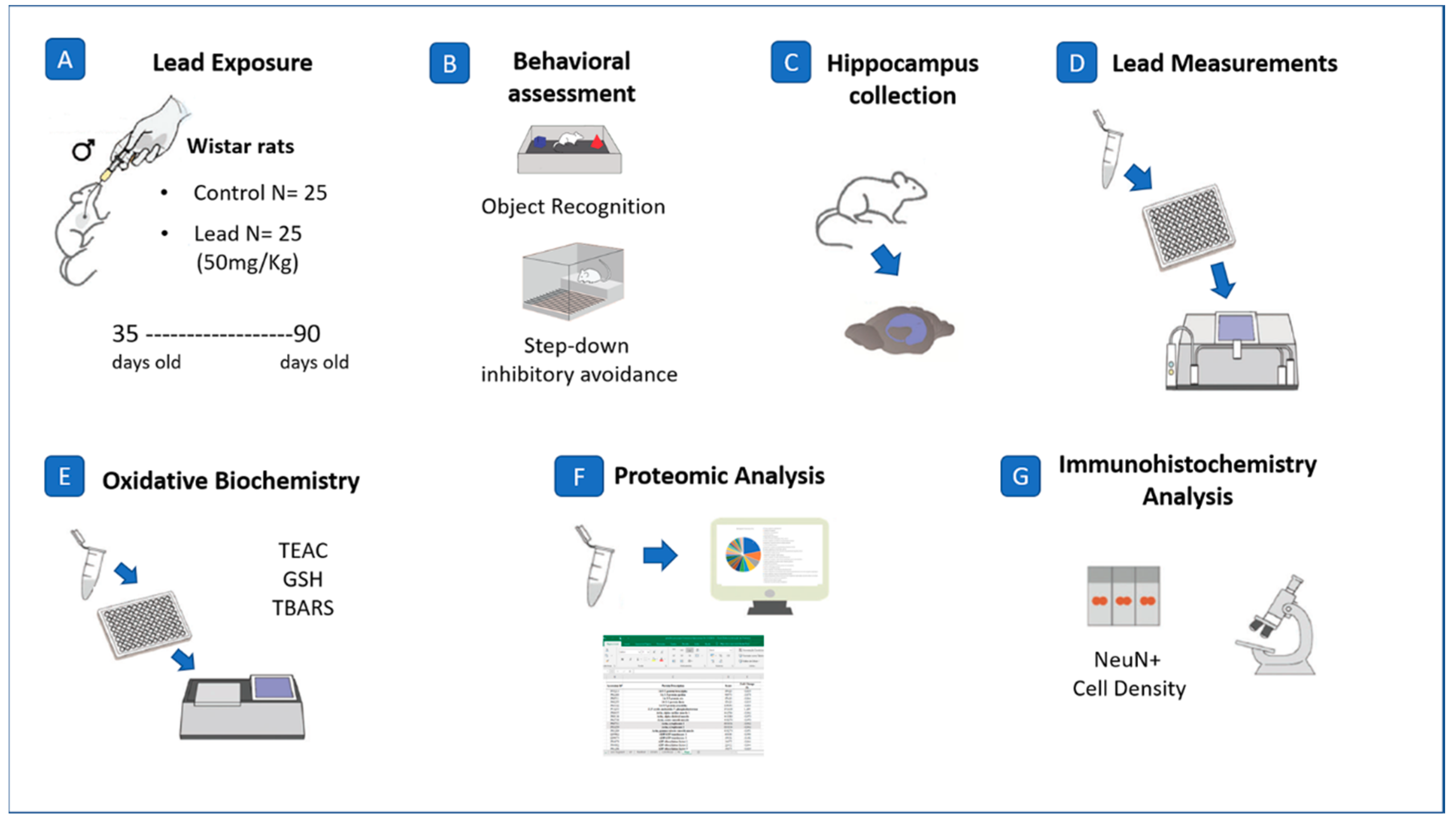
4. Materials and Methods
4.1. Experimental Animals
4.2. Lead Exposure and Body Mass Registration
4.3. Behavioral Assays
4.3.1. Object Recognition
4.3.2. Step-Down Inhibitory Avoidance Test
4.4. Quantification of Pb in Neural Parenchyma
4.5. Oxidative Biochemistry Analyses
4.5.1. Obtaining and Preparing Samples
4.5.2. Determination of Trolox Equivalent Antioxidant Capacity (TEAC)
4.5.3. Determination of Glutathione
4.5.4. Thiobarbituric Acid Reactive Substances (TBARS)
4.6. Proteomic Approach
4.6.1. Protein Extraction, Digestion and Purification
4.6.2. Mass Spectrometry and Bioinformatic Analyses
4.7. Perfusion, Histological and Immunohistochemistry Procedures
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CC | Calcium-calmodulin |
| CyC | Cytochrome C |
| GSH | Glutathione |
| HSP | Heat shock proteins |
| MDA | Malondialdehyde |
| MIP OES | Microwave-induced plasma-optical emission spectrometer |
| Pb | Lead |
| PbS | Galena |
| ROS | Reactive oxygen species |
| TBARS | Thiobarbituric acid reactive substances |
| TEAC | Trolox equivalent antioxidant capacity |
References
- Ge, Y.; Chen, L.; Sun, X.; Yin, Z.; Song, X.; Li, C.; Liu, J.; An, Z.; Yang, X.; Ning, H. Lead-induced changes of cytoskeletal protein is involved in the pathological basis in mice brain. Environ. Sci. Pollut. Res. Int. 2018, 25, 11746–11753. [Google Scholar] [CrossRef] [PubMed]
- Vanz, A.; Mirlean, N.; Baisch, P. Avaliação de poluição do ar por chumbo particulado: Uma abordagem geoquímica. Química Nova 2003, 26, 25–29. [Google Scholar] [CrossRef]
- Wang, T.; Guan, R.L.; Liu, M.C.; Shen, X.F.; Chen, J.Y.; Zhao, M.G.; Luo, W.J. Lead Exposure Impairs Hippocampus Related Learning and Memory by Altering Synaptic Plasticity and Morphology During Juvenile Period. Mol. Neurobiol. 2016, 53, 3740–3752. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhang, P.; Qiao, M.; Shao, J.; Li, H.; Xie, W. The effects of early life lead exposure on the expression of P2X7 receptor and synaptophysin in the hippocampus of mouse pups. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. (GMS) 2015, 30, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Lalith Kumar, V. Ameliorative effects of ferulic Acid against lead acetate-induced oxidative stress, mitochondrial dysfunctions and toxicity in prepubertal rat brain. Neurochem. Res. 2014, 39, 2501–2515. [Google Scholar] [CrossRef]
- Yun, S.; Wu, Y.; Niu, R.; Feng, C.; Wang, J. Effects of lead exposure on brain glucose metabolism and insulin signaling pathway in the hippocampus of rats. Toxicol. Lett. 2019, 310, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Paulis, M.G.; Hassan, O.A.; Abbass, M.F.; Mohammad, M.A.H. Structural and lipid peroxidation effects of lead on rat hippocampus and its attenuation by hydrogen rich water. J. Chem. Neuroanat. 2018, 91, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Liu, H.; Li, Q.; Wang, Q.; Zhang, W.; Chen, Y.; Wang, D.; Cai, Y. Effect of lead sulfide nanoparticles exposure on calcium homeostasis in rat hippocampus neurons. J. Inorg. Biochem. 2013, 126, 70–75. [Google Scholar] [CrossRef]
- Holdstock, J.S.; Mayes, A.R.; Isaac, C.L.; Gong, Q.; Roberts, N. Differential involvement of the hippocampus and temporal lobe cortices in rapid and slow learning of new semantic information. Neuropsychologia 2002, 40, 748–768. [Google Scholar] [CrossRef]
- Li, J.S.; Chao, Y.S. Electrolytic lesions of dorsal CA3 impair episodic-like memory in rats. Neurobiol. Learn. Mem. 2008, 89, 192–198. [Google Scholar] [CrossRef]
- Ezzyat, Y.; Olson, I.R. The medial temporal lobe and visual working memory: Comparisons across tasks, delays, and visual similarity. Cogn. Affect. Behav. Neurosci. 2008, 8, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Barkur, R.R.; Bairy, L.K. Assessment of oxidative stress in hippocampus, cerebellum and frontal cortex in rat pups exposed to lead (Pb) during specific periods of initial brain development. Biol. Trace Elem. Res. 2015, 164, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Lorente de Nó, R. Studies on the structure of the cerebral cortex. II. Continuation of the study of the ammonic system. J. Psychol. Neurol. (Lpz) 1934, 46, 113–177. [Google Scholar]
- Sengupta, P. The Laboratory Rat: Relating Its Age With Human’s. Int. J. Prev. Med. 2013, 4, 624–630. [Google Scholar] [PubMed]
- Anderson, D.W.; Mettil, W.; Schneider, J.S. Effects of low level lead exposure on associative learning and memory in the rat: Influences of sex and developmental timing of exposure. Toxicol. Lett. 2016, 246, 57–64. [Google Scholar] [CrossRef]
- Gu, H.; Wei, X.; Monnot, A.D.; Fontanilla, C.V.; Behl, M.; Farlow, M.R.; Zheng, W.; Du, Y. Lead exposure increases levels of β-amyloid in the brain and CSF and inhibits LRP1 expression in APP transgenic mice. Neurosci. Lett. 2011, 490, 16–20. [Google Scholar] [CrossRef]
- Gu, H.; Robison, G.; Hong, L.; Barrea, R.; Wei, X.; Farlow, M.R.; Pushkar, Y.N.; Du, Y.; Zheng, W. Increased β-amyloid deposition in Tg-SWDI transgenic mouse brain following in vivo lead exposure. Toxicol. Lett. 2012, 213, 211–219. [Google Scholar] [CrossRef]
- Leão, L.K.R.; Bittencourt, L.O.; Oliveira, A.C.; Nascimento, P.C.; Miranda, G.H.N.; Ferreira, R.O.; Nabiça, M.; Dantas, K.; Dionizio, A.; Cartágenes, S.; et al. Long-Term Lead Exposure Since Adolescence Causes Proteomic and Morphological Alterations in the Cerebellum Associated with Motor Deficits in Adult Rats. Int. J. Mol. Sci. 2020, 21, 3571. [Google Scholar] [CrossRef]
- Da Silva, D.R.F.; Bittencourt, L.O.; Aragão, W.A.B.; Nascimento, P.C.; Leão, L.K.R.; Oliveira, A.C.A.; Crespo-López, M.E.; Lima, R.R. Long-term exposure to lead reduces antioxidant capacity and triggers motor neurons degeneration and demyelination in spinal cord of adult rats. Ecotoxicol. Environ. Saf. 2020, 194, 110358. [Google Scholar] [CrossRef]
- Dobrakowski, M.; Pawlas, N.; Kasperczyk, A.; Kozłowska, A.; Olewińska, E.; Machoń-Grecka, A.; Kasperczyk, S. Oxidative DNA damage and oxidative stress in lead-exposed workers. Hum. Exp. Toxicol. 2017, 36, 744–754. [Google Scholar] [CrossRef]
- Bussche, J.V.; Soares, E.V. Lead induces oxidative stress and phenotypic markers of apoptosis in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2011, 90, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Reichmann, D.; Voth, W.; Jakob, U. Maintaining a Healthy Proteome during Oxidative Stress. Mol. Cell 2018, 69, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Agashe, V.R.; Hartl, F.U. Roles of molecular chaperones in cytoplasmic protein folding. Semin. Cell Dev. Biol. 2000, 11, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Arya, R.; Mallik, M.; Lakhotia, S.C. Heat shock genes—Integrating cell survival and death. J. Biosci. 2007, 32, 595–610. [Google Scholar] [CrossRef]
- Soti, C.; Csermely, P. Aging cellular networks: Chaperones as major participants. Exp. Gerontol. 2007, 42, 113–119. [Google Scholar] [CrossRef][Green Version]
- Jolly, C.; Morimoto, R.I. Role of the heat shock response and molecular chaperones in oncogenesis and cell death. J. Natl. Cancer Inst. 2000, 92, 1564–1572. [Google Scholar] [CrossRef]
- Sokolowska, I.; Woods, A.G.; Wagner, J.; Dorler, J.; Wormwood, K.; Thome, J.; Darie, C.C. Mass Spectrometry for Proteomics-Based Investigation of Oxidative Stress and Heat Shock Proteins. In Oxidative Stress Diagnostics, Prevention, and Therapy; American Chemical Society: Washington, DC, USA, 2011; Volume 1083, pp. 369–411. [Google Scholar]
- Witt, S.N. Hsp70 molecular chaperones and Parkinson’s disease. Biopolymers 2010, 93, 218–228. [Google Scholar] [CrossRef]
- Evgen’ev, M.B.; Krasnov, G.S.; Nesterova, I.V.; Garbuz, D.G.; Karpov, V.L.; Morozov, A.V.; Snezhkina, A.V.; Samokhin, A.N.; Sergeev, A.; Kulikov, A.M.; et al. Molecular Mechanisms Underlying Neuroprotective Effect of Intranasal Administration of Human Hsp70 in Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. JAD 2017, 59, 1415–1426. [Google Scholar] [CrossRef]
- Srinivasan, S.; Avadhani, N.G. Cytochrome c oxidase dysfunction in oxidative stress. Free Radic. Biol. Med. 2012, 53, 1252–1263. [Google Scholar] [CrossRef]
- Mylonas, C.; Kouretas, D. Lipid peroxidation and tissue damage. In Vivo (Athens, Greece) 1999, 13, 295–309. [Google Scholar]
- Zhang, H.H.; Zhang, X.Q.; Wang, W.Y.; Xue, Q.S.; Lu, H.; Huang, J.L.; Gui, T.; Yu, B.W. Increased synaptophysin is involved in inflammation-induced heat hyperalgesia mediated by cyclin-dependent kinase 5 in rats. PLoS ONE 2012, 7, e46666. [Google Scholar] [CrossRef] [PubMed]
- Valtorta, F.; Pennuto, M.; Bonanomi, D.; Benfenati, F. Synaptophysin: Leading actor or walk-on role in synaptic vesicle exocytosis? BioEssays News Rev. Mol. Cell. Dev. Biol. 2004, 26, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Alder, J.; Kanki, H.; Valtorta, F.; Greengard, P.; Poo, M.M. Overexpression of synaptophysin enhances neurotransmitter secretion at Xenopus neuromuscular synapses. J. Neurosci. Off. J. Soc. Neurosci. 1995, 15, 511–519. [Google Scholar] [CrossRef]
- Gordon, S.L.; Harper, C.B.; Smillie, K.J.; Cousin, M.A. A Fine Balance of Synaptophysin Levels Underlies Efficient Retrieval of Synaptobrevin II to Synaptic Vesicles. PLoS ONE 2016, 11, e0149457. [Google Scholar] [CrossRef]
- Simons, M.; Kramer, E.M.; Macchi, P.; Rathke-Hartlieb, S.; Trotter, J.; Nave, K.A.; Schulz, J.B. Overexpression of the myelin proteolipid protein leads to accumulation of cholesterol and proteolipid protein in endosomes/lysosomes: Implications for Pelizaeus-Merzbacher disease. J. Cell Biol. 2002, 157, 327–336. [Google Scholar] [CrossRef]
- Kristensson, K.; Holmes, K.V.; Duchala, C.S.; Zeller, N.K.; Lazzarini, R.A.; Dubois-Dalcq, M. Increased levels of myelin basic protein transcripts in virus-induced demyelination. Nature 1986, 322, 544–547. [Google Scholar] [CrossRef]
- Claudel, T.; Leibowitz, M.D.; Fiévet, C.; Tailleux, A.; Wagner, B.; Repa, J.J.; Torpier, G.; Lobaccaro, J.M.; Paterniti, J.R.; Mangelsdorf, D.J.; et al. Reduction of atherosclerosis in apolipoprotein E knockout mice by activation of the retinoid X receptor. Proc. Natl. Acad. Sci. USA 2001, 98, 2610–2615. [Google Scholar] [CrossRef]
- Mahley, R.W. Apolipoprotein E: Cholesterol transport protein with expanding role in cell biology. Science 1988, 240, 622–630. [Google Scholar] [CrossRef]
- Oikonomidi, A.; Tautvydaitė, D.; Gholamrezaee, M.M.; Henry, H.; Bacher, M.; Popp, J. Macrophage Migration Inhibitory Factor is Associated with Biomarkers of Alzheimer’s Disease Pathology and Predicts Cognitive Decline in Mild Cognitive Impairment and Mild Dementia. J. Alzheimer’s Dis. JAD 2017, 60, 273–281. [Google Scholar] [CrossRef]
- Khaibullin, T.; Ivanova, V.; Martynova, E.; Cherepnev, G.; Khabirov, F.; Granatov, E.; Rizvanov, A.; Khaiboullina, S. Elevated Levels of Proinflammatory Cytokines in Cerebrospinal Fluid of Multiple Sclerosis Patients. Front. Immunol. 2017, 8, 531. [Google Scholar] [CrossRef]
- Popp, J.; Bacher, M.; Kölsch, H.; Noelker, C.; Deuster, O.; Dodel, R.; Jessen, F. Macrophage migration inhibitory factor in mild cognitive impairment and Alzheimer’s disease. J. Psychiatr. Res. 2009, 43, 749–753. [Google Scholar] [CrossRef]
- Østergaard, C.; Benfield, T. Macrophage migration inhibitory factor in cerebrospinal fluid from patients with central nervous system infection. Crit. Care 2009, 13, R101. [Google Scholar] [CrossRef]
- Bittencourt, L.O.; Dionizio, A.; Nascimento, P.C.; Puty, B.; Leão, L.K.R.; Luz, D.A.; Silva, M.C.F.; Amado, L.L.; Leite, A.; Buzalaf, M.R.; et al. Proteomic approach underlying the hippocampal neurodegeneration caused by low doses of methylmercury after long-term exposure in adult rats. Met. Integr. Biomet. Sci. 2019, 11, 390–403. [Google Scholar] [CrossRef]
- Brahmachari, S.; Fung, Y.K.; Pahan, K. Induction of glial fibrillary acidic protein expression in astrocytes by nitric oxide. J. Neurosci. Off. J. Soc. Neurosci. 2006, 26, 4930–4939. [Google Scholar] [CrossRef] [PubMed]
- Sorci, G.; Riuzzi, F.; Arcuri, C.; Tubaro, C.; Bianchi, R.; Giambanco, I.; Donato, R. S100B protein in tissue development, repair and regeneration. World J. Biol. Chem. 2013, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ohtaki, N.; Kamitani, W.; Watanabe, Y.; Hayashi, Y.; Yanai, H.; Ikuta, K.; Tomonaga, K. Downregulation of an astrocyte-derived inflammatory protein, S100B, reduces vascular inflammatory responses in brains persistently infected with Borna disease virus. J. Virol. 2007, 81, 5940–5948. [Google Scholar] [CrossRef] [PubMed]
- Amaral, D.; Lavenex, P. Hippocampal neuroanatomy. In The Hippocampus Book; Oxford University Press: New York, NY, USA, 2007; pp. 37–114. [Google Scholar]
- Laroche, S.; Davis, S.; Jay, T.M. Plasticity at hippocampal to prefrontal cortex synapses: Dual roles in working memory and consolidation. Hippocampus 2000, 10, 438–446. [Google Scholar] [CrossRef]
- Eichenbaum, H.; Otto, T.; Cohen, N.J. Two functional components of the hippocampal memory system. Behav. Brain Sci. 1994, 17, 449–472. [Google Scholar] [CrossRef]
- Barker, G.R.; Warburton, E.C. Object-in-place associative recognition memory depends on glutamate receptor neurotransmission within two defined hippocampal-cortical circuits: A critical role for AMPA and NMDA receptors in the hippocampus, perirhinal, and prefrontal cortices. Cereb. Cortex 2015, 25, 472–481. [Google Scholar] [CrossRef]
- Silvers, J.M.; Harrod, S.B.; Mactutus, C.F.; Booze, R.M. Automation of the novel object recognition task for use in adolescent rats. J. Neurosci. Methods 2007, 166, 99–103. [Google Scholar] [CrossRef]
- Tanimizu, T.; Kenney, J.W.; Okano, E.; Kadoma, K.; Frankland, P.W.; Kida, S. Functional Connectivity of Multiple Brain Regions Required for the Consolidation of Social Recognition Memory. J. Neurosci. Off. J. Soc. Neurosci. 2017, 37, 4103–4116. [Google Scholar] [CrossRef] [PubMed]
- Rossato, J.I.; Bevilaqua, L.R.; Myskiw, J.C.; Medina, J.H.; Izquierdo, I.; Cammarota, M. On the role of hippocampal protein synthesis in the consolidation and reconsolidation of object recognition memory. Learn. Mem. 2007, 14, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.J.; Munchow, A.H.; Rios, L.M.; Zhang, G.; Asgeirsdóttir, H.N.; Stackman, R.W., Jr. The rodent hippocampus is essential for nonspatial object memory. Curr. Biol. CB 2013, 23, 1685–1690. [Google Scholar] [CrossRef]
- Chen, H.; Ma, T.; Ho, I.K. Effects of developmental lead exposure on inhibitory avoidance learning and glutamate receptors in rats. Environ. Toxicol. Pharmacol. 2001, 9, 185–191. [Google Scholar] [CrossRef]
- Teixeira, F.B.; Fernandes, R.M.; Farias-Junior, P.M.; Costa, N.M.; Fernandes, L.M.; Santana, L.N.; Silva-Junior, A.F.; Silva, M.C.; Maia, C.S.; Lima, R.R. Evaluation of the effects of chronic intoxication with inorganic mercury on memory and motor control in rats. Int. J. Environ. Res. Public Health 2014, 11, 9171–9185. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, F.B.; Santana, L.N.; Bezerra, F.R.; De Carvalho, S.; Fontes-Júnior, E.A.; Prediger, R.D.; Crespo-López, M.E.; Maia, C.S.; Lima, R.R. Chronic ethanol exposure during adolescence in rats induces motor impairments and cerebral cortex damage associated with oxidative stress. PLoS ONE 2014, 9, e101074. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.C.; Pereira, M.C.; Santana, L.N.; Fernandes, R.M.; Teixeira, F.B.; Oliveira, G.B.; Fernandes, L.M.; Fontes-Júnior, E.A.; Prediger, R.D.; Crespo-López, M.E.; et al. Chronic ethanol exposure during adolescence through early adulthood in female rats induces emotional and memory deficits associated with morphological and molecular alterations in hippocampus. J. Psychopharmacol. 2015, 29, 712–724. [Google Scholar] [CrossRef]
- Pires, V.A.; Pamplona, F.A.; Pandolfo, P.; Fernandes, D.; Prediger, R.D.; Takahashi, R.N. Adenosine receptor antagonists improve short-term object-recognition ability of spontaneously hypertensive rats: A rodent model of attention-deficit hyperactivity disorder. Behav. Pharmacol. 2009, 20, 134–145. [Google Scholar] [CrossRef]
- Ennaceur, A.; Delacour, J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav. Brain Res. 1988, 31, 47–59. [Google Scholar] [CrossRef]
- Izquierdo, I.; Medina, J.H.; Da-Cunha, C.; Wolfman, C.; Jerusalinsky, D.; Ferreira, M.B. Memory modulation by brain benzodiazepines. Braz. J. Med. Biol. Res. = Rev. Bras. Pesqui. Med. Biol. 1991, 24, 865–881. [Google Scholar]
- Walz, R.; Roesler, R.; Quevedo, J.; Rockenbach, I.C.; Amaral, O.B.; Vianna, M.R.; Lenz, G.; Medina, J.H.; Izquierdo, I. Dose-dependent impairment of inhibitory avoidance retention in rats by immediate post-training infusion of a mitogen-activated protein kinase kinase inhibitor into cortical structures. Behav. Brain Res. 1999, 105, 219–223. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Brazilian Society of Cardiology. Modified LDLs Dosage through Lipid Peroxidation: Correlation with Atherogenic Risk; Sociedade Brasileira de Cardiologia: Sao Paolo, Brazil, 1994; Volume 13, pp. 7–9. [Google Scholar]
- Dionizio, A.S.; Melo, C.G.S.; Sabino-Arias, I.T.; Ventura, T.M.S.; Leite, A.L.; Souza, S.R.G.; Santos, E.X.; Heubel, A.D.; Souza, J.G.; Perles, J.; et al. Chronic treatment with fluoride affects the jejunum: Insights from proteomics and enteric innervation analysis. Sci. Rep. 2018, 8, 3180. [Google Scholar] [CrossRef]
- Corrêa, M.G.; Bittencourt, L.O.; Nascimento, P.C.; Ferreira, R.O.; Aragão, W.A.B.; Silva, M.C.F.; Gomes-Leal, W.; Fernandes, M.S.; Dionizio, A.; Buzalaf, M.R.; et al. Spinal cord neurodegeneration after inorganic mercury long-term exposure in adult rats: Ultrastructural, proteomic and biochemical damages associated with reduced neuronal density. Ecotoxicol. Environ. Saf. 2020, 191, 110159. [Google Scholar] [CrossRef]
- Lima Leite, A.; Gualiume Vaz Madureira Lobo, J.; Barbosa da Silva Pereira, H.A.; Silva Fernandes, M.; Martini, T.; Zucki, F.; Sumida, D.H.; Rigalli, A.; Buzalaf, M.A.R. Proteomic analysis of gastrocnemius muscle in rats with streptozotocin-induced diabetes and chronically exposed to fluoride. PLoS ONE 2014, 9, e106646. [Google Scholar] [CrossRef]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.-H.; Pagès, F.; Trajanoski, Z.; Galon, J.J.B. ClueGO: A Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef]
- Santana, L.; Bittencourt, L.O.; Nascimento, P.C.; Fernandes, R.M.; Teixeira, F.B.; Fernandes, L.M.P.; Freitas Silva, M.C.; Nogueira, L.S.; Amado, L.L.; Crespo-Lopez, M.E.; et al. Low doses of methylmercury exposure during adulthood in rats display oxidative stress, neurodegeneration in the motor cortex and lead to impairment of motor skills. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. (GMS) 2019, 51, 19–27. [Google Scholar] [CrossRef]
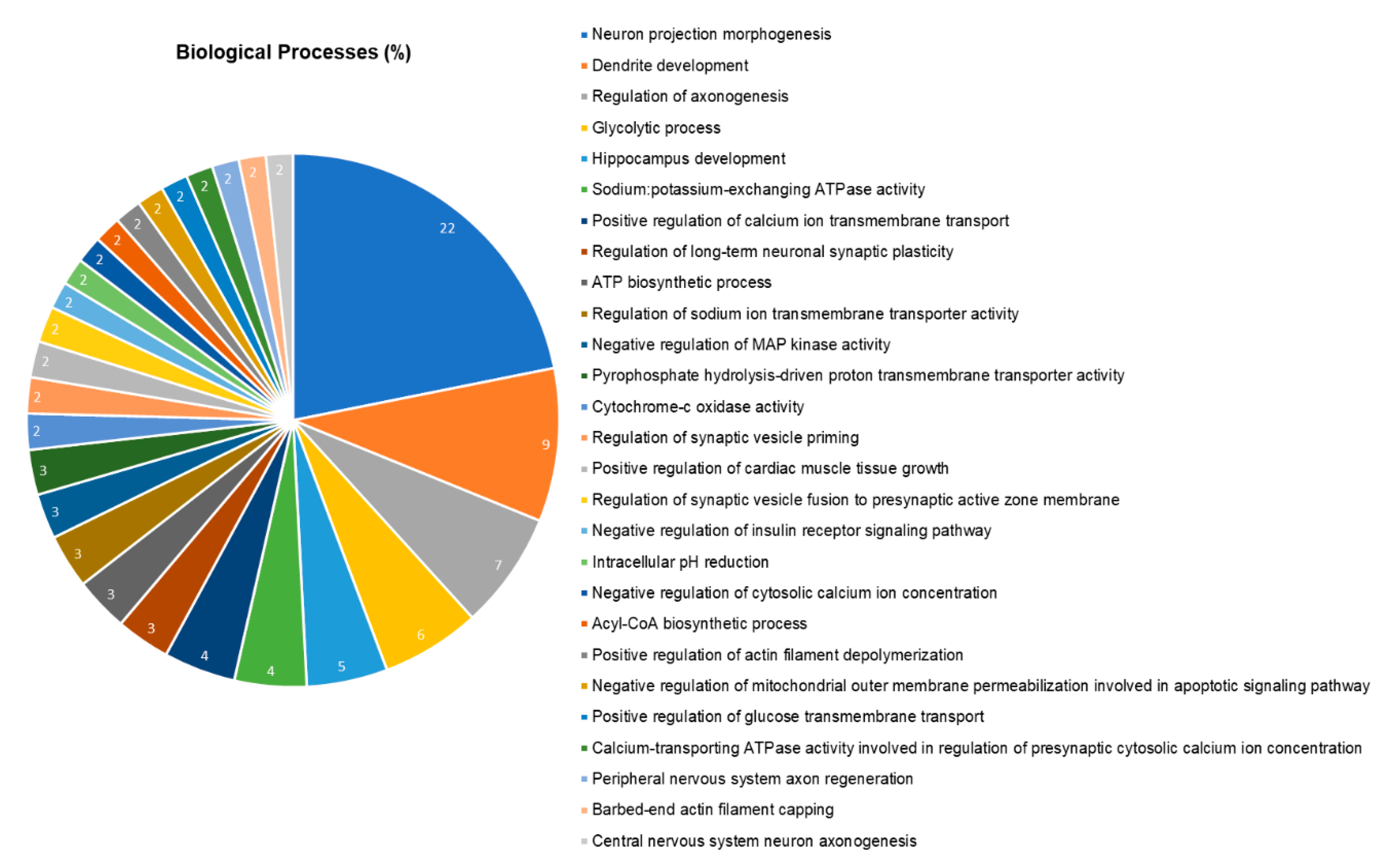
| Accession Id a | Protein Description | Score | Fold Change |
|---|---|---|---|
| Pb | |||
| P0DMW1 | Heat shock 70 kDa protein 1B | 4870 | −0.932 |
| P09951 | Synapsin-1 | 18,756 | −0.914 |
| P0DMW0 | Heat shock 70 kDa protein 1A | 4870 | −0.896 |
| P10719 | ATP synthase subunit beta, mitochondrial | 232,442 | −0.835 |
| P04631 | Protein S100-B | 205,290 | −0.835 |
| Q63537 | Synapsin-2 | 41,092 | −0.819 |
| P47861 | Synaptotagmin-5 | 2245 | −0.795 |
| P29101 | Synaptotagmin-2 | 2245 | −0.763 |
| P21707 | Synaptotagmin-1 | 20,604 | −0.748 |
| P10888 | Cytochrome c oxidase subunit 4 isoform 1, mitochondrial | 14,314 | −0.625 |
| P00406 | Cytochrome c oxidase subunit 2 | 42,862 | −0.538 |
| P47819 | Glial fibrillary acidic protein | 14,060 | 1.062 |
| P61765 | Syntaxin-binding protein 1 | 59,845 | 1.073 |
| P30904 | Macrophage migration inhibitory factor | 38,694 | 1.094 |
| P11275 | Calcium/calmodulin-dependent protein kinase type II subunit alpha | 181,954 | 1.105 |
| P15791 | Calcium/calmodulin-dependent protein kinase type II subunit delta | 62,682 | 1.105 |
| P08413 | Calcium/calmodulin-dependent protein kinase type II subunit beta | 80,840 | 1.127 |
| P11730 | Calcium/calmodulin-dependent protein kinase type II subunit gamma | 66,045 | 1.127 |
| P34058 | Heat shock protein HSP 90-beta | 16,345 | 1.197 |
| P10818 | Cytochrome c oxidase subunit 6A1, mitochondrial | 30,209 | 1.284 |
| P07825 | Synaptophysin | 42,961 | 1.310 |
| P32551 | Cytochrome b-c1 complex subunit 2, mitochondrial | 4014 | 1.323 |
| P02650 | Apolipoprotein E | 4894 | − |
| P21571 | ATP synthase-coupling factor 6, mitochondrial | 31,889 | + |
| Q7TNJ2 | ATP-binding cassette sub-family A member 7 | 3891 | + |
| P27139 | Carbonic anhydrase 2 | 12,017 | − |
| P12075 | Cytochrome c oxidase subunit 5B, mitochondrial | 23,587 | − |
| P24942 | Excitatory amino acid transporter 1 | 27,055 | − |
| P04906 | Glutathione S-transferase P | 9312 | + |
| + 219 proteins with different status of regulation | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves Oliveira, A.C.; Dionizio, A.; Teixeira, F.B.; Bittencourt, L.O.; Nonato Miranda, G.H.; Oliveira Lopes, G.; Varela, E.L.P.; Nabiça, M.; Ribera, P.; Dantas, K.; et al. Hippocampal Impairment Triggered by Long-Term Lead Exposure from Adolescence to Adulthood in Rats: Insights from Molecular to Functional Levels. Int. J. Mol. Sci. 2020, 21, 6937. https://doi.org/10.3390/ijms21186937
Alves Oliveira AC, Dionizio A, Teixeira FB, Bittencourt LO, Nonato Miranda GH, Oliveira Lopes G, Varela ELP, Nabiça M, Ribera P, Dantas K, et al. Hippocampal Impairment Triggered by Long-Term Lead Exposure from Adolescence to Adulthood in Rats: Insights from Molecular to Functional Levels. International Journal of Molecular Sciences. 2020; 21(18):6937. https://doi.org/10.3390/ijms21186937
Chicago/Turabian StyleAlves Oliveira, Ana Carolina, Aline Dionizio, Francisco Bruno Teixeira, Leonardo Oliveira Bittencourt, Giza Hellen Nonato Miranda, Géssica Oliveira Lopes, Everton L. P. Varela, Mariane Nabiça, Paula Ribera, Kelly Dantas, and et al. 2020. "Hippocampal Impairment Triggered by Long-Term Lead Exposure from Adolescence to Adulthood in Rats: Insights from Molecular to Functional Levels" International Journal of Molecular Sciences 21, no. 18: 6937. https://doi.org/10.3390/ijms21186937
APA StyleAlves Oliveira, A. C., Dionizio, A., Teixeira, F. B., Bittencourt, L. O., Nonato Miranda, G. H., Oliveira Lopes, G., Varela, E. L. P., Nabiça, M., Ribera, P., Dantas, K., Leite, A., Buzalaf, M. A. R., Monteiro, M. C., Maia, C. S. F., & Lima, R. R. (2020). Hippocampal Impairment Triggered by Long-Term Lead Exposure from Adolescence to Adulthood in Rats: Insights from Molecular to Functional Levels. International Journal of Molecular Sciences, 21(18), 6937. https://doi.org/10.3390/ijms21186937