Low-Dose Nicotine Activates EGFR Signaling via α5-nAChR and Promotes Lung Adenocarcinoma Progression
Abstract
1. Introduction
2. Results
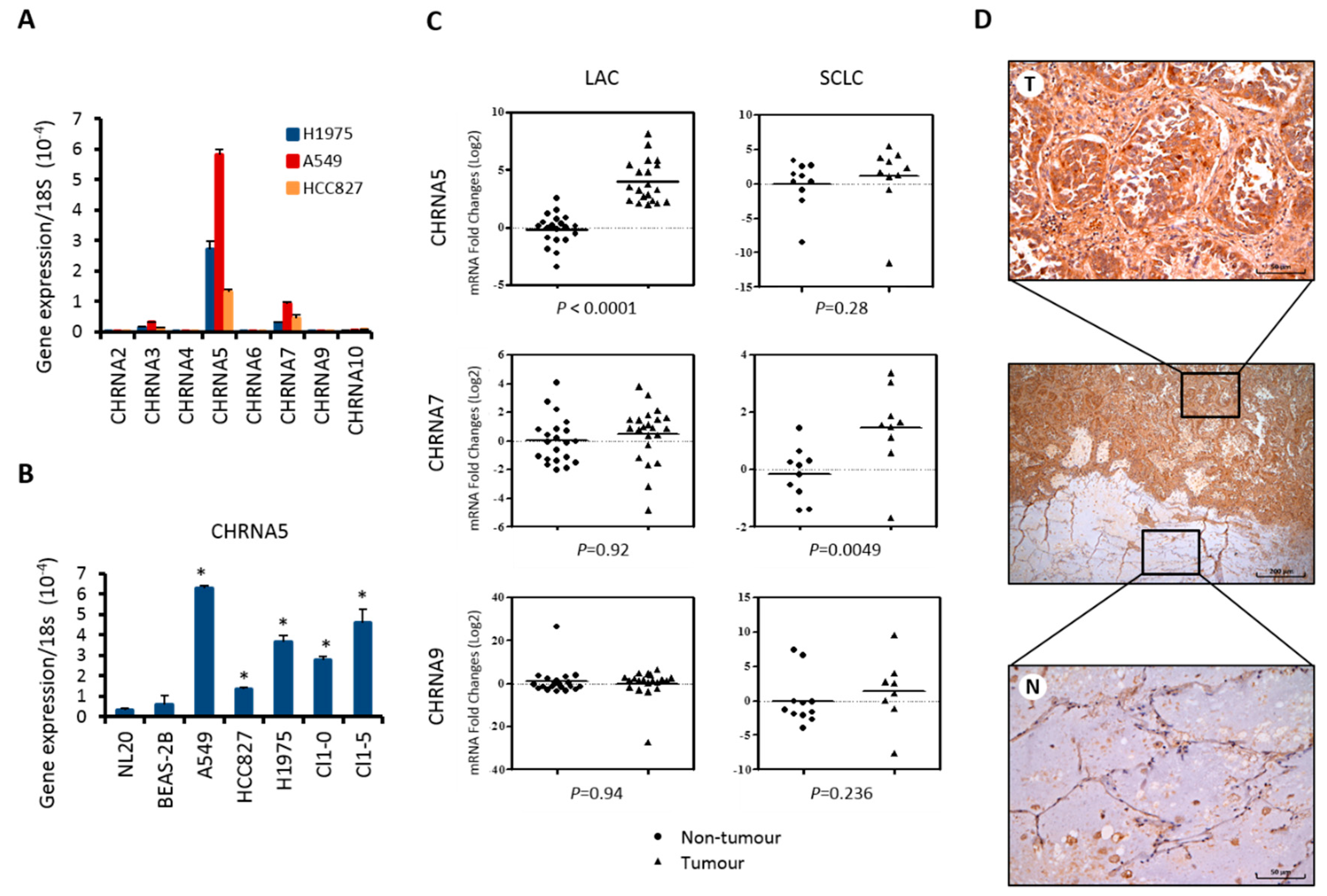
2.1. α5-nAChR Expression is Elevated in Lung Adenocarcinoma Cells and Clinical Tumor Tissue
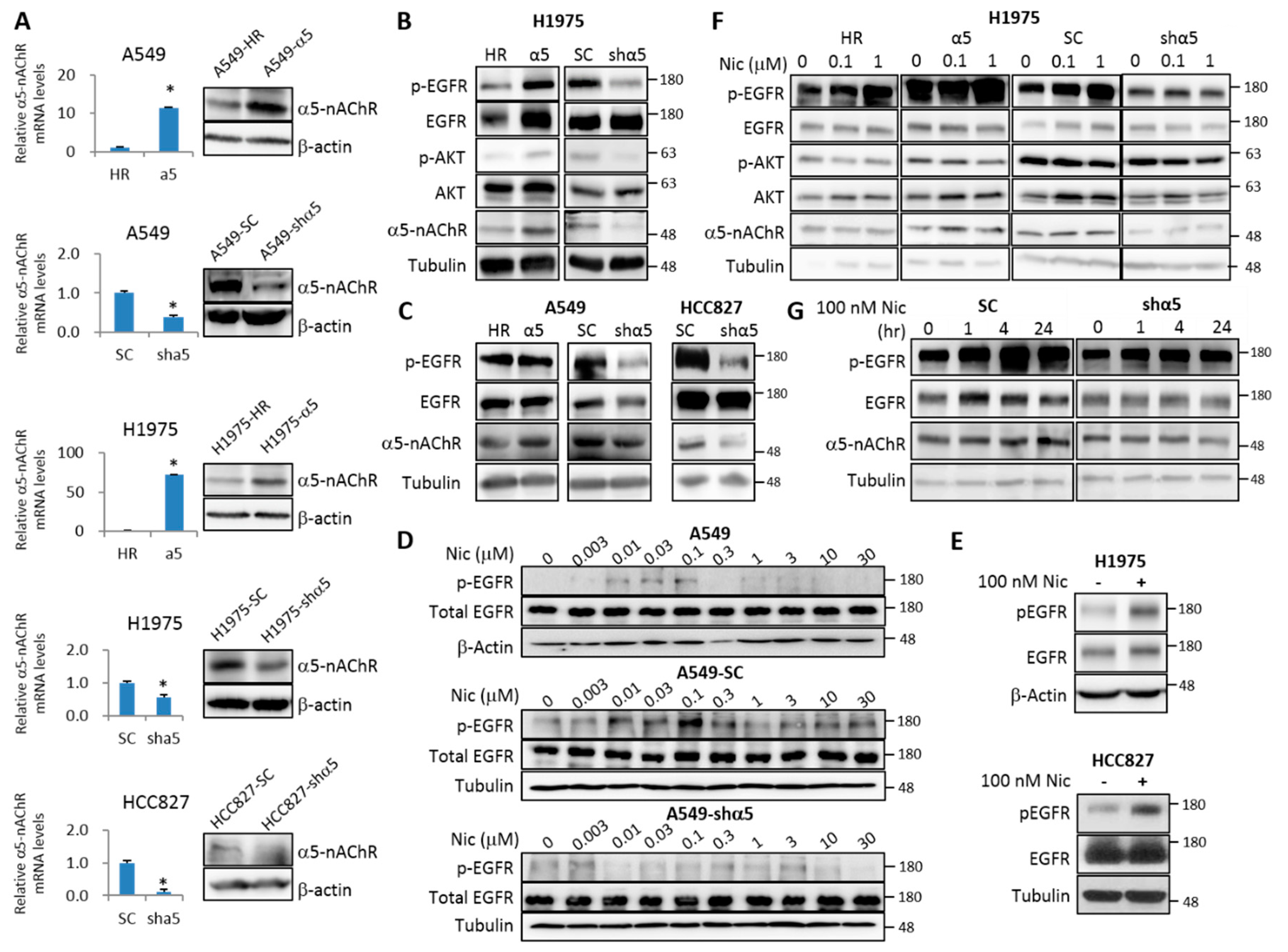
2.2. α5-nAChR Interacts with EGFR
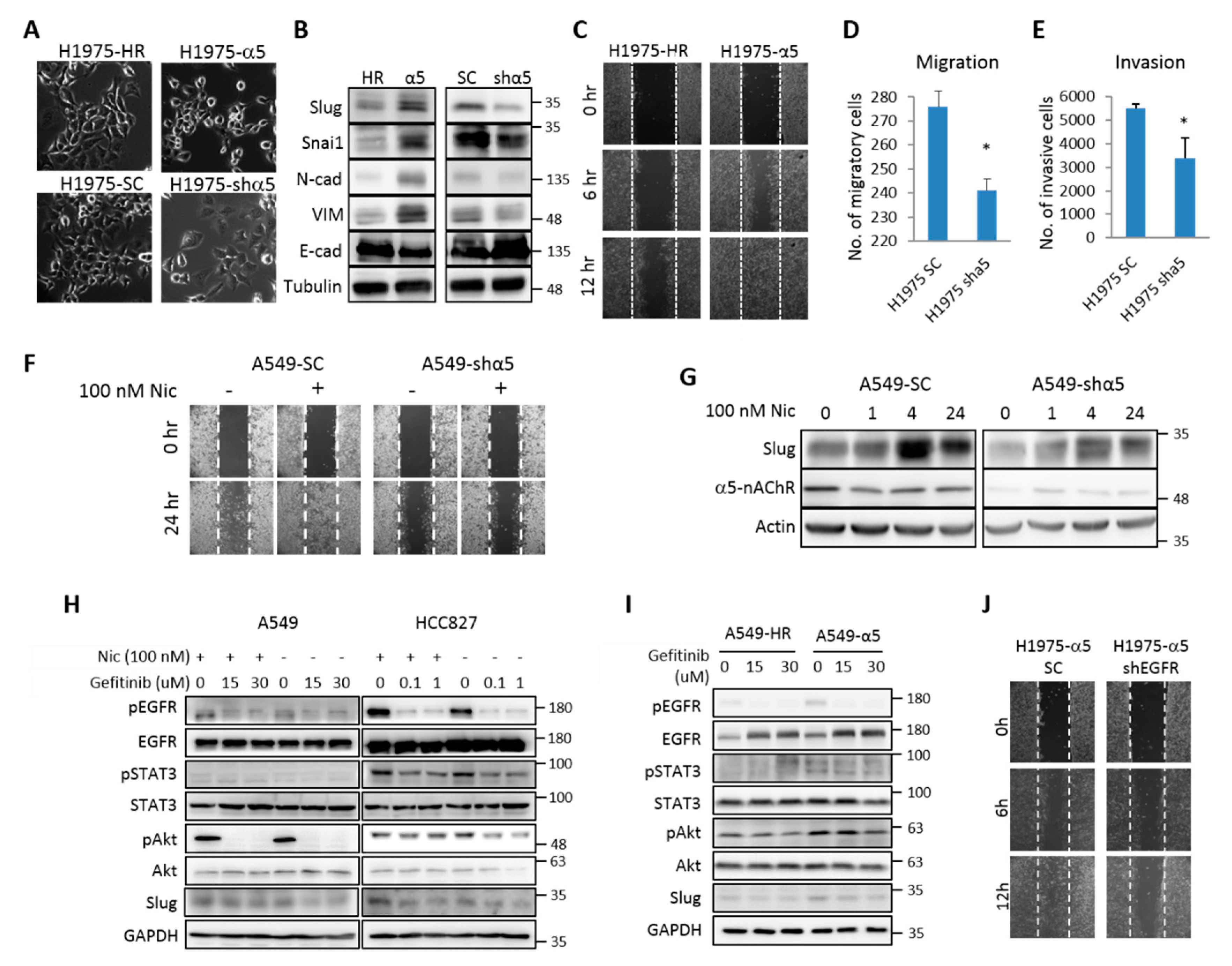
2.3. α5-nAChR is Essential for Low-Dose Nicotine-Regulated Cell Motility
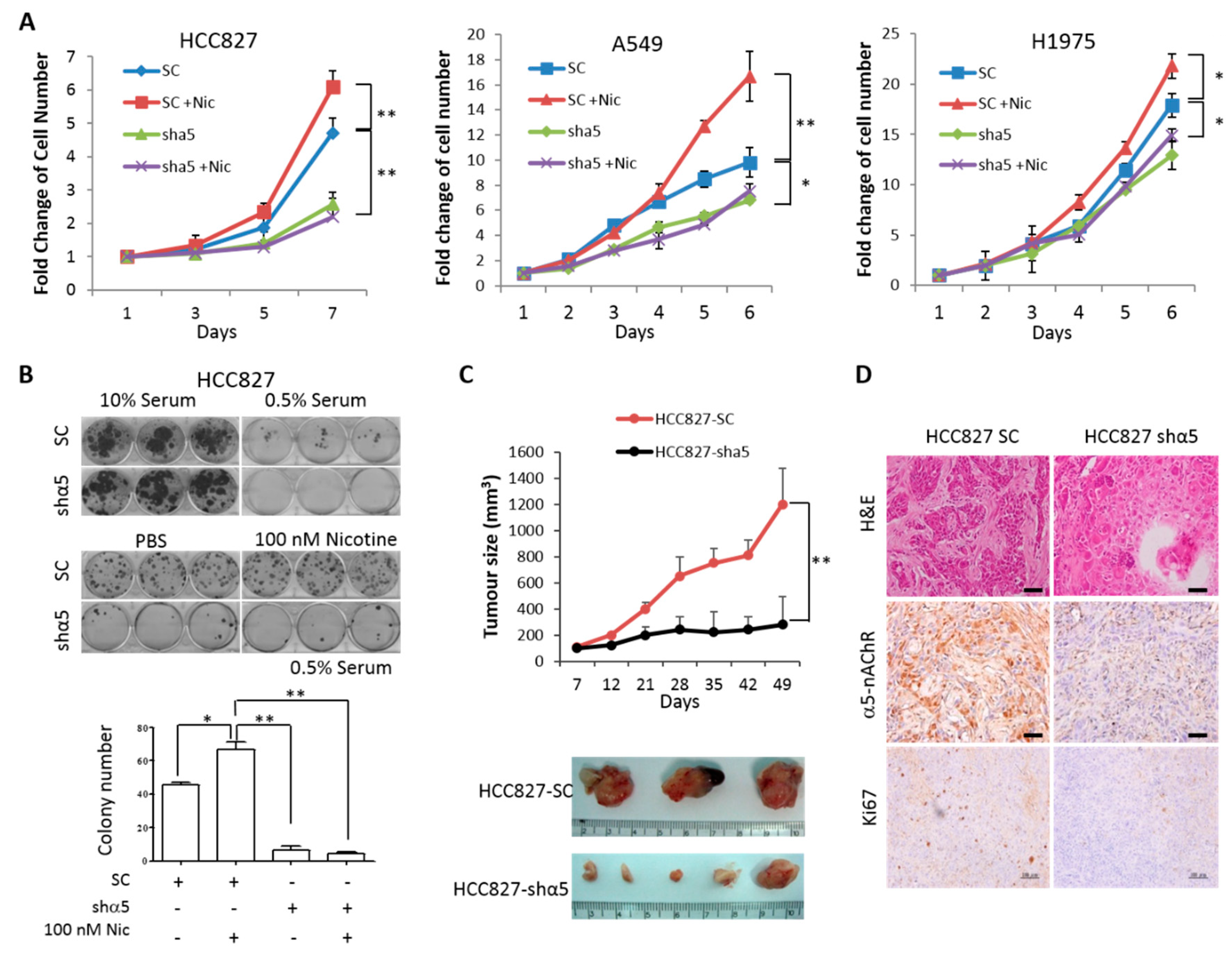
2.4. α5-nAChR Promotes LAC Tumor Progression In Vitro and In Vivo
2.5. α5-nAChR Expression Correlates with LAC Tumor Recurrence and Patient Survival
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Culture Condition
4.2. Expression Plasmid and shRNA
4.3. Western Blot
4.4. Animals and Tumor Cell Transplantation
4.5. Clinical Specimens and Tissue Microarray Construction
4.6. Immunohistochemistry and Assessment of Staining
4.7. Survival Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| LAC | lung adenocarcinoma |
| α-nAChR | nicotinic acetylcholine receptor subunit α |
| EGFR | epidermal growth factor receptor |
| NSCLC | non-small-cell lung cancer |
| nAChRs | nicotinic acetylcholine receptors |
| TKI | tyrosine kinase inhibitor |
References
- Jemal, A.; Siegel, R.; Ward, E.; Hao, Y.; Xu, J.; Thun, M.J. Cancer statistics, 2009. CA Cancer J. Clin. 2009, 59, 225–249. [Google Scholar] [CrossRef] [PubMed]
- Tuveson, D.A.; Jacks, T. Modeling human lung cancer in mice: Similarities and shortcomings. Oncogene 1999, 18, 5318–5324. [Google Scholar] [CrossRef] [PubMed]
- Barbone, F.; Bovenzi, M.; Cavallieri, F.; Stanta, G. Cigarette smoking and histologic type of lung cancer in men. Chest 1997, 112, 1474–1479. [Google Scholar] [CrossRef] [PubMed]
- Joya, X.; Manzano, C.; Alvarez, A.T.; Mercadal, M.; Torres, F.; Salat-Batlle, J.; Garcia-Algar, O. Transgenerational exposure to environmental tobacco smoke. Int. J. Environ. Res. Public Health 2014, 11, 7261–7274. [Google Scholar] [CrossRef] [PubMed]
- Hackshaw, A.K.; Law, M.R.; Wald, N.J. The accumulated evidence on lung cancer and environmental tobacco smoke. BMJ 1997, 315, 980–988. [Google Scholar] [CrossRef]
- Ko, Y.C.; Lee, C.H.; Chen, M.J.; Huang, C.C.; Chang, W.Y.; Lin, H.J.; Wang, H.Z.; Chang, P.Y. Risk factors for primary lung cancer among non-smoking women in Taiwan. Int. J. Epidemiol. 1997, 26, 24–31. [Google Scholar] [CrossRef]
- Chen, C.S.; Lee, C.H.; Hsieh, C.D.; Ho, C.T.; Pan, M.H.; Huang, C.S.; Tu, S.H.; Wang, Y.J.; Chen, L.C.; Chang, Y.J.; et al. Nicotine-induced human breast cancer cell proliferation attenuated by garcinol through down-regulation of the nicotinic receptor and cyclin D3 proteins. Breast Cancer Res. Treat. 2011, 125, 73–87. [Google Scholar] [CrossRef]
- Zheng, Y.; Ritzenthaler, J.D.; Roman, J.; Han, S. Nicotine stimulates human lung cancer cell growth by inducing fibronectin expression. Am. J. Respir. Cell Mol. Biol. 2007, 37, 681–690. [Google Scholar] [CrossRef]
- Diaz, F.; Raval, A.P. Simultaneous nicotine and oral contraceptive exposure alters brain energy metabolism and exacerbates ischemic stroke injury in female rats. J. Cereb. Blood Flow Metab. 2020, 271678X20925164. [Google Scholar] [CrossRef]
- Lunell, E.; Bergström, M.; Antoni, G.; Långström, B.; Nordberg, A. Nicotine deposition and body distribution from a nicotine inhaler and a cigarette studied with positron emission tomography. Clin. Pharmacol. Ther. 1996, 59, 593–594. [Google Scholar] [CrossRef]
- Jarvis, M.J.; Russell, M.A.; Feyerabend, C. Absorption of nicotine and carbon monoxide from passive smoking under natural conditions of exposure. Thorax 1983, 38, 829–833. [Google Scholar] [CrossRef] [PubMed]
- Schuller, H.M. Is cancer triggered by altered signalling of nicotinic acetylcholine receptors? Nat. Rev. Cancer 2009, 9, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Lam, D.C.; Girard, L.; Ramirez, R.; Chau, W.S.; Suen, W.S.; Sheridan, S.; Tin, V.P.; Chung, L.P.; Wong, M.P.; Shay, J.W.; et al. Expression of nicotinic acetylcholine receptor subunit genes in non-small-cell lung cancer reveals differences between smokers and nonsmokers. Cancer Res. 2007, 67, 4638–4647. [Google Scholar] [CrossRef] [PubMed]
- Bierut, L.J. Nicotine dependence and genetic variation in the nicotinic receptors. Drug Alcohol. Depend. 2009, 104 (Suppl. 1), S64–S69. [Google Scholar] [CrossRef]
- Benowitz, N.L. Nicotine addiction. N. Engl. J. Med. 2010, 362, 2295–2303. [Google Scholar] [CrossRef]
- Ramirez-Latorre, J.; Yu, C.R.; Qu, X.; Perin, F.; Karlin, A.; Role, L. Functional contributions of alpha5 subunit to neuronal acetylcholine receptor channels. Nature 1996, 380, 347–351. [Google Scholar] [CrossRef]
- Brown, R.W.; Collins, A.C.; Lindstrom, J.M.; Whiteaker, P. Nicotinic alpha5 subunit deletion locally reduces high-affinity agonist activation without altering nicotinic receptor numbers. J. Neurochem. 2007, 103, 204–215. [Google Scholar]
- Gallego, X.; Molas, S.; Amador-Arjona, A.; Marks, M.J.; Robles, N.; Murtra, P.; Armengol, L.; Fernandez-Montes, R.D.; Gratacos, M.; Pumarola, M.; et al. Overexpression of the CHRNA5/A3/B4 genomic cluster in mice increases the sensitivity to nicotine and modifies its reinforcing effects. Amino Acids 2012, 43, 897–909. [Google Scholar] [CrossRef]
- Gerzanich, V.; Wang, F.; Kuryatov, A.; Lindstrom, J. alpha 5 Subunit alters desensitization, pharmacology, Ca++ permeability and Ca++ modulation of human neuronal alpha 3 nicotinic receptors. Clin. Pharmacol. Ther. 1998, 286, 311–320. [Google Scholar]
- Samet, J.M.; Avila-Tang, E.; Boffetta, P.; Hannan, L.M.; Olivo-Marston, S.; Thun, M.J.; Rudin, C.M. Lung cancer in never smokers: Clinical epidemiology and environmental risk factors. Clin. Cancer Res. 2009, 15, 5626–5645. [Google Scholar] [CrossRef]
- Falvella, F.S.; Galvan, A.; Frullanti, E.; Spinola, M.; Calabro, E.; Carbone, A.; Incarbone, M.; Santambrogio, L.; Pastorino, U.; Dragani, T.A. Transcription deregulation at the 15q25 locus in association with lung adenocarcinoma risk. Clin. Cancer Res. 2009, 15, 1837–1842. [Google Scholar] [CrossRef] [PubMed]
- Spitz, M.R.; Amos, C.I.; Dong, Q.; Lin, J.; Wu, X. The CHRNA5-A3 region on chromosome 15q24-25.1 is a risk factor both for nicotine dependence and for lung cancer. J. Natl. Cancer Inst. 2008, 100, 1552–1556. [Google Scholar] [CrossRef] [PubMed]
- Berrettini, W.; Yuan, X.; Tozzi, F.; Song, K.; Francks, C.; Chilcoat, H.; Waterworth, D.; Muglia, P.; Mooser, V. Alpha-5/alpha-3 nicotinic receptor subunit alleles increase risk for heavy smoking. Mol. Psychiatry 2008, 13, 368–373. [Google Scholar] [CrossRef]
- Friedman, J.R.; Richbart, S.D.; Merritt, J.C.; Brown, K.C.; Nolan, N.A.; Akers, A.T.; Lau, J.K.; Robateau, Z.R.; Miles, S.L.; Dasgupta, P. Acetylcholine signaling system in progression of lung cancers. Pharmacol. Ther. 2019, 194, 222–254. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Chang, Y.C.; Chen, C.S.; Tu, S.H.; Wang, Y.J.; Chen, L.C.; Chang, Y.J.; Wei, P.L.; Chang, H.W.; Chang, C.H.; et al. Crosstalk between nicotine and estrogen-induced estrogen receptor activation induces alpha9-nicotinic acetylcholine receptor expression in human breast cancer cells. Breast Cancer Res. Treat. 2011, 129, 331–345. [Google Scholar] [CrossRef]
- Lee, C.H.; Huang, C.S.; Chen, C.S.; Tu, S.H.; Wang, Y.J.; Chang, Y.J.; Tam, K.W.; Wei, P.L.; Cheng, T.C.; Chu, J.S.; et al. Overexpression and activation of the alpha9-nicotinic receptor during tumorigenesis in human breast epithelial cells. J. Natl. Cancer Inst. 2010, 102, 1322–1335. [Google Scholar] [CrossRef]
- Lien, Y.C.; Wang, W.; Kuo, L.J.; Liu, J.J.; Wei, P.L.; Ho, Y.S.; Ting, W.C.; Wu, C.H.; Chang, Y.J. Nicotine promotes cell migration through alpha7 nicotinic acetylcholine receptor in gastric cancer cells. Ann. Surg Oncol 2011, 18, 2671–2679. [Google Scholar] [CrossRef]
- Chou, Y.T.; Lin, H.H.; Lien, Y.C.; Wang, Y.H.; Hong, C.F.; Kao, Y.R.; Lin, S.C.; Chang, Y.C.; Lin, S.Y.; Chen, S.J.; et al. EGFR Promotes Lung Tumorigenesis by Activating miR-7 through a Ras/ERK/Myc Pathway that Targets the Ets2 Transcriptional Repressor ERF. Cancer Res. 2010, 70, 8822–8831. [Google Scholar] [CrossRef]
- Singh, S.; Trevino, J.; Bora-Singhal, N.; Coppola, D.; Haura, E.; Altiok, S.; Chellappan, S.P. EGFR/Src/Akt signaling modulates Sox2 expression and self-renewal of stem-like side-population cells in non-small cell lung cancer. Mol. Cancer 2012, 11, 73. [Google Scholar] [CrossRef]
- Nishioka, T.; Kim, H.S.; Luo, L.Y.; Huang, Y.; Guo, J.; Chen, C.Y. Sensitization of epithelial growth factor receptors by nicotine exposure to promote breast cancer cell growth. Breast Cancer Res. 2011, 13, R113. [Google Scholar] [CrossRef]
- Goldman, M.J.; Craft, B.; Hastie, M.; Repečka, K.; McDade, F.; Kamath, A.; Banerjee, A.; Luo, Y.; Rogers, D.; Brooks, A.N.; et al. Visualizing and interpreting cancer genomics data via the Xena platform. Nat. Biotech. 2020, 38, 675–678. [Google Scholar] [CrossRef] [PubMed]
- Toh, C.K.; Gao, F.; Lim, W.T.; Leong, S.S.; Fong, K.W.; Yap, S.P.; Hsu, A.A.; Eng, P.; Koong, H.N.; Thirugnanam, A.; et al. Never-smokers with lung cancer: Epidemiologic evidence of a distinct disease entity. Int. J. Clin. Oncol. 2006, 24, 2245–2251. [Google Scholar] [CrossRef] [PubMed]
- Amos, C.I.; Wu, X.; Broderick, P.; Gorlov, I.P.; Gu, J.; Eisen, T.; Dong, Q.; Zhang, Q.; Gu, X.; Vijayakrishnan, J.; et al. Genome-wide association scan of tag SNPs identifies a susceptibility locus for lung cancer at 15q25.1. Nat. Genet. 2008, 40, 616–622. [Google Scholar] [CrossRef]
- Chen, Y.C.; Hsu, H.S.; Chen, Y.W.; Tsai, T.H.; How, C.K.; Wang, C.Y.; Hung, S.C.; Chang, Y.L.; Tsai, M.L.; Lee, Y.Y.; et al. Oct-4 expression maintained cancer stem-like properties in lung cancer-derived CD133-positive cells. PLoS ONE 2008, 3, e2637. [Google Scholar] [CrossRef]
- Thorgeirsson, T.E.; Geller, F.; Sulem, P.; Rafnar, T.; Wiste, A.; Magnusson, K.P.; Manolescu, A.; Thorleifsson, G.; Stefansson, H.; Ingason, A.; et al. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature 2008, 452, 638–642. [Google Scholar] [CrossRef]
- Wei, P.L.; Chang, Y.J.; Ho, Y.S.; Lee, C.H.; Yang, Y.Y.; An, J.; Lin, S.Y. Tobacco-specific carcinogen enhances colon cancer cell migration through alpha7-nicotinic acetylcholine receptor. Ann. Surg. 2009, 249, 978–985. [Google Scholar] [CrossRef]
- Brown, K.C.; Perry, H.E.; Lau, J.K.; Jones, D.V.; Pulliam, J.F.; Thornhill, B.A.; Crabtree, C.M.; Luo, H.; Chen, Y.C.; Dasgupta, P. Nicotine induces the up-regulation of the alpha7-nicotinic receptor (alpha7-nAChR) in human squamous cell lung cancer cells via the Sp1/GATA protein pathway. J. Biol. Chem. 2013, 288, 33049–33059. [Google Scholar] [CrossRef]
- Cheng, W.-L.; Chen, K.-Y.; Lee, K.-Y.; Feng, P.-H.; Wu, S.-M. Nicotinic-nAChR signaling mediates drug resistance in lung cancer. J. Cancer 2020, 11, 1125–1140. [Google Scholar] [CrossRef]
- Hsu, C.C.; Tsai, K.Y.; Su, Y.F.; Chien, C.Y.; Chen, Y.C.; Wu, Y.C.; Liu, S.Y.; Shieh, Y.S. α7-Nicotine acetylcholine receptor mediated nicotine induced cell survival and cisplatin resistance in oral cancer. Arch. Oral Biol. 2020, 111, 104653. [Google Scholar] [CrossRef]
- Witayateeraporn, W.; Arunrungvichian, K.; Pothongsrisit, S.; Doungchawee, J.; Vajragupta, O.; Pongrakhananon, V. α7-Nicotinic acetylcholine receptor antagonist QND7 suppresses non-small cell lung cancer cell proliferation and migration via inhibition of Akt/mTOR signaling. Biochem. Biophys. Res. Commun. 2020, 521, 977–983. [Google Scholar] [CrossRef]
- Schaal, C.M.; Bora-Singhal, N.; Kumar, D.M.; Chellappan, S.P. Regulation of Sox2 and stemness by nicotine and electronic-cigarettes in non-small cell lung cancer. Mol. Cancer 2018, 17, 149. [Google Scholar] [CrossRef] [PubMed]
- Cesario, A.; Russo, P.; Nastrucci, C.; Granone, P. Is alpha7-nAChR a Possible Target for Lung Cancer and Malignant Pleural Mesothelioma Treatment? Curr. Drug Targets 2012, 13, 688–694. [Google Scholar] [PubMed]
- Arredondo, J.; Chernyavsky, A.I.; Jolkovsky, D.L.; Pinkerton, K.E.; Grando, S.A. Receptor-mediated tobacco toxicity: Acceleration of sequential expression of alpha5 and alpha7 nicotinic receptor subunits in oral keratinocytes exposed to cigarette smoke. FASEB J. 2008, 22, 1356–1368. [Google Scholar] [CrossRef]
- Chen, R.J.; Ho, Y.S.; Guo, H.R.; Wang, Y.J. Long-term nicotine exposure-induced chemoresistance is mediated by activation of Stat3 and downregulation of ERK1/2 via nAChR and beta-adrenoceptors in human bladder cancer cells. Toxicol. Sci. 2010, 115, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jia, Y.; Zhang, Y.; Zhou, D.; Sun, H.; Ma, X. α5-nAChR contributes to epithelial-mesenchymal transition and metastasis by regulating Jab1/Csn5 signalling in lung cancer. J. Cell Mol. Med. 2020, 24, 2497–2506. [Google Scholar] [CrossRef]
- Dang, N.; Meng, X.; Qin, G.; An, Y.; Zhang, Q.; Cheng, X.; Huang, S. α5-nAChR modulates melanoma growth through the Notch1 signaling pathway. J. Cell Physiol. 2020. [Google Scholar] [CrossRef]
- Barik, J.; Wonnacott, S. Indirect modulation by alpha7 nicotinic acetylcholine receptors of noradrenaline release in rat hippocampal slices: Interaction with glutamate and GABA systems and effect of nicotine withdrawal. Mol. Pharmacol. 2006, 69, 618–628. [Google Scholar] [CrossRef]
- Mozayan, M.; Lee, T.J. Statins prevent cholinesterase inhibitor blockade of sympathetic alpha7-nAChR-mediated currents in rat superior cervical ganglion neurons. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H1737–H1744. [Google Scholar] [CrossRef]
- Wong, H.P.; Yu, L.; Lam, E.K.; Tai, E.K.; Wu, W.K.; Cho, C.H. Nicotine promotes cell proliferation via alpha7-nicotinic acetylcholine receptor and catecholamine-synthesizing enzymes-mediated pathway in human colon adenocarcinoma HT-29 cells. Toxicol. Appl. Pharmacol. 2007, 221, 261–267. [Google Scholar] [CrossRef]
- Schuller, H.M.; Tithof, P.K.; Williams, M.; Plummer, H. The tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone is a beta-adrenergic agonist and stimulates DNA synthesis in lung adenocarcinoma via beta-adrenergic receptor-mediated release of arachidonic acid. Cancer Res. 1999, 59, 4510–4515. [Google Scholar]
- Laag, E.; Majidi, M.; Cekanova, M.; Masi, T.; Takahashi, T.; Schuller, H.M. NNK activates ERK1/2 and CREB/ATF-1 via beta-1-AR and EGFR signaling in human lung adenocarcinoma and small airway epithelial cells. Int. J. Cancer 2006, 119, 1547–1552. [Google Scholar] [PubMed]
- Chu, Y.W.; Yang, P.C.; Yang, S.C.; Shyu, Y.C.; Hendrix, M.J.; Wu, R.; Wu, C.W. Selection of invasive and metastatic subpopulations from a human lung adenocarcinoma cell line. Am. J. Respir. Cell Mol. 1997, 17, 353–360. [Google Scholar] [CrossRef]
- Hong, C.F.; Chou, Y.T.; Lin, Y.S.; Wu, C.W. MAD2B, a novel TCF4-binding protein, modulates TCF4-mediated epithelial-mesenchymal transdifferentiation. J. Biol. Chem. 2009, 284, 19613–19622. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.C.; Chou, T.Y. Pulmonary neuroendocrine tumors: Study of 90 cases focusing on clinicopathological characteristics, immunophenotype, preoperative biopsy, and frozen section diagnoses. J. Surg. Oncol. 2014, 109, 280–286. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.-L.; Hsu, Y.-F.; Liu, C.-H.; Kuo, Y.-L.; Chen, Y.-C.; Yeh, Y.-C.; Ho, H.-L.; Wu, Y.-C.; Chou, T.-Y.; Wu, C.-W. Low-Dose Nicotine Activates EGFR Signaling via α5-nAChR and Promotes Lung Adenocarcinoma Progression. Int. J. Mol. Sci. 2020, 21, 6829. https://doi.org/10.3390/ijms21186829
Wang M-L, Hsu Y-F, Liu C-H, Kuo Y-L, Chen Y-C, Yeh Y-C, Ho H-L, Wu Y-C, Chou T-Y, Wu C-W. Low-Dose Nicotine Activates EGFR Signaling via α5-nAChR and Promotes Lung Adenocarcinoma Progression. International Journal of Molecular Sciences. 2020; 21(18):6829. https://doi.org/10.3390/ijms21186829
Chicago/Turabian StyleWang, Mong-Lien, Yi-Fan Hsu, Chih-Hsuan Liu, Ya-Ling Kuo, Yi-Chen Chen, Yi-Chen Yeh, Hsiang-Ling Ho, Yu-Chung Wu, Teh-Ying Chou, and Cheng-Wen Wu. 2020. "Low-Dose Nicotine Activates EGFR Signaling via α5-nAChR and Promotes Lung Adenocarcinoma Progression" International Journal of Molecular Sciences 21, no. 18: 6829. https://doi.org/10.3390/ijms21186829
APA StyleWang, M.-L., Hsu, Y.-F., Liu, C.-H., Kuo, Y.-L., Chen, Y.-C., Yeh, Y.-C., Ho, H.-L., Wu, Y.-C., Chou, T.-Y., & Wu, C.-W. (2020). Low-Dose Nicotine Activates EGFR Signaling via α5-nAChR and Promotes Lung Adenocarcinoma Progression. International Journal of Molecular Sciences, 21(18), 6829. https://doi.org/10.3390/ijms21186829




