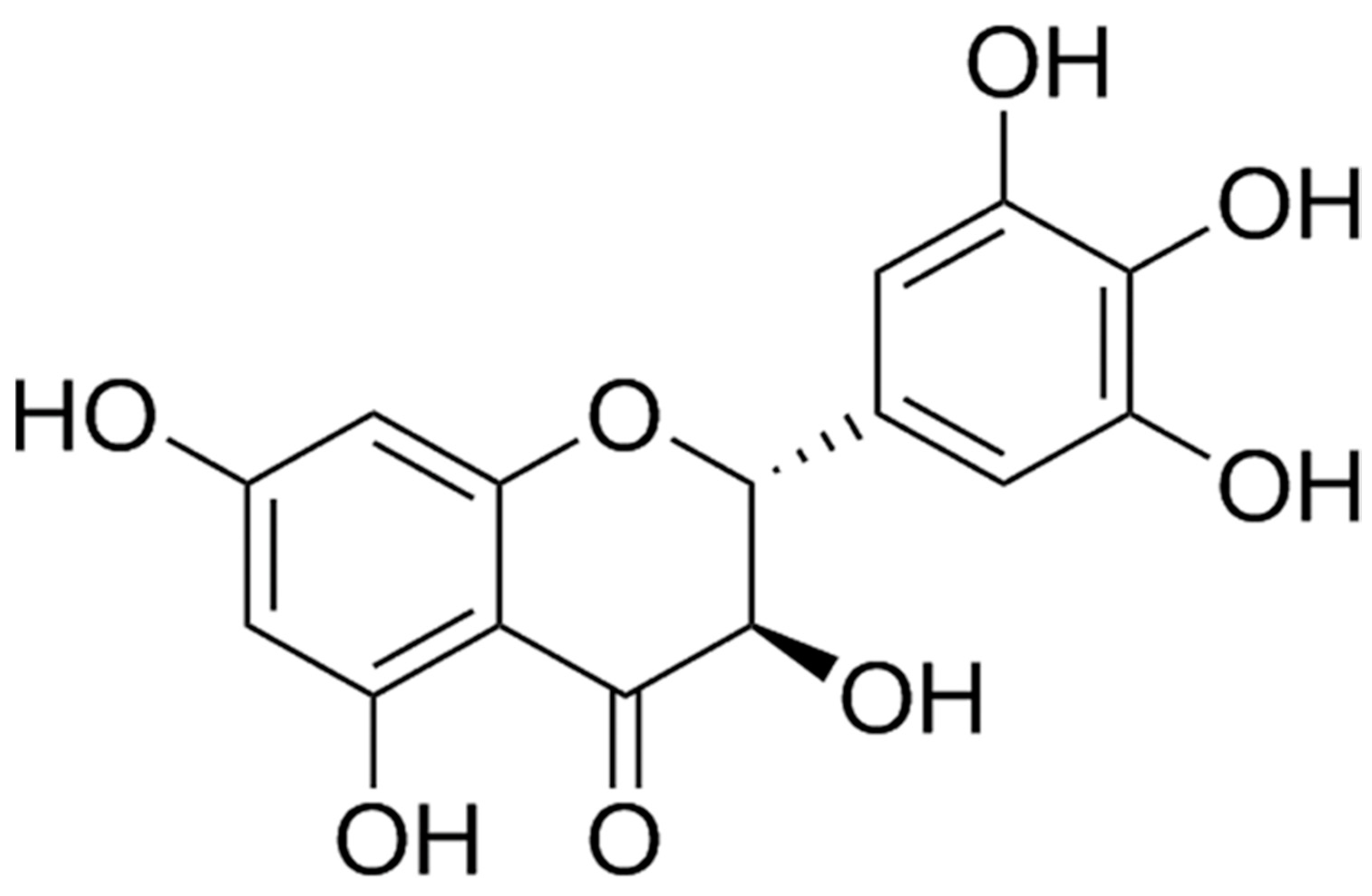
Dihydromyricetin Improves Endothelial Dysfunction in Diabetic Mice via Oxidative Stress Inhibition in a SIRT3-Dependent Manner
Abstract
1. Introduction
2. Results
2.1. DHY Decreased FBG and HbA1c Level in Diabetic Mice
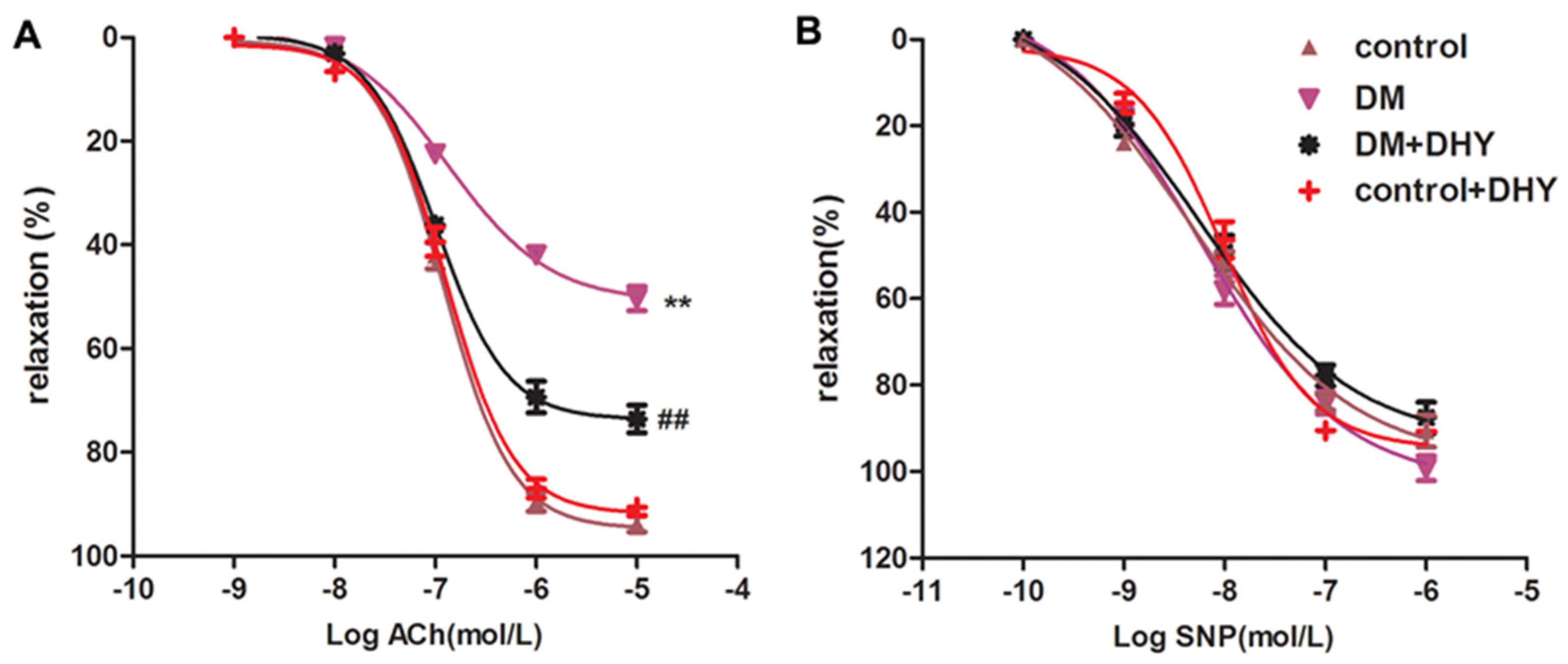
2.2. DHY Improved Endothelium-Dependent Relaxation of Thoracic Aorta in Diabetic Mice
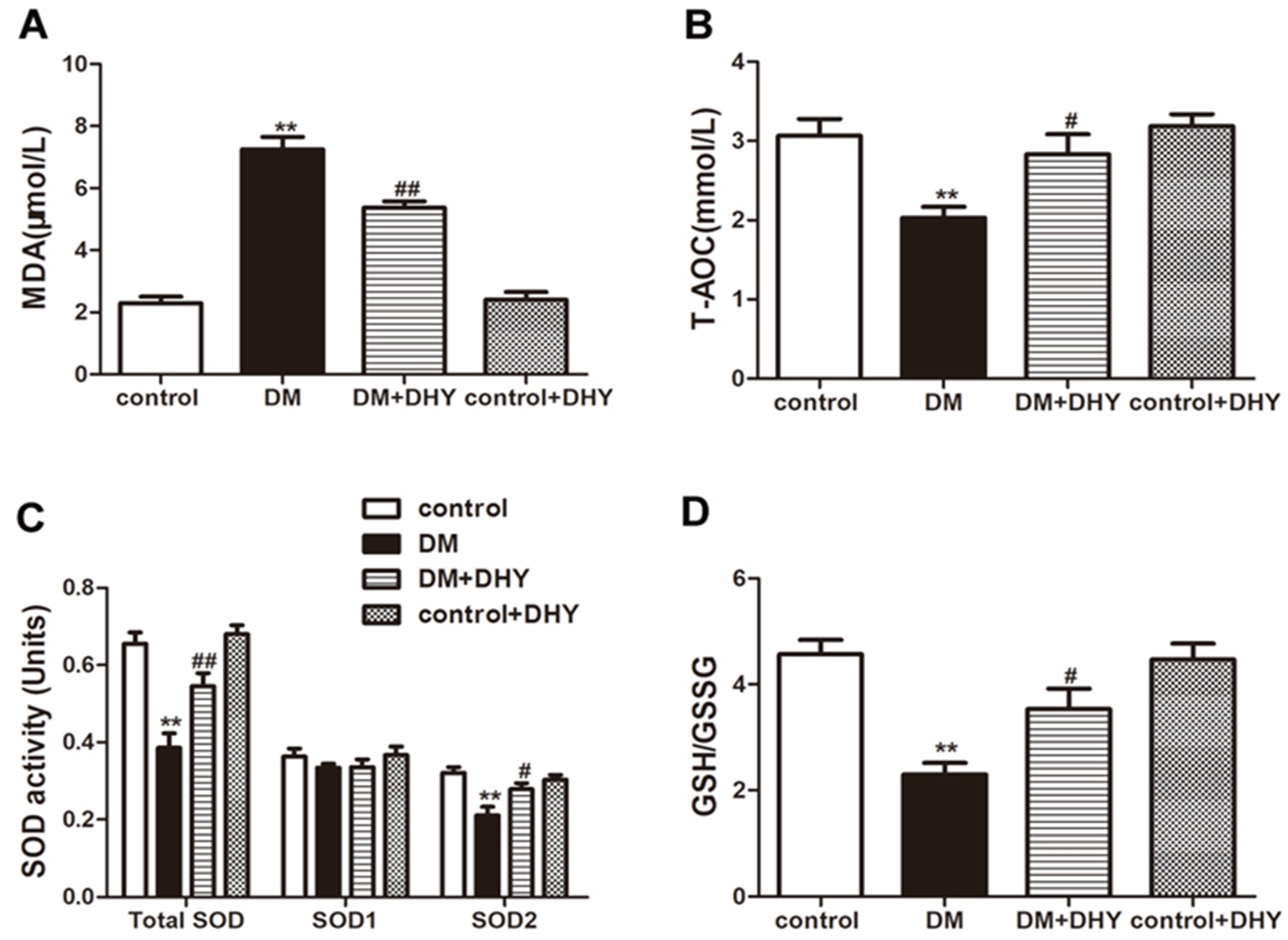
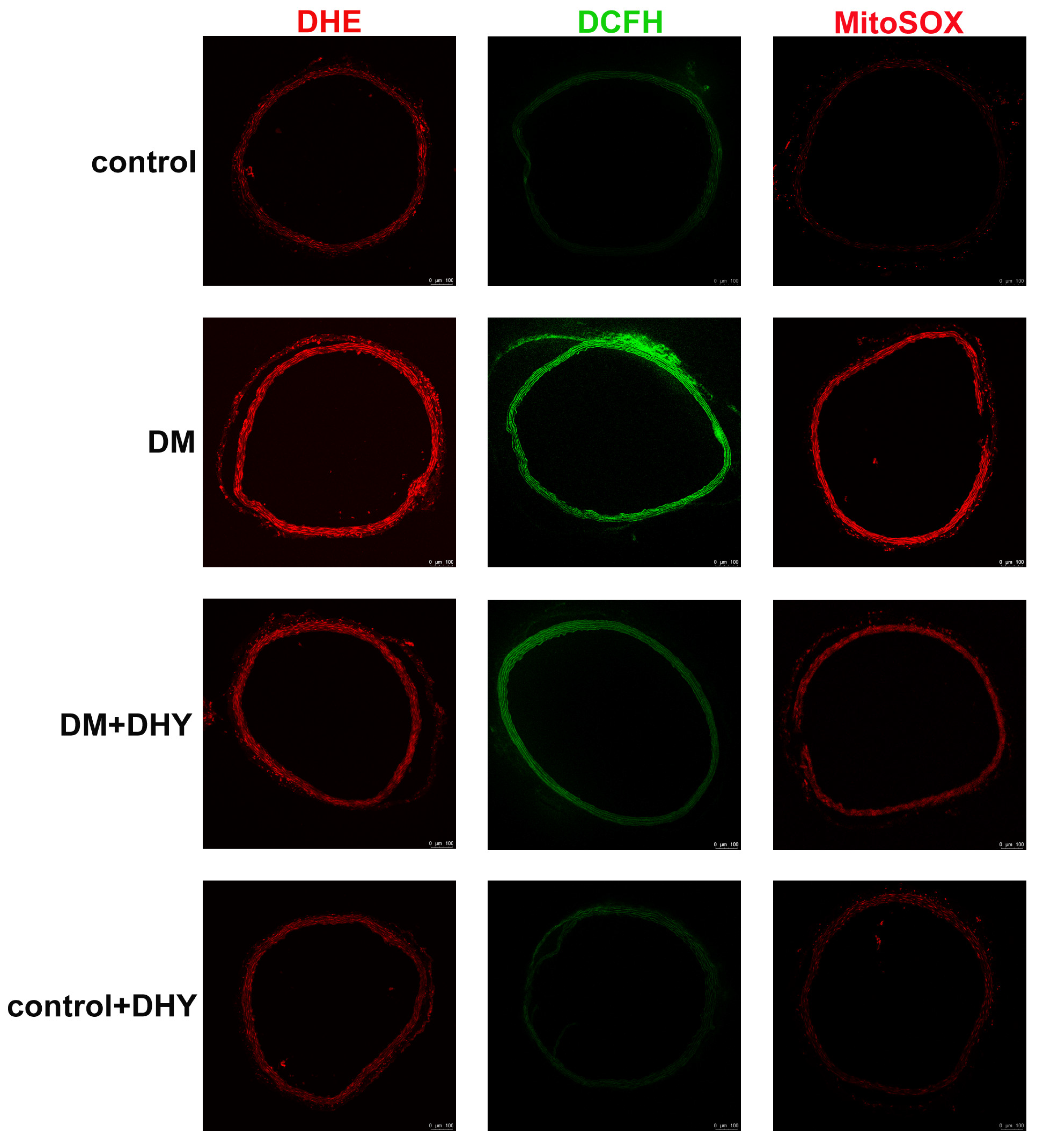
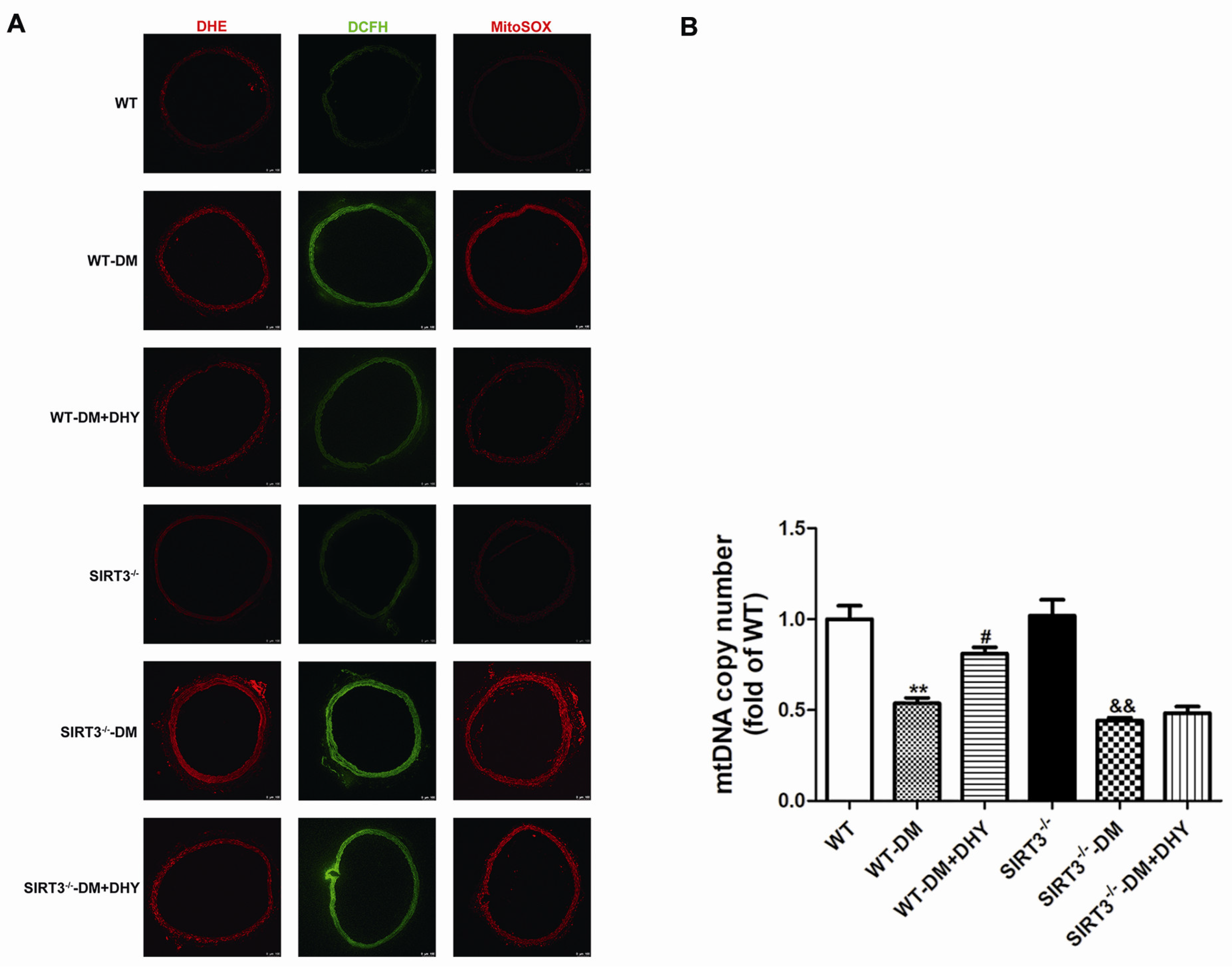
2.3. DHY Suppressed Oxidative Stress in Thoracic Aorta of Diabetic Mice
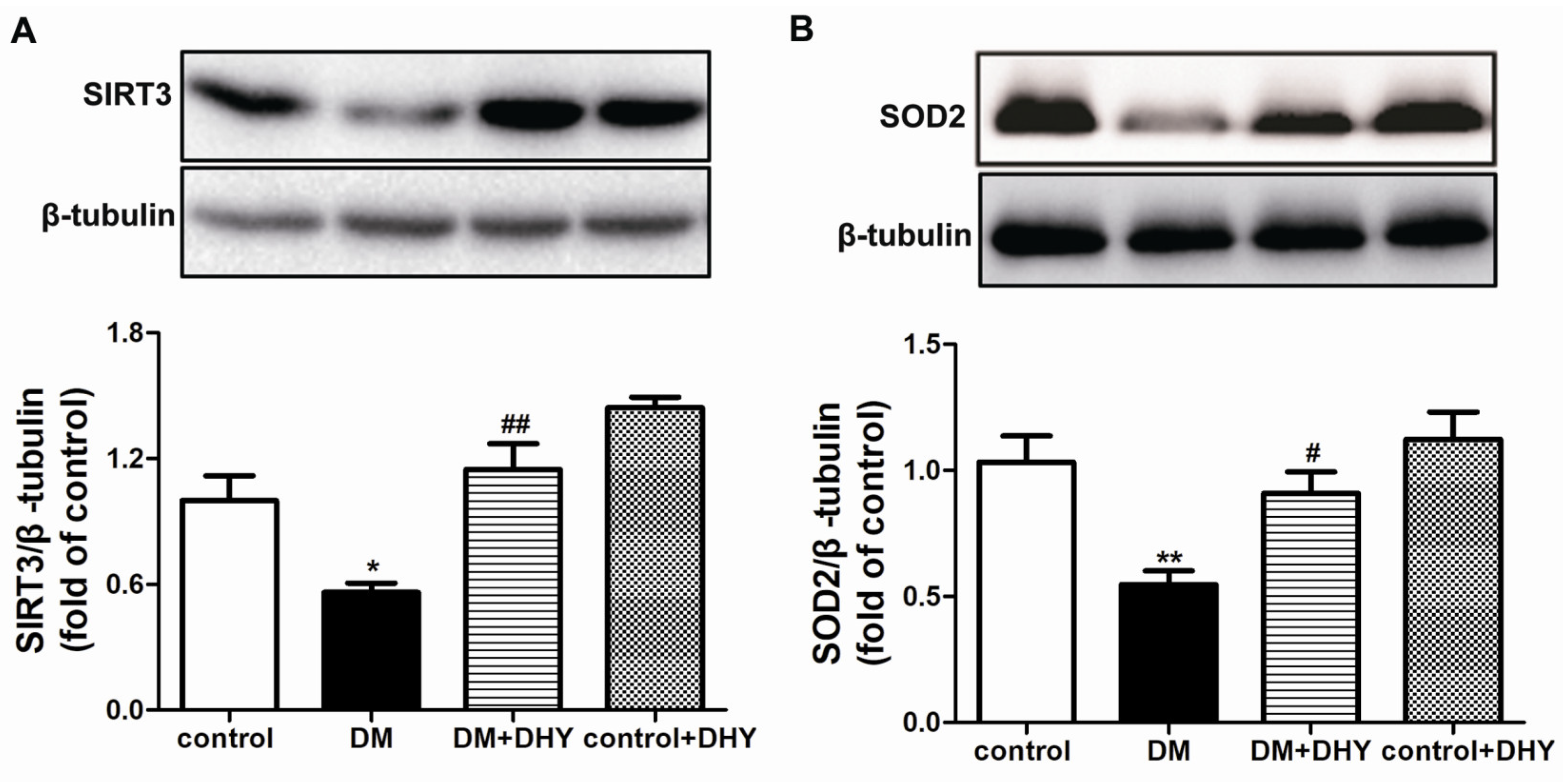
2.4. DHY Enhanced SIRT3 and SOD2 Protein Expression in Thoracic Aorta of Diabetic Mice
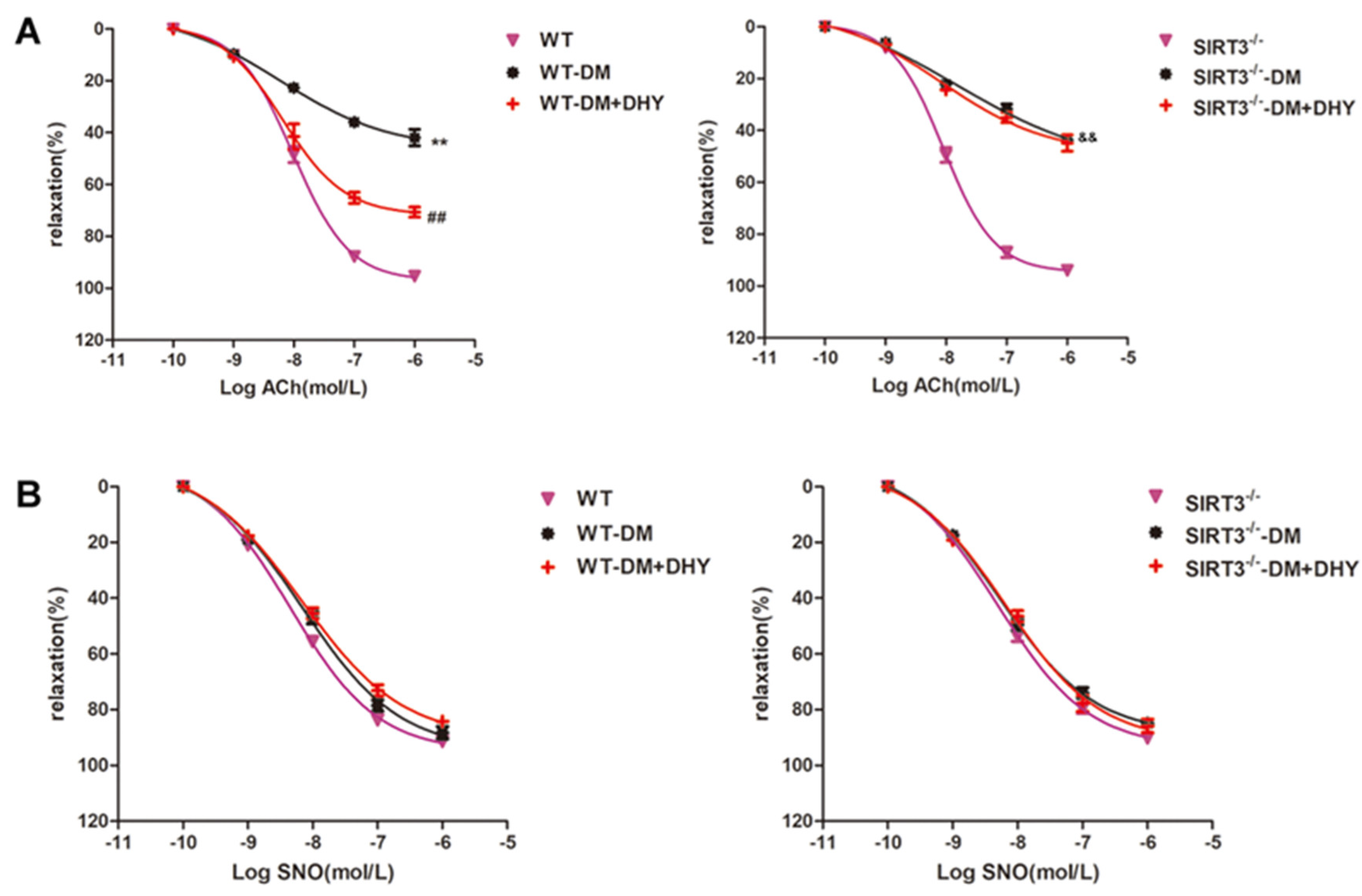
2.5. DHY Failed To Improve Endothelium-Dependent Relaxation of Thoracic Aorta in SIRT3-/- Mice with Diabetes
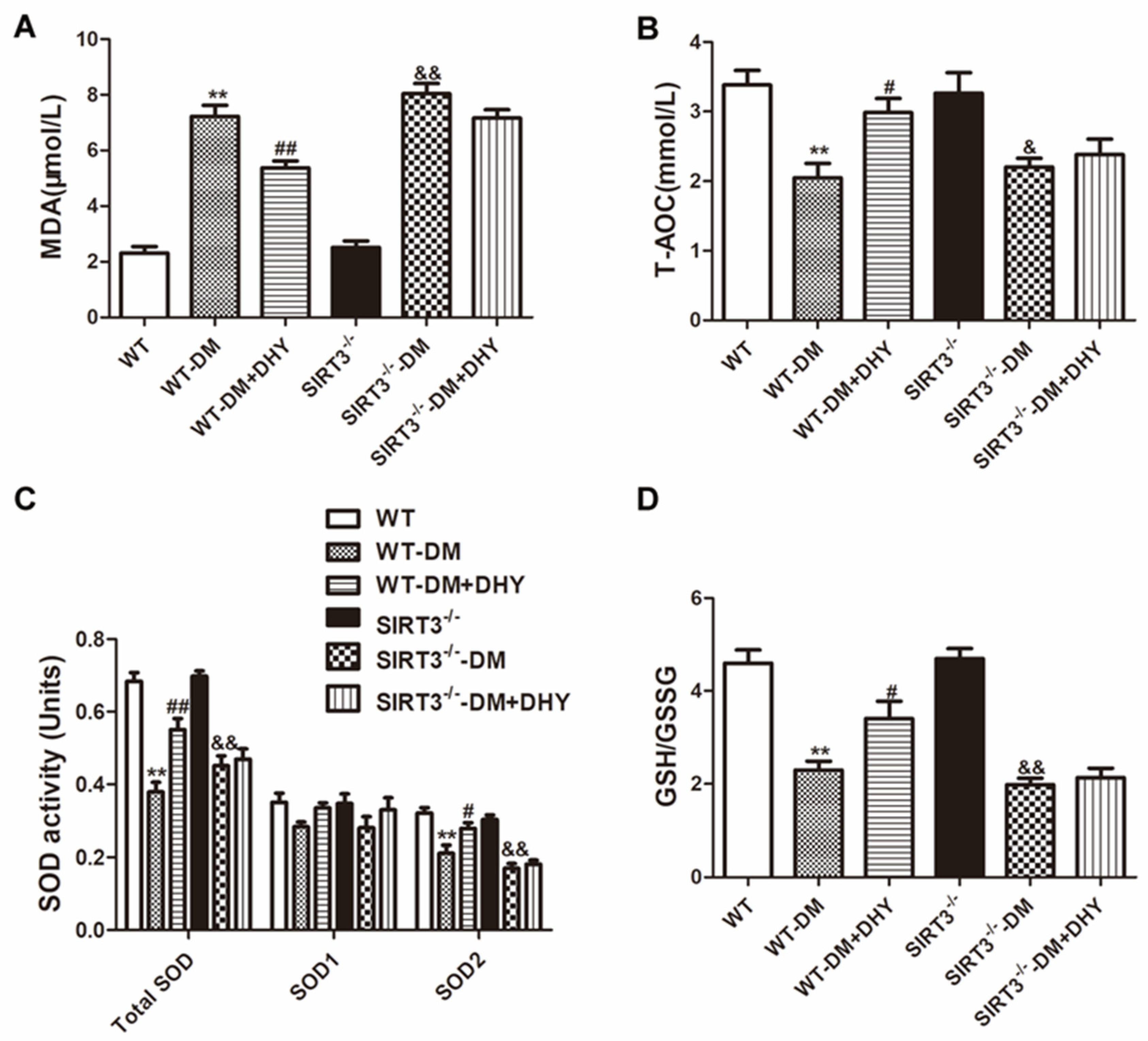
2.6. DHY Failed to Suppress Oxidative Stress of Thoracic Aorta in SIRT3-/- Mice with Diabetes
3. Discussion
4. Materials and Methods
4.1. Animal Treatment
4.2. Measurement of the Glycosylated Hemoglobin (HbA1c)
4.3. Assessment of Endothelium-Dependent Relaxations
4.4. Biochemaical Assessment
4.5. Measurement of Superoxide Formation in the Thoracic Aorta
4.6. Measurement of Intracellular ROS
4.7. Measurement of Mitochondrial ROS
4.8. Western Blot
4.9. Measurement of mtRNA Copy Number
4.10. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Ach | Acetylcholine |
| CAT | Catalase |
| CMC | Carboxymethylcellulose |
| DAPI | 2-(4-Amidinophenyl)-6-indolecarbamidine dihydrochloride |
| DCFH-DA | 2′, 7′-dichlorofluorescein diacetate |
| DHE | Dihydroethidium |
| DHY | Dihydromyricetin |
| DM | Diabetes mellitus |
| ELISA | enzyme-linked immunosorbent assay |
| FBG | Fasting blood glucose |
| GSH | glutathione |
| GSSG | Glutathione disulfide |
| HbA1c | Glycosylated hemoglobin |
| HG | High glucose |
| HMEC-1 | Human microvascular endothelial cell-1 |
| HUVECs | Human umbilical vein endothelial cells |
| MDA | Malondialdehyde |
| mtDNA | Mitochondrial Deoxyribonucleic Acid |
| NAC | N-acetyl-L-cysteine |
| OCT | Optimal cutting temperature |
| PVDF | Polyvinylidene difluoride |
| ROS | Reactive oxygen species |
| SDS-PAGE | Sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| SIRT3 | Sirtuin 3 |
| SNP | Sodium nitroprusside |
| SOD | Superoxide dismutase |
| STZ | Streptozocin |
| TAC | Transverse aortic constriction |
| T-AOC | Total antioxidant capacity |
| T2DM | Type 2 diabetes mellitus |
| WT | Wild type |
References
- Khan, R.M.M.; Chua, Z.J.Y.; Tan, J.C.; Yang, Y.; Liao, Z.; Zhao, Y. From Pre-Diabetes to Diabetes: Diagnosis, Treatments and Translational Research. Medicina (Kaunas) 2019, 55, 546. [Google Scholar] [CrossRef]
- Jia, W.; Weng, J.; Zhu, D.; Ji, L.; Lu, J.; Zhou, Z.; Zou, D.; Guo, L.; Ji, Q.; Chen, L.; et al. Standards of medical care for type 2 diabetes in China 2019. Diabetes Metab. Res. Rev. 2019, 35, e3158. [Google Scholar] [CrossRef] [PubMed]
- Dogruel, H.; Balci, M.K. Development of therapeutic options on type 2 diabetes in years: Glucagon-like peptide-1 receptor agonist’s role intreatment; from the past to future. World J. Diabetes 2019, 10, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Ajjan, R.A.; Schroeder, V. Role of complement in diabetes. Mol. Immunol. 2019, 114, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Avogaro, A.; Fadini, G.P. Microvascular complications in diabetes: A growing concern for cardiologists. Int. J. Cardiol. 2019, 291, 29–35. [Google Scholar] [CrossRef]
- Kurniawan, A.H.; Suwandi, B.H.; Kholili, U. Diabetic Gastroenteropathy: A Complication of Diabetes Mellitus. Acta Med. Indones. 2019, 51, 263–271. [Google Scholar]
- Eid, S.; Sas, K.M.; Abcouwer, S.F.; Feldman, E.L.; Gardner, T.W.; Pennathur, S.; Fort, P.E. New insights into the mechanisms of diabetic complications: Role of lipids and lipid metabolism. Diabetologia 2019, 62, 1539–1549. [Google Scholar] [CrossRef]
- Almourani, R.; Chinnakotla, B.; Patel, R.; Kurukulasuriya, L.R.; Sowers, J. Diabetes and Cardiovascular Disease: An Update. Curr. Diab. Rep. 2019, 19, 161. [Google Scholar] [CrossRef]
- Huynh, D.T.N.; Heo, K.S. Therapeutic targets for endothelial dysfunction in vascular diseases. Arch. Pharm. Res. 2019, 42, 848–861. [Google Scholar] [CrossRef]
- Shi, Y.; Vanhoutte, P.M. Macro- and microvascular endothelial dysfunction in diabetes. J. Diabetes 2017, 9, 434–449. [Google Scholar] [CrossRef]
- Knapp, M.; Tu, X.; Wu, R. Vascular endothelial dysfunction, a major mediator in diabetic cardiomyopathy. Acta Pharmacol. Sin. 2019, 40, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.C.; Liu, P.G.; Wang, C.; Zheng, A.D.; Wan, Z. Tacrolimus protects vascular endothelial cells from injuries caused by Ox-LDL by regulating endoplasmic reticulum stress. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 3966–3973. [Google Scholar] [PubMed]
- Yang, L.; Tang, L.; Dai, F.; Meng, G.; Yin, R.; Xu, X.; Yao, W. Raf-1/CK2 and RhoA/ROCK signaling promote TNF-alpha-mediated endothelial apoptosis via regulating vimentin cytoskeleton. Toxicology 2017, 389, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Xin, R.; An, D.; Li, Y.; Fu, J.; Huang, F.; Zhu, Q. Fenofibrate improves vascular endothelial function in diabetic mice. Biomed. Pharmacother. 2019, 112, 108722. [Google Scholar] [CrossRef]
- Xu, F.; Liu, Y.; Zhu, X.; Li, S.; Shi, X.; Li, Z.; Ai, M.; Sun, J.; Hou, B.; Cai, W.; et al. Protective Effects and Mechanisms of Vaccarin on Vascular Endothelial Dysfunction in Diabetic Angiopathy. Int. J. Mol. Sci. 2019, 20, 4587. [Google Scholar] [CrossRef]
- Zeng, Y.; Peng, Y.; Tang, K.; Wang, Y.Q.; Zhao, Z.Y.; Wei, X.Y.; Xu, X.L. Dihydromyricetin ameliorates foam cell formation via LXRalpha-ABCA1/ABCG1-dependent cholesterol efflux in macrophages. Biomed. Pharmacother. 2018, 101, 543–552. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Y.; Luo, H.; Sun, L.; Xu, M.; Yu, J.; Zhou, Q.; Meng, G.; Yang, S. Recent Update on the Pharmacological Effects and Mechanisms of Dihydromyricetin. Front. Pharmacol. 2018, 9, 1204. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, H.Q.; Sun, L.L.; Xu, M.T.; Yu, J.; Liu, L.L.; Zhang, J.Y.; Wang, Y.Q.; Wang, H.X.; Bao, X.F.; et al. Dihydromyricetin Attenuates Myocardial Hypertrophy Induced by Transverse Aortic Constriction via Oxidative Stress Inhibition and SIRT3 Pathway Enhancement. Int. J. Mol. Sci. 2018, 19, 2592. [Google Scholar] [CrossRef]
- Zeng, Y.; Hua, Y.Q.; Wang, W.; Zhang, H.; Xu, X.L. Modulation of SIRT1-mediated signaling cascades in the liver contributes to the amelioration of nonalcoholic steatohepatitis in high fat fed middle-aged LDL receptor knockout mice by dihydromyricetin. Biochem. Pharmacol. 2020, 175, 113927. [Google Scholar] [CrossRef]
- Meng, G.; Yang, S.; Chen, Y.; Yao, W.; Zhu, H.; Zhang, W. Attenuating effects of dihydromyricetin on angiotensin II-induced rat cardiomyocyte hypertrophy related to antioxidative activity in a NO-dependent manner. Pharm. Biol. 2015, 53, 904–912. [Google Scholar] [CrossRef]
- Hou, X.; Tong, Q.; Wang, W.; Xiong, W.; Shi, C.; Fang, J. Dihydromyricetin protects endothelial cells from hydrogen peroxide-induced oxidative stress damage by regulating mitochondrial pathways. Life Sci. 2015, 130, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.; Liu, J.; Liu, S.; Song, Q.; Liu, L.; Xie, L.; Han, Y.; Ji, Y. Hydrogen sulfide pretreatment improves mitochondrial function in myocardial hypertrophy via a SIRT3-dependent manner. Br. J. Pharmacol. 2018, 175, 1126–1145. [Google Scholar] [CrossRef]
- Li, X.; Song, S.; Xu, M.; Hua, Y.; Ding, Y.; Shan, X.; Meng, G.; Wang, Y. Sirtuin3 deficiency exacerbates carbon tetrachloride-induced hepatic injury in mice. J. Biochem. Mol. Toxicol. 2019, 33, e22249. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.K.; Chhabra, G.; Ndiaye, M.A.; Garcia-Peterson, L.M.; Mack, N.J.; Ahmad, N. The Role of Sirtuins in Antioxidant and Redox Signaling. Antioxid. Redox Signal. 2018, 28, 643–661. [Google Scholar] [CrossRef] [PubMed]
- Leger, T.; Azarnoush, K.; Traore, A.; Cassagnes, L.; Rigaudiere, J.P.; Jouve, C.; Pages, G.; Bouvier, D.; Sapin, V.; Pereira, B.; et al. Antioxidant and Cardioprotective Effects of EPA on Early Low-Severity Sepsis through UCP3 and SIRT3 Upholding of the Mitochondrial Redox Potential. Oxid. Med. Cell. Longev. 2019, 2019, 9710352. [Google Scholar] [CrossRef]
- Liu, T.; Ma, X.; Ouyang, T.; Chen, H.; Xiao, Y.; Huang, Y.; Liu, J.; Xu, M. Efficacy of 5-aminolevulinic acid-based photodynamic therapy against keloid compromised by downregulation of SIRT1-SIRT3-SOD2-mROS dependent autophagy pathway. Redox. Biol. 2019, 20, 195–203. [Google Scholar] [CrossRef]
- Kim, H.; Lee, Y.D.; Kim, H.J.; Lee, Z.H.; Kim, H.H. SOD2 and Sirt3 Control Osteoclastogenesis by Regulating Mitochondrial ROS. J. Bone. Miner. Res. 2017, 32, 397–406. [Google Scholar] [CrossRef]
- Dikalova, A.E.; Pandey, A.; Xiao, L.; Arslanbaeva, L.; Sidorova, T.; Lopez, M.G.; Billings, F.T., IV; Verdin, E.; Auwerx, J.; Harrison, D.G.; et al. Mitochondrial Deacetylase Sirt3 Reduces Vascular Dysfunction and Hypertension While Sirt3 Depletion in Essential Hypertension Is Linked to Vascular Inflammation and Oxidative Stress. Circ. Res. 2020, 126, 439–452. [Google Scholar] [CrossRef]
- Gu, Y.; Tang, X.; Xie, L.; Meng, G.; Ji, Y. Aliskiren improves endothelium-dependent relaxation of thoracic aorta by activating PI3K/Akt/eNOS signal pathway in SHR. Clin. Exp. Pharmacol. Physiol. 2016, 43, 450–458. [Google Scholar] [CrossRef]
- Winnik, S.; Gaul, D.S.; Siciliani, G.; Lohmann, C.; Pasterk, L.; Calatayud, N.; Weber, J.; Eriksson, U.; Auwerx, J.; Van Tits, L.J.; et al. Mild endothelial dysfunction in Sirt3 knockout mice fed a high-cholesterol diet: Protective role of a novel C/EBP-beta-dependent feedback regulation of SOD2. Basic Res. Cardiol. 2016, 111, 33. [Google Scholar] [CrossRef]
- Liu, H.; Xiang, H.; Zhao, S.; Sang, H.; Lv, F.; Chen, R.; Shu, Z.; Chen, A.F.; Chen, S.; Lu, H. Vildagliptin improves high glucose-induced endothelial mitochondrial dysfunction via inhibiting mitochondrial fission. J. Cell. Mol. Med. 2019, 23, 798–810. [Google Scholar] [CrossRef] [PubMed]
- Calabriso, N.; Gnoni, A.; Stanca, E.; Cavallo, A.; Damiano, F.; Siculella, L.; Carluccio, M.A. Hydroxytyrosol Ameliorates Endothelial Function under Inflammatory Conditions by Preventing Mitochondrial Dysfunction. Oxid. Med. Cell. Longev. 2018, 2018, 9086947. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Lehrke, M.; Marx, N. Diabetes Mellitus and Heart Failure. Am. J. Cardiol. 2017, 120, S37–S47. [Google Scholar] [CrossRef]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- Henning, R.J. Type-2 diabetes mellitus and cardiovascular disease. Future Cardiol. 2018, 14, 491–509. [Google Scholar] [CrossRef]
- Htay, T.; Soe, K.; Lopez-Perez, A.; Doan, A.H.; Romagosa, M.A.; Aung, K. Mortality and Cardiovascular Disease in Type 1 and Type 2 Diabetes. Curr. Cardiol. Rep. 2019, 21, 45. [Google Scholar] [CrossRef]
- Petak, S.; Sardhu, A. The Intersection of Diabetes and Cardiovascular Disease. Methodist Debakey Cardiovasc. J. 2018, 14, 249–250. [Google Scholar]
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. Pharmacol. 2015, 70, 5.47.1–5.47.20. [Google Scholar] [CrossRef]
- Nath, S.; Ghosh, S.K.; Choudhury, Y. A murine model of type 2 diabetes mellitus developed using a combination of high fat diet and multiple low doses of streptozotocin treatment mimics the metabolic characteristics of type 2 diabetes mellitus in humans. J. Pharmacol. Toxicol. Methods 2017, 84, 20–30. [Google Scholar] [CrossRef]
- Glastras, S.J.; Chen, H.; Teh, R.; McGrath, R.T.; Chen, J.; Pollock, C.A.; Wong, M.G.; Saad, S. Mouse Models of Diabetes, Obesity and Related Kidney Disease. PLoS ONE 2016, 11, e0162131. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhao, X.; Wan, J.; Ran, L.; Qin, Y.; Wang, X.; Gao, Y.; Shu, F.; Zhang, Y.; Liu, P.; et al. Dihydromyricetin improves glucose and lipid metabolism and exerts anti-inflammatory effects in nonalcoholic fatty liver disease: A randomized controlled trial. Pharmacol. Res. 2015, 99, 74–81. [Google Scholar] [CrossRef]
- He, J.; Zhang, J.; Dong, L.; Dang, X.; Wang, L.; Cheng, L.; Huang, Y. Dihydromyricetin Attenuates Metabolic Syndrome And Improves Insulin Sensitivity By Upregulating Insulin Receptor Substrate-1 (Y612) Tyrosine Phosphorylation In db/db Mice. Diabetes Metab. Syndr. Obes. 2019, 12, 2237–2249. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wan, J.; Lang, H.; Si, M.; Zhu, J.; Zhou, Y.; Mi, M. Dihydromyricetin delays the onset of hyperglycemia and ameliorates insulin resistance without excessive weight gain in Zucker diabetic fatty rats. Mol. Cell. Endocrinol. 2017, 439, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.T.; Zeng, Y.; Tang, K.; Chen, X.; Zhang, W.; Xu, X.L. Dihydromyricetin ameliorates atherosclerosis in LDL receptor deficient mice. Atherosclerosis 2017, 262, 39–50. [Google Scholar] [CrossRef]
- Yang, D.; Yang, Z.; Chen, L.; Kuang, D.; Zou, Y.; Li, J.; Deng, X.; Luo, S.; Luo, J.; He, J.; et al. Dihydromyricetin increases endothelial nitric oxide production and inhibits atherosclerosis through microRNA-21 in apolipoprotein E-deficient mice. J. Cell. Mol. Med. 2020, 24, 5911–5925. [Google Scholar] [CrossRef]
- Huang, B.; Li, Y.; Yao, Y.; Shu, W.; Chen, M. Dihydromyricetin from ampelopsis grossedentata protects against vascular neointimal formation via induction of TR3. Eur. J. Pharmacol. 2018, 838, 23–31. [Google Scholar] [CrossRef]
- Yang, D.; Tan, S.; Yang, Z.; Jiang, P.; Qin, C.; Yuan, Q.; Dang, R.; Yao, X.; Qu, J.; Lu, Q.; et al. Dihydromyricetin Attenuates TNF-alpha-Induced Endothelial Dysfunction through miR-21-Mediated DDAH1/ADMA/NO Signal Pathway. Biomed. Res. Int. 2018, 2018, 1047810. [Google Scholar]
- Ling, H.; Zhu, Z.; Yang, J.; He, J.; Yang, S.; Wu, D.; Feng, S.; Liao, D. Dihydromyricetin improves type 2 diabetes-induced cognitive impairment via suppressing oxidative stress and enhancing brain-derived neurotrophic factor-mediated neuroprotection in mice. Acta Biochim. Biophys. Sin. (Shanghai) 2018, 50, 298–306. [Google Scholar] [CrossRef]
- Song, Q.; Liu, L.; Yu, J.; Zhang, J.; Xu, M.; Sun, L.; Luo, H.; Feng, Z.; Meng, G. Dihydromyricetin attenuated Ang II induced cardiac fibroblasts proliferation related to inhibitory of oxidative stress. Eur. J. Pharmacol. 2017, 807, 159–167. [Google Scholar] [CrossRef]
- Liu, X.; Cao, K.; Lv, W.; Feng, Z.; Liu, J.; Gao, J.; Li, H.; Zang, W.; Liu, J. Punicalagin attenuates endothelial dysfunction by activating FoxO1, a pivotal regulating switch of mitochondrial biogenesis. Free Radic. Biol. Med. 2019, 135, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.S.; Hernandez-Cuervo, H.; Fukumoto, J.; Narala, V.R.; Saji, S.; Borra, M.; Alleyn, M.; Lin, M.; Soundararajan, R.; Lockey, R.; et al. Alda-1 attenuates hyperoxia-induced mitochondrial dysfunction in lung vascular endothelial cells. Aging (Albany NY) 2019, 11, 3909–3918. [Google Scholar] [CrossRef]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.S.; Kabir, M.T.; Jakaria, M.; Mamun, A.A.; Niaz, K.; Amran, M.S.; Barreto, G.E.; Ashraf, G.M. Endothelial PPARgamma Is Crucial for Averting Age-Related Vascular Dysfunction by Stalling Oxidative Stress and ROCK. Neurotox. Res. 2019, 36, 583–601. [Google Scholar] [CrossRef] [PubMed]
- Lau, Y.S.; Ling, W.C.; Murugan, D.; Mustafa, M.R. Boldine Ameliorates Vascular Oxidative Stress and Endothelial Dysfunction: Therapeutic Implication for Hypertension and Diabetes. J. Cardiovasc. Pharmacol. 2015, 65, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.R.; Jacobs, J.S.; Li, Q.; Gaddam, R.R.; Vikram, A.; Liu, J.; Kassan, M.; Irani, K.; Kumar, S. SUMO2 regulates vascular endothelial function and oxidative stress in mice. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H1292–H1300. [Google Scholar] [CrossRef]
- Tucker, M.A.; Fox, B.M.; Seigler, N.; Rodriguez-Miguelez, P.; Looney, J.; Thomas, J.; McKie, K.T.; Forseen, C.; Davison, G.W.; Harris, R.A. Endothelial Dysfunction in Cystic Fibrosis: Role of Oxidative Stress. Oxid. Med. Cell. Longev. 2019, 2019, 1629638. [Google Scholar] [CrossRef]
- Odegaard, A.O.; Jacobs, D.R., Jr.; Sanchez, O.A.; Goff, D.C., Jr.; Reiner, A.P.; Gross, M.D. Oxidative stress, inflammation, endothelial dysfunction and incidence of type 2 diabetes. Cardiovasc. Diabetol. 2016, 15, 51. [Google Scholar] [CrossRef]
- Liu, S.; Ai, Q.; Feng, K.; Li, Y.; Liu, X. The cardioprotective effect of dihydromyricetin prevents ischemia-reperfusion-induced apoptosis in vivo and in vitro via the PI3K/Akt and HIF-1alpha signaling pathways. Apoptosis 2016, 21, 1366–1385. [Google Scholar] [CrossRef]
- Wei, L.; Sun, X.; Qi, X.; Zhang, Y.; Li, Y.; Xu, Y. Dihydromyricetin Ameliorates Cardiac Ischemia/Reperfusion Injury through Sirt3 Activation. Biomed. Res. Int. 2019, 2019, 6803943. [Google Scholar] [CrossRef]
- Wang, J.; Wang, K.; Huang, C.; Lin, D.; Zhou, Y.; Wu, Y.; Tian, N.; Fan, P.; Pan, X.; Xu, D.; et al. SIRT3 Activation by Dihydromyricetin Suppresses Chondrocytes Degeneration via Maintaining Mitochondrial Homeostasis. Int. J. Biol. Sci. 2018, 14, 1873–1882. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Yang, J.; Hu, O.; Huang, J.; Ran, L.; Chen, M.; Zhang, Y.; Zhou, X.; Zhu, J.; Zhang, Q.; et al. Dihydromyricetin Ameliorates Nonalcoholic Fatty Liver Disease by Improving Mitochondrial Respiratory Capacity and Redox Homeostasis Through Modulation of SIRT3 Signaling. Antioxid. Redox. Signal. 2019, 30, 163–183. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hua, Y.-Y.; Zhang, Y.; Gong, W.-W.; Ding, Y.; Shen, J.-R.; Li, H.; Chen, Y.; Meng, G.-L. Dihydromyricetin Improves Endothelial Dysfunction in Diabetic Mice via Oxidative Stress Inhibition in a SIRT3-Dependent Manner. Int. J. Mol. Sci. 2020, 21, 6699. https://doi.org/10.3390/ijms21186699
Hua Y-Y, Zhang Y, Gong W-W, Ding Y, Shen J-R, Li H, Chen Y, Meng G-L. Dihydromyricetin Improves Endothelial Dysfunction in Diabetic Mice via Oxidative Stress Inhibition in a SIRT3-Dependent Manner. International Journal of Molecular Sciences. 2020; 21(18):6699. https://doi.org/10.3390/ijms21186699
Chicago/Turabian StyleHua, Yu-Yun, Yue Zhang, Wei-Wei Gong, Yue Ding, Jie-Ru Shen, Hua Li, Yun Chen, and Guo-Liang Meng. 2020. "Dihydromyricetin Improves Endothelial Dysfunction in Diabetic Mice via Oxidative Stress Inhibition in a SIRT3-Dependent Manner" International Journal of Molecular Sciences 21, no. 18: 6699. https://doi.org/10.3390/ijms21186699
APA StyleHua, Y.-Y., Zhang, Y., Gong, W.-W., Ding, Y., Shen, J.-R., Li, H., Chen, Y., & Meng, G.-L. (2020). Dihydromyricetin Improves Endothelial Dysfunction in Diabetic Mice via Oxidative Stress Inhibition in a SIRT3-Dependent Manner. International Journal of Molecular Sciences, 21(18), 6699. https://doi.org/10.3390/ijms21186699




