Abstract
High-energy and high-atom-number (HZE) space radiation poses an inevitable potential threat to astronauts on deep space exploration missions. Compared with low-LET radiation, high-energy and high-LET radiation in space is more efficient in inducing clustered DNA damage with more serious biological consequences, such as carcinogenesis, central nervous system injury and degenerative disease. Space radiation also causes epigenetic changes in addition to inducing damage at the DNA level. Considering the important roles of microRNAs in the regulation of biological responses of radiation, we systematically reviewed both expression profiling and functional studies relating to microRNAs responding to space radiation as well as to space compound environment. Finally, the directions for improvement of the research related to microRNAs responding to space radiation are proposed. A better understanding of the functions and underlying mechanisms of the microRNAs responding to space radiation is of significance to both space radiation risk assessment and therapy development for lesions caused by space radiation.
1. MicroRNAs
MicroRNAs (miRNAs) are a class of small single-stranded endogenous noncoding RNAs of 18-24 nucleotides at length, which are identified in various organisms, including mammals, plants, and many microorganisms. They are transcribed from the genomes by RNA polymerase II or RNA polymerase III, capable of suppressing the expression of a large number of genes by binding to the 3′-untranslated region (3′-UTR) of their transcripts [1].
Early studies have found that most of miRNA genes are located in the intergenic region 1 kb away from the annotated genes, while some of miRNA genes are located in the introns of known genes and share the transcriptional elements with their host genes. It is reported that about 50% of miRNA gene is located closely to other miRNAs as a cluster [2,3]. Most miRNA genes have their own promoters and the clustered miRNAs come from the polycistronic transcripts [4,5]. Most of miRNAs are transcribed from the genomes by RNA polymerase II or RNA polymerase III under strict regulation [4]. It was found by Bradley that 70% of the mammalian miRNA genes were located in a specific transcription unit, and 117 of 232 miRNAs were located in the introns, of which 90 were located in the introns of protein-encoding genes, and 27 were located in non-coding RNAs in the introns. Thus, miRNAs can be divided into three types according to their location: the exonic miRNAs located in the non-coding transcription units, the intronic miRNAs located in the non-coding transcription units, and the intronic miRNAs in the protein encoding units [6].
The primary transcription product of a miRNA gene is primary miRNA (pri-miRNA), which is thousands of nucleotides long and contains a hairpin structure. Once the primary miRNA is produced, its 5′ end is added with a methylation hat while the 3′ end is polyadenylated [7]. The modified primary miRNA is first cut by the nuclear ribonuclease III (RNase III) Drosha in the nucleus to produce about 70 nucleotides long precursor miRNA molecules (precursor miRNA, pre-miRNA) [8]. It is generally believed that the residual flanking sequence will be degraded in the nucleus, but its specific function remains to be clarified. Drosha is a conserved protein of 160 kDa size containing two series of RNase III domains (RIIIDs) and a double stranded RNA binding domain (dsRBD) [9], which in the human body associates with DiGeorge syndrome critical region gene 8 (DGCR8) to form a microprocessor complex of 650 kDa [10]. At present, it is believed that DGCR8 can assist Drosha to identify the substrate, and the determinant of the specificity for substrate recognition is the structure of pri-miRNA [11,12,13].
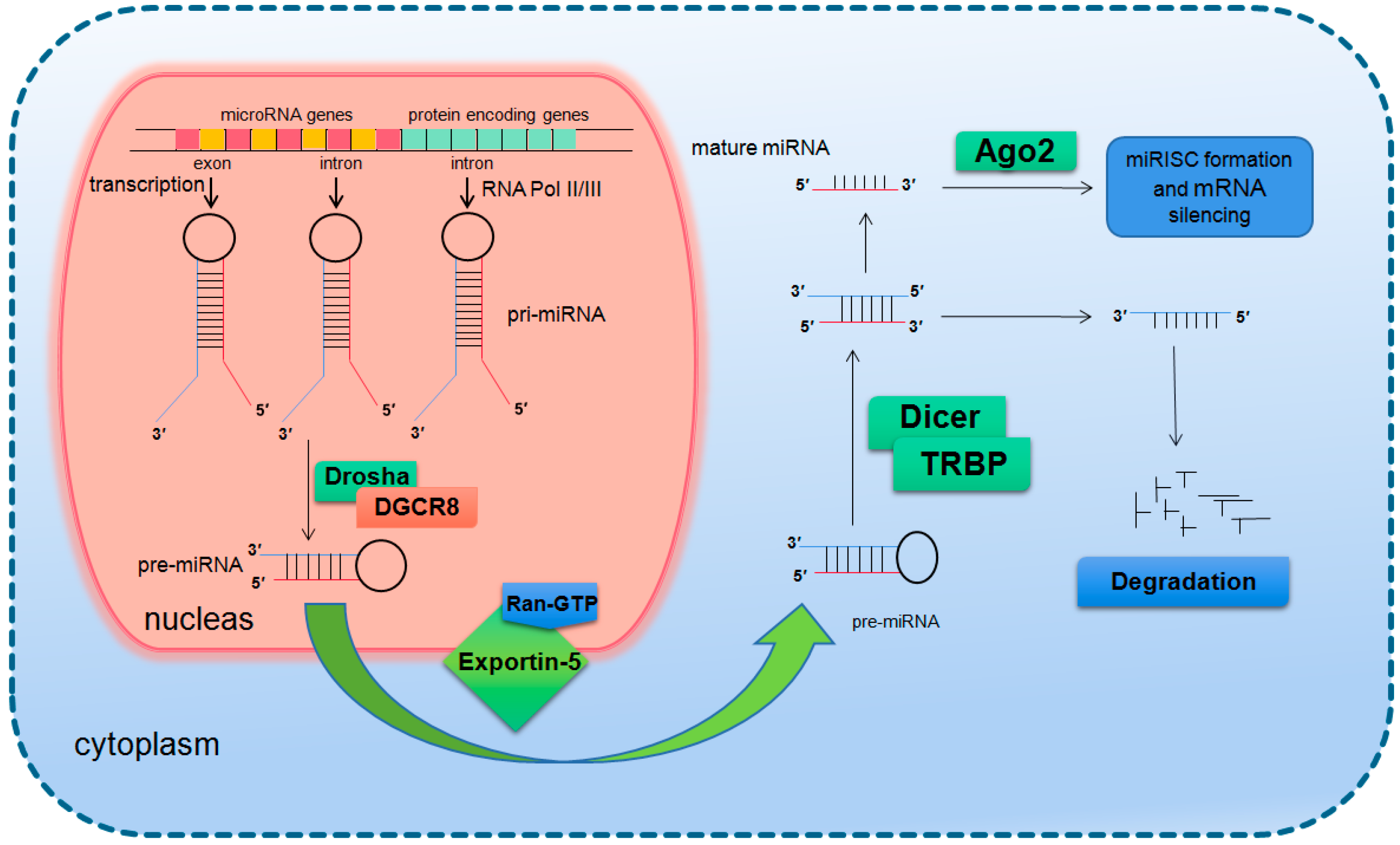
In mammals, the two-step splicing of pri-miRNA occurs in the nucleus and cytoplasm (Figure 1). The nuclear export of a pre-miRNA is mediated by nuclear transport receptor exportin-5. It combines pre-miRNA and co-factor Ran-GTP in the nucleus. Once exported, GTP is hydrolyzed to GDP, resulting in the release of pre-miRNA from the transport complex. When exportin-5 is knocked-down, the amounts of both pre-miRNA and mature miRNA in the cytoplasm decrease, but the pre-miRNA in the nucleus does not accumulate, which indicates that pre-miRNA is extremely unstable in the nucleus or gets stabilized by binding with exportin-5 [14,15]. The homologues of Drosha and DGCR8/Pasha were not found in plants and yeast. The study in Arabidopsis found the nucleoprotein Dcl1 was one of the Dicer-like proteins and was involved in processing miRNA [16,17].
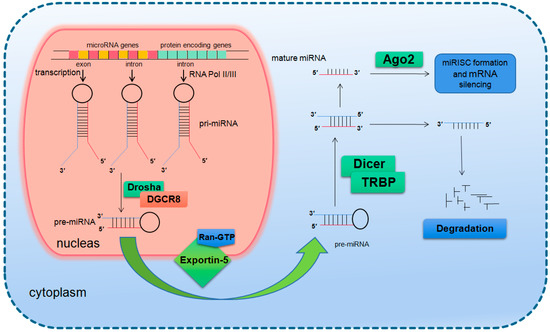
Figure 1.
Basic miRNA processing pathway.
After being exported to the cytoplasm, the pre-miRNA is further spliced by RNase III nuclease Dicer to generate ~22 nt double stranded RNA molecules. It is reported that the knockout of Dicer in Caenorhabditis elegans (C. elegans) causes the accumulation of pre-miRNA and the decrease of mature miRNA [18,19], providing direct evidence for Dicer as a RNA enzyme in the maturation process of miRNA. Dicer is about 200 kDa of the multi-domain protein, which contains two RIIIDs domains and a dsRBD domain like Drosha, and the difference is that Dicer contains a long N short sequence, including a DEAD-BOX RNA helicase domain, a DUF283 domain and a PAZ domain. The PAZ domain is also found in the Argonaute family of proteins, which specifically binds to the 3′ terminal of small RNA molecules [20,21]. There is only one kind of Dicer in mammals and nematodes, which plays a key role in the biogenesis of siRNA and miRNA [22,23]. The miRNA dimer is subsequently integrated into the complex called miRNP (ribonucleoprotein complex containing miRNA) or miRISC (miRNA-induced silencing complex). The life span of miRNA dimer is short. Once combined with Ago protein, the double strands are quickly unraveled, a chain is stabilized and the other is lost. The retention or degradation of the chain depends on its thermodynamic stability [24,25,26]. The mammalian Dicer/Ago/miRNA complex also interacts with a number of other proteins, such as Gemin3, Gemin4, Mov10, and GW182 [27,28,29,30].
Since the first miRNA Lin-4 was identified in nematode in 1993 as a development regulation gene, the number of miRNAs found in all species has reached more than 38,000 and the number of human miRNAs also exceeded 2600 (http://www.mirbase.org/). It is presumed that more than 60% of the human genes are regulated by miRNAs [31,32]. Precious studies suggest that miRNAs regulate gene expression through translational inhibition and/or degradation of target mRNAs [33,34]. There is no final conclusion on which mechanism will play a major role. It is speculated that the number, type and location of mismatch in miRNA/mRNA dimers as well as the cell type determines the triggering of degradation or translation inhibition [35,36]. A miRNA interacts with its target genes to form a regulatory network, which acts in almost all life processes. It has been shown by many studies that miRNAs are a key group of important modulators in biological processes and play important roles in development [37,38], organogenesis [39,40], cell proliferation and differentiation [41], cell cycle regulation [41,42], cell apoptosis [43,44,45], aging [46,47,48], pathogenesis [49,50], cellular response to stresses [51,52,53,54], etc.
As a key modulator in radiation response of human and other organisms, miRNAs have been found to respond to various radiation (both ionizing radiation (IR) and non-ionizing radiation) as well as to regulate a lot of genes involved in the cellular radiation response [55,56,57]. As a key radiation type confronted by the astronauts in the manned spaceflight missions, high-energy and high-Z (HZE) particles-related biological effects and the underlying mechanisms have attracted much attention from space radiobiologists. In particular research on HZE particle radiation-induced miRNAs and their roles in the regulation of the corresponding biological effects has made great progress.
2. Space Radiation and the Health Effects
With the deepening of space exploration, the duration of astronauts’ permanence in space will become longer and longer. The safety evaluation of space explorations beyond the low Earth orbit (LEO) inevitably is one of the most important topics in space science since the radiation levels exceed those routinely received by terrestrial radiation workers, or astronauts in near-Earth orbits such as the International Space Station (ISS). Because of the lack of atmosphere and magnetic field and thereby important shielding properties the only shielding measure is the spacecraft bulkhead and high energy space radiation is an unavoidable danger for astronauts in manned space missions.
Basically, there are three main sources of space radiation: (1) Trapped low Earth orbit (LEO) radiation, in which the main components are protons and electrons with relatively low energy. (2) Incidental and high dose radiation from solar particle events (SPEs). Solar flares emit a large number of high-energy protons, and a small amount of α particles and heavy ions. (3) Low dose rate galactic cosmic rays (GCR). The protons account for about 85%, followed by 14% α particles and 1% of heavy ions. Although low in dose and dose rate, GCR is highly energetic and powerfully penetrating. There are no shielding materials capable of blocking it completely. Exposure of astronauts to GCR causes clustered DNA damages that are difficult to repair, which are strongly lethal, mutagenic and carcinogenic [58,59,60]. Heavy ions are high in ionization density (linear energy transfer, LET), although their abundance is low [61,62,63]. In addition to the abovementioned radiation, secondary particles produced by these primary particles through the shielding materials form a secondary radiation environment, including photons, electrons, protons and neutrons, which can also lead to biological effects such as DNA damage, gene mutation, cell transformation, and other biological effects [64].
The human experience in space flights is only about six decades old, inaugurated by the first complete Earth orbit flight by Yuri Gagarin, and currently continuing on the International Space Station. To date, manned missions have been limited to near-Earth orbits, with the moon as our farthest destination from Earth. Historical space radiation career exposures for astronauts from all NASA missions through December 1999 (including early Mercury, Gemini, STS, and Apollo Missions) involved total exposures of less than about 20 mSv [65]. With the advent of Skylab and MIR, total career exposure levels increased to a maximum of nearly 200 mSv. It is estimated that astronauts will be exposed to 1.84 ± 0.30 mSv/day of GCR in interplanetary space and 0.64 ± 0.12 mSv/day of GCR on the Mars surface, amounting to a total mission dose equivalent of ~1.01 Sv for a round trip to Mars with 180 days (each way) cruise, and 500 days stay on the Mars surface for a particular solar cycle [66]. Besides, radiation tissue equivalent doses deposited in blood-forming organs when encountering a large SPE may reach 1.93 Sv even behind 5 g/cm2 Al shielding [67]. Missions into deep space, due to the requisite longer duration of the planned missions, may pose greater risks due to the increased potential for exposure to complex radiation fields comprised of a broad range of radiation types and energies from cosmic and unpredictable solar sources. Ionizing radiation prevalent in space such as protons, carbon, argon and iron ions covers both a broad range of energies and a highly diverse range of radiation quality. LETs of these particles range from <10 to >200 keV/µm. Protons are more prevalent with relatively low LET values, whereas the iron ions are relatively rare, but with high LET values. Dose rate measured in LEO is of the order of 1 mSv/day, but will be higher on missions to Mars as mentioned above. It is an enormous and complex task to assess the biological and clinical effects of all possible space radiation scenarios. Reminders of the presence of low-flux particle radiation fields have graphically been visualized in light-flash phenomena experienced by many space travelers [68]. Evidence exists from the accelerator-based human exposures with muons [69], pions [70], helium ions [71], carbon ions [72], and nitrogen ions [73]. Visual phenomena have also been noted by human subjects on exposure to neutrons of various energies. Several human subjects saw a multitude of bright colorless flashes on exposure to neutrons, which were described as “a bunch of stars moving or blinking” and are similar to light flashes and streaks seen by astronauts on translunar flight. These phenomena are caused by interaction with retinal rods by proton recoils and by α particles released from neutron reactions with carbon and oxygen [74,75,76]. However, space radiation exposure influences multiple organs and physiological systems in complicated ways. NASA has classified the biomedical consequences into four risk areas [77]: (1) Degenerative tissue effects from radiation exposure, e.g., cardiovascular disease, cataract formation, and premature aging; (2) Radiation-induced carcinogenesis; (3) CNS (central nervous system) injury caused by radiation exposure, leading to deficits in cognitive and executive function, inducing fatigue, and degrading crew performance; (4) Radiation syndromes caused by SPE. The high doses of radiations from large SPEs induce acute radiation syndrome effects, such as nausea, emesis, haemorrhaging, or, possibly, even death. However, the underlying molecular mechanisms are still under considerations.
3. MiRNAs Involved in the Biological Responses to Space Radiation
Up to now, a number of studies have found that the biological responses to space radiation are related to miRNAs, as demonstrated in Table 1. Khan et al. examined the miRNA expression in selected mouse organs (testis, brain, and liver) exposed to whole-body proton irradiation (2 Gy). By bioinformatics analysis they revealed dysregulation of 14 miRNAs in mouse testis, 8 in liver, and 8 in brain and a possible mechanism of proton particle involvement in the onset of tumorigenesis [78]. Templin et al. studied the expression changes of miRNA derived from mouse blood using quantitative real-time polymerase chain reaction (qRT-PCR) in mice exposed to 600 MeV protons at doses of 0.5 or 1.0 Gy. They found 26 miRNAs were differentially expressed and mouse blood miRNA signatures are radiation type- and dose-dependent [79].

Table 1.
Studies relating to miRNAs responding to space radiation.
Besides the involvement of miRNAs in response to proton radiation, they were also found to participate in the biological responses to particles of higher LET, such as α particle and heavy ions. Kovalchuk et al. analyzed microRNAome changes in bystander tissues after 5.4 Gy α-particle microbeam irradiation of 3-D artificial human tissues using miRNA microarrays and found that miRNAs play a profound role in the manifestation of late radiation-induced bystander effect (RIBE) end points [80]. Chauhan et al. studied the miRNA expression patterns in three human cell lines (A549, THP-1 and HFL) exposed to 0.5 Gy, 1.0 Gy and 1.5 Gy of α-particles at a low dose-rate (0.98 ± 0.01 Gy/h), and found cell-specific responses of 13 miRNAs. Besides, bioinformatics analysis suggested the α-particle induced miRNA mapped to target genes related to ribosomal assembly, lung carcinoma development, TGF-β signaling, cell communication and keratin sulfate [81]. Nie et al. studied the malignant transformation of immortalized human bronchial epithelial cells (BEAS-2B) irradiated by 0.25 Gy α-particles using miRNA-mRNA networks. Sixty-eight miRNAs were found to be dysregulated, among which miR-107 and miR-494 were predicted to play a role in α-particles-mediated cellular malignant transformation processes by bioinformatics analysis [82].
Templin et al. also compared the miRNA expression signatures in peripheral blood of mice exposed to either γ-rays or 56Fe ions and found that miRNA expression signatures were radiation type-specific and dose- and time-dependent [83]. He et al. investigated the toxicity in testis of mice following enterocoelia irradiation with 2 Gy carbon ions by miRNA sequencing and bioinformatics analyses. Differentially expressed miRNAs were found to be involved in the regulation of metabolism, development, and reproduction [84]. Wei et al. analyzed miRNA expression profiles with miRNA PCR arrays at 24 h post heavy ion irradiation, and developed a universal model to predict the degree of exposure to different radiation types with high sensitivity and specificity based on five miRNAs (miR-183-5p, miR-9-3p, miR-200b-5p, miR-342-3p and miR-574-5p) that showed a significant response to 0.1–2 Gy of carbon-ion, iron-ion or X-rays [85]. In another study, this team exposed Kunming mice to different doses of carbon ions and X-rays and found two miRNAs (let-7a-5p, miR-200b-5p) in the serum of irradiated mice were up-regulated significantly and exhibited dose- (0.1~2 Gy) and time-dependence (6~72 h), which may serve as potential noninvasive indicators for space radiation [86].
In addition to the characteristic of being rich in high LET radiation, the effects of space radiation on astronauts are affected by a variety of other space environmental factors, such as microgravity, weak magnetic field, and short circadian rhythm. As shown in Table 2, several studies have been carried out to identify the miRNAs participating in the regulation of compound effect of space radiation and other space environment factors. Researchers from Padua University analyzed miRNA expression profile in human peripheral blood lymphocytes (PBL) incubated for 4 and 24 h in normal gravity (1 g) and in modeled microgravity (MMG) during the repair time after irradiation with 0.2 or 2 Gy of γ-rays. They found that MMG altered miRNA expression signature of irradiated PBL by decreasing the number of radio-responsive miRNAs. Integrated analyses of transcriptome and microRNAome suggested that modeled microgravity affected the DNA-damage response to irradiation [87]. Fu et al. analyzed RNA expression profiles in human lymphoblastoid TK6 cells incubated for 24 h under static or stimulated microgravity after 2 Gy γ-ray irradiation. Although no differentially-expressed miRNAs were identified under either simulated microgravity or irradiation conditions, both miR-15b and miR-221 were found to be differentially expressed under compound conditions with an interactive effect [88].

Table 2.
Studies relating to miRNAs responding to space compound environment.
Furthermore, some studies were also carried out to gain insight into the effects of spaceflight on miRNA expression profile. Some researchers from Dr. Yeqing Sun’s group explored the miRNA expression profile changes in space-flown C. elegans larvae that experienced the 16.5-day shuttle spaceflight on Shenzhou-8 spacecraft. 23 miRNAs were found to express differentially in spaceflight groups compared with the ground control group and were predicted to be involved in the regulation of developmental processes, growth and body morphogenesis, DNA damage response as well as biological behavioural responses [89,90]. Gao et al. from the same team also investigated miRNAome and mRNA expression in the ced-1 C. elegans mutant vs. the wild-type strain, both of which underwent spaceflight, spaceflight 1g-centrifuge control and ground control conditions during the Shenzhou-8 mission, and found differential miRNA expression increased from 43 (ground control condition) to 57 and 91 in spaceflight and spaceflight control conditions, respectively, suggesting several miRNAs responding to space radiation were deregulated by microgravity, which is consistent with the finding of Girardi’s group. Some of differentially expressed miRNAs were predicted to regulate apoptosis, neurogenesis larval development and ATP metabolism [91].
Considering the above studies, we can see that for the same cell or tissue type, the miRNA expression profile is radiation type-, dose- and time-dependent. The miRNA expression profiles are also significantly different in varying cell or tissue types even though exposed to the same radiation of the same dose. This suggests that we should consider radiation type, dose, biological samples, as well as sample collection time comprehensively when designing space radiation-related miRNA experiments. In addition, we note that both miR-150-5p and miR-342-3p are down-regulated in blood samples of different mouse models (C57BL/6 or Kunming mice) at 24 h after exposed to different radiation (0.5 Gy iron or carbon ions) [80,82], implying their important regulatory roles in biological effects of heavy ion radiation. Although a large number of miRNAs have been identified to be involved in the responses to space radiation, few functional experiments were carried out. Zhu et al. found that high LET IR promoted miR-21 expression through the EGFR/STAT3 pathway and miR-21 played an important role in high LET IR-induced carcinogenesis [92,93]. Zhang et al. found miR-21 promoted formation of high LET (Fe ions) irradiation-induced reactive oxygen species (ROS) by targeting both SOD3 and TNFα and contributed to an elevation of IR-induced cell transformation [94]. Wang et al. found that radiation didn’t induce any lung tumorigenesis in miR-21–/– mice while miR-21 knock-in mice showed high spontaneous lung tumor incidences. By further analysis, miR-21 was found to be upregulated by oxygen, silicon and iron ion irradiation and contribute to radiation-induced lung tumorigenesis, whereas its level in serum could be used as a biomarker for predicting high-LET radiation-induced lung tumorigenesis [95]. Kim et al. found high energy proton irradiation induced down-regulation of miR-31-5p in mouse serum while its inhibition protected human colonic epithelial cells against ionizing radiation in a hMLH1-dependent manner [96]. However, most studies involving space radiation-related miRNAs are limited to description of radiation-induced changes of miRNA profiles, the functions of the differentially expressed miRNAs and the underlying mechanisms in space radiation-induced biological effects are still not revealed.
4. Future Challenges
Increasing evidence shows that miRNAs function as significant molecules in the regulation of the response of living organisms to various kinds of space radiation. Based on the findings mentioned above, several specific miRNAs from varying kinds of exposed tissues have been associated with the space radiation response. Besides, it has been reported that serum miRNAs can be used as biomarkers in early stages after exposure to assess space radiation damage, and that five miRNAs (miR-183-5p, miR-9-3p, miR-200b-5, miR-342-3p, and miR-574-5p) present dose- and time-dependent changes upon different types of radiation exposure [85]. Thus, analyzing the expression profiling of miRNAs is expected to be conducive to assess risk of space radiation.
Space radiation cause expressional changes of different types of miRNAs, contributing to the change of the corresponding mRNAs, related genes and other regulatory factors, which pose a significant impact on various biological processes of an organism. Therefore, exploring the biological functions and the underlying action mechanisms of the differentially expressed miRNA is of great significance to the prediction of the medical results caused by space radiation. However, the biological importance of the miRNAs responding to space radiation is incompletely understood. In fact, most of the currently reported space radiation-related miRNA research is limited to the description of the expressional patterns of miRNAs (up- or down-regulation). The exact contribution of the differentially expressed miRNAs to the specific health effects of the space radiation is required to be deciphered for applying them for space radiation risk prediction and possible therapy of the lesions caused by space radiation, which depends on the target identification and tissue-specific functional analysis. Besides the focus on miRNA profiling in response of the organisms to space radiation, more attention should be paid to the profiling of mRNAs and proteins responding to space radiation at the same time since miRNAs play regulatory roles through translation inhibition or mRNA degradation of the target genes. Further, recent studies have shown miRNA is involved in competing endogenous RNA (ceRNA) regulatory network with long non-coding RNA (lncRNA) or circular RNA (circRNA). Taking these issues into account, new approaches in genomics, transcriptomics, proteomics, metabolomics and epigenomics should also be employed in the mechanistic study, which will help to elucidate the effects of space radiation and to develop personalized countermeasures more precisely.
Due to the nature and characteristics of the space radiation, space radiation-related miRNA studies should be improved in the following two aspects. First, more attention should be paid to the miRNAs involved in the space radiation-induce bystander effect. Considering the low dose/dose rate and low fluence characteristics of space radiation, most cells of the exposed living organisms will not be hit by the space high energy particles, thus the radiation-induce bystander effect plays a significant role in the space radiation-induced biological effects in this situation [97]. It has been found that several miRNAs play important roles in radiation-induced bystander effects [98,99], however, there is no reports about miRNAs functioning in the space radiation-induced bystander effects so far. Second, more studies should be performed to identify miRNAs participating in the regulation of compound effect of space radiation and other space environmental factors, such as microgravity, weak magnetic field, and short circadian rhythm. The interactive effects between the mixed radiation as well as space radiation and other space environmental factors have been observed in a number of studies [100,101,102,103]. A few researchers probed into the miRNA profiling changes in response to ionizing radiation and microgravity [88], while there is still lack of studies with all factor simulation of space environment, which is dependent on the building up of ground-based all factor space radiation simulation platform. As for the miRNA study relating to mixed space radiation, there are no reports so far. In conclusion, space radiation-related miRNA study has made significant achievements and is showing tremendous research values. However, there still is a lot of work to do for space radiobiologists all around the world before a better understanding of both functions and mechanisms of the space radiation-responding miRNAs could be obtained as well as these space radiation-responding miRNAs could be used as bona fide biomarkers for space radiation risk assessment.
Author Contributions
Conceptualization, W.H. and G.Z.; Writing—Original Draft Preparation, Y.Y., K.Z. and W.H.; Writing—Review and Editing, W.H. and G.Z.; Visualization, Y.Y. and W.H.; Supervision, G.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key R&D Program of China through MOST (Ministry of Science and Technology) (nos. 2018YFC0115704 and 2018YFC0115705), Collaborative Innovation Center of Radiological Medicine of Jiangsu Higher Education Institutions, and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bartel, D.P. MicroRNAs: Target Recognition and Regulatory Functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Lau, N.C.; Lim, L.P.; Weinstein, E.G.; Bartel, D.P. An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science 2001, 294, 858–862. [Google Scholar] [CrossRef] [PubMed]
- Mourelatos, Z.; Dostie, J.; Paushkin, S.; Sharma, A.; Charroux, B.; Abel, L.; Rappsilber, J.; Mann, M.; Dreyfuss, G. miRNPs: A novel class of ribonucleoproteins containing numerous microRNAs. Genes Dev. 2002, 16, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Hagedorn, C.H.; Cullen, B.R. Human microRNAs are processed from capped, polyadenylated transcripts that can also function as mRNAs. RNA 2004, 10, 1957–1966. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, M.; Han, J.; Yeom, K.H.; Lee, S.; Baek, S.H.; Kim, V.N. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004, 23, 4051–4060. [Google Scholar] [CrossRef]
- Rodriguez, R.E.; Mecchia, M.A.; Debernardi, J.M.; Schommer, C.; Weigel, D.; Palatnik, J.F. Control of cell proliferation in Arabidopsis thaliana by microRNA miR396. Development 2010, 137, 103–112. [Google Scholar] [CrossRef]
- Kim, V.N. MicroRNA biogenesis: Coordinated cropping and dicing. Nat. Rev. Mol. Cell. Biol. 2005, 6, 376–385. [Google Scholar] [CrossRef]
- Kim, S.; Song, M.L.; Min, H.; Hwang, I.; Baek, S.K.; Kwon, T.K.; Park, J.W. miRNA biogenesis-associated RNase III nucleases Drosha and Dicer are upregulated in colorectal adenocarcinoma. Oncol. Lett. 2017, 14, 4379–4383. [Google Scholar] [CrossRef]
- Pong, S.K.; Gullerova, M. Noncanonical functions of microRNA pathway enzymes—Drosha, DGCR8, Dicer and Ago proteins. FEBS Lett. 2018, 592, 2973–2986. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Park, J.; Dang, T.L.; Choi, Y.G.; Kim, V.N. Microprocessor depends on hemin to recognize the apical loop of primary microRNA. Nucleic Acids Res. 2018, 46, 5726–5736. [Google Scholar] [CrossRef]
- Spadotto, V.; Giambruno, R.; Massignani, E.; Mihailovich, M.; Maniaci, M.; Patuzzo, F.; Ghini, F.; Nicassio, F.; Bonaldi, T. PRMT1-mediated methylation of the microprocessor-associated proteins regulates microRNA biogenesis. Nucleic Acids Res. 2020, 48, 96–115. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, C.E.; Godfrey, J.D.; Shibakawa, A.; Bushell, M.; Bevan, C.L. A novel role for GSK3beta as a modulator of Drosha microprocessor activity and MicroRNA biogenesis. Nucleic Acids Res. 2017, 45, 2809–2828. [Google Scholar] [PubMed]
- Nguyen, T.L.; Nguyen, T.D.; Bao, S.; Li, S.; Nguyen, T.A. The internal loops in the lower stem of primary microRNA transcripts facilitate single cleavage of human Microprocessor. Nucleic Acids Res. 2020, 48, 2579–2593. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; He, J.; Pu, W.; Peng, Y. The Role of Exportin-5 in MicroRNA Biogenesis and Cancer. Genom. Proteom. Bioinform. 2018, 16, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Hessam, S.; Sand, M.; Skrygan, M.; Gambichler, T.; Bechara, F.G. Inflammation induced changes in the expression levels of components of the microRNA maturation machinery Drosha, Dicer, Drosha co-factor DGRC8 and Exportin-5 in inflammatory lesions of hidradenitis suppurativa patients. J. Dermatol. Sci. 2016, 82, 166–174. [Google Scholar] [CrossRef]
- Reis, R.S.; Eamens, A.L.; Roberts, T.H.; Waterhouse, P.M. Chimeric DCL1-Partnering Proteins Provide Insights into the MicroRNA Pathway. Front. Plant Sci. 2015, 6, 1201. [Google Scholar] [CrossRef]
- Song, L.; Han, M.H.; Lesicka, J.; Fedoroff, N. Arabidopsis primary microRNA processing proteins HYL1 and DCL1 define a nuclear body distinct from the Cajal body. Proc. Natl. Acad. Sci. USA 2007, 104, 5437–5442. [Google Scholar] [CrossRef]
- Nelson, C.; Ambros, V. Trans-splicing of the C. elegans let-7 primary transcript developmentally regulates let-7 microRNA biogenesis and let-7 family microRNA activity. Development 2019, 146, dev172031. [Google Scholar] [CrossRef]
- Zisoulis, D.G.; Kai, Z.S.; Chang, R.K.; Pasquinelli, A.E. Autoregulation of microRNA biogenesis by let-7 and Argonaute. Nature 2012, 486, 541–544. [Google Scholar] [CrossRef]
- Elkarhat, Z.; Elkhattabi, L.; Charoute, H.; Morjane, I.; Errouagui, A.; Carey, F.; Nasser, B.; Barakat, A.; Rouba, H. Identification of deleterious missense variants of human Piwi like RNA-mediated gene silencing 1 gene and their impact on PAZ domain structure, stability, flexibility and dimension: In silico analysis. J. Biomol. Struct. Dyn. 2019, 1–7. [Google Scholar] [CrossRef]
- Kandeel, M.; Kitade, Y. In silico molecular docking analysis of the human Argonaute 2 PAZ domain reveals insights into RNA interference. J. Comput.-Aided Mol. Des. 2013, 27, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, R.; Han, B.W.; Hung, J.H.; Xu, J.; Weng, Z.; Zamore, P.D. Dicer Partner Proteins Tune the Length of Mature miRNAs in Flies and Mammals. Cell 2012, 151, 912. [Google Scholar] [CrossRef] [PubMed]
- Betancur, J.G.; Tomari, Y. Dicer is dispensable for asymmetric RISC loading in mammals. RNA 2012, 18, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Garner, A.L.; Lorenz, D.A.; Sandoval, J.; Gallagher, E.E.; Kerk, S.A.; Kaur, T.; Menon, A. Tetracyclines as Inhibitors of Pre-microRNA Maturation: A Disconnection between RNA Binding and Inhibition. ACS Med. Chem. Lett. 2019, 10, 816–821. [Google Scholar] [CrossRef]
- Dallaire, P.; Tan, H.; Szulwach, K.; Ma, C.; Jin, P.; Major, F. Structural dynamics control the MicroRNA maturation pathway. Nucleic Acids Res. 2016, 44, 9956–9964. [Google Scholar] [CrossRef] [PubMed]
- Curtin, S.J.; Michno, J.M.; Campbell, B.W.; Gil-Humanes, J.; Mathioni, S.M.; Hammond, R.; Gutierrez-Gonzalez, J.J.; Donohue, R.C.; Kantar, M.B.; Eamens, A.L.; et al. MicroRNA Maturation and MicroRNA Target Gene Expression Regulation Are Severely Disrupted in Soybean dicer-like1 Double Mutants. G3 (Bethesda) 2015, 6, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Komiya, R.; Nonomura, K. Isolation and bioinformatic analyses of small RNAs interacting with germ cell-specific argonaute in rice. Methods Mol. Biol. 2014, 1093, 235–245. [Google Scholar]
- Till, S.; Lejeune, E.; Thermann, R.; Bortfeld, M.; Hothorn, M.; Enderle, D.; Heinrich, C.; Hentze, M.W.; Ladurner, A.G. A conserved motif in Argonaute-interacting proteins mediates functional interactions through the Argonaute PIWI domain. Nat. Struct. Mol. Biol. 2007, 14, 897–903. [Google Scholar] [CrossRef]
- Bridge, K.S.; Shah, K.M.; Li, Y.; Foxler, D.E.; Wong, S.C.K.; Miller, D.C.; Davidson, K.M.; Foster, J.G.; Rose, R.; Hodgkinson, M.R.; et al. Argonaute Utilization for miRNA Silencing Is Determined by Phosphorylation-Dependent Recruitment of LIM-Domain-Containing Proteins. Cell Rep. 2017, 20, 173–187. [Google Scholar] [CrossRef]
- Leung, A.K.; Sharp, P.A. Quantifying Argonaute proteins in and out of GW/P-bodies: Implications in microRNA activities. Adv. Exp. Med. Biol. 2013, 768, 165–182. [Google Scholar]
- Friedman, R.C.; Farh, K.K.; Burge, C.B.; Bartel, D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009, 19, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.P.; Lau, N.C.; Garrett-Engele, P.; Grimson, A.; Schelter, J.M.; Castle, J.; Bartel, D.P.; Linsley, P.S.; Johnson, J.M. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature 2005, 433, 769–773. [Google Scholar] [CrossRef]
- Miao, L.; Yao, H.; Li, C.; Pu, M.; Yao, X.; Yang, H.; Qi, X.; Ren, J.; Wang, Y. A dual inhibition: MicroRNA-552 suppresses both transcription and translation of cytochrome P450 2E1. Biochim. Biophys. Acta 2016, 1859, 650–662. [Google Scholar] [CrossRef] [PubMed]
- Subasic, D.; Brummer, A.; Wu, Y.; Pinto, S.M.; Imig, J.; Keller, M.; Jovanovic, M.; Lightfoot, H.L.; Nasso, S.; Goetze, S.; et al. Cooperative target mRNA destabilization and translation inhibition by miR-58 microRNA family in C. elegans. Genome Res. 2015, 25, 1680–1691. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, V.; Naqvi, A.R.; Uttamani, J.R.; Nares, S. MiRNA-Target Interaction Reveals Cell-Specific Post-Transcriptional Regulation in Mammalian Cell Lines. Int. J. Mol. Sci. 2016, 17, 72. [Google Scholar] [CrossRef]
- Aleman, L.M.; Doench, J.; Sharp, P.A. Comparison of siRNA-induced off-target RNA and protein effects. RNA 2007, 13, 385–395. [Google Scholar] [CrossRef]
- Mahmoodian Sani, M.R.; Hashemzadeh-Chaleshtori, M.; Saidijam, M.; Jami, M.S.; Ghasemi-Dehkordi, P. MicroRNA-183 Family in Inner Ear: Hair Cell Development and Deafness. J. Audiol. Otol. 2016, 20, 131–138. [Google Scholar] [CrossRef]
- Kittelmann, S.; McGregor, A.P. Modulation and Evolution of Animal Development through microRNA Regulation of Gene Expression. Genes 2019, 10, 321. [Google Scholar] [CrossRef]
- Hayashi, T.; Hoffman, M.P. Exosomal microRNA communication between tissues during organogenesis. RNA Biol. 2017, 14, 1683–1689. [Google Scholar] [CrossRef]
- Shrestha, A.; Mukhametshina, R.T.; Taghizadeh, S.; Vasquez-Pacheco, E.; Cabrera-Fuentes, H.; Rizvanov, A.; Mari, B.; Carraro, G.; Bellusci, S. MicroRNA-142 is a multifaceted regulator in organogenesis, homeostasis, and disease. Dev. Dyn. An Off. Publ. Am. Assoc. Anat. 2017, 246, 285–290. [Google Scholar]
- Sengupta, D.; Govindaraj, V.; Kar, S. Alteration in microRNA-17-92 dynamics accounts for differential nature of cellular proliferation. FEBS Lett. 2018, 592, 446–458. [Google Scholar] [CrossRef]
- Rissland, O.S.; Hong, S.J.; Bartel, D.P. MicroRNA destabilization enables dynamic regulation of the miR-16 family in response to cell-cycle changes. Mol. Cell 2011, 43, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Jin, Q.; Wang, X.; Li, Y. MicroRNA-802 Inhibits Cell Proliferation and Induces Apoptosis in Human Laryngeal Cancer by Targeting cAMP-Regulated Phosphoprotein 19. Cancer Manag. Res. 2020, 12, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Lu, S.; Hu, Y.; Guo, L.; Wu, X.; Liu, X.; Sun, Y. MicroRNA-135a Regulates VEGFC Expression and Promotes Luteinized Granulosa Cell Apoptosis in Polycystic Ovary Syndrome. Reprod. Sci. 2020, 27, 1436–1442. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xie, Y.; Yang, B.; Huang, S.; Zhang, Y.; Jia, Z.; Ding, G.; Zhang, A. MicroRNA-214 targets COX-2 to antagonize indoxyl sulfate (IS)-induced endothelial cell apoptosis. Apoptosis Int. J. Program. Cell Death 2020, 25, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Umansky, S. Aging and aging-associated diseases: A microRNA-based endocrine regulation hypothesis. Aging 2018, 10, 2557–2569. [Google Scholar] [CrossRef] [PubMed]
- Margolis, L.M.; Rivas, D.A. Potential Role of MicroRNA in the Anabolic Capacity of Skeletal Muscle with Aging. Exerc. Sport Sci. Rev. 2018, 46, 86–91. [Google Scholar] [CrossRef]
- McCormick, R.; Goljanek-Whysall, K. MicroRNA Dysregulation in Aging and Pathologies of the Skeletal Muscle. Int. Rev. cell Mol. Biol. 2017, 334, 265–308. [Google Scholar]
- Matsuyama, H.; Suzuki, H.I. Systems and Synthetic microRNA Biology: From Biogenesis to Disease Pathogenesis. Int. J. Mol. Sci. 2019, 21, 132. [Google Scholar] [CrossRef]
- Aziz, N.B.; Mahmudunnabi, R.G.; Umer, M.; Sharma, S.; Rashid, M.A.; Alhamhoom, Y.; Shim, Y.B.; Salomon, C.; Shiddiky, M.J.A. MicroRNAs in ovarian cancer and recent advances in the development of microRNA-based biosensors. Analyst 2020, 145, 2038–2057. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Ahmadi, Z.; Samarghandian, S.; Mohammadinejad, R.; Yaribeygi, H.; Sathyapalan, T.; Sahebkar, A. MicroRNA-mediated regulation of Nrf2 signaling pathway: Implications in disease therapy and protection against oxidative stress. Life Sci. 2020, 244, 117329. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, G.; Plantamura, I.; Cataldo, A.; Iorio, M.V. MicroRNA and Oxidative Stress Interplay in the Context of Breast Cancer Pathogenesis. Int. J. Mol. Sci. 2019, 20, 5143. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H. MicroRNA Networks Modulate Oxidative Stress in Cancer. Int. J. Mol. Sci. 2019, 20, 4497. [Google Scholar] [CrossRef] [PubMed]
- Biggar, K.K.; Storey, K.B. Functional impact of microRNA regulation in models of extreme stress adaptation. J. Mol. Cell. Biol. 2018, 10, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Hawkins, P.G.; Bi, N.; Dess, R.T.; Tewari, M.; Hearn, J.W.D.; Hayman, J.A.; Kalemkerian, G.P.; Lawrence, T.S.; Ten Haken, R.K.; et al. Serum MicroRNA Signature Predicts Response to High-Dose Radiation Therapy in Locally Advanced Non-Small Cell Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 107–114. [Google Scholar] [CrossRef]
- Halimi, M.; Asghari, S.M.; Sariri, R.; Moslemi, D.; Parsian, H. Cellular Response to Ionizing Radiation: A MicroRNA Story. Int. J. Mol. Cell. Med. 2012, 1, 178–184. [Google Scholar]
- Kraemer, A.; Anastasov, N.; Angermeier, M.; Winkler, K.; Atkinson, M.J.; Moertl, S. MicroRNA-mediated processes are essential for the cellular radiation response. Radiat. Res. 2011, 176, 575–586. [Google Scholar] [CrossRef]
- Tsao, D.; Kalogerinis, P.; Tabrizi, I.; Dingfelder, M.; Stewart, R.D.; Georgakilas, A.G. Induction and processing of oxidative clustered DNA lesions in Fe-56-Ion-irradiated human monocytes. Radiat. Res. 2007, 168, 87–97. [Google Scholar] [CrossRef]
- Zhang, X.R.; Ye, C.Y.; Sun, F.; Wei, W.J.; Hu, B.R.; Wang, J.F. Both Complexity and Location of DNA Damage Contribute to Cellular Senescence Induced by Ionizing Radiation. PloS ONE 2016, 11, e0155725. [Google Scholar] [CrossRef]
- Lorat, Y.; Brunner, C.U.; Schanz, S.; Jakob, B.; Taucher-Scholz, G.; Rube, C.E. Nanoscale analysis of clustered DNA damage after high-LET irradiation by quantitative electron microscopy—The heavy burden to repair. DNA Repair 2015, 28, 93–106. [Google Scholar] [CrossRef]
- Nelson, G.A. Space Radiation and Human Exposures, A Primer. Radiat. Res. 2016, 185, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Ikeda, H.; Yoshida, Y. Role of High-Linear Energy Transfer Radiobiology in Space Radiation Exposure Risks. Int. J. Part. Ther. 2018, 5, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Norbury, J.W.; Slaba, T.C.; Aghara, S.; Badavi, F.F.; Blattnig, S.R.; Clowdsley, M.S.; Heilbronn, L.H.; Lee, K.; Maung, K.M.; Mertens, C.J.; et al. Advances in space radiation physics and transport at NASA. Life Sci. Space Res. 2019, 22, 98–124. [Google Scholar] [CrossRef] [PubMed]
- Inozemtsev, K.O.; Kushin, V.V.; Stradi, A.; Ambrozova, I.; Kodaira, S.; Szabo, J.; Tolochek, R.V.; Shurshakov, V.A. Measurement of Different Components of Secondary Radiation Onboard International Space Station by Means of Passive Detectors. Radiat. Prot. Dosim. 2018, 181, 412–417. [Google Scholar] [CrossRef]
- Cucinotta, F.A.; Schimmerling, W.; Wilson, J.W.; Peterson, L.E.; Badhwar, G.D.; Saganti, P.B.; Dicello, J.F. Space radiation cancer risks and uncertainties for Mars missions. Radiat. Res. 2001, 156, 682–688. [Google Scholar] [CrossRef]
- Hassler, D.M.; Zeitlin, C.; Wimmer-Schweingruber, R.F.; Ehresmann, B.; Rafkin, S.; Eigenbrode, J.L.; Brinza, D.E.; Weigle, G.; Bottcher, S.; Bohm, E.; et al. Mars’ Surface Radiation Environment Measured with the Mars Science Laboratory’s Curiosity Rover. Science 2014, 343, 1244797. [Google Scholar] [CrossRef]
- Hellweg, C.E.; Baumstark-Khan, C. Getting ready for the manned mission to Mars: The astronauts’ risk from space radiation. Die Nat. 2007, 94, 517–526. [Google Scholar] [CrossRef]
- McNulty, P.J.; Pease, V.P.; Bond, V.P. Role of Cerenkov radiation in the eye-flashes observed by Apollo astronauts. Life Sci. Space Res. 1976, 14, 205–217. [Google Scholar]
- McNulty, P.J.; Pease, V.P.; Bond, V.P. Muon-induced visual sensations. J. Opt. Soc. Am. 1976, 66, 49–55. [Google Scholar] [CrossRef]
- McNulty, P.J.; Pease, V.P.; Bond, V.P. Visual sensations induced by Cerenkov radiation. Science 1975, 189, 453–454. [Google Scholar] [CrossRef]
- Tobias, S.; Abramson, T. Interaction among anxiety, stress, response mode, and familiarity of subject matter on achievement from programmed instruction. J. Educ. Psychol. 1971, 62, 357–364. [Google Scholar] [CrossRef] [PubMed]
- McNulty, P.J.; Pease, V.P.; Bond, V.P. Visual phenomena induced by relativistic carbon ions with and without Cerenkov radiation. Science 1978, 201, 341–343. [Google Scholar] [CrossRef]
- Budinger, T.F.; Lyman, J.T.; Tobias, C.A. Visual perception of accelerated nitrogen nuclei interacting with the human retina. Nature 1972, 239, 209–211. [Google Scholar] [CrossRef]
- Fremlin, J.H. Causality and tachyons. Nature 1970, 226, 194–195. [Google Scholar] [CrossRef] [PubMed]
- Budinger, T.F.; Bichsel, H.; Tobias, C.A. Visual phenomena noted by human subjects on exposure to neutrons of energies less than 25 million electron volts. Science 1971, 172, 868–870. [Google Scholar] [CrossRef] [PubMed]
- Charman, W.N.; Dennis, J.A.; Fazio, G.G.; Jelley, J.V. Visual sensations produced by single fast particles. Nature 1971, 230, 522–524. [Google Scholar] [CrossRef]
- Chancellor, J.C.; Scott, G.B.; Sutton, J.P. Space Radiation: The Number One Risk to Astronaut Health beyond Low Earth Orbit. Life (Basel) 2014, 4, 491–510. [Google Scholar] [CrossRef]
- Khan, S.Y.; Tariq, M.A.; Perrott, J.P.; Brumbaugh, C.D.; Kim, H.J.; Shabbir, M.I.; Ramesh, G.T.; Pourmand, N. Distinctive microRNA expression signatures in proton-irradiated mice. Mol. Cell. Biochem. 2013, 382, 225–235. [Google Scholar] [CrossRef]
- Templin, T.; Young, E.F.; Smilenov, L.B. Proton radiation-induced miRNA signatures in mouse blood: Characterization and comparison with 56Fe-ion and gamma radiation. Int. J. Radiat. Biol. 2012, 88, 531–539. [Google Scholar] [CrossRef]
- Kovalchuk, O.; Zemp, F.J.; Filkowski, J.N.; Altamirano, A.M.; Dickey, J.S.; Jenkins-Baker, G.; Marino, S.A.; Brenner, D.J.; Bonner, W.M.; Sedelnikova, O.A. microRNAome changes in bystander three-dimensional human tissue models suggest priming of apoptotic pathways. Carcinogenesis 2010, 31, 1882–1888. [Google Scholar] [CrossRef]
- Chauhan, V.; Howland, M.; Wilkins, R. Effects of alpha-Particle Radiation on MicroRNA Responses in Human Cell-Lines. Open Biochem. J. 2012, 6, 16–22. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nie, J.H.; Chen, Z.H.; Shao, C.L.; Pei, W.W.; Zhang, J.; Zhang, S.Y.; Jiao, Y.; Tong, J. Analysis of the miRNA-mRNA networks in malignant transformation BEAS-2B cells induced by alpha-particles. J. Toxicol. Environ. Health Part A 2016, 79, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Templin, T.; Amundson, S.A.; Brenner, D.J.; Smilenov, L.B. Whole mouse blood microRNA as biomarkers for exposure to gamma-rays and (56)Fe ion. Int. J. Radiat. Biol. 2011, 87, 653–662. [Google Scholar] [CrossRef]
- He, Y.; Zhang, Y.; Li, H.; Zhang, H.; Li, Z.; Xiao, L.; Hu, J.; Ma, Y.; Zhang, Q.; Zhao, X. Comparative Profiling of MicroRNAs Reveals the Underlying Toxicological Mechanism in Mice Testis Following Carbon Ion Radiation. Dose-Response A Publ. Int. Hormesis Soc. 2018, 16, 1559325818778633. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; He, J.; Wang, J.; Ding, N.; Wang, B.; Lin, S.; Zhang, X.; Hua, J.; Li, H.; Hu, B. Serum microRNAs as Early Indicators for Estimation of Exposure Degree in Response to Ionizing Irradiation. Radiat. Res. 2017, 188, 342–354. [Google Scholar] [CrossRef]
- Wei, W.J.; Wang, J.F.; He, J.P.; Xie, X.D. Serum microRNA as noninvasive indicator for space radiation. Acta Astronaut. 2018, 152, 101–104. [Google Scholar] [CrossRef]
- Girardi, C.; De Pitta, C.; Casara, S.; Sales, G.; Lanfranchi, G.; Celotti, L.; Mognato, M. Analysis of miRNA and mRNA expression profiles highlights alterations in ionizing radiation response of human lymphocytes under modeled microgravity. PLoS ONE 2012, 7, e31293. [Google Scholar] [CrossRef]
- Fu, H.; Su, F.; Zhu, J.; Zheng, X.; Ge, C. Effect of simulated microgravity and ionizing radiation on expression profiles of miRNA, lncRNA, and mRNA in human lymphoblastoid cells. Life Sci. Space Res. 2020, 24, 1–8. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, D.; Zhao, L.; Zhang, M.; Sun, Y.Q. Effects of microgravity on DNA damage response in Caenorhabditis elegans during Shenzhou-8 spaceflight. Int. J. Radiat. Biol. 2015, 91, 531–539. [Google Scholar] [CrossRef]
- Xu, D.; Gao, Y.; Huang, L.; Sun, Y. Changes in miRNA expression profile of space-flown Caenorhabditis elegans during Shenzhou-8 mission. Life Sci. Space Res. 2014, 1, 44–52. [Google Scholar] [CrossRef]
- Gao, Y.; Li, S.; Xu, D.; Wang, J.; Sun, Y. Changes in apoptotic microRNA and mRNA expression profiling in Caenorhabditis elegans during the Shenzhou-8 mission. J. Radiat. Res. 2015, 56, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yu, X.Y.; Fu, H.J.; Wang, H.Y.; Wang, P.; Zheng, X.F.; Wang, Y. MicroRNA-21 is involved in ionizing radiation-promoted liver carcinogenesis. Int. J. Clin. Exp. Med. 2010, 3, 211–222. [Google Scholar] [PubMed]
- Shi, Y.; Zhang, X.M.; Tang, X.B.; Wang, P.; Wang, H.C.; Wang, Y. MiR-21 is Continually Elevated Long-Term in the Brain after Exposure to Ionizing Radiation. Radiat. Res. 2012, 177, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.M.; Ng, W.L.; Wang, P.; Tian, L.L.; Werner, E.; Wang, H.C.; Doetsch, P.; Wang, Y. MicroRNA-21 Modulates the Levels of Reactive Oxygen Species by Targeting SOD3 and TNF alpha. Cancer Res. 2012, 72, 4707–4713. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, X.; Wang, P.; Wang, X.; Farris, A.B., 3rd; Wang, Y. Lessons learned using different mouse models during space radiation-induced lung tumorigenesis experiments. Life Sci. Space Res. 2016, 9, 48–55. [Google Scholar] [CrossRef]
- Kim, S.B.; Zhang, L.; Barron, S.; Shay, J.W. Inhibition of microRNA-31-5p protects human colonic epithelial cells against ionizing radiation. Life Sci. Space Res. 2014, 1, 67–73. [Google Scholar] [CrossRef]
- Barcellos-Hoff, M.H.; Mao, J.H. HZE Radiation Non-Targeted Effects on the Microenvironment That Mediate Mammary Carcinogenesis. Front. Oncol. 2016, 6, 57. [Google Scholar] [CrossRef]
- Hu, W.; Xu, S.; Yao, B.; Hong, M.; Wu, X.; Pei, H.; Chang, L.; Ding, N.; Gao, X.; Ye, C.; et al. MiR-663 inhibits radiation-induced bystander effects by targeting TGFB1 in a feedback mode. RNA Biol. 2014, 11, 1189–1198. [Google Scholar] [CrossRef]
- Xu, S.; Wang, J.; Ding, N.; Hu, W.; Zhang, X.; Wang, B.; Hua, J.; Wei, W.; Zhu, Q. Exosome-mediated microRNA transfer plays a role in radiation-induced bystander effect. RNA Biol. 2015, 12, 1355–1363. [Google Scholar] [CrossRef]
- Moreno-Villanueva, M.; Wong, M.; Lu, T.; Zhang, Y.; Wu, H. Interplay of space radiation and microgravity in DNA damage and DNA damage response. NPJ Microgravity 2017, 3, 14. [Google Scholar] [CrossRef]
- Ramadan, S.S.; Sridharan, V.; Koturbash, I.; Miousse, I.R.; Hauer-Jensen, M.; Nelson, G.A.; Boerma, M. A priming dose of protons alters the early cardiac cellular and molecular response to 56Fe irradiation. Life Sci. Space Res. 2016, 8, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Yatagai, F.; Honma, M.; Dohmae, N.; Ishioka, N. Biological effects of space environmental factors: A possible interaction between space radiation and microgravity. Life Sci. Space Res. 2019, 20, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Bennett, P.V.; Cutter, N.C.; Sutherland, B.M. Proton-HZE-particle sequential dual-beam exposures increase anchorage-independent growth frequencies in primary human fibroblasts. Radiat. Res. 2006, 166, 488–494. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).