Involvement of Netrin/Unc-5 Interaction in Ciliary Beating and in Pattern Formation of the Ciliary Band-Associated Strand (CBAS) in the Sea Urchin, Hemicentrotus pulcherrimus
Abstract
1. Introduction
2. Results
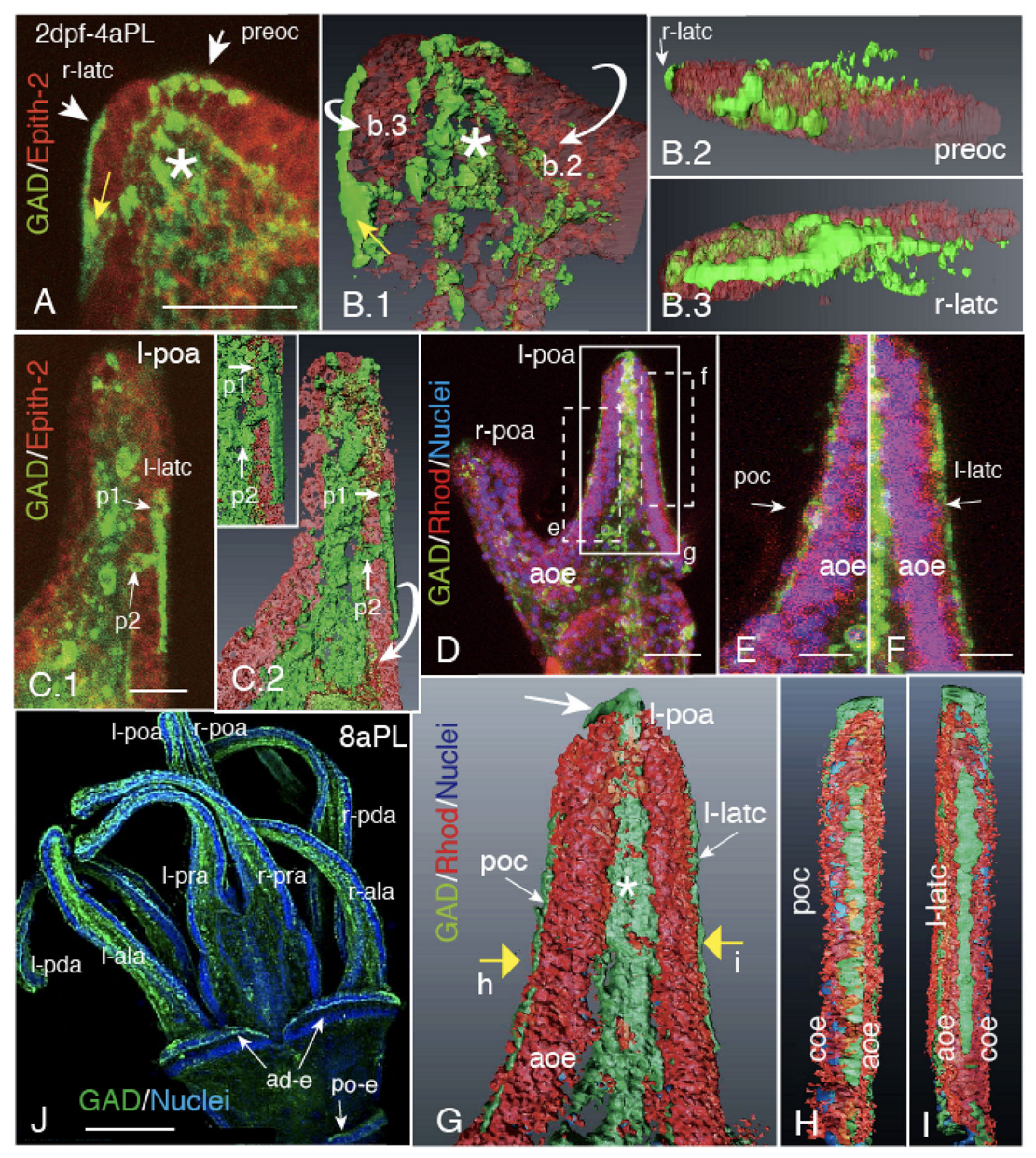
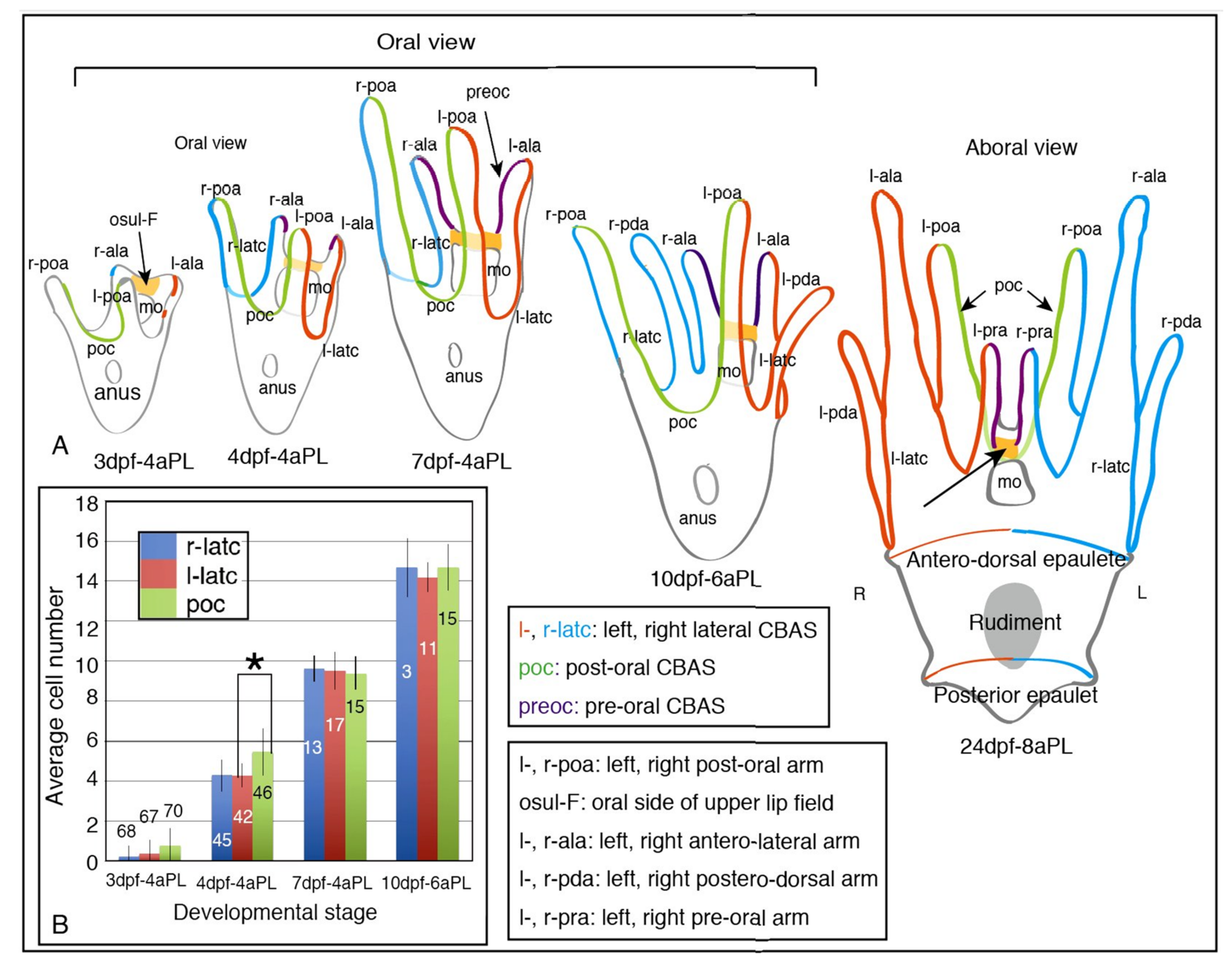
2.1. Blastocoelar GAD-Expressing Cell Egression onto the Apical Surface of Larval Arms and Lining Up for CBAS Patterning
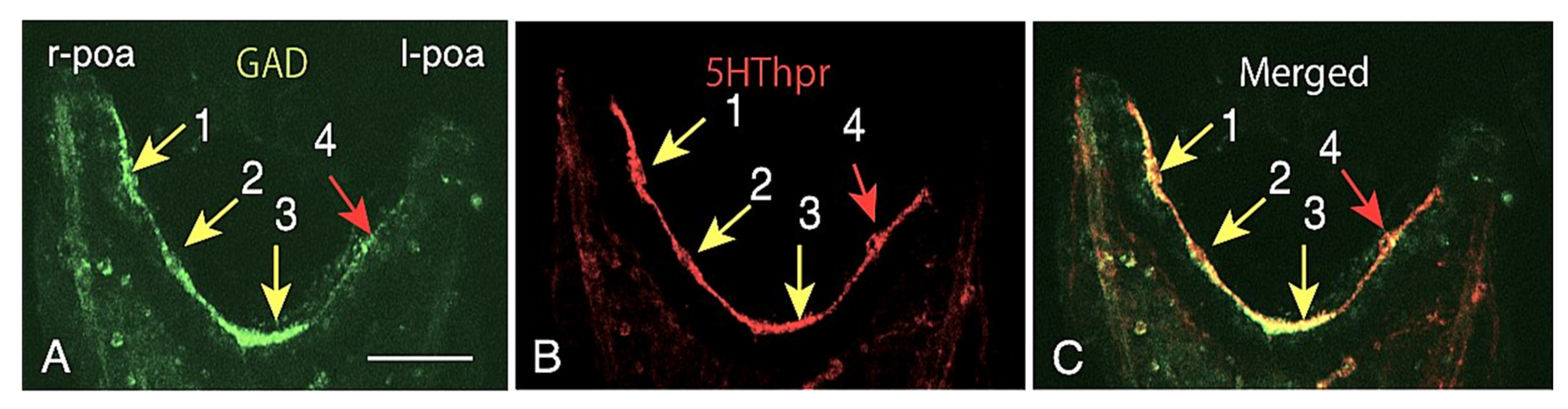
2.2. Heterogeneous Protein Expression among CBAS Component Cells
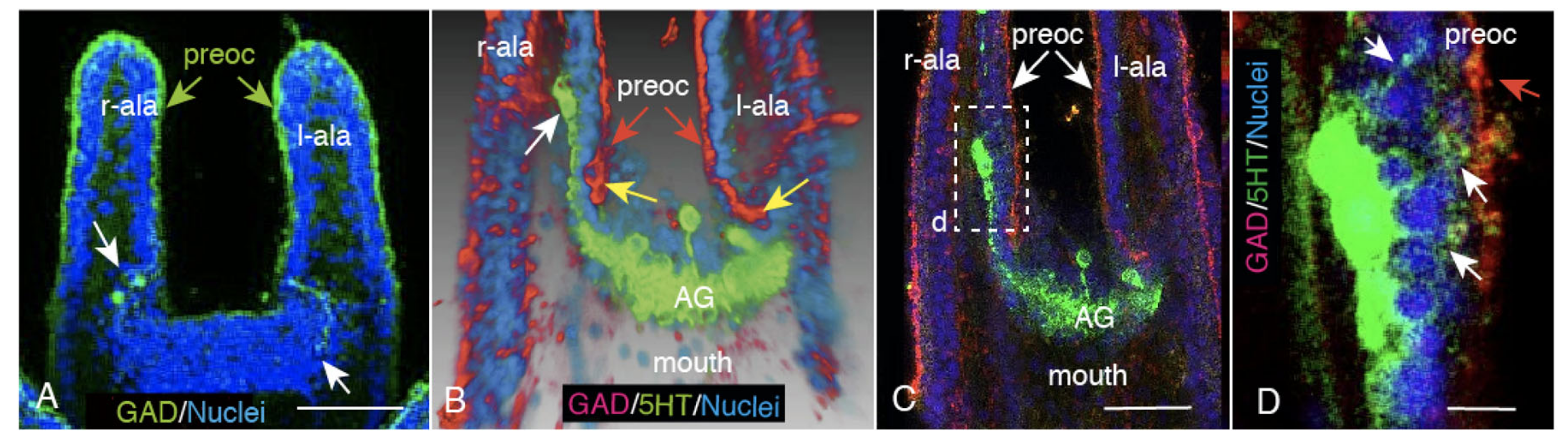
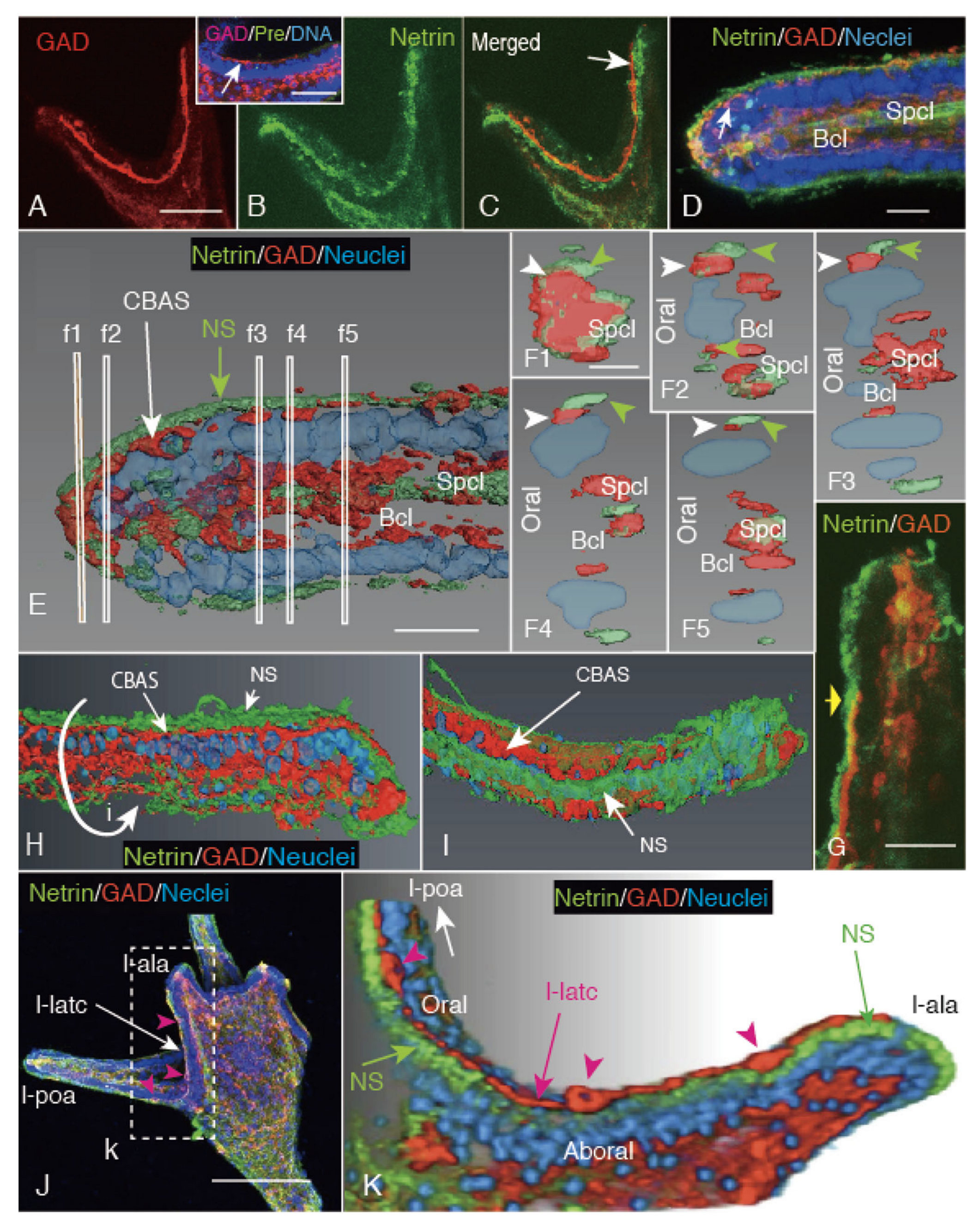
2.3. CBAS Locates between the Netrin Stripe on the Aboral Side and the Netrin-Free Oral Side of the Circumoral Ectoderm
2.4. Netrin Stripe along the Ciliary Band
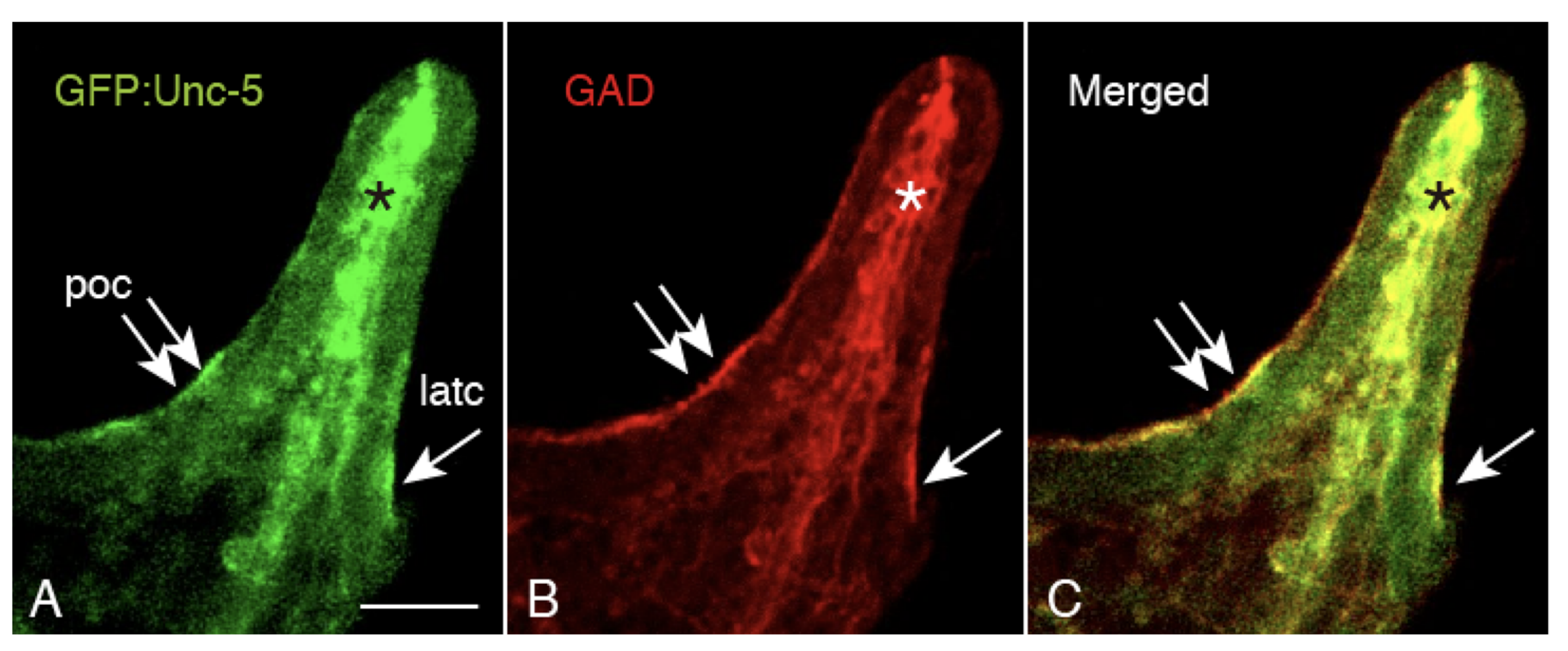
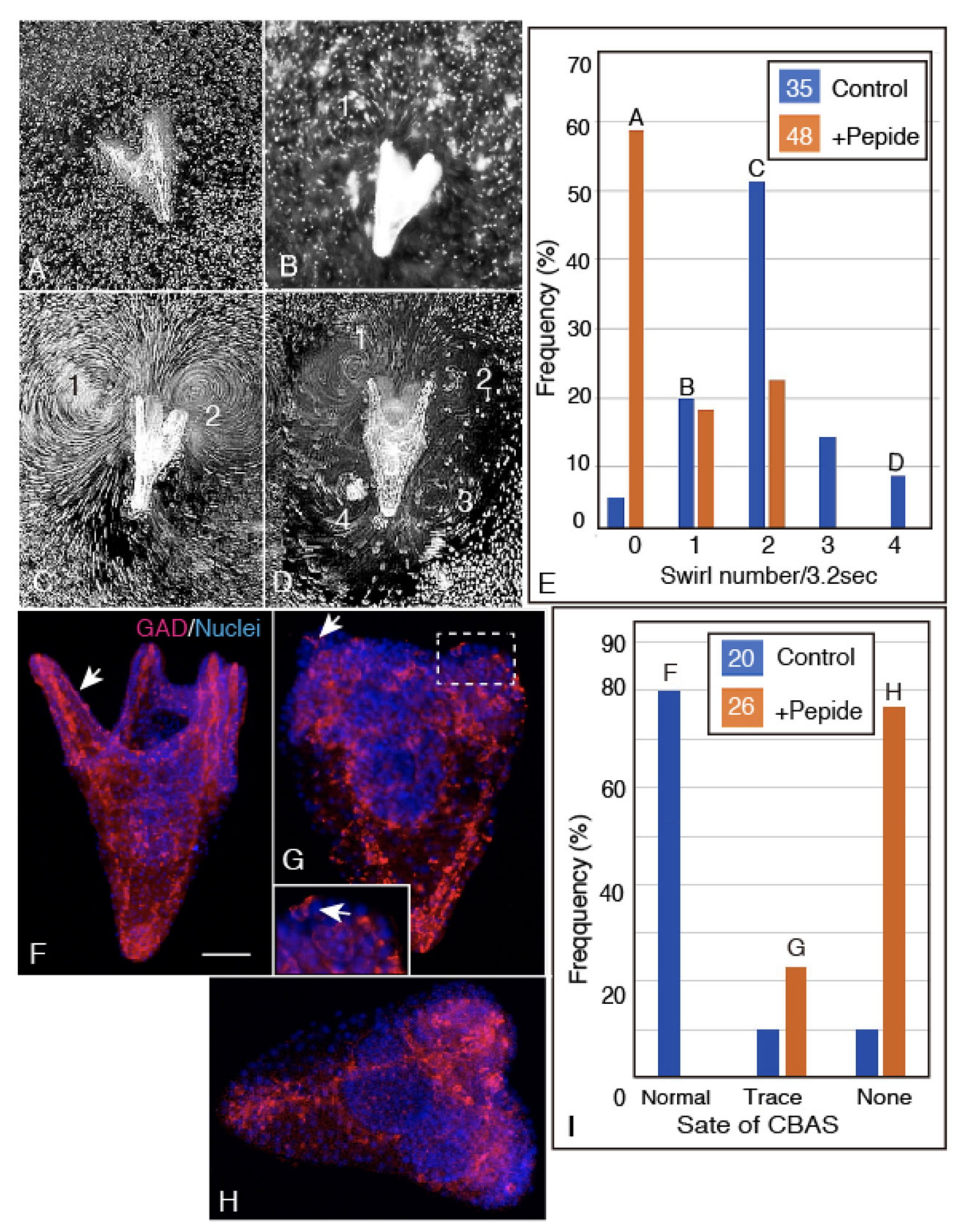
2.5. Unc-5 Expression in the CBAS on the Oral Side of the Netrin Stripe and Involvement of the Netrin/Unc-5 Interaction in Larval Ciliary Beating and CBAS Pattern Formation
3. Discussion
3.1. Blastocoelar GADC Egression onto the Apical Surface of the Larval Arms and Lining Up for the CBAS Patterning
3.2. Apical Surface Netrin as an Egression and CBAS Extension Signaling
3.3. Netrin Stripe along the Ciliary Band
4. Materials and Methods
4.1. Larval Preparation
4.2. Whole-Mount Immunohistochemistry
4.3. 3D Image Reconstruction
4.4. Counting the Number of GADCs in Each CBAS of Larvae
4.5. Generation of GFP-Tagged Hp-Unc-5v2
- R-v2: 5′-TCACCGAATTCATGAGACGCCGAAGTGCCGG-3′
- R-v2: 5′-ACTCGCTCGAGCYGTACATGGCCTTCAAACA-3′
4.6. Intervention for Proper Patterning of CBAS by the Unc-5 Binding Ig Domain Peptide of Hp-Netrin
4.7. Swirling Track Pattern Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| GADC | Glutamate decarboxylase-expressing cell |
| dpf | Days post-fertilization |
| CBAS | Ciliary band associated strand |
| r-, l-ala | Right or left antero-lateral arm |
| r-, l-latc | Right or left lateral CBAS |
| poc | Post-oral CBAS |
| preoc | Preoral CBAS |
| CLSM | Confocal laser scanning microscopy |
| DRD-1 | Dopamine decarboxylase D-1 |
| WMIHC | Whole-mount immunohistochemistry |
| GFP:Unc-5 | Green fluorescein tagged Unc-5 |
References
- Maruyama, Y. Development of swimming behavior in sea urchin embryos. I. Dev. Biol. 1981, 215, 163–171. [Google Scholar]
- Katow, H.; Katow, T.; Abe, K.; Ooka, S.; Kiyomoto, M.; Hamanaka, G. Mesomere-derived glutamate decarboxylase- expressing blastocoelar mesenchyme cells of sea urchin larvae. Biol. Open 2014, 3, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Katow, H.; Abe, K.; Katow, T.; Zamani, A.; Abe, H. Development of the GABA-ergic signaling system and its role in larval swimming in sea urchin. J. Exp. Biol. 2013, 216, 1704–1716. [Google Scholar] [CrossRef] [PubMed]
- Katow, H.; Katow, T.; Yoshida, H.; Kiyomoto, M.; Uemura, I. Immunohistochemical and ultrastructural properties of the larval ciliary band-associated strand in the sea urchin Hemicentrotus pulcherrimus. Front. Zool. 2016, 13, 27. [Google Scholar] [CrossRef][Green Version]
- Katow, H.; Katow, T.; Kiyomto, M. The ontogeny of synaptophysin expression patterns on the GABAergic ciliary band-associated strand during larval development of the sea urchin, Hemicentrotus pulcherrimus A. Agassiz, 1864. Zoomorphology 2019, 138, 117–126. [Google Scholar] [CrossRef]
- Wheeler, J.D.; Chan, K.Y.K.; Andderson, E.J.; Mullineaux, L.S. Ontogenetic changes in larval swimming and orientation of pre-competent sea urchin Arbacia punctulata in turbulence. J. Exp. Biol. 2016, 219, 1303–1310. [Google Scholar] [CrossRef]
- Metaxas, A.; Young, C.M. Responses of echinoid larvae to food patches of different algal densities. Mar. Biol. 1998, 130, 433–445. [Google Scholar] [CrossRef]
- Katow, H.; Yoshida, H.; Kiyomoto, M. Initial report of γ-aminobutyric acidergic locomotion regulatory system and its 3-mercaptopropionic acid-sensitivity in metamorphic juvenile of sea urchin, Hemicentrotus pulcherrimus. Sci. Rep. UK 2020, 10, 778. [Google Scholar] [CrossRef]
- Florey, E.; Cahill, M.A.; Rathmayer, M. Excitatory actions of GABA and acetylcholine in sea urchin tube feet. Comp. Biochem. Phys. C. 1975, 51, 5–12. [Google Scholar] [CrossRef]
- Devlin, C.L. The Pharmacology of Gamma-Aminobutyric Acid and Acetylcholine Receptors at the Echinoderm Neuromuscular Junction. J. Exp. Biol. 2001, 204, 887–896. [Google Scholar]
- Nakajima, Y.; Kaneko, H.; Murray, G.; Burke, R.D. Divergent patterns of neural development in larval echinoids and asteroids. Evol. Develop. E 2004, 6, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Burke, R.D.; Angerer, L.M.; Elphick, M.R.; Humphrey, G.W.; Yaguchi, S.; Kiyama, T.; Liang, T.S.; Mu, X.; Agca, C.; Klein, W.H.; et al. A Genomic View of the Sea Urchin Nervous System. Dev. Biol. 2006, 300, 434–460. [Google Scholar] [CrossRef] [PubMed]
- Yaguchi, S.; Kanoh, K.S.; Amemiya, S.; Katow, H. Initial analysis of immunochemical cell surface properties, location and formation of the serotonergic apical ganglion in sea urchin embryos. Dev. Growth Differ. 2000, 42, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Katow, T.; Ooka, S.; Katow, H. Unc-5/netrin-mediated axonal projection during larval serotonergic nervous system formation in the sea urchin, Hemicentrotus pulcherrimus. Int. Natl. J. Dev. Biol. 2013, 57, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.-s.; Wadsworth, W.G. Identification of domains of Netrin UNC-6 that mediate attractive and repulsive guidance and responses from cells and growth cones. J. Neurosc. 2002, 22, 7080–7087. [Google Scholar] [CrossRef]
- Zamani, A. Role of Cytokinetic Factors in the Formation of Epaulets, a Set of Ciliary Bands in the Trunk Regions, During Late Pluteus Stages in Sea Urchin. Master’s Thesis, Tohoku University, Sendai, Japan, 2012. [Google Scholar]
- Katow, H. Spatio-temporal expression of a netrin homolog in the sea urchin Hemicentrotus pulcherrimus (HpNetrin) during serotonergic axon extension. Int. J. Dev. Biol. 2008, 52, 1077–1088. [Google Scholar] [CrossRef]
- Katow, H.; Suyemitsu, T.; Ooka, S.; Yaguchi, J.; Jin-nai, T.; Kuwahara, I.; Katow, T.; Yaguchi, S.; Abe, H. Development of a dopaminergic system in sea urchin embryos and larvae. J. Exp. Biol. 2010, 213, 2808–2819. [Google Scholar] [CrossRef]
- Suyemitsu, T. Role of the dopamine receptor on the metamorphosis of sea urchin larvae. SUCRA 2007, 5, 666–667. [Google Scholar]
- Giudice, G. Developmental Biology of the Sea Urchin Embryo; Academic Press: New York, NY, USA; London, UK, 1973. [Google Scholar]
- Slota, L.A.; Miranda, E.M.; McClay, D.R. Spatial and temporal patterns of gene expression during neurogenesis in the sea urchin Lytechinus variegatus. Evol. Dev. 2019, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Grandin, M.; Meier, M.; Delcros, J.G.; Nikodemus, D.; Reuten, R.; Patel, T.R.; Goldschneider, D.; Orriss, G.; Krahn, N.; Boussouar, A.; et al. Structural decoding of the Netrin-1/UNC5 Interaction and it’s therapeutical implications in cancers. Cancer Cell 2016, 29, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Yaguchi, S.; Katow, H. Expression of tryptophan 5-hydroxylase gene during sea urchin neurogenesis and role of serotonergic nervous system in larval behavior. J. Comp. Neurol. 2003, 466, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, K. Normal development to metamorphosis. In The Sea Urchin Embryo. Biochemistry and Morphogenesis; Czihak, G., Ed.; Springer: Berlin, Germany, 1975; pp. 177–232. [Google Scholar]
- Katayama, Y.; Battista, M.; Kao, W.M.; Hidalgo, A.; Peired, A.J.; Thomas, S.A.; Frenette, P.S. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell 2006, 124, 407–421. [Google Scholar] [CrossRef] [PubMed]
- Beltz, B.S.; Zhang, Y.; Benton, J.L.; Sandeman, D.C. Adult neurogenesis in the decapod crustacean brain: A hematopoietic connection? Eur. J. Neurosci. 2011, 34, 870–883. [Google Scholar] [CrossRef]
- Katow, H.; Solursh, M. Ultrastructure of primary mesenchyme cell ingression in the sea urchin Lytechinus pictus. J. Exp. Zool. 1980, 213, 231–246. [Google Scholar] [CrossRef]
- Shook, D.; Keller, R. Mechanisms, mechanics and function of epithelial–mesenchymal transitions in early development. Mech. Devel. 2003, 120, 1351–1383. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.M.; Tymoczko, J.L.; Stryer, L. Biochemistry, 5th ed.; W. H. Freeman: New York, NY, USA, 2002. [Google Scholar]
- Kinjo, S.; Kiyomoto, M.; Yamamoto, T.; Ikeo, K.; Yaguchi, S. HpBase: A genome database of a sea urchin, Hemicentrotus pulcherrimus. Dev. Growth Differ. 2018, 60, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Slivko-Koltchik, G.A.; Kuznetsov, V.P.; Panchin, Y.V. Are there gap junctions without connexins or pannexins? BMC Evol. Biol. 2019, 19 (Suppl. 1), 46. [Google Scholar] [CrossRef]
- Yazaki, I.; Dale, B.; Tosti, E. Functional gap junctions in the early sea urchin embryo are localized to the vegetal pole. Dev. Biol. 1999, 212, 503–510. [Google Scholar] [CrossRef][Green Version]
- Yang, Y.; Lee, W.S.; Tang, X.; Wadsworth, W.G. Extracellular matrix regulates UNC-6 (netrin) axon guidance by controlling the direction of intracellular UNC-40 (DCC) outgrowth activity. PLoS ONE 2014, 9, e97258. [Google Scholar] [CrossRef]
- Rentzsch, F.; Layden, M.; Manuel, M. The cellular and molecular basis of cnidarian neurogenesis. Wiley Interdiscip. Rev. Dev. Biol. 2017, 6, e257. [Google Scholar] [CrossRef]
- Mäkinen, M.E.-L.; Ylä-Outinen, L.; Narkilahti, S. GABA and gap junctions in the development of synchronized activity in human pluripotent stem cell-derived neural networks. Front. Cell. Neurosci. 2018, 12, 56. [Google Scholar] [CrossRef]
- Paredes, I.; Himmels, P.; de Almodóvar, C.R. Neurovascular Communication During CNS Development. Dev. Cell. 2018, 45, 10–32. [Google Scholar] [CrossRef]
- Meijers, R.; Smock, R.G.; Zhang, Y.; Wang, J.-H. Netrin synergizes signaling and adhesion through DCC. Trends Biochem. Sci. 2020, 45. [Google Scholar] [CrossRef] [PubMed]
- Dominichi, C.; Moreno-Bravo, J.A.; Puiggros, S.R.; Pappeneau, Q.; Rama, N.; Vieugue, P.; Bernet, A.; Mehlen, P.; Chédotal, A. Floor plate-derived netrin-1 is dispensable for commissural axon guidance. Nature 2017, 545, 350–354. [Google Scholar] [CrossRef]
- Kruger, R.P.; Lee, J.; Li, W.; Guan, K.-L. Mapping netrin receptor binding reveals domains of Unc5 regulating its tyrosine phosphorylation. J. Neurosci. 2004, 24, 10826–10834. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.L.; Correia, J.P.; Kennedy, T.E. Netrins: Versatile extracellular cues with diverse funtions. Development 2011, 138, 2153–2169. [Google Scholar] [CrossRef] [PubMed]
- Boyer, N.P.; Gupton, S.L. Revising Netrin-1: One who guides (Axons). Front. Cell. Neurosci. 2018, 12, 221. [Google Scholar] [CrossRef]
- Guthrie, S. Axon guidance: Netrin receptors are revealed. Curr. Biol. 1997, 7, R6–R9, ISSN 0960-9822. [Google Scholar] [CrossRef][Green Version]
- Strähle, U.; Fischer, N.; Blader, P. Expression and regulation of a netrin homologue in the zebrafish embryo. Mech. Develop. 1997, 62, 147–160. [Google Scholar] [CrossRef]
- Lee, J.-R.; Kuo, D.-H. Heterochronically expressed midline netrin was recruited to guide mesoderm migration in epibolic gastrulation of the leech. bioRxiv 2019. [Google Scholar] [CrossRef]
- Asakura, T.; Waga, N.; Ogura, K.; Goshima, Y. Genes required for cellular UNC-6/Netrin localization in Caenorhabditis elegans. Genetics 2010, 185, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Hiramoto, M.; Hiromi, Y.; Giniger, E.; Hotta, Y. 2000 The Drosophila Netrin receptor Frazzled guides axons by controlling Netrin distribution. Nature 2000, 406, 886–889. [Google Scholar] [CrossRef] [PubMed]
- Katow, H.; Yaguchi, D.S.; Kiyomoto, M.; Washio, M. The 5-HT receptor cell is a new member of secondary mesenchyme cell descendants and forms a major blastocoelar network in sea urchin larvae. Mech. Develop. 2004, 121, 325–337. [Google Scholar] [CrossRef] [PubMed]


| Primary Antibody | Applied Dilution | References |
|---|---|---|
| Hp-GAD rabbit | 1:500 | [14] |
| Epith-2 mAb mouse | 1:100 | [13] |
| 5HT rabbit (S5545) | 1:1000 | Sigma-Aldrich, St. Louse, MO, USA |
| Hp-Netrin rabbit | 1:50 | [17] |
| Hp-Dopamine receptor D1 mouse | 1:500 | [18,19] |
| Hp-5HThpr mouse | 1:200 | [4,47] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katow, H.; Abe, K.; Katow, T.; Yoshida, H.; Kiyomoto, M. Involvement of Netrin/Unc-5 Interaction in Ciliary Beating and in Pattern Formation of the Ciliary Band-Associated Strand (CBAS) in the Sea Urchin, Hemicentrotus pulcherrimus. Int. J. Mol. Sci. 2020, 21, 6587. https://doi.org/10.3390/ijms21186587
Katow H, Abe K, Katow T, Yoshida H, Kiyomoto M. Involvement of Netrin/Unc-5 Interaction in Ciliary Beating and in Pattern Formation of the Ciliary Band-Associated Strand (CBAS) in the Sea Urchin, Hemicentrotus pulcherrimus. International Journal of Molecular Sciences. 2020; 21(18):6587. https://doi.org/10.3390/ijms21186587
Chicago/Turabian StyleKatow, Hideki, Kouki Abe, Tomoko Katow, Hiromi Yoshida, and Masato Kiyomoto. 2020. "Involvement of Netrin/Unc-5 Interaction in Ciliary Beating and in Pattern Formation of the Ciliary Band-Associated Strand (CBAS) in the Sea Urchin, Hemicentrotus pulcherrimus" International Journal of Molecular Sciences 21, no. 18: 6587. https://doi.org/10.3390/ijms21186587
APA StyleKatow, H., Abe, K., Katow, T., Yoshida, H., & Kiyomoto, M. (2020). Involvement of Netrin/Unc-5 Interaction in Ciliary Beating and in Pattern Formation of the Ciliary Band-Associated Strand (CBAS) in the Sea Urchin, Hemicentrotus pulcherrimus. International Journal of Molecular Sciences, 21(18), 6587. https://doi.org/10.3390/ijms21186587




