Halogen-Bonded Guanine Base Pairs, Quartets and Ribbons
Abstract
1. Introduction
2. Results and Discussion
2.1. Method Comparison
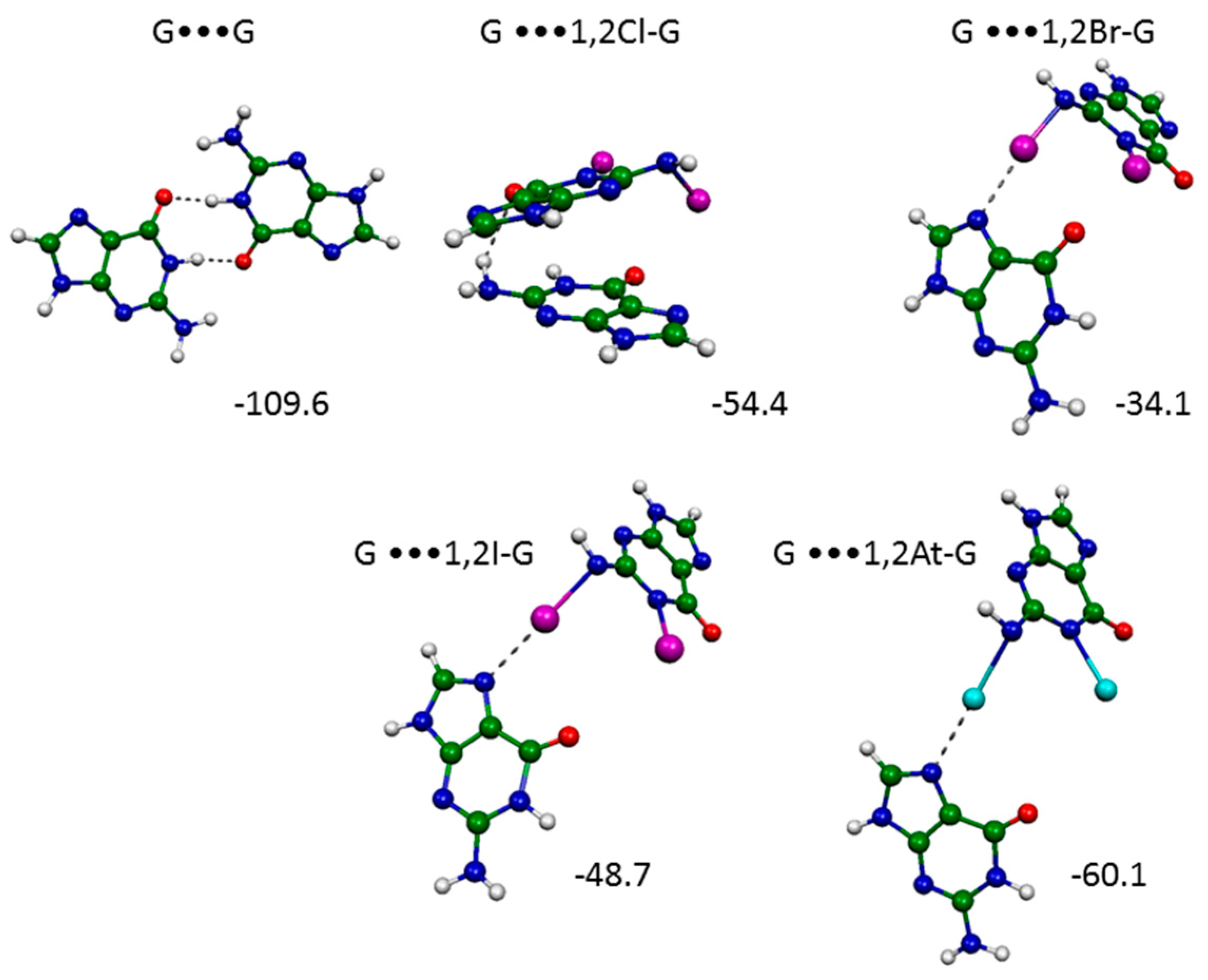
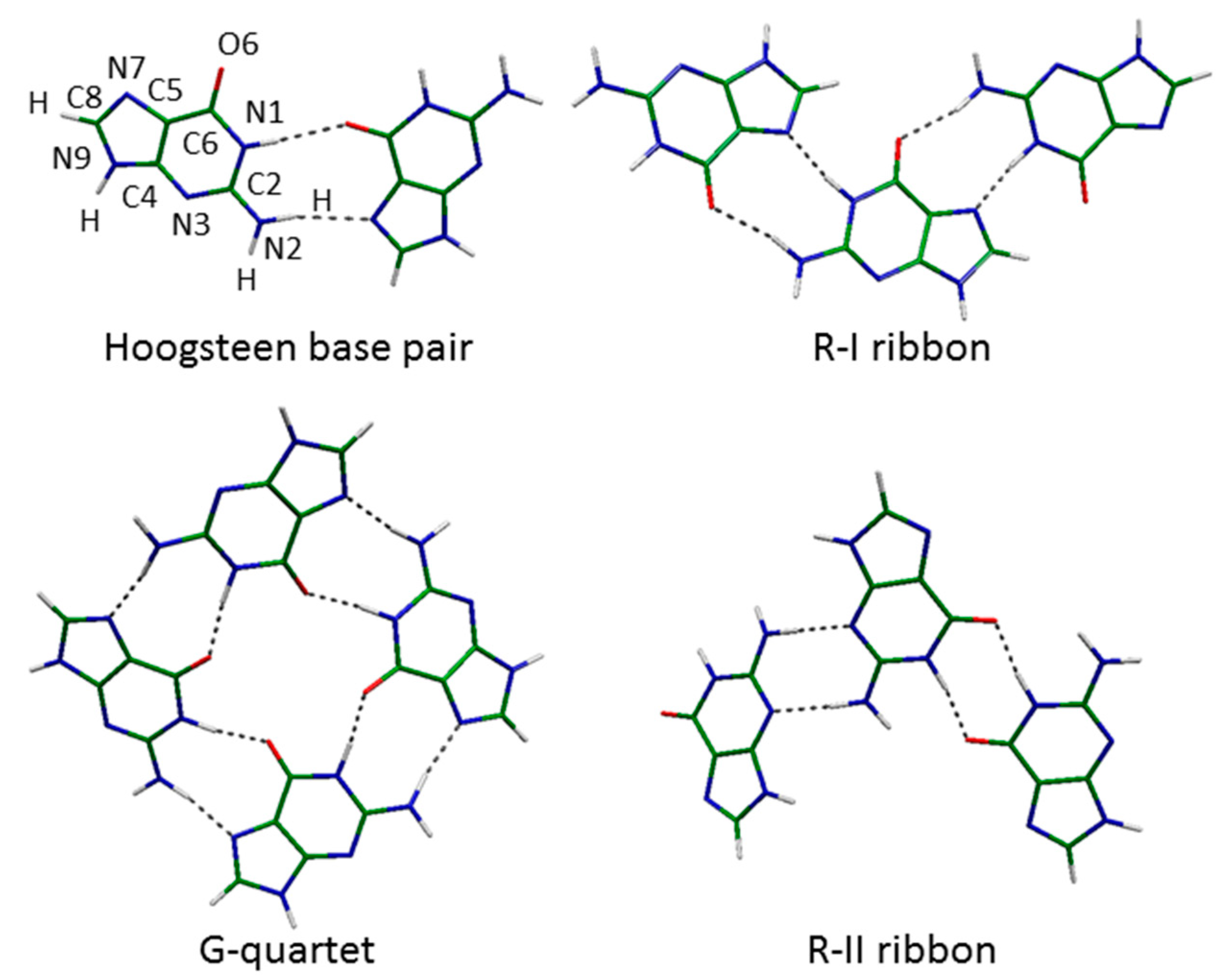
2.2. Hoogsteen Base Pair
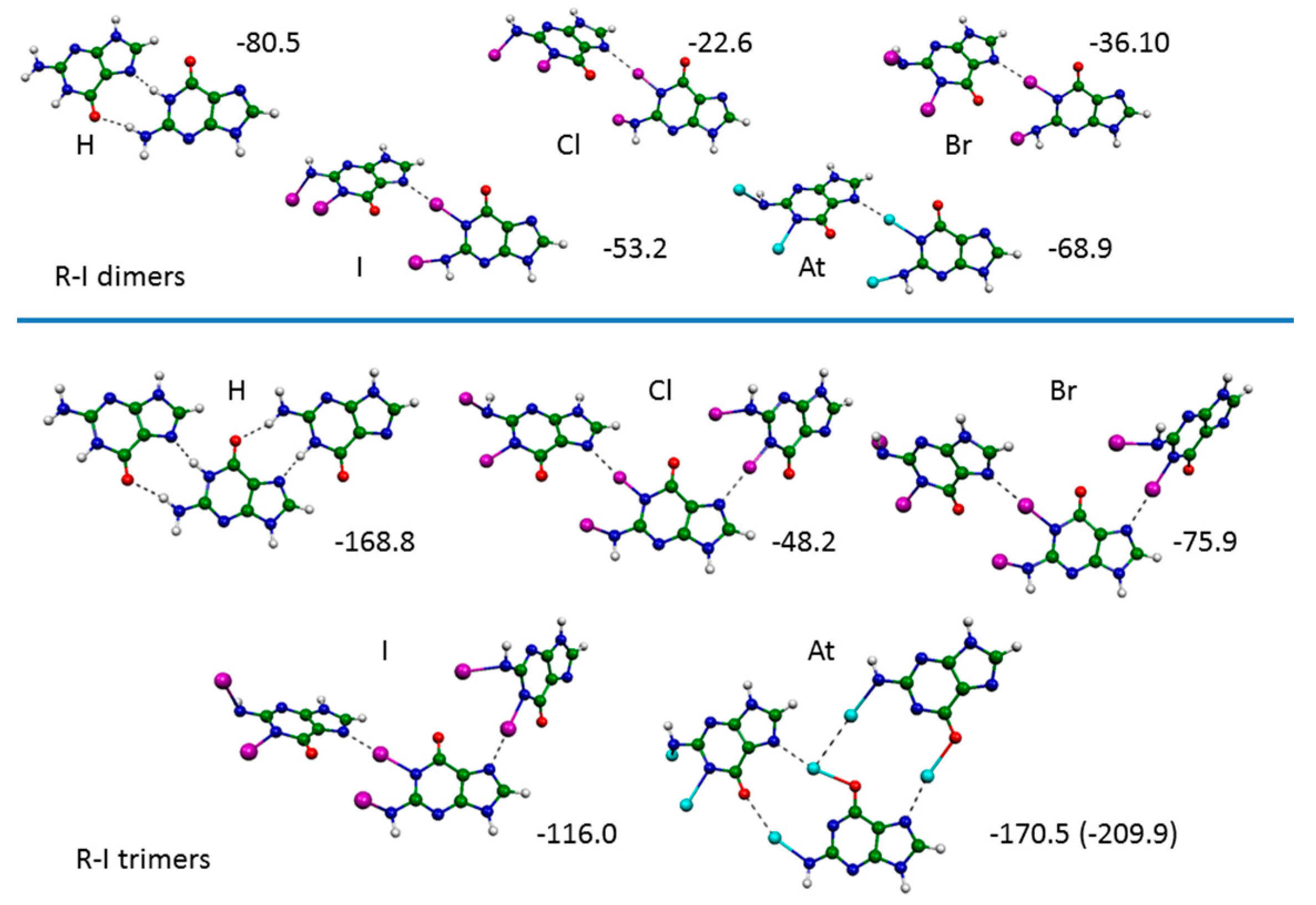
2.3. Ribbons R-I
2.4. Ribbons R-II
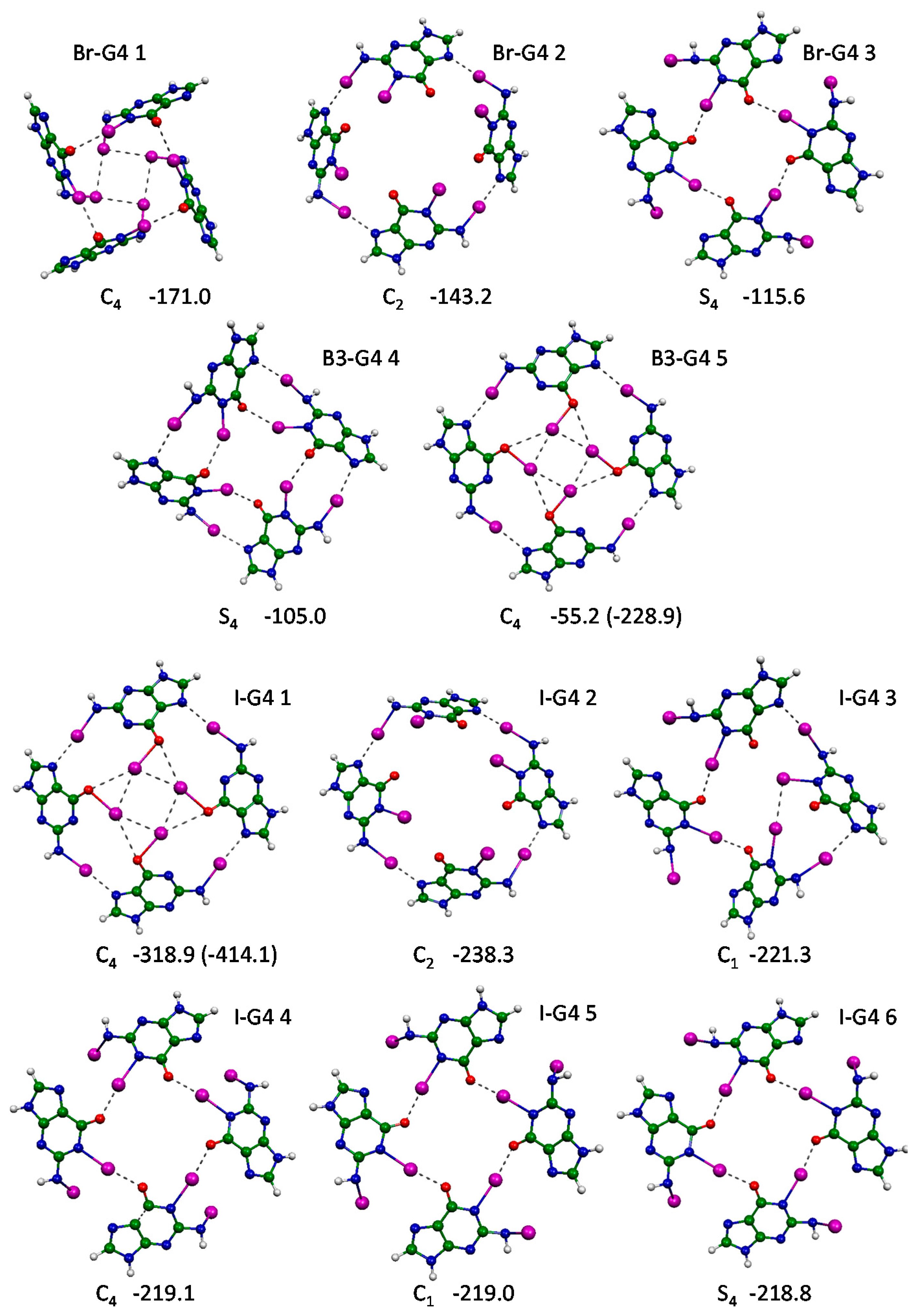
2.5. G-Quartets
3. Methodology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The halogen bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef] [PubMed]
- Kolář, M.H.; Hobza, P. Computer modeling of halogen bonds and other σ-hole interactions. Chem. Rev. 2016, 116, 5155–5187. [Google Scholar] [CrossRef] [PubMed]
- Legon, A.C. The halogen bond: An interim perspective. Phys. Chem. Chem. Phys. 2010, 12, 7736–7747. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Lane, P.; Concha, M.; Ma, Y.; Murray, J. An overview of halogen bonding. J. Mol. Model. 2007, 13, 305–311. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S. Halogen bonding: An interim discussion. Chem. Phys. Chem. 2013, 14, 278–294. [Google Scholar] [CrossRef]
- Wilcken, R.; Zimmermann, M.O.; Lange, A.; Joerger, A.C.; Boeckler, F.M. Principles and applications of halogen bonding in medicinal chemistry and chemical biology. J. Med. Chem. 2013, 56, 1363–1388. [Google Scholar] [CrossRef]
- Wolters, L.P.; Schyman, P.; Pavan, M.J.; Jorgensen, W.L.; Bickelhaupt, F.M.; Kozuch, S. The many faces of halogen bonding: A review of theoretical models and methods. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2014, 4, 523–540. [Google Scholar] [CrossRef]
- Cates, E.L.; van Mourik, T. Halogen bonding with the halogenabenzene bird structure, halobenzene, and halocyclopentadiene. J. Comput. Chem. 2019, 40, 2111–2118. [Google Scholar] [CrossRef]
- Saccone, M.; Catalano, L. Halogen Bonding beyond Crystals in Materials Science. J. Phys. Chem. B 2019, 123, 9281–9290. [Google Scholar] [CrossRef]
- Otero-de-la-Roza, A.; LeBlanc, L.M.; Johnson, E.R. Dispersion XDM with Hybrid Functionals: Delocalization Error and Halogen Bonding in Molecular Crystals. J. Chem. Theory Comput. 2019, 15, 4933–4944. [Google Scholar] [CrossRef]
- Auffinger, P.; Hays, F.A.; Westhof, E.; Ho, P.S. Halogen bonds in biological molecules. Proc. Natl. Acad. Sci. USA 2004, 101, 16789–16794. [Google Scholar] [CrossRef] [PubMed]
- Metrangolo, P.; Resnati, G. Halogen versus hydrogen. Science 2008, 321, 918–919. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density functional thermochemistry. 3. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Parker, A.J.; Stewart, J.; Donald, K.J.; Parish, C.A. Halogen bonding in DNA base pairs. J. Am. Chem. Soc. 2012, 134, 5165–5172. [Google Scholar] [CrossRef]
- Dapprich, S.; Komiromi, K.S.; Byun, K.; Morokuma, K.; Frisch, M.J. A new ONIOM implementation in Gaussian 98. Part I. The calculation of energies, gradients, vibrational frequencies and electric field derivatives. J. Mol. Struct. THEOCHEM 1999, 461, 1–21. [Google Scholar] [CrossRef]
- Xu, L.; Sang, P.; Zou, J.-W.; Xu, M.-B.; Li, X.-M.; Yu, Q.-S. Evaluation of nucleotide C–BrO–P contacts from ONIOM calculations: Theoretical insight into halogen bonding in nucleic acids. Chem. Phys. Lett. 2011, 509, 175–180. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Møller, C.; Plesset, M.S. Note on an approximation treatment for many-electron systems. Phys. Rev. 1934, 46, 618–622. [Google Scholar] [CrossRef]
- Frontera, A.; Bauzá, A. Halogen bonds in protein nucleic acid recognition. J. Chem. Theory Comp. 2020, 16, 4744–4752. [Google Scholar] [CrossRef] [PubMed]
- Kolář, M.H.; Tabarrini, O. Halogen bonding in nucleic acid complexes. J. Med. Chem. 2017, 60, 8681–8690. [Google Scholar] [CrossRef] [PubMed]
- Voth, A.R.; Hays, F.A.; Ho, P.S. Directing macromolecular conformation through halogen bonds. Proc. Natl. Acad. Sci. USA 2007, 104, 6188–6193. [Google Scholar] [CrossRef] [PubMed]
- Paragi, G.; Guerra, C.F. Cooperativity in the self-assembly of the guanine nucleobase into quartet and ribbon structures on surfaces. Chem. Eur. J. 2017, 23, 3042–3050. [Google Scholar] [CrossRef]
- Otero, R.; Schöck, M.; Molina, L.M.; Lægsgaard, E.; Stensgaard, I.; Hammer, B.; Besenbacher, F. Guanine quartet networks stabilized by cooperative hydrogen bonds. Angew. Chem. Int. Ed. 2005, 44, 2270–2275. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, T.; Grepioni, F.; Manet, I.; Mariani, P.; Masiero, S.; Mezzina, E.; Pieraccini, S.; Saturni, L.; Spada, G.P.; Gottarelli, G. Gel-like lyomesophases formed in organic solvents by self-assembled guanine ribbons. Chem. Eur. J. 2002, 8, 2143–2152. [Google Scholar] [CrossRef]
- Gottarelli, G.; Masiero, S.; Mezzina, E.; Pieraccini, S.; Rabe, J.P.; Samorí, P.; Spada, G.P. The self-assembly of lipophilic guanosine derivatives in solution and on solid surfaces. Chem. Eur. J. 2000, 6, 3242–3248. [Google Scholar] [CrossRef]
- Gottarelli, G.; Masiero, S.; Mezzina, E.; Spada, G.P.; Mariani, P.; Recanatini, M. The self-assembly of a lipophilic deoxyguanosine derivative and the formation of a liquid-crystalline phase in hydrocarbon solvents. Helv. Chim. Acta 1998, 81, 2078–2092. [Google Scholar] [CrossRef]
- Xu, Z.; Yang, Z.; Liu, Y.; Lu, Y.; Chen, K.; Zhu, W. Halogen bond: Its role beyond drug–target binding affinity for drug discovery and development. J. Chem. Inf. Model. 2014, 54, 69–78. [Google Scholar] [CrossRef]
- Clark, T.; Hennemann, M.; Murray, J.; Politzer, P. Halogen bonding: The σ-hole. J. Mol. Model. 2007, 13, 291–296. [Google Scholar] [CrossRef]
- Hogan, S.W.L.; van Mourik, T. Halogen bonding in mono- and dihydrated halobenzene. J. Comput. Chem. 2019, 40, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Hogan, S.W.L.; van Mourik, T. Corrigendum: Competition between hydrogen and halogen bonding in halogenated 1-methyluracil: Water systems. J. Comput. Chem. 2017, 38, 933. [Google Scholar] [CrossRef] [PubMed]
- Hogan, S.W.L.; van Mourik, T. Competition between hydrogen and halogen bonding in halogenated 1-methyluracil: Water systems. J. Comput. Chem. 2016, 37, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Fleig, T.; Sadlej, A.J. Electric dipole polarizabilities of the halogen atoms in 2P1/2 and 2P3/2 states: Scalar relativistic and two-component configuration-interaction calculations. Phys. Rev. A 2002, 65, 032506. [Google Scholar] [CrossRef]
- Guo, N.; Maurice, R.; Teze, D.; Graton, J.; Champion, J.; Montavon, G.; Galland, N. Experimental and computational evidence of halogen bonds involving astatine. Nat. Chem. 2018, 10, 428–434. [Google Scholar] [CrossRef]
- Liu, L.; Guo, N.; Champion, J.; Graton, J.; Montavon, G.; Galland, N.; Maurice, R. Towards a stronger halogen bond involving astatine: Unexpected adduct with Bu3PO stabilized by hydrogen bonding. Chem. Eur. J. 2020, 26, 3713–3717. [Google Scholar] [CrossRef]
- Bhattacharyya, D.; Koripella, S.C.; Mitra, A.; Rajendran, V.B.; Sinha, B. Theoretical analysis of noncanonical base pairing interactions in RNA molecules. J. Biosci. 2007, 32, 809–825. [Google Scholar] [CrossRef]
- Roy, A.; Panigrahi, S.; Bhattacharyya, M.; Bhattacharyya, D. Structure, stability, and dynamics of canonical and noncanonical base pairs: Quantum chemical studies. J. Phys. Chem. B 2008, 112, 3786–3796. [Google Scholar] [CrossRef]
- van Mourik, T.; Gdanitz, R.J. A critical note on density functional theory studies on rare-gas dimers. J. Chem. Phys. 2002, 116, 9620–9623. [Google Scholar] [CrossRef]
- Egli, M.; Lubini, P.; Pallan, P.S. The long and winding road to the structure of homo-DNA. Chem. Soc. Rev. 2007, 36, 31–45. [Google Scholar] [CrossRef]
- Eschenmoser, A.; Loewenthal, E. Chemistry of potentially prebiological natural products. Chem. Soc. Rev. 1992, 21, 1–16. [Google Scholar] [CrossRef]
- Chawla, M.; Abdel-Azeim, S.; Oliva, R.; Cavallo, L. Higher order structural effects stabilizing the reverse Watson–Crick guanine-cytosine base pair in functional RNAs. Nucleic Acid Res. 2013, 42, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Halder, A.; Bhattacharya, S.; Bhattacharyya, D.; Mitra, A. Reverse Watson-Crick purine-purine base pairs—The Sharp-turn motif and other structural consequences in functional RNAs. BioRchiv 2017, 098723. [Google Scholar] [CrossRef]
- Burge, S.; Parkinson, G.N.; Hazel, P.; Todd, A.K.; Neidle, S. Quadruplex DNA: Sequence, topology and structure. Nucleic Acids Res. 2006, 34, 5402–5415. [Google Scholar] [CrossRef]
- Meyer, M.; Steinke, T.; Brandl, M.; Sühnel, J. Density functional study of guanine and uracil quartets and of guanine quartet/metal ion complexes. J. Comput. Chem. 2001, 22, 109–124. [Google Scholar] [CrossRef]
- van Mourik, T.; Dingley, A.J. Characterization of the monovalent ion position and hydrogen bond network in guanine quartets by DFT calculations of NMR parameters. Chem. Eur. J. 2005, 11, 6064–6079. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Becke, A.D.; Johnson, E.R. A density-functional model of the dispersion interaction. J. Chem. Phys. 2005, 123, 154101. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- van der Wijst, T.; Fonseca Guerra, C.; Swart, M.; Bickelhaupt, F.M.; Lippert, B. A ditopic ion-pair receptor based on stacked nucleobase quartets. Angew. Chem. Int. Ed. 2009, 48, 3285–3287. [Google Scholar] [CrossRef] [PubMed]
- Boys, S.F.; Bernardi, F. Calculation of small molecular interactions by differences of separate total energies—Some procedures with reduced errors. Mol. Phys. 1970, 19, 553–566. [Google Scholar] [CrossRef]
- Neese, F. “The ORCA program system”, Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system, version 4.0, Wiley Interdiscip. Rev. Comput. Mol. Sci. 2018, 8, e1327. [Google Scholar]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef]
- Periodic Table-Astatine. Available online: http://www.rsc.org/periodic-table/element/85/astatine (accessed on 1 May 2020).
- CRC. Handbook of Chemistry and Physics, 98th ed.; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Dennington, R.; Keith, T.; Millam, J. Gauss View; Semichem, Inc.: Shawnee Mission, KS, USA, 2000. [Google Scholar]
- Sarr, S.; Graton, J.; Montavon, G.; Pilmé, J.; Galland, N. On the interplay between charge-shift bonding and halogen bonding. Chem. Phys. Chem. 2020, 21, 240–250. [Google Scholar] [CrossRef]



| Geometry | Energy | ΔECP | BSSE | R(I•••N) | R(I•••O) | ∠(NI•••N) | ∠(NI•••O) |
|---|---|---|---|---|---|---|---|
| BLYP-D3/SVP | BLYP-D3/SVP | −55.5 | −13.5 | 2.58 | 3.78 | 178 | 113 |
| BLYP-D3/SVP | BLYP-D3/TZVP | −53.2 | −2.1 | ||||
| BLYP-D3/TZVP | BLYP-D3/TZVP | −53.0 | −2.0 | 2.61 | 3.94 | 177 | 110 |
| BLYP-D3/SVP | B3LYP-D3/TZVP | −54.7 | −1.8 | ||||
| B3LYP-D3/TZVP | B3LYP-D3/TZVP | −53.2 | −1.7 | 2.60 | 3.98 | 177 | 107 |
| System | R(X•••N) | R(X•••O) | vdW(X•••N) | vdW(X•••O) | ∠(NX•••N) | ∠(NX•••O) |
|---|---|---|---|---|---|---|
| G•••1,2Br-G | 2.59 | 3.26 | 0.76 | 0.97 | 173.1 | 137.2 |
| G•••1,2I-G | 2.60 | 3.99 | 0.74 | 1.14 | 179.5 | 122.3 |
| G•••1,2At-G | 2.63 | 4.34 | 0.74 | 1.23 | 179.4 | 117.8 |
| System | R(X•••N) | R(X•••O) | vdW(X•••N) | vdW(X•••O) | ∠(NX•••N) | ∠(NX•••O) |
|---|---|---|---|---|---|---|
| (1,2Cl-G)2 | 2.58 | 3.24 | 0.78 | 0.99 | 178 | 135 |
| (1,2Br-G)2 | 2.57 | 3.47 | 0.76 | 1.03 | 179 | 124 |
| (1,2I-G)32 | 2.58 | 3.78 | 0.73 | 1.08 | 178 | 113 |
| (1,2At-G)2 | 2.60 | 3.74 | 0.73 | 1.06 | 177 | 111 |
| (1,2Cl-G)3 | 2.54/2.54 | 3.47/3.35 | 0.78/0.77 | 1.06/1.03 | 176/178 | 125/135 |
| (1,2Br-G)3 | 2.52/2.51 | 3.45/3.34 | 0.74/0.74 | 1.02/0.99 | 178/178 | 124/126 |
| (1,2I-G)33 | 2.53/2.52 | 3.76/3.67 | 0.72/0.71 | 1.07/1.05 | 178/179 | 109/116 |
| (1,2At-G)3 | 2.52/2.47 | 2.77/3.53 | 0.73/0.69 | 0.78/1.00 | 143/146 | 161/138 |
| System | R(X•••N) | R(X•••O) | vdW(X•••N) | vdW(X•••O) | ∠(NX•••N) | ∠(NX•••O) |
|---|---|---|---|---|---|---|
| Dimer 1 | ||||||
| (1,2Cl-G)2 | 2.83 | 0.86 | 166 | |||
| (1,2Br-G)2 | 2.85 | 0.84 | 163 | |||
| (1,2I-G)2 | 2.65/4.52 | 0.75/1.28 | 177/97 | |||
| (1,2At-G)2 | 2.55/4.19 | 0.71/1.17 | 178/91 | |||
| Dimer 2 | ||||||
| (1,2Cl-G)2 | 2.75 | 0.84 | 166 | |||
| (1,2Br-G)2 | 2.79 | 0.83 | 161 | |||
| (1,2I-G)2 | 2.94 | 0.84 | 155 | |||
| (1,2At-G)2 | 2.86 | 0.81 | 153 | |||
| (1,2Cl-G)3 | 2.86/2.81 | 2.72/2.77 | 0.87/0.85 | 0.83/0.85 | 165/167 | 167/165 |
| (1,2Br-G)3 | 2.82/2.85 | 2.74/2.83 | 0.83/0.84 | 0.81/0.84 | 165/160 | 163/159 |
| (1,2I-G)3 | 2.63/4.50 | 2.73/3.23 | 0.74/1.28 | 0.78/0.92 | 177/97 | 153/147 |
| (1,2At-G)3 | 2.56/4.16 | 2.68/3.13 | 0.72/1.17 | 0.76/0.88 | 178/91 | 160/146 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thornton, N.J.; van Mourik, T. Halogen-Bonded Guanine Base Pairs, Quartets and Ribbons. Int. J. Mol. Sci. 2020, 21, 6571. https://doi.org/10.3390/ijms21186571
Thornton NJ, van Mourik T. Halogen-Bonded Guanine Base Pairs, Quartets and Ribbons. International Journal of Molecular Sciences. 2020; 21(18):6571. https://doi.org/10.3390/ijms21186571
Chicago/Turabian StyleThornton, Nicholas J., and Tanja van Mourik. 2020. "Halogen-Bonded Guanine Base Pairs, Quartets and Ribbons" International Journal of Molecular Sciences 21, no. 18: 6571. https://doi.org/10.3390/ijms21186571
APA StyleThornton, N. J., & van Mourik, T. (2020). Halogen-Bonded Guanine Base Pairs, Quartets and Ribbons. International Journal of Molecular Sciences, 21(18), 6571. https://doi.org/10.3390/ijms21186571







