Multiple Known Mechanisms and a Possible Role of an Enhanced Immune System in Bt-Resistance in a Field Population of the Bollworm, Helicoverpa zea: Differences in Gene Expression with RNAseq
Abstract
1. Introduction
2. Results
2.1. Cry1Ac Susceptibility Bioassays
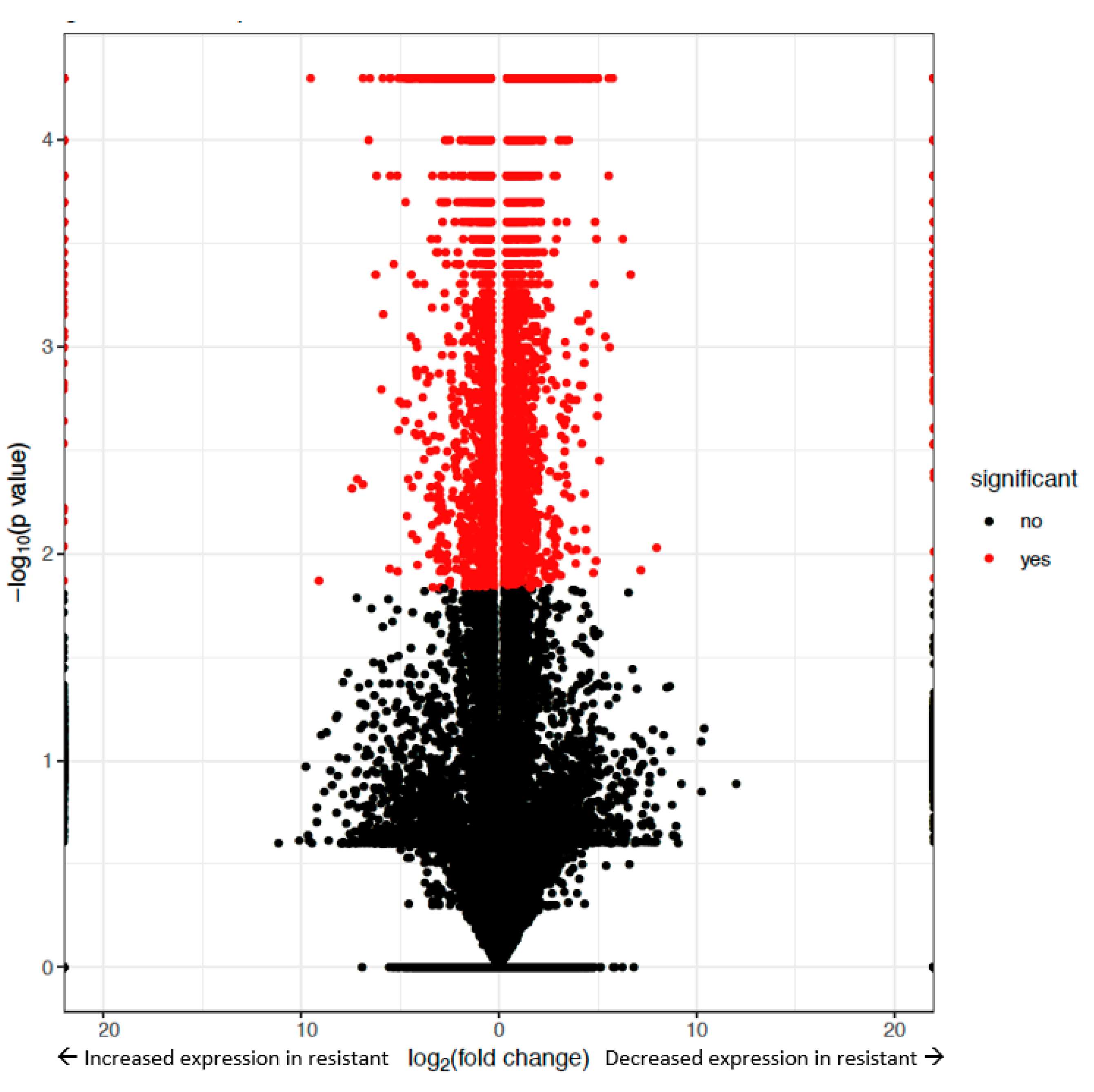
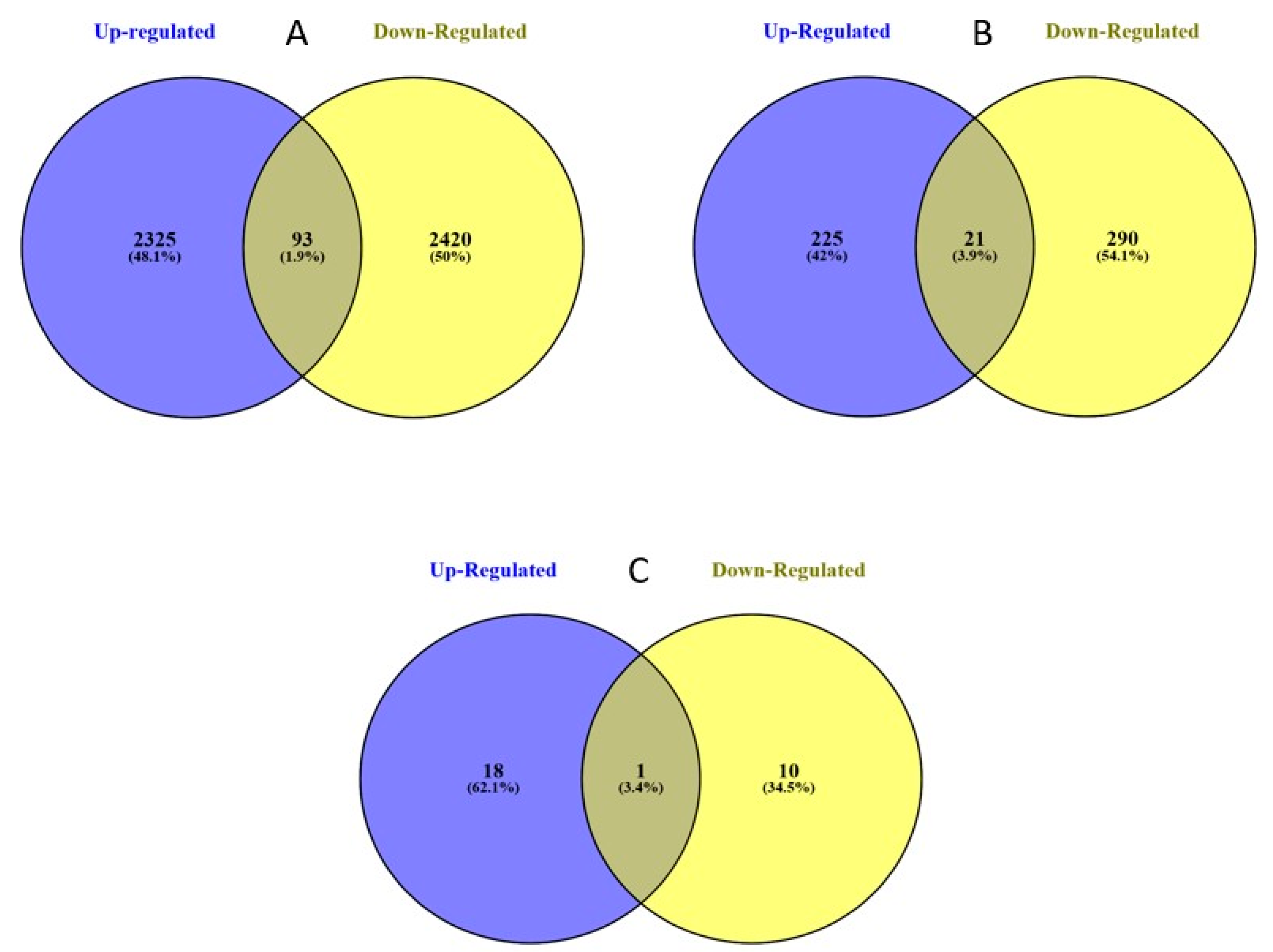
2.2. Genome-Wide Differential Expression in Helicoverpa zea
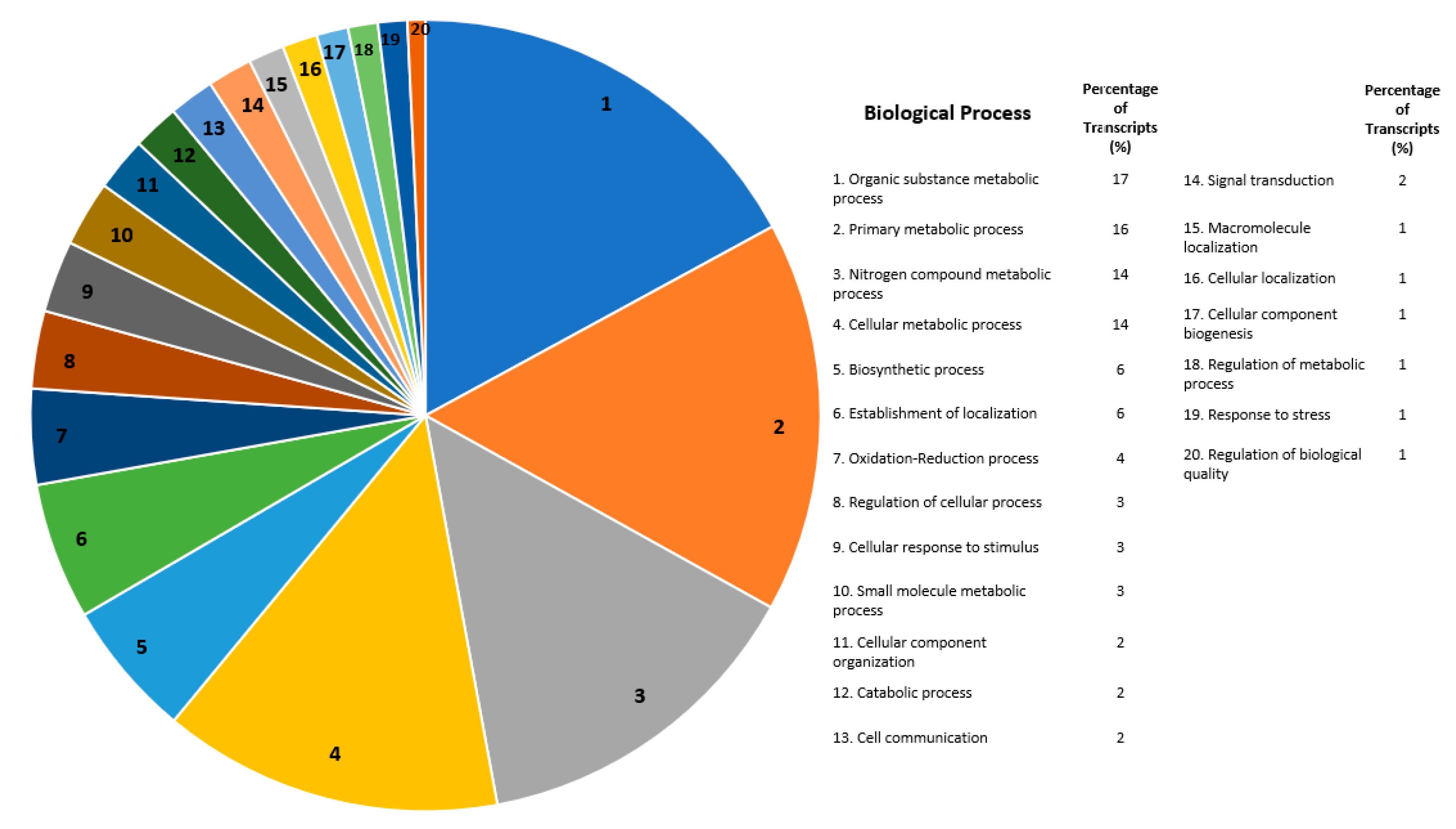
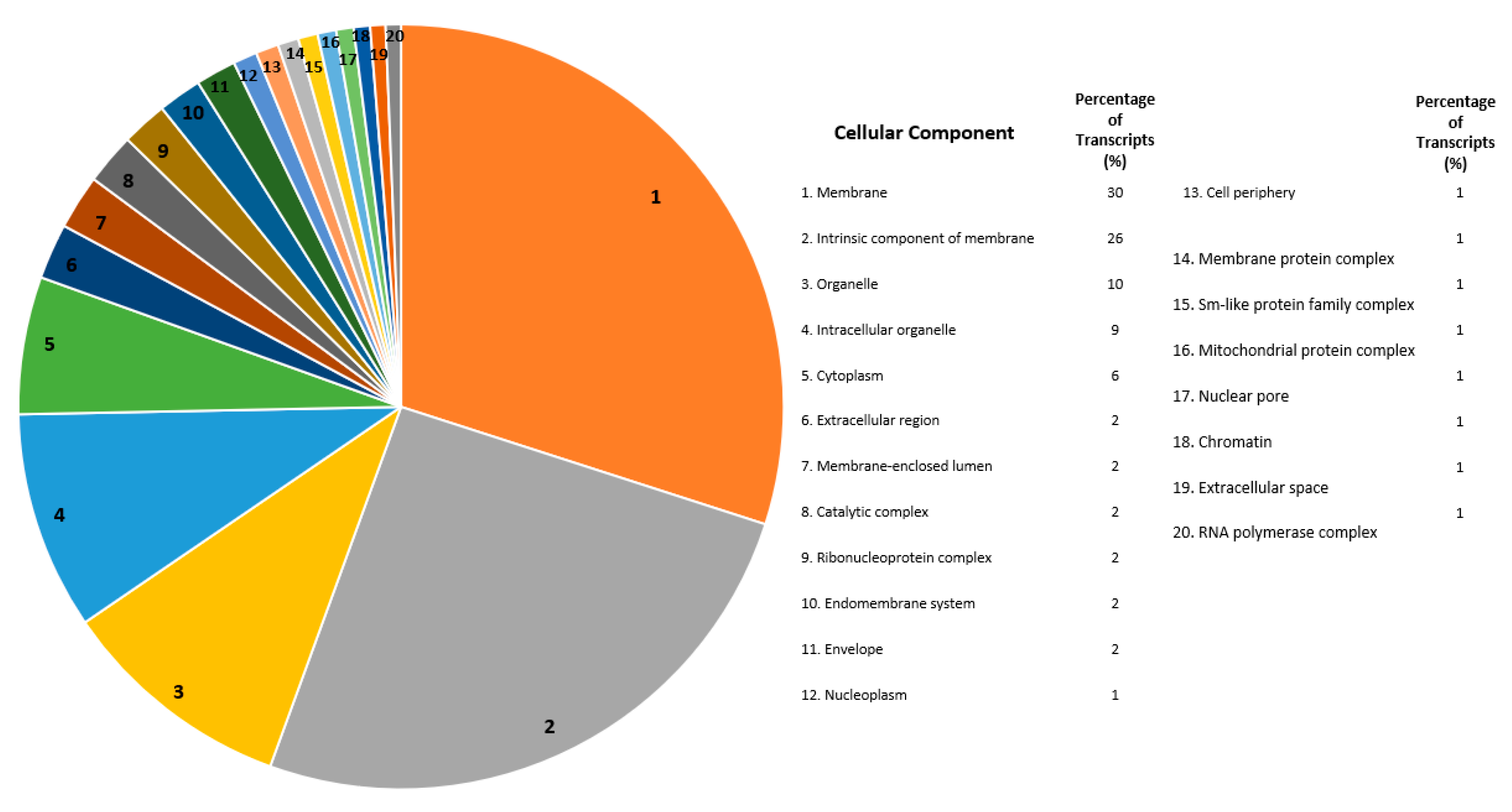
2.3. Global Gene Functional Annotations for Differentially Expressed Transcripts
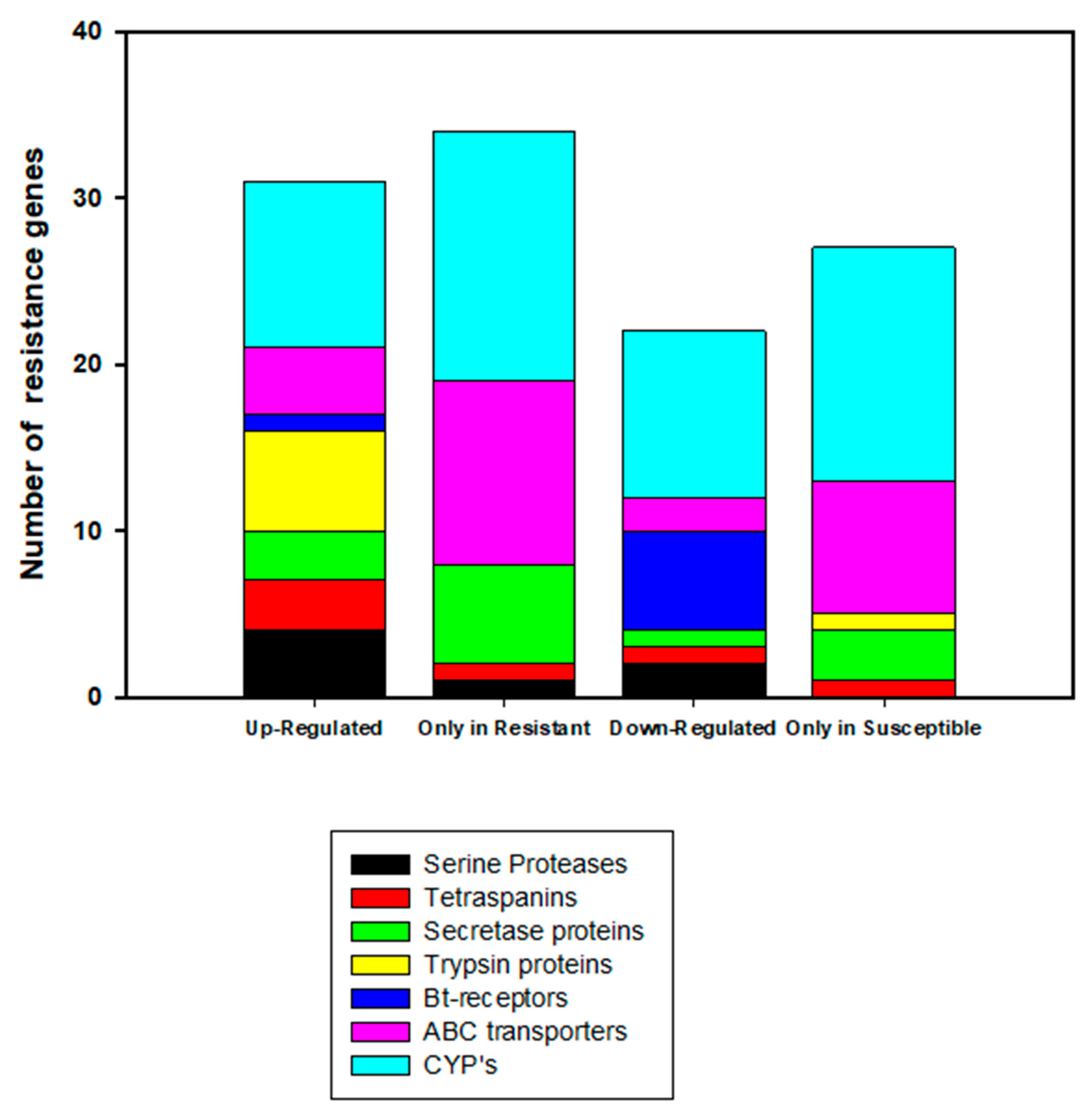
2.4. Bt-Resistance Associated Differential Expression
2.5. Differential Expression of Immune Function Associated Transcripts
3. Discussion
3.1. Resistant Versus Susceptible Bollworms
3.2. Differential Expression Between Bt Resistant Versus Susceptible Bollworms
3.3. Differential Expression of Cytochrome P450s in Bt-Resistant Helicoverpa zea
3.4. Genome Characterization of Global Differentially Expressed Transcripts
3.5. Role of Proteases, Receptors, and Transporters in Resistance
3.6. Role of Insect Immunity in Resistance
4. Materials and Methods
4.1. Sample Collection and Preparation
4.2. Cry1Ac Susceptibility Bioassays
4.3. RNA Extraction
4.4. RNA Sequencing
4.5. Transcript Assembly and Quality Control
4.6. Data Analysis and Figure Construction
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABC | ATP-binding cassette transporter |
| Bt | Bacillus thuringiensis |
| CYP | Cytochrome P450 |
| GMO | Genetically modified organism |
| H. zea | Helicoverpa zea |
| IMD | Immune deficiency |
| PGRP | Peptidoglycan receptor protein |
| TSPAN | Tetraspanin |
References
- USDA. Pests and Diseases of Cotton; United States Department of Agriculture: Washington, DC, USA, 1925.
- University of California Agriculture. UC IPM Pest Management Guidelines: Cotton. UC ANR Publication 3444. Available online: http://ipm.ucanr.edu/PMG/r114300511.html (accessed on 19 June 2019).
- Fleming, D.; Musser, F.; Reisig, D.; Greene, J.; Taylor, S.; Parajulee, M.; Lorenz, G.; Catchot, A.; Gore, J.; Kerns, D.; et al. Effects of transgenic Bacillus thuringiensis cotton on insecticide use, heliothine counts, plant damage, and cotton yield: A meta-analysis, 1996–2015. PLoS ONE 2018, 13, e0200131. [Google Scholar] [CrossRef]
- Zhu, B.; Xu, M.; Shi, H.; Gao, X.; Liang, P. Genome-wide identification of lncRNAs associated with chlorantraniliprole resistance in diamondback moth Plutella xylostella (L.). BMC Genom. 2017, 18, 380. [Google Scholar] [CrossRef]
- Palma, L.; Muñoz, D.; Berry, C.; Murillo, J.; Caballero, P. Bacillus thuringiensis toxins: An overview of their biocidal activity. Toxins 2014, 6, 3296–3325. [Google Scholar] [CrossRef]
- Qaim, M.; De Janvry, A. Bt cotton and pesticide use in Argentina: Economic and environmental effects. Environ. Dev. Econ. 2005, 10, 179–200. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (EPA). EPA’s Regulation of Bacillus thuringiensis (Bt) Crops. Available online: https://archive.epa.gov/pesticides/biopesticides/web/html/regofbtcrops.html (accessed on 19 June 2019).
- Statista. Cotton Production by Country Worldwide in 2018/2019. Available online: https://www.statista.com/statistics/263055/cotton-production-worldwide-by-top-countries/ (accessed on 19 June 2019).
- Bravo, A.; Gill, S.S.; Soberón, M. Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon 2007, 49, 423–435. [Google Scholar] [CrossRef]
- Moar, W.; Roush, R.; Shelton, A.; Ferre, J.; MacIntosh, S.; Leonard, B.R.; Abel, C. Field-evolved resistance to Bt toxins. Nat. Biotechnol. 2008, 26, 1072. [Google Scholar] [CrossRef]
- Tabashnik, B.E.; Van Rensburg, J.; Carrière, Y. Field-evolved insect resistance to Bt crops: Definition, theory, and data. J. Econ. Entomol. 2009, 102, 2011–2025. [Google Scholar] [CrossRef]
- Huang, F.; Qureshi, J.A.; Meagher, R.L., Jr.; Reisig, D.D.; Head, G.P.; Andow, D.A.; Ni, X.; Kerns, D.; Buntin, G.D.; Niu, Y. Cry1F resistance in fall armyworm Spodoptera frugiperda: Single gene versus pyramided Bt maize. PLoS ONE 2014, 9, e112958. [Google Scholar] [CrossRef]
- van Rensburg, J.B.J. First report of field resistance by the stem borer, Busseola fusca (Fuller) to Bt-transgenic maize. S. Afr. J. Plant Soil 2007, 24, 147–151. [Google Scholar] [CrossRef]
- Gassmann, A.J.; Petzold-Maxwell, J.L.; Keweshan, R.S.; Dunbar, M.W. Field-evolved resistance to Bt maize by western corn rootworm. PLoS ONE 2011, 6, e22629. [Google Scholar] [CrossRef] [PubMed]
- Gassmann, A.J.; Petzold-Maxwell, J.L.; Clifton, E.H.; Dunbar, M.W.; Hoffmann, A.M.; Ingber, D.A.; Keweshan, R.S. Field-evolved resistance by western corn rootworm to multiple Bacillus thuringiensis toxins in transgenic maize. Proc. Natl. Acad. Sci. USA 2014, 111, 5141–5146. [Google Scholar] [CrossRef] [PubMed]
- Dhurua, S.; Gujar, G.T. Field-evolved resistance to Bt toxin Cry1Ac in the pink bollworm, Pectinophora gossypiella (Saunders) (Lepidoptera: Gelechiidae), from India. Pest Manag. Sci. 2011, 67, 898–903. [Google Scholar] [CrossRef]
- Jackson, R.; Marcus, M.; Gould, F.; Bradley, J., Jr.; Van Duyn, J. Cross-resistance responses of Cry1Ac-selected Heliothis virescens (Lepidoptera: Noctuidae) to the Bacillus thuringiensis protein Vip3A. J. Econ. Entomol. 2007, 100, 180–186. [Google Scholar] [CrossRef]
- Gould, F.; Anderson, A.; Jones, A.; Sumerford, D.; Heckel, D.G.; Lopez, J.; Micinski, S.; Leonard, R.; Laster, M. Initial frequency of alleles for resistance to Bacillus thuringiensis toxins in field populations of Heliothis virescens. Proc. Natl. Acad. Sci. USA 1997, 94, 3519–3523. [Google Scholar] [CrossRef] [PubMed]
- Tabashnik, B.E.; Fabrick, J.A.; Unnithan, G.C.; Yelich, A.J.; Masson, L.; Zhang, J.; Bravo, A.; Soberón, M. Efficacy of genetically modified Bt toxins alone and in combinations against pink bollworm resistant to Cry1Ac and Cry2Ab. PLoS ONE 2013, 8, e80496. [Google Scholar] [CrossRef]
- Tabashnik, B.E.; Zhang, M.; Fabrick, J.A.; Wu, Y.; Gao, M.; Huang, F.; Wei, J.; Zhang, J.; Yelich, A.; Unnithan, G.C. Dual mode of action of Bt proteins: Protoxin efficacy against resistant insects. Sci. Rep. 2015, 5, 15107. [Google Scholar] [CrossRef]
- Dively, G.P.; Venugopal, P.D.; Finkenbinder, C. Field-evolved resistance in corn earworm to Cry proteins expressed by transgenic sweet corn. PLoS ONE 2016, 11, e0169115. [Google Scholar] [CrossRef]
- Yang, F.; González, J.C.S.; Williams, J.; Cook, D.C.; Gilreath, R.T.; Kerns, D.L. Occurrence and ear damage of Helicoverpa zea on transgenic Bacillus thuringiensis maize in the field in Texas, U.S. and its susceptibility to Vip3A protein. Toxins 2019, 11, 102. [Google Scholar] [CrossRef]
- Soberón, M.; Pardo, L.; Muñóz-Garay, C.; Sánchez, J.; Gómez, I.; Porta, H.; Bravo, A. Pore formation by Cry toxins. In Proteins Membrane Binding and Pore Formation; Anderluh, G., Lakey, J., Eds.; Landes Bioscience: Austin, TX, USA, 2010; pp. 127–142. [Google Scholar]
- Pardo-Lopez, L.; Soberon, M.; Bravo, A. Bacillus thuringiensis insecticidal three domain Cry toxins: Mode of action, insect resistance and consequences for crop protection. FEMS Microbiol. Rev. 2013, 37, 3–22. [Google Scholar] [CrossRef]
- Jurat-Fuentes, J.L.; Karumbaiah, L.; Jakka, S.R.K.; Ning, C.; Liu, C.; Wu, K.; Jackson, J.; Gould, F.; Blanco, C.; Portilla, M. Reduced levels of membrane-bound alkaline phosphatase are common to lepidopteran strains resistant to Cry toxins from Bacillus thuringiensis. PLoS ONE 2011, 6, e17606. [Google Scholar] [CrossRef]
- Gahan, L.J.; Pauchet, Y.; Vogel, H.; Heckel, D.G. An ABC transporter mutation is correlated with insect resistance to Bacillus thuringiensis Cry1Ac toxin. PLoS Genet. 2010, 6, e1001248. [Google Scholar] [CrossRef] [PubMed]
- Atsumi, S.; Miyamoto, K.; Yamamoto, K.; Narukawa, J.; Kawai, S.; Sezutsu, H.; Kobayashi, I.; Uchino, K.; Tamura, T.; Mita, K. Single amino acid mutation in an ATP-binding cassette transporter gene causes resistance to Bt toxin Cry1Ab in the silkworm, Bombyx mori. Proc. Natl. Acad. Sci. USA 2012, 109, E1591–E1598. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Wang, J.; Guan, F.; Zhang, J.; Yu, S.; Liu, S.; Xue, Y.; Li, L.; Wu, S.; Wang, X. Dominant point mutation in a tetraspanin gene associated with field-evolved resistance of cotton bollworm to transgenic Bt cotton. Proc. Natl. Acad. Sci. USA 2018, 115, 11760–11765. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wei, J.; Ni, X.; Zhang, J.; Jurat-Fuentes, J.L.; Fabrick, J.A.; Carrière, Y.; Tabashnik, B.E.; Li, X. Decreased Cry1Ac activation by midgut proteases associated with Cry1Ac resistance in Helicoverpa zea. Pest Manag. Sci. 2019, 75, 1019–1106. [Google Scholar] [CrossRef] [PubMed]
- Reisig, D.D.; Huseth, A.S.; Bacheler, J.S.; Aghaee, M.A.; Braswell, L.; Burrack, H.J.; Flanders, K.; Greene, J.K.; Herbert, D.A.; Jacobson, A. Long-term empirical and observational evidence of practical Helicoverpa zea resistance to cotton with pyramided Bt toxins. J. Econ. Entomol. 2018, 111, 1824–1833. [Google Scholar] [CrossRef] [PubMed]
- Conesa, A.; Götz, S.; García-Gómez, J.M.; Terol, J.; Talón, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef]
- Sayyed, A.H.; Moores, G.; Crickmore, N.; Wright, D.J. Cross-resistance between a Bacillus thuringiensis Cry toxin and non-Bt insecticides in the Diamondback Moth. Pest Manag. Sci. 2008, 64, 813–819. [Google Scholar] [CrossRef]
- Hafeez, M.; Liu, S.; Jan, S.; Shi, L.; Fernández-Grandon, G.M.; Gulzar, A.; Ali, B.; Rehman, M.; Wang, M. Knock-Down of gossypol-Inducing cytochrome P450 genes reduced deltamethrin sensitivity in Spodoptera exigua (Hübner). Int. J. Mol. Sci. 2019, 20, 2248. [Google Scholar] [CrossRef]
- Chen, A.; Zhang, H.; Shan, T.; Shi, X.; Gao, X. The overexpression of three cytochrome P450 genes CYP6CY14, CYP6CY22 and CYP6UN1 contributed to metabolic resistance to Dinotefuran in melon/cotton Aphid, Aphis Gossypii Glover. Pestic. Biochem. Physiol. 2020, 167, 104601. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, D.; Zhou, F.; Wang, G.; An, C. Identification of immunity-related genes in Ostrinia furnacalis against entomopathogenic fungi by RNA-seq analysis. PLoS ONE 2014, 9, e86436. [Google Scholar]
- Cao, X.; He, Y.; Hu, Y.; Wang, Y.; Chen, Y.R.; Bryant, B.; Clem, R.J.; Schwartz, L.M.; Blissard, G.; Jiang, H. The immune signaling pathways of Manduca sexta. Insect Biochem. Mol. Biol. 2015, 62, 64–74. [Google Scholar] [CrossRef]
- Liu, A.; Huang, X.; Gong, L.; Guo, Z.; Zhang, Y.; Yang, Z. Characterization of immune-related PGRP gene expression and phenoloxidase activity in Cry1Ac-susceptible and -resistant Plutella xylostella (L.). Pestic. Biochem. Physiol. 2019, 160, 79–86. [Google Scholar] [CrossRef]
- Xia, X.; Sun, B.; Gurr, G.M.; Vasseur, L.; Xue, M.; You, M. Gut microbiota mediate insecticide resistance in the diamondback moth, Plutella xylostella (L.). Front Microbiol. 2018, 9, 25. [Google Scholar] [CrossRef]
- Carriere, Y.; Degain, B.; Unnithan, G.C.; Harpold, V.S.; Li, X.; Tabashnik, B.E. Seasonal Declines in Cry1Ac and Cry2Ab concentration in maturing cotton favor faster evolution of resistance to pyramided Bt cotton in Helicoverpa zea (Lepidoptera: Noctuidae). J. Econ. Entomol. 2019, 112, 2907–2914. [Google Scholar] [CrossRef]
- Welch, K.L.; Unnithan, G.C.; Degain, B.A.; Wei, J.; Zhang, J.; Li, X.; Tabashnik, B.E.; Carriere, Y. Cross-resistance to toxins used in pyramided Bt crops and resistance to Bt sprays in helicoverpa zea. J. Invetrebr. Pathol. 2015, 132, 149–156. [Google Scholar] [CrossRef]
- Kullman, S.W.; Mattingly, C.J.; Meyer, J.N.; Whitehead, A. Perspectives on informatics in toxicology. In A Textbook of Modern Toxicology, 4th ed.; Hodgson, E., Ed.; Wiley: Hoboken, NJ, USA, 2010; pp. 593–605. [Google Scholar]
- Santos-Garcia, D.; Mestre-Rincon, N.; Zchori-Fein, E.; Morin, S. Inside Out: Microbiota dynamics during host-Plant adaption of whiteflies. ISME J. 2020, 14, 847–856. [Google Scholar] [CrossRef]
- Crava, C.M.; Brutting, C.; Baldwin, I.T. Transcriptome profiling reveals differential gene expression of detoxification enzymes in a hemimetabolous tobacco pest after feeding on jasmonate-silenced Nicotiana attenuata plants. BMC Genom. 2016, 17, 1005. [Google Scholar] [CrossRef]
- Mao, W.; Schuler, M.A.; Berenbaum, M.R. Task-related differential expression of four cytochrome P450 genes in honeybee appendages. Insect Mol. Biol. 2015, 24, 582–588. [Google Scholar] [CrossRef]
- Thorpe, P.; Escudero-Martinez, C.M.; Eves-van den Akker, S.; Bos, J.I.B. Transcriptional changes in the aphid species Myzus cerasi under different host and environmental conditions. Insect Mol. Biol. 2020, 29, 271–282. [Google Scholar] [CrossRef]
- Lei, Y.; Zhu, X.; Xie, W.; Wu, Q.; Wang, S.; Guo, Z.; Xu, B.; Li, X.; Zhou, X.; Zhang, Y. Midgut transcriptome response to a cry toxin in the Diamondback Moth, Plutella Xylostella (Lepidoptera: Plutellidae). Gene 2014, 533, 180–187. [Google Scholar] [CrossRef]
- Wei, J.; Yang, S.; Chen, L.; Liu, X.; Du, M.; An, S.; Liang, G. Transcriptomic Responses to Different Cry1Ac Selection Stresses in Helicoverpa armigera. Front Physiol. 2018, 9, 1653. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.N.; Wang, Y.Q.; Wang, Z.Y.; Hu, B.J.; Ling, Y.H.; He, K.L. Transcriptome differences between Cry1Ab resistant and susceptible strains of Asian corn borer. BMC Genom. 2015, 16, 173. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Kang, S.; Zhou, J.; Sun, D.; Guo, L.; Qin, J.; Zhu, L.; Bai, Y.; Ye, F.; Akami, M.; et al. Reduced Expression of a Novel Midgut Trypsin Gene Involved in Protoxin Activation Correlates with Cry1Ac Resistance in a Laboratory-Selected Strain of Plutella xylostella (L.). Toxins 2020, 12, 76. [Google Scholar] [CrossRef]
- Leinonen, R.; Sugawara, H.; Shumway, M. International Nucleotide Sequence Database Collaboration. The sequence read archive. Nucleic Acids Res. 2011, 39, D19–D21. [Google Scholar] [CrossRef] [PubMed]
- Babraham Bioinformatics: FastQC: A Quality Control Tool for High Throughput Sequence Data. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 8 January 2019).
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Haas, B.J.; Papanicolaou, A.; Yassour, M.; Grabherr, M.; Blood, P.D.; Bowden, J.; Couger, M.B.; Eccles, D.; Li, B.; Lieber, M.; et al. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat. Protoc. 2013, 8, 1494–1512. [Google Scholar] [CrossRef]
- Kumar, S.; Jones, M.; Koutsovoulos, G.; Clarke, M.; Blaxter, M. Blobology: Exploring raw genome data for contaminants, symbionts and parasites using taxon-annotated GC-coverage plots. Front. Genet. 2013, 4, 237. [Google Scholar] [CrossRef]
- Rago, A.; Gilbert, D.G.; Choi, J.H.; Sackton, T.B.; Wang, X.; Kelkar, Y.D.; Werren, J.H.; Colbourne, J.K. OGS2: Genome re-annotation of the jewel wasp Nasonia vitripennis. BMC Genom. 2016, 17, 678. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Trapnell, C.; Hendrickson, D.; Sauvageau, S.; Goff, L.; Rinn, J.L.; Pachter, L. Differential analysis of gene regulation at transcript resolution with RNA-seq. Nat. Biotechnol. 2013, 31, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential gene and transcripts expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2012. [Google Scholar]


| Gene a | Gene ID b | General Function c | Log2 Fold Increase d | |
|---|---|---|---|---|
| 1 | Hzea.19884 | WD repeat-containing protein on Y chromosome-like | Bromodomain protein | +9.08472 |
| 2 | Hzea.21942 | BAC, pupae DNA | Pupal protein | +7.16661 |
| 3 | Hzea.16694 | BAC, pupae DNA | Pupal protein | +6.87974 |
| 4 | Hzea.24399 | allergen Api m 6-like | Serine Type Endopeptidase, protease inhibitor | +6.86176 |
| 5 | Hzea.23153 | BAC, pupae DNA | Pupal protein | +6.51622 |
| 6 | Hzea.2403 | KGHa033C10 carboxyl/choline esterase | Dietary detoxification, hormone/pheromone degradation, neurodevelopment | +6.17351 |
| 7 | Hzea.15968 | fatty acid binding protein | Binding and transfer of fatty acids | +5.93447 |
| 8 | Hzea.27515 | zonadhesin-like | Facilitates binding of sperm to egg | +5.87046 |
| 9 | Hzea.2907 | sphingolipid delta(4)-desaturase/C4-monooxygenase DES2 | Degenerative spermatocyte protein | +5.83895 |
| 10 | Hzea.2119 | serine/threonine-protein kinase dyrk1 | Phosphorylation of serines and threonines | +5.51915 |
| 11 | Hzea.8474 | sorbitol dehydrogenase-like | Sorbitol metabolism | +5.51262 |
| 12 | Hzea.3274 | E3 ubiquitin-protein ligase Siah1-like | Proteosome mediated protein degradation | +5.46839 |
| 13 | Hzea.4257 | trypsin 3A1-like | Intestinal protein degradation | +5.31623 |
| 14 | Hzea.23538 | transmembrane protease serine 9-like | Serine protein cleavage | +5.13044 |
| 15 | Hzea.32418 | zinc finger protein 266-like | DNA binding domain protein | +5.0701 |
| 16 | Hzea.18297 | heat shock protein Hsp-12.2-like | Cellular stress response protein | +5.02824 |
| 17 | Hzea.18477 | AY2 tetraspanin 1 (TSPAN1) | Protein stabilization, cell signaling pathways, associated with BT resistance | +4.91784 |
| 18 | Hzea.13946 | facilitated trehalose transporter Tret1 | Trehalose transport from fat body | +4.73271 |
| 19 | Hzea.10453 | gamma-secretase subunit pen-2 | Cleavage of transmembrane proteins | +4.70584 |
| 20 | Hzea.31473 | JP123 retrotransposon HaRT3 | Replication | +4.6664 |
| 21 | Hzea.16029 | nose resistant to fluoxetine protein 6-like | Uptake of lipids and xenobiotics from intestines | +4.64037 |
| 22 | Hzea.1396 | BAC, pupae DNA | Pupal protein | +4.5819 |
| 23 | Hzea.15497 | chymotrypsin-1-like | Intestinal protein degradation | +4.44894 |
| 24 | Hzea.14146 | cytochrome P450 6B5-like | Xenobiotic metabolism | +4.4463 |
| 25 | Hzea.8807 | BAC, pupae DNA | Pupal protein | +4.43852 |
| 26 | Hzea.20434 | stabilizer of axonemal microtubules 2 | Microtubule binding | +4.42253 |
| 27 | Hzea.16622 | deoxyribose-phosphate aldolase | Deoxyribose phosphate catalysis | +4.37083 |
| 28 | Hzea.9448 | cytochrome P450 337B2v2 | Xenobiotic metabolism, associated with insecticide resistance | +4.23841 |
| 29 | Hzea.31690 | H/ACA ribonucleoprotein complex subunit 1-like | Ribosome biogenesis and telomere maintenance | +4.14773 |
| 30 | Hzea.8806 | protein lethal(2)essential for life-like | Embryonic development | +4.14618 |
| 31 | Hzea.27511 | JP151 retrotransposon HaRT2 | Replication | +4.14218 |
| 32 | Hzea.14487 | BAC 79L08 | Unknown | +4.13627 |
| 33 | Hzea.15502 | trypsin-like protease | Intestinal protein degradation | +4.12705 |
| 34 | Hzea.29377 | cytochrome P450 337B3v1 | Xenobiotic metabolism, associated with insecticide resistance | +4.10688 |
| 35 | Hzea.17522 | JP151 retrotransposon HaRT2 | Replication | +4.10241 |
| 36 | Hzea.1891 | odorant receptor Or1-like | Odorant receptor | +4.07168 |
| 37 | Hzea.25785 | BAC, pupae DNA | Pupal protein | +4.04936 |
| 38 | Hzea.391 | cytochrome P450 337B2v2 | Xenobiotic metabolism, associated with insecticide resistance | +4.0049 |
| 39 | Hzea.2400 | trypsin-1-like | Intestinal protein degradation | +3.94767 |
| 40 | Hzea.11080 | neuroblastoma-amplified sequence-like | Vesicle binding/transport | +3.89792 |
| 41 | Hzea.18643 | H/ACA ribonucleoprotein complex subunit 1-like | Ribosome biogenesis and telomere maintenance | +3.89234 |
| 42 | Hzea.30733 | NAD-dependent protein deacylase sirtuin-5, mitochondrial-like | Protein Deacylation | +3.78646 |
| 43 | Hzea.10806 | BAC, pupae DNA | Pupal protein | +3.77303 |
| 44 | Hzea.2665 | BAC, pupae DNA | Pupal protein | +3.76731 |
| 45 | Hzea.15446 | fas-binding factor 1 homolog | Binding protein, cell stabilization | +3.75496 |
| 46 | Hzea.19761 | BAC, pupae DNA | Pupal protein | +3.67297 |
| 47 | Hzea.12185 | trypsin, alkaline C-like | Intestinal protein degradation | +3.62558 |
| 48 | Hzea.23659 | BAC, pupae DNA | Pupal protein | +3.57875 |
| 49 | Hzea.29138 | zinc finger protein OZF-like | DNA binding domain protein | +3.56187 |
| 50 | Hzea.15307 | pancreatic triacylglycerol lipase-like | Lipid metabolism | +3.50668 |
| Gene a | Gene ID b | General Function c | Log2 Fold Decrease d | |
|---|---|---|---|---|
| 1 | Hzea.11969 | cytochrome P450 337B3v1 | Xenobiotic metabolism, associated with insecticide resistance | −7.18074 |
| 2 | Hzea.27562 | family 31 glucosidase KIAA1161-like | Glucose metabolism | −6.66764 |
| 3 | Hzea.17125 | protein ALP1-like | Cytoskeletal development | −6.26055 |
| 4 | Hzea.29678 | tudor domain-containing protein 7A | Post-transcriptional modification | −5.60295 |
| 5 | Hzea.812 | cytochrome P450 337B3v1 | Xenobiotic metabolism, associated with insecticide resistance | −5.56349 |
| 6 | Hzea.6938 | splicing factor 3B subunit 4-like | Gene Splicing | −5.09605 |
| 7 | Hzea.12845 | putative nuclease HARBI1 | Nucleic Acid cleavage | −5.01358 |
| 8 | Hzea.31102 | cuticle protein-like | Cuticle structural protein | −4.96903 |
| 9 | Hzea.15432 | transcription factor Adf-1-like | Adh gene expression regulation | −4.93912 |
| 10 | Hzea.28236 | ATP-binding cassette sub-family G member 1 | ABC transporter protein | −4.92069 |
| 11 | Hzea.14683 | BAC, pupae DNA | Pupal Protein | −4.82383 |
| 12 | Hzea.12475 | isolate AD2 clone 1 microsatellite D47 | Unknown | −4.78341 |
| 13 | Hzea.7219 | BAC, egg DNA | Oval DNA | −4.77338 |
| 14 | Hzea.4118 | multiple inositol polyphosphate phosphatase 1-like | Regulates cellular inositol levels | −4.63011 |
| 15 | Hzea.28234 | serine/threonine-protein kinase RIO1 | Ribosomal subunit maturation | −4.61965 |
| 16 | Hzea.15388 | zinc finger protein 628-like | DNA binding domain protein | −4.61078 |
| 17 | Hzea.13573 | glucose dehydrogenase [FAD, quinone]-like | Glucose metabolism | −4.56858 |
| 18 | Hzea.811 | BAC 33J17 cytochrome P450 337B3v1 | Xenobiotic metabolism, associated with insecticide resistance | −4.54212 |
| 19 | Hzea.17013 | small nuclear ribonucleoprotein F | Pre-mRNA splicing | −4.54025 |
| 20 | Hzea.32168 | UDP-glucuronosyltransferase 2B4-like | Glucuronidation catalysis | −4.51753 |
| 21 | Hzea.4237 | KGHa033C10 carboxyl/choline esterase CCE001f | Dietary detoxification, hormone/pheromone degradation, neurodevelopment | −4.4949 |
| 22 | Hzea.21278 | facilitated trehalose transporter Tret1-like | Trehalose transport from fat body | −4.46546 |
| 23 | Hzea.25419 | putative nuclease HARBI1 | Nucleic Acid cleavage | −4.41916 |
| 24 | Hzea.9745 | BAC 18J13 cytochrome P450 337B2v2 and cytochrome P450 337B1v1 | Xenobiotic metabolism, associated with insecticide resistance | −4.32618 |
| 25 | Hzea.13850 | BAC, pupae DNA | Pupal Protein | −4.30511 |
| 26 | Hzea.12890 | fatty acid synthase-like | Fatty acid synthesis | −4.27779 |
| 27 | Hzea.4150 | phospholipase A2-like | Fatty acid cleavage | −4.24604 |
| 28 | Hzea.3034 | BAC, pupae DNA | Pupal Protein | −4.20592 |
| 29 | Hzea.13370 | cytochrome P450 6B6 | Xenobiotic metabolism | −4.19982 |
| 30 | Hzea.11776 | cuticle protein 65 | Cuticle structural protein | −4.10268 |
| 31 | Hzea.4423 | lipase member K-like | Lipid metabolism | −3.98569 |
| 32 | Hzea.23288 | cuticle protein 1 | Cuticle structural protein | −3.97585 |
| 33 | Hzea.25418 | putative nuclease HARBI1 | Nucleic Acid cleavage | −3.95527 |
| 34 | Hzea.6856 | enoyl-CoA delta isomerase 1, mitochondrial-like | Fatty acid oxidation | −3.95465 |
| 35 | Hzea.4455 | dihydrofolate reductase | Dihydrofolic reduction | −3.931 |
| 36 | Hzea.21115 | zinc finger BED domain-containing protein 1-like | DNA binding domain protein | −3.9258 |
| 37 | Hzea.14952 | guanine nucleotide-binding protein G(q) subunit alpha | Guanine binding | −3.8824 |
| 38 | Hzea.20452 | cytochrome P450 337B2v2 | Xenobiotic metabolism, associated with insecticide resistance | −3.8696 |
| 39 | Hzea.13504 | protein deltex | Cell communication, Notch pathway | −3.86954 |
| 40 | Hzea.11171 | organic cation transporter protein-like | Transport protein, cations | −3.81687 |
| 41 | Hzea.14992 | alkaline phosphatase 2 | BT receptor in intestines | −3.77113 |
| 42 | Hzea.30431 | BAC, pupae DNA | Pupal Protein | −3.75267 |
| 43 | Hzea.7746 | cytochrome P450 337B2v2 | Xenobiotic metabolism, associated with insecticide resistance | −3.71829 |
| 44 | Hzea.5603 | UDP-glucuronosyltransferase 2B7-like | Glucuronidation catalysis | −3.71518 |
| 45 | Hzea.21691 | BAC, pupae DNA | Pupal Protein | −3.69295 |
| 46 | Hzea.17647 | beta-secretase 1-like | Protein Cleavage | −3.64879 |
| 47 | Hzea.32280 | tyrosine--tRNA ligase | Ligation of tRNA and tyrosine, translation | −3.6039 |
| 48 | Hzea.23371 | CD209 antigen-like protein 2 | Pathogen recognition receptor | −3.59733 |
| 49 | Hzea.14510 | BAC, pupae DNA | Pupal Protein | −3.5325 |
| 50 | Hzea.21503 | polyribonucleotide nucleotidyltransferase 1 | Transferase protein | −3.48953 |
| Gene a | Gene ID b | General Function c | Log2 Fold Change d | |
|---|---|---|---|---|
| Tetraspanins | Hzea.18477 | Tetraspanin 1 | Protein stabilization, cell signaling pathways, associated with BT resistance | +4.92 |
| Hzea.11255 | Tetraspanin 1 | Protein stabilization, cell signaling pathways, associated with BT resistance | +3.37 | |
| Hzea.498 | Tetraspanin 1 | Protein stabilization, cell signaling pathways, associated with BT resistance | +2.88 | |
| Serine Proteases | Hzea.23538 | Serine Protease 9-like | Protein cleavage | +5.13 |
| Hzea.24399 | allergen Api m 6-like | Serine Type Endopeptidase | +6.86176 | |
| Hzea.7824 | Serine Protease Snake-like | Protein cleavage | +3.1 | |
| Hzea.5128 | Serine Protease 9-like | Protein cleavage | +2.4 | |
| Hzea.16515 | Serine Protease 3-like | Protein cleavage | +2.23 | |
| Secretase Proteins | Hzea.10453 | Gamma-Secretase | Cleavage of transmembrane proteins | +4.7 |
| Hzea.30068 | Beta-Secretase 1-like | Protein Cleavage | +3.04 | |
| Hzea.27773 | Beta-Secretase 1-like | Protein cleavage | +2.07 | |
| Trypsin Proteins | Hzea.15497 | Chymotrypsin 1-like | Intestinal protein cleavage | +4.44 |
| Hzea.4257 | Trypsin 3A1-like | Intestinal protein cleavage | +5.31 | |
| Hzea.15502 | Trypsin-like Protease | Intestinal protein cleavage | +4.12 | |
| Hzea.2400 | trypsin 1-like | Intestinal protein cleavage | +3.94 | |
| Hzea.12185 | Trypsin, Alkaline C like | Intestinal protein cleavage | +3.62 | |
| Hzea.12186 | Trypsin, Alkaline C like | Intestinal protein cleavage | +2.17 | |
| Bt-Receptors | Hzea.14992 | Alkaline Phosphatase 2 | Intestinal receptor for Cry1Ac | −3.77 |
| Hzea.17125 | Alkaline Phosphatase 1 like | Intestinal receptor for Cry1Ac | −6.26 | |
| Hzea.11178 | Mutant Cadherin (BtR) | Intestinal receptor for Cry1Ac | +2.93 | |
| Hzea.18127 | Cadherin-like | Intestinal receptor for Cry1Ac | −2.29 | |
| Hzea.20 | Caherin-r15 | Intestinal receptor for Cry1Ac | −2.89 | |
| Hzea.8058 | Aminopeptidase 1D | Intestinal receptor for Cry1Ac | −2.02 | |
| Hzea.16397 | Aminopeptidase 1 | Intestinal receptor for Cry1Ac | −2.21 | |
| Peptidases | Hzea.12825 | Carboxypeptidase B like | Peptide cleavage | +3.04 |
| Hzea.7667 | Carboxypeptidase B like | Peptide cleavage | −2.03 | |
| Transporters | Hzea.29517 | Multidrug Resistance protein 1 like | Efflux transporter | +2.22 |
| Hzea.3344 | Multidrug Resistance protein 4 like | Efflux transporter | −2.06 | |
| Hzea.9148 | ABC Transporter ABCC3 | Transporter protein | +3.36 | |
| Hzea.10318 | ABC Transporter ABCC3 | Transporter protein | +3.06 | |
| Hzea.13698 | ABC Transporter ABCC2 | Transporter protein | +2.6 | |
| Hzea.13541 | ABC Transporter ABCC3 | Transporter protein | +2.5 | |
| Chitin | Hzea.21994 | Chitin Synthase A & B | Chitin synthesis | +3.11 |
| Hzea.18297 | Heat Shock Protein 12.2 | Cellular stress response | +5.02 | |
| Metabolic transcripts | Hzea.9448 | CYP337B2v2 & CYP337B1v1 | Xenobiotic metabolism, associated with resistance to insecticides | +4.23 |
| Hzea.29377 | CYP337B3v1 | Xenobiotic metabolism, associated with resistance to insecticides | +4.1 | |
| Hzea.391 | CYP337B2v2 & CYP337B1v1 | Xenobiotic metabolism, associated with resistance to insecticides | +4 | |
| Hzea.20633 | CYP337B3v1 | Xenobiotic metabolism, associated with resistance to insecticides | +3.37 | |
| Hzea.6595 | CYP367A2 | Xenobiotic metabolism | +2.43 | |
| Hzea.1468 | CYP4AU1 | Xenobiotic metabolism | +2.31 | |
| Hzea.13660 | CYP337B3v1 | Xenobiotic metabolism, associated with resistance to insecticides | +2.22 | |
| Hzea.18411 | CYP421A5 | Xenobiotic metabolism | +2.16 | |
| Hzea.16306 | CYP337B3v1 | Xenobiotic metabolism, associated with resistance to insecticides | +2.04 | |
| Hzea.29950 | CYP337B3v1 | Xenobiotic metabolism, associated with resistance to insecticides | +2.01 | |
| Hzea.2403 | carboxyl/choline esterase CCE001f | Xenobiotic metabolism, associated with resistance to insecticides | +6.17351 | |
| Protease | Hzea.3274 | E3 ubiquitin-protein ligase Siah1-like | Proteosome mediated protein degradation | +5.46839 |
| Gene a | Gene ID b | Strain | General Function c | Log2 Fold Change d |
|---|---|---|---|---|
| Hzea.27139 | Peptidoglycan (PGN) Recognition Protein C | Resistant | IMD immune pathway | +1.86 |
| Hzea.24180 | lysM & PGN binding protein 2 | Resistant | IMD immune pathway | +1.43 |
| Hzea.15134 | PGN recognition protein LB | Resistant | IMD immune pathway | +1.11 |
| Hzea.8213 | NF-kappa-beta p110 subunit | Resistant | IMD immune pathway | +0.88 |
| Hzea.3860 | NF-kappa-beta binding protein | Resistant | IMD immune pathway | +0.74 |
| Hzea.11065 | Immunoglobulin binding protein | Resistant | IMD immune pathway | +3.41 |
| Hzea.15446 | Fas-binding factor 1 | Resistant | IMD immune pathway | +3.75496 |
| Hzea.18171 | Transforming Growth Factor Beta 1 | Resistant | IMD immune pathway | +0.545215 |
| Hzea.22952 | Transforming Growth Factor Beta 1 Receptor | Resistant | IMD immune pathway | +0.486983 |
| Hzea.12555 | TGF-Beta Activated Kinase 1 | Susceptible | IMD immune pathway | −0.584251 |
| Hzea.2642 | Cecropin 1 | Susceptible | Antibacterial protein | −3.39285 |
| Hzea.5285 | Lysozyme-like | Resistant | Antimicrobial protein | +0.984174 |
| Hzea.8918 | Lysozyme-like | Susceptible | Antimicrobial protein | −0.469066 |
| Hzea.5268 | Lysozyme | Susceptible | Antimicrobial protein | −3.34181 |
| Hzea.19610 | Putative Defense Protein Hdd11 | Resistant | Pathogen Defense | +1.11573 |
| Hzea.28790 | Beta-1,3-glucanase protein | Resistant | Pathogen recognition protein | +1.47469 |
| Hzea.30418 | Nodulin 75 like | Resistant | General Immune response | +1.55155 |
| Hzea.5226 | Adaptor molecule Crk | Resistant | Immune signaling | +0.598283 |
| Hzea.5227 | Adaptor molecule Crk | Susceptible | Immune signaling | −0.79207 |
| Hzea.8567 | Autophagy protein 5 | Resistant | Programmed cell death, removes unnecessary cells. Involved in immunity | +0.984792 |
| Hzea.23318 | Autophagy Related protein 16-1 | Resistant | Programmed cell death, removes unnecessary cells. Involved in immunity | +0.918917 |
| Hzea.21156 | Autophagy Related protein 13 | Resistant | Programmed cell death, removes unnecessary cells. Involved in immunity | +0.80002 |
| Hzea.25537 | Autophagy Related protein 2 homolog A | Susceptible | Programmed cell death, removes unnecessary cells. Involved in immunity | −0.690981 |
| Hzea.25536 | Autophagy related protein 2 homolog A | Susceptible | Programmed cell death, removes unnecessary cells. Involved in immunity | −1.00944 |
| Hzea.25507 | Autophagy related protein 2 homolog A | Only in Resistant | Programmed cell death, removes unnecessary cells. Involved in immunity | N/A |
| Hzea.2633 | C-type lectin | Susceptible | Immune response to pathogens | −1.07 |
| Hzea.19146 | Death associated protein kinase 1 | Resistant | Toll immune pathway | +0.678001 |
| Hzea.15107 | Toll like receptor 3 | Resistant | Toll immune pathway | +1.29648 |
| Hzea.4906 | Toll like protein | Resistant | Toll immune pathway | +0.327495 |
| Hzea.17194 | Signaling intermediate in Toll pathway | Susceptible | Toll immune pathway | −0.83 |
| Hzea.24906 | CD63 Antigen | Resistant | General Immune response | +1.08759 |
| Hzea.23769 | Antigen 8 | Resistant | General Immune response | +0.582441 |
| Hzea.25130 | CD109 antigen like | Resistant | General Immune response | +0.529484 |
| Hzea.24900 | CD63 antigen like | Resistant | General Immune response | +0.513073 |
| Hzea.25569 | H13 Antigen | Resistant | General Immune response | +0.334133 |
| Hzea.24905 | Antigen CD53 like | Susceptible | General Immune response | −0.8215 |
| Hzea.8177 | cell nuclear antigen | Susceptible | General Immune response | −1.15965 |
| Hzea.23371 | CD209 antigen like protein 2 | Susceptible | General Immune response | −3.59733 |
| Hzea.9589 | CD63 antigen | Only in Susceptible | General Immune response | N/A |
| Hzea.11558 | CD109 antigen | Susceptible | General Immune response | −1.03885 |
| Hzea.8646 | tyrosine kinase hopscotch | Resistant | JAK/STAT pathway | +1.10303 |
| Hzea.19419 | signal transducer and activator of transcription 5B (STAT5B) | Resistant | JAK/STAT pathway | +0.589054 |
| Hzea.17134 | SH3 domain-containing kinase-binding protein 1 | Resistant | JAK/STAT pathway | +0.676851 |
| Hzea.3426 | E3 SUMO-protein ligase PIAS3 | Susceptible | JAK/STAT pathway | −0.79 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lawrie, R.D.; Mitchell III, R.D.; Deguenon, J.M.; Ponnusamy, L.; Reisig, D.; Pozo-Valdivia, A.D.; Kurtz, R.W.; Roe, R.M. Multiple Known Mechanisms and a Possible Role of an Enhanced Immune System in Bt-Resistance in a Field Population of the Bollworm, Helicoverpa zea: Differences in Gene Expression with RNAseq. Int. J. Mol. Sci. 2020, 21, 6528. https://doi.org/10.3390/ijms21186528
Lawrie RD, Mitchell III RD, Deguenon JM, Ponnusamy L, Reisig D, Pozo-Valdivia AD, Kurtz RW, Roe RM. Multiple Known Mechanisms and a Possible Role of an Enhanced Immune System in Bt-Resistance in a Field Population of the Bollworm, Helicoverpa zea: Differences in Gene Expression with RNAseq. International Journal of Molecular Sciences. 2020; 21(18):6528. https://doi.org/10.3390/ijms21186528
Chicago/Turabian StyleLawrie, Roger D., Robert D. Mitchell III, Jean Marcel Deguenon, Loganathan Ponnusamy, Dominic Reisig, Alejandro Del Pozo-Valdivia, Ryan W. Kurtz, and R. Michael Roe. 2020. "Multiple Known Mechanisms and a Possible Role of an Enhanced Immune System in Bt-Resistance in a Field Population of the Bollworm, Helicoverpa zea: Differences in Gene Expression with RNAseq" International Journal of Molecular Sciences 21, no. 18: 6528. https://doi.org/10.3390/ijms21186528
APA StyleLawrie, R. D., Mitchell III, R. D., Deguenon, J. M., Ponnusamy, L., Reisig, D., Pozo-Valdivia, A. D., Kurtz, R. W., & Roe, R. M. (2020). Multiple Known Mechanisms and a Possible Role of an Enhanced Immune System in Bt-Resistance in a Field Population of the Bollworm, Helicoverpa zea: Differences in Gene Expression with RNAseq. International Journal of Molecular Sciences, 21(18), 6528. https://doi.org/10.3390/ijms21186528






