Osteoclasts’ Ability to Generate Trenches Rather Than Pits Depends on High Levels of Active Cathepsin K and Efficient Clearance of Resorption Products
Abstract
1. Introduction
2. Results
2.1. OCs in Pit or Trench Mode Are Differently Sensitive to Drugs Affecting Collagenolysis, Acidification, and Endocytosis
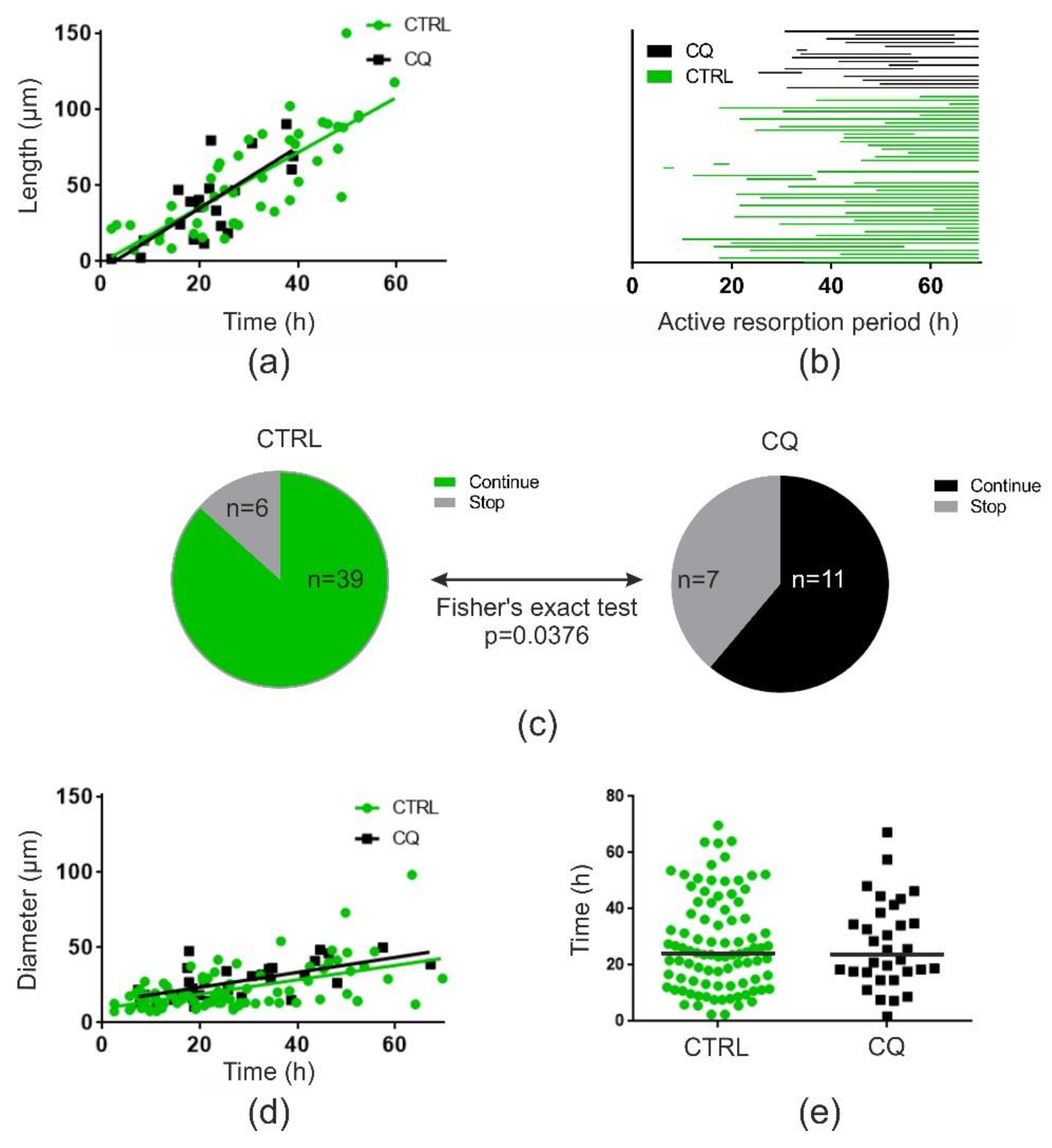
2.2. Chloroquine (at 10 or 20 µM) Does Not Affect Resorption Depth but Shortens Trenches Significantly
2.3. Chloroquine (10 µM) Does Not Affect Resorption Speed but Induces a Premature Stop of Resorption by OCs in Trench Mode
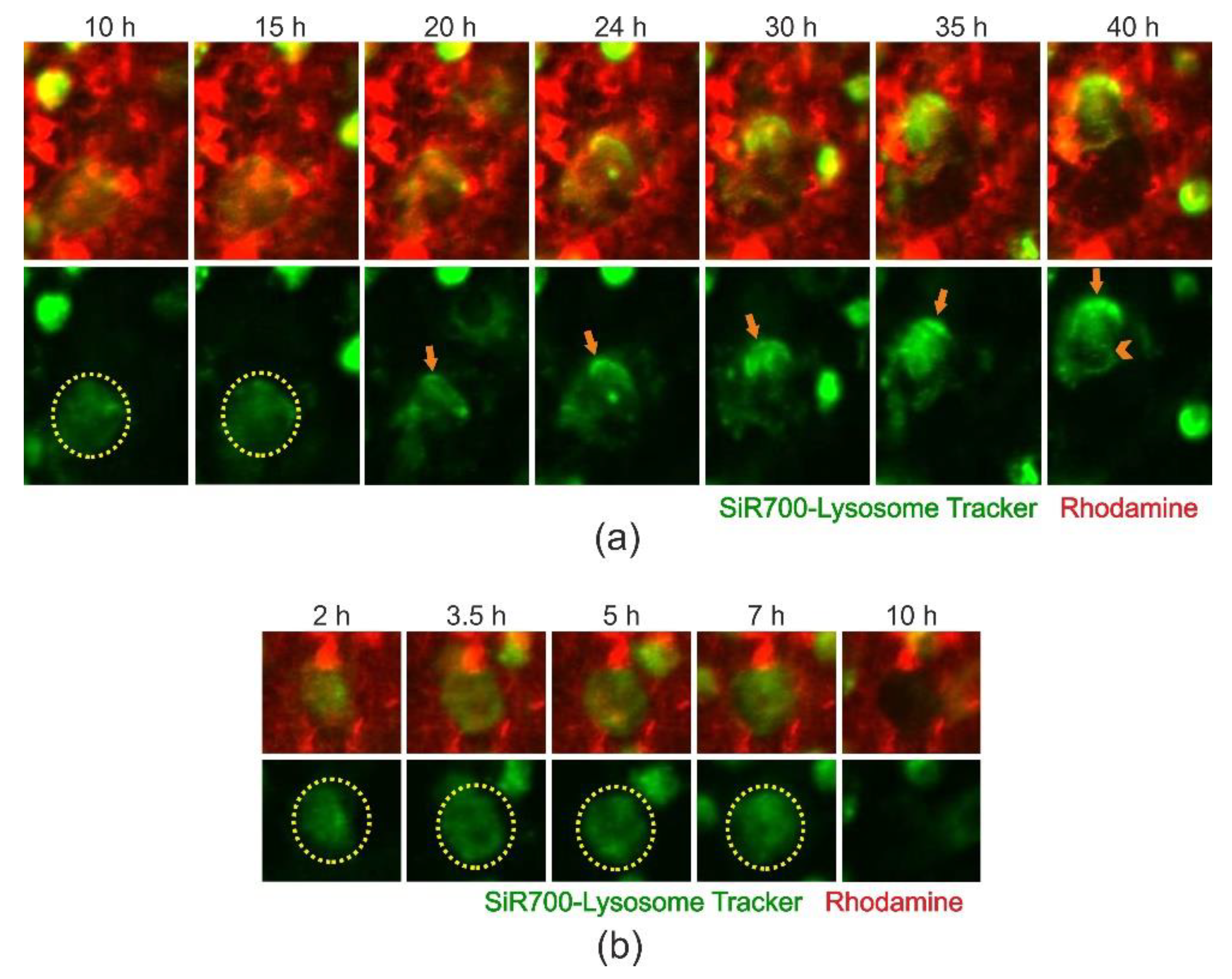
2.4. Cathepsin D-Containing Lysosomes Appear at the Resorptive Front of Trench-Forming OCs and Are Transported Towards the Distal End
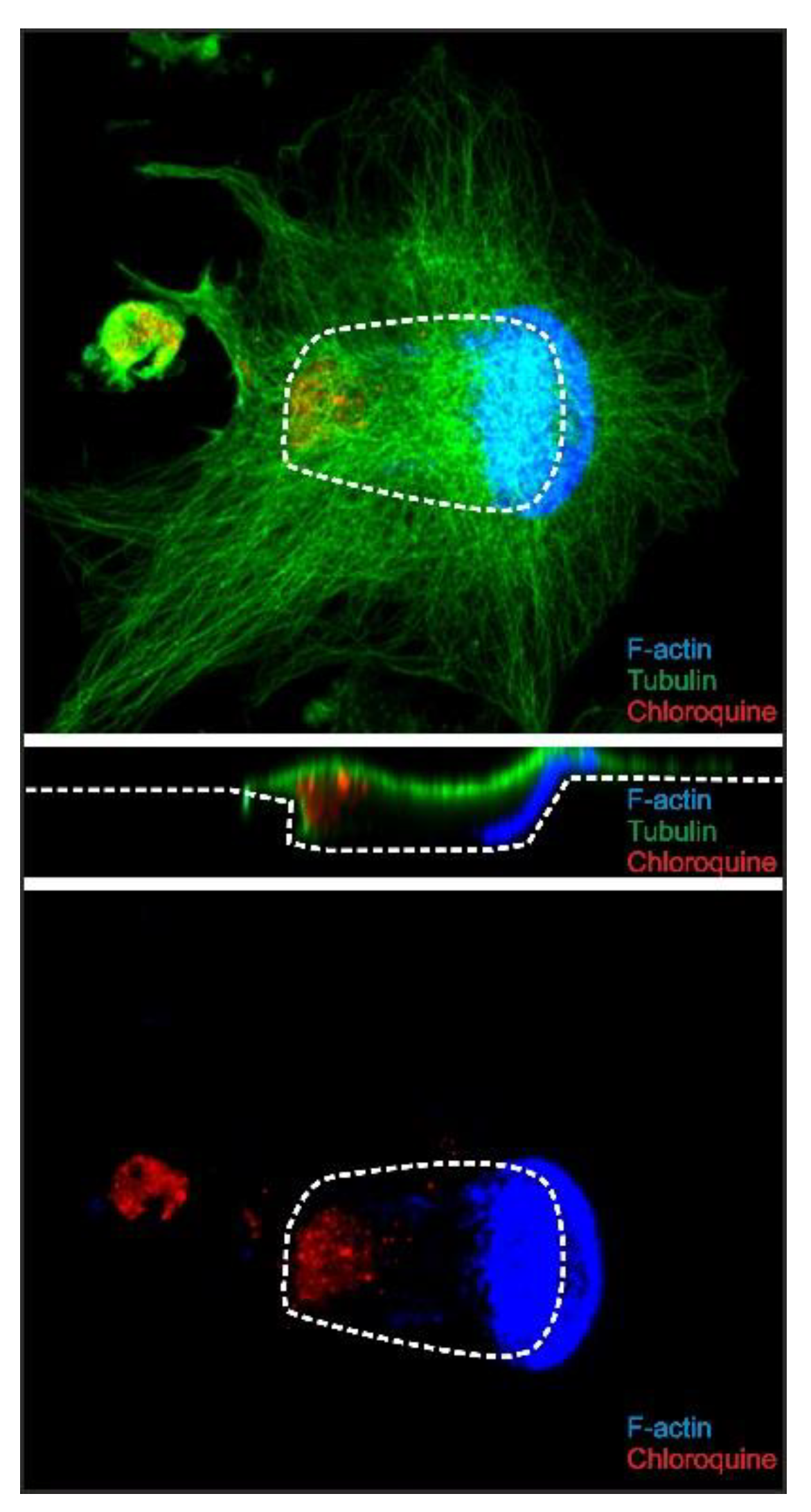
2.5. Chloroquine Accumulates in the Distal End of Trench-Forming OCs
3. Discussion
3.1. High Levels of Cathepsin K Are Needed for Initiating Trench Formation: Th Role of Extracellular Collagenolysis
3.2. Involvement of Clathrin-Mediated Endocytosis in Both Pit- and Trench-Forming OCs
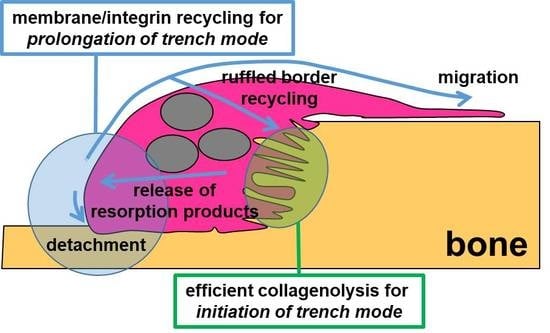
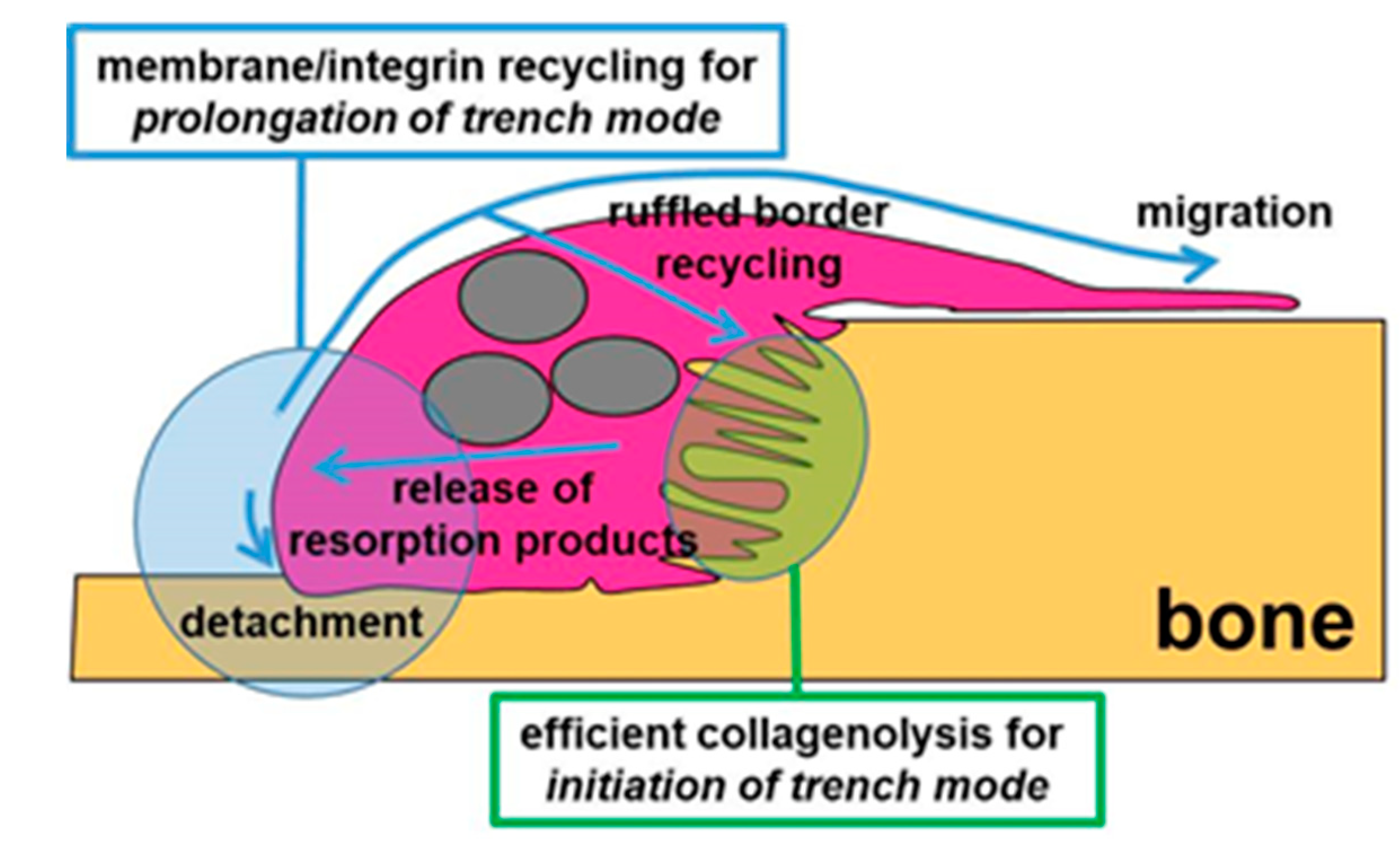
3.3. A Mechanism Integrating Release of Resorption Products and Membrane/Integrin Recycling Is Needed for Prolonged Simultaneous Resorption and Migration
4. Materials and Methods
4.1. Generation of OCs
4.2. Osteoclastic Bone Resorption Assay
4.3. Quantification of Bone Resorption
4.4. IF Staining and Confocal Microscopy of Bone-Resorbing OCs
4.5. Time-Lapse Recording of Bone-Resorbing OCs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Søe, K.; Delaisse, J.-M.; Borggaard, X.G. Osteoclast formation at the bone marrow/bone surface interface: Importance of structural elements, matrix, and intercellular communication. Semin. Cell Dev. Biol. 2020, S1084-9521(19)30260-5. [Google Scholar]
- Feng, X.; Teitelbaum, S.L. Osteoclasts: New Insights. Bone Res. 2013, 1, 11–26. [Google Scholar] [PubMed]
- Garnero, P.; Borel, O.; Byrjalsen, I.; Ferreras, M.; Drake, F.H.; McQueney, M.S.; Foged, N.T.; Delmas, P.D.; Delaisse, J.M. The Collagenolytic Activity of Cathepsin K Is Unique among Mammalian Proteinases. J. Biol. Chem. 1998, 273, 32347–32352. [Google Scholar] [CrossRef] [PubMed]
- Saftig, P.; Hunziker, E.; Wehmeyer, O.; Jones, S.; Boyde, A.; Rommerskirch, W.; Moritz, J.D.; Schu, P.; von Figura, K. Impaired osteoclastic bone resorption leads to osteopetrosis in cathepsin-K-deficient mice. Proc. Natl. Acad. Sci. USA 1998, 95, 13453–13458. [Google Scholar] [CrossRef] [PubMed]
- Panwar, P.; Du, X.; Sharma, V.; Lamour, G.; Castro, M.; Li, H.; Bromme, D. Effects of cysteine proteases on the structural and mechanical properties of collagen fibers. J. Biol.Chem. 2013, 288, 5940–5950. [Google Scholar] [CrossRef]
- Aguda, A.H.; Panwar, P.; Du, X.; Nguyen, N.T.; Brayer, G.D.; Brömme, D. Structural basis of collagen fiber degradation by cathepsin K. Proc. Natl. Acad. Sci. USA 2014, 111, 17474–17479. [Google Scholar] [CrossRef]
- Luxenburg, C.; Winograd-Katz, S.; Addadi, L.; Geiger, B. Involvement of actin polymerization in podosome dynamics. J. Cell Sci. 2012, 125, 1666–1672. [Google Scholar] [CrossRef]
- Van Den Dries, K.; Linder, S.; Maridonneau-parini, I.; Poincloux, R. Probing the mechanical landscape—New insights into podosome architecture and mechanics. J. Cell Sci. 2019, 132, jcs236828. [Google Scholar] [CrossRef] [PubMed]
- Ng, P.Y.; Patricia Ribet, A.B.; Pavlos, N.J. Membrane trafficking in osteoclasts and implications for osteoporosis. Biochem. Soc. Trans. 2019, 47, 639–650. [Google Scholar] [CrossRef]
- Georgess, D.; Machuca-Gayet, I.; Blangy, A.; Jurdic, P. Podosome organization drives osteoclast-mediated bone resorption. Cell Adh. Migr. 2014, 8, 192–204. [Google Scholar] [CrossRef]
- Lakkakorpi, P.T.; Vaananen, H.K. Kinetics of the osteoclast cytoskeleton during the resorption cycle in vitro. J. Bone Miner. Res. 1991, 6, 817–826. [Google Scholar] [CrossRef]
- Novack, D.V.; Faccio, R. Osteoclast motility: Putting the brakes on bone resorption. Ageing Res. Rev. 2009, 10, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Saltel, F.; Destaing, O.; Bard, F.; Eichert, D.; Jurdic, P. Apatite-mediated actin dynamics in resorbing osteoclasts. Mol. Biol. Cell 2004, 15, 5231–5241. [Google Scholar] [CrossRef] [PubMed]
- Vives, V.; Laurin, M.; Cres, G.; Larrousse, P.; Morichaud, Z.; Noel, D.; Cote, J.F.; Blangy, A. The Rac1 exchange factor Dock5 is essential for bone resorption by osteoclasts. J. Bone Miner. Res. 2011, 26, 1099–1110. [Google Scholar] [CrossRef]
- Søe, K.; Delaissé, J.-M. Time-lapse reveals that osteoclasts can move across the bone surface while resorbing. J. Cell Sci. 2017, 130, 2026–2035. [Google Scholar] [CrossRef]
- Søe, K.; Delaisse, J.-M. Glucocorticoids maintain human osteoclasts in the active mode of their resorption cycle. J. Bone Miner. Res. 2010, 25, 2184–2192. [Google Scholar] [CrossRef] [PubMed]
- Søe, K.; Merrild, D.M.H.; Delaissé, J.-M. Steering the osteoclast through the demineralization-collagenolysis balance. Bone 2013, 56, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Panwar, P.; Søe, K.; Guido, R.V.; Bueno, R.V.C.C.; Delaisse, J.-M.; Bromme, D. A novel approach to inhibit bone resorption: Exosite inhibitors against cathepsin K. Br. J. Pharm. 2016, 173, 396–410. [Google Scholar] [CrossRef]
- Møller, A.M.J.; Füchtbauer, E.-M.; Brüel, A.; Andersen, T.L.; Borggaard, X.G.; Pavlos, N.J.; Thomsen, J.S.; Pedersen, F.S.; Delaisse, J.-M.; Søe, K. Septins are critical regulators of osteoclastic bone resorption. Sci. Rep. 2018, 8, 13016. [Google Scholar] [CrossRef]
- Møller, A.M.J.; Delaisse, J.-M.; Bechmann, T.; Madsen, J.S.; Volmer, L.M.; Søe, K. Human osteoclasts generated from different individuals show a highly variable sensitivity to zoledronic acid in vitro—this sensitivity relates to in vivo characteristics of each individual. J. Bone Oncol. 2018, 13, 41. [Google Scholar]
- Møller, A.; Delaisse, J.; Olesen, J.; Madsen, J.; Canto, L.; Bechmann, T.; Rogatto, S.; Søe, K. Aging and menopause reprogram osteoclast precursors for aggressive bone resorption. Bone Res. 2020, 8, 27. [Google Scholar] [CrossRef]
- Merrild, D.M.H.; Pirapaharan, D.C.; Andreasen, C.M.; Kjaersgaard-Andersen, P.; Møller, A.M.J.; Ding, M.; Delaisse, J.-M.; Søe, K. Pit- and trench-forming osteoclasts: A distinction that matters. Bone Res. 2015, 3, 15032. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, J.Y.; Chauret, N.; Cromlish, W.; Desmarais, S.; Duong le, T.; Falgueyret, J.P.; Kimmel, D.B.; Lamontagne, S.; Leger, S.; LeRiche, T. The discovery of odanacatib (MK-0822), a selective inhibitor of cathepsin K. Bioorg. Med. Chem. Lett. 2008, 18, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Yayon, A.; Ginsburg, H. Chloroquine inhibits the degredation of endocytic vesicles in human malaria parasites. Cell Biol. Int. Rep. 1983, 7, 895. [Google Scholar] [CrossRef]
- Wibo, M.; Poole, B. Protein degradation in cultured cells: II. the uptake of chloroquine by rat fibroblasts and the inhibition of cellular protein degradation and cathepsin B1. J. Cell Biol. 1974, 63, 430–440. [Google Scholar] [CrossRef] [PubMed]
- De Duve, C.; De Barsy, T.; Poole, B.; Trouet, A.; Tulkens, P.; Van Hoof, F. Lysosomotropic agents. Biochem. Pharm. 1974, 23, 2495–2531. [Google Scholar] [CrossRef]
- Von Kleist, L.; Stahlschmidt, W.; Bulut, H.; Gromova, K.; Puchkov, D.; Robertson, M.J.; MacGregor, K.A.; Tomilin, N.; Pechstein, A.; Chau, N.; et al. Role of the clathrin terminal domain in regulating coated pit dynamics revealed by small molecule inhibition. Cell 2011, 146, 471–484. [Google Scholar] [CrossRef]
- Boyde, A.; Maconnachie, E.; Reid, S.A.; Delling, G.; Mundy, G.R. Scanning electron microscopy in bone pathology: Review of methods, potential and applications. Scan. Electron. Microsc. 1986, 4, 1537–1554. [Google Scholar]
- Dempster, D.W.; Shane, E.; Horbert, W.; Lindsay, R. A simple method for correlative light and scanning electron microscopy of human iliac crest bone biopsies: Qualitative observations in normal and osteoporotic subjects. J. Bone Miner. Res. 1986, 1, 15–21. [Google Scholar] [CrossRef]
- Gentzsch, C.; Delling, G.; Kaiser, E. Microstructural classification of resorption lacunae and perforations in human proximal femora. Calcif. Tissue Int. 2003, 72, 698–709. [Google Scholar] [CrossRef]
- Jones, S.J.; Boyde, A.; Ali, N.N.; Maconnachie, E. A Review of Bone Cell and Substratum Interactions—An Illustration of the Role of Scanning Electron-Microscopy. Scanning 1985, 7, 5–24. [Google Scholar] [CrossRef]
- Sun, X.; Driscoll, M.K.; Guven, C.; Das, S.; Parent, C.A.; Fourkas, J.T.; Losert, W. Asymmetric nanotopography biases cytoskeletal dynamics and promotes unidirectional cell guidance. Proc. Natl. Acad. Sci. USA 2015, 112, 12557–12562. [Google Scholar] [CrossRef] [PubMed]
- Panwar, P.; Xue, L.; Søe, K.; Srivastava, K.; Law, S.; Delaisse, J.-M.J.M.; Brömme, D. An Ectosteric Inhibitor of Cathepsin K Inhibits Bone Resorption in Ovariectomized Mice. J. Bone Miner. Res. 2017, 32, 2415–2430. [Google Scholar] [CrossRef] [PubMed]
- Leung, P.; Pickarski, M.; Zhuo, Y.; Masarachia, P.J.; Duong, L.T. The effects of the cathepsin K inhibitor odanacatib on osteoclastic bone resorption and vesicular trafficking. Bone 2011, 49, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Ejiri, S.; Yanagisawa, S.; Ozawa, H. Regulation of osteoclast polarization. Odontology 2007, 95, 1–9. [Google Scholar] [CrossRef]
- Everts, V.; Delaisse, J.M.; Korper, W.; Jansen, D.C.; Tigchelaar-Gutter, W.; Saftig, P.; Beertsen, W. The bone lining cell: Its role in cleaning Howship’s lacunae and initiating bone formation. J. Bone Min. Res. 2002, 17, 77–90. [Google Scholar] [CrossRef]
- Chambers, T.J.; Darby, J.A.; Fuller, K. Mammalian collagenase predisposes bone surfaces to osteoclastic resorption. Cell Tissue Res. 1985, 241, 671–675. [Google Scholar] [CrossRef]
- Salo, J.; Lehenkari, P.; Mulari, M.; Metsikkö, K.; Väänänen, H.K. Removal of osteoclast bone resorption products by transcytosis. Science 1997, 276, 270–273. [Google Scholar] [CrossRef]
- Nesbitt, S.A.; Horton, M.A. Trafficking of matrix collagens through bone-resorbing osteoclasts. Science 1997, 276, 266–269. [Google Scholar] [CrossRef]
- Mulari, M.T.K.; Zhao, H.; Lakkakorpi, P.T.; Vaananen, H.K. Osteoclast Ruffled Border Has Distinct Subdomains for Secretion and Degraded Matrix Uptake. Traffic 2003, 4, 113–125. [Google Scholar] [CrossRef]
- von Kleist, L.; Haucke, V. At the crossroads of chemistry and cell biology: Inhibiting membrane traffic by small molecules. Traffic 2012, 13, 495–504. [Google Scholar] [CrossRef]
- Brunet, S.; Sardon, T.; Zimmerman, T.; Wittmann, T.; Pepperkok, R.; Karsenti, E.; Vernos, I. Characterization of the TPX2 domains involved in microtubule nucleation and spindle assembly in Xenopus egg extracts. Mol. Biol. Cell 2004, 15, 5318–5328. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Sung, T.; Lin, N.; Abraham, R.T.; Jessen, B.A. Lysosomal adaptation: How cells respond to lysosomotropic compounds. PLoS ONE 2017, 12, e0173771. [Google Scholar] [CrossRef] [PubMed]
- Stenbeck, G.; Horton, M.A. A new specialized cell-matrix interaction in actively resorbing osteoclasts. J. Cell Sci. 2000, 113, 1577–1587. [Google Scholar] [PubMed]
- Stenbeck, G.; Horton, M.A. Endocytic trafficking in actively resorbing osteoclasts. J. Cell Sci. 2004, 117, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Coxon, F.P.; Taylor, A. Vesicular trafficking in osteoclasts. Semin. Cell Dev. Biol. 2008, 19, 424–433. [Google Scholar] [CrossRef]
- Hirvonen, M.J.; Fagerlund, K.; Lakkakorpi, P.P.; Väänänen, H.K.; Mulari, M.T.K. Novel perspectives on the transcytotic route in osteoclasts. BoneKEy Rep. 2013, 2, 306. [Google Scholar] [CrossRef]
- Salo, J.; Metsikko, K.; Palokangas, H.; Lehenkari, P.; Vaananen, H.K. Bone-resorbing osteoclasts reveal a dynamic division of basal plasma membrane into two different domains. J. Cell Sci. 1996, 109, 301. [Google Scholar]
- Vääräniemi, J.; Halleen, J.M.; Kaarlonen, K.; Ylipahkala, H.; Alatalo, S.L.; Andersson, G.; Kaija, H.; Vihko, P.; Väänänen, H.K. Intracellular machinery for matrix degradation in bone-resorbing osteoclasts. J. Bone Miner. Res. 2004, 19, 1432–1440. [Google Scholar] [CrossRef]
- Palokangas, H.; Mulari, M.; Vaananen, H.K. Endocytic pathway from the basal plasma membrane to the ruffled border membrane in bone-resorbing osteoclasts. J. Cell Sci. 1997, 110, 1767. [Google Scholar]
- Florey, O.; Gammoh, N.; Kim, S.E.; Jiang, X.; Overholtzer, M. V-ATPase and osmotic imbalances activate endolysosomal LC3 lipidation. Autophagy 2015, 11, 88–99. [Google Scholar] [CrossRef]
- Yoon, Y.H.; Cho, K.S.; Hwang, J.J.; Lee, S.-J.; Choi, J.A.; Koh, J.-Y. Induction of lysosomal dilatation, arrested autophagy, and cell death by chloroquine in cultured ARPE-19 cells. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6030–6037. [Google Scholar] [CrossRef] [PubMed]
- Mauthe, M.; Orhon, I.; Rocchi, C.; Zhou, X.; Luhr, M.; Hijlkema, K.-J.; Coppes, R.P.; Engedal, N.; Mari, M.; Reggiori, F. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy 2018, 14, 1435–1455. [Google Scholar] [CrossRef] [PubMed]
- Mulari, M.T.K.; Nars, M.; Laitala-Leinonen, T.; Kaisto, T.; Metsikkö, K.; Sun, Y.; Väänänen, H.K. Recombinant VSV G proteins reveal a novel raft-dependent endocytic pathway in resorbing osteoclasts. Exp. Cell Res. 2008, 314, 1641–1651. [Google Scholar] [CrossRef]
- Wheal, B.D.; Beach, R.J.; Tanabe, N.; Dixon, S.J.; Sims, S.M. Subcellular elevation of cytosolic free calcium is required for osteoclast migration. J. Bone Miner. Res. 2014, 29, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Ishihara, A.; Oxford, G.; Johnson, B.; Jacobson, K. Regulation of cell movement is mediated by stretch-activated calcium channels. Nature 1999, 400, 382–386. [Google Scholar] [CrossRef]
- Lawson, M.A.; Maxfield, F.R. Ca(2+)- and calcineurin-dependent recycling of an integrin to the front of migrating neutrophils. Nature 1995, 377, 75–79. [Google Scholar] [CrossRef]
- Bretscher, M.S. Moving Membrane up to the Front of Migrating Cells. Cell 1996, 85, 465–467. [Google Scholar] [CrossRef]
- Paul, N.R.; Jacquemet, G.; Caswell, P.T. Endocytic Trafficking of Integrins in Cell Migration. Curr. Biol. 2015, 25, R1092–R1105. [Google Scholar] [CrossRef]
- Søe, K.; Andersen, T.L.; Hobolt-Pedersen, A.S.; Bjerregaard, B.; Larsson, L.I.; Delaisse, J.-M. Involvement of human endogenous retroviral syncytin-1 in human osteoclast fusion. Bone 2011, 48, 837–846. [Google Scholar] [CrossRef]
- Panwar, P.; Law, S.; Jamroz, A.; Azizi, P.; Zhang, D.; Ciufolini, M.; Brömme, D. Tanshinones that selectively block the collagenase activity of cathepsin K provide a novel class of ectosteric antiresorptive agents for bone. Br. J. Pharm. 2018, 175, 902–923. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borggaard, X.G.; Pirapaharan, D.C.; Delaissé, J.-M.; Søe, K. Osteoclasts’ Ability to Generate Trenches Rather Than Pits Depends on High Levels of Active Cathepsin K and Efficient Clearance of Resorption Products. Int. J. Mol. Sci. 2020, 21, 5924. https://doi.org/10.3390/ijms21165924
Borggaard XG, Pirapaharan DC, Delaissé J-M, Søe K. Osteoclasts’ Ability to Generate Trenches Rather Than Pits Depends on High Levels of Active Cathepsin K and Efficient Clearance of Resorption Products. International Journal of Molecular Sciences. 2020; 21(16):5924. https://doi.org/10.3390/ijms21165924
Chicago/Turabian StyleBorggaard, Xenia G., Dinisha C. Pirapaharan, Jean-Marie Delaissé, and Kent Søe. 2020. "Osteoclasts’ Ability to Generate Trenches Rather Than Pits Depends on High Levels of Active Cathepsin K and Efficient Clearance of Resorption Products" International Journal of Molecular Sciences 21, no. 16: 5924. https://doi.org/10.3390/ijms21165924
APA StyleBorggaard, X. G., Pirapaharan, D. C., Delaissé, J.-M., & Søe, K. (2020). Osteoclasts’ Ability to Generate Trenches Rather Than Pits Depends on High Levels of Active Cathepsin K and Efficient Clearance of Resorption Products. International Journal of Molecular Sciences, 21(16), 5924. https://doi.org/10.3390/ijms21165924