Effects of Aerobic Exercise on Tau and Related Proteins in Rats with the Middle Cerebral Artery Occlusion
Abstract
1. Introduction
2. Result
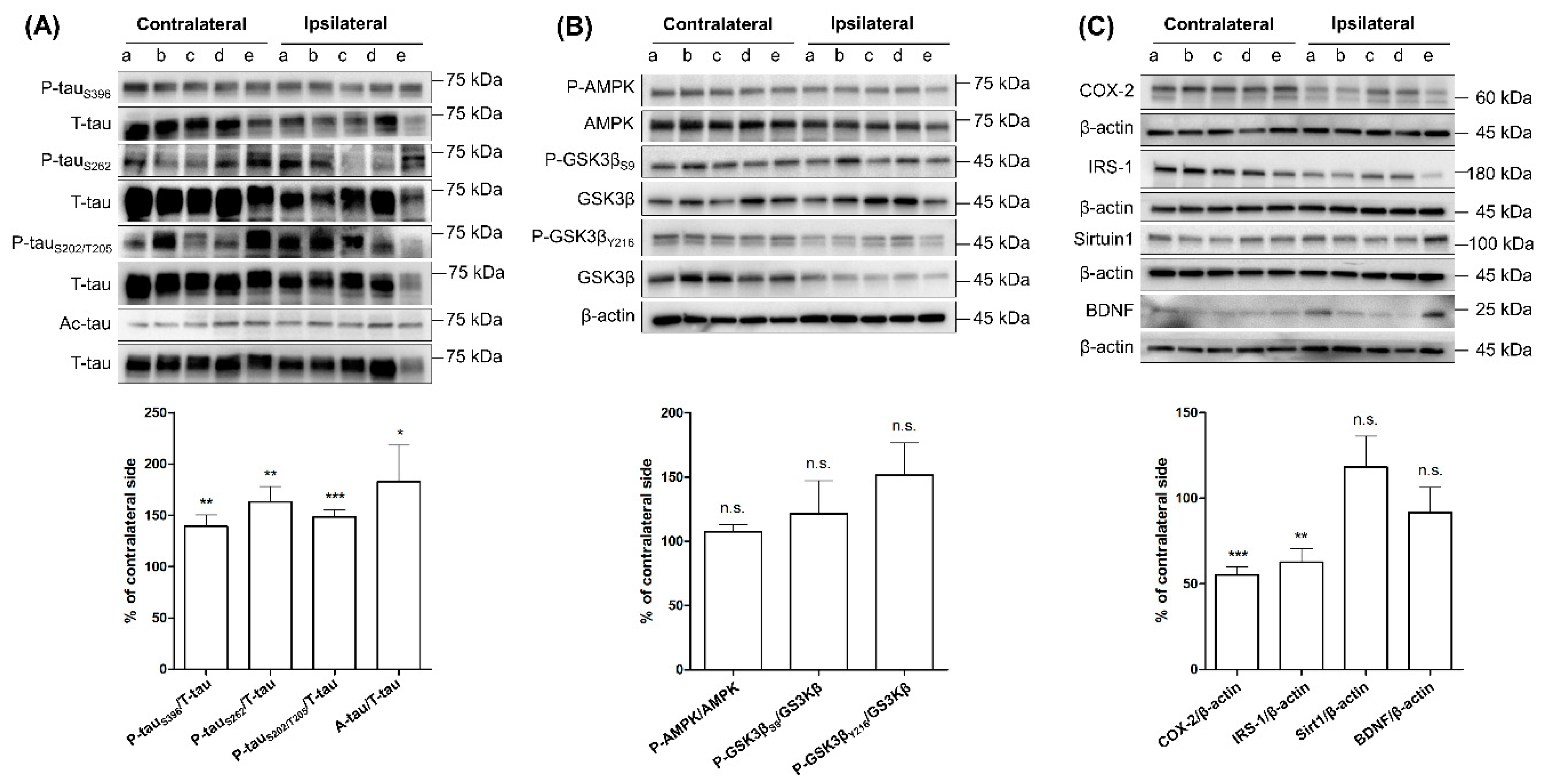
2.1. Comparing the Levels of Tau Phosphorylation, Acetylation, and Tau-Related Proteins between Ipsilateral and Contralateral Cortices of SG Groups after Three-Month Aerobic Exercise
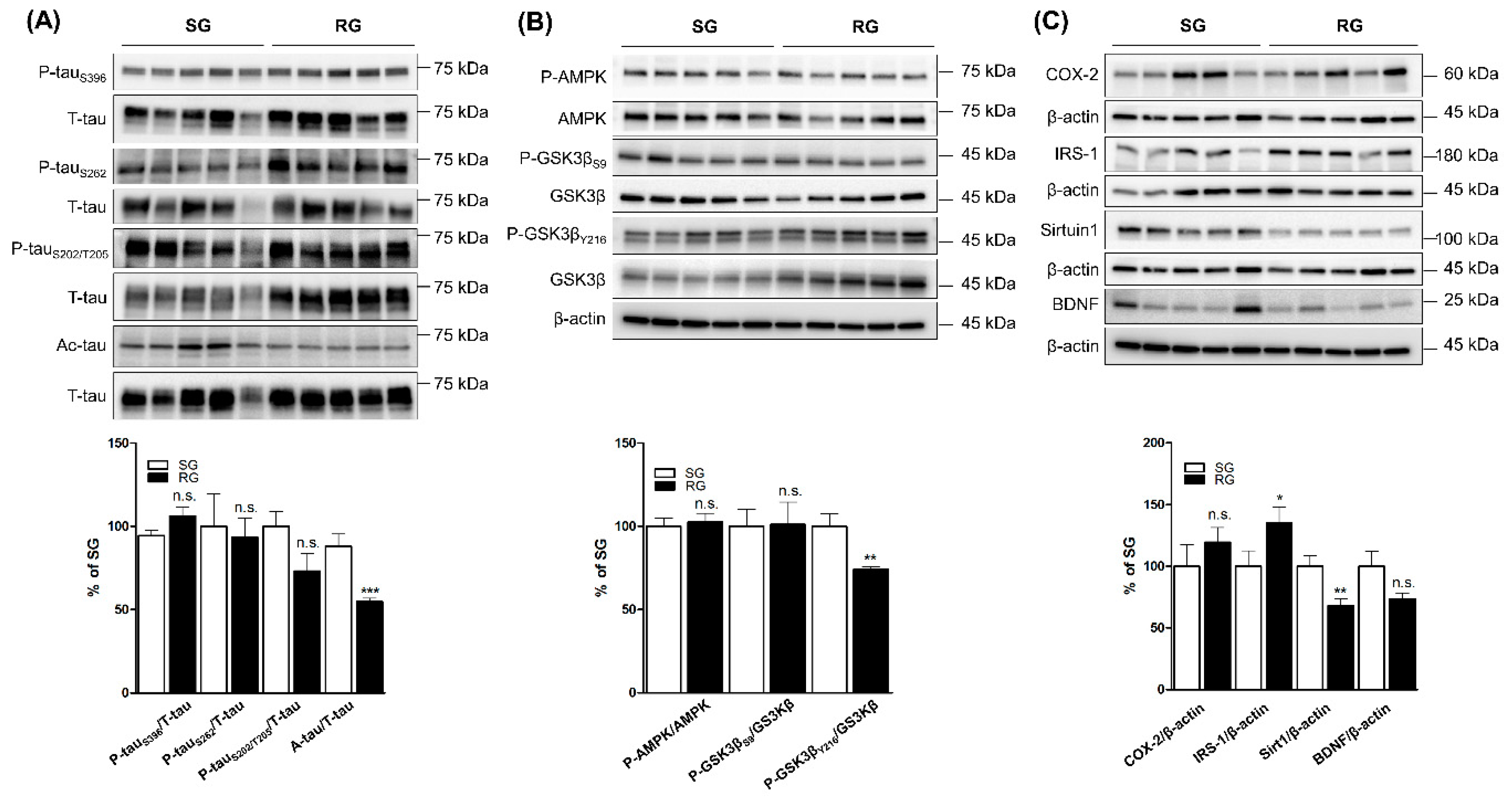
2.2. Comparing the Levels of Tau Phosphorylation, Acetylation, and Tau-Related Proteins between Ipsilateral and Contralateral Cortices of RG Groups after Three-Month Aerobic Exercise
2.3. Comparing the Levels of Tau Phosphorylation, Acetylation and Tau-Related Proteins in Ipsilateral Cortices of RG with SG Groups after Three-Month Aerobic Exercise
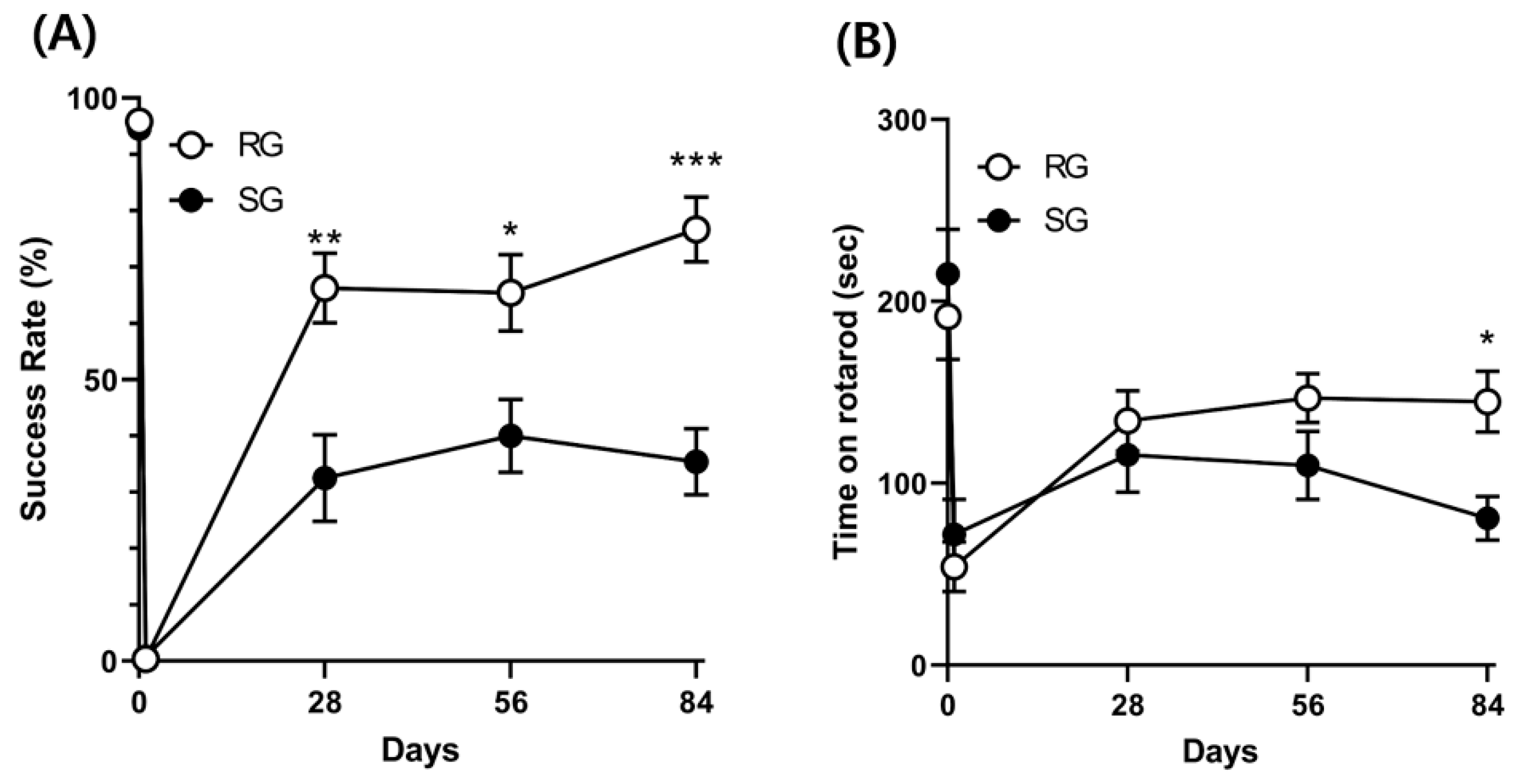
2.4. Improvements in Motor and Balance Functions Caused by Aerobic Training (12 Rats per Group)
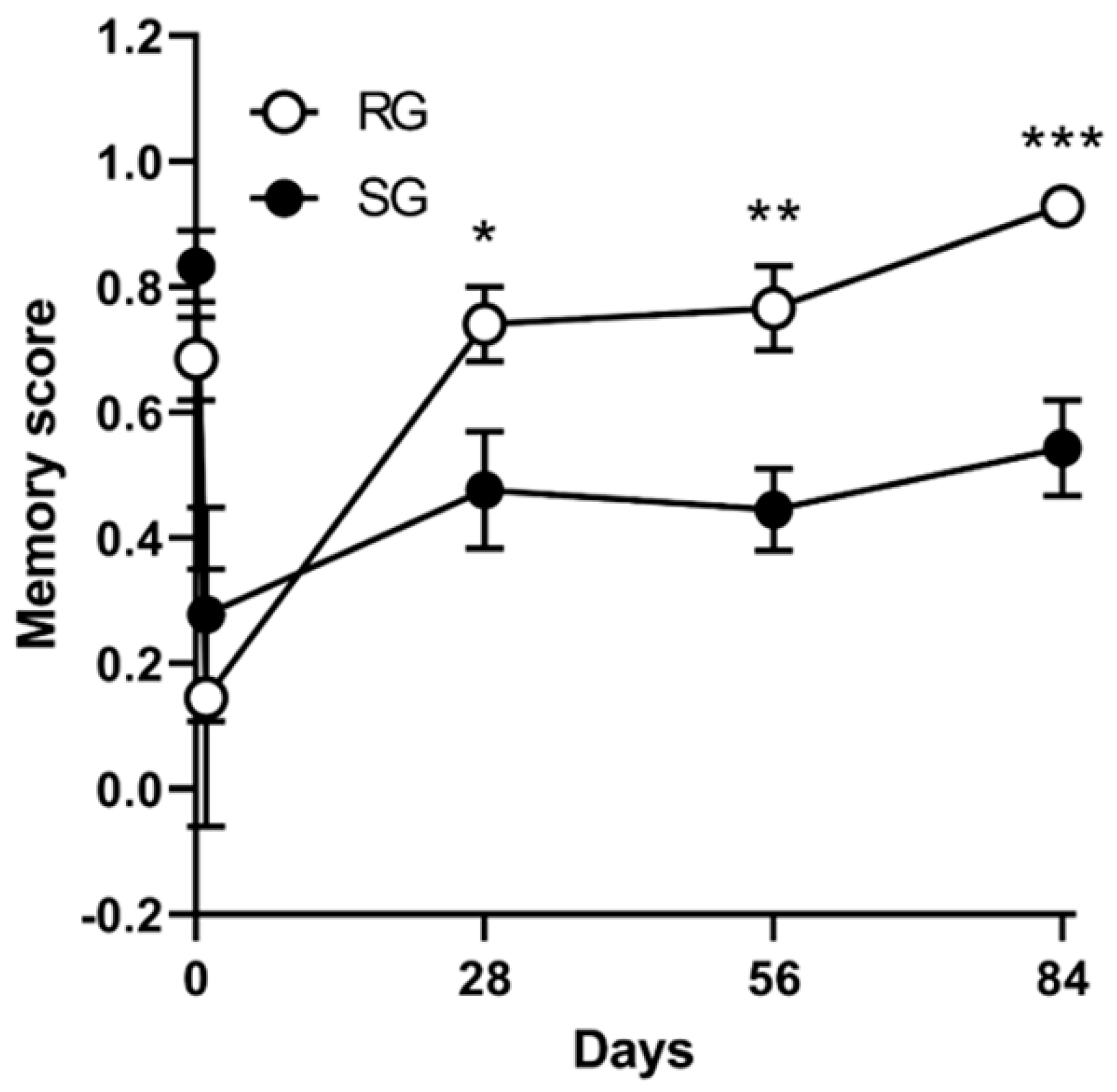
2.5. Improvements in Memory Functions Caused by Aerobic Training (12 Rats per Group)
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Induction of MCAO Infarction
4.3. Western Blot
4.4. The Single Pellet Reaching (SPR) Test
4.5. Rotarod Test
4.6. The Radial Maze Test
4.7. Treadmill Aerobic Exercise Training
4.8. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Iqbal, K.; Liu, F.; Gong, C.-X.; Grundke-Iqbal, I. Tau in Alzheimer Disease and Related Tauopathies. Curr. Alzheimer Res. 2010, 7, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Min, S.W.; Cho, S.H.; Zhou, Y.; Schroeder, S.; Haroutunian, V.; Seeley, W.W.; Huang, E.J.; Shen, Y.; Masliah, E.; Mukherjee, C.; et al. Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron 2010, 67, 953–966. [Google Scholar] [CrossRef] [PubMed]
- Cook, C.; Stankowski, J.N.; Carlomagno, Y.; Stetler, C.; Petrucelli, L. Acetylation: A new key to unlock tau’s role in neurodegeneration. Alzheimer’s Res. Ther. 2014, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.; Knudsen, G.M.; Maeda, S.; Trinidad, J.C.; Ioanoviciu, A.; Burlingame, A.L.; Mucke, L. Tau post-translational modifications in wild-type and human amyloid precursor protein transgenic mice. Nat. Neurosci. 2015, 18, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Trzeciakiewicz, H.; Tseng, J.H.; Wander, C.M.; Madden, V.; Tripathy, A.; Yuan, C.X.; Cohen, T.J. A dual pathogenic mechanism links tau acetylation to sporadic tauopathy. Sci. Rep. 2017, 7, 44102. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, H. Tau as a potential therapeutic target for ischemic stroke. Aging 2019, 11, 12827–12843. [Google Scholar] [CrossRef]
- Kim, T.H.; Mehta, S.L.; Kaimal, B.; Lyons, K.; Dempsey, R.J.; Vemuganti, R. Poststroke induction of α-synuclein mediates ischemic brain damage. J. Neurosci. 2016, 36, 7055–7065. [Google Scholar] [CrossRef]
- Surgucheva, I.; Newell, K.L.; Burns, J.; Surguchov, A. New α- and γ-synuclein immunopathological lesions in human brain. Acta Neuropathol. Commun. 2013, 2, 132. [Google Scholar] [CrossRef]
- Surgucheva, I.; He, S.; Rich, M.C.; Sharma, R.; Ninkina, N.N.; Stahel, P.F.; Surguchov, A. Role of synucleins in traumatic brain injury—An experimental in vitro and in vivo study in mice. Mol. Cell. Neurosci. 2014, 63, 114–123. [Google Scholar] [CrossRef]
- Joa, K.L.; Moon, S.; Kim, J.H.; Shin, D.W.; Lee, K.H.; Jung, S.; Kim, M.O.; Kim, C.H.; Jung, H.Y.; Kang, J.H. Effects of task-specific rehabilitation training on tau modification in rat with photothrombotic cortical ischemic damage. Neurochem. Int. 2017, 108, 309–317. [Google Scholar] [CrossRef]
- Green, A.R.; Odergren, T.; Ashwood, T. Animal models of stroke: Do they have value for discovering neuroprotective agents? Trends Pharmacol. Sci. 2003, 24, 402–408. [Google Scholar] [CrossRef]
- Keci, A.; Tani, K.; Xhema, J. Role of rehabilitation in neural plasticity. Open Access Maced. J. Med. Sci. 2019, 7, 1540–1547. [Google Scholar] [CrossRef]
- Fluri, F.; Schuhmann, M.K.; Kleinschnitz, C. Animal models of ischemic stroke and their application in clinical research. Drug Des. Dev. Ther. 2015, 9, 3445–3454. [Google Scholar]
- Li, F.; Geng, X.; Huber, C.; Stone, C.; Ding, Y. In Search of a Dose: The Functional and Molecular Effects of Exercise on Post-stroke Rehabilitation in Rats. Front. Cell. Neurosci. 2020, 14, 1–12. [Google Scholar] [CrossRef]
- Ohia-Nwoko, O.; Montazari, S.; Lau, Y.S.; Eriksen, J.L. Long-term treadmill exercise attenuates tau pathology in P301S tau transgenic mice. Mol. Neurodegener. 2014, 9, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.M.; Klakotskaia, D.; Ajit, D.; Weisman, G.A.; Wood, W.G.; Sun, G.Y.; Serfozo, P.; Simonyi, A.; Schachtman, T.R. Beneficial effects of dietary EGCG and voluntary exercise on behavior in an Alzheimer’s disease mouse model. J. Alzheimer’s Dis. 2015, 44, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.K.; Vidoni, E.D.; Johnson, D.K.; Van Sciver, A.; Mahnken, J.D.; Honea, R.A.; Wilkins, H.M.; Brooks, W.M.; Billinger, S.A.; Swerdlow, R.H.; et al. Aerobic exercise for Alzheimer’s disease: A randomized controlled pilot trial. PLoS ONE 2017, 12, e0170547. [Google Scholar] [CrossRef]
- Leys, D.; Hénon, H.; Mackowiak-Cordoliani, M.A.; Pasquier, F. Poststroke dementia. Lancet Neurol. 2005, 4, 752–759. [Google Scholar] [CrossRef]
- Wen, Y.; Yang, S.; Liu, R.; Simpkins, J.W. Transient cerebral ischemia induces site-specific hyperphosphorylation of tau protein. Brain Res. 2004, 1022, 30–38. [Google Scholar] [CrossRef]
- Chen, W.W.; Zhang, X.; Huang, W.J. Role of physical exercise in alzheimer’s disease. Biomed. Rep. 2016, 4, 403–407. [Google Scholar] [CrossRef]
- Medhat, E.; Rashed, L.; Abdelgwad, M.; Aboulhoda, B.E.; Khalifa, M.M.; El-Din, S.S. Exercise enhances the effectiveness of vitamin D therapy in rats with Alzheimer’s disease: Emphasis on oxidative stress and inflammation. Metab. Brain Dis. 2020, 35, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Yuede, C.M.; Zimmerman, S.D.; Dong, H.; Kling, M.J.; Bero, A.W.; Holtzman, D.M.; Timson, B.F.; Csernansky, J.G. Effects of voluntary and forced exercise on plaque deposition, hippocampal volume, and behavior in the Tg2576 mouse model of Alzheimer’s disease. Neurobiol. Dis. 2009, 35, 426–432. [Google Scholar] [CrossRef]
- Ittner, A.; Chua, S.W.; Bertz, J.; Volkerling, A.; Van Der Hoven, J.; Gladbach, A.; Przybyla, M.; Bi, M.; Van Hummel, A.; Stevens, C.H.; et al. Site-specific phosphorylation of tau inhibits amyloid-β toxicity in Alzheimer’s mice. Science 2016, 354, 904–908. [Google Scholar] [CrossRef] [PubMed]
- Domise, M.; Didier, S.; Marinangeli, C.; Zhao, H.; Chandakkar, P.; Buée, L.; Viollet, B.; Davies, P.; Marambaud, P.; Vingtdeux, V. AMP-activated protein kinase modulates tau phosphorylation and tau pathology in vivo. Sci. Rep. 2016, 6, 26758. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Paudel, H.K. Glycogen synthase kinase 3β phosphorylates Alzheimer’s disease-specific Ser396 of microtubule-associated protein tau by a sequential mechanism. Biochemistry 2006, 45, 3125–3133. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.A.; Souder, D.C.; Miller, K.N.; Clark, J.P.; Sagar, A.K.; Eliceiri, K.W.; Puglielli, L.; Beasley, T.M.; Anderson, R.M. GSK3β Regulates Brain Energy Metabolism. Cell Rep. 2018, 23, 1922–1931. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, Y.; Zhang, M.; Chang, J.; Zeng, Z.; Kou, X.; Chen, N. Exercise Attenuates Brain Aging by Rescuing Down-Regulated Wnt/β-Catenin Signaling in Aged Rats. Front. Aging Neurosci. 2020, 12, 1–12. [Google Scholar] [CrossRef]
- Cho, J.; Shin, M.K.; Kim, D.; Lee, I.; Kim, S.; Kang, H. Treadmill Running Reverses Cognitive Declines due to Alzheimer Disease. Med. Sci. Sports Exerc. 2015, 47, 1814–1824. [Google Scholar] [CrossRef]
- Wen, Y.; Yang, S.H.; Liu, R.; Perez, E.J.; Brun-Zinkernagel, A.M.; Koulen, P.; Simpkins, J.W. Cdk5 is involved in NFT-like tauopathy induced by transient cerebral ischemia in female rats. Biochim. Biophys. Acta Mol. Basis Dis. 2007, 1772, 473–483. [Google Scholar] [CrossRef]
- Gordon-Krajcer, W.; Kozniewska, E.; Lazarewicz, J.W.; Ksiezak-Reding, H. Differential changes in phosphorylation of tau at PHF-1 and 12E8 epitopes during brain ischemia and reperfusion in gerbils. Neurochem. Res. 2007, 32, 729–737. [Google Scholar] [CrossRef]
- Uchihara, T.; Nakamura, A.; Arai, T.; Ikeda, K.; Tsuchiya, K. Microglial Tau Undergoes Phosphorylation-Independent Modification after Ischemia. Glia 2004, 45, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Morioka, M.; Kawano, T.; Yano, S.; Kai, Y.; Tsuiki, H.; Yoshinaga, Y.; Matsumoto, J.; Maeda, T.; Hamada, J.-I.; Yamamoto, H.; et al. Hyperphosphorylation at serine 199/202 of tau factor in the gerbil hippocampus after transient forebrain ischemia. Biochem. Biophys. Res. Commun. 2006, 347, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Julien, C.; Tremblay, C.; Émond, V.; Lebbadi, M.; Salem, N.; Bennett, D.A.; Calon, F. Sirtuin 1 reduction parallels the accumulation of tau in alzheimer disease. J. Neuropathol. Exp. Neurol. 2009, 68, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Sallam, N.; Laher, I. Exercise modulates oxidative stress and inflammation in aging and cardiovascular diseases. Oxid. Med. Cell. Longev. 2016, 2016, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Neumann, S.; Boothman-Burrell, L.; Gowing, E.K.; Jacobsen, T.A.; Ahring, P.K.; Young, S.L.; Sandager-Nielsen, K.; Clarkson, A.N. The Delta-Subunit Selective GABAA Receptor Modulator, DS2, Improves Stroke Recovery via an Anti-inflammatory Mechanism. Front. Neurosci. 2019, 13, 1–13. [Google Scholar] [CrossRef]
- Lovatel, G.A.; Bertoldi, K.; Elsner, V.R.; Vanzella, C.; Moysés, F.d.S.; Spindler, C.; Funck, V.R.; Pereira, L.M.; de Oliveira, C.V.; Oliveira, M.S.; et al. Time-dependent effects of treadmill exercise on aversive memory and cyclooxygenase pathway function. Neurobiol. Learn. Mem. 2012, 98, 182–187. [Google Scholar] [CrossRef]
- Stopford, C.L.; Thompson, J.C.; Neary, D.; Richardson, A.M.T.; Snowden, J.S. Working memory, attention, and executive function in Alzheimer’s disease and frontotemporal dementia. Cortex 2012, 48, 429–446. [Google Scholar] [CrossRef]
- Pedroso, R.V.; Coelho, F.G.d.M.; Santos-Galduróz, R.F.; Costa, J.L.R.; Gobbi, S.; Stella, F. Balance, executive functions and falls in elderly with Alzheimer’s disease (AD): A longitudinal study. Arch. Gerontol. Geriatr. 2012, 54, 348–351. [Google Scholar] [CrossRef]
- Gashaj, V.; Oberer, N.; Mast, F.W.; Roebers, C.M. Individual differences in basic numerical skills: The role of executive functions and motor skills. J. Exp. Child Psychol. 2019, 182, 187–195. [Google Scholar] [CrossRef]
- Trueman, R.C.; Harrison, D.J.; Dwyer, D.M.; Dunnett, S.B.; Hoehn, M.; Farr, T.D. A Critical Re-Examination of the Intraluminal Filament MCAO Model: Impact of External Carotid Artery Transection. Transl. Stroke Res. 2011, 2, 651–661. [Google Scholar] [CrossRef]
- Longa, E.Z.; Weinstein, P.R.; Carlson, S.; Cummins, R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989, 20, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Moon, S.; Paik, J.H.; Shin, D.W.; Kim, L.S.; Park, C.S.; Ha, J.; Kang, J.H. Activation of the 5′-AMP-activated protein kinase in the cerebral cortex of young senescence-accelerated P8 mice and association with GSK3β- and PP2A-dependent inhibition of p-tau396 expression. J. Alzheimer’s Dis. 2015, 46, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Whishaw, I.Q.; Pellis, S.M.; Gorny, B.; Kolb, B.; Tetzlaff, W. Proximal and distal impairments in rat forelimb use in reaching follow unilateral pyramidal tract lesions. Behav. Brain Res. 1993, 56, 59–76. [Google Scholar] [CrossRef]
- Whishaw, I.Q. Lateralization and reaching skill related: Results and implications from a large sample of Long-Evans rats. Behav. Brain Res. 1992, 52, 45–48. [Google Scholar] [CrossRef]
- Shabani, M.; Larizadeh, M.H.; Parsania, S.; Hajali, V.; Shojaei, A. Evaluation of destructive effects of exposure to cisplatin during developmental stage: No profound evidence for sex differences in impaired motor and memory performance. Int. J. Neurosci. 2012, 122, 439–448. [Google Scholar] [CrossRef]
- Okada, A.; Kato, A.; Kamei, C. Effects of histamine H1 receptor antagonists on delayed spatial win-shift task in rats. Eur. J. Pharmacol. 2010, 631, 24–27. [Google Scholar] [CrossRef]
- Richter, S.H.; Zeuch, B.; Lankisch, K.; Gass, P.; Durstewitz, D.; Vollmayr, B. Where Have I Been? Where Should I Go? Spatial Working Memory on a Radial Arm Maze in a Rat Model of Depression. PLoS ONE 2013, 8, e62458. [Google Scholar] [CrossRef]
- Jia, J.; Hu, Y.S.; Wu, Y.; Liu, G.; Yu, H.X.; Zheng, Q.P.; Zhu, D.N.; Xia, C.M.; Cao, Z.J. Pre-ischemic treadmill training affects glutamate and gamma aminobutyric acid levels in the striatal dialysate of a rat model of cerebral ischemia. Life Sci. 2009, 84, 505–511. [Google Scholar] [CrossRef]

| Antibodies | Dilution | Manufacturers | Source |
|---|---|---|---|
| Phospho-tau (Ser396) | 1:1000 | Santa Cruz (sc-12414) | Rabbit |
| Phospho-tau (Ser262) | 1:500 | Invitrogen (44-750G) | Rabbit |
| Phospho-tau (Ser202, Thr205) | 1:1000 | Thermo Scientific (MN1020) | Mouse |
| Ac-tau | 1:500 | Anaspec (As56077) | Rabbit |
| T-tau (tau-5) | 1:1000 | Thermo Scientific *AHB0042) | Mouse |
| Phospho-AMPKα (Thr172) | 1:1000 | Cell signaling (2535) | Rabbit |
| AMPKα | 1:1000 | Cell signaling (2532) | Rabbit |
| Phospho-GSK3βS9 | 1:1000 | Cell signaling (9336) | Rabbit |
| Phospho-GSK3βY216 | 1:1000 | Santa Cruz (sc-135653) | Rabbit |
| GSK3β | 1:1000 | Cell signaling (9315) | Rabbit |
| COX-2 | 1:1000 | Abcam (Ab15191) | Rabbit |
| IRS-1 | 1:1000 | Cell signaling (2382) | Rabbit |
| Sirtuin1 | 1:1000 | Cell signaling (9475) | Rabbit |
| BDNF | 1:1000 | Santa Cruz (sc-65514) | Mouse |
| β-actin | 1:1000 | Pro-sci (3779) | Rabbit |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mankhong, S.; Kim, S.; Moon, S.; Lee, K.-H.; Jeon, H.-E.; Hwang, B.-H.; Beak, J.-W.; Joa, K.-L.; Kang, J.-H. Effects of Aerobic Exercise on Tau and Related Proteins in Rats with the Middle Cerebral Artery Occlusion. Int. J. Mol. Sci. 2020, 21, 5842. https://doi.org/10.3390/ijms21165842
Mankhong S, Kim S, Moon S, Lee K-H, Jeon H-E, Hwang B-H, Beak J-W, Joa K-L, Kang J-H. Effects of Aerobic Exercise on Tau and Related Proteins in Rats with the Middle Cerebral Artery Occlusion. International Journal of Molecular Sciences. 2020; 21(16):5842. https://doi.org/10.3390/ijms21165842
Chicago/Turabian StyleMankhong, Sakulrat, Sujin Kim, Sohee Moon, Kyoung-Hee Lee, Hyeong-Eun Jeon, Byeong-Hun Hwang, Jong-Won Beak, Kyung-Lim Joa, and Ju-Hee Kang. 2020. "Effects of Aerobic Exercise on Tau and Related Proteins in Rats with the Middle Cerebral Artery Occlusion" International Journal of Molecular Sciences 21, no. 16: 5842. https://doi.org/10.3390/ijms21165842
APA StyleMankhong, S., Kim, S., Moon, S., Lee, K.-H., Jeon, H.-E., Hwang, B.-H., Beak, J.-W., Joa, K.-L., & Kang, J.-H. (2020). Effects of Aerobic Exercise on Tau and Related Proteins in Rats with the Middle Cerebral Artery Occlusion. International Journal of Molecular Sciences, 21(16), 5842. https://doi.org/10.3390/ijms21165842





