The Effect of Different Immunization Cycles of a Recombinant Mucin1-Maltose-Binding Protein Vaccine on T Cell Responses to B16-MUC1 Melanoma in Mice
Abstract
1. Introduction
2. Results
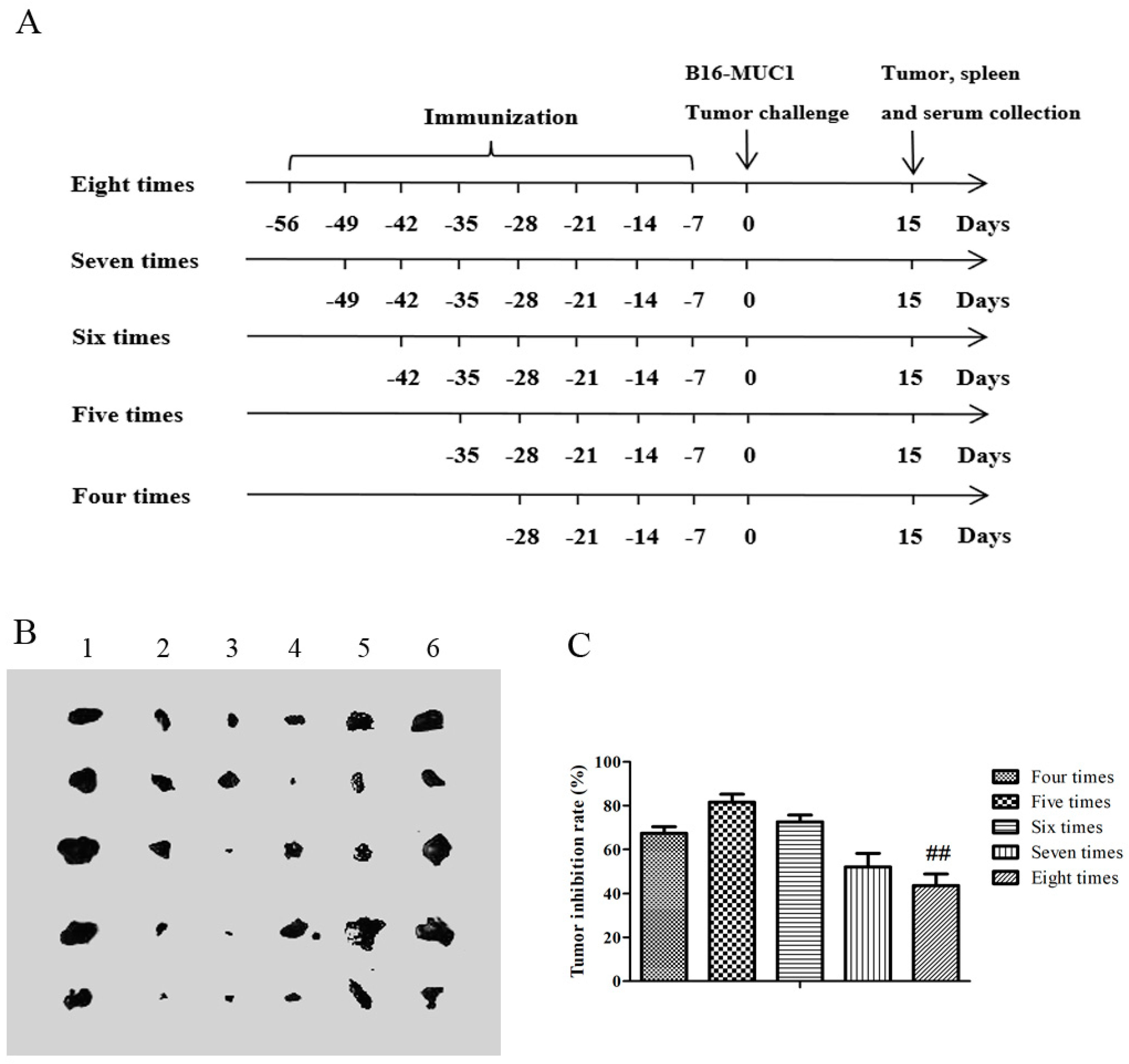
2.1. The Recombinant MUC1-MBP Vaccine Inhibited B16-MUC1 Melanoma Growth in a Preventive Mouse Model
2.2. Five Immunizations with the Recombinant MUC1-MBP Vaccine Induced Stronger T Cellular Immune Responses than Eight Immunizations in the Preventive Mouse Model
2.3. Five Immunizations with the Recombinant MUC1-MBP Vaccine More Significantly Upregulated CD4+ T and CD8+ T cells Both in the Spleen and the Tumor Microenvironment (TME) Than Eight Immunizations in the Preventive Mouse Model
2.4. Five Immunizations with the Recombinant MUC1-MBP Vaccine More Significantly Increased the Th1 and Tc1 Cell Populations and Decreased the Th17 Cell Population in the Spleen Than Eight Immunizations in the Preventive Mouse Model
2.5. Five Immunizations with the Recombinant MUC1-MBP Vaccine More Significantly Decreased the MDSC Population and Increased the CD8/MDSC Ratio in Both the Spleen and the Tumor Microenvironment Than Eight Immunizations in the Preventive Mouse Model
2.6. Five Immunizations with the Recombinant MUC1-MBP Vaccine Induced T Cell Immune Responses Against B16-MUC1 Melanoma Growth Than Eight Immunizations in the Therapeutic Tumor-Bearing Mouse Model
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. Immunization
4.3. Tumor Protection in a Prophylactic Model
4.4. ELISA for MUC1-Specific Immunoglobulin Subclasses
4.5. MUC1 Specific Cell Proliferation and Th1 Activity Assay
4.6. MUC1-Specific CTL Cytotoxicity Assay
4.7. Isolation of Tumor Infiltrating Lymphocytes (TILs)
4.8. Flow Cytometry Analysis
4.9. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MUC1-MBP | Recombinant mucin1-maltose-binding protein |
| TAA | Tumor-associated antigen |
| DC | Dendritic cell |
| MBP | Maltose-binding protein |
| APC | Antigen-presenting cells |
| B16-MUC1 | Human MUC1-overexpressing mouse melanoma B16 cells |
| MDSCs | Myeloid derived suppressor cells |
| CTL | Cytotoxic T lymphocyte |
| RTCA | Real-time cell analyser |
| TME | Tumor microenvironment |
| TILs | Tumor infiltrating lymphocytes |
| NC | Negative control |
| CI | Cell index |
| ELISA | Enzyme-linked immunosorbent assay |
| CD8/MDSC | CD8+ T cells/MDSCs |
| NK | Natural killer |
| MUC1 | Mucin-1 |
References
- Song, Q.; Zhang, C.D.; Wu, X.H. Therapeutic cancer vaccines: From initial findings to prospects. Immunol. Lett. 2018, 196, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Mkrtichyan, M.; Najjar, Y.G.; Raulfs, E.C.; Abdalla, M.Y.; Samara, R.; Rotem-Yehudar, R.; Cook, L.; Khleif, S.N. Anti-PD-1 synergizes with cyclophosphamide to induce potent anti-tumor vaccine effects through novel mechanisms. Eur. J. Immunol. 2011, 41, 2977–2986. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Liu, G.; Ni, W.; Zhang, N.; Jie, J.; Xie, F.; Tai, G. The Combination of MBP and BCG-Induced Dendritic Cell Maturation through TLR2/TLR4 Promotes Th1 Activation In Vitro and Vivo. Mediat. Inflamm. 2017, 2017, 1953680. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhang, Y.; Zhang, N.; Ni, W.; Jie, J.; Jiang, L.; Tai, G. Escherichia coli maltose-binding protein (MBP) activates mouse Th1 through TLR2-mediated MyD88-dependent pathway and TLR4-mediated TRIF-dependent pathway. Int. Immunopharmacol. 2017, 50, 338–344. [Google Scholar] [CrossRef]
- Wang, W.; Yuan, H.Y.; Liu, G.M.; Ni, W.H.; Wang, F.; Tai, G.X. Escherichia coli Maltose-Binding Protein Induces M1 Polarity of RAW264.7 Macrophage Cells via a TLR2- and TLR4-Dependent Manner. Int. J. Mol. Sci. 2015, 16, 9896–9909. [Google Scholar] [CrossRef]
- Ni, W.; Zhang, Q.; Liu, G.; Wang, F.; Yuan, H.; Guo, Y.; Zhang, X.; Xie, F.; Li, Q.; Tai, G. Escherichia coli maltose-binding protein activates mouse peritoneal macrophages and induces M1 polarization via TLR2/4 in vivo and in vitro. Int. Immunopharmacol. 2014, 21, 171–180. [Google Scholar] [CrossRef]
- Zhang, Q.; Ni, W.; Zhao, X.; Wang, F.; Gao, Z.; Tai, G. Synergistic antitumor effects of Escherichia coli maltose binding protein and Bacillus Calmette-Guerin in a mouse lung carcinoma model. Immunol. Lett. 2011, 136, 108–113. [Google Scholar] [CrossRef]
- Kang, Q.Z.; Duan, G.C.; Fan, Q.T.; Xi, Y.L. Fusion expression of Helicobacter pylori neutrophil-activating protein in E.coli. World J. Gastroenterol. 2005, 11, 454–456. [Google Scholar] [CrossRef]
- Jie, J.; Zhang, Y.; Zhou, H.; Zhai, X.; Zhang, N.; Yuan, H.; Ni, W.; Tai, G. CpG ODN1826 as a Promising Mucin1-Maltose-Binding Protein Vaccine Adjuvant Induced DC Maturation and Enhanced Antitumor Immunity. Int. J. Mol. Sci. 2018, 19, 920. [Google Scholar] [CrossRef]
- Bauer, S.; Kirschning, C.J.; Hacker, H.; Redecke, V.; Hausmann, S.; Akira, S.; Wagner, H.; Lipford, G.B. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc. Natl. Acad. Sci. USA 2001, 98, 9237–9242. [Google Scholar] [CrossRef]
- Lee, K.W.; Kim, D.S.; Kwon, H.J. CG sequence- and phosphorothioate backbone modification-dependent activation of the NF-kappaB-responsive gene expression by CpG-oligodeoxynucleotides in human RPMI 8226 B cells. Mol. Immunol. 2004, 41, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, G.; Weeratna, R.D.; Ballas, Z.K.; Payette, P.; Blackwell, S.; Suparto, I.; Rasmussen, W.L.; Waldschmidt, M.; Sajuthi, D.; Purcell, R.H.; et al. Delineation of a CpG phosphorothioate oligodeoxynucleotide for activating primate immune responses in vitro and in vivo. J. Immunol. 2000, 164, 1617–1624. [Google Scholar] [CrossRef] [PubMed]
- Shivahare, R.; Vishwakarma, P.; Parmar, N.; Yadav, P.K.; Haq, W.; Srivastava, M.; Gupta, S.; Kar, S. Combination of liposomal CpG oligodeoxynucleotide 2006 and miltefosine induces strong cell-mediated immunity during experimental visceral leishmaniasis. PLoS ONE 2014, 9, e94596. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Xu, H.; Lu, W.H.; Zhu, L.; Yu, Y.H.; Hong, F.Z. In vitro and in vivo evaluations of human papillomavirus type 16 (HPV16)-derived peptide-loaded dendritic cells (DCs) with a CpG oligodeoxynucleotide (CpG-ODN) adjuvant as tumor vaccines for immunotherapy of cervical cancer. Arch. Gynecol. Obstet. 2014, 289, 155–162. [Google Scholar] [CrossRef]
- Amemiya, K.; Meyers, J.L.; Rogers, T.E.; Fast, R.L.; Bassett, A.D.; Worsham, P.L.; Powell, B.S.; Norris, S.L.; Krieg, A.M.; Adamovicz, J.J. CpG oligodeoxynucleotides augment the murine immune response to the Yersinia pestis F1-V vaccine in bubonic and pneumonic models of plague. Vaccine 2009, 27, 2220–2229. [Google Scholar] [CrossRef]
- Babaer, D.; Amara, S.; McAdory, B.S.; Johnson, O.; Myles, E.L.; Zent, R.; Rathmell, J.C.; Tiriveedhi, V. Oligodeoxynucleotides ODN 2006 and M362 Exert Potent Adjuvant Effect through TLR-9/-6 Synergy to Exaggerate Mammaglobin-A Peptide Specific Cytotoxic CD8+T Lymphocyte Responses against Breast Cancer Cells. Cancers 2019, 11, 672. [Google Scholar] [CrossRef]
- Millward, M.; Underhill, C.; Lobb, S.; McBurnie, J.; Meech, S.J.; Gomez-Navarro, J.; Marshall, M.A.; Huang, B.; Mather, C.B. Phase I study of tremelimumab (CP-675 206) plus PF-3512676 (CPG 7909) in patients with melanoma or advanced solid tumours. Br. J. Cancer 2013, 108, 1998–2004. [Google Scholar] [CrossRef]
- Li, F.; Zhao, Y.; Wei, L.; Li, S.; Liu, J. Tumor-infiltrating Treg, MDSC, and IDO expression associated with outcomes of neoadjuvant chemotherapy of breast cancer. Cancer Biol. Ther. 2018, 19, 695–705. [Google Scholar] [CrossRef]
- Duraiswamy, J.; Kaluza, K.M.; Freeman, G.J.; Coukos, G. Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors. Cancer Res. 2013, 73, 3591–3603. [Google Scholar] [CrossRef]
- D’Alterio, C.; Buoncervello, M.; Ierano, C.; Napolitano, M.; Portella, L.; Rea, G.; Barbieri, A.; Luciano, A.; Scognamiglio, G.; Tatangelo, F.; et al. Targeting CXCR4 potentiates anti-PD-1 efficacy modifying the tumor microenvironment and inhibiting neoplastic PD-1. J. Exp. Clin. Cancer Res. 2019, 38, 432. [Google Scholar] [CrossRef]
- Martinez-Saez, N.; Peregrina, J.M.; Corzana, F. Principles of mucin structure: Implications for the rational design of cancer vaccines derived from MUC1-glycopeptides. Chem. Soc. Rev. 2017, 46, 7154–7175. [Google Scholar] [CrossRef] [PubMed]
- Kessler, A.; Sollie, S.; Karagiannis, S.N.; Walldius, G.; Hammar, N.; Van Hemelrijck, M. Serum IgG Is Associated With Risk of Melanoma in the Swedish AMORIS Study. Front. Oncol. 2019, 9, 1095. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Zhang, Y.; Cai, H.; Zhou, F.; Gao, H.; Deng, L.; Li, R. A PD-L1-Based Cancer Vaccine Elicits Antitumor Immunity in a Mouse Melanoma Model. Mol. Ther. Oncolytics 2019, 14, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.C.; Williams, M.A.; Bevan, M.J. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat. Immunol. 2004, 5, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Tondini, E.; Arakelian, T.; Oosterhuis, K.; Camps, M.; van Duikeren, S.; Han, W.; Arens, R.; Zondag, G.; van Bergen, J.; Ossendorp, F. A poly-neoantigen DNA vaccine synergizes with PD-1 blockade to induce T cell-mediated tumor control. Oncoimmunology 2019, 8, 1652539. [Google Scholar] [CrossRef]
- Kodumudi, K.N.; Ramamoorthi, G.; Snyder, C.; Basu, A.; Jia, Y.; Awshah, S.; Beyer, A.P.; Wiener, D.; Lam, L.; Zhang, H.; et al. Sequential Anti-PD1 Therapy Following Dendritic Cell Vaccination Improves Survival in a HER2 Mammary Carcinoma Model and Identifies a Critical Role for CD4 T Cells in Mediating the Response. Front. Immunol. 2019, 10, 1939. [Google Scholar] [CrossRef]
- Tian, H.; Shi, G.; Wang, Q.; Li, Y.; Yang, Q.; Li, C.; Yang, G.; Wu, M.; Xie, Q.; Zhang, S.; et al. A novel cancer vaccine with the ability to simultaneously produce anti-PD-1 antibody and GM-CSF in cancer cells and enhance Th1-biased antitumor immunity. Signal Transduct. Target. Ther. 2016, 1, 16025. [Google Scholar] [CrossRef]
- Giraldo, N.A.; Becht, E.; Vano, Y.; Petitprez, F.; Lacroix, L.; Validire, P.; Sanchez-Salas, R.; Ingels, A.; Oudard, S.; Moatti, A.; et al. Tumor-Infiltrating Peripheral Blood T-cell Immunophenotypes Predict Early Relapse in Localized Clear Cell Renal Cell Carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 4416–4428. [Google Scholar] [CrossRef]
- Hoesli, R.; Birkeland, A.C.; Rosko, A.J.; Issa, M.; Chow, K.L.; Michmerhuizen, N.L.; Mann, J.E.; Chinn, S.B.; Shuman, A.G.; Prince, M.E.; et al. Proportion of CD4 and CD8 tumor infiltrating lymphocytes predicts survival in persistent/recurrent laryngeal squamous cell carcinoma. Oral Oncol. 2018, 77, 83–89. [Google Scholar] [CrossRef]
- Vesely, M.D.; Kershaw, M.H.; Schreiber, R.D.; Smyth, M.J. Natural innate and adaptive immunity to cancer. Annu. Rev. Immunol. 2011, 29, 235–271. [Google Scholar] [CrossRef]
- Fu, Q.; Chen, N.; Ge, C.; Li, R.; Li, Z.; Zeng, B.; Li, C.; Wang, Y.; Xue, Y.; Song, X.; et al. Prognostic value of tumor-infiltrating lymphocytes in melanoma: A systematic review and meta-analysis. Oncoimmunology 2019, 8, 1593806. [Google Scholar] [CrossRef]
- Gatti-Mays, M.E.; Redman, J.M.; Donahue, R.N.; Palena, C.; Madan, R.A.; Karzai, F.; Bilusic, M.; Sater, H.A.; Marte, J.L.; Cordes, L.M.; et al. A Phase I Trial Using a Multitargeted Recombinant Adenovirus 5 (CEA/MUC1/Brachyury)-Based Immunotherapy Vaccine Regimen in Patients with Advanced Cancer. Oncologist 2019. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.H. T helper 17 (Th17) cells and interleukin-17 (IL-17) in cancer. Arch. Pharmacal Res. 2019, 42, 549–559. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Li, H.; Yusuf, N.; Elmets, C.A.; Li, J.; Mountz, J.D.; Xu, H. IL-17 promotes tumor development through the induction of tumor promoting microenvironments at tumor sites and myeloid-derived suppressor cells. J. Immunol. 2010, 184, 2281–2288. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yi, T.; Kortylewski, M.; Pardoll, D.M.; Zeng, D.; Yu, H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J. Exp. Med. 2009, 206, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Martin-Orozco, N.; Muranski, P.; Chung, Y.; Yang, X.O.; Yamazaki, T.; Lu, S.; Hwu, P.; Restifo, N.P.; Overwijk, W.W.; Dong, C. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity 2009, 31, 787–798. [Google Scholar] [CrossRef]
- Ankathatti Munegowda, M.; Deng, Y.; Mulligan, S.J.; Xiang, J. Th17 and Th17-stimulated CD8(+) T cells play a distinct role in Th17-induced preventive and therapeutic antitumor immunity. Cancer Immunol. Immunother. 2011, 60, 1473–1484. [Google Scholar] [CrossRef]
- Gabrilovich, D.I. Myeloid-Derived Suppressor Cells. Cancer Immunol. Res. 2017, 5, 3–8. [Google Scholar] [CrossRef]
- Paluskievicz, C.M.; Cao, X.; Abdi, R.; Zheng, P.; Liu, Y.; Bromberg, J.S. T Regulatory Cells and Priming the Suppressive Tumor Microenvironment. Front. Immunol. 2019, 10, 2453. [Google Scholar] [CrossRef]
- Kumar, V.; Patel, S.; Tcyganov, E.; Gabrilovich, D.I. The Nature of Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. Trends Immunol. 2016, 37, 208–220. [Google Scholar] [CrossRef]
- Ma, P.; Beatty, P.L.; McKolanis, J.; Brand, R.; Schoen, R.E.; Finn, O.J. Circulating Myeloid Derived Suppressor Cells (MDSC) That Accumulate in Premalignancy Share Phenotypic and Functional Characteristics With MDSC in Cancer. Front. Immunol. 2019, 10, 1401. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Wang, E.; Nielsen, M.B.; Wunderlich, J.; Migueles, S.; Connors, M.; Steinberg, S.M.; Rosenberg, S.A.; Marincola, F.M. Increased vaccine-specific T cell frequency after peptide-based vaccination correlates with increased susceptibility to in vitro stimulation but does not lead to tumor regression. J. Immunol. 1999, 163, 6292–6300. [Google Scholar]
- LaCelle, M.G.; Jensen, S.M.; Fox, B.A. Partial CD4 depletion reduces regulatory T cells induced by multiple vaccinations and restores therapeutic efficacy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009, 15, 6881–6890. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.C.; Chianese-Bullock, K.A.; Petroni, G.R.; Schaefer, J.T.; Brill, L.B., 2nd; Molhoek, K.R.; Deacon, D.H.; Patterson, J.W.; Slingluff, C.L., Jr. The vaccine-site microenvironment induced by injection of incomplete Freund’s adjuvant, with or without melanoma peptides. J. Immunother. 2012, 35, 78–88. [Google Scholar] [CrossRef] [PubMed]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; Zhang, Z.; Liu, G.; Jiang, M.; Wang, J.; Liu, Y.; Tai, G. The Effect of Different Immunization Cycles of a Recombinant Mucin1-Maltose-Binding Protein Vaccine on T Cell Responses to B16-MUC1 Melanoma in Mice. Int. J. Mol. Sci. 2020, 21, 5810. https://doi.org/10.3390/ijms21165810
Zhou H, Zhang Z, Liu G, Jiang M, Wang J, Liu Y, Tai G. The Effect of Different Immunization Cycles of a Recombinant Mucin1-Maltose-Binding Protein Vaccine on T Cell Responses to B16-MUC1 Melanoma in Mice. International Journal of Molecular Sciences. 2020; 21(16):5810. https://doi.org/10.3390/ijms21165810
Chicago/Turabian StyleZhou, Hongyue, Zenan Zhang, Guomu Liu, Mengyu Jiang, Jingjing Wang, Yu Liu, and Guixiang Tai. 2020. "The Effect of Different Immunization Cycles of a Recombinant Mucin1-Maltose-Binding Protein Vaccine on T Cell Responses to B16-MUC1 Melanoma in Mice" International Journal of Molecular Sciences 21, no. 16: 5810. https://doi.org/10.3390/ijms21165810
APA StyleZhou, H., Zhang, Z., Liu, G., Jiang, M., Wang, J., Liu, Y., & Tai, G. (2020). The Effect of Different Immunization Cycles of a Recombinant Mucin1-Maltose-Binding Protein Vaccine on T Cell Responses to B16-MUC1 Melanoma in Mice. International Journal of Molecular Sciences, 21(16), 5810. https://doi.org/10.3390/ijms21165810




