Distinct Cell Surface Expression Patterns of N-Glycosylation Site Mutants of AMPA-Type Glutamate Receptor under the Homo-Oligomeric Expression Conditions
Abstract
1. Introduction
2. Results
2.1. Distinct Roles of GluA1 and GluA2 N-Glycans on Cell Surface Expression Levels
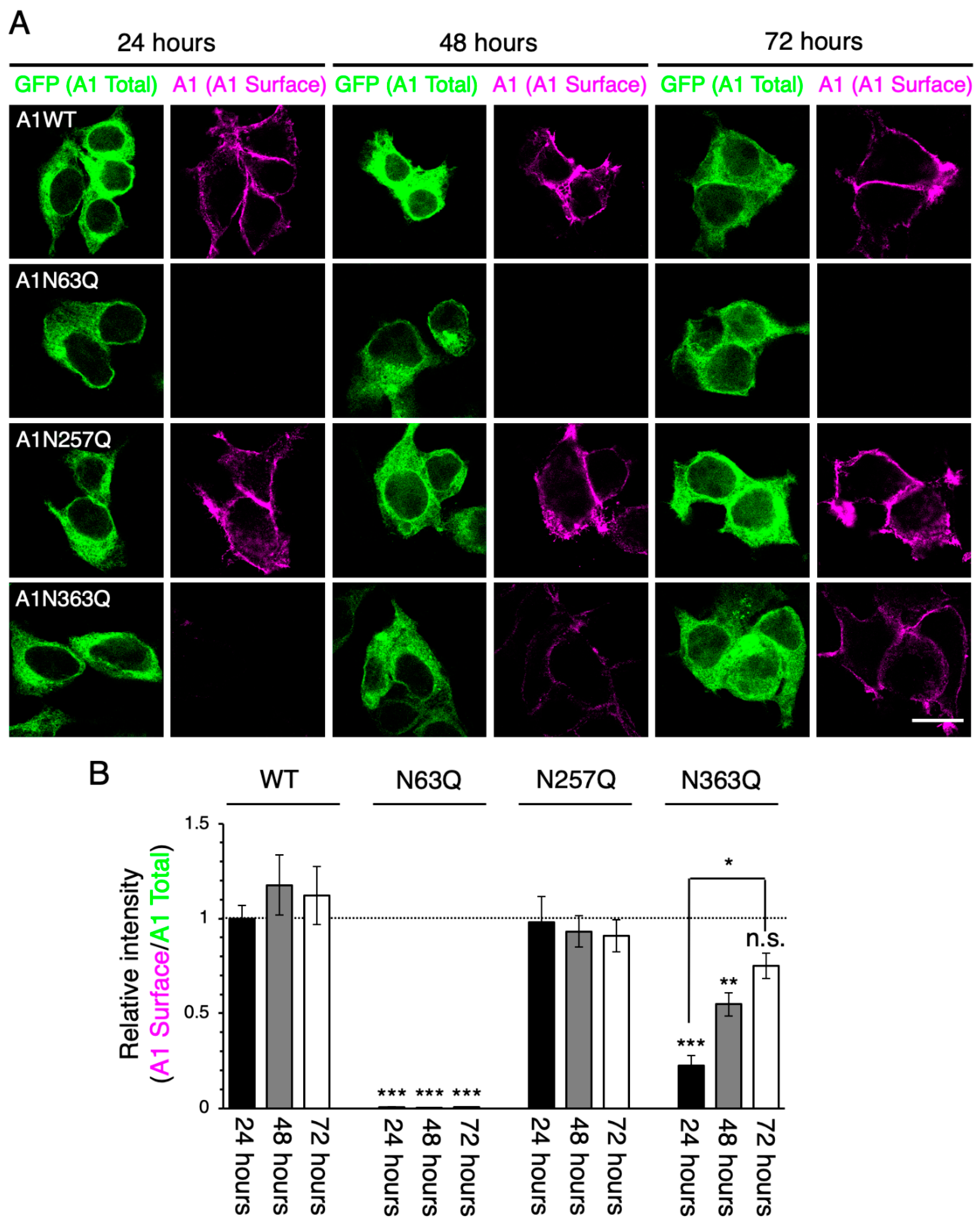
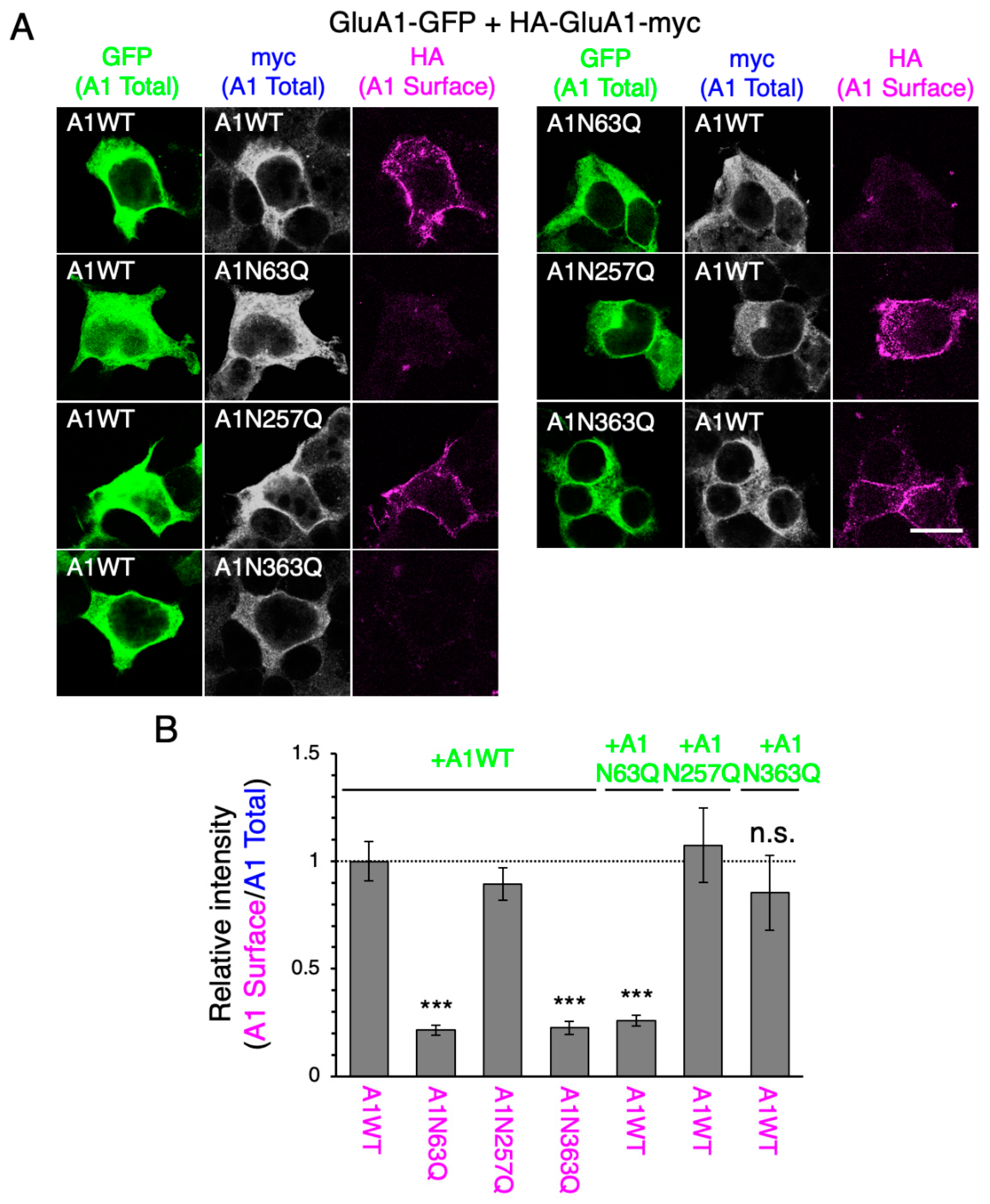
2.2. N-Glycan at GluA1N63 Is Essential for GluA1 Cell Surface Expression
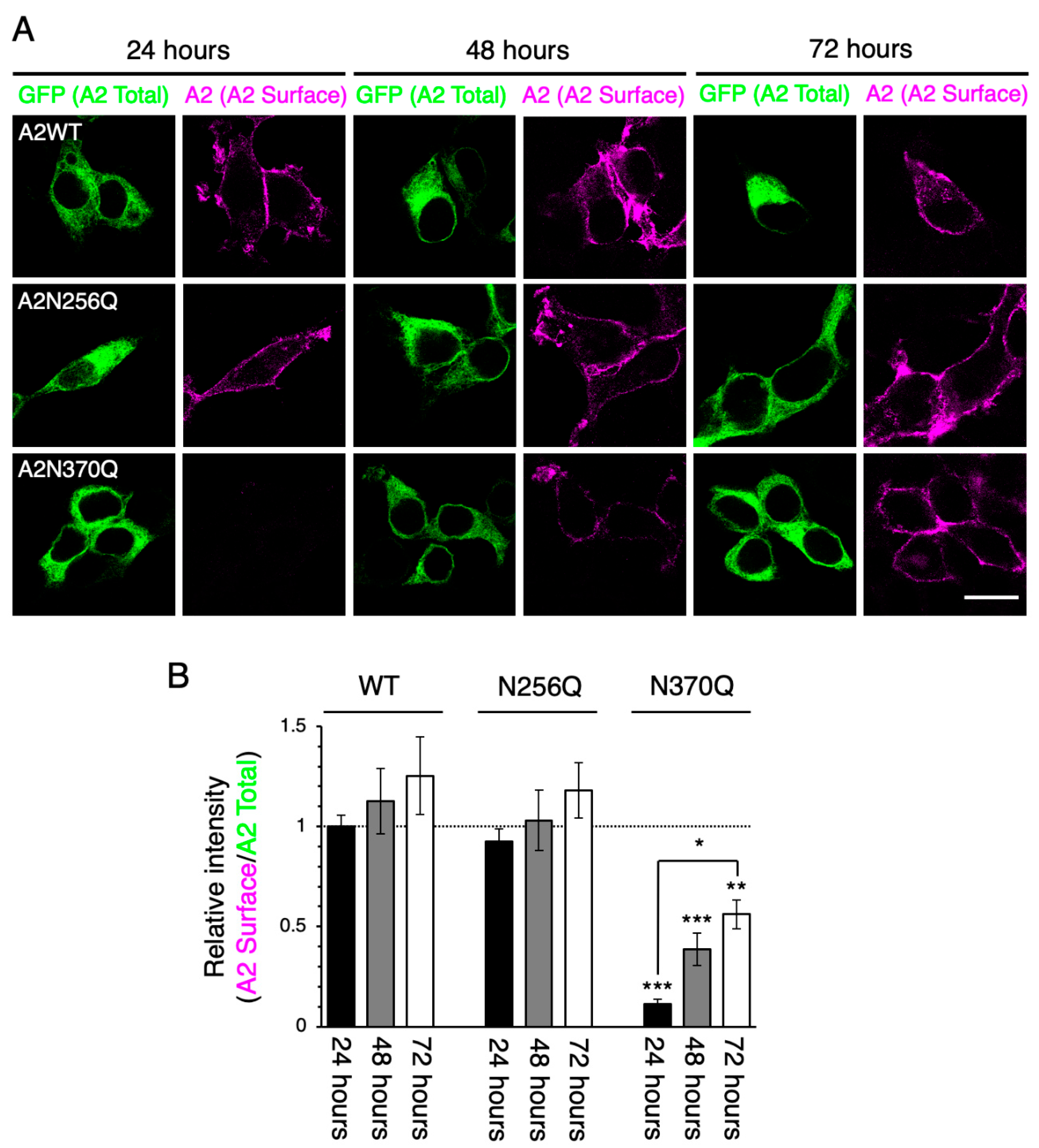
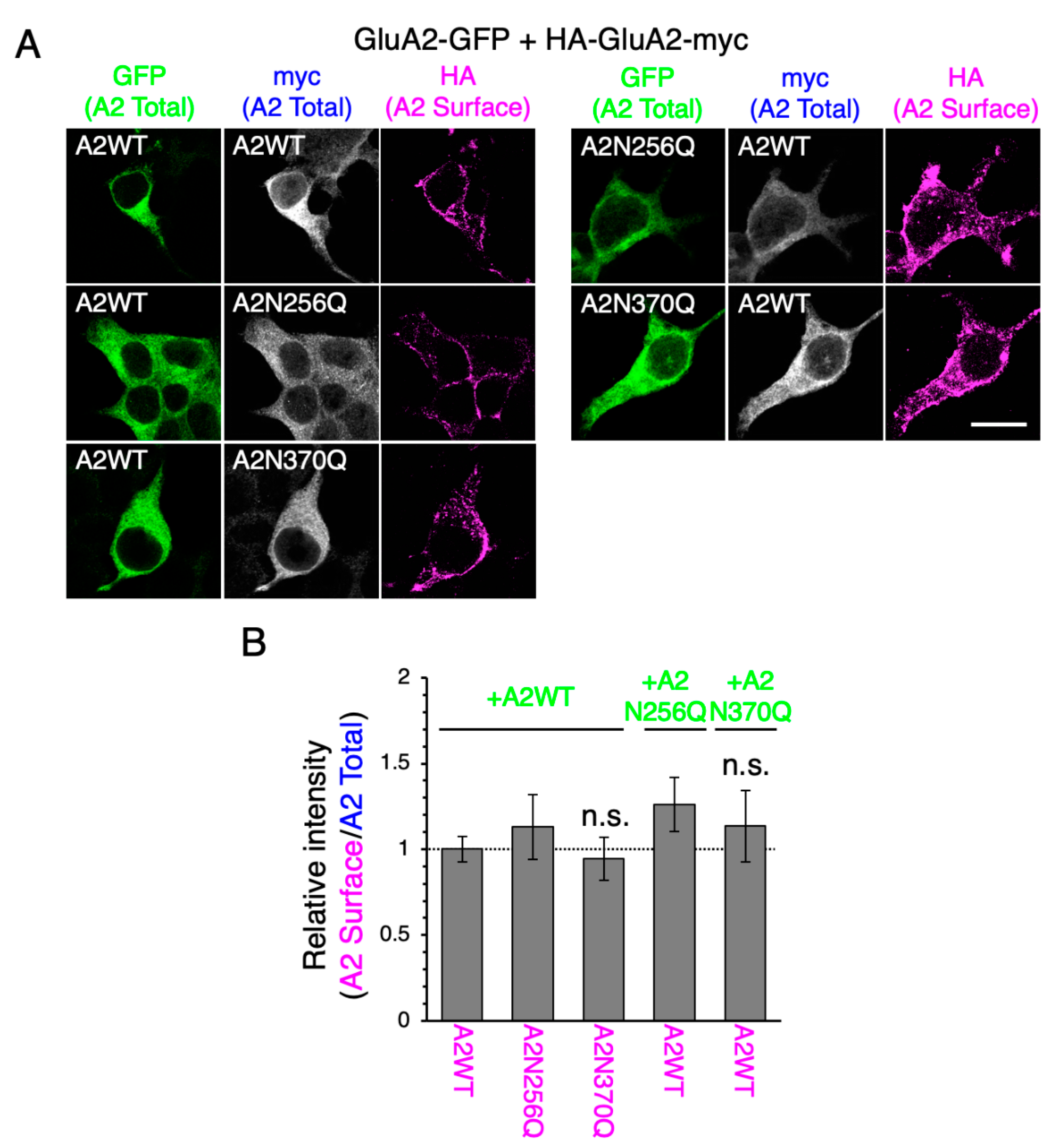
2.3. GluA2WT Enhances the ER-Exit Rate of GluA2N370Q
3. Discussion
4. Materials and Methods
4.1. Plasmids
4.2. Cell Culture and Transfection
4.3. Immunostaining
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Huganir, R.L.; Nicoll, R.A. AMPARs and synaptic plasticity: The last 25 years. Neuron 2013, 80, 704–717. [Google Scholar] [CrossRef]
- Henley, J.M.; Wilkinson, K.A. Synaptic AMPA receptor composition in development, plasticity and disease. Nat. Rev. Neurosci. 2016, 17, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Greger, I.H.; Khatri, L.; Ziff, E.B. RNA editing at arg607 controls AMPA receptor exit from the endoplasmic reticulum. Neuron 2002, 34, 759–772. [Google Scholar] [CrossRef]
- Wright, A.; Vissel, B. The essential role of AMPA receptor GluR2 subunit RNA editing in the normal and diseased brain. Front. Mol. Neurosci. 2012, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Shi, Y.; Jackson, A.C.; Bjorgan, K.; During, M.J.; Sprengel, R.; Seeburg, P.H.; Nicoll, R.A. Subunit composition of synaptic AMPA receptors revealed by a single-cell genetic approach. Neuron 2009, 62, 254–268. [Google Scholar] [CrossRef]
- Fortin, D.A.; Davare, M.A.; Srivastava, T.; Brady, J.D.; Nygaard, S.; Derkach, V.A.; Soderling, T.R. Long-term potentiation-dependent spine enlargement requires synaptic Ca2+-permeable AMPA receptors recruited by CaM-kinase I. J. Neurosci. 2010, 30, 11565–11575. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, X.; Zhou, Q. Perisynaptic GluR2-lacking AMPA receptors control the reversibility of synaptic and spines modifications. Proc. Natl. Acad. Sci. USA 2010, 107, 11999–12004. [Google Scholar]
- Park, P.; Kang, H.; Sanderson, T.M.; Bortolotto, Z.A.; Georgiou, J.; Zhuo, M.; Kaang, B.; Collingridge, G.L. The role of calcium-permeable AMPARs in long-term potentiation at principal neurons in the rodent hippocampus. Front. Synaptic Neurosci. 2018, 10, 42. [Google Scholar] [CrossRef]
- Pick, J.E.; Ziff, E.B. Regulation of AMPA receptor trafficking and exit from the endoplasmic reticulum. Mol. Cell Neurosci. 2018, 91, 3–9. [Google Scholar] [CrossRef]
- Schwenk, J.; Boudkkazi, S.; Kocylowski, M.K.; Brechet, A.; Zolles, G.; Bus, T.; Costa, K.; Kollewe, A.; Jordan, J.; Bank, J.; et al. An ER assembly line of AMPA-receptors controls excitatory neurotransmission and its plasticity. Neuron 2019, 104, 680–692. [Google Scholar] [CrossRef]
- Greger, I.H.; Ziff, E.B.; Penn, A.C. Molecular determinants of AMPA receptor subunit assembly. Trends Neurosci. 2007, 30, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Möykkynen, T.; Coleman, S.K.; Semenov, A.; Keinänen, K. The N-terminal domain modulates α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor desensitization. J. Biol. Chem. 2014, 289, 13197–13205. [Google Scholar] [CrossRef] [PubMed]
- Everts, I.; Villmann, C.; Hollmann, M. N-Glycosylation is not a prerequisite for glutamate receptor function but is essential for lectin modulation. Mol. Pharmacol. 1997, 52, 861–873. [Google Scholar] [CrossRef] [PubMed]
- Tucholski, J.; Pinner, A.L.; Simmons, M.S.; Meador-Woodruff, J.H. Evolutionarily Conserved Pattern of AMPA ReceptorSubunit Glycosylation in Mammalian Frontal Cortex. PLoS ONE 2014, 9, e94255. [Google Scholar] [CrossRef]
- Hanus, C.; Geptin, H.; Tushev, G.; Garg, S.; Alvarez-Castelao, B.; Sambandan, S.; Kochen, L.; Hafner, A.; Langer, J.D.; Schuman, E.M. Unconventional secretory processing diversifies neuronal ion channel properties. eLife 2016, 5, e20609. [Google Scholar] [CrossRef]
- Morita, I.; Kakuda, S.; Takeuchi, Y.; Itoh, S.; Kawasaki, N.; Kizuka, Y.; Kawasaki, T.; Oka, S. HNK-1 glyco-epitope regulates the stability of the glutamate receptor subunit GluR2 on the neuronal cell surface. J. Biol. Chem. 2009, 284, 30209–30217. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Morise, J.; Morita, I.; Takematsu, H.; Oka, S. Role of site-specific N-glycans expressed on GluA2 in the regulation of cell surface expression of AMPA-type glutamate receptors. PLoS ONE 2015, 10, e0135644. [Google Scholar] [CrossRef]
- Morita, I.; Kakuda, S.; Takeuchi, Y.; Kawasaki, T.; Oka, S. HNK-1 (human natural killer-1) glyco-epitope is essential for normal spine morphogenesis in developing hippocampal neurons. Neuroscience 2009, 164, 1685–1694. [Google Scholar] [CrossRef]
- Yamamoto, S.; Oka, S.; Inoue, M.; Shimuta, M.; Manabe, T.; Takahashi, H.; Miyamoto, M.; Asano, M.; Sakagami, J.; Sudo, K.; et al. Mice deficient in nervous system-specific carbohydrate epitope HNK-1 exhibit impaired synaptic plasticity and spatial learning. J. Biol. Chem. 2002, 277, 27227–27231. [Google Scholar] [CrossRef]
- Kandel, M.B.; Yamamoto, S.; Midorikawa, R.; Morise, J.; Wakazono, Y.; Oka, S.; Takamiya, K. N-glycosylation of the AMPA-type glutamate receptor regulates cell surface expression and tetramer formation affecting channel function. J. Neurochem. 2018, 147, 730–747. [Google Scholar] [CrossRef] [PubMed]
- Shanks, N.F.; Maruo, T.; Farina, A.N.; Ellisman, M.H.; Nakagawa, T. Contribution of the global subunit structure and stargazin on the maturation of AMPA receptors. J. Neurosci. 2010, 30, 2728–2740. [Google Scholar] [CrossRef] [PubMed]
- Coleman, S.K.; Hou, Y.; Willibald, M.; Semenov, A.; Möykkynen, T.; Keinänenm, K. Aggregation limits surface expression of homomeric GluA3 receptors. J. Biol. Chem. 2016, 291, 8784–8794. [Google Scholar] [CrossRef] [PubMed]
- Midorikawa, R.; Takakura, D.; Morise, J.; Wakazono, Y.; Kawasaki, N.; Oka, S.; Takamiya, K. Monitoring the glycosylation of α-amino-3-hydroxy-5-methyl-4-isoxazole-propionate-type glutamate receptors using specific antibodies reveals a novel regulatory mechanism of N-glycosylation occupancy by molecular chaperones in mice. J. Neurochem. 2020, 153, 567–585. [Google Scholar] [CrossRef] [PubMed]
- Leuschner, W.D.; Hock, W. Subtype-specific assembly of a-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor subunits is mediated by their N-terminal domains. J. Biol. Chem. 1999, 274, 16907–16916. [Google Scholar] [CrossRef]
- Lee, S.H.; Simonetta, A.; Sheng, M. Subunit rules governing the sorting of internalized AMPA receptors in hippocampal neurons. Neuron 2004, 43, 221–236. [Google Scholar] [CrossRef]
- Hastie, P.; Ulbrich, M.H.; Wang, H.L.; Arant, R.J.; Lau, A.G.; Zhang, Z.; Isacoff, E.Y.; Chen, L. AMPA receptor/TARP stoichiometry visualized by single molecule subunit counting. Proc. Natl. Acad. Sci. USA 2013, 110, 5163–5168. [Google Scholar] [CrossRef]
- Gan, Q.; Dai, J.; Zhou, H.X.; Wollmuth, L.P. The transmembrane domain mediates tetramerization of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors. J. Biol. Chem. 2016, 291, 6595–6606. [Google Scholar] [CrossRef]
- Sinitskiy, A.V.; Stanley, N.H.; Hackos, D.H.; Hanson, J.E.; Sellers, B.D.; Pande, V.S. Computationally discovered potentiating role of glycans on NMDA receptors. Sci. Rep. 2017, 7, 44578. [Google Scholar] [CrossRef]
- Subedi, G.P.; Sinitskiy, A.V.; Roberts, J.T.; Patel, K.R.; Pande, V.S.; Barb, A.W. Intradomain interactions in an NMDA receptor fragment mediate N-glycan processing and conformational sampling. Structure 2019, 27, 55–65. [Google Scholar] [CrossRef]
- Morise, J.; Suzuki, K.G.N.; Kitagawa, A.; Wakazono, Y.; Takamiya, K.; Tsunoyama, T.A.; Nemoto, Y.L.; Takematsu, H.; Kusumi, A.; Oka, S. AMPA receptors in the synapse turnover by monomer diffusion. Nat. Commun. 2019, 10, 5245. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morise, J.; Yamamoto, S.; Midorikawa, R.; Takamiya, K.; Nonaka, M.; Takematsu, H.; Oka, S. Distinct Cell Surface Expression Patterns of N-Glycosylation Site Mutants of AMPA-Type Glutamate Receptor under the Homo-Oligomeric Expression Conditions. Int. J. Mol. Sci. 2020, 21, 5101. https://doi.org/10.3390/ijms21145101
Morise J, Yamamoto S, Midorikawa R, Takamiya K, Nonaka M, Takematsu H, Oka S. Distinct Cell Surface Expression Patterns of N-Glycosylation Site Mutants of AMPA-Type Glutamate Receptor under the Homo-Oligomeric Expression Conditions. International Journal of Molecular Sciences. 2020; 21(14):5101. https://doi.org/10.3390/ijms21145101
Chicago/Turabian StyleMorise, Jyoji, Saki Yamamoto, Ryosuke Midorikawa, Kogo Takamiya, Motohiro Nonaka, Hiromu Takematsu, and Shogo Oka. 2020. "Distinct Cell Surface Expression Patterns of N-Glycosylation Site Mutants of AMPA-Type Glutamate Receptor under the Homo-Oligomeric Expression Conditions" International Journal of Molecular Sciences 21, no. 14: 5101. https://doi.org/10.3390/ijms21145101
APA StyleMorise, J., Yamamoto, S., Midorikawa, R., Takamiya, K., Nonaka, M., Takematsu, H., & Oka, S. (2020). Distinct Cell Surface Expression Patterns of N-Glycosylation Site Mutants of AMPA-Type Glutamate Receptor under the Homo-Oligomeric Expression Conditions. International Journal of Molecular Sciences, 21(14), 5101. https://doi.org/10.3390/ijms21145101




