Abstract
Alzheimer disease (AD) is one of the most common neurodegenerative diseases, affecting middle-aged and elderly individuals worldwide. AD pathophysiology involves the accumulation of beta-amyloid plaques and neurofibrillary tangles in the brain, along with chronic neuroinflammation and neurodegeneration. Physical exercise (PE) is a beneficial non-pharmacological strategy and has been described as an ally to combat cognitive decline in individuals with AD. However, the molecular mechanisms that govern the beneficial adaptations induced by PE in AD are not fully elucidated. MicroRNAs are small non-coding RNAs involved in the post-transcriptional regulation of gene expression, inhibiting or degrading their target mRNAs. MicroRNAs are involved in physiological processes that govern normal brain function and deregulated microRNA profiles are associated with the development and progression of AD. It is also known that PE changes microRNA expression profile in the circulation and in target tissues and organs. Thus, this review aimed to identify the role of deregulated microRNAs in the pathophysiology of AD and explore the possible role of the modulation of microRNAs as a molecular mechanism involved in the beneficial actions of PE in AD.
1. Introduction
Alzheimer disease (AD) is the leading cause of dementia, being defined as a progressive neurodegenerative disease with neuropathological hallmarks: Neurofibrillary tangles and beta-amyloid plaques [1]. Neurofibrillary tangles are formed by hyperphosphorylated Tau protein, which loses its physiological role in promoting microtubule stability in neurons [2,3]. Plaques are aggregates of beta amyloid (Aβ) oligomers, which are highly toxic to the central nervous system (CNS) and promote neuroinflammation [4,5]. Considering that AD’s prevalence is expected to grow, it is crucial to increase pathophysiological knowledge, to identify novel biomarkers for early diagnosis, and to develop direct therapeutic interventions to slow disease progression.
Physical exercise (PE) has been reported to reduce memory loss [6], and sedentarism has been recognized as a modifiable risk factor for the development of AD [7]. The results of a recent meta-analysis indicate that different PE programs have a beneficial impact on patients with dementia, leading to significant improvements in cognitive function, memory, and ability to perform daily activities [8]. The mechanisms involved in the benefits promoted by PE in AD remain not fully understood.
It is known that PE has antioxidant [9] and anti-inflammatory actions that contribute to neuroprotection and cognitive improvements seen in animal models and AD patients [10]. In AD mouse models, PE modulated neuroinflammation, improved memory [11,12,13], reduced neuronal death, and increased the expression of antioxidant enzymes [14]. Moreover, PE has also been reported to attenuate the accumulation of Aβ plaques in the brain, increase hippocampal volume, and improve behavior in a Tg2576 AD mouse model [15].
PE modulates the profile of microRNA expression detected in body fluids, tissues, and organs. The hypothesis that modulation of microRNA expression by PE is mechanistically linked to the improvements reported in cognitive function in the context of AD, however, has not been sufficiently explored to date [16,17,18]. MicroRNAs (miRNAs) are small, single-stranded non-coding RNAs that play key roles in the post-transcriptional regulation of gene expression [19]. MiRNAs bind to the 3′-untranslated region (3′UTR) of target mRNAs [20], leading to degradation, deadenylation, or inhibition of translation [21].
A single miRNA can regulate different targets, and it is estimated that miRNAs regulate approximately 60% of all human protein coding genes by post-transcriptional mechanisms [22]. Therefore, miRNAs are involved in several biological processes, including apoptosis [23], cell metabolism, homeostasis [24], differentiation [25], cell cycle [26], growth [27], cell survival [28], cell proliferation, and migration [29]. In addition, the involvement of miRNAs has also been demonstrated in pathological processes of different diseases, such as heart failure [30], arterial hypertension [31], type 2 diabetes mellitus, obesity [32], Chagas disease cardiomyopathy [33], and AD [34].
In this review, we aimed to evaluate the evidence supporting the role of deregulated miRNA profiles in the pathogenesis of AD and the potential beneficial actions of PE-induced miRNAs in physiological brain functions. Overlaps in the miRNAs reported to be deregulated in AD and induced by PE were also evaluated and discussed.
2. Biogenesis of MiRNAs
MiRNA biogenesis is a complex process that begins in the nucleus, with transcription, and finishes in the cytoplasm, with the maturation step [35,36]. In the nucleus, miRNAs are transcribed by RNA polymerase II into a precursor molecule, referred to as the primary transcript (pri-miRNA), which contains one or more sequences in a stem-loop structure [37,38]. The pri-miRNA undergoes two cleavages by Drosha, a class 2 RNase III-like enzyme, [39,40,41] in conjunction with DGCR8 (DiGeorge syndrome chromosomal region 8) [42,43,44]. This processing leads to the conversion of the pri-miRNA into a stem-loop intermediate structure with approximately 70 nucleotides called precursor (pre-) miRNA [22]. The pre-miRNA is exported to the cytoplasm by Exportin 5, being targeted by Dicer, a member of ribonuclease III (RNase III) family [19,45,46,47,48]. Dicer recognizes the pre-miRNA hairpin and cuts it into a smaller miRNA duplex, of approximately 22 nucleotides [49].
After being processed by Dicer, the mature miRNA is incorporated into the miRNA-induced silencing complex (miRISC), the final effector complex. The remaining strand exhibits less stable base pairing and associates with Argonaute (AGO) proteins, components of RISC that interact with miRNAs, as downstream effectors in the processes of translational repression through mRNA degradation or deadenylation [50,51,52,53].
Although several microRNAs with altered expression have been reported in AD, their function and mechanisms of regulation remain to be fully clarified. Accumulating evidence shows that microRNAs play a critical role in the pathogenesis of AD, being involved in the Aβ hypothesis (process regulation of Aβ production and clearance) and in the Tau hypothesis (imbalance between Tau phosphorylation and dephosphorylation in the formation of neurofibrillary tangles) [54]. Other microRNAs are involved in the regulation of neurogenesis, neuroinflammation, and insulin cascade, and may also contribute to the disease pathogenesis, as described in the next sections.
3. MiRNAs and AD Pathophysiology
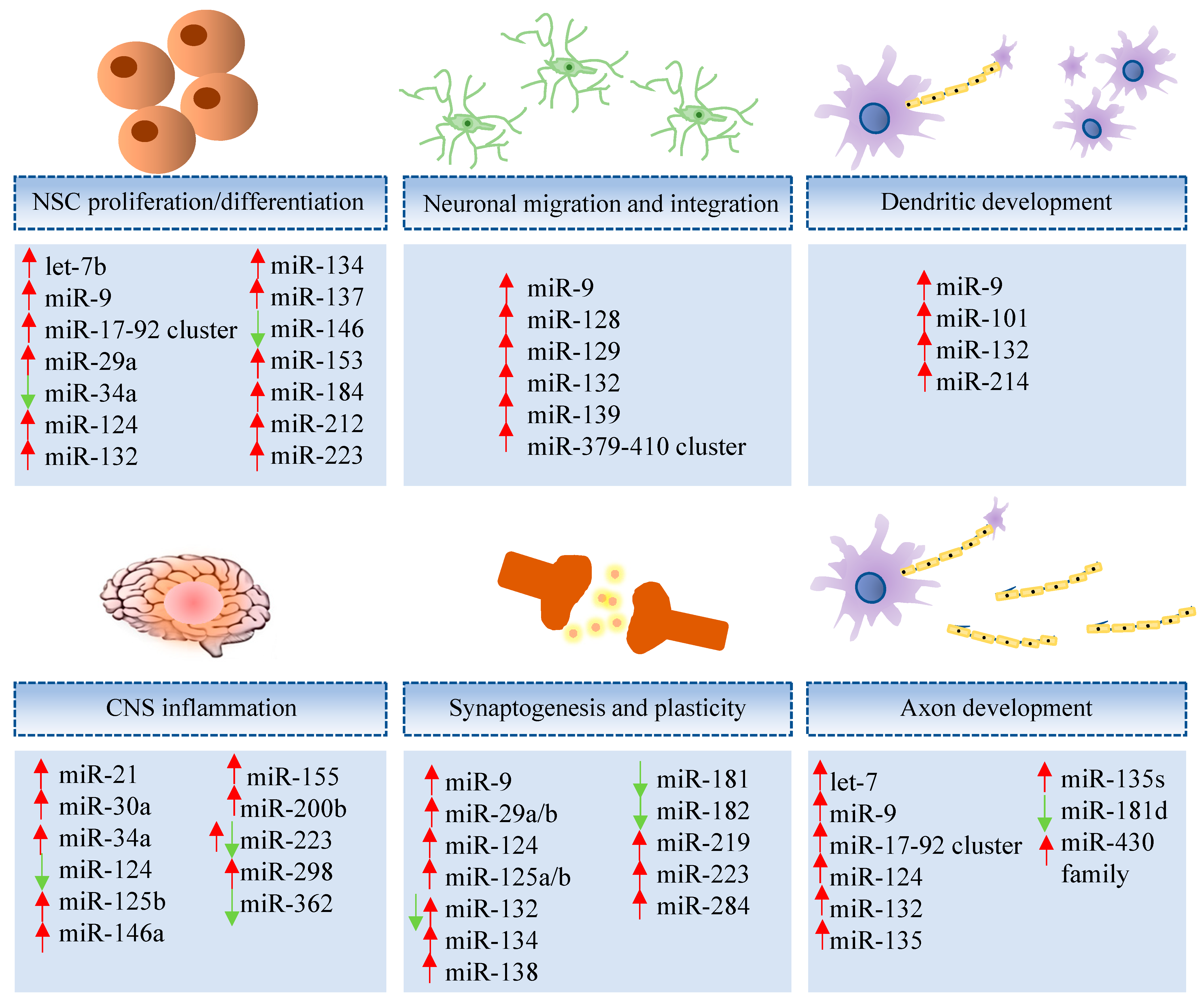
MiRNAs play key roles in brain development, neuron differentiation, and synaptic plasticity [55], as illustrated in Figure 1. Moreover, deregulation of miRNAs is found in neurodegenerative diseases, including AD [56], involving processes of synaptogenesis, ion channel expression, inflammation, and neurodegeneration [57]. A review of microRNAs, from their role in AD, is found below and listed (Supplementary Tables S1 and S2).

Figure 1.
Representative miRNAs involved in neural development and function. Let-7b [58,59], miR-9 [58,59], miR-17-92 cluster [60], miR-29a [61], miR-34a [62], miR-124 [58], miR-132 [63], miR-134 [64], miR-137 [65], miR-146 [66], miR-153 [67], miR-184 [58], miR-212 [68], miR-223 [69], miR-9 [70], miR-128 [71], miR-129 [72], miR-132 [70], miR-139 [73], miR-379-410 cluster [74], miR-9 [75], miR-101 [76], miR-132 [77], miR-214 [78], miR-21 [79], miR-30a [80], miR-34a [81], miR-124 [81], miR-125b [82], miR-146a [83], miR-155 [81], miR-200b [79], miR-223 [57], miR-298 [79], miR-362 [79], miR-9 [84], miR-29a/b [57], miR-124 [84], miR-125a/b [64], miR-132 [64], miR-134 [64], miR-138 [58], miR-181 [85], miR-182 [86], miR-219 [87], miR-223 [57], miR-284 [88], let-7 [89,90], miR-9 [91], miR-17-92 cluster [60], miR-124 [84], miR-132 [92], miR-135 [93], miR-135s [93], miR-181d [94], miR-430 family [95].
3.1. MiRNAs in the Regulation of Neurogenesis
A decline of adult neurogenesis has been reported in brain samples obtained from AD patients when compared to controls [96]. Impaired neurogenesis seen in AD is probably the result of a process that compromises the ability for neural stem cell self-renewal, and deregulation of miRNAs is one of the mechanisms underlying age-related decline in neurogenesis, altering the normal function, self-renewal, differentiation, and survival of neural stem cells (NSCs).
Many are the reports of miRNAs that regulate NSC function, including miR-184 and miR-137 that, upon overexpression, increase NSC proliferation, and miR-124, miR-9, and lethal-7b (let-7b), which are associated with neural differentiation. MiR-223 was shown to induce neuronal morphogenesis in both human embryonic stem cells and adult mouse dentate gyrus NSCs [69]. The pro-neurogenic role of the miRNA-17-92 cluster was demonstrated in mice, as its ablation was associated with impaired adult hippocampal neurogenesis and loss of cognitive function [60].
MiR-9 is a brain-enriched miRNA that is expressed in the neurogenic areas of the embryonic and adult brain. During NSC differentiation, miR-9 expression is increased while TLX is decreased. This negative feedback loop could be regulating NSC proliferation and differentiation [58]. TLX also regulates transcription of miR-137 [65] and let-7 plays an important role in hippocampal neurogenesis, memory, and learning [59].
Notch pathway activation is required for normal NSC function and different miRNAs were linked to this regulation. MiR-184 and miR-34a are involved in NSCs’ maintenance, increasing self-renewal by regulation of Numbl, an inhibitor of Notch signaling. Knockdown of endogenous miR-34a in murine neural progenitor cells and NSCs resulted in increased expression of Numbl, NeuroD1, and Mash1 along with decreased Notch1 expression [62,97]. A recent study in aged mice showed that miR-153 is downregulated in the hippocampus, and that miR-153 overexpression increased neurogenesis by interfering with the Notch signaling pathway [67].
The adult brain physiologically expresses miR-106b, miR-93, and miR-25 (members of the miR-106b~25 cluster), which target Forkhead box O3 (FoxO3), a member of the forkhead transcription factors that orchestrates gene programs involved with insulin/insulin-like growth factor (IGF) signaling. FoxO3 targeting by miR-106b increases neural progenitor cells and NSC proliferation, including neuronal differentiation in vitro, with implications for tissue homeostasis and aging [98].
Members of the let-7 family (let-7a and let-7b) have been identified as important regulators of NSCs’ fate [99,100]. Let-7 regulates Hmga2, a member of the high-mobility group A (HMGA) that is downregulated in NSCs with aging, reducing the proliferative capacity of both fetal and adult NSCs in mice [89]. Furthermore, it was demonstrated that miR-145 regulates the differentiation of NSCs by modulating the Sox2-Lin28/let-7 signaling pathway in vitro [90].
Some miRNAs are regulated in response to neuronal activity, including miR-134, which is modulated by myocyte enhancer factor 2 (MEF2) in response to membrane depolarization. MiR-132 is induced by cAMP response elements (CREB) activation and targets methyl CpG binding protein 2 (MECP2) [63]. MECP2 also modulates miR-137 and miR-15a expressions [101,102]. MEF2 plays an important role in neuron survival [103] and deficiency in MECP2 protein leads to neuronal dysfunction [104].
In cortical neurogenesis, let-7b, miR-9, miR-124, and miR-134 are recruited to control the behavior of cortical progenitors. The involvement of miR-9 and miR-132 in neurite outgrowth has been described in embryonic mouse neocortex [70]. The absence of miR-132, along with miR-212, was shown to induce Tau aggregation and impair cognitive skills in an experimental model of AD. Additionally, high expression levels of these miRNAs were found in postmortem frontal cortex tissue from AD patients, suggesting possible involvement in the disease pathogenesis, as higher miR-132 and miR-212 levels were associated with improved AD patient cognitive scores [68].
3.2. MiRNAs and AD Neuroinflammation
A group of miRNAs displays neuroprotective functions and their deregulation plays critical roles in the pathogenesis of neurodegeneration. Some of these miRNAs are also involved in the regulation of inflammatory processes in neurological diseases, including miR-146a, one of the first miRNA found upregulated in AD patients’ brain tissues and in in vitro AD model [66]. Indeed, neuroinflammation plays a key role in AD pathogenesis [105,106] and increased secretion of proinflammatory cytokines leads to Tau hyperphosphorylation. These processes result from neuroinflammatory cascades, activation of microglia [106] and astrocytes, and their repercussions in glia-neuron interactions [107].
Brain inflammation appears to have a dual function, playing a neuroprotective role during an acute-phase response and protecting the brain from pathogens and neurotoxic agents and promoting tissue repair [108,109], but becomes detrimental when a chronic response is mounted [110]. Accumulated Aβ oligomers in AD trigger microglia activation through toll-like receptors, leading to chronic inflammation. Overexpression of miR-34a, which was described in AD human retinal tissues and in vitro model, leads to inhibition of Triggering receptor expressed on myeloid cells 2 (TREM2) expression in microglia, interfering with clearance of the oligomers [111,112]. Activation of the Nuclear factor-κB (NFκB) pathway in brain cells is associated with increased expression of inflammation-associated miRNAs as well as miR-155, miR-124, and miR-146a [81,113,114].
To note, miR-155 expression has been observed to be altered in brain tissue from patients with AD [115] as it displays a regulatory role in T-cell functions and, thus, AD progression [116]. Similarly, miR-124 is a potent negative regulator of Beta-Secretase 1 (BACE1) in the cellular AD phenotype and might be involved in the pathogenesis of AD [117]. MiR-146a and miR-181a are implicated in the mechanisms of AD progression as they are increased in patients with mild cognitive impairment who converted to AD. Their alterations are correlated to amyloid beta cerebrospinal fluid (CSF) concentration and associated to neuroimaging features [118].
Other miRNAs have been reported to be abnormally expressed and be closely correlated with the pathogenesis of AD, as follows. Let-7 family overexpression binds to TLR7 in AD-promoting neuroinflammation and compromises neuronal survival [119]. MiR-181 dysregulation in astrocytes may also contribute to the disease pathogenesis, since it has been linked to synaptic dysfunction [120]. In contrast, miR-21 plays a pivotal act in neuroinflammation as it can exert protective roles in AD, which might be dependent on Programmed Cell Death 4/Phosphoinositide 3-kinases/Protein kinase B/Glycogen synthase kinase 3 (PDCD4/PI3K/AKT/GSK-3β) signaling pathway in vitro [121].
3.3. MiRNAs, Insulin Resistance, and AD
Insulin resistance (IR) is central for the development of type 2 diabetes [122] and a growing body of evidence indicates that it is also a critical process for AD development, being associated with a failure in PI3K pathway activation [5,123]. Amyloid fibrils are polymers [124] produced by a precursor membrane protein called amyloid precursor protein (APP) [125]. APP cleavage by β- and Υ-secretases lead to the production of cytotoxic Aβ peptide with 40 or 42 amino acids (Aβ40-Aβ42) [126,127]. Aβ oligomers are associated with the development of neuronal insulin resistance (IR) [128].
It has been demonstrated that miR-29 family is the microglial modulators of the insulin-like growth factor-1 (IGF-1) and fractalkine ligand (CX3CL1) [129], supporting the hypothesis that impairment of IR and IGF-1 mediates cognitive impairment in AD [130]. MiR-375, miR-101, and miR-802 are involved in the regulation of gene expression in patients with type 2 diabetes [131] and are also present in mouse models of AD [132,133]. Increased levels of Aβ40-Aβ42 are usually seen in association with downregulation of miR-195 in AD [18]. At the early stages of AD in vivo, it has been reported that miR-132/212 are also downregulated [134].
Insulin receptors are found in CNS synapses in areas related to cognitive function and memory, such as the cerebral cortex and the hippocampus [135]. Insulin receptors can activate several different protein substrates, such as the insulin receptor substrate (IRS) family [136,137]. IRS-1 mediates glucose metabolism in AD [5,138], while IRS-2 mediates IR in type 2 diabetes [139]. However, it has been reported that miR-7 is overexpressed in the cerebellum and frontal cortex of AD patients, reducing the expression of IRS-2, impairing the insulin signaling pathway [140], and possibly modulating Aβ metabolism during disease progression. It has been reported that miR-126 is involved with micro- and macrovascular complications in type 2 diabetes [16], and this miRNA was found overexpressed in the cortex and hippocampus in a mouse model of AD [132,141]. MiR-126 regulates IRS-1 gene, reducing its expression, impairing the signaling pathway of PI3K-AKT, and, consequently, contributing to the development of IR.
Increased expression of miR-200b or -c diminishes secretion of the Aβ oligomers and, consequently, reduces IRS-1 phosphorylation in serine residues, which is associated with cognitive impairment and memory loss in AD [142,143,144]. Exacerbated IRS-1 phosphorylation on serine residues may also induce dephosphorylation of protein kinase B (AKT) by inhibiting pro-survival signaling pathways and activation of glycogen synthase kinase-3β (GSK-3β), a cellular pathway that leads to neuronal apoptosis [145]. GSK-3β, in turn, inhibits the induction of the autophagosome to the lysosome and stimulates the insertion of the autophagosome to the multivesicular body (MVB). MVB incorporates autophagosome, endosome containing some proteins, and exosomes containing proteins, DNA, mRNAs, and miRNAs, which later will be fused to the plasma membrane and are released into the extracellular medium in the form of extracellular vesicles (EVs) [146]. The EVs act in intercellular communication and are associated with the propagation of proteins such as APP and Tau that participate strongly in the pathophysiological process of AD. EVs are also related with the spread of several miRNAs that regulate gene expression at the post-transcriptional level, modulating many signaling pathways in AD [147].
Tumor necrosis factor (TNF-α) is highly expressed by microglia and astrocytes and activation of the TNF-α pathway contributes to IRS-1 phosphorylation, inhibiting PI3K pathway in AD [5]. Inflammatory responses mediated by microglial and astrocytes’ activation can be regulated by miR-21, which will contribute to avoid cognitive impairment and memory loss [148]. Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor alpha (IkBa), IκB kinase (IKK) and c-Jun N-terminal kinase (JNK) can be also activated in AD and lead to inhibition of IRS-1 phosphorylation in tyrosine residues with the concomitant increase of TNF-α [2]. Impaired insulin signaling pathway is linked to neuronal inflammation [4], synaptic loss, neurodegeneration, decreased neurogenesis, changes in behavior, cognitive impairment, and memory loss [149,150]. Conversely, memory enhancement was seen through the inhibition of miR-124, with concomitant downregulation of several genes [151].
3.4. MiRNAs and Aβ Production
Deregulation of miRNAs that target APP and β-site amyloid precursor protein cleaving enzyme 1 (BACE1) can lead to increased production and accumulation of Aβ peptides in the brain, with consequent synaptic failure, neurotoxicity, and dementia [152]. Different miRNAs have been reported to be involved in the regulation of Aβ expression, including miR-153 [153], miR-101 [154], miR-16 [155], miR-193b [156], miR-17, miR-20a, miR-106b, and miR-132, among others. [157].
Aβ peptides are fragments derived from APP, and miRNAs participate in the regulation of APP gene expression. MiR-101, miR-153, and miR-384 inhibit APP expression and, consequently, reduce the levels of Aβ peptide. These microRNAs were reported to be downregulated in vitro and AD patients [153,154,158]. MiR-29a, miR-29b-1, miR-29c, and miR-339-5p, which regulate BACE1, were found downregulated in AD, leading to BACE1 upregulation, with increased APP cleavage and Aβ accumulation in vitro, in vivo, and in the AD patients’ brain [61,159,160]. MiR-132 and miR-212 have also been found downregulated in AD patients and in 3xTg-AD mice, increasing the production of Aβ oligomers and senile plaque formation, while contributing to loss of cognitive function [161].
MiR-16, miR-101, miR-106a/b, miR-147, and miR-160a function as APP suppressors [133,162,163]. APP expression levels may influence the risk of AD [164]. Recently, it was described that miR-346 binds to the 5 ′ UTR of the APP mRNA, increasing its expression and translation, with consequent increase of Aβ production, leading to a cascade of redox stress and inflammation in vitro and in AD patients’ brain tissues [165]. These findings suggest that therapeutic modulation of the expression of miRNAs involved in the regulation of APP gene may impact Aβ accumulation.
One of the mechanisms associated with the accumulation of Aβ is the sequential proteolytic cleavage of full-length APP by BACE1. In vitro studies demonstrated that miR-29 is involved in the regulation of APP and BACE1 expression. In AD in vitro model, miR-29b negatively regulates BACE1 and subsequent Aβ peptides’ levels [166]. BACE1 is the aspartic-acid protease initiator for the formation of Aβ, and expression levels have been detected in AD brains. Several miRNAs have been reported to regulate BACE1 expression, including miR-339, miR-298, miR-328, miR-107, miR-200a, miR-124, miR-195, and miR-188. It was demonstrated that loss of miR-29 cluster increased BACE1 protein levels in AD [163]. Reduced miR-107 in AD patients’ brain neocortex samples negatively correlated with BACE1 [167].
MicroRNAs are also involved in the process of synaptic failure induced by Aβ. It was reported that inhibition of the expression of members of the miR-34 family could improve cognitive functions in the Aβ transgenic mice model and in cultured neurons exposed to Aβ [168,169]. MiR-188-5p was also demonstrated to restore the synaptic dysfunction and cognitive impairment evaluated in Aβ transgenic mice [170].
3.5. MiRNAs and Tau Hyperphosphorylation in AD
Hyperphosphorylated Tau protein is one of the hallmarks of AD and results from an imbalance between the activity of related kinases and phosphatases [171]. The accumulation of neurofibrillary tangles associated with tau hyperphosphorylation induces neuronal apoptosis, neurotoxicity, proteolysis, fibrillization, and neurodegeneration, impairing different brain functions, leading to AD [172] and cognitive deficits [173].
It was demonstrated in vivo that Tau expression is directly regulated by miR-137, miR-132, and miR-212. Interestingly, deletion of miR-132/212 in mice was associated with increased Tau phosphorylation and accumulation [134]. In brain tissues from AD patients, miR-219 was downregulated and associated with neurodegeneration, while in vitro assays confirmed Tau as its direct target [174].
Overexpression of miR-125b in hippocampal neurons is associated with changes in dendritic spine morphology and function [175]. In the context of AD, miR-125b is overexpressed, reducing the expression of dual-specific phosphatase 6 (DUSP6), protein phosphatase 1 catalytic subunit alpha isoform (PPP1CA), and B-cell lymphoma 2-like protein 2 (Bcl-W), resulting in the increase of p35, cdk5, and p44/42- mitogen-activated protein kinase (MAPK) signaling, hyperphosphorylation of Tau protein, and neurotoxicity, contributing to cognitive deficits in AD [173].
Increased miR-922 expression found in AD contributes to Tau phosphorylation, suppressing ubiquitin carboxy-terminal hydrolase L1 (UCHL1) in vitro [176], a protein involved in the maintenance of synaptic and cognitive function [177]. Similarly, overexpression of miR-146a in both in vitro (SH-SY5Y cells treated with Aβ1-42) and in a transgenic mouse model of AD suppressed the expression of rho-associated, coiled-coil-containing protein kinase 1 (ROCK1), reducing the phosphatase and tensin homologue (PTEN) phosphorylation, which contributes to GSK3 phosphorylation, finally promoting an exacerbation of phosphorylation of Tau protein. The accumulation of Tau in these models leads to the formation of forming neurofibrillary tangles, generating neuronal death, and favoring the progression of AD [178].
MiR-219, which showed reduced expression in post-mortem brain tissues obtained from AD patients, was associated with increased Tau gene expression and protein accumulation in the brain [174]. Likewise, reduced miR-106b expression in brain tissue samples from patients with AD was associated with increased expression of the proto-oncogene tyrosine protein kinase (FYN) gene, which has several biological functions from brain development to neuroinflammation, plasticity, and synaptic function and also contributes to Tau phosphorylation [179]. MiR-132 is also reduced in AD, being associated with increased expression of inositol 1,4,5-triphosphate 3 kinase B (ITPKB) and augmented Tau phosphorylation in the hippocampus of mice, favoring disease progression and cognitive dysfunction [180].
Another mechanism of epigenetic regulation of gene expression, histone deacetylation mediated by the histone deacetylases (HDACs), plays an important function in memory and synaptic plasticity [181]. The expression of HDACs is increased in AD precisely in the entorhinal cortex and in the hippocampus, contributing to increased cognitive deficits [182]. Interestingly, increased HDAC2 expression causes deacetylation of the hepatocyte nuclear factor 4a (HNF4A) transcriptional factor, inhibiting its expression and reducing the expression of miR-101b, which targets 5′ adenosine monophosphate-activated protein kinase (AMPK). Thus, AMPK is overexpressed, inducing tauopathy, promoting the progression of AD in a mouse model and comorbidities associated [183].
3.6. Circulating MiRNAs in AD
Stable extracellular miRNAs are present in body fluids and, therefore, circulating miRNAs have emerged as potential biomarkers for diagnosis, staging, and progress monitoring of various diseases. In AD, deregulated circulating miRNAs that are representative of the subjacent pathological process in the brain tissue could be utilized to support early diagnostics and to monitor disease progression. MiRNAs extracted from peripheral blood, serum, plasma, whole blood, or exosomes have been explored as biomarker candidates in AD [184] (Supplementary Table S3).
It is noteworthy that a circulating miRNA signature not necessarily correlates with brain tissue miRNA expression and underlying disease pathology. A systematic meta-analysis assessment was performed using 147 independent datasets across 107 publications. Deregulation of 32 miRNAs in the brain, CSF, and blood was reported. Five miRNAs exhibited significant differential expression in both brain and blood: The hsa-miR-181c-5p and hsa-miR-29c-3p (downregulated) and hsa-miR-125b-5p, hsa-miR-146a-5p, and hsa-miR-223-3p were upregulated in brain and downregulated in blood [185]. A review method was used to evaluate 8098 miRNAs in AD and controls from 26 studies. They found six downregulated miRNAs in AD patients: miR-107, miR-125b, miR-146a, miR-181c, miR-29b, and miR-342 [186,187].
Deregulated miRNAs in AD patients’ serum samples include miR-137, miR-181c, miR-9, and miR-29a/b [188]. The same miRNAs were also found downregulated in AD patients’ brain samples, showing a negative correlation with ceramide and Serine palmitoyltransferase, long chain base subunit 1/2 (SPTLC1/2) protein levels in the neocortex [189]. A genome-wide serum miRNA expression analysis identified six differentially expressed miRNAs in the serum of AD patients: miR-98-5p, miR-885-5p, miR-483-3p, miR-342-3p, miR-191-5p, and let-7d-5p [190]. Evaluation of 84 selected miRNAs in serum samples revealed downregulation of miR-125b, miR-23a, and miR-26b in AD patients [191]. Reduced levels of serum miR-125b was also found in AD. The same study reported miR-181c downregulation and miR-9 upregulation in serum samples of AD patients [192].
Interestingly, a study demonstrated that miR-384 regulates both APP and β-secretase expression. Downregulated miR-384 was found in CSF and serum compared with controls. In addition, the authors reported lower levels of this miRNA in blood samples from patients with AD dementia compared with mild cognitive impairment. [158].
Other studies evaluated miRNA expression profile in the CSF and exosome as a potential biomarker of AD. In the CSF, deregulation of several miRNAs including miR15b, miR-34a, miR-142, miR-146a, miR-125b, miR-545, and miR-29 family were found in AD patients compared to control subjects [193,194]. The expression levels of miR-193b, miR-135a, and miR-200b were associated with APP [156,195]. APP has an important function in the brain. However, the process responsible for the metabolism of APP to Aβ has not been well understood [196]. Six miRNAs were identified in plasma and CSF of AD patients as potential markers: miR-9, miR-29a, miR-29b, miR-34a, miR-125b, and miR-146a [197].
It was reported that circulating let-7d-5p, let-7g-5p, miR-15b-5p, miR-142-3p, miR-191-5p, miR-301a-3p, and miR-545-3p were upregulated in plasma samples from AD patients compared with normal controls [198]. Deregulation of miR-107 and miR-650 found in plasma samples of AD patients was associated with Aβ metabolism, contributing to pathological processes [199].
Finally, it was demonstrated that apolipoprotein E gene allele e4 is correlated with miR-146a levels in brain/plasma in mice and associated with increased AD risk [200]. Apolipoprotein E (ApoE) acts as a major lipid carrier in the brain and the isoform ApoE e4 has been identified as a strong AD genetic risk factor [201].
4. Physical Exercise
4.1. Beneficial Actions of Physical Exercise in AD
PE is currently recommended as a non-pharmacological measure for preventing cognitive decline [149,202,203]. Some studies have supported a role for PE in counteracting the pathophysiology determinants of AD and reducing the risk of memory loss in elderly, attenuating AD development [6,7]. Mechanisms reported involve enhanced brain plasticity [204], increased brain-derived neurotrophic factor (BDNF) [205], improved brain metabolism [206], and changes in the microbiome [207].
PE has been linked to improvement of cognitive status in AD patients [208] and attenuation of cognitive decline in aged individuals that express the APOE ε4 allele [209] and are predisposed to develop AD [210]. Glucose homeostasis is extremely important to synaptic plasticity and PE can contribute to manage it [211] and to inhibit oxidative stress [212].
Neuroprotective roles exerted by PE involve antioxidant and anti-inflammatory actions [9]. PE-induced reduction of neuroinflammation and improvements in memory were reported in AD experimental models [11,12,13]. Interestingly, transgenic AD mouse model submitted to an aerobic PE in the running wheel for three weeks showed reduced TNF-α and interleukin-1β (IL-1β) expression and decreased Aβ deposition in the brain [213]. Resistance training in 3xTg-AD mice also reduced the accumulation of Aβ and Tau protein, reduced expression of TNF-α and IL-1β, increased expression of IL-6, IL-10, Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), and fibroblast growth factor 21 (FGF-21), increased the expression of structural synaptic proteins, and improved cognitive function. These benefits may also be associated with AKT, GSK-3β, and JNK signaling pathways that were modulated by PE [214].
Converging with these findings, other studies report that PE mediates neuroprotection, increases antioxidant enzymes [14], decreases the expression of Aβ-42, Cox-2 and Caspase-3, cytochrome-c, and Bax, the phosphorylation of JNK, p38MAPK, and Tau, and exacerbated phosphorylation of extracellular signal-regulated kinase (ERK), PI3K, AKT, GSK-3α/β, NGF, BDNF, phospho-CREB, superoxide dismutase 1 (SOD-1), superoxide dismutase 2 (SOD-2), 70 kilodalton heat shock protein (HSP-70), and B-cell lymphoma 2 (BCL-2). Glucose, insulin, and corticosterone levels were also reduced after PE [15]. In an AD model, PE restored levels of non-protein thiols, preventing the accumulation of reactive oxygen species (ROS), increased SOD activity, prevented restored normal activity of glutathione reductase, glutathione peroxidase, and glutathione transferase, and suppressed TNF-α and IL-1β [215].
In healthy individuals, PE stimulates a better functioning of the PI3K pathway [123,216] through both insulin-dependent and -independent pathways [217,218]. PE also activates the β-oxidative pathway to degrade fat in order to produce energy [219]. Carnitine palmitoyl transferase (CPT) is a key enzyme complex in fat oxidation. Gene expression and the activity of the CPT complex is enhanced in muscle fibers of individuals who exercise [220]. CPT gene expression is modulated by peroxisome proliferator-activated receptor alpha (PPAR-α) and a co-activator (PGC-1α) [220]. PGC-1α leads to the production of fibronectin type III domain-containing 5 (FNDC5), which is cleaved at the C terminal to produce irisin [221].
Irisin is a myokine produced in the skeletal muscle during PE which correlated with increased IGF-1 levels in humans [222] and other growth factors in mice [223]. Irisin plays a role in synaptic plasticity, neurogenesis, cognitive function, and memory, but has unidentified receptors in the brain [139,149]. Irisin is supposed to be positively correlated to BDNF and IRS-1 activation in tyrosine residues in humans with AD, which may underline PE-mediated improvement of insulin resistance, neuroinflammation, and cognitive function in AD [149].
4.2. Modulation of MiRNAs by PE
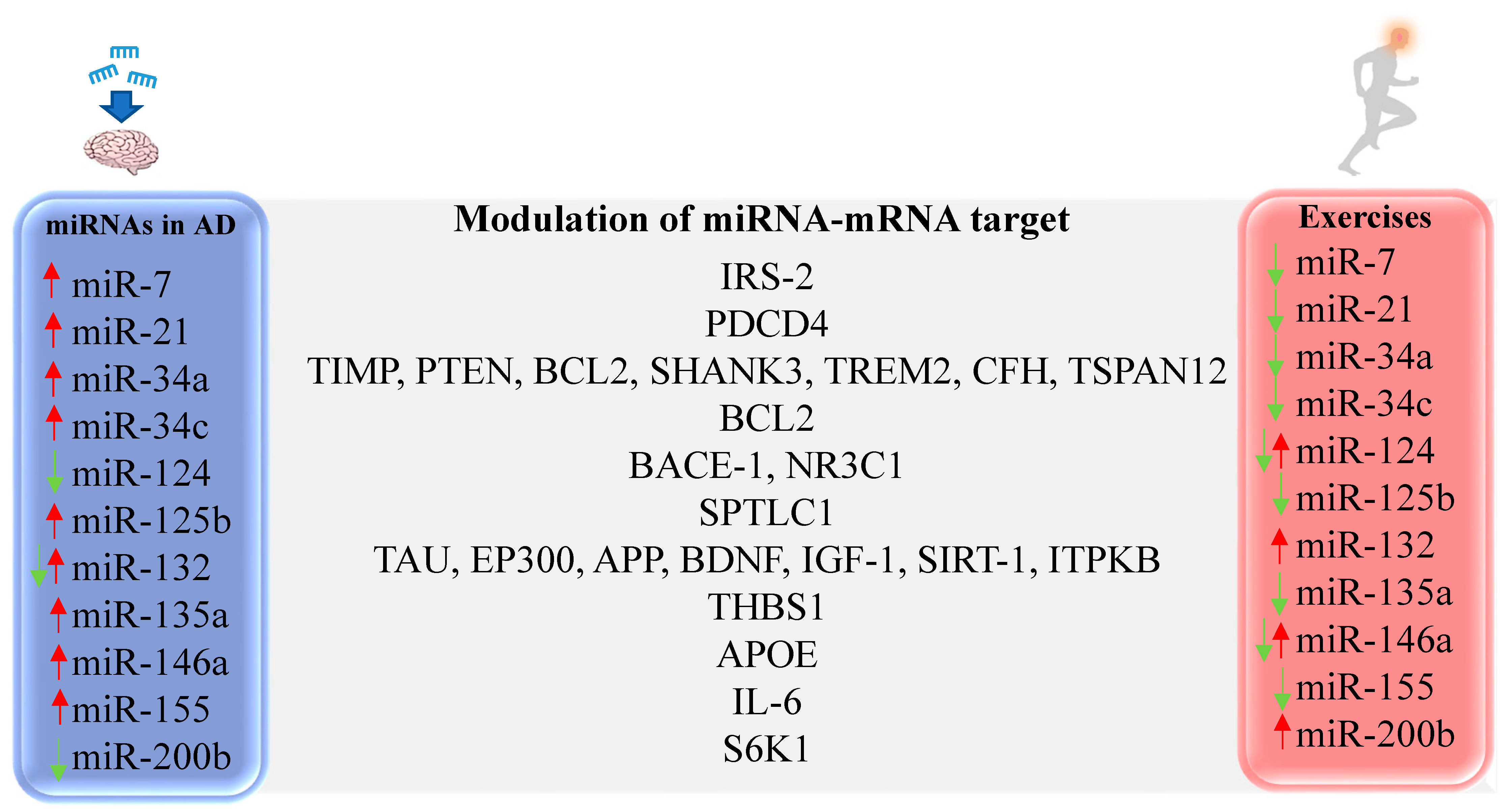
Several studies have evaluated the role of PE in modulating miRNA expression both in the periphery and in the CNS, but the vast majority was performed in healthy individuals (Figure 2). An experimental study evaluated the effects of voluntary aerobic PE in the Senescence Accelerated Mouse-Prone 8 (SAMP8) mouse model of AD. While increased miR-132 was found in the hippocampus of untreated SAMP8 mice, PE was able to downregulate miRNA expression and reduce the accumulation of APP, inhibiting hippocampal degeneration and improving cognitive function [224]. In other preclinical studies, the expression of miR-132 was found reduced, which was also shown in AD patients and in vitro AD model [134,161,225,226]. Other studies have shown miR-132 upregulation in the brain after eight weeks of swimming training [227] and in trained human subjects [228,229,230]. Specificities of the evaluated model and time points may justify the discrepancies.
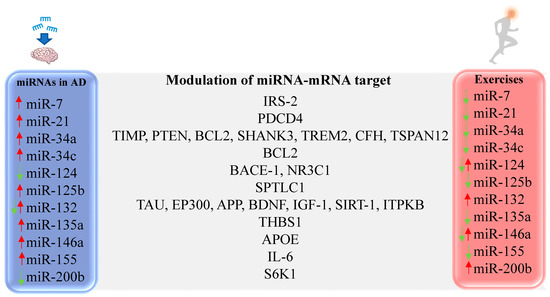
Figure 2.
Representative miRNAs with altered expression in AD and differentially expressed after PE. IRS-2 [231,232], PDCD4 [121], TIMP [233], PTEN [233], BCL2 [234], SHANK3 [235], TREM2 [235], CFH [235], TSPAN12 [235], BCL2 [236], BACE-1 [237], NR3C1 [238], SPTLC1 [239], TAU [134], EP300 [240], APP [224], BDNF [227], IGF-1 [227], SIRT-1 [161], ITPKB [180], THBS1 [241], APOE [200], IL-6 [242,243], S6K1 [142].
MiRNAs associated with fatty acid metabolism and biosynthesis signaling pathways, like miR-15b, miR-148b, miR-338, and miR-766, were also previously studied. MiR-15b is reduced in an in vitro AD model [244] and in brain samples of AD patients, leading to increased BACE-1 levels [245]. Chronic aerobic PE was able to increase hippocampal expression of miR-15b in SAMP8 mice [246], giving mechanistic support to the finding that PE reduces the expression of BACE-1 and decreases the accumulation of Aβ protein in the brain. Circulating miR-148b is increased in the blood of AD patients [187], but in healthy subjects, chronic aerobic PE reduced its expression [247]. MiR-338 circulating is downregulated in the plasma of AD patients [248], but PE increased expression of this miRNA in healthy subjects [228,247]. MiR-766 circulating is overexpressed in cerebral-spinal fluid of AD patients [249] but is reduced in plasma samples of healthy subjects after chronic PE [247].
MiR-124 was found downregulated in brain samples from AD patients [250] and in an in vitro AD model [237], in association with increased BACE-1 expression. Aerobic PE by chronic treadmill running [251] or chronic running wheel [252] increased miR-124 expression in the hippocampus and cerebral cortex, respectively, of healthy animals, which could contribute to reduce BACE-1 expression and Aβ accumulation.
Overexpression of miR-7 in AD plays an important role in Aβ metabolism by decreasing IRS-2 expression and suppressing insulin signaling pathway [140]. Meanwhile, after acute aerobic PE in a cycle ergometer, reduced expression of miR-7 was found in human serum samples [231]. Chronic swimming reduces IR in a type 2 diabetes mouse model by inducing miR-382 overexpression, which regulates resistin [253]. MiR-382 expression is reduced in brain samples of AD patients [250], bringing the hypothesis that IR may also be suppressed in AD by PE through regulation of miR-382.
Impaired insulin signaling and IR increases Aβ-mediated inflammatory process by modulating the IRS/PI3K/AKT/GSK3β signaling pathway [232], inducing neuroinflammation in AD [254,255]. In this context, increased activity of the transcription factor NFκB promotes elevation of miR-146a expression in brain tissues of AD patients [256], but acute resistance PE induced reduction of miR-146a expression [257] and the same result was obtained after three months of basketball training [258].
MiR-155 overexpression in brain samples of transgenic mouse model of AD was associated with activation of astrocytes and microglia, favoring increased expression of inflammatory mediators, including IL-6 [242]. In contrast, mice trained with endurance PE reduced expression of miR-155 and IL-6 expression was observed after six weeks of training [243].
MiR-137 is downregulated in both hippocampus and cerebral cortex of transgenic AD mice [259] and in serum samples of AD patients [188], contributing to Tau upregulation and phosphorylation. In contrast, hippocampal samples of mice that performed chronic aerobic PE with a running wheel showed elevation of miR-137 expression, improving memory in mice [260].
Overexpression of miR-125b is seen in AD and also favors Tau hyperphosphorylation [173]. However, it was observed that individuals who performed acute cycle ergometer exercise obtained reduction of miR-125b expression [228], a possible molecular mechanism to improve cognitive aspects and reduce neurotoxicity.
In the same perspective, miR-146a has been consistently found overexpressed in an in vitro AD model (SH-SY5Y cells treated with Aβ1-42), in a transgenic mouse model of AD [178], and in CSF, serum, and hippocampal samples of AD patients [261,262,263]. Individuals that underwent either acute resistance training or chronic basketball training reduced circulating levels of miR-146a [257,258]. This may be another signaling pathway for reducing Tau phosphorylation and that suppresses the formation of neurofibrillary tangles, minimizing neuronal death.
However, individuals who have done rowing training and after a marathon run, types of aerobic PE, had increased expression of miR-146a in serum and plasma samples, respectively [264,265], demonstrating that different types of training, the timing of the analysis, the protocol utilized, and type of sample can lead to divergent results regarding miRNAs’ expression patterns.
4.3. Overlaps Between MiRNA Signatures in AD and Physical Exercise
A number of studies have evaluated the impact of different modalities of PE in circulating and brain miRNA signatures (Supplementary Tables S4 and S5). Due to the lack of available results of PE-induced miRNA changes in AD subjects, we evaluated the commonalities between the lists of differentially expressed miRNAs reported in AD studies and in studies evaluating miRNA regulation by PE, mostly performed in healthy individuals. Overlaps were built, taking into account the lists of differentially expressed miRNAs, regardless of the pattern of expression (i.e., upregulated or downregulated) and Venn diagrams were constructed (Figure 3 and Figure 4).
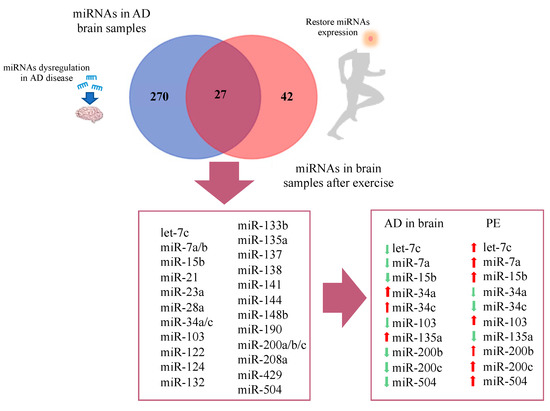
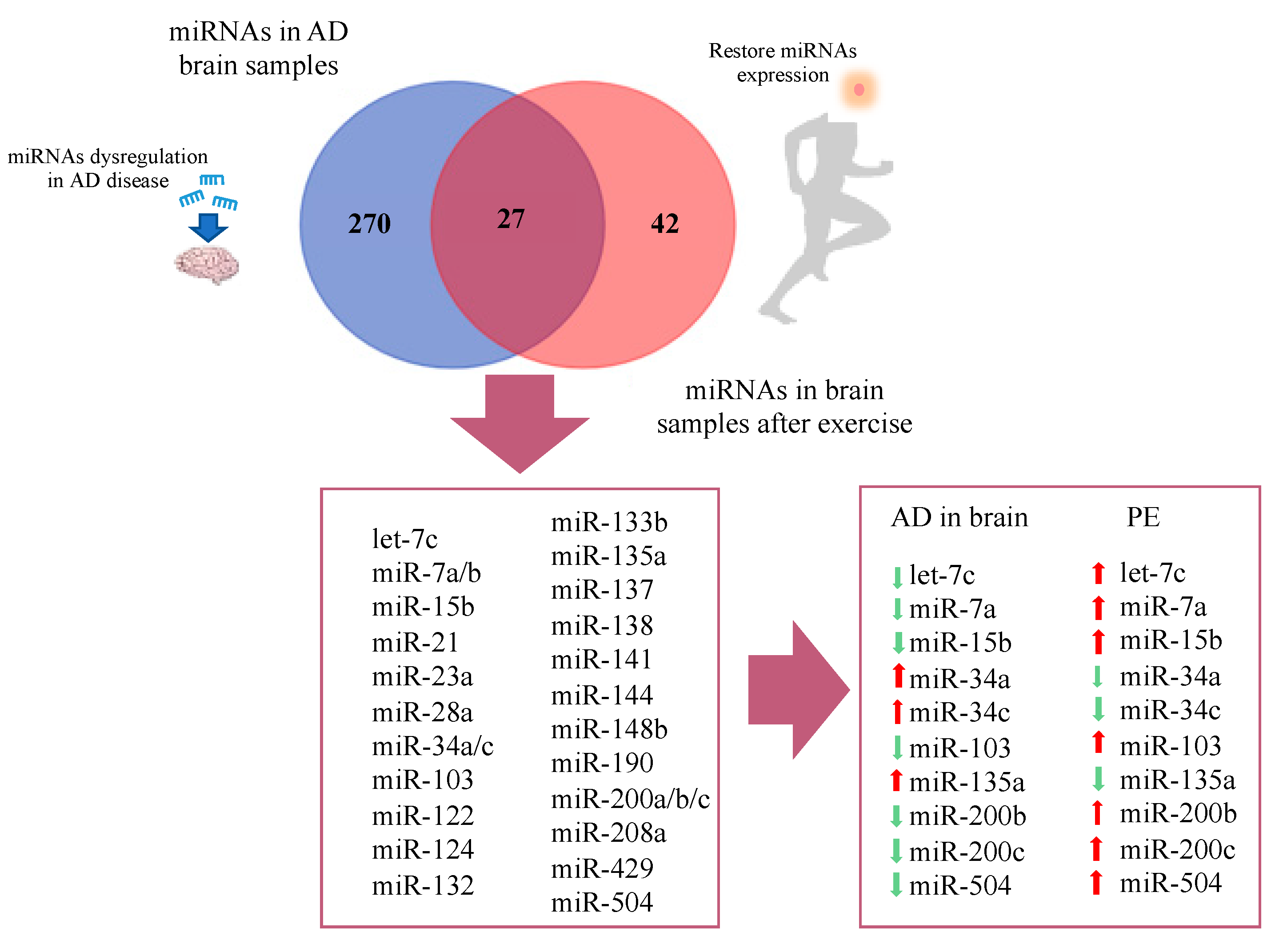
Figure 3.
Venn diagram representing overlap of miRNAs in AD brain and restored miRNAs after physical exercise.
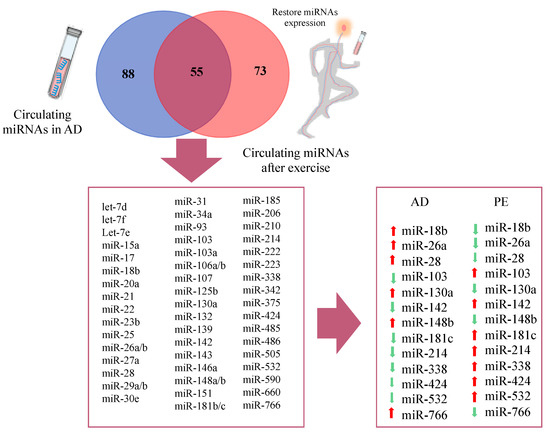
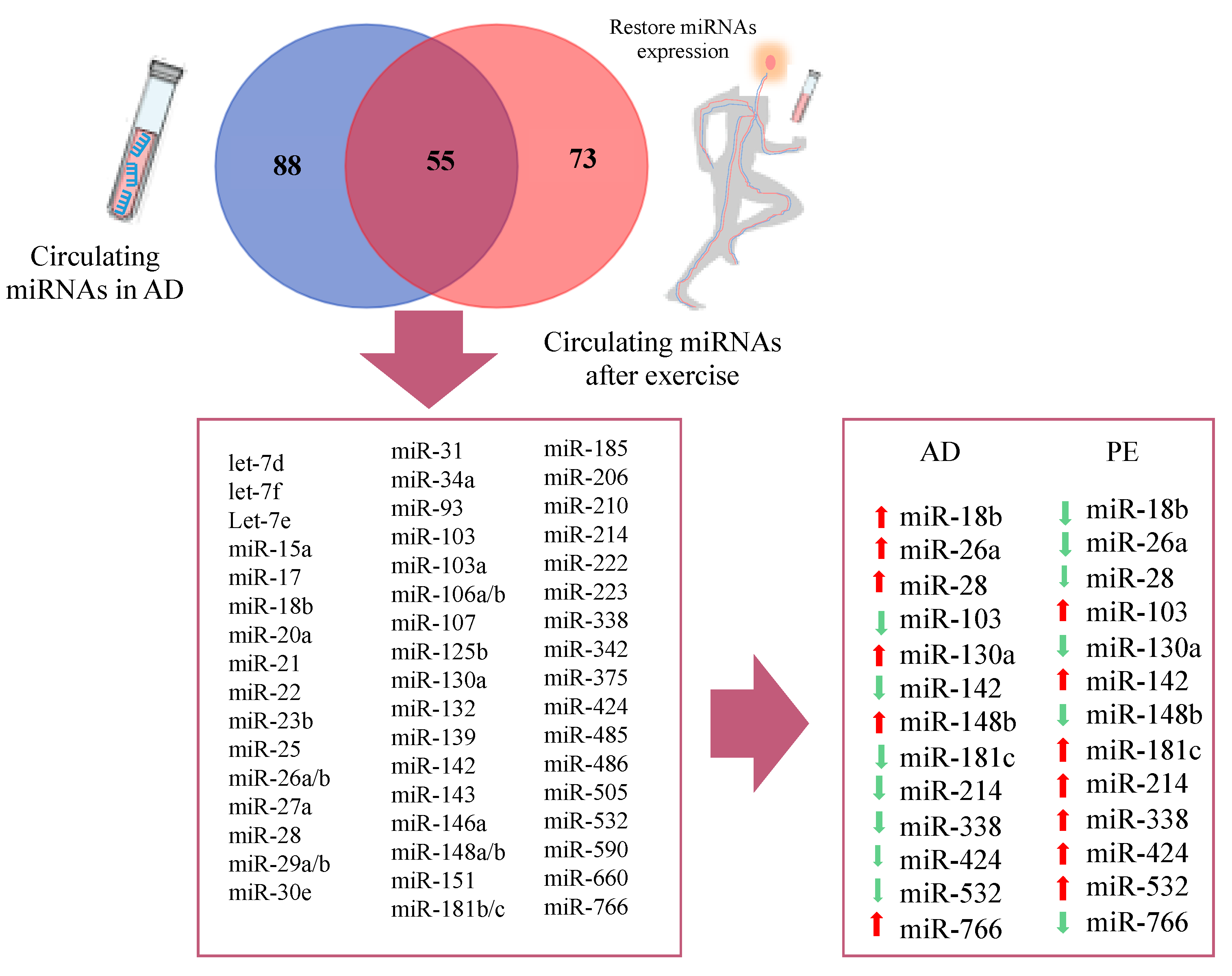
Figure 4.
Venn diagram of circulating miRNAs in Alzheimer’s disease and restored miRNAs after physical exercise.
Regarding studies evaluating brain tissue, a total of 270 differentially expressed miRNAs were reported exclusively in AD studies and 42 in PE studies, while 27 miRNAs were found overlapping AD and PE studies (Figure 3). The overlapping miRNAs were: let-7c, miR-7a, miR-7b, miR-15b, miR-21, miR-23a, miR-28a, miR-34a, miR-34c, miR-103, miR-122, miR-124, miR-132, miR-133b, miR-135a, miR-137, miR-138, miR-141, miR-144, miR-148b, miR-190, miR-200a, miR-200b, miR-200c, miR-208a, miR-429, and miR-504. Interestingly, from this list, 10 miRNAs showed opposite expression pattern comparing AD and PE studies, namely let-7c, miR-7a, miR-15b, miR-34a, miR-34c, miR-103, miR135a, miR-200b, miR-200c, and miR-504 (Figure 3).
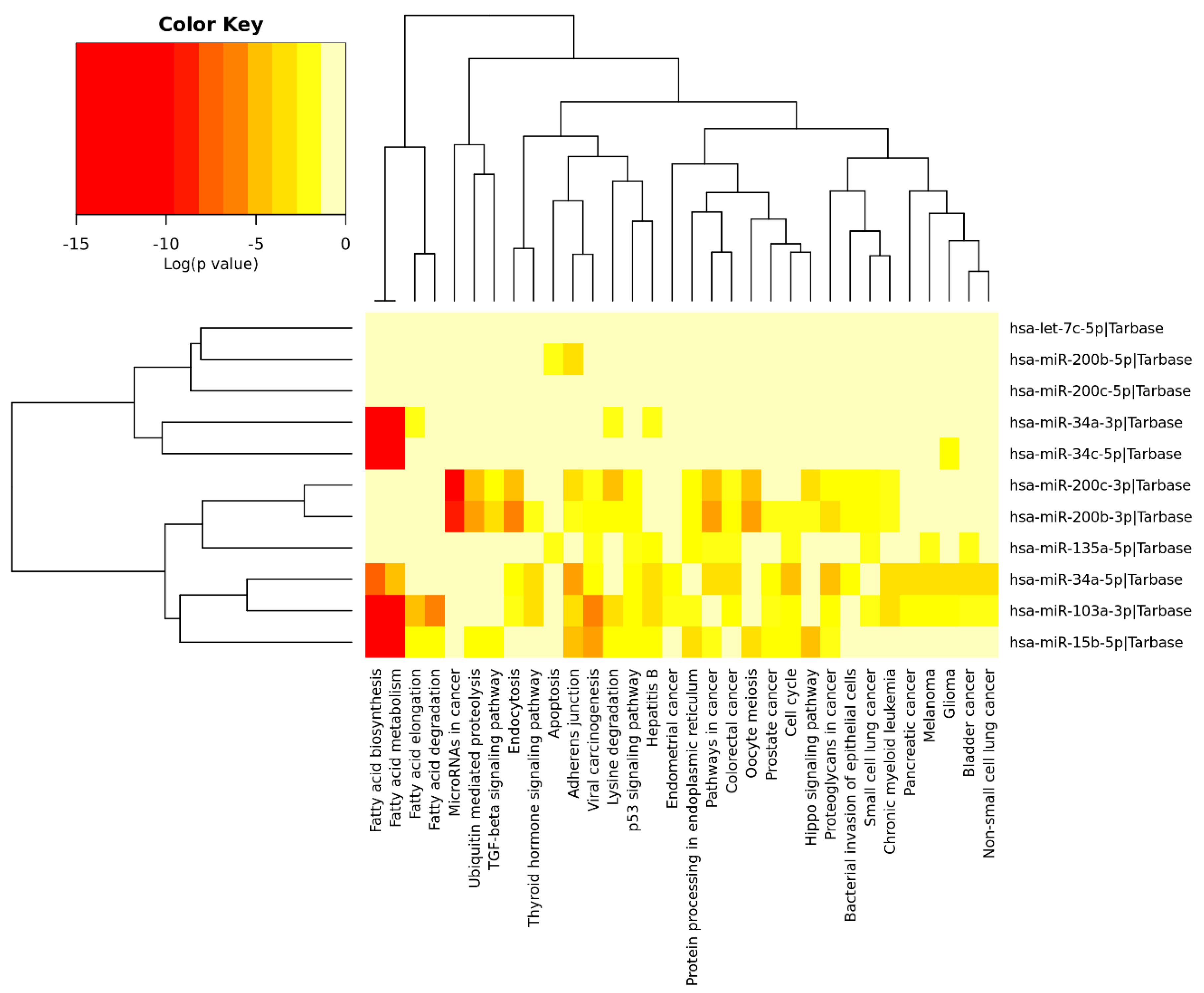
We performed an exploratory analysis of these 10 miRNAs by DIANA-miRPath v.3.0 integrated with TarBase v.7.0 and KEGG pathways. This database can determine the pathways that are regulated by multiple miRNAs using the hypergeometric test. Pathway enrichment analysis demonstrated association of these miRNAs with the following processes (excluding cancer and infectious disease-related processes): Fatty acid metabolism, ubiquitin-mediated proteolysis, protein processing in endoplasmic reticulum, endocytosis, cell cycle, adherens junctions, apoptosis, lysine degradation, p53 signaling pathway, hippo pathway, and transforming growth factor-β (TGF-β) pathway (Figure 5). Some of the identified pathways have been previously extensively studied in the context of AD. For instance, dysregulation of unsaturated fatty acid metabolism seems to be connected to AD severity [266], and PE induces changes in fatty acid metabolism, as well as modifying the expression of inflammatory adipokines and increased lipolysis in white adipose tissue [267], including elevating the expression of enzymes associated with the metabolism of polyunsaturated fatty acids [268].

Figure 5.
Heatmap clustering of miRNAs (brain samples) in Alzheimer’s disease vs. physical exercise and significant pathways’ interaction.
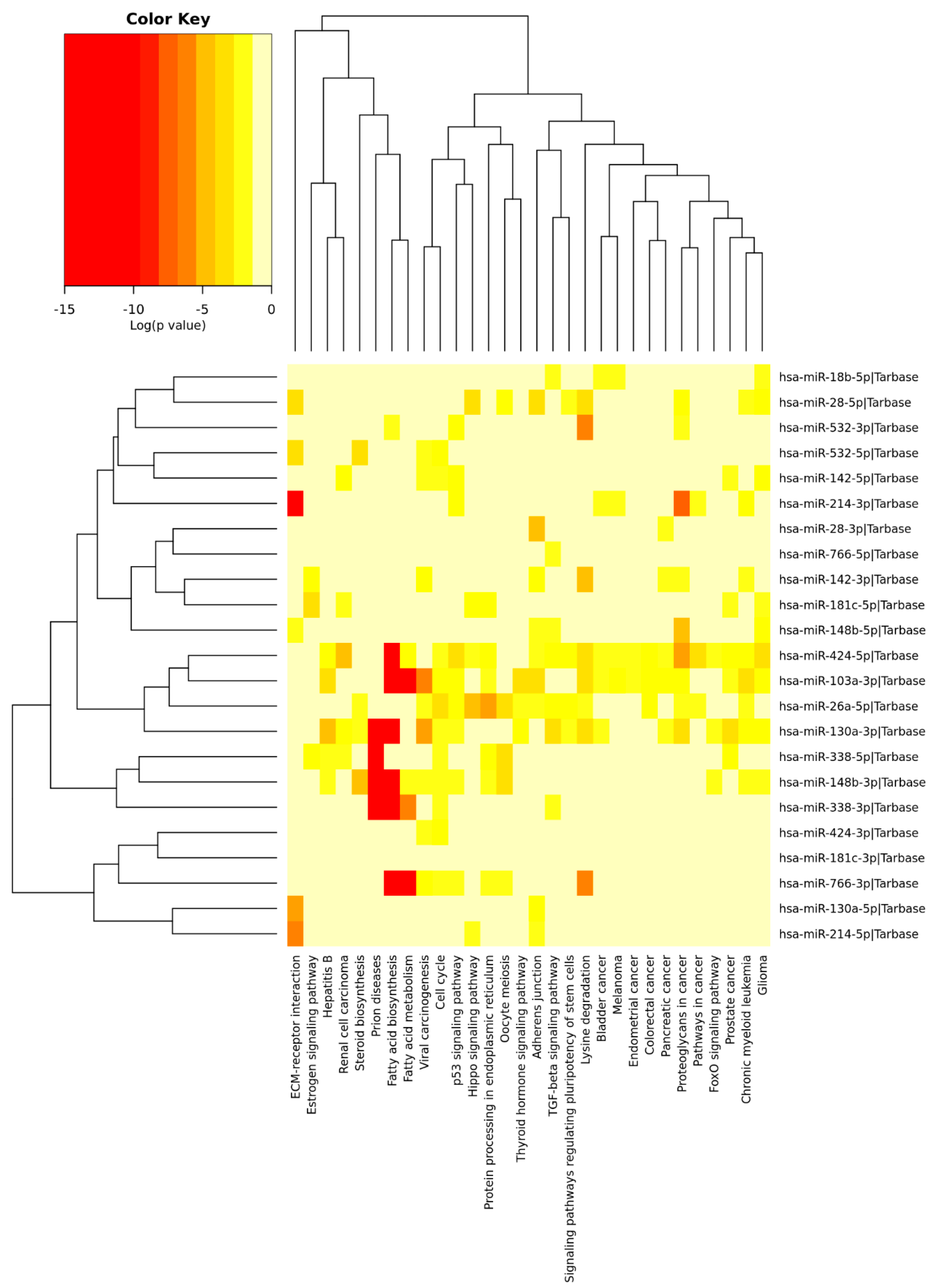
Similar analysis was performed for circulating miRNAs reported in AD studies and PE studies. A total of 88 differentially expressed circulating miRNAs were reported exclusively in AD studies and 73 in PE studies, while 55 miRNAs were found overlapping AD and PE studies (Figure 4), from which 13 showed opposite expression patterns: miR-18b, miR-26a, miR-28, miR-103, miR-130a, miR-142, miR-148b, miR-181c, miR-214, miR-338, miR-424, miR-532, and miR-766. Although a different list of miRNAs was found when compared to brain miRNAs, similar signaling pathways were found (Figure 6). Interestingly, miR-103 was found in both circulating and brain miRNA analyses. In a recent study, miR-103 was shown to target A disintegrin and metalloproteinase domain-containing protein 10 (ADAM10), which is the most important enzyme with α-secretase activity involved in the processing of APP and the formation of amyloid plaques [269], Supplementary Tables S1–S5 ([233,234,235,236,238,239,240,241,270,271,272,273,274,275,276,277,278,279,280,281,282,283,284,285,286,287,288,289,290,291,292,293,294,295,296,297,298,299,300,301,302,303,304,305,306,307,308,309,310,311,312,313,314,315,316,317,318,319,320,321,322,323,324,325,326,327,328,329,330,331,332,333,334,335,336,337,338,339,340,341,342,343,344,345,346,347,348,349,350,351,352,353,354,355,356,357,358,359,360,361,362,363,364,365,366,367,368,369,370,371,372,373,374,375,376,377,378,379,380,381,382]).
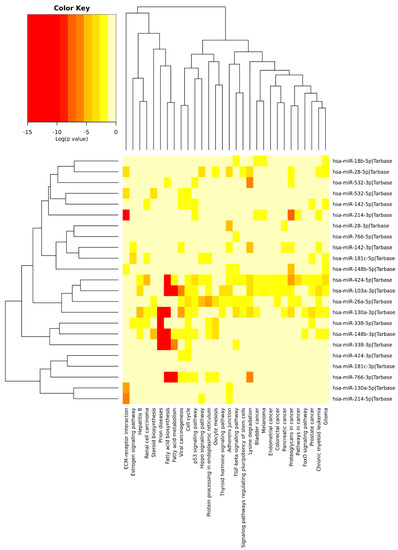
Figure 6.
Heatmap clustering of circulating miRNAs in Alzheimers’ disease (AD) vs. physical exercise (PE) and significant pathways’ interaction.
5. Conclusions
MiRNA deregulation participates in different aspects of AD pathogenesis. Although PE is a non-pharmacological measure recommended for AD patients, the mechanisms involved in the associated benefits are not fully understood. Current evidence is insufficient to prove but can provide significant biological plausibility to support a possible role for PE in counteracting some of the deregulated miRNAs found in AD, and to generate hypotheses regarding which integrated pathway clusters may be involved. PE may act on restoring a miRNA profile that supports normal NSC function, brain metabolism, and inflammatory status, among others. Further studies, however, are required to advance this knowledge. Finally, the study of miRNAs involved in the beneficial role of PE in AD may also contribute to identify biomarkers and to develop new drugs and therapies for the disease in the future.
Supplementary Materials
Supplementary materials can be found at https://www.mdpi.com/1422-0067/21/14/4977/s1.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–Brasil (CAPES)–Finance Code 001.
Acknowledgments
The authors acknowledge Postgraduate Program in Medicine and Health, Faculty of Medicine of Bahia, Federal University of Bahia, Salvador, Bahia, Brazil.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ferreira, S.T.; Vieira, M.N.N.; De Felice, F.G. Soluble protein oligomers as emerging toxins in Alzheimer’s and other amyloid diseases. IUBMB Life 2007, 59, 332–345. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.T.; Klein, W.L. The Aβ oligomer hypothesis for synapse failure and memory loss in Alzheimer’s disease. Neurobiol. Learn. Mem. 2011, 96, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Mandelkow, E.M.; Mandelkow, E. Tau in Alzheimer’s disease. Trends Cell Biol. 1998, 8, 425–427. [Google Scholar] [CrossRef]
- Ferreira, S.T.; Clarke, J.R.; Bomfim, T.R.; De Felice, F.G. Inflammation, defective insulin signaling, and neuronal dysfunction in Alzheimer’s disease. Alzheimer’s Dement. 2014, 10, S76–S83. [Google Scholar] [CrossRef]
- Lourenco, M.V.; Clarke, J.R.; Frozza, R.L.; Bomfim, T.R.; Forny-Germano, L.; Batista, A.F.; Sathler, L.B.; Brito-Moreira, J.; Amaral, O.B.; Silva, C.A.; et al. TNF-α mediates PKR-dependent memory impairment and brain IRS-1 inhibition induced by Alzheimer’s β-amyloid oligomers in mice and monkeys. Cell Metab. 2013, 18, 831–843. [Google Scholar] [CrossRef]
- Kramer, A.F.; Erickson, K.I.; Colcombe, S.J. Neural Changes Associated with Training Exercise, Cognition, and the Aging Brain. J. Appl. Physiol. 2006, 101, 1237–1242. [Google Scholar] [CrossRef]
- Ballard, C.; Gauthier, S.; Corbett, A.; Brayne, C.; Aarsland, D.; Jones, E. Alzheimer’s disease. Lancet 2011, 377, 1019–1031. [Google Scholar] [CrossRef]
- Forbes, D.; Forbes, S.C.; Blake, C.M.; Thiessen, E.J.; Forbes, S. Exercise programs for people with dementia. Cochrane Database Syst. Rev. 2015, 2015. [Google Scholar] [CrossRef]
- Banerjee, A.K.; Mandal, A.; Chanda, D.; Chakraborti, S. Oxidant, antioxidant and physical exercise. Mol. Cell. Biochem. 2003, 253, 307–312. [Google Scholar] [CrossRef]
- Stranahan, A.M.; Martin, B.; Maudsley, S. Anti-Inflammatory Effects of Physical Activity in Relationship to Improved Cognitive Status in Humans and Mouse Models of Alzheimers Disease. Curr. Alzheimer Res. 2012, 9, 86–92. [Google Scholar] [CrossRef]
- Parachikova, A.; Nichol, K.E.; Cotman, C.W. Short-term exercise in aged Tg2576 mice alters neuroinflammation and improves cognition. Neurobiol. Dis. 2008, 30, 121–129. [Google Scholar] [CrossRef] [PubMed]
- García-Mesa, Y.; López-Ramos, J.C.; Giménez-Llort, L.; Revilla, S.; Guerra, R.; Gruart, A.; Laferla, F.M.; Cristòfol, R.; Delgado-García, J.M.; Sanfeliu, C. Physical exercise protects against Alzheimer’s disease in 3xTg-AD mice. J. Alzheimer’s Dis. 2011, 24, 421–454. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011, 11, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Um, H.S.; Kang, E.B.; Koo, J.H.; Kim, H.T.; Lee, J.; Kim, E.J.; Yang, C.H.; An, G.Y.; Cho, I.H.; Cho, J.Y. Treadmill exercise represses neuronal cell death in an aged transgenic mouse model of Alzheimer’s disease. Neurosci. Res. 2011, 69, 161–173. [Google Scholar] [CrossRef]
- Yuede, C.M.; Zimmerman, S.D.; Dong, H.; Kling, M.J.; Bero, A.W.; Holtzman, D.M.; Timson, B.F.; Csernansky, J.G. Effects of voluntary and forced exercise on plaque deposition, hippocampal volume, and behavior in the Tg2576 mouse model of Alzheimer’s disease. Neurobiol. Dis. 2009, 35, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Caria, A.C.I.; Nonaka, C.K.V.; Pereira, C.S.; Soares, M.B.P.; Macambira, S.G.; de Freitas Souza, B.S. Exercise training-induced changes in microRNAs: Beneficial regulatory effects in hypertension, type 2 diabetes, and obesity. Int. J. Mol. Sci. 2018, 19, 3608. [Google Scholar] [CrossRef] [PubMed]
- Muljo, S.A.; Kanellopoulou, C.; Aravind, L. MicroRNA targeting in mammalian genomes: Genes and mechanisms. Wiley Interdiscip. Rev. Syst. Biol. Med. 2010, 2, 148–161. [Google Scholar] [CrossRef]
- Shaik, M.M.; Tamargo, I.A.; Abubakar, M.B.; Kamal, M.A.; Greig, N.H.; Gan, S.H. The role of microRNAs in Alzheimer’s disease and their therapeutic potentials. Genes 2018, 9, 174. [Google Scholar] [CrossRef]
- Baek, D.; Villén, J.; Shin, C.; Camargo, F.D.; Gygi, S.P.; Bartel, D.P. The impact of microRNAs on protein output. Nature 2008, 455, 64–71. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef]
- Huntzinger, E.; Izaurralde, E. Gene silencing by microRNAs: Contributions of translational repression and mRNA decay. Nat. Rev. Genet. 2011, 12, 99–110. [Google Scholar] [CrossRef]
- Krol, J.; Loedige, I.; Filipowicz, W. The widespread regulation of microRNA biogenesis, function and decay. Nat. Rev. Genet. 2010, 11, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Tony, H.; Meng, K.; Wu, B.; Yu, A.; Zeng, Q.; Yu, K.; Zhong, Y. MicroRNA-208a Dysregulates Apoptosis Genes Expression and Promotes Cardiomyocyte Apoptosis during Ischemia and Its Silencing Improves Cardiac Function after Myocardial Infarction. Mediat. Inflamm. 2015, 2015, 479123. [Google Scholar] [CrossRef] [PubMed]
- Dumortier, O.; Hinault, C.; Van Obberghen, E. MicroRNAs and metabolism crosstalk in energy homeostasis. Cell Metab. 2013, 18, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Ivey, K.N.; Srivastava, D. MicroRNAs as Regulators of Differentiation and Cell Fate Decisions. Cell Stem Cell 2010, 7, 36–41. [Google Scholar] [CrossRef]
- Mens, M.M.J.; Ghanbari, M. Cell Cycle Regulation of Stem Cells by MicroRNAs. Stem Cell Rev. Rep. 2018, 14, 309–322. [Google Scholar] [CrossRef]
- Yuan, M.; Cristina, A.; Da Silva, A.L.; Arnold, A.; Okeke, L.; Ames, H.; Correa-Cerro, L.S.; Adelita Vizcaino, M.; Ho, C.-Y.; Eberhart, C.G.; et al. MicroRNA (miR) 125b regulates cell growth and invasion in pediatric low grade glioma. Sci. Rep. 2018, 8, 12506. [Google Scholar] [CrossRef]
- Leivonen, S.-K.; Icay, K.; Jäntti, K.; Siren, I.; Liu, C.; Alkodsi, A.; Cervera, A.; Ludvigsen, M.; Jacques Hamilton-Dutoit, S.; D’amore, F.; et al. MicroRNAs regulate key cell survival pathways and mediate chemosensitivity during progression of diffuse large B-cell lymphoma. Blood Cancer J. 2017, 7, 654. [Google Scholar] [CrossRef]
- Ling, C.; Wang, X.; Zhu, J.; Tang, H.; Du, W.; Zeng, Y.; Sun, L.; Huang, J.A.; Liu, Z. MicroRNA-4286 promotes cell proliferation, migration, and invasion via PTEN regulation of the PI3K/Akt pathway in non-small cell lung cancer. Cancer Med. 2019, 8, 3520–3531. [Google Scholar] [CrossRef]
- Verjans, R.; Derks, W.J.A.; Korn, K.; Sönnichsen, B.; van Leeuwen, R.E.W.; Schroen, B.; van Bilsen, M.; Heymans, S. Functional Screening Identifies MicroRNAs as Multi-Cellular Regulators of Heart Failure. Sci. Rep. 2019, 9, 6055. [Google Scholar] [CrossRef]
- Bátkai, S.; Thum, T. MicroRNAs in hypertension: Mechanisms and therapeutic targets. Curr. Hypertens. Rep. 2012, 14, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Dehwah, M.A.; Xu, A.; Huang, Q. MicroRNAs and type 2 diabetes/obesity. J. Genet. Genom. 2012, 39, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, C.K.V.; Macêdo, C.T.; Cavalcante, B.R.R.; De Alcântara, A.C.; Silva, D.N.; Bezerra, M.D.R.; Caria, A.C.I.; Tavora, F.R.F.; Neto, J.D.D.S.; Noya-Rabelo, M.M.; et al. Circulating miRNAs as potential biomarkers associated with cardiac remodeling and fibrosis in chagas disease cardiomyopathy. Int. J. Mol. Sci. 2019, 20, 4064. [Google Scholar] [CrossRef] [PubMed]
- Delay, C.; Mandemakers, W.; Hébert, S.S. MicroRNAs in Alzheimer’s disease. Neurobiol. Dis. 2012, 46, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Kim, V.N.; Han, J.; Siomi, M.C. Biogenesis of small RNAs in animals. Nat. Rev. Mol. Cell Biol. 2009, 10, 126–139. [Google Scholar] [CrossRef]
- Ha, M.; Kim, V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol 2014, 15, 509–524. [Google Scholar] [CrossRef]
- Chiang, H.R.; Schoenfeld, L.W.; Ruby, J.G.; Auyeung, V.C.; Spies, N.; Baek, D.; Johnston, W.K.; Russ, C.; Luo, S.; Babiarz, J.E.; et al. Mammalian microRNAs: Experimental evaluation of novel and previously annotated genes. Genes Dev. 2010, 24, 992–1009. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, M.; Han, J.; Yeom, K.H.; Lee, S.; Baek, S.H.; Kim, V.N. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004, 23, 4051–4060. [Google Scholar] [CrossRef]
- Ma, H.; Wu, Y.; Choi, J.G.; Wu, H. Lower and upper stem-single-stranded RNA junctions together determine the Drosha cleavage site. Proc. Natl. Acad. Sci. USA 2013, 110, 20687–20692. [Google Scholar] [CrossRef]
- Davis, B.N.; Hilyard, A.C.; Nguyen, P.H.; Lagna, G.; Hata, A. Smad proteins bind a conserved RNA sequence to promote microRNA maturation by Drosha. Mol. Cell 2010, 39, 373–384. [Google Scholar] [CrossRef]
- Davis, B.N.; Hilyard, A.C.; Lagna, G.; Hata, A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature 2008, 454, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Medvid, R.; Melton, C.; Jaenisch, R.; Blelloch, R. DGCR8 is essential for microRNA biogenesis and silencing of embryonic stem cell self-renewal. Nat. Genet. 2007, 39, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Denli, A.M.; Tops, B.B.; Plasterk, R.H.; Ketting, R.F.; Hannon, G.J. Processing of primary microRNAs by the Microprocessor complex. Nature 2004, 432, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Gregory, R.I.; Yan, K.P.; Amuthan, G.; Chendrimada, T.; Doratotaj, B.; Cooch, N.; Shiekhattar, R. The Microprocessor complex mediates the genesis of microRNAs. Nature 2004, 432, 235–240. [Google Scholar] [CrossRef]
- Okada, C.; Yamashita, E.; Lee, S.J.; Shibata, S.; Katahira, J.; Nakagawa, A.; Yoneda, Y.; Tsukihara, T. A high-resolution structure of the pre-microRNA nuclear export machinery. Science 2009, 326, 1275–1279. [Google Scholar] [CrossRef]
- Zeng, Y.; Cullen, B.R. Structural requirements for pre-microRNA binding and nuclear export by Exportin 5. Nucleic Acids Res. 2004, 32, 4776–4785. [Google Scholar] [CrossRef]
- Yi, R.; Qin, Y.; Macara, I.G.; Cullen, B.R. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev. 2003, 17, 3011–3016. [Google Scholar] [CrossRef]
- Ketting, R.F.; Fischer, S.E.; Bernstein, E.; Sijen, T.; Hannon, G.J.; Plasterk, R.H. Dicer functions in RNA interference and in synthesis of small RNA involved in developmental timing in C. elegans. Genes Dev. 2001, 15, 2654–2659. [Google Scholar] [CrossRef]
- Hutvágner, G.; McLachlan, J.; Pasquinelli, A.E.; Bálint, E.; Tuschl, T.; Zamore, P.D. A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science 2001, 293, 834–838. [Google Scholar] [CrossRef]
- Horman, S.R.; Janas, M.M.; Litterst, C.; Wang, B.; MacRae, I.J.; Sever, M.J.; Morrissey, D.V.; Graves, P.; Luo, B.; Umesalma, S.; et al. Akt-mediated phosphorylation of argonaute 2 downregulates cleavage and upregulates translational repression of MicroRNA targets. Mol. Cell 2013, 50, 356–367. [Google Scholar] [CrossRef]
- Taylor, D.W.; Ma, E.; Shigematsu, H.; Cianfrocco, M.A.; Noland, C.L.; Nagayama, K.; Nogales, E.; Doudna, J.A.; Wang, H.W. Substrate-specific structural rearrangements of human Dicer. Nat. Struct. Mol. Biol. 2013, 20, 662–670. [Google Scholar] [CrossRef]
- Chakravarthy, S.; Sternberg, S.H.; Kellenberger, C.A.; Doudna, J.A. Substrate-specific kinetics of Dicer-catalyzed RNA processing. J. Mol. Biol. 2010, 404, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Song, J.J.; Smith, S.K.; Hannon, G.J.; Joshua-Tor, L. Crystal structure of Argonaute and its implications for RISC slicer activity. Science 2004, 305, 1434–1437. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.-P.; Xie, Y.; Meng, X.-Y.; Kang, J.-S. History and progress of hypotheses and clinical trials for Alzheimer’s disease. Signal Transduct. Target. Ther. 2019, 4, 29. [Google Scholar] [CrossRef]
- Bielefeld, P.; Mooney, C.; Henshall, D.C.; Fitzsimons, C.P. miRNA-Mediated Regulation of Adult Hippocampal Neurogenesis; Implications for Epilepsy. Brain Plast. 2016, 3, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Tan, E.K.; Zeng, L. Micrornas and neurodegenerative diseases. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2015; Volume 888, pp. 51–70. [Google Scholar]
- Hu, Z.; Li, Z. miRNAs in Synapse Development and Synaptic Plasticity. Curr. Opin. Neurobiol. 2017, 45, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhao, X.; Hsieh, J.; Wichterle, H.; Impey, S.; Banerjee, S.; Neveu, P.; Kosik, K.S. MicroRNA regulation of neural stem cells and neurogenesis. J. Neurosci. 2010, 30, 14931–14936. [Google Scholar] [CrossRef]
- Murai, K.; Qu, Q.; Sun, G.Q.; Ye, P.; Li, W.; Asuelime, G.; Sun, E.; Tsai, G.E.; Shi, Y. Nuclear receptor TLX stimulates hippocampal neurogenesis and enhances learning and memory in a transgenic mouse model. Proc. Natl. Acad. Sci. USA 2014, 111, 9115–9120. [Google Scholar] [CrossRef]
- Pan, W.L.; Chopp, M.; Fan, B.; Zhang, R.; Wang, X.; Hu, J.; Zhang, X.M.; Zhang, Z.G.; Liu, X.S. Ablation of the microRNA-17-92 cluster in neural stem cells diminishes adult hippocampal neurogenesis and cognitive function. FASEB J. 2019, 33, 5257–5267. [Google Scholar] [CrossRef]
- Hébert, S.S.; Horré, K.; Nicolaï, L.; Papadopoulou, A.S.; Mandemakers, W.; Silahtaroglu, A.N.; Kauppinen, S.; Delacourte, A.; De Strooper, B. Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer’s disease correlates with increased BACE1/β-secretase expression. Proc. Natl. Acad. Sci. USA 2008, 105, 6415–6420. [Google Scholar] [CrossRef]
- Fineberg, S.K.; Datta, P.; Stein, C.S.; Davidson, B.L. MiR-34a represses Numbl in murine neural progenitor cells and antagonizes neuronal differentiation. PLoS ONE 2012, 7, e38562. [Google Scholar] [CrossRef]
- Magill, S.T.; Cambronne, X.A.; Luikart, B.W.; Lioy, D.T.; Leighton, B.H.; Westbrook, G.L.; Mandel, G.; Goodman, R.H. MicroRNA-132 regulates dendritic growth and arborization of newborn neurons in the adult hippocampus. Proc. Natl. Acad. Sci. USA 2010, 107, 20382–20387. [Google Scholar] [CrossRef] [PubMed]
- Bredy, T.W.; Lin, Q.; Wei, W.; Baker-Andresen, D.; Mattick, J.S. MicroRNA regulation of neural plasticity and memory. Neurobiol. Learn. Mem. 2011, 96, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Ye, P.; Murai, K.; Lang, M.F.; Li, S.; Zhang, H.; Li, W.; Fu, C.; Yin, J.; Wang, A.; et al. MiR-137 forms a regulatory loop with nuclear receptor TLX and LSD1 in neural stem cells. Nat. Commun. 2011, 2, 529. [Google Scholar] [CrossRef] [PubMed]
- Lukiw, W.J.; Zhao, Y.; Jian, G.C. An NF-κB-sensitive micro RNA-146a-mediated inflammatory circuit in alzheimer disease and in stressed human brain cells. J. Biol. Chem. 2008, 283, 31315–31322. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Zhao, J.; Chang, S.; Sun, Q.; Liu, N.; Dong, J.; Chen, Y.; Yang, D.; Ye, D.; Liu, X.; et al. MicroRNA-153 improves the neurogenesis of neural stem cells and enhances the cognitive ability of aged mice through the notch signaling pathway. Cell Death Differ. 2020, 27, 808–825. [Google Scholar] [CrossRef]
- Hadar, A.; Milanesi, E.; Walczak, M.; Puzianowska-Kuźnicka, M.; Kuźnicki, J.; Squassina, A.; Niola, P.; Chillotti, C.; Attems, J.; Gozes, I.; et al. SIRT1, miR-132 and miR-212 link human longevity to Alzheimer’s Disease. Sci. Rep. 2018, 8, 8465. [Google Scholar] [CrossRef]
- Harraz, M.M.; Xu, J.-C.; Guiberson, N.; Dawson, T.M.; Dawson, V.L. MiR-223 regulates the differentiation of immature neurons. Mol. Cell. Ther. 2014, 2, 18. [Google Scholar] [CrossRef]
- Clovis, Y.M.; Enard, W.; Marinaro, F.; Huttner, W.B.; de Pietri Tonelli, D. Convergent repression of Foxp2 3′UTR by miR-9 and miR-132 in embryonic mouse neocortex: Implications for radial migration of neurons. Development 2012, 139, 3332–3342. [Google Scholar] [CrossRef]
- Franzoni, E.; Booker, S.A.; Parthasarathy, S.; Rehfeld, F.; Grosser, S.; Srivatsa, S.; Fuchs, H.; Tarabykin, V.; Vida, I.; Wulczyn, F.G. miR-128 regulates neuronal migration, outgrowth and intrinsic excitability via the intellectual disability gene Phf6. Elife 2015, 4, e04263. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, X.; Chen, P.; Ruan, X.; Liu, W.; Li, Y.; Sun, C.; Hou, L.; Yin, B.; Qiang, B.; et al. MicroRNA-129 modulates neuronal migration by targeting Fmr1 in the developing mouse cortex. Cell Death Dis. 2019, 10, 287. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Jiang, J.; Zheng, G.; Chen, J.; Lu, H.; Guo, H.; Wu, C. miR-139-5p modulates cortical neuronal migration by targeting Lis1 in a rat model of focal cortical dysplasia. Int. J. Mol. Med. 2014, 33, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Vo, N.K.; Cambronne, X.A.; Goodman, R.H. MicroRNA pathways in neural development and plasticity. Curr. Opin. Neurobiol. 2010, 20, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Giusti, S.A.; Vogl, A.M.; Brockmann, M.M.; Vercelli, C.A.; Rein, M.L.; Trümbach, D.; Wurst, W.; Cazalla, D.; Stein, V.; Deussing, J.M.; et al. MicroRNA-9 controls dendritic development by targeting REST. Elife 2014, 3, e02755. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Fernandes, C.C.; Ewell, L.A.; John, D.; Romoli, B.; Curia, G.; Taylor, S.R.; Frady, E.P.; Jensen, A.B.; Liu, J.C.; et al. MicroRNA-101 Regulates Multiple Developmental Programs to Constrain Excitation in Adult Neural Networks. Neuron 2016, 92, 1337–1351. [Google Scholar] [CrossRef] [PubMed]
- Pathania, M.; Torres-Reveron, J.; Yan, L.; Kimura, T.; Lin, T.V.; Gordon, V.; Teng, Z.-Q.; Zhao, X.; Fulga, T.A.; Van Vactor, D.; et al. miR-132 Enhances Dendritic Morphogenesis, Spine Density, Synaptic Integration, and Survival of Newborn Olfactory Bulb Neurons. PLoS ONE 2012, 7, e38174. [Google Scholar] [CrossRef]
- Irie, K.; Tsujimura, K.; Nakashima, H.; Nakashima, K. MicroRNA-214 promotes dendritic development by targeting the schizophrenia-associated gene quaking (Qki). J. Biol. Chem. 2016, 291, 13891–13904. [Google Scholar] [CrossRef]
- Madathil, S.K.; Nelson, P.T.; Saatman, K.E.; Wilfred, B.R. MicroRNAs in CNS injury: Potential roles and therapeutic implications. BioEssays 2011, 33, 21–26. [Google Scholar] [CrossRef]
- Fang, X.; Sun, D.; Wang, Z.; Yu, Z.; Liu, W.; Pu, Y.; Wang, D.; Huang, A.; Liu, M.; Xiang, Z.; et al. MiR-30a Positively Regulates the Inflammatory Response of Microglia in Experimental Autoimmune Encephalomyelitis. Neurosci. Bull. 2017, 33, 603–615. [Google Scholar] [CrossRef]
- Su, W.; Aloi, M.S.; Garden, G.A. MicroRNAs mediating CNS inflammation: Small regulators with powerful potential. Brain Behav. Immun. 2016, 52, 1–8. [Google Scholar] [CrossRef]
- Parisi, C.; Napoli, G.; Pelegrin, P.; Volonté, C. M1 and M2 Functional Imprinting of Primary Microglia: Role of P2X7 Activation and miR-125b. Mediat. Inflamm. 2016, 2016, 2989548. [Google Scholar] [CrossRef] [PubMed]
- Alexandrov, P.N.; Dua, P.; Lukiw, W.J. Up-Regulation of miRNA-146a in Progressive, Age-Related Inflammatory Neurodegenerative Disorders of the Human CNS. Front. Neurol. 2014, 5, 181. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Yu, C.; Wang, Y.; Liu, L.; Zhang, K.; Fang, C.; Liu, F.; Bian, G.; Song, B.; Yang, A.; et al. miR-9 and miR-124 synergistically affect regulation of dendritic branching via the AKT/GSK3β pathway by targeting Rap2a. Sci. Rep. 2016, 6, 26781. [Google Scholar] [CrossRef] [PubMed]
- Kos, A.; Olde Loohuis, N.; Meinhardt, J.; van Bokhoven, H.; Kaplan, B.B.; Martens, G.J.; Aschrafi, A. MicroRNA-181 promotes synaptogenesis and attenuates axonal outgrowth in cortical neurons. Cell. Mol. Life Sci. 2016, 73, 3555–3567. [Google Scholar] [CrossRef]
- Griggs, E.M.; Young, E.J.; Rumbaugh, G.; Miller, C.A. MicroRNA-182 regulates amygdala-dependent memory formation. J. Neurosci. 2013, 33, 1734–1740. [Google Scholar] [CrossRef]
- Pusic, A.D.; Kraig, R.P. Youth and environmental enrichment generate serum exosomes containing miR-219 that promote CNS myelination. Glia 2014, 62, 284–299. [Google Scholar] [CrossRef]
- Beal, E. MicroRNA: MicroRNAs have receptor subunits in a bind. Nat. Rev. Neurosci. 2009, 10, 469. [Google Scholar] [CrossRef]
- Nishino, J.; Kim, I.; Chada, K.; Morrison, S.J. Hmga2 promotes neural stem cell self-renewal in young but not old mice by reducing p16Ink4a and p19Arf Expression. Cell 2008, 135, 227–239. [Google Scholar] [CrossRef]
- Morgado, A.L.; Rodrigues, C.M.P.; Solá, S. MicroRNA-145 Regulates Neural Stem Cell Differentiation through the Sox2-Lin28/let-7 Signaling Pathway. Stem Cells 2016, 34, 1386–1395. [Google Scholar] [CrossRef]
- Dajas-Bailador, F.; Bonev, B.; Garcez, P.; Stanley, P.; Guillemot, F.; Papalopulu, N. MicroRNA-9 regulates axon extension and branching by targeting Map1b in mouse cortical neurons. Nat. Neurosci. 2012, 15, 697–699. [Google Scholar] [CrossRef]
- Hancock, M.L.; Preitner, N.; Quan, J.; Flanagan, J.G. MicroRNA-132 is enriched in developing axons, locally regulates Rasa1 mRNA, and promotes axon extension. J. Neurosci. 2014, 34, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Van Battum, E.Y.; Verhagen, M.G.; Vangoor Fujita, V.R.Y.; Derijck, A.A.H.A.; O’Duibhir, E.; Giuliani, G.; De Gunst, T.; Adolfs, Y.; Lelieveld, D.; Egan, D.; et al. An image-based miRNA screen identifies miRNA-135s as regulators of CNS axon growth and regeneration by targeting krüppel-like factor 4. J. Neurosci. 2018, 38, 613–630. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Pan, L.; Wei, M.; Wang, Q.; Liu, W.W.; Wang, N.; Jiang, X.Y.; Zhang, X.; Bao, L. FMRP-Mediated Axonal Delivery of miR-181d Regulates Axon Elongation by Locally Targeting Map1b and Calm1. Cell Rep. 2015, 13, 2794–2807. [Google Scholar] [CrossRef] [PubMed]
- Rowan, A. Development: MicroRNAs and brain morphogenesis. Nat. Rev. Neurosci. 2005, 6, 499. [Google Scholar] [CrossRef]
- Moreno-Jiménez, E.P.; Flor-García, M.; Terreros-Roncal, J.; Rábano, A.; Cafini, F.; Pallas-Bazarra, N.; Ávila, J.; Llorens-Martín, M. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat. Med. 2019, 25, 554–560. [Google Scholar] [CrossRef]
- Liu, C.; Teng, Z.-Q.; Santistevan, N.J.; Szulwach, K.E.; Guo, W.; Peng, J.; Zhao, X. Epigenetic regulation of miR-184 by MBD1 governs neural stem cell proliferation and differentiation. Cell Stem Cell 2010, 6, 433–444. [Google Scholar] [CrossRef]
- Brett, J.O.; Renault, V.M.; Rafalski, V.A.; Webb, A.E.; Brunet, A. The microRNA cluster miR-106b~25 regulates adult neural stem/progenitor cell proliferation and neuronal differentiation. Aging (Albany NY) 2011, 3, 108–124. [Google Scholar] [CrossRef]
- Schwamborn, J.C.; Berezikov, E.; Knoblich, J.A. The TRIM-NHL Protein TRIM32 Activates MicroRNAs and Prevents Self-Renewal in Mouse Neural Progenitors. Cell 2009, 136, 913–925. [Google Scholar] [CrossRef]
- Nicklas, S.; Okawa, S.; Hillje, A.L.; González-Cano, L.; Del Sol, A.; Schwamborn, J.C. The RNA helicase DDX6 regulates cell-fate specification in neural stem cells via miRNAs. Nucleic Acids Res. 2015, 43, 2638–2654. [Google Scholar] [CrossRef]
- Gao, Y.; Su, J.; Guo, W.; Polich, E.D.; Magyar, D.P.; Xing, Y.; Li, H.; Smrt, R.D.; Chang, Q.; Zhao, X. Inhibition of miR-15a promotes BDNF expression and rescues dendritic maturation deficits in MeCP2-deficient neurons HHS Public Access. Stem Cells 2015, 33, 1618–1629. [Google Scholar] [CrossRef]
- Smrt, R.D.; Szulwach, K.E.; Pfeiffer, R.L.; Li, X.; Guo, W.; Pathania, M.; Teng, Z.-Q.; Luo, Y.; Peng, J.; Bordey, A.; et al. MicroRNA miR-137 regulates neuronal maturation by targeting ubiquitin ligase Mind Bomb-1. Stem Cells 2010, 28, 1060–1070. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, S.I.; Nakamura, T.; Cieplak, P.; Chan, S.F.; Kalashnikova, E.; Liao, L.; Saleem, S.; Han, X.; Clemente, A.; Nutter, A.; et al. S-nitrosylation-mediated redox transcriptional switch modulates neurogenesis and neuronal cell death. Cell Rep. 2014, 8, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Irier, H.A.; Jin, P. Dynamics of DNA methylation in aging and Alzheimer’s disease. DNA Cell Biol. 2012, 31, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Calsolaro, V.; Edison, P. Neuroinflammation in Alzheimer’s disease: Current evidence and future directions. Alzheimer’s Dement. 2016, 12, 719–732. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Okello, A.A.; Brooks, D.J.; Edison, P. Longitudinal influence of microglial activation and amyloid on neuronal function in Alzheimer’s disease. Brain 2015, 138, 3685–3698. [Google Scholar] [CrossRef]
- Guzman-Martinez, L.; Maccioni, R.B.; Andrade, V.; Navarrete, L.P.; Pastor, M.G.; Ramos-Escobar, N. Neuroinflammation as a common feature of neurodegenerative disorders. Front. Pharmacol. 2019, 10, 1008. [Google Scholar] [CrossRef]
- Klein, R.S.; Garber, C.; Howard, N.; Author, N.I. Infectious immunity in the central nervous system and brain function HHS Public Access Author manuscript. Nat. Immunol. 2017, 18, 132–141. [Google Scholar] [CrossRef]
- Yong, H.Y.F.; Rawji, K.S.; Ghorbani, S.; Xue, M.; Yong, V.W. The benefits of neuroinflammation for the repair of the injured central nervous system. Cell. Mol. Immunol. 2019, 16, 540–546. [Google Scholar] [CrossRef]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2018, 4, 575–590. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Zhao, Y.; Dua, P.; Rogaev, E.I.; Lukiw, W.J. MicroRNA-34α-mediated down-regulation of the microglial-enriched triggering receptor and phagocytosis-sensor TREM2 in age-related macular degeneration. PLoS ONE 2016, 11, e0150211. [Google Scholar] [CrossRef]
- Zhao, Y.; Bhattacharjee, S.; Jones, B.M.; Dua, P.; Alexandrov, P.N.; Hill, J.M.; Lukiw, W.J. Regulation of TREM2 expression by an NF-kB-sensitive miRNA-34a. Neuroreport 2013, 24, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Doxaki, C.; Kampranis, S.C.; Eliopoulos, A.G.; Spilianakis, C.; Tsatsanis, C. Coordinated Regulation of miR-155 and miR-146a Genes during Induction of Endotoxin Tolerance in Macrophages. J. Immunol. 2015, 195, 5750–5761. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Li, Y.; Li, M.; Deng, G.; Wu, X.; Zeng, J.; Hao, X.; Wang, X.; Liu, J.; Cho, W.C.S.; et al. MicroRNA-124 negatively regulates TLR signaling in alveolar macrophages in response to mycobacterial infection. Mol. Immunol. 2014, 62, 150–158. [Google Scholar] [CrossRef]
- Culpan, D.; Kehoe, P.G.; Love, S. Tumour necrosis factor-α (TNF-α) and miRNA expression in frontal and temporal neocortex in Alzheimer’s disease and the effect of TNF-α on miRNA expression in vitro. Int. J. Mol. Epidemiol. Genet. 2011, 2, 156–162. [Google Scholar] [PubMed]
- Song, J.; Lee, J.E. MiR-155 is involved in Alzheimer’s disease by regulating T lymphocyte function. Front. Aging Neurosci. 2015, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- An, F.; Gong, G.; Wang, Y.; Bian, M.; Yu, L.; Wei, C. MiR-124 acts as a target for Alzheimer’s disease by regulating BACE1. Oncotarget 2017, 8, 114065–114071. [Google Scholar] [CrossRef] [PubMed]
- Ansari, A.; Maffioletti, E.; Milanesi, E.; Marizzoni, M.; Frisoni, G.B.; Blin, O.; Richardson, J.C.; Bordet, R.; Forloni, G.; Gennarelli, M.; et al. miR-146a and miR-181a are involved in the progression of mild cognitive impairment to Alzheimer’s disease. Neurobiol. Aging 2019, 82, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Slota, J.A.; Booth, S.A. MicroRNAs in neuroinflammation: Implications in disease pathogenesis, biomarker discovery and therapeutic applications. Non-Coding RNA 2019, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, M.; Lehtonen, S.; Jaronen, M.; Goldsteins, G.; Hämäläinen, R.H.; Koistinaho, J. Astrocyte alterations in neurodegenerative pathologies and their modeling in human induced pluripotent stem cell platforms. Cell. Mol. Life Sci. 2019, 76, 2739–2760. [Google Scholar] [CrossRef]
- Feng, M.G.; Liu, C.F.; Chen, L.; Feng, W.B.; Liu, M.; Hai, H.; Lu, J.M. MiR-21 attenuates apoptosis-triggered by amyloid-β via modulating PDCD4/ PI3K/AKT/GSK-3β pathway in SH-SY5Y cells. Biomed. Pharmacother. 2018, 101, 1003–1007. [Google Scholar] [CrossRef]
- Reaven, G. Insulin resistance, type 2 diabetes mellitus, and cardiovascular disease: The end of the beginning. Circulation 2005, 112, 3030–3032. [Google Scholar] [CrossRef] [PubMed]
- Plum, L.; Schubert, M.; Brüning, J.C. The role of insulin receptor signaling in the brain. Trends Endocrinol. Metab. 2005, 16, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Glenner, G.G.; Wong, C.W. Alzheimer’s disease: Initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 1984, 120, 885–890. [Google Scholar] [CrossRef]
- Koo, E.H.; Sisodia, S.S.; Archer, D.R.; Martin, L.J.; Weidemann, A.; Beyreuther, K.; Fischer, P.; Masters, C.L.; Price, D.L. Precursor of amyloid protein in Alzheimer disease undergoes fast anterograde axonal transport. Proc. Natl. Acad. Sci. USA 1990, 87, 1561–1565. [Google Scholar] [CrossRef]
- Tanzi, R.E.; Haines, J.L.; Watkins, P.C.; Stewart, G.D.; Wallace, M.R.; Hallewell, R.; Wong, C.; Wexler, N.S.; Conneally, P.M.; Gusella, J.F. Genetic linkage map of human chromosome 21. Genomics 1988, 3, 129–136. [Google Scholar] [CrossRef]
- Vassar, R.; Kovacs, D.M.; Yan, R.; Wong, P.C. The β-secretase enzyme BACE in health and Alzheimer’s disease: Regulation, cell biology, function, and therapeutic potential. J. Neurosci. 2009, 29, 12787–12794. [Google Scholar] [CrossRef]
- Brito-Moreira, J.; Lourenco, M.V.; Oliveira, M.M.; Ribeiro, F.C.; Ledo, J.H.; Diniz, L.P.; Vital, J.F.S.; Magdesian, M.H.; Melo, H.M.; Barros-Aragão, F.; et al. Interaction of amyloid-β (Aβ) oligomers with neurexin 2α and neuroligin 1 mediates synapse damage and memory loss in mice. J. Biol. Chem. 2017, 292, 7327–7337. [Google Scholar] [CrossRef]
- Femminella, G.D.; Ferrara, N.; Rengo, G. The emerging role of microRNAs in Alzheimer’s disease. Front. Physiol. 2015, 6, 40. [Google Scholar] [CrossRef]
- Folch, J.; Olloquequi, J.; Ettcheto, M.; Busquets, O.; Sánchez-López, E.; Cano, A.; Espinosa-Jiménez, T.; García, M.L.; Beas-Zarate, C.; Casadesús, G.; et al. The Involvement of Peripheral and Brain Insulin Resistance in Late Onset Alzheimer’s Dementia. Front. Aging Neurosci. 2019, 11, 236. [Google Scholar] [CrossRef]
- Higuchi, C.; Nakatsuka, A.; Eguchi, J.; Teshigawara, S.; Kanzaki, M.; Katayama, A.; Yamaguchi, S.; Takahashi, N.; Murakami, K.; Ogawa, D.; et al. Identification of circulating miR-101, miR-375 and miR-802 as biomarkers for type 2 diabetes. Metabolism 2015, 64, 489–497. [Google Scholar] [CrossRef]
- Barak, B.; Shvarts-Serebro, I.; Modai, S.; Gilam, A.; Okun, E.; Michaelson, D.M.; Mattson, M.P.; Shomron, N.; Ashery, U. Opposing actions of environmental enrichment and Alzheimer’s disease on the expression of hippocampal microRNAs in mouse models. Transl. Psychiatry 2013, 3, e304–e313. [Google Scholar] [CrossRef]
- Vilardo, E.; Barbato, C.; Ciotti, M.T.; Cogoni, C.; Ruberti, F. MicroRNA-101 regulates amyloid precursor protein expression in hippocampal neurons. J. Biol. Chem. 2010, 285, 18344–18351. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.Y.; Hernandez-Rapp, J.; Jolivette, F.; Lecours, C.; Bisht, K.; Goupil, C.; Dorval, V.; Parsi, S.; Morin, F.; Planel, E.; et al. MiR-132/212 deficiency impairs tau metabolism and promotes pathological aggregation in vivo. Hum. Mol. Genet. 2015, 24, 6721–6735. [Google Scholar] [CrossRef] [PubMed]
- Abbott, M.A.; Wells, D.G.; Fallon, J.R. The insulin receptor tyrosine kinase substrate p58/53 and the insulin receptor are components of CNS synapses. J. Neurosci. 1999, 19, 7300–7308. [Google Scholar] [CrossRef]
- Folli, F.; Saad, M.J.A.; Backer, J.M.; Kahn, C.R. Insulin stimulation of phosphatidylinositol 3-kinase activity and association with insulin receptor substrate 1 in liver and muscle of the intact rat. J. Biol. Chem. 1992, 267, 22171–22177. [Google Scholar] [PubMed]
- Cai, D.; Dhe-Paganon, S.; Melendez, P.A.; Lee, J.; Shoelson, S.E. Two new substrates in insulin signaling, IRS5/DOK4 and IRS6/DOK5. J. Biol. Chem. 2003, 278, 25323–25330. [Google Scholar] [CrossRef] [PubMed]
- Bomfim, T.R.; Forny-Germano, L.; Sathler, L.B.; Brito-Moreira, J.; Houzel, J.-C.; Decker, H.; Silverman, M.A.; Kazi, H.; Melo, H.M.; Mcclean, P.L.; et al. An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer’s disease-associated Aβ oligomers. J. Clin. Investig. 2012, 122, 1239–1253. [Google Scholar] [CrossRef]
- De Sousa, R.A.L. Brief report of the effects of the aerobic, resistance, and high-intensity interval training in type 2 diabetes mellitus individuals. Int. J. Diabetes Dev. Ctries. 2018, 38, 138–145. [Google Scholar] [CrossRef]
- Fernández-de Frutos, M.; Galán-Chilet, I.; Goedeke, L.; Kim, B.; Pardo-Marqués, V.; Pérez-García, A.; Herrero, J.I.; Fernández-Hernando, C.; Kim, J.; Ramírez, C.M. MicroRNA 7 Impairs Insulin Signaling and Regulates Aβ Levels through Posttranscriptional Regulation of the Insulin Receptor. Mol. Cell. Biol. 2019, 39, 1–19. [Google Scholar] [CrossRef]
- Kim, W.; Noh, H.; Lee, Y.; Jeon, J.; Shanmugavadivu, A.; McPhie, D.L.; Kim, K.S.; Cohen, B.M.; Seo, H.; Sonntag, K.C. MiR-126 Regulates Growth Factor Activities and Vulnerability to Toxic Insult in Neurons. Mol. Neurobiol. 2016, 53, 95–108. [Google Scholar] [CrossRef]
- Higaki, S.; Muramatsu, M.; Matsuda, A.; Matsumoto, K.; Satoh, J.I.; Michikawa, M.; Niida, S. Defensive effect of microRNA-200b/c against amyloid-beta peptide-induced toxicity in Alzheimer’s disease models. PLoS ONE 2018, 13, e0196929. [Google Scholar] [CrossRef] [PubMed]
- Arnold, S.E.; Lucki, I.; Brookshire, B.R.; Carlson, G.C.; Browne, C.A.; Kazi, H.; Bang, S.; Choi, B.R.; Chen, Y.; McMullen, M.F.; et al. High fat diet produces brain insulin resistance, synaptodendritic abnormalities and altered behavior in mice. Neurobiol. Dis. 2014, 67, 79–87. [Google Scholar] [CrossRef] [PubMed]
- de la Monte, S.M. Insulin Resistance and Neurodegeneration: Progress Towards the Development of New Therapeutics for Alzheimer’s Disease. Drugs 2017, 77, 47–65. [Google Scholar] [CrossRef] [PubMed]
- Neves, F.S.; Marques, P.T.; Barros-Aragão, F.; Nunes, N.B.; Venancio, A.M.; Cozachenco, D.; Frozza, R.L.; Passos, G.F.; Costa, R.; de Oliveira, J.; et al. Brain-defective insulin signaling is associated to late cognitive impairment in post-septic mice. Mol. Neurobiol. 2018, 55, 435–444. [Google Scholar] [CrossRef]
- Watson, L.S.; Hamlett, E.D.; Stone, T.D.; Sims-Robinson, C. Neuronally derived extracellular vesicles: An emerging tool for understanding Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 22. [Google Scholar] [CrossRef]
- Paolicelli, R.C.; Bergamini, G.; Rajendran, L. Cell-to-cell Communication by Extracellular Vesicles: Focus on Microglia. Neuroscience 2019, 405, 148–157. [Google Scholar] [CrossRef]
- Cui, G.; Wu, J.; Mou, F.; Xie, W.; Wang, F.; Wang, Q.; Fang, J.; Xu, Y.; Dong, Y.; Liu, J.; et al. Exosomes derived from hypoxia-preconditioned mesenchymal stromal cells ameliorate cognitive decline by rescuing synaptic dysfunction and regulating inflammatory responses in APP/PS1 mice. FASEB J. 2018, 32, 654–668. [Google Scholar] [CrossRef]
- Lourenco, M.V.; Frozza, R.L.; de Freitas, G.B.; Zhang, H.; Kincheski, G.C.; Ribeiro, F.C.; Gonçalves, R.A.; Clarke, J.R.; Beckman, D.; Staniszewski, A.; et al. Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s models. Nat. Med. 2019, 25, 165–175. [Google Scholar] [CrossRef]
- Nichols, M.R.; St-Pierre, M.; Wendeln, A.; Makoni, N.J.; Gouwens, L.K.; Garrad, E.C.; Sohrabi, M.; Neher, J.J.; Tremblay, M.; Combs, C.K. Inflammatory mechanisms in neurodegeneration. J. Neurochem. 2019, 149, 562–581. [Google Scholar] [CrossRef]
- Jash, K.; Gondaliya, P.; Kirave, P.; Kulkarni, B.; Sunkaria, A.; Kalia, K. Cognitive dysfunction: A growing link between diabetes and Alzheimer’s disease. Drug Dev. Res. 2020, 81, 144–164. [Google Scholar] [CrossRef]
- Patel, N.; Hoang, D.; Miller, N.; Ansaloni, S.; Huang, Q.; Rogers, J.T.; Lee, J.C.; Saunders, A.J. MicroRNAs can regulate human APP levels. Mol. Neurodegener. 2008, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Long, J.M.; Ray, B.; Lahiri, D.K. MicroRNA-153 physiologically inhibits expression of amyloid-β precursor protein in cultured human fetal brain cells and is dysregulated in a subset of Alzheimer disease patients. J. Biol. Chem. 2012, 287, 31298–31310. [Google Scholar] [CrossRef]
- Long, J.M.; Lahiri, D.K. MicroRNA-101 downregulates Alzheimer’s amyloid-β precursor protein levels in human cell cultures and is differentially expressed. Biochem. Biophys. Res. Commun. 2011, 404, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Yuan, K.; Tong, X.; Hu, J.; Song, Z.; Zhang, G.; Fang, X.; Zhang, W. MiR-16 attenuates β-amyloid-induced neurotoxicity through targeting β-site amyloid precursor protein-cleaving enzyme 1 in an Alzheimer’s disease cell model. Neuroreport 2018, 29, 1365–1372. [Google Scholar] [CrossRef]
- Liu, C.G.; Song, J.; Zhang, Y.Q.; Wang, P.C. MicroRNA-193b is a regulator of amyloid precursor protein in the blood and cerebrospinal fluid derived exosomal microRNA-193b is a biomarker of Alzheimer’s disease. Mol. Med. Rep. 2014, 10, 2395–2400. [Google Scholar] [CrossRef] [PubMed]
- Amakiri, N.; Kubosumi, A.; Tran, J.; Reddy, P.H. Amyloid beta and micrornas in Alzheimer’s disease. Front. Neurosci. 2019, 13, 430. [Google Scholar] [CrossRef]
- Liu, C.G.; Wang, J.L.; Li, L.; Wang, P.C. MicroRNA-384 regulates both amyloid precursor protein and β-secretase expression and is a potential biomarker for Alzheimer’s disease. Int. J. Mol. Med. 2014, 34, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Long, J.M.; Ray, B.; Lahiri, D.K. MicroRNA-339-5p down-regulates protein expression of β-site amyloid precursor protein-cleaving enzyme 1 (BACE1) in human primary brain cultures and is reduced in brain tissue specimens of alzheimer disease subjects. J. Biol. Chem. 2014, 289, 5184–5198. [Google Scholar] [CrossRef]
- Yang, G.; Song, Y.; Zhou, X.; Deng, Y.; Liu, T.; Weng, G.; Yu, D.; Pan, S. MicroRNA-29c targets β-site amyloid precursor protein-cleaving enzyme 1 and has a neuroprotective role in vitro and in vivo. Mol. Med. Rep. 2015, 12, 3081–3088. [Google Scholar] [CrossRef]
- Hernandez-Rapp, J.; Rainone, S.; Goupil, C.; Dorval, V.; Smith, P.Y.; Saint-Pierre, M.; Vallée, M.; Planel, E.; Droit, A.; Calon, F.; et al. MicroRNA-132/212 deficiency enhances Aβ production and senile plaque deposition in Alzheimer’s disease triple transgenic mice. Sci. Rep. 2016, 6, 30953. [Google Scholar] [CrossRef]
- Liu, W.; Liu, C.; Zhu, J.; Shu, P.; Yin, B.; Gong, Y.; Qiang, B.; Yuan, J.; Peng, X. MicroRNA-16 targets amyloid precursor protein to potentially modulate Alzheimer’s-associated pathogenesis in SAMP8 mice. Neurobiol. Aging 2012, 33, 522–534. [Google Scholar] [CrossRef]
- Hébert, S.S.; Horré, K.; Nicolaï, L.; Bergmans, B.; Papadopoulou, A.S.; Delacourte, A.; De Strooper, B. MicroRNA regulation of Alzheimer’s Amyloid precursor protein expression. Neurobiol. Dis. 2009, 33, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Lewczuk, P.; Kamrowski-Kruck, H.; Peters, O.; Heuser, I.; Jessen, F.; Popp, J.; Bürger, K.; Hampel, H.; Frölich, L.; Wolf, S.; et al. Soluble amyloid precursor proteins in the cerebrospinal fluid as novel potential biomarkers of Alzheimer’s disease: A multicenter study. Mol. Psychiatry 2010, 15, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Long, J.M.; Maloney, B.; Rogers, J.T.; Lahiri, D.K. Novel upregulation of amyloid-β precursor protein (APP) by microRNA-346 via targeting of APP mRNA 5′-untranslated region: Implications in Alzheimer’s disease. Mol. Psychiatry 2019, 24, 345–363. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.A.; Tomás, J.F.; Queiroz, J.A.; Figueiras, A.R.; Sousa, F. Recombinant pre-miR-29b for Alzheimer’s disease therapeutics. Sci. Rep. 2016, 6, 19946. [Google Scholar] [CrossRef] [PubMed]
- Nelson, P.T.; Wang, W.X. MiR-107 is reduced in Alzheimer’s disease brain neocortex: Validation study. J. Alzheimer’s Dis. 2010, 21, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Wang, H.; Chen, K.; Cheng, P.; Gao, S.; Liu, J.; Li, X.; Sun, X. MicroRNA-34c Downregulation Ameliorates Amyloid-β-Induced Synaptic Failure and Memory Deficits by Targeting VAMP2. J. Alzheimer’s Dis. 2015, 48, 673–686. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, P.; Wang, X.; Yao, J.; Zhuang, S. miR-34a deficiency in APP/PS1 mice promotes cognitive function by increasing synaptic plasticity via AMPA and NMDA receptors. Neurosci. Lett. 2018, 670, 94–104. [Google Scholar] [CrossRef]
- Lee, K.; Kim, H.; An, K.; Kwon, O.B.; Park, S.; Cha, J.H.; Kim, M.H.; Lee, Y.; Kim, J.H.; Cho, K.; et al. Replenishment of microRNA-188-5p restores the synaptic and cognitive deficits in 5XFAD Mouse Model of Alzheimer’s Disease. Sci. Rep. 2016, 6, 34433. [Google Scholar] [CrossRef]
- Šimić, G.; Babić Leko, M.; Wray, S.; Harrington, C.; Delalle, I.; Jovanov-Milošević, N.; Bažadona, D.; Buée, L.; de Silva, R.; Di Giovanni, G.; et al. Tau protein hyperphosphorylation and aggregation in alzheimer’s disease and other tauopathies, and possible neuroprotective strategies. Biomolecules 2016, 6, 6. [Google Scholar] [CrossRef]
- Ma, X.; Liu, L.; Meng, J. MicroRNA-125b promotes neurons cell apoptosis and Tau phosphorylation in Alzheimer’s disease. Neurosci. Lett. 2017, 661, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Banzhaf-Strathmann, J.; Benito, E.; May, S.; Arzberger, T.; Tahirovic, S.; Kretzschmar, H.; Fischer, A.; Edbauer, D. Micro RNA-125b induces tau hyperphosphorylation and cognitive deficits in Alzheimer’s disease. EMBO J. 2014, 33, 1667–1680. [Google Scholar] [CrossRef] [PubMed]
- Santa-Maria, I.; Alaniz, M.E.; Renwick, N.; Cela, C.; Fulga, T.A.; Van Vactor, D.; Tuschl, T.; Clark, L.N.; Shelanski, M.L.; McCabe, B.D.; et al. Dysregulation of microRNA-219 promotes neurodegeneration through post-transcriptional regulation of tau. J. Clin. Investig. 2015, 125, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Edbauer, D.; Neilson, J.R.; Foster, K.A.; Wang, C.F.; Seeburg, D.P.; Batterton, M.N.; Tada, T.; Dolan, B.M.; Sharp, P.A.; Sheng, M. Regulation of Synaptic Structure and Function by FMRP-Associated MicroRNAs miR-125b and miR-132. Neuron 2010, 65, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.B.; Wu, L.; Xiong, R.; Wang, L.L.; Zhang, B.; Wang, C.; Li, H.; Liang, L.; Chen, S.D. MicroRNA-922 promotes tau phosphorylation by downregulating ubiquitin carboxy-terminal hydrolase L1 (UCHL1) expression in the pathogenesis of Alzheimer’s disease. Neuroscience 2014, 275, 232–237. [Google Scholar] [CrossRef]
- Gong, B.; Cao, Z.; Zheng, P.; Vitolo, O.V.; Liu, S.; Staniszewski, A.; Moolman, D.; Zhang, H.; Shelanski, M.; Arancio, O. Ubiquitin Hydrolase Uch-L1 Rescues β-Amyloid-Induced Decreases in Synaptic Function and Contextual Memory. Cell 2006, 126, 775–788. [Google Scholar] [CrossRef]
- Wang, G.; Huang, Y.; Wang, L.L.; Zhang, Y.F.; Xu, J.; Zhou, Y.; Lourenco, G.F.; Zhang, B.; Wang, Y.; Ren, R.J.; et al. MicroRNA-146a suppresses ROCK1 allowing hyperphosphorylation of tau in Alzheimer’s disease. Sci. Rep. 2016, 6, 26697. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, J.; Lu, G. miR-106b inhibits tau phosphorylation at Tyr18 by targeting Fyn in a model of Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2016, 478, 852–857. [Google Scholar] [CrossRef]
- Salta, E.; Sierksma, A.; Vanden Eynden, E.; De Strooper, B. miR-132 loss de-represses ITPKB and aggravates amyloid and TAU pathology in Alzheimer’s brain. EMBO Mol. Med. 2016, 8, 1005–1018. [Google Scholar] [CrossRef]
- Guan, J.S.; Haggarty, S.J.; Giacometti, E.; Dannenberg, J.H.; Joseph, N.; Gao, J.; Nieland, T.J.F.; Zhou, Y.; Wang, X.; Mazitschek, R.; et al. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature 2009, 459, 55–60. [Google Scholar] [CrossRef]
- Gräff, J.; Rei, D.; Guan, J.S.; Wang, W.Y.; Seo, J.; Hennig, K.M.; Nieland, T.J.F.; Fass, D.M.; Kao, P.F.; Kahn, M.; et al. An epigenetic blockade of cognitive functions in the neurodegenerating brain. Nature 2012, 483, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Tang, H.; Li, X.Y.; Deng, M.F.; Wei, N.; Wang, X.; Zhou, Y.F.; Wang, D.Q.; Fu, P.; Wang, J.Z.; et al. Targeting the HDAC2/HNF-4A/miR-101b/AMPK Pathway Rescues Tauopathy and Dendritic Abnormalities in Alzheimer’s Disease. Mol. Ther. 2017, 25, 752–764. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Li, J.; Huang, L.; Chen, X.; Li, D.; Wang, T.; Hu, C.; Xu, J.; Zhang, C.; Zen, K.; et al. Serum MicroRNA Profiles Serve as Novel Biomarkers for the Diagnosis of Alzheimer’s Disease. Dis. Markers 2015, 2015, 625659. [Google Scholar] [CrossRef] [PubMed]
- Takousis, P.; Sadlon, A.; Schulz, J.; Wohlers, I.; Dobricic, V.; Middleton, L.; Lill, C.M.; Perneczky, R.; Bertram, L. Differential expression of microRNAs in Alzheimer’s disease brain, blood, and cerebrospinal fluid. Alzheimer’s Dement. 2019, 15, 1468–1477. [Google Scholar] [CrossRef] [PubMed]
- Fransquet, P.D.; Ryan, J. Micro RNA as a potential blood-based epigenetic biomarker for Alzheimer’s disease. Clin. Biochem. 2018, 58, 5–14. [Google Scholar] [CrossRef]
- Satoh, J.I.; Kino, Y.; Niida, S. MicroRNA-Seq data analysis pipeline to identify blood biomarkers for alzheimer’s disease from public data. Biomark. Insights 2015, 10, 21–31. [Google Scholar] [CrossRef]
- Geekiyanage, H.; Jicha, G.A.; Nelson, P.T.; Chan, C. Blood serum miRNA: Non-invasive biomarkers for Alzheimer’s disease. Exp. Neurol. 2012, 235, 491–496. [Google Scholar] [CrossRef]
- Geekiyanage, H.; Chan, C. Micro RNA-137/181c regulates serine palmitoyltransferase and in turn amyloid β novel targets in sporadic Alzheimer’s disease. J. Neurosci. 2011, 31, 14820–14830. [Google Scholar] [CrossRef]
- Tan, L.; Yu, J.T.; Tan, M.S.; Liu, Q.Y.; Wang, H.F.; Zhang, W.; Jiang, T.; Tan, L. Genome-wide serum microRNA expression profiling identifies serum biomarkers for Alzheimer’s disease. J. Alzheimer’s Dis. 2014, 40, 1017–1027. [Google Scholar] [CrossRef]
- Galimberti, D.; Villa, C.; Fenoglio, C.; Serpente, M.; Ghezzi, L.; Cioffi, S.M.G.; Arighi, A.; Fumagalli, G.; Scarpini, E. Circulating miRNAs as potential biomarkers in alzheimer’s disease. J. Alzheimer’s Dis. 2014, 42, 1261–1267. [Google Scholar] [CrossRef]
- Tan, L.; Yu, J.T.; Liu, Q.Y.; Tan, M.S.; Zhang, W.; Hu, N.; Wang, Y.L.; Sun, L.; Jiang, T.; Tan, L. Circulating miR-125b as a biomarker of Alzheimer’s disease. J. Neurol. Sci. 2014, 336, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Lusardi, T.A.; Phillips, J.I.; Wiedrick, J.T.; Harrington, C.A.; Lind, B.; Lapidus, J.A.; Quinn, J.F.; Saugstad, J.A. MicroRNAs in human cerebrospinal fluid as biomarkers for Alzheimer’s disease. J. Alzheimer’s Dis. 2017, 55, 1223–1233. [Google Scholar] [CrossRef] [PubMed]
- Cosín-Tomás, M.; Antonell, A.; Lladó, A.; Alcolea, D.; Fortea, J.; Ezquerra, M.; Lleó, A.; Martí, M.J.; Pallàs, M.; Sanchez-Valle, R.; et al. Plasma miR-34a-5p and miR-545-3p as Early Biomarkers of Alzheimer’s Disease: Potential and Limitations. Mol. Neurobiol. 2017, 54, 5550–5562. [Google Scholar] [CrossRef]
- Liu, C.G.; Wang, J.L.; Li, L.; Xue, L.X.; Zhang, Y.Q.; Wang, P.C. MicroRNA-135a and -200b, potential Biomarkers for Alzheimer’s disease, regulate β secretase and amyloid precursor protein. Brain Res. 2014, 1583, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; Levine, H. Alzheimer’s disease and the amyloid-β peptide. J. Alzheimer’s Dis. 2010, 19, 311–323. [Google Scholar] [CrossRef]
- Kiko, T.; Nakagawa, K.; Tsuduki, T.; Furukawa, K.; Arai, H.; Miyazawa, T. MicroRNAs in plasma and cerebrospinal fluid as potential markers for Alzheimer’s disease. J. Alzheimer’s Dis. 2014, 39, 253–259. [Google Scholar] [CrossRef]
- Kumar, P.; Dezso, Z.; MacKenzie, C.; Oestreicher, J.; Agoulnik, S.; Byrne, M.; Bernier, F.; Yanagimachi, M.; Aoshima, K.; Oda, Y. Circulating miRNA Biomarkers for Alzheimer’s Disease. PLoS ONE 2013, 8, e69807. [Google Scholar] [CrossRef]
- Prendecki, M.; Florczak-Wyspianska, J.; Kowalska, M.; Ilkowski, J.; Grzelak, T.; Bialas, K.; Kozubski, W.; Dorszewska, J. APOE genetic variants and apoE, miR-107 and miR-650 levels in Alzheimer’s disease. Folia Neuropathol. 2019, 57, 106–116. [Google Scholar] [CrossRef]
- Teter, B.; Ladu, M.J.; Sullivan, P.M.; Frautschy, S.A.; Cole, G.M. Apolipoprotein e isotype-dependent modulation of microRNA-146a in plasma and brain. Neuroreport 2016, 27, 791–795. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, Y.; Mok, K.Y.; Kwok, T.C.Y.; Mok, V.C.T.; Guo, Q.; Ip, F.C.; Chen, Y.; Mullapudi, N.; Weiner, M.W.; et al. Non-coding variability at the APOE locus contributes to the Alzheimer’s risk. Nat. Commun. 2019, 10, 3310. [Google Scholar] [CrossRef]
- Nagamatsu, L.S.; Flicker, L.; Kramer, A.F.; Voss, M.W.; Erickson, K.I.; Hsu, C.L.; Liu-Ambrose, T. Exercise is medicine, for the body and the brain. Br. J. Sports Med. 2014, 48, 943–944. [Google Scholar] [CrossRef] [PubMed]
- Brini, S.; Sohrabi, H.R.; Peiffer, J.J.; Karrasch, M.; Hämäläinen, H.; Martins, R.N.; Fairchild, T.J. Physical Activity in Preventing Alzheimer’s Disease and Cognitive Decline: A Narrative Review. Sports Med. 2018, 48, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Lucia, A.; Ruiz, J.R. Exercise is beneficial for patients with alzheimer’s disease: A call for action. Br. J. Sports Med. 2011, 45, 468–469. [Google Scholar] [CrossRef] [PubMed]
- Devenney, K.E.; Guinan, E.M.; Kelly, Á.M.; Mota, B.C.; Walsh, C.; Olde Rikkert, M.; Schneider, S.; Lawlor, B. Acute high-intensity aerobic exercise affects brain-derived neurotrophic factor in mild cognitive impairment: A randomised controlled study. BMJ Open Sport Exerc. Med. 2019, 5, e000499. [Google Scholar] [CrossRef]
- Radak, Z.; Hart, N.; Sarga, L.; Koltai, E.; Atalay, M.; Ohno, H.; Boldogh, I. Exercise plays a preventive role against Alzheimer’s disease. J. Alzheimer’s Dis. 2010, 20, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Abraham, D.; Feher, J.; Scuderi, G.L.; Szabo, D.; Dobolyi, A.; Cservenak, M.; Juhasz, J.; Ligeti, B.; Pongor, S.; Gomez-Cabrera, M.C.; et al. Exercise and probiotics attenuate the development of Alzheimer’s disease in transgenic mice: Role of microbiome. Exp. Gerontol. 2019, 115, 122–131. [Google Scholar] [CrossRef]
- Du, Z.; Li, Y.; Li, J.; Zhou, C.; Li, F.; Yang, X. Physical activity can improve cognition in patients with alzheimer’s disease: A systematic review and meta-analysis of randomized controlled trials. Clin. Interv. Aging 2018, 13, 1593–1603. [Google Scholar] [CrossRef]
- Schuit, A.J.; Feskens, E.J.M.; Launer, L.J.; Kromhout, D. Physical activity and cognitive decline, the role of the apolipoprotein e4 allele. Med. Sci. Sports Exerc. 2001, 33, 772–777. [Google Scholar] [CrossRef]
- Liu, C.C.; Kanekiyo, T.; Xu, H.; Bu, G. Apolipoprotein e and Alzheimer disease: Risk, mechanisms and therapy. Nat. Rev. Neurol. 2013, 9, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Freitas, D.A.; Rocha-Vieira, E.; Soares, B.A.; Nonato, L.F.; Fonseca, S.R.; Martins, J.B.; Mendonça, V.A.; Lacerda, A.C.; Massensini, A.R.; Poortamns, J.R.; et al. High intensity interval training modulates hippocampal oxidative stress, BDNF and inflammatory mediators in rats. Physiol. Behav. 2018, 184, 6–11. [Google Scholar] [CrossRef]
- Freitas, D.A.; Rocha-Vieira, E.; De Sousa, R.A.L.; Soares, B.A.; Rocha-Gomes, A.; Chaves Garcia, B.C.; Cassilhas, R.C.; Mendonça, V.A.; Camargos, A.C.R.; De Gregorio, J.A.M.; et al. High-intensity interval training improves cerebellar antioxidant capacity without affecting cognitive functions in rats. Behav. Brain Res. 2019, 376, 112181. [Google Scholar] [CrossRef] [PubMed]
- Nichol, K.E.; Poon, W.W.; Parachikova, A.I.; Cribbs, D.H.; Glabe, C.G.; Cotman, C.W. Exercise alters the immune profile in Tg2576 Alzheimer mice toward a response coincident with improved cognitive performance and decreased amyloid. J. Neuroinflamm. 2008, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chu, J.M.T.; Yan, T.; Zhang, Y.; Chen, Y.; Chang, R.C.C.; Wong, G.T.C. Short-term resistance exercise inhibits neuroinflammation and attenuates neuropathological changes in 3xTg Alzheimer’s disease mice. J. Neuroinflam. 2020, 17, 4. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.C.; Filho, C.B.; Goes, A.T.R.; Del Fabbro, L.; De Gomes, M.G.; Savegnago, L.; Oliveira, M.S.; Jesse, C.R. Neuroprotective effect of physical exercise in a mouse model of alzheimer’s disease induced by β-Amyloid1-40 peptide. Neurotox. Res. 2013, 24, 148–163. [Google Scholar] [CrossRef]
- Grice, B.A.; Mason, C.C.; Weil, E.J.; Knowler, W.C.; Pomeroy, J. The relationship between insulin sensitivity and maximal oxygen uptake is confounded by method of adjustment for body composition. Diabetes Vasc. Dis. Res. 2013, 10, 530–535. [Google Scholar] [CrossRef]
- Molsted, S.; Eiken, P.; Andersen, J.L.; Eidemak, I.; Harrison, A.P. Interleukin-6 and Vitamin D Status during High-Intensity Resistance Training in Patients with Chronic Kidney Disease. Biomed. Res. Int. 2014, 2014, 176190. [Google Scholar] [CrossRef]
- Olesen, J.; Ringholm, S.; Nielsen, M.M.; Brandt, C.T.; Pedersen, J.T.; Halling, J.F.; Goodyear, L.J.; Pilegaard, H. Role of PGC-1α in exercise training- and resveratrol-induced prevention of age-associated inflammation. Exp. Gerontol. 2013, 48, 1274–1284. [Google Scholar] [CrossRef]
- Turner, N.; Cooney, G.J.; Kraegen, E.W.; Bruce, C.R. Fatty acid metabolism, energy expenditure and insulin resistance in muscle. J. Endocrinol. 2014, 220, T61–T79. [Google Scholar] [CrossRef]
- Yamashita, A.S.; Lira, F.S.; Lima, W.P.; Carnevali, L.C.; Gonçalves, D.C.; Tavares, F.L.; Seelaender, M.C.L. Influence of aerobic physical training in the motochondrial transport of long chain fatty acids in the skeletal muscle: Role of the carnitine palmitoil transferase. Rev. Bras. Med. Esporte 2008, 14, 150–154. [Google Scholar] [CrossRef]
- Reza, M.M.; Subramaniyam, N.; Sim, C.M.; Ge, X.; Sathiakumar, D.; McFarlane, C.; Sharma, M.; Kambadur, R. Irisin is a pro-myogenic factor that induces skeletal muscle hypertrophy and rescues denervation-induced atrophy. Nat. Commun. 2017, 8, 1104. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.Y.; Panagiotou, G.; Mougios, V.; Brinkoetter, M.; Vamvini, M.T.; Schneider, B.E.; Mantzoros, C.S. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism 2012, 61, 1725–1738. [Google Scholar] [CrossRef] [PubMed]
- Shan, T.; Liang, X.; Bi, P.; Kuang, S. Myostatin knockout drives browning of white adipose tissue through activating the AMPK-PGC1-Fndc5 pathway in muscle. FASEB J. 2013, 27, 1981–1989. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Liu, Y.; Zhan, Z.; Wang, X. MicroRNA-132 is associated with the cognition improvement following voluntary exercise in SAMP8 mice. Brain Res. Bull. 2018, 140, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.B.; Unmehopa, U.; Bossers, K.; Hu, Y.T.; Verwer, R.; Balesar, R.; Zhao, J.; Bao, A.M.; Swaab, D. MicroRNA-132 and early growth response-1 in nucleus basalis of Meynert during the course of Alzheimer’s disease. Brain 2016, 139, 908–921. [Google Scholar] [CrossRef]
- Wang, Y.; Veremeyko, T.; Wong, A.H.K.; El Fatimy, R.; Wei, Z.; Cai, W.; Krichevsky, A.M. Downregulation of miR-132/212 impairs S-nitrosylation balance and induces tau phosphorylation in Alzheimer’s disease. Neurobiol. Aging 2017, 51, 156–166. [Google Scholar] [CrossRef]
- Habibi, P.; Babri, S.; Ahmadiasl, N.; Yousefi, H. Effects of genistein and swimming exercise on spatial memory and expression of microRNA 132, BDNF, and IGF-1 genes in the hippocampus of ovariectomized rats. Iran. J. Basic Med. Sci. 2017, 20, 856–862. [Google Scholar] [CrossRef]
- Radom-Aizik, S.; Zaldivar, F.; Leu, S.Y.; Adams, G.R.; Oliver, S.; Cooper, D.M. Effects of Exercise on microRNA Expression in Young Males Peripheral Blood Mononuclear Cells. Clin. Transl. Sci. 2012, 5, 32–38. [Google Scholar] [CrossRef]
- de Gonzalo-Calvo, D.; Dávalos, A.; Montero, A.; García-González, Á.; Tyshkovska, I.; González-Medina, A.; Soares, S.M.A.; Martínez-Camblor, P.; Casas-Agustench, P.; Rabadán, M.; et al. Circulating inflammatory miRNA signature in response to different doses of aerobic exercise. J. Appl. Physiol. 2015, 119, 124–134. [Google Scholar] [CrossRef] [PubMed]
- de Gonzalo-Calvo, D.; Dávalos, A.; Fernández-Sanjurjo, M.; Amado-Rodríguez, L.; Díaz-Coto, S.; Tomás-Zapico, C.; Montero, A.; García-González, Á.; Llorente-Cortés, V.; Heras, M.E.; et al. Circulating microRNAs as emerging cardiac biomarkers responsive to acute exercise. Int. J. Cardiol. 2018, 264, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Barber, J.L.; Zellars, K.N.; Barringhaus, K.G.; Bouchard, C.; Spinale, F.G.; Sarzynski, M.A. The Effects of Regular Exercise on Circulating Cardiovascular-related MicroRNAs. Sci. Rep. 2019, 9, 7527. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.Q.; Townsend, M. Insulin resistance and amyloidogenesis as common molecular foundation for type 2 diabetes and Alzheimer’s disease. Biochim. Biophys. Acta—Mol. Basis Dis. 2009, 1792, 482–496. [Google Scholar] [CrossRef]
- Cogswell, J.; Ward, J.; Taylor, I.; Waters, M.; Shi, Y.; Cannon, B.; Kelnar, K.; Kemppainen, J.; Brown, D.; Chen, C.; et al. Identification of miRNA Changes in Alzheimer’s.pdf. J. Alzheimer’s Dis. 2008, 14, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, P.; Zhu, H.; Xu, Y.; Ma, C.; Dai, X.; Huang, L.; Liu, Y.; Zhang, L.; Qin, C. miR-34a, a microRNA up-regulated in a double transgenic mouse model of Alzheimer’s disease, inhibits bcl2 translation. Brain Res. Bull. 2009, 80, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Jaber, V.R.; LeBeauf, A.; Sharfman, N.M.; Lukiw, W.J. MicroRNA-34a (miRNA-34a) mediated down-regulation of the post-synaptic cytoskeletal element SHANK3 in sporadic Alzheimer’s disease (AD). Front. Neurol. 2019, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Zovoilis, A.; Agbemenyah, H.Y.; Agis-Balboa, R.C.; Stilling, R.M.; Edbauer, D.; Rao, P.; Farinelli, L.; Delalle, I.; Schmitt, A.; Falkai, P.; et al. MicroRNA-34c is a novel target to treat dementias. EMBO J. 2011, 30, 4299–4308. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Wang, J.; Zhang, X.; Geng, Y.; Hu, Z.; Rudd, J.A.; Ling, S.; Chen, W.; Han, S. The miR-124 regulates the expression of BACE1/β-secretase correlated with cell death in Alzheimer’s disease. Toxicol. Lett. 2012, 209, 94–105. [Google Scholar] [CrossRef]
- Pan-Vazquez, A.; Rye, N.; Ameri, M.; McSparron, B.; Smallwood, G.; Bickerdyke, J.; Rathbone, A.; Dajas-Bailador, F.; Toledo-Rodriguez, M. Impact of voluntary exercise and housing conditions on hippocampal glucocorticoid receptor, miR-124 and anxiety. Mol. Brain 2015, 8, 40. [Google Scholar] [CrossRef]
- Sethi, P.; Lukiw, W.J. Micro-RNA abundance and stability in human brain: Specific alterations in Alzheimer’s disease temporal lobe neocortex. Neurosci. Lett. 2009, 459, 100–104. [Google Scholar] [CrossRef]
- Patrick, E.; Rajagopal, S.; Wong, H.K.A.; McCabe, C.; Xu, J.; Tang, A.; Imboywa, S.H.; Schneider, J.A.; Pochet, N.; Krichevsky, A.M.; et al. Dissecting the role of non-coding RNAs in the accumulation of amyloid and tau neuropathologies in Alzheimer’s disease. Mol. Neurodegener. 2017, 12, 51. [Google Scholar] [CrossRef]
- Ko, C.Y.; Chu, Y.Y.; Narumiya, S.; Chi, J.Y.; Furuyashiki, T.; Aoki, T.; Wang, S.M.; Chang, W.C.; Wang, J.M. The CCAAT/enhancer-binding protein delta/miR135a/thrombospondin 1 axis mediates PGE2-induced angiogenesis in Alzheimer’s disease. Neurobiol. Aging 2015, 36, 1356–1368. [Google Scholar] [CrossRef]
- Guedes, J.R.; Custódia, C.M.; Silva, R.J.; de Almeida, L.P.; de Lima, M.C.P.; Cardoso, A.L. Early miR-155 upregulation contributes to neuroinflammation in Alzheimer’s disease triple transgenic mouse model. Hum. Mol. Genet. 2014, 23, 6286–6301. [Google Scholar] [CrossRef] [PubMed]
- Virtual Health Sciences Library. The Effect of Endurance Training on MiR-155 Expression, STAT3 Gene Expression, and Interleukin 6 Protein in Mice with Breast Cancer. Available online: https://vlibrary.emro.who.int/imemr/the-effect-of-endurance-training-on-mir-155-expression-stat3-gene-expression-and-interleukin-6-protein-in-mice-with-breast-cancer-2/ (accessed on 6 April 2020).
- Li, J.; Wang, H. MiR-15b reduces amyloid-β accumulation in SH-SY5Y cell line through targetting NF-κB signaling and BACE1. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef]
- Gong, G.; An, F.; Wang, Y.; Bian, M.; Yu, L.J.; Wei, C. miR-15b represses BACE1 expression in sporadic Alzheimer’s disease. Oncotarget 2017, 8, 91551–91557. [Google Scholar] [CrossRef] [PubMed]
- Cosín-Tomás, M.; Alvarez-López, M.J.; Sanchez-Roige, S.; Lalanza, J.F.; Bayod, S.; Sanfeliu, C.; Pallàs, M.; Escorihuela, R.M.; Kaliman, P. Epigenetic alterations in hippocampus of SAMP8 senescent mice and modulation by voluntary physical exercise. Front. Aging Neurosci. 2014, 6, 51. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.; Åkerström, T.; Rinnov, A.; Yfanti, C.; Scheele, C.; Pedersen, B.K.; Laye, M.J. The miRNA plasma signature in response to acute aerobic exercise and endurance training. PLoS ONE 2014, 9, e87308. [Google Scholar] [CrossRef] [PubMed]
- Lugli, G.; Cohen, A.M.; Bennett, D.A.; Shah, R.C.; Fields, C.J.; Hernandez, A.G.; Smalheiser, N.R. Plasma exosomal miRNAs in persons with and without Alzheimer disease: Altered expression and prospects for biomarkers. PLoS ONE 2015, 10, e0139233. [Google Scholar] [CrossRef]
- Denk, J.; Boelmans, K.; Siegismund, C.; Lassner, D.; Arlt, S.; Jahn, H. MicroRNA profiling of CSF reveals potential biomarkers to detect Alzheimer’s disease. PLoS ONE 2015, 10, e0126423. [Google Scholar] [CrossRef]
- Wang, W.X.; Huang, Q.; Hu, Y.; Stromberg, A.J.; Nelson, P.T. Patterns of microRNA expression in normal and early Alzheimer’s disease human temporal cortex: White matter versus gray matter. Acta Neuropathol. 2011, 121, 193–205. [Google Scholar] [CrossRef]
- Mojtahedi, S.; Kordi, M.R.; Hosseini, S.E.; Omran, S.F.; Soleimani, M. Effect of treadmill running on the expression of genes that are involved in neuronal differentiation in the hippocampus of adult male rats. Cell Biol. Int. 2013, 37, 276–283. [Google Scholar] [CrossRef]
- Miao, W.; Bao, T.H.; Han, J.H.; Yin, M.; Yan, Y.; Wang, W.W.; Zhu, Y.H. Voluntary exercise prior to traumatic brain injury alters miRNA expression in the injured mouse cerebral cortex. Braz. J. Med. Biol. Res. 2015, 48, 433–439. [Google Scholar] [CrossRef]
- Liu, S.X.; Zheng, F.; Xie, K.L.; Xie, M.R.; Jiang, L.J.; Cai, Y. Exercise Reduces Insulin Resistance in Type 2 Diabetes Mellitus via Mediating the lncRNA MALAT1/MicroRNA-382-3p/Resistin Axis. Mol. Ther.—Nucleic Acids 2019, 18, 34–44. [Google Scholar] [CrossRef]
- Shih, R.H.; Wang, C.Y.; Yang, C.M. NF-kappaB signaling pathways in neurological inflammation: A mini review. Front. Mol. Neurosci. 2015, 8, 77. [Google Scholar] [CrossRef] [PubMed]
- Lyman, M.; Lloyd, D.G.; Ji, X.; Vizcaychipi, M.P.; Ma, D. Neuroinflammation: The role and consequences. Neurosci. Res. 2014, 79, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.G.; Li, Y.Y.; Zhao, Y.; Bhattacharjee, S.; Lukiw, W.J. Differential regulation of Interleukin-1 Receptor-associated Kinase-1 (IRAK-1) and IRAK-2 by microRNA-146a and NF-κB in stressed human astroglial cells and in Alzheimer disease. J. Biol. Chem. 2010, 285, 38951–38960. [Google Scholar] [CrossRef] [PubMed]
- Sawada, S.; Kon, M.; Wada, S.; Ushida, T.; Suzuki, K.; Akimoto, T. Profiling of Circulating MicroRNAs after a Bout of Acute Resistance Exercise in Humans. PLoS ONE 2013, 8, e70823. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yao, M.; Zhou, Q.; Cheng, Y.; Che, L.; Xu, J.; Xiao, J.; Shen, Z.; Bei, Y. Dynamic regulation of circulating microRNAs during acute exercise and long-term exercise training in basketball athletes. Front. Physiol. 2018, 9, 282. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, B.; Chen, J.; Sui, Y.; Ren, L.; Li, J.; Zhang, H.; Guo, L.; Sun, X. Micro-RNA-137 inhibits tau hyperphosphorylation in alzheimer’s disease and targets the CACNA1C gene in transgenic mice and human neuroblastoma SH-SY5Y cells. Med. Sci. Monit. 2018, 24, 5635–5644. [Google Scholar] [CrossRef]
- Jessop, P.; Toledo-Rodriguez, M. Hippocampal TET1 and TET2 expression and DNA hydroxymethylation are affected by physical exercise in aged mice. Front. Cell Dev. Biol. 2018, 6, 45. [Google Scholar] [CrossRef]
- Alexandrov, P.N.; Dua, P.; Hill, J.M.; Bhattacharjee, S.; Zhao, Y.; Lukiw, W.J. MicroRNA (miRNA) speciation in Alzheimer’s disease (AD) cerebrospinal fluid (CSF) and extracellular fluid (ECF). Int. J. Biochem. Mol. Biol. 2012, 3, 365–373. [Google Scholar]
- Wu, Y.; Xu, J.; Xu, J.; Cheng, J.; Jiao, D.; Zhou, C.; Dai, Y.; Chen, Q. Lower serum levels of miR-29c-3p and miR-19b-3p as biomarkers for Alzheimer’s disease. Tohoku J. Exp. Med. 2017, 242, 129–136. [Google Scholar] [CrossRef]
- Arena, A.; Iyer, A.M.; Milenkovic, I.; Kovacs, G.G.; Ferrer, I.; Perluigi, M.; Aronica, E. Developmental Expression and Dysregulation of miR-146a and miR-155 in Down’s Syndrome and Mouse Models of Down’s Syndrome and Alzheimer’s Disease. Curr. Alzheimer Res. 2017, 14, 1305–1317. [Google Scholar] [CrossRef]
- Baggish, A.L.; Hale, A.; Weiner, R.B.; Lewis, G.D.; Systrom, D.; Wang, F.; Wang, T.J.; Chan, S.Y. Dynamic regulation of circulating microRNA during acute exhaustive exercise and sustained aerobic exercise training. J. Physiol. 2011, 589, 3983–3994. [Google Scholar] [CrossRef] [PubMed]
- Baggish, A.L.; Park, J.; Min, P.-K.; Isaacs, S.; Parker, B.A.; Thompson, P.D.; Troyanos, C.; D’Hemecourt, P.; Dyer, S.; Thiel, M.; et al. Rapid upregulation and clearance of distinct circulating microRNAs after prolonged aerobic exercise. J. Appl. Physiol. 2014, 116, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Snowden, S.G.; Ebshiana, A.A.; Hye, A.; An, Y.; Pletnikova, O.; O’Brien, R.; Troncoso, J.; Legido-Quigley, C.; Thambisetty, M. Association between fatty acid metabolism in the brain and Alzheimer disease neuropathology and cognitive performance: A nontargeted metabolomic study. PLoS Med. 2017, 14, e1002266. [Google Scholar] [CrossRef] [PubMed]
- de Souza Teixeira, A.A.; Lira, F.S.; Pimentel, G.D.; de Souza, C.O.; Batatinha, H.; Biondo, L.A.; Yamashita, A.S.; Lima Júnior, E.A.; Rosa Neto, J.C. Aerobic exercise modulates the free fatty acids and inflammatory response during obesity and cancer cachexia. Crit. Rev. Eukaryot. Gene Expr. 2016, 26, 187–198. [Google Scholar] [CrossRef]
- Mika, A.; Macaluso, F.; Barone, R.; Di Felice, V.; Sledzinski, T. Effect of Exercise on Fatty Acid Metabolism and Adipokine Secretion in Adipose Tissue. Front. Physiol. 2019, 10, 26. [Google Scholar] [CrossRef]
- Augustin, R.; Endres, K.; Reinhardt, S.; Kuhn, P.H.; Lichtenthaler, S.F.; Hansen, J.; Wurst, W.; Trümbach, D. Computational identification and experimental validation of microRNAs binding to the Alzheimer-related gene ADAM10. BMC Med. Genet. 2012, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.X.; Rajeev, B.W.; Stromberg, A.J.; Ren, N.; Tang, G.; Huang, Q.; Rigoutsos, I.; Nelson, P.T. The expression of microRNA miR-107 decreases early in Alzheimer’s disease and may accelerate disease progression through regulation of β-site amyloid precursor protein-cleaving enzyme 1. J. Neurosci. 2008, 28, 1213–1223. [Google Scholar] [CrossRef] [PubMed]
- Boissonneault, V.; Plante, I.; Rivest, S.; Provost, P. MicroRNA-298 and microRNA-328 regulate expression of mouse β-amyloid precursor protein-converting enzyme 1. J. Biol. Chem. 2009, 284, 1971–1981. [Google Scholar] [CrossRef]
- Schonrock, N.; Ke, Y.D.; Humphreys, D.; Staufenbiel, M.; Ittner, L.M.; Preiss, T.; Götz, J. Neuronal microrna deregulation in response to Alzheimer’s disease amyloid-β. PLoS ONE 2010, 5, e11070. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, J.; Zong, Y.; Xu, Y.; Deng, W.; Zhu, H.; Liu, Y.; Ma, C.; Huang, L.; Zhang, L.; et al. MiR-106b aberrantly expressed in a double transgenic mouse model for Alzheimer’s disease targets TGF-β type II receptor. Brain Res. 2010, 1357, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Hennessey, T.; Flynt, A.; Lai, E.; Flint Beal, M.; Lin, M.T. MicroRNA-Related Cofilin Abnormality in Alzheimer’s Disease. PLoS ONE 2010, 5, e15546. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Cui, J.G.; Hill, J.M.; Bhattacharjee, S.; Zhao, Y.; Lukiw, W.J. Increased expression of miRNA-146a in Alzheimer’s disease transgenic mouse models. Neurosci. Lett. 2011, 487, 94–98. [Google Scholar] [CrossRef][Green Version]
- Zhu, H.C.; Wang, L.M.; Wang, M.; Song, B.; Tan, S.; Teng, J.F.; Duan, D.X. MicroRNA-195 downregulates Alzheimer’s disease amyloid-β production by targeting BACE1. Brain Res. Bull. 2012, 88, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.T.; Chu, K.; Jung, K.H.; Kim, J.H.; Huh, J.Y.; Yoon, H.; Park, D.K.; Lim, J.Y.; Kim, J.M.; Jeon, D.; et al. MiR-206 regulates brain-derived neurotrophic factor in Alzheimer disease model. Ann. Neurol. 2012, 72, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Zhu, H.; Xu, Y.; Huang, L.; Ma, C.; Deng, W.; Liu, Y.; Qin, C. MicroRNA-153 negatively regulates the expression of amyloid precursor protein and amyloid precursor-like protein 2. Brain Res. 2012, 1455, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Croce, N.; Gelfo, F.; Ciotti, M.T.; Federici, G.; Caltagirone, C.; Bernardini, S.; Angelucci, F. NPY modulates miR-30a-5p and BDNF in opposite direction in an in vitro model of Alzheimer disease: A possible role in neuroprotection? Mol. Cell. Biochem. 2013, 376, 189–195. [Google Scholar] [CrossRef]
- Cheng, C.; Li, W.; Zhang, Z.; Yoshimura, S.; Hao, Q.; Zhang, C.; Wang, Z. MicroRNA-144 is regulated by activator protein-1 (AP-1) and decreases expression of alzheimer disease-related a disintegrin and metalloprotease 10 (ADAM10). J. Biol. Chem. 2013, 288, 13748–13761. [Google Scholar] [CrossRef]
- Wong, H.K.A.; Veremeyko, T.; Patel, N.; Lemere, C.A.; Walsh, D.M.; Esau, C.; Vanderburg, C.; Krichevsky, A.M. De-repression of FOXO3a death axis by microRNA-132 and -212 causes neuronal apoptosis in Alzheimer’s disease. Hum. Mol. Genet. 2013, 22, 3077–3092. [Google Scholar] [CrossRef]
- Hu, Y.K.; Wang, X.; Li, L.; Du, Y.H.; Ye, H.T.; Li, C.Y. MicroRNA-98 induces an Alzheimer’s disease-like disturbance by targeting insulin-like growth factor 1. Neurosci. Bull. 2013, 29, 745–751. [Google Scholar] [CrossRef]
- Absalon, S.; Kochanek, D.M.; Raghavan, V.; Krichevsky, A.M. MiR-26b, upregulated in Alzheimer’s disease, activates cell cycle entry, Tau-phosphorylation, and apoptosis in postmitotic neurons. J. Neurosci. 2013, 33, 14645–14659. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, Q.; Niu, J.; Lu, K.; Xie, B.; Cui, D.; Xu, S. Screening of microRNAs associated with Alzheimer’s disease using oxidative stress cell model and different strains of senescence accelerated mice. J. Neurol. Sci. 2014, 338, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hu, M.; Teng, Z.; Tang, Y.P.; Chen, C. Synaptic and cognitive improvements by inhibition of 2-AG metabolism are through upregulation of microRNA-188-3p in a mouse model of Alzheimer’s disease. J. Neurosci. 2014, 34, 14919–14933. [Google Scholar] [CrossRef] [PubMed]
- Tian, N.; Cao, Z.; Zhang, Y. MiR-206 decreases brain-derived neurotrophic factor levels in a transgenic mouse model of Alzheimer’s disease. Neurosci. Bull. 2014, 30, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Wu, Q.; Ye, X.; Xiong, Y.; Zhu, J.; Xu, J.; Diao, Y.; Zhang, D.; Wang, M.; Qiu, J.; et al. Genome-wide analysis of miRNA signature in the APPswe/PS1ΔE9 mouse model of Alzheimer’s disease. PLoS ONE 2014, 9, e101725. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Ortiz, C.J.; Baglietto-Vargas, D.; Martinez-Coria, H.; Laferla, F.M.; Kitazawa, M. Upregulation of miR-181 decreases c-Fos and SIRT-1 in the hippocampus of 3xTg-AD mice. J. Alzheimer’s Dis. 2014, 42, 1229–1238. [Google Scholar] [CrossRef]
- Kong, Y.; Wu, J.; Yuan, L. MicroRNA Expression Analysis of Adult-Onset Drosophila Alzheimer’s Disease Model. Curr. Alzheimer Res. 2014, 11, 882–891. [Google Scholar] [CrossRef]
- Wang, X.; Tan, L.; Lu, Y.; Peng, J.; Zhu, Y.; Zhang, Y.; Sun, Z. MicroRNA-138 promotes tau phosphorylation by targeting retinoic acid receptor alpha. FEBS Lett. 2015, 589, 726–729. [Google Scholar] [CrossRef]
- Lei, X.; Lei, L.; Zhang, Z.; Zhang, Z.; Cheng, Y. Downregulated miR-29c correlates with increased BACE1 expression in sporadic Alzheimer’s disease. Int. J. Clin. Exp. Pathol. 2015, 8, 1565–1574. [Google Scholar]
- Zong, Y.; Yu, P.; Cheng, H.; Wang, H.; Wang, X.; Liang, C.; Zhu, H.; Qin, Y.; Qin, C. miR-29c regulates NAV3 protein expression in a transgenic mouse model of Alzheimer’s disease. Brain Res. 2015, 1624, 95–102. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Y.; Zhu, H.; Ma, C.; Dai, X.; Qin, C. Downregulated microRNA-222 is correlated with increased p27Kip1 expression in a double transgenic mouse model of Alzheimer’s disease. Mol. Med. Rep. 2015, 12, 7687–7692. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Wu, J.; Zhang, D.; Wan, C.; Yuan, L. The Role of miR-124 in Drosophila Alzheimer’s Disease Model by Targeting Delta in Notch Signaling Pathway. Curr. Mol. Med. 2015, 15, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chu, W.; Gong, L.; Gao, X.; Wang, W. MicroRNA-26b is upregulated in a double transgenic mouse model of Alzheimer’s disease and promotes the expression of amyloid-β by targeting insulin-like growth factor 1. Mol. Med. Rep. 2016, 13, 2809–2814. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhang, R.; Lu, K.; Yu, W.; Xie, B.; Cui, D.; Jiang, L.; Zhang, Q.; Xu, S. Deregulation of miRNA-181c potentially contributes to the pathogenesis of AD by targeting collapsin response mediator protein 2 in mice. J. Neurol. Sci. 2016, 367, 3–10. [Google Scholar] [CrossRef]
- Zhang, Y.; Lv, X.; Liu, C.; Gao, S.; Ping, H.; Wang, J.; Wang, P. MiR-214-3p attenuates cognition defects via the inhibition of autophagy in SAMP8 mouse model of sporadic Alzheimer’s disease. Neurotoxicology 2016, 56, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Micheli, F.; Palermo, R.; Talora, C.; Ferretti, E.; Vacca, A.; Napolitano, M. Regulation of proapoptotic proteins Bak1 and p53 by miR-125b in an experimental model of Alzheimer’s disease: Protective role of 17β-estradiol. Neurosci. Lett. 2016, 629, 234–240. [Google Scholar] [CrossRef]
- Sarkar, S.; Jun, S.; Rellick, S.; Quintana, D.D.; Cavendish, J.Z.; Simpkins, J.W. Expression of microRNA-34a in Alzheimer’s disease brain targets genes linked to synaptic plasticity, energy metabolism, and resting state network activity. Brain Res. 2016, 1646, 139–151. [Google Scholar] [CrossRef]
- Li, S.; Yan, Y.; Jiao, Y.; Gao, Z.; Xia, Y.; Kong, L.; Yao, Y.; Tao, Z.; Song, J.; Yan, Y.; et al. Neuroprotective Effect of Osthole on Neuron Synapses in an Alzheimer’s Disease Cell Model via Upregulation of MicroRNA-9. J. Mol. Neurosci. 2016, 60, 71–81. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.; Wang, L.; Zhang, Y.; Chen, L. miR-98-5p Acts as a Target for Alzheimer’s Disease by Regulating Aβ Production through Modulating SNX6 Expression. J. Mol. Neurosci. 2016, 60, 413–420. [Google Scholar] [CrossRef]
- Li, W.; Li, X.; Xin, X.; Kan, P.C.; Yan, Y. MicroRNA-613 regulates the expression of brain-derived neurotrophic factor in Alzheimer’s disease. Biosci. Trends 2016, 10, 372–377. [Google Scholar] [CrossRef]
- Zhang, R.; Zhou, H.; Jiang, L.; Mao, Y.; Cui, X.; Xie, B.; Cui, D.; Wang, H.; Zhang, Q.; Xu, S. MiR-195 dependent roles of mitofusin2 in the mitochondrial dysfunction of hippocampal neurons in SAMP8 mice. Brain Res. 2016, 1652, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Bao, J.S.; Su, J.H.; Huang, W. MicroRNA-139 modulates Alzheimer’s-associated pathogenesis in SAMP8 mice by targeting cannabinoid receptor type 2. Genet. Mol. Res. 2017, 16. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Li, Z.; Zhao, L.; Zhao, W. Simvastatin ameliorate memory deficits and inflammation in clinical and mouse model of Alzheimer’s disease via modulating the expression of miR-106b. Biomed. Pharmacother. 2017, 92, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Li, L.; Cui, C.; Zhao, Z.; Song, G. Overexpression of let-7a increases neurotoxicity in a PC12 cell model of Alzheimer’s disease via regulating autophagy. Exp. Ther. Med. 2017, 14, 3688–3698. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Min, L.; Guo, Q.D.; Zhang, J.X.; Jiang, H.L.; Shao, S.; Xing, J.G.; Yin, L.L.; Liu, J.H.; Liu, R.; et al. Profiling MicroRNA from Brain by Microarray in a Transgenic Mouse Model of Alzheimer’s Disease. Biomed. Res. Int. 2017, 2017, 8030369. [Google Scholar] [CrossRef]
- Zhang, Q.S.; Liu, W.; Lu, G.X. miR-200a-3p promotes β-Amyloid-induced neuronal apoptosis through down-regulation of SIRT1 in Alzheimer’s disease. J. Biosci. 2017, 42, 397–404. [Google Scholar] [CrossRef]
- Wang, X.; Liu, D.; Huang, H.Z.; Wang, Z.H.; Hou, T.Y.; Yang, X.; Pang, P.; Wei, N.; Zhou, Y.F.; Dupras, M.J.; et al. A Novel MicroRNA-124/PTPN1 Signal Pathway Mediates Synaptic and Memory Deficits in Alzheimer’s Disease. Biol. Psychiatry 2018, 83, 395–405. [Google Scholar] [CrossRef]
- Manzine, P.R.; Pelucchi, S.; Horst, M.A.; Vale, F.A.C.; Pavarini, S.C.I.; Audano, M.; Mitro, N.; Di Luca, M.; Marcello, E.; Cominetti, M.R. MicroRNA 221 Targets ADAM10 mRNA and Is Downregulated in Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 61, 113–123. [Google Scholar] [CrossRef]
- Shi, R.; Zhang, S.; Cheng, G.; Yang, X.; Zhao, N.; Chen, C. Ginsenoside Rg1 and Acori Graminei Rhizoma Attenuates Neuron Cell Apoptosis by Promoting the Expression of miR-873-5p in Alzheimer’s Disease. Neurochem. Res. 2018, 43, 1529–1538. [Google Scholar] [CrossRef]
- Jin, Y.; Tu, Q.; Liu, M. MicroRNA-125b regulates Alzheimer’s disease through SphK1 regulation. Mol. Med. Rep. 2018, 18, 2373–2380. [Google Scholar] [CrossRef]
- Wu, B.W.; Wu, M.S.; Guo, J.D. Effects of microRNA-10a on synapse remodeling in hippocampal neurons and neuronal cell proliferation and apoptosis through the BDNF-TrkB signaling pathway in a rat model of Alzheimer’s disease. J. Cell. Physiol. 2018, 233, 5281–5292. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zhang, R.; Yuan, Q.; Gao, Y.; Yang, M.Q.; Zhang, C.; Huang, J.; Sun, Y.; Yang, W.; Yang, J.Y.; et al. The emerging role of microRNA-4487/6845-3p in Alzheimer’s disease pathologies is induced by Aβ25-35 triggered in SH-SY5Y cell. BMC Syst. Biol. 2018, 12, 119. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, W.; Yi, Y.; Tong, Q. miR-219-5p inhibits tau phosphorylation by targeting TTBK1 and GSK-3β in Alzheimer’s disease. J. Cell. Biochem. 2019, 120, 9936–9946. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Zhou, Y.; Zhang, R.; Wu, K.; Lu, Y.; Li, Y.; Duan, R.; Yao, Y.; Zhu, D.; Jia, Y. MicroRNA let-7f-5p promotes bone marrow mesenchymal stem cells survival by targeting caspase-3 in Alzheimer disease model. Front. Neurosci. 2018, 12, 333. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Z.F.; Li, W.; Hong, H.; Chen, J.; Tian, Y.; Liu, Z.Y. Protective effects of microRNA-330 on amyloid β-protein production, oxidative stress, and mitochondrial dysfunction in Alzheimer’s disease by targeting VAV1 via the MAPK signaling pathway. J. Cell. Biochem. 2018, 119, 5437–5448. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Liang, X.; Yao, Y.; Xiao, H.; Shi, Y.; Yang, J. Osthole attenuates APP-induced Alzheimer’s disease through up-regulating miRNA-101a-3p. Life Sci. 2019, 225, 117–131. [Google Scholar] [CrossRef]
- Cosín-Tomás, M.; Álvarez-López, M.J.; Companys-Alemany, J.; Kaliman, P.; González-Castillo, C.; Ortuño-Sahagún, D.; Pallàs, M.; Griñán-Ferré, C. Temporal integrative analysis of mRNA and microRNAs expression profiles and epigenetic alterations in female SAMP8, a model of age-related cognitive decline. Front. Genet. 2018, 9, 596. [Google Scholar] [CrossRef]
- Song, Y.; Hu, M.; Zhang, J.; Teng, Z.; Chen, C. A novel mechanism of synaptic and cognitive impairments mediated via microRNA-30b in Alzheimer’s disease. EBioMedicine 2019, 39, 409–421. [Google Scholar] [CrossRef]
- Yang, Q.; Zhao, Q.; Yin, Y. miR-133b is a potential diagnostic biomarker for Alzheimer’s disease and has a neuroprotective role. Exp. Ther. Med. 2019, 18, 2711–2718. [Google Scholar] [CrossRef]
- Baby, N.; Alagappan, N.; Dheen, S.T.; Sajikumar, S. MicroRNA-134-5p inhibition rescues long-term plasticity and synaptic tagging/capture in an Aβ(1–42)-induced model of Alzheimer’s disease. Aging Cell 2020, 19, e13046. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Liu, P.; Bai, H.; Li, X.; Xiao, J.; Yuan, Q.; Geng, S.; Yin, H.; Zhang, H.; et al. MicroRNA-128 knockout inhibits the development of Alzheimer’s disease by targeting PPARγ in mouse models. Eur. J. Pharmacol. 2019, 843, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.Z.; Zhao, Y.; Chen, H.Z. MicroRNA-98 reduces amyloid β-protein production and improves oxidative stress and mitochondrial dysfunction through the Notch signaling pathway via HEY2 in Alzheimer’s disease mice. Int. J. Mol. Med. 2019, 43, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Qin, L.; Tang, B. MicroRNAs in Alzheimer’s disease. Front. Genet. 2019, 10, 153. [Google Scholar] [CrossRef] [PubMed]
- Lukiw, W.J. Micro-RNA speciation in fetal, adult and Alzheimer’s disease hippocampus. Neuroreport 2007, 18, 297–300. [Google Scholar] [CrossRef]
- Shioya, M.; Obayashi, S.; Tabunoki, H.; Arima, K.; Saito, Y.; Ishida, T.; Satoh, J. Aberrant microRNA expression in the brains of neurodegenerative diseases: MiR-29a decreased in Alzheimer disease brains targets neurone navigator 3. Neuropathol. Appl. Neurobiol. 2010, 36, 320–330. [Google Scholar] [CrossRef]
- Bekris, L.M.; Lutz, F.; Montine, T.J.; Yu, C.E.; Tsuang, D.; Peskind, E.R.; Leverenz, J.B. MicroRNA in Alzheimer’s disease: An exploratory study in brain, cerebrospinal fluid and plasma. Biomarkers 2013, 18, 455–466. [Google Scholar] [CrossRef]
- Müller, M.; Kuiperij, H.B.; Claassen, J.A.; Küsters, B.; Verbeek, M.M. MicroRNAs in Alzheimer’s disease: Differential expression in hippocampus and cell-free cerebrospinal fluid. Neurobiol. Aging 2014, 35, 152–158. [Google Scholar] [CrossRef]
- Mezache, L.; Mikhail, M.; Garofalo, M.; Nuovo, G.J. Reduced miR-512 and the elevated expression of its targets cFLIP and MCL1 localize to neurons with hyperphosphorylated tau protein in Alzheimer disease. Appl. Immunohistochem. Mol. Morphol. 2015, 23, 615–623. [Google Scholar] [CrossRef]
- Zhang, C.; Lu, J.; Liu, B.; Cui, Q.; Wang, Y. Primate-specific miR-603 is implicated in the risk and pathogenesis of Alzheimer’s disease. Aging (Albany NY) 2016, 8, 272–290. [Google Scholar] [CrossRef][Green Version]
- Moncini, S.; Lunghi, M.; Valmadre, A.; Grasso, M.; Del Vescovo, V.; Riva, P.; Denti, M.A.; Venturin, M. The miR-15/107 Family of MicroRNA Genes Regulates CDK5R1/p35 with Implications for Alzheimer’s Disease Pathogenesis. Mol. Neurobiol. 2017, 54, 4329–4342. [Google Scholar] [CrossRef]
- Pichler, S.; Gu, W.; Hartl, D.; Gasparoni, G.; Leidinger, P.; Keller, A.; Meese, E.; Mayhaus, M.; Hampel, H.; Riemenschneider, M. The miRNome of Alzheimer’s disease: Consistent downregulation of the miR-132/212 cluster. Neurobiol. Aging 2017, 50, 167.e1–167.e10. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Vijayan, M.; Reddy, P.H. MicroRNA-455-3p as a potential peripheral biomarker for Alzheimer’s disease. Hum. Mol. Genet. 2017, 26, 3808–3822. [Google Scholar] [CrossRef]
- Akhter, R.; Shao, Y.; Shaw, M.; Formica, S.; Khrestian, M.; Leverenz, J.B.; Bekris, L.M. Regulation of ADAM10 by miR-140-5p and potential relevance for Alzheimer’s disease. Neurobiol. Aging 2018, 63, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Reddy, P.H. MicroRNA-455-3p as a potential biomarker for Alzheimer’s disease: An update. Front. Aging Neurosci. 2018, 10, 41. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wu, Y.; Gu, M.; Zhang, Y. MiR-342-5p decreases ankyrin G levels in Alzheimer’s disease transgenic mouse models. Cell Rep. 2014, 6, 264–270. [Google Scholar] [CrossRef]
- Garza-Manero, S.; Arias, C.; Bermúdez-Rattoni, F.; Vaca, L.; Zepeda, A. Identification of age- and disease-related alterations in circulating miRNAS in a mouse model of Alzheimer’s disease. Front. Cell. Neurosci. 2015, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Ye, X.; Xiong, Y.; Zhu, H.; Miao, J.; Zhang, W.; Wan, J. The protective role of microRNA-200c in Alzheimer’s disease pathologies is induced by beta amyloid-triggered endoplasmic reticulum stress. Front. Mol. Neurosci. 2016, 9, 140. [Google Scholar] [CrossRef]
- Zhang, Y.; Xing, H.; Guo, S.; Zheng, Z.; Wang, H.; Xu, D. MicroRNA-135b has a neuroprotective role via targeting of β-site APP-cleaving enzyme 1. Exp. Ther. Med. 2016, 12, 809–814. [Google Scholar] [CrossRef]
- Hong, H.; Li, Y.; Su, B. Identification of circulating miR-125b as a potential biomarker of Alzheimer’s disease in APP/PS1 transgenic mouse. J. Alzheimer’s Dis. 2017, 59, 1449–1458. [Google Scholar] [CrossRef]
- Wang, L.; Liu, J.; Wang, Q.; Jiang, H.; Zeng, L.; Li, Z.; Liu, R. MicroRNA-200a-3p Mediates Neuroprotection in Alzheimer-Related Deficits and Attenuates Amyloid-Beta Overproduction and Tau Hyperphosphorylation via Coregulating BACE1 and PRKACB. Front. Pharmacol. 2019, 10, 806. [Google Scholar] [CrossRef]
- Schipper, H.M.; Maes, O.C.; Chertkow, H.M.; Wang, E. MicroRNA Expression in Alzheimer Blood Mononuclear Cells. Gene Regul. Syst. Biol. 2007, 1, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Leidinger, P.; Backes, C.; Deutscher, S.; Schmitt, K.; Mueller, S.C.; Frese, K.; Haas, J.; Ruprecht, K.; Paul, F.; Stähler, C.; et al. A blood based 12-miRNA signature of Alzheimer disease patients. Genome Biol. 2013, 14, R78. [Google Scholar] [CrossRef] [PubMed]
- Frigerio, C.S.; Lau, P.; Salta, E.; Tournoy, J.; Bossers, K.; Vandenberghe, R.; Wallin, A.; Bjerke, M.; Zetterberg, H.; Blennow, K.; et al. Reduced expression of hsa-miR-27a-3p in CSF of patients with Alzheimer disease. Neurology 2013, 81, 2103–2106. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Doecke, J.D.; Sharples, R.A.; Villemagne, V.L.; Fowler, C.J.; Rembach, A.; Martins, R.N.; Rowe, C.C.; Macaulay, S.L.; Masters, C.L.; et al. Prognostic serum miRNA biomarkers associated with Alzheimer’s disease shows concordance with neuropsychological and neuroimaging assessment. Mol. Psychiatry 2015, 20, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, S.; Chertkow, H.; Schipper, H.M.; Yuan, Z.; Shetty, V.; Jenkins, S.; Jones, T.; Wang, E. Increased microRNA-34c abundance in Alzheimer’s disease circulating blood plasma. Front. Mol. Neurosci. 2014, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Tiribuzi, R.; Crispoltoni, L.; Porcellati, S.; Di Lullo, M.; Florenzano, F.; Pirro, M.; Bagaglia, F.; Kawarai, T.; Zampolini, M.; Orlacchio, A.; et al. miR128 up-regulation correlates with impaired amyloid β(1–42) degradation in monocytes from patients with sporadic Alzheimer’s disease. Neurobiol. Aging 2014, 35, 345–356. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, A.; Xia, C.; Lin, Q.; Chen, C. A single nucleotide polymorphism in primary-microRNA-146a reduces the expression of mature microRNA-146a in patients with Alzheimer’s disease and is associated with the pathogenesis of Alzheimer’s disease. Mol. Med. Rep. 2015, 12, 4037–4042. [Google Scholar] [CrossRef]
- Van Harten, A.C.; Mulders, J.; Scheltens, P.; Van Der Flier, W.M.; Oudejans, C.B.M. Differential Expression of MicroRNA in Cerebrospinal Fluid as a Potential Novel Biomarker for Alzheimer’s Disease. J. Alzheimer’s Dis. 2015, 47, 243–252. [Google Scholar] [CrossRef]
- Ren, R.J.; Zhang, Y.F.; Dammer, E.B.; Zhou, Y.; Wang, L.; Liu, X.H.; Feng, B.L.; Jiang, G.X.; Chen, S.D.; Wang, G.; et al. Peripheral Blood MicroRNA Expression Profiles in Alzheimer’s Disease: Screening, Validation, Association with Clinical Phenotype and Implications for Molecular Mechanism. Mol. Neurobiol. 2016, 53, 5772–5781. [Google Scholar] [CrossRef]
- Gui, Y.X.; Liu, H.; Zhang, L.S.; Lv, W.; Hu, X.Y. Altered microRNA profiles in cerebrospinal fluid exosome in Parkinson disease and Alzheimer disease. Oncotarget 2015, 6, 37043–37053. [Google Scholar] [CrossRef]
- Dangla-Valls, A.; Molinuevo, J.L.; Altirriba, J.; Sánchez-Valle, R.; Alcolea, D.; Fortea, J.; Rami, L.; Balasa, M.; Muñoz-García, C.; Ezquerra, M.; et al. CSF MicroRNA Profiling in Alzheimer’s Disease: A Screening and Validation Study. Mol. Neurobiol. 2017, 54, 6647–6654. [Google Scholar] [CrossRef]
- Guedes, J.R.; Santana, I.; Cunha, C.; Duro, D.; Almeida, M.R.; Cardoso, A.M.; de Lima, M.C.P.; Cardoso, A.L. MicroRNA deregulation and chemotaxis and phagocytosis impairment in Alzheimer’s disease. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2016, 3, 7–17. [Google Scholar] [CrossRef]
- Yllmaz, Ş.G.; Erdal, M.E.; Özge, A.A.; Sungur, M.A. Can Peripheral MicroRNA Expression Data Serve as Epigenomic (Upstream) Biomarkers of Alzheimer’s Disease? Omics J. Integr. Biol. 2016, 20, 456–461. [Google Scholar] [CrossRef]
- Müller, M.; Jäkel, L.; Bruinsma, I.B.; Claassen, J.A.; Kuiperij, H.B.; Verbeek, M.M. MicroRNA-29a Is a Candidate Biomarker for Alzheimer’s Disease in Cell-Free Cerebrospinal Fluid. Mol. Neurobiol. 2016, 53, 2894–2899. [Google Scholar] [CrossRef] [PubMed]
- Bao, T.; Miao, W.; Han, J.; Yin, M.; Yan, Y.; Wang, W.; Zhu, Y. Spontaneous Running Wheel Improves Cognitive Functions of Mouse Associated with miRNA Expressional Alteration in Hippocampus Following Traumatic Brain Injury. J. Mol. Neurosci. 2014, 54, 622–629. [Google Scholar] [CrossRef]
- Hu, T.; Zhou, F.J.; Chang, Y.F.; Li, Y.S.; Liu, G.C.; Hong, Y.; Chen, H.L.; Xiyang, Y.B.; Bao, T.H. miR21 is Associated with the Cognitive Improvement Following Voluntary Running Wheel Exercise in TBI Mice. J. Mol. Neurosci. 2015, 57, 114–122. [Google Scholar] [CrossRef]
- Kou, X.; Li, J.; Liu, X.; Chang, J.; Zhao, Q.; Jia, S.; Fan, J.; Chen, N. Swimming attenuates D-galactose-induced brain aging via suppressing miR-34a-mediated autophagy impairment and abnormal mitochondrial dynamics. J. Appl. Physiol. 2017, 122, 1462–1469. [Google Scholar] [CrossRef]
- Fernandes, J.; Vieira, A.S.; Kramer-Soares, J.C.; Da Silva, E.A.; Lee, K.S.; Lopes-Cendes, I.; Arida, R.M. Hippocampal microRNA-mRNA regulatory network is affected by physical exercise. Biochim. Biophys. Acta —Gen. Subj. 2018, 1862, 1711–1720. [Google Scholar] [CrossRef]
- Mohammadipoor-Ghasemabad, L.; Sangtarash, M.H.; Esmaeili-Mahani, S.; Sheibani, V.; Sasan, H.A. Abnormal hippocampal miR-1b expression is ameliorated by regular treadmill exercise in the sleep-deprived female rats. Iran. J. Basic Med. Sci. 2019, 22, 485–490. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, A.; Wang, Y.; Hu, S.; Zhang, R.; Qian, S. Genome-wide identification of brain miRNAs in response to high-intensity intermittent swimming training in Rattus norvegicus by deep sequencing. BMC Mol. Biol. 2019, 20, 3. [Google Scholar] [CrossRef]
- Pons-Espinal, M.; Gasperini, C.; Marzi, M.J.; Braccia, C.; Armirotti, A.; Pötzsch, A.; Walker, T.L.; Fabel, K.; Nicassio, F.; Kempermann, G.; et al. MiR-135a-5p Is Critical for Exercise-Induced Adult Neurogenesis. Stem Cell Rep. 2019, 12, 1298–1312. [Google Scholar] [CrossRef]
- Radom-Aizik, S.; Zaldivar, F.; Oliver, S.; Galassetti, P.; Cooper, D.M. Evidence for microRNA involvement in exercise-associated neutrophil gene expression changes. J. Appl. Physiol. 2010, 109, 252–261. [Google Scholar] [CrossRef]
- Aoi, W.; Ichikawa, H.; Mune, K.; Tanimura, Y.; Mizushima, K.; Naito, Y.; Yoshikawa, T. Muscle-enriched microRNA miR-486 decreases in circulation in response to exercise in young men. Front. Physiol. 2013, 4, 80. [Google Scholar] [CrossRef]
- Radom-Aizik, S.; Zaldivar, F.; Haddad, F.; Cooper, D.M. Impact of brief exercise on peripheral blood NK cell gene and microRNA expression in young adults. J. Appl. Physiol. 2013, 114, 628–636. [Google Scholar] [CrossRef]
- Banzet, S.; Chennaoui, M.; Girard, O.; Racinais, S.; Drogou, C.; Chalabi, H.; Koulmann, N. Changes in circulating microRNAs levels with exercise modality. J. Appl. Physiol. 2013, 115, 1237–1244. [Google Scholar] [CrossRef]
- Uhlemann, M.; Möbius-Winkler, S.; Fikenzer, S.; Adam, J.; Redlich, M.; Möhlenkamp, S.; Hilberg, T.; Schuler, G.C.; Adams, V. Circulating microRNA-126 increases after different forms of endurance exercise in healthy adults. Eur. J. Prev. Cardiol. 2014, 21, 484–491. [Google Scholar] [CrossRef]
- Mooren, F.C.; Viereck, J.; Krüger, K.; Thum, T. Circulating microRNAs as potential biomarkers of aerobic exercise capacity. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H557–H563. [Google Scholar] [CrossRef]
- Gomes, C.P.C.; Oliveira-Jr, G.P.; Madrid, B.; Almeida, J.A.; Franco, O.L.; Pereira, R.W. Circulating miR-1, miR-133a, and miR-206 levels are increased after a half-marathon run. Biomarkers 2014, 19, 585–589. [Google Scholar] [CrossRef]
- Radom-Aizik, S.; Zaldivar, F.P.; Haddad, F.; Cooper, D.M. Impact of brief exercise on circulating monocyte gene and microRNA expression: Implications for atherosclerotic vascular disease. Brain. Behav. Immun. 2014, 39, 121–129. [Google Scholar] [CrossRef]
- Zhang, T.; Birbrair, A.; Wang, Z.-M.; Messi, M.L.; Marsh, A.P.; Leng, I.; Nicklas, B.J.; Delbono, O. Improved knee extensor strength with resistance training associates with muscle specific miRNAs in older adults. Exp. Gerontol. 2015, 62, 7–13. [Google Scholar] [CrossRef]
- Dias, R.G.; Silva, M.S.M.; Duarte, N.E.; Bolani, W.; Alves, C.R.; Lemos, J.R., Jr.; da Silva, J.L.; de Oliveira, P.A.; Alves, G.B.; de Oliveira, E.M.; et al. PBMCs express a transcriptome signature predictor of oxygen uptake responsiveness to endurance exercise training in men. Physiol. Genom. 2015, 47, 13–23. [Google Scholar] [CrossRef]
- Liu, X.; Xiao, J.; Zhu, H.; Wei, X.; Platt, C.; Damilano, F.; Xiao, C.; Bezzerides, V.; Boström, P.; Che, L.; et al. miR-222 is necessary for exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell Metab. 2015, 21, 584–595. [Google Scholar] [CrossRef]
- Clauss, S.; Wakili, R.; Hildebrand, B.; Kääb, S.; Hoster, E.; Klier, I.; Martens, E.; Hanley, A.; Hanssen, H.; Halle, M.; et al. MicroRNAs as Biomarkers for Acute Atrial Remodeling in Marathon Runners (The miRathon Study—A Sub-Study of the Munich Marathon Study). PLoS ONE 2016, 11, e0148599. [Google Scholar] [CrossRef]
- Min, P.-K.; Park, J.; Isaacs, S.; Taylor, B.A.; Thompson, P.D.; Troyanos, C.; D’Hemecourt, P.; Dyer, S.; Chan, S.Y.; Baggish, A.L. Influence of statins on distinct circulating microRNAs during prolonged aerobic exercise. J. Appl. Physiol. 2016, 120, 711–720. [Google Scholar] [CrossRef]
- Cui, S.F.; Wang, C.; Yin, X.; Tian, D.; Lu, Q.J.; Zhang, C.Y.; Chen, X.; Ma, J.Z. Similar Responses of Circulating MicroRNAs to Acute High-Intensity Interval Exercise and Vigorous-Intensity Continuous Exercise. Front. Physiol. 2016, 7, 102. [Google Scholar] [CrossRef]
- Margolis, L.M.; Rivas, D.A.; Berrone, M.; Ezzyat, Y.; Young, A.J.; McClung, J.P.; Fielding, R.A.; Pasiakos, S.M. Prolonged Calorie Restriction Downregulates Skeletal Muscle mTORC1 Signaling Independent of Dietary Protein Intake and Associated MicroRNA Expression. Front. Physiol. 2016, 7, 445. [Google Scholar] [CrossRef]
- Denham, J.; Prestes, P.R. Muscle-Enriched MicroRNAs Isolated from Whole Blood Are Regulated by Exercise and Are Potential Biomarkers of Cardiorespiratory Fitness. Front. Genet. 2016, 7, 196. [Google Scholar] [CrossRef]
- Gomes, J.L.; Fernandes, T.; Soci, U.P.; Silveira, A.C.; Barretti, D.L.; Negrão, C.E.; Oliveira, E.M. Obesity Downregulates MicroRNA-126 Inducing Capillary Rarefaction in Skeletal Muscle: Effects of Aerobic Exercise Training. Oxid. Med. Cell. Longev. 2017, 2017, 2415246. [Google Scholar] [CrossRef]
- Zhang, T.; Brinkley, T.E.; Liu, K.; Feng, X.; Marsh, A.P.; Kritchevsky, S.; Zhou, X.; Nicklas, B.J. Circulating MiRNAs as biomarkers of gait speed responses to aerobic exercise training in obese older adults. Aging (Albany NY) 2017, 9, 900–913. [Google Scholar] [CrossRef]
- Horak, M.; Zlamal, F.; Iliev, R.; Kucera, J.; Cacek, J.; Svobodova, L.; Hlavonova, Z.; Kalina, T.; Slaby, O.; Bienertova-Vasku, J. Exercise-induced circulating microRNA changes in athletes in various training scenarios. PLoS ONE 2018, 13, e0191060. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).