Embryo–Maternal Interactions Underlying Reproduction in Mammals
Abstract
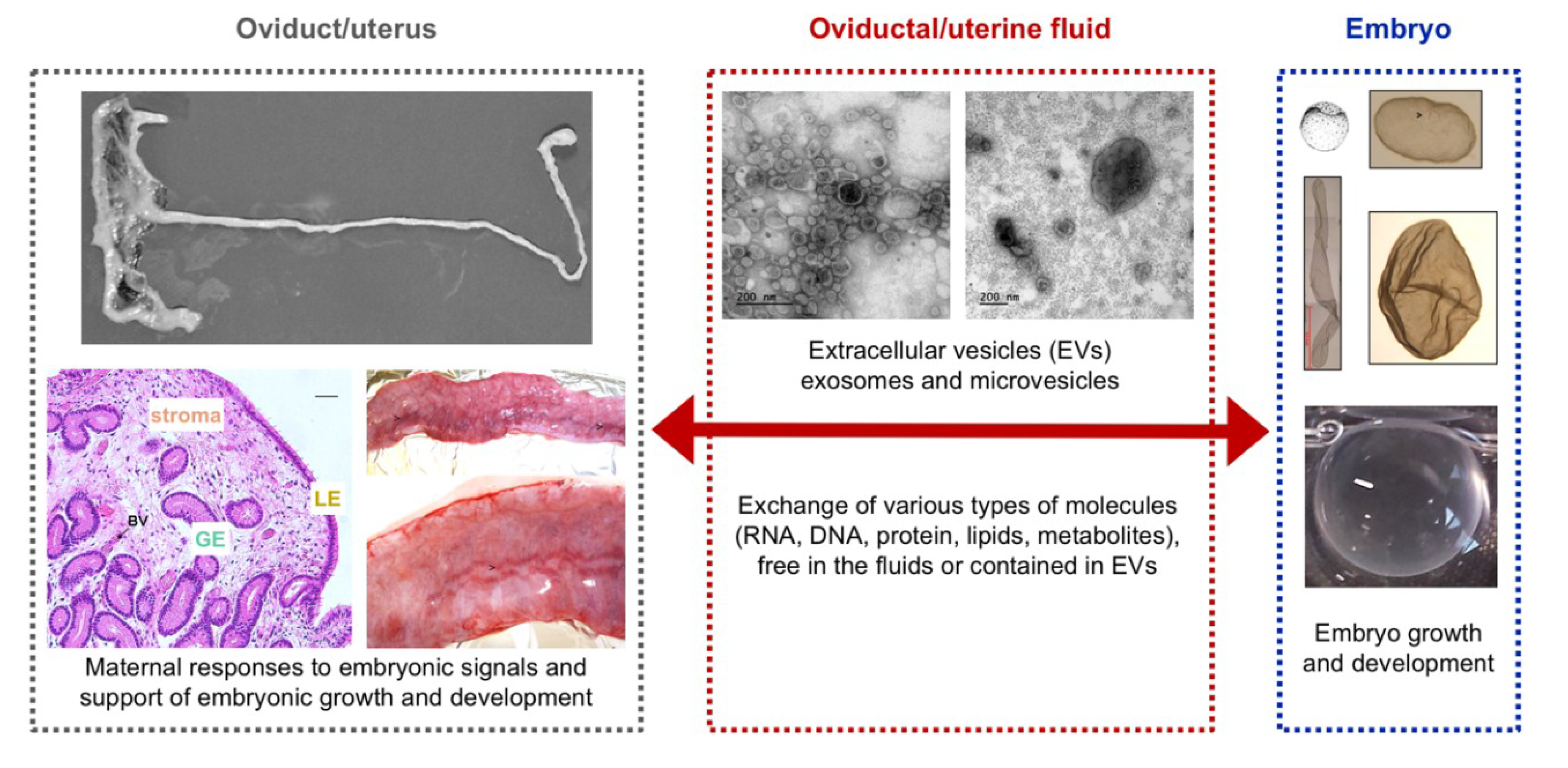
1. Introduction
2. The Oviduct
3. The Uterus
4. The Embryo
5. The Extracellular Vesicles
6. The Impact of the Diseases and Stress on the Establishment of Pregnancy.
7. Conclusions
Funding
Conflicts of Interest
References
- Bauersachs, S.; Wolf, E. Uterine responses to the preattachment embryo in domestic ungulates: Recognition of pregnancy and preparation for implantation. Ann. Rev. Anim. Biosci. 2015, 3, 489–511. [Google Scholar] [CrossRef]
- Rizos, D.; Maillo, V.; Sanchez-Calabuig, M.J.; Lonergan, P. The Consequences of Maternal-Embryonic Cross Talk During the Periconception Period on Subsequent Embryonic Development. Adv. Exp. Med. Biol. 2017, 1014, 69–86. [Google Scholar] [CrossRef]
- Sanchez, J.M.; Mathew, D.J.; Passaro, C.; Fair, T.; Lonergan, P. Embryonic maternal interaction in cattle and its relationship with fertility. Reprod. Domest. Anim. 2018, 53 (Suppl 2), 20–27. [Google Scholar] [CrossRef] [PubMed]
- Spencer, T.E.; Hansen, T.R. Implantation and Establishment of Pregnancy in Ruminants. Adv. Anat. Embryol. Cell Biol. 2015, 216, 105–135. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Bernal, M.A.; Fazleabas, A.T. Physiologic Events of Embryo Implantation and Decidualization in Human and Non-Human Primates. Int. J. Mol. Sci. 2020, 21, 1973. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, H.; Ono, M.; Sato, Y.; Imakawa, K.; Iizuka, T.; Kagami, K.; Fujiwara, T.; Horie, A.; Tani, H.; Hattori, A.; et al. Promoting Roles of Embryonic Signals in Embryo Implantation and Placentation in Cooperation with Endocrine and Immune Systems. Int. J. Mol. Sci. 2020, 21, 1885. [Google Scholar] [CrossRef] [PubMed]
- Bridi, A.; Perecin, F.; Silveira, J.C.d. Extracellular Vesicles Mediated Early Embryo–Maternal Interactions. Int. J. Mol. Sci. 2020, 21, 1163. [Google Scholar] [CrossRef]
- Alminana, C.; Bauersachs, S. Extracellular vesicles: Multi-signal messengers in the gametes/embryo-oviduct cross-talk. Theriogenology 2020. [Google Scholar] [CrossRef]
- Alminana, C.; Bauersachs, S. Extracellular Vesicles in the Oviduct: Progress, Challenges and Implications for the Reproductive Success. Bioengineering (Basel) 2019, 6, 32. [Google Scholar] [CrossRef]
- Saint-Dizier, M.; Schoen, J.; Chen, S.; Banliat, C.; Mermillod, P. Composing the Early Embryonic Microenvironment: Physiology and Regulation of Oviductal Secretions. Int. J. Mol. Sci. 2020, 21, 223. [Google Scholar] [CrossRef]
- Banliat, C.; Tsikis, G.; Labas, V.; Teixeira-Gomes, A.-P.; Com, E.; Lavigne, R.; Pineau, C.; Guyonnet, B.; Mermillod, P.; Saint-Dizier, M. Identification of 56 Proteins Involved in Embryo–Maternal Interactions in the Bovine Oviduct. Int. J. Mol. Sci. 2020, 21, 466. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Alonso, B.; Maillo, V.; Acuña, O.S.; López-Úbeda, R.; Torrecillas, A.; Simintiras, C.A.; Sturmey, R.; Avilés, M.; Lonergan, P.; Rizos, D. Spatial and Pregnancy-Related Changes in the Protein, Amino Acid, and Carbohydrate Composition of Bovine Oviduct Fluid. Int. J. Mol. Sci. 2020, 21, 1681. [Google Scholar] [CrossRef] [PubMed]
- Canha-Gouveia, A.; Paradela, A.; Ramos-Fernández, A.; Prieto-Sánchez, M.T.; Sánchez-Ferrer, M.L.; Corrales, F.; Coy, P. Which Low-Abundance Proteins are Present in the Human Milieu of Gamete/Embryo Maternal Interaction? Int. J. Mol. Sci. 2019, 20, 5305. [Google Scholar] [CrossRef] [PubMed]
- Chiumia, D.; Hankele, A.-K.; Groebner, A.E.; Schulke, K.; Reichenbach, H.-D.; Giller, K.; Zakhartchenko, V.; Bauersachs, S.; Ulbrich, S.E. Vascular Endothelial Growth Factor A and VEGFR-1 Change during Preimplantation in Heifers. Int. J. Mol. Sci. 2020, 21, 544. [Google Scholar] [CrossRef]
- Chiumia, D.; Schulke, K.; Groebner, A.E.; Waldschmitt, N.; Reichenbach, H.-D.; Zakhartchenko, V.; Bauersachs, S.; Ulbrich, S.E. Initiation of Conceptus Elongation Coincides with an Endometrium Basic Fibroblast Growth Factor (FGF2) Protein Increase in Heifers. Int. J. Mol. Sci. 2020, 21, 1584. [Google Scholar] [CrossRef] [PubMed]
- Eozenou, C.; Lesage-Padilla, A.; Mauffré, V.; Healey, G.D.; Camous, S.; Bolifraud, P.; Giraud-Delville, C.; Vaiman, D.; Shimizu, T.; Miyamoto, A.; et al. FOXL2 is a Progesterone Target Gene in the Endometrium of Ruminants. Int. J. Mol. Sci. 2020, 21, 1478. [Google Scholar] [CrossRef]
- Kaczynski, P.; Bauersachs, S.; Baryla, M.; Goryszewska, E.; Muszak, J.; Grzegorzewski, W.J.; Waclawik, A. Estradiol-17β-Induced Changes in the Porcine Endometrial Transcriptome In Vivo. Int. J. Mol. Sci. 2020, 21, 890. [Google Scholar] [CrossRef]
- Gibson, C.; de Ruijter-Villani, M.; Bauersachs, S.; Stout, T.A.E. Asynchronous Embryo Transfer Followed by Comparative Transcriptomic Analysis of Conceptus Membranes and Endometrium Identifies Processes Important to the Establishment of Equine Pregnancy. Int. J. Mol. Sci. 2020, 21, 2562. [Google Scholar] [CrossRef]
- Salilew-Wondim, D.; Gebremedhn, S.; Hoelker, M.; Tholen, E.; Hailay, T.; Tesfaye, D. The Role of MicroRNAs in Mammalian Fertility: From Gametogenesis to Embryo Implantation. Int. J. Mol. Sci. 2020, 21, 585. [Google Scholar] [CrossRef]
- Kaczmarek, M.M.; Najmula, J.; Guzewska, M.M.; Przygrodzka, E. MiRNAs in the Peri-Implantation Period: Contribution to Embryo–Maternal Communication in Pigs. Int. J. Mol. Sci. 2020, 21, 2229. [Google Scholar] [CrossRef]
- Smits, K.; Gansemans, Y.; Tilleman, L.; Van Nieuwerburgh, F.; Van De Velde, M.; Gerits, I.; Ververs, C.; Roels, K.; Govaere, J.; Peelman, L.; et al. Maternal Recognition of Pregnancy in the Horse: Are MicroRNAs the Secret Messengers? Int. J. Mol. Sci. 2020, 21, 419. [Google Scholar] [CrossRef]
- Klein, C.; Scoggin, K.E.; Ealy, A.D.; Troedsson, M.H. Transcriptional profiling of equine endometrium during the time of maternal recognition of pregnancy. Biol. Reprod. 2010, 83, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Merkl, M.; Ulbrich, S.E.; Otzdorff, C.; Herbach, N.; Wanke, R.; Wolf, E.; Handler, J.; Bauersachs, S. Microarray analysis of equine endometrium at days 8 and 12 of pregnancy. Biol. Reprod. 2010, 83, 874–886. [Google Scholar] [CrossRef] [PubMed]
- Dini, P.; El-Sheikh Ali, H.; Carossino, M.; Loux, S.C.; Esteller-Vico, A.; Scoggin, K.E.; Daels, P.; Ball, B.A. Expression Profile of the Chromosome 14 MicroRNA Cluster (C14MC) Ortholog in Equine Maternal Circulation throughout Pregnancy and Its Potential Implications. Int. J. Mol. Sci. 2019, 20, 6285. [Google Scholar] [CrossRef]
- Seo, H.; Bazer, F.W.; Burghardt, R.C.; Johnson, G.A. Immunohistochemical Examination of Trophoblast Syncytialization during Early Placentation in Sheep. Int. J. Mol. Sci. 2019, 20, 4530. [Google Scholar] [CrossRef] [PubMed]
- Spencer, T.E.; Ott, T.L.; Bazer, F.W. tau-Interferon: Pregnancy recognition signal in ruminants. Proc. Soc. Exp. Biol. Med. 1996, 213, 215–229. [Google Scholar] [CrossRef]
- Malo Estepa, I.; Tinning, H.; Rosas Vasconcelos, E.J.; Fernandez-Fuertes, B.; Sánchez, J.M.; Burns, G.W.; Spencer, T.E.; Lonergan, P.; Forde, N. Protein Synthesis by Day 16 Bovine Conceptuses during the Time of Maternal Recognition of Pregnancy. Int. J. Mol. Sci. 2020, 21, 2870. [Google Scholar] [CrossRef]
- Gatien, J.; Mermillod, P.; Tsikis, G.; Bernardi, O.; Janati Idrissi, S.; Uzbekov, R.; Le Bourhis, D.; Salvetti, P.; Almiñana, C.; Saint-Dizier, M. Metabolomic Profile of Oviductal Extracellular Vesicles across the Estrous Cycle in Cattle. Int. J. Mol. Sci. 2019, 20, 6339. [Google Scholar] [CrossRef]
- Bauersachs, S.; Mermillod, P.; Almiñana, C. The Oviductal Extracellular Vesicles’ RNA Cargo Regulates the Bovine Embryonic Transcriptome. Int. J. Mol. Sci. 2020, 21, 1303. [Google Scholar] [CrossRef]
- Alminana, C.; Tsikis, G.; Labas, V.; Uzbekov, R.; da Silveira, J.C.; Bauersachs, S.; Mermillod, P. Deciphering the oviductal extracellular vesicles content across the estrous cycle: Implications for the gametes-oviduct interactions and the environment of the potential embryo. BMC Genom. 2018, 19, 622. [Google Scholar] [CrossRef]
- Canisso, I.F.; Segabinazzi, L.G.T.M.; Fedorka, C.E. Persistent Breeding-Induced Endometritis in Mares—A Multifaceted Challenge: From Clinical Aspects to Immunopathogenesis and Pathobiology. Int. J. Mol. Sci. 2020, 21, 1432. [Google Scholar] [CrossRef] [PubMed]
- Schindler, M.; Pendzialek, S.M.; Grybel, K.; Seeling, T.; Navarrete Santos, A. Metabolic Profiling in Blastocoel Fluid and Blood Plasma of Diabetic Rabbits. Int. J. Mol. Sci. 2020, 21, 919. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Trakooljul, N.; Schoen, J.; Chen, S. Does Maternal Stress Affect the Early Embryonic Microenvironment? Impact of Long-Term Cortisol Stimulation on the Oviduct Epithelium. Int. J. Mol. Sci. 2020, 21, 443. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bauersachs, S.; Almiñana, C. Embryo–Maternal Interactions Underlying Reproduction in Mammals. Int. J. Mol. Sci. 2020, 21, 4872. https://doi.org/10.3390/ijms21144872
Bauersachs S, Almiñana C. Embryo–Maternal Interactions Underlying Reproduction in Mammals. International Journal of Molecular Sciences. 2020; 21(14):4872. https://doi.org/10.3390/ijms21144872
Chicago/Turabian StyleBauersachs, Stefan, and Carmen Almiñana. 2020. "Embryo–Maternal Interactions Underlying Reproduction in Mammals" International Journal of Molecular Sciences 21, no. 14: 4872. https://doi.org/10.3390/ijms21144872
APA StyleBauersachs, S., & Almiñana, C. (2020). Embryo–Maternal Interactions Underlying Reproduction in Mammals. International Journal of Molecular Sciences, 21(14), 4872. https://doi.org/10.3390/ijms21144872




