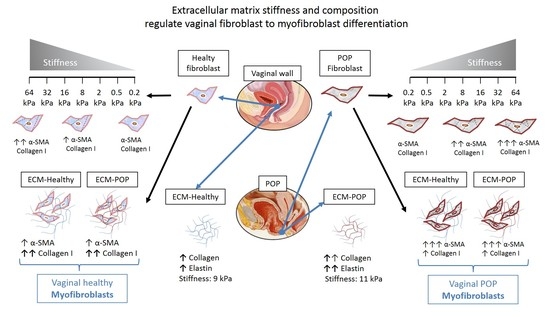
Extracellular Matrix Stiffness and Composition Regulate the Myofibroblast Differentiation of Vaginal Fibroblasts
Abstract
1. Introduction
2. Results
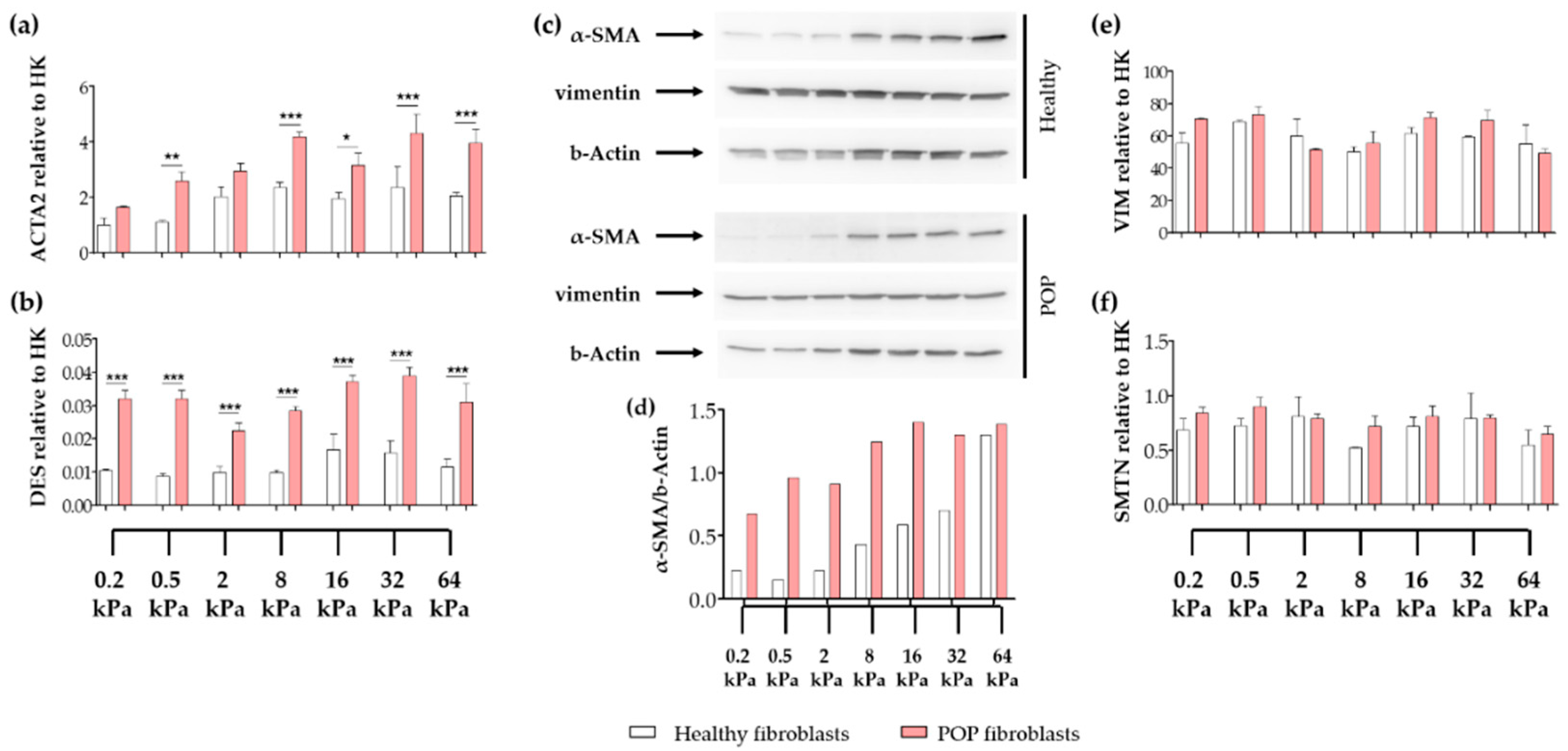
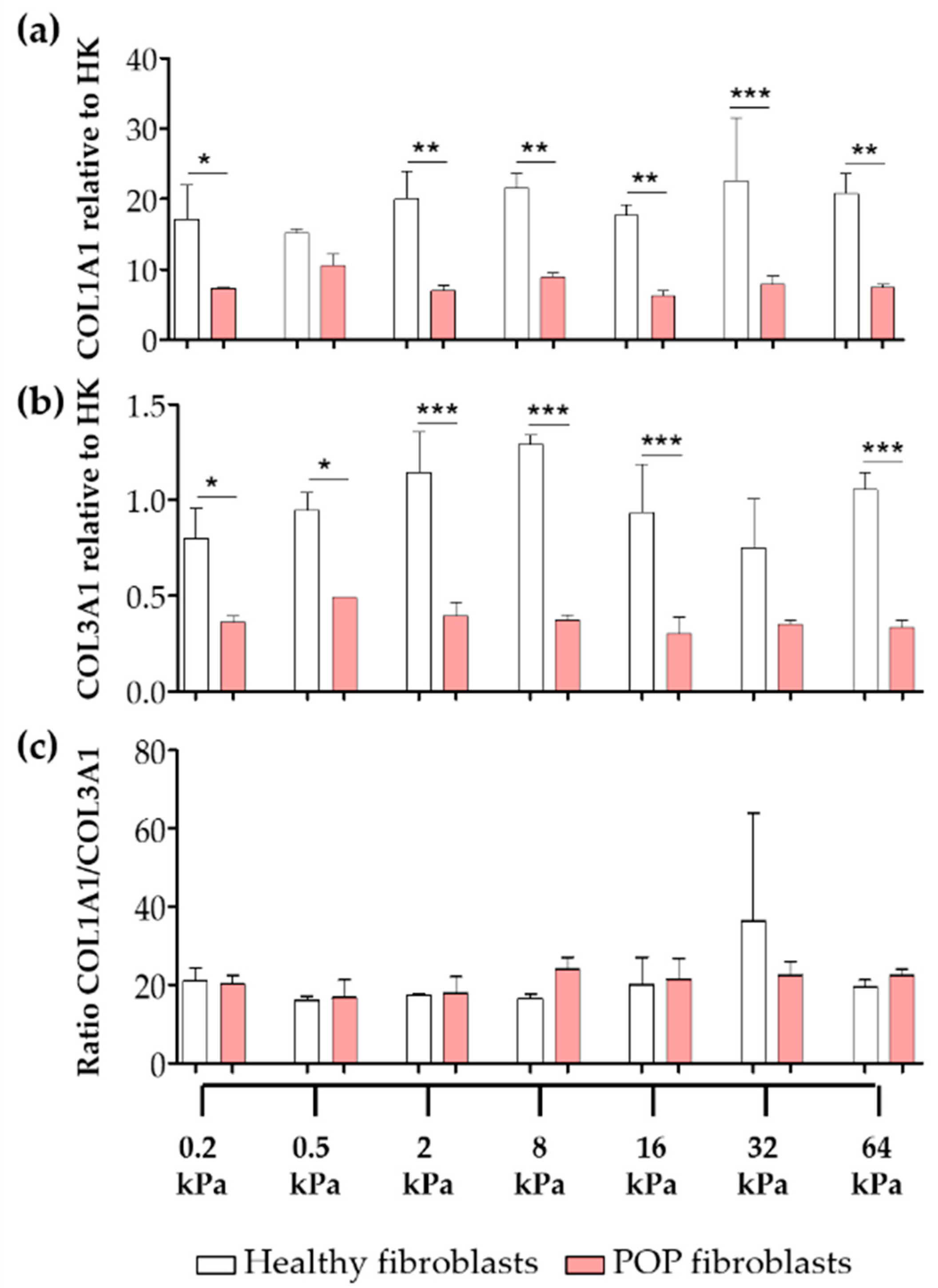
2.1. Effect of Micro-Environments’ Stiffness on Vaginal Fibroblasts to Myofibroblast Differentiation
2.2. Biochemical and Biomechanical Characterization of Vaginal Extracellular Matrix (ECM)
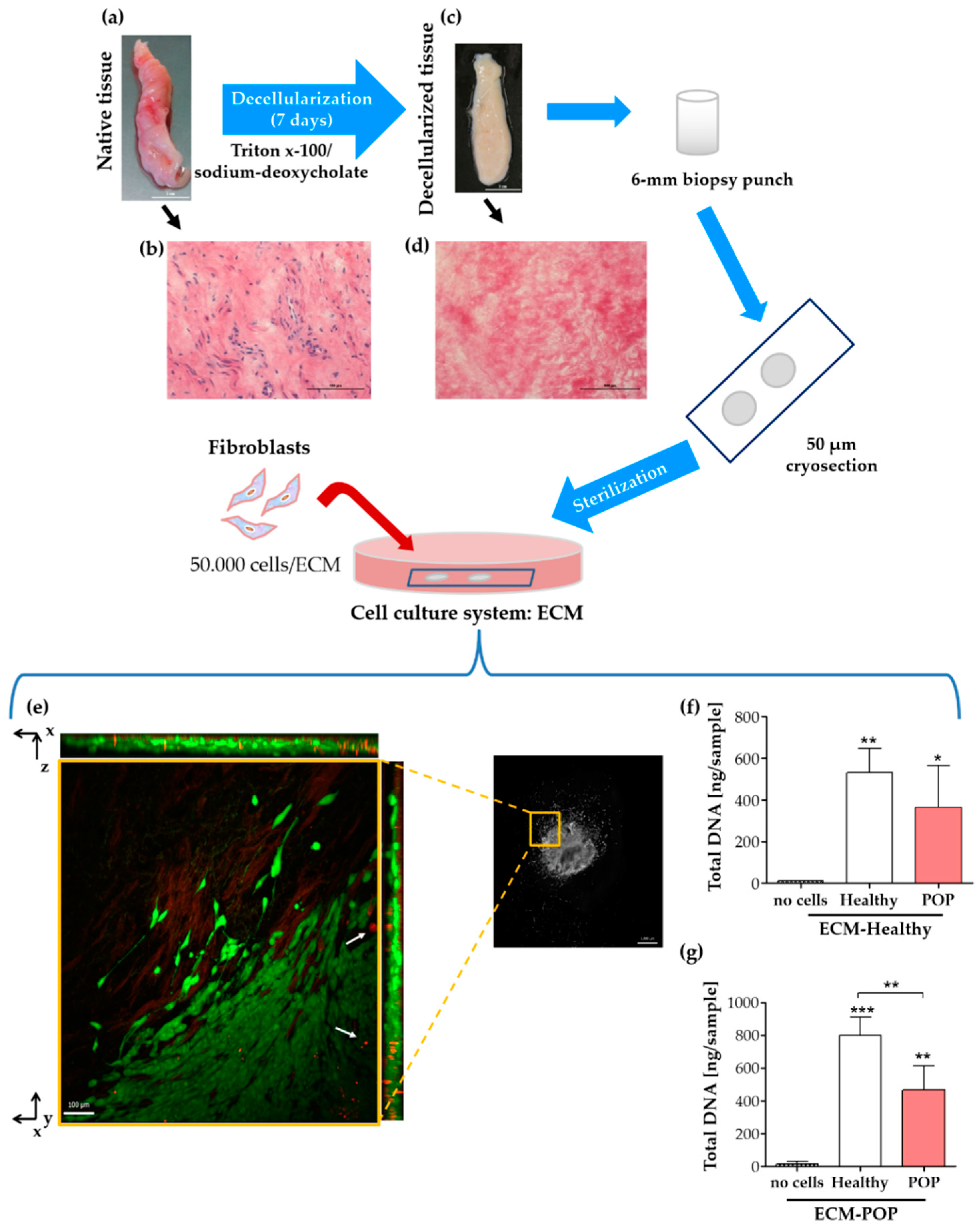
2.3. Vaginal Extracellular Matrix (ECM) Can Be Successfully Used as A Cell Culture System for Vaginal Fibroblasts
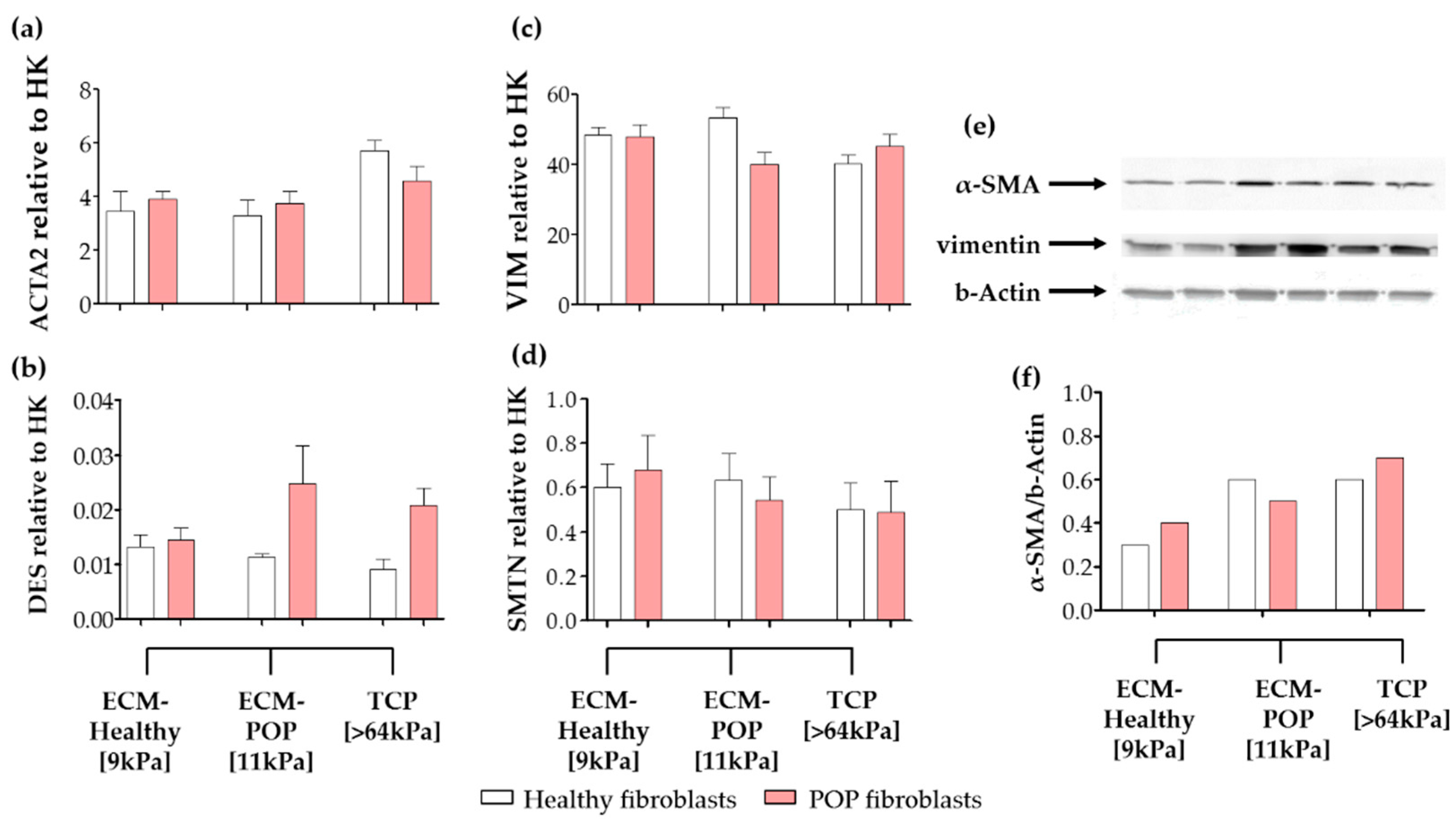
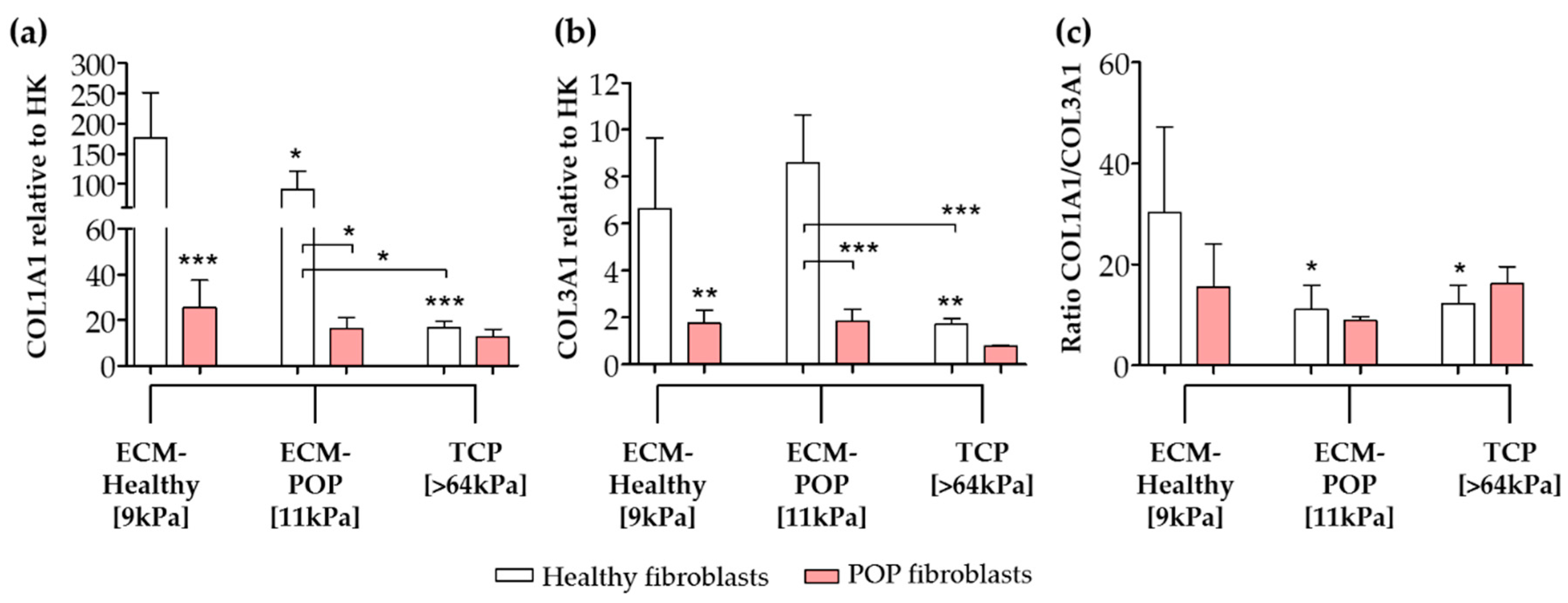
2.4. Effect of Vaginal Extracellular Matrix (ECM) on Vaginal Fibroblasts’ Expression of Proteins Involved in Myofibroblast Differentiation
3. Discussion
4. Materials and Methods
4.1. Biopsy Collection, Cell Isolation and Tissue Decellularization
4.2. Matrices with Different Stiffness for Cell Culture
4.3. Haematoxylin and Eosin Staining (H&E)
4.4. Biochemical Analyses of Collagen
4.5. Biochemical Analyses of Elastin
4.6. Biochemical Analyses of Glycosaminoglycans (GAG)
4.7. ECM for Cell Culture: Preparation and Reseeding
4.8. ECM Surface Micro-Stiffness
4.9. Total DNA
4.10. Quantitative RT-PCR
4.11. Western Blot Analysis
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | One-Way Analysis of Variance |
| α-SMA | α-Smooth Muscle Actin |
| Cp | Crossing-point |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| DNA | Deoxyribonucleic Acid |
| ECM | Extra Cellular Matrix |
| FCS | Foetal Calf Serum |
| FDA | Food and Drug Administration |
| GAG | Glycosaminoglycans |
| HK | House-Keeping |
| HP | Hydroxylysinepyridinoline |
| HPLC | High Performance Liquid Chromatography |
| H&E | Haematoxylin and Eosin |
| LP | Lysylpyridinoline |
| PBS | Phosphate-Buffered Saline |
| PBST | Phosphate-Buffered Saline-Tween |
| POP | Pelvic Organ Prolapse |
| RNA | Ribonucleic Acid |
| RT-PCR | Real Time-Polymerase Chain Reaction |
| SD | Standard Deviation |
| TCP | Tissue Culture Plate |
References
- Xue, M.; Jackson, C.J. Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv. Wound Care 2015, 4, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Tomasek, J.J.; Gabbiani, G.; Hinz, B.; Chaponnier, C.; Brown, R.A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 2002, 3, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Lensen, E.J.; Withagen, M.I.; Kluivers, K.B.; Milani, A.L.; Vierhout, M.E. Surgical treatment of pelvic organ prolapse: A historical review with emphasis on the anterior compartment. Int. Urogynecol. J. 2013, 24, 1593–1602. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Zapata, A.M.; Feola, A.J.; Heesakkers, J.; de Graaf, P.; Blaganje, M.; Sievert, K.-D. Biomechanical properties of the pelvic floor and its relation to pelvic floor disorders. Eur. Urol. Suppl. 2018, 17, 80–90. [Google Scholar] [CrossRef]
- Meyer, S.; Achtari, C.; Hohlfeld, P.; Juillerat-Jeanneret, L. The contractile properties of vaginal myofibroblasts: Is the myofibroblasts contraction force test a valuable indication of future prolapse development? Int. Urogynecol. J. 2008, 19, 1399–1403. [Google Scholar] [CrossRef]
- Ruiz-Zapata, A.M.; Kerkhof, M.H.; Zandieh-Doulabi, B.; Brolmann, H.A.; Smit, T.H.; Helder, M.N. Functional characteristics of vaginal fibroblastic cells from premenopausal women with pelvic organ prolapse. Mol. Hum. Reprod. 2014, 20, 1135–1143. [Google Scholar] [CrossRef]
- Ruiz-Zapata, A.M.; Kerkhof, M.H.; Zandieh-Doulabi, B.; Brolmann, H.A.; Smit, T.H.; Helder, M.N. Fibroblasts from women with pelvic organ prolapse show differential mechanoresponses depending on surface substrates. Int. Urogynecol. J. 2013, 24, 1567–1575. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Z.; Lu, D.; Xu, Q. Effects of mechanical stretching on the morphology and cytoskeleton of vaginal fibroblasts from women with pelvic organ prolapse. Int. J. Mol. Sci. 2015, 16, 9406–9419. [Google Scholar] [CrossRef]
- Kufaishi, H.; Alarab, M.; Drutz, H.; Lye, S.; Shynlova, O. Static mechanical loading influences the expression of extracellular matrix and cell adhesion proteins in vaginal cells derived from premenopausal women with severe pelvic organ prolapse. Reprod. Sci. 2016, 23, 978–992. [Google Scholar] [CrossRef]
- Ruiz-Zapata, A.M.; Kerkhof, M.H.; Ghazanfari, S.; Zandieh-Doulabi, B.; Stoop, R.; Smit, T.H.; Helder, M.N. Vaginal fibroblastic cells from women with pelvic organ prolapse produce matrices with increased stiffness and collagen content. Sci. Rep. 2016, 6, 22971. [Google Scholar] [CrossRef]
- Liang, R.; Abramowitch, S.; Knight, K.; Palcsey, S.; Nolfi, A.; Feola, A.; Stein, S.; Moalli, P.A. Vaginal degeneration following implantation of synthetic mesh with increased stiffness. BJOG Int. J. Obstet. Gynaecol. 2013, 120, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Administration, F.D. Urogynecologic Surgical Mesh Implants. 2019. Available online: https://www.fda.gov/medical-devices/implants-and-prosthetics/urogynecologic-surgical-mesh-implants (accessed on 29 May 2020).
- Jallah, Z.; Liang, R.; Feola, A.; Barone, W.; Palcsey, S.; Abramowitch, S.D.; Yoshimura, N.; Moalli, P. The impact of prolapse mesh on vaginal smooth muscle structure and function. BJOG Int. J. Obstet. Gynaecol. 2016, 123, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- Emmerson, S.; Mukherjee, S.; Melendez-Munoz, J.; Cousins, F.; Edwards, S.L.; Karjalainen, P.; Ng, M.; Tan, K.S.; Darzi, S.; Bhakoo, K.; et al. Composite mesh design for delivery of autologous mesenchymal stem cells influences mesh integration, exposure and biocompatibility in an ovine model of pelvic organ prolapse. Biomaterials 2019, 225, 119495. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yang, N.; Fiore, V.F.; Barker, T.H.; Sun, Y.; Morris, S.W.; Ding, Q.; Thannickal, V.J.; Zhou, Y. Matrix stiffness-induced myofibroblast differentiation is mediated by intrinsic mechanotransduction. Am. J. Respir. Cell Mol. Biol. 2012, 47, 340–348. [Google Scholar] [CrossRef]
- Abramowitch, S.D.; Feola, A.; Jallah, Z.; Moalli, P.A. Tissue mechanics, animal models, and pelvic organ prolapse: A review. Eur. J. Obs. Gynecol. Reprod. Biol. 2009, 144 (Suppl. 1), S146–S158. [Google Scholar] [CrossRef]
- Badiou, W.; Granier, G.; Bousquet, P.J.; Monrozies, X.; Mares, P.; de Tayrac, R. Comparative histological analysis of anterior vaginal wall in women with pelvic organ prolapse or control subjects. A pilot study. Int. Urogynecol. J. 2008, 19, 723–729. [Google Scholar] [CrossRef]
- Jackson, S.R.; Avery, N.C.; Tarlton, J.F.; Eckford, S.D.; Abrams, P.; Bailey, A.J. Changes in metabolism of collagen in genitourinary prolapse. Lancet 1996, 347, 1658–1661. [Google Scholar] [CrossRef]
- Moalli, P.A.; Shand, S.H.; Zyczynski, H.M.; Gordy, S.C.; Meyn, L.A. Remodeling of vaginal connective tissue in patients with prolapse. Obs. Gynecol. 2005, 106, 953–963. [Google Scholar] [CrossRef]
- Mosier, E.; Lin, V.K.; Zimmern, P. Extracellular matrix expression of human prolapsed vaginal wall. Neurourol. Urodyn. 2010, 29, 582–586. [Google Scholar] [CrossRef]
- Zong, W.; Stein, S.E.; Starcher, B.; Meyn, L.A.; Moalli, P.A. Alteration of vaginal elastin metabolism in women with pelvic organ prolapse. Obs. Gynecol. 2010, 115, 953–961. [Google Scholar] [CrossRef]
- de Landsheere, L.; Blacher, S.; Munaut, C.; Nusgens, B.; Rubod, C.; Noel, A.; Foidart, J.M.; Cosson, M.; Nisolle, M. Changes in elastin density in different locations of the vaginal wall in women with pelvic organ prolapse. Int. Urogynecol. J. 2014, 25, 1673–1681. [Google Scholar] [CrossRef] [PubMed]
- Kerkhof, M.H.; Ruiz-Zapata, A.M.; Bril, H.; Bleeker, M.C.; Belien, J.A.; Stoop, R.; Helder, M.N. Changes in tissue composition of the vaginal wall of premenopausal women with prolapse. Am. J. Obs. Gynecol. 2014, 210, e161–e169. [Google Scholar] [CrossRef] [PubMed]
- Karam, J.A.; Vazquez, D.V.; Lin, V.K.; Zimmern, P.E. Elastin expression and elastic fibre width in the anterior vaginal wall of postmenopausal women with and without prolapse. BJU Int. 2007, 100, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Alarab, M.; Kufaishi, H.; Lye, S.; Drutz, H.; Shynlova, O. Expression of extracellular matrix-remodeling proteins is altered in vaginal tissue of premenopausal women with severe pelvic organ prolapse. Reprod. Sci. 2014, 21, 704–715. [Google Scholar] [CrossRef]
- Hinz, B. The myofibroblast: Paradigm for a mechanically active cell. J. Biomech. 2010, 43, 146–155. [Google Scholar] [CrossRef]
- Grant, C.A.; Twigg, P.C.; Tobin, D.J. Static and dynamic nanomechanical properties of human skin tissue using atomic force microscopy: Effect of scarring in the upper dermis. Acta Biomater. 2012, 8, 4123–4129. [Google Scholar] [CrossRef]
- Hayakawa, T.; Hashimoto, Y.; Myokei, Y.; Aoyama, H.; Izawa, Y. Changes in type of collagen during the development of human post-burn hypertrophic scars. Clin. Chim. Acta 1979, 93, 119–125. [Google Scholar] [CrossRef]
- Ewies, A.A.; Al-Azzawi, F.; Thompson, J. Changes in extracellular matrix proteins in the cardinal ligaments of post-menopausal women with or without prolapse: A computerized immunohistomorphometric analysis. Hum. Reprod. 2003, 18, 2189–2195. [Google Scholar] [CrossRef]
- Gabriel, B.; Watermann, D.; Hancke, K.; Gitsch, G.; Werner, M.; Tempfer, C.; zur Hausen, A. Increased expression of matrix metalloproteinase 2 in uterosacral ligaments is associated with pelvic organ prolapse. Int. Urogynecol. J. 2006, 17, 478–482. [Google Scholar] [CrossRef]
- Wang, S.; Lu, D.; Zhang, Z.; Jia, X.; Yang, L. Effects of mechanical stretching on the morphology of extracellular polymers and the mRNA expression of collagens and small leucine-rich repeat proteoglycans in vaginal fibroblasts from women with pelvic organ prolapse. PLoS ONE 2018, 13, e0193456. [Google Scholar] [CrossRef]
- Rensen, S.S.; Doevendans, P.A.; van Eys, G.J. Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Neth. Heart J. 2007, 15, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Ismail, S. Three Countries Ban the Use of Vaginal Mesh in Prolapse Surgery. IUGA: The Official Newsletter. Volume 12, Issue 4, 2017. Released January 19, 2018. Available online: https://static1.squarespace.com/static/52a626cfe4b0e076e263cf7c/t/5a66207608522991e0716303/1516642424430/IUGA+mesh+ban+statement.pdf (accessed on 29 May 2020).
- Fitzpatrick, J.C.; Clark, P.M.; Capaldi, F.M. Effect of decellularization protocol on the mechanical behavior of porcine descending aorta. Int. J. Biomater. 2010. [Google Scholar] [CrossRef] [PubMed]
- Heinz, A.; Jung, M.C.; Duca, L.; Sippl, W.; Taddese, S.; Ihling, C.; Rusciani, A.; Jahreis, G.; Weiss, A.S.; Neubert, R.H.; et al. Degradation of tropoelastin by matrix metalloproteinases--cleavage site specificities and release of matrikines. FEBS J. 2010, 277, 1939–1956. [Google Scholar] [CrossRef]
- Schmelzer, C.E.; Jung, M.C.; Wohlrab, J.; Neubert, R.H.; Heinz, A. Does human leukocyte elastase degrade intact skin elastin? FEBS J. 2012, 279, 4191–4200. [Google Scholar] [CrossRef] [PubMed]
- Heinz, A.; Schrader, C.U.; Baud, S.; Keeley, F.W.; Mithieux, S.M.; Weiss, A.S.; Neubert, R.H.; Schmelzer, C.E. Molecular-level characterization of elastin-like constructs and human aortic elastin. Matrix Biol. 2014, 38, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xin, L.; Shan, B.; Chen, W.; Xie, M.; Yuen, D.; Zhang, W.; Zhang, Z.; Lajoie, G.A.; Ma, B. PEAKS DB: De novo sequencing assisted database search for sensitive and accurate peptide identification. Mol. Cell. Proteom. 2012, 11, M111 010587. [Google Scholar] [CrossRef]
- Ghazanfari, S.; Driessen-Mol, A.; Sanders, B.; Dijkman, P.E.; Hoerstrup, S.P.; Baaijens, F.P.; Bouten, C.V. In Vivo collagen remodeling in the vascular wall of decellularized stented tissue-engineered heart valves. Tissue Eng. Part A 2015, 21, 2206–2215. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
| Feature | ECM-Healthy | ECM-POP | p Value |
|---|---|---|---|
| Collagen a | 84.3 (± 15.63) | 110.0 (± 8.69) | 0.0099 ** |
| Pyridinolines per collagen molecule (HP + LP)/triple helix | 0.11 (± 0.03) | 0.14 (± 0.02) | 0.0525 |
| Pentosidine/triple helix | 0.002 (± 0.0009) | 0.001 (± 0.0006) | 0.0606 |
| GAG b | 3.7 (± 0.43) | 3.6 (± 1.05) | 0.8471 |
| Elastin c | 3.4 (± 0.67) | 6.5 (± 1.37) | 0.0020 ** |
| Desmosine/Isodesmosine | 17.6 (± 7.54) | 20.7 (± 2.14) | 0.4068 |
| Effective Young modulus (kPa) | 8.9 (± 4.26) | 11.2 (± 5.37) | 0.0123 * |
| Target Gene | Oligonucleotide Sequence | |
|---|---|---|
| ACTA2 | Forward Reverse | 5′ CCTGACTGAGCGTGGCTATT 3′ 5′ GATGAAGGATGGCTGGAACA 3′ |
| COL1A1 | Forward Reverse | 5′ TCCAACGAGATCGAGATCC 3′ 5′ AAGCCGAATTCCTGGTCT 3′ |
| COL3A1 | Forward Reverse | 5′ GATCCGTTCTCTGCGATGAC 3′ 5′ AGTTCTGAGGACCAGTAGGG 3′ |
| DES | Forward Reverse | 5′ TGCATGAAGAGGAGATCCGTG 3′ 5′ GGCAGTGAGGTCTGGCTTAG 3′ |
| HPRT | Forward Reverse | 5′ GCTGACCTGCTGGATTACAT 3′ 5′ CTTGCGACCTTGACCATCT 3′ |
| SMTN | Forward Reverse | 5′ CTATGGGCAGCTTAGCCCTC 3′ 5′ CATGGGTCTCCGCAGATGAG 3′ |
| VIM | Forward Reverse | 5′ GGCTCAGATTCAGGAACAGC 3′ 5′ GCTTCAACGGCAAAGTTCTC 3′ |
| YWHAZ | Forward Reverse | 5′ GATGAAGCCATTGCTGAACTTG 3′ 5′ CTATTTGTGGGACAGCATGGA 3′ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Zapata, A.M.; Heinz, A.; Kerkhof, M.H.; van de Westerlo-van Rijt, C.; Schmelzer, C.E.H.; Stoop, R.; Kluivers, K.B.; Oosterwijk, E. Extracellular Matrix Stiffness and Composition Regulate the Myofibroblast Differentiation of Vaginal Fibroblasts. Int. J. Mol. Sci. 2020, 21, 4762. https://doi.org/10.3390/ijms21134762
Ruiz-Zapata AM, Heinz A, Kerkhof MH, van de Westerlo-van Rijt C, Schmelzer CEH, Stoop R, Kluivers KB, Oosterwijk E. Extracellular Matrix Stiffness and Composition Regulate the Myofibroblast Differentiation of Vaginal Fibroblasts. International Journal of Molecular Sciences. 2020; 21(13):4762. https://doi.org/10.3390/ijms21134762
Chicago/Turabian StyleRuiz-Zapata, Alejandra M., Andrea Heinz, Manon H. Kerkhof, Cindy van de Westerlo-van Rijt, Christian E. H. Schmelzer, Reinout Stoop, Kirsten B. Kluivers, and Egbert Oosterwijk. 2020. "Extracellular Matrix Stiffness and Composition Regulate the Myofibroblast Differentiation of Vaginal Fibroblasts" International Journal of Molecular Sciences 21, no. 13: 4762. https://doi.org/10.3390/ijms21134762
APA StyleRuiz-Zapata, A. M., Heinz, A., Kerkhof, M. H., van de Westerlo-van Rijt, C., Schmelzer, C. E. H., Stoop, R., Kluivers, K. B., & Oosterwijk, E. (2020). Extracellular Matrix Stiffness and Composition Regulate the Myofibroblast Differentiation of Vaginal Fibroblasts. International Journal of Molecular Sciences, 21(13), 4762. https://doi.org/10.3390/ijms21134762