Lymphocystis Disease Virus (Iridoviridae) Enters Flounder (Paralichthys olivaceus) Gill Cells via a Caveolae-Mediated Endocytosis Mechanism Facilitated by Viral Receptors
Abstract
1. Introduction
2. Results
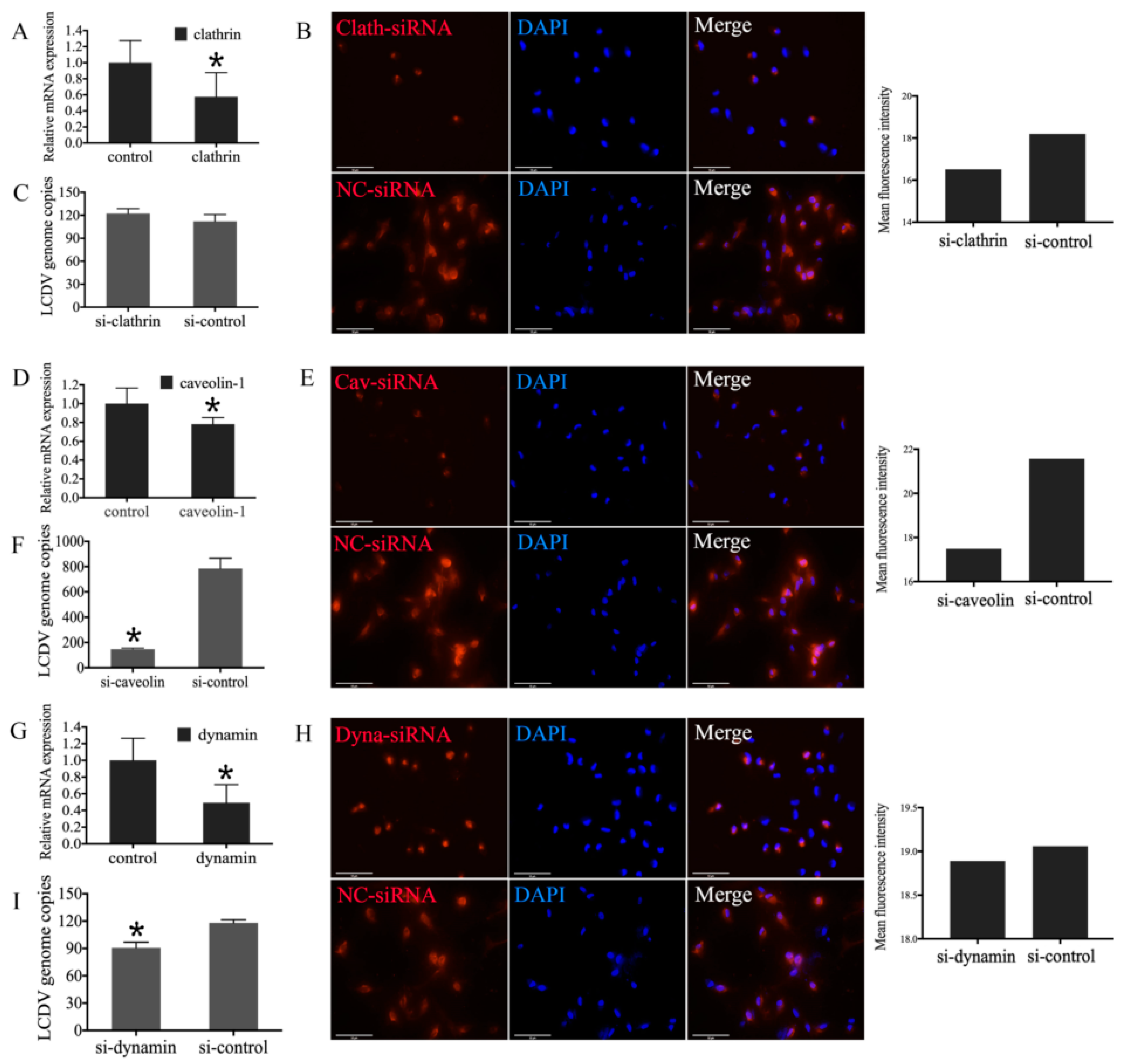
2.1. Specificity of Mouse Anti-Clathrin, Anti-Caveolin, and Anti-Dynamin Polyclonal Antibodies
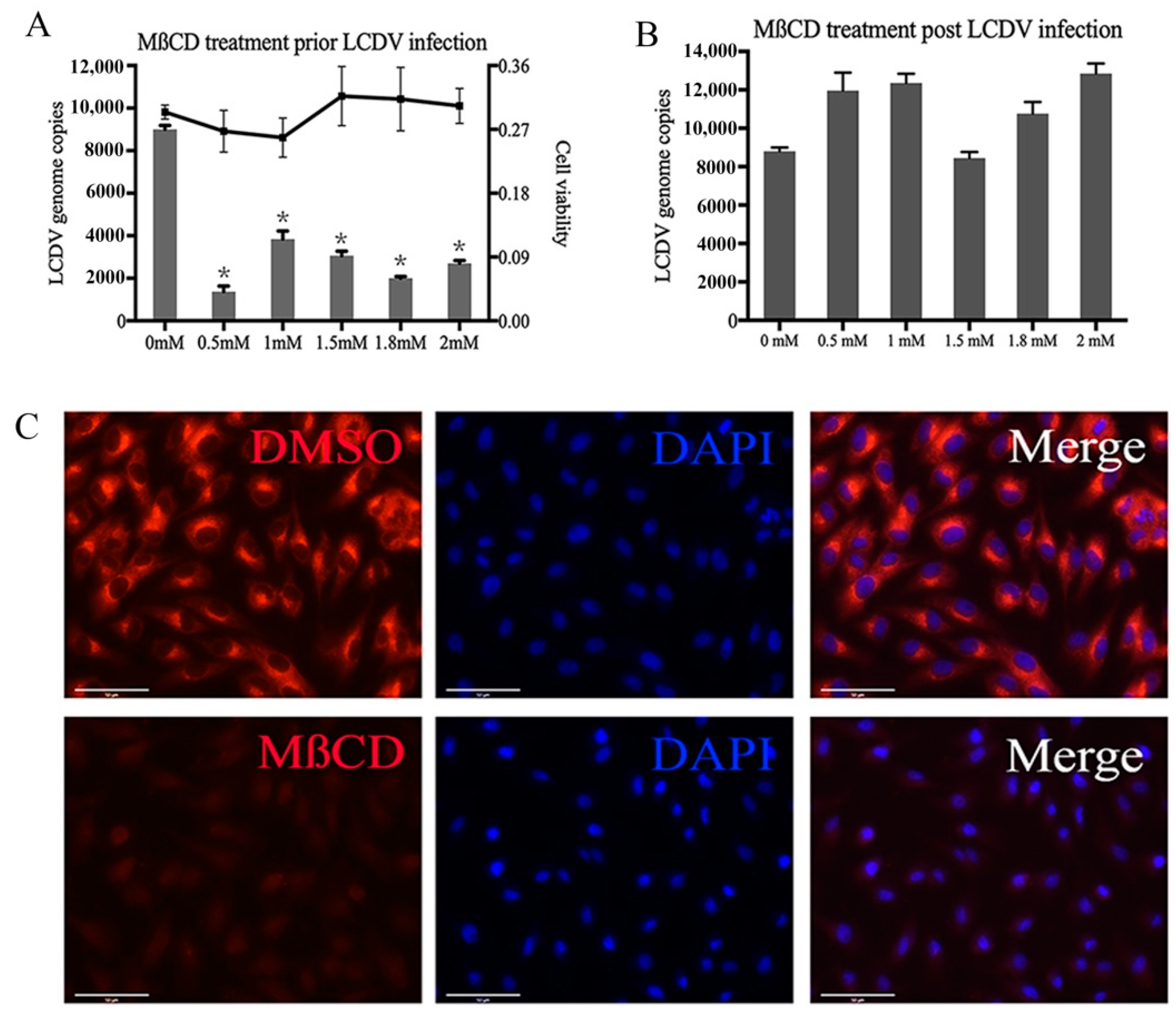
2.2. LCDV Entry Requires Membrane Cholesterol
2.3. LCDV Entry into FG Cells Depends on Dynamin and Microtubules
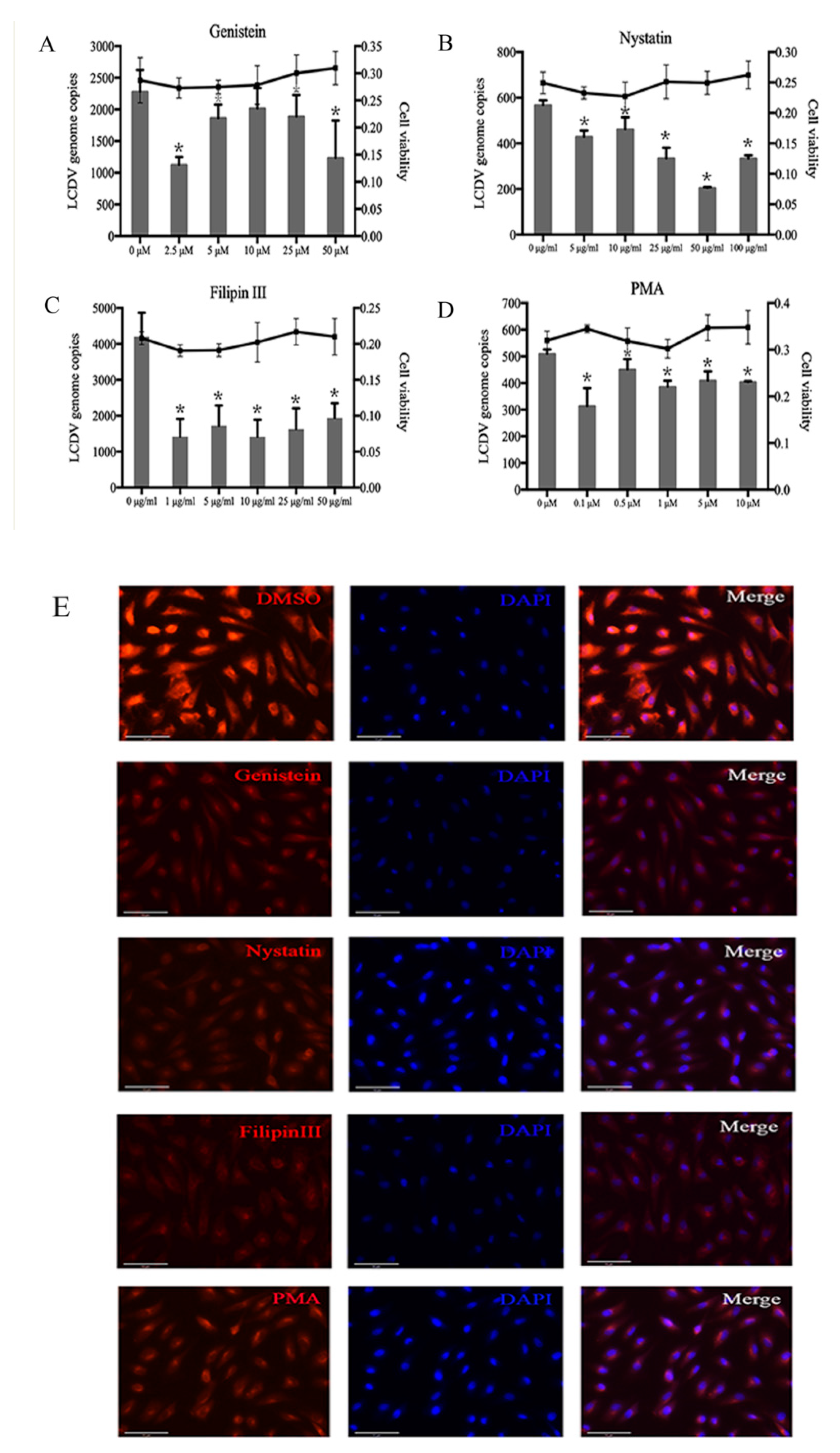
2.4. LCDV Entry Is Caveolae-Dependent
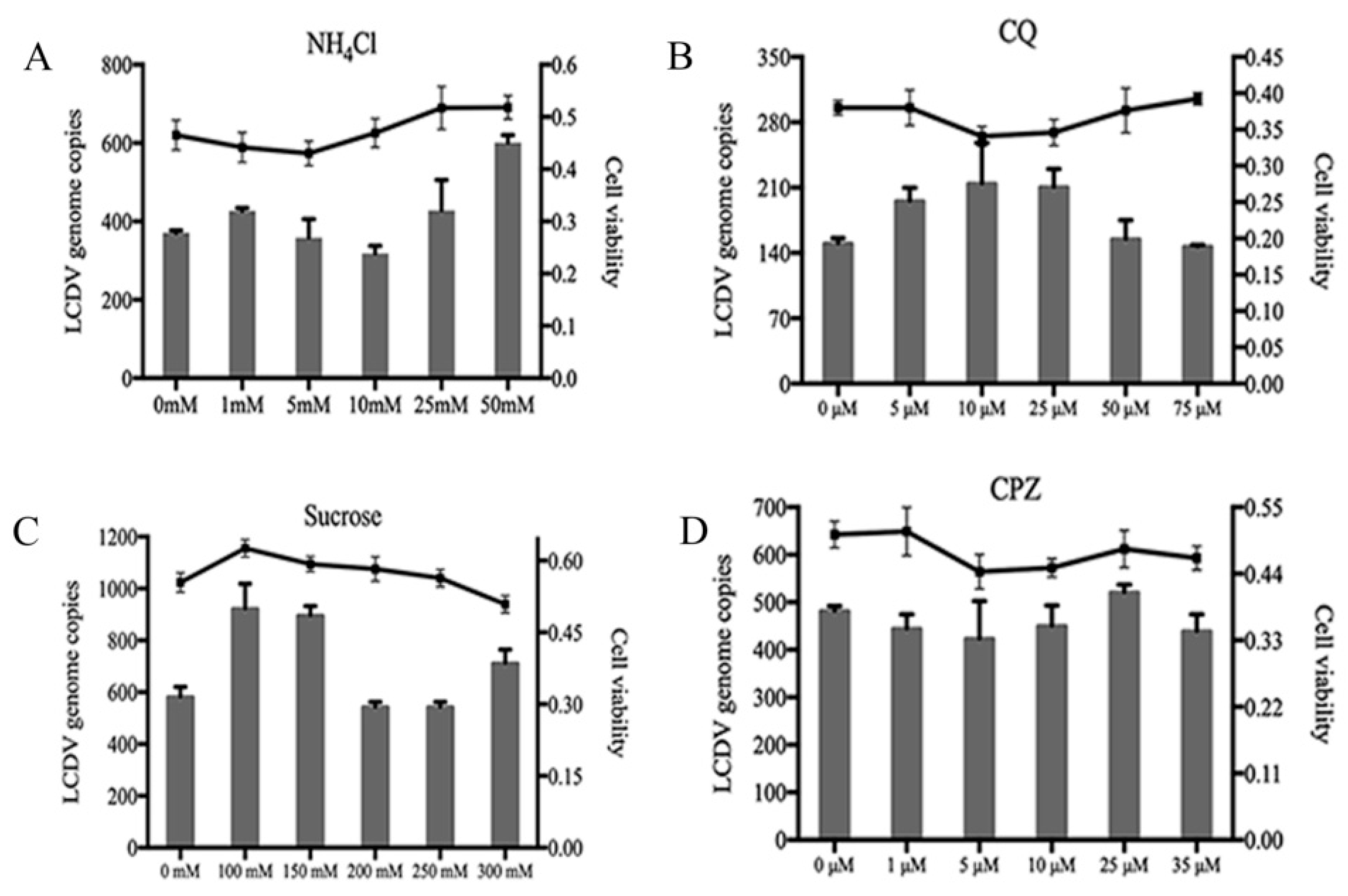
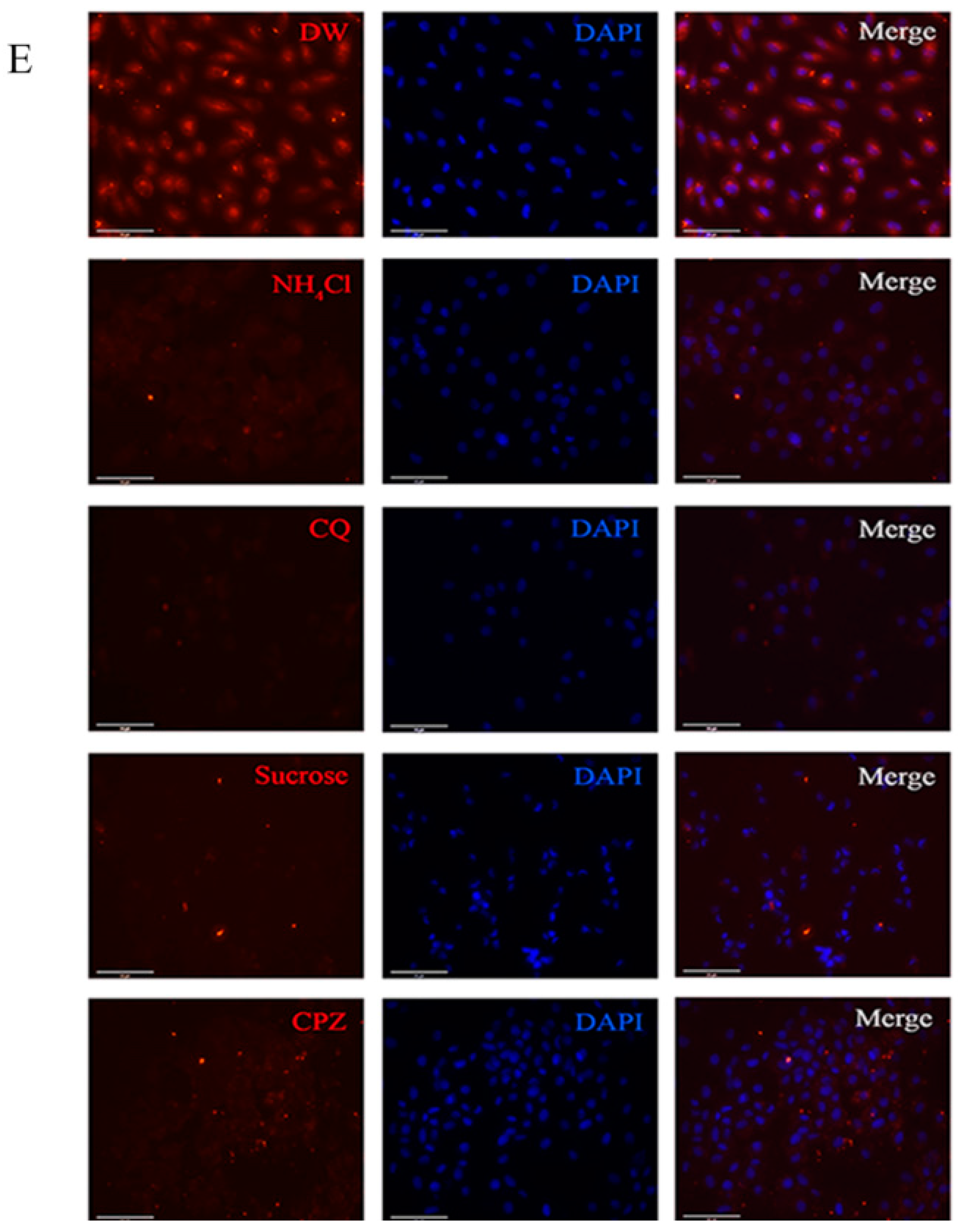
2.5. LCDV Entry Is pH- and Clathrin-Independent
2.6. LCDV Entry Is Macropinocytosis-Independent
2.7. Influence of Clathrin, Caveolin-1, and Dynamin Knockdown in FG Cells on LCDV Infection
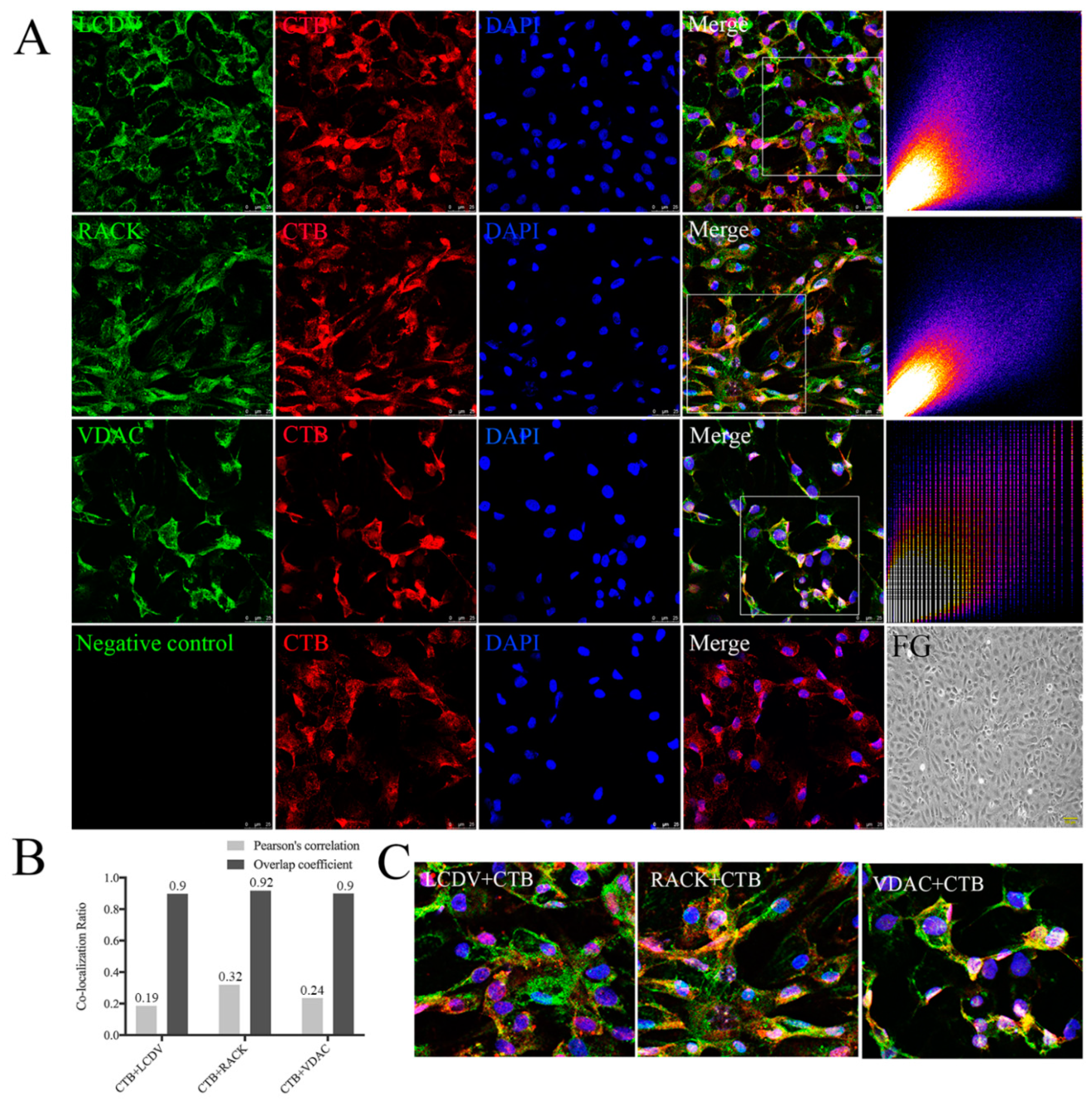
2.8. Co-Localization of LCDV with RACK1 and VDAC2
2.9. Co-Localization of CTB with LCDV, VDAC2, and RACK1
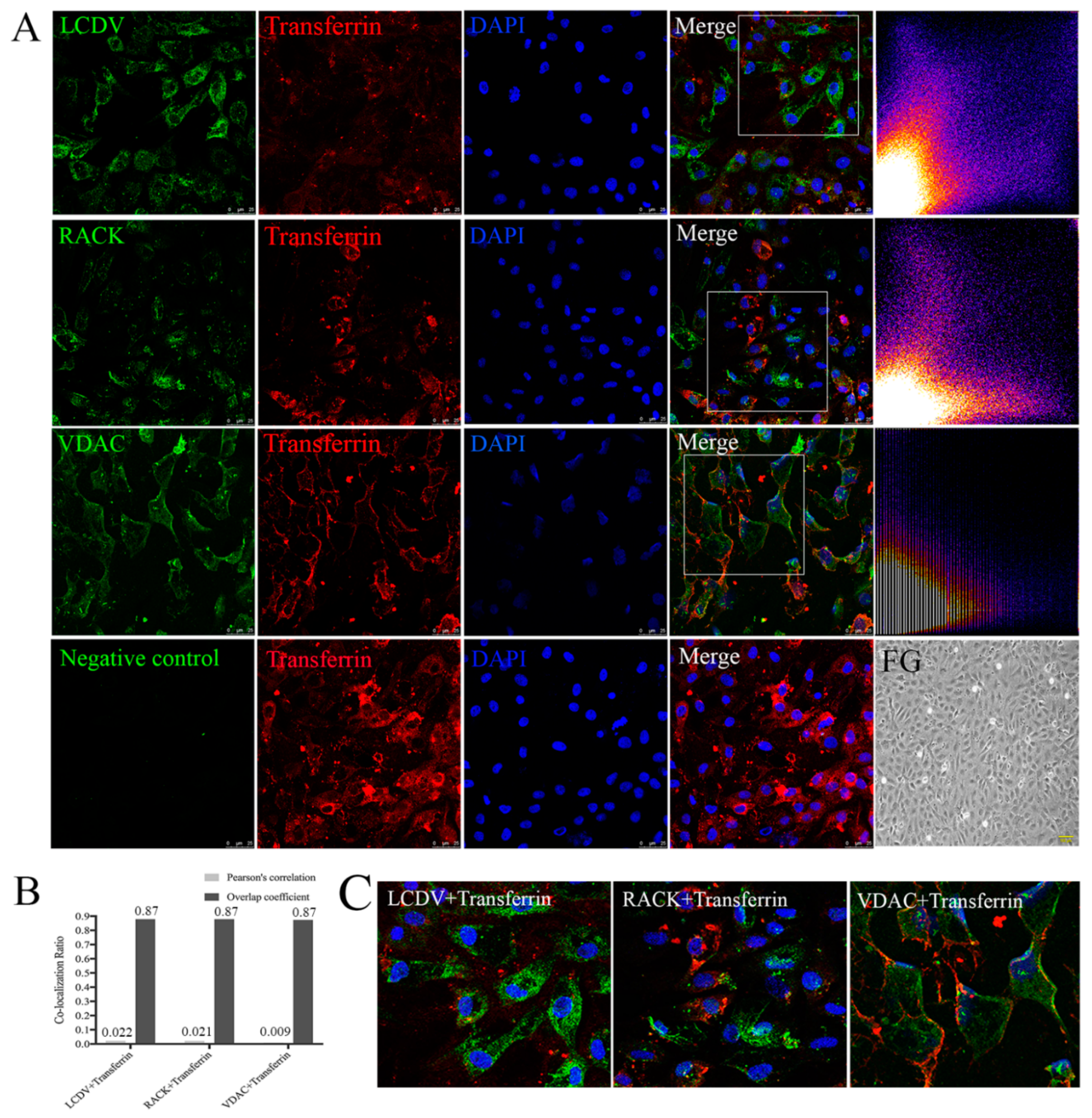
2.10. LCDV, VDAC2, and RACK1 Are Not Co-Localized with Transferrin
2.11. Co-Localization of LCDV, VDAC2, and RACK1 with Caveolin
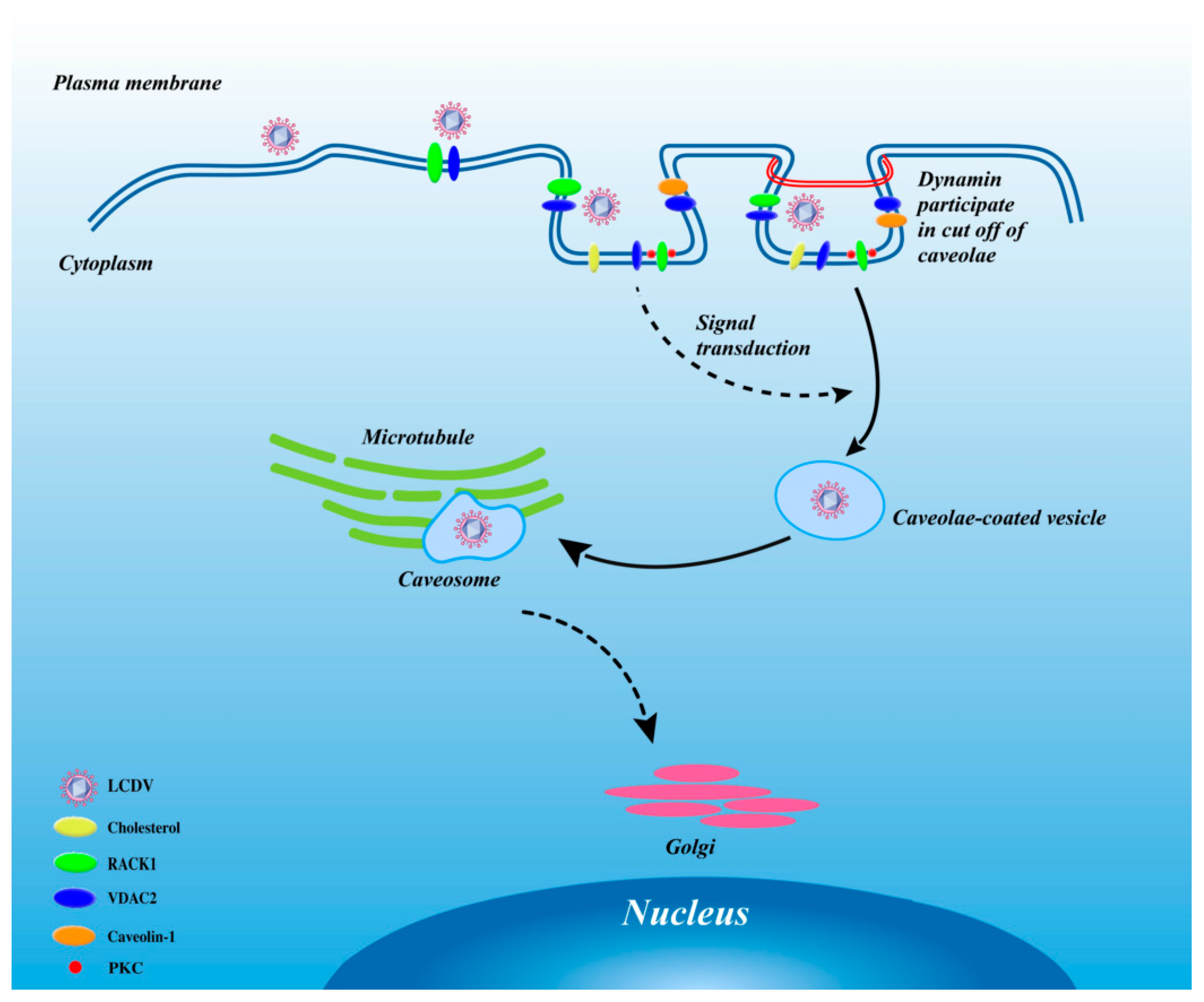
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Cells, Virus and Antibodies
4.3. Production and Specificity Analysis of Mouse Anti-Clathrin, Anti-Caveolin, and Anti-Dynamin Polyclonal Antibodies
4.4. Drug Treatment and Cytotoxicity Assay
4.5. RNAi Knockdown of Clathrin, Caveolin-1, and Dynamin
4.6. Laser Scanning Confocal Immunofluorescence Microscopy
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Berthiaume, L.; Alain, R.; Robin, J. Morphology and ultrastructure of lymphocystis disease virus, a fish iridovirus, grown in tissue culture. J. Virol. 1984, 135, 10–19. [Google Scholar] [CrossRef]
- Schnitzler, P.; Handermann, M.; Szépe, O.; Darai, G. The primary structure of the thymidine kinase gene of fish lymphocystis disease virus. Virology 1991, 182, 835–840. [Google Scholar] [CrossRef]
- Kitamura, S.-I.; Ko, J.-Y.; Lee, W.-L.; Kim, S.-R.; Song, J.-Y.; Kim, D.-K.; Jung, S.-J.; Oh, M.-J. Seasonal prevalence of lymphocystis disease virus and aquabirnavirus in Japanese flounder, Paralichthys olivaceus and blue mussel, Mytilus galloprovincialis. Aquaculture 2007, 266, 26–31. [Google Scholar] [CrossRef]
- Hossain, M.; Kim, S.-R.; Kitamura, S.-I.; Kim, D.-W.; Jung, S.-J.; Nishizawa, T.; Yoshimizu, M.; Oh, M.-J. Lymphocystis disease virus persists in the epidermal tissues of olive flounder, Paralichthys olivaceus (Temminch & Schlegel), at low temperatures. J. Fish Dis. 2009, 32, 699–703. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.-Y.; Wu, Z.-H.; Jian, J.-C.; Lu, Y.-S.; Sun, X.-Q. Analysis of the genetic diversity of the lymphocystis virus and its evolutionary relationship with its hosts. Virus Genes 2011, 43, 358–366. [Google Scholar] [CrossRef]
- Sheng, X.; Wu, R.; Tang, X.; Xing, J.; Zhan, W. Tissue Localization of Lymphocystis Disease Virus (LCDV) Receptor-27.8 kDa and Its Expression Kinetics Induced by the Viral Infection in Turbot (Scophthalmus maximus). Int. J. Mol. Sci. 2015, 16, 26506–26519. [Google Scholar] [CrossRef]
- Schnitzler, P.; Darai, G. Identification of the gene encoding the major capsid protein of fish lymphocystis disease virus. J. Gen. Virol. 1993, 74, 2143–2150. [Google Scholar] [CrossRef]
- Huang, X.; Huang, Y.; Xu, L.; Wei, S.; Ouyang, Z.; Feng, J.; Qin, Q. Identification and characterization of a novel lymphocystis disease virus isolate from cultured grouper in China. J. Fish Dis. 2014, 38, 379–387. [Google Scholar] [CrossRef]
- Carballo, C.; Castro, D.; Borrego, J.J.; Manchado, M. Gene expression profiles associated with lymphocystis disease virus (LCDV) in experimentally infected Senegalese sole (Solea senegalensis). Fish Shellfish Immunol. 2017, 66, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Valverde, E.J.; Borrego, J.J.; Sarasquete, C.; Ortiz-Delgado, J.B.; Castro, D. Target organs for lymphocystis disease virus replication in gilthead seabream (Sparus aurata). Vet. Res. 2017, 48, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Sheng, X.; Xing, J.; Tang, X.; Zhan, W. Identification of a 27.8 kDa protein from flounder gill cells involved in lymphocystis disease virus binding and infection. Dis. Aquat. Org. 2011, 94, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.-L.; Li, H.; Miao, H.-Z. The establishment and partial characterization of a continuous fish cell line FG-9307 from the gill of flounder Paralichthys olivaceus. Aquaculture 1997, 156, 327–333. [Google Scholar] [CrossRef]
- Zhong, Y.; Fei, C.; Tang, X.; Zhan, W.; Sheng, X. A 32 kDa viral attachment protein of lymphocystis disease virus (LCDV) specifically interacts with a 27.8 kDa cellular receptor from flounder (Paralichthys olivaceus). J. Gen. Virol. 2017, 98, 1477–1488. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Tang, X.; Sheng, X.; Xing, J.; Zhan, W. Development and Characterization of Monoclonal Antibodies to the 32 kDa Viral Attachment Protein of Lymphocystis Disease Virus and Their Neutralizing Ability in Vitro. Int. J. Mol. Sci. 2018, 19, 2536. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Tang, X.; Sheng, X.; Xing, J.; Zhan, W. Voltage-Dependent Anion Channel Protein 2 (VDAC2) and Receptor of Activated Protein C Kinase 1 (RACK1) Act as Functional Receptors for Lymphocystis Disease Virus Infection. J. Virol. 2019, 93, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Bàthori, G.; Parolini, I.; Tombola, F.; Szabò, I.; Messina, A.; Oliva, M.; De Pinto, V.; Lisanti, M.; Sargiacomo, M.; Zoratti, M. Porin Is Present in the Plasma Membrane Where It Is Concentrated in Caveolae and Caveolae-related Domains. J. Biol. Chem. 1999, 274, 29607–29612. [Google Scholar] [CrossRef] [PubMed]
- Marin, R.; Ramírez, C.; Gonzalez, M.D.; González-Muñoz, E.; Zorzano, A.; Camps, M.; Alonso, R.; Díaz, M. Voltage-dependent anion channel (VDAC) participates in amyloid beta-induced toxicity and interacts with plasma membrane estrogen receptor α in septal and hippocampal neurons. Mol. Membr. Biol. 2007, 24, 148–160. [Google Scholar] [CrossRef] [PubMed]
- De Pinto, V.; Messina, A.; Lane, D.; Lawen, A. Voltage-dependent anion-selective channel (VDAC) in the plasma membrane. FEBS Lett. 2010, 584, 1793–1799. [Google Scholar] [CrossRef]
- Smart, E.J.; Graf, G.A.; McNiven, M.A.; Sessa, W.C.; Engelman, J.A.; Scherer, P.E.; Okamoto, T.; Lisanti, M.P. Caveolins, Liquid-Ordered Domains, and Signal Transduction. Mol. Cell. Biol. 1999, 19, 7289–7304. [Google Scholar] [CrossRef]
- Mochly-Rosen, D.; Gordon, A.S. Anchoring proteins for protein kinase C: A means for isozyme selectivity. FASEB J. 1998, 12, 35–42. [Google Scholar] [CrossRef]
- Mercer, J.; Schelhaas, M.; Helenius, A. Virus Entry by Endocytosis. Annu. Rev. Biochem. 2010, 79, 803–833. [Google Scholar] [CrossRef]
- Ou, X.; Liu, Y.; Lei, X.; Li, P.; Mi, D.; Ren, L.; Guo, L.; Guo, R.; Chen, T.; Hu, J.; et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020, 11, 1620. [Google Scholar] [CrossRef]
- Grove, J.; Marsh, M. The cell biology of receptor-mediated virus entry. J. Cell Biol. 2011, 195, 1071–1082. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Helenius, A. Virus entry at a glance. J. Cell Sci. 2013, 126, 1289–1295. [Google Scholar] [CrossRef] [PubMed]
- Marsh, M.; Helenius, A. Virus entry into animal cells. Adv. Virus Res. 1989, 36, 107–151. [Google Scholar]
- Robinson, M.S. Adaptable adaptors for coated vesicles. Trends Cell Biol. 2004, 14, 167–174. [Google Scholar] [CrossRef]
- Pelkmans, L.; Fava, E.; Grabner, H.; Hannus, M.; Habermann, B.; Krausz, E.; Zerial, M. Genome-wide analysis of human kinases in clathrin- and caveolae/raft-mediated endocytosis. Nature 2005, 436, 78–86. [Google Scholar] [CrossRef]
- Mousavi, S.A.; Malerod, L.; Berg, T.; Kjeken, R. Clathrin-dependent endocytosis. Biochem. J. 2004, 377, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Marsh, M.; Helenius, A. Virus Entry: Open Sesame. Cell 2006, 124, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Anderson, H.A.; Chen, Y.; Norkin, L.C. Bound simian virus 40 translocates to caveolin-enriched membrane domains, and its entry is inhibited by drugs that selectively disrupt caveolae. Mol. Biol. Cell 1996, 7, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Stang, E.; Kartenbeck, J.; Parton, R. Major histocompatibility complex class I molecules mediate association of SV40 with caveolae. Mol. Biol. Cell 1997, 8, 47–57. [Google Scholar] [CrossRef]
- Pelkmans, L.; Kartenbeck, J.; Helenius, A. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular transport pathway to the ER. Nat. Cell Biol. 2001, 3, 473–483. [Google Scholar] [CrossRef]
- Patel, H.H.; Murray, F.; Insel, P.A. Caveolae as organizers of pharmacologically relevant signal transduction molecules. Annu. Rev. Pharmacol. 2008, 48, 359–391. [Google Scholar] [CrossRef] [PubMed]
- Lisanti, M.P.; Tang, Z.; Scherer, P.E.; Kübler, E.; Koleske, A.J.; Sargiacomo, M. Caveolae, transmembrane signalling and cellular transformation. Mol. Membr. Biol. 1995, 12, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.-J.; Wu, Y.-Y.; Yang, L.-S.; Yang, X.-B.; He, J.; Mi, S.; Jia, K.-T.; Weng, S.-P.; Yu, X.-Q.; He, J.; et al. Infectious Spleen and Kidney Necrosis Virus (a Fish Iridovirus) Enters Mandarin Fish Fry Cells via Caveola-Dependent Endocytosis. J. Virol. 2011, 86, 2621–2631. [Google Scholar] [CrossRef] [PubMed]
- Simpson, C.; Yamauchi, Y. Microtubules in Influenza Virus Entry and Egress. Viruses 2020, 12, 117. [Google Scholar] [CrossRef] [PubMed]
- Mercer, J.; Helenius, A. Virus entry by macropinocytosis. Nat. Cell Biol. 2009, 11, 510–520. [Google Scholar] [CrossRef]
- Swanson, J.A. Shaping cups into phagosomes and macropinosomes. Nat. Rev. Mol. Cell Biol. 2008, 9, 639–649. [Google Scholar] [CrossRef]
- Ghigo, É.; Kartenbeck, J.; Lien, P.; Pelkmans, L.; Capo, C.; Mege, J.-L.; Raoult, D. Ameobal Pathogen Mimivirus Infects Macrophages through Phagocytosis. PLoS Pathog. 2008, 4, e1000087. [Google Scholar] [CrossRef]
- Rojek, J.M.; Perez, M.; Kunz, S. Cellular entry of lymphocytic choriomeningitis virus. J. Virol. 2008, 82, 1505–1517. [Google Scholar] [CrossRef]
- Pelkmans, L.; Helenius, A. Endocytosis via caveolae. Traffic 2002, 3, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Mayle, K.M.; Le, A.M.; Kamei, D.T. The intracellular trafficking pathway of transferrin. Biochim. Biophys. Acta 2012, 1820, 264–281. [Google Scholar] [CrossRef] [PubMed]
- Chinchar, V.G.; Yu, K.H.; Jancovich, J.K. The Molecular Biology of Frog Virus 3 and other Iridoviruses Infecting Cold-Blooded Vertebrates. Viruses 2011, 3, 1959–1985. [Google Scholar] [CrossRef] [PubMed]
- Chinchar, V.G.; Waltzek, T.B. Ranaviruses: Not Just for Frogs. PLoS Pathog. 2014, 10, e1003850. [Google Scholar] [CrossRef]
- Wang, S.; Huang, X.; Huang, Y.; Hao, X.; Xu, H.; Cai, M.; Wang, H.; Qin, Q. Entry of a Novel Marine DNA Virus, Singapore Grouper Iridovirus, into Host Cells Occurs via Clathrin-Mediated Endocytosis and Macropinocytosis in a pH-Dependent Manner. J. Virol. 2014, 88, 13047–13063. [Google Scholar] [CrossRef]
- Chazal, N.; Gerlier, D. Virus Entry, Assembly, Budding, and Membrane Rafts. Microbiol. Mol. Biol. Rev. 2003, 67, 226–237. [Google Scholar] [CrossRef]
- Smith, A.E.; Helenius, A. How Viruses Enter Animal Cells. Science 2004, 304, 237–242. [Google Scholar] [CrossRef]
- Braunwald, J.; Nonnenmacher, H.; Tripier-Darcy, F. Ultrastructural and Biochemical Study of Frog Virus 3 Uptake by BHK-21 Cells. J. Gen. Virol. 1985, 66, 283–293. [Google Scholar] [CrossRef]
- Guo, C.-J.; Liu, N.; Wu, Y.-Y.; Yang, X.-B.; Yang, L.-S.; Mi, S.; Huang, Y.-X.; Luo, Y.-W.; Jia, K.-T.; Liu, Z.-Y.; et al. Entry of Tiger Frog Virus (an Iridovirus) into HepG2 Cells via a pH-Dependent, Atypical, Caveola-Mediated Endocytosis Pathway. J. Virol. 2011, 85, 6416–6426. [Google Scholar] [CrossRef]
- Zhang, Q.-Y.; Xiao, F.; Xie, J.; Li, Z.-Q.; Gui, J.-F. Complete Genome Sequence of Lymphocystis Disease Virus Isolated from China. J. Virol. 2004, 78, 6982–6994. [Google Scholar] [CrossRef]
- He, L.-B.; Ke, F.; Wang, J.; Gao, X.-C.; Zhang, Q.-Y. Rana grylio virus (RGV) envelope protein 2L: Subcellular localization and essential roles in virus infectivity revealed by conditional lethal mutant. J. Gen. Virol. 2014, 95, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.Y.; Shih, W.L.; Huang, W.R.; Chi, P.I.; Wu, M.H.; Liu, H.-J. Bovine Ephemeral Fever Virus Uses a Clathrin-Mediated and Dynamin 2-Dependent Endocytosis Pathway That Requires Rab5 and Rab7 as Well as Microtubules. J. Virol. 2012, 86, 13653–13661. [Google Scholar] [CrossRef]
- Zhang, Y.-N.; Liu, Y.-Y.; Xiao, F.-C.; Liu, C.-C.; Liang, X.-D.; Chen, J.; Zhou, J.; Baloch, A.S.; Kan, L.; Zhou, B.; et al. Rab5, Rab7, and Rab11 Are Required for Caveola-Dependent Endocytosis of Classical Swine Fever Virus in Porcine Alveolar Macrophages. J. Virol. 2018, 92, e00797-18. [Google Scholar] [CrossRef] [PubMed]
- Hernaez, B.; Alonso, C. Dynamin- and Clathrin-Dependent Endocytosis in African Swine Fever Virus Entry. J. Virol. 2009, 84, 2100–2109. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.-J.; Liu, C.-C.; Zhou, J.; Wang, S.-Q.; Gao, Z.-C.; Zhang, X.-M.; Zhou, B.; Chen, P.-Y. Entry of Classical Swine Fever Virus into PK-15 Cells via a pH-, Dynamin-, and Cholesterol-Dependent, Clathrin-Mediated Endocytic Pathway That Requires Rab5 and Rab7. J. Virol. 2016, 90, 9194–9208. [Google Scholar] [CrossRef]
- Liu, P.; Rudick, M.; Anderson, R.G.W. Multiple Functions of Caveolin-1. J. Biol. Chem. 2002, 277, 41295–41298. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Yang, Y.-T.; Yu, S.-L.; Hsiao, K.-N.; Liu, C.-C.; Sia, C.; Chow, Y.-H. Caveolar Endocytosis Is Required for Human PSGL-1-Mediated Enterovirus 71 Infection. J. Virol. 2013, 87, 9064–9076. [Google Scholar] [CrossRef]
- Frampton, A.R.; Stolz, D.B.; Uchida, H.; Goins, W.F.; Cohen, J.B.; Glorioso, J.C. Equine Herpesvirus 1 Enters Cells by Two Different Pathways, and Infection Requires the Activation of the Cellular Kinase ROCK1. J. Virol. 2007, 81, 10879–10889. [Google Scholar] [CrossRef]
- Nabi, I.R.; Le, P.U. Caveolae/raft-dependent endocytosis. J. Cell Biol. 2003, 161, 673–677. [Google Scholar] [CrossRef]
- Rust, M.J.; Lakadamyali, M.; Zhang, F.; Zhuang, X. Assembly of endocytic machinery around individual influenza viruses during viral entry. Nat. Struct. Mol. Biol. 2004, 11, 567–573. [Google Scholar] [CrossRef]
- De Vries, E.; Tscherne, D.M.; Wienholts, M.J.; Cobos-Jiménez, V.; Scholte, F.E.M.; García-Sastre, A.; Rottier, P.J.M.; De Haan, C.A.M. Dissection of the Influenza A Virus Endocytic Routes Reveals Macropinocytosis as an Alternative Entry Pathway. PLoS Pathog. 2011, 7, e1001329. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Tang, X.; Sheng, X.; Zhan, W. Relationship between Expression of Cellular Receptor-27.8kDa and Lymphocystis Disease Virus (LCDV) Infection. PLoS ONE 2015, 10, e0127940. [Google Scholar] [CrossRef] [PubMed]
- Gerold, G.; Moeller, R.; Pietschmann, T. Hepatitis C virus entry: Protein interactions and fusion determinants governing productive hepatocyte invasion. Cold Spring Harb. Perspect. Med. 2020, 10, a036830. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-W.; Lin, H.-Y.; Tsou, Y.-L.; Ebenezer, C.; Hsiao, K.-N.; Shao, H.-Y.; Liu, C.-C.; Si, C.; Chong, P.; Chow, Y.-H. Human SCARB2 mediated entry and endocytosis of EV71. PLoS ONE 2012, 7, e30507. [Google Scholar] [CrossRef]
- Kornilova, E. Receptor-mediated endocytosis and cytoskeleton. Biochemistry 2014, 79, 865–878. [Google Scholar] [CrossRef]
- Matsuda, M.; Suzuki, R.; Kataoka, C.; Watashi, K.; Aizaki, H.; Kato, N.; Matsuura, Y.; Suzuki, T.; Wakita, T. Alternative endocytosis pathway for productive entry of hepatitis C virus. J. Gen. Virol. 2014, 95, 2658–2667. [Google Scholar] [CrossRef]
- Coyne, C.B.; Bergelson, J.M. Virus-Induced Abl and Fyn Kinase Signals Permit Coxsackievirus Entry through Epithelial Tight Junctions. Cell 2006, 124, 119–131. [Google Scholar] [CrossRef]
- Torgersen, M.L.; Skretting, G.; Van Deurs, B.; Sandvig, K. Internalization of cholera toxin by different endocytic mechanisms. J. Cell Sci. 2001, 114, 3737–3747. [Google Scholar]
- Wu, R.; Sheng, X.; Tang, X.; Xing, J.; Zhan, W. Transcriptome Analysis of Flounder (Paralichthys olivaceus) Gill in Response to Lymphocystis Disease Virus (LCDV) Infection: Novel Insights into Fish Defense Mechanisms. Int. J. Mol. Sci. 2018, 19, 160. [Google Scholar] [CrossRef]
- Hayer, A.; Stoeber, M.; Ritz, D.; Engel, S.; Meyer, H.; Helenius, A. Caveolin-1 is ubiquitinated and targeted to intralumenal vesicles in endolysosomes for degradation. J. Cell Biol. 2010, 191, 615–629. [Google Scholar] [CrossRef]
- Guo, M.; Chen, K.; Lv, Z.; Shao, Y.; Zhang, W.; Zhao, X.; Li, C. Bcl-2 mediates coelomocytes apoptosis by suppressing cytochrome c release T in Vibrio splendidus challenged Apostichopus japonicus. Dev. Comp. Immunol. 2020, 103, 103533. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, M.; Lv, Z.; Zhang, W.; Shao, Y.; Zhao, X.; Li, C. Fas-associated death domain (FADD) in sea cucumber (Apostichopus japonicus): Molecular cloning, characterization and pro-apoptotic function analysis. Dev. Comp. Immunol. 2020, 108, 103673. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, L.; Huang, X.; Wang, S.; Huang, Y.; Qin, Q. Fish Cholesterol 25-Hydroxylase Inhibits Virus Replication via Regulating Interferon Immune Response or Affecting Virus Entry. Front. Immunol. 2019, 10, 322. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Wang, M.; Xing, J.; Zhan, W. Monoclonal antibodies against 27.8 kDa protein receptor efficiently block lymphocystis disease virus infection in flounder Paralichthys olivaceus gill cells. Dis. Aquat. Org. 2012, 100, 19–27. [Google Scholar] [CrossRef] [PubMed]





| Primer/siRNA/Peptide | Primer Sequence (5′–3′) | Use |
|---|---|---|
| qCaveolin-F | GGACCCCAAGCACATAAACG | RT-PCR (caveolin mRNA expression) |
| qCaveolin-R qClathrin-F qClathrin-R qDynamin-F qDynamin-R beta-actin-F beta-actin-R | GGACAGGATGGCGAAGAAGAT CTCGGGTATCATTGGGGTCA GACGGATGGTGTCTGGGGTA ACGCCCTGTCTCAAATGTATCG AAGCCTATGAAGTCCTCGTGGTT CACTGTGCCCATCTACGAG CCATCTCCTGCTCGAAGTC | RT-PCR (caveolin mRNA expression) RT-PCR (clathrin mRNA expression) RT-PCR (clathrin mRNA expression) RT-PCR (dynamin mRNA expression) RT-PCR (dynamin mRNA expression) RT-PCR (internal control) RT-PCR (internal control) |
| LCDV-038F | TCTTGTTCAGCATTTACTTCTCGGC | RT-PCR (LCDV copy number) |
| LCDV-038R | TCTTCTCCTTTAGATGATTTCCC | RT-PCR (LCDV copy number) |
| Clathrin-siRNA Caveolin-siRNA | GCAACCAAAUGUUCACCAATT CCUUCACCGUCACCAAGUATT | RNA interference assay RNA interference assay |
| Dynamin-siRNA NC-siRNA | GCCCGUAGACAUUGAGCAUTT UUCUCCGAACGUGUCACGUTT | RNA interference assay RNA interference assay |
| Clathrin peptide | MADPNTPIRRPISAC | Production of mouse polyclonal antibody against clathrin |
| Caveolin-1 peptide | NIYKPNNKDMDNDSC | Production of mouse polyclonal antibody against caveolin-1 |
| Dynamin peptide | CPGVPRRPAPRRNQW | Production of mouse polyclonal antibody against dynamin |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheng, X.; Zhong, Y.; Zeng, J.; Tang, X.; Xing, J.; Chi, H.; Zhan, W. Lymphocystis Disease Virus (Iridoviridae) Enters Flounder (Paralichthys olivaceus) Gill Cells via a Caveolae-Mediated Endocytosis Mechanism Facilitated by Viral Receptors. Int. J. Mol. Sci. 2020, 21, 4722. https://doi.org/10.3390/ijms21134722
Sheng X, Zhong Y, Zeng J, Tang X, Xing J, Chi H, Zhan W. Lymphocystis Disease Virus (Iridoviridae) Enters Flounder (Paralichthys olivaceus) Gill Cells via a Caveolae-Mediated Endocytosis Mechanism Facilitated by Viral Receptors. International Journal of Molecular Sciences. 2020; 21(13):4722. https://doi.org/10.3390/ijms21134722
Chicago/Turabian StyleSheng, Xiuzhen, Ying Zhong, Jing Zeng, Xiaoqian Tang, Jing Xing, Heng Chi, and Wenbin Zhan. 2020. "Lymphocystis Disease Virus (Iridoviridae) Enters Flounder (Paralichthys olivaceus) Gill Cells via a Caveolae-Mediated Endocytosis Mechanism Facilitated by Viral Receptors" International Journal of Molecular Sciences 21, no. 13: 4722. https://doi.org/10.3390/ijms21134722
APA StyleSheng, X., Zhong, Y., Zeng, J., Tang, X., Xing, J., Chi, H., & Zhan, W. (2020). Lymphocystis Disease Virus (Iridoviridae) Enters Flounder (Paralichthys olivaceus) Gill Cells via a Caveolae-Mediated Endocytosis Mechanism Facilitated by Viral Receptors. International Journal of Molecular Sciences, 21(13), 4722. https://doi.org/10.3390/ijms21134722





