Relevance of Interactions between Starch-based Coatings and Plum Fruit Surfaces: A Physical-Chemical Analysis
Abstract
1. Introduction
2. Results and Discussion
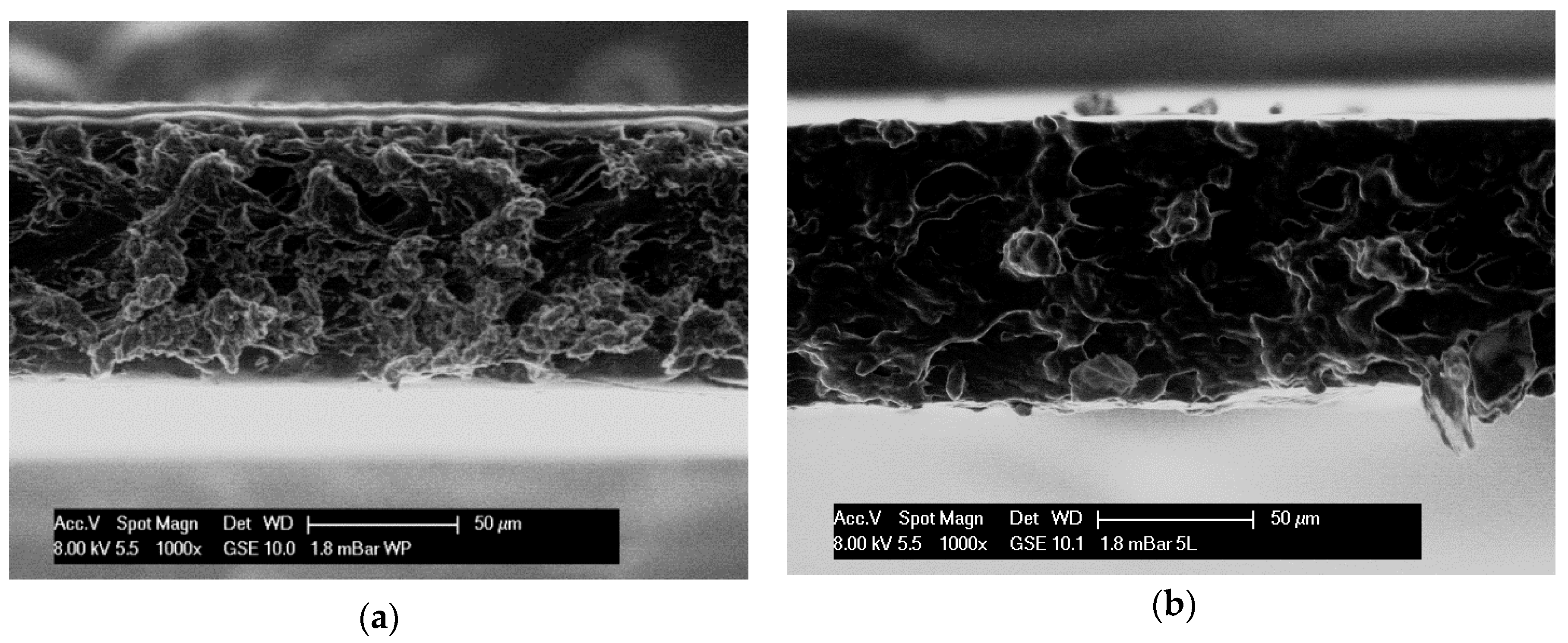
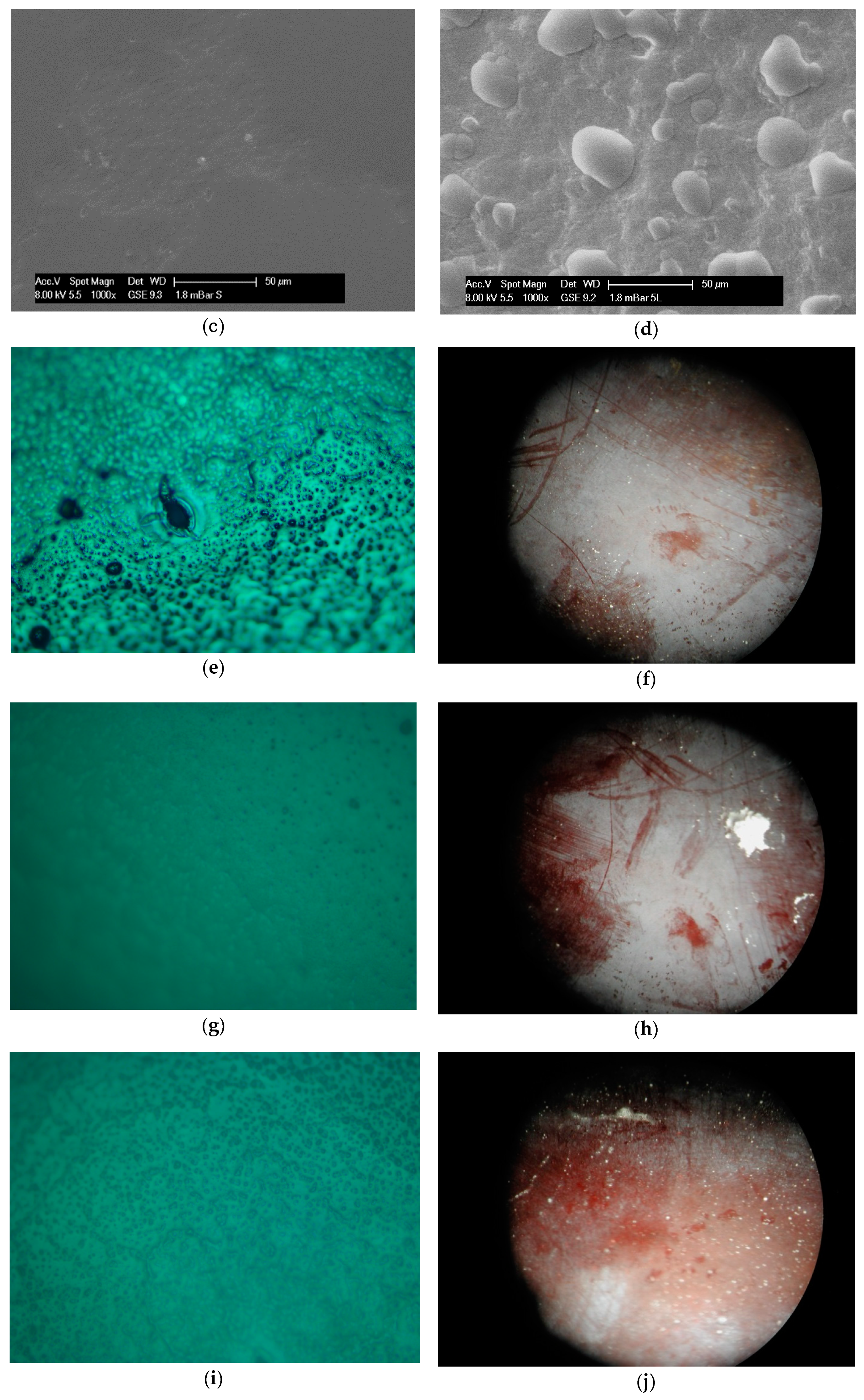
2.1. Structural and Visual Properties of Films and Coatings Applied onto the Surface of Plums
2.2. Surface Properties
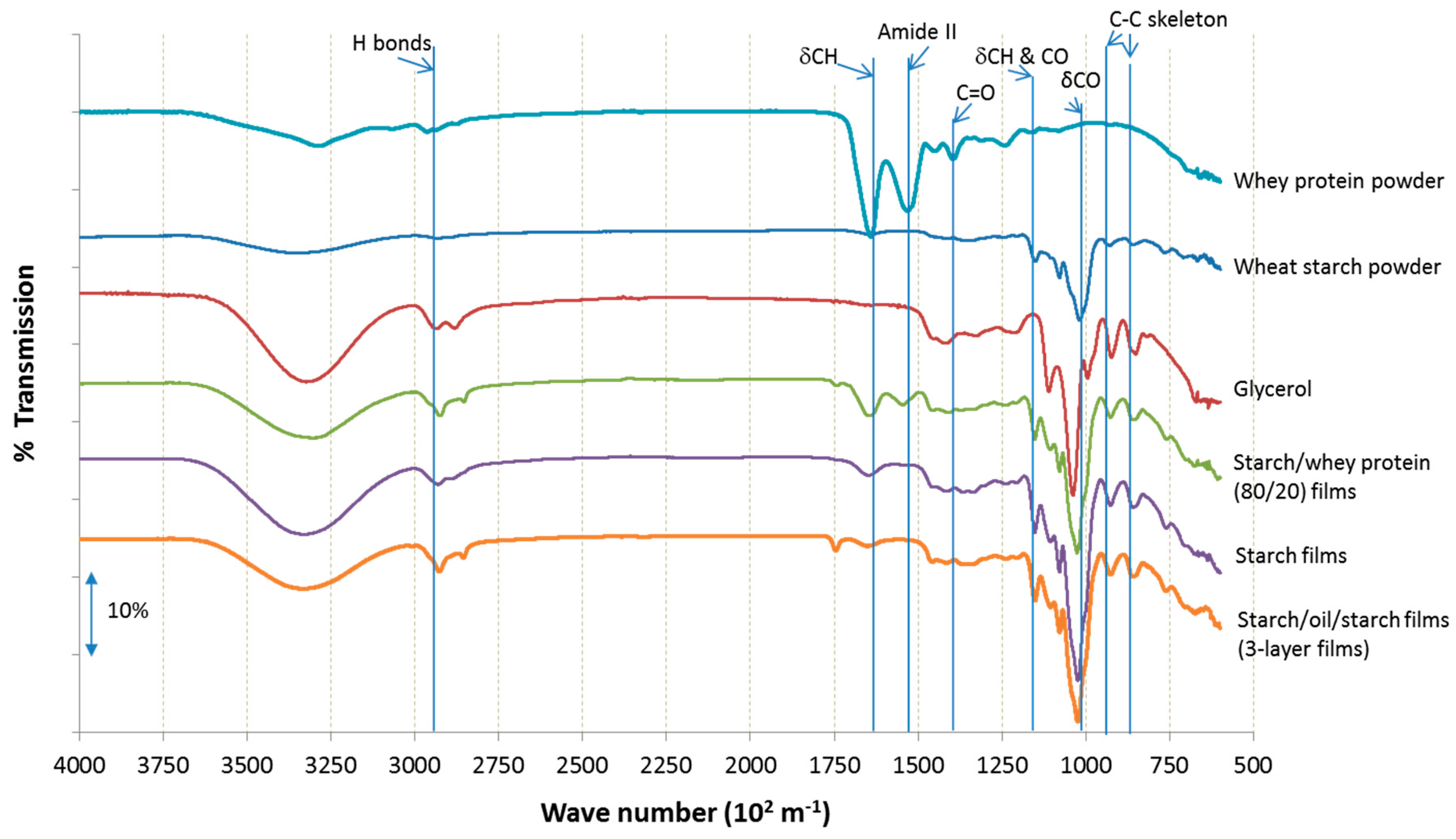
2.3. Molecular Interactions Involved in Coatings and Films Related to Surface Properties
3. Materials and Methods
3.1. Materials
3.2. Preparation of Starch and Whey Protein Edible Films and Coatings
3.3. Coating Thickness
3.4. Film Thickness
3.5. Surface Properties Measurements
3.5.1. Contact Angle Determination
3.5.2. Critical Surface Tension
3.5.3. Surface Tension
3.5.4. Adhesion, Cohesion, and Spreading Coefficient
3.6. Transport and Solubility Properties
3.6.1. Water Content of Films
3.6.2. Absorption Index
3.6.3. Solubility in Water
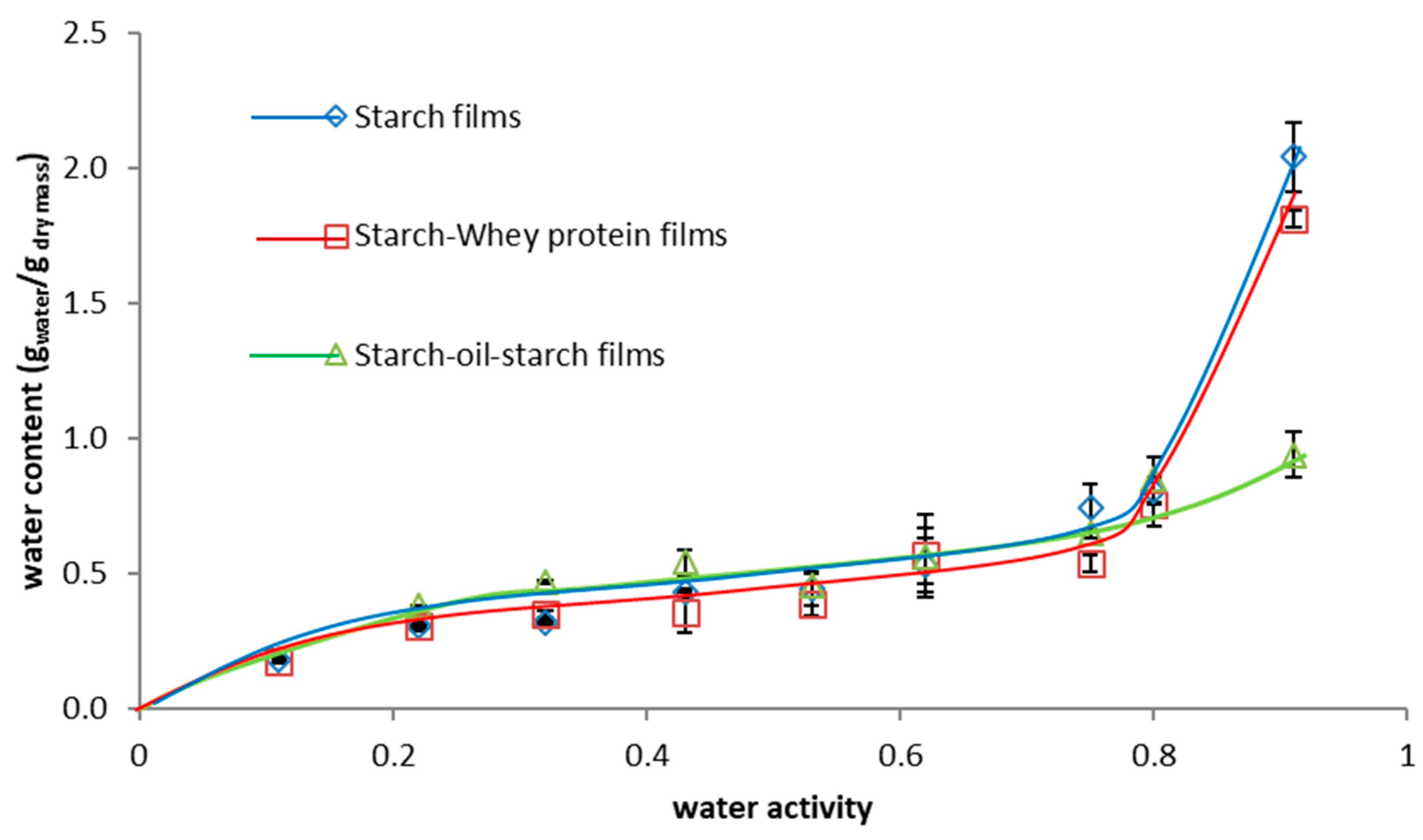
3.6.4. Moisture Sorption Isotherm
3.7. Water Vapour (WVP) and Oxygen (OP) Permeabilities of Films
3.8. Color Parameters
3.9. Microstructure Observations
3.10. Microscopy Observations
3.11. Fourier Transform Infrared with Attenuated Total Reflection (FTIR-ATR)
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Alves, V.D.; Mali, S.; Beléia, A.; Grossman, M.V.E. Effect of glycerol and amylose enrichment on cassava starch film properties. J. Food Eng. 2007, 78, 941–946. [Google Scholar] [CrossRef]
- Khalil, H.P.S.A.; Banerjee, A.; Saurabh, C.K.; Tye, Y.Y.; Suriani, A.B.; Mohamed, A.; Karim, A.A.; Rizal, S.; Paridah, M.T. Biodegradable Films for Fruits and Vegetables Packaging Application: Preparation and Properties. Food Eng. Rev. 2018, 10, 139–153. [Google Scholar] [CrossRef]
- Tihminlioglu, F.; DoğanAtik, I.; Özen, B. Water vapour and oxygen-barrier performance of corn-zein coated polypropylene films. J. Food Eng. 2010, 96, 342–347. [Google Scholar] [CrossRef]
- Garcia, L.C.; Pereira, L.M.; De Luca Sarantopoulos, C.I.G.; Hubinger, M.D. Selection of an Edible Starch Coating for Minimally Processed Strawberry. Food Bioprocess and Technol. 2010, 3, 834–842. [Google Scholar] [CrossRef]
- Eum, H.L.; Hwang, D.K.; Linke, M.; Lee, S.K.; Zude, M. Influence of edible coating on quality of plum (Prunussalicina Lindl. cv. ‘Sapphire’). European Food Researchand Technol. 2009, 229, 427–434. [Google Scholar] [CrossRef]
- Koch, K.; Gillgren, T.; Stading, M.; Andersson, R. Mechanical and structural properties of solution-cast high amylose maize starch films. Int. J. Biol. Macromol. 2010, 46, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Hou, H.; Guo, P.; Dong, H. Effects of extrusion and glycerol content on properties of oxidized and acetylated corn starch-based films. Carbohyd. Poly. 2012, 87, 707–712. [Google Scholar] [CrossRef]
- Ghanbarzadeh, B.; Almasi, H.; Entezami, A.A. Improving the barrier and mechanical properties of corn starch-based edible films: Effect of citric acid and carboxymethylcellulose. Ind. Crop Prod. 2011, 33, 229–235. [Google Scholar] [CrossRef]
- Jiménez, A.; Fabra, M.J.; Talens, P.; Chiralt, A. Effect of sodium caseinate on properties and ageing behaviour of corn starch based films. Food Hydrocolloids 2013, 29, 265–271. [Google Scholar] [CrossRef]
- Reinoso, E.; Mittal, G.S.; Lim, L.-T. Influence of Whey Protein Composite Coatings on Plum (Prunus domestica L.) Fruit Quality. Food Bioprocess Technol. 2008, 1, 314–325. [Google Scholar] [CrossRef]
- Baldwin, E.A.; Hagenmaier, R.; Bai, J. Edible Coatings and Films to Improve food Quality, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Basiak, E.; Lenart, A.; Debeaufort, F. Effect of oil lamination and starch content on structural and functional properties of starch-based films. Food Chemistry 2015, 195, 56–63. [Google Scholar] [CrossRef]
- Tharanathan, R.N. Biodegradable films and composite coatings: Past, present and future. Trends Food Sci. Technol. 2003, 14, 71–78. [Google Scholar] [CrossRef]
- Labuza, T.P.; Altunakar, B. Water Activity Prediction and Moisture Sorption Isotherms. In Water Activity in Foods: Fundamentals and Applications; Barbosa-Canovas, G.V., Fontana, A.J., Schmidt, S.J., Labuza, T.P., Eds.; Blackwell Publishing: Hoboken, NJ, USA, 2007; pp. 109–154. [Google Scholar]
- Pérez-Gallardo, A.; Bello-Pérez, L.A.; García-Almendárez, B.; Montejano-Gaitán, G.; Barbosa-Cánovas, G.V.; Regalado, C. Effect of structural characteristics of modified waxy corn starches on rheological properties, film-forming solutions, and on water vapour permeability, solubility, and opacity of films. Starch-Stärke 2011, 64, 27–36. [Google Scholar] [CrossRef]
- Linke, M.; Geyer, M. Condensation dynamics in plastic film packaging of fruit and vegetables. J. Food Eng. 2013, 116, 144–154. [Google Scholar] [CrossRef]
- Cordeiro, S.Z.; Simas, N.K.; de Oliveira Arruda, R.; Sato, A. Composition of epicuticular wax layer of two species of Mandevilla (Apocynoideae, Apocynaceae) from Rio de Janeiro, Brazil. Biochem. Syst. Ecol. 2011, 39, 198–202. [Google Scholar] [CrossRef]
- Knoche, M.; Peschel, S. Deposition and Strain of the Cuticle of Developing European Plum Fruit. J. Am. Soc. Hortic. Sci. 2007, 132, 597–602. [Google Scholar] [CrossRef]
- Banks, N.H.; Dadzie, B.K.; Cleland, D.J. Reducing gas exchange of fruits with surface coatings. Post. Biol. Technol. 1993, 3, 269–284. [Google Scholar] [CrossRef]
- Storey, R.; Price, W.E. Microstructure of the skin of d’Agen plums. Sci. Hortic. 1999, 81, 279–286. [Google Scholar] [CrossRef]
- Navarro-Tarazaga, M.L.; Massa, A.; Pérez-Gago, M.B. Effect of beeswax content on hydroxypropyl methylcellulose-based edible film properties and postharvest quality of coated plums (Cv. Angeleno). LWT- Food Sci. Technol. 2011, 44, 2328–2334. [Google Scholar] [CrossRef]
- Bickelmann, U. Vermarktungsnormen für Obst und Gemüse (In English: Marketing Standards for Fruit and Vegetables); AID: Weinanbau, Germany, 2008. (In German) [Google Scholar]
- Wisuthiphaet, N.; Damerow, L.; Blanke, M.M. Non-destructive detection of the wax bloom on European plum during post-harvest handling. J. Food Eng. 2014, 140, 46–51. [Google Scholar] [CrossRef]
- Baker, E.A. Chemistry and Morphology of Plant Epicuticular Waxes. In The Plant Cuticle; Price, D.F., Cutler, K.L., Alvin, C.E., Eds.; Springer: Berlin/Heidelberg, Germany, 1982; pp. 115–123. [Google Scholar]
- Judd, D.B. Chromaticity sensibility to stimulus differences. J. Optic. Soc. Am. 1931, 22, 72–108. [Google Scholar] [CrossRef]
- Mahy, M.; Van Eycken, L.; Oosterlinck, A. Evaluation of Uniform Color Spaces Developed after the Adoption of CIELAB and CIELUV. Color Res. Appl. 1994, 19, 105–121. [Google Scholar]
- Valero, D.; Díaz-Mula, H.M.; Zapata, P.J.; Guillén, F.; Martínez-Romero, D.; Castillo, S.; Serrano, M. Effects of alginate edible coating on preserving fruit quality in four plum cultivars during postharvest storage. Post. Biol. Technol. 2013, 77, 1–6. [Google Scholar] [CrossRef]
- Basiak, E.; Galus, S.; Lenart, A. Characterization of composite edible films based on wheat starch and whey-protein isolate. Int. J. Food Sci. Technol. 2015, 50, 372–380. [Google Scholar] [CrossRef]
- Basiak, E.; Lenart, A.; Debeaufort, F. Effects of carbohydrate/protein ratio effects on microstructure, barrier and sorption properties, of wheat starch–whey protein blend edible films. Int. J. Sci. Food Agri. 2017, 97, 858–867. [Google Scholar] [CrossRef]
- Basiak, E.; Lenart, A.; Debeaufort, F. Role of starch nature on the physico-chemical properties of edible films. Int. J. Biol. Macromol. 2017, 98, 348–356. [Google Scholar] [CrossRef]
- Madigan, M.T.; Martinko, J.M.; Bender, K.S.; Buckley, D.H.; Stahl, D.A.; Brock, T. Brock Biology of Microorganisms, 14th ed.; Pearson Education Inc.: San Francisco, CA, USA, 2014. [Google Scholar]
- Tortora, G.J.; Funke, B.R.; Case, C.L. Microbiology. An Introduction, 12th ed.; Pearson Education Inc.: San Francisco, CA, USA, 2015. [Google Scholar]
- Rux, G.; Mahajan, P.V.; Geyer, M.; Linke, M.; Pant, A.; Saengerlaub, S.; Caleb, O.J. Application of humidity-regulating tray for packaging of mushrooms. Post. Biol. Technol. 2015, 108, 102–110. [Google Scholar] [CrossRef]
- Elzayat, H.E.E.; Moline, H.E. Effect of Some Gas Mixtures on Storage Quality of Plums. J. Food Quality 1995, 18, 511–519. [Google Scholar] [CrossRef]
- Liang, J.; Ludescher, R.D. Effects of glycerol on the molecular ability and hydrogen bond network in starch matrix. Carbohyd. Poly. 2015, 115, 401–407. [Google Scholar] [CrossRef]
- Basiak, E.; Lenart, A.; Debeaufort, F. How glycerol and water contents affect the structural and functional properties of starch-based edible films. Polymers 2018, 10, 412. [Google Scholar] [CrossRef]
- Zaleska, H.; Ring, S.; Tomasik, P. Elektrosynthesis of potato starch-whey protein isolate complexes. Carbohyd. Poly. 2001, 45, 89–94. [Google Scholar] [CrossRef]
- Souza, R.C.R.; Andrade, C.T. Investigation of the gelatinization and extrusion processes of corn starch. Adv. Poly. Technol. 2002, 21, 17–24. [Google Scholar] [CrossRef]
- Stuart, B. Infrared Spectroscopy: Fundamentals and Applications; John Wiley & Sons: New York, NY, USA, 2004; pp. 113–135. [Google Scholar]
- Farjami, T.; Madadlou, A.; Labbafi, M. Characteristics of the bulk hydrogels made of the citric acid cross-linked whey protein microgels. Food Hydrocolloids 2015, 50, 159–165. [Google Scholar] [CrossRef]
- Casal, H.L.; Kohler, U.; Mantsch, H.H. Structural and conformational changes of β-lactoglobulin B: An infrared spectroscopic study of the effect of pH and temperature. BBA- Protein Structures 1988, 957, 11–20. [Google Scholar] [CrossRef]
- Barth, A. Infrared spectroscopy of proteins. Bioch. Biophys. Acta-Gen. Subjects 2007, 1767, 1073–1101. [Google Scholar] [CrossRef]
- Jackson, M.; Mantsch, H.H. The use and misuse of FTIR spectroscopy in the determination of protein structure. Crit. Rev. Biochem. Mol. Biol. 1995, 30, 95–120. [Google Scholar] [CrossRef]
- Mohammadian, M.; Madadlou, A. Characterization of fibrillated antioxidant whey protein hydrolysate and comparison with fibrillated protein solution. Food Hydrocolloid. 2016, 52, 221–230. [Google Scholar] [CrossRef]
- Hussain, R.; Gaiani, C.; Jeandel, C.; Ghanbaja, J.; Scher, J. Combined effect of heat treatment and ionic strength on the functionality of whey proteins. J. Dairy Sci. 2012, 95, 6260–6273. [Google Scholar] [CrossRef]
- Guerrero, P.; Retegi, A.; Gabilondo, N.; de la Caba, K. Mechanical and thermal properties of soy protein films processed by casting and compression. J. Food Eng. 2010, 100, 145–151. [Google Scholar] [CrossRef]
- Young, T. An Essay on the Cohesion of Fluids. Philosoph. Transact. Royal Soc. London 1805, 95, 65–87. [Google Scholar] [CrossRef]
- Karbowiak, T.; Debeaufort, F.; Voilley, A. Wetting properties at the surface of iota-carrageenan-based edible films. J. Colloid. Interface Sci. 2006, 294, 400–410. [Google Scholar] [CrossRef]
- Debeaufort, F.; Martin-Polo, M.; Voilley, A. Polarity and structure affect water vapour permeability of model edible films. J. Food Sci. 1993, 58, 428–434. [Google Scholar] [CrossRef]
- Song, B.; Springer, J. Determination of Interfacial Tension from the Profile of a Pendant Drop Using Computer-Aided Image Processing: 1. Theoretical. J. Colloid. Interface Sci. 1996, 184, 64–76. [Google Scholar]
- Song, B.; Springer, J. Determination of Interfacial Tension from the Profile of a Pendant Drop Using Computer-Aided Image Processing: 2. Experimental. J. Interface Sci 1996, 184, 77–91. [Google Scholar]
- Zisman, W.A. Contact Angle, Wettability and Adhesion; Fowkes, F.M., Ed.; American Chemical Society: Washington, DC, USA, 1964; pp. 1–51. [Google Scholar]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Poly. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Dupre, A. Théorie Mécanique de La Chaleur (The Mechanical Theory of Heat); Gauthier-Villan: Paris, France, 1869. (In French) [Google Scholar]
- Gontard, D.; Duchaz, C.; Cuq, J.; Guilbert, S. Edible composite films of wheat gluten and lipids: Water vapour permeability and other physical properties. Int. J. Food Sci. Technol. 1994, 29, 39–50. [Google Scholar] [CrossRef]
- ISO 9932. Standard Test Methods for Water Vapor Transmission of Materials; Method E96–80; ASTM International: West Conshohocken, PA, USA, 1990. [Google Scholar]
- ISO 15105-1. Standard Test Methods Using a GDP-C for Gas Permeability, Permeation; American Society for Testing and Materials: West Conshohocken, PA, USA, 2007. [Google Scholar]
- Gennadios, A.; Weller, C.L.; Hanna, M.A.; Froning, G.W. Mechanical and barrier properties of egg albumen films. J. Food Eng. 1996, 61, 585–589. [Google Scholar] [CrossRef]
- Sobral, P.J.; dos Santos, J.S.; Garcia, F.T. Effect of protein and plasticizer concentration in film forming solutions on physical properties of edible films based on muscle proteins of a Thai Tilapia. J. Food Eng. 2005, 70, 93–100. [Google Scholar] [CrossRef]
| Surface | L | a | b | ΔE | C |
|---|---|---|---|---|---|
| Uncoated plums (control) | 54.43 ± 4.90 a | 2.23 ± 0.37 b | −8.18 ± 1.73 a | - | 8.48 b |
| Starch film | 96.20 ± 0.31 b | 0.32 ± 0.10 a | 3.72 ± 0.61 b | 1.52 a | 4.02 a |
| Starch-whey protein (80/20) films | 95.13 ± 0.32 b | 0.46 ± 0.11 a | 4.36 ± 0.73 b | 2.59 b | 5.08 a |
| Starch coated plums | 54.45 ± 11.04 a | 4.61 ± 1.37 c | −8.93 ± 3.31 a | 2.49 b | 10.04 b |
| Starch-whey protein (80/20) coated plums | 54.01 ± 11.42 a | 3.64 ± 1.21 c | −7.76 ± 3.34 a | 1.53 a | 8.57 b |
| Film Characteristics | Film Composition | ||||
|---|---|---|---|---|---|
| Starch-Whey Protein (80/20) | Starch | Starch-Oil-Starch (3-layer) | |||
| Thickness (µm) | 79.7 ± 11.2 b | 80.8 ± 12.59 b | 27.7 ± 5.69 a | ||
| Surface properties | Contact angle (o) | Water (γL = 72.8 mNm−1) | 61 b | 43 a | 77 c |
| Surface free energy (m N m−1) | γSD | 38.0 a,b | 35.4 a | 40.6 b | |
| γSP | 22.9 b | 24.9 b | 15.5 a | ||
| γS | 60.9 b | 60.3 b | 56.1 a | ||
| WA (mJ m−2) | 125.86 b | 126.80 b | 115.12 a | ||
| WC (mJ m−2) | 121.80 b | 120.56 b | 112.12 a | ||
| WS (mJ m−2) | −4.06 a | −6.24 b | −3.00 a | ||
| Transport and solubility properties | Swelling index (%) | 53.49 ± 1.86 c | 39.20 ± 1.43 b | 34.91 ± 1.39 a | |
| Water content (kg water kg−1dm) | 2.04 ± 0.05 a | 3.24 ± 0.50 c | 2.69 ± 0.21 b,c | ||
| Solubility in water (%) | 13.06 ± 0.18 b | 19.67 ± 0.17 c | 10.70 ± 0.72 a | ||
| Water vapour permeability (10−10 g m−1 s−1 Pa−1) | 33–0% RH | 4.99 ± 0.06 b | 5.24 ± 0.26 b | 0.57 ±0.46 a | |
| 75–30% RH | 6.37 ± 1.03 c | 7.70 ± 0.85 c | 3.55 ± 2.80 b | ||
| 100–30% RH | 7.16 ± 0.54 c | 7.87 ± 0.65 c | 3.40 ± 0.31 b | ||
| Oxygen permeability (10−14 cm3 m−1 s−1 Pa−1) | 53% RH | 7.44 ± 1.07 c | 7.23 ± 1.00 c | 0.96 ± 0.02 a | |
| 75% RH | 11.69 ± 0.86 d | 7.41 ± 1.39 c | 1.12 ± 0.05 b | ||
| Film | Wheat Starch (S) | Whey Protein Isolate (WPI) | Rapeseed Oil (O) | Glycerol | Water |
|---|---|---|---|---|---|
| (g) | (g) | (g) | (g) | (g) | |
| Starch | 5 | 0 | 0 | 2.5 | 95 |
| starch-whey proteins (80/20) | 4 | 1 | 0 | 2.5 | 95 |
| Starch-oil-starch (3-layer) | 5 | 0 | 3 | 2.5 | 95 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basiak, E.; Geyer, M.; Debeaufort, F.; Lenart, A.; Linke, M. Relevance of Interactions between Starch-based Coatings and Plum Fruit Surfaces: A Physical-Chemical Analysis. Int. J. Mol. Sci. 2019, 20, 2220. https://doi.org/10.3390/ijms20092220
Basiak E, Geyer M, Debeaufort F, Lenart A, Linke M. Relevance of Interactions between Starch-based Coatings and Plum Fruit Surfaces: A Physical-Chemical Analysis. International Journal of Molecular Sciences. 2019; 20(9):2220. https://doi.org/10.3390/ijms20092220
Chicago/Turabian StyleBasiak, Ewelina, Martin Geyer, Frédéric Debeaufort, Andrzej Lenart, and Manfred Linke. 2019. "Relevance of Interactions between Starch-based Coatings and Plum Fruit Surfaces: A Physical-Chemical Analysis" International Journal of Molecular Sciences 20, no. 9: 2220. https://doi.org/10.3390/ijms20092220
APA StyleBasiak, E., Geyer, M., Debeaufort, F., Lenart, A., & Linke, M. (2019). Relevance of Interactions between Starch-based Coatings and Plum Fruit Surfaces: A Physical-Chemical Analysis. International Journal of Molecular Sciences, 20(9), 2220. https://doi.org/10.3390/ijms20092220







