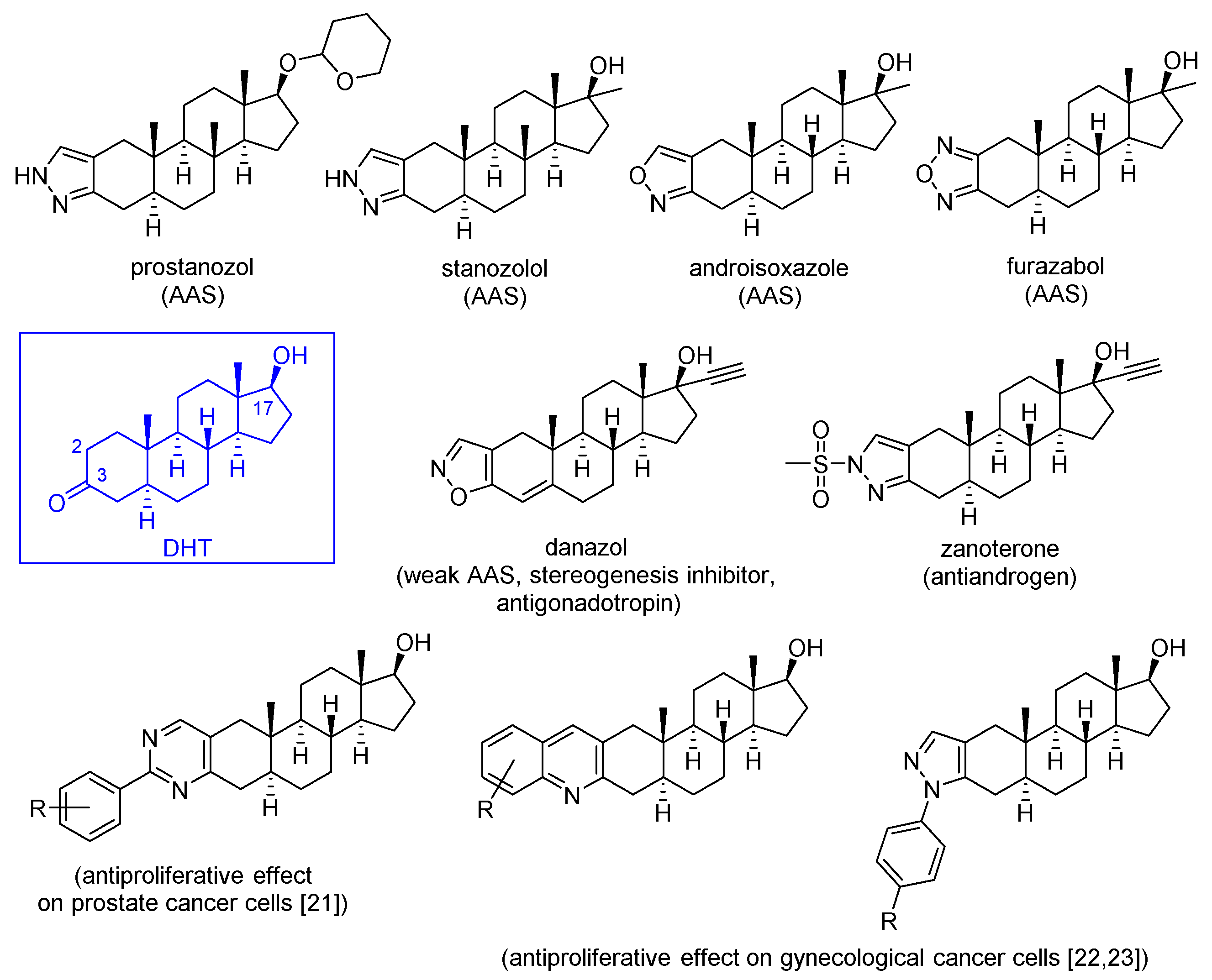
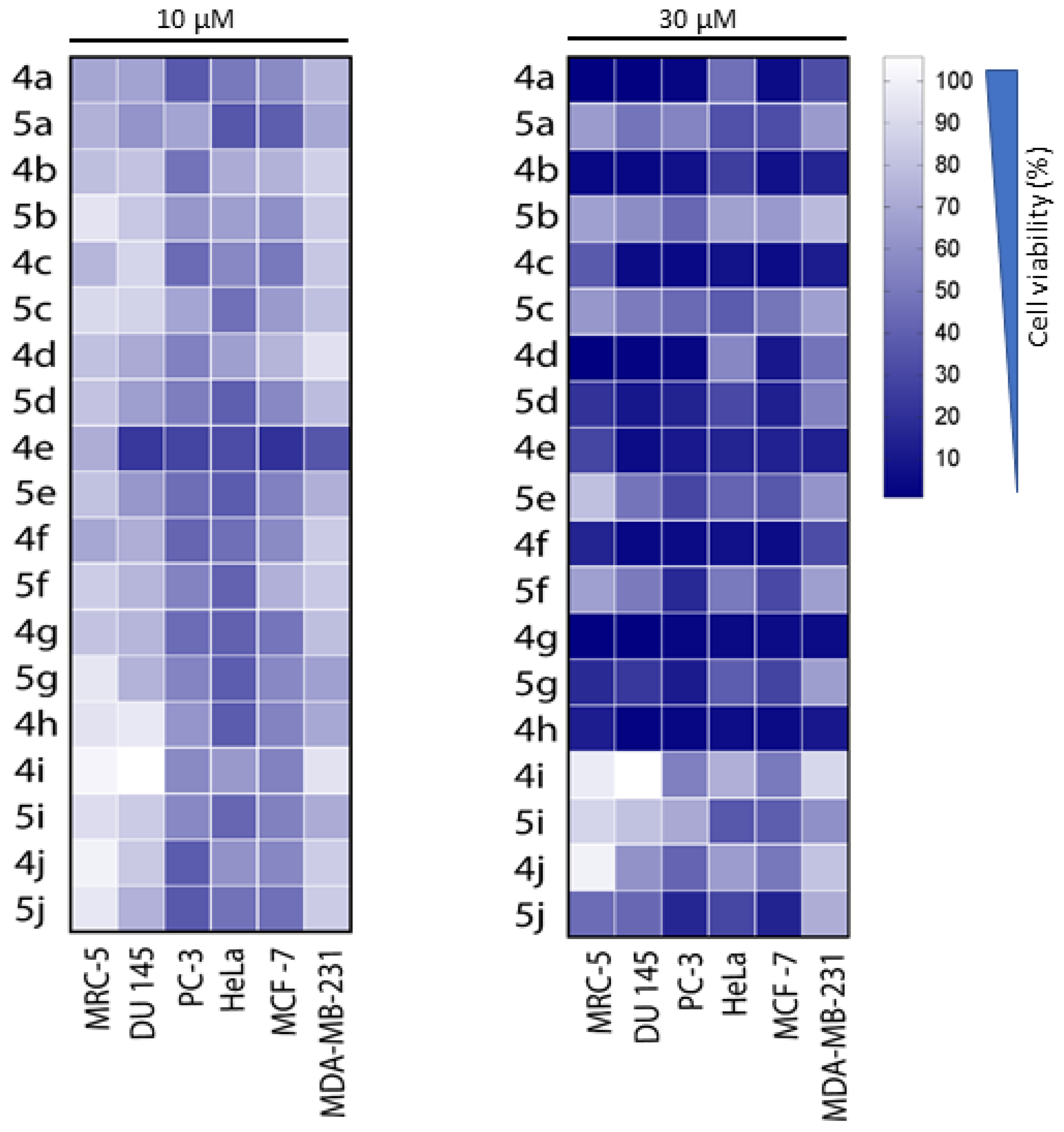
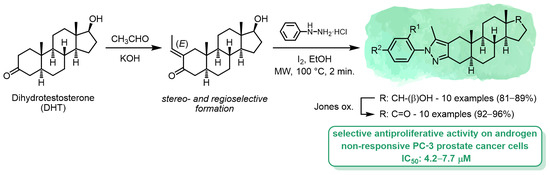
3.1. Chemistry
3.1.1. General Information
Reagents and materials were purchased from commercial suppliers (TCI, Tokyo, Japan; Alfa Aesar, Haverhill, MA, USA and Sigma-Aldrich Corporation, St. Louis, MO, USA). All solvents were dried and purified according to standard procedures. MW-assisted reactions were carried out with a CEM Discover SP instrument (CEM Corporation, Matthews, NC, USA) using a maximum power of 200 W with dynamic control program. Thin layer chromatography (TLC) was carried out on Kieselgel-G (Si 254 F, Merck KGaA, Darmstadt, Germany) plates (0.25 mm thick). The spots were detected by spraying with phosphomolybdic acid (5%) in aqueous phosphoric acid (50%) or visualized with UV light 254 nm. The products were purified with preparative column chromatography on Merck silica gel 60, 40–63 μm (Merck KGaA). Melting points (Mps) were measured on an SRS Optimelt digital device (Stanford Research Systems Inc, Sunnyvale, CA, USA). Elementary analysis data were obtained with a PerkinElmer CHN analyzer model 2400 (PerkinElmer Inc, Waltham, MA, USA). NMR spectra were recorded at 298 K with a Bruker DRX 500 instrument (Bruker, Billerica, MA, USA). Chemical shifts are reported in ppm (δ scale) and coupling constants (J) in Hz. The 1H resonance signals are indicated as a singlet (s), a doublet (d), a double doublet (dd), a triplet (t), a triplet of doublets (td) or a multiplet (m). 13C NMR spectra are 1H-decoupled. The J-MOD pulse sequence was applied to determine multiplicities. An HPLC/MSD system was used for automated flow injection analyses. Components of the system: An Agilent 1100 micro vacuum degasser (Agilent Technologies, Santa Clara, CA, USA) a quaternary pump, a micro-well plate autoinjector and a 1946A MSD equipped with an electrospray ion source (ESI) operated in positive ion mode. ESI parameters: Nebulizing gas N2, at 35 psi; drying gas N2, at 350 °C and 12 L/min; capillary voltage 3000 V; fragmentor voltage 70 V. The MSD was operated in scan mode with a mass range of m/z 60−620. Samples (0.2 μL) were injected directly into the solvent flow (0.3 mL/min) of CH3CN/H2O 70:30 (v/v) supplemented with 0.1% formic acid with automated needle wash. Agilent LC/MSD Chemstation software (C.01.08, Agilent Technologies Inc) was used to control the system.
3.1.2. Synthesis of 2-Ethylidene-DHT (1)
DHT (2.9 g, 10 mmol) and KOH (561 mg, 10 mmol) were dissolved in absolute EtOH (40 mL), and the homogenous solution was cooled to approximately −10 °C in an ice bath containing sodium chloride. (Cooling was applied during the whole procedure.) Afterward, acetaldehyde (1.01 mL, 18 mmol) was added to the mixture and it was stirred at the given temperature. After 1.5 h, a further amount of acetaldehyde (0.25 mL, 4.5 mmol) was added to the reaction mixture and it was stirred for 2 h. After completion of the reaction, the pH was adjusted to around 7 with 1 M H2SO4 solution in EtOH, and the mixture was evaporated in vacuo. The residual oil was dissolved in EtOAc and washed with water (2 x 10 mL), then the combined organic layers were dried over anhydrous Na2SO4 and evaporated in vacuo. The crude product was purified by column chromatography with EtOAc/CH2Cl2 = 2:98. Yield: 2.2 g (white solid); Mp 137–141 °C; Anal. Calcd. for C21H32O2 (316.49): C, 79.70; H, 10.19. Found: C, 79.84; H, 10.25. 1H NMR (CDCl3, 500 MHz): δ 0.76 (s, 3H, 18-H3), 0.81 (s, 3H, 19-H3), 0.83–1.01 (overlapping m, 3H), 1.11 (m, 1H), 1.18–1.31 (overlapping m, 2H), 1.35–1.47 (overlapping m, 4H), 1.58–1.70 (overlapping m, 5H), 1.72 (dd, 3H, J = 7.2 Hz, J = 2.2 Hz, 21-H3), 1.84 (d, 1H, J = 15.2 Hz, 1α-H), 1.87 (m, 1H), 2.06 (m, 1H, 16α-H), 2.14 (dd, 1H, J = 18.5 Hz, J = 13.2 Hz, 4β-H), 2.33 (dd, 1H, J = 18.5 Hz, J = 5.2 Hz, 4α-H), 2.76 (d, 1H, J = 15.2 Hz, 1β-H), 3.65 (t, 1H, J = 8.6 Hz, 17α-H), 6.74 (m, 1H, 20-H); 13C NMR (CDCl3, 125 MHz): δ 11.0 (C-18), 11.8 (C-19), 13.6 (C-21), 20.9 (C-11), 23.4 (C-15), 28.4 (C-6), 30.5 (C-16), 31.0 (C-7), 35.4 (C-8), 35.6 (C-10), 36.6 (C-12), 39.7 (C-1), 42.5 (C-5), 42.7 (C-4), 42.8 (C-13), 50.9 (C-14), 53.7 (C-9), 81.8 (C-17), 135.7 (C-20), 136.1 (C-2), 200.8 (C-3); ESI-MS 317 [M+H]+.
3.1.3. General Procedure for the One-Pot Synthesis of Ring A-Condensed Pyrazoles 4a–j under MW Irradiation (Method C)
To a solution of 1 (187 mg, 0.60 mmol) in absolute EtOH (5 mL), I2 (152 mg, 0.6 mmol) and (substituted) phenylhydrazine hydrochloride (2a–j, 1.20 mmol) were added and the mixture was irradiated in a closed vessel at 100 °C for 2 min. After completion of the reaction, the mixture was poured into saturated aqueous solution of Na2S2O3 (10 mL) and extracted with CH2Cl2 (3× 10 mL). The combined organic layers were dried over anhydrous Na2SO4 and concentrated in vacuo. The crude product was purified by column chromatography with EtOAc/CH2Cl2 = 5:95.
17β-Hydroxy-1′-phenyl-5′-methylpyrazolo[3′,4′:3,2]-5α-androstane (4a)
According to the general procedure, phenylhydrazine hydrochloride (2a, 174 mg) was used. Yield: 206 mg (85%, white solid); Mp 241–245 °C; Anal. Calcd. for C27H36N2O (404.60): C, 80.15; H, 8.97. Found: C, 80.02; H, 8.90. 1H NMR (CDCl3, 500 MHz): δ 0.77 (s, 3H, 18-H3), 0.81 (s, 3H, 19-H3), 0.84–1.02 (overlapping m, 3H, 9α-H, 7α-H and 14α-H), 1.11 (td, 1H, J = 12.7, J = 3.7 Hz, 12α-H), 1.27 (m, 1H, 15β-H), 1.33–1.51 (overlapping m, 4H, 6β-H, 8β-H, 16β-H and 11β-H), 1.53–1.66 (overlapping m, 3H, 5α-H, 15α-H and 6α-H), 1.67–1.75 (overlapping m, 2H, 11α-H and 7β-H), 1.86 (m, 1H, 12β-H), 2.05 (d + m, 2H, J = 15.0 Hz, 1α-H and 16α-H), 2.21 (s, 3H, 5′-CH3), 2.34 (dd, 1H, J = 16.8 Hz, J = 11.9 Hz, 4β-H), 2.52 (d, 1H, J = 15.0 Hz, 1β-H), 2.67 (dd, 1H, J = 16.8 Hz, J = 5.1 Hz, 4α-H), 3.65 (t, 1H, J = 8.6 Hz, 17α-H), 7.32 (m, 1H, 4″-H), 7.43 (d, 4H, J = 4.4 Hz, 2″-H, 3″-H, 5″-H and 6″-H); 13C NMR (CDCl3, 125 MHz): δ 10.9 (5′-CH3), 11.0 (C-18), 11.8 (C-19), 20.8 (C-11), 23.4 (C-15), 27.4 (C-4), 29.2 (C-6), 30.5 (C-16), 31.3 (C-7), 34.8 (C-1), 35.8 (C-8), 36.2 (C-10), 36.7 (C-12), 42.4 (C-5), 42.8 (C-13), 50.9 (C-14), 54.0 (C-9), 81.9 (C-17), 115.1 (C-2), 124.5 (2C, C-2″ and C-6″), 127.1 (C-4″), 129.0 (2C, C-3″ and C-5″), 135.4 (C-5′), 139.6 (C-1″), 148.4 (C-3); ESI-MS 405 [M+H]+.
17β-Hydroxy-1′-(2″-tolyl)-5′-methylpyrazolo[3′,4′:3,2]-5α-androstane (4b)
According to the general procedure, 2-tolylhydrazine hydrochloride (2b, 190 mg) was used. Yield: 221 mg (88%, white solid); Mp 225–227 °C; Anal. Calcd. for C28H38N2O (418.63): C, 80.34; H, 9.15. Found: C, 80.27; H, 9.22. 1H NMR (CDCl3, 500 MHz): δ 0.77 (s, 3H, 18-H3), 0.81 (s, 3H, 19-H3), 0.85–1.01 (overlapping m, 3H, 9α-H, 7α-H and 14α-H), 1.11 (td, 1H, J = 13.0, J = 3.7 Hz, 12α-H), 1.27 (m, 1H, 15β-H), 1.35–1.51 (overlapping m, 4H, 6β-H, 8β-H, 16β-H and 11β-H), 1.56–1.65 (overlapping m, 3H, 5α-H, 15α-H and 6α-H), 1.67–1.76 (overlapping m, 2H, 11α-H and 7β-H), 1.86 (m, 1H, 12β-H), 1.95 (s, 3H), 2.04 (s, 3H), 2.08 (d + m, 2H, J = 15.2 Hz, 1α-H and 16α-H), 2.21 (s, 3H, 5′-CH3), 2.34 (dd, 1H, J = 16.2 Hz, J = 12.4 Hz, 4β-H), 2.52 (d, 1H, J = 15.2 Hz, 1β-H), 2.67 (dd, 1H, J = 16.2 Hz, J = 4.5 Hz, 4α-H), 3.65 (t, 1H, J = 7.8 Hz, 17α-H), 7.19–7.33 (overlapping m, 4H, 3″-H, 4″-H, 5″-H and 6″-H); 13C NMR (CDCl3, 125 MHz): δ 9.7 (5′-CH3), 11.1 (C-18), 11.8 (C-19), 17.3 (2″-CH3), 20.8 (C-11), 23.4 (C-15), 27.5 (C-4), 29.3 (C-6), 30.6 (C-16), 31.4 (C-7), 34.8 (C-1), 35.8 (C-8), 36.3 (C-10), 36.7 (C-12), 42.5 (C-5), 42.8 (C-13), 51.0 (C-14), 54.2 (C-9), 81.9 (C-17), 113.1 (C-2), 126.3 (CH), 128.0 (CH), 128.8 (CH), 130.8 (CH), 136.1, 136.2 and 138.6 (C-5′, C-1″ and C-2″), 147.8 (C-3); ESI-MS 419 [M+H]+.
17β-Hydroxy-1′-(4″-tolyl)-5′-methylpyrazolo[3′,4′:3,2]-5α-androstane (4c)
According to the general procedure, 4-tolylhydrazine hydrochloride (2c, 190 mg) was used. Yield: 206 mg (82%, white solid); Mp 247–248 °C; Anal. Calcd. for C28H38N2O (418.63): C, 80.34; H, 9.15. Found: C, 80.45; H, 9.24. 1H NMR (CDCl3, 500 MHz): δ 0.77 (s, 3H, 18-H3), 0.81 (s, 3H, 19-H3), 0.83–1.01 (overlapping m, 3H, 9α-H, 7α-H and 14α-H), 1.11 (td, 1H, J = 12.7, J = 3.5 Hz, 12α-H), 1.28 (m, 1H, 15β-H), 1.34–1.50 (overlapping m, 4H, 6β-H, 8β-H, 16β-H and 11β-H), 1.54–1.66 (overlapping m, 3H, 5α-H, 15α-H and 6α-H), 1.67–1.76 (overlapping m, 2H, 11α-H and 7β-H), 1.86 (m, 1H, 12β-H), 2.06 (d + m, 2H, J = 15.0 Hz, 1α-H and 16α-H), 2.18 (s, 3H, 5′-CH3), 2.34 (dd, 1H, J = 16.8 Hz, J = 12.3 Hz, 4β-H), 2.38 (4″-CH3), 2.51 (d, 1H, J = 15.0 Hz, 1β-H), 2.67 (dd, 1H, J = 16.8 Hz, J = 4.8 Hz, 4α-H), 3.65 (t, 1H, J = 8.4 Hz, 17α-H), 7.22 (d, 2H, J = 8.1 Hz, 3″-H and 5″-H), 7.30 (d, 2H, J = 8.1 Hz, 2″-H and 6″-H); 13C NMR (CDCl3, 125 MHz): δ 10.8 (5′-CH3), 11.0 (C-18), 11.8 (C-19), 20.8 (C-11), 21.0 (4″-CH3), 23.4 (C-15), 27.5 (C-4), 29.2 (C-6), 30.6 (C-16), 31.4 (C-7), 34.9 (C-1), 35.8 (C-8), 36.3 (C-10), 36.8 (C-12), 42.5 (C-5), 42.8 (C-13), 51.0 (C-14), 54.1 (C-9), 81.9 (C-17), 114.7 (C-2), 124.5 (2C, C-2″ and C-6″), 129.5 (2C, C-3″ and C-5″), 136.9, 137.2 and 137.4 (C-5′, C-1″ and C-4″), 148.2 (C-3); ESI-MS 419 [M+H]+.
17β-Hydroxy-1′-(2″,4″-dimethylphenyl)-5′-methylpyrazolo[3′,4′:3,2]-5α-androstane (4d)
According to the general procedure, 2,4-dimethylphenylhydrazine hydrochloride (2d, 207 mg) was used. Yield: 208 mg (80%, white solid); Mp 209–211 °C; Anal. Calcd. for C29H40N2O (432.65): C, 80.51; H, 9.32. Found: C, 80.40; H, 9.21. 1H NMR (CDCl3, 500 MHz): δ 0.77 (s, 3H, 18-H3), 0.81 (s, 3H, 19-H3), 0.84–1.02 (overlapping m, 3H, 9α-H, 7α-H and 14α-H), 1.11 (td, 1H, J = 12.7, J = 3.5 Hz, 12α-H), 1.28 (m, 1H, 15β-H), 1.34–1.51 (overlapping m, 4H, 6β-H, 8β-H, 16β-H and 11β-H), 1.55–1.65 (overlapping m, 3H, 5α-H, 15α-H and 6α-H), 1.66–1.75 (overlapping m, 2H, 11α-H and 7β-H), 1.85 (m, 1H, 12β-H), 1.93 (s, 3H), 1.98 (s, 3H), 2.07 (d + m, 2H, J = 15.0 Hz, 1α-H and 16α-H), 2.32 (m, 1H, 4β-H), 2.35 (s, 3H, 4″-CH3), 2.50 (d, 1H, J = 15.0 Hz, 1β-H), 2.65 (dd, 1H, J = 16.6 Hz, J = 4.4 Hz, 4α-H), 3.64 (t, 1H, J = 8.4 Hz, 17α-H), 7.01–7.10 (overlapping m, 3H, 3″-H, 5″-H and 6″-H); 13C NMR (CDCl3, 125 MHz): δ 9.7 (5′-CH3), 11.1 (C-18), 11.7 (C-19), 17.2 (2″-CH3), 20.8 (C-11), 21.1 (4″-CH3), 23.4 (C-15), 27.5 (C-4), 29.3 (C-6), 30.6 (C-16), 31.4 (C-7), 34.8 (C-1), 35.8 (C-8), 36.3 (C-10), 36.8 (C-12), 42.5 (C-5), 42.8 (C-13), 51.0 (C-14), 54.2 (C-9), 81.9 (C-17), 113.0 (C-2), 126.9, 127.7 and 131.4 (C-3″, C-5″ and C-6″), 135.8, 135.9, 136.2 and 138.6 (C-5′, C-1″, C-2″ and C-4″), 147.7 (C-3); ESI-MS 433 [M+H]+.
17β-Hydroxy-1′-(4″-methoxyphenyl)-5′-methylpyrazolo[3′,4′:3,2]-5α-androstane (4e)
According to the general procedure, 4-methoxyphenylhydrazine hydrochloride (2e, 210 mg) was used. Yield: 232 mg (89%, white solid); Mp 257–259 °C; Anal. Calcd. for C28H38N2O2 (434.62): C, 77.38; H, 8.81. Found: C, 77.49; H, 8.89. 1H NMR (CDCl3, 500 MHz): δ 0.77 (s, 3H, 18-H3), 0.80 (s, 3H, 19-H3), 0.84–1.02 (overlapping m, 3H, 9α-H, 7α-H and 14α-H), 1.11 (td, 1H, J = 12.7, J = 3.2 Hz, 12α-H), 1.28 (m, 1H, 15β-H), 1.33–1.50 (overlapping m, 4H, 6β-H, 8β-H, 16β-H and 11β-H), 1.52–1.65 (overlapping m, 3H, 5α-H, 15α-H and 6α-H), 1.67–1.75 (overlapping m, 2H, 11α-H and 7β-H), 1.86 (m, 1H, 12β-H), 2.06 (d + m, 2H, J = 15.0 Hz, 1α-H and 16α-H), 2.15 (s, 3H, 5′-CH3), 2.34 (dd, 1H, J = 16.5 Hz, J = 12.2 Hz, 4β-H), 2.50 (d, 1H, J = 15.0 Hz, 1β-H), 2.66 (dd, 1H, J = 16.5 Hz, J = 4.7 Hz, 4α-H), 3.65 (t, 1H, J = 8.4 Hz, 17α-H), 3.83 (4″-OMe), 6.94 (d, 2H, J = 8.4 Hz, 3″-H and 5″-H), 7.32 (d, 2H, J = 8.4 Hz, 2″-H and 6″-H); 13C NMR (CDCl3, 125 MHz): δ 10.7 (5′-CH3), 11.0 (C-18), 11.8 (C-19), 20.8 (C-11), 23.4 (C-15), 27.5 (C-4), 29.2 (C-6), 30.6 (C-16), 31.4 (C-7), 34.9 (C-1), 35.8 (C-8), 36.3 (C-10), 36.8 (C-12), 42.5 (C-5), 42.8 (C-13), 51.0 (C-14), 54.1 (C-9), 55.5 (4″-OMe), 81.9 (C-17), 114.1 (2C, C-2″ and C-6″), 114.4 (C-2), 126.1 (2C, C-3″ and C-5″), 133.1 (C-1″), 135.3 (C-5′), 148.0 (C-3), 158.6 (C-4″); ESI-MS 435 [M+H]+.
17β-Hydroxy-1′-(4″-fluorophenyl)-5′-methylpyrazolo[3′,4′:3,2]-5α-androstane (4f)
According to the general procedure, 4-fluorophenylhydrazine hydrochloride (2f, 195 mg) was used. Yield: 208 mg 82%, (white solid); Mp 244–246 °C; Anal. Calcd. for C27H35FN2O (422.59): C, 76.74; H, 8.35. Found: C, 76.85; H, 8.22. 1H NMR (CDCl3, 500 MHz): δ 0.77 (s, 3H, 18-H3), 0.80 (s, 3H, 19-H3), 0.84–1.01 (overlapping m, 3H, 9α-H, 7α-H and 14α-H), 1.11 (td, 1H, J = 12.8, J = 4.2 Hz, 12α-H), 1.28 (m, 1H, 15β-H), 1.34–1.50 (overlapping m, 4H, 6β-H, 8β-H, 16β-H and 11β-H), 1.53–1.65 (overlapping m, 3H, 5α-H, 15α-H and 6α-H), 1.66–1.75 (overlapping m, 2H, 11α-H and 7β-H), 1.86 (m, 1H, 12β-H), 2.06 (d + m, 2H, J = 15.1 Hz, 1α-H and 16α-H), 2.17 (s, 3H, 5′-CH3), 2.33 (dd, 1H, J = 16.8 Hz, J = 12.1 Hz, 4β-H), 2.51 (d, 1H, J = 15.1 Hz, 1β-H), 2.66 (dd, 1H, J = 16.5 Hz, J = 5.2 Hz, 4α-H), 3.64 (t, 1H, J = 8.5 Hz, 17α-H), 7.12 (t, 2H, J = 8.5 Hz, 3″-H and 5″-H), 7.39 (m, 2H, 2″-H and 6″-H); 13C NMR (CDCl3, 125 MHz): δ 10.7 (5′-CH3), 11.0 (C-18), 11.8 (C-19), 20.8 (C-11), 23.4 (C-15), 27.4 (C-4), 29.2 (C-6), 30.5 (C-16), 31.3 (C-7), 34.8 (C-1), 35.8 (C-8), 36.3 (C-10), 36.8 (C-12), 42.5 (C-5), 42.8 (C-13), 51.0 (C-14), 54.1 (C-9), 81.9 (C-17), 115.0 (C-2), 115.8 (2C, J = 23.0 Hz, C-3″ and C-5″), 126.4 (2C, J = 8.5 Hz, C-2″ and C-6″), 135.3 (C-5′), 136.1 (C-1″), 148.6 (C-3), 161.4 (J = 246.6 Hz, C-4″); ESI-MS 423 [M+H]+.
17β-Hydroxy-1′-(4″-chlorophenyl)-5′-methylpyrazolo[3′,4′:3,2]-5α-androstane (4g)
According to the general procedure, 4-chlorophenylhydrazine hydrochloride (2g, 215 mg) was used. Yield: 219 mg (83%, white solid); Mp 260–262 °C; Anal. Calcd. for C27H35ClN2O (439.04): C, 73.87; H, 8.04. Found: C, 73.73; H, 8.09. 1H NMR (CDCl3, 500 MHz): δ 0.77 (s, 3H, 18-H3), 0.79 (s, 3H, 19-H3), 0.83–1.01 (overlapping m, 3H, 9α-H, 7α-H and 14α-H), 1.11 (td, 1H, J = 12.8, J = 4.1 Hz, 12α-H), 1.27 (m, 1H, 15β-H), 1.33–1.50 (overlapping m, 4H, 6β-H, 8β-H, 16β-H and 11β-H), 1.53–1.65 (overlapping m, 3H, 5α-H, 15α-H and 6α-H), 1.66–1.75 (overlapping m, 2H, 11α-H and 7β-H), 1.86 (m, 1H, 12β-H), 2.05 (d + m, 2H, J = 15.0 Hz, 1α-H and 16α-H), 2.20 (s, 3H, 5′-CH3), 2.33 (dd, 1H, J = 16.8 Hz, J = 12.1 Hz, 4β-H), 2.51 (d, 1H, J = 15.0 Hz, 1β-H), 2.65 (dd, 1H, J = 16.5 Hz, J = 5.2 Hz, 4α-H), 3.64 (t, 1H, J = 8.6 Hz, 17α-H), 7.35–7.41 (overlapping m, 4H, 2″-H, 3″-H, 5″-H and 6″-H); 13C NMR (CDCl3, 125 MHz): δ 10.9 (5′-CH3), 11.0 (C-18), 11.8 (C-19), 20.8 (C-11), 23.4 (C-15), 27.4 (C-4), 29.2 (C-6), 30.5 (C-16), 31.3 (C-7), 34.8 (C-1), 35.8 (C-8), 36.2 (C-10), 36.7 (C-12), 42.5 (C-5), 42.8 (C-13), 50.9 (C-14), 54.1 (C-9), 81.9 (C-17), 115.4 (C-2), 125.5 (2C, C-2″ and C-6″), 129.1 (2C, C-3″ and C-5″), 132.5 (C-4″), 135.2 (C-5′), 138.5 (C-1″), 149.0 (C-3); ESI-MS 440 [M+H]+.
17β-Hydroxy-1′-(4″-bromophenyl)-5′-methylpyrazolo[3′,4′:3,2]-5α-androstane (4h)
According to the general procedure, 4-bromophenylhydrazine hydrochloride (2h, 268 mg) was used. Yield: 232 mg (80%, white solid); Mp 256–259 °C; Anal. Calcd. for C27H35BrN2O (483.49): C, 67.07; H, 7.30. Found: C, 67.16; H, 7.38. 1H NMR (CDCl3, 500 MHz): δ 0.77 (s, 3H, 18-H3), 0.79 (s, 3H, 19-H3), 0.83–1.01 (overlapping m, 3H, 9α-H, 7α-H and 14α-H), 1.11 (td, 1H, J = 12.7, J = 3.5 Hz, 12α-H), 1.27 (m, 1H, 15β-H), 1.33–1.50 (overlapping m, 4H, 6β-H, 8β-H, 16β-H and 11β-H), 1.52–1.64 (overlapping m, 3H, 5α-H, 15α-H and 6α-H), 1.66–1.75 (overlapping m, 2H, 11α-H and 7β-H), 1.86 (m, 1H, 12β-H), 2.05 (d + m, 2H, J = 15.0 Hz, 1α-H and 16α-H), 2.20 (s, 3H, 5′-CH3), 2.32 (dd, 1H, J = 16.6 Hz, J = 12.4 Hz, 4β-H), 2.51 (d, 1H, J = 15.0 Hz, 1β-H), 2.66 (dd, 1H, J = 16.6 Hz, J = 4.9 Hz, 4α-H), 3.64 (t, 1H, J = 8.6 Hz, 17α-H), 7.32 (d, 2H, J = 8.5 Hz, 3″-H and 5″-H), 7.55 (d, 2H, J = 8.5 Hz, 2″-H and 6″-H); 13C NMR (CDCl3, 125 MHz): δ 11.0 (5′-CH3), 11.0 (C-18), 11.8 (C-19), 20.8 (C-11), 23.4 (C-15), 27.4 (C-4), 29.2 (C-6), 30.6 (C-16), 31.3 (C-7), 34.8 (C-1), 35.8 (C-8), 36.2 (C-10), 36.8 (C-12), 42.4 (C-5), 42.8 (C-13), 50.9 (C-14), 54.1 (C-9), 81.9 (C-17), 115.5 (C-2), 120.4 (C-4″), 125.8 (2C, C-2″ and C-6″), 132.0 (2C, C-3″ and C-5″), 135.2 (C-5′), 138.9 (C-1″), 149.0 (C-3); ESI-MS 484 [M+H]+.
17β-Hydroxy-1′-(4″-cyanophenyl)-5′-methylpyrazolo[3′,4′:3,2]-5α-androstane (4i)
According to the general procedure, 4-cianophenylhydrazine hydrochloride (2i, 204 mg) was used. Yield: 209 mg (81%, white solid); Mp 247–250 °C; Anal. Calcd. for C28H35N3O (429.61): C, 78.28; H, 8.21. Found: C, 78.12; H, 8.15. 1H NMR (CDCl3, 500 MHz): δ 0.77 (s, 3H, 18-H3), 0.79 (s, 3H, 19-H3), 0.84–1.02 (overlapping m, 3H, 9α-H, 7α-H and 14α-H), 1.11 (td, 1H, J = 12.7, J = 3.6 Hz, 12α-H), 1.28 (m, 1H, 15β-H), 1.33–1.51 (overlapping m, 4H, 6β-H, 8β-H, 16β-H and 11β-H), 1.52–1.65 (overlapping m, 3H, 5α-H, 15α-H and 6α-H), 1.67–1.76 (overlapping m, 2H, 11α-H and 7β-H), 1.86 (m, 1H, 12β-H), 2.06 (d + m, 2H, J = 15.3 Hz, 1α-H and 16α-H), 2.29 (s, 3H, 5′-CH3), 2.34 (dd, 1H, J = 16.9 Hz, J = 12.4 Hz, 4β-H), 2.53 (d, 1H, J = 15.3 Hz, 1β-H), 2.67 (dd, 1H, J = 16.9 Hz, J = 5.0 Hz, 4α-H), 3.64 (t, 1H, J = 8.5 Hz, 17α-H), 7.61 (d, 2H, J = 8.9 Hz, 2″-H and 6″-H), 7.72 (d, 2H, J = 8.9 Hz, 3″-H and 5″-H); 13C NMR (CDCl3, 125 MHz): δ 11.0 (C-18), 11.5 (5′-CH3), 11.8 (C-19), 20.8 (C-11), 23.4 (C-15), 27.4 (C-4), 29.2 (C-6), 30.6 (C-16), 31.3 (C-7), 34.7 (C-1), 35.8 (C-8), 36.2 (C-10), 36.7 (C-12), 42.3 (C-5), 42.8 (C-13), 50.9 (C-14), 54.0 (C-9), 81.9 (C-17), 109.8 (C-4″), 117.0 (C-2), 118.4 (CN), 123.7 (2C, C-2″ and C-6″), 133.1 (2C, C-3″ and C-5″), 135.5 (C-5′), 143.4 (C-1″), 150.3 (C-3); ESI-MS 430 [M+H]+.
17β-Hydroxy-1′-(4″-nitrophenyl)-5′-methylpyrazolo[3′,4′:3,2]-5α-androstane (4j)
According to the general procedure, 4-nitrophenylhydrazine hydrochloride (2j, 228 mg) was used. Yield: 229 mg (85%, yellow solid); Mp 238–240 °C; Anal. Calcd. for C27H35N3O3 (449.60): C, 72.13; H, 7.85. Found: C, 72.04; H, 7.76. 1H NMR (CDCl3, 500 MHz): δ 0.77 (s, 3H, 18-H3), 0.80 (s, 3H, 19-H3), 0.84–1.02 (overlapping m, 3H, 9α-H, 7α-H and 14α-H), 1.12 (td, 1H, J = 12.7, J = 3.6 Hz, 12α-H), 1.28 (m, 1H, 15β-H), 1.33–1.51 (overlapping m, 4H, 6β-H, 8β-H, 16β-H and 11β-H), 1.53–1.66 (overlapping m, 3H, 5α-H, 15α-H and 6α-H), 1.67–1.76 (overlapping m, 2H, 11α-H and 7β-H), 1.87 (m, 1H, 12β-H), 2.06 (d + m, 2H, J = 15.0 Hz, 1α-H and 16α-H), 2.32 (s, 3H, 5′-CH3), 2.34 (m, 1H, 4β-H), 2.54 (d, 1H, J = 15.0 Hz, 1β-H), 2.67 (dd, 1H, J = 17.0 Hz, J = 5.1 Hz, 4α-H), 3.64 (t, 1H, J = 8.5 Hz, 17α-H), 7.67 (d, 2H, J = 9.0 Hz, 2″-H and 6″-H), 8.30 (d, 2H, J = 9.0 Hz, 3″-H and 5″-H); 13C NMR (CDCl3, 125 MHz): δ 11.0 (C-18), 11.6 (5′-CH3), 11.8 (C-19), 20.8 (C-11), 23.4 (C-15), 27.4 (C-4), 29.2 (C-6), 30.5 (C-16), 31.3 (C-7), 34.7 (C-1), 35.8 (C-8), 36.2 (C-10), 36.7 (C-12), 42.3 (C-5), 42.8 (C-13), 50.9 (C-14), 54.0 (C-9), 81.9 (C-17), 117.0 (C-2), 123.2 (2C, C-2″ and C-6″), 124.7 (2C, C-3″ and C-5″), 135.7 (C-5′), 145.0 and 145.4 (C-1″ and C-4″), 150.7 (C-3); ESI-MS 450 [M+H]+.
3.1.4. General Procedure for the Synthesis of Compounds 5a–j by Jones Oxidation
Compound 4a–j (0.25 mmol) was dissolved in acetone (10 mL) and Jones reagent (0.2 mL) was added dropwise into the solution, which was then stirred at room temperature for 20 min, and after the given reaction time diluted with water (15 mL). The precipitate that formed was extracted with CH2Cl2 (3× 10 mL), and the combined organic phases were washed with water (10 mL), then dried over anhydrous Na2SO4 and concentrated in vacuo. The crude product was purified by column chromatography with EtOAc/CH2Cl2 = 2:98.
1′-Phenyl-5′-methylpyrazolo[3′,4′:3,2]-5α-androst-17-one (5a)
According to the general procedure, 4a (101 mg) was used. Yield: 96 mg (96%, white solid); Mp 247–250 °C; Anal. Calcd. for C27H34N2O (402.58): C, 80.55; H, 8.51. Found: C, 80.60; H, 8.59. 1H NMR (CDCl3, 500 MHz): δ 0.83 (s, 3H, 19-H3), 0.90 (s, 3H, 18-H3), 0.94 (m, 1H), 1.04 (m, 1H), 1.27–1.35 (overlapping m, 2H), 1.37–1.63 (overlapping m, 5H), 1.69 (m, 1H), 1.79 (m, 1H), 1.83–1.89 (overlapping m, 2H), 1.98 (m, 1H), 2.08 (d + m, 2H, J = 15.2 Hz, 1α-H and 16α-H), 2.21 (s, 3H, 5′-CH3), 2.36 (dd, 1H, J = 16.7 Hz, J = 12.1 Hz, 4β-H), 2.46 (dd, 1H, J = 19.2 Hz, J = 8.8 Hz, 16β-H), 2.52 (d, 1H, J = 15.2 Hz, 1β-H2), 2.69 (dd, 1H, J = 16.7 Hz, J = 5.1 Hz, 4α-H), 7.31 (m, 1H, 4″-H), 7.43 (d, 4H, J = 4.4 Hz, 2″-H, 3″-H, 5″-H and 6″-H); 13C NMR (CDCl3, 125 MHz): δ 10.9 (5′-CH3), 11.7 (C-19), 13.7 (C-18), 20.5 (C-11), 21.8 (C-15), 27.5 (C-4), 29.1 (C-6), 30.6 (C-12), 31.6 (C-7), 34.8 (C-1), 35.3 (C-8), 35.8 (C-16), 36.4 (C-10), 42.5 (C-5), 47.6 (C-13), 51.4 (C-14), 54.1 (C-9), 114.8 (C-2), 124.5 (2C, C-2″ and C-6″), 127.0 (C-4″), 128.9 (2C, C-3″ and C-5″), 135.2 (C-5′), 139.9 (C-1″), 148.4 (C-3), 221.2 (C-17); ESI-MS 403 [M+H]+.
1′-(2″-Tolyl)-5′-methylpyrazolo[3′,4′:3,2]-5α-androst-17-one (5b)
According to the general procedure, 4b (105 mg) was used. Yield: 97 mg (93%, white solid); Mp 245–250 °C; Anal. Calcd. for C28H36N2O (416.61): C, 80.73; H, 8.71. Found: C, 80.61; H, 8.77. 1H NMR (CDCl3, 500 MHz): δ 0.83 (s, 3H, 19-H3), 0.90 (s, 3H, 18-H3), 0.94 (m, 1H), 1.04 (m, 1H), 1.27–1.35 (overlapping m, 2H), 1.37–1.64 (overlapping m, 5H), 1.68 (m, 1H), 1.79 (m, 1H), 1.83–1.89 (overlapping m, 2H), 1.95 (s, 3H), 1.98 (m, 1H), 2.03 (s, 3H), 2.09 (d + m, 2H, J = 15.1 Hz, 1α-H and 16α-H), 2.36 (dd, 1H, J = 16.5 Hz, J = 12.2 Hz, 4β-H), 2.46 (dd, 1H, J = 19.2 Hz, J = 8.8 Hz, 16β-H), 2.52 (d, 1H, J = 15.1 Hz, 1β-H2), 2.68 (dd, 1H, J = 16.5 Hz, J = 4.9 Hz, 4α-H), 7.19–7.32 (overlapping m, 4H, 3″-H, 4″-H, 5″-H and 6″-H); 13C NMR (CDCl3, 125 MHz): δ 9.7 (5′-CH3), 11.7 (C-19), 13.7 (C-18), 17.3 (2″-CH3), 20.5 (C-11), 21.8 (C-15), 27.5 (C-4), 29.1 (C-6), 30.7 (C-12), 31.6 (C-7), 34.8 (C-1), 35.3 (C-8), 35.8 (C-16), 36.4 (C-10), 42.5 (C-5), 47.6 (C-13), 51.4 (C-14), 54.1 (C-9), 112.9 (C-2), 126.3 (CH), 127.9 (CH), 128.7 (CH), 130.7 (CH), 136.1, 136.2 and 138.8 (C-5′, C-1″ and C-2″), 147.7 (C-3), 221.2 (C-17); ESI-MS 417 [M+H]+.
1′-(4″-Tolyl)-5′-methylpyrazolo[3′,4′:3,2]-5α-androst-17-one (5c)
According to the general procedure, 4c (105 mg) was used. Yield: 96 mg (92%, white solid); Mp 241–243 °C; Anal. Calcd. for C28H36N2O (416.61): C, 80.73; H, 8.71. Found: C, 80.79; H, 8.78. 1H NMR (CDCl3, 500 MHz): δ 0.83 (s, 3H, 19-H3), 0.90 (s, 3H, 18-H3), 0.93 (m, 1H), 1.04 (m, 1H), 1.28–1.35 (overlapping m, 2H), 1.38–1.63 (overlapping m, 5H), 1.69 (m, 1H), 1.79 (m, 1H), 1.84–1.90 (overlapping m, 2H), 1.98 (m, 1H), 2.09 (d + m, 2H, J = 15.0 Hz, 1α-H and 16α-H), 2.18 (s, 3H, 5′-CH3), 2.36 (m, 1H, 4β-H), 2.38 (4″-CH3), 2.46 (dd, 1H, J = 19.3 Hz, J = 9.0 Hz, 16β-H), 2.52 (d, 1H, J = 15.0 Hz, 1β-H2), 2.70 (dd, 1H, J = 16.7 Hz, J = 4.6 Hz, 4α-H), 7.23 (d, 2H, J = 8.0 Hz, 3″-H and 5″-H), 7.30 (d, 2H, J = 8.0 Hz, 2″-H and 6″-H); 13C NMR (CDCl3, 125 MHz): δ 10.8 (5′-CH3), 11.8 (C-19), 13.7 (C-18), 20.5 (C-11), 21.0 (4″-CH3), 21.8 (C-15), 27.4 (C-4), 29.0 (C-6), 30.6 (C-12), 31.6 (C-7), 34.8 (C-1), 35.3 (C-8), 35.8 (C-16), 36.4 (C-10), 42.5 (C-5), 47.6 (C-13), 51.4 (C-14), 54.1 (C-9), 114.5 (C-2), 124.5 (2C, C-2″ and C-6″), 129.5 (2C, C-3″ and C-5″), 136.9, 137.0 and 137.3 (C-5′, C-1″ and C-4″), 148.0 (C-3), 221.2 (C-17); ESI-MS 417 [M+H]+.
1′-(2″,4″-Dimethylphenyl)-5′-methylpyrazolo[3′,4′:3,2]-5α-androst-17-one (5d)
According to the general procedure, 4d (108 mg) was used. Yield: 101 mg (94%, white solid); Mp 173–176 °C; Anal. Calcd. for C29H38N2O (430.64): C, 80.88; H, 8.89. Found: C, 80.98; H, 8.93. 1H NMR (CDCl3, 500 MHz): δ 0.82 (s, 3H, 19-H3), 0.90 (s, 3H, 18-H3), 0.94 (m, 1H), 1.04 (m, 1H), 1.28–1.35 (overlapping m, 2H), 1.37–1.63 (overlapping m, 5H), 1.68 (m, 1H), 1.79 (m, 1H), 1.84–1.90 (overlapping m, 2H), 1.94 (s, 3H), 1.99 (s + m, 4H), 2.09 (d + m, 2H, J = 15.0 Hz, 1α-H and 16α-H), 2.35 (s + m, 4H, 4″-CH3 and 4β-H), 2.46 (dd, 1H, J = 19.5 Hz, J = 9.0 Hz, 16β-H), 2.51 (d, 1H, J = 15.0 Hz, 1β-H2), 2.68 (dd, 1H, J = 16.7 Hz, J = 4.5 Hz, 4α-H), 7.01–7.10 (overlapping m, 3H, 3″-H, 5″-H and 6″-H); 13C NMR (CDCl3, 125 MHz): δ 9.7 (5′-CH3), 11.7 (C-19), 13.7 (C-18), 17.2 (2″-CH3), 20.5 (C-11), 21.1 (4″-CH3), 21.8 (C-15), 27.5 (C-4), 29.1 (C-6), 30.7 (C-12), 31.6 (C-7), 34.8 (C-1), 35.3 (C-8), 35.8 (C-16), 36.4 (C-10), 42.4 (C-5), 47.6 (C-13), 51.4 (C-14), 54.1 (C-9), 112.8 (C-2), 127.0, 127.6 and 131.4 (C-3″, C-5″ and C-6″), 135.8, 135.9, 136.3 and 138.7 (C-5′, C-1″, C-2″ and C-4″), 147.4 (C-3), 221.2 (C-17); ESI-MS 431 [M+H]+.
1′-(4″-Methoxyphenyl)-5′-methylpyrazolo[3′,4′:3,2]-5α-androst-17-one (5e)
According to the general procedure, 4e (109 mg) was used. Yield: 99 mg (92%, white solid); Mp 225–226 °C; Anal. Calcd. for C28H36N2O2 (432.61): C, 77.74; H, 8.39. Found: C, 77.68; H, 8.48. 1H NMR (CDCl3, 500 MHz): δ 0.82 (s, 3H, 19-H3), 0.90 (s, 3H, 18-H3), 0.93 (m, 1H), 1.04 (m, 1H), 1.27–1.34 (overlapping m, 2H), 1.37–1.63 (overlapping m, 5H), 1.68 (m, 1H), 1.78 (m, 1H), 1.83–1.89 (overlapping m, 2H), 1.98 (m, 1H), 2.08 (d + m, 2H, J = 15.0 Hz, 1α-H and 16α-H), 2.15 (s, 3H, 5′-CH3), 2.36 (dd, 1H, J = 16.6 Hz, J = 12.3 Hz, 4β-H), 2.46 (dd, 1H, J = 19.4 Hz, J = 9.0 Hz, 16β-H), 2.51 (d, 1H, J = 15.2 Hz, 1β-H2), 2.70 (dd, 1H, J = 16.6 Hz, J = 4.8 Hz, 4α-H), 3.83 (4″-OMe), 6.94 (d, 2H, J = 8.8 Hz, 3″-H and 5″-H), 7.32 (d, 2H, J = 8.8 Hz, 2″-H and 6″-H); 13C NMR (CDCl3, 125 MHz): δ 10.6 (5′-CH3), 11.8 (C-19), 13.7 (C-18), 20.5 (C-11), 21.8 (C-15), 27.4 (C-4), 29.0 (C-6), 30.6 (C-12), 31.6 (C-7), 34.8 (C-1), 35.3 (C-8), 35.8 (C-16), 36.4 (C-10), 42.4 (C-5), 47.6 (C-13), 51.4 (C-14), 54.1 (C-9), 55.5 (4″-OMe), 114.1 (2C, C-2″ and C-6″), 114.2 (C-2), 126.2 (2C, C-3″ and C-5″), 132.3 (C-1″), 135.5 (C-5′), 147.7 (C-3), 158.7 (C-4″), 221.2 (C-17); ESI-MS 433 [M+H]+.
1′-(4″-Fluorophenyl)-5′-methylpyrazolo[3′,4′:3,2]-5α-androst-17-one (5f)
According to the general procedure, 4f (106 mg) was used. Yield: 100 mg (95%, white solid); Mp 208–210 °C; Anal. Calcd. for C27H33FN2O (420.57): C, 77.11; H, 7.91. Found: C, 77.23; H, 7.99. 1H NMR (CDCl3, 500 MHz): δ 0.81 (s, 3H, 19-H3), 0.90 (s, 3H, 18-H3), 0.93 (m, 1H), 1.03 (m, 1H), 1.27–1.34 (overlapping m, 2H), 1.37–1.61 (overlapping m, 5H), 1.68 (m, 1H), 1.78 (m, 1H), 1.83–1.89 (overlapping m, 2H), 1.97 (m, 1H), 2.08 (d + m, 2H, J = 15.0 Hz, 1α-H and 16α-H), 2.17 (s, 3H, 5′-CH3), 2.36 (dd, 1H, J = 16.6 Hz, J = 12.3 Hz, 4β-H), 2.45 (dd, 1H, J = 19.2 Hz, J = 8.9 Hz, 16β-H), 2.51 (d, 1H, J = 15.2 Hz, 1β-H2), 2.67 (dd, 1H, J = 16.6 Hz, J = 4.9 Hz, 4α-H), 7.11 (t, 2H, J = 8.4 Hz, 3″-H and 5″-H), 7.38 (m, 2H, 2″-H and 6″-H); 13C NMR (CDCl3, 125 MHz): δ 10.6 (5′-CH3), 11.7 (C-19), 13.7 (C-18), 20.5 (C-11), 21.8 (C-15), 27.4 (C-4), 29.0 (C-6), 30.6 (C-12), 31.6 (C-7), 34.8 (C-1), 35.3 (C-8), 35.8 (C-16), 36.3 (C-10), 42.4 (C-5), 47.6 (C-13), 51.4 (C-14), 54.0 (C-9), 114.8 (C-2), 115.5 (2C, J = 22.8 Hz, C-3″ and C-5″), 126.3 (2C, J = 8.5 Hz, C-2″ and C-6″), 135.4 (C-5′), 136.1 (C-1″), 148.4 (C-3), 161.5 (J = 247.1 Hz, C-4″), 221.2 (C-17); ESI-MS 421 [M+H]+.
1′-(4″-Chlorophenyl)-5′-methylpyrazolo[3′,4′:3,2]-5α-androst-17-one (5g)
According to the general procedure, 4g (110 mg) was used. Yield: 102 mg (93%, white solid); Mp 200–203 °C; Anal. Calcd. for C27H33ClN2O (437.02): C, 74.21; H, 7.61. Found: C, 74.09; H, 7.67. 1H NMR (CDCl3, 500 MHz): δ 0.81 (s, 3H, 19-H3), 0.90 (s, 3H, 18-H3), 0.93 (m, 1H), 1.04 (m, 1H), 1.27–1.35 (overlapping m, 2H), 1.37–1.62 (overlapping m, 5H), 1.69 (m, 1H), 1.78 (m, 1H), 1.83–1.89 (overlapping m, 2H), 1.98 (m, 1H), 2.08 (d + m, 2H, J = 15.0 Hz, 1α-H and 16α-H), 2.20 (s, 3H, 5′-CH3), 2.35 (dd, 1H, J = 16.8 Hz, J = 12.1 Hz, 4β-H), 2.46 (dd, 1H, J = 19.2 Hz, J = 8.9 Hz, 16β-H), 2.52 (d, 1H, J = 15.0 Hz, 1β-H2), 2.69 (dd, 1H, J = 16.8 Hz, J = 5.1 Hz, 4α-H), 7.35–7.42 (overlapping m, 4H, 2″-H, 3″-H, 5″-H and 6″-H); 13C NMR (CDCl3, 125 MHz): δ 10.9 (5′-CH3), 11.7 (C-19), 13.7 (C-18), 20.5 (C-11), 21.8 (C-15), 27.4 (C-4), 29.0 (C-6), 30.6 (C-12), 31.6 (C-7), 34.7 (C-1), 35.3 (C-8), 35.8 (C-16), 36.3 (C-10), 42.4 (C-5), 47.6 (C-13), 51.4 (C-14), 54.0 (C-9), 115.2 (C-2), 125.5 (2C, C-2″ and C-6″), 129.1 (2C, C-3″ and C-5″), 132.7 (C-4″), 135.4 (C-5′), 138.4 (C-1″), 148.7 (C-3), 221.2 (C-17); ESI-MS 438 [M+H]+.
1′-(4″-Bromophenyl)-5′-methylpyrazolo[3′,4′:3,2]-5α-androst-17-one (5h)
According to the general procedure, 4h (121 mg) was used. Yield: 110 mg (92%, white solid); Mp 239–242 °C; Anal. Calcd. for C27H33BrN2O (481.48): C, 67.35; H, 6.91. Found: C, 67.50; H, 6.99. 1H NMR (CDCl3, 500 MHz): δ 0.81 (s, 3H, 19-H3), 0.90 (s, 3H, 18-H3), 0.93 (m, 1H), 1.04 (m, 1H), 1.27–1.34 (overlapping m, 2H), 1.37–1.62 (overlapping m, 5H), 1.69 (m, 1H), 1.78 (m, 1H), 1.84–1.89 (overlapping m, 2H), 1.98 (m, 1H), 2.07 (d + m, 2H, J = 15.0 Hz, 1α-H and 16α-H), 2.20 (s, 3H, 5′-CH3), 2.34 (dd, 1H, J = 16.7 Hz, J = 12.4 Hz, 4β-H), 2.46 (dd, 1H, J = 19.3 Hz, J = 8.9 Hz, 16β-H), 2.52 (d, 1H, J = 15.0 Hz, 1β-H2), 2.68 (dd, 1H, J = 16.7 Hz, J = 5.1 Hz, 4α-H), 7.32 (d, 2H, J = 8.6 Hz, 3″-H and 5″-H), 7.55 (d, 2H, J = 8.6 Hz, 2″-H and 6″-H); 13C NMR (CDCl3, 125 MHz): δ 11.0 (5′-CH3), 11.7 (C-19), 13.7 (C-18), 20.5 (C-11), 21.8 (C-15), 27.4 (C-4), 29.0 (C-6), 30.6 (C-12), 31.6 (C-7), 34.8 (C-1), 35.3 (C-8), 35.8 (C-16), 36.3 (C-10), 42.4 (C-5), 47.6 (C-13), 51.4 (C-14), 54.0 (C-9), 115.3 (C-2), 120.4 (C-4″), 125.8 (2C, C-2″ and C-6″), 132.0 (2C, C-3″ and C-5″), 135.2 (C-5′), 139.0 (C-1″), 148.9 (C-3), 221.2 (C-17); ESI-MS 482 [M+H]+.
1′-(4″-Cyanophenyl)-5′-methylpyrazolo[3′,4′:3,2]-5α-androst-17-one (5i)
According to the general procedure, 4i (107 mg) was used. Yield: 98 mg (92%, white solid); Mp 234–237 °C; Anal. Calcd. for C28H33N3O (427.59): C, 78.65; H, 7.78. Found: C, 78.76; H, 7.87. 1H NMR (CDCl3, 500 MHz): δ 0.81 (s, 3H, 19-H3), 0.90 (s, 3H, 18-H3), 0.94 (m, 1H), 1.05 (m, 1H), 1.27–1.35 (overlapping m, 2H), 1.37–1.62 (overlapping m, 5H), 1.70 (m, 1H), 1.79 (m, 1H), 1.84–1.90 (overlapping m, 2H), 1.98 (m, 1H), 2.08 (d + m, 2H, J = 15.1 Hz, 1α-H and 16α-H), 2.29 (s, 3H, 5′-CH3), 2.35 (dd, 1H, J = 16.8 Hz, J = 12.4 Hz, 4β-H), 2.47 (dd, 1H, J = 19.2 Hz, J = 8.7 Hz, 16β-H), 2.54 (d, 1H, J = 15.1 Hz, 1β-H2), 2.69 (dd, 1H, J = 16.8 Hz, J = 5.1 Hz, 4α-H), 7.61 (d, 2H, J = 8.6 Hz, 3″-H and 5″-H), 7.72 (d, 2H, J = 8.6 Hz, 2″-H and 6″-H); 13C NMR (CDCl3, 125 MHz): δ 11.5 (5′-CH3), 11.8 (C-19), 13.7 (C-18), 20.5 (C-11), 21.8 (C-15), 27.4 (C-4), 29.0 (C-6), 30.6 (C-12), 31.6 (C-7), 34.7 (C-1), 35.3 (C-8), 35.8 (C-16), 36.3 (C-10), 42.3 (C-5), 47.6 (C-13), 51.4 (C-14), 54.0 (C-9), 109.8 (C-4″), 116.7 (C-2), 118.4 (CN), 123.7 (2C, C-2″ and C-6″), 133.1 (2C, C-3″ and C-5″), 135.4 (C-5′), 143.5 (C-1″), 150.3 (C-3); 221.1 (C-17); ESI-MS 428 [M+H]+.
1′-(4″-Nitrophenyl)-5′-methylpyrazolo[3′,4′:3,2]-5α-androst-17-one (5j)
According to the general procedure, 4j (112 mg) was used. Yield: 107 mg (96%, yellow solid); Mp 140–143 °C; Anal. Calcd. for C27H33N3O3 (447.58): C, 72.46; H, 7.43. Found: C, 72.55; H, 7.34. 1H NMR (CDCl3, 500 MHz): δ 0.81 (s, 3H, 19-H3), 0.90 (s, 3H, 18-H3), 0.94 (m, 1H), 1.05 (m, 1H), 1.27–1.35 (overlapping m, 2H), 1.37–1.62 (overlapping m, 5H), 1.70 (m, 1H), 1.79 (m, 1H), 1.84–1.90 (overlapping m, 2H), 1.98 (m, 1H), 2.08 (d + m, 2H, J = 15.1 Hz, 1α-H and 16α-H), 2.33 (s, 3H, 5′-CH3), 2.35 (m, 1H, 4β-H), 2.46 (dd, 1H, J = 19.1 Hz, J = 8.7 Hz, 16β-H), 2.54 (d, 1H, J = 15.1 Hz, 1β-H2), 2.69 (dd, 1H, J = 16.8 Hz, J = 4.7 Hz, 4α-H), 7.66 (d, 2H, J = 8.9 Hz, 2″-H and 6″-H), 8.30 (d, 2H, J = 8.9 Hz, 3″-H and 5″-H); 13C NMR (CDCl3, 125 MHz): δ 11.7 (5′-CH3), 11.8 (C-19), 13.7 (C-18), 20.5 (C-11), 21.8 (C-15), 27.4 (C-4), 29.0 (C-6), 30.5 (C-12), 31.6 (C-7), 34.7 (C-1), 35.3 (C-8), 35.8 (C-16), 36.3 (C-10), 42.3 (C-5), 47.6 (C-13), 51.3 (C-14), 54.0 (C-9), 117.1 (C-2), 123.1 (2C, C-2″ and C-6″), 124.7 (2C, C-3″ and C-5″), 135.5 (C-5′), 145.2 and 145.3 (C-1″ and C-4″), 150.6 (C-3); 221.1 (C-17); ESI-MS 448 [M+H]+.