Saccharomyces cerevisiae as a Tool to Investigate Plant Potassium and Sodium Transporters
Abstract
1. Introduction
2. Functional Complementation as an Approach to Identify and Characterize Plant K+/Na+ Channels and Transporters
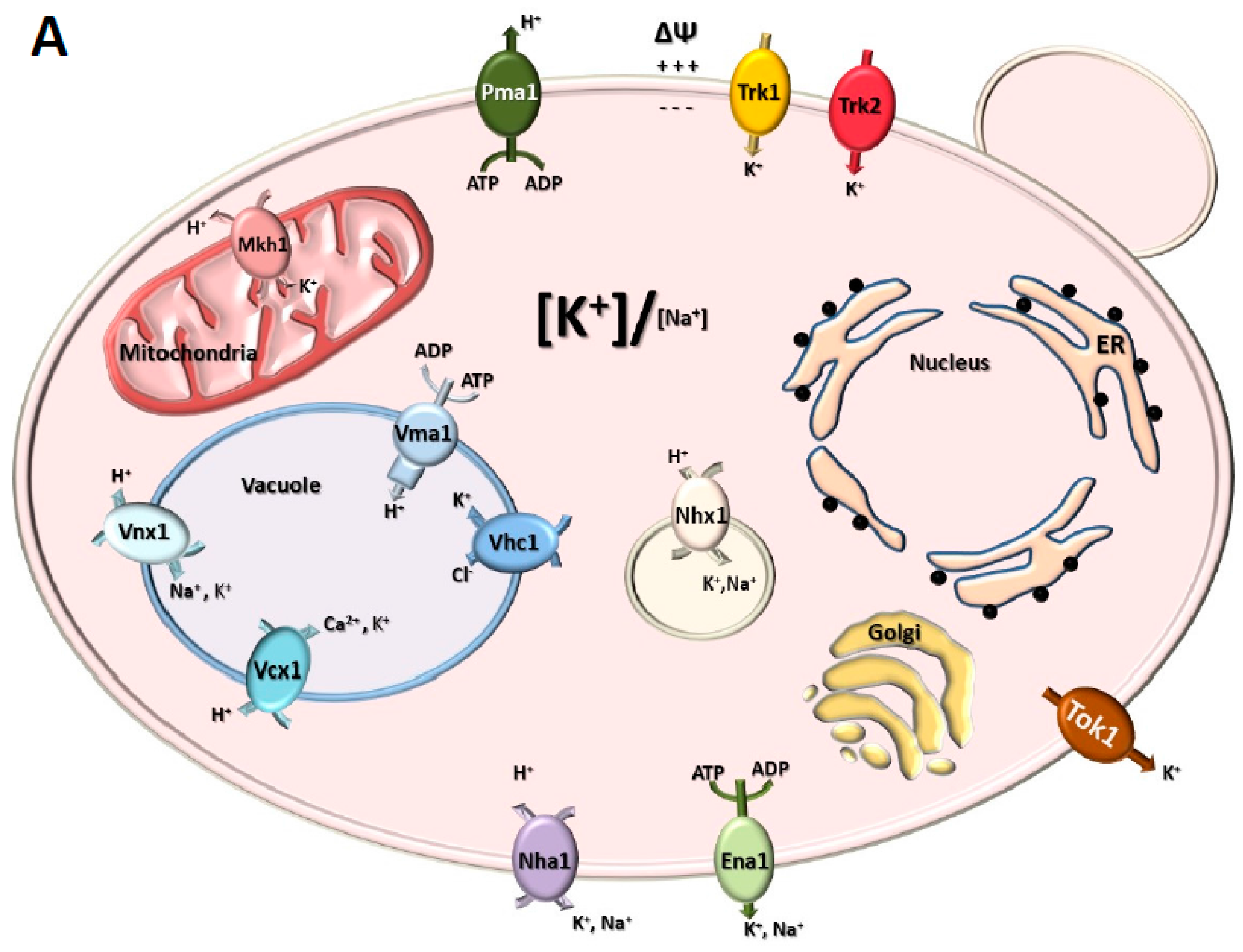
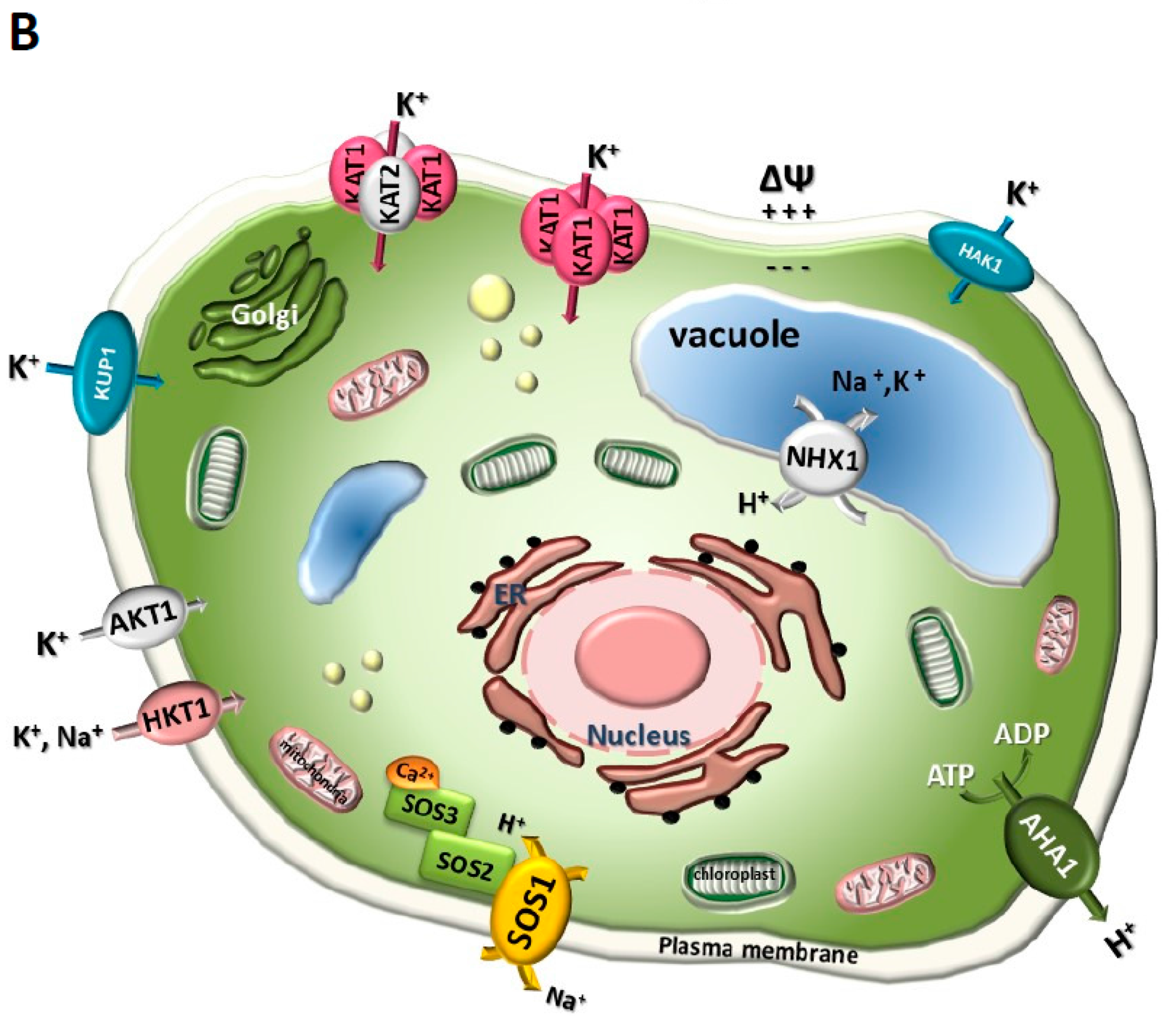
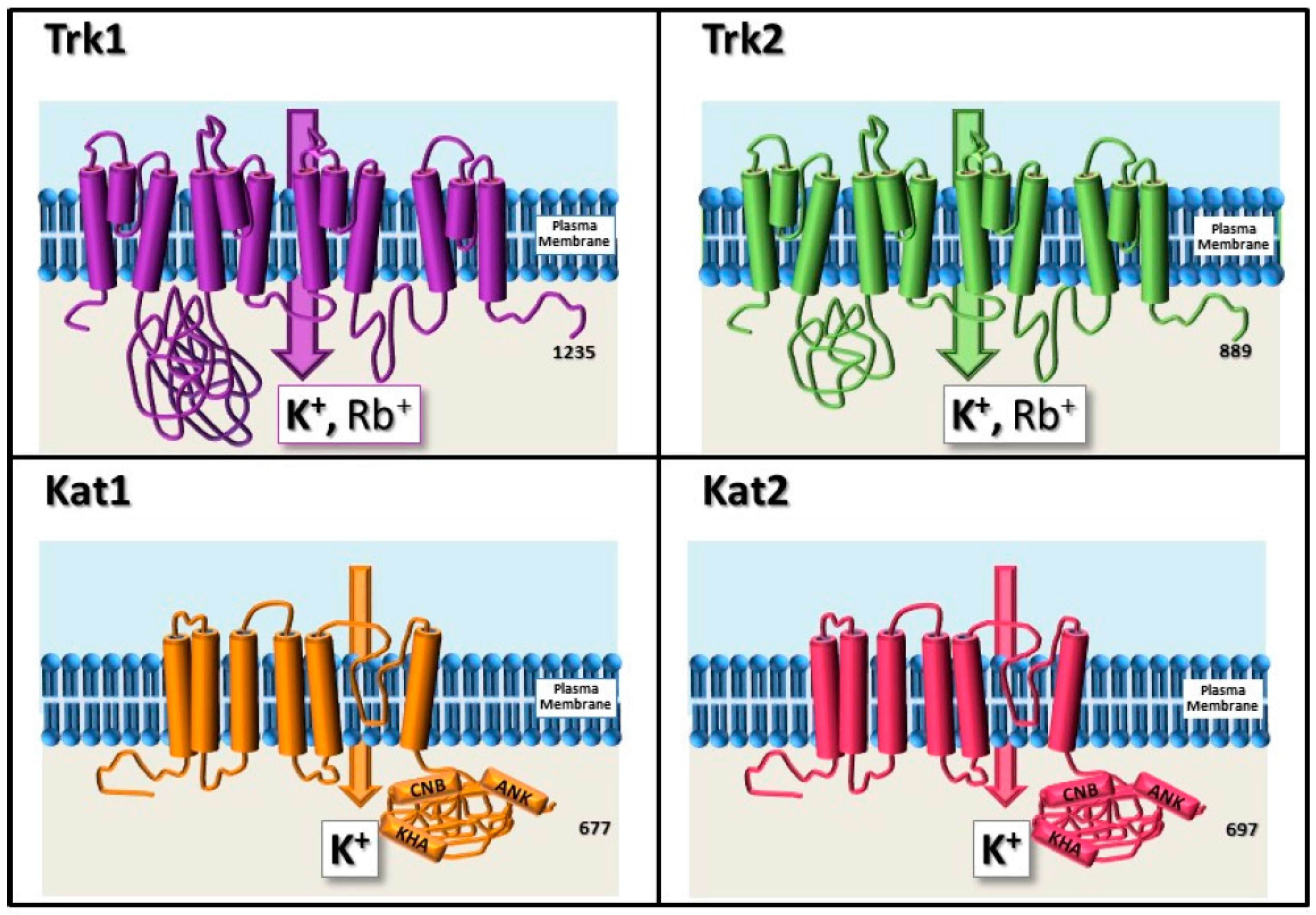
3. Milestones in the Identification of K+ Channels and Transporters in Plants
4. High-Throughput and Directed Protein-Protein Interaction Assays Used to Identify Plant K+/Na+ Transporter Regulators
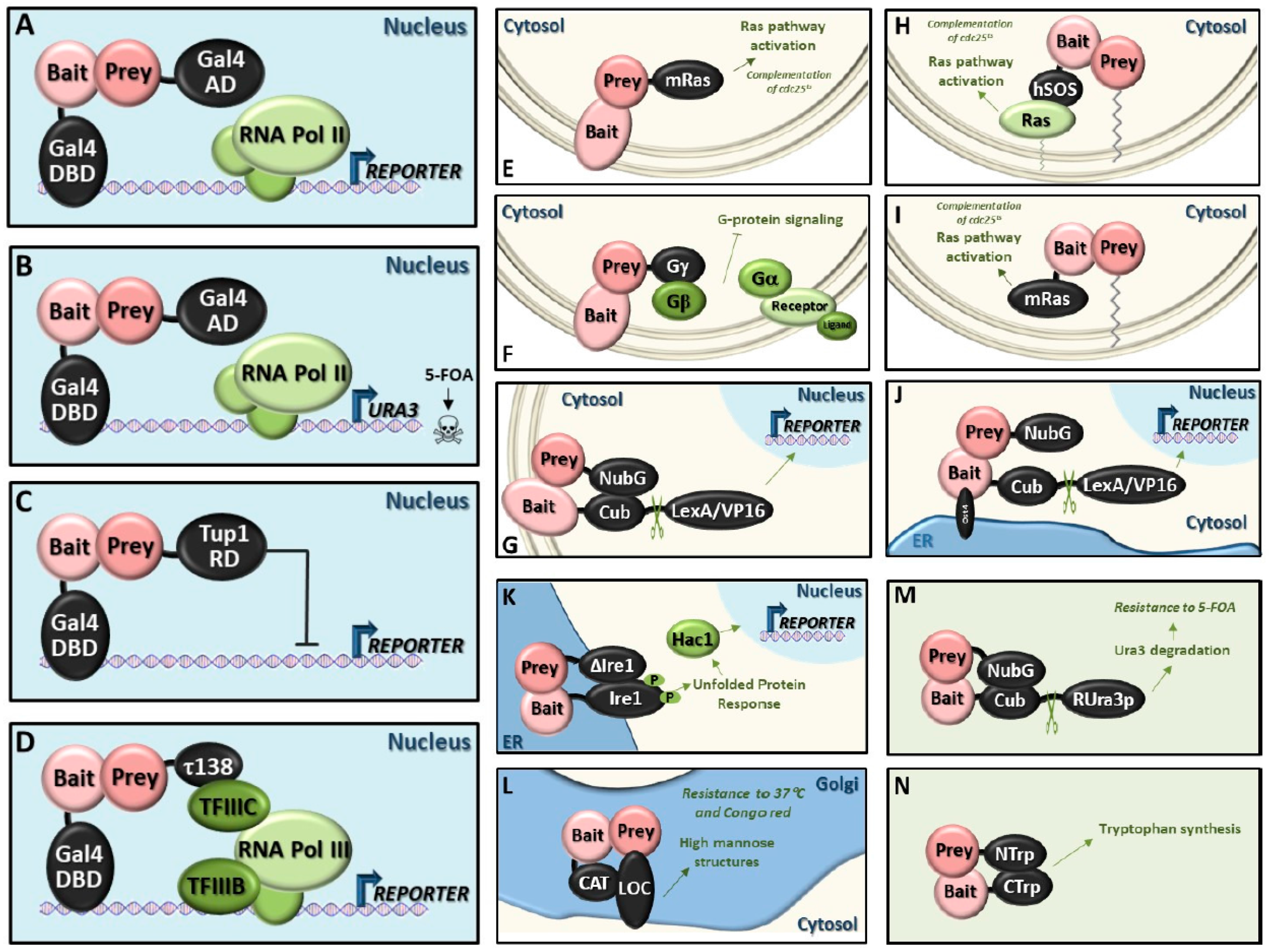
5. State-of-the-Art of the Available Techniques for Detecting Protein–Protein Interactions in Yeast
5.1. Classic Y2H System (Y2H)
5.2. Reverse Y2H System (rY2H)
5.3. Repressed Transactivator System (RTA)
5.4. RNA Polymerase III System (Pol III)
5.5. Small-G-Protein-Based Methods
- -
- SOS recruitment system (SRS): One protein is fused to a modified human Sos protein (hSos), which can functionally replace its yeast counterpart Cdc25, but only if it is targeted to the plasma membrane. The other protein is fused to the C-terminus of the v-Src myristoylation sequence, which targets proteins to the membrane. Interactions between both proteins leads to the recruitment of hSos to the membrane and activation of the yeast Ras pathway that complements the temperature‑sensitive cdc25 mutation at the restrictive temperature (36 °C) [139].Advantages: Can be applied to cytosolic proteins that are unable to enter the nucleus or that require post-translational modifications in the cytoplasm.
- -
- Ras recruitment system (RRS): The principle is similar to the SRS, however, hSos is substituted by a mutant form of mammalian Ras (mRas). This Ras protein (Ras(61)ΔF), is a constitutively active form of mammalian Ras, which lacks the CAAX box required for its lipid modification and subsequent localization to the plasma membrane [140]. The bait protein is fused to this version of mRas. The prey proteins are fused to a membrane localization sequence. In this way, if the bait and prey proteins interact, the constitutively active form of Ras is recruited to the plasma membrane and can complement the cdc25 temperature-sensitive mutant.Advantages: Reduction of false positives; furthermore, the smaller size of mRas compared with hSOS reduces the steric hindrance problem observed with the large hSos protein.
- -
- Reverse Ras recruitment system (rRRS): The principle is similar to the RRS, however, mRas is fused to the prey protein and the bait protein contains its own membrane localization signal or is an intrinsic membrane protein [138].Advantages: Can be applied to membrane proteins.
5.6. Heterotrimeric G-Protein Fusion System
5.7. Screening for Interactions Between Extracellular Proteins (SCINEX-P)
5.8. Golgi Y2H System (GY2H)
5.9. Dual-Bait System
5.10. Split-Ubiquitin System
- -
- Membrane split-ubiquitin system (MbY2H): The bait protein needs to be excluded from the nucleus and the topology must be such that the fusions are in the cytosol. This system has been successfully applied to integral membrane proteins. It can also be used for proteins that are resident in other membrane systems or those that have lipid modifications [135].Advantages: Can be applied to membrane proteins.
- -
- Cytosolic split-ubiquitin system (CytoY2H): The same strategy as that used for the MbY2H system is employed but, in this case, the bait protein does not have to be a membrane-bound protein by itself, because the integral ER membrane protein Ost4 is added at the N-terminal end of the bait to impede its entry into the nucleus (reviewed in [152,153,154]).Advantages: Can be applied to cytosolic proteins that require post-translational modifications in the cytoplasm.
- -
- Generally applicable split-ubiquitin system: In this case, the transcription factor is replaced by the reporter protein Ura3, with an arginine residue (R-Ura3) between Ura3 and Cub. After interaction of bait and prey, which can reside in membranes or the cytosol, Ura3 is cleaved off and it is quickly degraded due to the exposed N-terminal arginine residue. Consequently, the cells become resistant to 5-FOA. The R-Ura3 method is especially suitable for finding transcription factor partners, both activators and repressors (reviewed in [153]).Advantages: Careful optimization of 5-FOA levels reduces false discovery rates.
5.11. Split-Trp System
5.12. Split-mDHFR System
6. Interactors of K+/Na+ Transporters/Channels Detected Using Protein–Protein Interaction Techniques in Yeast
7. Reconstitution of Functional Plant Ion Transport Systems in Yeast: The SOS Pathway Paradigm
8. Identification of Plant Genes Involved in K+/Na+ Homeostasis by Heterologous Expression
Author Contributions
Acknowledgements
Conflicts of Interest
References
- Epstein, E. Mechanisms of Ion Transport through Plant Cell Membranes. Int. Rev. Cytol. 1973, 34, 123–168. [Google Scholar]
- Kochian, L.V.; Lucas, W.J. Potassium Transport in Roots. Adv. Bot. Res. 1989, 15, 136–151. [Google Scholar]
- Schroeder, J.I. Knockout of the guard cell K+out channel and stomatal movements. Proc. Natl. Acad. Sci. USA 2003, 100, 4976–4977. [Google Scholar] [CrossRef]
- Hurst, A.C.; Meckel, T.; Tayefeh, S.; Thiel, G.; Homann, U. Trafficking of the plant potassium inward rectifier KAT1 in guard cell protoplasts of Vicia faba. Plant J. 2004, 37, 391–397. [Google Scholar] [CrossRef]
- Nieves-Cordones, M.; Al Shiblawi, F.R.; Sentenac, H. Roles and Transport of Sodium and Potassium in Plants. In The Alkali Metal Ions: Their Role for Life. Metal Ions in Life Science; Springer International Publishing: Zurich, Switzerland, 2016; pp. 291–324. [Google Scholar]
- BRAG, H. The Influence of Potassium on the Transpiration Rate and Stomatal Opening in Triticum aestivum andPisum sativum. Physiol. Plant. 1972, 26, 250–257. [Google Scholar] [CrossRef]
- Mohd Zain, N.A.; Ismail, M.R. Effects of potassium rates and types on growth, leaf gas exchange and biochemical changes in rice (Oryza sativa) planted under cyclic water stress. Agric. Water Manag. 2016, 164, 83–90. [Google Scholar] [CrossRef]
- Hooymans, J.J.M. The influence of the transpiration rate on uptake and transport of potassium ions in barley plants. Planta 1969, 88, 369–371. [Google Scholar] [CrossRef]
- Ohnishi, J.-I.; Flugge, U.-I.; Heldt, H.W.; Kanai, R. Involvement of Na+ in Active Uptake of Pyruvate in Mesophyll Chloroplasts of Some C4 Plants : Na+/Pyruvate Cotransport. Plant Physiol. 1990, 94, 950–959. [Google Scholar] [CrossRef]
- Amtmann, A.; Sanders, D. Mechanisms of Na+ Uptake by Plant Cells. Adv. Bot. Res. 1998, 29, 75–112. [Google Scholar]
- Horie, T.; Costa, A.; Kim, T.H.; Han, M.J.; Horie, R.; Leung, H.Y.; Miyao, A.; Hirochika, H.; An, G.; Schroeder, J.I. Rice OsHKT2;1 transporter mediates large Na+ influx component into K+-starved roots for growth. EMBO J 2007, 26, 3003–3014. [Google Scholar] [CrossRef]
- Wu, H. Plant salt tolerance and Na+ sensing and transport. Crop J. 2018, 6, 215–225. [Google Scholar] [CrossRef]
- Pyo, Y.J.; Gierth, M.; Schroeder, J.I.; Cho, M.H. High-affinity K(+) transport in Arabidopsis: AtHAK5 and AKT1 are vital for seedling establishment and postgermination growth under low-potassium conditions. Plant Physiol. 2010, 153, 863–875. [Google Scholar] [CrossRef]
- Maathuis, F.J.; Sanders, D. Mechanism of high-affinity potassium uptake in roots of Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 1994, 91, 9272–9276. [Google Scholar] [CrossRef]
- Anderson, J.A.; Huprikar, S.S.; Kochian, L.V.; Lucas, W.J.; Gaber, R.F. Functional expression of a probable Arabidopsis thaliana potassium channel in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 1992, 89, 3736–3740. [Google Scholar] [CrossRef]
- Sentenac, H.; Bonneaud, N.; Minet, M.; Lacroute, F.; Salmon, J.M.; Gaymard, F.; Grignon, C. Cloning and expression in yeast of a plant potassium ion transport system. Science 1992, 256, 663–665. [Google Scholar] [CrossRef]
- Rodríguez-Rosales, M.P.; Gálvez, F.J.; Huertas, R.; Aranda, M.N.; Baghour, M.; Cagnac, O.; Venema, K. Plant NHX cation/proton antiporters. Plant Signal. Behav. 2009, 4, 265–276. [Google Scholar] [CrossRef]
- Venema, K.; Belver, A.; Marín-Manzano, M.C.; Rodríguez-Rosales, M.P.; Donaire, J.P. A novel intracellular K+/H+ antiporter related to Na+/H+ antiporters is important for K+ ion homeostasis in plants. J. Biol. Chem. 2003, 278, 22453–22459. [Google Scholar] [CrossRef]
- Gassmann, W.; Schroeder, J.I. Inward-rectifying K+ channels in root hairs of wheat. Plant Physiol. 1994, 105, 1399–1408. [Google Scholar] [CrossRef]
- Cao, Y.; Ward, J.M.; Kelly, W.B.; Ichida, A.M.; Gaber, R.F.; Anderson, J.A.; Uozumi, N.; Schroeder, J.I.; Crawford, N.M. Multiple genes, tissue specificity, and expression-dependent modulationcontribute to the functional diversity of potassium channels in Arabidopsis thaliana. Plant Physiol. 1995, 109, 1093–1106. [Google Scholar] [CrossRef]
- Müller-Röber, B.; Ellenberg, J.; Provart, N.; Willmitzer, L.; Busch, H.; Becker, D.; Dietrich, P.; Hoth, S.; Hedrich, R. Cloning and electrophysiological analysis of KST1,an inward rectifying K+ channel expressed in potato guard cells. EMBO J. 1995, 14, 2409–2416. [Google Scholar] [CrossRef]
- Lebaudy, A.; Véry, A.A.; Sentenac, H. K+ channel activity in plants: Genes, regulations and functions. FEBS Lett. 2007, 581, 2357–2366. [Google Scholar] [CrossRef]
- Véry, A.A.; Nieves-Cordones, M.; Daly, M.; Khan, I.; Fizames, C.; Sentenac, H. Molecular biology of K+ transport across the plant cell membrane: What do we learn from comparison between plant species? J. Plant Physiol. 2014, 171, 748–769. [Google Scholar] [CrossRef]
- Dreyer, I.; Uozumi, N. Potassium channels in plant cells. FEBS J. 2011, 278, 4293–4303. [Google Scholar] [CrossRef]
- Zimmermann, S.; Sentenac, H. Plant ion channels: From molecular structures to physiological functions. Curr. Opin. Plant Biol. 1999, 2, 477–482. [Google Scholar] [CrossRef]
- Fu, H.H.; Luan, S. AtKuP1: A dual-affinity K+ transporter from Arabidopsis. Plant Cell 1998, 10, 63–73. [Google Scholar]
- Schachtman, D.P.; Schroeder, J.I.; Lucas, W.J.; Anderson, J.A.; Gaber, R.F. Expression of an inward-rectifying potassium channel by the Arabidopsis KAT1 cDNA. Science 1992, 258, 1654–1658. [Google Scholar] [CrossRef]
- Gaymard, F.; Cerutti, M.; Horeau, C.; Lemaillet, G.; Urbach, S.; Ravallec, M.; Devauchelle, G.; Sentenac, H.; Thibaud, J.B. The baculovirus/insect cell system as an alternative to Xenopus oocytes. First characterization of the AKT1 K+channel from Arabidopsis thaliana. J. Biol. Chem. 1996, 271, 22863–22870. [Google Scholar] [CrossRef][Green Version]
- Su, H.; Balderas, E.; Vera-Estrella, R.; Golldack, D.; Quigley, F.; Zhao, C.; Pantoja, O.; Bohnert, H.J. Expression of the cation transporter McHKT1 in a halophyte. Plant Mol. Biol. 2003, 52, 967–980. [Google Scholar] [CrossRef]
- Paynter, J.J.; Andres-Enguix, I.; Fowler, P.W.; Tottey, S.; Cheng, W.; Enkvetchakul, D.; Bavro, V.N.; Kusakabe, Y.; Sansom, M.S.P.; Robinson, N.J.; et al. Functional complementation and genetic deletion studies of KirBac channels: Activatory mutations highlight gating-sensitive domains. J. Biol. Chem. 2010, 285, 40754–40761. [Google Scholar] [CrossRef]
- Jan, Y.N.; Bichet, D.; Lin, Y.-F.; Ibarra, C.A.; Huang, C.S.; Yi, B.A.; Jan, Y.N.; Jan, L.Y. Evolving potassium channels by means of yeast selection reveals structural elements important for selectivity. Proc. Natl. Acad. Sci. USA 2004, 101, 4441–4446. [Google Scholar]
- Zaks-Makhina, E.; Kim, Y.; Aizenman, E.; Levitan, E.S. Novel neuroprotective K+ channel inhibitor identified by high-throughput screening in yeast. Mol. Pharmacol. 2004, 65, 214–219. [Google Scholar] [CrossRef]
- Paynter, J.J.; Sarkies, P.; Andres-Enguix, I.; Tucker, S.J. Genetic selection of activatory mutations in KcsA. Channels 2008, 2, 413–418. [Google Scholar] [CrossRef]
- Yenush, L. Potassium and Sodium Transport in Yeast. In Yeast Membrane Transport, Advances in Experimental Medicine and Biology; Ramos, J., Sychrová, H., Kschischo, M., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 187–228. [Google Scholar]
- Gaber, R.F.; Styles, C.A.; Fink, G.R. TRK1 encodes a plasma membrane protein required for high-affinity potassium transport in Saccharomyces cerevisiae. Mol. Cell. Biol. 1988, 8, 2848–2859. [Google Scholar] [CrossRef]
- Ko, C.H.; Gaber, R.F. TRK1 and TRK2 encode structurally related K+ transporters in Saccharomyces cerevisiae. Mol. Cell. Biol. 1991, 11, 4266–4273. [Google Scholar] [CrossRef]
- Ko, C.H.; Buckley, A.M.; Gaber, R.F. TRK2 is required for low affinity K+transport in Saccharomyces cerevisiae. Genetics 1990, 125, 305–312. [Google Scholar]
- Durell, S.R.; Guy, H.R. Structural models of the KtrB, TrkH, and Trk1,2 symporters based on the structure of the KcsA K(+) channel. Biophys. J. 1999, 77, 789–807. [Google Scholar] [CrossRef]
- Haro, R.; Rodríguez-Navarro, A. Functional analysis of the M2(D) helix of the TRK1 potassium transporter of Saccharomyces cerevisiae. Biochim. Biophys. Acta 2003, 1613, 1–6. [Google Scholar] [CrossRef]
- Kuroda, T.; Bihler, H.; Bashi, E.; Slayman, C.L.; Rivetta, A. Chloride channel function in the yeast TRK-potassium transporters. J. Membr. Biol. 2004, 198, 177–192. [Google Scholar] [CrossRef]
- Zayats, V.; Stockner, T.; Pandey, S.K.; Wörz, K.; Ettrich, R.; Ludwig, J. A refined atomic scale model of the Saccharomyces cerevisiae K+-translocation protein Trk1p combined with experimental evidence confirms the role of selectivity filter glycines and other key residues. Biochim. Biophys. Acta Biomembr. 2015, 1848, 1183–1195. [Google Scholar] [CrossRef][Green Version]
- Haro, R.; Rodríguez-Navarro, A. Molecular analysis of the mechanism of potassium uptake through the TRK1 transporter of Saccharomyces cerevisiae. Biochim. Biophys. Acta 2002, 1564, 114–122. [Google Scholar] [CrossRef]
- Ariño, J.; Ramos, J.; Sychrová, H. Alkali Metal Cation Transport and Homeostasis in Yeasts. Microbiol. Mol. Biol. Rev. 2010, 74, 95–120. [Google Scholar] [CrossRef]
- Ruiz, A.; Arino, J. Function and regulation of the Saccharomyces cerevisiae ENA sodium ATPase system. Eukaryot. Cell 2007, 6, 2175–2183. [Google Scholar] [CrossRef] [PubMed]
- Haro, R.; Garciadeblas, B.; Rodríguez-Navarro, A. A novel P-type ATPase from yeast involved in sodium transport. FEBS Lett. 1991, 291, 189–191. [Google Scholar] [CrossRef]
- Wieland, J.; Nitsche, A.M.; Strayle, J.; Steiner, H.; Rudolph, H.K. The PMR2 gene cluster encodes functionally distinct isoforms of a putative Na+ pump in the yeast plasma membrane. EMBO J 1995, 14, 3870–3882. [Google Scholar] [CrossRef]
- Benito, B.; Quintero, F.J.; Rodríguez-Navarro, A. Overexpression of the sodium ATPase of Saccharomyces cerevisiae: Conditions for phosphorylation from ATP and Pi. Biochim. Biophys. Acta 1997, 1328, 214–226. [Google Scholar] [CrossRef]
- Benito, B.; Garciadeblás, B.; Rodríguez-Navarro, A. Potassium- or sodium-efflux ATPase, a key enzyme in the evolution of fungi. Microbiology 2002, 148, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Palmgren, M.G.; Nissen, P. P-type ATPases. Annu. Rev. Biophys. 2011, 40, 243–266. [Google Scholar] [CrossRef]
- Bañuelos, M.A.; Sychrová, H.; Bleykasten-Grosshans, C.; Souciet, J.L.; Potier, S. The Nha1 antiporter of Saccharomyces cerevisiae mediates sodium and potassium efflux. Microbiology 1998, 144 (Pt 1), 2749–2758. [Google Scholar]
- Nakamura, N.; Tanaka, S.; Teko, Y.; Mitsui, K.; Kanazawa, H. Four Na+/H+ exchanger isoforms are distributed to Golgi and post-Golgi compartments and are involved in organelle pH regulation. J. Biol. Chem. 2005, 280, 1561–1572. [Google Scholar] [CrossRef]
- Ohgaki, R.; Nakamura, N.; Mitsui, K.; Kanazawa, H. Characterization of the ion transport activity of the budding yeast Na+/H+ antiporter, Nha1p, using isolated secretory vesicles. Biochim. Biophys. Acta 2005, 1712, 185–196. [Google Scholar] [CrossRef]
- Ketchum, K.A.; Joiner, W.J.; Sellers, A.J.; Kaczmarek, L.K.; Goldstein, S.A. A new family of outwardly rectifying potassium channel proteins with two pore domains in tandem. Nature 1995, 376, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Maresova, L.; Urbankova, E.; Gaskova, D.; Sychrova, H. Measurements of plasma membrane potential changes in Saccharomyces cerevisiae cells reveal the importance of the Tok1 channel in membrane potential maintenance. FEMS Yeast Res. 2006, 6, 1039–1046. [Google Scholar] [CrossRef]
- Ahmed, A.; Sesti, F.; Ilan, N.; Shih, T.M.; Sturley, S.L.; Goldstein, S.A. A molecular target for viral killer toxin: TOK1 potassium channels. Cell 1999, 99, 283–291. [Google Scholar] [CrossRef]
- Cagnac, O.; Leterrier, M.; Yeager, M.; Blumwald, E. Identification and characterization of Vnx1p, a novel type of vacuolar monovalent cation/H+ antiporter of Saccharomyces cerevisiae. J. Biol. Chem. 2007, 282, 24284–24293. [Google Scholar] [CrossRef]
- Petrezselyova, S.; Kinclova-Zimmermannova, O.; Sychrova, H. Vhc1, a novel transporter belonging to the family of electroneutral cation-Cl(−) cotransporters, participates in the regulation of cation content and morphology of Saccharomyces cerevisiae vacuoles. Biochim. Biophys. Acta 2013, 1828, 623–631. [Google Scholar] [CrossRef] [PubMed]
- André, B.; Scherens, B. The yeast YBR235w gene encodes a homolog of the mammalian electroneutral Na(+)-(K+)-C1- cotransporter family. Biochem. Biophys. Res. Commun. 1995, 217, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Nass, R.; Rao, R. Novel localization of a Na+/H+ exchanger in a late endosomal compartment of yeast. Implications for vacuole biogenesis. J. Biol. Chem. 1998, 273, 21054–21060. [Google Scholar] [CrossRef] [PubMed]
- Maresova, L.; Sychrova, H. Physiological characterization of Saccharomyces cerevisiae kha1 deletion mutants. Mol. Microbiol. 2005. [Google Scholar] [CrossRef]
- Nowikovsky, K.; Froschauer, E.M.; Zsurka, G.; Samaj, J.; Reipert, S.; Kolisek, M.; Wiesenberger, G.; Schweyen, R.J. The LETM1/YOL027 gene family encodes a factor of the mitochondrial K+ homeostasis with a potential role in the Wolf-Hirschhorn syndrome. J. Biol. Chem. 2004, 279, 30307–30315. [Google Scholar] [CrossRef]
- Rodríguez-Navarro, A. Potassium transport in fungi and plants. Biochim. Biophys. Acta 2000, 1469, 1–30. [Google Scholar] [CrossRef]
- Madrid, R.; Gómez, M.J.; Ramos, J.; Rodríguez-Navarro, A. Ectopic potassium uptake in trk1 trk2 mutants of Saccharomyces cerevisiae correlates with a highly hyperpolarized membrane potential. J. Biol. Chem. 1998, 273, 14838–14844. [Google Scholar] [CrossRef]
- Anderson, J.A.; Nakamura, R.L.; Gaber, R.F. Heterologous expression of K+ channels in Saccharomyces cerevisiae: Strategies for molecular analysis of structure and function. Symp. Soc. Exp. Biol. 1994, 48, 85–97. [Google Scholar]
- Lebaudy, A.; Pascaud, F.; Véry, A.A.; Alcon, C.; Dreyer, I.; Thibaud, J.B.; Lacombe, B. Preferential KAT1-KAT2 heteromerization determines inward K+ current properties in Arabidopsis guard cells. J. Biol. Chem. 2010, 285, 6265–6274. [Google Scholar] [CrossRef]
- Ache, P.; Becker, D.; Ivashikina, N.; Dietrich, P.; Roelfsema, M.R.G.; Hedrich, R. GORK, a delayed outward rectifier expressed in guard cells of Arabidopsis thaliana, is a K+-selective, K+-sensing ion channel. FEBS Lett. 2000, 486, 93–98. [Google Scholar] [CrossRef]
- Schroeder, J.I.; Allen, G.J.; Hugouvieux, V.; Kwak, J.M.; Waner, D. GUARD CELL SIGNAL TRANSDUCTION. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 627–658. [Google Scholar] [CrossRef]
- Choe, H.; Sackin, H.; Palmer, L.G. Permeation Properties of Inward-Rectifier Potassium Channels and Their Molecular Determinants. J. Gen. Physiol. 2000, 115, 391–404. [Google Scholar] [CrossRef]
- Jiang, Y.; Lee, A.; Chen, J.; Ruta, V.; Cadene, M.; Chait, B.T.; MacKinnon, R. X-ray structure of a voltage-dependent K+ channel. Nature 2003, 423, 33–41. [Google Scholar] [CrossRef]
- Long, S.B.; Campbell, E.B.; Mackinnon, R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science 2005, 309, 897–903. [Google Scholar] [CrossRef]
- Pilot, G.; Lacombe, B.; Gaymard, F.; Chérel, I.; Boucherez, J.; Thibaud, J.B.; Sentenac, H. Guard Cell Inward K+ Channel Activity in Arabidopsis Involves Expression of the Twin Channel Subunits KAT1 and KAT2. J. Biol. Chem. 2001, 276, 3215–3221. [Google Scholar] [CrossRef]
- Saito, S.; Hoshi, N.; Zulkifli, L.; Widyastuti, S.; Goshima, S.; Dreyer, I.; Uozumi, N. Identification of regions responsible for the function of the plant K+ channels KAT1 and AKT2 in Saccharomyces cerevisiae and Xenopus laevis oocytes. Channels 2017, 11, 510–516. [Google Scholar] [CrossRef]
- Nakamura, R.L.; Anderson, J.A.; Gaber, R.F. Determination of key structural requirements of a K+ channel pore. J. Biol. Chem. 1997, 272, 1011–1018. [Google Scholar] [CrossRef]
- Nakamura, R.L.; Gaber, R.F. Ion selectivity of the Kat1 K+ channel pore. Mol. Membr. Biol. 2009, 26, 293–308. [Google Scholar] [CrossRef]
- Kochian, L.V.; Garvin, D.F.; Shaff, J.E.; Chilcott, T.C.; Lucas, W.J. Towards an understanding of the molecular basis of plants K+ transport: Characterization of cloned K+ transport cDNAs. Plant Soil 1993, 155, 115–118. [Google Scholar] [CrossRef]
- Lai, H.C.; Grabe, M.; Yuh, N.J.; Lily, Y.J. The S4 voltage sensor packs against the pore domain in the KAT1 voltage-gated potassium channel. Neuron 2005, 47, 395–406. [Google Scholar] [CrossRef]
- Su, Y.-H. Regulation by External K+ in a Maize Inward Shaker Channel Targets Transport Activity in the High Concentration Range. PLANT CELL ONLINE 2005, 17, 1532–1548. [Google Scholar] [CrossRef]
- Obata, T.; Kitamoto, H.K.; Nakamura, A.; Fukuda, A.; Tanaka, Y. Rice Shaker Potassium Channel OsKAT1 Confers Tolerance to Salinity Stress on Yeast and Rice Cells. Plant Physiol. 2007, 144, 1978–1985. [Google Scholar] [CrossRef]
- Hirsch, R.E.; Lewis, B.D.; Spalding, E.P.; Sussman, M.R. A role for the AKT1 potassium channel in plant nutrition. Science 1998, 280, 918–921. [Google Scholar] [CrossRef]
- Ahn, S.J.; Shin, R.; Schachtman, D.P. Expression of KT/KUP Genes in Arabidopsis and the Role of Root Hairs in K+ Uptake. Plant Physiol. 2004, 134, 1135–1145. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, W.-H. Potassium transport and signaling in higher plants. Annu. Rev. Plant Biol. 2013, 64, 451–476. [Google Scholar] [CrossRef]
- Gierth, M.; Mäser, P.; Schroeder, J.I. The potassium transporter AtHAK5 functions in K(+) deprivation-induced high-affinity K(+) uptake and AKT1 K(+) channel contribution to K(+) uptake kinetics in Arabidopsis roots. Plant Physiol. 2005, 137, 1105–1114. [Google Scholar] [CrossRef]
- Ros, R.; Lemaillet, G.; Fonrouge, A.G.; Daram, P.; Enjuto, M.; Salmon, J.M.; Thibaud, J.B.; Sentenac, H. Molecular determinants of the Arabidopsis AKT1 K+ channel ionic selectivity investigated by expression in yeast of randomly mutated channels. Physiol. Plant. 2002, 105, 459–468. [Google Scholar] [CrossRef]
- Marten, I.; Gaymard, F.; Lemaillet, G.; Thibaud, J.B.; Sentenac, H.; Hedrich, R. Functional expression of the plant K+ channel KAT1 in insect cells. FEBS Lett. 1996, 380, 229–232. [Google Scholar] [CrossRef]
- Zimmermann, S.; Talke, I.; Ehrhardt, T.; Nast, G.; Müller-Röber, B. Characterization of SKT1, an Inwardly Rectifying Potassium Channel from Potato, by Heterologous Expression in Insect Cells. Plant Physiol. 1998, 116, 879–890. [Google Scholar] [CrossRef]
- Philippar, K.; Fuchs, I.; Lüthen, H.; Hoth, S.; Bauer, C.S.; Haga, K.; Thiel, G.; Ljung, K.; Sandberg, G.; Böttger, M.; et al. Auxin-induced K+ channel expression represents an essential step in coleoptile growth and gravitropism. Proc. Natl. Acad. Sci. USA 1999, 96, 12186–12191. [Google Scholar] [CrossRef]
- Hartje, S.; Zimmermann, S.; Klonus, D.; Mueller-Roeber, B. Functional characterisation of LKT1, a K+ uptake channel from tomato root hairs, and comparison with the closely related potato inwardly rectifying K+ channel SKT1 after expression in Xenopus oocytes. Planta 2000, 210, 723–731. [Google Scholar] [CrossRef]
- Boscari, A.; Clément, M.; Volkov, V.; Golldack, D.; Hybiak, J.; Miller, A.J.; Amtmann, A.; Fricke, W. Potassium channels in barley: Cloning, functional characterization and expression analyses in relation to leaf growth and development. Plant Cell Environ. 2009, 32, 1761–1777. [Google Scholar] [CrossRef]
- Schleyer, M.; Bakker, E.P. Nucleotide sequence and 3′-end deletion studies indicate that the K+- uptake protein kup from Escherichia coli is composed of a hydrophobic core linked to a large and partially essential hydrophilic C terminus. J. Bacteriol. 1993, 175, 6925–6931. [Google Scholar] [CrossRef]
- Bañuelos, M.A.; Klein, R.D.; Alexander-Bowman, S.J.; Rodríguez-Navarro, A. A potassium transporter of the yeast Schwanniomyces occidentalis homologous to the Kup system of Escherichia coli has a high concentrative capacity. EMBO J. 1995, 14, 3021–3027. [Google Scholar] [CrossRef]
- Santa-María, G.E.; Rubio, F.; Dubcovsky, J.; Rodríguez-Navarro, A. The HAK1 gene of barley is a member of a large gene family and encodes a high-affinity potassium transporter. Plant Cell 1997, 9, 2281–2289. [Google Scholar] [CrossRef]
- Nieves-Cordones, M.; Alemán, F.; Martínez, V.; Rubio, F. The Arabidopsis thaliana HAK5 K+ transporter is required for plant growth and K+ acquisition from low K+ solutions under saline conditions. Mol. Plant 2010, 3, 326–333. [Google Scholar] [CrossRef]
- Rubio, F.; Santa-María Guillermo, E.; Rodríguez-Navarro, A. Cloning of Arabidopsis and barley cDNAs encoding HAK potassium transporters in root and shoot cells. Physiol. Plant. 2001, 109, 34–43. [Google Scholar] [CrossRef]
- Kim, E.J.; Kwak, J.M.; Uozumi, N.; Schroeder, J.I. AtKUP1: An Arabidopsis gene encoding high-affinity potassium transport activity. Plant Cell 1998, 10, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Quintero, F.J.; Blatt, M.R. A new family of K+ transporters from Arabidopsis that are conserved across phyla. FEBS Lett. 1997, 415, 206–211. [Google Scholar] [CrossRef]
- Rigas, S.; Debrosses, G.; Haralampidis, K.; Vicente-Agullo, F.; Feldmann, K.A.; Grabov, A.; Dolan, L.; Hatzopoulos, P. TRH1 encodes a potassium transporter required for tip growth in Arabidopsis root hairs. Plant Cell 2001, 13, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Tenorio-Berrío, R.; Pérez-Alonso, M.M.; Vicente-Carbajosa, J.; Martín-Torres, L.; Dreyer, I.; Pollmann, S. Identification of two auxin-regulated potassium transporters involved in seed maturation. Int. J. Mol. Sci. 2018, 19, 2132. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Luo, W.; Lin, W.; Ma, L.; Kabir, M.H. Model of cation transportation mediated by high-affinity potassium transporters (HKTs) in higher plants. Biol. Proced. Online 2015, 17, 1. [Google Scholar] [CrossRef]
- Maser, P.; Hosoo, Y.; Goshima, S.; Horie, T.; Eckelman, B.; Yamada, K.; Yoshida, K.; Bakker, E.P.; Shinmyo, A.; Oiki, S.; et al. Glycine residues in potassium channel-like selectivity filters determine potassium selectivity in four-loop-per-subunit HKT transporters from plants. Proc. Natl. Acad. Sci. USA 2002, 99, 6428–6433. [Google Scholar] [CrossRef]
- Hamamoto, S.; Horie, T.; Hauser, F.; Deinlein, U.; Schroeder, J.I.; Uozumi, N. HKT transporters mediate salt stress resistance in plants: From structure and function to the field. Curr. Opin. Biotechnol. 2015, 32, 113–120. [Google Scholar] [CrossRef]
- Schachtman, D.P.; Schroeder, J.I. Structure and transport mechanism of a high-affinity potassium uptake transporter from higher plants. Nature 1994, 370, 655–658. [Google Scholar] [CrossRef]
- Rubio, F.; Gassmann, W.; Schroeder, J.I. Sodium-driven potassium uptake by the plant potassium transporter HKT1 and mutations conferring salt tolerance. Science 1995, 270, 1660–1663. [Google Scholar] [CrossRef]
- Gassmann, W.; Rubio, F.; Schroeder, J.I. Alkali cation selectivity of the wheat root high-affinity potassium transporter HKT1. Plant J. 1996, 10, 869–882. [Google Scholar] [CrossRef]
- Uozumi, N.; Kim, E.J.; Rubio, F.; Yamaguchi, T.; Muto, S.; Tsuboi, A.; Bakker, E.P.; Nakamura, T.; Schroeder, J.I. The Arabidopsis HKT1 gene homolog mediates inward Na(+) currents in xenopus laevis oocytes and Na(+) uptake in Saccharomyces cerevisiae. Plant Physiol. 2000, 122, 1249–1259. [Google Scholar] [CrossRef]
- Rubio, F.; Schwarz, M.; Gassmann, W.; Schroeder, J.I. Genetic selection of mutations in the high affinity K+ transporter HKT1 that define functions of a loop site for reduced Na+ permeability and increased Na+ tolerance. J. Biol. Chem. 1999, 274, 6839–6847. [Google Scholar] [CrossRef]
- Sunarpi; Horie, T.; Motoda, J.; Kubo, M.; Yang, H.; Yoda, K.; Horie, R.; Chan, W.Y.; Leung, H.Y.; Hattori, K.; et al. Enhanced salt tolerance mediated by AtHKT1 transporter-induced Na unloading from xylem vessels to xylem parenchyma cells. Plant J 2005, 44, 928–938. [Google Scholar] [CrossRef]
- Fairbairn, D.J.; Liu, W.; Schachtman, D.P.; Gomez-Gallego, S.; Day, S.R.; Teasdale, R.D. Characterisation of two distinct HKT1-like potassium transporters from Eucalyptus camaldulensis. Plant Mol. Biol. 2000, 43, 515–525. [Google Scholar] [CrossRef]
- Horie, T.; Yoshida, K.; Nakayama, H.; Yamada, K.; Oiki, S.; Shinmyo, A. Two types of HKT transporters with different properties of Na+ and K+ transport in Oryza sativa. Plant J. 2001, 27, 129–138. [Google Scholar] [CrossRef]
- Wang, T.B.; Gassmann, W.; Rubio, F.; Schroeder, J.I.; Glass, A.D. Rapid Up-regulation of HKT1, a high-affinity potassium transporter gene, in roots of barley and wheat following withdrawal of potassium. Plant Physiol. 1998, 118, 651–659. [Google Scholar] [CrossRef]
- Garciadeblás, B.; Senn, M.E.; Bañuelos, M.A.; Rodríguez-Navarro, A. Sodium transport and HKT transporters: The rice model. Plant J. 2003, 34, 788–801safi. [Google Scholar] [CrossRef]
- Safiarian, M.J.; Pertl-Obermeyer, H.; Lughofer, P.; Hude, R.; Bertl, A.; Obermeyer, G. Lost in traffic? The K+ channel of lily pollen, LilKT1, is detected at the endomembranes inside yeast cells, tobacco leaves, and lily pollen. Front. Plant Sci. 2015, 6, 47. [Google Scholar] [CrossRef]
- Mouline, K.; Véry, A.A.; Gaymard, F.; Boucherez, J.; Pilot, G.; Devic, M.; Bouchez, D.; Thibaud, J.B.; Sentenac, H. Pollen tube development and competitive ability are impaired by disruption of a Shaker K+ channel in Arabidopsis. Genes Dev. 2002, 16, 339–350. [Google Scholar] [CrossRef]
- Bihler, H. TPK1 Is a Vacuolar Ion Channel Different from the Slow-Vacuolar Cation Channel. Plant Physiol. 2005, 139, 417–424. [Google Scholar] [CrossRef]
- Hamamoto, S.; Marui, J.; Matsuoka, K.; Higashi, K.; Igarashi, K.; Nakagawa, T.; Kuroda, T.; Mori, Y.; Murata, Y.; Nakanishi, Y.; et al. Characterization of a tobacco TPK-type K+ channel as a novel tonoplast K+ channel using yeast tonoplasts. J. Biol. Chem. 2008, 283, 1911–1920. [Google Scholar] [CrossRef]
- Goldstein, S.A.N.; Bockenhauer, D.; O’Kelly, I.; Zilberberg, N. Potassium leak channels and the KCNK family of two-p-domain subunits. Nat. Rev. Neurosci. 2001, 2, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.J.; Honoré, E. Properties and modulation of mammalian 2P domain K+ channels. Trends Neurosci. 2001, 24, 339–346. [Google Scholar] [CrossRef]
- Becker, D.; Geiger, D.; Dunkel, M.; Roller, A.; Bertl, A.; Latz, A.; Carpaneto, A.; Dietrich, P.; Roelfsema, M.R.; Voelker, C.; et al. AtTPK4, an Arabidopsis tandem-pore K+ channel, poised to control the pollen membrane voltage in a pH- and Ca2+-dependent manner. Proc. Natl. Acad. Sci. USA 2004, 101, 15621–15626. [Google Scholar] [CrossRef] [PubMed]
- Apse, M.P.; Aharon, G.S.; Snedden, W.A.; Blumwald, E. Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science 1999, 285, 1256–1258. [Google Scholar] [CrossRef] [PubMed]
- Gaxiola, R.A.; Rao, R.; Sherman, A.; Grisafi, P.; Alper, S.L.; Fink, G.R. The Arabidopsis thaliana proton transporters, AtNhx1 and Avp1, can function in cation detoxification in yeast. Proc. Natl. Acad. Sci. USA 1999, 96, 1480–1485. [Google Scholar] [CrossRef]
- Bassil, E.; Blumwald, E.; Nakano, R.; Ohto, M.; Liang, Y.-C.; Ushijima, K.; Esumi, T.; Tajima, H.; Belmonte, M.; Coku, A. The Arabidopsis Na+/H+ Antiporters NHX1 and NHX2 Control Vacuolar pH and K+ Homeostasis to Regulate Growth, Flower Development, and Reproduction. Plant Cell 2011, 23, 3482–3497. [Google Scholar] [CrossRef]
- Bassil, E.; Ohto, M.A.; Esumi, T.; Tajima, H.; Zhu, Z.; Cagnac, O.; Belmonte, M.; Peleg, Z.; Yamaguchi, T.; Blumwald, E. The Arabidopsis intracellular Na+/H+ antiporters NHX5 and NHX6 are endosome associated and necessary for plant growth and development. Plant Cell 2011, 23, 224–239. [Google Scholar] [CrossRef]
- Xia, T.; Apse, M.P.; Aharon, G.S.; Blumwald, E. Identification and characterization of a NaCl-inducible vacuolar Na+/H+antiporter in Beta vulgaris. Physiol. Plant. 2002, 116, 206–212. [Google Scholar] [CrossRef]
- Wu, C.; Gao, X.; Kong, X.; Zhao, Y.; Zhang, H. Molecular Cloning and Functional Analysis of a Na+/H+ Antiporter Gene ThNHX1 from a Halophytic Plant Thellungiella halophila. Plant Mol. Biol. Report. 2009, 27, 1–12. [Google Scholar] [CrossRef]
- Cosentino, C.; Alberio, L.; Thiel, G.; Moroni, A. Yeast-based screening system for the selection of functional light-driven K+ channels. Methods Mol. Biol. 2017, 1596, 271–285. [Google Scholar]
- Plugge, B.; Gazzarrini, S.; Nelson, M.; Cerana, R.; Van Etten, J.L.; Derst, C.; DiFrancesco, D.; Moroni, A.; Thiel, G. A potassium channel protein encoded by chlorella virus PBCV-1. Science 2000, 287, 1641–1644. [Google Scholar] [CrossRef]
- Xu, K.; Zhang, H.; Blumwald, E.; Xia, T. A novel plant vacuolar Na+/H+ antiporter gene evolved by DNA shuffling confers improved salt tolerance in yeast. J. Biol. Chem. 2010, 285, 22999–23006. [Google Scholar] [CrossRef]
- Fields, S.; Song, O. A novel genetic system to detect protein-protein interactions. Nature 1989, 340, 245–246. [Google Scholar] [CrossRef]
- Vidal, M.; Brachmann, R.K.; Fattaey, A.; Harlow, E.; Boeke, J.D. Reverse two-hybrid and one-hybrid systems to detect dissociation of protein-protein and DNA-protein interactions. Proc. Natl. Acad. Sci. USA 1996, 93, 10315–10320. [Google Scholar] [CrossRef]
- Leanna, C.A.; Hannink, M. The reverse two-hybrid system: A genetic scheme for selection against specific protein/protein interactions. Nucleic Acids Res. 1996, 24, 3341–3347. [Google Scholar] [CrossRef]
- Licitra, E.J.; Liu, J.O. A three-hybrid system for detecting small ligand-protein receptor interactions. Proc. Natl. Acad. Sci. USA 1996, 93, 12817–12821. [Google Scholar] [CrossRef]
- Serebriiskii, I.; Khazak, V.; Golemis, E.A. A two-hybrid dual bait system to discriminate specificity of protein interactions. J. Biol. Chem. 1999, 274, 17080–17087. [Google Scholar] [CrossRef]
- Hirst, M.; Ho, C.; Sabourin, L.; Rudnicki, M.; Penn, L.; Sadowski, I. A two-hybrid system for transactivator bait proteins. Proc. Natl. Acad. Sci. USA 2001, 2001, 8726–8731. [Google Scholar] [CrossRef]
- Petrascheck, M.; Castagna, F.; Barberis, A. Two-hybrid selection assay to identify proteins interacting with polymerase II transcription factors and regulators. Biotechniques 2001, 30, 296–298,300,302. [Google Scholar] [CrossRef] [PubMed]
- Johnsson, N.; Varshavsky, A. Split ubiquitin as a sensor of protein interactions in vivo. Proc. Natl. Acad. Sci. USA 1994, 91, 10340–10344. [Google Scholar] [CrossRef] [PubMed]
- Stagljar, I.; Korostensky, C.; Johnsson, N.; te Heesen, S. A genetic system based on split-ubiquitin for the analysis of interactions between membrane proteins in vivo. Proc. Natl. Acad. Sci. USA 1998, 95, 5187–5192. [Google Scholar] [CrossRef] [PubMed]
- Gisler, S.M.; Kittanakom, S.; Fuster, D.; Wong, V.; Bertic, M.; Radanovic, T.; Hall, R.A.; Murer, H.; Biber, J.; Markovich, D.; et al. Monitoring Protein-Protein Interactions between the Mammalian Integral Membrane Transporters and PDZ-interacting Partners Using a Modified Split-ubiquitin Membrane Yeast Two-hybrid System. Mol. Cell. Proteomics 2008, 7, 1362–1377. [Google Scholar] [CrossRef] [PubMed]
- Ehrhard, K.N.; Jacoby, J.J.; Fu, X.Y.; Jahn, R.; Dohlman, H.G. Use of G-protein fusions to monitor integral membrane protein-protein interactions in yeast. Nat. Biotechnol. 2000, 18, 1075–1079. [Google Scholar] [CrossRef]
- Hubsman, M.; Yudkovsky, G.; Aronheim, A. A novel approach for the identification of protein-protein interaction with integral membrane proteins. Nucleic Acids Res. 2001, 29, E18. [Google Scholar] [CrossRef]
- Aronheim, A.; Zandi, E.; Hennemann, H.; Elledge, S.J.; Karin, M. Isolation of an AP-1 repressor by a novel method for detecting protein-protein interactions. Mol. Cell. Biol. 1997, 17, 3094–3102. [Google Scholar] [CrossRef]
- Broder, Y.C.; Katz, S.; Aronheim, A. The ras recruitment system, a novel approach to the study of protein-protein interactions. Curr. Biol. 1998, 8, 1121–1124. [Google Scholar] [CrossRef]
- Mockli, N.; Deplazes, A.; Hassa, P.O.; Zhang, Z.; Peter, M.; Hottiger, M.O.; Stagljar, I.; Auerbach, D. Yeast split-ubiquitin-based cytosolic screening system to detect interactions between transcriptionally active proteins. Biotechniques 2007, 42, 725–730. [Google Scholar] [CrossRef]
- Boder, E.T.; Wittrup, K.D. Yeast surface display for screening combinatorial polypeptide libraries. Nat. Biotechnol. 1997, 15, 553–557. [Google Scholar] [CrossRef]
- Urech, D.M.; Lichtlen, P.; Barberis, A. Cell growth selection system to detect extracellular and transmembrane protein interactions. Biochim. Biophys. Acta 2003, 1622, 117–127. [Google Scholar] [CrossRef]
- Dube, D.H.; Li, B.; Greenblatt, E.J.; Nimer, S.; Raymond, A.K.; Kohler, J.J. A two-hybrid assay to study protein interactions within the secretory pathway. PLoS ONE 2010, 5, e15648. [Google Scholar] [CrossRef] [PubMed]
- Laser, H.; Bongards, C.; Schuller, J.; Heck, S.; Johnsson, N.; Lehming, N. A new screen for protein interactions reveals that the Saccharomyces cerevisiae high mobility group proteins Nhp6A/B are involved in the regulation of the GAL1 promoter. Proc. Natl. Acad. Sci. USA 2000, 97, 13732–13737. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, J.N.; Campbell-Valois, F.X.; Michnick, S.W. Oligomerization domain-directed reassembly of active dihydrofolate reductase from rationally designed fragments. Proc. Natl. Acad. Sci. USA 1998, 95, 12141–12146. [Google Scholar] [CrossRef]
- Ozawa, T.; Kaihara, A.; Sato, M.; Tachihara, K.; Umezawa, Y. Split luciferase as an optical probe for detecting protein-protein interactions in mammalian cells based on protein splicing. Anal. Chem. 2001, 73, 2516–2521. [Google Scholar] [CrossRef] [PubMed]
- Tafelmeyer, P.; Johnsson, N.; Johnsson, K. Transforming a (beta/alpha)8--barrel enzyme into a split-protein sensor through directed evolution. Chem. Biol. 2004, 11, 681–689. [Google Scholar] [CrossRef]
- Hu, C.-D.; Chinenov, Y.; Kerppola, T.K. Visualization of interactions among bZIP and Rel family proteins in living cells using bimolecular fluorescence complementation. Mol. Cell. 2002, 9, 789–798. [Google Scholar] [CrossRef]
- Magliery, T.J.; Wilson, C.G.M.; Pan, W.; Mishler, D.; Ghosh, I.; Hamilton, A.D.; Regan, L. Detecting protein-protein interactions with a green fluorescent protein fragment reassembly trap: Scope and mechanism. J. Am. Chem. Soc. 2005, 127, 146–157. [Google Scholar] [CrossRef]
- Xing, S.; Wallmeroth, N.; Berendzen, K.W.; Grefen, C. Techniques for the Analysis of Protein-Protein Interactions in Vivo. Plant Physiol. 2016, 171, 727–758. [Google Scholar] [CrossRef]
- Moosavi, B.; Mousavi, B.; Yang, W.-C.; Yang, G.-F. Yeast-based assays for detecting protein-protein/drug interactions and their inhibitors. Eur. J. Cell Biol. 2017, 96, 529–541. [Google Scholar] [CrossRef]
- Stynen, B.; Tournu, H.; Tavernier, J.; Van Dijck, P. Diversity in genetic in vivo methods for protein-protein interaction studies: From the yeast two-hybrid system to the mammalian split-luciferase system. Microbiol. Mol. Biol. Rev. 2012, 76, 331–382. [Google Scholar] [CrossRef]
- Bruckner, A.; Polge, C.; Lentze, N.; Auerbach, D.; Schlattner, U. Yeast two-hybrid, a powerful tool for systems biology. Int. J. Mol. Sci. 2009, 10, 2763–2788. [Google Scholar] [CrossRef] [PubMed]
- Gunde, T.; Tanner, S.; Auf der Maur, A.; Petrascheck, M.; Barberis, A. Quenching accumulation of toxic galactose-1-phosphate as a system to select disruption of protein-protein interactions in vivo. Biotechniques 2004, 37, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Obrdlik, P.; El-Bakkoury, M.; Hamacher, T.; Cappellaro, C.; Vilarino, C.; Fleischer, C.; Ellerbrok, H.; Kamuzinzi, R.; Ledent, V.; Blaudez, D.; et al. K+ channel interactions detected by a genetic system optimized for systematic studies of membrane protein interactions. Proc. Natl. Acad. Sci. USA 2004, 101, 12242–12247. [Google Scholar] [CrossRef] [PubMed]
- Raquet, X.; Eckert, J.H.; Muller, S.; Johnsson, N. Detection of altered protein conformations in living cells. J. Mol. Biol. 2001, 305, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Karasawa, S.; Miyawaki, A.; Tsumuraya, T.; Fujii, I. Novel screening system for protein-protein interactions by bimolecular fluorescence complementation in Saccharomyces cerevisiae. J. Biosci. Bioeng. 2011, 111, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Cherel, I.; Gaillard, I. The Complex Fine-Tuning of K(+) Fluxes in Plants in Relation to Osmotic and Ionic Abiotic Stresses. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef] [PubMed]
- Bregante, M.; Yang, Y.; Formentin, E.; Carpaneto, A.; Schroeder, J.I.; Gambale, F.; Lo Schiavo, F.; Costa, A. KDC1, a carrot Shaker-like potassium channel, reveals its role as a silent regulatory subunit when expressed in plant cells. Plant Mol. Biol. 2008, 66, 61–72. [Google Scholar] [CrossRef]
- Sklodowski, K.; Riedelsberger, J.; Raddatz, N.; Riadi, G. The receptor-like pseudokinase MRH1 interacts with the voltage- gated potassium channel AKT2. Nat. Publ. Gr. 2017, 1–12. [Google Scholar] [CrossRef]
- Zhang, A.; Ren, H.-M.; Tan, Y.-Q.; Qi, G.-N.; Yao, F.-Y.; Wu, G.-L.; Yang, L.-W.; Hussain, J.; Sun, S.-J.; Wang, Y.-F. S-type Anion Channels SLAC1 and SLAH3 Function as Essential Negative Regulators of Inward K+ Channels and Stomatal Opening in Arabidopsis. Plant Cell 2016, 28, 949–955. [Google Scholar] [CrossRef]
- Rosas-Santiago, P.; Lagunas-Gomez, D.; Barkla, B.J.; Vera-Estrella, R.; Lalonde, S.; Jones, A.; Frommer, W.B.; Zimmermannova, O.; Sychrova, H.; Pantoja, O. Identification of rice cornichon as a possible cargo receptor for the Golgi-localized sodium transporter OsHKT1;3. J. Exp. Bot. 2015, 66, 2733–2748. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Karnik, R.; Wang, Y.; Wallmeroth, N.; Blatt, M.R.; Grefen, C. The Arabidopsis R-SNARE VAMP721 Interacts with KAT1 and KC1 K+ Channels to Moderate K+ Current at the Plasma Membrane. Plant Cell 2015, 27, 1697–1717. [Google Scholar] [CrossRef] [PubMed]
- Honsbein, A.; Sokolovski, S.; Grefen, C.; Campanoni, P.; Pratelli, R.; Paneque, M.; Chen, Z.; Johansson, I.; Blatt, M.R. A tripartite SNARE-K+ channel complex mediates in channel-dependent K+ nutrition in Arabidopsis. Plant Cell 2009, 21, 2859–2877. [Google Scholar] [CrossRef] [PubMed]
- Grefen, C.; Chen, Z.; Honsbein, A.; Donald, N.; Hills, A.; Blatt, M.R. A novel motif essential for SNARE interaction with the K(+) channel KC1 and channel gating in Arabidopsis. Plant Cell 2010, 22, 3076–3092. [Google Scholar] [CrossRef] [PubMed]
- Osakabe, Y.; Arinaga, N.; Umezawa, T.; Katsura, S.; Nagamachi, K.; Tanaka, H.; Ohiraki, H.; Yamada, K.; Seo, S.U.; Abo, M.; et al. Osmotic stress responses and plant growth controlled by potassium transporters in Arabidopsis. Plant Cell 2013, 25, 609–624. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Lan, W.; Kim, B.; Li, L.; Cheong, Y.H.; Pandey, G.K.; Lu, G.; Buchanan, B.B.; Luan, S. A protein phosphorylation / dephosphorylation network regulates a plant potassium channel. Proc. Natl. Acad. Sci. USA 2007, 104, 15959–15964. [Google Scholar] [CrossRef] [PubMed]
- Pilot, G.; Gaymard, F.; Mouline, K.; Chérel, I.; Sentenac, H. Regulated expression of Arabidopsis Shaker K+ channel genes involved in K+ uptake and distribution in the plant. Plant Mol. Biol. 2003, 51, 773–787. [Google Scholar] [CrossRef]
- Ren, X.; Qi, G.; Feng, H.; Zhao, S.; Zhao, S.; Wang, Y.; Wu, W. Calcineurin B-like protein CBL10 directly interacts with AKT1 and modulates K + homeostasis in Arabidopsis. Plant J. 2013, 74, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Kim, B.G.; Cheong, Y.H.; Pandey, G.K.; Luan, S. A Ca(2)+ signaling pathway regulates a K(+) channel for low-K response in Arabidopsis. Proc. Natl. Acad. Sci. USA 2006, 103, 12625–12630. [Google Scholar] [CrossRef]
- Xu, J.; Li, H.D.; Chen, L.Q.; Wang, Y.; Liu, L.L.; He, L.; Wu, W.H. A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis. Cell 2006, 125, 1347–1360. [Google Scholar] [CrossRef]
- Geiger, D.; Becker, D.; Vosloh, D.; Gambale, F.; Palme, K.; Rehers, M.; Anschuetz, U.; Dreyer, I.; Hedrich, R. Heteromeric At KC1·AKT1 Channels in Arabidopsis Roots Facilitate Growth under K + -limiting Conditions. J. Biol. Chem. 2009, 284, 21288–21295. [Google Scholar]
- Ardie, S.W.; Nishiuchi, S.; Liu, S.; Takano, T. Ectopic expression of the K+ channel beta subunits from Puccinellia tenuiflora (KPutB1) and rice (KOB1) alters K+ homeostasis of yeast and Arabidopsis. Mol. Biotechnol. 2011, 48, 76–86. [Google Scholar] [CrossRef]
- Held, K.; Pascaud, F.; Eckert, C.; Gajdanowicz, P.; Hashimoto, K. Calcium-dependent modulation and plasma membrane targeting of the AKT2 potassium channel by the CBL4 / CIPK6 calcium sensor / protein kinase complex. Nat. Publ. Gr. 2011, 21, 1116–1130. [Google Scholar] [CrossRef]
- Cherel, I. Physical and Functional Interaction of the Arabidopsis K+ Channel AKT2 and Phosphatase AtPP2CA. PLANT CELL ONLINE 2002, 14, 1133–1146. [Google Scholar] [CrossRef]
- Vranova, E.; Tahtiharju, S.; Sriprang, R.; Willekens, H.; Heino, P.; Palva, E.T.; Inze, D.; Van Camp, W. The AKT3 potassium channel protein interacts with the AtPP2CA protein phosphatase 2C. J. Exp. Bot. 2001, 52, 181–182. [Google Scholar] [CrossRef]
- Lefoulon, C.; Boeglin, M.; Moreau, B.; Véry, A.; Szponarski, W.; Dauzat, M.; Michard, E.; Gaillard, I.; Chérel, I. The Arabidopsis AtPP2CA Protein Phosphatase Inhibits the GORK K+ Efflux Channel and Exerts a Dominant Suppressive Effect on Phosphomimetic-activating Mutations. J. Biol. Chem. 2016, 291, 6521–6533. [Google Scholar] [CrossRef]
- Dreyer, I.; Poree, F.; Schneider, A.; Mittelstadt, J.; Bertl, A.; Sentenac, H.; Thibaud, J.-B.; Mueller-Roeber, B. Assembly of plant Shaker-like K(out) channels requires two distinct sites of the channel alpha-subunit. Biophys. J. 2004, 87, 858–872. [Google Scholar] [CrossRef]
- Liu, L.; Zheng, C.; Kuang, B.; Wei, L.; Yan, L.; Wang, T. Receptor-Like Kinase RUPO Interacts with Potassium Transporters to Regulate Pollen Tube Growth and Integrity in Rice. PLoS Genet. 2016, 12, e1006085. [Google Scholar] [CrossRef]
- Naso, A.; Dreyer, I.; Pedemonte, L.; Testa, I.; Gomez-porras, J.L.; Usai, C.; Mueller-rueber, B.; Diaspro, A.; Gambale, F.; Picco, C. The Role of the C-Terminus for Functional Heteromerization of the Plant Channel KDC1. Biophys. J. 2009, 96, 4063–4074. [Google Scholar] [CrossRef][Green Version]
- Boneh, U.; Biton, I.; Schwartz, A.; Ben-Ari, G. Characterization of the ABA signal transduction pathway in Vitis vinifera. Plant Sci. 2012, 187, 89–96. [Google Scholar] [CrossRef]
- Hwang, H.; Yoon, J.; Kim, H.Y.; Min, M.K.; Kim, J.-A.; Choi, E.-H.; Lan, W.; Bae, Y.-M.; Luan, S.; Cho, H.; et al. Unique Features of Two Potassium Channels, OsKAT2 and OsKAT3, Expressed in Rice Guard Cells. PLoS ONE 2013, 8, e72541. [Google Scholar] [CrossRef]
- Ehrhardt, T.; Zimmermann, S.; Muller-Rober, B. Association of plant K+(in) channels is mediated by conserved C-termini and does not affect subunit assembly. FEBS Lett. 1997, 409, 166–170. [Google Scholar] [CrossRef]
- Daras, G.; Rigas, S.; Tsitsekian, D.; Iacovides, T.A.; Hatzopoulos, P. Potassium transporter TRH1 subunits assemble regulating root-hair elongation autonomously from the cell fate determination pathway. Plant Sci. 2015, 231, 131–137. [Google Scholar] [CrossRef]
- Sato, A.; Sato, Y.; Fukao, Y.; Fujiwara, M.; Umezawa, T.; Shinozaki, K.; Hibi, T.; Taniguchi, M.; Miyake, H.; Goto, D.B.; et al. Threonine at position 306 of the KAT1 potassium channel is essential for channel activity and is a target site for ABA-activated SnRK2/OST1/SnRK2.6 protein kinase. Biochem. J. 2009, 424, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Acharya, B.R.; Jeon, B.W.; Zhang, W.; Assmann, S.M. Open Stomata 1 (OST1) is limiting in abscisic acid responses of Arabidopsis guard cells. New Phytol 2013, 200, 1049–1063. [Google Scholar] [CrossRef] [PubMed]
- Honsbein, A.; Blatt, M.R.; Grefen, C. A molecular framework for coupling cellular volume and osmotic solute transport control. J. Exp. Bot. 2011, 62, 2363–2370. [Google Scholar] [CrossRef] [PubMed]
- Sokolovski, S.; Hills, A.; Gay, R.A.; Blatt, M.R. Functional interaction of the SNARE protein NtSyp121 in Ca2+ channel gating, Ca2+ transients and ABA signalling of stomatal guard cells. Mol. Plant 2008, 1, 347–358. [Google Scholar] [CrossRef]
- Zhu, J.K.; Liu, J.; Xiong, L. Genetic analysis of salt tolerance in arabidopsis. Evidence for a critical role of potassium nutrition. Plant Cell 1998, 10, 1181–1191. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Quintero, F.J.; Pardo, J.M.; Zhu, J.-K. The putative plasma membrane Na(+)/H(+) antiporter SOS1 controls long-distance Na(+) transport in plants. Plant Cell 2002, 14, 465–477. [Google Scholar] [CrossRef]
- Guo, Y.; Halfter, U.; Ishitani, M.; Zhu, J.K. Molecular characterization of functional domains in the protein kinase SOS2 that is required for plant salt tolerance. Plant Cell 2001, 13, 1383–1400. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, J.K. A calcium sensor homolog required for plant salt tolerance. Science 1998, 280, 1943–1945. [Google Scholar] [CrossRef]
- Shi, H.; Xiong, L.; Stevenson, B.; Lu, T.; Zhu, J.-K. The Arabidopsis salt overly sensitive 4 mutants uncover a critical role for vitamin B6 in plant salt tolerance. Plant Cell 2002, 14, 575–588. [Google Scholar] [CrossRef]
- Shi, H.; Zhu, J.-K. SOS4, A Pyridoxal Kinase Gene, Is Required for Root Hair Development in Arabidopsis. Plant Physiol. 2002, 129, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Rueschhoff, E.E.; Gillikin, J.W.; Sederoff, H.W.; Daub, M.E. The SOS4 pyridoxal kinase is required for maintenance of vitamin B6-mediated processes in chloroplasts. Plant Physiol. Biochem. 2013, 63, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Quintero, F.J.; Ohta, M.; Shi, H.; Zhu, J.-K.; Pardo, J.M. Reconstitution in yeast of the Arabidopsis SOS signaling pathway for Na+ homeostasis. Proc. Natl. Acad. Sci. USA 2002, 99, 9061–9066. [Google Scholar] [CrossRef]
- Gong, D.; Guo, Y.; Schumaker, K.; Zhu, J.; Zhang, J.; Fuglsang, A.T.; Palmgren, M.G.; Wu, W.; Guo, Y. The SOS3 Family of Calcium Sensors and SOS2 Family of Protein Kinases in Arabidopsis. Plant Physiol. 2011, 134, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Quan, R.; Lin, H.; Mendoza, I.; Zhang, Y.; Cao, W.; Yang, Y.; Shang, M.; Chen, S.; Pardo, J.M.; Guo, Y. SCABP8/CBL10, a Putative Calcium Sensor, Interacts with the Protein Kinase SOS2 to Protect Arabidopsis Shoots from Salt Stress. PLANT CELL ONLINE 2007, 19, 1415–1431. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Lin, H.; Chen, S.; Wu, Y.; Zhang, J.; Fuglsang, A.T.; Palmgren, M.G.; Wu, W.; Guo, Y. Phosphorylation of SOS3-like calcium-binding proteins by their interacting SOS2-like protein kinases is a common regulatory mechanism in Arabidopsis. Plant Physiol. 2011, 156, 2235–2243. [Google Scholar] [CrossRef]
- Lin, H.; Yang, Y.; Quan, R.; Mendoza, I.; Wu, Y.; Du, W.; Zhao, S.; Schumaker, K.S.; Pardo, J.M.; Guo, Y. Phosphorylation of SOS3-LIKE CALCIUM BINDING PROTEIN8 by SOS2 protein kinase stabilizes their protein complex and regulates salt tolerance in Arabidopsis. Plant Cell 2009, 21, 1607–1619. [Google Scholar] [CrossRef]
- Qiu, Q.-S.; Guo, Y.; Quintero, F.J.; Pardo, J.M.; Schumaker, K.S.; Zhu, J.-K. Regulation of vacuolar Na+/H+ exchange in Arabidopsis thaliana by the salt-overly-sensitive (SOS) pathway. J. Biol. Chem. 2004, 279, 207–215. [Google Scholar] [CrossRef]
- Cheng, N.-H.; Pittman, J.K.; Zhu, J.-K.; Hirschi, K.D. The protein kinase SOS2 activates the Arabidopsis H(+)/Ca(2+) antiporter CAX1 to integrate calcium transport and salt tolerance. J. Biol. Chem. 2004, 279, 2922–2926. [Google Scholar] [CrossRef]
- Quintero, F.J.; Martinez-Atienza, J.; Villalta, I.; Jiang, X.; Kim, W.-Y.; Ali, Z.; Fujii, H.; Mendoza, I.; Yun, D.-J.; Zhu, J.-K.; et al. Activation of the plasma membrane Na/H antiporter Salt-Overly-Sensitive 1 (SOS1) by phosphorylation of an auto-inhibitory C-terminal domain. Proc. Natl. Acad. Sci. USA 2011, 108, 2611–2616. [Google Scholar] [CrossRef]
- de Dios Barajas-Lopez, J.; Moreno, J.R.; Gamez-Arjona, F.M.; Pardo, J.M.; Punkkinen, M.; Zhu, J.-K.; Quintero, F.J.; Fujii, H. Upstream kinases of plant SnRKs are involved in salt stress tolerance. Plant J. 2018, 93, 107–118. [Google Scholar] [CrossRef]
- Song, A.; Lu, J.; Jiang, J.; Chen, S.; Guan, Z.; Fang, W.; Chen, F. Isolation and characterisation of Chrysanthemum crassum SOS1, encoding a putative plasma membrane Na+/H+ antiporter. Plant Biol. 2012, 14, 706–713. [Google Scholar] [CrossRef]
- Garciadeblás, B.; Haro, R.; Benito, B. Cloning of two SOS1 transporters from the seagrass Cymodocea nodosa. SOS1 transporters from Cymodocea and Arabidopsis mediate potassium uptake in bacteria. Plant Mol. Biol. 2007, 63, 479–490. [Google Scholar] [CrossRef]
- Jarvis, D.E.; Ryu, C.-H.; Beilstein, M.A.; Schumaker, K.S. Distinct Roles for SOS1 in the Convergent Evolution of Salt Tolerance in Eutrema salsugineum and Schrenkiella parvula. Mol. Biol. Evol. 2014, 31, 2094–2107. [Google Scholar] [CrossRef]
- Zhao, X.; Wei, P.; Liu, Z.; Yu, B.; Shi, H. Soybean Na+/H+ antiporter GmsSOS1 enhances antioxidant enzyme activity and reduces Na+ accumulation in Arabidopsis and yeast cells under salt stress. Acta Physiol. Plant. 2017, 39, 19. [Google Scholar] [CrossRef]
- Martínez-Atienza, J.; Jiang, X.; Garciadeblas, B.; Mendoza, I.; Zhu, J.-K.; Pardo, J.M.; Quintero, F.J. Conservation of the salt overly sensitive pathway in rice. Plant Physiol. 2007, 143, 1001–1012. [Google Scholar] [CrossRef]
- Takahashi, R.; Liu, S.; Takano, T. Isolation and characterization of plasma membrane Na+/H+ antiporter genes from salt-sensitive and salt-tolerant reed plants. J. Plant Physiol. 2009, 166, 301–309. [Google Scholar] [CrossRef]
- Fraile-Escanciano, A.; Kamisugi, Y.; Cuming, A.C.; Rodríguez-Navarro, A.; Benito, B. The SOS1 transporter of Physcomitrella patens mediates sodium efflux in planta. New Phytol. 2010, 188, 750–761. [Google Scholar] [CrossRef]
- Tang, R.-J.; Liu, H.; Bao, Y.; Lv, Q.-D.; Yang, L.; Zhang, H.-X. The woody plant poplar has a functionally conserved salt overly sensitive pathway in response to salinity stress. Plant Mol. Biol. 2010, 74, 367–380. [Google Scholar] [CrossRef]
- Zhou, Y.; Yin, X.; Duan, R.; Hao, G.; Guo, J.; Jiang, X. SpAHA1 and SpSOS1 Coordinate in Transgenic Yeast to Improve Salt Tolerance. PLoS ONE 2015, 10, e0137447. [Google Scholar] [CrossRef] [PubMed]
- Huertas, R.; Olías, R.; Eljakaoui, Z.; Gálvez, F.J.; Li, J.; De Morales, P.A.; Belver, A.; Rodríguez-Rosales, M.P. Overexpression of SlSOS2 (SlCIPK24) confers salt tolerance to transgenic tomato. Plant Cell Environ. 2012, 35, 1467–1482. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Jiang, X.; Zhan, K.; Cheng, X.; Chen, X.; Pardo, J.M.; Cui, D. Functional characterization of a wheat plasma membrane Na+/H+ antiporter in yeast. Arch. Biochem. Biophys. 2008, 473, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Feki, K.; Quintero, F.J.; Pardo, J.M.; Masmoudi, K. Regulation of durum wheat Na+/H+ exchanger TdSOS1 by phosphorylation. Plant Mol. Biol. 2011, 76, 545–556. [Google Scholar] [CrossRef]
- Serrano, R.; Kielland-Brandt, M.C.; Fink, G.R. Yeast plasma membrane ATPase is essential for growth and has homology with (Na+ + K+), K+- and Ca2+-ATPases. Nature 1986, 319, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Harper, J.F.; Surowy, T.K.; Sussman, M.R. Molecular cloning and sequence of cDNA encoding the plasma membrane proton pump (H+-ATPase) of Arabidopsis thaliana (cation pumps/nucleotide sequence/amino add homology/oligonucleotide screening/transmembrane segments). Proc. Natl. Acad. Sci. USA 1989, 86, 1234–1238. [Google Scholar] [CrossRef] [PubMed]
- Palmgren, M.G.; Christensen, G. Complementation in situ of the yeast plasma membrane H+-ATPase gene pmal by an H+-ATPase gene from a heterologous species. FEBS Lett. 1993, 317, 216–222. [Google Scholar] [CrossRef]
- Palmgren, M.G.; Christensen, G. Functional comparisons between plant plasma membrane H+-ATPase isoforms expressed in yeast. J. Biol. Chem. 1994, 269, 3027–3033. [Google Scholar] [PubMed]
- Baunsgaard, L.; Venema, K.; Axelsen, K.B.; Villalba, J.M.; Welling, A.; Wollenweber, B.; Palmgren, M.G. Modified plant plasma membrane H+-ATPase with improved transport coupling efficiency identified by mutant selection in yeast. Plant J. 1996, 10, 451–458. [Google Scholar] [CrossRef]
- Santiago, J.; Dupeux, F.; Betz, K.; Antoni, R.; Gonzalez-Guzman, M.; Rodriguez, L.; Márquez, J.A.; Rodriguez, P.L. Structural insights into PYR/PYL/RCAR ABA receptors and PP2Cs. Plant Sci. 2012, 182, 3–11. [Google Scholar] [CrossRef]
- Cutler, S.R.; Rodriguez, P.L.; Finkelstein, R.R.; Abrams, S.R. Abscisic Acid: Emergence of a Core Signaling Network. Annu. Rev. Plant Biol. 2010, 61, 651–679. [Google Scholar] [CrossRef] [PubMed]
- Pizzio, G.A.; Rodriguez, L.; Antoni, R.; Gonzalez-Guzman, M.; Yunta, C.; Merilo, E.; Kollist, H.; Albert, A.; Rodriguez, P.L. The PYL4 A194T mutant uncovers a key role of PYR1-LIKE4/PROTEIN PHOSPHATASE 2CA interaction for abscisic acid signaling and plant drought resistance. Plant Physiol. 2013, 163, 441–455. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, L.; Gonzalez-Guzman, M.; Diaz, M.; Rodrigues, A.; Izquierdo-Garcia, A.C.; Peirats-Llobet, M.; Fernandez, M.A.; Antoni, R.; Fernandez, D.; Marquez, J.A.; et al. C2-Domain Abscisic Acid-Related Proteins Mediate the Interaction of PYR/PYL/RCAR Abscisic Acid Receptors with the Plasma Membrane and Regulate Abscisic Acid Sensitivity in Arabidopsis. Plant Cell 2014, 26, 4802–4820. [Google Scholar] [CrossRef]
- Belda-Palazon, B.; Rodriguez, L.; Fernandez, M.A.; Castillo, M.-C.; Anderson, E.M.; Gao, C.; Gonzalez-Guzman, M.; Peirats-Llobet, M.; Zhao, Q.; De Winne, N.; et al. FYVE1/FREE1 Interacts with the PYL4 ABA Receptor and Mediates Its Delivery to the Vacuolar Degradation Pathway. Plant Cell 2016, 28, 2291–2311. [Google Scholar] [CrossRef]
- Okamoto, M.; Cutler, S.R. Chemical Control of ABA Receptors to Enable Plant Protection Against Water Stress. Methods Mol. Biol. 2018, 1795, 127–141. [Google Scholar]
- Shen, Y.; Shen, L.; Shen, Z.; Jing, W.; Ge, H.; Zhao, J.; Zhang, W. The potassium transporter OsHAK21 functions in the maintenance of ion homeostasis and tolerance to salt stress in rice. Plant Cell Env. 2015, 38, 2766–2779. [Google Scholar] [CrossRef] [PubMed]
- Gaxiola, R.; de Larrinoa, I.F.; Villalba, J.M.; Serrano, R. A novel and conserved salt-induced protein is an important determinant of salt tolerance in yeast. EMBO J. 1992, 11, 3157–3164. [Google Scholar] [CrossRef]
- Yenush, L.; Mulet, J.M.; Ariño, J.; Serrano, R. The Ppz protein phosphatases are key regulators of K+ and pH homeostasis: Implications for salt tolerance, cell wall integrity and cell cycle progression. EMBO J. 2002, 21, 920–929. [Google Scholar] [CrossRef]
- Mulet, J.M.; Leube, M.P.; Kron, S.J.; Rios, G.; Fink, G.R.; Serrano, R. A novel mechanism of ion homeostasis and salt tolerance in yeast: The Hal4 and Hal5 protein kinases modulate the Trk1-Trk2 potassium transporter. Mol. Cell. Biol. 1999, 19, 3328–3337. [Google Scholar] [CrossRef] [PubMed]
- Mendizabal, I.; Rios, G.; Mulet, J.M.; Serrano, R.; de Larrinoa, I.F. Yeast putative transcription factors involved in salt tolerance. FEBS Lett. 1998, 425, 323–328. [Google Scholar] [CrossRef]
- Rios, G.; Cabedo, M.; Rull, B.; Yenush, L.; Serrano, R.; Mulet, J.M. Role of the yeast multidrug transporter Qdr2 in cation homeostasis and the oxidative stress response. FEMS Yeast Res. 2013, 13, 97–106. [Google Scholar] [CrossRef]
- Bordas, M.; Montesinos, C.; Dabauza, M.; Salvador, A.; Roig, L.A.; Serrano, R.; Moreno, V. Transfer of the yeast salt tolerance gene HAL1 to Cucumis melo L. cultivars and in vitro evaluation of salt tolerance. Transgenic Res. 1997, 6, 41–50. [Google Scholar] [CrossRef]
- Gisbert, C.; Rus, A.M.; Bolarín, M.C.; López-Coronado, J.M.; Arrillaga, I.; Montesinos, C.; Caro, M.; Serrano, R.; Moreno, V. The yeast HAL1 gene improves salt tolerance of transgenic tomato. Plant Physiol. 2000, 123, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Forment, J.; Naranjo, M.A.; Roldán, M.; Serrano, R.; Vicente, O. Expression of Arabidopsis SR-like splicing proteins confers salt tolerance to yeast and transgenic plants. Plant J. 2002, 30, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Chen, A.-P.; Zhong, N.-Q.; Wang, F.; Wang, H.-Y.; Xia, G.-X. Functional screening of salt stress-related genes from Thellungiella halophila using fission yeast system. Physiol. Plant. 2007, 129, 671–678. [Google Scholar] [CrossRef]
- Minet, M.; Dufour, M.E.; Lacroute, F. Complementation of Saccharomyces cerevisiae auxotrophic mutants by Arabidopsis thaliana cDNAs. Plant J. 1992, 2, 417–422. [Google Scholar]
- Naranjo, M.A.; Forment, J.; Roldán, M.; Serrano, R.; Vicente, O. Overexpression of Arabidopsis thaliana LTL1, a salt-induced gene encoding a GDSL-motif lipase, increases salt tolerance in yeast and transgenic plants. Plant Cell Env. 2006, 29, 1890–1900. [Google Scholar] [CrossRef]
- Elledge, S.J.; Mulligan, J.T.; Ramer, S.W.; Spottswood, M.; Davis, R.W. lambdaYES: A multifunctional cDNA expression vector for the isolation of genes by complmentation of yeast and E. coli mutations. Proc. Natl. Acad. Sci. USA 1991, 88, 1731–1735. [Google Scholar] [CrossRef]
- Lippuner, V.; Cyert, M.S.; Gasser, C.S. Two classes of plant cDNA clones differentially complement yeast calcineurin mutants and increase salt tolerance of wild-type yeast. J. Biol. Chem. 1996, 271, 12859–12866. [Google Scholar] [CrossRef]
- Li, J.; Sun, X.; Yu, G.; Jia, C.; Liu, J.; Pan, H. Generation and Analysis of Expressed Sequence Tags (ESTs) from Halophyte Atriplex canescens to Explore Salt-Responsive Related Genes. Int. J. Mol. Sci. 2014, 15, 11172–11189. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Li, J.; Sun, X.; Liu, Y.; Wang, X.; Zhang, H.; Pan, H. Exploration for the Salinity Tolerance-Related Genes from Xero-Halophyte Atriplex canescens Exploiting Yeast Functional Screening System. Int. J. Mol. Sci. 2017, 18, 2444. [Google Scholar] [CrossRef] [PubMed]
- Kanhonou, R.; Serrano, R.; Palau, R.R. A catalytic subunit of the sugar beet protein kinase CK2 is induced by salt stress and increases NaCl tolerance in Saccharomyces cerevisiae. Plant Mol. Biol. 2001, 47, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Rausell, A.; Kanhonou, R.; Yenush, L.; Serrano, R.; Ros, R. The translation initiation factor eIF1A is an important determinant in the tolerance to NaCl stress in yeast and plants. Plant J. 2003, 34, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Serrano, R.; Montesinos, C.; Gaxiola, R.; Ríos, G.; Forment, J.; Leube, M.; Mulet, J.M.; Naranjo, M.A.; Roldán, M.; Vicente, O.; et al. Functional genomics of salt tolerance: The yeast overexpression approach. Acta Hortic. 2003, 609, 31–38. [Google Scholar] [CrossRef]
- Mulet, J.M.; Alemany, B.; Ros, R.; Calvete, J.J.; Serrano, R. Expression of a plant serine O-acetyltransferase in Saccharomyces cerevisiae confers osmotic tolerance and creates an alternative pathway for cysteine biosynthesis. Yeast 2004, 21, 303–312. [Google Scholar] [CrossRef]
- Mulet Salort, J.M.; Sanz Molinero, A.I.; Serrano Salom, R. Comprising Expression of Haemoglobin from Arabidopsis Basel (CH). U.S. Patent S371, 9 March 2010. [Google Scholar]
- Porcel, R.; Bustamante, A.; Ros, R.; Serrano, R.; Mulet Salort, J.M. BvCOLD1: A novel aquaporin from sugar beet (Beta vulgaris L.) involved in boron homeostasis and abiotic stress. Plant Cell Environ. 2018, 41, 2844–2857. [Google Scholar] [CrossRef]
- Zheng, J.-X.; Zhang, H.; Su, H.-X.; Xia, K.-F.; Jian, S.-G.; Zhang, M. Ipomoea pes-caprae IpASR Improves Salinity and Drought Tolerance in Transgenic Escherichia coli and Arabidopsis. Int. J. Mol. Sci. 2018, 19, 2252. [Google Scholar] [CrossRef]
- Eswaran, N.; Parameswaran, S.; Sathram, B.; Anantharaman, B.; Raja Krishna Kumar, G.; Tangirala, S.J. Yeast functional screen to identify genetic determinants capable of conferring abiotic stress tolerance in Jatropha curcas. BMC Biotechnol. 2010, 10, 23. [Google Scholar] [CrossRef]
- Kumar, R.; Mustafiz, A.; Sahoo, K.K.; Sharma, V.; Samanta, S.; Sopory, S.K.; Pareek, A.; Singla-Pareek, S.L. Functional screening of cDNA library from a salt tolerant rice genotype Pokkali identifies mannose-1-phosphate guanyl transferase gene (OsMPG1) as a key member of salinity stress response. Plant Mol. Biol. 2012, 79, 555–568. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, C.; Tan, Z.; Liu, J.; Zhuang, L.; Yang, Z.; Huang, B. Functional Identification and Characterization of Genes Cloned from Halophyte Seashore Paspalum Conferring Salinity and Cadmium Tolerance. Front. Plant Sci. 2016, 7, 102. [Google Scholar] [CrossRef]
- Patankar, H.V.; Al-Harrasi, I.; Al-Yahyai, R.; Yaish, M.W. Identification of Candidate Genes Involved in the Salt Tolerance of Date Palm (Phoenix dactylifera L.) Based on a Yeast Functional Bioassay. DNA Cell Biol. 2018, 37, 524–534. [Google Scholar] [CrossRef]
- Nakahara, Y.; Sawabe, S.; Kainuma, K.; Katsuhara, M.; Shibasaka, M.; Suzuki, M.; Yamamoto, K.; Oguri, S.; Sakamoto, H. Yeast functional screen to identify genes conferring salt stress tolerance in Salicornia europaea. Front. Plant Sci. 2015, 6, 920. [Google Scholar] [CrossRef]
- Gangadhar, B.H.; Yu, J.W.; Sajeesh, K.; Park, S.W. A systematic exploration of high-temperature stress-responsive genes in potato using large-scale yeast functional screening. Mol. Genet. Genomics 2014, 289, 185–201. [Google Scholar] [CrossRef]
- Gangadhar, B.H.; Sajeesh, K.; Venkatesh, J.; Baskar, V.; Abhinandan, K.; Yu, J.W.; Prasad, R.; Mishra, R.K. Enhanced Tolerance of Transgenic Potato Plants Over-Expressing Non-specific Lipid Transfer Protein-1 (StnsLTP1) against Multiple Abiotic Stresses. Front. Plant Sci. 2016, 7, 1228. [Google Scholar] [CrossRef]
- Kim, M.-J.; Lim, G.-H.; Kim, E.-S.; Ko, C.-B.; Yang, K.-Y.; Jeong, J.-A.; Lee, M.-C.; Kim, C.S. Abiotic and biotic stress tolerance in Arabidopsis overexpressing the multiprotein bridging factor 1a (MBF1a) transcriptional coactivator gene. Biochem. Biophys. Res. Commun. 2007, 354, 440–446. [Google Scholar] [CrossRef]
- Chen, Y.; Zong, J.; Tan, Z.; Li, L.; Hu, B.; Chen, C.; Chen, J.; Liu, J. Systematic mining of salt-tolerant genes in halophyte-Zoysia matrella through cDNA expression library screening. Plant Physiol. Biochem. 2015, 89, 44–52. [Google Scholar] [CrossRef]
- Bissoli, G.; Niñoles, R.; Fresquet, S.; Palombieri, S.; Bueso, E.; Rubio, L.; García-Sánchez, M.J.; Fernández, J.A.; Mulet, J.M.; Serrano, R. Peptidyl-prolyl cis-trans isomerase ROF2 modulates intracellular pH homeostasis in Arabidopsis. Plant J. 2012, 70, 704–716. [Google Scholar] [CrossRef]
- Wang, C.; Burzio, L.A.; Koch, M.S.; Silvanovich, A.; Bell, E. Purification, characterization and safety assessment of the introduced cold shock protein B in DroughtGard maize. Regul. Toxicol. Pharmacol. 2015, 71, 164–173. [Google Scholar] [CrossRef]
- Cabral, K.M.; Almeida, M.S.; Valente, A.P.; Almeida, F.C.; Kurtenbach, E. Production of the active antifungal Pisum sativum defensin 1 (Psd1) in Pichia pastoris: Overcoming the inefficiency of the STE13 protease. Protein Expr. Purif. 2003, 31, 115–122. [Google Scholar] [CrossRef]
- Sigoillot, M.; Brockhoff, A.; Lescop, E.; Poirier, N.; Meyerhof, W.; Briand, L. Optimization of the production of gurmarin, a sweet-taste-suppressing protein, secreted by the methylotrophic yeast Pichia pastoris. Appl. Microbiol. Biotechnol. 2012, 96, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Opekarová, M.; Tanner, W. Specific lipid requirements of membrane proteins--a putative bottleneck in heterologous expression. Biochim. Biophys. Acta 2003, 1610, 11–22. [Google Scholar] [CrossRef]
- Farrokhi, N.; Hrmova, M.; Burton, R.A.; Fincher, G.B. Heterologous and cell free protein expression systems. Methods Mol. Biol 2009, 513, 175–198. [Google Scholar]
- Veitia, R.A.; Caburet, S.; Birchler, J.A. Mechanisms of Mendelian dominance. Clin. Genet. 2018, 93, 419–428. [Google Scholar] [CrossRef] [PubMed]
| Mutant Name | Genetic Background | Relevant Genotype |
|---|---|---|
| WΔ3 | W303-1A | MAT a ade2-1 canl-100 trpl-1 ura3-1 trk1::LEU2 trk2::HIS3 |
| CY162 | R757 | MAT a ura3–52 his3Δ200 his44–15 trk1Δtrk2::pCK64 |
| 9.3 | W303-1A | MAT a ena1-4Δ:HIS3::ena4Δ leu2 ura3–1 trp1–1 ade2–1 trk1Δ trk2::pCK64 |
| SGY1528 | W303-1A | MAT a ade2-1 canl-100 his3-11,15 leu2-3,112 trpl-1 ura3-1 trk1::HIS3 trk2::TRP1 |
| BYT12 | BY4741 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 trk1Δ::loxP trk2Δ::loxP |
| PLY240 | JRY379 | MAT a his3-Δ200 leu2-3,112 trp1-Δ901 ura3-52 suc2-Δ9 trk1Δ51 trk2Δ50::kanMX |
| PLY246 | JRY379 | MAT a his3-Δ200 leu2-3,112 trp1-Δ901 ura3-52 suc2-Δ9 trk1Δ51 trk2Δ50::kanMX tok1Δ1::HIS3 |
| Possible Baits | Year | Technique | Response | Cellular Compartment * | References | |
|---|---|---|---|---|---|---|
| Proteins capable of entering nucleus | Non-transactivating proteins | 1989 | Classic Y2H system (Y2H) | Transcriptional activation | Nucleus | [127] |
| 1996 | Reverse Y2H system (rY2H) | Transcriptional activation | Nucleus | [128,129] | ||
| 1996 | Yeast three-hybrid system (Y3H) | Transcriptional activation | Nucleus | [130] | ||
| 1999 | Dual-bait system | Transcriptional activation | Nucleus | [131] | ||
| Transactivating proteins | 2001 | Repressed transactivator system (RTA) | Inhibition of transcriptional activation | Nucleus | [132] | |
| 2001 | RNA polymerase III system (Pol III) | Transcriptional activation | Nucleus | [133] | ||
| Membrane proteins | 1998 | Membrane split-ubiquitin system (MbY2H) | Transcriptional activation | Membrane periphery | [134,135,136] | |
| 2000 | Heterotrimeric G-protein fusion system | Inhibition of protein G signaling | Membrane periphery | [137] | ||
| 2001 | Reverse Ras recruitment system (rRRS) | Ras signaling | Membrane periphery | [138] | ||
| Cytosolic proteins | 1997 | SOS recruitment system (SRS) | Ras signaling | Membrane periphery | [139] | |
| 1998 | Ras recruitment system (RRS) | Ras signaling | Membrane periphery | [140] | ||
| 2007 | Cytosolic split-ubiquitin system (CytoY2H) | Transcriptional activation | Endoplasmic reticulum membrane periphery | [134,141] | ||
| Extracellular and secretory pathway proteins | 1997 | Yeast surface system (YS2H) | Extracellular surface | [142] | ||
| 2003 | SCINEX-P system | Downstream signaling & transcriptional activation | Endoplasmic reticulum | [143] | ||
| 2010 | Golgi Y2H system (GY2H) | Och1 activity | Golgi lumen | [144] | ||
| Nuclear, membrane and cytosolic proteins | 1994 | Generally applicable split-ubiquitin system | Uracil auxotrophy and 5-FOA resistance | Cytosol | [134,145] | |
| 1998 | Split-mDHFR system | DHFR activity | Native compartment | [146] | ||
| 2001 | Split-luciferase system | Luminescent signal | Native compartment | [147] | ||
| 2004 | Split-Trp system | Trp1 activity | Cytosol; Native compartment | [148] | ||
| 2005 | Split-FP system | Fluorescent signal | Native compartment | [149,150] | ||
| Na+/K+ Transporter/Channel | Interactors | Technique | References |
|---|---|---|---|
| AKT1 | KAT1, AtKC1 | MbY2H, mating-based | [156] |
| KDC1 | MbY2H | [160] | |
| AKT2 | MRH1/MDIS2 | MbY2H, mating-based | [161] |
| SLAC1 | MbY2H, mating-based | [162] | |
| OsHKT1 | OsCNIH1 | MbY2H, mating-based | [163] |
| KAT1 | KAT1, AKT1, PUP11 | MbY2H, mating-based | [156] |
| SLAC1 | MbY2H, mating-based | [162] | |
| VAMP721 | MbY2H, mating-based | [164] | |
| KAT2 | SLAC1 | MbY2H, mating-based | [162] |
| AtKC1 | AKT1, NRT2.7, ROP1 | MbY2H, mating-based | [156] |
| SLAC1 | MbY2H, mating-based | [162] | |
| SYP121 | MbY2H, mating-based | [165,166] | |
| VAMP721 | MbY2H, mating-based | [164] | |
| KDC1 | AKT1 | MbY2H | [160] |
| KUP6 | SnRK2.6 , SnRK2.2 | MbY2H, mating-based | [167] |
| AKT1 | AIP1, CIPK6, CIPK16 | Y2H | [168] |
| AKT1, AKT2, AtKC1 | Y2H | [169] | |
| CBL10 (CBL5, CBL7) | Y2H | [170] | |
| CIPK23 | Y2H | [171,172,173] | |
| Y2H competition assay | [170] | ||
| AKT1, OsAKT1, PutAKT1 | KPutB1, OsKOB1 | Y2H | [174] |
| AKT2 | AKT1, AKT2, AtKC1 | Y2H | [169] |
| CIPK6 | Y2H | [175] | |
| MRH1/MDIS2 | Y2H Matchmaker Gold | [161] | |
| PP2CA | Y2H | [176] | |
| AKT3 | AtPP2CA | Y2H | [177] |
| GORK | AtPP2CA | Y2H | [178] |
| GORK, SKOR | Y2H | [179] | |
| OsHAK1 | OsRUPO | Y2H | [180] |
| KAT1 | KDC1 | Y2H | [181] |
| VvKAT1 | VvSnRK2.4 | Y2H | [182] |
| OsKAT2 | OsKAT2, OsKAT3 | Y2H | [183] |
| OsKAT3 | OsKAT2, OsKAT3 | Y2H | [183] |
| AtKC1 | AKT1 | Y2H | [169] |
| KDC1 | KAT1 | Y2H | [181] |
| OsKOB1 | AKT1, OsAKT1, PutAKT1 | Y2H | [174] |
| KPutB1 | AKT1, OsAKT1, PutAKT1 | Y2H | [174] |
| KST1 | SKT2, SKT3 | Y2H | [184] |
| SKOR | SKOR, GORK | Y2H | [179] |
| SKT2 | KST1 | Y2H | [184] |
| SKT3 | KST1 | Y2H | [184] |
| TRH1 | TRH1 | Y2H | [185] |
| Oligomer | References | |
|---|---|---|
| AKT1 | AKT1, AKT2 | [169] |
| KAT1 | [156] | |
| AtKC1 | [156,169] | |
| KDC1 | [160] | |
| AKT1, OsAKT1, PutAKT1 | KPutB1, OsKOB1 | [174] |
| AKT2 | AKT1, AKT2 | [169] |
| AtKC1 | [169] | |
| GORK | GORK, SKOR | [179] |
| KAT1 | AKT1 | [156] |
| KAT1 | [156] | |
| KDC1 | [181] | |
| OsKAT2 | OsKAT2, OsKAT3 | [183] |
| OsKAT3 | OsKAT2, OsKAT3 | [183] |
| AtKC1 | AKT1 | [156,169] |
| KDC1 | AKT1 | [160] |
| KAT1 | [181] | |
| OsKOB1 | AKT1, OsAKT1, PutAKT1 | [174] |
| KPutB1 | AKT1, OsAKT1, PutAKT1 | [174] |
| KST1 | SKT2, SKT3 | [184] |
| SKOR | SKOR, GORK | [179] |
| SKT2 | KST1 | [184] |
| SKT3 | KST1 | [184] |
| TRH1 | TRH1 | [185] |
| Na+/K+ Transporter/Channel | Regulatory Protein | Na+/K+ Transporter/Channel Regulation | References |
|---|---|---|---|
| AKT1 | AIP1 | Reduces AKT1 activity | [168] |
| CBL10 (CBL5, CBL7) | Impairs AKT1 activity | [170] | |
| CIPK6, CIPK16 | Phosphorylates and activates AKT1 | [168] | |
| CIPK23 | Phosphorylates and activates AKT1 | [170,171,172,173] | |
| AKT2 | CIPK6 | Upon interaction with CIPK6, CBL4 mediates ER-to-PM translocation of AKT2 and enhances AKT2 activity | [175] |
| MRH1/MDIS2 | [161] | ||
| PP2CA | Dephosphorylates and inhibits AKT2, regulated by ABA signaling | [176] | |
| SLAC1 | [162] | ||
| AKT3 | AtPP2CA | [177] | |
| GORK | AtPP2CA | Dephosphorylation-independent inactivation of GORK | [178] |
| OsHAK1 | OsRUPO | Disruption of RUPO leads to K+ over-accumulation in pollen | [180] |
| OsHKT1 | OsCNIH1 | Golgi-localization of OsHKT1 | [163] |
| KAT1 | PUP11 | [156] | |
| SLAC1 | Inhibits KAT1 activity | [162] | |
| VAMP721 | Suppresses KAT1 and KC1 activity | [164] | |
| VvKAT1 | VvSnRK2.4 | [182] | |
| KAT2 | SLAC1 | [162] | |
| AtKC1 | NRT2.7 | K+ is known to increase nitrate (NO3− ) uptake from soil | [156] |
| ROP1 | Actin filament reorganization affects K+ channel activities in stomata. ROP1 regulates pollen tip growth | [156] | |
| SLAC1 | [162] | ||
| SYP121 | Promotes KAT1 activity, in the presence of KC1 | [165,166] | |
| VAMP721 | Suppresses KAT1 and KC1 activity | [164] | |
| KUP6 | SnRK2.6, SnRK2.2 | SnRK2.6 phosphorylates KUP6, regulated by ABA signaling (drought stress) | [167] |
| Plant Species | Genes Characterized | Reference |
|---|---|---|
| Arabidopsis thaliana | SOS1-3 | [197] |
| Chrysanthemum crassum | SOS1 | [206] |
| Cymodocea nodosa | SOS1 | [207] |
| Eutrema salsugineum | SOS1 | [208] |
| Glycine max | SOS1 | [209] |
| Oryza sativa | SOS1-3 | [210] |
| Phragmites australis Trinius | SOS1 | [211] |
| Physcomitrella patens | SOS1 | [212] |
| Populus trichocarpa | SOS1-3 | [213] |
| Schrenkiella parvula | SOS1 | [208] |
| Sesuvium portulacastrum | SOS1 | [214] |
| Solanum lycopersicum | SOS2 | [215] |
| Triticum aestivum | SOS1 | [216] |
| Triticum durum | SOS1 | [217] |
| Plant Species | cDNA Library [Reference] | Yeast Strain | Screening Conditions | Isolated Genes | Reference |
|---|---|---|---|---|---|
| Arabidopsis thaliana | Leaves from Arabidopsis seedlings [239] | ena1-4 (W303-1A) | 50 mM LiCl | AtRCY1 (K/T-cyclin with SR domain of splicing proteins) AtSRL1 (SR domain of splicing proteins) | [237] |
| AtLTL1 (GDSL-motif lipase) | [240] | ||||
| [241] | cna1cna2YPH499 | 200 mM NaCl | AtSTZ1 (Zinc finger protein) AtSTO1 (B-Box domain protein 24) | [242] | |
| Atriplex canescens | Young leaves and stems treated with 400 mM NaCl [243] | WT (INVSc1) | 2 M NaCl | KJ026992 (Cyclophilin) KJ027014 (Glycine-rich protein) KJ027023 (Cytochrome P450) KJ027035 (Temperature-induced lipocalin) KJ027049 (Cysteine proteinase A494) KJ027057 (Alanine aminotransferase 2) KJ027061 (Hexose transporter) KJ027088 (RNA-binding family protein) KJ027102 (Cysteine proteinases) KJ027110 (calmodulin1) | [244] |
| Beta vulgaris | Leafs from salt stressed plants [245] | ena1-4 nha1(W303-1A) | 150 mM NaCl | BvCK2 (catalytic subunit of the casein kinase) | [245] |
| BveIF1A (Translation initiation factor) | [246] | ||||
| BvSATO1 (RNA binding protein with RGG and RE/D motifs) BvSATO2 (homologous to SATO1) BvSATO4 (RNA binding protein) BvSATO5 (RNA binding protein) BvU2AF (U2snRNP AF protein) | [247] | ||||
| gpd1(W303-1A) | 1 M Sorbitol | BvSAT1 (Serine acetyl trasferase 1) | [248] | ||
| BvGLB2 (Type II non symbiotic plant hemoglobin) | [249] | ||||
| WT (W303-1A) | 10 °C | BvCOLD1 (TIP-like aquaporin) | [250] | ||
| Ipomoea pes-caprae | Growing leaves, shoots and roots [251] | ena1-4 nha1 nhx1(W303-1A) | 75 mM NaCl | MF680587 (putative abscisic acid, stress, and ripening-induced protein (ASR)) MF680589 (nudix hydrolase, chloroplastic) MF680592 (F-box protein At5g46170-like) MF680597 (fructokinase) MF680602 (adenosylhomocysteinase 1) MF680603 (peptide upstream protein) MF680604 (catalase) MF680608 (40S ribosomal protein S25) MF680611 (phosphomannomutase) MF765747 (dnaj protein-like protein) MF680614 (phytosulfokines-like) MF680616 (sugar carrier protein C) MF680619 (abscisic acid 8′-hydroxylase 4) KX426069 (dehydrin) | [251] |
| Jatropha curcas | 3-4 week seedlings treated with 150 mM NaCl [252] | shs-2 (UV-generated mutant in the BY4741 background) | 750 mM NaCl | FJ489601 (Allene oxide cyclase) FJ489602 (Thioredoxin H-type (TRX-h)) FJ489603 (Metallothionein) FJ489604 (Heterotrophic ferredoxin) FJ489605 (Defensin) FJ489606 (Calmodulin-7 (CAM-7)) FJ489608 (S18.A ribosomal protein) FJ489609 (60S ribosomal protein L18a) FJ489611 (Unknown protein) FJ619041 (Membrane protein -2) FJ619045 (Profilin-like protein) FJ619048 (Copper chaperone) FJ619052 (Annexin-like protein) FJ619053 (Al-induced protein) FJ619055 (60S ribosomal protein L39) FJ619056 (Ribosomal protein L37) FJ619057 (Ribosomal protein L15) FJ623457 (40S ribosomal protein S15) FJ623458 (40S ribosomal S18) | [252] |
| Oryza sativa | Leaves from seedlings treated with different abiotic stresses [253] | WT (AH109) | 900 mM NaCl | OsMPG1 (mannose-1-phosphate guanyl transferase gene) | [253] |
| Paspalum vaginatum | Cultivated stolons treated with 250 mM NaCl [254] | ena1-4(G19) | 500 mM NaCl | KT203435 (Uncharacterized protein) KT203436 (Iron-regulated transporter) KT203439 (Early light-induced protein) KT203440 (14-3-3-Like protein) KT203441 (Class 1 HSP) KT203442 (Cysteine synthase) KT203443 (Aldo-ketoreductase) KT203444 (L-Ascorbate peroxidase 2) KT203447 (Nop14-like family protein) KT203450 (Protein IQ-DOMAIN 14-like) KT203451 (Metacaspase-5-like) | [254] |
| Phoenix datilifera | Salt-treated roots [255] | WT (INvSc1) | 2 M NaCl | XM_008806660.2 (11S globulin seed storage protein 2-like) XM_008805834.2 (ABC transporter) XM_008780694.2 (Aquaporin PIP1-2) XM_008793314.1 (Aquaporin PIP2-4-like) XM_008779330.1 (Cysteine desulfurase) XM_008802947.2 (Cytochrome b5-like) XM_008783561.2 (Cytospin-A-like 1) XM_008797620.2 (Hexokinase-2-like) XM_008780092.2 (Mavicyanin-like 1) XM_008814440.2 (Peroxidase 3-like) XM_008786338.2 (Peroxidase 3-like 2) XR_604439.2 (Uncharacterized) XM_008800513.2 (Uncharacterized) XM_017846106.1 (Uncharacterized) XM_017846788.1 (Uncharacterized) | [255] |
| Salicornia europaea | 3-month-old plants | WT (BY4741) | 1.6 M NaCl | SeNN8 (Similar to FKBP5) SeNN24 (Thaumatin like protein) SeNN43 (Unknown protein) | [256] |
| Solanum tuberosum | Plants grown in vitro and subjected to heat shock at 35 °C [257] | WT (BY4741) | 39 °C | StnsLTP1 (Non-specific Lipid Transfer Protein-1) | [258] |
| Zea mays | Maize kernels | Not indicated | Not indicated | MBF1a (Multiprotein bridging factor 1a transcriptional coactivator) | [259] |
| Zoysia matrella | Cultivated stolons treated with 300 mM NaCl | ena1-4 (G19) | 500 mM NaCl | KM265171 (Uncharacterized protein) KM265174 (C2H2-type Zinc finger protein) KM265176 (Unknown protein) KM265177 (Alcohol dehydrogenase 1) KM265179 (Protein disulfide isomerase) KM265182 (Glyoxylate reductase) KM265183 (Serine carboxypeptidase) | [260] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Locascio, A.; Andrés-Colás, N.; Mulet, J.M.; Yenush, L. Saccharomyces cerevisiae as a Tool to Investigate Plant Potassium and Sodium Transporters. Int. J. Mol. Sci. 2019, 20, 2133. https://doi.org/10.3390/ijms20092133
Locascio A, Andrés-Colás N, Mulet JM, Yenush L. Saccharomyces cerevisiae as a Tool to Investigate Plant Potassium and Sodium Transporters. International Journal of Molecular Sciences. 2019; 20(9):2133. https://doi.org/10.3390/ijms20092133
Chicago/Turabian StyleLocascio, Antonella, Nuria Andrés-Colás, José Miguel Mulet, and Lynne Yenush. 2019. "Saccharomyces cerevisiae as a Tool to Investigate Plant Potassium and Sodium Transporters" International Journal of Molecular Sciences 20, no. 9: 2133. https://doi.org/10.3390/ijms20092133
APA StyleLocascio, A., Andrés-Colás, N., Mulet, J. M., & Yenush, L. (2019). Saccharomyces cerevisiae as a Tool to Investigate Plant Potassium and Sodium Transporters. International Journal of Molecular Sciences, 20(9), 2133. https://doi.org/10.3390/ijms20092133







