Attenuating Diabetic Vascular and Neuronal Defects by Targeting P2rx7
Abstract
1. Introduction
2. Results
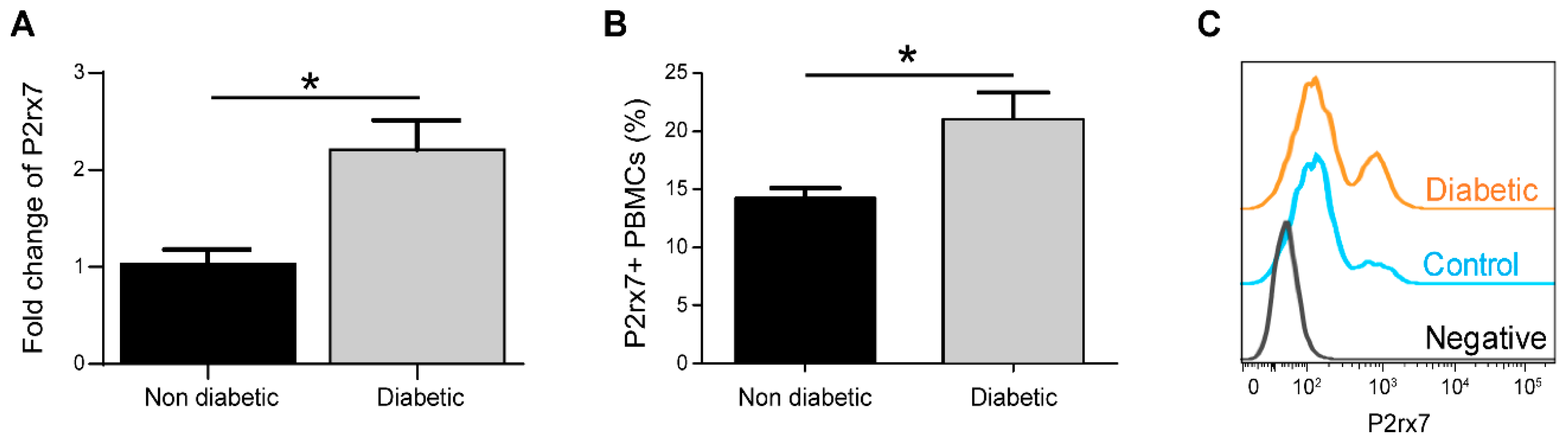
2.1. P2rx7 is Upregulated in Diabetic Mice
2.2. The Effect of 3TC on Diabetes and Circulating Immune Cells
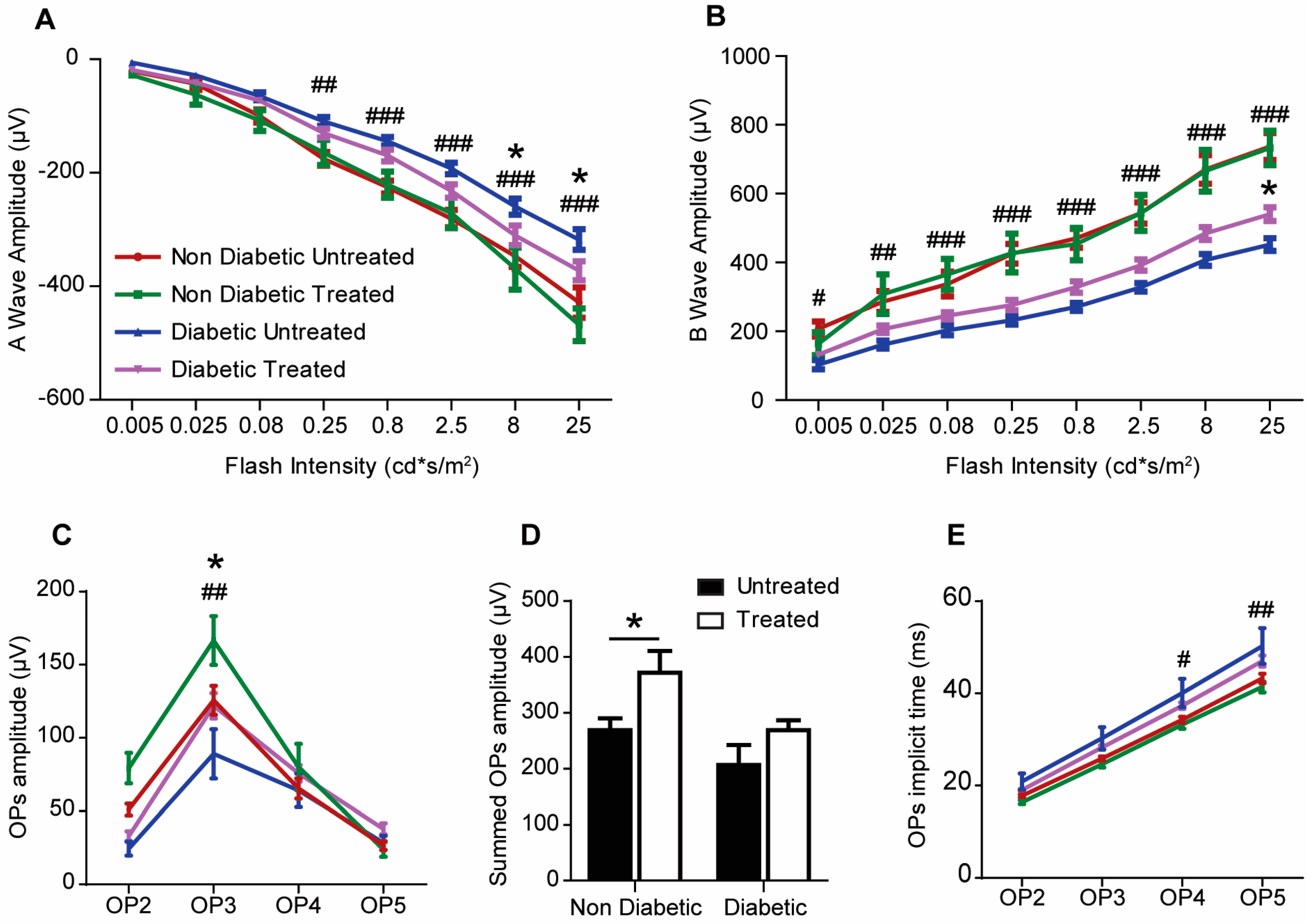
2.3. The Effect of 3TC on Visual Function
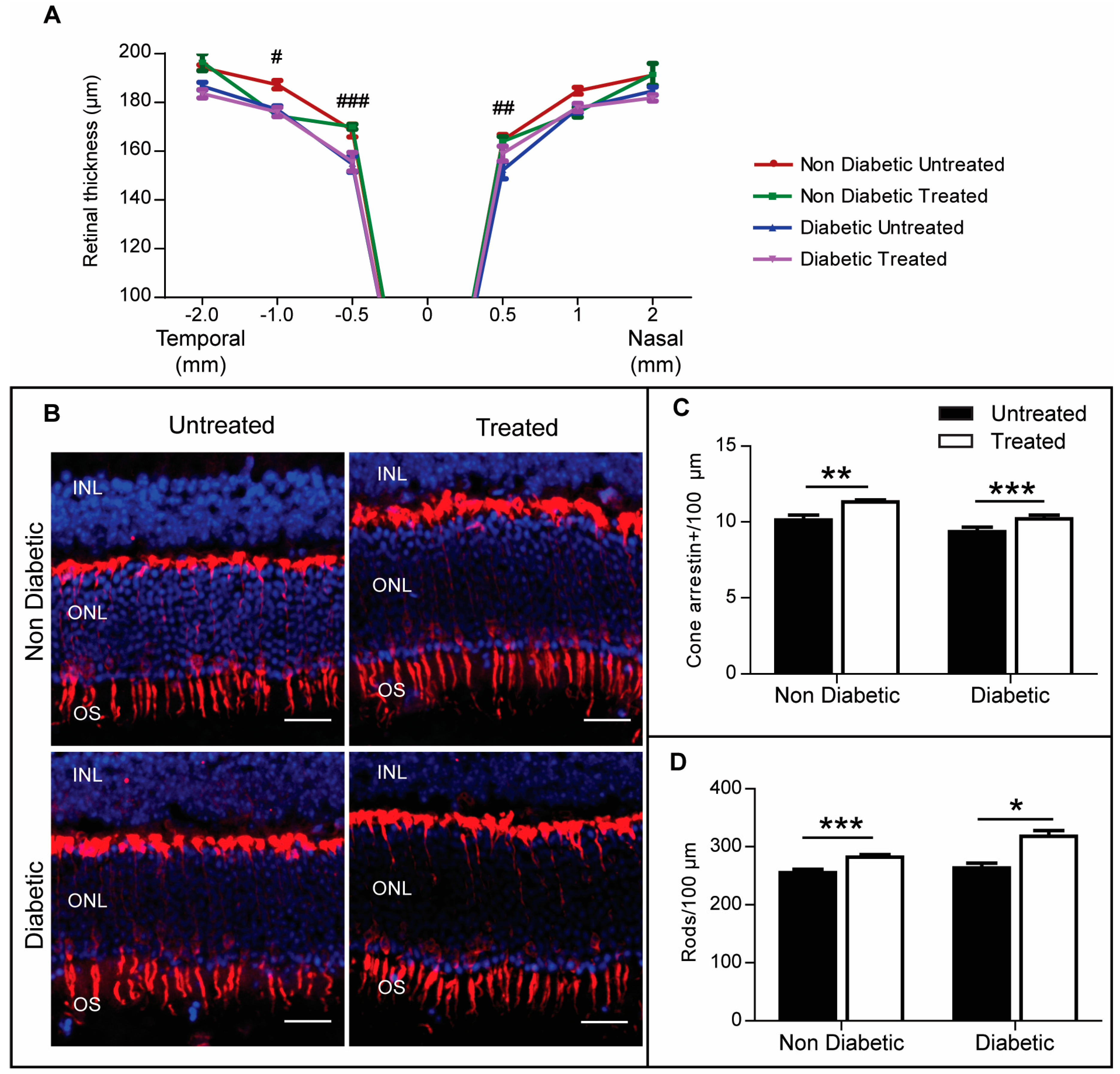
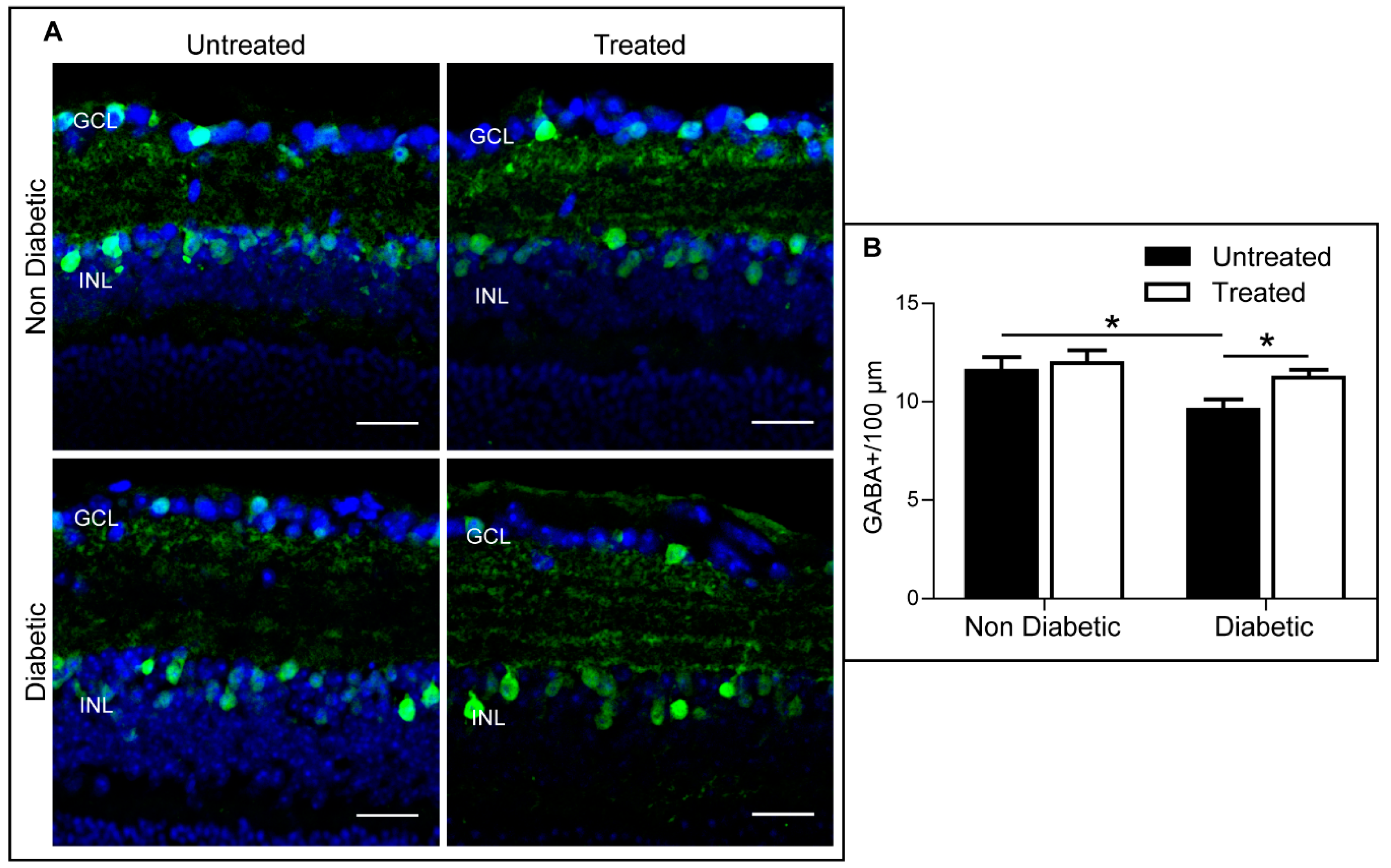
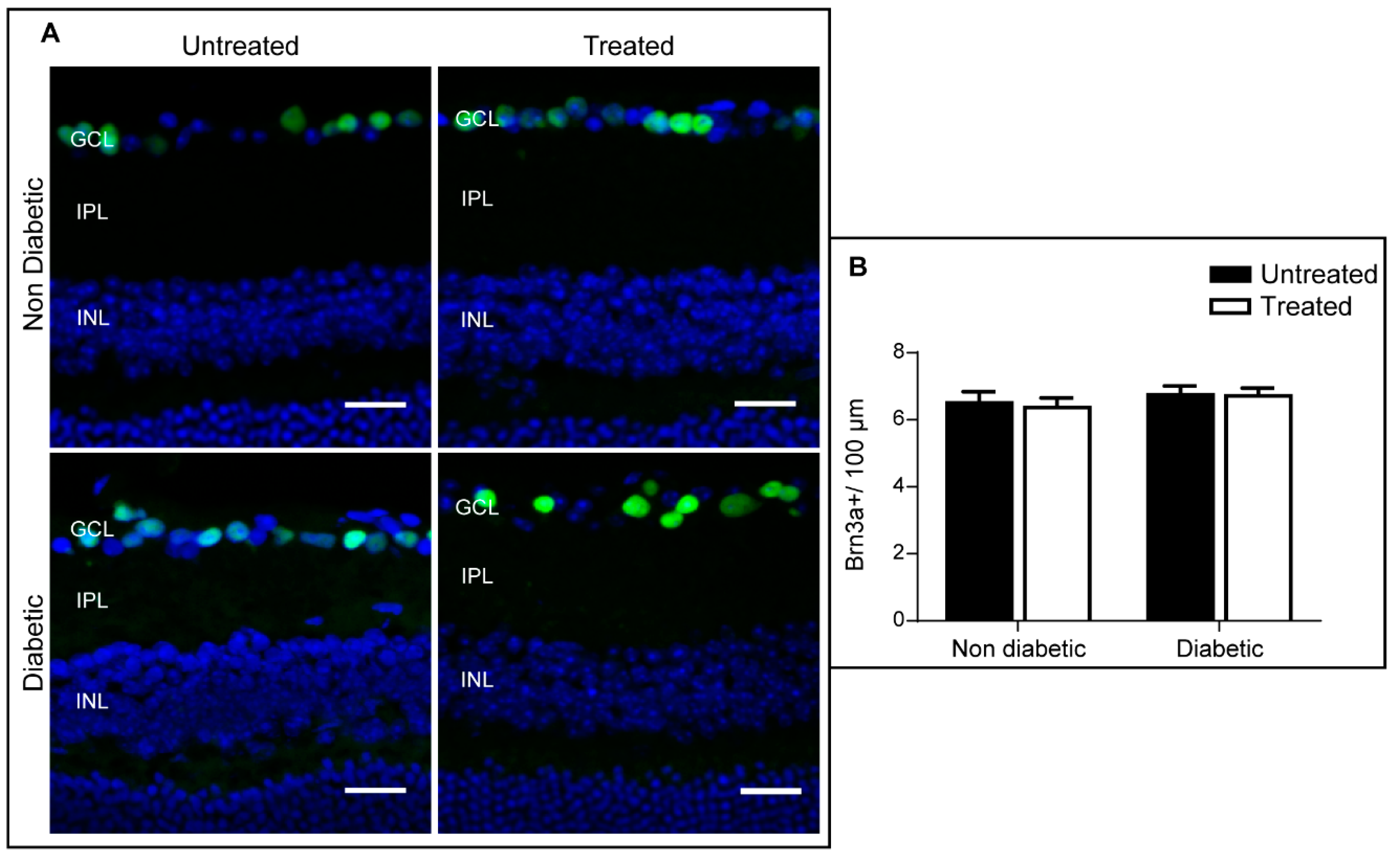
2.4. The Effect of 3TC on Diabetes-Induced Retinal Neurodegeneration
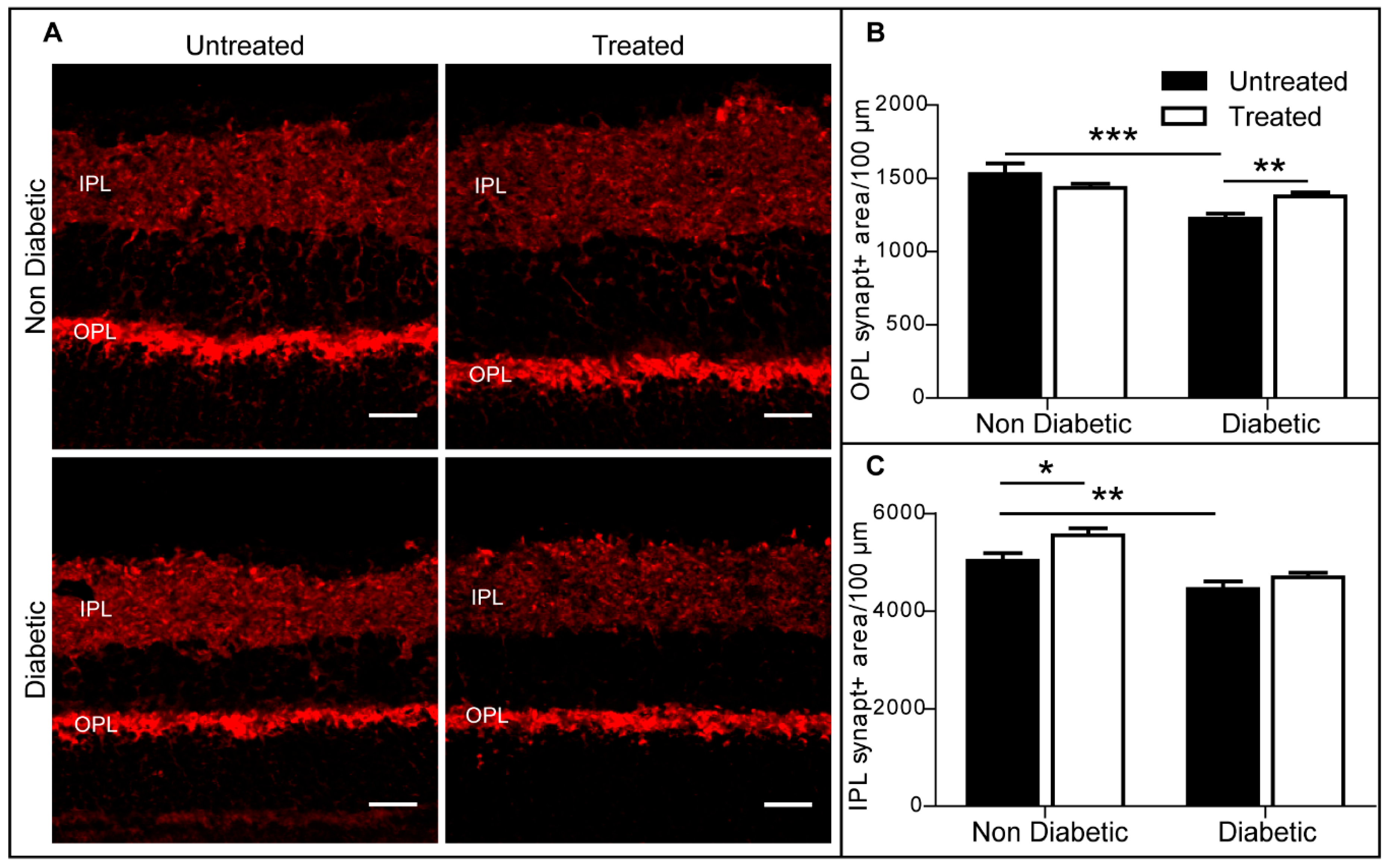
2.5. The Effect of 3TC on Retinal Synaptophysin Expression in Control and Diabetic Mice
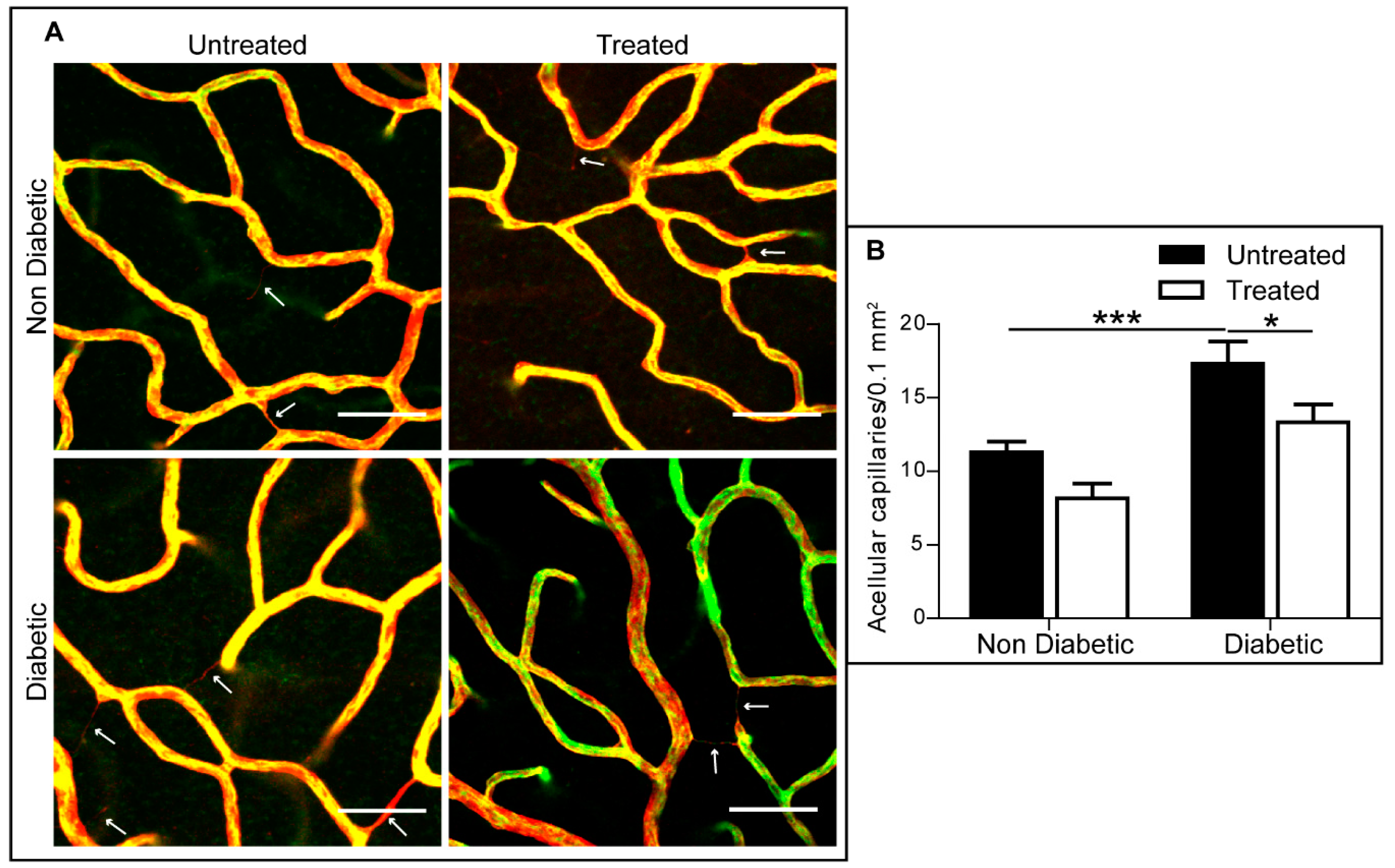
2.6. The Effect of 3TC on Diabetes-Induced Acellular Capillaries
3. Discussion
4. Methods
4.1. Animals
4.2. STZ-Mediated Type 1 Diabetes
4.3. 3TC Administration
4.4. Electroretinography
4.5. Spectral Domain Optical Coherence Tomography (SD-OCT)
4.6. Flow Cytometry
4.7. Immunofluorescence Staining
4.8. Real Time qRT-PCR
4.9. Retinal Cell Quantifications
4.10. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability
References
- Resnikoff, S.; Pascolini, D.; Etya’ale, D.; Kocur, I.; Pararajasegaram, R.; Pokharel, G.P.; Mariotti, S.P. Global Data on Visual Impairment in the Year 2002. Bull. World Health Organ. 2004, 82, 844–851. [Google Scholar]
- Stitt, A.W.; Curtis, T.M.; Chen, M.; Medina, R.J.; McKay, G.J.; Jenkins, A.; Gardiner, T.A.; Lyons, T.J.; Hammes, H.P.; Simo, R.; et al. The Progress in Understanding and Treatment of Diabetic Retinopathy. Prog. Retin. Eye Res. 2016, 51, 156–186. [Google Scholar] [CrossRef] [PubMed]
- Ewing, F.M.; Deary, I.J.; Strachan, M.W.; Frier, B.M. Seeing Beyond Retinopathy in Diabetes: Electrophysiological and Psychophysical Abnormalities and Alterations in Vision. Endocr. Rev. 1998, 19, 462–476. [Google Scholar] [CrossRef]
- Tzekov, R.; Arden, G.B. The Electroretinogram in Diabetic Retinopathy. Surv. Ophthalmol. 1999, 44, 53–60. [Google Scholar] [CrossRef]
- Klemp, K.; Sander, B.; Brockhoff, P.B.; Vaag, A.; Lund-Andersen, H.; Larsen, M. The Multifocal ERG in Diabetic Patients without Retinopathy during Euglycemic Clamping. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2620–2626. [Google Scholar] [CrossRef]
- Shirao, Y.; Kawasaki, K. Electrical Responses from Diabetic Retina. Prog. Retin. Eye Res. 1998, 17, 59–76. [Google Scholar] [CrossRef]
- Duh, E.J.; Sun, J.K.; Stitt, A.W. Diabetic Retinopathy: Current Understanding, Mechanisms, and Treatment Strategies. JCI Insight 2017, 2. [Google Scholar] [CrossRef]
- Du, Y.; Veenstra, A.; Palczewski, K.; Kern, T.S. Photoreceptor Cells are Major Contributors to Diabetes-Induced Oxidative Stress and Local Inflammation in the Retina. Proc. Natl. Acad. Sci. USA 2013, 110, 16586–16591. [Google Scholar] [CrossRef] [PubMed]
- Sohn, E.H.; van Dijk, H.W.; Jiao, C.; Kok, P.H.; Jeong, W.; Demirkaya, N.; Garmager, A.; Wit, F.; Kucukevcilioglu, M.; van Velthoven, M.E.; et al. Retinal Neurodegeneration may Precede Microvascular Changes Characteristic of Diabetic Retinopathy in Diabetes Mellitus. Proc. Natl. Acad. Sci. USA 2016, 113, E2655–E2664. [Google Scholar] [CrossRef]
- Young, C.N.J.; Gorecki, D.C. P2RX7 Purinoceptor as a Therapeutic Target-the Second Coming? Front. Chem. 2018, 6, 248. [Google Scholar] [CrossRef]
- Sluyter, R. The P2X7 Receptor. Adv. Exp. Med. Biol. 2017, 1051, 17–53. [Google Scholar]
- Illes, P.; Khan, T.M.; Rubini, P. Neuronal P2X7 Receptors Revisited: Do they really Exist? J. Neurosci. 2017, 37, 7049–7062. [Google Scholar] [CrossRef]
- Ishii, K.; Kaneda, M.; Li, H.; Rockland, K.S.; Hashikawa, T. Neuron-Specific Distribution of P2X7 Purinergic Receptors in the Monkey Retina. J. Comp. Neurol. 2003, 459, 267–277. [Google Scholar] [CrossRef]
- Portillo, J.C.; Lopez Corcino, Y.; Miao, Y.; Tang, J.; Sheibani, N.; Kern, T.S.; Dubyak, G.R.; Subauste, C.S. CD40 in Retinal Muller Cells Induces P2X7-Dependent Cytokine Expression in Macrophages/Microglia in Diabetic Mice and Development of Early Experimental Diabetic Retinopathy. Diabetes 2017, 66, 483–493. [Google Scholar] [CrossRef]
- Solini, A.; Chiozzi, P.; Morelli, A.; Adinolfi, E.; Rizzo, R.; Baricordi, O.R.; Di Virgilio, F. Enhanced P2X7 Activity in Human Fibroblasts from Diabetic Patients: A Possible Pathogenetic Mechanism for Vascular Damage in Diabetes. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1240–1245. [Google Scholar] [CrossRef]
- Coutinho-Silva, R.; Parsons, M.; Robson, T.; Lincoln, J.; Burnstock, G. P2X and P2Y Purinoceptor Expression in Pancreas from Streptozotocin-Diabetic Rats. Mol. Cell. Endocrinol. 2003, 204, 141–154. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; Bergamaschi, C.T.; Fernandes, M.J.; Paredes-Gamero, E.J.; Buri, M.V.; Ferreira, A.T.; Araujo, S.R.; Punaro, G.R.; Maciel, F.R.; Nogueira, G.B.; et al. P2X7 Receptor in the Kidneys of Diabetic Rats Submitted to Aerobic Training Or to N-Acetylcysteine Supplementation. PLoS ONE 2014, 9, e97452. [Google Scholar] [CrossRef]
- Kneer, K.; Green, M.B.; Meyer, J.; Rich, C.B.; Minns, M.S.; Trinkaus-Randall, V. High Fat Diet Induces Pre-Type 2 Diabetes with Regional Changes in Corneal Sensory Nerves and Altered P2X7 Expression and Localization. Exp. Eye Res. 2018, 175, 44–55. [Google Scholar] [CrossRef]
- Solini, A.; Menini, S.; Rossi, C.; Ricci, C.; Santini, E.; Blasetti Fantauzzi, C.; Iacobini, C.; Pugliese, G. The Purinergic 2X7 Receptor Participates in Renal Inflammation and Injury Induced by High-Fat Diet: Possible Role of NLRP3 Inflammasome Activation. J. Pathol. 2013, 231, 342–353. [Google Scholar] [CrossRef]
- Solini, A.; Chiozzi, P.; Falzoni, S.; Morelli, A.; Fellin, R.; Di Virgilio, F. High Glucose Modulates P2X7 Receptor-Mediated Function in Human Primary Fibroblasts. Diabetologia 2000, 43, 1248–1256. [Google Scholar] [CrossRef]
- Brandle, U.; Kohler, K.; Wheeler-Schilling, T.H. Expression of the P2X7-Receptor Subunit in Neurons of the Rat Retina. Brain Res. Mol. Brain Res. 1998, 62, 106–109. [Google Scholar] [CrossRef]
- Sakamoto, K.; Endo, K.; Suzuki, T.; Fujimura, K.; Kurauchi, Y.; Mori, A.; Nakahara, T.; Ishii, K. P2X7 Receptor Antagonists Protect Against N-Methyl-D-Aspartic Acid-Induced Neuronal Injury in the Rat Retina. Eur. J. Pharmacol. 2015, 756, 52–58. [Google Scholar] [CrossRef]
- Yan, Z.; Li, S.; Liang, Z.; Tomic, M.; Stojilkovic, S.S. The P2X7 Receptor Channel Pore Dilates Under Physiological Ion Conditions. J. Gen. Physiol. 2008, 132, 563–573. [Google Scholar] [CrossRef]
- Surprenant, A.; Rassendren, F.; Kawashima, E.; North, R.A.; Buell, G. The Cytolytic P2Z Receptor for Extracellular ATP Identified as a P2X Receptor (P2X7). Science 1996, 272, 735–738. [Google Scholar] [CrossRef]
- Sugiyama, T.; Kobayashi, M.; Kawamura, H.; Li, Q.; Puro, D.G. Enhancement of P2X7-Induced Pore Formation and Apoptosis: An Early Effect of Diabetes on the Retinal Microvasculature. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1026–1032. [Google Scholar] [CrossRef]
- Fowler, B.J.; Gelfand, B.D.; Kim, Y.; Kerur, N.; Tarallo, V.; Hirano, Y.; Amarnath, S.; Fowler, D.H.; Radwan, M.; Young, M.T.; et al. Nucleoside Reverse Transcriptase Inhibitors Possess Intrinsic Anti-Inflammatory Activity. Science 2014, 346, 1000–1003. [Google Scholar] [CrossRef]
- Jiang, L.H. HIV Drug Nucleoside Reverse Transcriptase Inhibitors as Promising Anti-Inflammation Therapeutics by Targeting P2X7-Dependent Large Pore Formation: One Stone for Two Birds? Front. Pharmacol. 2015, 6, 38. [Google Scholar] [CrossRef]
- Mizutani, T.; Fowler, B.J.; Kim, Y.; Yasuma, R.; Krueger, L.A.; Gelfand, B.D.; Ambati, J. Nucleoside Reverse Transcriptase Inhibitors Suppress Laser-Induced Choroidal Neovascularization in Mice. Investig. Ophthalmol. Vis. Sci. 2015, 56, 7122–7129. [Google Scholar] [CrossRef]
- Loukovaara, S.; Sahanne, S.; Jalkanen, S.; Yegutkin, G.G. Increased Intravitreal Adenosine 5′-Triphosphate, Adenosine 5’-Diphosphate and Adenosine 5’-Monophosphate Levels in Patients with Proliferative Diabetic Retinopathy. Acta Ophthalmol. 2015, 93, 67–73. [Google Scholar] [CrossRef]
- Aplin, F.P.; Luu, C.D.; Vessey, K.A.; Guymer, R.H.; Shepherd, R.K.; Fletcher, E.L. ATP-Induced Photoreceptor Death in a Feline Model of Retinal Degeneration. Investig. Ophthalmol. Vis. Sci. 2014, 55, 8319–8329. [Google Scholar] [CrossRef]
- Notomi, S.; Hisatomi, T.; Kanemaru, T.; Takeda, A.; Ikeda, Y.; Enaida, H.; Kroemer, G.; Ishibashi, T. Critical Involvement of Extracellular ATP Acting on P2RX7 Purinergic Receptors in Photoreceptor Cell Death. Am. J. Pathol. 2011, 179, 2798–2809. [Google Scholar] [CrossRef]
- Xu, H.; Chen, M. Targeting the Complement System for the Management of Retinal Inflammatory and Degenerative Diseases. Eur. J. Pharmacol. 2016, 787, 94–104. [Google Scholar] [CrossRef]
- Rubsam, A.; Parikh, S.; Fort, P.E. Role of Inflammation in Diabetic Retinopathy. Int. J. Mol. Sci. 2018, 19, 942. [Google Scholar] [CrossRef]
- El-Asrar, A.M. Role of Inflammation in the Pathogenesis of Diabetic Retinopathy. Middle East Afr. J. Ophthalmol. 2012, 19, 70–74. [Google Scholar] [CrossRef]
- Kern, T.S.; Barber, A.J. Retinal Ganglion Cells in Diabetes. J. Physiol. 2008, 586, 4401–4408. [Google Scholar] [CrossRef]
- Ng, D.S.; Chiang, P.P.; Tan, G.; Cheung, C.G.; Cheng, C.Y.; Cheung, C.Y.; Wong, T.Y.; Lamoureux, E.L.; Ikram, M.K. Retinal Ganglion Cell Neuronal Damage in Diabetes and Diabetic Retinopathy. Clin. Exp. Ophthalmol. 2016, 44, 243–250. [Google Scholar] [CrossRef]
- Hombrebueno, J.R.; Chen, M.; Penalva, R.G.; Xu, H. Loss of Synaptic Connectivity, Particularly in Second Order Neurons is a Key Feature of Diabetic Retinal Neuropathy in the Ins2Akita Mouse. PLoS ONE 2014, 9, e97970. [Google Scholar] [CrossRef]
- Kurihara, T.; Ozawa, Y.; Nagai, N.; Shinoda, K.; Noda, K.; Imamura, Y.; Tsubota, K.; Okano, H.; Oike, Y.; Ishida, S. Angiotensin II Type 1 Receptor Signaling Contributes to Synaptophysin Degradation and Neuronal Dysfunction in the Diabetic Retina. Diabetes 2008, 57, 2191–2198. [Google Scholar] [CrossRef]
- Kolesnikov, A.V.; Fan, J.; Crouch, R.K.; Kefalov, V.J. Age-Related Deterioration of Rod Vision in Mice. J. Neurosci. 2010, 30, 11222–11231. [Google Scholar] [CrossRef]
- Chen, M.; Forrester, J.V.; Xu, H. Dysregulation in Retinal Para-Inflammation and Age-Related Retinal Degeneration in CCL2 Or CCR2 Deficient Mice. PLoS ONE 2011, 6, e22818. [Google Scholar] [CrossRef][Green Version]

| Antibody | Dilution | Source | Retinal Localisation |
|---|---|---|---|
| Cone arrestin | 1/1000 | Merck Millipore | Cone photoreceptors |
| GABA | 1/500 | Sigma | GABAergic amacrine cells |
| Brn3a | 1/50 | SantaCruz | Ganglion cells |
| Synaptophysin | 1/200 | Abcam | Presynaptic elements |
| Isolectin B4 | 1/50 | Vector Labs | Endothelial cells |
| Collagen IV | 1/75 | Bio Rad | Basal lamina in blood vessels |
| Donkey anti-rabbit Alexa Fluor 488 | 1/200 | Jackson ImmunoResearch | Secondary Antibody |
| Donkey anti-rabbit Alexa Fluor 594 | 1/200 | Jackson ImmunoResearch | Secondary Antibody |
| Donkey anti-goat Alexa Fluor 546 | 1/200 | Jackson ImmunoResearch | Secondary Antibody |
| Streptavidin Alexa Fluor 488 | 1/100 | Life Technologies | Secondary Antibody |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavlou, S.; Augustine, J.; Cunning, R.; Harkin, K.; Stitt, A.W.; Xu, H.; Chen, M. Attenuating Diabetic Vascular and Neuronal Defects by Targeting P2rx7. Int. J. Mol. Sci. 2019, 20, 2101. https://doi.org/10.3390/ijms20092101
Pavlou S, Augustine J, Cunning R, Harkin K, Stitt AW, Xu H, Chen M. Attenuating Diabetic Vascular and Neuronal Defects by Targeting P2rx7. International Journal of Molecular Sciences. 2019; 20(9):2101. https://doi.org/10.3390/ijms20092101
Chicago/Turabian StylePavlou, Sofia, Josy Augustine, Rónán Cunning, Kevin Harkin, Alan W. Stitt, Heping Xu, and Mei Chen. 2019. "Attenuating Diabetic Vascular and Neuronal Defects by Targeting P2rx7" International Journal of Molecular Sciences 20, no. 9: 2101. https://doi.org/10.3390/ijms20092101
APA StylePavlou, S., Augustine, J., Cunning, R., Harkin, K., Stitt, A. W., Xu, H., & Chen, M. (2019). Attenuating Diabetic Vascular and Neuronal Defects by Targeting P2rx7. International Journal of Molecular Sciences, 20(9), 2101. https://doi.org/10.3390/ijms20092101







