Macrocyclic Compounds for Drug and Gene Delivery in Immune-Modulating Therapy
Abstract
:1. Introduction
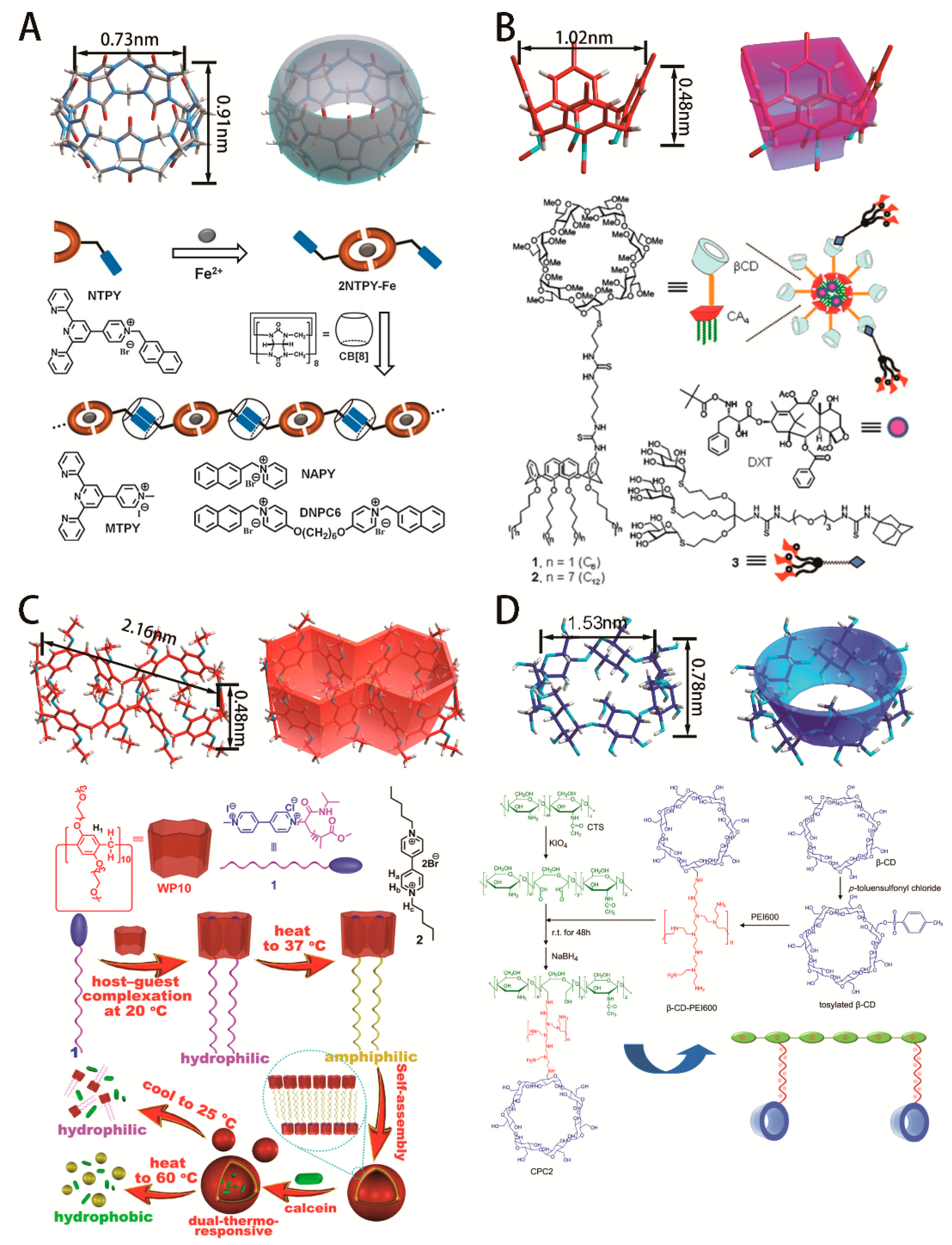
2. Macrocyclic Compounds
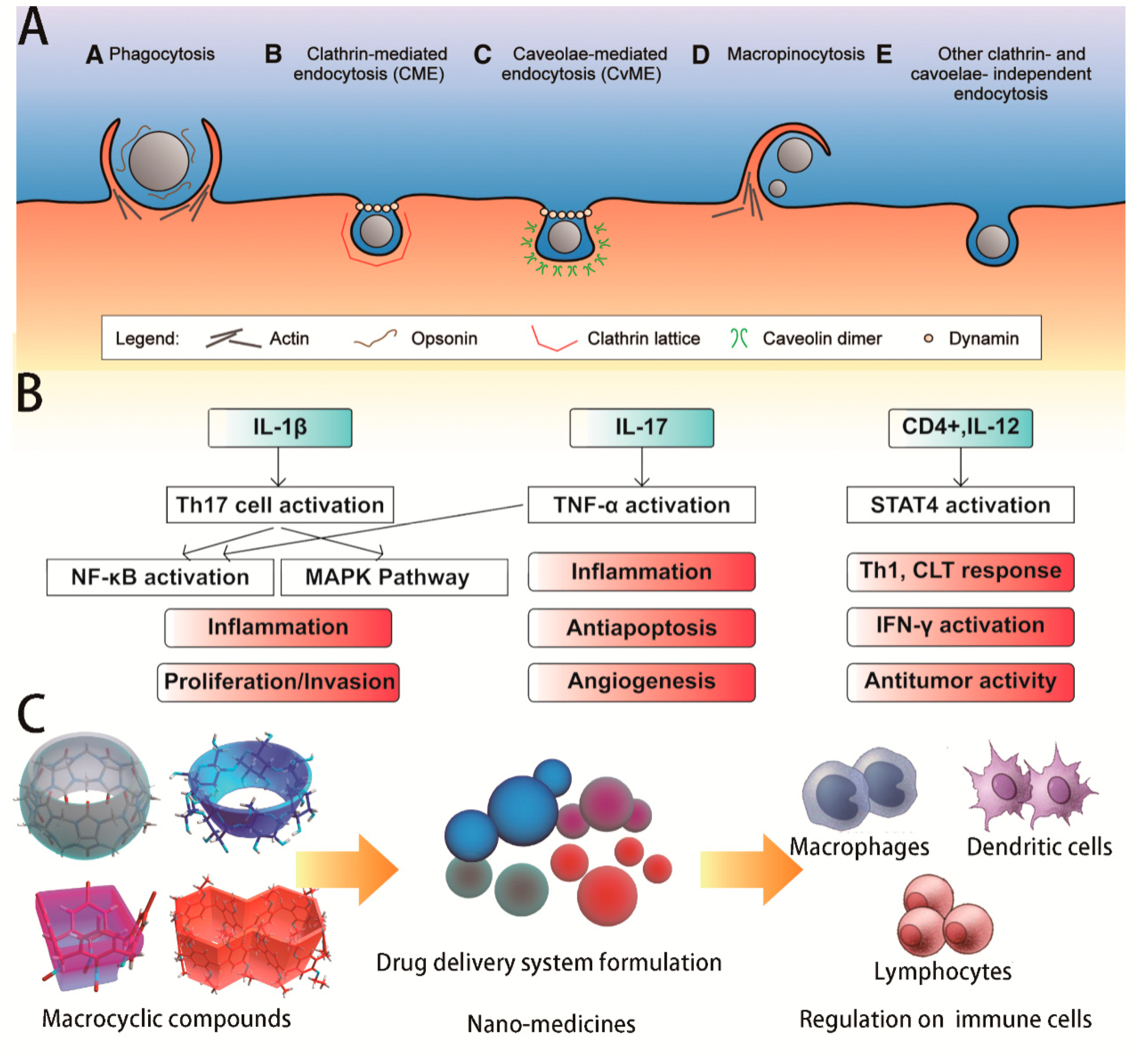
3. Biological Immunity Mechanisms in Vitro
3.1. Cell Uptake and Drug Delivery
3.2. Regulatory Cytokines and Signaling Pathways
3.3. Regulation of Macrophage Reprogramming
3.4. Inducing the Maturation and Activation of Dendritic Cells and T Helper Lymphocytes
4. Immune Therapy in Vivo
4.1. Cancer Treatment
4.2. Treatment of Cardiovascular and Other Diseases
5. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Chen, H.B.; Gu, Z.J.; An, H.W.; Chen, C.Y.; Chen, J.; Cui, R.; Chen, S.Q.; Chen, W.H.; Chen, X.S.; Chen, X.Y.; et al. Precise nanomedicine for intelligent therapy of cancer. Sci. China Chem. 2018, 61, 1503–1552. [Google Scholar] [CrossRef]
- Yang, G.B.; Phua, S.Z.F.; Bindra, A.K.; Zhao, Y.L. Degradability and Clearance of Inorganic Nanoparticles for Biomedical Applications. Adv. Mater. 2019, 1805730. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.H.; Kroll, A.V.; Gao, W.W.; Zhang, L.F. Cell Membrane Coating Nanotechnology. Adv. Mater. 2018, 30, 1706759. [Google Scholar] [CrossRef]
- Maruf, A.; Wang, Y.; Yin, T.Y.; Huang, J.L.; Wang, N.; Durkan, C.; Tan, Y.H.; Wu, W.; Wang, G.X. Atherosclerosis Treatment with Stimuli-Responsive Nanoagents: Recent Advances and Future Perspectives. Adv. Healthc. Mater. 2019, 1900036. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.L.; Choi, Y.; Kim, J. Mesoporous Silica as a Versatile Platform for Cancer Immunotherapy. Adv. Mater. 2018, 1803953. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.K.; Mishra, H.; Ekielski, A.; Talegaonkar, S.; Vaidya, B. Zinc oxide nanoparticles: A promising nanomaterial for biomedical applications. Drug Discov. Today 2017, 22, 1825–1834. [Google Scholar] [CrossRef]
- Mishra, H.; Mishra, P.K.; Ekielski, A.; Jaggi, M.; Iqbal, Z.; Talegaonkar, S. Melanoma treatment: From conventional to nanotechnology. J. Cancer Res. Clin. Oncol. 2018, 144, 2283–2302. [Google Scholar] [CrossRef]
- Bhandari, J.; Mishra, H.; Mishra, P.K.; Wimmer, R.; Ahmad, F.J.; Talegaonkar, S. Cellulose nanofiber aerogel as a promising biomaterial for customized oral drug delivery. Int. J. Nanomed. 2017, 12, 2021–2031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Yu, G.C.; Huang, F.H. Supramolecular chemotherapy based on host–guest molecular recognition: A novel strategy in the battle against cancer with a bright future. Chem. Soc. Rev. 2017, 46, 7021–7053. [Google Scholar] [CrossRef]
- Harada, A.; Takashima, Y.; Yamaguchi, H. Cyclodextrin-based supramolecular polymers. Chem. Soc. Rev. 2009, 38, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Harada, A. Cyclodextrin-Based Molecular Machines. Acc. Chem. Res. 2001, 34, 456–464. [Google Scholar] [CrossRef]
- Harada, A.; Takashima, Y.; Nakahata, M. Supramolecular Polymeric Materials via Cyclodextrin−Guest Interactions. Acc. Chem. Res. 2014, 47, 2128–2140. [Google Scholar] [CrossRef]
- Bogdan, A.R.; Davies, N.L.; James, K. Comparison of diffusion coefficients for matched pairs of macrocyclic and linear molecules over a drug-like molecular weight range. Org. Biomol. Chem. 2011, 9, 7727–7733. [Google Scholar] [CrossRef] [PubMed]
- Mallinson, J.; Collins, I. Macrocycles in new drug discovery. Future Med. Chem. 2012, 4, 1409–1438. [Google Scholar] [CrossRef] [PubMed]
- Driggers, E.M.; Hale, S.P.; Lee, J.B.; Terrett, N.K. The exploration of macrocycles for drug discovery-an underexploited structural class. Nat. Rev. Drug Discov. 2008, 7, 608–624. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Huang, Z.H.; Tan, X.X.; Wang, Z.Q.; Zhang., X. Cucurbit[8]uril-based supramolecular polymers: Promoting supramolecular polymerization by metal-coordination. Chem. Commun. 2013, 49, 5766–5768. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Yerga, L.; Lomazzi, M.; Sansone, F.; Mellet, C.O.; Casnatib, A.; Ferna´ndez, J.M.G. Glycoligand-targeted core-shell nanosphereswith tunable drug release profiles from calixarene-cyclodextrin heterodimers. Chem. Commun. 2014, 50, 7440–7443. [Google Scholar] [CrossRef] [PubMed]
- Chi, X.D.; Yu, G.C.; Shao, L.; Chen, J.Z.; Huang, F.H. A Dual-Thermoresponsive Gemini-Type Supra-amphiphilic Macromolecular [3] Pseudorotaxane Based on Pillar[10]arene/Paraquat Cooperative Complexation. J. Am. Chem. Soc. 2016, 138, 3168–3174. [Google Scholar] [CrossRef]
- Ping, Y.; Liu, C.D.; Zhang, Z.X.; Liu, L.K.; Chen, J.H.; Li, J. Chitosan-graft-(PEI-β-cyclodextrin) copolymers and their supramolecular PEGylation for DNA and siRNA delivery. Biomaterials 2011, 32, 8328–8341. [Google Scholar] [CrossRef]
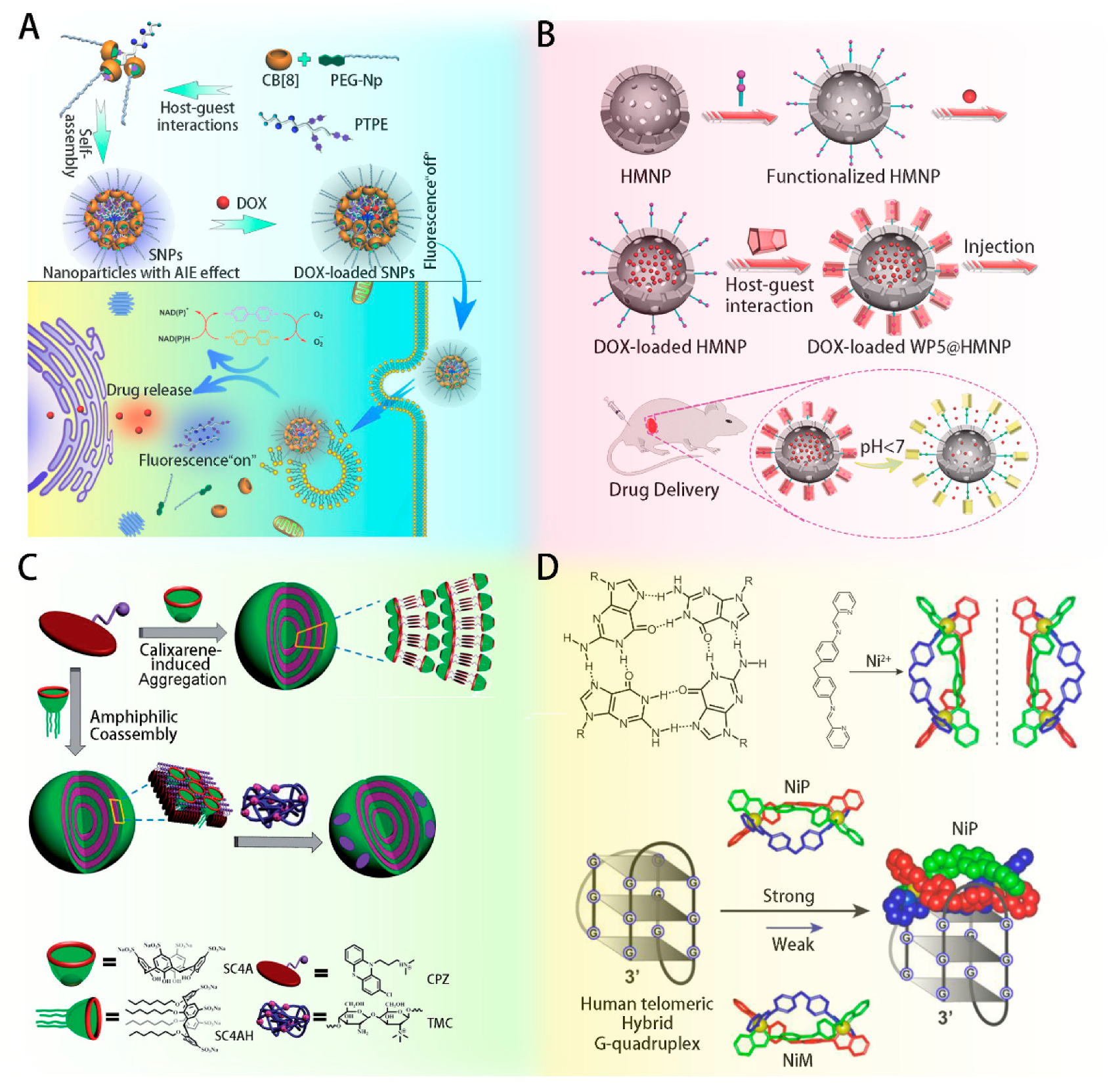
- Wu, D.; Li, Y.; Yang, J.; Shen, J.; Zhou, J.; Hu, Q.L.; Yu, G.C.; Tang, G.P.; Chen, X.Y. Supramolecular Nanomedicine Constructed from Cucurbit[8]uril-Based Amphiphilic Brush Copolymer for Cancer Therapy. ACS Appl. Mater. Interfaces 2017, 9, 44392–44401. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, Y.; Zhao, R.B.; Shao, L.; Tang, R.K.; Huang, F.H. Improved in vivo tumor therapy via host–guest complexation. J. Mater. Chem. B. 2016, 4, 2691–2696. [Google Scholar] [CrossRef]
- Qin, Z.B.; Guo, D.S.; Gao, X.N.; Liu, Y. Supra-amphiphilic aggregates formed by psulfonatocalix[4]arenes and the antipsychotic drug chlorpromazine. Soft Matter. 2014, 10, 2253–2263. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.S.; Zhao, C.Q.; Sun, Y.H.; Ren, J.S.; Qu, X.G. Metallo-supramolecular complexes enantioselectively eradicate cancer stem cells in vivo. J. Am. Chem. Soc. 2017, 139, 16201–16209. [Google Scholar] [CrossRef]
- Masson, E.; Ling, X.X.; Joseph, R.; Kyeremeh-Mensah, L.; Lu, X.Y. Cucurbituril chemistry: A tale of supramolecular success. RSC Adv. 2012, 2, 1213–1247. [Google Scholar] [CrossRef]
- Lee, J.W.; Heo, S.W.; Lee, S.J.C.; Ko, J.Y.; Kim, H.; Kim, H.I. Probing Conformational Changes of Ubiquitin by Host–Guest Chemistry Using Electrospray Ionization Mass Spectrometry. J. Am. Soc. Mass Spectrom 2013, 24, 21–29. [Google Scholar] [CrossRef]
- Wang, Y.X.; Guo, D.S.; Cao, Y.; Liu, Y. Phosphatase-responsive amphiphilic calixarene assembly. RSC Adv. 2013, 3, 8058–8063. [Google Scholar] [CrossRef]
- Okamatsu, A.; Motoyama, K.; Onodera, R.; Higashi, T.; Koshigoe, T.; Shimada, Y.; Hattori, K.; Takeuchi, T.; Arima, H. Folate-Appended β-Cyclodextrin as a Promising Tumor Targeting Carrier for Antitumor Drugs in Vitro and in Vivo. Bioconjugate Chem. 2013, 24, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, Y.B.; Xing, H.B.; Guo, J.L.; Yuan, P.; Tang, G.P. Synergistic Enhancement of Lung Cancer Therapy Through Nanocarrier-Mediated Sequential Delivery of Superantigen and Tyrosin Kinase Inhibitor. Adv. Funct. Mater. 2014, 24, 5482–5492. [Google Scholar] [CrossRef]
- Rodell, C.B.; Arlauckas, S.P.; Cuccarese, M.F.; Garris, C.S.; Li, R.; Ahmed, M.S.; Kohler, R.H.; Pittet, M.J.; Weissleder, R. TLR7/8-agonist-loaded nanoparticles promote the polarization of tumour-associated macrophages to enhance cancer immunotherapy. Nat. Biomed. Eng. 2018, 2, 578–588. [Google Scholar] [CrossRef]
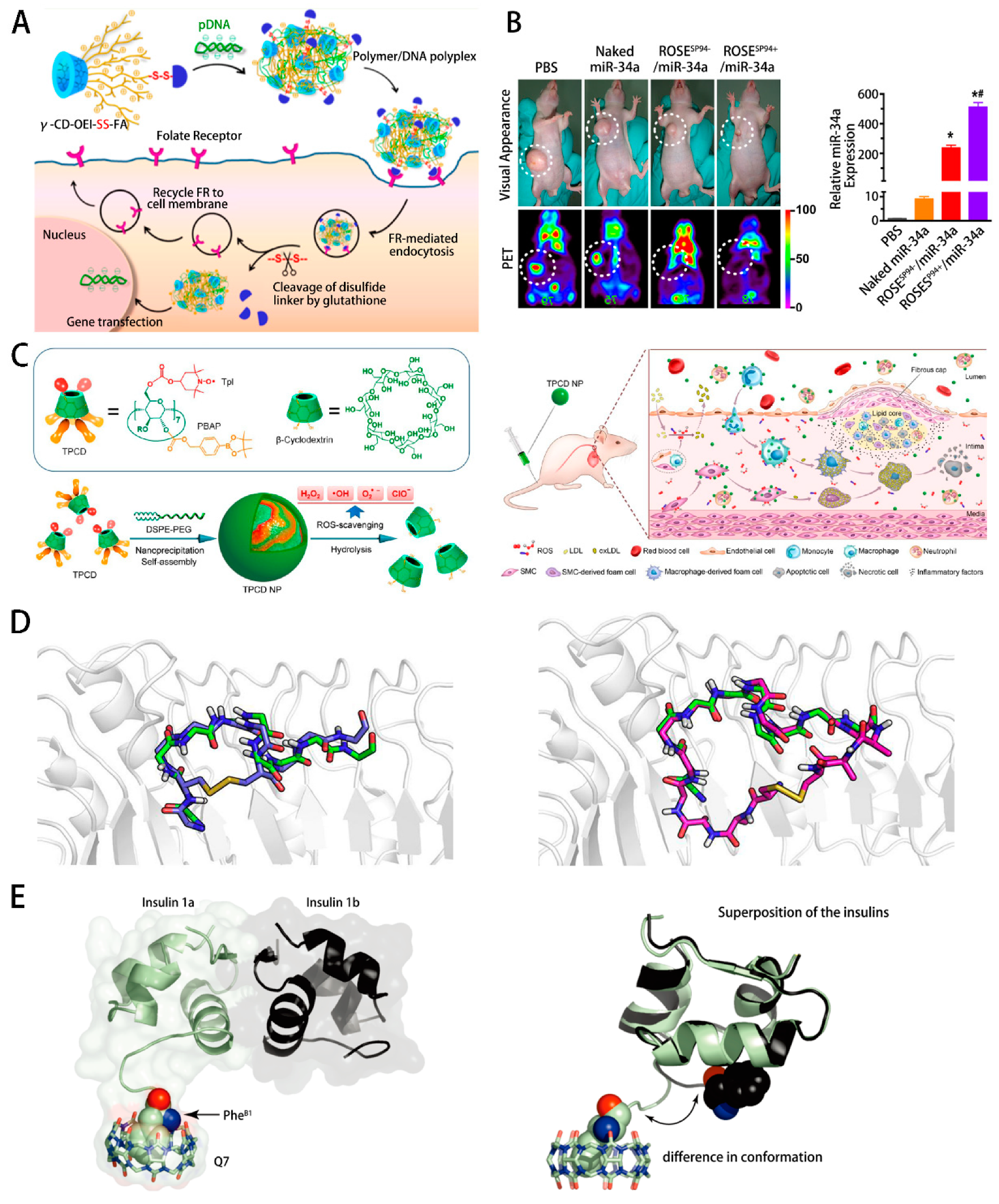
- Ping, Y.; Hu, Q.D.; Tang, G.P.; Li, J. FGFR-targeted gene delivery mediated by supramolecular assembly between β-cyclodextrin-crosslinked PEI and redox-sensitive PEG. Biomaterials 2013, 34, 6482–6494. [Google Scholar] [CrossRef]
- Hu, Q.L.; Wu, M.; Fang, C.; Cheng, C.Y.; Zhao, M.M.; Fang, W.H.; Chu, P.K.; Ping, Y.; Tang, G.P. Engineering Nanoparticle-Coated Bacteria as Oral DNA Vaccines for Cancer Immunotherapy. Nano Lett. 2015, 15, 2732–2739. [Google Scholar] [CrossRef]
- Hu, Q.D.; Wang, K.; Sun, X.; Li, Y.; Fu, Q.H.; Liang, T.B.; Tang, G.P. A redox-sensitive, oligopeptide-guided, self-assembling, and efficiency-enhanced (ROSE) system for functional delivery of microRNA therapeutics for treatment of hepatocellular carcinoma. Biomaterials 2016, 104, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.X.; Jia, Y.; Li, X.D.; Hu, Y.Q.; Li, X.H. Facile Engineering of Biocompatible Materials with pH-Modulated Degradability. Adv. Mater. 2011, 23, 3035–3040. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.L.; Wei, Y.L.; Chen, K.; Zhang, X.J.; Xu, X.Q.; Shi, Q.; Han, S.L.; Chen, X.; Gong, H.; Li, X.H. Biocompatible Reactive Oxygen Species (ROS) Responsive Nanoparticles as Superior Drug Delivery Vehicles. Adv. Healthcare Mater. 2015, 4, 69–76. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Li, L.L.; Zhao, W.B.; Dou, Y.; An, H.J.; Tao, H.; Xu, X.Q.; Jia, Y.; Lu, S.; Zhang, J.X. Targeted Therapy of Atherosclerosis by a Broad-Spectrum Reactive Oxygen Species Scavenging Nanoparticle with Intrinsic Anti-inflammatory Activity. ACS Nano 2018, 12, 8943–8960. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, R.J.; Li, C.W.; Tao, H.; Dou, Y.; Wang, Y.Q.; Hu, H.Y.; Zhang, J.X. A Targeting Nanotherapy for Abdominal Aortic Aneurysms. J. Am. Coll. Cardiol. 2018, 72, 2591–2605. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.D.; Liu, J.; Zhang, W.H.; Stashko, M.A.; Nichols, J.; Miley, M.J.; Norris-Drouin, J.; Chen, Z.L.; Machius, M.; DeRyckere, D.; et al. Design and Synthesis of Novel Macrocyclic Mer Tyrosine Kinase Inhibitors. ACS Med. Chem. Lett. 2016, 7, 1044–1049. [Google Scholar] [CrossRef] [Green Version]
- Hillaireau, H.; Couvreur, P. Nanocarriers’ entry into the cell: Relevance to drug delivery. Cell. Mol. Life Sci. 2009, 66, 2873–2896. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, M.; Ceballos-Olvera, I.; Barrio, L.D.; Re, F. Role of the Inflammasome, IL-1β, and IL-18 in Bacterial Infections. Sci. World J. 2011, 11, 2037–2050. [Google Scholar] [CrossRef] [Green Version]
- Netea, M.G.; van de Veerdonk, F.L.; van der Meer, J.M.; Dinarello, C.; Joosten, L.B. Inflammasome-Independent Regulation of IL-1-Family Cytokines. Annu. Rev. Immunol. 2015, 33, 49–77. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; Ghosh, S. Signaling to NF-κB. Genes Dev. 2004, 18, 2195–2224. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.T.; Verma, I.M. NF-κB Regulation in the immune system. Nat. Rev. Immunol. 2002, 2, 725–734. [Google Scholar] [CrossRef]
- Chang, L.F.; Karin, M. Mammalian MAP kinase signalling cascades. Nature 2001, 410, 37–40. [Google Scholar] [CrossRef]
- Roux, P.P.; Blenis, J. ERK and p38 MAPK-Activated Protein Kinases: A Family of Protein Kinases with Diverse Biological Functions. Microbiol. Mol. Biol. Rev. 2004, 68, 320–344. [Google Scholar] [CrossRef]
- Kolls, J.K.; Linde, A. Interleukin-17 Family Members and Inflammation. Immunity 2004, 21, 467–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zola, H.; Swart, B.; Nicholson, I.; Aasted, B.; Bensussan, A.; Boumsell, L.; Buckley, C.; Clark, G.; Drbal, K.; Engel, P.; et al. CD molecules 2005: Human cell differentiation molecules. Blood 2005, 106, 3123–3126. [Google Scholar] [CrossRef]
- Zimmer, S.; Grebe, A.; Bakke, S.S.; Bode, N.; Halvorsen, B.; Ulas, T.; Skjelland, M.; Nardo, D.D.; Labzin, L.I.; Kerksiek, A.; et al. Cyclodextrin promotes atherosclerosis regression via macrophage reprogramming. Sci. Transl. Med. 2016, 8, 333ra50. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Yun, C.H.; Han, S.H. Induction of Dendritic cell Maturation and activation by a Potential adjuvant, 2-hydroxypropyl-β-cyclodextrin. Front. Immunol. 2016, 7, 435. [Google Scholar] [CrossRef]
- Onishi, M.; Ozasa, K.; Kobiyama, K.; Ohata, K.; Kitano, M.; Taniguchi, K.; Homma, T.; Kobayashi, M.; Sato, A.; Katakai, Y.; et al. Hydroxypropyl-β-Cyclodextrin Spikes Local Inflammation That Induces Th2 Cell and T Follicular Helper Cell Responses to the Coadministered Antigen. J. Immunol. 2015, 194, 2673–2682. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.W.; Jin, W.H.; Wu, G.S.; Zhao, Y.; Jin, X.; Hu, X.R.; Zhou, J.; Tang, G.P.; Chu, P.K. Rare-earth-incorporated polymeric vector for enhanced gene delivery. Biomaterials 2014, 35, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Arai, K.; Lee, F.; Miyajima, A.; Miyatake, S.; Arai, N.; Yokota, T. Cytokines: Coordinators of immune and inflammatory responses. Annu. Rev. Biochem. 1990, 59, 783C836. [Google Scholar] [CrossRef]
- Stenken1, J.A.; Poschenrieder, A.J. Bioanalytical Chemistry of Cytokines-A Review. Anal. Chim. Acta 2015, 853, 95–115. [Google Scholar] [CrossRef] [PubMed]
- Matassoli, F.L.; Leão, I.C.; Bezerra, B.B.; Pollard, R.B.; Lütjohann, D.; Hildreth, J.E.K.; de Arruda, L.B. Hydroxypropyl-Beta-Cyclodextrin Reduces Inflammatory Signaling from Monocytes: Possible Implications for Suppression of HIV Chronic Immune Activation. mSphere 2018, 3, e00497-18. [Google Scholar] [CrossRef]
- Takeda, K.; Akira, S. Toll-like receptors in innate immunity. Int. Immunol. 2005, 17, 1–14. [Google Scholar] [CrossRef]
- Wajant, H.; Pfizenmaier, K.; Scheurich, P. Tumor necrosis factor signaling. Cell Death Differ. 2003, 10, 45–65. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.T.; Tang, Y.Q.; He, X.; Yan, J.; Wang, C.H.; Feng, X.L. Self-Assembled Raspberry-Like Core/Satellite Nanoparticles for Anti-Inflammatory Protein Delivery. ACS Appl. Mater. Interfaces 2017, 9, 6902–6907. [Google Scholar] [CrossRef]
- Lo, H.W.; Hung, M.C. Nuclear EGFR signalling network in cancers: Linking EGFR pathway to cell cycle progression, nitric oxide pathway and patient survival. Br. J. Cancer 2006, 94, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Schlessinger, J. Cell Signaling by Receptor Tyrosine Kinases. Cell 2000, 103, 211–225. [Google Scholar] [CrossRef] [Green Version]
- Yu, Q.L.; Watson, R.R.; Marchalonis, J.J.; Larson, D.F. A role for T lymphocytes in mediating cardiac diastolic function. Am J Physiol Heart Circ Physiol. 2005, 289, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Moser, B.; Schaerli, P.; Loetscher, P. CXCR5+ T cells: Follicular homing takes center stage in T-helper-cell responses. Trends Immunol. 2002, 23, 250–254. [Google Scholar] [CrossRef]
- Shende, P.K.; Gaud, R.S.; Bakal, R.; Patil, D. Effect of inclusion complexation of meloxicam with β-cyclodextrin and β-cyclodextrin-based nanosponges on solubility, in vitro release and stability studies. Colloids Surf. B 2015, 136, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Puglisi, A.; Rizzarelli, E.; Vecchio, G.; Iacovino, R.; Benedetti, E.; Pedone, C.; Saviano, M. Crystal and Molecular Structure of β-Cyclodextrins Functionalized with the Anti-Inflammatory Drug Etodolac. Biopolymers 2009, 91, 1227–1235. [Google Scholar] [CrossRef]
- Akkari, A.C.S.; Campos, E.V.R.; Keppler, A.F.; Fraceto, L.F.; de Paula, E.; Tófoli, G.R.; de Araujo, D.R. Budesonide-hydroxypropyl-β-cyclodextrin inclusion complex in binary poloxamer 407/403 system for ulcerative colitis treatment: A physico-chemical study from micelles to hydrogels. Colloids Surf. B 2016, 138, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Venuti, V.; Cannavà, C.; Cristiano, M.C.; Fresta, M.; Majolino, D.; Paolino, D.; Stancanelli, R.; Tommasini, S.; Ventura, C.A. A characterization study of resveratrol/sulfobutyl ether-β-cyclodextrin inclusion complex and in vitro anticancer activity. Colloids Surf. B 2014, 115, 22–28. [Google Scholar] [CrossRef]
- Benyettou, F.; Alhashimi, M.; O’Connor, M.; Pasricha, R.; Brandel, J.; Traboulsi, H.; Mazher, J.; Olsen, J.C.; Trabolsi, A. Sequential Delivery of Doxorubicin and Zoledronic Acid to Breast Cancer Cells by CB[7]-Modified Iron Oxide Nanoparticles. ACS Appl. Mater. Interfaces 2017, 9, 40006–40016. [Google Scholar] [CrossRef] [PubMed]
- Shangguan, L.Q.; Chen, Q.; Shi, B.B.; Huang, F.H. Enhancing the solubility and bioactivity of anticancer drug tamoxifen by water-soluble pillar[6]arene-based host–guest complexation. Chem. Commun. 2017, 53, 9749–9752. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.C.; Yu, W.; Mao, Z.W.; Gao, C.Y.; Huang, F.H. A Pillararene-Based Ternary Drug-Delivery System with Photo controlled Anticancer Drug Release. Small 2015, 11, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Geraci, C.; Consoli, G.M.L.; Granata, G.; Galante, E.; Palmigiano, A.; Pappalardo, M.; Di Puma, S.D.; Spadaro, A. First Self-Adjuvant Multicomponent Potential Vaccine Candidates byTethering of Four or Eight MUC1 Antigenic Immunodominant PDTRPUnits on a Calixarene Platform: Synthesis and Biological Evaluation. Bioconjugate Chem. 2013, 24, 1710–1720. [Google Scholar] [CrossRef]
- Zhao, F.; Yin, H.; Zhang, Z.X.; Li, J. Folic Acid Modified Cationic γ-Cyclodextrin-oligoethylenimine Star Polymer with Bioreducible Disulfide Linker for Efficient Targeted Gene Delivery. Biomacromolecules 2013, 14, 476–484. [Google Scholar] [CrossRef]
- Gao, M.; London, N.; Cheng, K.; Tamura, R.; Jin, J.L.; Schueler-Furman, O.; Yin, H. Rationally designed macrocyclic peptides as synergistic agonists of LPS-induced inflammatory response. Tetrahedron 2014, 70, 7664–7668. [Google Scholar] [CrossRef] [Green Version]
- Chinai, J.M.; Taylor, A.B.; Ryno, L.M.; Hargreaves, N.D.; Morris, C.A.; Hart, P.J.; Urbach, A.R. Molecular Recognition of Insulin by a Synthetic Receptor. J. Am. Chem. Soc. 2011, 133, 8810–8813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilely, K.; Bakke, S.S.; Palarasah, Y.; Skjoedt, M.O.; Bartels, E.D.; Espevik, T.; Garred, P. Alpha-cyclodextrin inhibits cholesterol crystal-induced complement mediated inflammation: A potential new compound for treatment of atherosclerosis. Atherosclerosis 2019, 283, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Ramirez, C.M.; Miller, A.M.; Repa, J.J.; Turley, S.D.; Dietschy, J.M. Cyclodextrin overcomes the transport defect in nearly every organ of NPC1 mice leading to excretion of sequestered cholesterol as bile acid. J. Lipid Res. 2010, 51, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Zadmard, R.; Alavijeh, N.S. Protein surface recognition by calixarenes. RSC Adv. 2014, 4, 41529–41542. [Google Scholar] [CrossRef]
- Giuliani, M.; Morbioli, I.; Sansone, F.; Casnati, A. Moulding calixarenes for biomacromolecule targeting. Chem. Commun. 2015, 51, 14140–14159. [Google Scholar] [CrossRef]
- Gordo, S.; Martos, V.; Santos, E.; Mene´ndez, M.; Bo, C.; Giralt, E.; de Mendoza, J. Stability and structural recovery of the tetramerization domain of p53-R337H mutant induced by a designed templating ligand. Proc. Natl. Acad. Sci. USA 2008, 105, 16426–16431. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Jia, S.R.; Wang, W.; Yuan, Z.; Ravoo, B.J.; Guo, D.S. Heteromultivalent peptide recognition by co-assembly of cyclodextrin and calixarene amphiphiles enables inhibition of amyloid fibrillation. Nat. Chem. 2019, 11, 86–93. [Google Scholar] [CrossRef] [PubMed]
| Macrocyclic Compound | Drug | Use | Method | Outcome | Ref. |
|---|---|---|---|---|---|
| β-CyD | MC11 peptide | SKOV-3 cancer | Physical mixture | Targeted FGFRs and improved transfection efficiency | [30] |
| β-CyD | pDNA | B16 melanoma tumor | Physical mixture | Promoted infection efficiency and immunotherapy, including T cell activation and cytokine production | [31] |
| β-CyD | Meloxicam | Infectious arthritis | Physical mixture or kneading | Enhanced the solubility and stability of meloxicam for anti-inflammatory and analgesic effects | [61] |
| β-CyD | Etodolac | Infectious arthritis | Physical mixture | Anti-inflammatory | [62] |
| 2-hydroxypropyl-β-CyD | MicroRNA-34a | HCC-LM3 tumor | Lyophilization | Redox-sensitive, oligopeptide-guided, self-assembling, efficiency-enhanced system for the treatment of hepatocellular carcinoma | [32] |
| 2-hydroxypropyl-β-CyD | Atherosclerosis | Increased oxysterol production and promoted macrophage reprogramming | [47] | ||
| 2-hydroxypropyl-β-CyD | Budesonide | Ulcerative colitis | Lyophilization | Improved the solubility of budesonide and benefited the treatment of ulcerative colitis | [63] |
| sulfobutyl ether-β-CyD | Resveratrol | MCF-7 tumor | Freeze-drying | Increased the solubility and anticancer activity of resveratrol | [64] |
| cucurbit[8]uril | Doxorubicin | HeLa tumor | Lyophilization | Self-imaging and controllable drug release ability | [20] |
| cucurbit[7]uril | Zoledronic and Doxorubicin | MCF-7 tumor | Physical mixture | Sequential delivery of doxorubicin/zoledronic acid to breast cancer cells | [65] |
| pillar[6]arene | Tamoxifen | MCF-7 and Hela tumor | Physical mixture | Enhanced the solubility and bioactivity of the poorly water-soluble anticancer drug tamoxifen | [66] |
| pillar[6]arene | Chlorambucil | A549 tumor | Physical mixture | Photocontrolled anticancer drug release | [67] |
| calix[8]arenes with glycosylation | MCF-7 tumor | Physical mixture | Prevented tumor migration and proliferation | [68] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, H.; Wang, J.; Li, Z.; Tang, G. Macrocyclic Compounds for Drug and Gene Delivery in Immune-Modulating Therapy. Int. J. Mol. Sci. 2019, 20, 2097. https://doi.org/10.3390/ijms20092097
Bai H, Wang J, Li Z, Tang G. Macrocyclic Compounds for Drug and Gene Delivery in Immune-Modulating Therapy. International Journal of Molecular Sciences. 2019; 20(9):2097. https://doi.org/10.3390/ijms20092097
Chicago/Turabian StyleBai, Hongzhen, Jianwei Wang, Zhongbao Li, and Guping Tang. 2019. "Macrocyclic Compounds for Drug and Gene Delivery in Immune-Modulating Therapy" International Journal of Molecular Sciences 20, no. 9: 2097. https://doi.org/10.3390/ijms20092097
APA StyleBai, H., Wang, J., Li, Z., & Tang, G. (2019). Macrocyclic Compounds for Drug and Gene Delivery in Immune-Modulating Therapy. International Journal of Molecular Sciences, 20(9), 2097. https://doi.org/10.3390/ijms20092097




