A New Approach for Glyco-Functionalization of Collagen-Based Biomaterials
Abstract
1. Introduction
2. Results
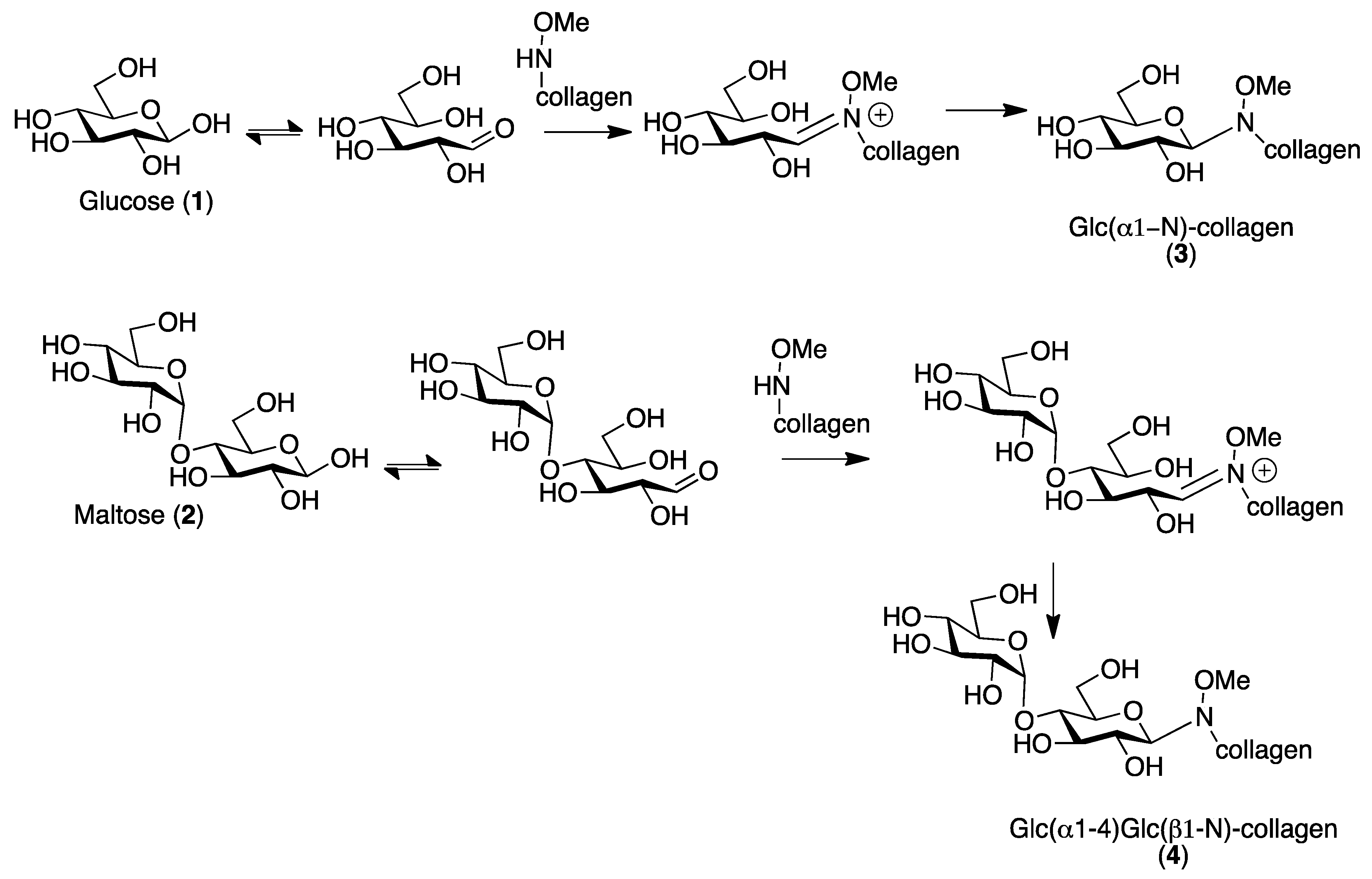
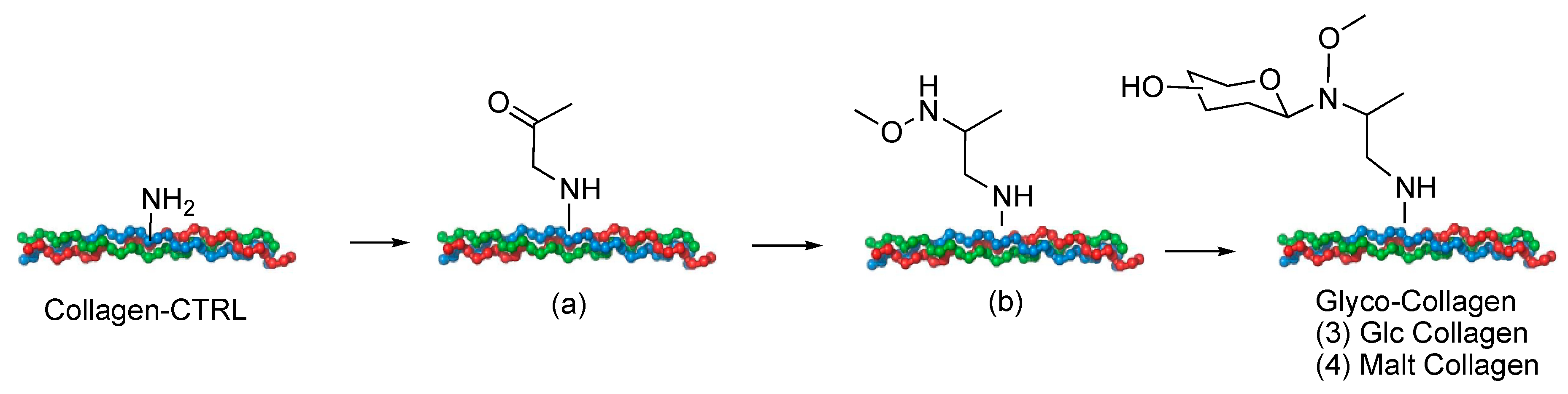
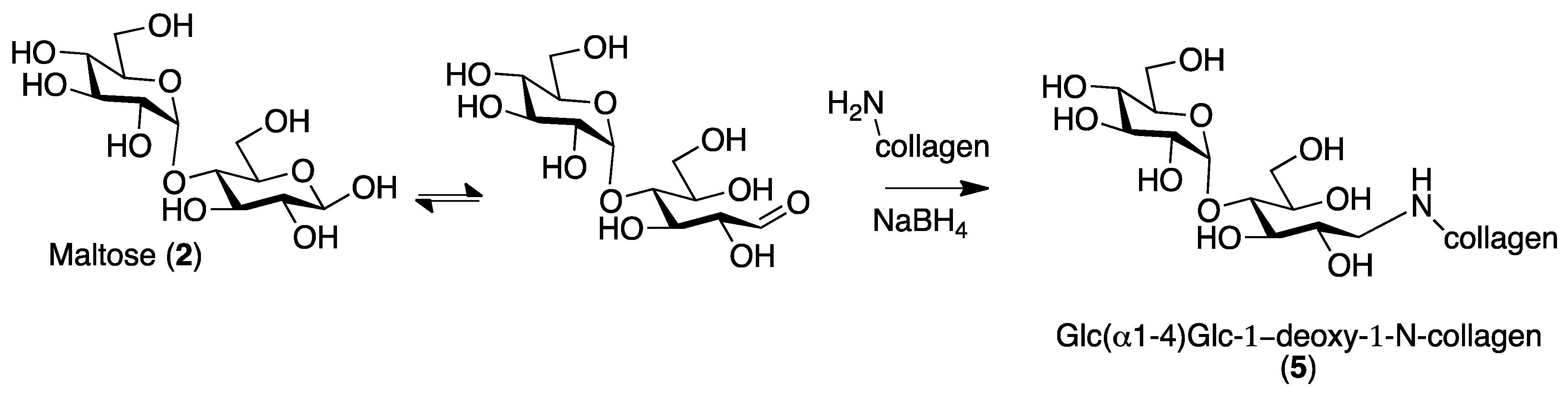
2.1. Collagen Matrix Neoglycosylation
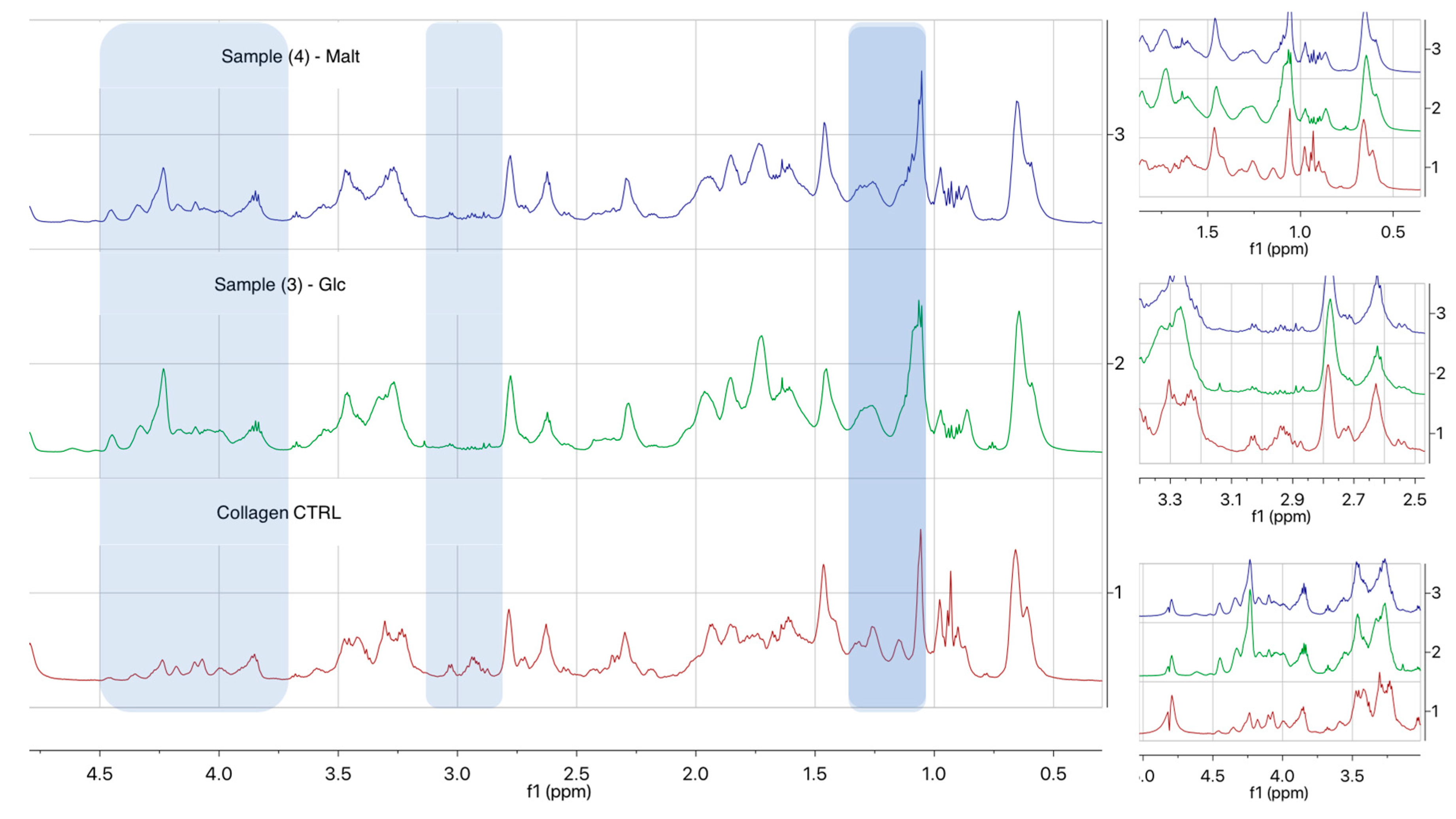
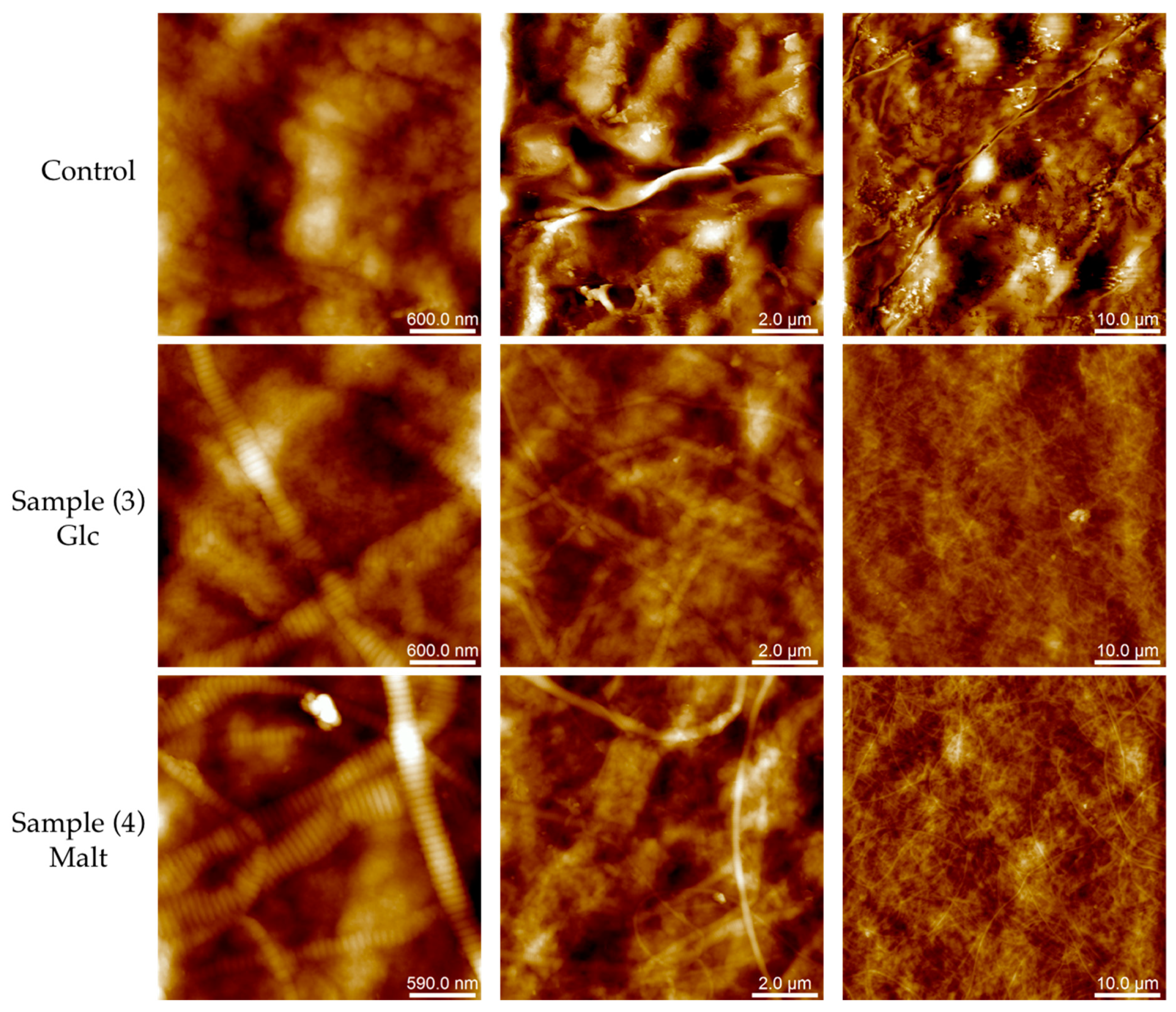
2.2. Biomolecular and Morphological Characterization
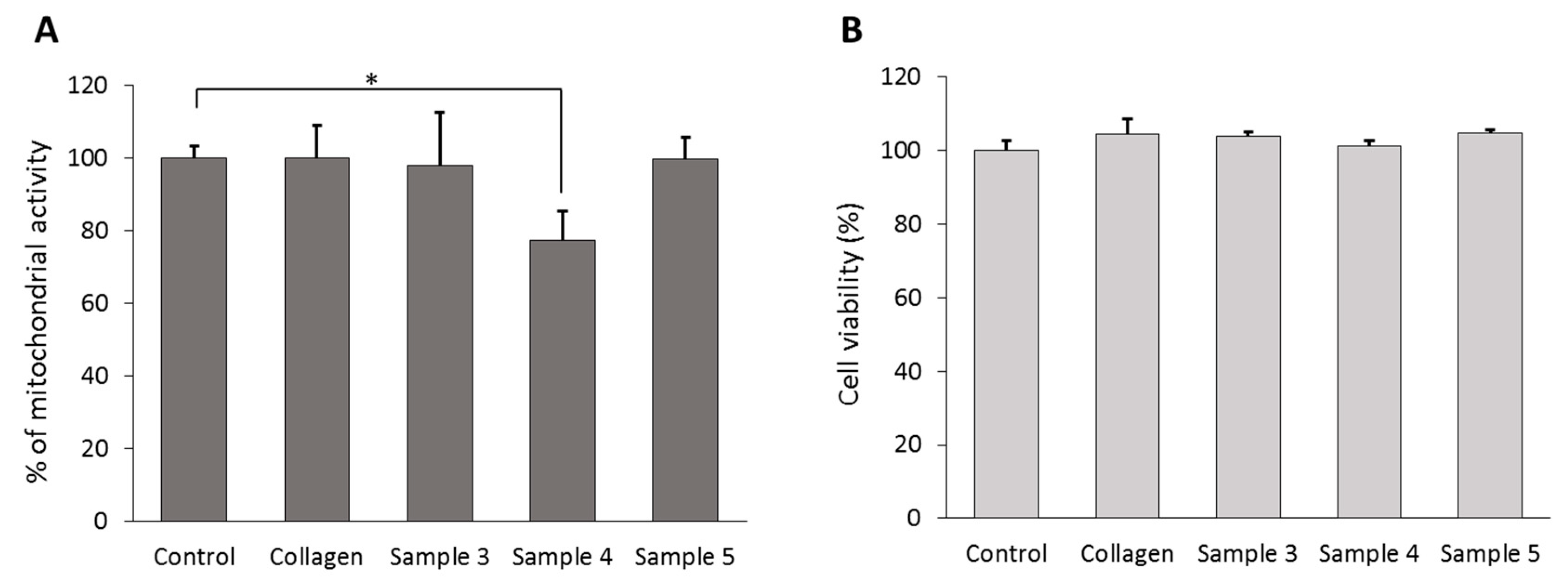
2.3. The Effect of Collagen on Cell Viability and Differentiation
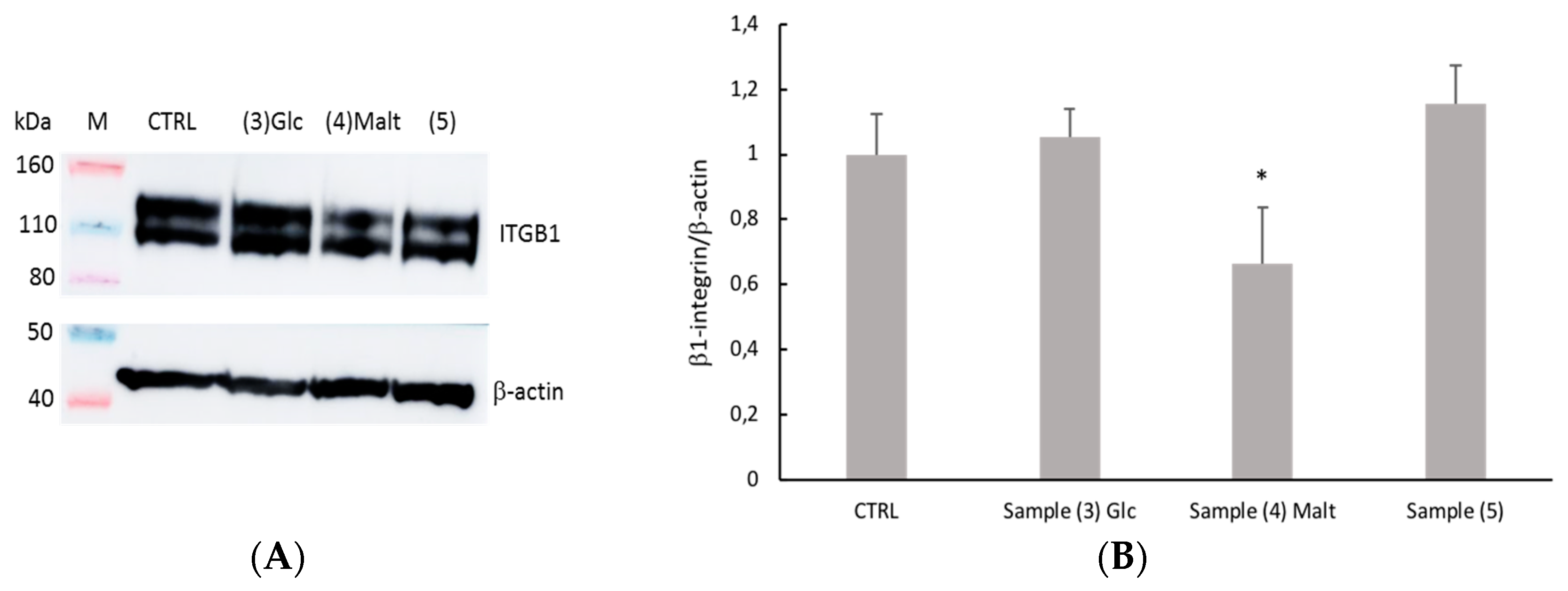
2.4. Evaluation of Cell-Collagen Adhesion
3. Discussion
4. Materials and Methods
4.1. General Materials
4.2. Collagen Samples Preparation
General Procedures
4.3. Ninhydrin Assay
4.4. Phenol–Sulfuric Acid Assay
4.5. FT-IR
4.6. 1H NMR
4.7. Atomic Force Microscopy (AFM)
4.8. Biocompatibility of Collagen Films
4.9. Immunofluorescence Labeling
4.10. Integrin-β-1 Immunostaining
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| NMR | Nuclear Magnetic Resonance |
| DOSY | Diffusion Ordered SpectroscopY |
| FT-IR | Fourier Fourier Transform Infrared Spectroscopy |
| AFM | Atomic Force Microscopy |
| Malt | Maltose |
| Glc | Glucose |
References
- Khademhosseini, A.; Langer, R. A decade of progress in tissue engineering. Nat. Protoc. 2016, 11, 1775. [Google Scholar] [CrossRef] [PubMed]
- Lutolf, M.P.; Hubbell, J.A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 2005, 23, 47. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Ohtsubo, K.; Marth, J.D. Glycosylation in cellular mechanisms of health and disease. Cell 2006, 126, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Sampaolesi, S.; Nicotra, F.; Russo, L. Glycans in nanomedicine, impact and perspectives. Future Med. Chem. 2019, 11, 43–60. [Google Scholar] [CrossRef] [PubMed]
- Russo, L.; Cipolla, L. Glycomics: New Challenges and Opportunities in Regenerative Medicine. Chem. A Eur. J. 2016, 22, 13380–13388. [Google Scholar] [CrossRef] [PubMed]
- Russo, L.; Sgambato, A.; Lecchi, M.; Pastori, V.; Raspanti, M.; Natalello, A.; Doglia, S.M.; Nicotra, F.; Cipolla, L. Neoglucosylated collagen matrices drive neuronal cells to differentiate. ACS Chem. Neurosci. 2014, 5, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Peri, F.; Nicotra, F. Chemoselective ligation in glycochemistry. Chem. Commun. 2004, 6, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Russo, L.; Gautieri, A.; Raspanti, M.; Taraballi, F.; Nicotra, F.; Vesentini, S.; Cipolla, L. Carbohydrate-functionalized collagen matrices: Design and characterization of a novel neoglycosylated biomaterial. Carbohydr. Res. 2014, 389, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Masuko, T.; Minami, A.; Iwasaki, N.; Majima, T.; Nishimura, S.I.; Lee, Y.C. Carbohydrate analysis by a phenol-sulfuric acid method in microplate format. Anal. Biochem. 2005, 339, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ramstrom, O.; Yan, M. A photochemically initiated chemistry for coupling underivatized carbohydrates to gold nanoparticles. J. Mater. Chem. 2009, 19, 8944–8949. [Google Scholar] [CrossRef] [PubMed]
- Durka, M.; Buffet, K.; Iehl, J.; Holler, M.; Nierengarten, J.F.; Taganna, J.; Bouckaert, J.; Vincent, S.P. The functional valency of dodecamannosylated fullerenes with Escherichia coli FimH--towards novel bacterial antiadhesives. Chem. Commun. 2011, 47, 1321–1323. [Google Scholar] [CrossRef] [PubMed]
- De Campos Vidal, B.; Mello, M.L.S. Collagen type I amide I band infrared spectroscopy. Micron 2011, 42, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Hunt, J.A.; Fawcett, S.; D’sa, R.; Akhtar, R.; Curran, J.M. The optimization and production of stable homogeneous amine enriched surfaces with characterized nanotopographical properties for enhanced osteoinduction of mesenchymal stem cells. J. Biomed. Mater. Res. A 2018, 106, 1862–1877. [Google Scholar] [CrossRef] [PubMed]
- Russo, L.; Sgambato, A.; Giannoni, P.; Quarto, R.; Vesentini, S.; Gautieri, A.; Cipolla, L. Response of osteoblast-like MG63 on neoglycosylated collagen matrices. Medchemcomm 2014, 5, 1208–1212. [Google Scholar] [CrossRef]
- Stylianou, A.; Yova, D. Surface nanoscale imaging of collagen thin films by Atomic Force Microscopy. Mater. Sci. Eng. C. Mater. Biol. Appl. 2013, 33, 2947–2957. [Google Scholar] [CrossRef] [PubMed]
- Stylianou, A. Atomic Force Microscopy for Collagen-Based Nanobiomaterials. J. Nanomater. 2017, 2017, 1–14. [Google Scholar] [CrossRef]
- Mizuno, K.; Adachi, E.; Imamura, Y.; Katsumata, O.; Hayashi, T. The fibril structure of type V collagen triple-helical domain. Micron 2001, 32, 317–323. [Google Scholar] [CrossRef]
- Rýglová, Š.; Braun, M.; Suchý, T. Collagen and Its Modifications—Crucial Aspects with Concern to Its Processing and Analysis. Macromol. Mater. Eng. 2017, 302, 1600460. [Google Scholar] [CrossRef]
- Mouw, J.K.; Ou, G.; Weaver, V.M. Extracellular matrix assembly: a multiscale deconstruction. Nat. Rev. Mol. Cell Biol. 2014, 15, 771–785. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figuereido, I.; Paiotta, A.; Dal Magro, R.; Tinelli, F.; Corti, R.; Re, F.; Cassina, V.; Caneva, E.; Nicotra, F.; Russo, L. A New Approach for Glyco-Functionalization of Collagen-Based Biomaterials. Int. J. Mol. Sci. 2019, 20, 1747. https://doi.org/10.3390/ijms20071747
Figuereido I, Paiotta A, Dal Magro R, Tinelli F, Corti R, Re F, Cassina V, Caneva E, Nicotra F, Russo L. A New Approach for Glyco-Functionalization of Collagen-Based Biomaterials. International Journal of Molecular Sciences. 2019; 20(7):1747. https://doi.org/10.3390/ijms20071747
Chicago/Turabian StyleFiguereido, Ines, Alice Paiotta, Roberta Dal Magro, Francesca Tinelli, Roberta Corti, Francesca Re, Valeria Cassina, Enrico Caneva, Francesco Nicotra, and Laura Russo. 2019. "A New Approach for Glyco-Functionalization of Collagen-Based Biomaterials" International Journal of Molecular Sciences 20, no. 7: 1747. https://doi.org/10.3390/ijms20071747
APA StyleFiguereido, I., Paiotta, A., Dal Magro, R., Tinelli, F., Corti, R., Re, F., Cassina, V., Caneva, E., Nicotra, F., & Russo, L. (2019). A New Approach for Glyco-Functionalization of Collagen-Based Biomaterials. International Journal of Molecular Sciences, 20(7), 1747. https://doi.org/10.3390/ijms20071747