The Molecular Mechanism of Vitamin E as a Bone-Protecting Agent: A Review on Current Evidence
Abstract
1. Introduction
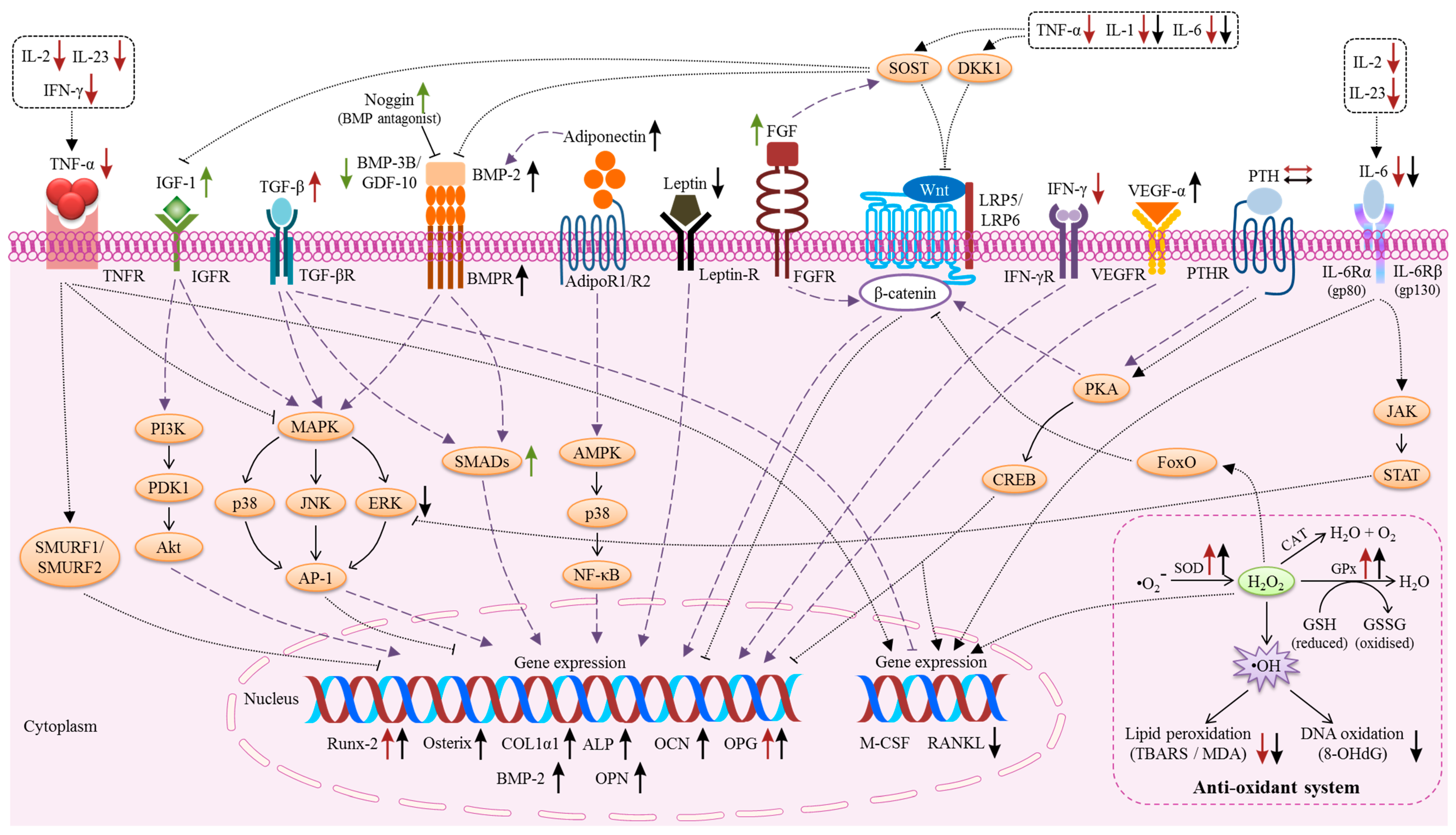
2. The Effects of Vitamin E on Bone Cells
3. Molecular Actions of Vitamin E on Bone
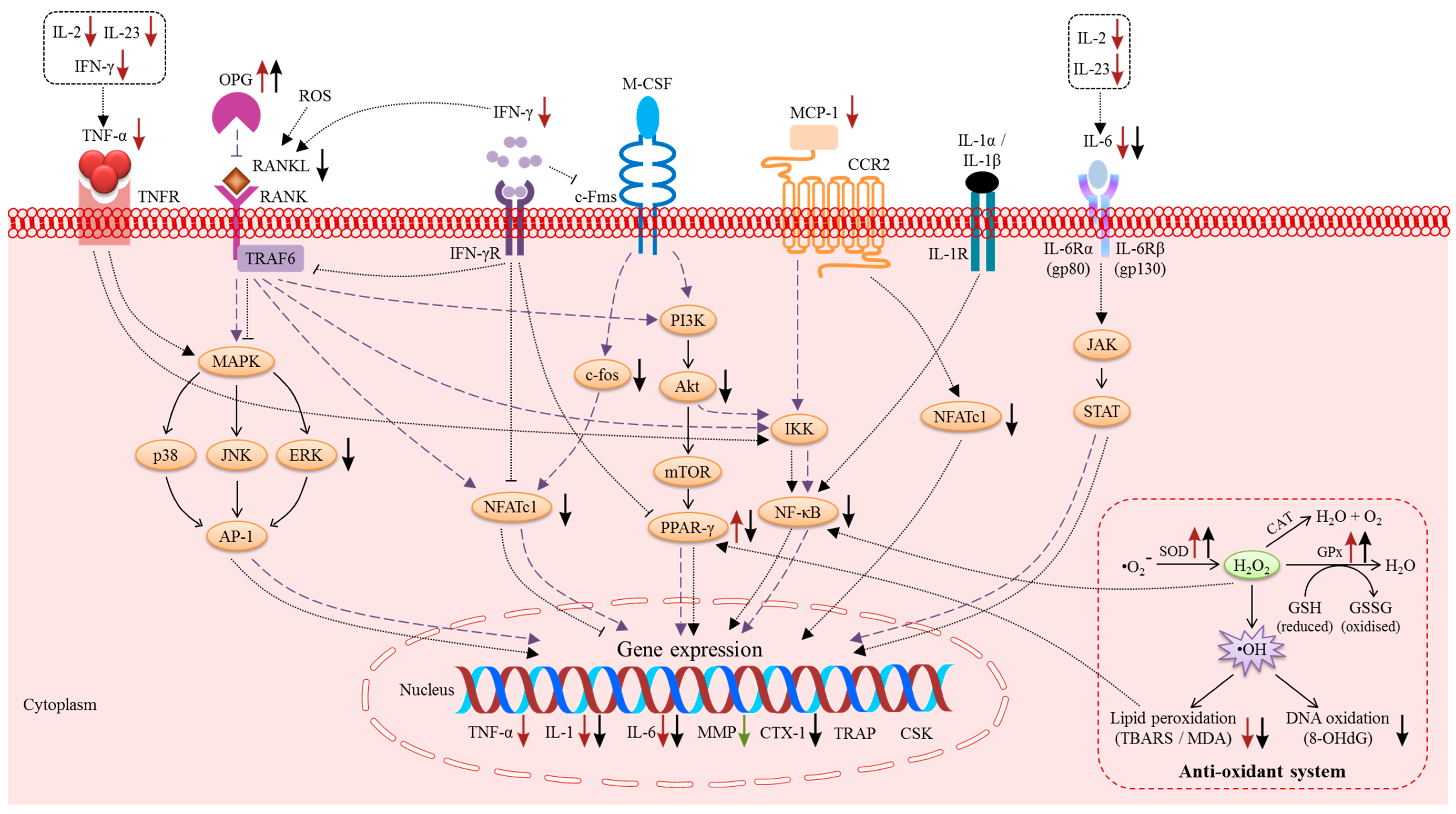
3.1. The Macrophage-Colony Stimulating Factor (M-CSF) and RANK/RANKL/OPG Pathway
3.2. Pro-Inflammatory Cytokines
3.3. Reactive Oxygen Species (ROS) and the Anti-Oxidant System
3.4. Growth Factors
3.5. Hormones
3.6. MicroRNA
4. Future Outlook and Conclusion
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 8-OHdG | 8-hydroxy-2′-deoxyguanosine |
| Akt | Protein kinase B |
| ALP | Alkaline phosphatase |
| AMPK | Adenosine monophosphate-activated protein kinase |
| AP-1 | Activator protein-1 |
| BFR | Bone formation rate |
| BMD | Bone mineral density |
| BMP | Bone morphogenetic protein |
| BMPR1B | Bone morphogenic protein receptor type 1B |
| BSP | Bone sialoprotein |
| BV | Bone volume |
| CAT | Catalase |
| c-Fos | Fos proto-oncogene |
| c-Fms | Colony-stimulating factor-1 receptor |
| COL1α1 | Collagen type 1 alpha 1 |
| CREB | cAMP-response element-binding |
| CTX | Carboxyl-terminal telopeptide of type I collagen |
| DC-STAMP | Dendritic cell-specific transmembrane protein |
| DKK1 | Dickkopf-related protein 1 |
| DNA | Deoxyribonucleic acid |
| ERK | Extracellular signal-regulated kinases |
| ES | Eroded surface |
| FGF | Fibroblast growth factor |
| FoxO | Forkhead Box O |
| FRAP | Ferric reducing ability of plasma |
| GDF-10 | Growth differentiation factor-10 |
| GPx | Glutathione peroxidase |
| GSH | Reduced glutathione |
| GSK3β | Glycogen synthase kinase-3 beta |
| H2O | Water |
| H2O2 | Hydrogen peroxide |
| HMWFGF-2 | High molecular weight isoform of fibroblast growth factor-2 |
| hs-CRP | High-sensitivity C-reactive protein |
| IFN | Interferon |
| IGF-1 | Insulin-like growth factor-1 |
| IGFR | Insulin-like growth factor-1 receptor |
| IL | Interleukin |
| IRS1 | Insulin receptor substrate 1 |
| JAK | Janus kinase |
| JNK | c-Jun N-terminal kinase |
| LMWFGF-2 | Low molecular weight isoform of fibroblast growth factor-2 |
| LRP5 | Low-density lipoprotein receptor-related protein 5 |
| MAPK | Mitogen-activated protein kinase |
| MCP-1 | Monocyte chemoattractant protein-1 |
| M-CSF | Macrophage-colony stimulating factor |
| MDA | Malondialdehyde |
| MetS | Metabolic syndrome |
| Micro-CT | Micro-computed tomography |
| mRNA | Messenger ribonucleic acid |
| MSCs | Mesenchymal stem cells |
| NFATc1 | Nuclear factor of activated T-cells cytoplasmic 1 |
| NF-κB | Nuclear factor-kappa B |
| NTX | N-terminal telopeptide |
| O2•− | Superoxide radical anion |
| O2 | Oxygen |
| Ob.N | Osteoblast number |
| OCN | Osteocalcin |
| Oc.N | Osteoclast number |
| ODF | Osteoclast differentiation factor |
| OPG | Osteoprotegerin |
| OSX | Osterix |
| P1NP | Procollagen I intact N-terminal propeptide |
| PDK1 | Phosphoinositide-dependent kinase 1 |
| PI3K | Phosphatidylinositol-3-kinase |
| PIP2 | Phosphatidylinositol-4,5-diphosphate |
| PIP3 | Phosphatidylinositol-3,4,5-trisphosaphate |
| PKA | Protein kinase A |
| PPAR-γ | Peroxisome proliferator-activated receptor-gamma |
| PTH | Parathyroid hormone |
| PYD | Pyridinoline |
| Runx-2 | Runt-related transcription factor-2 |
| Satb2 | Special AT-rich sequence-binding protein |
| SEM | Scanning electron microscope |
| SHC | Src homolog and collagen protein |
| SMAD | Suppressor of mothers against decapentaplegic |
| SMURF1 | Suppressor of mothers against decapentaplegic ubiquitylation regulatory factor |
| SOD | Superoxide dismutase |
| SOST | Sclerostin |
| SPP1 | Secreted phosphoprotein 1 |
| sRANKL | Soluble receptor activator of nuclear factor kappa-B ligand |
| STAT | Signal transducers and activators of transcription |
| T3 | Tocotrienol |
| TBARS | Thiobarbituric acid reactive substances |
| Tb.N | Trabecular number |
| Tb.Th | Trabecular thickness |
| Tcf | T cell factor |
| TF | Tocopherol |
| TGF-β | Transforming growth factor-beta |
| TNF-α | Tumour necrosis factor-alpha |
| TRAF6 | Tumour necrosis factor receptor-associated factor 6 |
| TRANCE | Tumour necrosis factor related activation-induced cytokine |
| RANK | Receptor activator of nuclear factor kappa-B |
| RANKL | Receptor activator of nuclear factor kappa-B ligand |
| ROS | Reactive oxygen species |
| VEGF | Vascular endothelial growth factor |
| Wnt | Wingless-related integration site |
References
- Karsenty, G.; Wagner, E.F. Reaching a genetic and molecular understanding of skeletal development. Dev. Cell 2002, 2, 389–406. [Google Scholar] [CrossRef]
- Kenkre, J.S.; Bassett, J. The bone remodelling cycle. Ann. Clin. Biochem. 2018, 55, 308–327. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; McDonald, J.M. Disorders of bone remodeling. Ann. Rev. Pathol. 2011, 6, 121–145. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.Y.; Ima-Nirwana, S. The effects of alpha-tocopherol on bone: A double-edged sword? Nutrients 2014, 6, 1424–1441. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.Y.; Ima-Nirwana, S. The biological effects of tocotrienol on bone: A review on evidence from rodent models. Drug Des. Devel. Ther. 2015, 9, 2049–2061. [Google Scholar] [CrossRef] [PubMed]
- Chai, S.C.; Wei, C.I.; Brummel-Smith, K.; Arjmandi, B.H. The role of vitamin E in reversing bone loss. Aging Clin. Exp. Res. 2008, 20, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Feresin, R.G.; Johnson, S.A.; Elam, M.L.; Kim, J.-S.; Khalil, D.A.; Lucas, E.A.; Smith, B.J.; Payton, M.E.; Akhter, M.P.; Arjmandi, B.H. Effects of Vitamin E on Bone Biomechanical and Histomorphometric Parameters in Ovariectomized Rats. J. Osteoporos. 2013, 2013, 9. [Google Scholar] [CrossRef]
- Fujita, K.; Iwasaki, M.; Ochi, H.; Fukuda, T.; Ma, C.; Miyamoto, T.; Takitani, K.; Negishi-Koga, T.; Sunamura, S.; Kodama, T.; et al. Vitamin E decreases bone mass by stimulating osteoclast fusion. Nat. Med. 2012, 18, 589–594. [Google Scholar] [CrossRef]
- Shuid, A.N.; Mehat, Z.; Mohamed, N.; Muhammad, N.; Soelaiman, I.N. Vitamin E exhibits bone anabolic actions in normal male rats. J. Bone Miner. Metab. 2010, 28, 149–156. [Google Scholar] [CrossRef]
- Arjmandi, B.H.; Juma, S.; Beharka, A.; Bapna, M.S.; Akhter, M.; Meydani, S.N. Vitamin E improves bone quality in the aged but not in young adult male mice. J. Nutr. Biochem. 2002, 13, 543–549. [Google Scholar] [CrossRef]
- Mehat, M.Z.; Shuid, A.N.; Mohamed, N.; Muhammad, N.; Soelaiman, I.N. Beneficial effects of vitamin E isomer supplementation on static and dynamic bone histomorphometry parameters in normal male rats. J. Bone Miner. Metab. 2010, 28, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Norazlina, M.; Ima-Nirwana, S.; Abul Gapor, M.T.; Abdul Kadir Khalid, B. Tocotrienols are needed for normal bone calcification in growing female rats. Asia. Pac. J. Clin. Nutr. 2002, 11, 194–199. [Google Scholar] [CrossRef]
- Abdul-Majeed, S.; Mohamed, N.; Soelaiman, I.N. Effects of tocotrienol and lovastatin combination on osteoblast and osteoclast activity in estrogen-deficient osteoporosis. Evid. Based Complement. Alternat. Med. 2012, 2012, 960742. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Majeed, S.; Mohamed, N.; Soelaiman, I.N. The use of delta-tocotrienol and lovastatin for anti-osteoporotic therapy. Life Sci. 2015, 125, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Ding, Y.; Peng, Y.; Wu, Y.; Fan, J.; Li, W.; Yang, R.; Yang, M.; Fu, Q. gamma-Tocotrienol protects against ovariectomy-induced bone loss via mevalonate pathway as HMG-CoA reductase inhibitor. Bone 2014, 67, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.Y.; Abdul-Majeed, S.; Mohamed, N.; Ima-Nirwana, S. The Effects of Tocotrienol and Lovastatin Co-Supplementation on Bone Dynamic Histomorphometry and Bone Morphogenetic Protein-2 Expression in Rats with Estrogen Deficiency. Nutrients 2017, 9, 143. [Google Scholar] [CrossRef] [PubMed]
- Soelaiman, I.N.; Ming, W.; Abu Bakar, R.; Hashnan, N.A.; Mohd Ali, H.; Mohamed, N.; Muhammad, N.; Shuid, A.N. Palm tocotrienol supplementation enhanced bone formation in oestrogen-deficient rats. Int. J. Endocrinol. 2012, 2012, 532862. [Google Scholar] [CrossRef]
- Chin, K.Y.; Abdul-Majeed, S.; Fozi, N.F.; Ima-Nirwana, S. Annatto tocotrienol improves indices of bone static histomorphometry in osteoporosis due to testosterone deficiency in rats. Nutrients 2014, 6, 4974–4983. [Google Scholar] [CrossRef]
- Chin, K.Y.; Ima-Nirwana, S. Effects of annatto-derived tocotrienol supplementation on osteoporosis induced by testosterone deficiency in rats. Clin. Interv. Aging 2014, 9, 1247–1259. [Google Scholar] [CrossRef]
- Chin, K.Y.; Gengatharan, D.; Mohd Nasru, F.S.; Khairussam, R.A.; Ern, S.L.; Aminuddin, S.A.; Ima-Nirwana, S. The Effects of Annatto Tocotrienol on Bone Biomechanical Strength and Bone Calcium Content in an Animal Model of Osteoporosis Due to Testosterone Deficiency. Nutrients 2016, 8, 808. [Google Scholar] [CrossRef]
- Ima-Nirwana, S.; Kiftiah, A.; Zainal, A.; Norazlina, M.; Gapor, M.; Khalid, B. Palm vitamin E prevents osteoporosis in orchidectomized growing male rats. Nat. Prod. Sci. 2000, 6, 155–160. [Google Scholar]
- Hermizi, H.; Faizah, O.; Ima-Nirwana, S.; Ahmad Nazrun, S.; Norazlina, M. Beneficial effects of tocotrienol and tocopherol on bone histomorphometric parameters in sprague-dawley male rats after nicotine cessation. Calcif. Tissue Int. 2009, 84, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Norazlina, M.; Hermizi, H.; Faizah, O.; Nazrun, A.S.; Norliza, M.; Ima-Nirwana, S. Vitamin E reversed nicotine-induced toxic effects on bone biochemical markers in male rats. Arch. Med. Sci. 2010, 6, 505–512. [Google Scholar] [CrossRef]
- Norazlina, M.; Maizatul-Neza, J.; Azarina, A.; Nazrun, A.S.; Norliza, M.; Ima-Nirwana, S. Effects of vitamin E on receptor activator of nuclear factor kappa B ligand (RANKL) and osteoprotegerin (OPG) in rats treated with nicotine. Med. J. Malays. 2010, 65, 14–17. [Google Scholar]
- Zakaria, S.; Mat-Husain, S.Z.; Ying-Hwey, K.; Xin-Kai, K.; Mohd-Badawi, A.; Abd-Ghani, N.A.; Aziz, M.A.; Mohamed, N. Vitamin E improved bone strength and bone minerals in male rats given alcohol. Iran J. Basic Med. Sci. 2017, 20, 1360–1367. [Google Scholar] [PubMed]
- Ahmad, N.S.; Khalid, B.A.; Luke, D.A.; Ima Nirwana, S. Tocotrienol offers better protection than tocopherol from free radical-induced damage of rat bone. Clin. Exp. Pharmacol. Physiol. 2005, 32, 761–770. [Google Scholar] [CrossRef]
- Ima-Nirwana, S.; Suhaniza, S. Effects of tocopherols and tocotrienols on body composition and bone calcium content in adrenalectomized rats replaced with dexamethasone. J. Med. Food 2004, 7, 45–51. [Google Scholar] [CrossRef]
- Ima-Nirwana, S.; Fakhrurazi, H. Palm vitamin E protects bone against dexamethasone-induced osteoporosis in male rats. Med. J. Malays. 2002, 57, 136–144. [Google Scholar]
- Mohamad, N.V.; Ima-Nirwana, S.; Chin, K.Y. Effect of tocotrienol from Bixa orellana (annatto) on bone microstructure, calcium content, and biomechanical strength in a model of male osteoporosis induced by buserelin. Drug Des. Devel. Ther. 2018, 12, 555–564. [Google Scholar] [CrossRef]
- Mohamad, N.V.; Soelaiman, I.N.; Chin, K.Y. Effects of tocotrienol from Bixa orellana (annatto) on bone histomorphometry in a male osteoporosis model induced by buserelin. Biomed. Pharmacother. 2018, 103, 453–462. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.Y.; Suhaimi, F.H.; Ahmad, F.; Ima-Nirwana, S. The Effects of Vitamin E from Elaeis guineensis (Oil Palm) in a Rat Model of Bone Loss Due to Metabolic Syndrome. Int. J. Environ. Res. Public Health 2018, 15, 1828. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.K.; Chin, K.Y.; Suhaimi, F.H.; Ahmad, F.; Ima-Nirwana, S. Exploring the potential of tocotrienol from Bixa orellana as a single agent targeting metabolic syndrome and bone loss. Bone 2018, 116, 8–21. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.-Y.; Suhaimi, F.H.; Ahmad, F.; Ima-Nirwana, S. The effects of palm tocotrienol on metabolic syndrome and bone loss in male rats induced by high-carbohydrate high-fat diet. J. Funct. Foods 2018, 44, 246–254. [Google Scholar] [CrossRef]
- Muhammad, N.; Luke, D.A.; Shuid, A.N.; Mohamed, N.; Soelaiman, I.N. Two different isomers of vitamin e prevent bone loss in postmenopausal osteoporosis rat model. Evid. Based Complement. Alternat. Med. 2012, 2012, 161527. [Google Scholar] [CrossRef]
- Norazlina, M.; Ima-Nirwana, S.; Gapor, M.T.; Khalid, B.A. Palm vitamin E is comparable to alpha-tocopherol in maintaining bone mineral density in ovariectomised female rats. Exp. Clin. Endocrinol. Diabetes 2000, 108, 305–310. [Google Scholar] [CrossRef]
- Soeta, S.; Higuchi, M.; Yoshimura, I.; Itoh, R.; Kimura, N.; Aamsaki, H. Effects of vitamin E on the osteoblast differentiation. J. Vet. Med. Sci. 2010, 72, 951–957. [Google Scholar] [CrossRef]
- Ahn, K.H.; Jung, H.K.; Jung, S.E.; Yi, K.W.; Park, H.T.; Shin, J.H.; Kim, Y.T.; Hur, J.Y.; Kim, S.H.; Kim, T. Microarray analysis of gene expression during differentiation of human mesenchymal stem cells treated with vitamin E in vitro into osteoblasts. Kor. J. Bone Metab. 2011, 18, 23–32. [Google Scholar]
- Urban, K.; Hohling, H.J.; Luttenberg, B.; Szuwart, T.; Plate, U. An in vitro study of osteoblast vitality influenced by the vitamins C and E. Head Face Med. 2012, 8, 25. [Google Scholar] [CrossRef]
- Kim, H.-N.; Lee, J.-H.; Jin, W.J.; Lee, Z.H. α-Tocopheryl Succinate Inhibits Osteoclast Formation by Suppressing Receptor Activator of Nuclear Factor-kappaB Ligand (RANKL) Expression and Bone Resorption. J. Bone Metab. 2012, 19, 111–120. [Google Scholar] [CrossRef]
- Johnson, S.A.; Feresin, R.G.; Soung do, Y.; Elam, M.L.; Arjmandi, B.H. Vitamin E suppresses ex vivo osteoclastogenesis in ovariectomized rats. Food Funct. 2016, 7, 1628–1633. [Google Scholar] [CrossRef]
- Abd Manan, N.; Mohamed, N.; Shuid, A.N. Effects of Low-Dose versus High-Dose gamma-Tocotrienol on the Bone Cells Exposed to the Hydrogen Peroxide-Induced Oxidative Stress and Apoptosis. Evid. Based Complement. Alternat. Med. 2012, 2012, 680834. [Google Scholar] [CrossRef]
- Xu, W.; He, P.; He, S.; Cui, P.; Mi, Y.; Yang, Y.; Li, Y.; Zhou, S. Gamma-Tocotrienol Stimulates the Proliferation, Differentiation, and Mineralization in Osteoblastic MC3T3-E1 Cells. J. Chem. 2018, 2018, 9. [Google Scholar] [CrossRef]
- Wan Hasan, W.N.; Abd Ghafar, N.; Chin, K.Y.; Ima-Nirwana, S. Annatto-derived tocotrienol stimulates osteogenic activity in preosteoblastic MC3T3-E1 cells: A temporal sequential study. Drug Des. Devel. Ther. 2018, 12, 1715–1726. [Google Scholar] [CrossRef]
- Brooks, R.; Kalia, P.; Ireland, D.C.; Beeton, C.; Rushton, N. Direct inhibition of osteoclast formation and activity by the vitamin E isomer gamma-tocotrienol. Int. J. Vitam. Nutr. Res. 2011, 81, 358–367. [Google Scholar] [CrossRef]
- Song, L.-S.; Zhang, Z.-X.; Wang, Y.; Liu, Y.; Zhang, R.; Lu, L.-j. Effects of nano-emulsion preparations of tocopherols and tocotrienols on oxidative stress and osteoblast differentiation. Arch. Biol. Sci. 2017, 69, 149–156. [Google Scholar] [CrossRef]
- Soelaiman, I.N.; Yong, C.K.; Suhana, E.; Ramli, M. Effects of individual tocotrienol isomers on bone cells in a 3D cell culture system. J. Oil Palm Environ. Health 2018, 9, 57–63. [Google Scholar]
- Lacey, D.; Timms, E.; Tan, H.-L.; Kelley, M.; Dunstan, C.; Burgess, T.; Elliott, R.; Colombero, A.; Elliott, G.; Scully, S. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998, 93, 165–176. [Google Scholar] [CrossRef]
- Yasuda, H.; Shima, N.; Nakagawa, N.; Yamaguchi, K.; Kinosaki, M.; Mochizuki, S.-i.; Tomoyasu, A.; Yano, K.; Goto, M.; Murakami, A. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc. Natl. Acad. Sci. USA 1998, 95, 3597–3602. [Google Scholar] [CrossRef]
- Arai, F.; Miyamoto, T.; Ohneda, O.; Inada, T.; Sudo, T.; Brasel, K.; Miyata, T.; Anderson, D.M.; Suda, T. Commitment and differentiation of osteoclast precursor cells by the sequential expression of c-Fms and receptor activator of nuclear factor κB (RANK) receptors. J. Exp. Med. 1999, 190, 1741–1754. [Google Scholar] [CrossRef]
- Hsu, H.; Lacey, D.L.; Dunstan, C.R.; Solovyev, I.; Colombero, A.; Timms, E.; Tan, H.-L.; Elliott, G.; Kelley, M.J.; Sarosi, I. Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc. Natl. Acad. Sci. USA 1999, 96, 3540–3545. [Google Scholar] [CrossRef]
- Kong, Y.Y.; Yoshida, H.; Sarosi, I.; Tan, H.L.; Timms, E.; Capparelli, C.; Morony, S.; Oliveira-dos-Santos, A.J.; Van, G.; Itie, A.; et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 1999, 397, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sarosi, I.; Yan, X.-Q.; Morony, S.; Capparelli, C.; Tan, H.-L.; McCabe, S.; Elliott, R.; Scully, S.; Van, G. RANK is the intrinsic hematopoietic cell surface receptor that controls osteoclastogenesis and regulation of bone mass and calcium metabolism. Proc. Natl. Acad. Sci. USA 2000, 97, 1566–1571. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.E.; Ralston, S.H.; Marken, J.; Bell, C.; MacPherson, H.; Wallace, R.G.; van Hul, W.; Whyte, M.P.; Nakatsuka, K.; Hovy, L. Mutations in TNFRSF11A, affecting the signal peptide of RANK, cause familial expansile osteolysis. Nat. Genet. 2000, 24, 45. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.H.; Nakashima, T.; Sanchez, O.H.; Kozieradzki, I.; Komarova, S.V.; Sarosi, I.; Morony, S.; Rubin, E.; Sarao, R.; Hojilla, C.V.; et al. Regulation of cancer cell migration and bone metastasis by RANKL. Nature 2006, 440, 692–696. [Google Scholar] [CrossRef] [PubMed]
- Hanada, R.; Hanada, T.; Sigl, V.; Schramek, D.; Penninger, J.M. RANKL/RANK—beyond bones. J. Mol. Med. 2011, 89, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Yao, Z.; Li, F.; Zhang, Q.; Badell, I.R.; Schwarz, E.M.; Takeshita, S.; Wagner, E.F.; Noda, M.; Matsuo, K. NF-κB p50 and p52 regulate receptor activator of NF-κB ligand (RANKL) and tumor necrosis factor-induced osteoclast precursor differentiation by activating c-Fos and NFATc1. J. Biol. Chem. 2007, 282, 18245–18253. [Google Scholar] [CrossRef]
- Takayanagi, H.; Kim, S.; Koga, T.; Nishina, H.; Isshiki, M.; Yoshida, H.; Saiura, A.; Isobe, M.; Yokochi, T.; Inoue, J.-i. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell 2002, 3, 889–901. [Google Scholar] [CrossRef]
- Asagiri, M.; Sato, K.; Usami, T.; Ochi, S.; Nishina, H.; Yoshida, H.; Morita, I.; Wagner, E.F.; Mak, T.W.; Serfling, E. Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J. Exp. Med. 2005, 202, 1261–1269. [Google Scholar] [CrossRef]
- Pettit, A.R.; Ji, H.; von Stechow, D.; Müller, R.; Goldring, S.R.; Choi, Y.; Benoist, C.; Gravallese, E.M. TRANCE/RANKL knockout mice are protected from bone erosion in a serum transfer model of arthritis. Am. J. Pathol. 2001, 159, 1689–1699. [Google Scholar] [CrossRef]
- Fuller, K.; Wong, B.; Fox, S.; Choi, Y.; Chambers, T.J. TRANCE is necessary and sufficient for osteoblast-mediated activation of bone resorption in osteoclasts. J. Exp. Med. 1998, 188, 997–1001. [Google Scholar] [CrossRef]
- Lacey, D.L.; Tan, H.L.; Lu, J.; Kaufman, S.; Van, G.; Qiu, W.; Rattan, A.; Scully, S.; Fletcher, F.; Juan, T. Osteoprotegerin ligand modulates murine osteoclast survival in vitro and in vivo. Am. J. Pathol. 2000, 157, 435–448. [Google Scholar] [CrossRef]
- Theoleyre, S.; Wittrant, Y.; Tat, S.K.; Fortun, Y.; Redini, F.; Heymann, D. The molecular triad OPG/RANK/RANKL: Involvement in the orchestration of pathophysiological bone remodeling. Cytokine Ggrowth F. Rev. 2004, 15, 457–475. [Google Scholar] [CrossRef]
- Kearns, A.E.; Khosla, S.; Kostenuik, P.J. Receptor activator of nuclear factor kappaB ligand and osteoprotegerin regulation of bone remodeling in health and disease. Endocr. Rev. 2008, 29, 155–192. [Google Scholar] [CrossRef] [PubMed]
- Simonet, W.S.; Lacey, D.L.; Dunstan, C.R.; Kelley, M.; Chang, M.S.; Luthy, R.; Nguyen, H.Q.; Wooden, S.; Bennett, L.; Boone, T.; et al. Osteoprotegerin: A novel secreted protein involved in the regulation of bone density. Cell 1997, 89, 309–319. [Google Scholar] [CrossRef]
- Bucay, N.; Sarosi, I.; Dunstan, C.R.; Morony, S.; Tarpley, J.; Capparelli, C.; Scully, S.; Tan, H.L.; Xu, W.; Lacey, D.L.; et al. Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes. Dev. 1998, 12, 1260–1268. [Google Scholar] [CrossRef]
- Bolon, B.; Carter, C.; Daris, M.; Morony, S.; Capparelli, C.; Hsieh, A.; Mao, M.; Kostenuik, P.; Dunstan, C.R.; Lacey, D.L. Adenoviral delivery of osteoprotegerin ameliorates bone resorption in a mouse ovariectomy model of osteoporosis. Mol. Ther. 2001, 3, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Capparelli, C.; Morony, S.; Warmington, K.; Adamu, S.; Lacey, D.; Dunstan, C.R.; Stouch, B.; Martin, S.; Kostenuik, P.J. Sustained antiresorptive effects after a single treatment with human recombinant osteoprotegerin (OPG): A pharmacodynamic and pharmacokinetic analysis in rats. J. Bone Miner. Res. 2003, 18, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Bekker, P.J.; Holloway, D.; Nakanishi, A.; Arrighi, M.; Leese, P.T.; Dunstan, C.R. The effect of a single dose of osteoprotegerin in postmenopausal women. J. Bone Miner. Res. 2001, 16, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, H.N.; Yang, D.; Jung, K.; Kim, H.M.; Kim, H.H.; Ha, H.; Lee, Z.H. Trolox prevents osteoclastogenesis by suppressing RANKL expression and signaling. J. Biol. Chem. 2009, 284, 13725–13734. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.; Lee, J.H.; Kim, H.N.; Lee, Z.H. alpha-Tocotrienol inhibits osteoclastic bone resorption by suppressing RANKL expression and signaling and bone resorbing activity. Biochem. Biophys. Res. Commun. 2011, 406, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.L.; Yang, S.; Tomison, M.D.; Romero, A.W.; Felton, C.K.; Mo, H. Tocotrienol supplementation suppressed bone resorption and oxidative stress in postmenopausal osteopenic women: A 12-week randomized double-blinded placebo-controlled trial. Osteoporos. Int. 2018, 29, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.C.; Duthie, G.G.; Aucott, L.S.; Macdonald, H.M. Vitamin E homologues alpha- and gamma-tocopherol are not associated with bone turnover markers or bone mineral density in peri-menopausal and post-menopausal women. Osteoporos. Int. 2016, 27, 2281–2290. [Google Scholar] [CrossRef] [PubMed]
- Redlich, K.; Smolen, J.S. Inflammatory bone loss: Pathogenesis and therapeutic intervention. Nat. Rev. Drug Discov. 2012, 11, 234–250. [Google Scholar] [CrossRef]
- Lacativa, P.G.S.; Farias, M.L.F.d. Osteoporosis and inflammation. Arq. Bras. Endocrinol. Metab. 2010, 54, 123–132. [Google Scholar] [CrossRef]
- AlFadhli, S. The interleukin-23/interleukin-17 axis and the role of Treg/Th17 cells in rheumatoid arthritis and joint destruction. OA Arthr. 2013, 1, 5–11. [Google Scholar]
- Khovidhunkit, W.; Epstein, S. Cytokines and Osteoporosis. In Growth Factors and Cytokines in Health and Disease; Leroith, D., Bondy, C., Eds.; Elsevier Inc.: Atlanta, GA, USA, 1997; pp. 459–497. [Google Scholar]
- Vidal, C.; Bermeo, S.; Li, W.; Huang, D.; Kremer, R.; Duque, G. Interferon gamma inhibits adipogenesis in vitro and prevents marrow fat infiltration in oophorectomized mice. Stem Cells 2012, 30, 1042–1048. [Google Scholar] [CrossRef]
- Maruhashi, T.; Kaifu, T.; Yabe, R.; Seno, A.; Chung, S.H.; Fujikado, N.; Iwakura, Y. DCIR maintains bone homeostasis by regulating IFN-gamma production in T cells. J. Immunol. 2015, 194, 5681–5691. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, J.; Shi, Z.; Jules, J.; Xu, D.; Luo, S.; Wei, S.; Feng, X. Molecular mechanisms of the biphasic effects of interferon-γ on osteoclastogenesis. J. Interferon Cytokine Res. 2012, 32, 34–45. [Google Scholar] [CrossRef]
- Xiong, Q.; Zhang, L.; Ge, W.; Tang, P. The roles of interferons in osteoclasts and osteoclastogenesis. Joint Bone Spine 2016, 83, 276–281. [Google Scholar] [CrossRef]
- Kohara, H.; Kitaura, H.; Fujimura, Y.; Yoshimatsu, M.; Morita, Y.; Eguchi, T.; Masuyama, R.; Yoshida, N. IFN-gamma directly inhibits TNF-alpha-induced osteoclastogenesis in vitro and in vivo and induces apoptosis mediated by Fas/Fas ligand interactions. Immunol. Lett. 2011, 137, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Tian, L.; Luo, G.; Yu, X. Interferon-Gamma-Mediated Osteoimmunology. Front. Immunol. 2018, 9, 1508. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Grassi, F.; Ryan, M.R.; Terauchi, M.; Page, K.; Yang, X.; Weitzmann, M.N.; Pacifici, R. IFN-gamma stimulates osteoclast formation and bone loss in vivo via antigen-driven T cell activation. J. Clin. Investig. 2007, 117, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Lee, M.S.; Lee, C.H.; Kim, H.Y.; Chae, S.U.; Kwak, H.B.; Oh, J. Effect of interferon-gamma on the fusion of mononuclear osteoclasts into bone-resorbing osteoclasts. BMB Rep. 2012, 45, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interferon Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.; Ninomiya, K.; Sonoda, K.H.; Miyauchi, Y.; Hoshi, H.; Iwasaki, R.; Miyamoto, H.; Yoshida, S.; Sato, Y.; Morioka, H.; et al. MCP-1 expressed by osteoclasts stimulates osteoclastogenesis in an autocrine/paracrine manner. Biochem. Biophys. Res. Commun. 2009, 383, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Yagi, M.; Miyamoto, T.; Sawatani, Y.; Iwamoto, K.; Hosogane, N.; Fujita, N.; Morita, K.; Ninomiya, K.; Suzuki, T.; Miyamoto, K.; et al. DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J. Exp. Med. 2005, 202, 345–351. [Google Scholar] [CrossRef]
- Muhammad, N.; Luke, D.A.; Shuid, A.N.; Mohamed, N.; Soelaiman, I.N. Tocotrienol supplementation in postmenopausal osteoporosis: Evidence from a laboratory study. Clinics 2013, 68, 1338–1343. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.Y.; Suhaimi, F.H.; Ahmad, F.; Ima-Nirwana, S. Effects of metabolic syndrome on bone mineral density, histomorphometry and remodelling markers in male rats. PLoS ONE 2018, 13, e0192416. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.Y.; Suhaimi, F.H.; Ahmad, F.; Jamil, N.A.; Ima-Nirwana, S. Osteoporosis is associated with metabolic syndrome induced by high-carbohydrate high-fat diet in a rat model. Biomed. Pharmacother. 2018, 98, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.L.; Kaur, G.; Wanders, D.; Sharma, S.; Tomison, M.D.; Ramalingam, L.; Chung, E.; Moustaid-Moussa, N.; Mo, H.; Dufour, J.M. Annatto-extracted tocotrienols improve glucose homeostasis and bone properties in high-fat diet-induced type 2 diabetic mice by decreasing the inflammatory response. Sci. Rep. 2018, 8, 11377. [Google Scholar] [CrossRef] [PubMed]
- Norazlina, M.; Lee, P.L.; Lukman, H.I.; Nazrun, A.S.; Ima-Nirwana, S. Effects of vitamin E supplementation on bone metabolism in nicotine-treated rats. Singap. Med. J. 2007, 48, 195–199. [Google Scholar]
- Roberts, C.K.; Sindhu, K.K. Oxidative stress and metabolic syndrome. Life Sci. 2009, 84, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Domazetovic, V.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Oxidative stress in bone remodeling: Role of antioxidants. Clin. C. Miner. Bone Metab. 2017, 14, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Baek, K.H.; Oh, K.W.; Lee, W.Y.; Lee, S.S.; Kim, M.K.; Kwon, H.S.; Rhee, E.J.; Han, J.H.; Song, K.H.; Cha, B.Y.; et al. Association of oxidative stress with postmenopausal osteoporosis and the effects of hydrogen peroxide on osteoclast formation in human bone marrow cell cultures. Calcif. Tissue Int. 2010, 87, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.; Han, L.; Martin-Millan, M.; Plotkin, L.I.; Stewart, S.A.; Roberson, P.K.; Kousteni, S.; O’Brien, C.A.; Bellido, T.; Parfitt, A.M.; et al. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J. Biol. Chem. 2007, 282, 27285–27297. [Google Scholar] [CrossRef]
- Almeida, M.; Han, L.; Martin-Millan, M.; O’Brien, C.A.; Manolagas, S.C. Oxidative stress antagonizes Wnt signaling in osteoblast precursors by diverting beta-catenin from T cell factor- to forkhead box O-mediated transcription. J. Biol. Chem. 2007, 282, 27298–27305. [Google Scholar] [CrossRef]
- Storz, P. Forkhead homeobox type O transcription factors in the responses to oxidative stress. Antioxid. Redox Signal 2011, 14, 593–605. [Google Scholar] [CrossRef]
- Ferre, P. The biology of peroxisome proliferator-activated receptors: Relationship with lipid metabolism and insulin sensitivity. Diabetes 2004, 53, S43–S50. [Google Scholar] [CrossRef]
- Schafer, M.; Werner, S. Oxidative stress in normal and impaired wound repair. Pharmacol. Res. 2008, 58, 165–171. [Google Scholar] [CrossRef]
- Ostman, B.; Michaelsson, K.; Helmersson, J.; Byberg, L.; Gedeborg, R.; Melhus, H.; Basu, S. Oxidative stress and bone mineral density in elderly men: Antioxidant activity of alpha-tocopherol. Free Radic. Biol. Med. 2009, 47, 668–673. [Google Scholar] [CrossRef]
- Ruiz-Ramos, M.; Vargas, L.A.; Fortoul Van der Goes, T.I.; Cervantes-Sandoval, A.; Mendoza-Nunez, V.M. Supplementation of ascorbic acid and alpha-tocopherol is useful to preventing bone loss linked to oxidative stress in elderly. J. Nutr. Health Aging 2010, 14, 467–472. [Google Scholar] [CrossRef]
- Xu, H.; Watkins, B.A.; Seifert, M.F. Vitamin E stimulates trabecular bone formation and alters epiphyseal cartilage morphometry. Calcif. Tissue Int. 1995, 57, 293–300. [Google Scholar] [CrossRef]
- Smith, B.J.; Lucas, E.A.; Turner, R.T.; Evans, G.L.; Lerner, M.R.; Brackett, D.J.; Stoecker, B.J.; Arjmandi, B.H. Vitamin E provides protection for bone in mature hindlimb unloaded male rats. Calcif. Tissue Int. 2005, 76, 272–279. [Google Scholar] [CrossRef]
- Turk, C.; Halici, M.; Guney, A.; Akgun, H.; Sahin, V.; Muhtaroglu, S. Promotion of fracture healing by vitamin E in rats. J. Int. Med. Res. 2004, 32, 507–512. [Google Scholar] [CrossRef]
- Shuid, A.N.; Mohamad, S.; Muhammad, N.; Fadzilah, F.M.; Mokhtar, S.A.; Mohamed, N.; Soelaiman, I.N. Effects of alpha-tocopherol on the early phase of osteoporotic fracture healing. J. Orthop. Res. 2011, 29, 1732–1738. [Google Scholar] [CrossRef]
- Maniam, S.; Mohamed, N.; Shuid, A.N.; Soelaiman, I.N. Palm tocotrienol exerted better antioxidant activities in bone than alpha-tocopherol. Basic Clin. Pharmacol. Toxicol. 2008, 103, 55–60. [Google Scholar] [CrossRef]
- Nazrun, A.; Khairunnur, A.; Norliza, M.; Norazlina, M.; Nirwana, I. Effects of palm tocotrienols on oxidative stress and bone strength in ovariectomised rats. Med. Health 2008, 3, 247–255. [Google Scholar]
- Devescovi, V.; Leonardi, E.; Ciapetti, G.; Cenni, E. Growth factors in bone repair. Chir. Organi. Mov. 2008, 92, 161–168. [Google Scholar] [CrossRef]
- Lieberman, J.R.; Daluiski, A.; Einhorn, T.A. The Role of Growth Factors in the Repair of Bone: Biology and Clinical Applications. JBJS 2002, 84, 1032–1044. [Google Scholar] [CrossRef]
- Ibrahim, N.; Mohamed, N.; Soelaiman, I.N.; Shuid, A.N. The Effects of Targeted Deliveries of Lovastatin and Tocotrienol on Ossification-Related Gene Expressions in Fracture Healing in an Osteoporosis Rat Model. Int. J. Environ. Res. Public Health 2015, 12, 12958–12976. [Google Scholar] [CrossRef] [PubMed]
- Abukhadir, S.S.A.; Mohamed, N.; Makpol, S.; Muhammad, N. Effects of Palm Vitamin E on Bone-Formation-Related Gene Expression in Nicotine-Treated Rats. Evid. Based Complement. Alternat. Med. 2012, 2012, 5. [Google Scholar] [CrossRef] [PubMed]
- Tennant, K.G.; Leonard, S.W.; Wong, C.P.; Iwaniec, U.T.; Turner, R.T.; Traber, M.G. High-Dietary Alpha-Tocopherol or Mixed Tocotrienols Have No Effect on Bone Mass, Density, or Turnover in Male Rats During Skeletal Maturation. J. Med. Food 2017, 20, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Deng, C.; Li, Y.P. TGF-beta and BMP signaling in osteoblast differentiation and bone formation. Int. J. Biol. Sci. 2012, 8, 272–288. [Google Scholar] [CrossRef]
- Wu, M.; Chen, G.; Li, Y.P. TGF-beta and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016, 4, 16009. [Google Scholar] [CrossRef] [PubMed]
- Cho, T.J.; Gerstenfeld, L.C.; Einhorn, T.A. Differential temporal expression of members of the transforming growth factor beta superfamily during murine fracture healing. J. Bone Miner. Res. 2002, 17, 513–520. [Google Scholar] [CrossRef]
- Mundy, G.R. Regulation of bone formation by bone morphogenetic proteins and other growth factors. Clin. Orthop. Relat. Res. 1996, 324, 24–28. [Google Scholar] [CrossRef]
- Hagihara, M.; Endo, M.; Hata, K.; Higuchi, C.; Takaoka, K.; Yoshikawa, H.; Yamashita, T. Neogenin, a receptor for bone morphogenetic proteins. J. Biol. Chem. 2011, 286, 5157–5165. [Google Scholar] [CrossRef]
- Burridge, K.; Chrzanowska-Wodnicka, M. Focal adhesions, contractility, and signaling. Ann. Rev. Cell Dev. Biol. 1996, 12, 463–518. [Google Scholar] [CrossRef]
- Zigmond, S.H. Signal transduction and actin filament organization. Curr. Opin. Cell Biol. 1996, 8, 66–73. [Google Scholar] [CrossRef]
- Ibrahim, N.; Mohamed, N.; Shuid, A.N. Update on statins: Hope for osteoporotic fracture healing treatment. Curr. Drug Targets 2013, 14, 1524–1532. [Google Scholar] [CrossRef]
- Coxon, F.P.; Helfrich, M.H.; Van’t Hof, R.; Sebti, S.; Ralston, S.H.; Hamilton, A.; Rogers, M.J. Protein geranylgeranylation is required for osteoclast formation, function, and survival: Inhibition by bisphosphonates and GGTI-298. J. Bone Miner. Res. 2000, 15, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Van beek, E.; Lowik, C.; van der Pluijm, G.; Papapoulos, S. The role of geranylgeranylation in bone resorption and its suppression by bisphosphonates in fetal bone explants in vitro: A clue to the mechanism of action of nitrogen-containing bisphosphonates. J. Bone Miner. Res. 1999, 14, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.B.; Li, Y.; Schneider, A.; Yu, W.; Rajendren, G.; Iqbal, J.; Yamamoto, M.; Alam, M.; Brunet, L.J.; Blair, H.C.; et al. Impaired osteoblastic differentiation, reduced bone formation, and severe osteoporosis in noggin-overexpressing mice. J. Clin. Investig. 2003, 112, 924–934. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.A. Vascular endothelial growth factor, a potent and selective angiogenic agent. J. Biol. Chem. 1996, 271, 603–606. [Google Scholar] [CrossRef]
- Ferrara, N.; Gerber, H.P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Deckers, M.M.; Karperien, M.; van der Bent, C.; Yamashita, T.; Papapoulos, S.E.; Lowik, C.W. Expression of vascular endothelial growth factors and their receptors during osteoblast differentiation. Endocrinology 2000, 141, 1667–1674. [Google Scholar] [CrossRef] [PubMed]
- Street, J.; Bao, M.; de Guzman, L.; Bunting, S.; Peale, F.V.; Ferrara, N.; Steinmetz, H.; Hoeffel, J.; Cleland, J.L.; Daugherty, A.; et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc. Natl. Acad. Sci. USA 2002, 99, 9656–9661. [Google Scholar] [CrossRef]
- Deckers, M.M.; van Bezooijen, R.L.; van der Horst, G.; Hoogendam, J.; van Der Bent, C.; Papapoulos, S.E.; Lowik, C.W. Bone morphogenetic proteins stimulate angiogenesis through osteoblast-derived vascular endothelial growth factor A. Endocrinology 2002, 143, 1545–1553. [Google Scholar] [CrossRef]
- Kempen, D.H.; Lu, L.; Heijink, A.; Hefferan, T.E.; Creemers, L.B.; Maran, A.; Yaszemski, M.J.; Dhert, W.J. Effect of local sequential VEGF and BMP-2 delivery on ectopic and orthotopic bone regeneration. Biomaterials 2009, 30, 2816–2825. [Google Scholar] [CrossRef]
- Gospodarowicz, D. Fibroblast growth factor. Chemical structure and biologic function. Clin. Orthop. Relat. Res. 1990, 231–248. [Google Scholar]
- Sorensen, V.; Nilsen, T.; Wiedlocha, A. Functional diversity of FGF-2 isoforms by intracellular sorting. Bioessays 2006, 28, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Tensho, K.; Nakaya, H.; Nawata, M.; Okabe, T.; Wakitani, S. Low dose fibroblast growth factor-2 (FGF-2) enhances bone morphogenetic protein-2 (BMP-2)-induced ectopic bone formation in mice. Bone 2005, 36, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Fei, Y.; Xiao, L.; Doetschman, T.; Coffin, D.J.; Hurley, M.M. Fibroblast growth factor 2 stimulation of osteoblast differentiation and bone formation is mediated by modulation of the Wnt signaling pathway. J. Biol. Chem. 2011, 286, 40575–40583. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.Y.; Ima-Nirwana, S.; Mohamed, I.N.; Hanapi Johari, M.; Ahmad, F.; Mohamed Ramli, E.S.; Wan Ngah, W.Z. Insulin-like growth factor-1 is a mediator of age-related decline of bone health status in men. Aging Male 2014, 17, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.C.; Zang, H.Y.; Guo, L.X.; Xue, H.B.; Liu, X.D.; Bai, Y.B.; Ma, Y.Z. The PI3K/AKT cell signaling pathway is involved in regulation of osteoporosis. J. Recept Signal Transduct. Res. 2015, 35, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Kim, M.-G.; Leem, K.-H. Osteogenic Activity of Collagen Peptide via ERK/MAPK Pathway Mediated Boosting of Collagen Synthesis and Its Therapeutic Efficacy in Osteoporotic Bone by Back-Scattered Electron Imaging and Microarchitecture Analysis. Molecules 2013, 18, 15474–15489. [Google Scholar] [CrossRef] [PubMed]
- Nakasaki, M.; Yoshioka, K.; Miyamoto, Y.; Sasaki, T.; Yoshikawa, H.; Itoh, K. IGF-I secreted by osteoblasts acts as a potent chemotactic factor for osteoblasts. Bone 2008, 43, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Gensure, R.C.; Gardella, T.J.; Juppner, H. Parathyroid hormone and parathyroid hormone-related peptide, and their receptors. Biochem. Biophys. Res. Commun. 2005, 328, 666–678. [Google Scholar] [CrossRef] [PubMed]
- Potts, J.T. Parathyroid hormone: Past and present. J. Endocrinol. 2005, 187, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Jilka, R.L.; O’Brien, C.A.; Bartell, S.M.; Weinstein, R.S.; Manolagas, S.C. Continuous elevation of PTH increases the number of osteoblasts via both osteoclast-dependent and -independent mechanisms. J. Bone Miner. Res. 2010, 25, 2427–2437. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.C.; Sakata, T.; Pfleger, L.L.; Bencsik, M.; Halloran, B.P.; Bikle, D.D.; Nissenson, R.A. PTH differentially regulates expression of RANKL and OPG. J. Bone Miner. Res. 2004, 19, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Jilka, R.L.; Manolagas, S.C.; O’Brien, C.A. Parathyroid hormone stimulates receptor activator of NFkappa B ligand and inhibits osteoprotegerin expression via protein kinase A activation of cAMP-response element-binding protein. J. Biol. Chem. 2002, 277, 48868–48875. [Google Scholar] [CrossRef]
- Li, X.; Qin, L.; Bergenstock, M.; Bevelock, L.M.; Novack, D.V.; Partridge, N.C. Parathyroid hormone stimulates osteoblastic expression of MCP-1 to recruit and increase the fusion of pre/osteoclasts. J. Biol. Chem. 2007, 282, 33098–33106. [Google Scholar] [CrossRef] [PubMed]
- Ogura, K.; Iimura, T.; Makino, Y.; Sugie-Oya, A.; Takakura, A.; Takao-Kawabata, R.; Ishizuya, T.; Moriyama, K.; Yamaguchi, A. Short-term intermittent administration of parathyroid hormone facilitates osteogenesis by different mechanisms in cancellous and cortical bone. Bone Rep. 2016, 5, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Xu, Y.; Fu, Q.; He, M. Parathyroid hormone regulates osteoblast differentiation in a Wnt/beta-catenin-dependent manner. Mol. Cell. Biochem. 2011, 355, 211–216. [Google Scholar] [CrossRef]
- Bellido, T.; Ali, A.A.; Gubrij, I.; Plotkin, L.I.; Fu, Q.; O’Brien, C.A.; Manolagas, S.C.; Jilka, R.L. Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: A novel mechanism for hormonal control of osteoblastogenesis. Endocrinology 2005, 146, 4577–4583. [Google Scholar] [CrossRef]
- Norazlina, M.; Chua, C.W.; Ima-Nirwana, S. Vitamin E deficiency reduced lumbar bone calcium content in female rats. Med. J. Malays. 2004, 59, 623–630. [Google Scholar]
- Holloway, W.R.; Collier, F.M.; Aitken, C.J.; Myers, D.E.; Hodge, J.M.; Malakellis, M.; Gough, T.J.; Collier, G.R.; Nicholson, G.C. Leptin inhibits osteoclast generation. J. Bone Miner. Res. 2002, 17, 200–209. [Google Scholar] [CrossRef]
- Barbour, K.E.; Zmuda, J.M.; Boudreau, R.; Strotmeyer, E.S.; Horwitz, M.J.; Evans, R.W.; Kanaya, A.M.; Harris, T.B.; Cauley, J.A. The effects of adiponectin and leptin on changes in bone mineral density. Osteoporos. Int. 2012, 23, 1699–1710. [Google Scholar] [CrossRef]
- Kocyigit, H.; Bal, S.; Atay, A.; Koseoglu, M.; Gurgan, A. Plasma leptin values in postmenopausal women with osteoporosis. Bosn. J. Basic Med. Sci. 2013, 13, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Tariq, S.; Baig, M.; Tariq, S.; Shahzad, M. Association of serum leptin with bone mineral density in postmenopausal osteoporotic females. Gynecol. Endocrinol. 2017, 33, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Ducy, P.; Amling, M.; Takeda, S.; Priemel, M.; Schilling, A.F.; Beil, F.T.; Shen, J.; Vinson, C.; Rueger, J.M.; Karsenty, G. Leptin inhibits bone formation through a hypothalamic relay: A central control of bone mass. Cell 2000, 100, 197–207. [Google Scholar] [CrossRef]
- Takeda, S.; Elefteriou, F.; Levasseur, R.; Liu, X.; Zhao, L.; Parker, K.L.; Armstrong, D.; Ducy, P.; Karsenty, G. Leptin regulates bone formation via the sympathetic nervous system. Cell 2002, 111, 305–317. [Google Scholar] [CrossRef]
- Chen, X.X.; Yang, T. Roles of leptin in bone metabolism and bone diseases. J. Bone Miner. Metab. 2015, 33, 474–485. [Google Scholar] [CrossRef] [PubMed]
- Astudillo, P.; Rios, S.; Pastenes, L.; Pino, A.M.; Rodriguez, J.P. Increased adipogenesis of osteoporotic human-mesenchymal stem cells (MSCs) characterizes by impaired leptin action. J. Cell Biochem. 2008, 103, 1054–1065. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Lee, C.Y.; Chen, M.Y.; Tsai, H.C.; Hsu, H.C.; Tang, C.H. Adiponectin increases BMP-2 expression in osteoblasts via AdipoR receptor signaling pathway. J. Cell Physiol. 2010, 224, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Challa, T.D.; Rais, Y.; Ornan, E.M. Effect of adiponectin on ATDC5 proliferation, differentiation and signaling pathways. Mol. Cell Endocrinol. 2010, 323, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Moore, B.T.; Xiao, P. MiRNA in bone diseases. MicroRNA 2013, 2, 20–31. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, R.L.; Croce, C.M.; Stein, J.L.; Lian, J.B.; van Wijnen, A.J.; Stein, G.S. A program of microRNAs controls osteogenic lineage progression by targeting transcription factor Runx2. Proc. Natl. Acad. Sci. USA 2011, 108, 9863–9868. [Google Scholar] [CrossRef]
- Li, J.; He, X.; Wei, W.; Zhou, X. MicroRNA-194 promotes osteoblast differentiation via downregulating STAT1. Biochem. Biophys. Res. Commun. 2015, 460, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Qi, J.; Huang, P.; Jiang, M.; Zhou, Q.; Zhou, H.; Kang, H.; Qian, N.; Yang, Q.; Guo, L.; et al. MicroRNA-17/20a inhibits glucocorticoid-induced osteoclast differentiation and function through targeting RANKL expression in osteoblast cells. Bone 2014, 68, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, J.H.; Kim, I.; Lee, J.; Seong, S.; Park, Y.W.; Kim, N. MicroRNA-26a regulates RANKL-induced osteoclast formation. Mol. Cells 2015, 38, 75–80. [Google Scholar] [PubMed]
- Zhao, H.; Zhang, J.; Shao, H.; Liu, J.; Jin, M.; Chen, J.; Huang, Y. Transforming growth factor beta1/Smad4 signaling affects osteoclast differentiation via regulation of miR-155 expression. Mol. Cells 2017, 40, 211–221. [Google Scholar] [PubMed]
- Gaedicke, S.; Zhang, X.; Schmelzer, C.; Lou, Y.; Doering, F.; Frank, J.; Rambach, G. Vitamin E dependent microRNA regulation in rat liver. FEBS Lett. 2008, 582, 3542–3546. [Google Scholar] [CrossRef]
- Tang, X.; Xu, M.; Li, Z.; Pan, Q.; Fu, J. Effects of vitamin E on expressions of eight microRNAs in the liver of Nile tilapia (Oreochromis niloticus). Fish Shellfish. Immunol. 2013, 6, 1470–1475. [Google Scholar] [CrossRef]
- Nakashima, K.; Zhou, X.; Kunkel, G.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; de Crombrugghe, B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 2002, 108, 17–29. [Google Scholar] [CrossRef]
- Tang, W.; Li, Y.; Osimiri, L.; Zhang, C. Osteoblast-specific transcription factor osterix (Osx) is an upstream regulator of satb2 during bone formation. J. Biol. Chem. 2011, 286, 32995–33002. [Google Scholar] [CrossRef]
- Zhang, J.; Tu, Q.; Grosschedl, R.; Kim, M.S.; Griffin, T.; Drissi, H.; Yang, P.; Chen, J. Roles of SATB2 in osteogenic differentiation and bone regeneration. Tissue Eng. Part A 2011, 17, 1767–1776. [Google Scholar] [CrossRef]
- Uto-Kondo, H.; Ohmori, R.; Kiyose, C.; Kishimoto, Y.; Saito, H.; Igarashi, O.; Kondo, K. Tocotrienol suppresses adipocyte differentiation and Akt phosphorylation in 3T3-L1 preadipocytes. J. Nutr. 2009, 139, 51–57. [Google Scholar] [CrossRef]
| Targets | References | Targets | References |
|---|---|---|---|
| Cytokines | Kinases | ||
| IFN-γ ↓ | [92] | Akt ↓ | [171] |
| IL-1 ↓ ↓ | [23,26,32,33,89,93] | ERK ↓ | [71] |
| IL-2 ↓ | [92] | Enzymes | |
| IL-6 ↓ ↓ | [23,26,32,33,89,93] | GPx ↑ ↑ | [107,108,109] |
| IL-23 ↓ | [92] | SOD ↑ ↑ | [107,109] |
| TNF-α ↓ | [92] | Chemokines | |
| RANKL ↓ | [15,19,70,72] | MCP-1 ↓ | [92] |
| Growth factors | Hormones | ||
| TGF-β1 ↑ | [37] | Leptin ↓ | [31,32] |
| FGF-2 ↑ | [114] | Adiponectin ↑ | [32] |
| VEGF-α ↑ | [112] | Receptors | |
| GDF-10 / BMP-3B ↓ | [114] | OPG ↑ ↑ | [15,24] |
| IGF-1 ↑ | [10] | BMPR1B ↑ | [114] |
| Oxidative stress markers | Bone markers | ||
| 8-OHdG ↓ | [72] | NTX ↓ | [72] |
| TBARS/MDA ↓ ↓ | [104,106,108,109] | ALP ↑ | [43] |
| FRAP ↑ | [105] | OCN ↑ | [15,43] |
| Transcription factors | CTX-1 ↓ | [15,17] | |
| Osterix ↑ | [15,43,113] | Genes | |
| Runx-2 ↑ ↑ | [15,37,112,113] | BMP-2 ↑ | [16,112,113] |
| PPAR-γ ↑ ↓ | [19,160] | ALPL ↑ | [19] |
| NFAT2 ↓ | [70] | SPP1 (osteopontin) ↑ | [19] |
| NFATc1 ↓ | [71] | Noggin ↑ | [114] |
| NF-κB ↓ | [71] | SMAD5 ↓ | [114] |
| Protein | c-Fos ↓ | [70,71] | |
| COL1α1 ↑ | [19,43] | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, S.K.; Mohamad, N.-V.; Ibrahim, N.‘I.; Chin, K.-Y.; Shuid, A.N.; Ima-Nirwana, S. The Molecular Mechanism of Vitamin E as a Bone-Protecting Agent: A Review on Current Evidence. Int. J. Mol. Sci. 2019, 20, 1453. https://doi.org/10.3390/ijms20061453
Wong SK, Mohamad N-V, Ibrahim N‘I, Chin K-Y, Shuid AN, Ima-Nirwana S. The Molecular Mechanism of Vitamin E as a Bone-Protecting Agent: A Review on Current Evidence. International Journal of Molecular Sciences. 2019; 20(6):1453. https://doi.org/10.3390/ijms20061453
Chicago/Turabian StyleWong, Sok Kuan, Nur-Vaizura Mohamad, Nurul ‘Izzah Ibrahim, Kok-Yong Chin, Ahmad Nazrun Shuid, and Soelaiman Ima-Nirwana. 2019. "The Molecular Mechanism of Vitamin E as a Bone-Protecting Agent: A Review on Current Evidence" International Journal of Molecular Sciences 20, no. 6: 1453. https://doi.org/10.3390/ijms20061453
APA StyleWong, S. K., Mohamad, N.-V., Ibrahim, N. ‘I., Chin, K.-Y., Shuid, A. N., & Ima-Nirwana, S. (2019). The Molecular Mechanism of Vitamin E as a Bone-Protecting Agent: A Review on Current Evidence. International Journal of Molecular Sciences, 20(6), 1453. https://doi.org/10.3390/ijms20061453






