MicroRNA Sequencing Revealed Citrus Adaptation to Long-Term Boron Toxicity through Modulation of Root Development by miR319 and miR171
Abstract
1. Introduction
2. Results
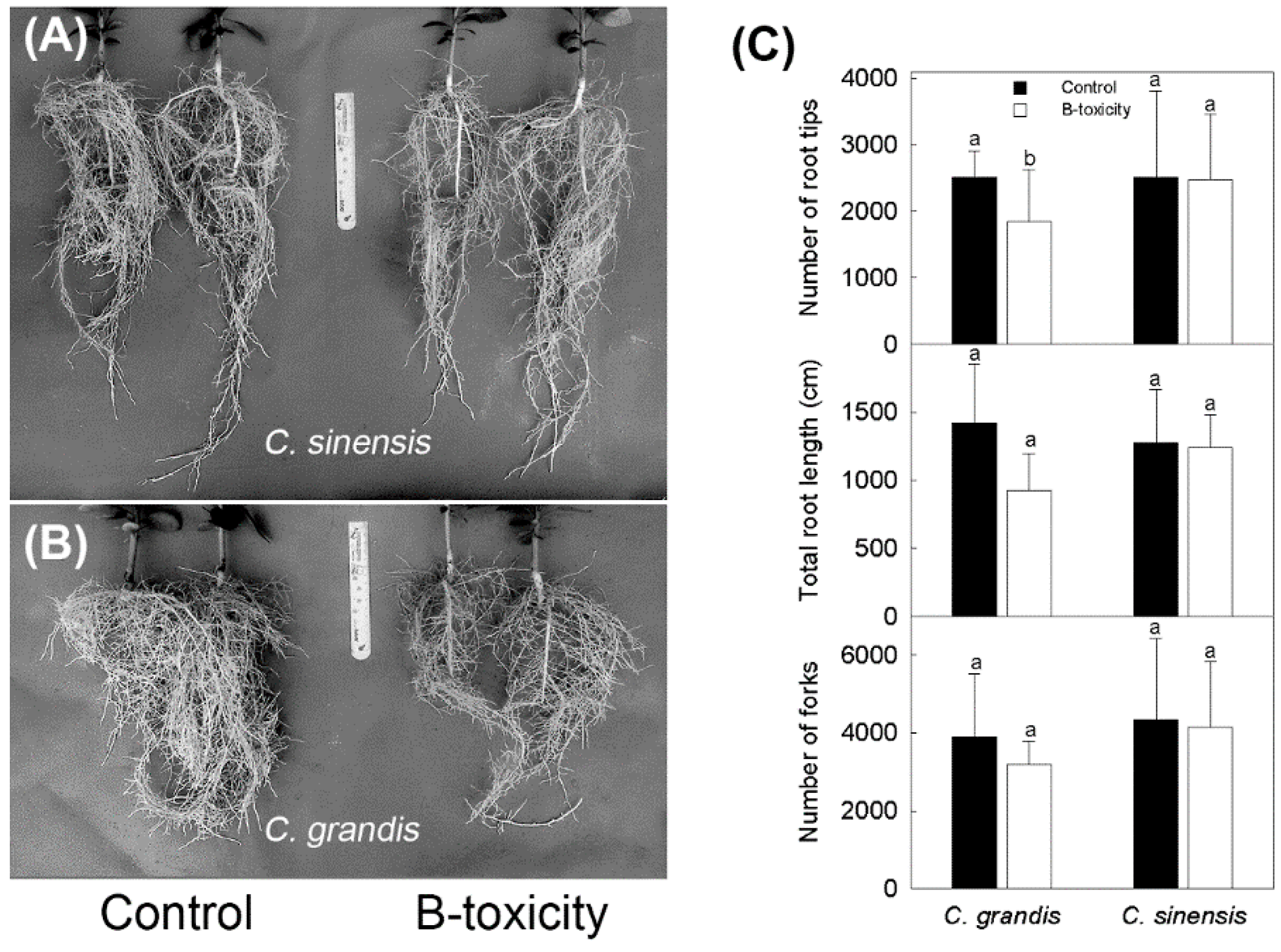
2.1. B-Toxic Treatment on Root Development
2.2. miRNA Sequencing Data Processing, Annotation, and Primary Analysis
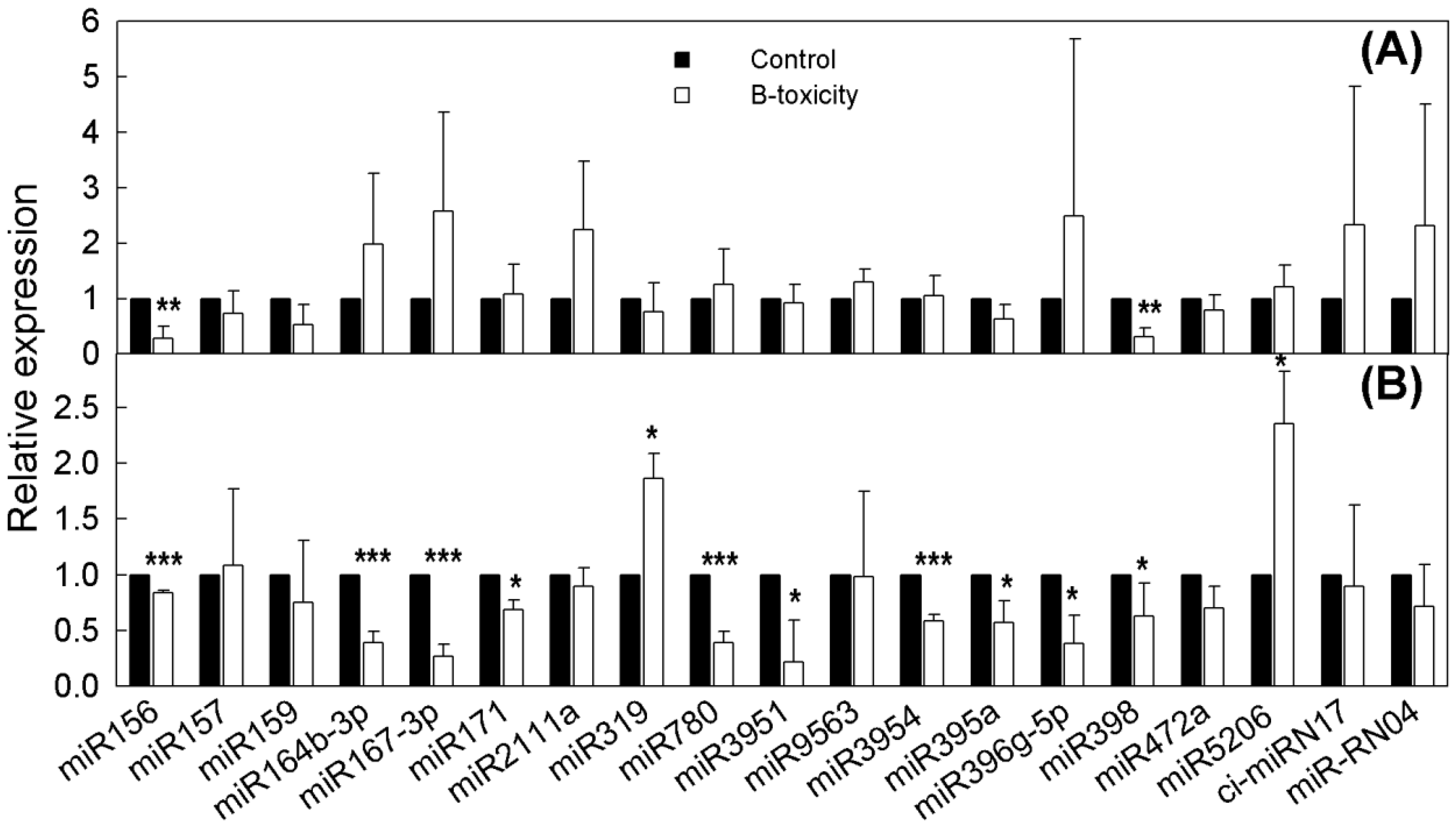
2.3. Differential Expressions of miRNAs in Response to B Toxicity
2.4. Prediction and GO Analysis of Targets for DE miRNAs
2.5. qRT-PCR Relative Expression Analysis of DE miRNAs and Target Genes
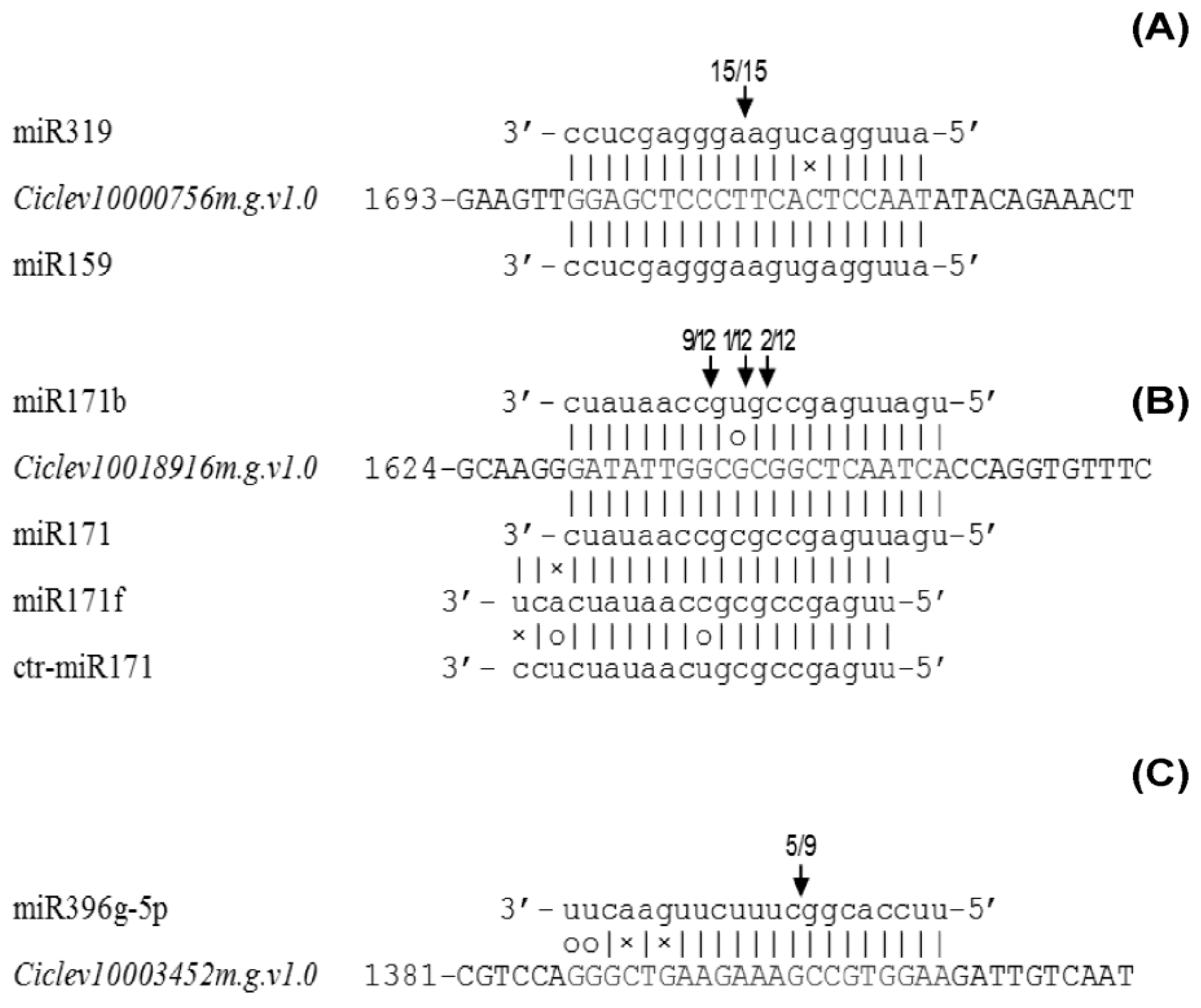
2.6. Experimental Validation of miRNA-Mediated Cleavage of Target Genes
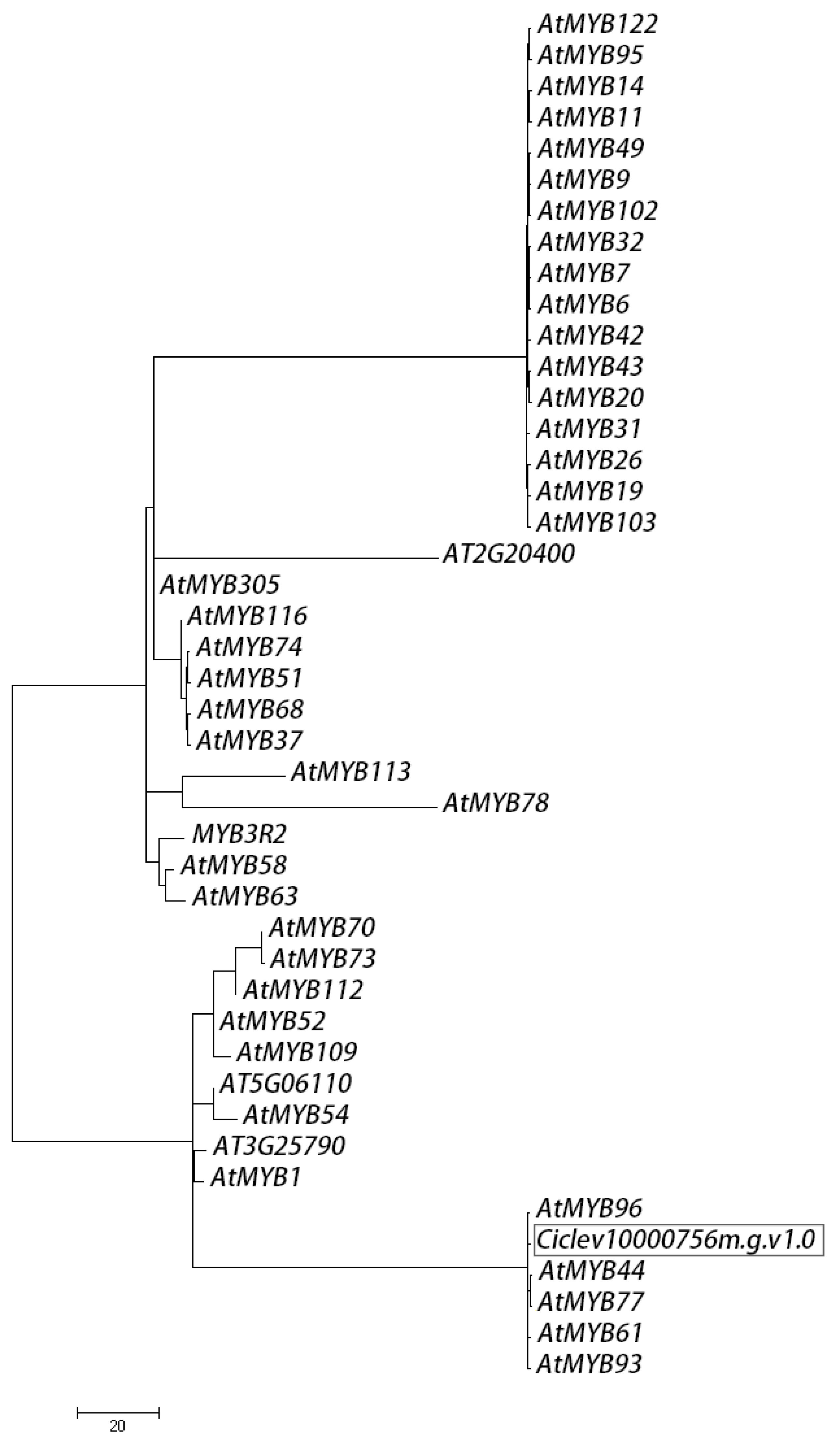
2.7. Bioinformatics of Ciclev10000756m.g.v1.0 Encoded Protein
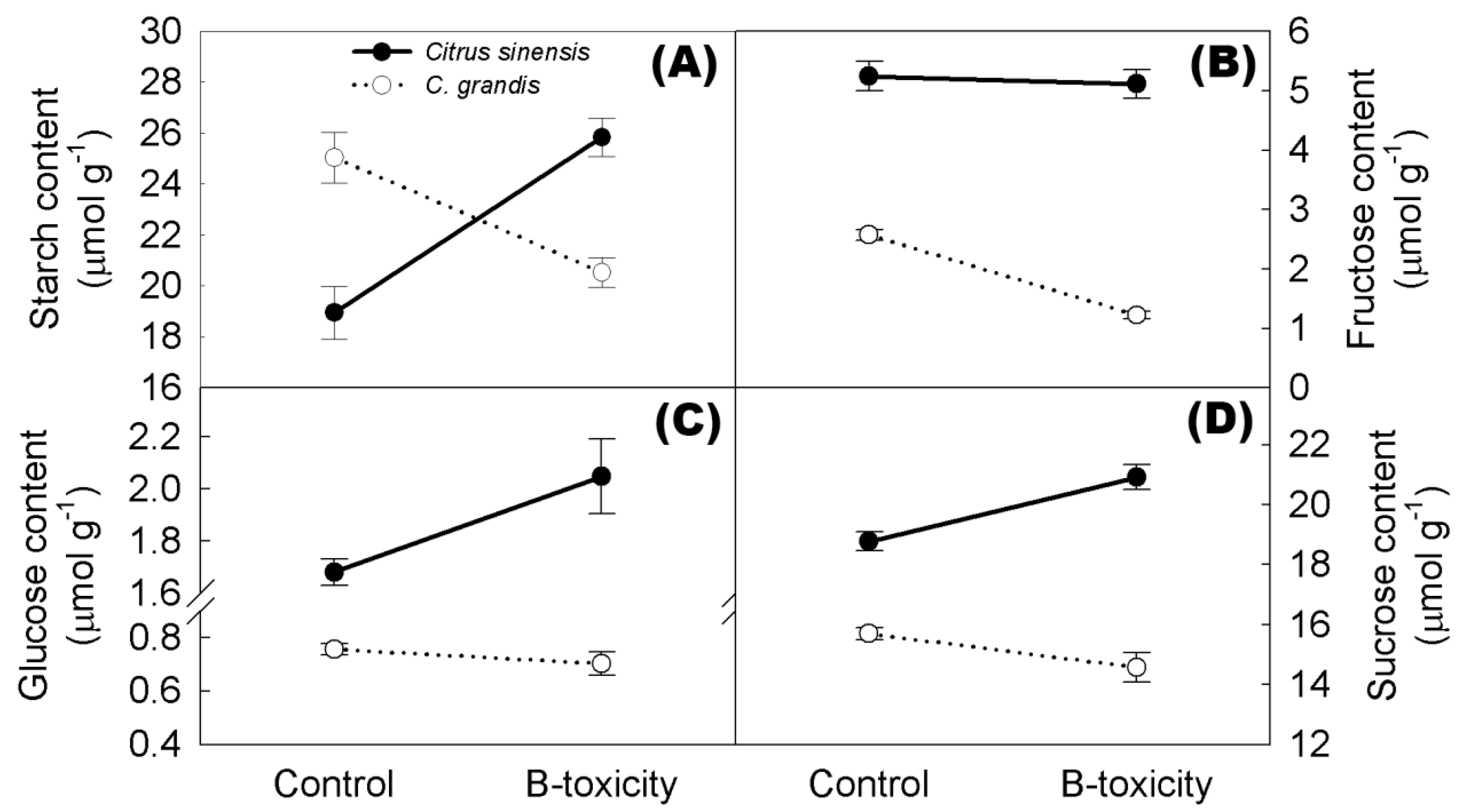
2.8. Non-Structural Carbohydrates in Citrus Roots
3. Discussion
3.1. Tissue-Specific Expression of miRNAs in Response to B Toxicity
3.2. Validation of miRNA Targets
3.3. B-Toxicity-Responsive miRNAs in Citrus Roots
3.4. Adaptive Responses of Citrus Roots to Long-Term B Toxicity
4. Materials and Methods
4.1. Plant Culture and B Treatments
4.2. Determination of Root Traits
4.3. RNA Preparation
4.4. Library Construction, Illumina Sequencing, and Data Processing
4.5. Analysis of Differentially Expressed (DE) miRNAs
4.6. Prediction and GO Analysis of miRNA Target Genes
4.7. QRT-PCR of DE miRNAs and Candidate Targets
4.8. Validation of miRNA Targets via RNA Ligase-Mediated 5′-RACE
4.9. Sequence Analysis and Phylogeny of MYBs
4.10. Measurements of Sugar Contents
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| B | Boron |
| DE | Differentially expressed |
| MYB | Myeloblastosis |
| PCD | Programmed cell death |
| RACE | Rapid-amplification of cDNA ends |
References
- Durbak, A.R.; Phillips, K.A.; Pike, S.; O’Neill, M.A.; Mares, J.; Gallavotti, A.; Malcomber, S.T.; Gassmann, W.; McSteen, P. Transport of boron by the tassel-less1 aquaporin is critical for vegetative and reproductive development in maize. Plant Cell 2014, 26, 2978–2995. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, M.; Tabi, Z.; Galli, M.; Malcomber, S.; Buck, A.; Muszynski, M.; Gallavotti, A. The boron efflux transporter ROTTEN EAR is required for maize inflorescence development and fertility. Plant Cell 2014, 26, 2962–2977. [Google Scholar] [CrossRef]
- Princi, M.P.; Lupini, A.; Araniti, F.; Longo, C.; Mauceri, A.; Sunseri, F.; Abenavoli, M.R. Boron Toxicity and Tolerance in Plants; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 115–147. [Google Scholar]
- Marschner, H. Diagnosis of deficiency and toxicity of mineral nutrients. In Mineral Nutrition of Higher Plants; Academic Press: London, UK, 1995; pp. 461–479. [Google Scholar]
- Nable, R.O.; Bañuelos, G.S.; Paull, J.G. Boron toxicity. Plant Soil 1997, 193, 181–198. [Google Scholar] [CrossRef]
- Martinez-Cuenca, M.R.; Martinez-Alcantara, B.; Quinones, A.; Ruiz, M.; Iglesias, D.J.; Primo-Millo, E.; Forner-Giner, M.A. Physiological and molecular responses to excess boron in Citrus macrophylla W. PLoS ONE 2015, 10, e0134372. [Google Scholar] [CrossRef] [PubMed]
- Papadakis, I.E.; Dimassi, K.N.; Bosabalidis, A.M.; Therios, I.N.; Patakas, A.; Giannakoula, A. Effects of B excess on some physiological and anatomical parameters of ‘Navelina’ orange plants grafted on two rootstocks. Environ. Exp. Bot. 2004, 51, 247–257. [Google Scholar] [CrossRef]
- Huang, J.-H.; Cai, Z.-J.; Wen, S.-X.; Guo, P.; Ye, X.; Lin, G.-Z.; Chen, L.-S. Effects of boron toxicity on root and leaf anatomy in two Citrus species differing in boron tolerance. Trees 2014, 28, 1653–1666. [Google Scholar] [CrossRef]
- Li, Y.; Han, M.Q.; Lin, F.; Ten, Y.; Lin, J.; Zhu, D.H.; Guo, P.; Weng, Y.B.; Chen, L.S. Soil chemical properties, ‘Guanximiyou’ pummelo leaf mineral nutrient status and fruit quality in the southern region of Fujian province, China. J. Soil Sci. Plant Nutr. 2015, 15, 615–628. [Google Scholar] [CrossRef]
- Reid, R.J.; Hayes, J.E.; Post, A.; Stangoulis, J.C.R.; Graham, R.D. A critical analysis of the causes of boron toxicity in plants. Plant Cell Environ. 2004, 27, 1405–1414. [Google Scholar] [CrossRef]
- Aquea, F.; Federici, F.; Moscoso, C.; Vega, A.; Jullian, P.; Haseloff, J.; Arce-Johnson, P. A molecular framework for the inhibition of Arabidopsis root growth in response to boron toxicity. Plant Cell Environ. 2012, 35, 719–734. [Google Scholar] [CrossRef]
- Papadakis, I.E.; Dimassi, K.N.; Therios, I.N.; Papadakis, I.E.; Dimassi, K.N.; Therios, I.N. Response of two citrus genotypes to six boron concentrations: Concentration and distribution of nutrients, total absorption, and nutrient use efficiency. Aust. J. Agric. Res. 2003, 54, 571–580. [Google Scholar] [CrossRef]
- Sang, W.; Huang, Z.R.; Yang, L.T.; Guo, P.; Ye, X.; Chen, L.S. An investigation of boron-toxicity in leaves of two citrus species differing in boron-tolerance using comparative proteomics. J. Proteom. 2015, 123, 128–146. [Google Scholar] [CrossRef]
- Guo, P.; Qi, Y.P.; Yang, L.T.; Ye, X.; Jiang, H.X.; Huang, J.H.; Chen, L.S. cDNA-AFLP analysis reveals the adaptive responses of citrus to long-term boron-toxicity. BMC Plant Biol. 2014, 14, 284. [Google Scholar] [CrossRef]
- Llave, C.; Xie, Z.; Kasschau, K.D.; Carrington, J.C. Cleavage of Scarecrow-like mRNA targets directed by a class of Arabidopsis miRNA. Science 2002, 297, 2053–2056. [Google Scholar] [CrossRef]
- Li, S.; Liu, L.; Zhuang, X.; Yu, Y.; Liu, X.; Cui, X.; Ji, L.; Pan, Z.; Cao, X.; Mo, B.; et al. MicroRNAs inhibit the translation of target mRNAs on the endoplasmic reticulum in Arabidopsis. Cell 2013, 153, 562–574. [Google Scholar] [CrossRef]
- Lu, Y.B.; Yang, L.T.; Qi, Y.P.; Li, Y.; Li, Z.; Chen, Y.B.; Huang, Z.R.; Chen, L.S. Identification of boron-deficiency-responsive microRNAs in Citrus sinensis roots by Illumina sequencing. BMC Plant Biol. 2014, 14, 123. [Google Scholar] [CrossRef]
- Ozhuner, E.; Eldem, V.; Ipek, A.; Okay, S.; Sakcali, S.; Zhang, B.; Boke, H.; Unver, T. Boron stress responsive microRNAs and their targets in barley. PLoS ONE 2013, 8, e59543. [Google Scholar] [CrossRef]
- Huang, J.H.; Qi, Y.P.; Wen, S.X.; Guo, P.; Chen, X.M.; Chen, L.S. Illumina microRNA profiles reveal the involvement of miR397a in Citrus adaptation to long-term boron toxicity via modulating secondary cell-wall biosynthesis. Sci. Rep. 2016, 6, 22900. [Google Scholar] [CrossRef]
- Axtell, M.J.; Snyder, J.A.; Bartel, D.P. Common functions for diverse small RNAs of land plants. Plant Cell 2007, 19, 1750–1769. [Google Scholar] [CrossRef]
- Zhu, Q.H.; Spriggs, A.; Matthew, L.; Fan, L.; Kennedy, G.; Gubler, F.; Helliwell, C. A diverse set of microRNAs and microRNA-like small RNAs in developing rice grains. Genome Res. 2008, 18, 1456–1465. [Google Scholar] [CrossRef]
- Pobezinsky, L.A.; Etzensperger, R.; Jeurling, S.; Alag, A.; Kadakia, T.; McCaughtry, T.M.; Kimura, M.Y.; Sharrow, S.O.; Guinter, T.I.; Feigenbaum, L.; et al. Let-7 microRNAs target the lineage-specific transcription factor PLZF to regulate terminal NKT cell differentiation and effector function. Nat. Immunol. 2015, 16, 517. [Google Scholar] [CrossRef]
- Moldovan, D.; Spriggs, A.; Yang, J.; Pogson, B.J.; Dennis, E.S.; Wilson, I.W. Hypoxia-responsive microRNAs and trans-acting small interfering RNAs in Arabidopsis. J. Exp. Bot. 2010, 61, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Seo, P.J.; Xiang, F.; Qiao, M.; Park, J.Y.; Lee, Y.N.; Kim, S.G.; Lee, Y.H.; Park, W.J.; Park, C.M. The MYB96 transcription factor mediates abscisic acid signaling during drought stress response in Arabidopsis. Plant Physiol. 2009, 151, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Zhao, Y.; Gao, J.; Xiang, C.; Zhu, J.K. The ABA receptor PYL9 together with PYL8 plays an important role in regulating lateral root growth. Sci. Rep. 2016, 6, 27177. [Google Scholar] [CrossRef] [PubMed]
- Shin, R.; Burch, A.Y.; Huppert, K.A.; Tiwari, S.B.; Murphy, A.S.; Guilfoyle, T.J.; Schachtman, D.P. The Arabidopsis transcription factor MYB77 modulates auxin signal transduction. Plant Cell 2007, 19, 2440–2453. [Google Scholar] [CrossRef]
- Romano, J.M.; Dubos, C.; Prouse, M.B.; Wilkins, O.; Hong, H.; Poole, M.; Kang, K.Y.; Li, E.; Douglas, C.J.; Western, T.L.; et al. AtMYB61, an R2R3-MYB transcription factor, functions as a pleiotropic regulator via a small gene network. New Phytol. 2012, 195, 774–786. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, D.J.; Voss, U.; Harding, S.A.; Fannon, J.; Moody, L.A.; Yamada, E.; Swarup, K.; Nibau, C.; Bassel, G.W.; Choudhary, A.; et al. AtMYB93 is a novel negative regulator of lateral root development in Arabidopsis. New Phytol. 2014, 203, 1194–1207. [Google Scholar] [CrossRef] [PubMed]
- Papadakis, I.E.; Dimassi, K.N.; Bosabalidis, A.M.; Therios, I.N.; Patakas, A.; Giannakoula, A. Boron toxicity in ‘Clementine’ mandarin plants grafted on two rootstocks. Plant Sci. 2004, 166, 539–547. [Google Scholar] [CrossRef]
- Guo, P.; Qi, Y.P.; Yang, L.T.; Ye, X.; Huang, J.H.; Chen, L.S. Long-term boron-excess-induced alterations of gene profiles in roots of two citrus species differing in boron-tolerance revealed by cDNA-AFLP. Front. Plant Sci. 2016, 7, 898. [Google Scholar] [CrossRef]
- Sang, W.; Huang, Z.R.; Qi, Y.P.; Yang, L.T.; Guo, P.; Chen, L.S. Effects of high toxic boron concentration on protein profiles in roots of two Citrus species differing in boron-tolerance revealed by a 2-DE based MS approach. Front. Plant Sci. 2017, 8, 180. [Google Scholar] [CrossRef]
- Xu, Z.; Zhong, S.; Li, X.; Li, W.; Rothstein, S.J.; Zhang, S.; Bi, Y.; Xie, C. Genome-wide identification of microRNAs in response to low nitrate availability in maize leaves and roots. PLoS ONE 2011, 6, e28009. [Google Scholar] [CrossRef]
- Colaiacovo, M.; Subacchi, A.; Bagnaresi, P.; Lamontanara, A.; Cattivelli, L.; Faccioli, P. A computational-based update on microRNAs and their targets in barley (Hordeum vulgare L.). BMC Genom. 2010, 11, 595. [Google Scholar] [CrossRef]
- Liang, G.; Yang, F.; Yu, D. MicroRNA395 mediates regulation of sulfate accumulation and allocation in Arabidopsis thaliana. Plant J. 2010, 62, 1046–1057. [Google Scholar] [CrossRef]
- Ergle, D.R. Effect of low nitrogen and sulfur supply on their accumulation in the cotton plant. Bot. Gaz. 1953, 114, 417–426. [Google Scholar] [CrossRef]
- Fismes, J.; Vong, P.C.; Guckert, A.; Frossard, E. Influence of sulfur on apparent N-use efficiency, yield and quality of oilseed rape (Brassica napus L.) grown on a calcareous soil. Eur. J. Agron. 2000, 12, 127–141. [Google Scholar] [CrossRef]
- Castillo, M.C.; Leon, J. Expression of the beta-oxidation gene 3-ketoacyl-CoA thiolase 2 (KAT2) is required for the timely onset of natural and dark-induced leaf senescence in Arabidopsis. J. Exp. Bot. 2008, 59, 2171–2179. [Google Scholar] [CrossRef]
- Achard, P.; Herr, A.; Baulcombe, D.C.; Harberd, N.P. Modulation of floral development by a gibberellin-regulated microRNA. Development 2004, 131, 3357–3365. [Google Scholar] [CrossRef]
- Schwab, R.; Palatnik, J.F.; Riester, M.; Schommer, C.; Schmid, M.; Weigel, D. Specific effects of microRNAs on the plant transcriptome. Dev. Cell 2005, 8, 517–527. [Google Scholar] [CrossRef]
- Palatnik, J.F.; Allen, E.; Wu, X.; Schommer, C.; Schwab, R.; Carrington, J.C.; Weigel, D. Control of leaf morphogenesis by microRNAs. Nature 2003, 425, 257. [Google Scholar] [CrossRef]
- Palatnik, J.F.; Wollmann, H.; Schommer, C.; Schwab, R.; Boisbouvier, J.; Rodriguez, R.; Warthmann, N.; Allen, E.; Dezulian, T.; Huson, D.; et al. Sequence and expression differences underlie functional specialization of Arabidopsis microRNAs miR159 and miR319. Dev. Cell 2007, 13, 115–125. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, L.; Lim, J.Y.; Kim, T.; Pyo, Y.; Sung, S.; Shin, C.; Qiao, H. Phosphorylation of CBP20 links microRNA to root growth in the ethylene response. PLoS Genet. 2016, 12, e1006437. [Google Scholar] [CrossRef]
- Hsieh, L.C.; Lin, S.I.; Shih, A.C.; Chen, J.W.; Lin, W.Y.; Tseng, C.Y.; Li, W.H.; Chiou, T.J. Uncovering small RNA-mediated responses to phosphate deficiency in Arabidopsis by deep sequencing. Plant Physiol. 2009, 151, 2120–2132. [Google Scholar] [CrossRef]
- Addo-Quaye, C.; Eshoo, T.W.; Bartel, D.P.; Axtell, M.J. Endogenous siRNA and miRNA targets identified by sequencing of the Arabidopsis degradome. Curr. Biol. 2008, 18, 758–762. [Google Scholar] [CrossRef]
- Di Laurenzio, L.; Diller, J.; Malamy, J.; Pysh, L.; Helariutta, Y.; Freshour, G.; Hahn, M.; Feldmann, K.; Benfey, P. The SCARECROW gene regulates an asymmetric cell division that is essential for generating the radial organization of the Arabidopsis root. Cell 1996, 86, 423–433. [Google Scholar] [CrossRef]
- Cui, H.; Levesque, M.P.; Vernoux, T.; Jung, J.W.; Paquette, A.J.; Gallagher, K.L.; Wang, J.Y.; Blilou, I.; Scheres, B.; Benfey, P.N. An Evolutionarily conserved mechanism delimiting SHR movement defines a single layer of endodermis in plants. Science 2007, 316, 421–425. [Google Scholar] [CrossRef]
- Lynch, J.P. Root Architecture and Nutrient Acquisition. In Nutrient Acquisition by Plants; BassiriRad, H., Ed.; Springer: Berlin, Germany, 2015; pp. 147–183. [Google Scholar]
- Ruiz, M.; Quinones, A.; Martinez-Alcantara, B.; Aleza, P.; Morillon, R.; Navarro, L.; Primo-Millo, E.; Martinez-Cuenca, M.R. Tetraploidy enhances boron-excess tolerance in Carrizo Citrange (Citrus sinensis L. Osb. x Poncirus trifoliata L. Raf.). Front. Plant Sci. 2016, 7, 701. [Google Scholar] [CrossRef]
- Dubos, C.; Willment, J.; Huggins, D.; Grant, G.H.; Campbell, M.M. Kanamycin reveals the role played by glutamate receptors in shaping plant resource allocation. Plant J. 2005, 43, 348–355. [Google Scholar] [CrossRef]
- Newman, L.J.; Perazza, D.E.; Juda, L.; Campbell, M.M. Involvement of the R2R3-MYB, AtMYB61, in the ectopic lignification and dark-photomorphogenic components of the det3 mutant phenotype. Plant J. 2004, 37, 239–250. [Google Scholar] [CrossRef]
- Pornaro, C.; Macolino, S.; Menegon, A.; Richardson, M. WinRHIZO technology for measuring morphological traits of Bermudagrass stolons. Agron. J. 2017, 109, 3007. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef]
- Allen, E.; Xie, Z.; Gustafson, A.M.; Carrington, J.C. microRNA-directed phasing during trans-acting siRNA biogenesis in plants. Cell 2005, 121, 207–221. [Google Scholar] [CrossRef]
- Xie, R.; Zhang, J.; Ma, Y.; Pan, X.; Dong, C.; Pang, S.; He, S.; Deng, L.; Yi, S.; Zheng, Y.; et al. Combined analysis of mRNA and miRNA identifies dehydration and salinity responsive key molecular players in citrus roots. Sci. Rep. 2017, 7, 42094. [Google Scholar] [CrossRef]
- Song, C.; Wang, C.; Zhang, C.; Korir, N.K.; Yu, H.; Ma, Z.; Fang, J. Deep sequencing discovery of novel and conserved microRNAs in trifoliate orange (Citrus trifoliata). BMC Genom. 2010, 11, 431. [Google Scholar] [CrossRef]
- Han, S.; Chen, L.S.; Jiang, H.X.; Smith, B.R.; Yang, L.T.; Xie, C.Y. Boron deficiency decreases growth and photosynthesis, and increases starch and hexoses in leaves of citrus seedlings. J. Plant Physiol. 2008, 165, 1331–1341. [Google Scholar] [CrossRef]
| C. grandis | C. sinensis | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B-Toxic Treated | Control | B-Toxic Treated | Control | |||||||||
| Replicate 1 | Replicate 2 | Replicate 3 | Replicate 1 | Replicate 2 | Replicate 3 | Replicate 1 | Replicate 2 | Replicate 3 | Replicate 1 | Replicate 2 | Replicate 3 | |
| Clean read counts | 17,034,957 | 13,517,774 | 12,734,477 | 20,753,601 | 17,185,770 | 16,082,626 | 12,685,529 | 14,237,047 | 15,683,586 | 11,708,012 | 13,599,502 | 13,102,318 |
| rRNA | 41.27% | 47.68% | 44.15% | 43.45% | 34.82% | 44.06% | 46.80% | 42.51% | 45.47% | 50.73% | 42.24% | 53.03% |
| scRNA | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% |
| snRNA | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% |
| snoRNA | 0.18% | 0.16% | 0.17% | 0.11% | 0.17% | 0.18% | 0.14% | 0.17% | 0.20% | 0.14% | 0.17% | 0.13% |
| tRNA | 2.88% | 2.65% | 3.26% | 2.84% | 2.92% | 2.45% | 2.74% | 2.70% | 3.34% | 3.00% | 3.10% | 2.93% |
| Repbase | 0.07% | 0.07% | 0.09% | 0.06% | 0.07% | 0.08% | 0.08% | 0.08% | 0.12% | 0.08% | 0.08% | 0.08% |
| Unannotated | 55.60% | 49.44% | 52.33% | 53.54% | 62.02% | 53.23% | 50.24% | 54.53% | 50.87% | 46.05% | 54.42% | 43.84% |
| miRNAs | Target ID | Annotation | Relative Expression (Mean ± SD) | |
|---|---|---|---|---|
| C. sinensis | C. grandis | |||
| miR156 | Ciclev10032171m.g.v1.0 | GTPase domain | 1.15 ± 0.55 | 1.39 ± 1.23 |
| Ciclev10031270m.g.v1.0 | SBP domain | 0.80 ± 0.19 * | 0.91 ± 0.18 | |
| Ciclev10031391m.g.v1.0 | SBP domain | 0.58 ± 0.39 * | 0.72 ± 0.59 | |
| Ciclev10020532m.g.v1.0 | SBP domain | 0.54 ± 0.43 * | 0.96 ± 1.26 | |
| Ciclev10031834m.g.v1.0 | SBP domain | 0.57 ± 0.38 * | 1.54 ± 1.40 | |
| Ciclev10011938m.g.v1.0 | SBP domain | 0.47 ± 0.41 * | 1.05 ± 0.45 | |
| Ciclev10004527m.g.v1.0 | Protein kinase domain | 0.88 ± 0.40 * | 0.84 ± 0.46 | |
| miR171 | Ciclev10019083m.g.v1.0 | SCARECROW-like protein | 1.09 ± 0.32 | 1.44 ± 0.50 |
| Ciclev10019094m.g.v1.0 | SCARECROW-like protein | 1.27 ± 0.24 * | 1.25 ± 0.42 | |
| Ciclev10018916m.g.v1.0 | SCARECROW-like protein | 1.36 ± 0.78 | 1.44 ± 0.87 | |
| miR319 | Ciclev10014986m.g.v1.0 | TCP (TEOSINTE BRANCHED1/CYCLOIDEA/PROLIFERATING CELL FACTOR) transcription factor | 1.57 ± 0.95 | 0.97 ± 0.70 |
| miR319/miR159 | Ciclev10000756m.g.v1.0 | MYB transcription factor | 1.32 ± 0.86 | 0.40 ± 0.22 * |
| miR3951 | Ciclev10028368m.g.v1.0 | Leucine rich repeats | 0.89 ± 0.50 | 0.90 ± 0.65 |
| miR396g-5p | Ciclev10019529m.g.v1.0 | WRC (Trp–Arg–Cys) growth-regulating factor 3 | 1.29 ± 0.91 | 2.17 ± 1.42 |
| Ciclev10003452m.g.v1.0 | Cation transporting ATPase | 1.32 ± 0.95 | 0.33 ± 0.26 * | |
| miR398 | Ciclev10009458m.g.v1.0 | Plastocyanin-like domain | 3.33 ± 2.35 * | 1.52 ± 0.98 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.-H.; Lin, X.-J.; Zhang, L.-Y.; Wang, X.-D.; Fan, G.-C.; Chen, L.-S. MicroRNA Sequencing Revealed Citrus Adaptation to Long-Term Boron Toxicity through Modulation of Root Development by miR319 and miR171. Int. J. Mol. Sci. 2019, 20, 1422. https://doi.org/10.3390/ijms20061422
Huang J-H, Lin X-J, Zhang L-Y, Wang X-D, Fan G-C, Chen L-S. MicroRNA Sequencing Revealed Citrus Adaptation to Long-Term Boron Toxicity through Modulation of Root Development by miR319 and miR171. International Journal of Molecular Sciences. 2019; 20(6):1422. https://doi.org/10.3390/ijms20061422
Chicago/Turabian StyleHuang, Jing-Hao, Xiong-Jie Lin, Ling-Yuan Zhang, Xian-Da Wang, Guo-Cheng Fan, and Li-Song Chen. 2019. "MicroRNA Sequencing Revealed Citrus Adaptation to Long-Term Boron Toxicity through Modulation of Root Development by miR319 and miR171" International Journal of Molecular Sciences 20, no. 6: 1422. https://doi.org/10.3390/ijms20061422
APA StyleHuang, J.-H., Lin, X.-J., Zhang, L.-Y., Wang, X.-D., Fan, G.-C., & Chen, L.-S. (2019). MicroRNA Sequencing Revealed Citrus Adaptation to Long-Term Boron Toxicity through Modulation of Root Development by miR319 and miR171. International Journal of Molecular Sciences, 20(6), 1422. https://doi.org/10.3390/ijms20061422





