Efficacy of Valganciclovir Treatment Depends on the Severity of Hearing Dysfunction in Symptomatic Infants with Congenital Cytomegalovirus Infection
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
2.2. Baseline Results of ABRs and Classification Based on the Hearing Severity
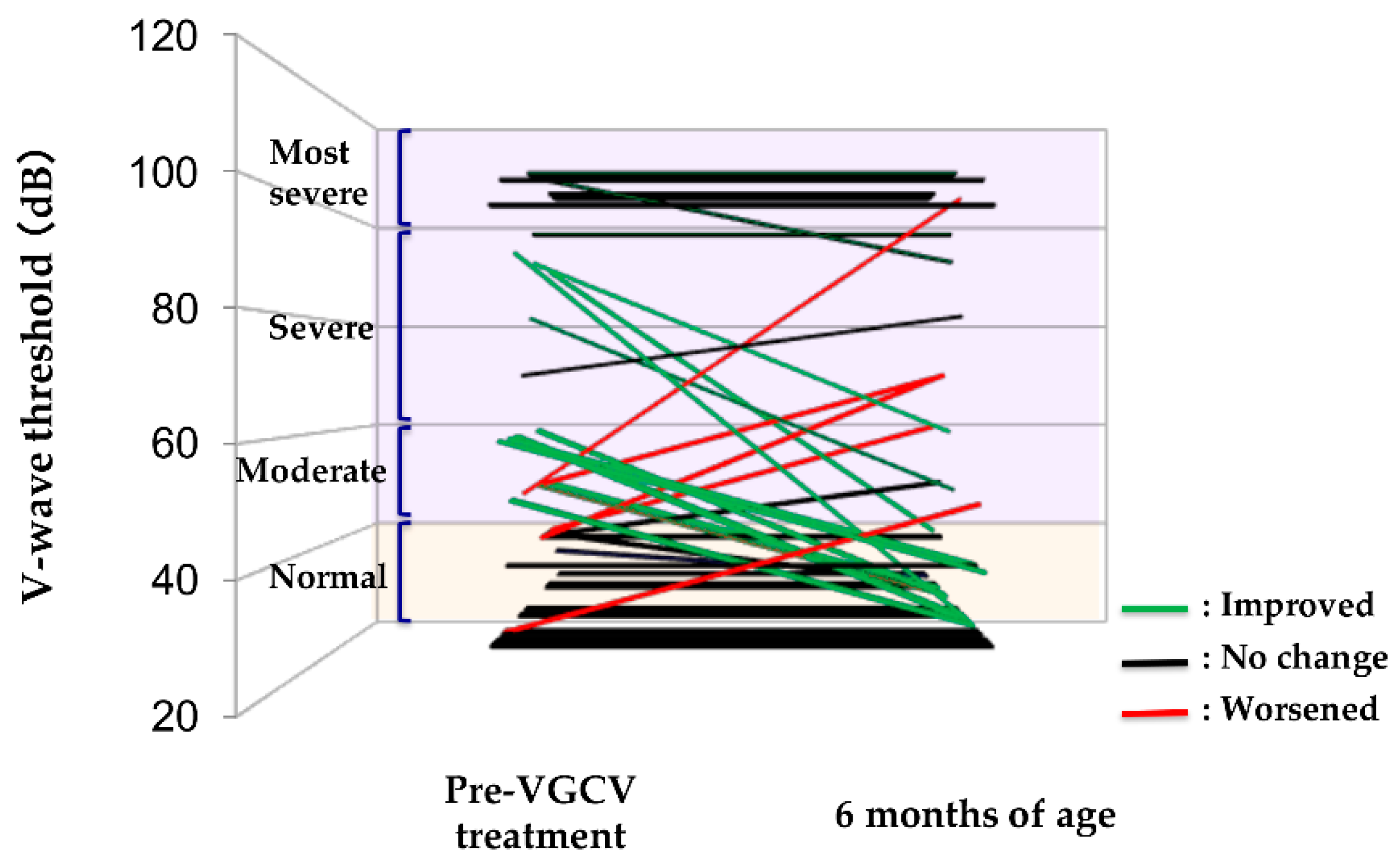
2.3. Efficacy of VGCV Treatment on Hearing
2.4. Differences in Therapeutic Efficacy Due to VGCV Treatment Duration
2.5. Number of Improved Ears after VGCV Treatment Based on Hearing Severity in Abnormal Ears
3. Discussion
- (1)
- Treatment duration was modified midway through the study period in July 2015, from 6 weeks of oral VGCV (32 mg/kg/day) to 6 months. However, ethical considerations about choosing the optimal treatment option for our patients dictated this decision, based on the evidence presented by Kimberlin et al. in 2015 supporting the superiority of 6-month versus 6-week regimens of VGCV [4]. As indicated in a review article by Rawlinson et al. [14], antiviral courses longer than 6 months are not yet currently recommended, given the paucity of evidence that infants receive any additional benefit. This led us to conclude that the 6-month regimen was best supported by the current consensus in the field.
- (2)
- Our decision to measure our main outcome at 6 months after birth could only capture the relatively short-term effects. It is critical for future studies to pursue longer-term follow-up; this would enable the assessment of any deterioration or progressive deafness. However, we achieved our original goal of assessing VGCV’s effects at a consistent time point for all patients. Hearing assessments during long-term follow-up can be affected by other factors besides CMV infection, such as otitis media. Therefore, we believe our choice of 6 months after birth as the assessment time point was appropriate for focusing on VGCV’s efficacy in treating congenital CMV infection.
- (3)
- Despite the impressive length of our study, spanning around 9 years, our subgroups were relatively small after classifying them by hearing severity. We consider this final limitation as characteristic of any single-center prospective studies for this subject with congenital CMV infection.
4. Materials and Methods
4.1. Study Design and Patients
4.2. Diagnosing Congenital CMV Infection
- (1)
- A positive result of a filter paper-based screening to detect CMV DNA in urine, a method developed previously by our team [15] or a presence of clinical symptoms and findings of congenital CMV infection;
- (2)
4.3. Definition of Symptomatic Congenital CMV Infection
4.4. Measuring CMV Viral Load in Blood and Urine
4.5. Definition of Hearing Dysfunction
4.6. VGCV Treatment Protocols
4.7. Efficacy Assessment
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| VGCV | Valganciclovir |
| CMV | Cytomegalovirus |
| GCV | Ganciclovir |
| IV | Intravenous |
| ABR | Auditory brainstem response |
| WBC | White blood cell |
| CT | Computed tomography |
| MRI | Magnetic resonance imaging |
| CN | Copy number |
| G-CSF | Granulocyte colony-stimulating factor |
References
- Morton, C.C.; Nance, W.E. Newborn hearing screening—A silent revolution. N. Engl. J. Med. 2006, 354, 2151–2164. [Google Scholar] [CrossRef] [PubMed]
- Kimberlin, D.W.; Lin, C.Y.; Sanchez, P.J.; Demmler, G.J.; Dankner, W.; Shelton, M.; Jacobs, R.F.; Vaudr, W.; Pass, R.F.; Kiell, J.M.; et al. Effect of ganciclovir therapy on hearing in symptomatic congenital cytomegalovirus disease involving the central nervous system: A randomized, controlled trial. J. Pediatr. 2003, 143, 16–25. [Google Scholar] [CrossRef]
- Amir, J.; Wolf, D.G.; Levy, I. Treatment of symptomatic congenital cytomegalovirus infection with intravenous ganciclovir followed by long-term oral valganciclovir. Eur. J. Pediatr. 2010, 169, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Kimberlin, D.W.; Jester, P.M.; Sanchez, P.J.; Ahmed, A.; Arav-Boger, R.; Michaels, M.G.; Ashouri, N.; Englund, J.A.; Estrada, B.; Jacobs, R.F.; et al. Valganciclovir for symptomatic congenital cytomegalovirus disease. N. Engl. J. Med. 2015, 372, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Bilavsky, E.; Shahar-Nissan, K.; Pardo, J.; Attias, J.; Amir, J. Hearing outcome of infants with congenital cytomegalovirus and hearing impairment. Arch. Dis. Child. 2016, 101, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Goderis, J.; De Leenheer, E.; Smets, K.; Van Hoecke, H.; Keymeulen, A.; Dhooge, I. Hearing loss and congenital CMV infection: A systematic review. Pediatrics 2014, 134, 972–982. [Google Scholar] [CrossRef] [PubMed]
- Whitley, R.J.; Cloud, G.; Gruber, W.; Storch, G.A.; Demmler, G.J.; Jacobs, R.F.; Dankner, W.; Spector, S.A.; Starr, S.; Pass, R.F.; et al. Ganciclovir treatment of symptomatic congenital cytomegalovirus infection: Results of a phase II study. J. Infect. Dis. 1997, 175, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, G.; Garofoli, F.; Villani, P.; Tizzoni, M.; Angelini, M.; Cusato, M.; Bollani, L.; De Silvestri, A.; Regazzi, M.; Stronati, M. Oral valganciclovir treatment in newborns with symptomatic congenital cytomegalovirus infection. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 1465–1470. [Google Scholar] [CrossRef] [PubMed]
- Lanari, M.; Lazzarotto, T.; Venturi, V.; Papa, I.; Gabrielli, L.; Guerra, B.; Landini, M.P.; Faldella, G. Neonatal cytomegalovirus blood load and risk of sequelae in symptomatic and asymptomatic congenitally infected newborns. Pediatrics 2006, 117, e76–e83. [Google Scholar] [CrossRef] [PubMed]
- Marsico, C.; Aban, I.; Kuo, H.; James, S.H.; Sanchez, P.J.; Ahmed, A.; Arav-Boger, R.; Michaels, M.G.; Ashouri, N.; Englund, J.A.; et al. Blood viral load in symptomatic congenital cytomegalovirus infection. J. Infect. Dis. 2018. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.A.; Novak, Z.; Fowler, K.B.; Arora, N.; Britt, W.J.; Boppana, S.B. Cytomegalovirus blood viral load and hearing loss in young children with congenital infection. Pediatr. Infect. Dis. J. 2009, 28, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Teissier, N.; Delezoide, A.L.; Mas, A.E.; Khung-Savatovsky, S.; Bessières, B.; Nardelli, J.; Vauloup-Fellous, C.; Picone, O.; Houhou, N.; Oury, J.F.; et al. Inner ear lesions in congenital cytomegalovirus infection of human fetuses. Acta Neuropathol. 2011, 122, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Teissier, N.; Bernard, S.; Quesnel, S.; Van Den Abbeele, T. Audiovestibular consequences of congenital cytomegalovirus infection. Eur. Ann. Otorhinolaryngol. Head. Neck. Dis. 2016, 133, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Rawlinson, W.D.; Boppana, S.B.; Fowler, K.B.; Kimberlin, D.W.; Lazzarotto, T.; Alain, S.; Daly, K.; Doutré, S.; Gibson, L.; Giles, M.L.; et al. Congenital cytomegalovirus infection in pregnancy and the neonate: Consensus recommendations for prevention, diagnosis, and therapy. Lancet Infect. Dis. 2017, 17, e177–e188. [Google Scholar] [CrossRef]
- Koyano, S.; Inoue, N.; Oka, A.; Moriuchi, H.; Asano, K.; Ito, Y.; Yamada, H.; Yoshikawa, T.; Suzutani, T. Screening for congenital cytomegalovirus infection using newborn urine samples collected on filter paper: Feasibility and outcomes from a multicentre study. BMJ Open 2011, 1, e000118. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Morioka, I.; Koda, T.; Nakamachi, Y.; Okazaki, Y.; Noguchi, Y.; Ogi, M.; Chikahira, M.; Tanimura, K.; Ebina, Y.; et al. Low total IgM values and high cytomegalovirus loads in the blood of newborns with symptomatic congenital cytomegalovirus infection. J. Perinat. Med. 2015, 43, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Morioka, I.; Oda, M.; Kobayashi, Y.; Nakamachi, Y.; Kawano, S.; Nagasaka, M.; Koda, T.; Yokota, T.; Morikawa, S.; et al. Quantitative evaluation of ventricular dilatation using computed tomography in infants with congenital cytomegalovirus infection. Brain Dev. 2014, 36, 10–15. [Google Scholar] [CrossRef]
- Nishida, K.; Morioka, I.; Nakamachi, Y.; Kobayashi, Y.; Imanishi, T.; Kawano, S.; Iwatani, S.; Koda, T.; Deguchi, M.; Tanimura, K.; et al. Neurological outcomes in symptomatic congenital cytomegalovirus-infected infants after introduction of newborn urine screening and antiviral treatment. Brain Dev. 2016, 38, 209–216. [Google Scholar] [CrossRef]
- Itabashi, K.; Miura, F.; Uehara, R.; Nakamura, Y. New Japanese neonatal anthropometric charts for gestational age at birth. Pediatr. Int. 2014, 56, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Japan Audiological Society. Classification of Hearing Dysfunction. Available online: https://audiology-japan.jp/audiology-japan/wp-content/uploads/2014/12/a1360e77a580a13ce7e259a406858656.pdf (accessed on 20 January 2019). (In Japanese).
- Jin, Y.; Shinjo, Y.; Sakai, Y.; Kaga, K. Normalization or improvement of auditory brainstem responses in infants and children who were suspected deafness. Otol. Jpn. 2006, 16, 171–177. (In Japanese) [Google Scholar]
| Symptomatic Infants with Congenital CMV Infection | n = 26 |
|---|---|
| Gestational age, weeks | 37 (31–40) |
| Birth weight, g | 2268 (940–3312) |
| Male | 8 (31) |
| Any symptoms | 26 (100) |
| Microcephaly | 8 (31) |
| Hepatosplenomegaly/hepatitis | 10 (38) |
| Thrombocytopenia | 12 (46) |
| Brain image abnormality | 23 (88) |
| Eye complications | 7 (27) |
| Hearing dysfunction | 21 (81) |
| Duration of VGCV treatment | |
| 6 weeks/6 months | 20 (77)/6 (23) |
| Age when treatment started, days after birth | 12 (4–105) |
| Adverse event of VGCV therapy 1 | 11 (42) |
| Neutropenia | 10 (38) |
| Thrombocytopenia | 2 (8) |
| Genital bleeding | 1 (4) |
| Impetigo | 1 (4) |
| Hypocalcemia | 1 (4) |
| CMV load in blood before VGCV treatment, copies/106 WBC | 7.15 × 102 (2.2 × 102–1.7 × 105) |
| CMV load in urine before VGCV treatment, copies/mL | 5.25 × 107 (1.9 × 104–2.4 × 109) |
| Residual CMV in blood at the time when VGCV treatment finished | 3 (12) |
| Residual of CMV in urine at the time when VGCV treatment finished | 6 (23) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohyama, S.; Morioka, I.; Fukushima, S.; Yamana, K.; Nishida, K.; Iwatani, S.; Fujioka, K.; Matsumoto, H.; Imanishi, T.; Nakamachi, Y.; et al. Efficacy of Valganciclovir Treatment Depends on the Severity of Hearing Dysfunction in Symptomatic Infants with Congenital Cytomegalovirus Infection. Int. J. Mol. Sci. 2019, 20, 1388. https://doi.org/10.3390/ijms20061388
Ohyama S, Morioka I, Fukushima S, Yamana K, Nishida K, Iwatani S, Fujioka K, Matsumoto H, Imanishi T, Nakamachi Y, et al. Efficacy of Valganciclovir Treatment Depends on the Severity of Hearing Dysfunction in Symptomatic Infants with Congenital Cytomegalovirus Infection. International Journal of Molecular Sciences. 2019; 20(6):1388. https://doi.org/10.3390/ijms20061388
Chicago/Turabian StyleOhyama, Shohei, Ichiro Morioka, Sachiyo Fukushima, Keiji Yamana, Kosuke Nishida, Sota Iwatani, Kazumichi Fujioka, Hisayuki Matsumoto, Takamitsu Imanishi, Yuji Nakamachi, and et al. 2019. "Efficacy of Valganciclovir Treatment Depends on the Severity of Hearing Dysfunction in Symptomatic Infants with Congenital Cytomegalovirus Infection" International Journal of Molecular Sciences 20, no. 6: 1388. https://doi.org/10.3390/ijms20061388
APA StyleOhyama, S., Morioka, I., Fukushima, S., Yamana, K., Nishida, K., Iwatani, S., Fujioka, K., Matsumoto, H., Imanishi, T., Nakamachi, Y., Deguchi, M., Tanimura, K., Iijima, K., & Yamada, H. (2019). Efficacy of Valganciclovir Treatment Depends on the Severity of Hearing Dysfunction in Symptomatic Infants with Congenital Cytomegalovirus Infection. International Journal of Molecular Sciences, 20(6), 1388. https://doi.org/10.3390/ijms20061388






