Matrix Metalloproteinases in Pulmonary and Central Nervous System Tuberculosis—A Review
Abstract
1. Introduction
MMPs
2. Methods of Investigation
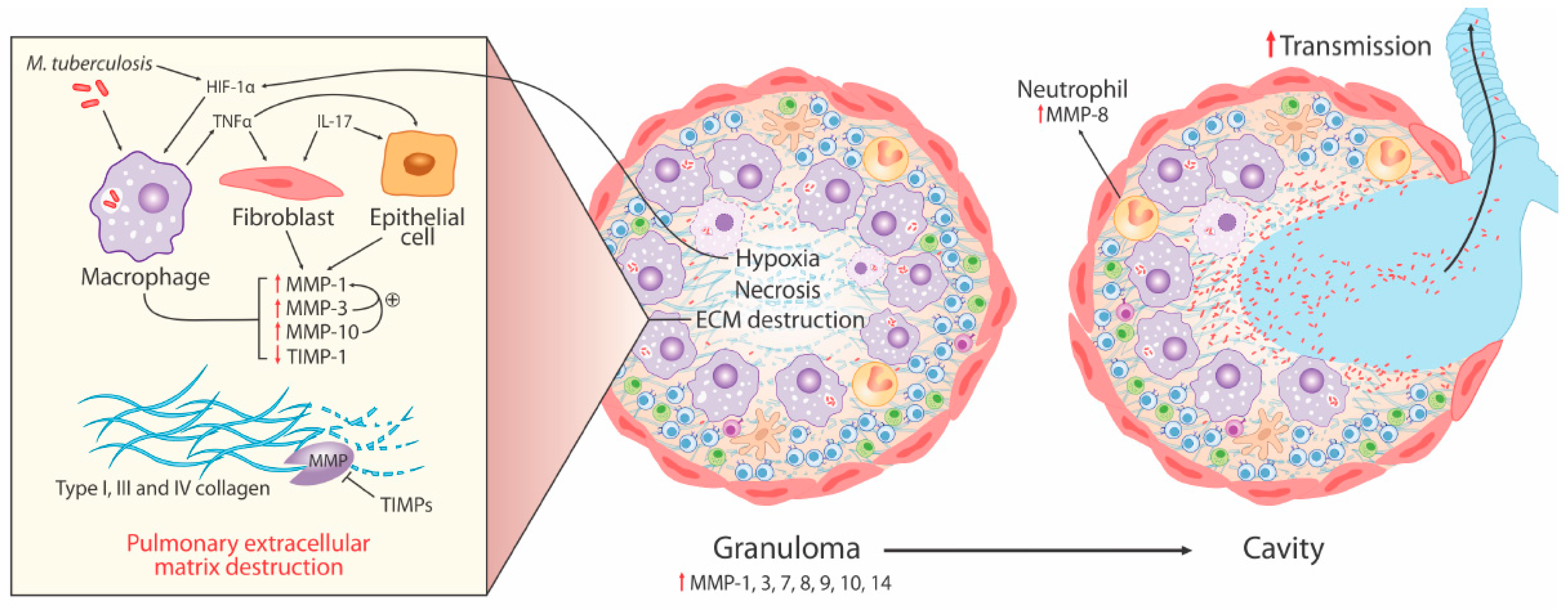
3. MMPs in pTB
- Elevated MMP concentrations (including MMP-1, -2, -8, -9) are consistently reported in respiratory fluids [sputum, broncho-alveolar lavage (BAL), and pleural fluid] from TB patients compared to patients with respiratory symptoms and/or healthy controls. Increased MMPs are associated with various markers of pTB disease severity, most significantly MMP-1 with sputum smear status, radiographic disease extent, and cavitation number in human pTB [28].
- Significantly increased MMP gene expression is found in human respiratory cells (alveolar macrophages, bronchial epithelial cells, and fibroblasts) and macrophages in response to Mtb infection and/or stimulation by conditioned media from Mtb-infected monocytes (CoMtb), resulting in increased MMP secretion [19,22,56]. Specifically, intracellular signaling involving p38 and extracellular signal-regulated kinase (ERK) mitogen-activated protein kinase pathway (MAPK) are important for MMP upregulation in macrophages in response to Mtb infection.
- Genetic associations implicate MMPs in TB disease risk (2G/2G MMP-1 genotype), endobronchial TB and tracheobronchial stenosis (1G MMP-1 allele), TB dissemination (MMP-9 1562C/C genotype), and post-TB chronic lung fibrosis (MMP-1 G-1607GG polymorphism).
- Whilst some animal models of tuberculosis have failed to fully replicate the spectrum of human pTB disease, following Mtb infection in human MMP-1-expressing transgenic mice, pathology was found to be more similar to pTB in humans with increased alveolar tissue damage and collagen destruction compared to wild type mice [27].
3.1. Collagen Degradation is an Early Pathological Event Promoted by Cell-Matrix Adhesion in TB
3.2. Neutrophil-Derived MMP-8 Drives TB Immunopathology
3.3. Hypoxia Drives Collagenase Activity in pTB
3.4. MMP and Cytokine Networks Enhance Tissue Damage in TB
3.5. Intracellular Regulation of MMP Activity in pTB
3.6. MMPs as Biomarkers of TB Disease
3.7. MMPs in TB-IRIS
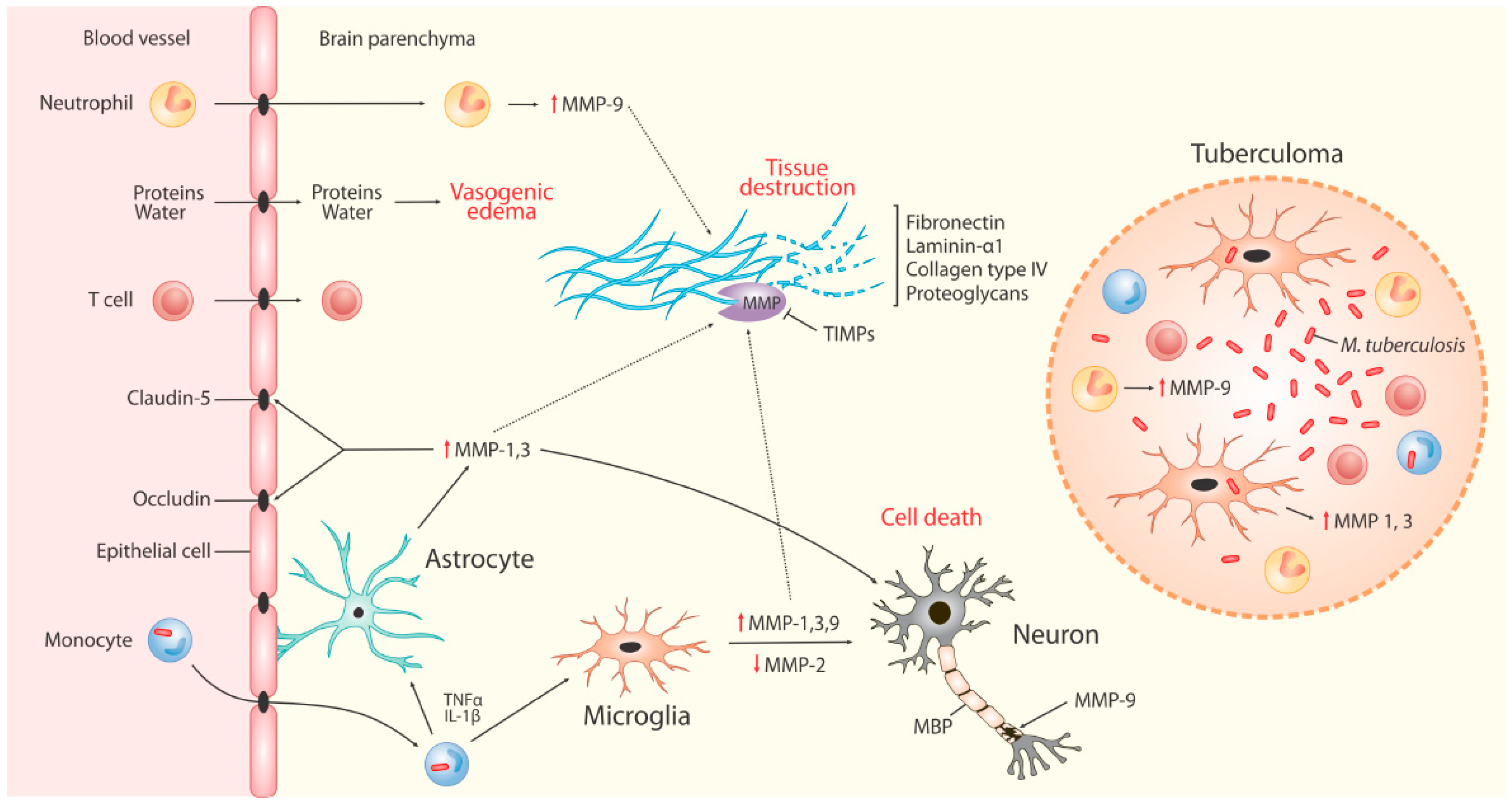
4. MMPs in TBM
4.1. The Blood Brain Barrier (BBB)
4.2. MMPs in Neuro-Inflammation
4.3. MMPs in TBM
4.4. MMPs in TBM-IRIS
4.5. Adults and Children
5. Treatment
5.1. MMP Inhibition in pTB
5.2. MMP-Inhibition in CNS TB
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organisation. Global Tuberculosis Report; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Wilkinson, R.J.; Rohlwink, U.; Misra, U.K.; van Crevel, R.; Thi Hoang Mai, N.; Dooley, K.E.; Caws, M.; Figaji, A.; Savic, R.; Solomons, R.; et al. Tuberculous meningitis. Nat. Rev. Neurol. 2017, 13, 581. [Google Scholar] [CrossRef] [PubMed]
- Elkington, P.T.; Ugarte-Gil, C.A.; Friedland, J.S. Matrix metalloproteinases in tuberculosis. Eur. Respir. J. 2011, 38, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Loffek, S.; Schilling, O.; Franzke, C.W. Series “matrix metalloproteinases in lung health and disease”: Biological role of matrix metalloproteinases: A critical balance. Eur. Respir. J. 2011, 38, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Sternlicht, M.D.; Werb, Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2001, 17, 463–516. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg GARosenberg, G.A. Matrix metalloproteinases in neuroinflammation. Glia 2002, 40, 130. [Google Scholar] [CrossRef] [PubMed]
- Visse, R.; Nagase, H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circul. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef]
- Lopez-Avila, V.; Spencer, J.V. Methods for detection of matrix metalloproteinases as biomarkers in cardiovascular disease. Clin. Med. Cardiol. 2008, 2, S484. [Google Scholar] [CrossRef]
- Candelario-Jalil, E.; Yang, Y.; Rosenberg, G. Diverse roles of matrix metalloproteinases and tissue inhibitors of metalloproteinases in neuroinflammation and cerebral ischemia. Neuroscience 2009, 158, 983–994. [Google Scholar] [CrossRef]
- Yong, V.W.; Power, C.; Forsyth, P.; Edwards, D.R. Metalloproteinases in biology and pathology of the nervous system. Nat. Rev. Neurosci. 2001, 2, 502. [Google Scholar] [CrossRef]
- Murphy, G. Tissue inhibitors of metalloproteinases. Genome Biol. 2011, 12, 233. [Google Scholar] [CrossRef]
- Green, J.A.; Friedland, J.S. Astrocyte-leucocyte interactions and the mechanisms regulating matrix degradation in CNS tuberculosis. Biochem. Soc. Trans. 2007, 35 Pt 4, 686–688. [Google Scholar] [CrossRef]
- Chen, P.; Abacherli, L.E.; Nadler, S.T.; Wang, Y.; Li, Q.; Parks, W.C. MMP7 shedding of syndecan-1 facilitates re-epithelialization by affecting α2β1 integrin activation. PLoS ONE 2009, 4, e6565. [Google Scholar] [CrossRef]
- Yong, V.W. Metalloproteinases: Mediators of pathology and regeneration in the CNS. Nat. Rev. Neurosci. 2005, 6, 931. [Google Scholar] [CrossRef]
- Vaillant, C.; Didier-Bazes, M.; Hutter, A.; Belin, M.F.; Thomasset, N. Spatiotemporal expression patterns of metalloproteinases and their inhibitors in the postnatal developing rat cerebellum. J. Neurosci. 1999, 19, 4994–5004. [Google Scholar] [CrossRef]
- Gonthier, B.; Koncina, E.; Satkauskas, S.; Perraut, M.; Roussel, G.; Aunis, D.; Kapfhammer, J.P.; Bagnard, D. A PKC-dependent recruitment of MMP-2 controls semaphorin-3A growth-promoting effect in cortical dendrites. PLoS ONE 2009, 4, e5099. [Google Scholar] [CrossRef]
- Ethell, I.M.; Ethell, D.W. Matrix metalloproteinases in brain development and remodeling: Synaptic functions and targets. J. Neurosci. Res. 2007, 85, 2813–2823. [Google Scholar] [CrossRef]
- Jung, K.; Klotzek, S.; Stephan, C.; Mannello, F.; Lein, M. Impact of blood sampling on the circulating matrix metalloproteinases 1, 2, 3, 7, 8, and 9. Clin. Chem. 2008, 54, 772–773. [Google Scholar] [CrossRef]
- Harris, J.E.; Nuttall, R.K.; Elkington, P.T.; Green, J.A.; Horncastel, D.E.; Graeber, M.B.; Edwards, D.R.; Friedland, J.S. Monocyte-astrocyte networks regulate matrix metalloproteinase gene expression and secretion in central nervous system tuberculosis in vitro and in vivo. J. Immunol. 2007, 178, 1199–1207. [Google Scholar] [CrossRef]
- Green, J.A.; Elkington, P.T.; Pennington, C.J.; Roncaroli, F.; Dholakia, S.; Moores, R.C.; Bullen, A.; Porter, J.C.; Agranoff, D.; Edawrds, D.R.; et al. Mycobacterium tuberculosis upregulates microglial matrix metalloproteinase-1 and -3 expression and secretion via NF-kappaB- and Activator Protein-1-dependent monocyte networks. J. Immunol. 2010, 184, 6492–6503. [Google Scholar] [CrossRef]
- Ong, C.W.; Pabisiak, P.J.; Brilha, S.; Singh, P.; Roncaroli, F.; Elkington, P.; Friedland, J.S. Complex regulation of neutrophil-derived MMP-9 secretion in central nervous system tuberculosis. J. Neuroinflam. 2017, 14, 31. [Google Scholar] [CrossRef]
- Elkington, P.T.; Nuttall, R.K.; Boyle, J.J.; O’Kane, C.M.; Horncastle, D.E.; Edwards, D.R.; Friedland, J.S. Mycobacterium tuberculosis, but not vaccine BCG, specifically upregulates matrix metalloproteinase-1. Am. J. Respir. Crit. Care Med. 2005, 172, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Elkington, P.T.; Emerson, J.E.; Lopez-Pascua, L.D.; O’Kane, C.M.; Horncastle, D.E.; Boyle, J.J.; Friedland, J.S. Mycobacterium tuberculosis up-regulates matrix metalloproteinase-1 secretion from human airway epithelial cells via a p38 MAPK switch. J. Immunol. 2005, 175, 5333–5340. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.P.; Wang, Y.M.; Wang, C.H.; He, C.C.; Lin, S.M.; Lin, H.C.; Liu, C.Y.; Huang, K.H.; Hsieh, L.L.; Huang, C.D. Matrix metalloproteinase-1 polymorphism in Taiwanese patients with endobronchial tuberculosis. Tuberculosis (Edinb). 2008, 88, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lin, H.; Lin, S.; Hunag, C.; Liu, C.; Huang, K.; Hsieh, L.; Chung, K.F.; Kuo, H. MMP-1 (-1607G) polymorphism as a risk factor for fibrosis after pulmonary tuberculosis in Taiwan. Int. J. Tuberc. Lung Dis. 2010, 14, 627–634. [Google Scholar] [PubMed]
- Ganachari, M.; Ruiz-Morales, J.A.; Gomez de la Torre Pretell, J.C.; Dinh, J.; Granados, J.; Flores-Villanueva, P.O. Joint effect of MCP-1 genotype GG and MMP-1 genotype 2G/2G increases the likelihood of developing pulmonary tuberculosis in BCG-vaccinated individuals. PLoS One. 2010, 5, e8881. [Google Scholar] [CrossRef] [PubMed]
- Elkington, P.; Shiomi, T.; Breen, R.; Nuttall, R.K.; Ugarte-Gil, C.A.; Walker, N.F.; Saraiva, L.; Pedersen, B.; Mauri, F.; Lipman, M.; et al. MMP-1 drives immunopathology in human tuberculosis and transgenic mice. J. Clin. Investig. 2011, 121, 1827–1833. [Google Scholar] [CrossRef]
- Walker, N.F.; Clark, S.O.; Oni, T.; Andreu, N.; Tezera, L.; Singh, S.; Saraiva, L.; Pederson, B.; Kelly, D.L.; Tree, J.A.; et al. Doxycycline and HIV infection suppress tuberculosis-induced matrix metalloproteinases. Am. J. Respir. Crit. Care Med. 2012, 185, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Ganachari, M.; Guio, H.; Zhao, N.; Flores-Villanueva, P.O. Host gene-encoded severe lung TB: From genes to the potential pathways. Genes Immun. 2012, 13, 605–620. [Google Scholar] [CrossRef]
- Seddon, J.; Kasprowicz, V.; Walker, N.F.; Yuen, H.M.; Sunpath, H.; Tezera, L.; Meintjes, G.; Wilkinson, R.J.; Bishai, W.R.; Friedland, J.S.; et al. Procollagen III N-terminal propeptide and desmosine are released by matrix destruction in pulmonary tuberculosis. J. Infect. Dis. 2013, 208, 1571–1579. [Google Scholar] [CrossRef]
- Ugarte-Gil, C.A.; Elkington, P.; Gilman, R.H.; Coronel, J.; Tezera, L.B.; Bernabe-Oritz, A.; Friedland, J.S.; Moore, D.A. Induced sputum MMP-1, -3 & -8 concentrations during treatment of tuberculosis. PLoS ONE 2013, 8, e61333. [Google Scholar]
- Kubler, A.; Luna, B.; Larsson, C.; Ammerman, N.C.; Andrade, B.B.; Orandle, M.; Bock, K.W.; Xu, Z.; Bagci, U.; Mollura, D.J.; et al. Mycobacterium tuberculosis dysregulates MMP/TIMP balance to drive rapid cavitation and unrestrained bacterial proliferation. J. Pathol. 2015, 235, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kubler, A.; Singh, U.K.; Singh, A.; Gardiner, H.; Prasad, R.; Elkington, P.T.; Firedland, J.S. Antimycobacterial drugs modulate immunopathogenic matrix metalloproteinases in a cellular model of pulmonary tuberculosis. Antimicrob. Agents Chemother. 2014, 58, 4657–4665. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Sheu, J.; Chen, R.; Hsiao, C.; Chou, Y.; Chung, C.; Hsiao, G. Mycobacterium tuberculosis upregulates TNF-α Expression via TLR2/ERK signaling and induces MMP-1 and MMP-9 production in human pleural mesothelial cells. PLoS ONE 2015, 10, e0137979. [Google Scholar] [CrossRef] [PubMed]
- Sathyamoorthy, T.; Sandhu, G.; Tezera, L.B.; Thomas, R.; Singhania, A.; Woelk, C.H.; Dimitrov, B.D.; Agranoff, D.; Evans, C.A.; Friedland, J.S.; et al. Gender-dependent differences in plasma matrix metalloproteinase-8 elevated in pulmonary tuberculosis. PLoS ONE 2015, 10, e0117605. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.R.; Tsai, C.J.; Wang, W.J.; Chuang, T.Y.; Yang, C.M.; Chang, L.Y.; Lin, C.K.; Wang, J.Y.; Shu, C.C.; Lee, L.N.; et al. Plasma Biomarkers Can Predict Treatment Response in Tuberculosis Patients: A Prospective Observational Study. Medicine (Baltimore) 2015, 94, e1628. [Google Scholar] [CrossRef] [PubMed]
- Andrade, B.B.; Pavan Kumar, N.; Amaral, E.P.; Riteau, N.; Mayer-Barber, K.D.; Tosh, K.W.; Maier, N.; Conceição, E.L.; Kubler, A.; Sridhar, R.; et al. Heme Oxygenase-1 Regulation of Matrix Metalloproteinase-1 Expression Underlies Distinct Disease Profiles in Tuberculosis. J. Immunol. 2015, 195, 2763–2773. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.W.; Elkington, P.T.; Brilha, S.; Ugarte-Gil, C.; Tome-Esteban, M.T.; Tezera, L.B.; Pabisiak, P.J.; Moores, R.C.; Sathyamoorthy, T.; Patel, V.; et al. Neutrophil-derived MMP-8 drives AMPK-dependent matrix destruction in human pulmonary tuberculosis. PLoS Pathogens 2015, 11, e1004917. [Google Scholar] [CrossRef]
- Sathyamoorthy, T.; Tezera, L.B.; Walker, N.F.; Brilha, S.; Saraiva, L.; Mauri, F.; Wilkinson, R.J.; Friedland, J.S.; Elkington, P.T. Membrane Type 1 Matrix Metalloproteinase Regulates Monocyte Migration and Collagen Destruction in Tuberculosis. J. Immunol. 2015, 195, 882–891. [Google Scholar] [CrossRef]
- Brilha, S.; Sathyamoorthy, T.; Stuttaford, L.H.; Walker, N.F.; Wilkinson, R.J.; Singh, S.; Moores, R.C.; Elkington, P.T.; Friedland, J.S. Early secretory antigenic target-6 drives matrix metalloproteinase-10 gene expression and secretion in tuberculosis. Am. J. Respir. Cell Mol. Biol. 2017, 56, 223–232. [Google Scholar]
- Fox, K.A.; Kirwan, D.E.; Whittington, A.M.; Krishnan, N.; Robertson, B.D.; Gilman, R.H.; Lopez, J.W.; Singh, S.; Porter, J.C.; Friedland, J.S. Platelets regulate pulmonary inflammation and tissue destruction in tuberculosis. Am. J. Respir. Crit. Care Med. 2018, 198, 245–255. [Google Scholar] [CrossRef]
- Singh, S.; Maniakis-Grivas, G.; Singh, U.K.; Asher, R.M.; Mauri, F.; Elkington, P.T.; Friedland, J.S. Interleukin-17 regulates matrix metalloproteinase activity in human pulmonary tuberculosis. J. Pathol. 2018, 244, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, E.; Umehara, F.; Hashiguchi, T.; Fujimoto, N.; Okada, Y.; Osame, M. Marked increase of matrix metalloproteinase 9 in cerebrospinal fluid of patients with fungal or tuberculous meningoencephalitis. J. Neurol. Sci. 2000, 173, 45–52. [Google Scholar] [CrossRef]
- Price, N.M.; Farrar, J.; Tran, T.T.; Nguyen, T.H.; Tran, T.H.; Friedland, J.S. Identification of a matrix-degrading phenotype in human tuberculosis in vitro and in vivo. J. Immunol. 2001, 166, 4223–4230. [Google Scholar] [CrossRef] [PubMed]
- Thwaites, G.E.; Simmons, C.P.; Than Ha Quyen, N.; Thi Hong Chau, T.; Phuong Mai, P.; Thi Dung, N.; Hoan Phu, N.; White, N.P.; Tinh Hien, T.; Farrar, J.J. Pathophysiology and prognosis in vietnamese adults with tuberculous meningitis. J. Infect. Dis. 2003, 188, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Kim, E.H.; Yang, W.S.; Ryu, H.; Cho, S.N.; Lee, B.I.; Heo, J.H. Persistent increase of matrix metalloproteinases in cerebrospinal fluid of tuberculous meningitis. J. Neurol. Sci. 2004, 220, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Green, J.A.; Tran, C.T.; Farrar, J.J.; Nguyen, M.T.; Dinh, S.X.; Ho, N.D.; Ly, C.V.; Tran, H.T.; Friedland, J.S.; Thwaites, G.E. Dexamethasone, cerebrospinal fluid matrix metalloproteinase concentrations and clinical outcomes in tuberculous meningitis. PLoS ONE 2009, 4, e7277. [Google Scholar] [CrossRef] [PubMed]
- Rai, D.; Garg, R.K.; Mahdi, A.A.; Jain, A.; Verma, R.; Tripathi, A.K.; Singh, M.K.; Malhotra, H.S.; Singh, G.P.; Ahmad, M.K. Cerebrospinal fluid cytokines and matrix metalloproteinases in human immunodeficiency seropositive and seronegative patients of tuberculous meningitis. Ann. Indian Acad. Neurol. 2014, 17, 171–178. [Google Scholar] [PubMed]
- Marais, S.; Wilkinson, K.A.; Lesosky, M.; Coussens, A.K.; Deffur, A.; Pepper, D.J.; Schutz, C.; Ismail, Z.; Meintjes, G.; Wilkinson, R.J. Neutrophil-associated central nervous system inflammation in tuberculous meningitis immune reconstitution inflammatory syndrome. Clin. Infect. Dis. 2014, 59, 1638–1647. [Google Scholar] [CrossRef]
- Majeed, S.; Singh, P.; Sharma, N.; Sharma, S. Role of matrix metalloproteinase-9 in progression of tuberculous meningitis: A pilot study in patients at different stages of the disease. BMC Infect. Dis. 2016, 16, 722. [Google Scholar] [CrossRef]
- Marais, S.; Lai, R.P.; Wilkinson, K.A.; Meintjes, G.; O’garra, A.; Wilkinson, R.J. Inflammasome Activation Underlying Central Nervous System Deterioration in HIV-Associated Tuberculosis. J. Infect. Dis. 2017, 215, 677–686. [Google Scholar]
- Mailankody, S.; Dangeti, G.V.; Soundravally, R.; Joseph, N.M.; Mandal, J.; Dutta, T.K.; Kadhiravan, T. Cerebrospinal fluid matrix metalloproteinase 9 levels, blood-brain barrier permeability, and treatment outcome in tuberculous meningitis. PLoS ONE 2017, 12, e0181262. [Google Scholar] [CrossRef]
- Li, Y.J.; Wilkinson, K.A.; Wilkinson, R.J.; Figaji, A.A.; Rohlwink, U.K. Elevated matrix metalloproteinases offer novel insight into their role in paediatric tuberculous meningitis. J. Pediatr. Infect. Dis. Soc. 2019. [Epub ahead of print]. [Google Scholar] [CrossRef]
- Dheda, K.; Lenders, L.; Magombedze, G.; Srivastava, S.; Raj, P.; Arning, E.; Ashcraft, P.; Bottiglieri, T.; Wainwright, H.; Pennel, T.; et al. Drug-Penetration Gradients Associated with Acquired Drug Resistance in Patients with Tuberculosis. Am. J. Respir. Crit. Care Med. 2018, 198, 1208–1219. [Google Scholar] [CrossRef]
- Ong, C.W.; Elkington, P.T.; Friedland, J.S. Tuberculosis, pulmonary cavitation, and matrix metalloproteinases. Am. J. Respir. Crit. Care Med. 2014, 190, 9–18. [Google Scholar] [CrossRef]
- Chang, J.C.; Wysocki, A.; Tchou-Wong, K.M.; Moskowitz, N.; Zhang, Y.; Rom, W.N. Effect of Mycobacterium tuberculosis and its components on macrophages and the release of matrix metalloproteinases. Thorax 1996, 51, 306–311. [Google Scholar] [CrossRef]
- Walker, N.F.; Wilkinson, K.A.; Meintjes, G.; Tezera, L.B.; Goliath, R.; Peyper, J.M.; Tadokera, R.; Opondo, C.; Coussens, A.K.; Wilkinson, R.J.; et al. Matrix Degradation in Human Immunodeficiency Virus Type 1–Associated Tuberculosis and Tuberculosis Immune Reconstitution Inflammatory Syndrome: A Prospective Observational Study. Clin. Infect. Dis. 2017, 65, 121–132. [Google Scholar] [CrossRef]
- Al Shammari, B.; Shiomi, T.; Tezera, L.; Bielecka, M.K.; Workman, V.; Sathyamoorthy, T.; Mauri, F.; Jayasinghe, S.N.; Robertson, B.D.; D’Armiento, J.; et al. The extracellular matrix regulates granuloma necrosis in tuberculosis. J. Infect. Dis. 2015, 212, 463–473. [Google Scholar] [CrossRef]
- Brilha, S.; Wysoczanski, R.; Whittington, A.M.; Friedland, J.S.; Porter, J.C. Monocyte Adhesion, Migration, and Extracellular Matrix Breakdown Are Regulated by Integrin alphaVbeta3 in Mycobacterium tuberculosis Infection. J. Immunol. 2017, 199, 982–991. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Ge, P.; Cao, D.; Miao, B.; Robertson, I.; Zhou, X.; Zhang, L.; Chen, H.; Guo, A. Tissue inhibitor of metalloproteinases 1, a novel biomarker of tuberculosis. Mol. Med. Rep. 2017, 15, 483–487. [Google Scholar] [CrossRef]
- Pavan Kumar, N.; Anuradha, R.; Andrade, B.B.; Suresh, N.; Ganesh, R.; Shankar, J.; Kumaraswami, V.; Nutman, T.B.; Babu, S. Circulating biomarkers of pulmonary and extrapulmonary tuberculosis in children. Clin. Vaccine Immunol. 2013, 20, 704–711. [Google Scholar] [CrossRef]
- Eum, S.Y.; Kong, J.H.; Hong, M.S.; Lee, Y.; Kim, J.; Hwang, S.; Cho, S.; Via, L.E.; Barry, C.E. Neutrophils are the predominant infected phagocytic cells in the airways of patients with active pulmonary TB. Chest 2010, 137, 122–128. [Google Scholar] [CrossRef]
- Lowe, D.M.; Redford, P.S.; Wilkinson, R.J.; O’Garra, A.; Martineau, A.R. Neutrophils in tuberculosis: Friend or foe? Trends Immunol. 2012, 33, 14–25. [Google Scholar] [CrossRef]
- Lowe, D.M.; Bandara, A.K.; Packe, G.E.; Barker, R.D.; Wilkinson, R.J.; Griffiths, C.J.; Martineau, A.R. Neutrophilia independently predicts death in tuberculosis. Eur. Respir. J. 2013, 42, 1752–1757. [Google Scholar] [CrossRef]
- Ravimohan, S.; Tamuhla, N.; Kung, S.J.; Nfanyana, K.; Steenhoff, A.P.; Gross, R.; Weissman, D.; Bisson, G.P. Matrix Metalloproteinases in Tuberculosis-Immune Reconstitution Inflammatory Syndrome and Impaired Lung Function Among Advanced HIV/TB Co-infected Patients Initiating Antiretroviral Therapy. EBioMedicine 2015, 3, 100–107. [Google Scholar] [CrossRef]
- Via, L.E.; Lin, P.L.; Ray, S.M.; Carrillo, J.; Allen, S.S.; Eum, S.Y.; Taylor, K.; Klein, E.; Manjunatha, U.; Gonzales, J.; et al. Tuberculous granulomas are hypoxic in guinea pigs, rabbits, and nonhuman primates. Infect. Immunol. 2008, 76, 2333–2340. [Google Scholar] [CrossRef]
- Belton, M.; Brilha, S.; Manavaki, R.; Mauri, F.; Nijran, K.; Hong, Y.T.; Patel, N.H.; Dembek, M.; Tezera, L.; Green, J.; et al. Hypoxia and tissue destruction in pulmonary TB. Thorax 2016, 71, 1145–1153. [Google Scholar] [CrossRef]
- Ong, C.W.; Fox, K.; Ettorre, A.; Elkington, P.T.; Friedland, J.S. Hypoxia increases neutrophil-driven matrix destruction after exposure to Mycobacterium tuberculosis. Sci. Rep. 2018, 8, 11475. [Google Scholar] [CrossRef]
- Matthews, K.; Deffur, A.; Ntsekhe, M.; Syed, F.; Russell, J.B.W.; Tibazarawa, K.; Wolske, J.; Brink, J.; Mayosi, B.M.; Wilkinson, R.J.; et al. A compartmentalized profibrotic immune response characterizes pericardial tuberculosis, irrespective of HIV-1 infection. Am. J. Respir. Crit. Care Med. 2015, 192, 1518–1521. [Google Scholar] [CrossRef]
- O’Kane, C.M.; Elkington, P.T.; Friedland, J.S. Monocyte-dependent oncostatin M and TNF-α synergize to stimulate unopposed matrix metalloproteinase-1/3 secretion from human lung fibroblasts in tuberculosis. Eur. J. Immunol. 2008, 38, 1321–1330. [Google Scholar] [CrossRef]
- Comstock, G.W.; Livesay, V.T.; Woolpert, S.F. The prognosis of a positive tuberculin reaction in childhood and adolescence. Am. J. Epidemiol. 1974, 99, 131–138. [Google Scholar] [CrossRef]
- Elkington, P.T.; Bateman, A.C.; Thomas, G.J.; Ottensmeier, C.H. Implications of Tuberculosis Reactivation after Immune Checkpoint Inhibition. Am. J. Respir. Crit. Care Med. 2018, 198, 1451–1453. [Google Scholar] [CrossRef]
- Barber, D.L.; Sakai, S.; Kudchadkar, R.R.; Fling, S.P.; Day, T.A.; Vergara, J.A.; Ashkin, D.; Cheng, J.H.; Lundgren, L.M.; Raabe, V.N.; et al. Tuberculosis following PD-1 blockade for cancer immunotherapy. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef]
- Singh, S.; Saraiva, L.; Elkington, P.T.; Friedland, J.S. Regulation of matrix metalloproteinase-1,-3, and-9 in Mycobacterium tuberculosis-dependent respiratory networks by the rapamycin-sensitive PI3K/p70S6K cascade. FASEB J. 2014, 28, 85–93. [Google Scholar] [CrossRef]
- Moores, R.C.; Brilha, S.; Schutgens, F.; Elkington, P.T.; Friedland, J.S. Epigenetic regulation of Matrix Metalloproteinase-1 and-3 expression in Mycobacterium tuberculosis infection. Front. Immunol. 2017, 8, 602. [Google Scholar] [CrossRef]
- Brace, P.T.; Tezera, L.B.; Bielecka, M.K.; Mellows, T.; Garay, D.; Tian, S.; Rand, L.; Green, J.; Jogai, S.; Steele, A.J.; et al. Mycobacterium tuberculosis subverts negative regulatory pathways in human macrophages to drive immunopathology. PLoS Pathogens 2017, 13, e1006367. [Google Scholar] [CrossRef]
- Sebastian, V.P.; Salazar, G.A.; Coronado-Arrazola, I.; Schultz, B.M.; Vallejos, O.P.; Berkowitz, L.; Alvarez-Lobos, M.M.; Riedel, C.A.; Kalergis, A.M.; Bueno, S.M. Heme Oxygenase-1 as a Modulator of Intestinal Inflammation Development and Progression. Front. Immunol. 2018, 9, 1956. [Google Scholar] [CrossRef]
- Coussens, A.K.; Wilkinson, R.J.; Nikolayevskyy, V.; Elkington, P.T.; Hanifa, Y.; Islam, K.; Timms, P.M.; Bothamley, G.H.; Claxton, A.P.; Packe, G.E.; et al. Ethnic variation in inflammatory profile in tuberculosis. PLoS Pathogens 2013, 9, e1003468. [Google Scholar] [CrossRef]
- Esmail, H.; Riou, C.; du Bruyn, E.; Lai, R.P.; Harley, Y.X.R.; Meintjes, G.; Wilkinson, K.A.; Wilkinson, R.J. The immune response to Mycobacterium tuberculosis in HIV-1-coinfected persons. Annu. Rev. Immunol. 2018, 36, 603–638. [Google Scholar] [CrossRef]
- Meintjes, G.; Lawn, S.D.; Scano, F.; Maartens, G.; French, M.A.; Worodria, W.; Elliott, J.H.; Murdoch, D.; Wilkinson, R.J.; Seyler, C.; et al. Tuberculosis-associated immune reconstitution inflammatory syndrome: Case definitions for use in resource-limited settings. Lancet Infect. Dis. 2008, 8, 516–523. [Google Scholar] [CrossRef]
- Walker, N.F.; Stek, C.; Wasserman, S.; Wilkinson, R.J.; Meintjes, G. The tuberculosis-associated immune reconstitution inflammatory syndrome: Recent advances in clinical and pathogenesis research. Curr. Opin. HIV AIDS 2018, 13, 512–521. [Google Scholar] [CrossRef]
- Meintjes, G.; Stek, C.; Blumenthal, L.; Thienemann, F.; Schutz, C.; Buyze, J.; Ravinetto, R.; van Loen, H.; Nair, A.; Jackson, A.; et al. Prednisone for the Prevention of Paradoxical Tuberculosis-Associated IRIS. N. Engl. J. Med. 2018, 379, 1915–1925. [Google Scholar] [CrossRef]
- Tadokera, R.; Meintjes, G.A.; Wilkinson, K.A.; Skolimowska, K.H.; Walker, N.; Friedland, J.S.; Maartens, G.; Elkington, P.T.; Wilkinson, R.J. Matrix metalloproteinases and tissue damage in HIV-tuberculosis immune reconstitution inflammatory syndrome. Eur. J. Immunol. 2014, 44, 127–136. [Google Scholar] [CrossRef]
- Meintjes, G.; Wilkinson, R.J.; Morroni, C.; Pepper, D.J.; Rebe, K.; Rangaka, M.X.; Oni, T.; Maartens, G. Randomized placebo-controlled trial of prednisone for paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome. AIDS 2010, 24, 2381–2390. [Google Scholar] [CrossRef]
- De Vries, H.E.; Kuiper, J.; de Boer, A.G.; Van Berkel, T.J.; Breimer, D.D. The blood-brain barrier in neuroinflammatory diseases. Pharmacol. Rev. 1997, 49, 143–155. [Google Scholar]
- Rosenberg, G.A.; Mun-Bryce, S. Matrix metalloproteinases in neuroinflammation and cerebral ischemia. In Proceedings of the Ernst Schering Research Foundation Workshop, Berlin, Heidelberg, Germany, 2004; pp. 1–16. [Google Scholar]
- Barr, T.L.; Latour, L.L.; Lee, K.Y.; Schaewe, T.L.; Luby, M.; Chang, G.S.; El-Zammer, Z.; Alam, S.; Hallenbeck, J.M.; Kidwell, C.S.; et al. Blood-brain barrier disruption in humans is independently associated with increased matrix metalloproteinase-9. Stroke 2010, 41, e123–e128. [Google Scholar] [CrossRef]
- Tayebjee, M.H.; Nadar, S.; Blann, A.D.; Beevers, D.G.; MacFadyen, R.J.; Lip, G.Y. Matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in hypertension and their relationship to cardiovascular risk and treatment: A substudy of the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT). Am. J. Hicsypertens. 2004, 17, 764–769. [Google Scholar] [CrossRef]
- Leppert, D.; Leib, S.; Grygar, C.; Miller, K.; Schaad, U.; Holländer, G. Matrix metalloproteinase (MMP)-8 and MMP-9 in cerebrospinal fluid during bacterial meningitis: Association with blood-brain barrier damage and neurological sequelae. Clin. Infect. Dis. 2000, 31, 80–84. [Google Scholar] [CrossRef]
- Green, R.C.; Berg, J.S.; Grody, W.W.; Kalia, S.S.; Korf, B.R.; Martin, C.L.; McGuire, A.L.; Nussbaum, R.L.; O-Daniel, J.M.; Ormond, K.E.; et al. ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. Genetics in Medicine. 2013, 15, 565–574. [Google Scholar] [CrossRef]
- Green, J.A.; Dholakia, S.; Janczar, K.; Ong, C.W.; Moores, R.; Fry, J.; Elkington, P.T.; Roncaroli, F.; Friedland, J.S. Mycobacterium tuberculosis-infected human monocytes down-regulate microglial MMP-2 secretion in CNS tuberculosis via TNFα, NFκB, p38 and caspase 8 dependent pathways. J. Neuroinflam. 2011, 8, 46. [Google Scholar] [CrossRef]
- Gurney, K.J.; Estrada, E.Y.; Rosenberg, G.A. Blood–brain barrier disruption by stromelysin-1 facilitates neutrophil infiltration in neuroinflammation. Neurobiol. Dis. 2006, 23, 87–96. [Google Scholar] [CrossRef]
- Asahi, M.; Wang, X.; Mori, T.; Sumii, T.; Jung, J.C.; Moskowitz, M.A.; Fin, M.E.; Lo, E.H. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J. Neurosci. 2001, 21, 7724–7732. [Google Scholar] [CrossRef]
- Price, N.M.; Gilman, R.H.; Uddin, J.; Recavarren, S.; Friedland, J.S. Unopposed matrix metalloproteinase-9 expression in human tuberculous granuloma and the role of TNF-alpha-dependent monocyte networks. J. Immunol. 2003, 171, 5579–5586. [Google Scholar] [CrossRef]
- Coussens, L.M.; Fingleton, B.; Matrisian, L.M. Matrix metalloproteinase inhibitors and cancer: Trials and tribulations. Science 2002, 295, 2387–2392. [Google Scholar] [CrossRef]
- Davis, A.G.; Rohlwink, U.K.; Proust, A.; Figaji, A.A.; Wilkinson, R.J. The pathogenesis of tuberculous meningitis. J. Leukoc. Biol. 2019, 105, 267–280. [Google Scholar] [CrossRef]
- Marais, S.; Meintjes, G.; Pepper, D.J.; Dodd, L.E.; Schutz, C.; Ismail, Z.; Wilkinson, K.A.; Wilkinson, R.J. Frequency, severity, and prediction of tuberculous meningitis immune reconstitution inflammatory syndrome. Clin. Infect. Dis. 2013, 56, 450–460. [Google Scholar] [CrossRef]
- Lai, R.P.; Meintjes, G.; Wilkinson, R.J. HIV-1 tuberculosis-associated immune reconstitution inflammatory syndrome. Semin. Immunopathol. 2016, 38, 185–198. [Google Scholar] [CrossRef]
- Marais, S.; Meintjes, G.; Lesosky, M.; Wilkinson, K.A.; Wilkinson, R.J. Interleukin-17 mediated differences in the pathogenesis of HIV-1-associated tuberculous and cryptococcal meningitis. AIDS 2016, 30, 395–404. [Google Scholar] [CrossRef]
- Van Well, G.T.; Paes, B.F.; Terwee, C.B.; Springer, P.; Roord, J.J.; Donald, P.R.; van Furth, A.M.; Schoeman, J.F. Twenty years of pediatric tuberculous meningitis: A retrospective cohort study in the Western Cape of South Africa. Pediatrics 2009, 123, e1–e8. [Google Scholar] [CrossRef]
- Van den Bos, F.; Terken, M.; Ypma, L.; Kimpen, J.L.; Nel, E.D.; Schaaf, H.S.; Schoeman, J.F.; Donald, P.R. Tuberculous meningitis and miliary tuberculosis in young children. Trop. Med. Int. Health 2004, 9, 309–313. [Google Scholar]
- Murase, S.; Lantz, C.L.; Kim, E.; Gupta, N.; Higgins, R.; Stopfer, M.; Hoffman, D.A.; Quinlan, E.M. Matrix metalloproteinase-9 regulates neuronal circuit development and excitability. Mol. Neurobiol. 2016, 53, 3477–3493. [Google Scholar] [CrossRef]
- Gonthier, B.; Nasarre, C.; Roth, L.; Perraut, M.; Thomasset, N.; Roussel, G.; Aunis, D.; Bagnard, D. Functional interaction between matrix metalloproteinase-3 and semaphorin-3C during cortical axonal growth and guidance. Cereb. Cortex 2006, 17, 1712–1721. [Google Scholar] [CrossRef]
- Van Hove, I.; Verslegers, M.; Buyens, T.; Delorme, N.; Lemmens, K.; Stroobants, S.; Gantois, I.; D’Hooge, R.; Moons, L. An aberrant cerebellar development in mice lacking matrix metalloproteinase-3. Mol. Neurobiol. 2012, 45, 17–29. [Google Scholar] [CrossRef]
- Ordonez, A.A.; Maiga, M.; Gupta, S.; Weinstein, E.A.; Bishai, W.R.; Jain, S.K. Novel adjunctive therapies for the treatment of tuberculosis. Curr. Mol. Med. 2014, 14, 385–395. [Google Scholar] [CrossRef]
- Stek, C.; Allwood, B.; Walker, N.F.; Wilkinson, R.J.; Lynen, L.; Meintjes, G. The Immune Mechanisms of Lung Parenchymal Damage in Tuberculosis and the Role of Host-Directed Therapy. Front. Microbiol. 2018, 9, 2603. [Google Scholar] [CrossRef]
- Tiberi, S.; du Plessis, N.; Walzl, G.; Vjecha, M.J.; Rao, M.; Ntoumi, F.; Mfinanga, S.; Kapata, N.; Mwaba, P.; McHugh, T.D.; et al. Tuberculosis: Progress and advances in development of new drugs, treatment regimens, and host-directed therapies. Lancet Infect. Dis. 2018, 18, e183–e198. [Google Scholar] [CrossRef]
- Schutz, C.; Davis, A.G.; Sossen, B.; Lai, R.P.; Ntsekhe, M.; Harley, Y.X.; Wilkinson, R.J. Corticosteroids as an adjunct to tuberculosis therapy. Expert Rev. Respir. Med. 2018, 12, 881–891. [Google Scholar] [CrossRef]
- Hanemaaijer, R.; Visser, H.; Koolwijk, P.; Sorsa, T.; Salo, T.; Golub, L.M.; van Hinsbergh, V.W. Inhibition of MMP synthesis by doxycycline and chemically modified tetracyclines (CMTs) in human endothelial cells. Adv. Dent. Res. 1998, 12, 114–118. [Google Scholar] [CrossRef]
- Henehan, M.; Montuno, M.; De Benedetto, A. Doxycycline as an anti-inflammatory agent: Updates in dermatology. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1800–1808. [Google Scholar] [CrossRef]
- Subbian, S.; Tsenova, L.; O’Brien, P.; Yang, G.; Koo, M.S.; Peixoto, B.; Fallows, D.; Zeldis, J.B.; Muller, G.; Kaplan, G. Phosphodiesterase-4 inhibition combined with isoniazid treatment of rabbits with pulmonary tuberculosis reduces macrophage activation and lung pathology. Am. J. Pathol. 2011, 179, 289–301. [Google Scholar] [CrossRef]
- Rothenberg, M.L.; Nelson, A.R.; Hande, K.R. New Drugs on the Horizon: Matrix Metalloproteinase Inhibitors. Oncologist 1998, 3, 271–274. [Google Scholar] [CrossRef]
- Hernandez-Pando, R.; Orozco, H.; Arriaga, K.; Pavön, L.; Rook, G. Treatment with BB-94, a broad spectrum inhibitor of zinc-dependent metalloproteinases, causes deviation of the cytokine profile towards Type-2 in experimental pulmonary tuberculosis in Balb/c mice. Int. J. Exp. Pathol. 2000, 81, 199–209. [Google Scholar] [CrossRef]
- Izzo, A.; Izzo, L.; Kasimos, J.; Majka, S. A matrix metalloproteinase inhibitor promotes granuloma formation during the early phase of Mycobacterium tuberculosis pulmonary infection. Tuberculosis 2004, 84, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.L.; Hattle, J.M.; Dreitz, S.A.; Troudt, J.M.; Izzo, L.S.; Basaraba, R.J.; Orme, I.M.; Matrisian, L.M.; Izzo, A.A. Role for matrix metalloproteinase 9 in granuloma formation during pulmonary Mycobacterium tuberculosis infection. Infect. Immun. 2006, 74, 6135–6144. [Google Scholar] [CrossRef] [PubMed]
- Urbanowski, M.E.; Ihms, E.A.; Bigelow, K.; Kübler, A.; Elkington, P.T.; Bishai, W.R. Repetitive aerosol exposure promotes cavitary tuberculosis and enables screening for targeted inhibitors of extensive lung destruction. J. Infect. Dis. 2018, 218, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Ordonez, A.A.; Pokkali, S.; Kim, S.; Carr, B.; Klunk, M.H.; Tong, L.; Saini, V.; Chang, Y.S.; McKevitt, M.; Smith, V.; et al. Adjunct antibody administration with standard treatment reduces relapse rates in a murine tuberculosis model of necrotic granulomas. PLoS ONE 2018, 13, e0197474. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, L.; Zimmerman, M.D.; Chen, K.Y.; Huang, L.; Fu, D.J.; Kaya, F.; Rakhilin, N.; Nazarova, E.V.; Bu, P.; et al. Matrix metalloproteinase inhibitors enhance the efficacy of frontline drugs against Mycobacterium tuberculosis. PLoS Pathogens 2018, 14, e1006974. [Google Scholar] [CrossRef] [PubMed]
- Ordonez, A.A.; Pokkali, S.; Sanchez-Bautista, J.; Klunk, M.H.; Urbanowski, M.E.; Kubler, A.; Bishai, W.R.; Elkington, P.T.; Jain, S.K. Matrix Metalloproteinase Inhibition in a Murine Model of Cavitary Tuberculosis Paradoxically Worsens Pathology. J. Infect. Dis. 2018, 219, 633–636. [Google Scholar] [CrossRef]
- Paul, R.; Lorenzl, S.; Koedel, U.; Sporer, B.; Vogel, U.; Frosch, M.; Pfister, H.W. Matrix metalloproteinases contribute to the blood—brain barrier disruption during bacterial meningitis. Ann. Neurol. 1998, 44, 592–600. [Google Scholar] [CrossRef]
- Majeed, S.; Radotra, B.D.; Sharma, S. Adjunctive role of MMP-9 inhibition along with conventional anti-tubercular drugs against experimental tuberculous meningitis. Int. J.Exp. Pathol. 2016, 97, 230–237. [Google Scholar] [CrossRef]
| Reference | CNS or pTB | Subjects | Samples | Method of Investigation | Analytes | Key Findings |
|---|---|---|---|---|---|---|
| Pulmonary TB (pTB) | ||||||
| Elkington et al. [22,23] | pTB | Adults (n = 6 proven pTB patients, 6 controls with cancer diagnosis) | Lung tissue from biopsy | Immunohistochemistry | MMP-1 MMP-7 | Study examined affected lung in Mtb vs unaffected lung in cancer patients
|
| Kuo et al. [24] | pTB | Adults (n = 101 confirmed pTB cases—38 with endobronchial TB, 68 without). All HIV negative | Blood | Genotyping | MMP-1 DNA (G-1607 GG) sequence single nucleotide polymorphisms | MMP-1 1G genotype was associated with endobronchial TB on bronchoscopy
|
| Wang et al. [25] | pTB | Adult (n = 98 pTB cases, 49 healthy controls). All HIV negative | Blood | Genotyping | MMP-1(G-1067GG) single nucleotide MMP-12(Asn357Ser), MMP-9(C-1562T) polymorphisms | MMP-1 (-1607G) polymorphism increased the risk of moderate and advanced lung fibrosis at one year in pTB cases—the odds increased by 3.80 and 6.02 fold, respectively for one copy and this remained after adjustment for age, sex, initial disease score on chest radiograph, sputum bacterial load, smoking status and presence of diabetes
|
| Ganachari et al. [26] | pTB | Adults (n = 894 pTB cases, 1039 PPD+ controls collected from 2 sites). All HIV negative | Blood | Genotyping Immunohistochemistry of lymph node samples for MMP-1 and MCP-1 | -2518A>G SNP in MCP-1 (rs1024611) -1607_1608insG variant in MMP-1 (rs1799750), and 42 genomic control SNPs |
|
| Elkington et al. [27] | pTB | Adults (n = 33 HIV uninfected pTB cases, 32 respiratory symptomatic controls | Induced sputum and BAL | Luminex (concentrations normalized to total protein) | MMP-1 MMP-2 MMP-3 MMP-7 MMP-8 MMP-9 MMP-12 TIMP-1 TIMP-4 |
|
| Walker et al. [28] | pTB | Adults (n = 23 pTB cases, 21 controls—mixed healthy and respiratory symptomatic). Mixed HIV status | Induced sputum | Luminex (concentrations normalized to total protein) | MMP-1 MMP-2 MMP-3 MMP-7 MMP-8 MMP-9 MMP-12 MMP-13 |
|
| Ganachari et al. [29] | pTB | Adults (n = 224 pTB cases, 42 controls). HIV negative | Blood | Genotyping | -2518A>G SNP in MCP-1 (rs1024611) -1607_1608insG variant in MMP-1 (rs1799750) and 42 genomic control SNPs |
|
| Seddon et al. [30] | pTB | Adults (n = 78). Mixed HIV status | Induced sputum Plasma | Luminex ELISA | MMP-1 MMP-2 MMP-3 MMP-7 MMP-8 MMP-9 MMP-10 Also PINP, PIIINP, PIIICP, CTX-I, CTX-III, EMMPRIN |
|
| Ugarte-Gil et al. [31] | pTB | Adults (n = 68 HIV negative pTB cases, 69 healthy controls) Longitudinal study, follow up at 2, 8 and 24 weeks | Induced sputum | Luminex (adjusted for total protein) ELISA | MMP-1 MMP-2 MMP-3 MMP-7 MMP-8 MMP-9 TIMP-1 TIMP-2 |
|
| Kubler et al. [32] | pTB | Adults (n = 97 pTB cases, 14 latent TB and 20 healthy controls without latent TB) | Plasma | ELISA | MMP-1 MMP-3 MMP-7 MMP-8 MMP-9 TIMP-1 TIMP-2 TIMP-3 TIMP-4 |
|
| Singh et al. [33] | pTB | Adults (n = 17 confirmed pTB cases, 18 respiratory symptomatic controls. All HIV uninfected) | BAL Fluid | Not specified | MMP-1 MMP-2 MMP-3 MMP-7 MMP-8 MMP-9 MMP-12 MMP-13 |
|
| Chen et al. [34] | Pleural TB | Adults (n = 18 TB pleuritis cases, 18 controls with congestive heart failure and pleural effusion) | Pleural fluid | ELISA | MMP-1 MMP-7 MMP-9 Also TNF-α |
|
| Sathyamoorthy et al. * [35] | pTB | Adults (n = 151 pTB cases, 109 symptomatic controls and 120 healthy controls) | Plasma | Luminex | MMP-1 MMP-3 MMP-7 MMP-8 MMP-9 MMP-10 MMP-12 MMP-13 |
|
| Lee et al. [36] | pTB | Adults (n = 167, HIV negative, culture-confirmed, drug sensitive pTB) | Blood | Luminex | MMP-1 MMP-3 MMP-8 MMP-9 MMP-12 Also cytokines and cytotoxic mediators |
|
| Andrade et al. * [37] | pTB | Brazilian adults (n = 63 active PTB, 15 individuals with LTBI, 10 healthy controls) Indian adults (n = 97 active PTB, 39 with LTBI, 40 uninfected healthy controls) North American adults (n = 18 culture-confirmed TB, 11 non-tuberculous mycobacteria infection [NTM], 48 pulmonary sarcoidosis) All HIV negative | Plasma | Luminex and ELISA | MMP-1 MMP-8 MMP-9 TIMP-1 TIMP-2 TIMP-3 TIMP-4 HO-1 and others |
|
| Ong et al. [38] | pTB | Adults (n = 5 pTB cases) Adults (n = 51 pTB cases, 57 healthy controls or a subset of 11 patients in each group for collagenase experiments). All HIV negative | Lung biopsies Induced sputum | Immunohistochemistry Luminex DQ collagen degradation assay | H&E and anti-neutrophil elastase MMP-8 MMP-9 (adjusted for total protein) Also myeloperoxidase (MPO) and neutrophil gelatinase associated lipocalin (NGAL) |
|
| Sathyamoorthy et al. [39] | pTB | Adults (n = 15 pTB cases, 10 controls Adults (n = 5 pTB cases, 5 controls) | Induced sputum Lung biopsy | RT-PCR Immunohistochemistry | MT-MMP-1 (MMP-14) |
|
| Brilha et al. [40] | pTB | Adult PTB vs control (respiratory symptomatic and healthy) South African cohort; Induced sputum, mixed serostatus as described in Walker et al. [28] Indian cohort: BAL as described in Singh et al. [33] Second South African adult cohort: Induced sputum for RNA (11 pTB patients and 17 healthy controls—all HIV negative) | Induced sputum and BAL | Luminex for MMP concentrations, RT-PCR for RNA | MMP-10 |
|
| Fox et al. [41] | pTB | Peruvian cohort: Plasma from adults (n = 50 pTB patients 50 and matched asymptomatic PPD negative controls) Indian cohort: BAL fluid from adults (n = 15 pTB patients and 15 matched respiratory symptomatic controls) | Plasma and BAL Fluid | Luminex | MMP-9 and platelet-derived growth factor (PDGF)-BB, RANTES, P-selectin, platelet factor-4 (PF4), Pentraxin-3 (PTX3) |
|
| Singh et al. [42] | pTB | Adults (n = 5 pTB cases, 5 non-TB controls | Lung tissue | Immunohistochemistry | MMP-3 IL-17 |
|
| Tuberculous meningitis (TBM) | ||||||
| Matsuura et al. [43] | CNS | Adults (n = 21 meningitis cases [7 TBM], 30 controls) | CSF | Gelatin zymography Immunohistochemistry (demonstrated immunoreactivity for MMP-9 and -2 for infiltrating mononuclear cells) | MMP-9 MMP-2 TIMP-1 TIMP-2 |
|
| Price et al. [44] | CNS | Human monocytic (THP-1) cells (in-vitro study) Adults (n = 23 TBM, 12 bacterial meningitis, 20 viral meningitis) | CSF | Northern Blot Western Blot Gelatin zymography ELISA (for TIMP-1) | MMP-9 TIMP-1 (MMP-2) | In-vitro study:
Human data (Represented as activity on zymogram and as MMP/CSF-leukocyte ratio):
|
| Thwaites et al. [45] | CNS | Adults (n = 21 TBM) | CSF Serum | ELISA | MMP-9 TIMP-1 Also several cytokines | Measured pre- and post-treatment analyte concentrations: All patients received streptomycin (20 mg/kg intramuscularly daily; maximum, 1 g) and an oral regimen of 5 mg/kg isoniazid, 10 mg/kg rifampicin, and 30 mg/ kg pyrazinamide for 3 months, followed by 3 drugs (isoniazid, rifampicin, and pyrazinamide) for 6 months
|
| Lee et al. [46] | CNS | Adults (n = 24 TBM, 23 acute aseptic meningitis, 10 controls [4 pTB and 6 non-inflammatory neurological disorders]) | CSF | ELISA Gelatin zymography | MMP-9 MMP-2 | Measured MMP concentrations early (<7 days after treatment) and late (after 7 days of treatment—range 10–106 days)
|
| Green et al. [47] | CNS | Adults (n = 37 TBM) | CSF | ELISA | MMP-1 MMP-2 MMP-3 MMP-7 MMP-8 MMP-9 MMP-10 TIMP-1 TIMP-2 TIMP-4 | Study compared the effect of dexamethasone on analyte concentrations relative to a placebo group. Concentrations were measured pre-treatment, on day 5 (3–8), day 30, 60, and 270
|
| Rai et al. [48] | CNS | Adults (n = 36 HIV negative, 28 HIV positive) | CSF | ELISA | MMP-2 MMP-9 |
MMP-2 (ng/mL)
MMP-9 (pg/mL)
|
| Marais et al. [49] | CNS | Adults (n = 34 HIV-associated TBM) Sampled longitudinally and stratified into TBM-IRIS vs TBM-non-IRIS | CSF serum | Luminex ELISA | MMP-1 MMP-2 MMP-3 MMP-7 MMP-9 MMP-10 MMP-12 MMP-13 TIMP-1 TIMP-2 Plus a large number of cytokines and chemokines. | CSF was analyzed 3–5 time points in HIV-TBM (n = 34) at TBM diagnosis, initiation of ART (day 14), 14 days after ART initiation, at presentation of TBM-IRIS, and 14 days thereafter. 40 mediators in CSF were compared to blood and between patients who developed TBM-IRIS (n = 16) vs those who did not (TBM-non-IRIS; n = 18)
|
| Majeed et al. [50] | CNS | C6 glioma cells (in-vitro study) Adults (n = 91 TBM cases, 16 controls) | CSF Serum | Zymography Reverse zymography | MMP-9 TIMP-1 | In-vitro study: Infected C6 glioma cells with Mtb, and treated them with MMP9-inhibitor (SB-3CT) and dexamethasone
|
| Marais et al. [51] | CNS | Adults (n = 34 as described above in Marais et al. [49]) | RNA from blood, Proteins from CSF and plasma | Microarray analysis (RNA) ELISA (CSF and plasma) | >47,000 probes |
|
| Mailankody et al. [52] | CNS | Adults (n = 40 TBM) | CSF | ELISA | MMP-9 TIMP-1 |
|
| Li et al. [53] | CNS | Children (n = 40 TBM, 8 controls) | CSF (lumbar & ventricular) Serum | Luminex | MMP-9 MMP-2 TIMP-1 TIMP-2 | First study to measure MMP (and TIMP) concentrations in children with TBM
|
| MMP Inhibitor | Animal Model | Treatment Started * | Combination Therapy | Results in Treated Group Compared to Controls | Reference | |||
|---|---|---|---|---|---|---|---|---|
| Lung CFU | Lung Pathology | Mortality | Other Findings in Treated Group | |||||
| Batimastat (BB-94) | Mouse (Balb/c) | Day 1 | No | NR | + | + | Lower TNF-α, IL-2 and IL-1α. Higher IL-4 | [113] |
| Day 30 | No | NR | = | = | No difference in TNF-α, IL-2 and IL-4. Higher IL-1α in pneumonic areas. | [113] | ||
| Mouse (C57BL/6) | Day 18 | No | − | −/= a | NR | Decreased leukocytes. No differences in IFN-γ, IL-4, IL-12, TNF-α and IL-10. Less CFU in blood. | [114] | |
| Day 1 | No | = | NR | NR | Less CFU in spleen and blood by day 14. | [115] | ||
| Day 7 | Isoniazid | − | NR | NR | [118] | |||
| Cipemastat (Ro 32-3555, Trocade) | Mouse (C3HeB/FeJ) | Day 1 | No | = | + | + | Higher rate of cavitation | [119] |
| Rabbit (New Zealand white) | Day 35 | No | NR | + | NR | Higher rate of cavitation. No differences in disease severity by gross pathology or histology | [116] | |
| Marimastat (BB-2516) | Mouse (C57BL/6) | Day 7 | No | = | = | NR | [118] | |
| Day 7 | Isoniazid | − | − | NR | Improved stability of blood vessels surrounding TB lesions | [118] | ||
| Prinomastat | Mouse (C57BL/6) | Day 7 | Isoniazid | = | NR | NR | [118] | |
| SB-3CT | Mouse (C57BL/6) | Day 7 | Isoniazid | − | NR | NR | [118] | |
| MMP-9 inhibitor I | Mouse (C57BL/6) | Day 7 | Isoniazid | − | NR | NR | [118] | |
| Anti MMP-9 antibody | Mouse (C3HeB/FeJ) | Day 42 | Rifampin, isoniazid and pyrazinamide | − | − | NR | Less relapse rates after 12 weeks of treatment | [117] |
| CC-3052 (PDE-4 inhibitor) b | Rabbit (New Zealand white) | Day 28 | No | = | + | NR | Worse disease compared to untreated controls | [111] |
| Day 28 | Isoniazid | − | − | NR | Less inflammation and fibrosis compared to isoniazid monotherapy controls | [111] | ||
| Doxycycline | Guinea pig | Day 14 | No | − | − | NR | No MMP-specific effect of doxycycline was identified | [28] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rohlwink, U.K.; Walker, N.F.; Ordonez, A.A.; Li, Y.J.; Tucker, E.W.; Elkington, P.T.; Wilkinson, R.J.; Wilkinson, K.A. Matrix Metalloproteinases in Pulmonary and Central Nervous System Tuberculosis—A Review. Int. J. Mol. Sci. 2019, 20, 1350. https://doi.org/10.3390/ijms20061350
Rohlwink UK, Walker NF, Ordonez AA, Li YJ, Tucker EW, Elkington PT, Wilkinson RJ, Wilkinson KA. Matrix Metalloproteinases in Pulmonary and Central Nervous System Tuberculosis—A Review. International Journal of Molecular Sciences. 2019; 20(6):1350. https://doi.org/10.3390/ijms20061350
Chicago/Turabian StyleRohlwink, Ursula K., Naomi F. Walker, Alvaro A. Ordonez, Yifan J. Li, Elizabeth W. Tucker, Paul T. Elkington, Robert J. Wilkinson, and Katalin A. Wilkinson. 2019. "Matrix Metalloproteinases in Pulmonary and Central Nervous System Tuberculosis—A Review" International Journal of Molecular Sciences 20, no. 6: 1350. https://doi.org/10.3390/ijms20061350
APA StyleRohlwink, U. K., Walker, N. F., Ordonez, A. A., Li, Y. J., Tucker, E. W., Elkington, P. T., Wilkinson, R. J., & Wilkinson, K. A. (2019). Matrix Metalloproteinases in Pulmonary and Central Nervous System Tuberculosis—A Review. International Journal of Molecular Sciences, 20(6), 1350. https://doi.org/10.3390/ijms20061350






