Autologous Platelet Concentrates in Treatment of Furcation Defects—A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Results
2.1. Study Characteristics
2.2. Meta-Analysis of Primary Outcomes
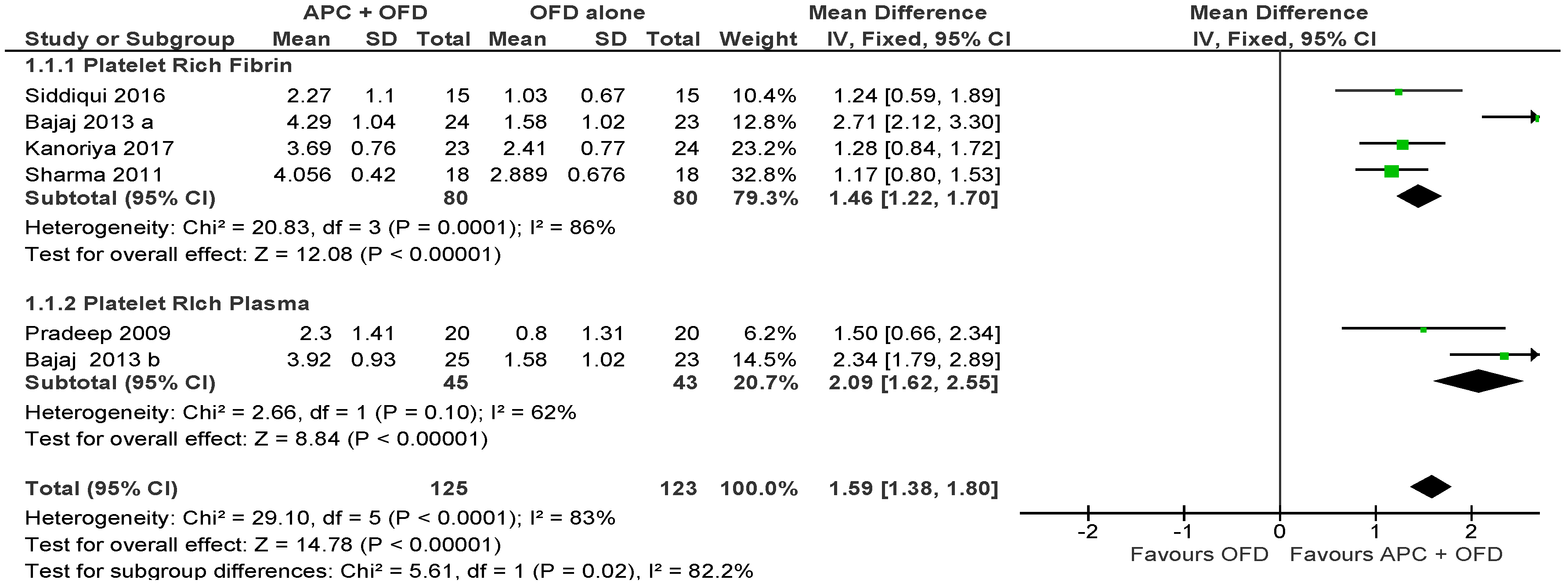
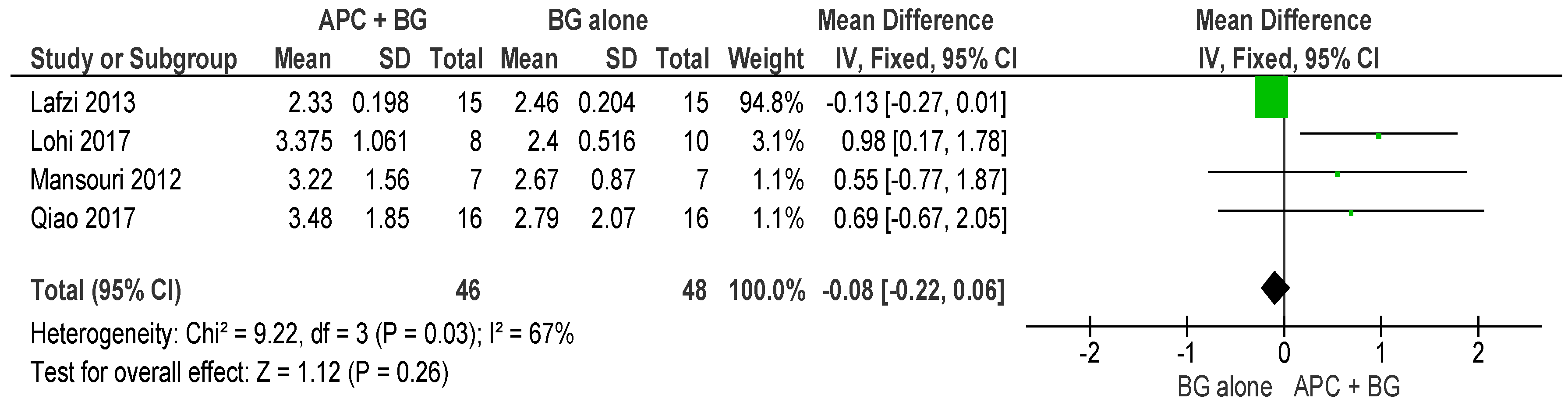
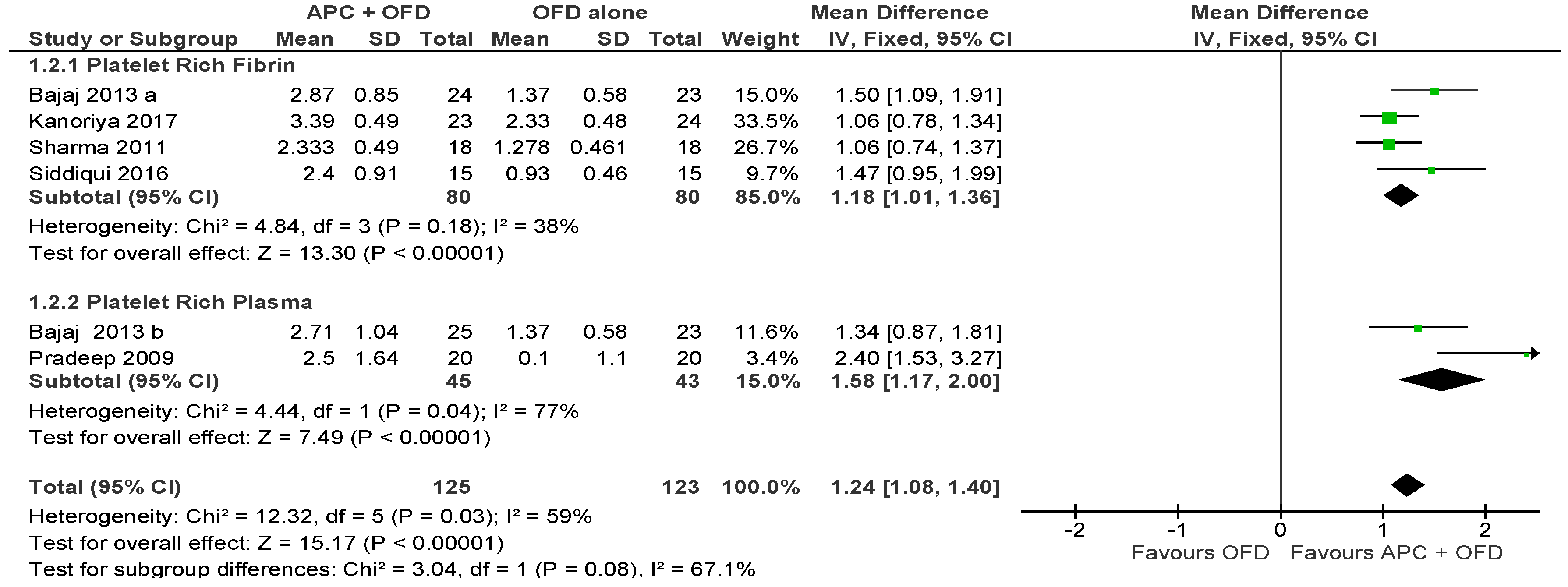
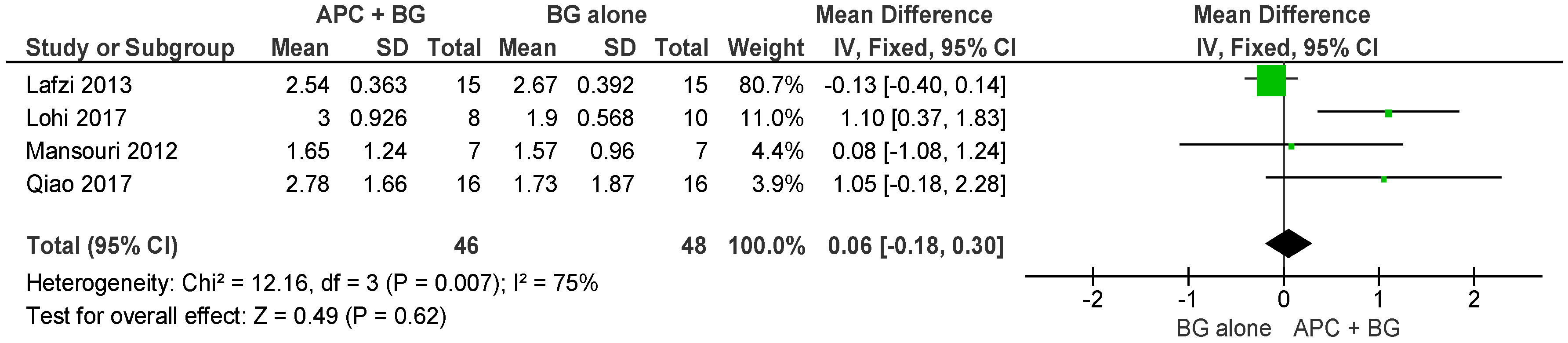
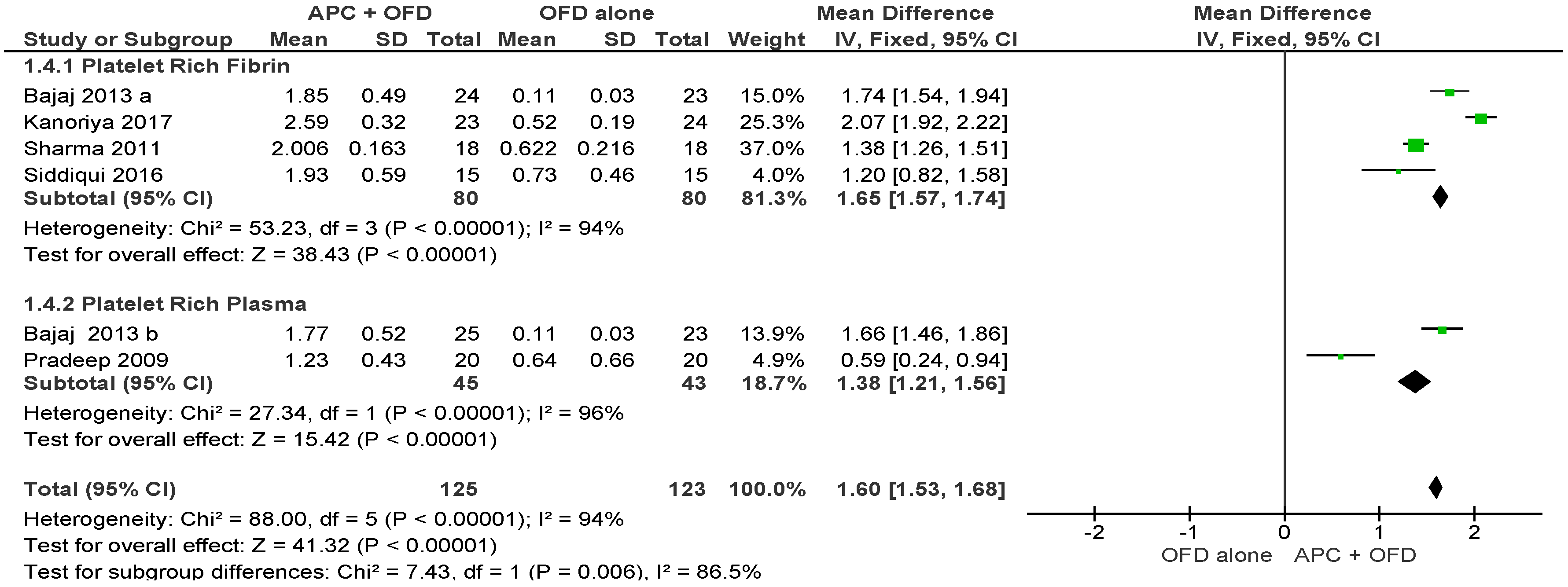
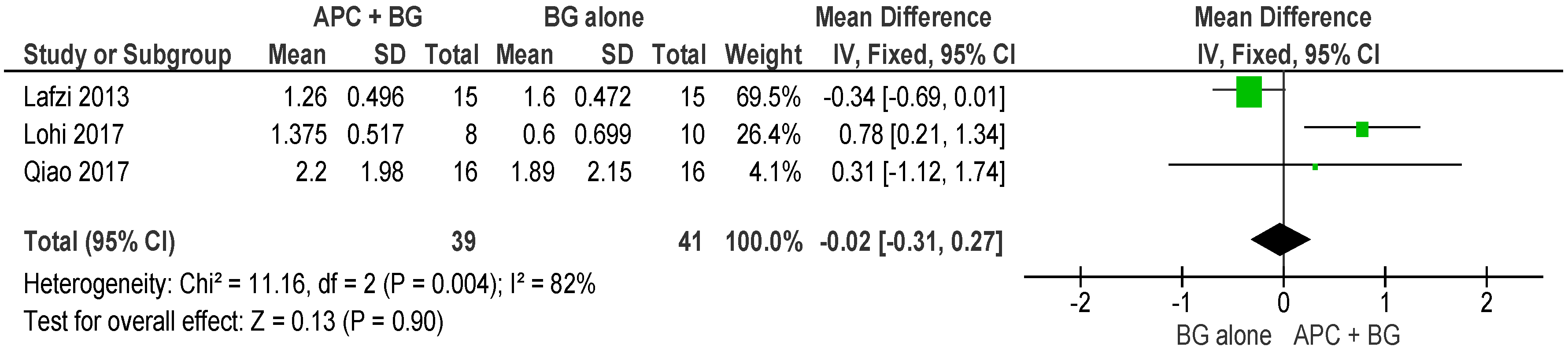
2.2.1. Probing Pocket Depth (PPD)
APC + OFD vs. OFD Alone (Figure 3)
APC + BG vs. BG Alone (Figure 4)
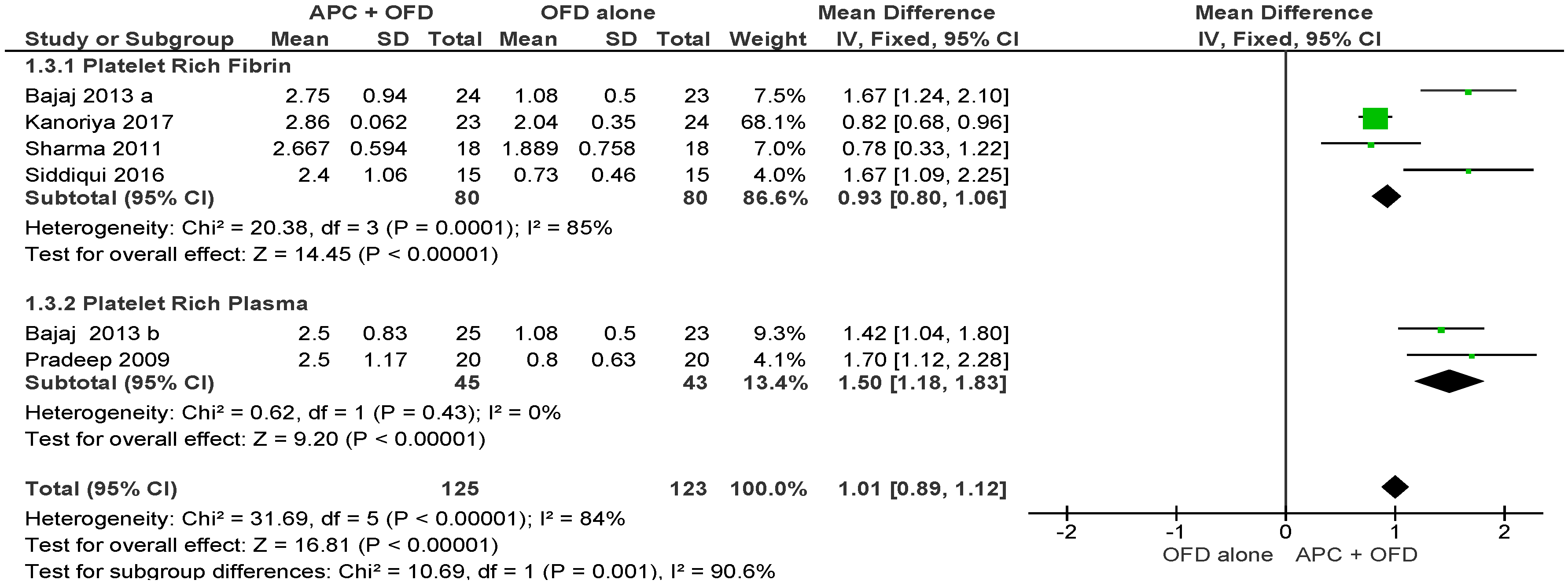
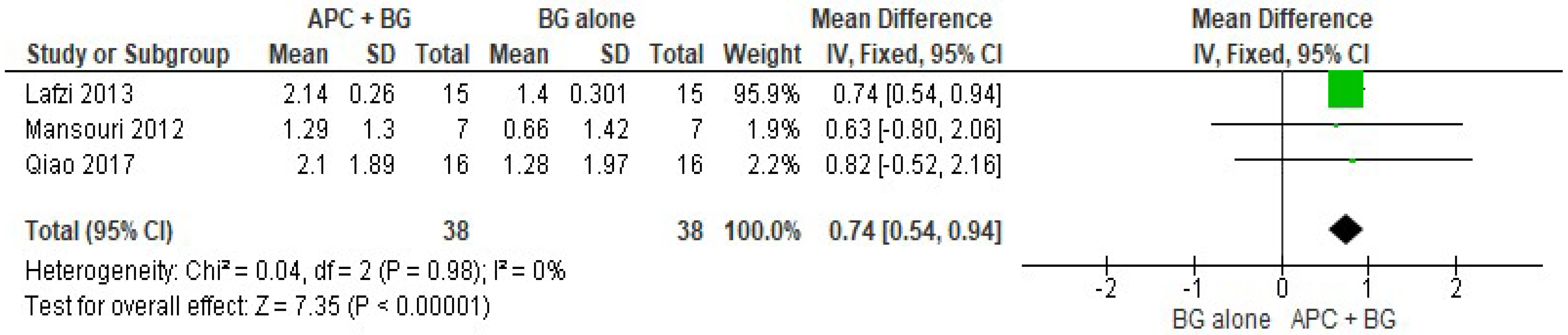
2.2.2. Vertical Clinical Attachment Level (VCAL)
APC + OFD vs. OFD Alone (Figure 5)
APC + BG vs. BG Alone (Figure 6)
2.2.3. Horizontal Clinical Attachment Level (HCAL)
APC + OFD vs. OFD Alone (Figure 7)
APC + BG vs. BG Alone (Figure 8)
2.2.4. Vertical Furcation Depth (VFD)
APC + OFD vs. OFD Alone (Figure 9)
APC + BG vs. BG Alone (Figure 10)
2.2.5. Horizontal Furcation Depth (HFD)
APC + OFD vs. OFD Alone (Figure 11)
APC + BG vs. BG Alone (Figure 12)
3. Discussion
4. Material and Methods
4.1. Research Question
4.2. Search Strategy
4.3. Inclusion Criteria
- Randomized clinical trials (RCT), either of a parallel group or of a split-mouth design;
- Presence of at least one experimental group in which APCs were clinically applied as an adjunct to surgical procedures alone or in combination with bone grafting materials or GTR procedures for the therapy of furcation defects;
- Presence of an appropriate control group, in which the same therapeutic procedures as those employed in at least one experimental group were clinically applied for the treatment of furcation defects, without the adjunctive effect of APCs;
- Patients included in the RCT should present with maxillary/mandibular Grade 2 or 3 furcation defects;
- Patients included in the RCT should have no systemic diseases nor taking medications that could potentially influence the outcome of periodontal therapy;
- The follow-up period had to be at least 6 months.
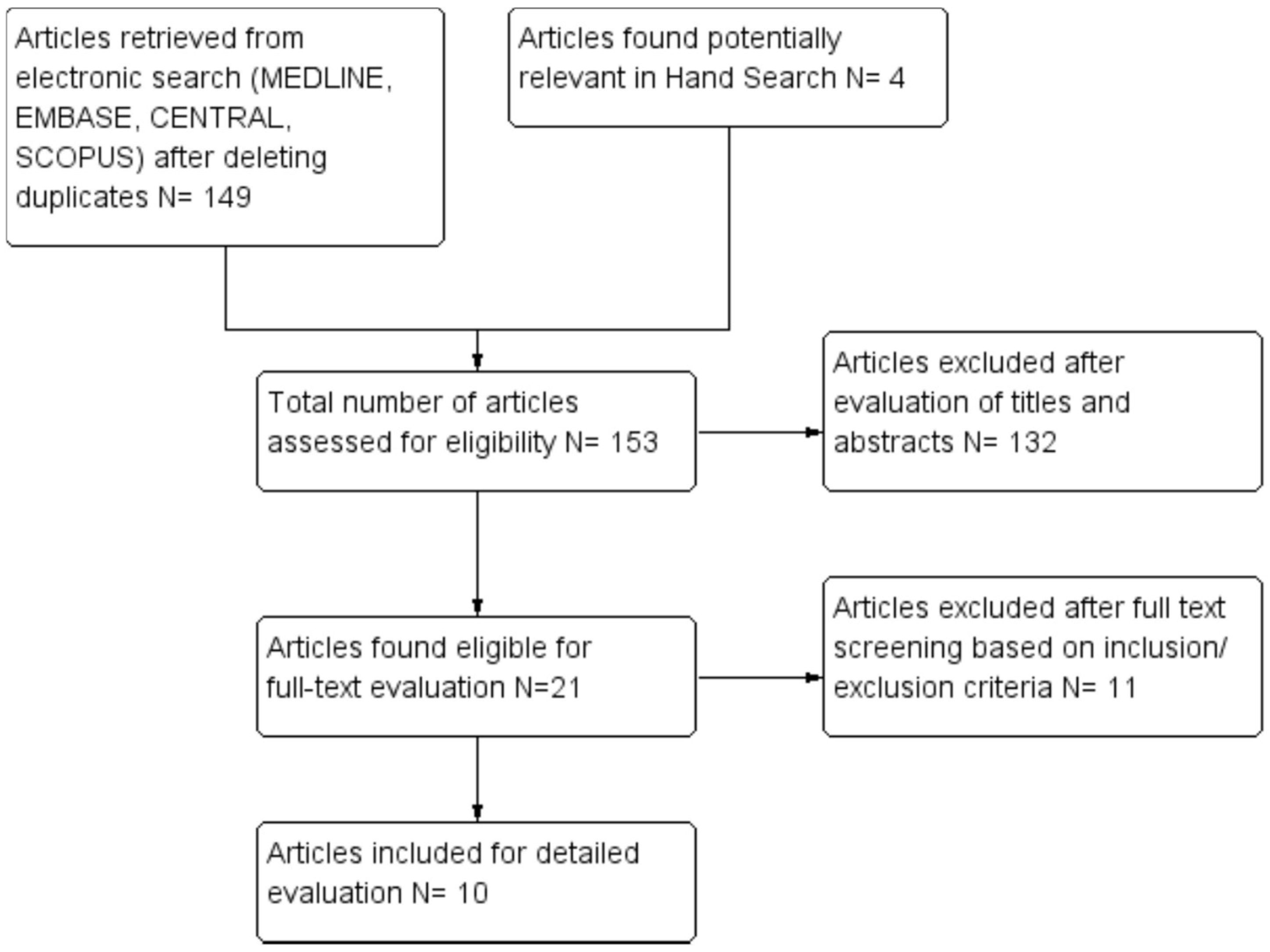
4.4. Selection of Studies
4.5. Data Extraction and Management
- Patients’ demographic characteristics
- Study design and sample size
- Type of platelet concentrate used (PRP, PRF, PRGF, CGF)
- Follow up duration
- source of funding and study setting
- Outcome variables, relative to baseline and post-operative defect characteristics (probing pocket depth (PPD), horizontal and vertical clinical attachment loss (HCAL, VCAL), horizontal and vertical furcation depth (HFD, VFD)
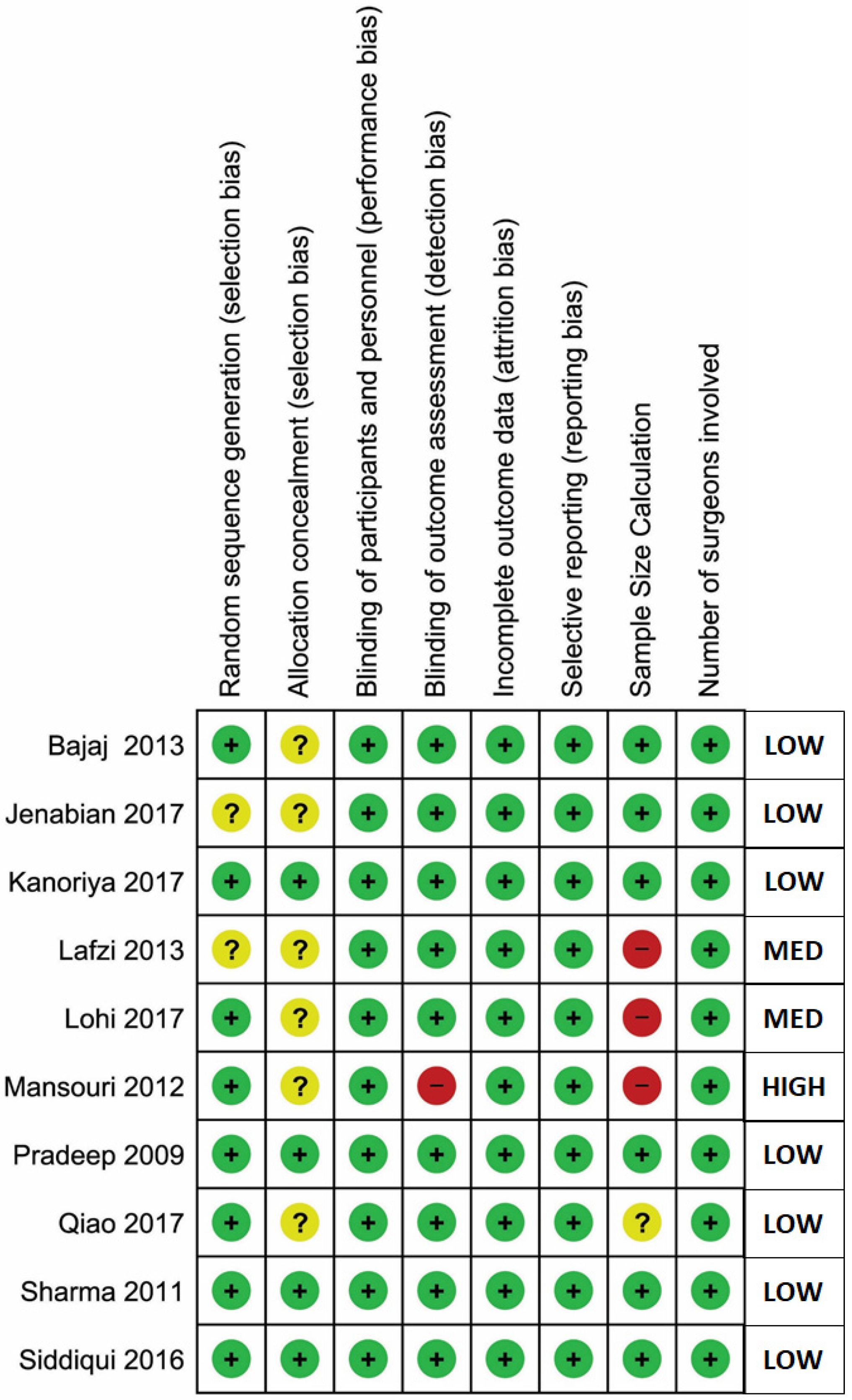
4.6. Risk of Bias Assessment
4.7. Data Synthesis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| APCs | Autologous platelet concentrates |
| PPD | Probing pocket depth |
| HCAL | Horizontal clinical attachment loss |
| VCAL | Vertical clinical attachment loss |
| HFD | Horizontal furcation depth |
| VFD | Vertical furcation depth |
| GTR | Guided tissue regeneration |
| rhBMP2 | Recombinant human bone morphogenetic protein-2 |
| rhPDGF | Recombinant human platelet-derived growth factor |
| TGF-β | Transforming growth factor beta |
| PRP | Platelet-rich plasma |
| PRF | Platelet-rich fibrin |
| PRGF | Plasma rich in growth factors |
| CGF | Concentrated growth factors |
| PRISMA | Preferred reporting items for systematic reviews and meta-analysis |
| RCT | Randomized clinical trials |
| CI | Confidence interval |
| OFD | Open flap debridement |
| BG | Bone graft |
References
- Cattabriga, M.; Pedrazzoli, V.; Wilson, T.G. The conservative approach in the treatment of furcation lesions. Periodontology 2000 2000, 22, 133–153. [Google Scholar]
- Bower, R.C. Furcation morphology relative to periodontal treatment. Furcation root surface anatomy. J. Periodontol. 1979, 50, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Pilloni, A.; Rojas, M.A. Furcation Involvement Classification: A Comprehensive Review and a New System Proposal. Dent. J. 2018, 6, 34. [Google Scholar]
- Glickman, I. Clinical Periodontology: Prevention, Diagnosis, and Treatment of Periodontal Disease in the Practice of General Dentistry, 4th ed.; Saunders: Philadelphia, PA, USA, 1972; ISBN 0-7216-4137-7. [Google Scholar]
- Martin, M.; Gantes, B.; Garrett, S.; Egelberg, J. Treatment of periodontal furcation defects. (I). Review of the literature and description of a regenerative surgical technique. J. Clin. Periodontol. 1988, 15, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, A.R.; Pai, S.; Garg, G.; Devi, P.; Shetty, S.K. A randomized clinical trial of autologous platelet-rich plasma in the treatment of mandibular degree II furcation defects. J. Clin. Periodontol. 2009, 36, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Pontoriero, R.; Lindhe, J.; Nyman, S.; Karring, T.; Rosenberg, E.; Sanavi, F. Guided tissue regeneration in the treatment of furcation defects in mandibular molars. A clinical study of degree III involvements. J. Clin. Periodontol. 1989, 16, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.-Z.; Ge, S.-H.; Sun, Q.-F.; Guo, H.-M.; Yang, P.-S. A pilot study evaluating the effect of recombinant human bone morphogenetic protein-2 and recombinant human beta-nerve growth factor on the healing of Class III furcation defects in dogs. J. Periodontol. 2010, 81, 1289–1298. [Google Scholar]
- Camelo, M.; Nevins, M.L.; Schenk, R.K.; Lynch, S.E.; Nevins, M. Periodontal regeneration in human Class II furcations using purified recombinant human platelet-derived growth factor-BB (rhPDGF-BB) with bone allograft. Int. J. Periodontics Restor. Dent. 2003, 23, 213–225. [Google Scholar]
- Synergistic Induction of Periodontal Tissue Regeneration by Binary Application of Human Osteogenic Protein-1 and Human Transforming Growth Factor-β. Available online: https://www.ncbi.nlm.nih.gov/pubmed/22142147 (accessed on 23 October 2018).
- Bajaj, P.; Pradeep, A.R.; Agarwal, E.; Rao, N.S.; Naik, S.B.; Priyanka, N.; Kalra, N. Comparative evaluation of autologous platelet-rich fibrin and platelet-rich plasma in the treatment of mandibular degree II furcation defects: A randomized controlled clinical trial. J. Periodontal Res. 2013, 48, 573–581. [Google Scholar]
- Jenabian, N.; Haghanifar, S.; Ehsani, H.; Zahedi, E.; Haghpanah, M. Guided tissue regeneration and platelet rich growth factor for the treatment of Grade II furcation defects: A randomized double-blinded clinical trial—A pilot study. Dent. Res. J. 2017, 14, 363–369. [Google Scholar]
- Kanoriya, D.; Pradeep, A.R.; Garg, V.; Singhal, S. Mandibular Degree II Furcation Defects Treatment with Platelet-Rich Fibrin and 1% Alendronate Gel Combination: A Randomized Controlled Clinical Trial. J. Periodontol. 2017, 88, 250–258. [Google Scholar] [CrossRef]
- Lohi, H.S.; Nayak, D.G.; Uppoor, A.S. Comparative Evaluation of the Efficacy of Bioactive Ceramic Composite Granules Alone and in Combination with Platelet Rich Fibrin in the Treatment of Mandibular Class II Furcation Defects: A Clinical and Radiographic Study. J. Clin. Diagn. Res. JCDR 2017, 11, ZC76–ZC80. [Google Scholar] [CrossRef] [PubMed]
- Lafzi, A.; Shirmohammadi, A.; Faramarzi, M.; Jabali, S.; Shayan, A. Clinical Comparison of Autogenous Bone Graft with and without Plasma Rich in Growth Factors in the Treatment of Grade II Furcation Involvement of Mandibular Molars. J. Dent. Res. Dent. Clin. Dent. Prospects 2013, 7, 22–29. [Google Scholar]
- Qiao, J.; Duan, J.Y.; Chu, Y.; Sun, C.Z. Effect of concentrated growth factors on the treatment of degree II furcation involvements of mandibular molars. Beijing Da Xue Xue Bao 2017, 49, 36–42. [Google Scholar] [PubMed]
- Sharma, A.; Pradeep, A.R. Autologous platelet-rich fibrin in the treatment of mandibular degree II furcation defects: A randomized clinical trial. J. Periodontol. 2011, 82, 1396–1403. [Google Scholar] [PubMed]
- Castro, A.B.; Meschi, N.; Temmerman, A.; Pinto, N.; Lambrechts, P.; Teughels, W.; Quirynen, M. Regenerative potential of leucocyte- and platelet-rich fibrin. Part A: Intra-bony defects, furcation defects and periodontal plastic surgery. A systematic review and meta-analysis. J. Clin. Periodontol. 2017, 44, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Del Fabbro, M.; Bortolin, M.; Taschieri, S.; Weinstein, R. Is platelet concentrate advantageous for the surgical treatment of periodontal diseases? A systematic review and meta-analysis. J. Periodontol. 2011, 82, 1100–1111. [Google Scholar] [CrossRef] [PubMed]
- Goldman, H. Therapy of the incipient bifurcation involvement. J. Periodontol. 1958, 29, 112–116. [Google Scholar]
- Staffileno, H.J. Surgical management of the furca invasion. Dent. Clin. N. Am. 1969, 13, 103–119. [Google Scholar]
- Hamp, S.E.; Nyman, S.; Lindhe, J. Periodontal treatment of multirooted teeth. Results after 5 years. J. Clin. Periodontol. 1975, 2, 126–135. [Google Scholar]
- Ramfjord, S.P.; Ash, M. Periodontology and Periodontics, 1st ed.; Saunders: Philadelphia, PA, USA, 1979; ISBN 13-978-0721674605. [Google Scholar]
- Ricchetti, P.A. A furcation classification based on pulp chamber-furcation relationships and vertical radiographic bone loss. Int. J. Periodontics Restor. Dent. 1982, 2, 50–59. [Google Scholar]
- Grant, D.; Stern, I.; Listgarten, M. Periodontics, 6th ed.; C.V. Mosby: St. Louis, IL, USA, 1988. [Google Scholar]
- Goldman, H.; Cohen, D. Periodontal Therapy, 6th ed.; C.V. Mosby: St. Louis, IL, USA, 1988; ISBN 13-9780801618741. [Google Scholar]
- Basaraba, N. Furcation invasions. In Periodontal Diseases; Lea and Febiger: Philadelphia, PA, USA, 1990; ISBN 13-978-0812110845. [Google Scholar]
- Nevins, M.; Cappetta, E. Treatment of maxillary furcations. In Periodontal Therapy—Clinical Approaches and Evidence of Success; Nevins, M., Mellonig, J.T., Eds.; Quintessence: Chicago, IL, USA, 1998; ISBN 13-978-0867153095. [Google Scholar]
- Walter, C.; Kaner, D.; Berndt, D.C.; Weiger, R.; Zitzmann, N.U. Three-dimensional imaging as a pre-operative tool in decision making for furcation surgery. J. Clin. Periodontol. 2009, 36, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, G.; Pontoriero, R.; Lindhe, J. Treatment of furcation—Involved teeth. In Clinical Periodontology and Implant Dentistry; Lindhe, J., Lang, N.P., Karring, T., Eds.; Munksgaard: Copenhagen, Denmark, 2012; Volume 2, pp. 823–847. ISBN 978-1-118-35561-9. [Google Scholar]
- Tal, H.; Lemmer, J. Furcal defects in dry mandibles. Part II: Severity of furcal defects. J. Periodontol. 1982, 53, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Eskow, R.N.; Kapin, S.H. Furcation invasions: Correlating a classification system with therapeutic considerations. Part I. Examination, diagnosis, and classification. Compend. Contin. Educ. Dent. 1984, 5, 479–483, 487. [Google Scholar] [PubMed]
- Tarnow, D.; Fletcher, P. Classification of the vertical component of furcation involvement. J. Periodontol. 1984, 55, 283–284. [Google Scholar] [CrossRef]
- Easley, J.R.; Drennan, G.A. Morphological classification of the furca. J. Can. Dent. Assoc. 1969, 35, 104–107. [Google Scholar] [PubMed]
- Fedi, P., Jr. The Periodontal Syllabus, 2nd ed.; Lea and Febiger: Philadelphia, PA, USA, 1985; ISBN 13-978-0781779722. [Google Scholar]
- Rosenberg, M. Management of osseous defects, furcation involvements, and periodontal-pulpal lesions. In Clinical Dentistry, Periodontal and Oral Surgery; Clark, J.W., Ed.; Harper and Row: Philadelphia, PA, USA, 1986. [Google Scholar]
- Hou, G.L.; Chen, Y.M.; Tsai, C.C.; Weisgold, A.S. A new classification of molar furcation involvement based on the root trunk and horizontal and vertical bone loss. Int. J. Periodontics Restor. Dent. 1998, 18, 257–265. [Google Scholar]
- Mehta, D.B.; Deshpande, N.C.; Dandekar, S.A. Comparative evaluation of platelet-rich fibrin membrane and collagen membrane along with demineralized freeze-dried bone allograft in Grade II furcation defects: A randomized controlled study. J. Indian Soc. Periodontol. 2018, 22, 322–327. [Google Scholar]
- Wanikar, I.; Rathod, S.; Kolte, A.P. Clinico-radiographic evaluation of 1% Alendronate gel as an adjunct and smart blood derivative platelet rich fibrin in grade II furcation defects. J. Periodontol. 2019, 90, 52–60. [Google Scholar] [CrossRef]
- Kaur, J.; Bathla, S.C. Regenerative potential of autologous platelet-rich fibrin with and without amnion membrane in the treatment of Grade-II furcation defects: A clinicoradiographic study. J. Indian Soc. Periodontol. 2018, 22, 235–242. [Google Scholar]
- Sharma, P.; Grover, H.S.; Masamatti, S.S.; Saksena, N. A clinicoradiographic assessment of 1% metformin gel with platelet-rich fibrin in the treatment of mandibular grade II furcation defects. J. Indian Soc. Periodontol. 2017, 21, 303–308. [Google Scholar] [CrossRef]
- Asimuddin, S.; Koduganti, R.R.; Panthula, V.N.R.; Jammula, S.P.; Dasari, R.; Gireddy, H. Effect of Autologous Platelet Rich Fibrin in Human Mandibular Molar Grade II Furcation Defects- A Randomized Clinical Trial. J. Clin. Diagn. Res. JCDR 2017, 11, ZC73–ZC77. [Google Scholar] [PubMed]
- Salaria, S.K.; Ghuman, S.K.; Kumar, S.; Sharma, G. Management of localized advance loss of periodontal support associated Grade II furcation and intrabony defect in chronic periodontitis patient through amalgamation of platelet-rich fibrin and hydroxyapatite bioactive glass composite granules. Contemp. Clin. Dent. 2016, 7, 405–408. [Google Scholar] [CrossRef]
- Biswas, S.; Sambashivaiah, S.; Kulal, R.; Bilichodmath, S.; Kurtzman, G.M. Comparative Evaluation of Bioactive Glass (Putty) and Platelet Rich Fibrin in Treating Furcation Defects. J. Oral Implantol. 2016, 42, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, A.R.; Karvekar, S.; Nagpal, K.; Patnaik, K.; Raju, A.; Singh, P. Rosuvastatin 1.2 mg In Situ Gel Combined with 1:1 Mixture of Autologous Platelet-Rich Fibrin and Porous Hydroxyapatite Bone Graft in Surgical Treatment of Mandibular Class II Furcation Defects: A Randomized Clinical Control Trial. J. Periodontol. 2016, 87, 5–13. [Google Scholar] [PubMed]
- Sandhu, G.K.; Khinda, P.K.; Gill, A.S.; Kalra, H.S. Surgical re-entry evaluation of regenerative efficacy of bioactive Gengigel® and platelet-rich fibrin in the treatment of grade II furcation: A novel approach. Contemp. Clin. Dent. 2015, 6, 570–573. [Google Scholar] [CrossRef] [PubMed]
- Mellonig, J.T.; del Pilar Valderrama, M.; Cochran, D.L. Histological and clinical evaluation of recombinant human platelet-derived growth factor combined with beta tricalcium phosphate for the treatment of human Class III furcation defects. Int. J. Periodontics Restor. Dent. 2009, 29, 169–177. [Google Scholar]
- Lekovic, V.; Camargo, P.M.; Weinlaender, M.; Vasilic, N.; Aleksic, Z.; Kenney, E.B. Effectiveness of a combination of platelet-rich plasma, bovine porous bone mineral and guided tissue regeneration in the treatment of mandibular grade II molar furcations in humans. J. Clin. Periodontol. 2003, 30, 746–751. [Google Scholar] [CrossRef]
- Siddiqui, Z.R.; Jhingran, R.; Bains, V.K.; Srivastava, R.; Madan, R.; Rizvi, I. Comparative evaluation of platelet-rich fibrin versus beta-tri-calcium phosphate in the treatment of Grade II mandibular furcation defects using cone-beam computed tomography. Eur. J. Dent. 2016, 10, 496–506. [Google Scholar]
- Mansouri, S.S.; Ghasemi, M.; Darmian, S.S.; Pourseyediyan, T. Treatment of Mandibular Molar Class II Furcation Defects in Humans with Bovine Porous Bone Mineral in Combination with Plasma Rich in Growth Factors. J. Dent. Tehran Iran 2012, 9, 41–49. [Google Scholar]
- Del Fabbro, M.; Lolato, A.; Panda, S.; Corbella, S.; Satpathy, A.; Das, A.C.; Kumar, M.; Taschieri, S. Methodological Quality Assessment of Systematic Reviews on Autologous Platelet Concentrates for the Treatment of Periodontal Defects. J. Evid. Based Dent. Pract. 2017, 17, 239–255. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Doraiswamy, J.; Malaiappan, S.; Varghese, S.S.; Del Fabbro, M. Additive effect of autologous platelet concentrates in treatment of intrabony defects: A systematic review and meta-analysis. J. Investig. Clin. Dent. 2016, 7, 13–26. [Google Scholar] [PubMed]
- Del Fabbro, M.; Karanxha, L.; Panda, S.; Bucchi, C.; Nadathur Doraiswamy, J.; Sankari, M.; Ramamoorthi, S.; Varghese, S.; Taschieri, S. Autologous platelet concentrates for treating periodontal infrabony defects. Cochrane Database Syst. Rev. 2018, 11, CD011423. [Google Scholar] [CrossRef]
- Mozzati, M.; Muzio, G.; Del Fabbro, M.; Pol, R.; D’Antico, S.; Mortellaro, C. Autologous Haemocomponents as Stimulators of Tissue Healing. (Emocomponenti Autologhi Come Stimolanti Della Guarigione Dei tessuti [Italian]), 1st ed.; Tueor Servizi SRL: Torino, Italy, 2017; ISBN 978-88-940334-9-6. [Google Scholar]
- Dohan Ehrenfest, D.M.; Pinto, N.R.; Pereda, A.; Jiménez, P.; Corso, M.D.; Kang, B.-S.; Nally, M.; Lanata, N.; Wang, H.-L.; Quirynen, M. The impact of the centrifuge characteristics and centrifugation protocols on the cells, growth factors, and fibrin architecture of a leukocyte- and platelet-rich fibrin (L-PRF) clot and membrane. Platelets 2018, 29, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Del Fabbro, M.; Corbella, S.; Ceresoli, V.; Ceci, C.; Taschieri, S. Plasma Rich in Growth Factors Improves Patients’ Postoperative Quality of Life in Maxillary Sinus Floor Augmentation: Preliminary Results of a Randomized Clinical Study. Clin. Implant Dent. Relat. Res. 2015, 17, 708–716. [Google Scholar] [PubMed]
- Del Fabbro, M.; Ceresoli, V.; Lolato, A.; Taschieri, S. Effect of platelet concentrate on quality of life after periradicular surgery: A randomized clinical study. J. Endod. 2012, 38, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Fabbro, M.D.; Bortolin, M.; Taschieri, S.; Ceci, C.; Weinstein, R.L. Antimicrobial properties of platelet-rich preparations. A systematic review of the current pre-clinical evidence. Platelets 2016, 27, 276–285. [Google Scholar] [PubMed]
- Drago, L.; Bortolin, M.; Vassena, C.; Taschieri, S.; Del Fabbro, M. Antimicrobial activity of pure platelet-rich plasma against microorganisms isolated from oral cavity. BMC Microbiol. 2013, 13, 47. [Google Scholar]


| Sl | Author | Year | Classification System |
|---|---|---|---|
| Horizontal Component | |||
| 1 | Goldman, H.M [20] | 1958 | Grade I: Incipient lesion; Grade II: Cul-de-sac lesion; Grade III: Through-and-through lesion |
| 2 | Staffileno, H.J. [21] | 1969 | Class I: Furcations with a soft tissue lesion extending to furcal level but with a minor degree of osseous destruction; Class II: Furcations with a soft tissue lesion and a variable degree of osseous destruction but not a through-and-through communication through the furca; Class III: Furcations with osseous destruction with through-and-through communication |
| 3 | Glickman, I. [4] | 1972 | Grade I: Incipient lesion. Suprabony pocket and slight bone loss in the furcation area. Grade II: Loss of interradicular bone and pocket formation but a portion of the alveolar bone and periodontal ligament remain intact. Grade III: Through-and-through lesion. Grade IV: Through-and-through lesion with gingival recession, leading to a clearly visible furcation area. |
| 4 | Hamp, S.E. et al. [22] | 1975 | Degree I: Horizontal attachment loss < 3 mm; Degree II: Horizontal attachment loss > 3 mm not encompassing the width of the furcation area; Degree III: Horizontal through-and-through destruction of the periodontal tissue in the furcation area. |
| 5 | Ramjford, S.P. et al. [23] | 1979 | Class I: Tissue destruction < 2 mm (1/3 of tooth width) into the furcation; Class II: Tissue destruction > 2 mm (>1/3 of tooth width); Class III: Through-and-through involvement. |
| 6 | Richietti, P.A. [24] | 1982 | Class I: 1 mm of horizontal invasion; Class Ia. 1–2 mm of horizontal invasion; Class II: 2–4 mm of horizontal invasion; Class IIa. 4–6 mm of horizontal invasion; Class III: >6 mm of horizontal invasion. |
| 7 | Grant, D.A. et al. [25] | 1988 | Class I: Involvement of the flute only; Class II: Involvement partially under the roof; Class III: Through-and-through loss. |
| 8 | Goldman, H.M. et al. [26] | 1988 | Degree I: Involves furcation entrance; Degree II: Involvement extends under the roof of furcation; Degree III: Through-and-through involvement. |
| 9 | Basaraba, N. [27] | 1990 | Class I: Initial furcation involvement; Class II: Partial furcation involvement; Class III: Communicating furcation involvement. |
| 10 | Nevins, M. et al. [28] | 1998 | Class I: Incipient or early loss of attachment; Class II: A deeper invasion and loss of attachment that does not extend to a complete invasion; Class III: Complete loss of periodontium extending from buccal to lingual surface. Diagnosed radiographically and clinically |
| 11 | Walter, C.et al [29] | 2009 | Modification of the Hamp et al. classification. Degree I: Horizontal attachment loss < 1/3 of the width of the tooth; Degree II: Horizontal loss of support > 3 mm, < 6 mm; Degree II–III: Horizontal loss of support > 6 mm, but not extending completely. Degree III: Horizontal through-and-through destruction. |
| 12 | Carnevale, G. et al. [30] | 2012 | Degree I: Horizontal attachment loss < 1/3; Degree II: Horizontal attachment loss > 1/3; Degree III: Horizontal through-and-through destruction. |
| Vertical Component | |||
| 1 | Tal, H. et al. [31] | 1982 | Furcal rating 1: Depth of the furcation is 0 mm; Furcal rating 2: Depth of the furcation is 1–2 mm; Furcal rating 3: Depth of the furcation is 3 mm; Furcal rating 4: Depth of the furcation is 4 mm or more. |
| 2 | Eskow, R.N. et al. [32] | 1984 | Furcation involvement grade 1 is classified as: Subclass A: Vertical destruction > 1/3; Subclass B: Vertical destruction of 2/3; Subclass C: Vertical destruction beyond the apical third of interradicular height. |
| 3 | Tarnow, D. et al. [33] | 1985 | For each class of horizontal classification (I–III), a subclass based on the vertical bone resorption was added: Subclass A: 0–3 mm; Subclass B: 4–6 mm; Subclass C: >7 mm. |
| Horizontal & Vertical Component (Combined) | |||
| 1 | Easley, J.R. et al. [34] | 1969 | Class I: Incipient involvement, but there is no horizontal component to the furca; Class II: Type 1. Horizontal attachment loss into the furcation; Type 2. Vertical attachment loss into the furcation; Class III: Through-and-through attachment loss into the furcation; Type 1. Horizontal attachment loss into the furcation; Type 2. Vertical attachment loss into the furcation. |
| 2 | Fedi, P.F. [35] | 1985 | Glickman + Hamp classifications: Grades are the same as Glickman’s classification (I–IV); Grade II is subdivided into degrees I and II; Degree I. Vertical bone loss 1–3 mm; Degree II. Vertical bone loss > 3 mm, but not communicate through-and-through. |
| 3 | Rosemberg, M.M. [36] | 1986 | Horizontal: Degree I: Probing < 4 mm; Degree II: Probing > 4 mm; Degree III: Two or three furcations classified as degree II are found. Vertical: Shallow: Slight lateral extension of an interradicular defect, from the center of the trifurcation in a horizontal direction; Deep: Internal furcation involvement but not penetrating the adjacent furcation. |
| 4 | Hou, G.L. et al. [37] | 1998 | Classification based on root trunk length and horizontal and vertical bone loss. Types of root trunk:
|
| Study & Year | Reason for Exclusion |
|---|---|
| Mehta et al. 2018 [38] | Use of Collagen Membrane along with DFDBA in control group |
| Wanikar et al. 2018 [39] | Both Control and Experimental group use PRF |
| Kaur et al. 2018 [40] | Both Control and Experimental group use PRF |
| Sharma et al. 2017 [41] | Both Control and Experimental group use PRF |
| Asimuddin et al. 2017 [42] | Comparison between use of PRF and Allograft + GTR |
| Salaria et al. 2016 [43] | Case Report |
| Biswas et al. 2016 [44] | Comparison between PRF and Bioactive Glass. |
| Pradeep et al. 2016 [45] | Both Control and Experimental group uses PRF |
| Sandhu et al. 2015 [46] | Case Report |
| Mellonig et al. 2009 [47] | Histological assessment |
| Lekovic et al. 2003 [48] | Comparison of PRP/BPBM/GTR versus OFD alone |
| Study & Year | Study Design | RCT Type | Treatment Comparison | N. Defects Test/Control | Age Range | Gender M/F | Follow Up |
|---|---|---|---|---|---|---|---|
| Kanoriya et al. 2017 [13] | RCT | Parallel | OFD vs. OFD + PRF | 26/26 | 30–50 (38) | 36/36 | 9 m |
| Siddiqui et al. 2016 [49] | RCT | Parallel | OFD vs. OFD + PRF | 17/17 | 30–50 | 24/7 | 6 m |
| Bajaj et al. 2013 [11] | RCT | Parallel | OFD vs. OFD + PRP OFD vs. OFD + PRF | 27/27 27/27 | 39.4 | 22/20 | 9 m |
| Sharma et al. 2011 [17] | RCT | Split Mouth | OFD vs. OFD + PRF | 18/18 | 34.2 | 10/8 | 9 m |
| Pradeep et al. 2009 [6] | RCT | Split Mouth | OFD vs. OFD + PRP | 20/20 | 42.8 | 10/10 | 6 m |
| Lohi et al. 2017 [14] | RCT | Parallel | BCCG + PRF vs. BCCG alone | 10/10 | 25–65 (43.05 + 10.73) | 12/4 | 6 m |
| Lafzi et al. 2013 [15] | RCT | Parallel | ABG + PRGF vs. ABG alone | 15/15 | NR | NR | 6 m |
| Mansouri et al. 2012 [50] | RCT | Split Mouth | BPBM + PRGF vs. BPBM alone | 7/7 | 44.7 + 11.2 | 4/3 | 6 m |
| Qiao et al. 2017 [16] | RCT | Parallel | BG + CGF vs. CGF alone | 15/16 | NR | 15/5 | 12 m |
| Jenabian et al. 2017 [12] | RCT | Split Mouth | GTR + PRGF vs. GTR alone | 8/8 | NR | NR | 6 m |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panda, S.; Karanxha, L.; Goker, F.; Satpathy, A.; Taschieri, S.; Francetti, L.; Chandra Das, A.; Kumar, M.; Panda, S.; Del Fabbro, M. Autologous Platelet Concentrates in Treatment of Furcation Defects—A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2019, 20, 1347. https://doi.org/10.3390/ijms20061347
Panda S, Karanxha L, Goker F, Satpathy A, Taschieri S, Francetti L, Chandra Das A, Kumar M, Panda S, Del Fabbro M. Autologous Platelet Concentrates in Treatment of Furcation Defects—A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences. 2019; 20(6):1347. https://doi.org/10.3390/ijms20061347
Chicago/Turabian StylePanda, Sourav, Lorena Karanxha, Funda Goker, Anurag Satpathy, Silvio Taschieri, Luca Francetti, Abhaya Chandra Das, Manoj Kumar, Sital Panda, and Massimo Del Fabbro. 2019. "Autologous Platelet Concentrates in Treatment of Furcation Defects—A Systematic Review and Meta-Analysis" International Journal of Molecular Sciences 20, no. 6: 1347. https://doi.org/10.3390/ijms20061347
APA StylePanda, S., Karanxha, L., Goker, F., Satpathy, A., Taschieri, S., Francetti, L., Chandra Das, A., Kumar, M., Panda, S., & Del Fabbro, M. (2019). Autologous Platelet Concentrates in Treatment of Furcation Defects—A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences, 20(6), 1347. https://doi.org/10.3390/ijms20061347








