Role of GirK Channels in Long-Term Potentiation of Synaptic Inhibition in an In Vivo Mouse Model of Early Amyloid-β Pathology
Abstract
1. Introduction
2. Results
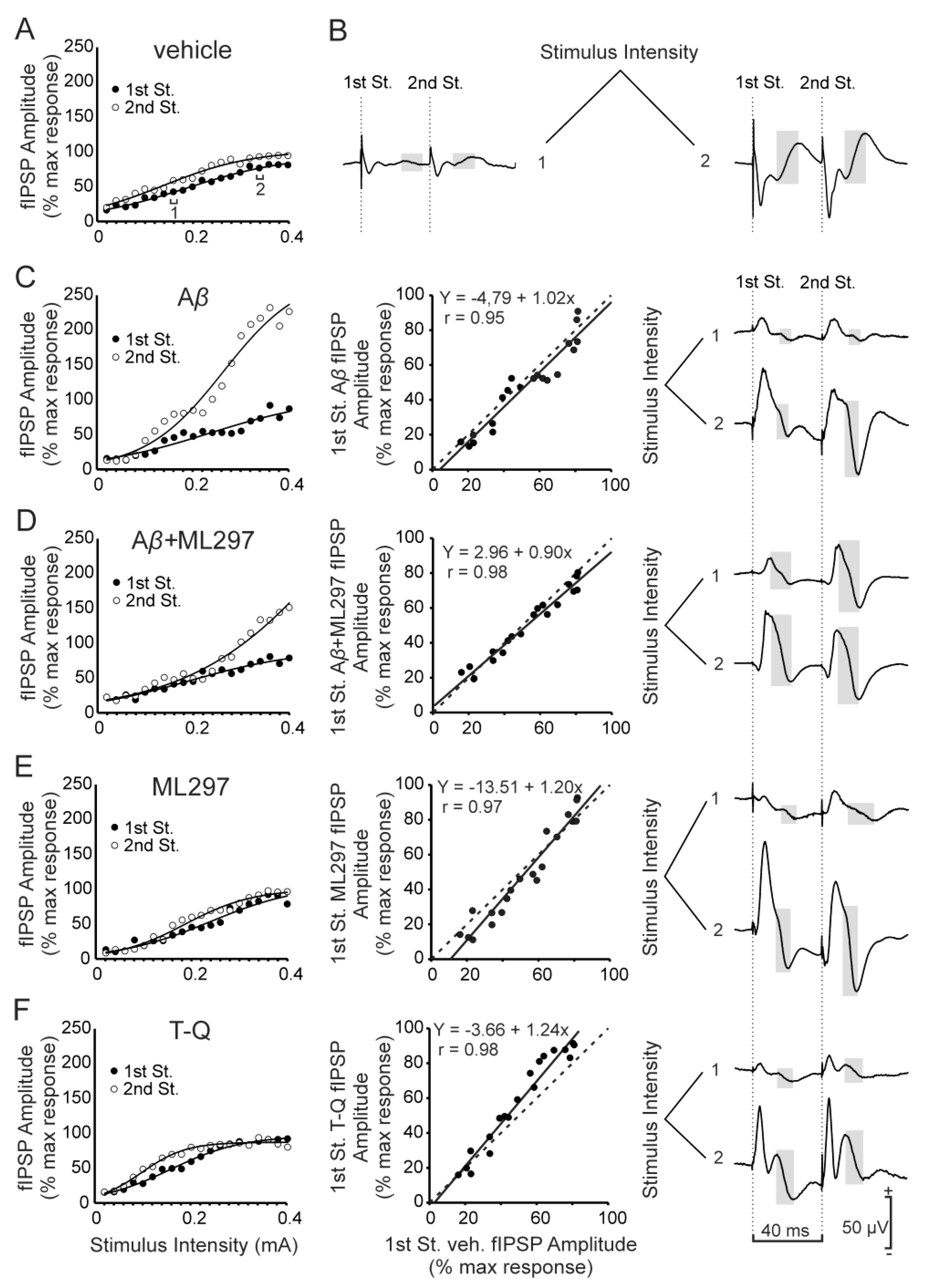
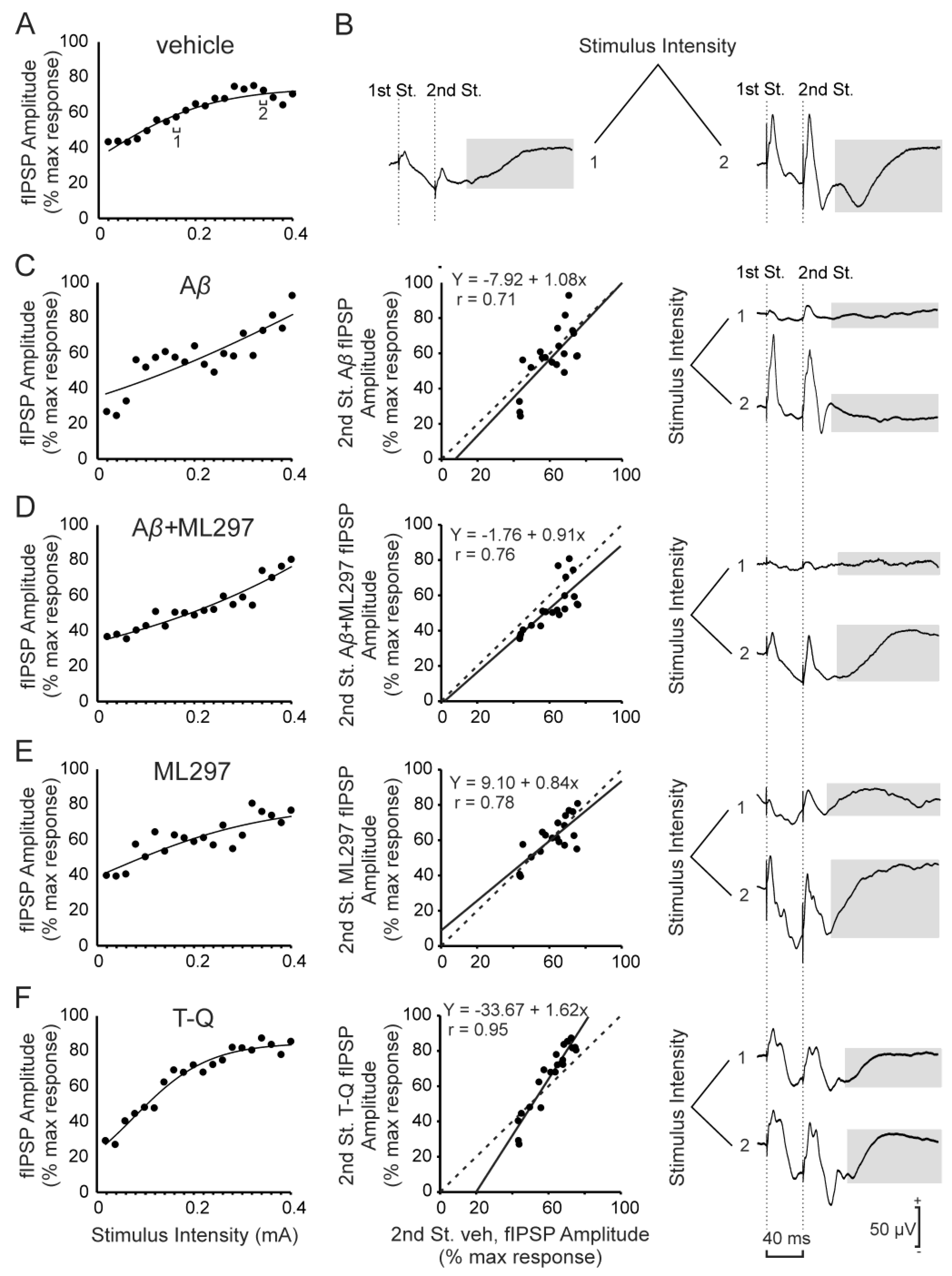
2.1. GABAA-Dependent Signaling is Disrupted by Aβ or GirK Blockage
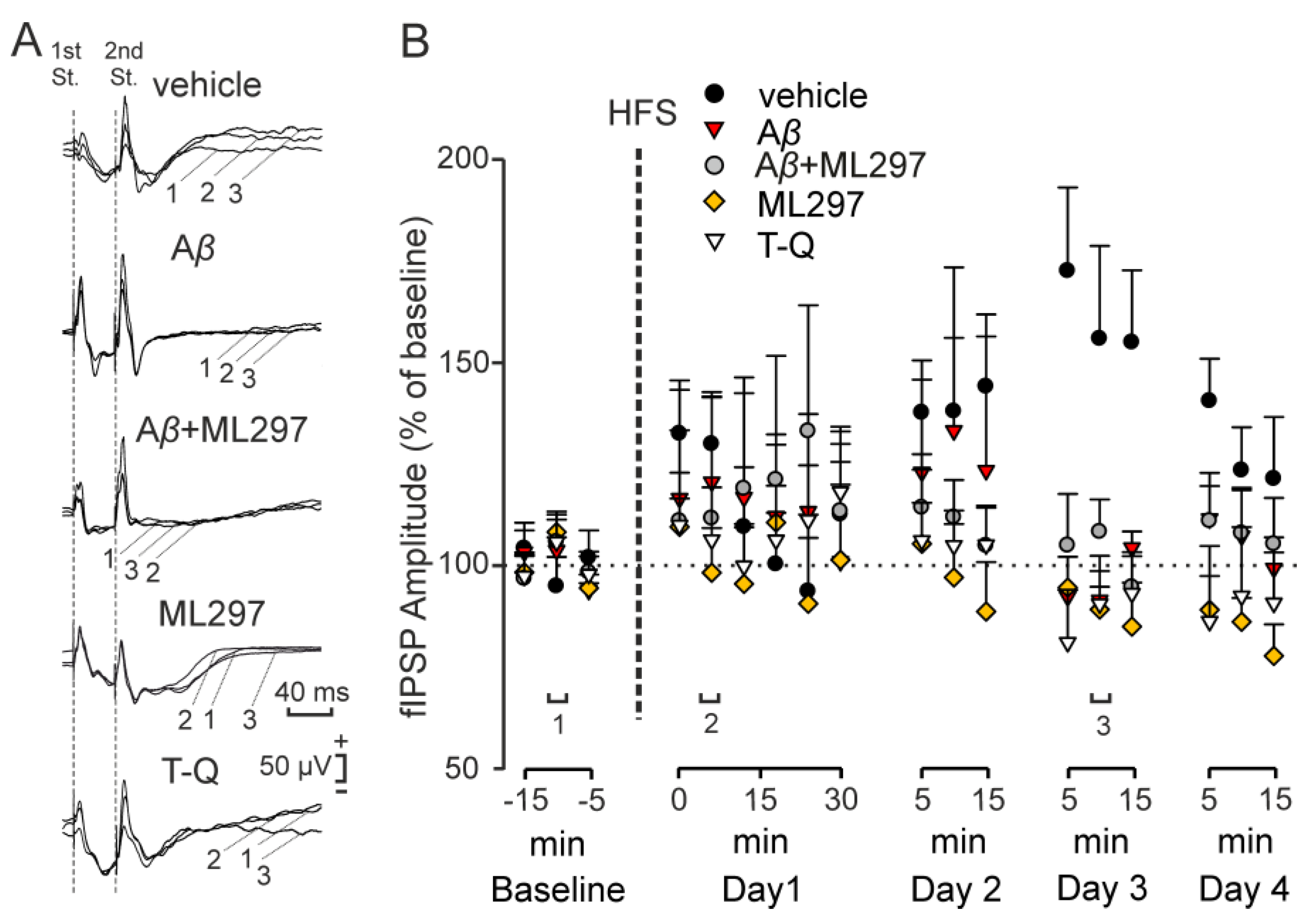
2.2. GirK-Dependent Signaling Enhancement Prevents the Decrease in Synaptic Inhibition Caused by Aβ
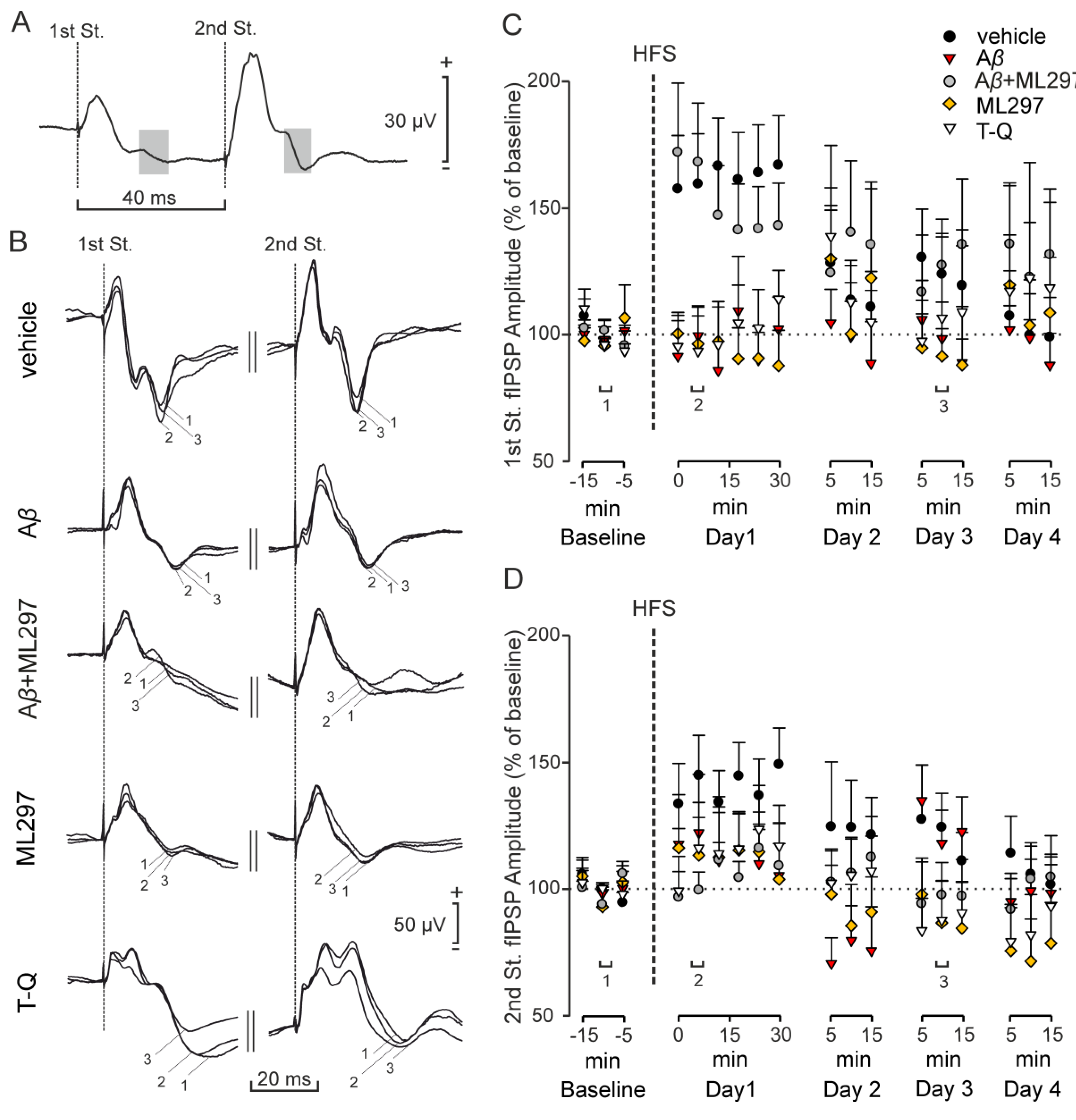
2.3. Inhibitory LTP Mirrors Excitatory LTP in the CA3–CA1 Dorsal Hippocampal Synapse
2.4. GirK-Dependent Signaling Undergoes LTP with a Late Appearance Latency after HFS
3. Discussion
3.1. Role of GirK Channels in the Excitability of the Dorsal Hippocampus of Alert Mice
3.2. GirK-Dependent Signaling Controls Synaptic Plasticity of Inhibitory Postsynaptic Responses in the Dorsal Hippocampus of Behaving Mice
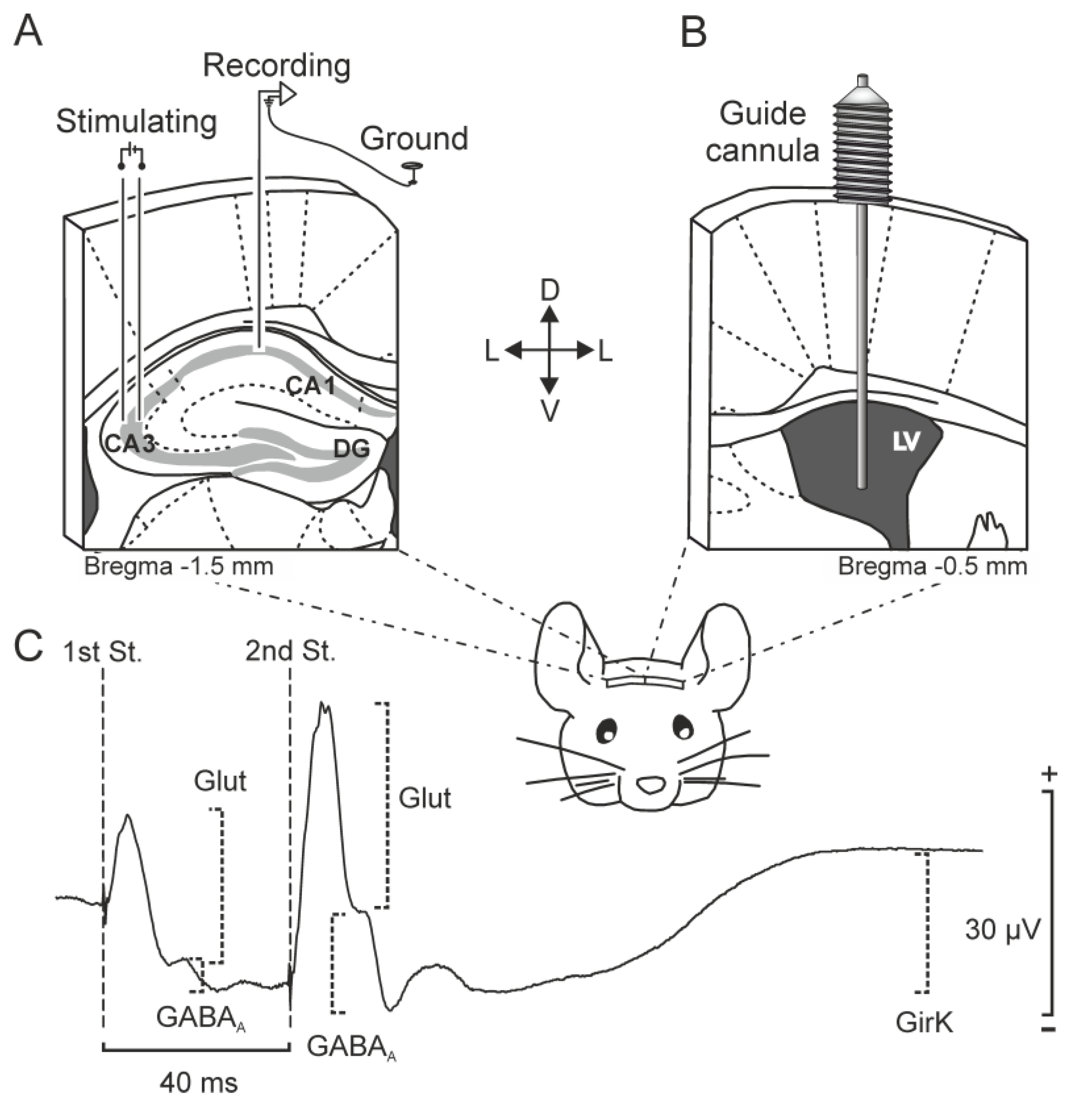
4. Materials and Methods
4.1. Subjects
4.2. Surgery for Chronic Recordings and ICV Injections in Alert Mice
4.3. Input/output Curves
4.4. Long-Term Potentiation
4.5. fIPSP Analysis
4.6. Drugs
4.7. Statistics
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Aβ | amyloid-β |
| AD | Alzheimer’s disease |
| CB1 | cannabinoid receptor type 1 |
| fEPSP | field excitatory postsynaptic potential |
| fIPSP | field inhibitory postsynaptic potential |
| fPSP | field postsynaptic potential |
| GirK | G-protein-gated potassium channels |
| GPCR | G-protein-coupled receptors |
| HFS | high-frequency stimulation |
| ICV | intracerebroventricular |
| LTD | long-term depression |
| LTP | long-term potentiation |
| NMDA | N-methyl-D-aspartate |
| TBS | theta burst stimulation |
| T-Q | tertiapin-Q |
References
- Palop, J.J.; Chin, J.; Roberson, E.D.; Wang, J.; Thwin, M.T.; Bien-Ly, N.; Yoo, J.; Ho, K.O.; Yu, G.Q.; Kreitzer, A.; et al. Aberrant Excitatory Neuronal Activity and Compensatory Remodeling of Inhibitory Hippocampal Circuits in Mouse Models of Alzheimer’s Disease. Neuron 2007, 55, 697–711. [Google Scholar] [CrossRef] [PubMed]
- Pini, L.; Pievani, M.; Bocchetta, M.; Altomare, D.; Bosco, P.; Cavedo, E.; Galluzzi, S.; Marizzoni, M.; Frisoni, G.B. Brain atrophy in Alzheimer’s Disease and aging. Ageing Res. Rev. 2016, 30, 25–48. [Google Scholar] [CrossRef] [PubMed]
- Palop, J.; Mucke, L. Amyloid-beta-induced neuronal dysfunction in Alzheimer’s disease: From synapses toward neural networks. Nat. Neurosci. 2010, 13, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Palop, J.; Mucke, L. Synaptic depression and aberrant excitatory network activity in alzheimer’s disease: Two faces of the same coin? Neuromol. Med. 2010, 12, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Busche, M.A.; Konnerth, A. Neuronal hyperactivity—A key defect in Alzheimer’s disease? BioEssays 2015, 37, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Busche, M.A.; Konnerth, A. Impairments of neural circuit function in Alzheimer’s disease. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2016, 371, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kurudenkandy, F.R.; Zilberter, M.; Biverstal, H.; Presto, J.; Honcharenko, D.; Stromberg, R.; Johansson, J.; Winblad, B.; Fisahn, A. Amyloid-β-Induced Action Potential Desynchronization and Degradation of Hippocampal Gamma Oscillations Is Prevented by Interference with Peptide Conformation Change and Aggregation. J. Neurosci. 2014, 34, 11416–11425. [Google Scholar] [CrossRef] [PubMed]
- Palop, J.J.; Mucke, L. Network abnormalities and interneuron dysfunction in Alzheimer disease. Nat. Rev. Neurosci. 2016, 17, 777–792. [Google Scholar] [CrossRef] [PubMed]
- Villette, V.; Poindessous-Jazat, F.; Simon, A.; Lena, C.; Roullot, E.; Bellessort, B.; Epelbaum, J.; Dutar, P.; Stephan, A. Decreased Rhythmic GABAergic Septal Activity and Memory-Associated θ Oscillations after Hippocampal Amyloid-β Pathology in the Rat. J. Neurosci. 2010, 30, 10991–11003. [Google Scholar] [CrossRef] [PubMed]
- Blennow, K.; de Leon, M.J.; Zetterberg, H. Alzheimer’s disease. Lancet 2006, 368, 387–403. [Google Scholar] [CrossRef]
- Walsh, D.M.; Klyubin, I.; Fadeeva, J.V.; Cullen, W.K.; Anwyl, R.; Wolfe, M.S.; Rowan, M.J.; Selkoe, D.J. Naturally secreted oligomers of amyloid β protein potently inhibit hippocampal long-term potentiation in vivo. Nature 2002, 416, 535. [Google Scholar] [CrossRef] [PubMed]
- Styr, B.; Slutsky, I. Imbalance between firing homeostasis and synaptic plasticity drives early-phase Alzheimer’s disease. Nat. Neurosci. 2018, 21, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Luján, R.; Marron Fernandez de Velasco, E.; Aguado, C.; Wickman, K. New insights into the therapeutic potential of Girk channels. Trends Neurosci. 2014, 37, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Jelacic, T.M.; Kennedy, M.E.; Wickman, K.; Clapham, D.E. Functional and biochemical evidence for G-protein-gated inwardly rectifying K+(GIRK) channels composed of GIRK2 and GIRK3. J. Biol. Chem. 2000, 275, 36211–36216. [Google Scholar] [CrossRef] [PubMed]
- Wickman, K.; Karschin, C.; Karschin, A.; Picciotto, M.R.; Clapham, D.E. Brain localization and behavioral impact of the G-protein-gated K+ channel subunit GIRK4. J. Neurosci. 2000, 20, 5608–5615. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Alacid, L.; Watanabe, M.; Molnár, E.; Wickman, K.; Luján, R. Developmental regulation of G protein-gated inwardly-rectifying K + (GIRK/Kir3) channel subunits in the brain. Eur. J. Neurosci. 2011, 34, 1724–1736. [Google Scholar] [CrossRef] [PubMed]
- Lüscher, C.; Slesinger, P.A. Emerging roles for G protein-gated inwardly rectifying potassium (GIRK) channels in health and disease. Nat. Rev. Neurosci. 2010, 11, 301. [Google Scholar] [CrossRef] [PubMed]
- Aguado, C.; Colón, J.; Ciruela, F.; Schlaudraff, F.; Cabañero, M.J.; Perry, C.; Watanabe, M.; Liss, B.; Wickman, K.; Luján, R. Cell type-specific subunit composition of G protein-gated potassium channels in the cerebellum. J. Neurochem. 2008, 105, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Koyrakh, L. Molecular and Cellular Diversity of Neuronal G-Protein-Gated Potassium Channels. J. Neurosci. 2005, 25, 11468–11478. [Google Scholar] [CrossRef] [PubMed]
- Lüscher, C.; Jan, L.Y.; Stoffel, M.; Malenka, R.C.; Nicoll, R.A. G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron 1997, 19, 687–695. [Google Scholar] [CrossRef]
- Hibino, H.; Inanobe, A.; Furutani, K.; Murakami, S.; Findlay, I.; Kurachi, Y. Inwardly Rectifying Potassium Channels: Their Structure, Function, and Physiological Roles. Physiol. Rev. 2010, 90, 291–366. [Google Scholar] [CrossRef] [PubMed]
- Isomoto, S.; Kondo, C.; Kurachi, Y. Inwardly rectifying potassium channels: Their molecular heterogeneity and function. Jpn. J. Physiol. 1997, 47, 11–39. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Johnston, D. A1 adenosine receptor-mediated GIRK channels contribute to the resting conductance of CA1 neurons in the dorsal hippocampus. J. Neurophysiol. 2015, 113, 2511–2523. [Google Scholar] [CrossRef] [PubMed]
- Glaaser, I.W.; Slesinger, P.A. Structural Insights into GIRK Channel Function. In International Review of Neurobiology; Academic Press: Cambridge, MA, USA, 2015; ISBN 9780128024584. [Google Scholar]
- Malik, R.; Johnston, D. Dendritic GIRK Channels Gate the Integration Window, Plateau Potentials, and Induction of Synaptic Plasticity in Dorsal But Not Ventral CA1 Neurons. J. Neurosci. 2017, 37, 3940–3955. [Google Scholar] [CrossRef] [PubMed]
- Bakker, A.; Krauss, G.L.; Albert, M.S.; Speck, C.L.; Jones, L.R.; Stark, C.E.; Yassa, M.A.; Bassett, S.S.; Shelton, A.L.; Gallagher, M. Reduction of Hippocampal Hyperactivity Improves Cognition in Amnestic Mild Cognitive Impairment. Neuron 2012, 74, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Busche, M.A.; Kekuš, M.; Adelsberger, H.; Noda, T.; Förstl, H.; Nelken, I.; Konnerth, A. Rescue of long-range circuit dysfunction in Alzheimer’s disease models. Nat. Neurosci. 2015, 18, 1623. [Google Scholar] [CrossRef] [PubMed]
- Verret, L.; Mann, E.O.; Hang, G.B.; Barth, A.M.I.; Cobos, I.; Ho, K.; Devidze, N.; Masliah, E.; Kreitzer, A.C.; Mody, I.; et al. Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in alzheimer model. Cell 2012, 149, 708–721. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rodríguez, I.; Temprano-Carazo, S.; Nájera, A.; Djebari, S.; Yajeya, J.; Gruart, A.; Delgado-García, J.M.; Jiménez-Díaz, L.; Navarro-López, J.D. Activation of G-protein-gated inwardly rectifying potassium (Kir3/GirK) channels rescues hippocampal functions in a mouse model of early amyloid-β pathology. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.S.; Shi, S.H.; Ule, J.; Ruggiu, M.; Barker, L.A.; Darnell, R.B.; Yuh, N.J.; Jan, L.Y. Common molecular pathways mediate long-term potentiation of synaptic excitation and slow synaptic inhibition. Cell 2005, 123, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Nava-Mesa, M.O.; Jiménez-Díaz, L.; Yajeya, J.; Navarro-Lopez, J.D. Amyloid-β induces synaptic dysfunction through G protein-gated inwardly rectifying potassium channels in the fimbria-CA3 hippocampal synapse. Front. Cell. Neurosci. 2013, 7, 117. [Google Scholar] [CrossRef] [PubMed]
- Vega-Flores, G.; Rubio, S.E.; Jurado-Parras, M.T.; Gómez-Climent, M.Á.; Hampe, C.S.; Manto, M.; Soriano, E.; Pascual, M.; Gruart, A.; Delgado-García, J.M. The GABAergic septohippocampal pathway is directly involved in internal processes related to operant reward learning. Cereb. Cortex 2014, 24, 2093–2107. [Google Scholar] [CrossRef] [PubMed]
- Vega-Flores, G.; Gruart, A.; Delgado-GarcÍa, J.M. Involvement of the gabaergic septo-hippocampal pathway in brain stimulation reward. PLoS ONE 2014, 9, e113787. [Google Scholar] [CrossRef] [PubMed]
- Fanselow, M.S.; Dong, H.W. Are the Dorsal and Ventral Hippocampus Functionally Distinct Structures? Neuron 2010, 65, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Nava-Mesa, M.O.; Jiménez-Díaz, L.; Yajeya, J.; Navarro-Lopez, J.D. GABAergic neurotransmission and new strategies of neuromodulation to compensate synaptic dysfunction in early stages of Alzheimer’s disease. Front. Cell. Neurosci. 2014, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Drake, C.T.; Bausch, S.B.; Milner, T.A.; Chavkin, C. GIRK1 immunoreactivity is present predominantly in dendrites, dendritic spines, and somata in the CA1 region of the hippocampus. Proc. Natl. Acad. Sci. USA 1997, 94, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Sebe, J.; Berger, A. GABAB modulation of GABAA and glycine receptor-mediated synaptic currents in hypoglossal motoneurons. Respir. Physiol. Neurobiol. 2004, 141, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, H.; Chen, Z.; Xu, H.; Bu, G.; Zheng, H. Implications of GABAergic neurotransmission in Alzheimer’s disease. Front. Aging Neurosci. 2016, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Potapenko, E.S.; Biancardi, V.C.; Zhou, Y.; Stern, J.E. Astrocytes Modulate a Postsynaptic NMDA-GABAA-Receptor Crosstalk in Hypothalamic Neurosecretory Neurons. J. Neurosci. 2013, 33, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Llamosas, N.; Ugedo, L.; Torrecilla, M. Inactivation of GIRK channels weakens the pre- and postsynaptic inhibitory activity in dorsal raphe neurons. Physiol. Rep. 2017. [Google Scholar] [CrossRef] [PubMed]
- Vezzani, A.; Sperk, G.; Colmers, W.F. Neuropeptide Y: Emerging evidence for a functional role in seizure modulation. Trends Neurosci. 1999, 22, 25–30. [Google Scholar] [CrossRef]
- Mayordomo-Cava, J.; Yajeya, J.; Navarro-López, J.D.; Jiménez-Díaz, L. Amyloid-β(25-35) Modulates the Expression of GirK and KCNQ Channel Genes in the Hippocampus. PLoS ONE 2015, 10, e0134385. [Google Scholar] [CrossRef] [PubMed]
- May, L.M.; Anggono, V.; Gooch, H.M.; Jang, S.E.; Matusica, D.; Kerbler, G.M.; Meunier, F.A.; Sah, P.; Coulson, E.J. G-Protein-Coupled inwardly Rectifying Potassium (GIRK) channel activation by the p75 neurotrophin receptor is required for amyloid β toxicity. Front. Neurosci. 2017, 11, 455. [Google Scholar] [CrossRef] [PubMed]
- Saura, C.A.; Parra-Damas, A.; Enriquez-Barreto, L. Gene expression parallels synaptic excitability and plasticity changes in Alzheimer’s disease. Front. Cell. Neurosci. 2015, 9, 318. [Google Scholar] [CrossRef] [PubMed]
- Foster, T.; Kyritsopoulos, C.; Kumar, A. Central role for NMDA receptors in redox mediated impairment of synaptic function during aging and Alzheimer’s disease. Behav. Brain Res. 2016, 322, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Puzzo, D.; Vitolo, O.; Trinchese, F.; Jacob, J.P.; Palmeri, A.; Arancio, O. Amyloid-β Peptide Inhibits Activation of the Nitric Oxide/cGMP/cAMP-Responsive Element-Binding Protein Pathway during Hippocampal Synaptic Plasticity. J. Neurosci. 2005, 25, 6887–6897. [Google Scholar] [CrossRef] [PubMed]
- Kalweit, A.N.; Yang, H.; Colitti-Klausnitzer, J.; Fülöp, L.; Bozsó, Z.; Penke, B.; Manahan-Vaughan, D. Acute intracerebral treatment with amyloid-beta (1–42) alters the profile of neuronal oscillations that accompany LTP induction and results in impaired LTP in freely behaving rats. Front. Behav. Neurosci. 2015, 9, 103. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.; Grigoryan, G.; Guy-David, L.; Tsoory, M.M.; Chen, A.; Reuveny, E. Trisomy of the G protein-coupled K+ channel gene, Kcnj6, affects reward mechanisms, cognitive functions, and synaptic plasticity in mice. Proc. Natl. Acad. Sci. USA 2012, 109, 2642–2647. [Google Scholar] [CrossRef] [PubMed]
- Victoria, N.C.; Marron Fernandez de Velasco, E.; Ostrovskaya, O.; Metzger, S.; Xia, Z.; Kotecki, L.; Benneyworth, M.A.; Zink, A.N.; Martemyanov, K.A.; Wickman, K. G Protein-Gated K+Channel Ablation in Forebrain Pyramidal Neurons Selectively Impairs Fear Learning. Biol. Psychiatry 2016, 80, 796–806. [Google Scholar] [CrossRef] [PubMed]
- Rozov, A.V.; Valiullina, F.F.; Bolshakov, A.P. Mechanisms of Long-Term Plasticity of Hippocampal GABAergic Synapses. Biochemistry 2017, 82, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Thorajak, P.; Pannangrong, W.; Welbat, J.U.; Chaijaroonkhanarak, W.; Sripanidkulchai, K.; Sripanidkulchai, B. Effects of aged garlic extract on cholinergic, glutamatergic and GABAergic systems with regard to cognitive impairment in Aβ-induced rats. Nutrients 2017, 9, 686. [Google Scholar] [CrossRef] [PubMed]
- Patenaude, C.; Chapman, C.A.; Bertrand, S.; Congar, P.; Lacaille, J.C. GABAB receptor- and metabotropic glutamate receptor-dependent cooperative long-term potentiation of rat hippocampal GABAA synaptic transmission. J. Physiol. 2003, 553, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Marsden, K.C.; Beattie, J.B.; Friedenthal, J.; Carroll, R.C. NMDA Receptor Activation Potentiates Inhibitory Transmission through GABA Receptor-Associated Protein-Dependent Exocytosis of GABAA Receptors. J. Neurosci. 2007, 27, 14326–14337. [Google Scholar] [CrossRef] [PubMed]
- Flores, C.E.; Nikonenko, I.; Mendez, P.; Fritschy, J.-M.; Tyagarajan, S.K.; Muller, D. Activity-dependent inhibitory synapse remodeling through gephyrin phosphorylation. Proc. Natl. Acad. Sci. USA 2015, 112, E65–E72. [Google Scholar] [CrossRef] [PubMed]
- Pennacchietti, F.; Vascon, S.; Nieus, T.; Rosillo, C.; Das, S.; Tyagarajan, S.K.; Diaspro, A.; Del Bue, A.; Petrini, E.M.; Barberis, A.; et al. Nanoscale Molecular Reorganization of the Inhibitory Postsynaptic Density Is a Determinant of GABAergic Synaptic Potentiation. J. Neurosci. 2017, 37, 1747–1756. [Google Scholar] [CrossRef] [PubMed]
- Tipps, M.E.; Buck, K.J. GIRK Channels: A Potential Link Between Learning and Addiction. In International Review of Neurobiology; Academic Press Inc.: Cambridge, MA, USA, 2015; Volume 123, pp. 239–277. ISBN 9780128024584. [Google Scholar]
- Chung, H.J.; Qian, X.; Ehlers, M.; Jan, Y.N.; Jan, L.Y. Neuronal activity regulates phosphorylation-dependent surface delivery of G protein-activated inwardly rectifying potassium channels. Proc. Natl. Acad. Sci. USA 2009, 106, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Ge, W.P.; Qian, X.; Wiser, O.; Jan, Y.N.; Jan, L.Y. G protein-activated inwardly rectifying potassium channels mediate depotentiation of long-term potentiation. Proc. Natl. Acad. Sci. USA 2009, 106, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J. Alzheimer’s disease is a synaptic failure. Science 2002, 298, 789–791. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Franklin, K.B.J. Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates, 2nd ed.; Academic Press: San Diego, CA, USA, 2001; ISBN 0125476361. [Google Scholar]
- Gruart, A.; Muñoz, M.D.; Delgado-García, J.M. Involvement of the CA3-CA1 Synapse in the Acquisition of Associative Learning in Behaving Mice. J. Neurosci. 2006, 26, 1077–1087. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.J.; Chen, Y.R. The coexistence of an equal amount of Alzheimer’s amyloid-β 40 and 42 forms structurally stable and toxic oligomers through a distinct pathway. FEBS J. 2014, 281, 2674–2687. [Google Scholar] [CrossRef] [PubMed]
- Jan, A.; Hartley, D.M.; Lashuel, H.A. Preparation and characterization of toxic Aβ aggregates for structural and functional studies in Alzheimer’s disease research. Nat. Protoc. 2010, 5, 1186–1209. [Google Scholar] [CrossRef] [PubMed]
- Kotecki, L.; Hearing, M.; McCall, N.M.; Marron Fernandez de Velasco, E.; Pravetoni, M.; Arora, D.; Victoria, N.C.; Munoz, M.B.; Xia, Z.; Slesinger, P.A.; et al. GIRK Channels Modulate Opioid-Induced Motor Activity in a Cell Type- and Subunit-Dependent Manner. J. Neurosci. 2015, 35, 7131–7142. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Rodríguez, I.; Gruart, A.; Delgado-García, J.M.; Jiménez-Díaz, L.; Navarro-López, J.D. Role of GirK Channels in Long-Term Potentiation of Synaptic Inhibition in an In Vivo Mouse Model of Early Amyloid-β Pathology. Int. J. Mol. Sci. 2019, 20, 1168. https://doi.org/10.3390/ijms20051168
Sánchez-Rodríguez I, Gruart A, Delgado-García JM, Jiménez-Díaz L, Navarro-López JD. Role of GirK Channels in Long-Term Potentiation of Synaptic Inhibition in an In Vivo Mouse Model of Early Amyloid-β Pathology. International Journal of Molecular Sciences. 2019; 20(5):1168. https://doi.org/10.3390/ijms20051168
Chicago/Turabian StyleSánchez-Rodríguez, Irene, Agnès Gruart, José María Delgado-García, Lydia Jiménez-Díaz, and Juan D. Navarro-López. 2019. "Role of GirK Channels in Long-Term Potentiation of Synaptic Inhibition in an In Vivo Mouse Model of Early Amyloid-β Pathology" International Journal of Molecular Sciences 20, no. 5: 1168. https://doi.org/10.3390/ijms20051168
APA StyleSánchez-Rodríguez, I., Gruart, A., Delgado-García, J. M., Jiménez-Díaz, L., & Navarro-López, J. D. (2019). Role of GirK Channels in Long-Term Potentiation of Synaptic Inhibition in an In Vivo Mouse Model of Early Amyloid-β Pathology. International Journal of Molecular Sciences, 20(5), 1168. https://doi.org/10.3390/ijms20051168




