Gamma-Tocotrienol Induces Apoptosis in Prostate Cancer Cells by Targeting the Ang-1/Tie-2 Signalling Pathway
Abstract
1. Introduction
2. Results
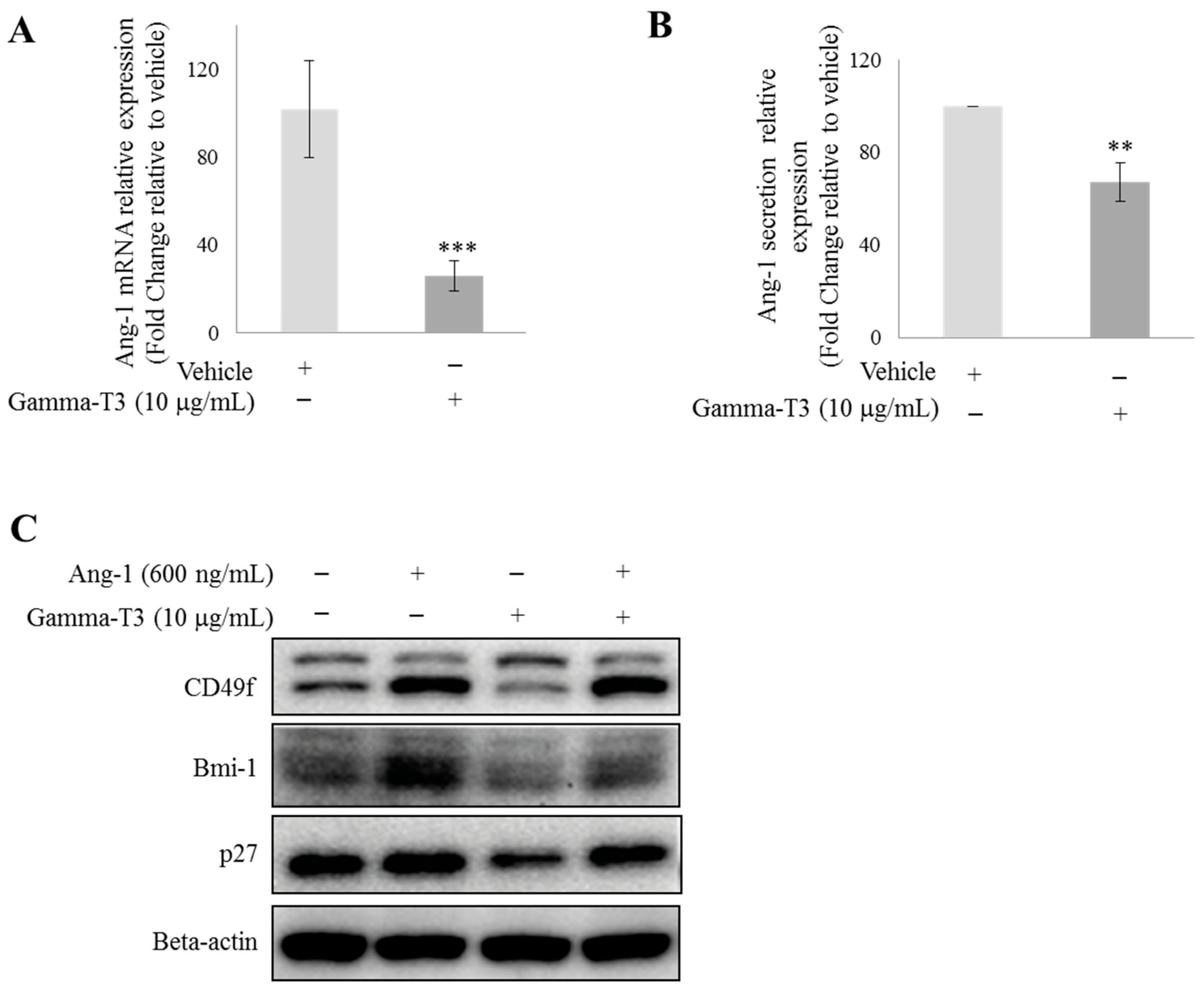
2.1. γ-T3 Downregulates Ang-1 Expression in Prostate Cancer Cells
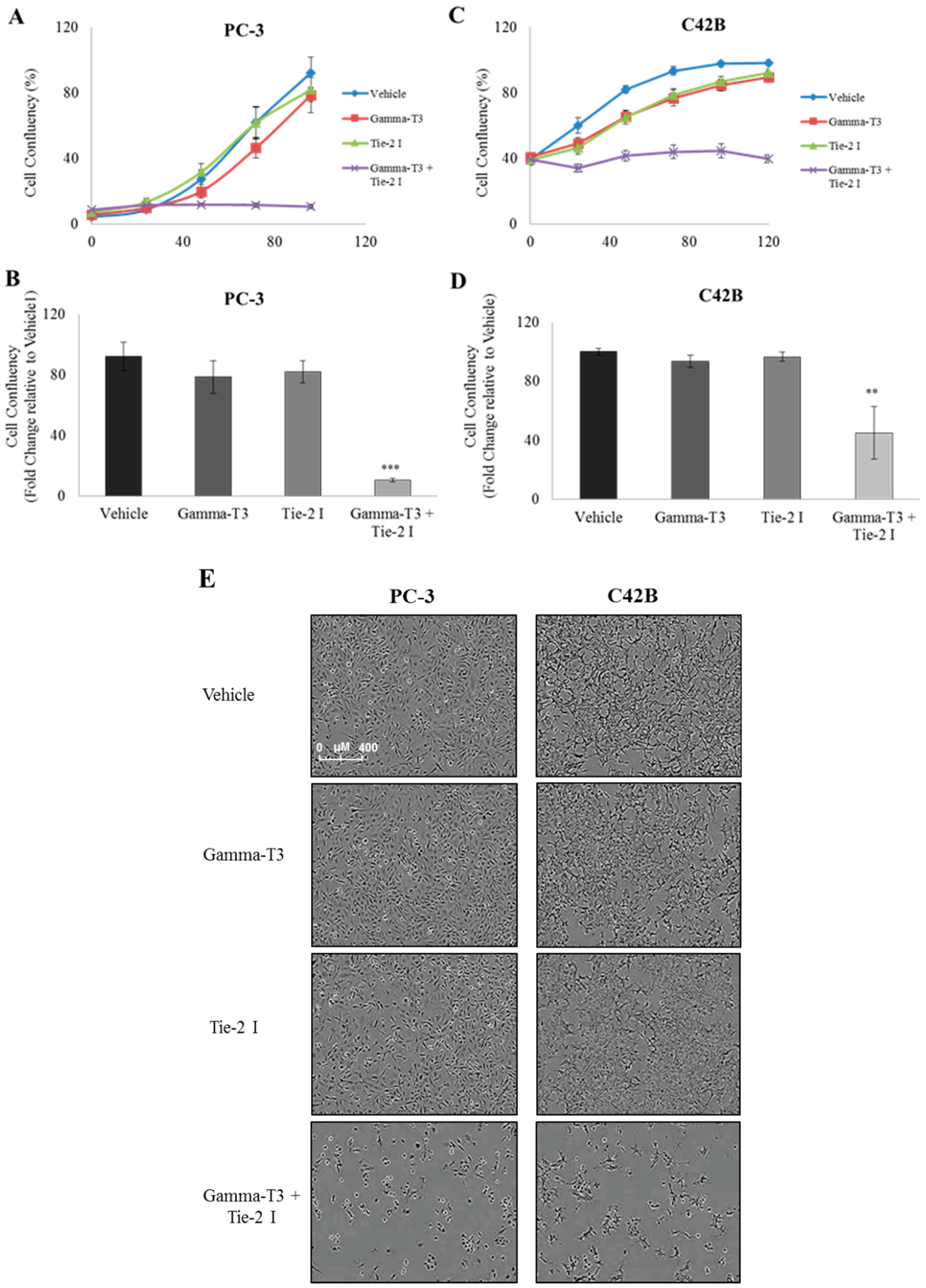
2.2. Synergistic Effect of γ-T3 and Tie-2 Inhibitor on Prostate Cancer Cell Growth
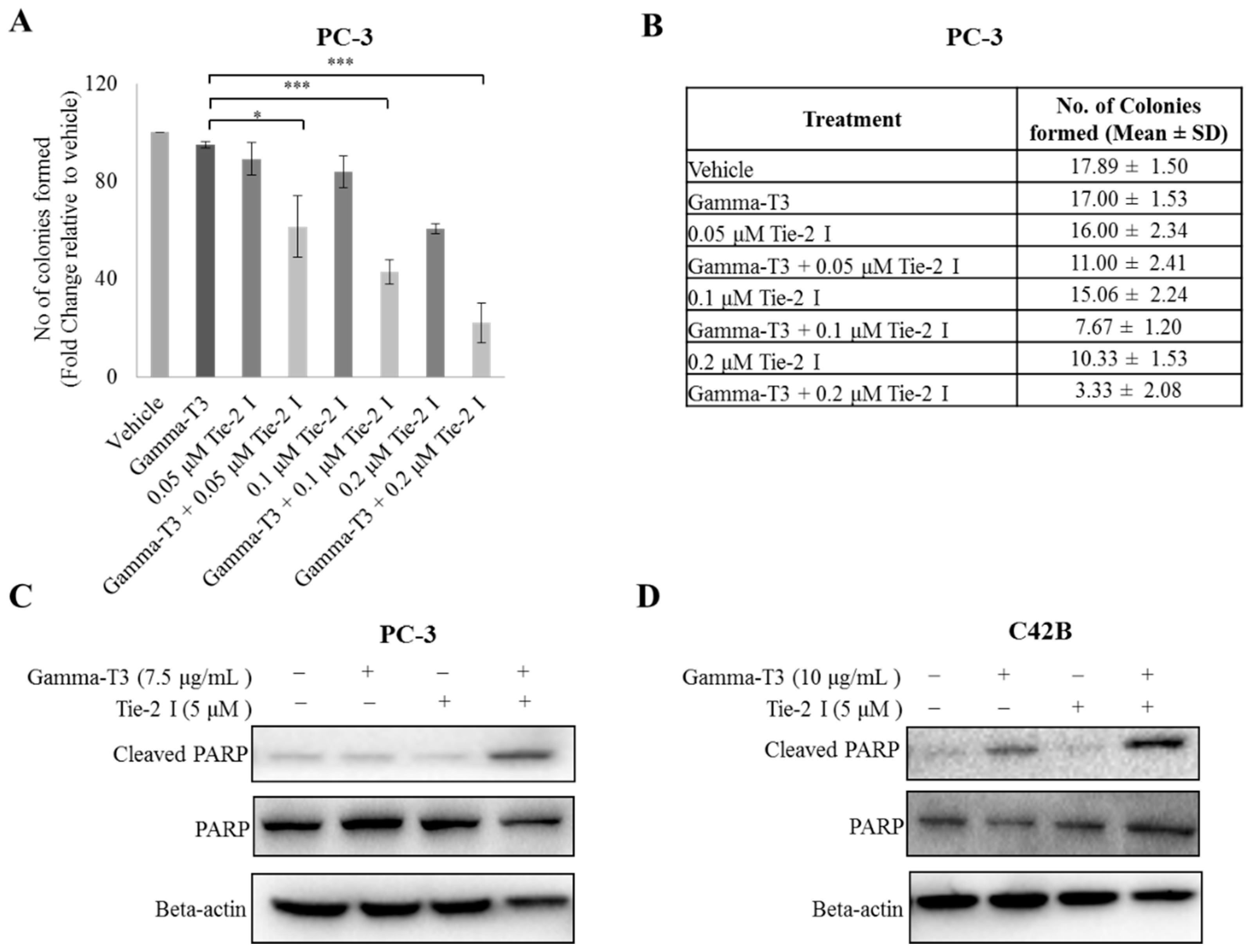
2.3. Inactivation of Tie-2 Enhances the Cytotoxic Effect of γ-T3
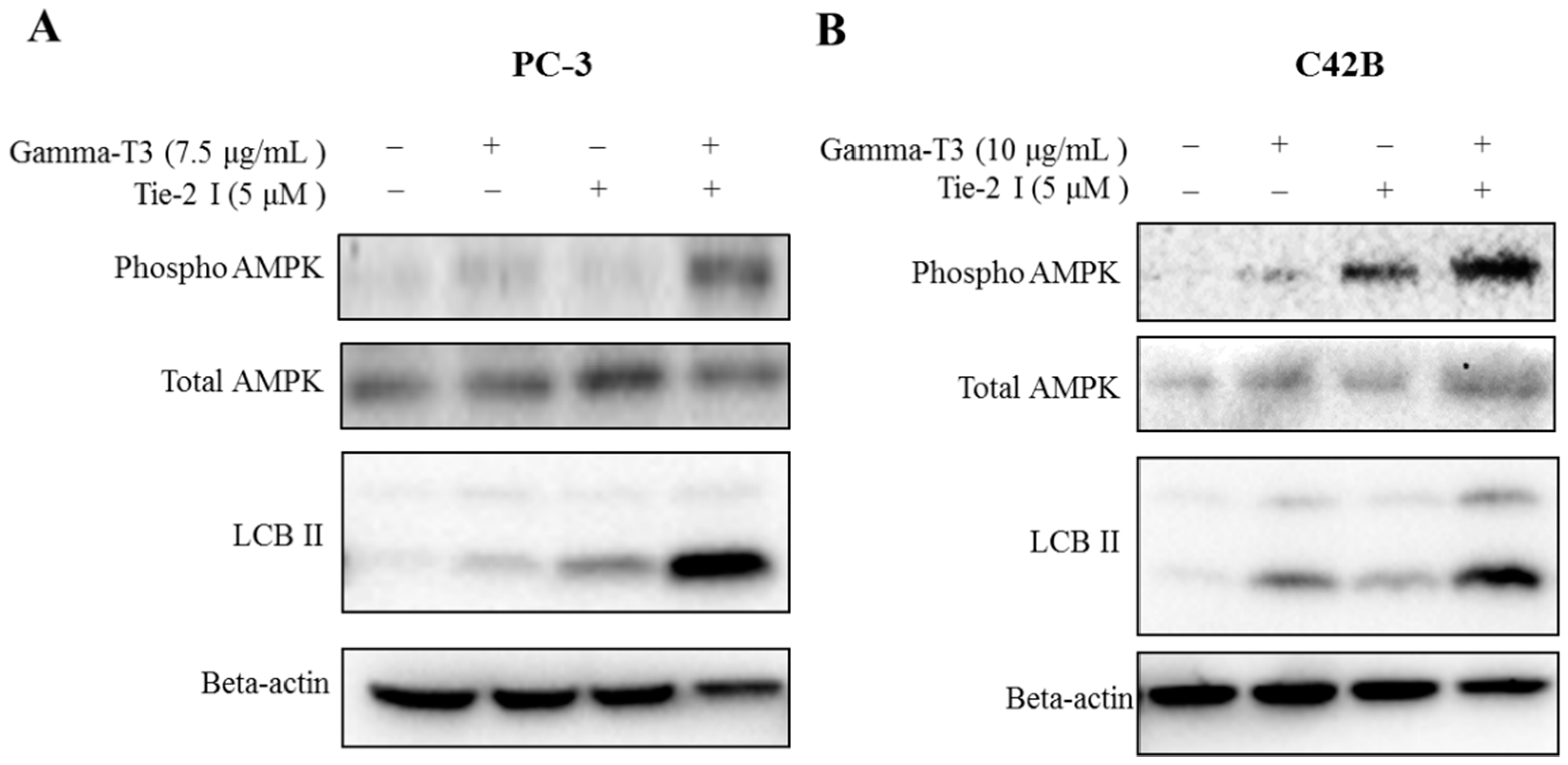
2.4. Combination of Tie-2 Inhibitor and γ-T3 Leads to the Activation of AMPK
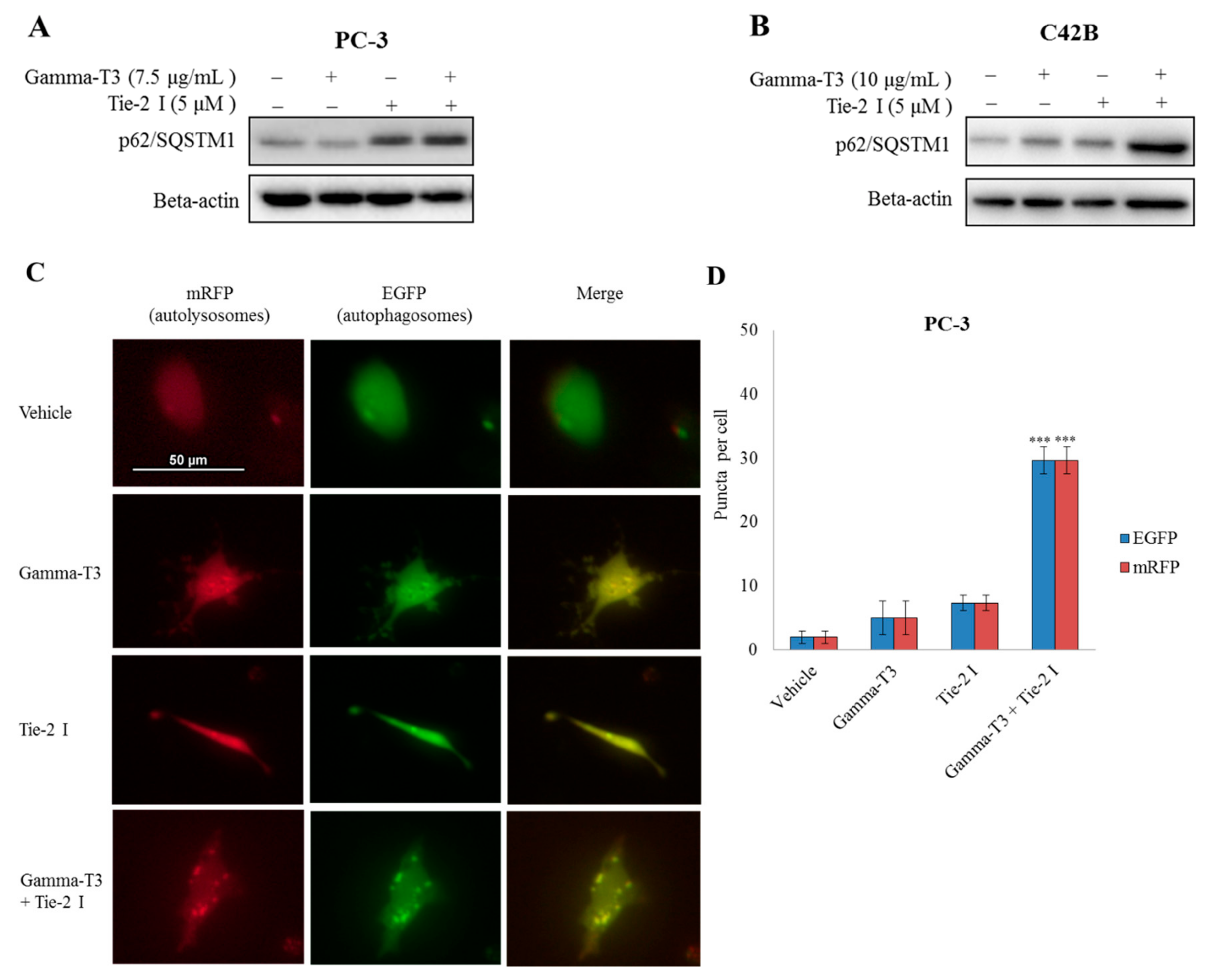
2.5. Inhibitory Effect of Tie-2 Inhibitor and γ-T3 on Autophagic Flux
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Culture Conditions
4.2. Antibodies and Reagents
4.3. qRT-PCR Analysis
4.4. Ang-1 ELISA
4.5. Western Blot
4.6. Cell Proliferation and Viability
4.7. Colony Formation Assay
4.8. Plasmid Transfection and Microscopy
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Ang-1 | Angiopoietin-1 |
| CM | Conditioned media |
| CSC | Cancer stem cell |
| FBS | Fetal bovine serum |
| γ-T3 | Gamma-tocotrienol |
| NF-κB | Factor-kapppa B |
| P/S | Penicillin-streptomycin |
| PMSF | Phenylmethylsulfonyl fluoride |
| PSP | Polysaccharopeptide |
| RPL32 | Ribosomal protein L32 |
| TP | Tocopherol |
| TRF | Tocotrienol-rich fraction |
References
- Cooperberg, M.R.; Broering, J.M.; Kantoff, P.W.; Carroll, P.R. Contemporary trends in low risk prostate cancer: Risk assessment and treatment. J. Urol. 2007, 178, S14–S19. [Google Scholar] [CrossRef] [PubMed]
- Lange, P.H.; Vessella, R.L. Mechanisms, hypotheses and questions regarding prostate cancer micrometastases to bone. Cancer Metastasis Rev. 1998, 17, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.E. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin. Cancer Res. 2006, 12, 6243S–6249S. [Google Scholar] [CrossRef] [PubMed]
- Ling, M.T.; Luk, S.U.; Al-Ejeh, F.; Khanna, K.K. Tocotrienol as a potential anticancer agent. Carcinogenesis 2012, 33, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Sato, C.; Kaneko, S.; Sato, A.; Virgona, N.; Namiki, K.; Yano, T. Combination Effect of delta-Tocotrienol and gamma-Tocopherol on Prostate Cancer Cell Growth. J. Nutr. Sci. Vitaminol. 2017, 63, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Palau, V.E.; Chakraborty, K.; Wann, D.; Lightner, J.; Hilton, K.; Brannon, M.; Stone, W.; Krishnan, K. γ-Tocotrienol induces apoptosis in pancreatic cancer cells by upregulation of ceramide synthesis and modulation of sphingolipid transport. BMC Cancer 2018, 18, 564. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Ma, K.; Liu, J.R.; Wang, H.X.; Tian, W.X.; Tu, Y.H.; Sun, W.G. gamma-tocotrienol inhibits the invasion and migration of human gastric cancer cells through downregulation of cyclooxygenase-2 expression. Oncol. Rep. 2018, 40, 999–1007. [Google Scholar] [PubMed]
- Srivastava, J.K.; Gupta, S. Tocotrienol-rich fraction of palm oil induces cell cycle arrest and apoptosis selectively in human prostate cancer cells. Biochem. Biophys. Res. Commun. 2006, 346, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Luk, S.U.; Yap, W.N.; Chiu, Y.T.; Lee, D.T.; Ma, S.; Lee, T.K.; Vasireddy, R.S.; Wong, Y.C.; Ching, Y.P.; Nelson, C.; et al. Gamma-tocotrienol as an effective agent in targeting prostate cancer stem cell-like population. Int. J. Cancer 2011, 128, 2182–2191. [Google Scholar] [CrossRef] [PubMed]
- Yap, W.N.; Chang, P.N.; Han, H.Y.; Lee, D.T.; Ling, M.T.; Wong, Y.C.; Yap, Y.L. Gamma-tocotrienol suppresses prostate cancer cell proliferation and invasion through multiple-signalling pathways. Br. J. Cancer 2008, 99, 1832–1841. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.E.; Rudder, B.; Phillips, R.B.; Whaley, S.G.; Stimmel, J.B.; Leesnitzer, L.M.; Lightner, J.; Dessus-Babus, S.; Duffourc, M.; Stone, W.L.; et al. γ-Tocotrienol induces growth arrest through a novel pathway with TGFbeta2 in prostate cancer. Free Radic. Biol. Med. 2011, 50, 1344–1354. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Sylvester, P.W. Tocotrienol-induced caspase-8 activation is unrelated to death receptor apoptotic signaling in neoplastic mammary epithelial cells. Exp. Biol. Med. 2004, 229, 745–755. [Google Scholar] [CrossRef]
- Liu, J.; Lau, E.Y.; Chen, J.; Yong, J.; Tang, K.D.; Lo, J.; Ng, I.O.; Lee, T.K.; Ling, M.T. Polysaccharopeptide enhanced the anti-cancer effect of gamma-tocotrienol through activation of AMPK. BMC Complement. Altern. Med. 2014, 14, 303. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, T.; Shibata, A.; Nakagawa, K.; Tsuzuki, T. Anti-angiogenic function of tocotrienol. Asia Pac. J. Clin. Nutr. 2008, 17, 253–256. [Google Scholar] [PubMed]
- Shibata, A.; Nakagawa, K.; Sookwong, P.; Tsuzuki, T.; Oikawa, S.; Miyazawa, T. Tumor anti-angiogenic effect and mechanism of action of delta-tocotrienol. Biochem. Pharmacol. 2008, 76, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Inokuchi, H.; Hirokane, H.; Tsuzuki, T.; Nakagawa, K.; Igarashi, M.; Miyazawa, T. Anti-angiogenic activity of tocotrienol. Biosci. Biotechnol. Biochem. 2003, 67, 1623–1627. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, S.; Sako, K.; Noda, K.; Zhang, J.; Minami, M.; Mochizuki, N. Angiopoietin-1/Tie2 receptor signaling in vascular quiescence and angiogenesis. Histol. Histopathol. 2010, 25, 387–396. [Google Scholar] [PubMed]
- Tang, K.D.; Holzapfel, B.M.; Liu, J.; Lee, T.K.; Ma, S.; Jovanovic, L.; An, J.; Russell, P.J.; Clements, J.A.; Hutmacher, D.W.; et al. Tie-2 regulates the stemness and metastatic properties of prostate cancer cells. Oncotarget 2015, 7, 2572–2584. [Google Scholar] [CrossRef] [PubMed]
- Zuazo-Gaztelu, I.; Casanovas, O. Unraveling the Role of Angiogenesis in Cancer Ecosystems. Front. Oncol. 2018, 8, 248. [Google Scholar] [CrossRef] [PubMed]
- Park, S.A.; Jeong, M.S.; Ha, K.T.; Jang, S.B. Structure and function of vascular endothelial growth factor and its receptor system. BMB Rep. 2018, 51, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Skobe, M.; Hawighorst, T.; Jackson, D.G.; Prevo, R.; Janes, L.; Velasco, P.; Riccardi, L.; Alitalo, K.; Claffey, K.; Detmar, M. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat. Med. 2001, 7, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Kajita, T.; Ohta, Y.; Kimura, K.; Tamura, M.; Tanaka, Y.; Tsunezuka, Y.; Oda, M.; Sasaki, T.; Watanabe, G. The expression of vascular endothelial growth factor C and its receptors in non-small cell lung cancer. Br. J. Cancer 2001, 85, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Furudoi, A.; Tanaka, S.; Haruma, K.; Kitadai, Y.; Yoshihara, M.; Chayama, K.; Shimamoto, F. Clinical significance of vascular endothelial growth factor C expression and angiogenesis at the deepest invasive site of advanced colorectal carcinoma. Oncology 2002, 62, 157–166. [Google Scholar] [CrossRef] [PubMed]
- O-charoenrat, P.; Rhys-Evans, P.; Eccles, S.A. Expression of vascular endothelial growth factor family members in head and neck squamous cell carcinoma correlates with lymph node metastasis. Cancer 2001, 92, 556–568. [Google Scholar] [CrossRef]
- De Silva, L.; Chuah, L.H.; Meganathan, P.; Fu, J.Y. Tocotrienol and cancer metastasis. Biofactors 2016, 42, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Teichert, M.; Milde, L.; Holm, A.; Stanicek, L.; Gengenbacher, N.; Savant, S.; Ruckdeschel, T.; Hasanov, Z.; Srivastava, K.; Hu, J.; et al. Pericyte-expressed Tie2 controls angiogenesis and vessel maturation. Nat. Commun. 2017, 8, 16106. [Google Scholar] [CrossRef] [PubMed]
- Satoh, N.; Yamada, Y.; Kinugasa, Y.; Takakura, N. Angiopoietin-1 alters tumor growth by stabilizing blood vessels or by promoting angiogenesis. Cancer Sci. 2008, 99, 2373–2379. [Google Scholar] [CrossRef] [PubMed]
- Wan Nazaimoon, W.M.; Khalid, B.A. Tocotrienols-rich diet decreases advanced glycosylation end-products in non-diabetic rats and improves glycemic control in streptozotocin-induced diabetic rats. Malays J. Pathol. 2002, 24, 77–82. [Google Scholar] [PubMed]
- Jiang, Q. Natural forms of vitamin E: Metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic. Biol. Med. 2014, 72, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Khanna, S.; Roy, S. Tocotrienols: Vitamin E beyond tocopherols. Life Sci. 2006, 78, 2088–2098. [Google Scholar] [CrossRef] [PubMed]
- Yap, W.N.; Zaiden, N.; Tan, Y.L.; Ngoh, C.P.; Zhang, X.W.; Wong, Y.C.; Ling, M.T.; Yap, Y.L. Id1, inhibitor of differentiation, is a key protein mediating anti-tumor responses of gamma-tocotrienol in breast cancer cells. Cancer Lett. 2010, 291, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K.; Aggarwal, B.B. γ-Tocotrienol suppresses growth and sensitises human colorectal tumours to capecitabine in a nude mouse xenograft model by down-regulating multiple molecules. Br. J. Cancer 2016, 115, 814–824. [Google Scholar] [CrossRef] [PubMed]
- Eitsuka, T.; Tatewaki, N.; Nishida, H.; Nakagawa, K.; Miyazawa, T. Synergistic Anticancer Effect of Tocotrienol Combined with Chemotherapeutic Agents or Dietary Components: A Review. Int. J. Mol. Sci. 2016, 17, 1605. [Google Scholar] [CrossRef] [PubMed]
- Mihaylova, M.M.; Shaw, R.J. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol. 2011, 13, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Chen, S.; Du, F.; Li, S.; Zhao, L.; Wang, X. Nutrient starvation elicits an acute autophagic response mediated by Ulk1 dephosphorylation and its subsequent dissociation from AMPK. Proc. Natl. Acad. Sci. USA 2011, 108, 4788–4793. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, R.V.; Parajuli, P.; Sylvester, P.W. γ-Tocotrienol-induced autophagy in malignant mammary cancer cells. Exp. Biol. Med. 2014, 239, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Su, M.; Xie, F.; Ai, J.; Ren, Y.; Zhang, J.; Guan, R.; He, W.; Gong, Y.; Guo, Y. Tetrandrine blocks autophagic flux and induces apoptosis via energetic impairment in cancer cells. Cell Death Dis. 2014, 5, e1123. [Google Scholar] [CrossRef] [PubMed]
- Kwan, P.S.; Lau, C.C.; Chiu, Y.T.; Man, C.; Liu, J.; Tang, K.D.; Wong, Y.C.; Ling, M.T. Daxx regulates mitotic progression and prostate cancer predisposition. Carcinogenesis 2013, 34, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.D.; Liu, J.; Jovanovic, L.; An, J.; Hill, M.M.; Vela, I.; Lee, T.K.; Ma, S.; Nelson, C.; Russell, P.J.; et al. Adipocytes promote prostate cancer stem cell self-renewal through amplification of the cholecystokinin autocrine loop. Oncotarget 2016, 7, 4939–4948. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, K.D.; Liu, J.; Russell, P.J.; Clements, J.A.; Ling, M.-T. Gamma-Tocotrienol Induces Apoptosis in Prostate Cancer Cells by Targeting the Ang-1/Tie-2 Signalling Pathway. Int. J. Mol. Sci. 2019, 20, 1164. https://doi.org/10.3390/ijms20051164
Tang KD, Liu J, Russell PJ, Clements JA, Ling M-T. Gamma-Tocotrienol Induces Apoptosis in Prostate Cancer Cells by Targeting the Ang-1/Tie-2 Signalling Pathway. International Journal of Molecular Sciences. 2019; 20(5):1164. https://doi.org/10.3390/ijms20051164
Chicago/Turabian StyleTang, Kai Dun, Ji Liu, Pamela J. Russell, Judith A. Clements, and Ming-Tat Ling. 2019. "Gamma-Tocotrienol Induces Apoptosis in Prostate Cancer Cells by Targeting the Ang-1/Tie-2 Signalling Pathway" International Journal of Molecular Sciences 20, no. 5: 1164. https://doi.org/10.3390/ijms20051164
APA StyleTang, K. D., Liu, J., Russell, P. J., Clements, J. A., & Ling, M.-T. (2019). Gamma-Tocotrienol Induces Apoptosis in Prostate Cancer Cells by Targeting the Ang-1/Tie-2 Signalling Pathway. International Journal of Molecular Sciences, 20(5), 1164. https://doi.org/10.3390/ijms20051164



