Physiological and Transcriptome Analyses of Early Leaf Senescence for ospls1 Mutant Rice (Oryza sativa L.) during the Grain-Filling Stage
Abstract
1. Introduction
2. Results
2.1. Characterization of Phenotype, Major Agronomic Traits, and Biochemical Changes of ospls1 Mutant during the Grain-Filling Stage
2.2. Differential Expressions of OsVHA-A and V-H+-ATPase Activities in ospls1 Mutant and Wild Type
2.3. Evaluation of RNA-Seq Reads and Mapping Results
2.4. Identification of Differentially Expressed Genes (DEGs) and Confirmation of Tag-Mapped Genes by Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.5. DEGs Significantly Enriched in Metabolic Pathways for the Two Rice Genotypes
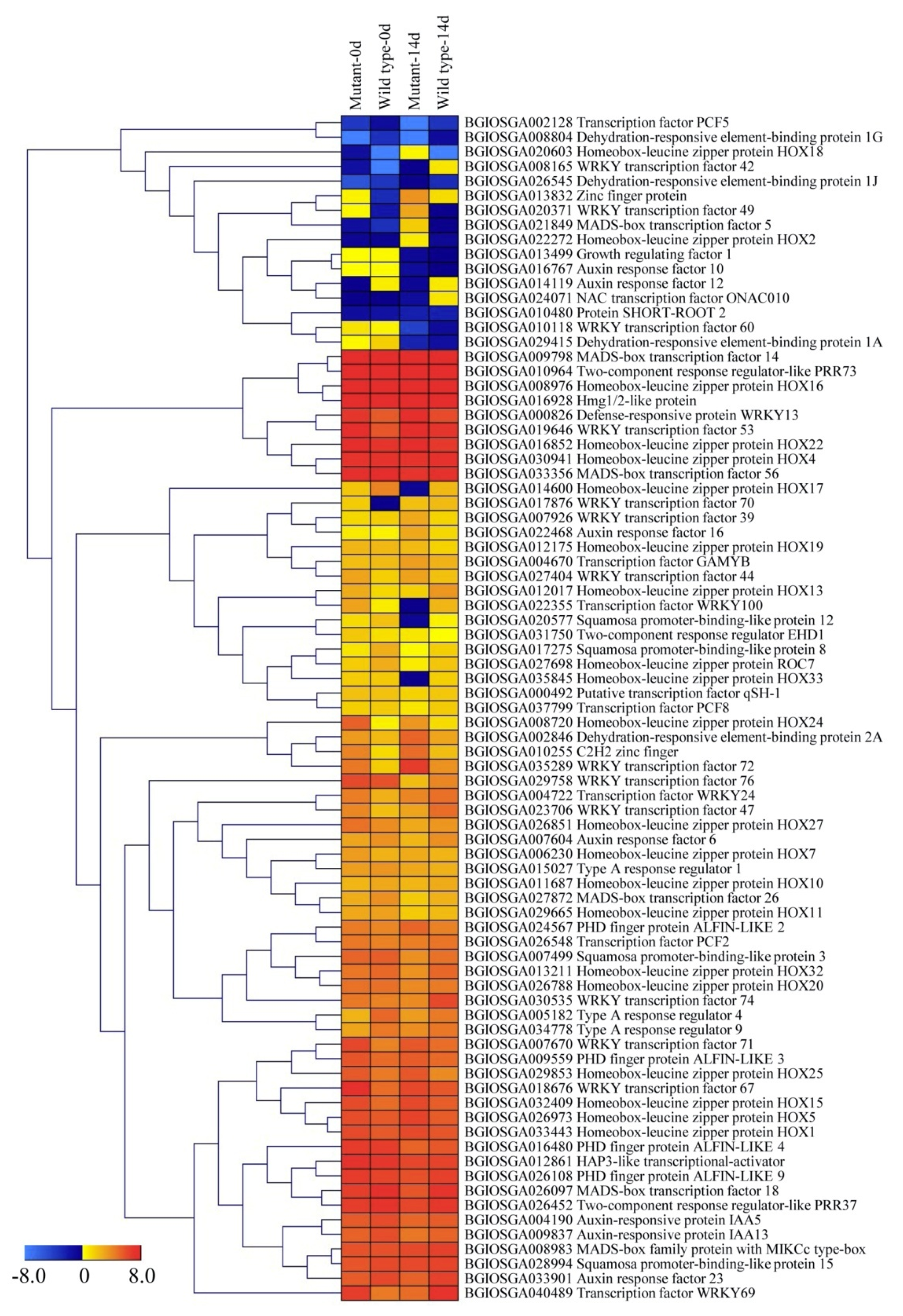
2.6. Transcription Factor (TF) Families in the DEGs of the Two Genotypes
2.7. DEGs Related to ATPase in the Two Genotypes
2.8. DEGs Associated with Hormone Signaling
2.9. DEGs Are Involved in the Antioxidative Metabolism and Cyanide-Resistant Respiration
2.10. DEGs Associated with the Regulation of Carbohydrate Metabolism in the ospls1 Mutant
2.11. DEGs Involved in the Hydrolysis and Autophagy in Senescing Leaves
3. Discussion
3.1. Deficiency of OsVHA-A Expression Led to Early Leaf Senescence in the ospls1 Mutant during the Grain-Filling Stage
3.2. TFs Are Highly Enriched during Leaf Senescence
3.3. Regulation of Antioxidative System during Early Leaf Senescence
3.4. Involvement of Hormone Signals during Early Leaf Senescence
3.5. Relationship between Carbohydrates Translocation and Leaf Senescence
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Measurement of Fv/Fv, Pn, Soluble Sugar and Protein, and V-H+-ATPase Activity
4.3. RNA Extraction and RNA-seq
4.4. Bioinformatics Analysis of RNA-seq Data and Identification of DGEs
4.5. qRT-PCR Validation
4.6. Functional Annotation, Gene Ontology (GO) Enrichment, and KEGG Analysis of DEGs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ABA | Abscisic acid |
| BKI1 | Brassinosteroid insensitive 1 kinase inhibitor 1 |
| BRI1 | Brassinosteroid insensitive 1 |
| BRs | Brassinosteroids |
| BZR1 | Brassinazole-resistant 1 |
| Cat | Catalase |
| CTKs | Cytokinins |
| DEGs | Differentially expressed genes |
| ET | Ethylene |
| FPKM | Fragments per kilobase of transcript sequence per million base pairs sequenced |
| Fv/Fm | Maximal quantum yield of PSII photochemistry |
| GO | Gene ontology |
| H2O2 | Hydrogen peroxide |
| KEGG | Kyoto encyclopedia of genes and genomes |
| KOBAS | The KEGG Orthology Based Annotation System |
| MDA | Malondialdehyde |
| 1O2 | Singlet oxygen |
| O2− | Superoxide anion radicals |
| OH∙ | Hydroxyl radicals |
| ospls1 | Oryza sativa premature leaf senescence 1 |
| Pn | Net photosynthesis rate |
| PPFD | Photosynthetic photon flux density |
| qRT-PCR | Quantitative real-time polymerase chain reaction |
| ROS | Reactive oxygen species |
| SA | Salicylic acid |
| SAGs | Senescence-associated genes |
| TFs | Transcription factors |
| TRC | Thioredoxin reductase |
| Trx | Thioredoxin |
| VHA-A | V-H+-ATPase subunit A |
References
- Lim, P.O.; Kim, H.J.; Nam, H.G. Leaf senescence. Annu. Rev. Plant Biol. 2007, 58, 115–136. [Google Scholar] [CrossRef] [PubMed]
- Podzimska-Sroka, D.; O’Shea, C.; Gregersen, P.L.; Skriver, K. NAC transcription factors in senescence: From molecular structure to function in crops. Plants 2015, 4, 412–448. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Kawasaki, T.; Wong, H.L.; Suharsono, U.; Hirano, H.; Shimamoto, K. Hyperphosphorylation of a mitochondrial protein, prohibitin, is induced by calyculin A in a rice lesion-mimic mutant cdr1. Plant Physiol. 2003, 132, 1861–1869. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.R.; Kim, H.J.; Nam, H.G.; Lim, P.O. Plant leaf senescence and death—Regulation by multiple layers of control and implications for aging in general. J. Cell Sci. 2013, 126, 4823–4833. [Google Scholar] [CrossRef] [PubMed]
- Hortensteiner, S.; Feller, U. Nitrogen metabolism and remobilization during senescence. J. Exp. Bot. 2002, 53, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Gross, B.L.; Zhao, Z. Archaeological and genetic insights into the origins of domesticated rice. Proc. Natl. Acad. Sci. USA 2014, 111, 6190–6197. [Google Scholar] [CrossRef] [PubMed]
- Gregersen, P.L.; Holm, P.B.; Krupinska, K. Leaf senescence and nutrient remobilisation in barley and wheat. Plant Biol. 2008, 10, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.Y.; Kuai, B.K.; Jia, J.Z.; Jing, H.C. Regulation of leaf senescence and crop genetic improvement. J. Integr. Plant Biol. 2012, 54, 936–952. [Google Scholar] [CrossRef] [PubMed]
- Distelfeld, A.; Avni, R.; Fischer, A.M. Senescence, nutrient remobilization, and yield in wheat and barley. J. Exp. Bot. 2014, 65, 3783–3798. [Google Scholar] [CrossRef] [PubMed]
- Quirino, B.F.; Noh, Y.S.; Himelblau, E.; Amasino, R.M. Molecular aspects of leaf senescence. Trends Plant Sci. 2000, 5, 278–282. [Google Scholar] [CrossRef]
- Leng, Y.; Ye, G.; Zeng, D. Genetic dissection of leaf senescence in rice. Int. J. Mol. Sci. 2017, 18, 2686. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Balazadeh, S.; Jaspert, N.; Arif, M.; Mueller-Roeber, B.; Maurina, V.G. Expression of ROS-responsive genes and transcription factors after metabolic formation of H2O2 in chloroplasts. Front. Plant Sci. 2012, 3, 234. [Google Scholar] [CrossRef] [PubMed]
- Jibran, R.; Hunter, A.; Dijkwel, P. Hormonal regulation of leaf senescence through integration of developmental and stress signals. Plant Mol. Biol. 2013, 82, 547–561. [Google Scholar] [CrossRef] [PubMed]
- Allu, A.D.; Soja, A.M.; Wu, A.; Szymanski, J.; Balazadeh, S. Salt stress and senescence: Identification of cross-talk regulatory components. J. Exp. Bot. 2014, 65, 3993–4008. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Wang, Y.; Zhu, Y.; Tang, J.; Hu, B.; Liu, L.; Ou, S.; Wu, H.; Sun, X.; Chu, J.; et al. OsNAP connects abscisic acid and leaf senescence by fine-tuning abscisic acid biosynthesis and directly targeting senescence-associated genes in rice. Proc. Natl. Acad. Sci. USA 2014, 111, 10013–10018. [Google Scholar] [CrossRef] [PubMed]
- Kluge, C.; Lahr, J.; Hanitzsch, M.; Bolte, S.; Golldack, D.; Dietz, K.J. New insight into the structure and regulation of the plant vacuolar H+-ATPase. J. Bioenerg. Biomembr. 2003, 35, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, K.; Krebs, M. The V-ATPase: Small cargo, large effects. Curr. Opin. Plant Biol. 2010, 13, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Marty, F. Plant vacuoles. Plant Cell 1999, 11, 587–600. [Google Scholar] [CrossRef] [PubMed]
- Kluge, C.; Seidel, T.; Bolte, S.; Sharma, S.S.; Hanitzsch, M.; Satiat-Jeunemaitre, B.; Ross, J.; Sauer, M.; Golldack, D.; Dietz, K.J. Subcellular distribution of the V-ATPase complex in plant cells, and in vivo localisation of the 100 kDa subunit VHA-a within the complex. BMC Cell Biol. 2004, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Dettmer, J.; Cchubert, D.; Calvo-Weimar, O.; Stierhof, Y.D.; Schmidt, R.; Schumacher, K. Essential role of the V-ATPase in male gametophyte development. Plant J. 2005, 41, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Niu, X.; Liu, J.; Xiao, F.; Cao, S.; Liu, Y. RNAi-directed downregulation of vacuolar H+-ATPase subunit A results in enhanced stomatal aperture and density in rice. PLoS ONE 2013, 8, e69046. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; He, X.L.; Zhao, Y.J.; Shen, Y.Z.; Huang, Z.J. Wheat vacuolar H+-ATPase subunit B cloning and its involvement in salt tolerance. Planta 2011, 234, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Gong, P.; Li, K.; Huang, F.; Cheng, F.; Pan, G. A single cytosine deletion in the OsPLS1 gene encoding vacuolar-type H+-ATPase subunit A1 leads to premature leaf senescence and seed dormancy in rice. J. Exp. Bot. 2016, 67, 2761–2776. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, F.; Lei, B.; Cao, Z.; Pan, G.; Cheng, F. Genotypic-dependent alteration in transcriptional expression of various CAT isoenzyme genes in esl mutant rice and its relation to H2O2-induced leaf senescence. Plant Growth Regul. 2014, 73, 237–248. [Google Scholar] [CrossRef]
- Lee, T.I.; Young, R.A. Transcription of eukaryotic protein-coding genes. Annu. Rev. Genet. 2000, 34, 77–137. [Google Scholar] [CrossRef] [PubMed]
- Rushton, P.J.; Somssich, I.E.; Ringler, P.; Shen, Q.J. WRKY transcription factors. Trends Plant Sci. 2010, 15, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Hong, Y.; Yin, M.; Li, C.; Zhang, C.; Grierson, D. A tomato HD-Zip homeobox protein, LeHB-1, plays an important role in floral organogenesis and ripening. Plant J. 2008, 55, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Donnelly, L.; Sun, D.; Rao, J.; Reid, M.S.; Jiang, C.Z. A petunia homeodomain-leucine zipper protein, PhHD-Zip, plays an important role in flower senescence. PLoS ONE 2014, 9, e88320. [Google Scholar] [CrossRef] [PubMed]
- Robatzek, S.; Somssich, I.E. A new member of the Arabidopsis WRKY transcription factor, AtWRKY6, is associated with both senescence- and defence-related processes. Plant J. 2001, 28, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Zentgraf, U. The antagonist function of Arabidopsis WRKY53 and ESR/ESP in leaf senescence is modulated by the jasmonic and salicylic acid equilibrium. Plant Cell 2007, 19, 819–830. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hu, Z.; Yao, Q.; Guo, X.; Nguyen, V.; Li, F.; Chen, G. A tomato MADS-box protein, SICMB1, regulates ethylene biosynthesis and carotenoid accumulation during fruit ripening. Sci. Rep. 2018, 8, 3413. [Google Scholar] [CrossRef] [PubMed]
- Ellis, C.M.; Nagpal, P.; Young, J.C.; Hagen, G.; Guilfoyle, T.J.; Reed, J.W. AUXIN RESPONSE FACTOR1 and AUXIN RESPONSE FACTOR2 regulate senescence and floral organ abscission in Arabidopsis thaliana. Development 2005, 132, 4563–4574. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zhou, J.J.; Zhang, J.Z. Aux/IAA gene family in plants: Molecular structure, regulation, and function. Int. J. Mol. Sci. 2018, 19, 259. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, M.; Oelmüller, R. WRKY transcription factor: Jack of many trades in plants. Plant Signal. Behav. 2014, 9, e27700. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Pang, C.; Fan, S.; Song, M.; Wei, H.; Yu, S. Global analysis of the Gossypium hirsutum L. Transcriptome during leaf senescence by RNA-seq. BMC Plant Biol. 2015, 15, 43. [Google Scholar] [CrossRef] [PubMed]
- Rogers, H.J. Is there an important role for reactive oxygen species and redox regulation during floral senescence? Plant Cell Environ. 2012, 35, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Salleh, F.M.; Mariotti, L.; Spadafora, N.D.; Price, A.M.; Picciarelli, P.; Wagstaff, C.; Lombardi, L.; Rogers, H. Interaction of plant growth regulators and reactive oxygen species to regulate petal senescence in wallflowers (Erysimum linifolium). BMC Plant Biol. 2016, 16, 77. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, F.; Zhao, Q.; Liu, J.; Cheng, F. Involvement of NADPH oxidase isoforms in the production of O2− manipulated by ABA in the senescing leaves of early-senescence-leaf (esl) mutant rice (Oryza sativa). PLoS ONE 2018, 13, e0190161. [Google Scholar] [CrossRef] [PubMed]
- Meyer, Y.; Buchanan, B.B.; Vignols, F.; Reichheld, J.P. Thioredoxins and glutaredoxins: Unifying elements in redox biology. Annu. Rev. Genet. 2009, 43, 335–367. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, L.R.; Froehlich, J.E.; Cruz, J.A.; Savage, L.J.; Kramer, D.M. Multi-level regulation of the chloroplast ATP synthase: The chloroplast NADPH thioredoxin reductase C (NTRC) is required for redox modulation specifically under low irradiance. Plant J. 2016, 87, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Thomas, H.; Stoddart, J.L. Leaf senescence. Ann. Rev. Plant Physiol. 1980, 31, 83–111. [Google Scholar] [CrossRef]
- Sarwat, M.; Naqvi, A.R.; Ahmad, P.; Ashraf, M.; Akram, N.A. Phytohormones and microRNAs as sensors and regulators of leaf senescence: Assigning macro roles to small molecules. Biotechnol. Adv. 2013, 31, 1153–1171. [Google Scholar] [CrossRef] [PubMed]
- Ye, N.; Jia, L.; Zhang, J. ABA signal in rice under stress conditions. Rice 2012, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Nambara, E.; Marion-Poll, A. Abscisic acid biosynthesis and catabolism. Annu. Rev. Plant Biol. 2005, 56, 165–185. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Dong, B.; Shiran, B.; Talbot, M.J.; Edlington, J.E.; Hughes, T.; White, R.G.; Gubler, F.; Dolferus, R. Control of abscisic acid catabolism and abscisic acid homeostasis is important for reproductive stage stress tolerance in cereals. Plant Physiol. 2011, 156, 647–662. [Google Scholar] [CrossRef] [PubMed]
- van der Graaff, E.; Schwacke, R.; Schneider, A.; Desimone, M.; Flügge, U.I.; Kunze, R. Transcription analysis of Arabidopsis membrane transporters and hormone pathways during developmental and induced leaf senescence. Plant Physiol. 2006, 141, 776–792. [Google Scholar] [CrossRef] [PubMed]
- Clouse, S.D.; Sasse, J.M. BRASSINOSTEROIDS: Essential regulators of plant growth and development. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 427–451. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Yuan, M.; Wang, R.; Yang, Y.; Wang, C.; Oses-Prieto, J.A.; Kim, T.W.; Zhou, H.W.; Deng, Z.; Gampala, S.S.; et al. PP2A activates brassinosteroid-responsive gene expression and plant growth by dephosphorylating BZR1. Nat. Cell Biol. 2011, 13, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Trivellini, A.; Cocetta, G.; Hunter, D.A.; Vernieri, P.; Ferrante, A. Spatial and temporal transcriptome changes occurring during flower opening and senescence of the ephemeral hibiscus flower, Hibiscus rosa-sinensis. J. Exp. Bot. 2016, 67, 5919–5931. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.; Zhang, J.H. Grain filling of cereals under soil drying. New Phytol. 2006, 169, 223–236. [Google Scholar] [CrossRef] [PubMed]
- van Doorn, W.G. Is petal senescence due to sugar starvation? Plant Physiol. 2004, 134, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Stitt, M. Coordination of carbon supply and plant growth. Plant Cell Environ. 2007, 30, 1126–1149. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, J.; Wang, Z.; Zhu, Q.; Liu, L. Activities of enzymes involved in sucrose-to-starch metabolism in rice grains subjected to water stress during filling. Field Crops Res. 2003, 81, 69–81. [Google Scholar] [CrossRef]
- Panda, D.; Sarkar, R.K. Natural leaf senescence: Probed by chlorophyll fluorescence, CO2 photosynthetic rate and antioxidant enzyme activities during grain filling in different rice cultivars. Physiol. Mol. Biol. Plants 2013, 19, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, F.; Lin, W.; Zhao, Q.; Liu, J.; Cheng, F. Carbon reserve and remobilization in leaf sheaths during the grain-filling stage in response to leaf early senescence. Acta Physiol. Plant. 2017, 39, 10. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle or protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef] [PubMed]

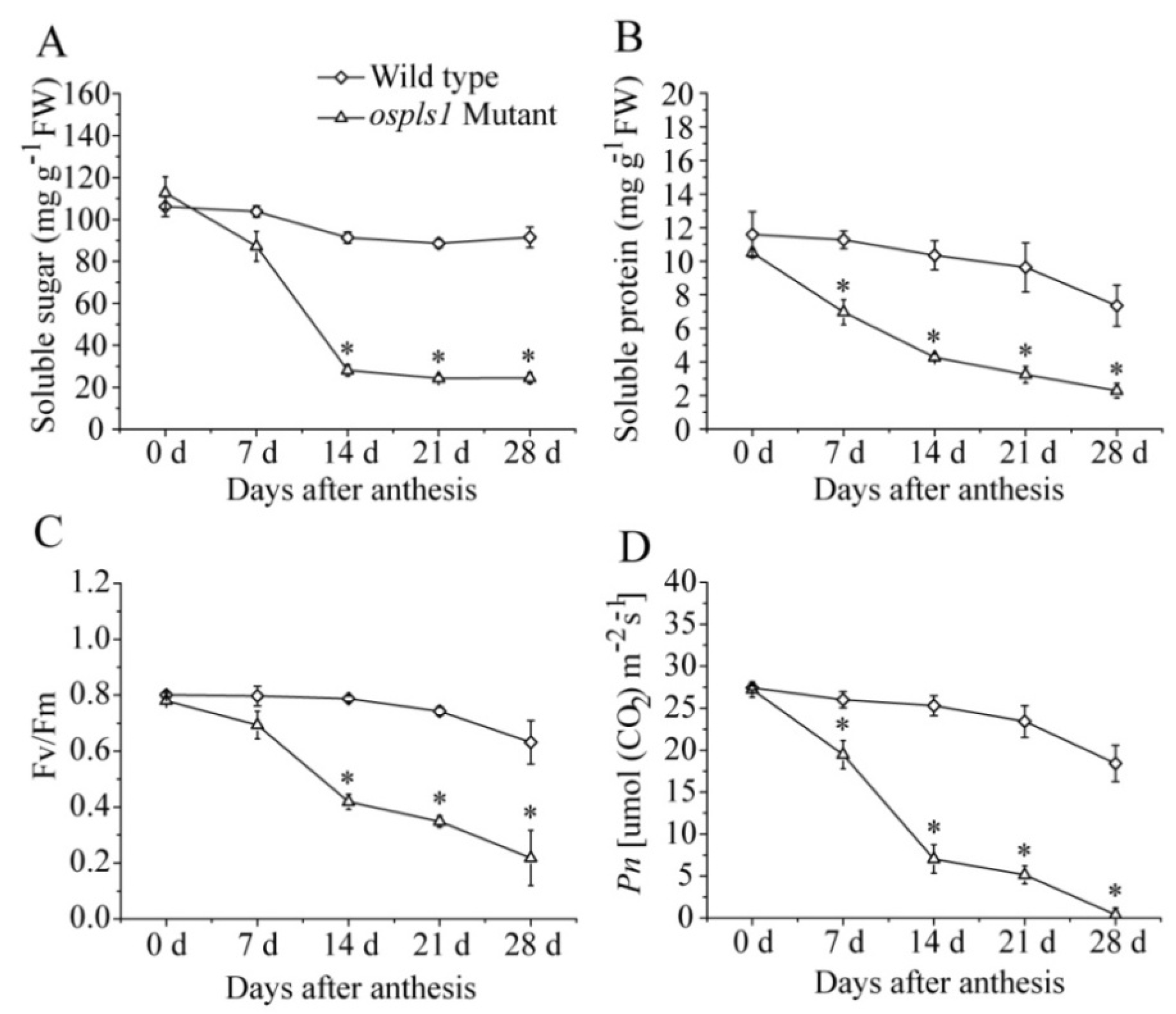
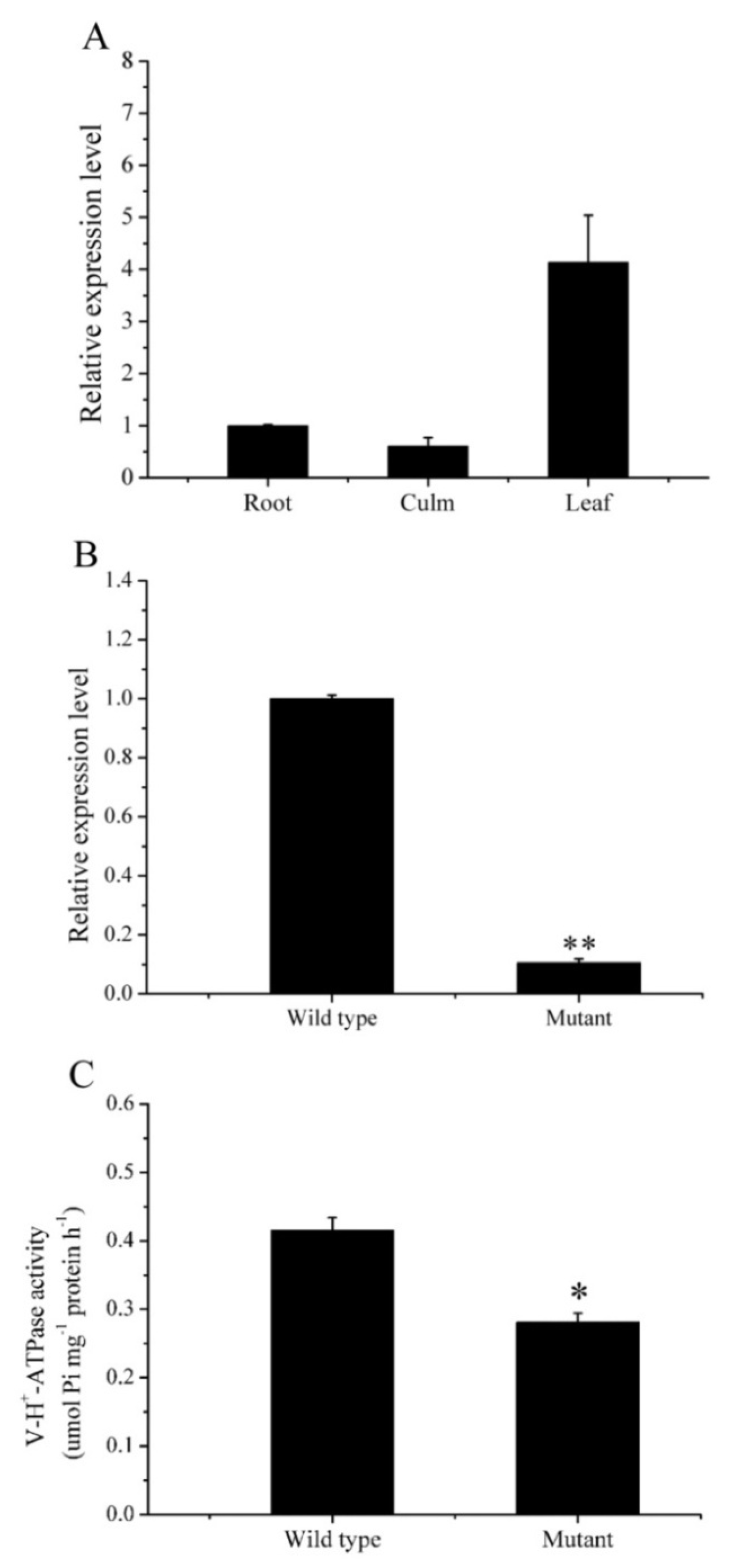
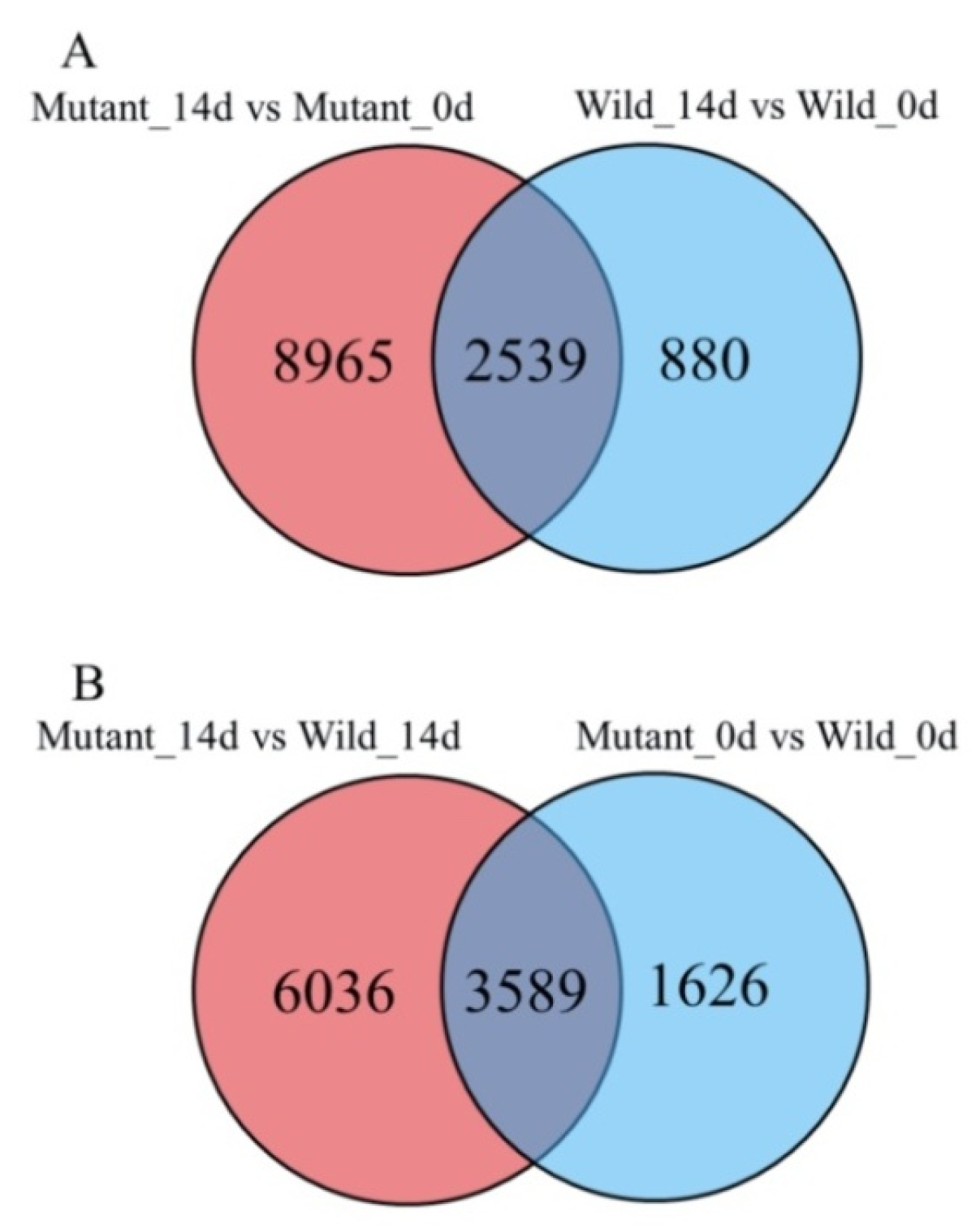
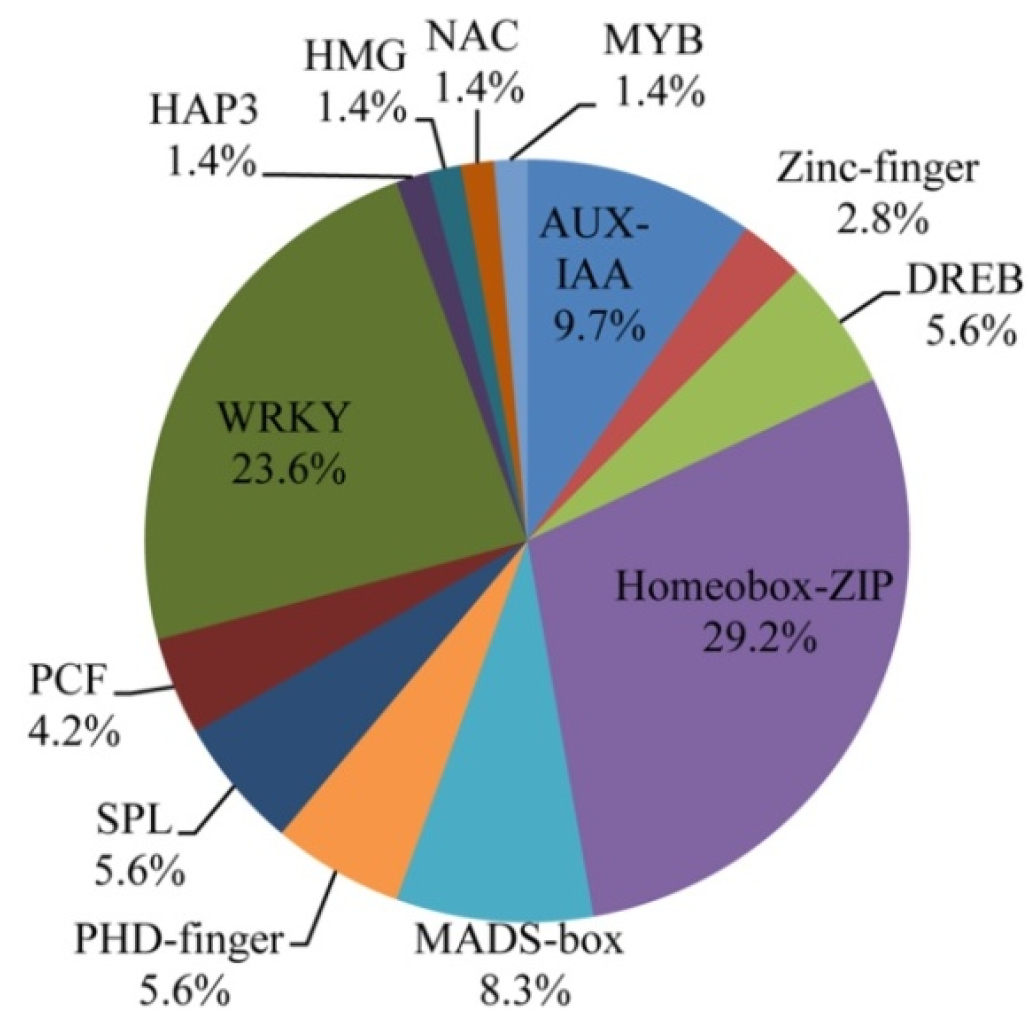
| Traits | 2015 | 2016 | ||||
|---|---|---|---|---|---|---|
| Wild Type | Mutant | Different Significance | Wild Type | Mutant | Different Significance | |
| Growth duration | 88.1 ± 2.1 | 86.6 ± 2.4 | ns | 89.6 ± 3.1 | 88.6 ± 2.4 | ns |
| Plant height (cm) | 109.3 ± 3.3 | 92.4 ± 2.2 | * | 110.8 ± 3.8 | 94.3 ± 3.6 | * |
| Available panicle number | 11.7 ± 3.8 | 6.3 ± 2.4 | ** | 11.1 ± 2.4 | 5.6 ± 0.8 | ** |
| Grain shape | 2.64 ± 0.02 | 2.58 ± 0.1 | ns | 2.61 ± 0.05 | 2.54 ± 0.08 | ns |
| Seed-setting rate (%) | 84.5 ± 3.9 | 41.8 ± 6.8 | ** | 82.2 ± 6.1 | 36.6 ±4.3 | ** |
| 1000-grain weight (g) | 23.37 ± 1.1 | 17.62 ± 1.6 | ** | 23.14 ± 0.6 | 18.1 ± 1.3 | ** |
| Yield per plant (g) | 53.0 ± 7.4 | 4.1 ± 1.8 | ** | 48.6 ± 9.5 | 3.8 ± 0.6 | ** |
| Harvest index | 0.44 ± 0.03 | 0.15 ± 0.03 | ** | 0.46 ± 0.02 | 0.19 ± 0.03 | ** |
| Sample | Raw Reads_count | Clean Reads_count | Total Mapped Reads_count | Mapped % | Uniquely Mapped Reads_count | Uniquely Mapped % |
|---|---|---|---|---|---|---|
| Mutant_0d1 | 50,966,886 | 49,401,112 | 39,881,950 | 80.73 | 38,567,821 | 78.07 |
| Mutant_0d2 | 60,634,598 | 58,467,030 | 47,637,811 | 81.48 | 46,087,811 | 78.83 |
| Mutant_0d3 | 51,135,352 | 49,444,022 | 39,862,112 | 80.62 | 38,601,162 | 78.07 |
| Wild_0d1 | 50,698,138 | 48,773,770 | 39,684,358 | 81.36 | 38,377,545 | 78.68 |
| Wild_0d2 | 46,710,304 | 44,703,316 | 36,126,426 | 80.81 | 34,969,393 | 78.23 |
| Wild_0d3 | 54,065,590 | 51,885,202 | 42,252,458 | 81.43 | 40,807,059 | 78.65 |
| Mutant_14d1 | 50,030,824 | 48,186,294 | 37,512,316 | 77.85 | 36,507,456 | 75.76 |
| Mutant_14d2 | 45,736,398 | 43,690,018 | 30,590,126 | 70.02 | 29,775,623 | 68.15 |
| Mutant_14d3 | 49,065,914 | 47,170,682 | 36,361,257 | 77.08 | 35,377,483 | 75.00 |
| Wild_14d1 | 62,344,682 | 60,065,524 | 48,768,418 | 81.19 | 47,309,394 | 78.76 |
| Wild_14d2 | 54,823,378 | 52,520,882 | 42,248,041 | 80.44 | 40,998,600 | 78.06 |
| Wild_14d3 | 48,894,006 | 46,946,312 | 38,265,388 | 81.51 | 37,104,074 | 79.04 |
| DEG Set | DEGs | Up-Regulated | Down-Regulated |
|---|---|---|---|
| Mutant_14d vs. Mutant_0d | 11,504 | 5312 | 6192 |
| Wild_14d vs. Wild_0d | 3419 | 1636 | 1783 |
| Mutant_0d vs. Wild_0d | 5215 | 3093 | 2122 |
| Mutant_14d vs. Wild_14d | 9625 | 4816 | 4809 |
| KEEG ID | KEEG Pathway (Down-Regulated) | Mutant | Wild Type | ||||
| Gene Number | Background Number | Corrected p-Value | Gene Number | Background Number | Corrected p-Value | ||
| osa01110 | Biosynthesis of secondary metabolites | 278 | 779 | 0.0028 | 82 | 779 | 0.0222 |
| osa00710 | Carbon fixation in photosynthetic organisms | 41 | 77 | 0.0285 | |||
| osa00195 | Photosynthesis | 40 | 79 | 0.0462 | |||
| osa00910 | Nitrogen metabolism | 10 | 27 | 0.0105 | |||
| osa00906 | Carotenoid biosynthesis | 8 | 26 | 0.0467 | |||
| KEEG ID | KEEG Pathway (Up-Regulated) | Mutant | Wild Type | ||||
| Gene Number | Background Number | Corrected p-Value | Gene Number | Background Number | Corrected p-Value | ||
| osa04626 | Plant-pathogen interaction | 29 | 130 | 0.0012 | |||
| osa00071 | Fatty acid degradation | 15 | 41 | 0.0012 | |||
| osa00941 | Flavonoid biosynthesis | 8 | 19 | 0.0305 | |||
| osa01110 | Biosynthesis of secondary metabolites | 95 | 779 | 0.0305 | |||
| osa00592 | alpha-Linolenic acid metabolism | 10 | 33 | 0.0305 | |||
| osa00520 | Amino sugar and nucleotide sugar metabolism | 20 | 103 | 0.0305 | |||
| Gene ID | Log2 (Fold Change 14d/0d) | Description | |
|---|---|---|---|
| Mutant | Wild Type | ||
| BGIOSGA009058 | −1.22 | ATP synthase | |
| BGIOSGA031512 | −2.22 | −1.03 | ATP synthase subunit b, chloroplastic |
| BGIOSGA004222 | 0.32 | ATP synthase subunit beta | |
| BGIOSGA017602 | 0.38 | ATP synthase subunit beta | |
| Novel01284 | −1.99 | putative ATPase | |
| BGIOSGA009328 | 0.59 | V-type proton ATPase subunit F | |
| BGIOSGA015836 | 0.61 | V-type proton ATPase subunit F | |
| Group | Gene ID | Log2 (Fold Change 14d/0d) | Description | |
|---|---|---|---|---|
| Mutant | Wild Type | |||
| Auxin/IAA | BGIOSGA004190 | −0.81 | −0.77 | Auxin-responsive protein IAA5 |
| BGIOSGA009837 | −1.07 | −1.00 | Auxin-responsive protein IAA13 | |
| BGIOSGA023979 | −1.24 | Probable indole-3-acetic acid-amido synthetase GH3.8 | ||
| BGIOSGA004826 | −1.22 | −1.13 | Putative AUX1-like permease | |
| CTKs | BGIOSGA015027 | −0.76 | −1.25 | Type A response regulator 1 |
| BRs | BGIOSGA029632 | −1.47 | Probable BRI1 kinase inhibitor 1 | |
| Novel00067 | −3.31 | Brassinazole-resistant 1 homolog 1 | ||
| ABA | Novel01323 | −0.78 | Putative abscisic acid-induced protein | |
| BGIOSGA026823 | −1.39 | −1.04 | Abscisic acid 8′-hydroxylase 2 | |
| BGIOSGA029635 | −1.13 | −1.14 | Abscisic acid 8′-hydroxylase 3 | |
| BGIOSGA016502 | −0.63 | −0.67 | Zeaxanthin epoxidase, chloroplastic | |
| Group | Gene ID | Log2 (Fold Change 14d/0d) | Description | |
|---|---|---|---|---|
| Mutant | Wild Type | |||
| Anti-oxidative metabolism | BGIOSGA011520 | −1.37 | −0.70 | Catalase |
| BGIOSGA007252 | 2.41 | 4.17 | Catalase isozyme A | |
| BGIOSGA023636 | 1.64 | Catalase isozyme B | ||
| BGIOSGA019625 | 0.52 | Superoxide dismutase | ||
| BGIOSGA022060 | −0.96 | Superoxide dismutase | ||
| BGIOSGA022277 | −0.77 | Superoxide dismutase | ||
| BGIOSGA029201 | −0.42 | Superoxide dismutase [Cu-Zn] | ||
| BGIOSGA025399 | −0.84 | Superoxide dismutase [Cu-Zn] | ||
| BGIOSGA023756 | 0.81 | Superoxide dismutase [Cu-Zn] | ||
| BGIOSGA020152 | −0.38 | Thioredoxin | ||
| BGIOSGA024701 | 0.34 | −0.74 | Thioredoxin H-type | |
| BGIOSGA021328 | −0.54 | −1.01 | Thioredoxin reductase | |
| BGIOSGA026313 | −0.61 | Thioredoxin reductase | ||
| Cyanide-resistant respiration | BGIOSGA014421 | 1.28 | 1.54 | Alternative oxidase |
| BGIOSGA014422 | 1.36 | Alternative oxidase | ||
| BGIOSGA005788 | −0.95 | Alternative oxidase | ||
| Group | Gene ID | Log2 (Fold Change 14d/0d) | Description | |
|---|---|---|---|---|
| Mutant | Wild Type | |||
| Hexose | BGIOSGA000339 | −0.64 | −0.68 | Fructokinase-1 |
| BGIOSGA004865 | −0.93 | Fructose-1,6-bisphosphatase, cytosolic | ||
| BGIOSGA027739 | −1.95 | Fructose-bisphosphate aldolase | ||
| BGIOSGA034421 | −1.88 | Fructose-bisphosphate aldolase | ||
| BGIOSGA019844 | 0.54 | Fructose-bisphosphate aldolase | ||
| BGIOSGA023247 | −2.52 | Fructose-bisphosphate aldolase | ||
| BGIOSGA017490 | −0.63 | Glucose-1-phosphate adenylyltransferase | ||
| BGIOSGA027135 | −0.67 | Glucose-1-phosphate adenylyltransferase | ||
| BGIOSGA009855 | −0.75 | Glucose-1-phosphate adenylyltransferase | ||
| BGIOSGA030039 | −0.95 | Glucose-1-phosphate adenylyltransferase | ||
| BGIOSGA024440 | 0.44 | Glucose-6-phosphate 1-dehydrogenase | ||
| BGIOSGA012859 | −1.66 | 0.76 | Glucose-6-phosphate 1-dehydrogenase | |
| BGIOSGA010851 | 1.09 | −1.43 | Glucose-6-phosphate 1-dehydrogenase | |
| BGIOSGA016632 | −0.61 | 1.29 | Glucose-6-phosphate 1-dehydrogenase | |
| BGIOSGA033719 | 1.41 | 1.42 | Mannose-6-phosphate isomerase | |
| BGIOSGA017373 | 0.53 | Phosphomannomutase | ||
| Sucrose | BGIOSGA000239 | −0.69 | Probable sucrose-phosphate synthase 1 | |
| BGIOSGA011194 | −0.67 | Trehalose-6-phosphate synthase 4 | ||
| BGIOSGA026976 | 2.47 | Trehalose-6-phosphate synthase 7 | ||
| BGIOSGA028759 | 2.15 | Trehalose-6-phosphate synthase 8 | ||
| BGIOSGA030372 | −0.55 | Beta-fructofuranosidase, insoluble isoenzyme 7 | ||
| BGIOSGA010570 | 2.45 | Sucrose synthase | ||
| BGIOSGA021739 | 0.40 | Sucrose synthase | ||
| BGIOSGA010770 | 1.56 | 0.87 | Sucrose synthase | |
| Starch | BGIOSGA013592 | −2.13 | 1.83 | UDP-glucose 6-dehydrogenase |
| BGIOSGA037342 | 0.75 | UDP-glucose 6-dehydrogenase | ||
| BGIOSGA031231 | −0.38 | UDP-glucose pyrophosphorylase | ||
| BGIOSGA021860 | −2.08 | −1.53 | Soluble starch synthase 1, chloroplastic | |
| BGIOSGA005631 | −1.96 | −1.19 | Soluble starch synthase II-2 | |
| BGIOSGA011829 | 1.46 | Beta-amylase | ||
| BGIOSGA033092 | 0.72 | Beta-amylase | ||
| BGIOSGA000478 | −0.46 | Phosphorylase | ||
| BGIOSGA009780 | −2.01 | −1.52 | Phosphorylase | |
| BGIOSGA004591 | 1.05 | Pectinesterase | ||
| Group | Gene ID | Log2 (Fold Change 14d/0d) | Description | |
|---|---|---|---|---|
| Mutant | Wild Type | |||
| Regulation of autophagy | BGIOSGA002187 | 1.05 | Autophagy-related protein 3 | |
| BGIOSGA024235 | 1.03 | Autophagy-related protein 8A | ||
| BGIOSGA014317 | 1.02 | Autophagy-related protein 8B | ||
| BGIOSGA028123 | 0.71 | Autophagy-related protein 8C | ||
| Proteolysis | BGIOSGA004666 | 0.45 | Proteasome subunit alpha type | |
| BGIOSGA008679 | 0.44 | Proteasome subunit alpha type | ||
| BGIOSGA011334 | 0.45 | Proteasome subunit alpha type | ||
| BGIOSGA017816 | 0.52 | Proteasome subunit alpha type | ||
| BGIOSGA026895 | 0.75 | Proteasome subunit alpha type-2 | ||
| BGIOSGA029124 | 0.43 | Proteasome subunit alpha type-7-A | ||
| BGIOSGA005534 | 0.43 | Proteasome subunit beta type | ||
| BGIOSGA021971 | 0.36 | Proteasome subunit beta type | ||
| BGIOSGA019302 | 0.50 | Proteasome subunit beta type | ||
| BGIOSGA029040 | 1.58 | Proteasome subunit beta type | ||
| BGIOSGA029445 | 0.39 | Proteasome subunit beta type | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Pan, X.; Guo, X.; Fan, K.; Lin, W. Physiological and Transcriptome Analyses of Early Leaf Senescence for ospls1 Mutant Rice (Oryza sativa L.) during the Grain-Filling Stage. Int. J. Mol. Sci. 2019, 20, 1098. https://doi.org/10.3390/ijms20051098
Li Z, Pan X, Guo X, Fan K, Lin W. Physiological and Transcriptome Analyses of Early Leaf Senescence for ospls1 Mutant Rice (Oryza sativa L.) during the Grain-Filling Stage. International Journal of Molecular Sciences. 2019; 20(5):1098. https://doi.org/10.3390/ijms20051098
Chicago/Turabian StyleLi, Zhaowei, Xinfeng Pan, Xiaodong Guo, Kai Fan, and Wenxiong Lin. 2019. "Physiological and Transcriptome Analyses of Early Leaf Senescence for ospls1 Mutant Rice (Oryza sativa L.) during the Grain-Filling Stage" International Journal of Molecular Sciences 20, no. 5: 1098. https://doi.org/10.3390/ijms20051098
APA StyleLi, Z., Pan, X., Guo, X., Fan, K., & Lin, W. (2019). Physiological and Transcriptome Analyses of Early Leaf Senescence for ospls1 Mutant Rice (Oryza sativa L.) during the Grain-Filling Stage. International Journal of Molecular Sciences, 20(5), 1098. https://doi.org/10.3390/ijms20051098





