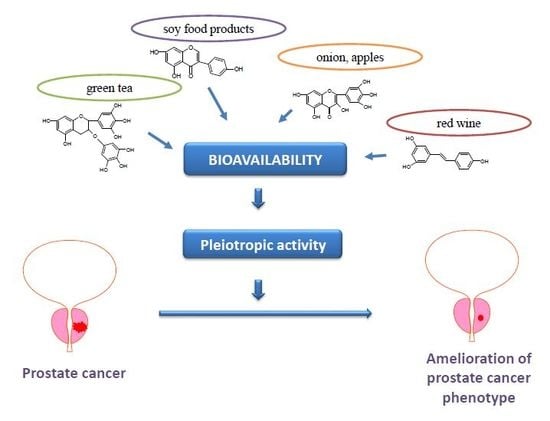
Molecular Mechanisms and Bioavailability of Polyphenols in Prostate Cancer
Abstract
1. Introduction
2. Molecular Mechanisms of Action of Polyphenols in Prostate Cancer
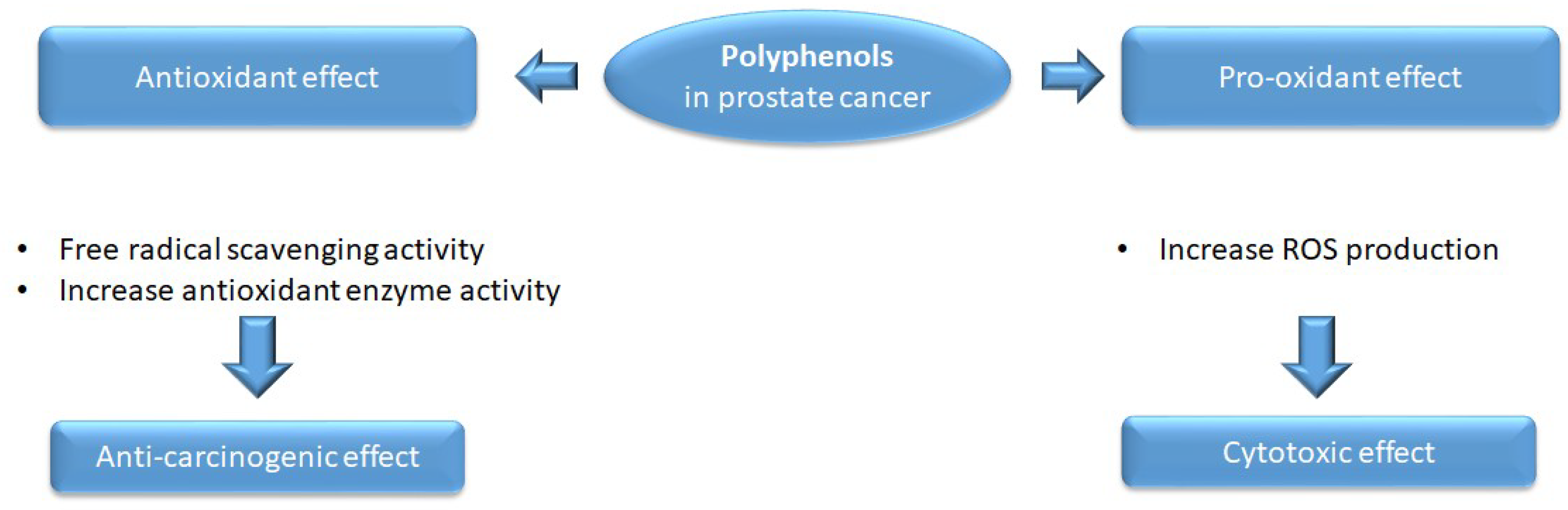
2.1. Antioxidant and Pro-Oxidant Activity
2.2. Proliferation and Survival
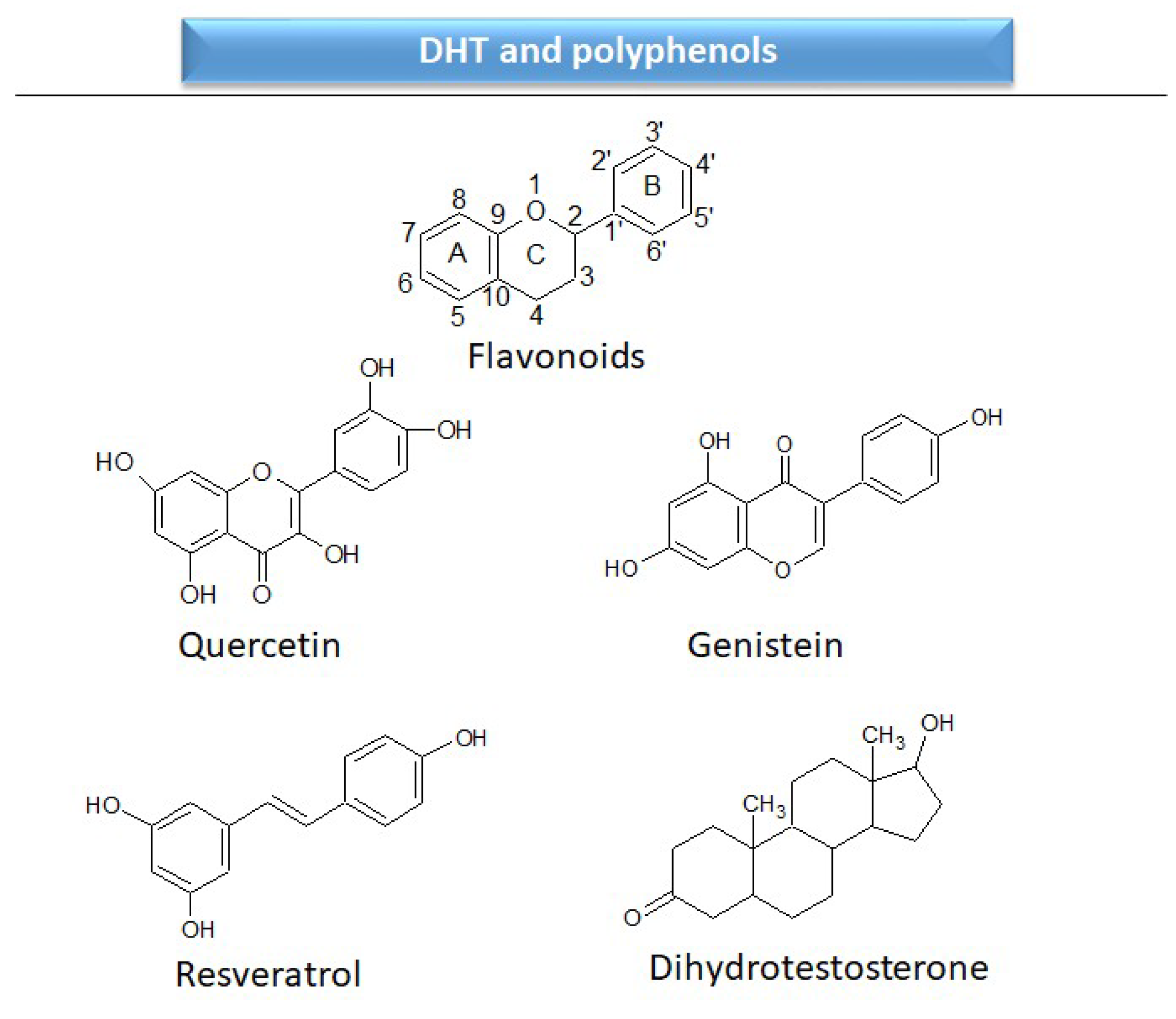
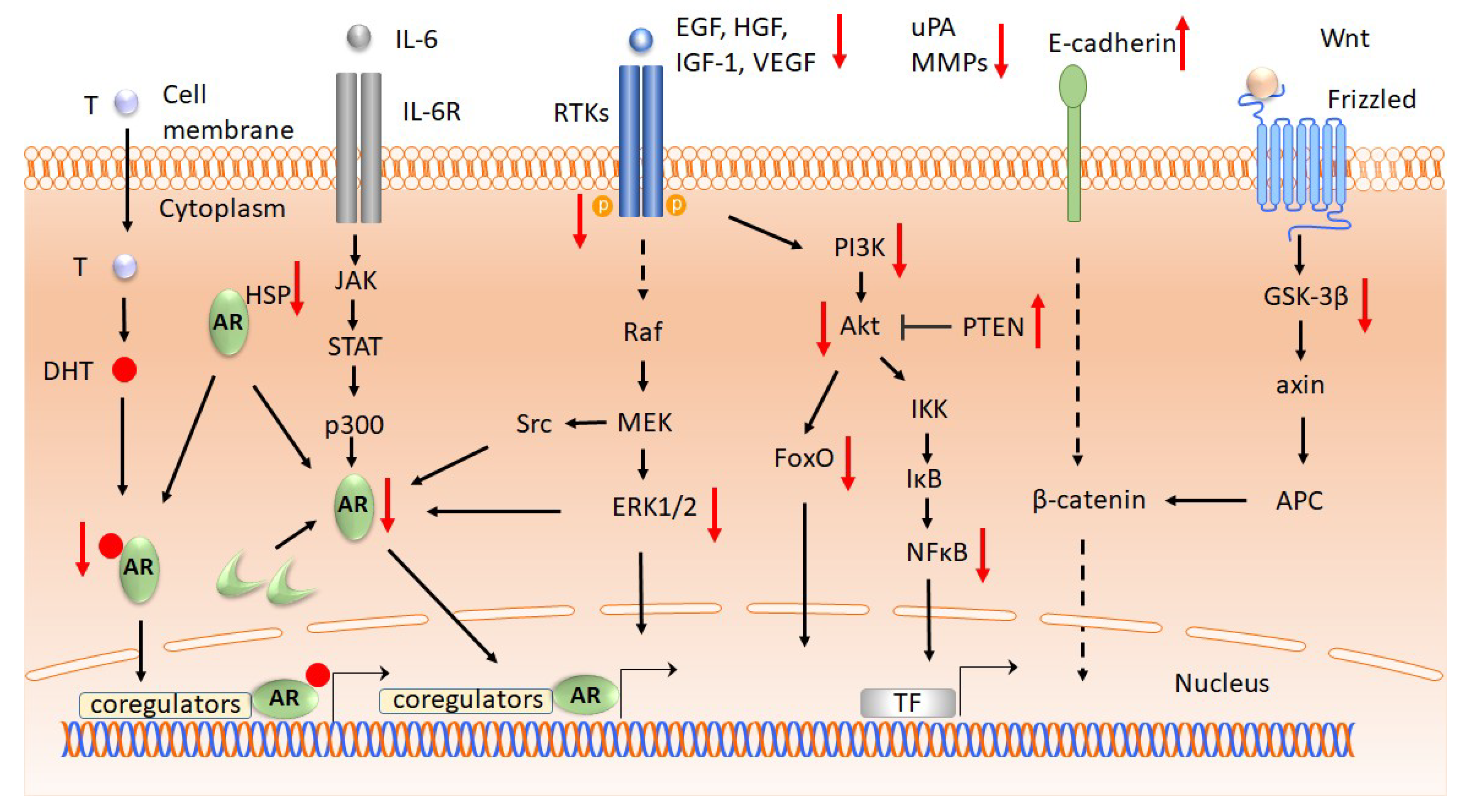
2.2.1. Androgen receptors
2.2.2. Growth Factors and Cytokine Receptors
2.2.3. Signal Transduction
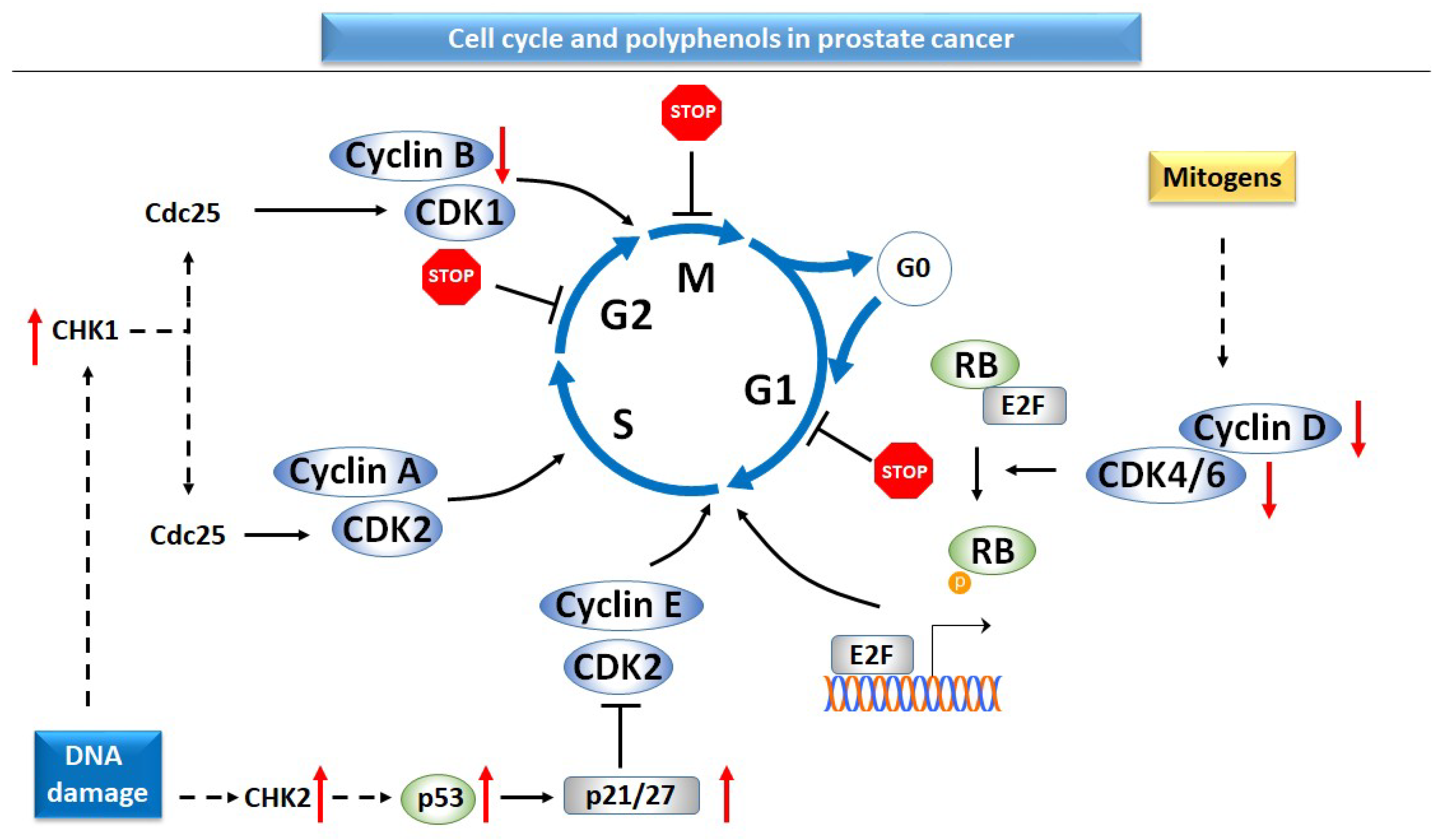
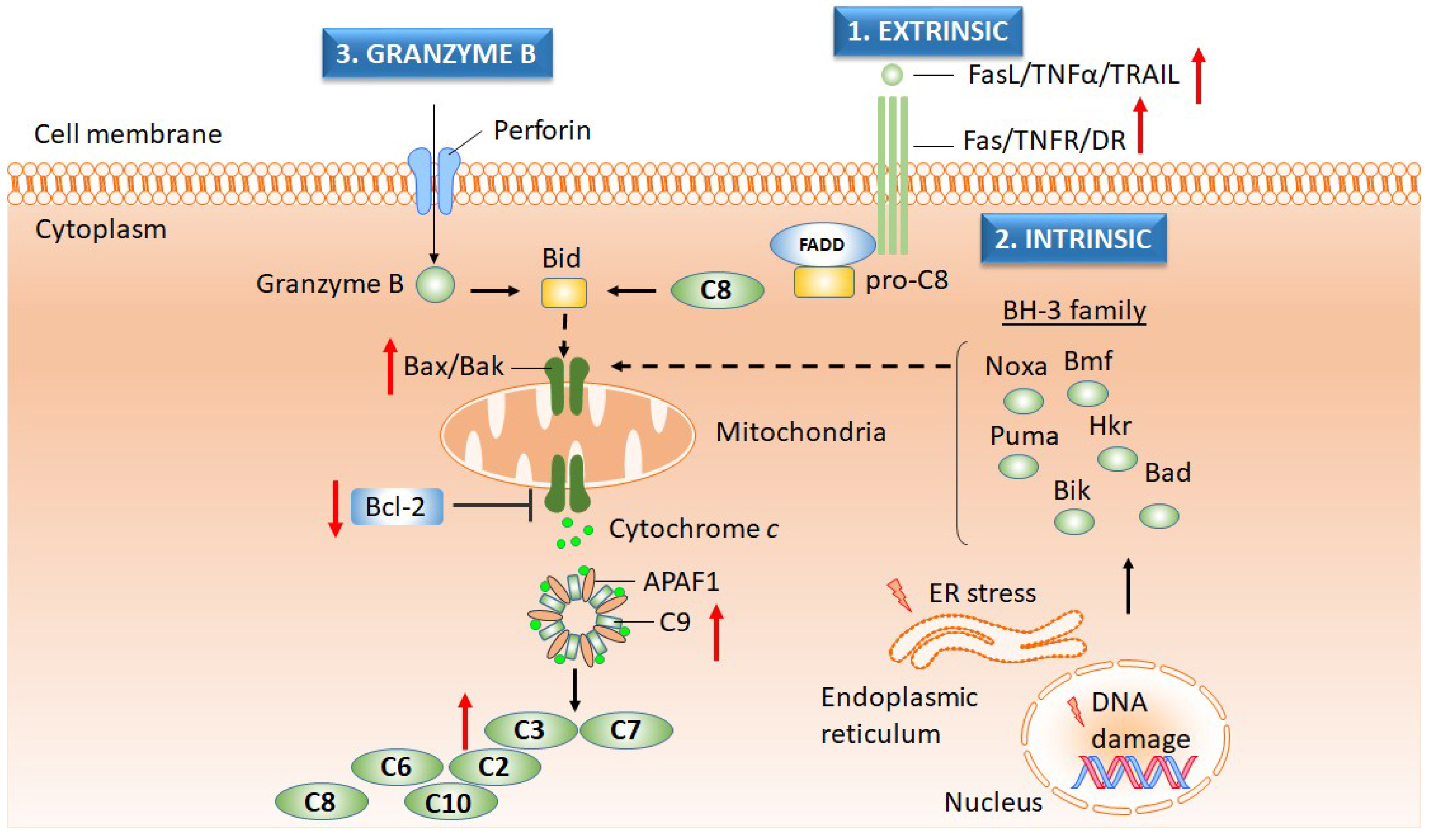
2.3. Cell Cycle and Apoptosis
2.3.1. Cell Cycle
2.3.2. Apoptosis
2.4. Invasion, Metastasis, and Angiogenesis
2.4.1. Invasion and Metastasis
2.4.2. Angiogenesis
2.5. Genomics and Epigenomics
2.5.1. Oncogenes
2.5.2. Tumor Suppressor Genes
2.5.3. DNA Methylation and Histone Modification
2.5.4. miRNA
3. Bioavailability of Polyphenols in Prostate Cancer
3.1. Role of Polyphenol Metabolites in Prostate Cancer
3.2. Strategies for Enhancing Polyphenol Bioavailability
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADT | androgen-deprivation therapy |
| Akt | Ak tymoma protein/PKB, protein kinase B |
| AR | androgen receptor |
| ATF | activating transcription factor |
| Bax | Bcl-2-associated X protein |
| Bcl-2 | B-cell lymphoma type 2 protein |
| Bcl-XL | Bcl-2 extra-large protein |
| BCRP | breast cancer resistance protein |
| BPH | benign prostate hyperplasia |
| BTG3 | B-cell translocation gene |
| caspase-3 | cysteine-aspartic acid protease 3 |
| CAT | catalase |
| cdc25 | cell cycle division protein 25 |
| CHK1 | checkpoint kinase 1 |
| CHOP | CCAAT-enhancer-binding protein homologous protein |
| c-Met/HGF | hepatocyte growth factor |
| CRPC | Castration-resistant prostate cancer |
| CYP450 | cytochrome 450 enzymes |
| DG5 | death receptor |
| DHT | dihydrotestosterone |
| EGCG | epigallocatechin gallate |
| EGF | epidermal growth factor |
| EGFR | epidermal growth factor receptor |
| ER | estrogen receptor |
| ERK1/2 | extracelluar signal-regulated kinases -1, -2 |
| FoxO | forkhead box O protein |
| GADD153 | growth arrest and DNA damage inducible protein 153 protein |
| GPx | glutathione peroxidase |
| GRB2 | growth factor receptor-bound protein 2 |
| GRP78 | glucose regulated protein of 78 kDa |
| GSK-3β | glycogen synthase kinase |
| GSR | glutathione reductase |
| GSTs | glutathione-S-transferases |
| HER2/ErbB2 | c-ErbB2 avian erythroblastic leukemia viral homolog 2 |
| CXCL-1, -2 | chemokine with CXC motif ligand -1 -2 |
| HSP90 | heat shock protein 90 |
| IGF-1 | insulin-like growth factor 1 |
| IL-6 | interleukin 6 |
| IκB | inhibitor of NF-κB |
| JNK | c-Jun N-terminal kinase |
| LPH | lactase phloridzin hydrolase |
| MAPK | mitogen activated protein kinase |
| miR | microRNA |
| MMP-2 | matrix metalloproteinase 2 |
| MRP | multidrug resistance-associated protein |
| mTOR | mammalian target of rapamycin |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| OPG | osteoprotegerin |
| p21/CIP1 | cyclin-dependent kinase inhibitor 1A/CDK-interacting protein 1 |
| p27/Kip1 | kinesin-like protein |
| PARP | poly(ADP-ribose) polymerase |
| PDK1 | phosphoinositide-dependent kinase-1 |
| P-gp | P-glycoprotein |
| PI3K | phosphatidylinositol 3-kinase |
| PKC | protein kinase C |
| PSA | prostate-specific antigen |
| PTEN | phosphatase and tensin homolog |
| RARβ | retinoic acid receptor beta |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase |
| SOS | son of sevenless |
| SULTs | sulphotransferases |
| TRAIL | TNF-related apoptosis-inducing ligand |
| TRAMP | transgenic adenocarcinoma of the mouse prostate |
| UGTs | diphospho-glucuronosyltransferases |
| uPA | urokinase-type plasminogen activator |
| VEGF | vascular endothelial factor; |
| c-Jun | avian sarcoma virus 17 homolog |
| ΔΨm | mitochondrial membrane potential |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Sathianathen, N.J.; Konety, B.R.; Crook, J.; Saad, F.; Lawrentschuk, N. Landmarks in prostate cancer. Nat. Rev. Urol. 2018, 15, 627–642. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2013. CA Cancer J. Clin. 2013, 63, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Pollock, P.A.; Ludgate, A.; Wassersug, R.J. In 2124, half of all men can count on developing prostate cancer. Curr. Oncol. 2015, 22, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Albertsen, P.C.; Hanley, J.A.; Fine, J. 20-year outcomes following conservative management of clinically localized prostate cancer. Jama 2005, 293, 2095–2101. [Google Scholar] [CrossRef] [PubMed]
- Moyad, M.A.; Carroll, P.R. Lifestyle recommendations to prevent prostate cancer, part II: Time to redirect our attention? Urol. Clin. North. Am. 2004, 31, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Moyad, M.A.; Carroll, P.R. Lifestyle recommendations to prevent prostate cancer, part I: Time to redirect our attention? The Urologic clinics of North America 2004, 31, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Wilt, T.J.; Brawer, M.K.; Jones, K.M.; Barry, M.J.; Aronson, W.J.; Fox, S.; Gingrich, J.R.; Wei, J.T.; Gilhooly, P.; Grob, B.M.; et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl. J. Med. 2012, 367, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Wilt, T.J.; Jones, K.M.; Barry, M.J.; Andriole, G.L.; Culkin, D.; Wheeler, T.; Aronson, W.J.; Brawer, M.K. Follow-up of Prostatectomy versus Observation for Early Prostate Cancer. N Engl. J. Med. 2017, 377, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.M.; Abate-Shen, C. Molecular genetics of prostate cancer: New prospects for old challenges. Genes Dev 2010, 24, 1967–2000. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.A.; Toivanen, R.; Risbridger, G.P. Stem cells in prostate cancer: Treating the root of the problem. Endocr. Relat. Cancer 2010, 17, R273–R285. [Google Scholar] [CrossRef] [PubMed]
- Abate-Shen, C.; Shen, M.M. Molecular genetics of prostate cancer. Genes Dev. 2000, 14, 2410–2434. [Google Scholar] [PubMed]
- Di Zazzo, E.; Galasso, G.; Giovannelli, P.; Di Donato, M.; Castoria, G. Estrogens and Their Receptors in Prostate Cancer: Therapeutic Implications. Front. Oncol. 2018, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Kucway, R.; Vicini, F.; Huang, R.; Stromberg, J.; Gonzalez, J.; Martinez, A. Prostate volume reduction with androgen deprivation therapy before interstitial brachytherapy. J. Urol. 2002, 167, 2443–2447. [Google Scholar] [CrossRef]
- Han, M.; Partin, A.W.; Zahurak, M.; Piantadosi, S.; Epstein, J.I.; Walsh, P.C. Biochemical (prostate specific antigen) recurrence probability following radical prostatectomy for clinically localized prostate cancer. J. Urol. 2003, 169, 517–523. [Google Scholar] [CrossRef]
- Keating, N.L.; Landrum, M.B.; Klabunde, C.N.; Fletcher, R.H.; Rogers, S.O.; Doucette, W.R.; Tisnado, D.; Clauser, S.; Kahn, K.L. Adjuvant chemotherapy for stage III colon cancer: Do physicians agree about the importance of patient age and comorbidity? J. Clin. Oncol. 2008, 26, 2532–2537. [Google Scholar] [CrossRef] [PubMed]
- Keating, N.L.; O’Malley, A.J.; Freedland, S.J.; Smith, M.R. Diabetes and cardiovascular disease during androgen deprivation therapy: Observational study of veterans with prostate cancer. J. Natl. Cancer Inst. 2010, 102, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Di Zazzo, E.; Galasso, G.; Giovannelli, P.; Di Donato, M.; Di Santi, A.; Cernera, G.; Rossi, V.; Abbondanza, C.; Moncharmont, B.; Sinisi, A.A.; et al. Prostate cancer stem cells: The role of androgen and estrogen receptors. Oncotarget 2016, 7, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Lee, E.J.; Madison, L.D.; Lazennec, G. Expression of estrogen receptor beta in prostate carcinoma cells inhibits invasion and proliferation and triggers apoptosis. FEBS Lett. 2004, 566, 169–172. [Google Scholar] [PubMed]
- Slusarz, A.; Jackson, G.A.; Day, J.K.; Shenouda, N.S.; Bogener, J.L.; Browning, J.D.; Fritsche, K.L.; MacDonald, R.S.; Besch-Williford, C.L.; Lubahn, D.B. Aggressive prostate cancer is prevented in ERalphaKO mice and stimulated in ERbetaKO TRAMP mice. Endocrinology 2012, 153, 4160–4170. [Google Scholar] [CrossRef] [PubMed]
- El Etreby, M.F.; Liang, Y.; Lewis, R.W. Induction of apoptosis by mifepristone and tamoxifen in human LNCaP prostate cancer cells in culture. Prostate 2000, 43, 31–42. [Google Scholar] [CrossRef]
- Price, D.; Stein, B.; Sieber, P.; Tutrone, R.; Bailen, J.; Goluboff, E.; Burzon, D.; Bostwick, D.; Steiner, M. Toremifene for the prevention of prostate cancer in men with high grade prostatic intraepithelial neoplasia: Results of a double-blind, placebo controlled, phase IIB clinical trial. J. Urol. 2006, 176, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Tannock, I.F.; de Wit, R.; Berry, W.R.; Horti, J.; Pluzanska, A.; Chi, K.N.; Oudard, S.; Theodore, C.; James, N.D.; Turesson, I.; et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl. J. Med. 2004, 351, 1502–1512. [Google Scholar] [CrossRef] [PubMed]
- Oh, W.K. The evolving role of estrogen therapy in prostate cancer. Clin. Prostate Cancer 2002, 1, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.E.; D’Amico, A.V. Progress and controversies: Radiation therapy for prostate cancer. CA Cancer J. Clin. 2014, 64, 389–407. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, S.; Attard, G.; Hudson, D.L. Prostate epithelial stem cells. Cell Prolif. 2005, 38, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Bonkhoff, H.; Remberger, K. Differentiation pathways and histogenetic aspects of normal and abnormal prostatic growth: A stem cell model. Prostate 1996, 28, 98–106. [Google Scholar] [CrossRef]
- Peisch, S.F.; Van Blarigan, E.L.; Chan, J.M.; Stampfer, M.J.; Kenfield, S.A. Prostate cancer progression and mortality: A review of diet and lifestyle factors. World J. Urol. 2017, 35, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Guarnido, O.; Urquiza-Salvat, N.; Saiz, M.; Lozano-Paniagua, D.; Rodrigo, L.; Pascual-Geler, M.; Lorente, J.A.; Alvarez-Cubero, M.J.; Rivas, A. Bioactive compounds of the Mediterranean diet and prostate cancer. Aging Male 2018, 21, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Manach, C.; Morand, C.; Remesy, C.; Jimenez, L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Croft, K.D. Dietary polyphenols: Antioxidants or not? Arch. Biochem. Biophys. 2016, 595, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Tsatsakis, A.; Mamoulakis, C.; Teodoro, M.; Briguglio, G.; Caruso, E.; Tsoukalas, D.; Margina, D.; Dardiotis, E.; Kouretas, D.; et al. Current evidence on the effect of dietary polyphenols intake on chronic diseases. Food Chem. Toxicol. 2017, 110, 286–299. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Sun, T.; Li, W.; Sun, X. Inhibiting effects of dietary polyphenols on chronic eye diseases. J. Funct. Foods 2017, 39, 186–197. [Google Scholar] [CrossRef]
- Caporali, A.; Davalli, P.; Astancolle, S.; D’Arca, D.; Brausi, M.; Bettuzzi, S.; Corti, A. The chemopreventive action of catechins in the TRAMP mouse model of prostate carcinogenesis is accompanied by clusterin over-expression. Carcinogenesis 2004, 25, 2217–2224. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, D.S.; Clubbs, E.A.; Ferruzzi, M.; Bomser, J.A. Epigallocatechin-3-gallate (EGCG) inhibits PC-3 prostate cancer cell proliferation via MEK-independent ERK1/2 activation. Chem. Biol. Interact. 2008, 171, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Abdal Dayem, A.; Choi, H.; Yang, G.-M.; Kim, K.; Saha, S.; Cho, S.-G. The anti-cancer effect of polyphenols against breast cancer and cancer stem cells: Molecular mechanisms. Nutrients 2016, 8, 581. [Google Scholar] [CrossRef] [PubMed]
- Amararathna, M.; Johnston, M.; Rupasinghe, H. Plant polyphenols as chemopreventive agents for lung cancer. Int. J. Mol. Sci 2016, 17, 1352. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.N.; Almoyad, M.; Huq, F. Polyphenols in colorectal cancer: Current state of knowledge including clinical trials and molecular mechanism of action. BioMed. Res. Int. 2018, 2018, 4154185. [Google Scholar] [CrossRef] [PubMed]
- Ignat, I.; Volf, I.; Popa, V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef] [PubMed]
- Khoddami, A.; Wilkes, M.A.; Roberts, T.H. Techniques for analysis of plant phenolic compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.-C.; Lin, C.-C.; Wu, H.-C.; Tsao, S.-M.; Hsu, C.-K. Apoptotic Effects of Protocatechuic Acid in Human Breast, Lung, Liver, Cervix, and Prostate Cancer Cells: Potential Mechanisms of Action. J. Agric. Food Chem. 2009, 57, 6468–6473. [Google Scholar] [CrossRef] [PubMed]
- Azrad, M.; Vollmer, R.T.; Madden, J.; Dewhirst, M.; Polascik, T.J.; Snyder, D.C.; Ruffin, M.T.; Moul, J.W.; Brenner, D.E.; Demark-Wahnefried, W. Flaxseed-derived enterolactone is inversely associated with tumor cell proliferation in men with localized prostate cancer. J. Med. Food 2013, 16, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Mandair, D.; Rossi, R.E.; Pericleous, M.; Whyand, T.; Caplin, M.E. Prostate cancer and the influence of dietary factors and supplements: A systematic review. Nutr. Metab. 2014, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-González, C.; Noé, V.; Izquierdo-Pulido, M. Walnut polyphenol metabolites, urolithins A and B, inhibit the expression of the prostate-specific antigen and the androgen receptor in prostate cancer cells. Food Funct. 2014, 5, 2922–2930. [Google Scholar] [CrossRef] [PubMed]
- Lall, R.; Syed, D.; Adhami, V.; Khan, M.; Mukhtar, H. Dietary polyphenols in prevention and treatment of prostate cancer. Int. J. Mol. Sci. 2015, 16, 3350–3376. [Google Scholar] [CrossRef] [PubMed]
- Itsumi, M.; Shiota, M.; Takeuchi, A.; Kashiwagi, E.; Inokuchi, J.; Tatsugami, K.; Kajioka, S.; Uchiumi, T.; Naito, S.; Eto, M. Equol inhibits prostate cancer growth through degradation of androgen receptor by S-phase kinase-associated protein 2. Cancer Sci. 2016, 107, 1022–1028. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Zhou, R.; Kong, Y.; Wang, J.; Xia, W.; Guo, J.; Liu, J.; Sun, H.; Liu, K.; Yang, J. S-equol, a secondary metabolite of natural anticancer isoflavone daidzein, inhibits prostate cancer growth in vitro and in vivo, though activating the Akt/FOXO3a pathway. Curr. Cancer Drug Targets 2016, 16, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Davalli, P.; Rizzi, F.; Caporali, A.; Pellacani, D.; Davoli, S.; Bettuzzi, S.; Brausi, M.; D’Arca, D. Anticancer activity of green tea polyphenols in prostate gland. Oxid. Med. Cell Longev. 2012, 2012, 984219. [Google Scholar] [CrossRef] [PubMed]
- Naponelli, V.; Ramazzina, I.; Lenzi, C.; Bettuzzi, S.; Rizzi, F. Green Tea Catechins for Prostate Cancer Prevention: Present Achievements and Future Challenges. Antioxidants 2017, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- van Duynhoven, J.; Vaughan, E.E.; Jacobs, D.M.; Kemperman, R.A.; van Velzen, E.J.; Gross, G.; Roger, L.C.; Possemiers, S.; Smilde, A.K.; Dore, J.; et al. Metabolic fate of polyphenols in the human superorganism. Proc. Natl. Acad. Sci. USA 2011, 108, 4531–4538. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Vance, T.M.; Chun, O.K. Estimated intake and major food sources of flavonoids among US adults: Changes between 1999–2002 and 2007–2010 in NHANES. Eur. J. Nutr. 2016, 55, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Tresserra-Rimbau, A.; Lamuela-Raventos, R.M.; Moreno, J.J. Polyphenols, food and pharma. Current knowledge and directions for future research. Biochem. Pharmacol. 2018, 156, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Oroian, M.; Escriche, I. Antioxidants: Characterization, natural sources, extraction and analysis. Food Res. Int. 2015, 74, 10–36. [Google Scholar] [CrossRef] [PubMed]
- Kallifatidis, G.; Hoy, J.J.; Lokeshwar, B.L. Bioactive natural products for chemoprevention and treatment of castration-resistant prostate cancer. Semin Cancer Biol. 2016, 40–41, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Sreekumar, S.; Sithul, H.; Muraleedharan, P.; Azeez, J.M.; Sreeharshan, S. Pomegranate fruit as a rich source of biologically active compounds. Biomed. Res. Int. 2014, 2014, 686921. [Google Scholar] [CrossRef] [PubMed]
- Vendrame, S.; Del Bo, C.; Ciappellano, S.; Riso, P.; Klimis-Zacas, D. Berry Fruit Consumption and Metabolic Syndrome. Antioxidants 2016, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Shrikanta, A.; Kumar, A.; Govindaswamy, V. Resveratrol content and antioxidant properties of underutilized fruits. J. Food Sci. Technol. 2015, 52, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Milder, I.E.J.; Feskens, E.J.M.; Arts, I.C.W.; de Mesquita, H.B.B.; Hollman, P.C.H.; Kromhout, D. Intake of the Plant Lignans Secoisolariciresinol, Matairesinol, Lariciresinol, and Pinoresinol in Dutch Men and Women. J. Nutr. 2005, 135, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Kocaadam, B.; Şanlier, N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2889–2895. [Google Scholar] [CrossRef] [PubMed]
- Semwal, R.B.; Semwal, D.K.; Combrinck, S.; Viljoen, A.M. Gingerols and shogaols: Important nutraceutical principles from ginger. Phytochemistry 2015, 117, 554–568. [Google Scholar] [CrossRef] [PubMed]
- Kampa, M.; Hatzoglou, A.; Notas, G.; Damianaki, A.; Bakogeorgou, E.; Gemetzi, C.; Kouroumalis, E.; Martin, P.-M.; Castanas, E.J.N. Wine antioxidant polyphenols inhibit the proliferation of human prostate cancer cell lines. Nutr. Cancer 2000, 37, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Serafini, M.; Ghiselli, A.; Ferro-Luzzi, A. In vivo antioxidant effect of green and black tea in man. Eur. J. Clin. Nutr. 1996, 50, 28–32. [Google Scholar] [PubMed]
- Basak, S.; Pookot, D.; Noonan, E.J.; Dahiya, R. Genistein down-regulates androgen receptor by modulating HDAC6-Hsp90 chaperone function. Mol. Cancer Ther. 2008, 7, 3195–3202. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.B.; DePrimo, S.E.; Whitfield, M.L.; Brooks, J.D. Resveratrol-induced gene expression profiles in human prostate cancer cells. Cancer Epidemiol. Biomarkers Prev. 2005, 14, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.C.; Yen, C.Y.; Wu, R.S.; Yang, J.S.; Lu, H.F.; Lu, K.W.; Lo, C.; Chen, H.Y.; Tang, N.Y.; Wu, C.C.; et al. The roles of endoplasmic reticulum stress and mitochondrial apoptotic signaling pathway in quercetin-mediated cell death of human prostate cancer PC-3 cells. Environ. Toxicol. 2014, 29, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Russell, P.J.; Kingsley, E.A. Human prostate cancer cell lines. Methods Mol. Med. 2003, 81, 21–39. [Google Scholar] [PubMed]
- Wu, X.; Gong, S.; Roy-Burman, P.; Lee, P.; Culig, Z. Current mouse and cell models in prostate cancer research. Endocr. Relat. Cancer 2013, 20, R155–R170. [Google Scholar] [CrossRef] [PubMed]
- Karna, P.; Chagani, S.; Gundala, S.R.; Rida, P.C.; Asif, G.; Sharma, V.; Gupta, M.V.; Aneja, R. Benefits of whole ginger extract in prostate cancer. Br. J. Nutr. 2012, 107, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Sobel, R.E.; Wang, Y.; Sadar, M.D. Molecular analysis and characterization of PrEC, commercially available prostate epithelial cells. Vitro Cell. Dev. Biol. -Animal 2006, 42, 33–39. [Google Scholar] [CrossRef]
- Chiyomaru, T.; Yamamura, S.; Zaman, M.S.; Majid, S.; Deng, G.; Shahryari, V.; Saini, S.; Hirata, H.; Ueno, K.; Chang, I.; et al. Genistein suppresses prostate cancer growth through inhibition of oncogenic microRNA-151. PLoS ONE 2012, 7, e43812. [Google Scholar] [CrossRef] [PubMed]
- Horoszewicz, J.S.; Leong, S.S.; Kawinski, E.; Karr, J.P.; Rosenthal, H.; Chu, T.M.; Mirand, E.A.; Murphy, G.P. LNCaP model of human prostatic carcinoma. Cancer Res. 1983, 43, 1809–1818. [Google Scholar] [PubMed]
- Veldscholte, J.; Ris-Stalpers, C.; Kuiper, G.; Jenster, G.; Berrevoets, C.; Claassen, E.; Van Rooij, H.; Trapman, J.; Brinkmann, A.; Mulder, E. A mutation in the ligand binding domain of the androgen receptor of human INCaP cells affects steroid binding characteristics and response to anti-androgens. Biochem. Biophys. Res. Commun. 1990, 173, 534–540. [Google Scholar] [CrossRef]
- Kaighn, M.; Narayan, K.S.; Ohnuki, Y.; Lechner, J.; Jones, L. Establishment and characterization of a human prostatic carcinoma cell line (PC-3). Investig. Urol. 1979, 17, 16–23. [Google Scholar]
- Tai, S.; Sun, Y.; Squires, J.M.; Zhang, H.; Oh, W.K.; Liang, C.Z.; Huang, J. PC3 is a cell line characteristic of prostatic small cell carcinoma. The Prostate 2011, 71, 1668–1679. [Google Scholar] [CrossRef] [PubMed]
- Pettaway, C.A.; Pathak, S.; Greene, G.; Ramirez, E.; Wilson, M.R.; Killion, J.J.; Fidler, I.J. Selection of highly metastatic variants of different human prostatic carcinomas using orthotopic implantation in nude mice. Clin. Cancer Res. 1996, 2, 1627–1636. [Google Scholar] [PubMed]
- Sheth, S.; Jajoo, S.; Kaur, T.; Mukherjea, D.; Sheehan, K.; Rybak, L.P.; Ramkumar, V. Resveratrol reduces prostate cancer growth and metastasis by inhibiting the Akt/MicroRNA-21 pathway. PLoS ONE 2012, 7, e51655. [Google Scholar] [CrossRef] [PubMed]
- Stone, K.R.; Mickey, D.D.; Wunderli, H.; Mickey, G.H.; Paulson, D.F. Isolation of a human prostate carcinoma cell line (DU 145). Int. J. Cancer 1978, 21, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Sramkoski, R.M.; Pretlow, T.G.; Giaconia, J.M.; Pretlow, T.P.; Schwartz, S.; Sy, M.-S.; Marengo, S.R.; Rhim, J.S.; Zhang, D.; Jacobberger, J.W. A new human prostate carcinoma cell line, 22Rv1. Vitro Cell. Dev. Biol. -Animal 1999, 35, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, S.; Doganlar, O.; Doganlar, Z.B.; Serttas, R.; Turkekul, K.; Dibirdik, I.; Bilir, A. The flavonoid apigenin reduces prostate cancer CD44(+) stem cell survival and migration through PI3K/Akt/NF-kappaB signaling. Life Sci. 2016, 162, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Machioka, K.; Izumi, K.; Kadono, Y.; Iwamoto, H.; Naito, R.; Makino, T.; Kadomoto, S.; Natsagdorj, A.; Keller, E.T.; Zhang, J. Establishment and characterization of two cabazitaxel-resistant prostate cancer cell lines. Oncotarget 2018, 9, 16185. [Google Scholar] [CrossRef] [PubMed]
- Klein, K.A.; Reiter, R.E.; Redula, J.; Moradi, H.; Zhu, X.L.; Brothman, A.R.; Lamb, D.J.; Marcelli, M.; Belldegrun, A.; Witte, O.N. Progression of metastatic human prostate cancer to androgen independence in immunodeficient SCID mice. Nat. Med. 1997, 3, 402. [Google Scholar] [CrossRef] [PubMed]
- Bello, D.; Webber, M.; Kleinman, H.; Wartinger, D.; Rhim, J. Androgen responsive adult human prostatic epithelial cell lines immortalized by human papillomavirus 18. Carcinogenesis 1997, 18, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Thalmann, G.N.; Anezinis, P.E.; Chang, S.-M.; Zhau, H.E.; Kim, E.E.; Hopwood, V.L.; Pathak, S.; von Eschenbach, A.C.; Chung, L.W. Androgen-independent cancer progression and bone metastasis in the LNCaP model of human prostate cancer. Cancer Res. 1994, 54, 2577–2581. [Google Scholar] [PubMed]
- Gao, X.; Schottker, B. Reduction-oxidation pathways involved in cancer development: A systematic review of literature reviews. Oncotarget 2017, 8, 51888–51906. [Google Scholar] [PubMed]
- Li, Y.; Che, M.; Bhagat, S.; Ellis, K.L.; Kucuk, O.; Doerge, D.R.; Abrams, J.; Cher, M.L.; Sarkar, F.H. Regulation of gene expression and inhibition of experimental prostate cancer bone metastasis by dietary genistein. Neoplasia 2004, 6, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, I.A.; Malik, A.; Adhami, V.M.; Asim, M.; Hafeez, B.B.; Sarfaraz, S.; Mukhtar, H. Green tea polyphenol EGCG sensitizes human prostate carcinoma LNCaP cells to TRAIL-mediated apoptosis and synergistically inhibits biomarkers associated with angiogenesis and metastasis. Oncogene 2008, 27, 2055–2063. [Google Scholar] [CrossRef] [PubMed]
- Vayalil, P.K.; Katiyar, S.K. Treatment of epigallocatechin-3-gallate inhibits matrix metalloproteinases-2 and -9 via inhibition of activation of mitogen-activated protein kinases, c-jun and NF-kappaB in human prostate carcinoma DU-145 cells. Prostate 2004, 59, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Perron, N.R.; Brumaghim, J.L. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef] [PubMed]
- Choe, E.; Min, D.B. Mechanisms of Antioxidants in the Oxidation of Foods. Compr. Rev. Food Sci. Food Saf. 2009, 8, 345–358. [Google Scholar] [CrossRef]
- Chaudhary, A.; Pechan, T.; Willett, K.L. Differential protein expression of peroxiredoxin I and II by benzo(a)pyrene and quercetin treatment in 22Rv1 and PrEC prostate cell lines. Toxicol. Appl. Pharmacol. 2007, 220, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Sorrenti, V.; Vanella, L.; Acquaviva, R.; Cardile, V.; Giofre, S.; Di Giacomo, C. Cyanidin induces apoptosis and differentiation in prostate cancer cells. Int. J. Oncol. 2015, 47, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.M.; Wu, Y.C.; Chia, Y.C.; Chang, F.R.; Hsu, H.K.; Hsieh, Y.C.; Chen, C.C.; Yuan, S.S. Gallic acid, a major component of Toona sinensis leaf extracts, contains a ROS-mediated anti-cancer activity in human prostate cancer cells. Cancer Lett. 2009, 286, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Russell, L.H., Jr.; Mazzio, E.; Badisa, R.B.; Zhu, Z.P.; Agharahimi, M.; Oriaku, E.T.; Goodman, C.B. Autoxidation of gallic acid induces ROS-dependent death in human prostate cancer LNCaP cells. Anticancer Res. 2012, 32, 1595–1602. [Google Scholar] [PubMed]
- Ward, A.B.; Mir, H.; Kapur, N.; Gales, D.N.; Carriere, P.P.; Singh, S. Quercetin inhibits prostate cancer by attenuating cell survival and inhibiting anti-apoptotic pathways. World J. Surg. Oncol. 2018, 16, 108. [Google Scholar] [CrossRef] [PubMed]
- Young, I.; Woodside, J. Antioxidants in health and disease. J. Clin. Pathol. 2001, 54, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Koike, H.; Matsui, H.; Ono, Y.; Hasumi, M.; Nakazato, H.; Okugi, H.; Sekine, Y.; Oki, K.; Ito, K.; et al. Genistein, a soy isoflavone, induces glutathione peroxidase in the human prostate cancer cell lines LNCaP and PC-3. Int. J. Cancer 2002, 99, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Sharmila, G.; Bhat, F.A.; Arunkumar, R.; Elumalai, P.; Raja Singh, P.; Senthilkumar, K.; Arunakaran, J. Chemopreventive effect of quercetin, a natural dietary flavonoid on prostate cancer in in vivo model. Clin. Nutr. 2014, 33, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Salganik, R.I. The benefits and hazards of antioxidants: Controlling apoptosis and other protective mechanisms in cancer patients and the human population. J. Am. Coll. Nutr. 2001, 20, 464S–472S; discussion 473S–475S. [Google Scholar] [CrossRef] [PubMed]
- Thomas, F.; Holly, J.M.; Persad, R.; Bahl, A.; Perks, C.M. Green tea extract (epigallocatechin-3-gallate) reduces efficacy of radiotherapy on prostate cancer cells. Urology 2011, 78, e15–e21. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Bolton, E.C.; Jones, J.O. Androgens and androgen receptor signaling in prostate tumorigenesis. J. Mol. Endocrinol. 2015, 54, R15–R29. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, M.E.; Huang, H.; Tindall, D.J. Androgen receptor signaling in androgen-refractory prostate cancer. J. Natl. Cancer Inst. 2001, 93, 1687–1697. [Google Scholar] [CrossRef] [PubMed]
- Shiota, M.; Yokomizo, A.; Naito, S. Oxidative stress and androgen receptor signaling in the development and progression of castration-resistant prostate cancer. Free Radic Biol. Med. 2011, 51, 1320–1328. [Google Scholar] [CrossRef] [PubMed]
- Boam, T. Anti-androgenic effects of flavonols in prostate cancer. Ecancermedicalscience 2015, 9, 585. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Zhu, T.; Parray, A.; Siddique, H.R.; Yang, W.; Saleem, M.; Bosland, M.C. Differential effects of genistein on prostate cancer cells depend on mutational status of the androgen receptor. PLoS ONE 2013, 8, e78479. [Google Scholar] [CrossRef] [PubMed]
- Benitez, D.A.; Pozo-Guisado, E.; Clementi, M.; Castellon, E.; Fernandez-Salguero, P.M. Non-genomic action of resveratrol on androgen and oestrogen receptors in prostate cancer: Modulation of the phosphoinositide 3-kinase pathway. Br. J. Cancer 2007, 96, 1595–1604. [Google Scholar] [CrossRef] [PubMed]
- Harada, N.; Murata, Y.; Yamaji, R.; Miura, T.; Inui, H.; Nakano, Y. Resveratrol down-regulates the androgen receptor at the post-translational level in prostate cancer cells. J. Nutr. Sci. Vitaminol. 2007, 53, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.H.; Zhu, W.; Young, C.Y. Resveratrol inhibits the expression and function of the androgen receptor in LNCaP prostate cancer cells. Cancer Res. 1999, 59, 5892–5895. [Google Scholar] [PubMed]
- Shi, W.F.; Leong, M.; Cho, E.; Farrell, J.; Chen, H.C.; Tian, J.; Zhang, D. Repressive effects of resveratrol on androgen receptor transcriptional activity. PLoS ONE 2009, 4, e7398. [Google Scholar] [CrossRef] [PubMed]
- Kai, L.; Levenson, A.S. Combination of resveratrol and antiandrogen flutamide has synergistic effect on androgen receptor inhibition in prostate cancer cells. Anticancer Res. 2011, 31, 3323–3330. [Google Scholar] [PubMed]
- Siddiqui, I.A.; Asim, M.; Hafeez, B.B.; Adhami, V.M.; Tarapore, R.S.; Mukhtar, H. Green tea polyphenol EGCG blunts androgen receptor function in prostate cancer. Faseb J. 2011, 25, 1198–1207. [Google Scholar] [CrossRef] [PubMed]
- Chuu, C.P.; Chen, R.Y.; Kokontis, J.M.; Hiipakka, R.A.; Liao, S. Suppression of androgen receptor signaling and prostate specific antigen expression by (-)-epigallocatechin-3-gallate in different progression stages of LNCaP prostate cancer cells. Cancer Lett. 2009, 275, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Lonergan, P.E.; Tindall, D.J. Overdiagnosis and overtreatment of prostate cancer. J. Carcinog. 2011, 10, 20. [Google Scholar] [PubMed]
- Huang, Y.; Jiang, X.; Liang, X.; Jiang, G. Molecular and cellular mechanisms of castration resistant prostate cancer. Oncol. Lett. 2018, 15, 6063–6076. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; MacLennan, G.T.; Fu, P.; Gupta, S. Apigenin attenuates insulin-like growth factor-I signaling in an autochthonous mouse prostate cancer model. Pharm. Res. 2012, 29, 1506–1517. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Romigh, T.; He, X.; Orloff, M.S.; Silverman, R.H.; Heston, W.D.; Eng, C. Resveratrol regulates the PTEN/AKT pathway through androgen receptor-dependent and -independent mechanisms in prostate cancer cell lines. Hum. Mol. Genet. 2010, 19, 4319–4329. [Google Scholar] [CrossRef] [PubMed]
- Killian, P.H.; Kronski, E.; Michalik, K.M.; Barbieri, O.; Astigiano, S.; Sommerhoff, C.P.; Pfeffer, U.; Nerlich, A.G.; Bachmeier, B.E. Curcumin inhibits prostate cancer metastasis in vivo by targeting the inflammatory cytokines CXCL1 and -2. Carcinogenesis 2012, 33, 2507–2519. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Xu, C.; Keum, Y.S.; Reddy, B.; Conney, A.; Kong, A.N. Inhibition of EGFR signaling in human prostate cancer PC-3 cells by combination treatment with beta-phenylethyl isothiocyanate and curcumin. Carcinogenesis 2006, 27, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Duhon, D.; Bigelow, R.L.; Coleman, D.T.; Steffan, J.J.; Yu, C.; Langston, W.; Kevil, C.G.; Cardelli, J.A. The polyphenol epigallocatechin-3-gallate affects lipid rafts to block activation of the c-Met receptor in prostate cancer cells. Mol. Carcinog. 2010, 49, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Giancotti, F.G. Deregulation of cell signaling in cancer. FEBS Lett. 2014, 588, 2558–2570. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.S. Cell signaling and cancer. Cancer Cell 2003, 4, 167–174. [Google Scholar] [CrossRef]
- Sever, R.; Brugge, J.S. Signal transduction in cancer. Cold Spring Harb. Perspect Med. 2015, 5, a006098. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Bhaskaran, N.; Babcook, M.A.; Fu, P.; Maclennan, G.T.; Gupta, S. Apigenin inhibits prostate cancer progression in TRAMP mice via targeting PI3K/Akt/FoxO pathway. Carcinogenesis 2014, 35, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Lamont, K.R.; Tindall, D.J. Minireview: Alternative activation pathways for the androgen receptor in prostate cancer. Mol. Endocrinol. 2011, 25, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, T.D. Introduction to NF-kappaB: Players, pathways, perspectives. Oncogene 2006, 25, 6680–6684. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. NF-kappaB, the first quarter-century: Remarkable progress and outstanding questions. Genes Dev. 2012, 26, 203–234. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.P.; Jiang, S.S.; Chuu, C.P. Caffeic acid phenethyl ester causes p21 induction, Akt signaling reduction, and growth inhibition in PC-3 human prostate cancer cells. PLoS ONE 2012, 7, e31286. [Google Scholar]
- Chuu, C.P.; Lin, H.P.; Ciaccio, M.F.; Kokontis, J.M.; Hause, R.J., Jr.; Hiipakka, R.A.; Liao, S.; Jones, R.B. Caffeic acid phenethyl ester suppresses the proliferation of human prostate cancer cells through inhibition of p70S6K and Akt signaling networks. Cancer Prev. Res. 2012, 5, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Deeb, D.; Jiang, H.; Gao, X.; Hafner, M.S.; Wong, H.; Divine, G.; Chapman, R.A.; Dulchavsky, S.A.; Gautam, S.C. Curcumin sensitizes prostate cancer cells to tumor necrosis factor-related apoptosis-inducing ligand/Apo2L by inhibiting nuclear factor-kappaB through suppression of IkappaBalpha phosphorylation. Mol. Cancer Ther. 2004, 3, 803–812. [Google Scholar] [PubMed]
- Liu, K.C.; Huang, A.C.; Wu, P.P.; Lin, H.Y.; Chueh, F.S.; Yang, J.S.; Lu, C.C.; Chiang, J.H.; Meng, M.; Chung, J.G. Gallic acid suppresses the migration and invasion of PC-3 human prostate cancer cells via inhibition of matrix metalloproteinase-2 and -9 signaling pathways. Oncol. Rep. 2011, 26, 177–184. [Google Scholar] [PubMed]
- Liu, C.M.; Kao, C.L.; Tseng, Y.T.; Lo, Y.C.; Chen, C.Y. Ginger Phytochemicals Inhibit Cell Growth and Modulate Drug Resistance Factors in Docetaxel Resistant Prostate Cancer Cell. Molecules 2017, 22, 1477. [Google Scholar] [CrossRef] [PubMed]
- Collins, K.; Jacks, T.; Pavletich, N.P. The cell cycle and cancer. Proc. Natl. Acad. Sci. USA 1997, 94, 2776–2778. [Google Scholar] [CrossRef] [PubMed]
- Otto, T.; Sicinski, P. Cell cycle proteins as promising targets in cancer therapy. Nat. Rev. Cancer 2017, 17, 93–115. [Google Scholar] [CrossRef] [PubMed]
- Sherr, C.J.; Bartek, J. Cell cycle–targeted cancer therapies. Annu Rev. Cancer Biol. 2017, 1, 41–57. [Google Scholar] [CrossRef]
- Williams, G.H.; Stoeber, K. The cell cycle and cancer. J. Pathol. 2012, 226, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wu, J.; Li, S.; Wang, X.; Liang, Z.; Xu, X.; Xu, X.; Hu, Z.; Lin, Y.; Chen, H.; et al. Apigenin inhibits migration and invasion via modulation of epithelial mesenchymal transition in prostate cancer. Mol. Med. Rep. 2015, 11, 1004–1008. [Google Scholar] [CrossRef] [PubMed]
- Raina, K.; Rajamanickam, S.; Deep, G.; Singh, M.; Agarwal, R.; Agarwal, C. Chemopreventive effects of oral gallic acid feeding on tumor growth and progression in TRAMP mice. Mol. Cancer Ther. 2008, 7, 1258–1267. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.J.; Wilson, L.; Jordan, M.A.; Nguyen, V.; Tang, J.; Smiyun, G. Hesperidin suppressed proliferations of both human breast cancer and androgen-dependent prostate cancer cells. Phytother. Res. 2010, 24, S15–S19. [Google Scholar] [CrossRef] [PubMed]
- Shirzad, M.; Beshkar, P.; Heidarian, E. The effects of hesperetin on apoptosis induction andinhibition of cell proliferation in the prostate cancer PC3 cells. J. HerbMed. Pharmacol. 2015, 4, 121–124. [Google Scholar]
- Kuwajerwala, N.; Cifuentes, E.; Gautam, S.; Menon, M.; Barrack, E.R.; Reddy, G.P. Resveratrol induces prostate cancer cell entry into s phase and inhibits DNA synthesis. Cancer Res. 2002, 62, 2488–2492. [Google Scholar] [PubMed]
- Rizzi, F.; Naponelli, V.; Silva, A.; Modernelli, A.; Ramazzina, I.; Bonacini, M.; Tardito, S.; Gatti, R.; Uggeri, J.; Bettuzzi, S. Polyphenon E(R), a standardized green tea extract, induces endoplasmic reticulum stress, leading to death of immortalized PNT1a cells by anoikis and tumorigenic PC3 by necroptosis. Carcinogenesis 2014, 35, 828–839. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; El-Deiry, W.S. Overview of cell death signaling pathways. Cancer Biol. Ther. 2005, 4, 139–163. [Google Scholar] [CrossRef] [PubMed]
- Nagata, S. Apoptosis and Clearance of Apoptotic Cells. Annu Rev. Immunol. 2018, 36, 489–517. [Google Scholar] [CrossRef] [PubMed]
- Portt, L.; Norman, G.; Clapp, C.; Greenwood, M.; Greenwood, M.T. Anti-apoptosis and cell survival: A review. Biochim. Biophys. Acta 2011, 1813, 238–259. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.S. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Gupta, S. Apigenin-induced prostate cancer cell death is initiated by reactive oxygen species and p53 activation. Free Radic Biol. Med. 2008, 44, 1833–1845. [Google Scholar] [CrossRef] [PubMed]
- Oishi, M.; Iizumi, Y.; Taniguchi, T.; Goi, W.; Miki, T.; Sakai, T. Apigenin sensitizes prostate cancer cells to Apo2L/TRAIL by targeting adenine nucleotide translocase-2. PLoS ONE 2013, 8, e55922. [Google Scholar] [CrossRef] [PubMed]
- Piantino, C.B.; Salvadori, F.A.; Ayres, P.P.; Kato, R.B.; Srougi, V.; Leite, K.R.; Srougi, M. An evaluation of the anti-neoplastic activity of curcumin in prostate cancer cell lines. Int. Braz. J. Urol. 2009, 35, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.C.; Cullen, S.P.; Martin, S.J. Apoptosis: Controlled demolition at the cellular level. Nat. Rev. Mol. Cell Biol. 2008, 9, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Hastak, K.; Gupta, S.; Ahmad, N.; Agarwal, M.K.; Agarwal, M.L.; Mukhtar, H. Role of p53 and NF-kappaB in epigallocatechin-3-gallate-induced apoptosis of LNCaP cells. Oncogene 2003, 22, 4851–4859. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Chung, J. Synergistic cell death by EGCG and ibuprofen in DU-145 prostate cancer cell line. Anticancer Res. 2007, 27, 3947–3956. [Google Scholar] [PubMed]
- Yang, J.; Yu, H.; Sun, S.; Zhang, L.; Das, U.N.; Ruan, H.; He, G.; Shen, S. Mechanism of free Zn(2+) enhancing inhibitory effects of EGCG on the growth of PC-3 cells: Interactions with mitochondria. Biol. Trace Elem. Res. 2009, 131, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.L.; He, G.Q.; Yu, H.N.; Yang, J.G.; Borthakur, D.; Zhang, L.C.; Shen, S.R.; Das, U.N. Free Zn(2+) enhances inhibitory effects of EGCG on the growth of PC-3 cells. Mol. Nutr. Food Res. 2008, 52, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, J.; Wei, T.; Ma, X.; Cheng, Q.; Huo, S.; Zhang, C.; Zhang, Y.; Duan, X.; Liang, X.J. Quercetin-loaded nanomicelles to circumvent human castration-resistant prostate cancer in vitro and in vivo. Nanoscale 2016, 8, 5126–5138. [Google Scholar] [CrossRef] [PubMed]
- Valastyan, S.; Weinberg, R.A. Tumor metastasis: Molecular insights and evolving paradigms. Cell 2011, 147, 275–292. [Google Scholar] [CrossRef] [PubMed]
- Leber, M.F.; Efferth, T. Molecular principles of cancer invasion and metastasis (review). Int. J. Oncol. 2009, 34, 881–895. [Google Scholar] [PubMed]
- Kalluri, R. EMT: When epithelial cells decide to become mesenchymal-like cells. J. Clin. Invest. 2009, 119, 1417–1419. [Google Scholar] [CrossRef] [PubMed]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Chen, S.; Xu, L.; Liu, Y.; Deb, D.K.; Platanias, L.C.; Bergan, R.C. Genistein inhibits p38 map kinase activation, matrix metalloproteinase type 2, and cell invasion in human prostate epithelial cells. Cancer Res. 2005, 65, 3470–3478. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sarkar, F.H. Down-regulation of invasion and angiogenesis-related genes identified by cDNA microarray analysis of PC3 prostate cancer cells treated with genistein. Cancer Lett. 2002, 186, 157–164. [Google Scholar] [CrossRef]
- Xu, L.; Bergan, R.C. Genistein inhibits matrix metalloproteinase type 2 activation and prostate cancer cell invasion by blocking the transforming growth factor beta-mediated activation of mitogen-activated protein kinase-activated protein kinase 2-27-kDa heat shock protein pathway. Mol. Pharmacol. 2006, 70, 869–877. [Google Scholar] [PubMed]
- Zhou, J.R.; Yu, L.; Zhong, Y.; Nassr, R.L.; Franke, A.A.; Gaston, S.M.; Blackburn, G.L. Inhibition of orthotopic growth and metastasis of androgen-sensitive human prostate tumors in mice by bioactive soybean components. Prostate 2002, 53, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Welti, J.; Loges, S.; Dimmeler, S.; Carmeliet, P. Recent molecular discoveries in angiogenesis and antiangiogenic therapies in cancer. J. Clin Invest. 2013, 123, 3190–3200. [Google Scholar] [CrossRef] [PubMed]
- Potente, M.; Gerhardt, H.; Carmeliet, P. Basic and therapeutic aspects of angiogenesis. Cell 2011, 146, 873–887. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Dabrosin, C.; Yin, X.; Fuster, M.M.; Arreola, A.; Rathmell, W.K.; Generali, D.; Nagaraju, G.P.; El-Rayes, B.; Ribatti, D.; et al. Broad targeting of angiogenesis for cancer prevention and therapy. Semin Cancer Biol. 2015, 35 Suppl, S224–s243. [Google Scholar] [CrossRef]
- Bergers, G.; Hanahan, D. Modes of resistance to anti-angiogenic therapy. Nat. Rev. Cancer 2008, 8, 592–603. [Google Scholar] [CrossRef] [PubMed]
- McLarty, J.; Bigelow, R.L.; Smith, M.; Elmajian, D.; Ankem, M.; Cardelli, J.A. Tea polyphenols decrease serum levels of prostate-specific antigen, hepatocyte growth factor, and vascular endothelial growth factor in prostate cancer patients and inhibit production of hepatocyte growth factor and vascular endothelial growth factor in vitro. Cancer Prev. Res. 2009, 2, 673–682. [Google Scholar]
- Pratheeshkumar, P.; Budhraja, A.; Son, Y.O.; Wang, X.; Zhang, Z.; Ding, S.; Wang, L.; Hitron, A.; Lee, J.C.; Xu, M.; et al. Quercetin inhibits angiogenesis mediated human prostate tumor growth by targeting VEGFR- 2 regulated AKT/mTOR/P70S6K signaling pathways. PLoS ONE 2012, 7, e47516. [Google Scholar] [CrossRef] [PubMed]
- Cantley, L.C.; Auger, K.R.; Carpenter, C.; Duckworth, B.; Graziani, A.; Kapeller, R.; Soltoff, S. Oncogenes and signal transduction. Cell 1991, 64, 281–302. [Google Scholar] [CrossRef]
- Mol, P.R.; Balan, A. Oncogenes as Therapeutic Targets in Cancer: A Review. IOSR J. Dent. Med Sci. 2013, 5, 46–56. [Google Scholar] [CrossRef]
- Felsher, D.W. Cancer revoked: Oncogenes as therapeutic targets. Nat. Rev. Cancer 2003, 3, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, I.B.; Joe, A. Oncogene addiction. Cancer Res. 2008, 68, 3077–3080. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhou, X.; Qi, G.; Guo, Y. Curcumin suppressed the prostate cancer by inhibiting JNK pathways via epigenetic regulation. J. Biochem. Mol. Toxicol. 2018, 32, e22049. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.Y.; Muller, W.J. Oncogenes and tumor suppressor genes. Cold Spring Harb. Perspect. Biol. 2010, 2, a003236. [Google Scholar] [CrossRef] [PubMed]
- Marshall, C.J. Tumor suppressor genes. Cell 1991, 64, 313–326. [Google Scholar] [CrossRef]
- Weinhold, B. Epigenetics: The science of change. Env. Health Perspect 2006, 114, A160–A167. [Google Scholar] [CrossRef] [PubMed]
- Mazzio, E.A.; Soliman, K.F. Basic concepts of epigenetics: Impact of environmental signals on gene expression. Epigenetics 2012, 7, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Ho, E.; Beaver, L.M.; Williams, D.E.; Dashwood, R.H. Dietary factors and epigenetic regulation for prostate cancer prevention. Adv. Nutr. 2011, 2, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.Y.; Kim, B.H.; Choi, M.; Yoo, E.J.; Moon, K.C.; Cho, Y.M.; Kim, D.; Kang, G.H. Hypermethylation of CpG island loci and hypomethylation of LINE-1 and Alu repeats in prostate adenocarcinoma and their relationship to clinicopathological features. J. Pathol. 2007, 211, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Marcu, M.G.; Jung, Y.J.; Lee, S.; Chung, E.J.; Lee, M.J.; Trepel, J.; Neckers, L. Curcumin is an inhibitor of p300 histone acetylatransferase. Med. Chem. 2006, 2, 169–174. [Google Scholar] [PubMed]
- Fang, M.Z.; Chen, D.; Sun, Y.; Jin, Z.; Christman, J.K.; Yang, C.S. Reversal of hypermethylation and reactivation of p16INK4a, RARbeta, and MGMT genes by genistein and other isoflavones from soy. Clin. Cancer Res. 2005, 11, 7033–7041. [Google Scholar] [CrossRef] [PubMed]
- Majid, S.; Dar, A.A.; Saini, S.; Chen, Y.; Shahryari, V.; Liu, J.; Zaman, M.S.; Hirata, H.; Yamamura, S.; Ueno, K.; et al. Regulation of minichromosome maintenance gene family by microRNA-1296 and genistein in prostate cancer. Cancer Res. 2010, 70, 2809–2818. [Google Scholar] [CrossRef] [PubMed]
- Vardi, A.; Bosviel, R.; Rabiau, N.; Adjakly, M.; Satih, S.; Dechelotte, P.; Boiteux, J.P.; Fontana, L.; Bignon, Y.J.; Guy, L.; et al. Soy phytoestrogens modify DNA methylation of GSTP1, RASSF1A, EPH2 and BRCA1 promoter in prostate cancer cells. In Vivo 2010, 24, 393–400. [Google Scholar] [PubMed]
- Kinney, S.R.M.; Zhang, W.; Pascual, M.; Greally, J.M.; Gillard, B.M.; Karasik, E.; Foster, B.A.; Karpf, A.R. Lack of evidence for green tea polyphenols as DNA methylation inhibitors in murine prostate. Cancer Prev. Res. 2009, 1940–6207. [Google Scholar]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- Wightman, B.; Ha, I.; Ruvkun, G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 1993, 75, 855–862. [Google Scholar] [CrossRef]
- Hayes, J.; Peruzzi, P.P.; Lawler, S. MicroRNAs in cancer: Biomarkers, functions and therapy. Trends Mol. Med. 2014, 20, 460–469. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Vanacore, D.; Boccellino, M.; Rossetti, S.; Cavaliere, C.; D’Aniello, C.; Di Franco, R.; Romano, F.J.; Montanari, M.; La Mantia, E.; Piscitelli, R.; et al. Micrornas in prostate cancer: An overview. Oncotarget 2017, 8, 50240–50251. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.Q.; Liu, M.; Li, W.; Che, J.P.; Wang, G.C.; Zheng, J.H. Combination of quercetin and hyperoside inhibits prostate cancer cell growth and metastasis via regulation of microRNA21. Mol. Med. Rep. 2015, 11, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Xing, N.; Chen, Y.; Mitchell, S.H.; Young, C.Y. Quercetin inhibits the expression and function of the androgen receptor in LNCaP prostate cancer cells. Carcinogenesis 2001, 22, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Young, C.Y.; Tian, Y.; Liu, Z.; Zhang, M.; Lou, H. Suppression of the androgen receptor function by quercetin through protein-protein interactions of Sp1, c-Jun, and the androgen receptor in human prostate cancer cells. Mol. Cell Biochem. 2010, 339, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Bektic, J.; Berger, A.P.; Pfeil, K.; Dobler, G.; Bartsch, G.; Klocker, H. Androgen receptor regulation by physiological concentrations of the isoflavonoid genistein in androgen-dependent LNCaP cells is mediated by estrogen receptor beta. Eur. Urol. 2004, 45, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Fritz, W.A.; Wang, J.; Eltoum, I.E.; Lamartiniere, C.A. Dietary genistein down-regulates androgen and estrogen receptor expression in the rat prostate. Mol. Cell Endocrinol. 2002, 186, 89–99. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary Intake and Bioavailability of Polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.L.; Shah, V.; Patnaik, R.; Adams, W.; Hussain, A.; Conner, D.; Mehta, M.; Malinowski, H.; Lazor, J.; Huang, S.M.; et al. Bioavailability and Bioequivalence: An FDA Regulatory Overview. Pharm. Res. 2001, 18, 1645–1650. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, M.; Filesi, C.; Vari, R.; Scazzocchio, B.; Masella, R. Bioavailability of the polyphenols: Status and controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef] [PubMed]
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; da Silva Pinto, M. Bioavailability of bioactive food compounds: A challenging journey to bioefficacy. Br. J. Clin. Pharmacol. 2013, 75, 588–602. [Google Scholar] [CrossRef] [PubMed]
- Velderrain-Rodriguez, G.R.; Palafox-Carlos, H.; Wall-Medrano, A.; Ayala-Zavala, J.F.; Chen, C.Y.; Robles-Sanchez, M.; Astiazaran-Garcia, H.; Alvarez-Parrilla, E.; Gonzalez-Aguilar, G.A. Phenolic compounds: Their journey after intake. Food Funct. 2014, 5, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [PubMed]
- Chiou, Y.-S.; Wu, J.-C.; Huang, Q.; Shahidi, F.; Wang, Y.-J.; Ho, C.-T.; Pan, M.-H. Metabolic and colonic microbiota transformation may enhance the bioactivities of dietary polyphenols. J. Funct. Foods 2014, 7, 3–25. [Google Scholar] [CrossRef]
- Li, Y.; Paxton, J.W. Oral Bioavailability and Disposition of Phytochemicals. In Phytochemicals—Bioactivities and Impact on Health; Rasooli, I., Ed.; InTech Europe: Rijeka, Croatia, 2011; pp. 118–138. [Google Scholar]
- Monagas, M.; Urpi-Sarda, M.; Sánchez-Patán, F.; Llorach, R.; Garrido, I.; Gómez-Cordovés, C.; Andres-Lacueva, C.; Bartolomé, B.J.F. Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites. Food Funct. 2010, 1, 233–253. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.R.; Andreadi, C.; Britton, R.G.; Horner-Glister, E.; Karmokar, A.; Sale, S.; Brown, V.A.; Brenner, D.E.; Singh, R.; Steward, W.P. Sulfate metabolites provide an intracellular pool for resveratrol generation and induce autophagy with senescence. Sci. Transl. Med. 2013, 5, 205ra133. [Google Scholar] [CrossRef] [PubMed]
- Vicinanza, R.; Zhang, Y.; Henning, S.M.; Heber, D. Pomegranate juice metabolites, ellagic acid and urolithin a, synergistically inhibit androgen-independent prostate cancer cell growth via distinct effects on cell cycle control and apoptosis. Evid. -Based Complementary Altern. Med. 2013, 2013, 247504. [Google Scholar] [CrossRef] [PubMed]
- Espín, J.C.; Larrosa, M.; García-Conesa, M.T.; Tomás-Barberán, F. Biological significance of urolithins, the gut microbial ellagic acid-derived metabolites: The evidence so far. Evid. -Based Complementary Altern. Med. 2013, 2013, 270418. [Google Scholar] [CrossRef] [PubMed]
- Henning, S.M.; Wang, P.; Carpenter, C.L.; Heber, D. Epigenetic effects of green tea polyphenols in cancer. Epigenomics 2013, 5, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Braune, A.; Blaut, M. Bacterial species involved in the conversion of dietary flavonoids in the human gut. Gut Microbes 2016, 7, 216–234. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed]
- Gaya, P.; Medina, M.; Sanchez-Jimenez, A.; Landete, J.M. Phytoestrogen Metabolism by Adult Human Gut Microbiota. Molecules 2016, 21, 1034. [Google Scholar] [CrossRef] [PubMed]
- Marin, L.; Miguelez, E.M.; Villar, C.J.; Lombo, F. Bioavailability of dietary polyphenols and gut microbiota metabolism: Antimicrobial properties. Biomed. Res. Int. 2015, 2015, 905215. [Google Scholar] [CrossRef] [PubMed]
- Ou, K.; Gu, L. Absorption and metabolism of proanthocyanidins. J. Funct. Foods 2014, 7, 43–53. [Google Scholar] [CrossRef]
- Romo-Vaquero, M.; García-Villalba, R.; González-Sarrías, A.; Beltrán, D.; Tomás-Barberán, F.A.; Espín, J.C.; Selma, M.V. Interindividual variability in the human metabolism of ellagic acid: Contribution of Gordonibacter to urolithin production. J. Funct. Foods 2015, 17, 785–791. [Google Scholar] [CrossRef]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Selma, M.V.; Beltrán, D.; García-Villalba, R.; Espín, J.C.; Tomás-Barberán, F.A. Description of urolithin production capacity from ellagic acid of two human intestinal Gordonibacter species. Food Funct. 2014, 5, 1779–1784. [Google Scholar] [CrossRef] [PubMed]
- Kapetanovic, I.M.; Muzzio, M.; Huang, Z.; Thompson, T.N.; McCormick, D.L. Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats. Cancer Chemother. Pharmacol. 2011, 68, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Stanisławska, I.J.; Granica, S.; Piwowarski, J.P.; Szawkało, J.; Wiązecki, K.; Czarnocki, Z.; Kiss, A.K. The Activity of Urolithin A and M4 Valerolactone, Colonic Microbiota Metabolites of Polyphenols, in a Prostate Cancer In Vitro Model. Planta Medica 2018, 85, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Vanella, L.; Di Giacomo, C.; Acquaviva, R.; Barbagallo, I.; Li Volti, G.; Cardile, V.; Abraham, N.G.; Sorrenti, V. Effects of ellagic acid on angiogenic factors in prostate cancer cells. Cancers 2013, 5, 726–738. [Google Scholar] [CrossRef] [PubMed]
- McCann, M.J.; Rowland, I.R.; Roy, N.C. The Anti-Proliferative Effects of Enterolactone in Prostate Cancer Cells: Evidence for the Role of DNA Licencing Genes, mi-R106b Cluster Expression, and PTEN Dosage. Nutrients 2014, 6, 4839–4855. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-Y.; Cui, J.; Zhang, Y.; Wang, Z.-L.; Chong, T.; Wang, Z.-M. Isoflavones and prostate cancer: A review of some critical issues. Chin. Med. J. 2016, 129, 341. [Google Scholar] [CrossRef] [PubMed]
- Frankenfeld, C.L. O-Desmethylangolensin: The Importance of Equol’s Lesser Known Cousin to Human Health. Adv. Nutr. 2011, 2, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Hamilton-Reeves, J.M.; Banerjee, S.; Banerjee, S.K.; Holzbeierlein, J.M.; Thrasher, J.B.; Kambhampati, S.; Keighley, J.; Van Veldhuizen, P. Short-term soy isoflavone intervention in patients with localized prostate cancer: A randomized, double-blind, placebo-controlled trial. PLoS ONE 2013, 8, e68331. [Google Scholar] [CrossRef] [PubMed]
- Piccolella, M.; Crippa, V.; Messi, E.; Tetel, M.J.; Poletti, A. Modulators of estrogen receptor inhibit proliferation and migration of prostate cancer cells. Pharmacol. Res. 2014, 79, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Liu, X.; Zhang, C.; Zeng, X. Food macromolecule based nanodelivery systems for enhancing the bioavailability of polyphenols. J. Food Drug Anal. 2017, 25, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Jäger, R.; Lowery, R.P.; Calvanese, A.V.; Joy, J.M.; Purpura, M.; Wilson, J.M. Comparative absorption of curcumin formulations. Nutr. J. 2014, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Lila, M.A.; Burton-Freeman, B.; Grace, M.; Kalt, W. Unraveling Anthocyanin Bioavailability for Human Health. Annu. Rev. Food Sci. Technol. 2016, 7, 375–393. [Google Scholar] [CrossRef] [PubMed]
- Petersen, B.; Egert, S.; Bosy-Westphal, A.; Muller, M.J.; Wolffram, S.; Hubbermann, E.M.; Rimbach, G.; Schwarz, K. Bioavailability of quercetin in humans and the influence of food matrix comparing quercetin capsules and different apple sources. Food Res. Int. 2016, 88, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Barnett, C.F.; Moreno-Ulloa, A.; Shiva, S.; Ramirez-Sanchez, I.; Taub, P.R.; Su, Y.; Ceballos, G.; Dugar, S.; Schreiner, G.; Villarreal, F. Pharmacokinetic, partial pharmacodynamic and initial safety analysis of (−)-epicatechin in healthy volunteers. Food Funct. 2015, 6, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; van der Hooft, J.J.; Crozier, A. Human studies on the absorption, distribution, metabolism, and excretion of tea polyphenols. Am. J. Clin. Nutr. 2013, 98, 1619S–1630S. [Google Scholar] [CrossRef] [PubMed]
- Holt, R.R.; Lazarus, S.A.; Sullards, M.C.; Zhu, Q.Y.; Schramm, D.D.; Hammerstone, J.F.; Fraga, C.G.; Schmitz, H.H.; Keen, C.L. Procyanidin dimer B2 [epicatechin-(4β-8)-epicatechin] in human plasma after the consumption of a flavanol-rich cocoa. Am. J. Clin. Nutr. 2002, 76, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Serra, A.; Macià, A.; Rubió, L.; Anglès, N.; Ortega, N.; Morelló, J.R.; Romero, M.-P.; Motilva, M.-J. Distribution of procyanidins and their metabolites in rat plasma and tissues in relation to ingestion of procyanidin-enriched or procyanidin-rich cocoa creams. Eur. J. Nutr. 2013, 52, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, I.A.; Mena, P.; Calani, L.; Borges, G.; Pereira-Caro, G.; Bresciani, L.; Del Rio, D.; Lean, M.E.; Crozier, A. New insights into the bioavailability of red raspberry anthocyanins and ellagitannins. Free Radic. Biol. Med. 2015, 89, 758–769. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Zhang, Y.; McKeever, R.; Henning, S.M.; Lee, R.-P.; Suchard, M.A.; Li, Z.; Chen, S.; Thames, G.; Zerlin, A. Pomegranate juice and extracts provide similar levels of plasma and urinary ellagitannin metabolites in human subjects. J. Med. Food 2008, 11, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Sergides, C.; Chirilă, M.; Silvestro, L.; Pitta, D.; Pittas, A. Bioavailability and safety study of resveratrol 500 mg tablets in healthy male and female volunteers. Exp. Ther. Med. 2016, 11, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Zick, S.M.; Djuric, Z.; Ruffin, M.T.; Litzinger, A.J.; Normolle, D.P.; Alrawi, S.; Feng, M.R.; Brenner, D.E. Pharmacokinetics of 6-gingerol, 8-gingerol, 10-gingerol, and 6-shogaol and conjugate metabolites in healthy human subjects. Cancer Epidemiol. Prev. Biomark. 2008, 17, 1930–1936. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.; Brown, N.M.; Zimmer-Nechemias, L.; Wolfe, B.; Jha, P.; Heubi, J.E.J.F. Metabolism of secoisolariciresinol-diglycoside the dietary precursor to the intestinally derived lignan enterolactone in humans. Food Funct. 2014, 5, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Aqil, F.; Munagala, R.; Jeyabalan, J.; Vadhanam, M.V. Bioavailability of phytochemicals and its enhancement by drug delivery systems. Cancer Lett. 2013, 334, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Puligundla, P.; Mok, C.; Ko, S.; Liang, J.; Recharla, N. Nanotechnological approaches to enhance the bioavailability and therapeutic efficacy of green tea polyphenols. J. Funct. Foods 2017, 34, 139–151. [Google Scholar] [CrossRef]
- Altamimi, M.A.; Elzayat, E.M.; Alshehri, S.M.; Mohsin, K.; Ibrahim, M.A.; Al Meanazel, O.T.; Shakeel, F.; Alanazi, F.K.; Alsarra, I.A. Utilizing spray drying technique to improve oral bioavailability of apigenin. Adv. Powder Technol. 2018, 29, 1676–1684. [Google Scholar] [CrossRef]
- de Vries, K.; Strydom, M.; Steenkamp, V. Bioavailability of resveratrol: Possibilities for enhancement. J. Herb. Med. 2018, 11, 71–77. [Google Scholar] [CrossRef]
- Bilia, A.R.; Isacchi, B.; Righeschi, C.; Guccione, C.; Bergonzi, M.C. Flavonoids loaded in nanocarriers: An opportunity to increase oral bioavailability and bioefficacy. Food Nutr. Sci. 2014, 5, 1212. [Google Scholar] [CrossRef]
- Ozkan, G.; Franco, P.; De Marco, I.; Xiao, J.; Capanoglu, E. A review of microencapsulation methods for food antioxidants: Principles, advantages, drawbacks and applications. Food Chem. 2019, 272, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Su, R.; Nie, S.; Sun, M.; Zhang, J.; Wu, D.; Moustaid-Moussa, N. Application of nanotechnology in improving bioavailability and bioactivity of diet-derived phytochemicals. J. Nutr. Biochem. 2014, 25, 363–376. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Misra, R.; Sahoo, S.K. Nanoparticles: A boon to drug delivery, therapeutics, diagnostics and imaging. Nanomedicine 2012, 8, 147–166. [Google Scholar] [CrossRef] [PubMed]
- Neves, A.R.; Lúcio, M.; Martins, S.; Lima, J.L.C.; Reis, S. Novel resveratrol nanodelivery systems based on lipid nanoparticles to enhance its oral bioavailability. Int. J. Nanomed. 2013, 8, 177. [Google Scholar]
- Dang, H.; Meng, M.H.W.; Zhao, H.; Iqbal, J.; Dai, R.; Deng, Y.; Lv, F. Luteolin-loaded solid lipid nanoparticles synthesis, characterization, & improvement of bioavailability, pharmacokinetics in vitro and vivo studies. J. Nanoparticle Res. 2014, 16, 2347. [Google Scholar]
- Kim, B.-K.; Cho, A.-R.; Park, D.-J. Enhancing oral bioavailability using preparations of apigenin-loaded W/O/W emulsions: In vitro and in vivo evaluations. Food Chem. 2016, 206, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Bi, C.; Chan, H.M.; Sun, S.; Zhang, Q.; Zheng, Y. Curcumin-loaded solid lipid nanoparticles have prolonged in vitro antitumour activity, cellular uptake and improved in vivo bioavailability. Colloids Surf. B 2013, 111, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Pinho, E.; Grootveld, M.; Soares, G.; Henriques, M. Cyclodextrins as encapsulation agents for plant bioactive compounds. Carbohydr. Polym. 2014, 101, 121–135. [Google Scholar] [CrossRef] [PubMed]
| Phenolic Compounds | Representative Compounds | Chemical Structure | Dietary Sources | Ref. |
| FLAVONOIDS | ||||
| Flavones | apigenin | 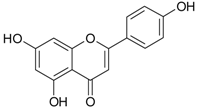 | oranges, lemons, apricots, apples, black currants, bananas, potatoes, spinach, onions, lettuce, beans, cereals | [53,54] |
| Flavonols | quercetin | 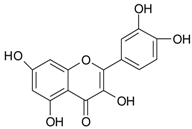 | ||
| Flavan-3-ols | epigallocatechin gallate (EGCG) |  | green/black tea | [53,54] |
| Isoflavones | genistein | 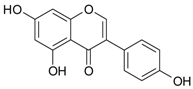 | soy milk, tofu, nattō | [30,55] |
| Anthocyanins | cyanidin |  | plums, grapes, elderberries, cherries | [55] |
| Proantho-cyanidins | proantho- cyanidin B |  | cranberries, grapes, walnuts, rice | [53] |
| NON-FLAVONOID | ||||
| Hydroxy-benzoic acids | gallic acid | 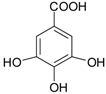 | blackcurrants, strawberries, raspberries, kiwi, cherry, plums | [30,55,56,57] |
| Hydroxy-cinnamic acids | caffeic acid phenethyl ester |  | artichoke, oregano, thyme, basil, coffee, mushrooms, medicinal plants | [55] |
| Ellagitannins (ellagic acid derivatives) | punicalagin | 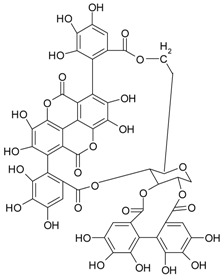 | berry fruits pomegranate | [58,59] |
| Stilbens | resveratrol | 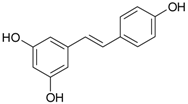 | grapes, mulberries | [60] |
| Lignans | secoisolarici-resinol | 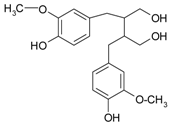 | sesame, flaxseeds | [61] |
| Other compounds | curcumin | 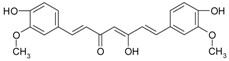 | Curcuma roots | [62] |
| gingerol | 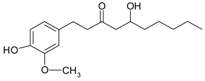 | fresh/dried ginger rhizomes | [63] | |
| Cell Type | Characteristics | References |
|---|---|---|
| Non-transformed prostate cell lines | ||
| PrEC | Normal prostate epithelial cells | [71,72] |
| RWPE-1 | Non-malignant epithelial prostate cell line | [73] |
| PCa cell lines | ||
| LNCaP | Androgen-responsive human prostate adenocarcinoma cell line; secrete PSA; low tumorigenicity in nude mice; have a mutated AR at T877A | [70,74,75] |
| PC-3 | Androgen-independent human prostate adenocarcinoma cell line; obtained from bone metastasis of prostate adenocarcinoma | [70,76,77] |
| PC-3M | Metastatic androgen-independent human prostate adenocarcinoma cell line | [78] |
| PC-3M-MM2 | Highly invasive androgen-independent human prostate adenocarcinoma cell line | [79] |
| DU-145 | Androgen-independent human prostate adenocarcinoma cell line; metastatic cell line isolated from brain | [80] |
| 22Rv1 | Castration-resistant PCa cell line with hyper-diploid DNA (50 chromosomes); 22Rv1 cells express PSA | [81] |
| prostate CSC | CSC isolated from PC-3 cells positive for CD44+ | [82] |
| CxR | Castration-resistant PCa cell line to cabazitaxel treatment (inhibitor of microtubule activity) | [83] |
| LAPC-4 | Androgen-responsive human prostate adenocarcinoma cell line; established from lymph node metastasis in xenograft models from patients with advance disease | [84] |
| WPE1-NB14, WPE1-NB11, WPE-NA22 | Cell lines of prostate adenocarcinoma; contain DNA from human papilloma virus 18 | [85] |
| C4-2, C4-2B | Metastatic androgen-independent human prostate sublines derived from LNCaP cells; able to develop bone metastasis | [86] |
| Cellular Effect | Polyphenol | Molecular Target | Cell Line/ Animal Model/ Clinical Trial | References |
|---|---|---|---|---|
| Antioxidant effect | Quercetin | ↓ROS ↑SOD, ↑CAT, ↑GPx, ↑GSR | Sprague‒Dawley rats | [100] |
| Genistein | ↑GPx | LNCaP, PC-3 cell lines | [99] | |
| EGGC | ↑SOD | DU-145 cell line | [101,102] | |
| Pro-oxidant effect | Apigenin | ↑ROS | 22Rv1 cell line | [150] |
| Quercetin | ↑ROS | DU-145 cell line | [97] | |
| Androgen and estrogen receptors | Quercetin | ↓AR | LNCaP cell line | [106,195,196] |
| Genistein | ↓AR (high doses of genistein) in correlation with ↓HSP90 | Sprague‒Dawley rats LNCaP cell line | [66,107,197,198] | |
| ↓ERα | ‒-Dawley rats | [198] | ||
| ↑AR (physiological doses of genistein) | PC-3 cells transfected with T877A-AR | [107] | ||
| Resveratrol | ↓AR | LNCaP cell line HeLa cells transfected with human AR | [108,109,110,111,112] | |
| ↓ERα | PC-3 cell line | [108] | ||
| EGCG | ↓AR, ↓ mRNA for AR | 22Rv1 tumor xenograft in nude mice | [113] | |
| Growth factors and cytokines receptors | Apigenin | ↓IGF-1 | TRAMP mice | [117] |
| Resveratrol | ↓EGFR, ↓HER2 | LNCaP, C4-2 cell lines | [118] | |
| Curcumin | ↓CXCL-1, -2 ↓EGFR (Tyr 845, Tyr 1068) | PC-3 cell line | [119,120] | |
| EGCG | ↓c-Met/HGF (Tyr1234/1235) | DU-145 cell line | [121] | |
| Signal transduction | Apigenin | ↓PI3K, ↓p-Akt (Ser473, Thr308), ↓ERK1/2, ↓p-FoxO (Ser253), ↓NF-κB | 22Rv1 TRAMP mice Prostate CSC (CD44+) isolated from PC-3 cells | [82,117,125,150] |
| CAPE | ↓ERK1/2, ↓p-Akt (Ser473), ↓p-mTOR (Ser2448, Ser24981), ↓p-GSK3α (Ser21), ↓p-GSK3β (Ser9), ↓p-PDK1 (Ser241) | LNCap, DU-145, PC-3 cell lines | [130,131] | |
| Curcumin | ↓NF-κB ↓p-PI3K, ↓p-Akt (Ser 473, Thr 208), ↓p-IκB | LNCaP, PC-3 cell lines | [119,120,132] | |
| Gallic acid | ↓SOS, ↓GRB2, ↓PKC, ↓NF-κB, ↓JNK, ↓ERK1/2, ↓p38-MAPK, ↓p-Akt | LNCaP, DU-145, PC-3 cell lines | [95] | |
| Gingerol | ↓MRP1 | PC-3 cell line | [134] | |
| EGCG | ↓NF-κB, ↓ERK1/2, ↓p-Akt | DU-145 cell line | [90,121] | |
| Resveratrol | ↓PI3K, ↓Akt, ↓GSK-3, ↑PTEN | LNCaP, PC-3 cell lines TRAMP mice | [108,118] | |
| Cell cycle | Apigenin | ↓cyclin D1 Arrest in G0/G1 or G2/M phase | LNCaP, PC-3 cell lines TRAMP mice | [125,139] |
| CAPE | ↓cyclin D1 ↓cyclin E | PC-3 cell line | [130] | |
| Gallic acid | G2/M arrest ↓cdc25 ↑CHK1, CHK2 | LNCaP, DU-145, PC-3 cell lines | [95] | |
| Gingerol | ↓cyclin D1, ↓cyclin E ↓CDK4 | Normal prostate epithelial cells (PrEC) PCa cell lines: LNCaP, DU-145, PC-3, C4-2, C4-2B | [71] | |
| EGCG | G0/G1 arrest or G2/M arrest—cell-line-dependent | LNCaP, DU-145, PC-3 cell lines | [144] | |
| Quercetin | G0/G1 arrest ↓CDK2, ↓cyclin E ↓cyclin D | PC-3 cell lines | [68] | |
| Apoptosis | Apigenin | ↑caspase-3, -8 ↓ΔΨm ↑cytochrome c ↓Bcl-2, ↓Bcl-XL ↑Bax ↑TRAIL, ↑DG5 | 22Rv1, PC-3 (p53-/-), PC-3(p53+/+) cell lines Prostate CSC (CD44+) isolated from PC-3 cells | [82,150,151] |
| Gallic acid | ↑cytochrome c ↑caspase-3, -8, -9 | LNCaP cell line | [96] | |
| Gingerol | ↑caspase-3, ↑PARP ↓Bcl-2 | Normal prostate epithelial cells (PrEC) PCa cell lines: LNCaP, DU-145, PC-3, C4-2, C4-2B | [71] | |
| EGCG | ↓Bcl-2, ↑Bax ↑caspase-3, -8, -9 ↑PARP ↑CHOP/GADD153 | LNCaP, DU-145, PC-3 cell lines | [144,154] | |
| Quercetin | ↓Bcl-2, ↑Bax ↑caspase-3, -8, -9 ↑ATF ↑GRP78 ↑GADD153 | PC-3 cell line | [68,158] | |
| Invasion and metastasis | Apigenin | ↓uPA, ↓MMP-2, ↓MMP-9 ↑E-cadherin ↓vimentin | DU-145 cell line TRAMP mice | [117,139] |
| EGCG, gallic acid, genistein | ↓MMPs | PC-3, DU-145 | [90,133,163,164,165] | |
| Angiogenesis | apigenin, genistein, quercetin, EGCG | ↓VEGF | PC-3 TRAMP mice Men of ages 18 to 75 years with PCa | [117,164,171,172] |
| Oncogenes and the coding proteins | Curcumin, EGCG | ↓c-Jun (Ser73) | LNCaP, DU-145 cell lines | [90,177] |
| Tumor suppressor genes and the coding proteins | Apigenin | ↑p53 ↑p27/Kip1 ↑p21/CIP1 | 22Rv1, LNCaP, PC-3 cell lines TRAMP mice | [125,150] |
| Curcumin | ↑p53 | PC-3 cell line | [119] | |
| EGCG | ↑p53, ↑p21/CIP1 | LNCaP cell line | [154] | |
| DNA methylation and histone modification | Curcumin | ↓p300-HAT ↓H3 acetylation | PC-3M cell line | [184] |
| Genistein | ↓DNA methylation of RARβ ↓BTG3 gene methylation | LNCaP, PC-3 cell lines | [185,186] | |
| miRNA | EGCG | ↓oncogenic miR-21 ↑tumor suppressor miR-330 | LNCaP, 22Rv1 cell lines | [113] |
| Genistein | ↓oncogenic miR-151 ↑tumor suppressor miR-574-3p | LNCaP, PC-3, DU-145 PCa cell lines RWPE-1 non-malignant epithelial prostate cell line | [73] | |
| Resveratrol | ↓oncogenic miR-21 | Highly invasive PC-3M-MM2, DU-145, LNCaP cell lines | [79] |
| Polyphenols | Metabolite | Cell Lines/ Animal Model/ Clinical Trial | Cellular Mechanism | Molecular Target | Reference |
|---|---|---|---|---|---|
| Ellagitannins | UA, EA | DU-145, PC-3 cell lines | Cell cycle | G2/M phase ↑cyclin B1/ cdc2 phosphorylation (UA) S phase ↓cyclin B1, cyclin D1 (EA) | [209] |
| Ellagitannins | UAA, UAB | LNCaP, PC-3 cell lines | Apoptosis AR | ↓Bcl-2 ↓AR expression ↓PSA | [46] |
| Ellagitannins and green tea catechins | UAA and M4 | LNCaP cell line | AR | ↓AR expression ↓PSA | [221] |
| Ellagitannins | EA | LNCaP cell line | Angiogenesis | ↓OH1, OH2 ↓VEGF, ↓OPG | [222] |
| Lignans | EL, ET | Phase II randomized control trial in PCa men awaiting prostatectomy | Angiogenesis Inhibition of proliferation | ↓VEGF, ↓Ki-67 | [44] |
| EL | RWPE-1, WPE1-NA22, WPE1-NB14, WPE1-NB11, WPE1-NB26, LNCaP cell lines | miRNA, tumor suppressor genes | ↑PTEN, ↓miR-106b cluster | [223] | |
| Isoflavones | equol | PC-3, DU-145, LNCaP, CxR, 22Rv1 cell lines | Signal transduction AR | ↑Akt/FOXO3a, ↓AR through Skp2 pathway | [48,49] |
| O-DA | LNCaP, LAPC-4 cell lines | AR | ↓AR | [225] | |
| Antho- cyanins | PrA | LNCaP cell lines | Apoptosis Angiogenesis Anti-inflamma-tory effects | ↑caspase -3, ↓membrane mitochondrial potential, ↓VEGF, ↓IL-6, IL-8 | [43] |
| Flavan-3-ols, proanthocya- nidins | Hipp 3-Hppp 4-Hpa | LNCaP cell line | Cell cycle | ↓cyclin B1 | [207] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costea, T.; Nagy, P.; Ganea, C.; Szöllősi, J.; Mocanu, M.-M. Molecular Mechanisms and Bioavailability of Polyphenols in Prostate Cancer. Int. J. Mol. Sci. 2019, 20, 1062. https://doi.org/10.3390/ijms20051062
Costea T, Nagy P, Ganea C, Szöllősi J, Mocanu M-M. Molecular Mechanisms and Bioavailability of Polyphenols in Prostate Cancer. International Journal of Molecular Sciences. 2019; 20(5):1062. https://doi.org/10.3390/ijms20051062
Chicago/Turabian StyleCostea, Teodora, Péter Nagy, Constanța Ganea, János Szöllősi, and Maria-Magdalena Mocanu. 2019. "Molecular Mechanisms and Bioavailability of Polyphenols in Prostate Cancer" International Journal of Molecular Sciences 20, no. 5: 1062. https://doi.org/10.3390/ijms20051062
APA StyleCostea, T., Nagy, P., Ganea, C., Szöllősi, J., & Mocanu, M.-M. (2019). Molecular Mechanisms and Bioavailability of Polyphenols in Prostate Cancer. International Journal of Molecular Sciences, 20(5), 1062. https://doi.org/10.3390/ijms20051062